Abstract
Due to intense industrialization and urbanization, air pollution has become a serious global concern as a hazard to human health. Epidemiological studies found that exposure to atmospheric particulate matter (PM) causes severe health problems in human and significant damage to the physiological systems. In recent days, PM exposure could be related as a carrier for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus transmission and Coronavirus disease 2019 (COVID-19) infection. Hence, it is important to understand the adverse effects of PM in human health. This review aims to provide insights on the detrimental effects of PM in various human health problems including respiratory, circulatory, nervous, and immune system along with their possible toxicity mechanisms. Overall, this review highlights the potential relationship of PM with several life-limiting human diseases and their significance for better management strategies.
Keywords: Aerosols, Health effects, Molecular mechanism, Oxidative stress, Organ damage
1. Introduction
In the transitional stages of industrialization and urbanization, the world faces serious problems caused by air pollution. Particularly, atmospheric particulate matter (PM) is extremely harmful to the human health and environment. Epidemiological studies have revealed that exposure to PM pollution can adversely affect the human, while their higher concentrations cause more significant harms (Sanchez et al., 2020). Further, there is a strong relationship between PM and Coronavirus disease 2019 (COVID-19) infection (Amoatey et al., 2020; Setti et al., 2020). PM enters the human body through respiration and the large particulates are filtered by the nose and upper respiratory tract. But most small particulates are deposited in the lungs and some of which can be transferred into the blood and enters different organs, thereby causing damage to multiple physiological systems, especially the respiratory, cardiovascular and nervous systems (Forman and Finch, 2018).
2. Atmospheric particulate matter
Atmospheric particulate matter refers to the sum of all liquid or solid particles present in the atmospheric environment with a wide range of unit particle sizes and different physical and chemical properties. A variety of PM is evenly distributed in the atmosphere, forming a relatively stable and complex suspension called the aerosol. Hence, PM is also called atmospheric aerosol (Pan et al., 2019). Based on the particle size, PM can be divided into following types such as total suspended particles (TSP), coarse particulate matter (PM10), fine particulate matter (PM2.5), and ultrafine particulate matter (PM1). TSP, PM10, PM2.5, and PM1 are referred to suspended atmospheric particles with an aerodynamic equivalent diameter of ≤100 μm, ≤10 μm, ≤ 2.5 μm, and ≤1.0 μm, respectively. The chemical composition of PM constantly changes with space and time (Fang et al., 2019). It mainly consists of heterogeneous mixtures, which includes, i) carbonaceous components such as organic carbon, elemental carbon, and a small amount of carbonates and carbonic acid; ii) inorganic ions; iii) polycyclic aromatic hydrocarbons (PAHs) and iv) metals like, Cd, Cu, Zn, V and Ni (Kim et al., 2015; Sun et al., 2018).
2.1. Sources of PM
The PM enter into the atmosphere from natural and anthropogenic sources. Primarily, the solid or liquid particles that are directly entered into the atmosphere during daily activities contribute to the major part. For instance, industrial activities, combustion of coal, vehicle exhaust emissions, mining, biomass burning, construction-generated dust and abrasion of brakes and tires (Kim et al., 2015; Chowdhury et al., 2018). In developing countries, traffic-related air pollution (TRAP) and solid fuel used for cooking and heating are the major sources of PM2.5 (Gautam et al., 2016; Morishita et al., 2019). The natural sources of PM are volcanoes, forest fires, dust storms, sea spray and vegetation (Kim et al., 2015). The photochemical reactions of gaseous contaminants in the atmosphere contribute to the secondary sources of PM (Xia et al., 2019). For example, nitrogen oxides, including nitric oxide and nitrous oxide, sulfur dioxide, and some organic gases, can produce nitrates, sulfates, and organic PM after a series of reactions in the atmosphere.
2.2. Health effects of PM in human
PM is one of the crucial air pollutants and found to cause a wide range of health effects in human (Manojkumar and Srimuruganandam, 2021). Epidemiological studies have revealed a strong association between PM and diseases involving multiple organ system (Cowell et al., 2019; Gu et al., 2020). For instance, the link between PM and the aggravation of respiratory illness such as, allergy and asthma has been well documented (Chowdhury et al., 2018). Besides, International Agency for Research on Cancer (IARC) has reported that PM in outdoor air pollution is a human carcinogen (IARC, 2016; Sun et al., 2018). Short term exposure to PM was significantly associated with acute cardiovascular risks like, myocardial infarction, arrhythmias, hypertension and metabolic syndrome (Chen et al., 2020a; Cowell et al., 2019). Furthermore, PM could also accelerate the risk of neurological effects, including Alzheimer's disease and dementia (Younan et al., 2020). It is apparent that the PM causes significant damage to various organ systems. Hence, this review highlights the health effects of PM along with underlying molecular mechanism in physiological systems such as respiratory, cardiovascular, nervous, and immune systems. In addition, the relationship between PM, SARS-CoV-2 transmission and COVID-19 infection was also reviewed.
3. Impact of PM on the respiratory system
3.1. Health effects
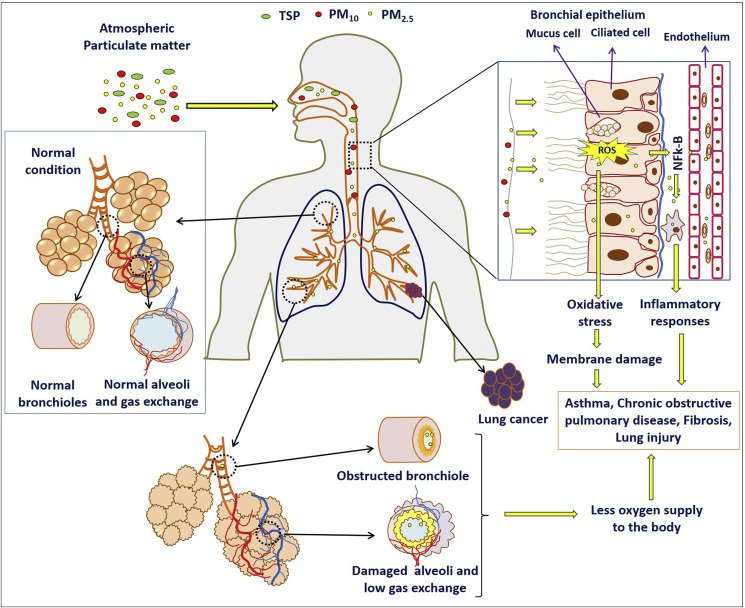
PM enters the human primarily through respiration and causes damage to the respiratory system. According to World Health Organization (WHO), an estimated more than 2 million deaths worldwide every year results from air pollution-caused injuries to the respiratory system (WHO, 2018a). Long-term exposure to PM can trigger various respiratory diseases including asthma, respiratory tract inflammation, and even lung cancer. Elevated TSP level is positively correlated with the incidence of respiratory diseases in children (Pan et al., 2010). Exposure to PM10 and PM2.5 have been reported to increase the risk of asthmatic children in Guadeloupe (Cadelis et al., 2014). Therefore, PM is a significant cause for the development of respiratory diseases such as asthma and lung cancer. The impact of PM in the respiratory system was summarized in Table 1 and Fig. 1 .
Table 1.
Health effects of PM on the respiratory system.
| S. No. | PM type | Country/Location | Period | Patients/Subject | Health effects | Reference |
|---|---|---|---|---|---|---|
| 1. | PM10, PM2.5 and PM1 | Oporto, Portugal | 2018–2019 | 65 mothers and their newborns | PM10 deposited in the head region, while PM2.5 and PM1 deposited in the pulmonary area | Madureira et al. (2020) |
| 2. | PM10, PM2.5 and PM1 | 7 Northeastern Chinese cities | 2012–2013 | 6740 children | Impaired lung function with significant impact on body mass index (BMI) | Xing et al. (2020) |
| 3. | PM10 and PM2.5 | 96 cities of China | 2013–2016 | Meteorological and hospital data | Increases the risk of COPD | Tian et al. (2020) |
| 4. | PM10 and PM2.5 | China | 2013–2018 | 69,491 patients | Increases the risk of respiratory system related diseases | Chang et al. (2020) |
| 5. | PM10 and PM2.5 | 4 Brazilian Southeast capitals | 2015–2018 | Meteorological and hospital data | Causes respiratory diseases | de Oliveira Fernandes et al. (2020) |
| 6. | PM10 | Taiwan | 2010–2012 | 120 children | Adverse effects on lung function | Yen et al. (2020) |
| 7. | PM10 | Bangkok, Thailand | 2013–2018 | Meteorological and hospital data | Respiratory diseases are associated with air pollution | Thongkum et al. (2020) |
| 8. | PM10 | England | 1991–1992 | 14,541 pregnant women with 13,963 children | Reductions in lung function in mid-childhood | Cai et al. (2020) |
| 9. | PM2.5 | Shanghai, China | 2012–2014 | 5281 participants | Decreases forced vital capacity (FVC), inspiration capacity (IC), and vital capacity (VC) with impaired lung function | Hou et al. (2020) |
| 10. | PM2.5 | Shenyang, China | 2015–2016 | 114 healthy volunteers | Causes imbalancein the oropharyngeal microbiota and impaired lung function in young people | Li et al. (2019a) |
| 11. | PM2.5 | United States | 2000–2018 | 7071 participants | Associated with increased emphysema | Wang et al. (2019a) |
| 12. | PM | Eastern Massachusetts | 2012–2014 | 81 COPD patients | Promotes systemic oxidative stress | Huang et al. (2020) |
Fig. 1.
Health effects of PM in respiratory system.
3.2. Toxicity mechanisms
Experimental evidences showed that exposure to PM can cause inflammation in lung tissues of rats, and the severity of inflammation tends to worsen as the dose increases (Xiao et al., 2007). The PM exposure causes various respiratory diseases mainly through the following mechanisms.
3.2.1. Free radical peroxidation damage
Heavy metal components and organic substances in PM2.5 can increase the free radical production and subsequent decrease of antioxidants level, which lead to lipid peroxidation in lung tissues (Pardo et al., 2019). The water-insoluble fraction of PM10 may induce the production of hydrogen peroxide and weaken the enzymatic antioxidant defense, thereby inducing oxidative damage in human lung epithelial A549 cells (Yi et al., 2014). The oxidative damage might be the leading cause of impairment to the respiratory system (Wu et al., 2019; Sun et al., 2020).
3.2.2. Inflammatory response
The inflammatory response is due to immune mechanism of the body in response to noxious stimuli. Long-term exposure of human lung epithelial cells to ambient PM10 can reduce the anti-inflammatory proteins and leads to the excessive release of inflammatory cytokines (Jeon et al., 2011). PM2.5 can stimulate the overexpression of multiple inflammatory cytokine-related genes (He et al., 2019; Zhao et al., 2019a). Excessive release of inflammatory cytokines can affect the pulmonary microenvironment, damage the lung tissues and reduce the lungs repair ability.
3.2.3. Imbalance of intracellular calcium homeostasis
Calcium (Ca) is an important second messenger that regulates the physiological functions of the body. Ca2+ plays a vital regulatory function after tissue injury in the respiratory system. PM2.5 disrupts the calcium ion homeostasis in the body, causing cell damage and apoptosis (Geng et al., 2006). PM2.5 increases the intracellular Ca2+ concentration in lymphocytes by regulating Ca2+-Mg2+-ATPase enzyme activities, resulting in imbalanced Ca2+ homeostasis and leads to an inflammatory response (Zhao et al., 2019b). In addition, the massive production of Ca2+ can cause a variety of subcellular damages, such as endoplasmic reticulum swelling, mitochondrial fission, and mitochondrial crista degeneration, eventually leading to cell death and affect the normal functioning of lung tissues (Guo et al., 2017).
3.2.4. Macrophage damage
After the PM entered into lungs, it stimulates alveolar macrophages to elicit a series of immune responses, which plays an important role in the induction of different diseases. PM10 exposure in macrophages can result in cytoskeletal changes and impairs the phagocytic capacity and motility of macrophages (Brown et al., 2004). PM2.5 can affect the cytoskeleton rearrangement by increasing the expression of PI3Kδ and inhibition of RhoA activity, thereby causing phagocytic dysfunction of macrophages in rats with chronic obstructive pulmonary disease (COPD) (Xia et al., 2017). Therefore, the damage to cytoskeletons may reduce the ability of macrophages to remove PM from the lungs, which resulted in lung damage.
3.2.5. Cell cycle dysregulation
The PM has a dual role in regulating cell cycle, i.e., initiating or inhibiting cell cycle arrest. PM10 can lead to abnormal cell death in lung tissues by inducing G0/G1 cell cycle arrest. PM2.5 modulates the expression of the p21 protein through the long noncoding RNA (LINC0034) to block the bronchial epithelial cells at the G2/M phase (Xu et al., 2017). PM2.5 can also arrest the G1 to S phase transition and the S phase checkpoint signaling pathway, which affects the cell survival. Furthermore, PM10 exposure may induce apoptosis evasion in lung cells by activating STAT3 via PKCζ and Src kinases, leading to lung cancer (Reyes-Zárate et al., 2016; Abbas et al., 2019).
4. Impact of PM on the cardiovascular system
4.1. Health effects
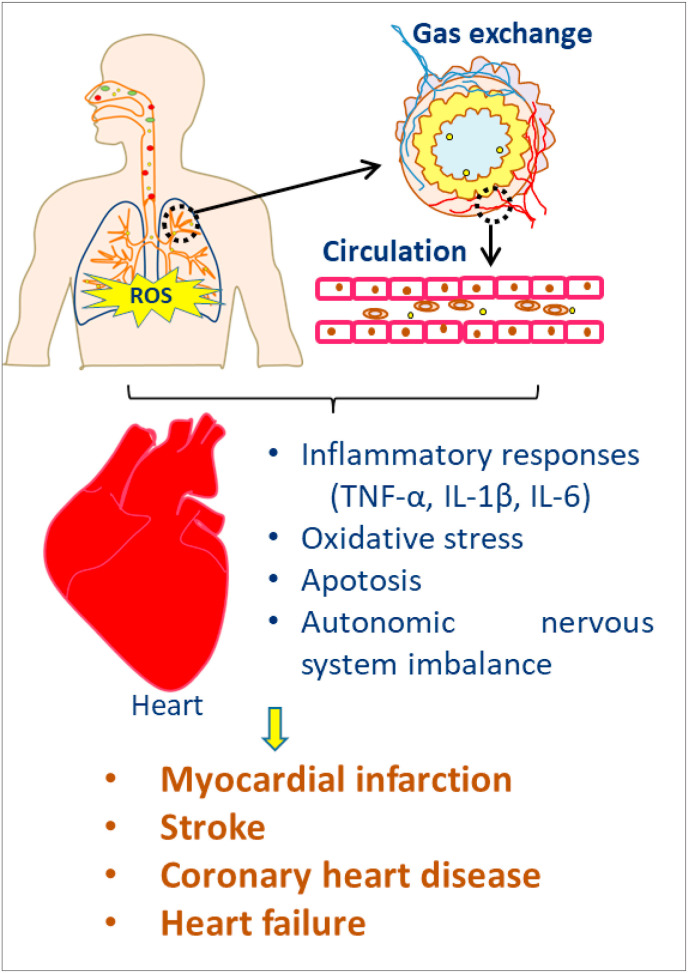
The cardiovascular system is one of the main targets of air pollution-induced toxic effects (Brook et al., 2010). Exposure to ambient PM can lead to various cardiovascular diseases (CVD), such as myocardial infarction, coronary heart disease, stroke, and cardiac failure. Cao et al. found that for every rise of 10 μg/m3 TSP, the risk of cardiovascular mortality increased by 0.9% (Cao et al., 2011). Other studies reported that the rise of 10 μg/m3 PM10 and PM2.5 could increase the cardiovascular and circulatory disease mortality by 0.55% and 1.22%, respectively (Guo et al., 2010; Yang et al., 2012). PM10 and PM2.5 can decrease the heart rate variability and increase blood pressure and thereby increases the risk of CVD in humans (Tofler and Muller, 2006). Table 2 and Fig. 2 showed the health impact of PM in the cardiovascular system.
Table 2.
Health effects of PM on the cardiovascular system.
| S. No. | PM type | Country/Location | Period | Patients/Subject | Health effects | Reference |
|---|---|---|---|---|---|---|
| 1. | PM2.5, PM2.5–10 and PM10, | United States | 2007–2017 | 165,675 participants | PM2.5 is associated with higher leukocyte count and reduced CD8+ T-cell proportions. Increased risk for CVD | Gondalia et al. (2020) |
| 2. | PM2.5, PM2.5–10 and PM10 | Taiwan | 2006–2011 | 90 Patients with prior myocardial infarction | Affects cardiac autonomic balance | Hung et al. (2020) |
| 3. | PM10 and PM2.5 | Yichang, China | 2015–2017 | 391,960 inpatient admissions | Increases respiratory disease and CVD hospital admissions | Yao et al. (2020a) |
| 4. | PM10 and PM2.5 | 9 cities in France, Iran and Italy | 2015–2016 | Meteorological and medical data | Increased risk of mortality for people with CVD and respiratory diseases | Sicard et al. (2019) |
| 5. | PM2.5 | Ann Arbor, Michigan | Two weeks | 50 participants | Worsened aortic hemodynamics and increased the risk for CVD | Morishita et al. (2019) |
| 6. | PM2.5 | Seoul, Korea | 2007 | 364 patients | Increased risk of rupture-prone coronary plaque | Yang et al. (2019) |
| 7. | PM2.5 | Allegheny County, Pennsylvania | 2007–2015 | 31,414 individuals with atrial Fibrillation (AF) | Associated with high risk of ischemic stroke | Rhinehart et al. (2020) |
| 8. | PM2.5 | Shanghai, China | 2014–2016 | 1,016,579 participants outpatients | Increased risk of cardiac arrhythmias | Yang et al. (2020) |
| 9. | PM2.5 | United States | 2000 | 565,477 participants | Increased mortality due to ischemic heart disease and stroke | Hayes et al. (2019) |
| 10 | PM2.5 | Swedish | 2018–2019 | 2927 participants | Increased dementia incidence via the heart failure and ischemic heart disease | Grande et al. (2020) |
| 11. | PM2.5 | China | 2016–2017 | 2337 patients in intensive cardiac care unit | High risk of acute non-cardiovascular critical illnesses | Chen et al. (2020a) |
| 12. | PM2.5 | North Carolina | 2004–2016 | 35,084 heart failure patients | Increased risk in individuals with cardiac failure | Ward-Caviness et al. (2020) |
| 13. | PM 2.5 | Boston | 2011–2012 | 237 maternal–infant pairs | Disrupt cardiac vagal tone during infancy, reduced autonomic flexibility | Cowell et al. (2019) |
| 14. | PM2.5 | China | 2013–2016 | Meteorological and medical data | Increased risks of CVD death | Xia et al. (2019) |
Fig. 2.
Health effects of PM in cardiovascular system.
4.2. Toxicity mechanisms
4.2.1. Inflammatory response
PM10 exposure increased the expression of tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and other inflammatory factors in rat myocardium, causing myocardial inflammatory responses and injuries to the cardiovascular system (Radan et al., 2019). Cui et al. have shown that PM2.5 exposure could significantly reduce the number of circulating endothelial progenitor cells in mice, induce serum inflammatory cytokines such as TNF-α and IL-1β, thereby leading to the occurrence and progression of CVD (Cui et al., 2015).
4.2.2. Oxidative stress
Cen et al. have found that PM10 induced cardiovascular toxicity in zebrafish larvae by increasing oxidative stress (Cen et al., 2020). When the fine PM is phagocytosed by macrophages, the extracellular concentrations of reactive oxygen species (ROS) will be greatly increased (Long et al., 2020). PM2.5 can also activate the Wnt/β-catenin signaling pathway (Zhou et al., 2010) and induces massive ROS production in the body. It leads to oxidative stress, cell membrane lipid peroxidation and subsequent damage to the cardiovascular system.
4.2.3. Apoptosis
PM2.5 can induce apoptosis in endothelial cells and cardiomyocytes and causing damage to the cardiovascular system. Endothelial cells are important interfaces in regulating the homeostasis of the cardiovascular system (Wang and Tang, 2020). An excessive increase in PM2.5 concentration can enhance the expression of cytochrome c, thereby promoting the activation of apoptotic proteins such as caspase-9 and caspase-3 and leading to apoptosis of endothelial cells (Dong et al., 2005). Moreover, PM2.5 may facilitate autophagy and apoptosis in human endothelial cells by inducing excessive endoplasmic reticulum stress in vivo (Wang and Tang, 2020). Cardiomyocytes are the cells that constitute most of the heart tissue. PM2.5 exposure can increase the phosphorylation levels of cardiac c-Jun NH2-terminal kinase (JNK) and p53, causing increased Bax (a downstream effector protein) and caspase-3 and decreased Bcl-2 levels to promote cardiomyocyte apoptosis (Wang et al., 2019b). Subsequently, the excessive activation of NF-κB by PM2.5 damages the heart function and increases the risk of myocardial infarction (Li et al., 2017).
4.2.4. Autonomic nervous system imbalance
The autonomic nervous system plays a vital role in regulating cardiac rate and its imbalance leads to heart rate variability which is considered as an important predictor of CVD. There is a correlation between PM exposure and reduction in heart rate variability (Mordukhovich et al., 2015). After PM2.5 exposure, the imbalance in cardiac autonomic nerve regulation might be related to the potential occurrence of CVD such as arrhythmia and ischemic heart disease (Xie et al., 2016). PM2.5 may change the autonomic tone and induce cardiac arrhythmia by activating the pulmonary reflex (Brook et al., 2004).
5. Impact of PM on the nervous system
5.1. Health effects
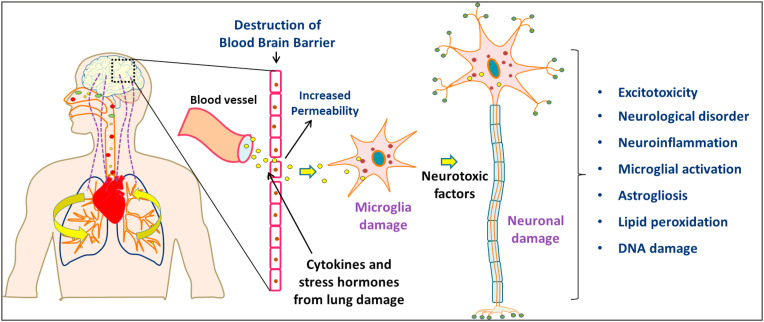
PM causes a significant impact on the human central nervous system (CNS). PM damages the developing brain and causes neuronal disorders (Costa et al., 2020). Maher et al. reported that PM was found in the brain of some residents in the metropolitan area of Mexico City which indicates that PM can enter the CNS (Maher et al., 2016). PM10 exposure affects the CNS that increases the risk of relapse in multiple sclerosis (a chronic neurological disease) (Roux et al., 2017). According to WHO, 93% of children are exposed to PM2.5 above guideline level, among them, 630 million belong to under 5 years and 1.8 billion belong to under 15 years age group (WHO, 2018b). Long-term exposure to ambient PM10 may increase the incidence of autism spectrum disorders in children (Yousefian et al., 2018). Many studies have shown that prenatal and early-childhood exposure to PM2.5 will cause psychomotor retardation (Guxens et al., 2014) and mental retardation (Jedrychowski et al., 2015). The health effects of PM on the nervous system were shown in Table 3 and Fig. 3 .
Table 3.
Health effects of PM on the nervous system.
| S. No. | PM type | Country/Location | Period | Patients/Subject | Health effects | Reference |
|---|---|---|---|---|---|---|
| 1. | PM10 and PM2.5 | Mexico | 2016–2017 | 120 children | Diminished olfactory identification performance and affected CNS function | Guarneros et al. (2020) |
| 2. | PM10 and PM2.5 | 13 cities of China | 2013–2015 | 111,842 hospital outpatients | Increases mental disorders exacerbations | Lu et al. (2020) |
| 3. | PM10 and PM2.5 | China | 2014–2015 | 16,601 anxiety hospital admissions | Worsens the risks of anxiety hospitalizations | Yue et al. (2019) |
| 4. | PM10 | Saxony, Germany | 2005–2014 | 1,126,014 individuals | Increased risk of anxiety and depression | Zhao et al. (2020) |
| 5. | PM2.5 | United Kingdom | 2006–2010 | 111,370 participants | Increases the risk of glaucoma via neurotoxic and/or vascular effects | Chua et al. (2019) |
| 6. | PM2.5 | Unites States | 1999–2010 | 998 participants | Increased risk for Alzheimer's disease | Younan et al. (2020) |
| 7. | PM2.5 | Boston, New York, Shanghai or Changsha | 2010–2014 | 135 first episode schizophrenia patients | Interacts with psychosis to reduce hippocampal volume | Worthington et al. (2020) |
| 8. | PM2.5 | Denmark | 1989–2014 | 21,057 cases | Increased risk factor for CNS tumors | Poulsen et al. (2020) |
| 9. | PM2.5 | Mexico | 2007–2011 | 509 mothers | Increased the risk of Postpartum depression and neuropsychological dysfunction in mothers | Niedzwiecki et al. (2020) |
| 10. | PM2.5 | Utah | 1986–2015 | 2444 pediatric patients and 13,459 young adult patients with cancer | Increases cancer mortality in pediatric lymphomas and CNS tumors | Ou et al. (2020a) |
| 11. | PM2.5 | Barcelona | 2012–2014 | 186 participants | Decreased corpus callosum volume in pre-adolescent children and behavior problems | Mortamais et al. (2019) |
| 12. | PM | West Virginian counties, USA | 2001–2005 | Public Health and Socioeconomic Data | Increased dementia mortality | Salm and Benson (2019) |
Fig. 3.
Health effects of PM in nervous system.
5.2. Toxicity mechanisms
Exposure to PM2.5 can impair the cerebral cortex growth in pregnant mice, which lead to anxiety, depression, and changes social behavior in their progeny (Zhang et al., 2018). Neuroinflammation and oxidative stress are two hypothetical biological mechanisms by which air pollutants adversely affect the brain. Neuroinflammation and oxidative stress-related markers have been significantly increased in individuals exposed to high level of PM, suggesting the association of air pollution-induced neurotoxicity, such as neurodevelopmental disorders and neurodegenerative diseases (Heneka et al., 2018; Butterfield and Halliwell, 2019). Moreover, PM can increase the penetrability of the blood–brain barrier (BBB), allowing the noxious substances quickly into the CNS and produce adverse effects.
5.2.1. Neuroinflammation
PM10 can activate the molecular signaling cascades of tissue inflammation (Woodward et al., 2017), resulting in the production of massive amounts of proinflammatory and inflammatory cytokines. These cytokines might then be released into the blood which interacts and disrupts the normal functions of BBB. Many cytokines, monocytes, and macrophages can enter the brain through the damaged BBB system and causes brain inflammation (Farina et al., 2013). Ehsanifar et al. (2019) have found that PM2.5 may increase inflammatory cytokines in the blood and thus cause the excessive activation of immune cells in the peripheral nervous system, resulting in inflammatory responses in the CNS and neurodegenerative disorders.
5.2.2. Oxidative stress
The trace metals, endotoxins, and other soluble compounds present in the PM10 can penetrate the fluid of the inner airway wall, interact with tissues, and induce ROS production through Fenton or Fenton-like reactions (Vidrio et al., 2008). Subsequently, ROS can react with lipids and proteins and alter their structures and functions. Since the brain is mainly composed of easily oxidizable lipids, PM can certainly cause brain damage. Long-term exposure to PM2.5 can cause oxidative damage, lipid peroxidation, morphological disruptions of neurons, and neuronal apoptosis in brain tissues (Angoa-Pérez et al., 2006).
5.2.3. Destruction of the blood–brain barrier
PM can break down the tight junctions between endothelial cells and increase the permeability of endothelial cell monolayers and the migration of monocytes. These effects indicate that exposure to PM compromises the integrity of the BBB which allows them to enter and damage the CNS (Shou et al., 2019). Biddlestone-Thorpe et al. has found that the PM2.5 can disrupt the cellular ultrastructure underlying the BBB and cause local microhemorrhage and microdamage (Biddlestone-Thorpe et al., 2012). As a result, harmful substances in the blood can pass through the BBB and damage the neurons, destabilizing the CNS microenvironment.
6. Impact of PM on the immune system
6.1. Health effects
Exposure to PM can compromise immune function in humans, reducing immunity and thereby inducing or aggravating various diseases. PM2.5 exposure may lead to disorders in T lymphocyte-mediated adaptive immune responses, causing injuries to the respiratory and cardiovascular systems (Zhao et al., 2013; Dobreva et al., 2015). A study assessed the relationship between PM and immune markers (CD4+ T lymphocytes and CD8+ T lymphocytes) has shown that chronic exposure to PM leads to airway inflammation and activation of the cellular or humoral immune system (Leonardi et al., 2000). In the Czech Republic, the degree of air pollution in the urban area has been negatively correlated with the number of CD4+ T lymphocytes and the low CD4+/CD8+ ratio in the blood of newborn umbilical cords (Hertz-Picciotto et al., 2002). Health effects of PM in immune system were reviewed in Table 4 .
Table 4.
Health effects of PM in the immune system.
| S. No. | PM type | Country/Location | Period | Patients/Subject | Health effects | Reference |
|---|---|---|---|---|---|---|
| 1. | PM10 and PM2.5 | Zhejiang and Shanxi, China | 2012–2014 | 120 participants | Increased mtDNA copy number and IL-5 concentration | Wang et al. (2020a) |
| 2. | PM10 and PM2.5 | Italy | 2014–2015 | 50 healthy adult volunteers | Impaired the immune system | Dolci et al. (2018) |
| 3. | PM10 and PM2.5 | Jinan, Shandong | 2016 | 163 and 110 school children from the polluted and control areas, respectively | Decreased C3 and C4 levels, and B lymphocyte count | Li et al. (2019b) |
| 4. | PM10 | South Korea | 2012–2013 | 100 participants | Impacts DNA methylation and immune responses | Lee et al. (2019) |
| 5. | PM2.5 | Mexico City | 2013–2017 | 35 residents | Alters functional immune cell responses and increased risk for Tuberculosis development | Torres et al. (2019) |
| 6. | PM2.5 | Japan | 2014–2015 | Cell line study | Activated antigen presenting cells and T-cells led to respiratory diseases | Chowdhury et al. (2018) |
| 7. | PM2.5 | Shanghai, China | 2016 | 43 volunteers | Saliva lysozyme (non-specific immune biomarker) was significantly inversely associated with indoor PM2.5 | Gao et al. (2019a, b) |
| 8. | PM2.5 | United States | 2012–2013 | 21 volunteers | Impairs critical antimycobacterial T cell immune functions | Ibironke et al. (2019) |
| 9. | PM2.5 | United States | 2003–2011 | 774 participants | Impacts DNA methylation and the human immune system | Gao et al. (2019a, b) |
| 10 | PM2.5 | 252 Chinese cities | 2013–2017 | 117,338,867 hospital admissions | Increased risk of diseases in the digestive, musculoskeletal, and genitourinary systems | Gu et al. (2020) |
6.2. Toxicity mechanism
Exposure to PM is closely related to the occurrence of immune system-related diseases. Xu et al. have reported that the severity of injury to the immune system in rats was positively correlated with the cumulative effect of the fine PM dose and exposure duration (Xu et al., 2008). Inflammation, oxidative damage, and apoptosis induced by PM exposure are the major mechanisms for the immune system damage.
6.2.1. Inflammatory response
PM directly enters the alveoli and interacts with the mucosal defense system. After, the alveolar macrophages identify the PM through the pattern recognition receptors and then a series of signal transduction mechanisms are activated to induce local or systemic inflammatory responses which leads to tissue damage (Sijan et al., 2015). Pope et al. (2016) has reported that PM2.5 may increase the IL-6, IL-8, and TNF-α levels in the peripheral blood and lungs of nonsmoking adolescents and cause systemic inflammatory and immune disorders.
6.2.2. Oxidative damage
Oxidative stress induced by PM2.5 causes significant increase in IL-4 and IL-13 (Th2-related cytokines) and a reduction in IFN-γ (Th1-related cytokine) in the rat cardiac muscles, which enhanced the allergic inflammatory responses (Zhao et al., 2012). PM2.5 damages the immune cells and alters cytokine secretion and causes disorders in the immune system (Kaur et al., 2011; Valavanidis et al., 2008).
6.2.3. Apoptosis
PM can upregulate pro-apoptotic genes, which controls the active cell death in tissues. For example, Reyes-Zárate et al. have found that PM10 exposure can induce G1/G0 arrest and induce the apoptosis of immune cells (Reyes-Zárate et al., 2016). After exposure to PM, microglia can activate the MAPK pathway to induce apoptosis and alter the expression of Toll-like receptors, T lymphocyte receptors, and B lymphocyte receptors as well as various proinflammatory cytokines and their receptors (Sama et al., 2007).
7. Impact of PM in COVID-19 infection
The COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). According to WHO, a total of 130,422,190 confirmed cases with 2,842,135 deaths were reported worldwide by COVID-19 infection as of April 05, 2021 (WHO, 2021). It has been confirmed that respiratory droplets with an aerodynamic diameter of 5–10 μm from the infected person could carry the virus and cause COVID-19 (WHO/Europe, 2020; WHO, 2020a; Amoatey et al., 2020). Many factors are associated with the spread of airborne COVID-19 infection (Srivastava, 2021). The potential transmission of SARS-CoV-2 through aerosols has confirmed the detection of viral RNA in a Wuhan hospital, China (Liu et al., 2020). It is well established that exposure to the PM could possibly carry different bacteria or viruses and pose harmful effects on human health (Zoran et al., 2020). PM could act as direct carrier and has prominent role in transmission of SARS-CoV-2 virus (Anand et al., 2021; Tung et al., 2021). For instance, Setti et al. (2020) have found the SARS-CoV-2 RNA on PM of Bergamo in Northern Italy. Further, PM2.5 produced from healthcare facilities could influence the presence of SARS-CoV-2 RNA in indoor environments (Nor et al., 2021). The impact of PM in SARS-CoV-2 transmission and COVID-19 infection was shown in Table 5 .
Table 5.
Relationship between PM and COVID-19 infection.
| S. No. | PM type | Country/Location | Period | Observation | Reference |
|---|---|---|---|---|---|
| 1. | PM10 and PM2.5 | China | January 26th - February 29th, 2020 | PM2.5 has the potential of COVID-19 transmission | Li et al. (2020) |
| 2. | PM10 and PM2.5 | China | January 23rd - February 29th, 2020 | Positively correlated with the risk of COVID-19 infection | Zhu et al. (2020) |
| 3. | PM10 and PM2.5 | China | January 25th - February 29th, 2020 | PM2.5 increases the risk of COVID-19 infection | Jiang et al. (2020) |
| 4. | PM10 and PM2.5 | China | Upto March 22nd, 2020 | Rise of every 10 of PM10 and PM2.5 was linked with increased COVID-19 mortality rate with 0.24% and 0.26%, respectively | Yao et al. (2020b) |
| 5 | PM10 and PM2.5 | United States | March 4th -April 24th, 2020 | Showed significant correlation between PM and COVID-19 | Bashir et al. (2020) |
| 6. | PM10 and PM2.5 | France | March 18th - April 27th, 2020 | Direct association with COVID-19 mortality | Magazzino et al. (2020) |
| 7. | PM10 and PM2.5 | Middle Eastern Countries | n.a. | Indoor burning could enable the possible spread of SARS-CoV-2 virus droplets | Amoatey et al. (2020) |
| 8. | PM10 and PM2.5 | Italy | Upto March 31, 2020 | Chronic exposure to PM2.5 causes alveolar ACE-2 receptor overexpression that links to COVID-19 infection | Frontera et al. (2020) |
| 9. | PM10 and PM2.5 | Italy | Upto April 27th, 2020 | Favors the transmission of SARS-CoV-2 infection | Fattorini and Regoli (2020) |
| 10. | PM10 | Italy | February 10th - February 29th, 2020 | Reported the presence of SARS-CoV-2 on PM | Setti et al. (2020) |
| 11. | PM10 | Italy | Upto April 7th, 2020 | Transmission of COVID-19 is mainly by the air pollution | Coccia (2020) |
| 12. | PM2.5 | United States | Upto April 22nd, 2020 | Increase of PM2.5 by 1 was connected with an 8% of increased COVID-19 fatality | Wu et al. (2020) |
| 13. | PM2.5 | New York | March 1st - April 20th, 2020 | PM2.5 was significantly associated COVID-19 infection, but not correlated with mortality | Adhikari and Yin (2020) |
Note: n.a. – data not available.
Several studies have suggested that SARS-CoV-2 can transmit through various indoor and outdoor aerosols, particularly by PM (Robotto et al., 2021; Senatore et al., 2021). Natural and anthropogenic sources such as industrial activities, domestic heating, and road transport in urban areas could contribute to PM production (Bontempi, 2020). In this context, Amoatey et al. (2020) have revealed that indoor burning could enable the possible transmission of SARS-CoV-2 virus droplets through PM. Recently, Wu et al. (2020) has demonstrated that an increase of PM2.5 by 1 could increase the COVID-19 mortality rate by 8%. Also, every rise of 10 PM10 and PM2.5 was the strongly linked with increased COVID-19 fatality rate by 0.24% and 0.26%, respectively (Yao et al., 2020b). Overall, it is evident that PM contributes to the COVID-19 infection and highlights the need for preventive actions.
7.1. Role of face masks in preventing PM and COVID-19 infection
Airborne transmission of infectious respiratory diseases involves the emission of microorganism-containing aerosols and droplets nuclei (≤5 μm in diameter) resulted from the evaporation of droplets or aerosolization from various expiratory activities such as breathing, talking, coughing, and sneezing (WHO, 2020c; Chen et al., 2021). It is well documented that the relationship between PM and COVID-19 infection by the airborne transmission of SARS-CoV-2 from the infected person through small droplets and particles (Setti et al., 2020; CDC, 2020b). In this context, several precautionary measures are being advised to the public for preventing COVID-19 infection and mortality rate, for example, social distancing, hand wash with soap and water or use of sanitizer, use of masks, travel restrictions, etc., (CDC, 2020c). Since, many patients are asymptomatic and unaware of their infection, using a facemask is a well-established strategy to control respiratory infection (Esposito et al., 2020).
Masks and respirators act as a physical barrier to respiratory droplets that expelled from infected individual (Chua et al., 2020). The proper and early use of facemask could prevent the COVID-19 transmission and it serves as dual preventive purpose of protecting oneself from getting the viral infection and protecting others (Abboah-Offei et al., 2021). The quality of facemasks plays major role in COVID-19 prevention and it depends on the filtration efficiency of particulates, bacteria and virus (Dharmaraj et al., 2021). Currently, three types of masks are being used to prevent COVID-19 infection which includes surgical masks, respirators and cloth masks. Surgical masks are non-woven triple layered structure with a middle filter efficient to prevent the infectious droplets of larger than 5 μm (Tcharkhtchi et al., 2021). Surgical masks are recommended to use not more than for 3–8 h. Respirators are one of the personal protective equipment (PPE) being in use among healthcare workers. N95 respirators filter about 0.3-μm sized particles including bacteria and viruses. Its efficiency is about 95% and intended to use for 2 h (Dharmaraj et al., 2021). Cloth masks are breathable non-certified or home-made masks has been used to prevent community spread. The advantage of cloth masks is cheap, reusable and easy to handle. However, the filtration, effectiveness, fit, and performance of cloth masks are inferior to medical masks and respirators (Chughtai et al., 2020). Facemasks are made from non-renewable and non-biodegradable petroleum-based polymers (Dharmaraj et al., 2021). The increased production and widespread usage of face masks raises concern of microplastic pollution in the environment due to improper disposal.
7.2. Health effects of COVID-19
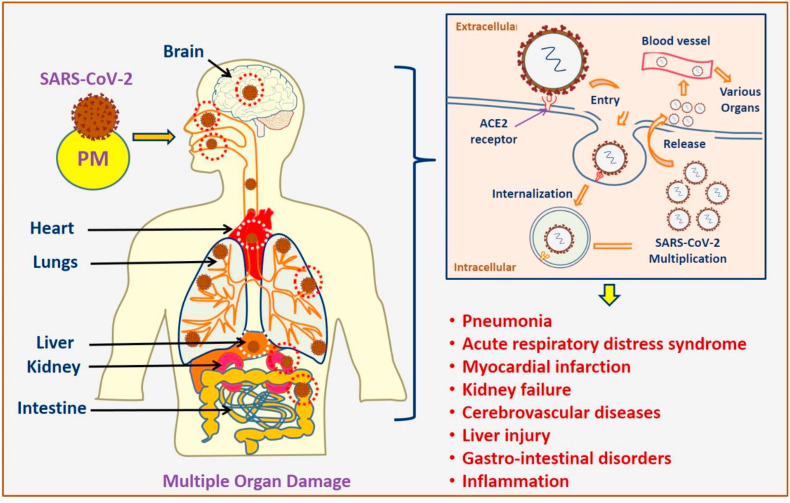
The clinical condition of COVID-19 infection is diverse, ranging from asymptomatic or severe with common symptoms like fever, cough, fatigue, diarrhea, sore throat, myalgia, loss of smell or taste and breathing difficulties (CDC, 2020a; WHO, 2020b). It can lead to life-threatening conditions in severe cases, including pneumonia, acute respiratory distress syndrome (ARDS), acute stroke, shock, myocardial infarction, kidney failure, and even death (Nalleballe et al., 2020). Fig. 4 shows the various health effects of COVID-19 infection in humans. SARS-CoV-2 infection primarily affects the pulmonary system. According to radiological examinations, symptoms like, pleural discharges, enlarged pulmonary vessels, lesions, lung opacities, and uncommon mediastinal lymphadenopathy can be used as the early indicator of lung injury (Albarello et al., 2020). There is an increased risk for CVD such as, myocarditis and myocardial injury as secondary consequences in infected patients (Deng et al. (2020). Patients with severe infection showed neurologic disorders such as impaired consciousness, acute cerebrovascular diseases and skeletal muscle injuries (Mao et al., 2020). The death rate was higher in old age groups and the persons with co-morbidities such as hypertension, diabetes, and CVD (Zhou et al., 2020). COVID-19 is also linked with high prevalence of psychological distress and posed mental health problems (Xiong et al., 2020).
Fig. 4.
Impact of PM on COVID-19 and its possible health effects.
7.3. Mechanisms
It is possible that PM exposure may increase the COVID-19 infection by facilitating attachment of SARS-CoV-2 through angiotensin-converting enzyme 2 (ACE2) receptor overexpression (Tung et al., 2021). SARS-CoV-2 is a β-coronavirus that has shown a strong affinity towards the ACE2 membrane receptor. ACE2 is found throughout the body, including lungs, nasal and oral mucosa, brain, kidney, heart, intestine, liver, and blood vessels (Behl et al., 2020; Ciaglia et al., 2020). PM10 exposure could upregulate the expression of ACE2 was reported in human respiratory epithelial and alveolar A549 cells (Miyashita et al., 2020). ACE2 is also expressed in myocytes and vascular endothelial cells and hence, there is a possibility of SARS-CoV-2 involvement in heart tissues, while interstitial mononuclear inflammatory infiltrates were found in cardiac tissues COVID-19 cases (Xu et al., 2020; Zhou et al., 2020). However, the relationship between the strong affinity of SARS-CoV-2 spike protein and ACE2 receptor towards infectivity is not well established (Chung et al., 2020). Whereas the binding of virus and ACE2 receptor lead to the entry of SARS-CoV-2 into the cell which in turn cause shedding and downregulation of ACE2 receptor (Verdecchia et al., 2020). The shedding of ACE2 is catalyzed by the action of a disintegrin and metalloproteinase (ADAM) 17 and ADAM 10, which releases the active form of soluble ACE2 into the plasma (Chung et al., 2020). ACE2 mediates the conversion of angiotensin II into angiotensin (1–7) through GPCR (G-protein coupled receptor) pathway. ACE2 and angiotensin (1–7) are involved in the protective functions of the body by anti-inflammatory and antioxidant actions (Behl et al., 2020). The downregulation of ACE2 reduces its protective action and worsening the angiotensin II effects. Hence, there is a strong relationship between SARS-CoV-2 and ACE2 in the COVID-19 disease severity. Angiotensin converting enzyme inhibitors (ACE inhibitors) are one of the current research area to find the potential therapeutic drug to control the infection (Ciaglia et al., 2020).
8. Other health impacts of PM
The urinary, digestive and reproductive systems are affected by exposure to high level of atmospheric pollutants (Kampa and Castanas, 2008; Somers, 2011). Some studies suggest that people with prenatal exposure to PM2.5 are susceptible to long-term metabolic syndrome (Wu et al., 2019). In addition, maternal exposure to PM2.5 is closely correlated with premature birth, low birth weight, stillbirth, and poor postpartum health (Tan et al., 2017; Klepac et al., 2018; Melody et al., 2019). Air pollution is also associated with the prevalence, morbidity, and mortality of diabetes mellitus. It can affect skin condition, induce acne, and accelerate skin aging (Schraufnagel et al., 2019).
9. Discussion
PM comprises of a complex and heterogeneous mixtures of pollutants from natural and anthropogenic activities (Abbas et al., 2019). PM are associated with many diseases in human such as, COPD, lung cancer, asthma, premature death, multiple sclerosis and other respiratory and CVD (Roux et al., 2017; Wang et al., 2020b). In this review, the impact of PM induced adverse health effects in human physiological systems were described with underlying mechanism at cellular and molecular levels. The human body consists of highly organized and coordinated network of organ systems. Any changes or disruption in one organ functions will affect the homeostasis of the human body by interfering the molecular signaling pathways. The predominant route of human exposure to PM is through the inhalation that enables them to enter and deposit in the airways and centriacinar regions of the lungs (Leikauf et al., 2020). Fine and ultrafine particulates can escape into the blood stream and affect the cardiovascular system. The sensory receptors on the alveolar surface may activate the autonomic nervous system that can immediately affect cardiovascular functions.
In lungs, epithelial cells and alveolar macrophages are involved in the detoxification process and subsequent removal of toxics via the lymphatic system based on the antioxidant molecules ratio (Alemayehu et al., 2020). While PM are directly or indirectly involved in the induction of oxidative and pro-inflammatory response in human bronchial epithelial cells (Abbas et al., 2019). Under oxidative stress condition, macrophages produce cytokines that can enter into circulation and regulates the inflammatory reaction (Sijan et al., 2015). There is a growing evidence that oxidative stress and inflammatory responses are the major cause of PM induced adverse effects in respiratory, cardiovascular, nervous, and immune systems (Cui et al., 2015; Wei and Tang, 2018; Son et al., 2020).
The pathogenesis of many diseases is believed to be due to the oxidative damage of biomolecules. Generally, free radicals or ROS is formed in the mitochondria and peroxisomes during the reduction of oxygen in electron transport chain. The imbalance of antioxidant molecules causes the inability to neutralize or remove the free radicals formed that leads to oxidative stress. Exposure to PM promotes the radical formation that can quickly reacts with biomolecules, such as fatty acids, proteins and nucleic acids and results in oxidative damage (Leikauf et al., 2020). Besides, PM (PM SRM1648a) exposure also affects the cellular activity by the accumulation of intracellular calcium level that induce the inflammatory response and even cell death (Maher et al., 2018). Subsequently, other toxicity mechanisms are activated such as inflammatory responses, apoptosis, macrophage damage, cell cycle dysregulation, etc., which are associated with the detrimental health effects (Ehsanifar et al., 2019).
Antioxidant supplements have been suggested as a preventive method to attenuate the PM induced adverse health effects (Son et al., 2020). Antioxidant molecules play a major role in relieve oxidative damage or cell stress caused by PM through mitochondrial and endoplasmic reticulum (ER) stress-mediated pathways (Wang et al., 2020b). For instance, Glutathione-S-transferase, an antioxidant defense enzyme is involved in detoxification of diesel exhaust particles in the respiratory tract (Meier-Girard et al., 2019). While the loss of GST protein increases the nasal allergic response in response to PM pollution (Schwartz et al., 2005). In this line, several studies have reported the preventive action of natural agents against oxidative damage. Recently, Radan et al. (2019) have reported the protective effect of gallic acid in PM induced cardiac dysfunction by lowering the oxidative stress and cytokine levels and improving the antioxidant enzymes. Nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor plays an important role in maintaining redox homeostasis and provide protective effects in PM induced toxicity (Pardo et al., 2019). Recently, Yang et al. (2021) has revealed the protective role of curcumin in PM2.5 induced oxidative stress and inflammation by reducing the ROS levels and enhancing Nrf2 expression that in turn regulating the downstream antioxidant enzymes encoding genes. Hence, the natural compounds with antioxidant, anti-inflammatory and ROS inhibiting property can be exploited for mitigating PM induced health disorders.
10. Regulations and control strategies for mitigating PM impacts
It is well documented that the PM exposure could affect human health and environment. According to WHO air quality guideline, about 15% of air pollution-related deaths can be reduced by the decrease of PM10 levels to 20 μg/m3 and PM2.5 levels to 10 μg/m3 (WHO, 2018c). While many countries have set the guideline values for the regulation of PM concentrations to minimize the health impacts (Table 6 ). However, the emission of PM concentrations was found to be much higher in developing countries (Kim et al., 2015) and exceeding the guideline levels.
Table 6.
Regulation guidelines for particulate matters (PM10 and PM2.5) in different countries.
| S. No. | Country |
Daily (μg/m3) |
Annual (μg/m3) |
Reference | ||
|---|---|---|---|---|---|---|
| PM10 | PM2.5 | PM10 | PM2.5 | |||
| 1. | China | 150 | 75 | 70 | 35 | Chen et al. (2020b) |
| 2. | India | 100 | 60 | 60 | 40 | NAAQS (2019) |
| 3. | United States | 150 | 35 | n.a. | 12 | EPA (2020) |
| 4. | European Union | 50 | n.a. | 40 | 25 | EEA (2020) |
| 5. | Australia | 50 | 25 | 25 | 8 | Air NEPM (2016) |
| 6. | Canada | n.a. | 27 | n.a. | 8.8 | Health Canada (2016) |
| 7. | Japan | n.a. | 35 | n.a. | 15 | Bota and Yamasaki (2020) |
| 8. | WHO | 50 | 25 | 20 | 10 | WHO (2018c) |
n.a. – data not available.
The primary step for reaching the regulatory standards can be the identification of emission-reduction targets and development of control strategies to achieve the targets (Ou et al., 2020b). Further, there is a correlation exists between the air pollutants and socio-economic factors (Chen et al., 2020b). The mitigation strategies that are advised to be technically and economically feasible to limit the negative impacts to air quality (Health Canada, 2016). Clean technologies that reduce the emission of air pollutants are being implemented in various industrial sectors, transport, urban planning, power generation and waste management, for instance, increased use of low-emission fuels and vehicles, combustion free power generations, making green cities, etc., (WHO, 2018c). Besides, the vehicular emissions can be controlled by decreasing the total number of old age vehicles. Furthermore, substantial health benefits can be attained by the substitution of high PM2.5 emission sources such as industrial coal and industrial liquids with cost-effective electricity use from renewable energy (solar and wind power) (Ou et al., 2020b). In this context, Liang et al. (2019) have reported that fleet electrification in China can decrease the fine PM concentration and provide more health benefits. Hence, the combination of clean power and electrified technologies are the key aspects to attain the PM reduction.
The important factors for mitigating PM pollution could be the investments in environmental protection and pollution control strategies. Moreover, the continuous monitoring of PM emission from various sources is essential for reviewing and updating the standards and regulations periodically, to ensure that they provide adequate health and environmental protection. Furthermore, the precautionary measures should be taken into consideration for the effective prevention and management of PM induced health issues in the highly polluted regions of the world.
11. Conclusion
The impact of atmospheric pollution on human health has become a hot topic in recent years including COVID-19. The negative impact of PM on human health is multifaceted. The PM first enters the human respiratory tract and triggers lung inflammation. Subsequently, it initiates systemic inflammatory responses, autonomic dysfunction, and a series of oxidative stress-induced injuries, which in turn causes severe damage to the human organ systems and functions. Oxidative stress and inflammatory responses are considered as the major mechanism involved in the PM induced adverse effects. A comprehensive understanding of the negative impact of PM on the human body and its associated mechanisms will help to develop a pollution control strategy to minimize the detrimental effects on public health.
Credit author contribution statement
Chengyue Zhu: Conceptualization, Writing – original draft, Writing – review & editing, Validation, Methodology, Data curation. Kannan Maharajan: Conceptualization, Writing – original draft, Writing – review & editing, Validation, Methodology, Data curation. Kechun Liu: Conceptualization, Supervision, Funding acquisition, Data curation, Writing – review & editing. Yun Zhang: Conceptualization, Supervision, Funding acquisition, Data curation, Writing – review & editing
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by Shandong Provincial Natural Science Foundation (ZR2020YQ60), Shandong-Chongqing Science and Technology Cooperation Project (2020LYXZ017), National Key R&D Program of China (2018YFC1707300), Science, Education and Industry Integration Innovation Pilot Project of Qilu University of Technology (Shandong Academy of Sciences) (2020KJC-ZD08).
References
- Abbas I., Badran G., Verdin A., Ledoux F., Roumie M., Lo Guidice J.M., Courcot D., Garcon G. In vitro evaluation of organic extractable matter from ambient PM2.5 using human bronchial epithelial BEAS-2B cells: cytotoxicity, oxidative stress, pro-inflammatory response, genotoxicity, and cell cycle deregulation. Environ. Res. 2019;171:510–522. doi: 10.1016/j.envres.2019.01.052. [DOI] [PubMed] [Google Scholar]
- Abboah-Offei M., Salifu Y., Adewale B., Bayuo J., Ofosu-Poku R., Opare-Lokko E.B.A. A rapid review of the use of face mask in preventing the spread of COVID-19. Int. J. Nurs. Stud. 2021;3:100013. doi: 10.1016/j.ijnsa.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A., Yin J. Short-term effects of ambient ozone, PM2. 5, and meteorological factors on COVID-19 confirmed cases and deaths in queens, New York. Int. J. Environ. Res. Publ. Health. 2020;17(11):4047. doi: 10.3390/ijerph17114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Air Nepm . 2016. Air National Environment Protection Measure. Australia State of the Environment, National Air Quality Standards. Ambient Air Quality (2016)https://soe.environment.gov.au/theme/ambient-air-quality/topic/2016/national-air-quality-standards [Google Scholar]
- Alemayehu Y.A., Asfaw S.L., Terfie T.A. Exposure to urban particulate matter and its association with human health risks. Environ. Sci. Pollut. Control Ser. 2020;27:27491–27506. doi: 10.1007/s11356-020-09132-1. [DOI] [PubMed] [Google Scholar]
- Albarello F., Pianura E., Di Stefano F., Cristofaro M., Petrone A., Marchioni L., Palazzolo C., Schininà V., Nicastri E., Petrosillo N., Campioni P. 2019-novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int. J. Infect. Dis. 2020;93:192–197. doi: 10.1016/j.ijid.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoatey P., Omidvarborna H., Baawain M.S., Al-Mamun A. Impact of building ventilation systems and habitual indoor incense burning on SARS-CoV-2 virus transmissions in Middle Eastern countries. Sci. Total Environ. 2020;733:139356. doi: 10.1016/j.scitotenv.2020.139356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Adelodun B., Pivato A., Suresh S., Indari O., Jakhmola S., Jha H.C., Jha P.K., Tripathi V., Di Maria F. A review of the presence of SARS-CoV-2 RNA in wastewater and airborne particulates and its use for virus spreading surveillance. Environ. Res. 2021;196:110929. doi: 10.1016/j.envres.2021.110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M., Jiang H., Rodríguez A.I., Lemini C., Levine R.A., Rivas-Arancibia S. Estrogen counteracts ozone-induced oxidative stress and nigral neuronal death. Neuroreport. 2006;17:629–633. doi: 10.1097/00001756-200604240-00014. [DOI] [PubMed] [Google Scholar]
- Bashir M.F., Bilal B.M., Komal B. Correlation between environmental pollution indicators and COVID-19 pandemic: a brief study in Californian context. Environ. Res. 2020:109652. doi: 10.1016/j.envres.2020.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl T., Kaur I., Bungau S., Kumar A., Uddin M.S., Kumar C., Pal G., Shrivastava K., Zengin G., Arora S. The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020;257:118075. doi: 10.1016/j.lfs.2020.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddlestone-Thorpe L., Marchi N., Guo K., Ghosh C., Janigro D., Valerie K., Yang H. Nanomaterial-mediated CNS delivery of diagnostic and therapeutic agents. Adv. Drug Deliv. Rev. 2012;64:605–613. doi: 10.1016/j.addr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020:109639. doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota E., Yamasaki S. vol. 156. OECD ENV/WKP(2020)3 (updated 9 March 2020); 2020. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/WKP(2020)3&docLanguage=En (Policies, Regulatory Framework and Enforcement for Air Quality Management: the Case of Japan – Environment Working Paper). [Google Scholar]
- Brook R.D., Franklin B., Cascio W., Hong Y., Howard G., Lipsett M., Luepker R., Mittleman M., Samet J., Smith S.C., Jr., Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American heart association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Brook R.D., Rajagopalan S., Pope C.A., Brook J.R., Bhatnagar A., Diez-Roux A.V., Holguin F., Hong Y.L., Luepker R.V., Mittleman M.A., Peters A., Siscovick D., Smith S.C., Whitsel L., Kaufman J.D. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brown D.M., Donaldson K., Stone V. Effects of PM10 in human peripheral blood monocytes and J774 macrophages. Respir. Res. 2004;5:29. doi: 10.1186/1465-9921-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadelis G., Tourres R., Molinie J. Short-term effects of the particulate pollutants contained in saharan dust on the visits of children to the emergency department due to asthmatic conditions in Guadeloupe (French archipelago of the caribbean) PloS One. 2014;9(3) doi: 10.1371/journal.pone.0091136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Hansell A.L., Granell R., Blangiardo M., Zottoli M., Fecht D., Gulliver J., Henderson A.J., Elliott P. Prenatal, early-life, and childhood exposure to air pollution and lung function: the ALSPAC cohort. Am. J. Respir. Crit. Care Med. 2020;202:112–123. doi: 10.1164/rccm.201902-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Yang C.X., Li J.X., Chen R.J., Chen B.H., Gu D.F., Kan H.D. Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J. Hazard Mater. 2011;186(2–3):1594–1600. doi: 10.1016/j.jhazmat.2010.12.036. [DOI] [PubMed] [Google Scholar]
- CDC . Symptoms of Coronavirus. 2020. Centre for disease control and prevention.https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html Accessed 24 October 2020. [Google Scholar]
- CDC . 2020. Centers for Disease Control and Prevention. How COVID-19 Spreads (Updated 28th October 2020)https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html [Google Scholar]
- CDC . 2020. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). How to Protect Yourself & Others (Updated 4th February 2021)https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html [Google Scholar]
- Cen J., Jia Z.L., Zhu C.Y., Wang X.F., Zhang F., Chen W.Y., Liu K.C., Li S.Y., Zhang Y. Particulate matter (PM10) induces cardiovascular developmental toxicity in zebrafish embryos and larvae via the ERS, Nrf2 and Wnt pathways. Chemosphere. 2020;250:126288. doi: 10.1016/j.chemosphere.2020.126288. [DOI] [PubMed] [Google Scholar]
- Chang Q., Zhang H., Zhao Y. Ambient air pollution and daily hospital admissions for respiratory system-related diseases in a heavy polluted city in Northeast China. Environ. Sci. Pollut. Res. Int. 2020;27:10055–10064. doi: 10.1007/s11356-020-07678-8. [DOI] [PubMed] [Google Scholar]
- Chen F., Liu Q., Huang B., Huang F., Li Y., Peng Y., Chen M. Association of fine particulate matter exposure with acute noncardiovascular critical illnesses and in-hospital outcomes in patients receiving intensive cardiac care. BMC Publ. Health. 2020;20:610. doi: 10.1186/s12889-020-08758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bai Y., Liu H., Alatalo J.M., Jiang B. Temporal variations in ambient air quality indicators in Shanghai municipality, China. Sci. Rep. 2020;10(1):11350. doi: 10.1038/s41598-020-68201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Jia P., Han J. Role of indoor aerosols for COVID-19 viral transmission: a review. Environ. Chem. Lett. 2021;1–18 doi: 10.1007/s10311-020-01174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury P.H., Okano H., Honda A., Kudou H., Kitamura G., Ito S., Ueda K., Takano H. Aqueous and organic extract of PM(2.5) collected in different seasons and cities of Japan differently affect respiratory and immune systems. Environ. Pollut. 2018;235:223–234. doi: 10.1016/j.envpol.2017.12.040. [DOI] [PubMed] [Google Scholar]
- Chua S.Y.L., Khawaja A.P., Morgan J., Strouthidis N., Reisman C., Dick A.D., Khaw P.T., Patel P.J., Foster P.J. The relationship between ambient atmospheric fine particulate matter (PM2.5) and glaucoma in a large community cohort. Invest. Ophthalmol. Vis. Sci. 2019;60:4915–4923. doi: 10.1167/iovs.19-28346. [DOI] [PubMed] [Google Scholar]
- Chua M.H., Cheng W., Goh S.S., Kong J., Li B., Lim J.Y., Mao L., Wang S., Xue K., Yang L., Ye E. Face masks in the new COVID-19 normal: materials, testing, and perspectives. AAAS Research. 2020;7286735 doi: 10.34133/2020/7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chughtai A.A., Seale H., Macintyre C.R. Effectiveness of cloth masks for protection against severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26(10) doi: 10.3201/eid2610.200948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M.K., Karnik S., Saef J., Bergmann C., Barnard J., Lederman M.M., Tilton J., Cheng F., Harding C.V., Young J.B., Mehta N. SARS-CoV-2 and ACE2: the biology and clinical data settling the ARB and ACEI controversy. EBioMedicine. 2020;58:102907. doi: 10.1016/j.ebiom.2020.102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaglia E., Vecchione C., Puca A.A. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front. Pediatr. 2020;8:206. doi: 10.3389/fped.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729:138474. doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L.G., Cole T.B., Dao K., Chang Y.C., Coburn J., Garrick J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020;210:107523. doi: 10.1016/j.pharmthera.2020.107523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell W.J., Brunst K.J., Malin A.J., Coull B.A., Gennings C., Kloog I., Lipton L., Wright R.O., Enlow M.B., Wright R.J. Prenatal exposure to PM2.5 and cardiac vagal tone during infancy: findings from a multiethnic birth cohort. Environ. Health Perspect. 2019;127:107007. doi: 10.1289/EHP4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Xie X., Jia F., He J., Li Z., Fu M., Hao H., Liu Y., Liu J.Z., Cowan P.J., Zhu H., Sun Q., Liu Z. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell. Physiol. Biochem. 2015;35:353–363. doi: 10.1159/000369701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Fernandes M.A., Andre O W.L., Maciel F.M., De Almeida Albuquerque T.T. Avoiding hospital admissions for respiratory system diseases by complying to the final Brazilian air quality standard: an estimate for Brazilian southeast capitals. Environ. Sci. Pollut. Res. Int. 2020;27:35889–35907. doi: 10.1007/s11356-020-07772-x. [DOI] [PubMed] [Google Scholar]
- Deng Q., Hu B., Zhang Y., Wang H., Zhou X., Hu W., Cheng Y., Yan J., Ping H., Zhou Q. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmaraj S., Ashokkumar V., Hariharan S., Manibharathi A., Show P.L., Tung C.C., Ngamcharussrivichai C. The COVID-19 pandemic face mask waste: a blooming threat to the marine environment. Chemosphere. 2021;272:129601. doi: 10.1016/j.chemosphere.2021.129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobreva Z.G., Kostadinova G.S., Popov B.N., Petkov G.S., Stanilova S.A. Proinflammatory and anti-inflammatory cytokines in adolescents from Southeast Bulgarian cities with different levels of air pollution. Toxicol. Ind. Health. 2015;31:1210–1217. doi: 10.1177/0748233713491812. [DOI] [PubMed] [Google Scholar]
- Dolci M., Favero C., Bollati V., Campo L., Cattaneo A., Bonzini M., Villani S., Ticozzi R., Ferrante P., Delbue S. Particulate matter exposure increases JC polyomavirus replication in the human host. Environ. Pollut. 2018;241:234–239. doi: 10.1016/j.envpol.2018.05.044. [DOI] [PubMed] [Google Scholar]
- Dong C., Song W.M., Shi Y.W. [Study on the oxidative injury of the vascular endothelial cell affected by PM2.5] Wei Sheng Yan Jiu. 2005;34:169–171. [PubMed] [Google Scholar]
- EEA . 2020. European Environment Agency. Air Quality Standards (Updated 23 Nov 2020)https://www.eea.europa.eu/themes/air/air-quality-concentrations/air-quality-standards [Google Scholar]
- Ehsanifar M., Tameh A.A., Farzadkia M., Kalantari R.R., Zavareh M.S., Nikzaad H., Jafari A.J. Exposure to nanoscale diesel exhaust particles: oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol. Environ. Saf. 2019;168:338–347. doi: 10.1016/j.ecoenv.2018.10.090. [DOI] [PubMed] [Google Scholar]
- EPA . 2020. Particulate Matter (PM) Pollution. National Ambient Air Quality Standards (NAAQS) for PM (Updated December 7, 2020)https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm [Google Scholar]
- Esposito S., Principi N., Leung C.C., Migliori G.B. Universal use of face masks for success against COVID-19: evidence and implications for prevention policies. Eur. Respir. J. 2020;55(6):2001260. doi: 10.1183/13993003.01260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G.C., Ho T.T., Chen Y.C., Zhuang Y.J., Kao C.L., Liang G.R. Sources and monthly and seasonal concentration variation study of atmospheric particulates and particles-bound PAEs. Environ. Geochem. Health. 2019;42:1863–1875. doi: 10.1007/s10653-019-00455-8. [DOI] [PubMed] [Google Scholar]
- Farina F., Sancini G., Battaglia C., Tinaglia V., Mantecca P., Camatini M., Palestini P. Milano summer particulate matter (PM10) triggers lung inflammation and extra pulmonary adverse events in mice. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0056636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman H.J., Finch C.E. A critical review of assays for hazardous components of air pollution. Free Radic. Biol. Med. 2018;117:202–217. doi: 10.1016/j.freeradbiomed.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera A., Cianfanelli L., Vlachos K., Landoni G., Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: the “double-hit” hypothesis. J. Infect. 2020;81(2):255–259. doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Colicino E., Shen J., Kioumourtzoglou M.A., Just A.C., Nwanaji-Enwerem J.C., Coull B., Lin X., Vokonas P., Zheng Y., Hou L., Schwartz J., Baccarelli A.A. Impacts of air pollution, temperature, and relative humidity on leukocyte distribution: an epigenetic perspective. Environ. Int. 2019;126:395–405. doi: 10.1016/j.envint.2019.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Xu Y., Cai Y., Shi J., Chen F., Lin Z., Chen T., Xia Y., Shi W., Zhao Z. Effects of filtered fresh air ventilation on classroom indoor air and biomarkers in saliva and nasal samples: a randomized crossover intervention study in preschool children. Environ. Res. 2019;179:108749. doi: 10.1016/j.envres.2019.108749. [DOI] [PubMed] [Google Scholar]
- Gautam S., Yadav A., Tsai C.J., Kumar P. A review on recent progress in observations, sources, classification and regulations of PM 2.5 in Asian environments. Environ. Sci. Pollut. Res. 2016;23(21):21165–21175. doi: 10.1007/s11356-016-7515-2. [DOI] [PubMed] [Google Scholar]
- Geng H., Meng Z., Zhang Q. In vitro responses of rat alveolar macrophages to particle suspensions and water-soluble components of dust storm PM(2.5) Toxicol. Vitro. 2006;20:575–584. doi: 10.1016/j.tiv.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Gondalia R., Holliday K.M., Baldassari A., Justice A.E., Stewart J.D., Liao D., Yanosky J.D., Engel S.M., Jordahl K.M., Bhatti P., Horvath S., Assimes T.L., Pankow J.S., Demerath E.W., Guan W., Fornage M., Bressler J., North K.E., Conneely K.N., Li Y., Hou L., Baccarelli A.A., Whitsel E.A. Leukocyte traits and exposure to ambient particulate matter air pollution in the women's health initiative and atherosclerosis risk in communities study. Environ. Health Perspect. 2020;128:17004. doi: 10.1289/EHP5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande G., Ljungman P.L.S., Eneroth K., Bellander T., Rizzuto D. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol. 2020;77:801–809. doi: 10.1001/jamaneurol.2019.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Shi Y., Zhu Y., Chen N., Wang H., Zhang Z., Chen T. Ambient air pollution and cause-specific risk of hospital admission in China: a nationwide time-series study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros M., L Pez-Rivera C., Gonsebatt M.E., Alcaraz-Zubeldia M., Hummel T., Schriever V.A., Valdez B., Hudson R. Metal-containing particulate matter and associated reduced olfactory identification ability in children from an area of high atmospheric exposure in Mexico city. Chem. Senses. 2020;45:45–58. doi: 10.1093/chemse/bjz071. [DOI] [PubMed] [Google Scholar]
- Guo Y., Barnett A.G., Zhang Y., Tong S., Yu W., Pan X. The short-term effect of air pollution on cardiovascular mortality in Tianjin, China: comparison of time series and case-crossover analyses. Sci. Total Environ. 2010;409:300–306. doi: 10.1016/j.scitotenv.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Guo Z., Hong Z., Dong W., Deng C., Zhao R., Xu J., Zhuang G., Zhang R. PM(2.5)-Induced oxidative stress and mitochondrial damage in the nasal mucosa of rats. Int. J. Environ. Res. Publ. Health. 2017;14(2):134. doi: 10.3390/ijerph14020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M., Garcia-Esteban R., Giorgis-Allemand L., Forns J., Badaloni C., Ballester F., Beelen R., Cesaroni G., Chatzi L., de Agostini M., de Nazelle A., Eeftens M., Fernandez M.F., Fernández-Somoano A., Forastiere F., Gehring U., Ghassabian A., Heude B., Jaddoe V.W., Klümper C., Kogevinas M., Krämer U., Larroque B., Lertxundi A., Lertxuni N., Murcia M., Navel V., Nieuwenhuijsen M., Porta D., Ramos R., Roumeliotaki T., Slama R., Sørensen M., Stephanou E.G., Sugiri D., Tardón A., Tiemeier H., Tiesler C.M., Verhulst F.C., Vrijkotte T., Wilhelm M., Brunekreef B., Pershagen G., Sunyer J. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology. 2014;25:636–647. doi: 10.1097/EDE.0000000000000133. [DOI] [PubMed] [Google Scholar]
- Hayes R.B., Lim C., Zhang Y., Cromar K., Shao Y., Reynolds H.R., Silverman D.T., Jones R.R., Park Y., Jerrett M., Ahn J., Thurston G.D. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int. J. Epidemiol. 2020;49:25–35. doi: 10.1093/ije/dyz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Ichinose T., Ito T., Toriba A., Yoshida S., Kaori S., Nishikawa M., Sun G.F., Shibamoto T. Investigation of inflammation inducing substances in PM2.5 particles by an elimination method using thermal decomposition. Environ. Toxicol. 2019;34:1137–1148. doi: 10.1002/tox.22816. [DOI] [PubMed] [Google Scholar]
- Health Canada . Health Canada; Ottawa, Ontario: 2016. Guidance for Evaluating Human Health Impacts in Environmental Assessment: Air Quality. Healthy Environments and Consumer Safety Branch.https://www.acee.gc.ca/050/documents/p80054/119376E.pdf [Google Scholar]
- Heneka M.T., McManus R.M., Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018;19:610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I., Dostál M., Dejmek J., Selevan S.G., Wegienka G., Gomez-Caminero A., Srám R.J. Air pollution and distributions of lymphocyte immunophenotypes in cord and maternal blood at delivery. Epidemiology. 2002;13:172–183. doi: 10.1097/00001648-200203000-00012. [DOI] [PubMed] [Google Scholar]
- Hou D., Ge Y., Chen C., Tan Q., Chen R., Yang Y., Li L., Wang J., Ye M., Li C., Meng X., Kan H., Cai J., Song Y. Associations of long-term exposure to ambient fine particulate matter and nitrogen dioxide with lung function: a cross-sectional study in China. Environ. Int. 2020;144:105977. doi: 10.1016/j.envint.2020.105977. [DOI] [PubMed] [Google Scholar]
- Huang S., Koutrakis P., Grady S.T., Vieira C.L.Z., Schwartz J.D., Coull B.A., Hart J.E., Laden F., Zhang J.J., Garshick E. Effects of particulate matter gamma radiation on oxidative stress biomarkers in COPD patients. J. Expo. Sci. Environ. Epidemiol. 2020 doi: 10.1038/s41370-020-0204-8. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C.S., Huang C.C., Pan S.C., Ma H.P., Huang C.C., Guo Y.L., Ho Y.L. Acute particulate matter exposure is associated with disturbances in heart rate complexity in patients with prior myocardial infarction. Sci. Total Environ. 2020;733:138842. doi: 10.1016/j.scitotenv.2020.138842. [DOI] [PubMed] [Google Scholar]
- IARC International Agency for Research on Cancer (IARC) monographs on the evaluation of carcinogenic risks to humans: outdoor air pollution. IARC Monogr. 2016;109 [PMC free article] [PubMed] [Google Scholar]
- Ibironke O., Carranza C., Sarkar S., Torres M., Choi H.T., Nwoko J., Black K., Quintana-Belmares R., Osornio-Vargas Á., Ohman-Strickland P., Schwander S. Urban air pollution particulates suppress human T-cell responses to Mycobacterium tuberculosis. Int. J. Environ. Res. Publ. Health. 2019;16(21):4112. doi: 10.3390/ijerph16214112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W.A., Perera F.P., Camann D., Spengler J., Butscher M., Mroz E., Majewska R., Flak E., Jacek R., Sowa A. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ. Sci. Pollut. Res. 2015;22:3631–3639. doi: 10.1007/s11356-014-3627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y.M., Son B.S., Lee M.Y. Proteomic identification of the differentially expressed proteins in human lung epithelial cells by airborne particulate matter. J. Appl. Toxicol. 2011;31:45–52. doi: 10.1002/jat.1566. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Wu X.J., Guan Y.J. Effect of ambient air pollutants and meteorological variables on COVID-19 incidence. Infect. Control Hosp. Epidemiol. 2020;41(9):1011–1015. doi: 10.1017/ice.2020.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa M., Castanas E. Human health effects of air pollution. Environ. Pollut. 2008;151:362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Kaur S., Rana S., Singh H.P., Batish D.R., Kohli R.K. Citronellol disrupts membrane integrity by inducing free radical generation. Z. Naturforsch C. J. Biosci. 2011;66:260–266. doi: 10.1515/znc-2011-5-609. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Kabir E., Kabir S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Klepac P., Locatelli I., Korošec S., Künzli N., Kukec A. Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. Environ. Res. 2018;167:144–159. doi: 10.1016/j.envres.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Lee M.K., Xu C.J., Carnes M.U., Nichols C.E., Ward J.M., Kwon S.O., Kim S.Y., Kim W.J., London S.J. Genome-wide DNA methylation and long-term ambient air pollution exposure in Korean adults. Clin. Epigenet. 2019;11:37. doi: 10.1186/s13148-019-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikauf G.D., Kim S.H., Jang A.S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020;52(3):329–337. doi: 10.1038/s12276-020-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi G.S., Houthuijs D., Steerenberg P.A., Fletcher T., Armstrong B., Antova T., Lochman I., Lochmanová A., Rudnai P., Erdei E., Musial J., Jazwiec-Kanyion B., Niciu E.M., Durbaca S., Fabiánová E., Koppová K., Lebret E., Brunekreef B., van Loveren H. Immune biomarkers in relation to exposure to particulate matter: a cross-sectional survey in 17 cities of Central Europe. Inhal. Toxicol. 2000;12(4):1–14. [PubMed] [Google Scholar]
- Li X., Geng J., Chen Y., Chen F., Liu C., Xu Q., Zhao J., Hu J., Xie J., Xu B. Exposure to particulate matter induces cardiomyocytes apoptosis after myocardial infarction through NFκB activation. Biochem. Biophys. Res. Commun. 2017;488:224–231. doi: 10.1016/j.bbrc.2017.05.047. [DOI] [PubMed] [Google Scholar]
- Li X., Sun Y., An Y., Wang R., Lin H., Liu M., Li S., Ma M., Xiao C. Air pollution during the winter period and respiratory tract microbial imbalance in a healthy young population in Northeastern China. Environ. Pollut. 2019;246:972–979. doi: 10.1016/j.envpol.2018.12.083. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang X., Zhang Z., Han L., Gong D., Li J., Wang T., Wang Y., Gao S., Duan H., Kong F. Air pollution exposure and immunological and systemic inflammatory alterations among schoolchildren in China. Sci. Total Environ. 2019;657:1304–1310. doi: 10.1016/j.scitotenv.2018.12.153. [DOI] [PubMed] [Google Scholar]
- Li H., Xu X.L., Dai D.W., Huang Z.Y., Ma Z., Guan Y.J. Air Pollution and temperature are associated with increased COVID-19 incidence: a time series study. Int. J. Infect. Dis. 2020;97:278–282. doi: 10.1016/j.ijid.2020.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Zhang S., Wu Y., Xing J., He X., Zhang K.M., Wang S., Hao J. Air quality and health benefits from fleet electrification in China. Nat. Sustain. 2019;2(10):962–971. [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Long Y.M., Yang X.Z., Yang Q.Q., Clermont A.C., Yin Y.G., Liu G.L., Hu L.G., Liu Q., Zhou Q.F., Liu Q.S., Ma Q.C., Liu Y.C., Cai Y. PM2.5 induces vascular permeability increase through activating MAPK/ERK signaling pathway and ROS generation. J. Hazard Mater. 2020;386:121659. doi: 10.1016/j.jhazmat.2019.121659. [DOI] [PubMed] [Google Scholar]
- Lu P., Zhang Y., Xia G., Zhang W., Xu R., Wang C., Guo Y., Li S. Attributable risks associated with hospital outpatient visits for mental disorders due to air pollution: a multi-city study in China. Environ. Int. 2020;143:105906. doi: 10.1016/j.envint.2020.105906. [DOI] [PubMed] [Google Scholar]
- Madureira J., Slezakova K., Silva A.I., Lage B., Mendes A., Aguiar L., Pereira M.C., Teixeira J.P., Costa C. Assessment of indoor air exposure at residential homes: inhalation dose and lung deposition of PM(10), PM(2.5) and ultrafine particles among newborn children and their mothers. Sci. Total Environ. 2020;717:137293. doi: 10.1016/j.scitotenv.2020.137293. [DOI] [PubMed] [Google Scholar]
- Magazzino C., Mele M., Schneider N. The relationship between air pollution and COVID-19-related deaths: an application to three French cities. Appl. Energy. 2020;279:115835. doi: 10.1016/j.apenergy.2020.115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher B.A., Ahmed I.A.M., Karloukovski V., MacLaren D.A., Foulds P.G., Allsop D., Mann D.M.A., Torres-Jardon R., Calderon-Garciduenas L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. U.S.A. 2016;113:10797–10801. doi: 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P., van Leyen K., Dey P.N., Honrath B., Dolga A., Methner A. The role of Ca2+ in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium. 2018;70:47–55. doi: 10.1016/j.ceca.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manojkumar N., Srimuruganandam B. Health effects of particulate matter in major Indian cities. Int. J. Environ. Health Res. 2021;31(3):258–270. doi: 10.1080/09603123.2019.1651257. [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Girard D., Delgado-Eckert E., Schaffner E., Schindler C., Künzli N., Adam M., Pichot V., Kronenberg F., Imboden M., Frey U., Probst-Hensch N. Association of long-term exposure to traffic-related PM10 with heart rate variability and heart rate dynamics in healthy subjects. Environ. Int. 2019;125:107–116. doi: 10.1016/j.envint.2019.01.031. [DOI] [PubMed] [Google Scholar]
- Melody S.M., Ford J., Wills K., Venn A., Johnston F.H. Maternal exposure to short-to medium-term outdoor air pollution and obstetric and neonatal outcomes: a systematic review. Environ. Pollut. 2019;244:915–925. doi: 10.1016/j.envpol.2018.10.086. [DOI] [PubMed] [Google Scholar]
- Miyashita L., Foley G., Semple S., Grigg J. Traffic-derived particulate matter and angiotensin-converting enzyme 2 expression in human airway epithelial cells. bioRxiv. 2020 doi: 10.1101/2020.05.15.097501. [DOI] [Google Scholar]
- Mordukhovich I., Coull B., Kloog I., Koutrakis P., Vokonas P., Schwartz J. Exposure to sub-chronic and long-term particulate air pollution and heart rate variability in an elderly cohort: the Normative Aging Study. Environ. Health. 2015;14:87. doi: 10.1186/s12940-015-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita M., Wang L., Speth K., Zhou N., Bard R.L., Li F., Brook J.R., Rajagopalan S., Brook R.D. Acute blood pressure and cardiovascular effects of near-roadway exposures with and without N95 respirators. Am. J. Hypertens. 2019;32:1054–1065. doi: 10.1093/ajh/hpz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M., Pujol J., Mart Nez-Vilavella G., Fenoll R., Reynes C., Sabatier R., Rivas I., Forns J., Vilor-Tejedor N., Alemany S., Cirach M., Alvarez-Pedrerol M., Nieuwenhuijsen M., Sunyer J. Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environ. Res. 2019;178:108734. doi: 10.1016/j.envres.2019.108734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAAQS . 2019. National Ambient Air Quality Standards. National Ambient Air Quality Status and Trends 2019. Central Pollution Control Board.https://cpcb.nic.in/upload/NAAQS_2019.pdf [Google Scholar]
- Nalleballe K., Onteddu S.R., Sharma R., Dandu V., Brown A., Jasti M., Yadala S., Veerapaneni K., Siddamreddy S., Avula A., Kapoor N. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav. Immun. 2020;88:71–74. doi: 10.1016/j.bbi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecki M.M., Rosa M.J., Solano-Gonz Lez M., Kloog I., Just A.C., Mart Nez-Medina S., Schnaas L., Tamayo-Ortiz M., Wright R.O., T Llez-Rojo M.M., Wright R.J. Particulate air pollution exposure during pregnancy and postpartum depression symptoms in women in Mexico City. Environ. Int. 2020;134:105325. doi: 10.1016/j.envint.2019.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nor N.S.M., Yip C.W., Ibrahim N., Jaafar M.H., Rashid Z.Z., Mustafa N., Abd Hamid H.H., Chandru K., Latif M.T., Saw P.E., Lin C.Y. Particulate matter (PM 2.5) as a potential SARS-CoV-2 carrier. Sci. Rep. 2021;11:2508. doi: 10.1038/s41598-021-81935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J.Y., Hanson H.A., Ramsay J.M., Kaddas H.K., Pope C.A., 3rd, Leiser C.L., Vanderslice J., Kirchhoff A.C. Fine particulate matter air pollution and mortality among pediatric, adolescent, and young adult cancer patients. Cancer Epidemiol. Biomark. Prev. 2020;29:1929–1939. doi: 10.1158/1055-9965.EPI-19-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y., West J.J., Smith S.J., Nolte C.G., Loughlin D.H. Air pollution control strategies directly limiting national health damages in the US. Nat. Commun. 2020;11(1):1–11. doi: 10.1038/s41467-020-14783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G.W., Zhang S.J., Feng Y.P., Takahashi K., Kagawa J., Yu L.Z., Wang P., Liu M.J., Liu Q.A., Hou S.W., Pan B.L., Li J.P. Air pollution and children's respiratory symptoms in six cities of Northern China. Respir. Med. 2010;104:1903–1911. doi: 10.1016/j.rmed.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Pan M.H., Carol L., Lednicky J.A., Eiguren-Fernandez A., Hering S., Fan Z.H., Wu C.Y. Determination of the distribution of infectious viruses in aerosol particles using water-based condensational growth technology and a bacteriophage MS2 model. Aerosol Sci. Technol. 2019;53:583–593. doi: 10.1080/02786826.2019.1581917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M., Xu F.F., Shemesh M., Qiu X.H., Barak Y., Zhu T., Rudich Y. Nrf2 protects against diverse PM2.5 components-induced mitochondrial oxidative damage in lung cells. Sci. Total Environ. 2019;669:303–313. doi: 10.1016/j.scitotenv.2019.01.436. [DOI] [PubMed] [Google Scholar]
- Pope C.A., 3rd, Bhatnagar A., McCracken J.P., Abplanalp W., Conklin D.J., O'Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ. Res. 2016;119:1204–1214. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen A.H., Hvidtfeldt U.A., S Rensen M., Puett R., Ketzel M., Brandt J., Geels C., Christensen J.H., Raaschou-Nielsen O. Intracranial tumors of the central nervous system and air pollution - a nationwide case-control study from Denmark. Environ. Health. 2020;19:81. doi: 10.1186/s12940-020-00631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radan M., Dianat M., Badavi M., Mard S.A., Bayati V., Goudarzi G. Gallic acid protects particulate matter (PM(10)) triggers cardiac oxidative stress and inflammation causing heart adverse events in rats. Environ. Sci. Pollut. Res. 2019;26:18200–18207. doi: 10.1007/s11356-019-05223-w. [DOI] [PubMed] [Google Scholar]
- Reyes-Zárate E., Sánchez-Pérez Y., Gutiérrez-Ruiz M.C., Chirino Y.I., Osornio-Vargas Á R., Morales-Bárcenas R., Souza-Arroyo V., García-Cuellar C.M. Atmospheric particulate matter (PM10) exposure-induced cell cycle arrest and apoptosis evasion through STAT3 activation via PKCζ and Src kinases in lung cells. Environ. Pollut. 2016;214:646–656. doi: 10.1016/j.envpol.2016.04.072. [DOI] [PubMed] [Google Scholar]
- Rhinehart Z.J., Kinnee E., Essien U.R., Saul M., Guhl E., Clougherty J.E., Magnani J.W. Association of fine particulate matter and risk of stroke in patients with atrial fibrillation. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robotto A., Quaglino P., Lembo D., Morello M., Brizio E., Bardi L., Civra A. SARS-CoV-2 and indoor/outdoor air samples: a methodological approach to have consistent and comparable results. Environ. Res. 2021;195:110847. doi: 10.1016/j.envres.2021.110847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux J., Bard D., Le Pabic E., Segala C., Reis J., Ongagna J.C., de Seze J., Leray E. Air pollution by particulate matter PM10 may trigger multiple sclerosis relapses. Environ. Res. 2017;156:404–410. doi: 10.1016/j.envres.2017.03.049. [DOI] [PubMed] [Google Scholar]
- Salm A.K., Benson M.J. Increased dementia mortality in West Virginia counties with mountaintop removal mining? Int. J. Environ. Res. Publ. Health. 2019;16(21):4278. doi: 10.3390/ijerph16214278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sama P., Long T.C., Hester S., Tajuba J., Parker J., Chen L.C., Veronesi B. The cellular and genomic response of an immortalized microglia cell line (BV2) to concentrated ambient particulate matter. Inhal. Toxicol. 2007;19:1079–1087. doi: 10.1080/08958370701628721. [DOI] [PubMed] [Google Scholar]
- Sanchez M., Milà C., Sreekanth V., Balakrishnan K., Sambandam S., Nieuwenhuijsen M., Kinra S., Marshall J.D., Tonne C. Personal exposure to particulate matter in peri-urban India: predictors and association with ambient concentration at residence. J. Expo. Sci. Environ. Epidemiol. 2020;30:596–605. doi: 10.1038/s41370-019-0150-5. [DOI] [PubMed] [Google Scholar]
- Schraufnagel D.E., Balmes J.R., Cowl C.T., De Matteis S., Jung S.H., Mortimer K., Perez-Padilla R., Rice M.B., Riojas-Rodriguez H., Sood A., Thurston G.D., To T., Vanker A., Wuebbles D.J. Air pollution and noncommunicable diseases: a review by the forum of international respiratory societies' environmental committee, Part 2: air pollution and organ systems. Chest. 2019;155:417–426. doi: 10.1016/j.chest.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Park S.K., O'Neill M.S., Vokonas P.S., Sparrow D., Weiss S., Kelsey K. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am. J. Respir. Crit. Care Med. 2005;172(12):1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatore V., Zarra T., Buonerba A., Choo K.H., Hasan S.W., Korshin G., Li C.W., Ksibi M., Belgiorno V., Naddeo V. Indoor versus outdoor transmission of SARS-COV-2: environmental factors in virus spread and underestimated sources of risk. Euro-Mediterr. J. Environ. Integr. 2021;6(30):1–9. doi: 10.1007/s41207-021-00243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Torboli V., Fontana F., Clemente L. SARS-Cov-2RNA found on particulate matter of Bergamo in northern Italy: first evidence. Environ. Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou Y., Huang Y., Zhu X., Liu C., Hu Y., Wang H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer's disease. Ecotoxicol. Environ. Saf. 2019;174:344–352. doi: 10.1016/j.ecoenv.2019.02.086. [DOI] [PubMed] [Google Scholar]
- Sicard P., Khaniabadi Y.O., Perez S., Gualtieri M., De Marco A. Effect of O(3), PM(10) and PM(2.5) on cardiovascular and respiratory diseases in cities of France, Iran and Italy. Environ. Sci. Pollut. Res. Int. 2019;26:32645–32665. doi: 10.1007/s11356-019-06445-8. [DOI] [PubMed] [Google Scholar]
- Sijan Z., Antkiewicz D.S., Heo J., Kado N.Y., Schauer J.J., Sioutas C., Shafer M.M. An in vitro alveolar macrophage assay for the assessment of inflammatory cytokine expression induced by atmospheric particulate matter. Environ. Toxicol. 2015;30:836–851. doi: 10.1002/tox.21961. [DOI] [PubMed] [Google Scholar]
- Somers C.M. Ambient air pollution exposure and damage to male gametes: human studies and in situ 'sentinel' animal experiments. Syst. Biol. Reprod. Med. 2011;57:63–71. doi: 10.3109/19396368.2010.500440. [DOI] [PubMed] [Google Scholar]
- Son E.S., Park J.W., Kim Y.J., Jeong S.H., Hong J.H., Kim S.H., Kyung S.Y. Effects of antioxidants on oxidative stress and inflammatory responses of human bronchial epithelial cells exposed to particulate matter and cigarette smoke extract. Toxicol. Vitro. 2020;67:104883. doi: 10.1016/j.tiv.2020.104883. [DOI] [PubMed] [Google Scholar]
- Srivastava A. COVID-19 and Air Pollution and Meteorology-an intricate relationship: a review. Chemosphere. 2021;263:128297. doi: 10.1016/j.chemosphere.2020.128297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Shi Y., Yang X., Zhao T., Duan J., Sun Z. DNA methylation: a critical epigenetic mechanism underlying the detrimental effects of airborne particulate matter. Ecotoxicol. Environ. Saf. 2018;161:173–183. doi: 10.1016/j.ecoenv.2018.05.083. [DOI] [PubMed] [Google Scholar]
- Sun B.Y., Shi Y.F., Li Y., Jiang J.J., Liang S., Duan J.C., Sun Z.W. Short-term PM2.5 exposure induces sustained pulmonary fibrosis development during post-exposure period in rats. J. Hazard Mater. 2020;385:121566. doi: 10.1016/j.jhazmat.2019.121566. [DOI] [PubMed] [Google Scholar]
- Tan Y., Yang R., Zhao J., Cao Z., Chen Y., Zhang B. The associations between air pollution and adverse pregnancy outcomes in China. Adv. Exp. Med. Biol. 2017;1017:181–214. doi: 10.1007/978-981-10-5657-4_8. [DOI] [PubMed] [Google Scholar]
- Thongkum W., Khiewkhern S., Thitisutthi S. Statistical analysis of air pollutants concentration and health information related to respiratory disease patients in bangkok, Thailand. Stud. Health Technol. Inf. 2020;272:399–402. doi: 10.3233/SHTI200579. [DOI] [PubMed] [Google Scholar]
- Tian F., Qi J., Wang L., Yin P., Qian Z.M., Ruan Z., Liu J., Liu Y., Mcmillin S.E., Wang C., Lin H., Zhou M. Differentiating the effects of ambient fine and coarse particles on mortality from cardiopulmonary diseases: a nationwide multicity study. Environ. Int. 2020;145:106096. doi: 10.1016/j.envint.2020.106096. [DOI] [PubMed] [Google Scholar]
- Tofler G.H., Muller J.E. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114:1863–1872. doi: 10.1161/CIRCULATIONAHA.105.596189. [DOI] [PubMed] [Google Scholar]
- Torres M., Carranza C., Sarkar S., Gonzalez Y., Osornio Vargas A., Black K., Meng Q., Quintana-Belmares R., Hernandez M., Angeles Garcia J.J.F., P Ramo-Figueroa V.H., I Iguez-Garcia M.A., Flores J.L., Zhang J.J., Gardner C.R., Ohman-Strickland P., Schwander S. Urban airborne particle exposure impairs human lung and blood Mycobacterium tuberculosis immunity. Thorax. 2019;74:675–683. doi: 10.1136/thoraxjnl-2018-212529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung N.T., Cheng P.C., Chi K.H., Hsiao T.C., Jones T., BéruBé K., Ho K.F., Chuang H.C. Particulate matter and SARS-CoV-2: a possible model of COVID-19 transmission. Sci. Total Environ. 2021;750:141532. doi: 10.1016/j.scitotenv.2020.141532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A., Fiotakis K., Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrio E., Jung H., Anastasio C. Generation of hydroxyl radicals from dissolved transition metals in surrogate lung fluid solutions. Atmos. Environ. 2008;42(1994):4369–4379. doi: 10.1016/j.atmosenv.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Aaron C.P., Madrigano J., Hoffman E.A., Angelini E., Yang J., Laine A., Vetterli T.M., Kinney P.L., Sampson P.D., Sheppard L.E., Szpiro A.A., Adar S.D., Kirwa K., Smith B., Lederer D.J., Diez-Roux A.V., Vedal S., Kaufman J.D., Barr R.G. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. Jama. 2019;322:546–556. doi: 10.1001/jama.2019.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Gan X., Li F., Chen Y., Fu W., Zhu X., Xu D., Long M., Xu D. PM(2.5) exposure induces more serious apoptosis of cardiomyocytes mediated by Caspase3 through JNK/P53 pathway in hyperlipidemic rats. Int. J. Biol. Sci. 2019;15:24–33. doi: 10.7150/ijbs.28633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Zhao J., Wang Y., Mao Y., Zhao X., Huang P., Liu Q., Ma Y., Yao Y., Yang Z., Yuan W., Cui W., Payne T.J., Li M.D. Genome-wide DNA methylation analysis reveals significant impact of long-term ambient air pollution exposure on biological functions related to mitochondria and immune response. Environ. Pollut. 2020;264:114707. doi: 10.1016/j.envpol.2020.114707. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu T., Meng T. Ambient particulate matter triggers dysfunction of subcellular structures and endothelial cell apoptosis through disruption of redox equilibrium and calcium homeostasis. J. Hazard Mater. 2020;394:122439. doi: 10.1016/j.jhazmat.2020.122439. [DOI] [PubMed] [Google Scholar]
- Wang Y., Tang M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci. Total Environ. 2020;710:136397. doi: 10.1016/j.scitotenv.2019.136397. [DOI] [PubMed] [Google Scholar]
- Ward-Caviness C.K., Weaver A.M., Buranosky M., Pfaff E.R., Neas L.M., Devlin R.B., Schwartz J., Di Q., Cascio W.E., Diaz-Sanchez D. Associations between long-term fine particulate matter exposure and mortality in heart failure patients. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2018. WHO Global Ambient Air Quality Database (Update 2018)https://www.who.int/airpollution/data/cities/en/ [Google Scholar]
- WHO . 2018. World Health Organisation, More than 90% of the World's Children Breathe Toxic Air Every Day. News Release, 29 October 2018, Geneva.https://www.who.int/news-room/detail/29-10-2018-more-than-90-of-the-world%E2%80%99s-children-breathe-toxic-air-every-day [Google Scholar]
- WHO . 2018. World Health Organisation, Ambient (outdoor) air pollution (2 May 2018)https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations: Scientific Brief. [Google Scholar]
- WHO . 2020. World Health Organization. Clinical Management of COVID-19 Disease, Interim Guidance (27 May 2020)https://www.who.int/publications/i/item/clinical-management-of-covid-19 [Google Scholar]
- WHO . 2020. World Health Organization. Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. (Updated 9th July 2020)https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions [Google Scholar]
- WHO . 2021. Weekly Operational Update on COVID-19 - 5 April 2021.https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---5-april-2021 [Google Scholar]
- WHO/Europe . WHO Regional Office for Europe Denmark; 2020. WHO Announces COVID-19 Outbreak a Pandemic. [Google Scholar]
- Woodward N.C., Levine M.C., Haghani A., Shirmohammadi F., Saffari A., Sioutas C., Morgan T.E., Finch C.E. Toll-like receptor 4 in glial inflammatory responses to air pollution in vitro and in vivo. J. Neuroinflammation. 2017;14:84. doi: 10.1186/s12974-017-0858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington M.A., Petkova E., Freudenreich O., Cather C., Holt D., Bello I., Diminich E., Tang Y., Ardekani B.A., Zeng B., Wu R., Fan X., Zhao J., Wang J., Goff D.C. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr. Res. 2020;218:63–69. doi: 10.1016/j.schres.2020.03.001. [DOI] [PubMed] [Google Scholar]
- Wu G., Brown J., Zamora M.L., Miller A., Satterfield M.C., Meininger C.J., Steinhauser C.B., Johnson G.A., Burghardt R.C., Bazer F.W., Li Y., Johnson N.M., Molina M.J., Zhang R. Adverse organogenesis and predisposed long-term metabolic syndrome from prenatal exposure to fine particulate matter. Proc. Natl. Acad. Sci. U.S.A. 2019;116:11590–11595. doi: 10.1073/pnas.1902925116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. 2020. Exposure to Air Pollution and COVID-19 Mortality in the United States. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q., Hu S.T., Zeng X.L., Bao H.R., Liu X.J. Mechanisms of cytoskeleton and PI3Kδ-RhoA in fine particulate matter deteriorating phagocytosis defect of alveolar macrophage in mice with chronic obstructive pulmonary disease. Zhonghua Yixue Zazhi. 2017;97:1893–1898. doi: 10.3760/cma.j.issn.0376-2491.2017.24.011. [DOI] [PubMed] [Google Scholar]
- Xia S.Y., Huang D.S., Jia H., Zhao Y., Li N., Mao M.Q., Lin H., Li Y.X., He W., Zhao L. Relationship between atmospheric pollutants and risk of death caused by cardiovascular and respiratory diseases and malignant tumors in Shenyang, China, from 2013 to 2016: an ecological research. Chin. Med. J. (Engl.) 2019;132:2269–2277. doi: 10.1097/CM9.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C., Guo L., Qi R., Xi S. [Effects of air mixed certain pollutants on the expression of CC16 and certain cytokine in pulmonary tissue of rats] Wei Sheng Yan Jiu. 2007;36:679–682. [PubMed] [Google Scholar]
- Xie Y., Bo L., Jiang S., Tian Z., Kan H., Li Y., Song W., Zhao J. Individual PM2.5 exposure is associated with the impairment of cardiac autonomic modulation in general residents. Environ. Sci. Pollut. Res. 2016;23:10255–10261. doi: 10.1007/s11356-015-5933-1. [DOI] [PubMed] [Google Scholar]
- Xing X., Hu L., Guo Y., Bloom M.S., Li S., Chen G., Yim S.H.L., Gurram N., Yang M., Xiao X., Xu S., Wei Q., Yu H., Yang B., Zeng X., Chen W., Hu Q., Dong G. Interactions between ambient air pollution and obesity on lung function in children: the Seven Northeastern Chinese Cities (SNEC) Study. Sci. Total Environ. 2020;699:134397. doi: 10.1016/j.scitotenv.2019.134397. [DOI] [PubMed] [Google Scholar]
- Xiong J., Lipsitz O., Nasri F., Lui L.M., Gill H., Phan L., Chen-Li D., Iacobucci M., Ho R., Majeed A., McIntyre R.S. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J. Affect. Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Huang N., Wang Q., Liu H. Study of ambient PM2.5 on the influence of the inflammation injury and the immune function of subchronic exposure rats. Wei Sheng Yan Jiu. 2008;37:423–428. [PubMed] [Google Scholar]
- Xu Y., Wu J., Peng X., Yang T., Liu M., Chen L., Dai X., Wang Z., Yang C., Yan B., Jiang Y. LncRNA LINC00341 mediates PM(2.5)-induced cell cycle arrest in human bronchial epithelial cells. Toxicol. Lett. 2017;276:1–10. doi: 10.1016/j.toxlet.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Resp. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Peng X., Huang W., Chen R., Xu Z., Chen B., Kan H. A time-stratified case-crossover study of fine particulate matter air pollution and mortality in Guangzhou, China. Int. Arch. Occup. Environ. Health. 2012;85:579–585. doi: 10.1007/s00420-011-0707-7. [DOI] [PubMed] [Google Scholar]
- Yang S., Lee S.P., Park J.B., Lee H., Kang S.H., Lee S.E., Kim J.B., Choi S.Y., Kim Y.J., Chang H.J. PM2.5 concentration in the ambient air is a risk factor for the development of high-risk coronary plaques. Eur. Heart. J. Cardiovasc. Imaging. 2019;20:1355–1364. doi: 10.1093/ehjci/jez209. [DOI] [PubMed] [Google Scholar]
- Yang M., Zhou R., Qiu X., Feng X., Sun J., Wang Q., Lu Q., Zhang P., Liu B., Li W., Chen M., Zhao Y., Mo B., Zhou X., Zhang X., Hua Y., Guo J., Bi F., Cao Y., Ling F., Shi S., Li Y.G. Artificial intelligence-assisted analysis on the association between exposure to ambient fine particulate matter and incidence of arrhythmias in outpatients of Shanghai community hospitals. Environ. Int. 2020;139:105745. doi: 10.1016/j.envint.2020.105745. [DOI] [PubMed] [Google Scholar]
- Yang S., Huang X.L., Chen J., Mao L.N., Liu X., Yuan W.S., Wu X.J., Luo G.W. Curcumin protects BEAS-2B cells from PM 2.5-induced oxidative stress and inflammation by activating NRF2/antioxidant response element pathways. Int. J. Mol. Med. 2021;47(4):45. doi: 10.3892/ijmm.2021.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C., Wang Y., Williams C., Xu C., Kartsonaki C., Lin Y., Zhang P., Yin P., Lam K.B.H. The association between high particulate matter pollution and daily cause-specific hospital admissions: a time-series study in Yichang, China. Environ. Sci. Pollut. Res. Int. 2020;27:5240–5250. doi: 10.1007/s11356-019-06734-2. [DOI] [PubMed] [Google Scholar]
- Yao Y., Pan J., Wang W., Liu Z., Kan H., Qiu Y., Meng X., Wang W. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci. Total Environ. 2020;741:140396. doi: 10.1016/j.scitotenv.2020.140396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen Y.C., Yang C.Y., Ho C.K., Yen P.C., Cheng Y.T., Mena K.D., Lee T.C., Chen P.S. Indoor ozone and particulate matter modify the association between airborne endotoxin and schoolchildren's lung function. Sci. Total Environ. 2020;705:135810. doi: 10.1016/j.scitotenv.2019.135810. [DOI] [PubMed] [Google Scholar]
- Yi S., Zhang F., Qu F., Ding W.J. Water-insoluble fraction of airborne particulate matter (PM10) induces oxidative stress in human lung epithelial A549 cells. Environ. Toxicol. 2014;29:226–233. doi: 10.1002/tox.21750. [DOI] [PubMed] [Google Scholar]
- Younan D., Petkus A.J., Widaman K.F., Wang X., Casanova R., Espeland M.A., Gatz M., Henderson V.W., Manson J.E., Rapp S.R., Sachs B.C., Serre M.L., Gaussoin S.A., Barnard R., Saldana S., Vizuete W., Beavers D.P., Salinas J.A., Chui H.C., Resnick S.M., Shumaker S.A., Chen J.C. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer's disease. Brain. 2020;143:289–302. doi: 10.1093/brain/awz348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefian F., Mahvi A.H., Yunesian M., Hassanvand M.S., Kashani H., Amini H. Long-term exposure to ambient air pollution and autism spectrum disorder in children: a case-control study in Tehran, Iran. Sci. Total Environ. 2018;643:1216–1222. doi: 10.1016/j.scitotenv.2018.06.259. [DOI] [PubMed] [Google Scholar]
- Yue J.L., Liu H., Li H., Liu J.J., Hu Y.H., Wang J., Lu L., Wang F. Association between ambient particulate matter and hospitalization for anxiety in China: a multicity case-crossover study. Int. J. Hyg Environ. Health. 2020;223:171–178. doi: 10.1016/j.ijheh.2019.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang T., Zheng X., Wang X., Zhao H., Wang T., Zhang H., Li W., Shen H., Yu L. Maternal exposure to PM(2.5) during pregnancy induces impaired development of cerebral cortex in mice offspring. Int. J. Mol. Sci. 2018;19(1):257. doi: 10.3390/ijms19010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Xie Y., Qian C., Li L., Jiang R., Kan H., Chen R., Song W. Imbalance of Th1 and Th2 cells in cardiac injury induced by ambient fine particles. Toxicol. Lett. 2012;208:225–231. doi: 10.1016/j.toxlet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Zhao J., Gao Z., Tian Z., Xie Y., Xin F., Jiang R., Kan H., Song W. The biological effects of individual-level PM(2.5) exposure on systemic immunity and inflammatory response in traffic policemen. Occup. Environ. Med. 2013;70:426–431. doi: 10.1136/oemed-2012-100864. [DOI] [PubMed] [Google Scholar]
- Zhao R.W., Guo Z.Q., Zhang R.X., Deng C.R., Dong W.Y., Zhuang G.S. The role of autophagy in PM2.5-induced inflammation in human nasal epithelial cells. Zhonghua er bi yan hou tou jing wai ke za zhi. 2019;54:510–516. doi: 10.3760/cma.j.issn.1673-0860.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Zhao H., Tong G., Liu J., Wang J., Zhang H., Bai J., Hou L., Zhang Z. IP3R and RyR channels are involved in traffic-related PM2. 5-induced disorders of calcium homeostasis. Toxicol. Ind. Health. 2019;35(5):339–348. doi: 10.1177/0748233719843763. [DOI] [PubMed] [Google Scholar]
- Zhao T., Tesch F., Markevych I., Baumbach C., Jan En C., Schmitt J., Romanos M., Nowak D., Heinrich J. Depression and anxiety with exposure to ozone and particulate matter: an epidemiological claims data analysis. Int. J. Hyg Environ. Health. 2020;228:113562. doi: 10.1016/j.ijheh.2020.113562. [DOI] [PubMed] [Google Scholar]
- Zhou T., Hu Y., Chen Y., Zhou K.K., Zhang B., Gao G.Q., Ma J.X. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010;51:4371–4379. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Hunag F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci. Total Environ. 2020;738:139825. doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]