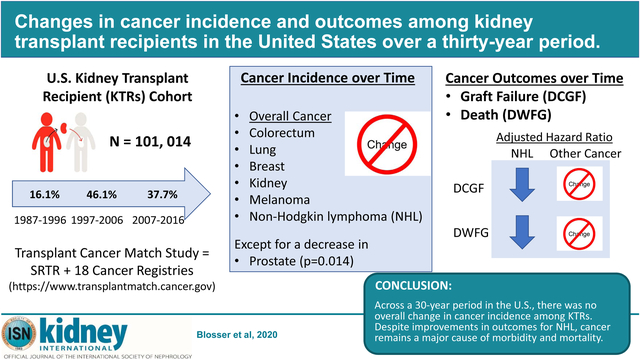
Abstract
Recipients of kidney transplants have elevated cancer risk compared with the general population. Improvements over time in transplant care and cancer treatment may have affected incidence and outcomes of cancer among recipients of kidney transplant. To evaluate this, we used linked United States transplant and cancer registry data to study 101,014 adult recipients of kidney transplants over three decades (1987–1996, 1997–2006, 2007–2016). Poisson regression was used to assess trends in incidence for cancer overall and seven common cancers. Associations of cancer with risk of death-censored graft failure (DCGF) and death with functioning graft (DWFG) were evaluated with Cox regression. We also estimated absolute risks of DCGF and graft failure following cancer for recipients transplanted in 2007–2016. There was no significant change in the incidence of cancer overall or for six common cancers in recipients across the 1987–2016 period. Only the incidence of prostate cancer significantly decreased across this period after multivariate adjustment. Among recipients of kidney transplants with non-Hodgkin lymphoma, there were significant declines over time in elevated risks for DCGF and DWFG but no significant changes for other combined cancers. For recipients transplanted in the most recent period (2007–2016), risks following cancer diagnosis remained high, with 38% experiencing DWFG and 14% graft failure within four years of diagnosis. Absolute risk of DWFG was especially high following lung cancer (78%), non-Hodgkin lymphoma (38%), melanoma (35%), and colorectal cancer (49%). Thus, across a 30-year period in the United States, there was no overall change in cancer incidence among recipients of kidney transplants. Despite improvements for non-Hodgkin lymphoma, cancer remains a major cause of morbidity and mortality.
Keywords: Cancer, Incidence, Graft Failure, Mortality, Kidney Transplant
Graphical Abstract
Introduction
Kidney transplantation is the preferred treatment for end-stage kidney disease (ESKD), but eligible patients have to wait months or years due to a scarcity of donor organs.1 Patient survival on the kidney transplant waitlist has improved over time, most notably for individuals over 55 years of age.1 Hence, older patients comprise an increasing proportion of kidney transplant recipients (KTRs), and they are living longer after transplantation.1
KTRs are at elevated risk of cancer compared with the general population.2,3 An important component of this elevation is explained by the effects of immunosuppressive medications, which are required to prevent graft rejection. Decreased immune surveillance results in loss of control of oncogenic viral infections, leading to development, for example, of non-Hodgkin lymphoma (NHL) caused by Epstein-Barr virus.2,3 Risk of certain other cancers, including melanoma, lung cancer, and kidney cancer, is also increased among KTRs, with contributions likely arising from immunosuppression as well as other factors including additional carcinogenic effects of medications or underlying ESKD.4–6 Risk is not increased to the same degree for other cancers that are common in the general population (e.g., colorectal cancer) and may actually be decreased for unclear reasons for certain other cancers (notably, prostate and breast cancers).2,3 Among KTRs, changes over time in cancer incidence could reflect factors unique to KTRs (e.g., changes in waitlist time or immunosuppressive medications) or trends in the general population.
Contemporary health care has improved post-transplant disease management, resulting in longer graft survival as well as a decrease in overall morbidity and mortality due to two of the leading causes of death -- cardiovascular disease and infection.1,7–9 Independent of whether there has been a change in cancer incidence, the improved survival of transplant recipients has resulted in an increased absolute risk for developing cancer.10 Cancer is the second or third leading cause of death in KTRs, and KTRs with early-onset cancers have a 3-fold higher risk of death in the first 3 years compared with those without cancer in the same period of time.11–13 The last decade has led to a burgeoning knowledge about the genetic and molecular complexity of cancer and the hopeful application of targeted therapies.14 Treatment of cancer in KTRs can be complicated by off-target effects, including rejection and graft failure.15
Among KTRs, failure of the transplanted kidney is associated with substantial morbidity, poor quality of life, and mortality.16–19 Patients who return to dialysis after graft failure have 3-fold greater risk of death compared with patients with a functioning graft, and they experience poorer quality of life and greater mortality than incident dialysis patients.16,19 There are multiple causes for graft failure, and the risk associated with cancer treatment is unknown. Clinicians often reduce the intensity of maintenance immunosuppressive medications in cancer patients to help control the malignancy, which may increase the risk of graft rejection and failure.20
The field of oncology is rapidly changing and post-transplant survival is improving. In the present study of KTRs in the United States, we assessed changes in the incidence of overall cancer along with seven common cancers and their association with graft failure, mortality, and the combined outcomes over the latest three decades.
Results
We evaluated a cohort of 101,014 KTRs, with 351,127 person-years of follow-up over 3 decades of transplantation (1987–2016). Most KTRs were male (60.2%). Recipients in this study were similar to the changing US KTR population (Table 1). In particular, there was an increase in age at transplantation over time (mean age 44.3, 48.6, and 50.9 years, respectively, for transplants in 1987–1996, 1997–2006, and 2007–2016) and growing racial/ethnic diversity. We also observed increasing body mass index and prevalence of ESKD due to diabetes mellitus or hypertension (Table 1). Across the 3 decades there were longer pre-transplant waiting times and increased living donation rates, along with more use of polyclonal or interleukin-2 receptor antibody induction and maintenance immunosuppression with tacrolimus and mycophenolate-based therapies. The use of corticosteroids for induction also increased, while use of corticosteroids for maintenance declined over time.
Table 1:
Demographic and clinical characteristics of kidney transplant recipients in the United States, by transplant year.
| 1987–1996 | 1997–2006 | 2007–2016 | Total | |
|---|---|---|---|---|
| Transplant recipients = | 16289 (16.1%) | 46595 (46.1%) | 38130 (37.7%) | 101014 (100.0%) |
| Person Years = | 64458 (18.4%) | 187658 (53.4%) | 99010 (28.2%) | 351127 (100.0%) |
| Gender | ||||
| Female | 6388 (39.2%) | 18614 (39.9%) | 15158 (39.8%) | 40160 (39.8%) |
| Male | 9901 (60.8%) | 27981 (60.1%) | 22972 (60.2%) | 60854 (60.2%) |
| Age in Years at Transplant, Mean (Standard Deviation) | 44.3 (12.8) | 48.6 (13.3) | 50.9 (13.5) | 48.7 (13.5) |
| Race/Ethnicity | ||||
| White, Non-Hispanic | 9579 (58.8%) | 23503 (50.4%) | 17685 (46.4%) | 50767 (50.3%) |
| Black, Non-Hispanic | 3676 (22.6%) | 11929 (25.6%) | 9418 (24.7%) | 25023 (24.8%) |
| Hispanic | 2064 (12.7%) | 7902 (17.0%) | 8005 (21.0%) | 17971 (17.8%) |
| Asian/Pacific Islander | 970 (6.0%) | 3261 (7.0%) | 3022 (7.9%) | 7253 (7.2%) |
| Body Mass Index | ||||
| < 18.5 | 829 (5.1%) | 1519 (3.3%) | 839 (2.2%) | 3187 (3.2%) |
| 18.5 – 24.9 | 8016 (49.2%) | 16989 (36.5%) | 11370 (29.8%) | 36375 (36.0%) |
| 25.0 – 29.9 | 4934 (30.3%) | 15697 (33.7%) | 12962 (34.0%) | 33593 (33.3%) |
| 30 – 34.9 | 1818 (11.2%) | 8448 (18.1%) | 8689 (22.8%) | 18955 (18.8%) |
| ≥ 35.0 | 692 (4.2%) | 3942 (8.5%) | 4270 (11.2%) | 8904 (8.8%) |
| Cause of ESKD | ||||
| Diabetes Mellitus | 3488 (21.4%) | 10910 (23.4%) | 10686 (28.0%) | 25084 (24.8%) |
| Hypertensive Nephrosclerosis | 2498 (15.3%) | 9686 (20.8%) | 8904 (23.4%) | 21088 (20.9%) |
| Glomerular Diseases | 5191 (31.9%) | 12586 (27.0%) | 9591 (25.2%) | 27368 (27.1%) |
| Polycystic Kidneys | 1537 (9.4%) | 4729 (10.1%) | 3944 (10.3%) | 10210 (10.1%) |
| Vascular Disease | 656 (4.0%) | 2080 (4.5%) | 816 (2.1%) | 3552 (3.5%) |
| Tubular/Interstitial Diseases | 632 (3.9%) | 1300 (2.8%) | 959 (2.5%) | 2891 (2.9%) |
| Congenital/Rare Familial/Metabolic Disorders | 525 (3.2%) | 1569 (3.4%) | 1027 (2.7%) | 3121 (3.1%) |
| Other/Unknown | 1762 (10.8%) | 3735 (8.0%) | 2203 (5.8%) | 7700 (7.6%) |
| Waiting Time for Transplant in Years, Mean (Standard Deviation) | 1.1 (1.2) | 2.4 (3.3) | 2.5 (2.3) | 2.2 (2.7) |
| Donor Type | ||||
| Deceased | 13948 (85.6%) | 32025 (68.7%) | 24267 (63.6%) | 70240 (69.5%) |
| Living | 2341 (14.4%) | 14570 (31.3%) | 13863 (36.4%) | 30774 (30.5%) |
| Induction Therapy | ||||
| Alemtuzumab | 0 (0%) | 2352 (5.0%) | 4983 (13.1%) | 7335 (7.3%) |
| IL-2 Receptor Antagonists | 57 (0.3%) | 14865 (31.9%) | 10338 (27.1%) | 25260 (25.0%) |
| Muromonab-CD3 | 2567 (15.8%) | 1141 (2.4%) | 72 (0.2%) | 3780 (3.7%) |
| Polyclonal Antibody | 2724 (16.7%) | 12518 (26.9%) | 17852 (46.8%) | 33094 (32.8%) |
| Corticosteroids | 6558 (40.3%) | 32970 (70.8%) | 25761 (67.6%) | 65289 (64.6%) |
| Maintenance Therapy | ||||
| Azathioprine | 10829 (66.5%) | 1360 (2.9%) | 92 (0.2%) | 12281 (12.2%) |
| Cyclosporine | 13885 (85.2%) | 15623 (33.5%) | 2424 (6.4%) | 31932 (31.6%) |
| Mycophenolate Mofetil | 2119 (13.0%) | 37042 (79.5%) | 35278 (92.5%) | 74439 (73.7%) |
| Tacrolimus | 1030 (6.3%) | 25911 (55.6%) | 30615 (80.3%) | 57556 (57.0%) |
| mTOR Inhibitors | 64 (0.4%) | 5471 (11.7%) | 1948 (5.1%) | 7483 (7.4%) |
| Corticosteroids | 15448 (94.8%) | 38570 (82.8%) | 25135 (65.9%) | 79153 (78.4%) |
This study includes data from 18 US cancer registries: California (years of coverage: 1988–2012), Colorado (1988–2014), Connecticut (1973–2009), Florida (1981–2009), Georgia (1995–2010), Hawaii (1973–2007), Illinois (1986–2013), Iowa (1973–2009), Kentucky (1995–2011), Michigan (1985–2009), North Carolina (1990–2010), New Jersey (1979–2010), New York (1976–2010), Ohio (1996–2015), Pennsylvania (1985–2013), Seattle-Puget Sound area of Washington State (1974–2014), Texas (1995–2014), Utah (1973–2008).
Abbreviations: ESKD end-stage kidney disease, IL-2 interleukin-2, mTOR mammalian target of rapamycin
Trends in cancer incidence in KTRs
Based on data in state/regional cancer registries, KTRs were linked to 3,378 first cancer diagnoses during follow-up. There was no significant change in overall cancer incidence over the three decades, both in unadjusted analyses and after adjustment for recipient and transplant characteristics and immunosuppression medication regimen (Table 2). Similarly, among seven selected cancer types, there was no change in the incidence of colorectal, lung, or breast cancers, or of melanoma or NHL (Table 2). In contrast, while there was no change in the incidence of prostate cancer in unadjusted analyses, there was a decrease over time after multivariate adjustment (adjusted incidence rate ratio [IRR] [95% confidence interval (CI)] 0.78 [0.46–1.31] and 0.48 [0.27–0.86] for 1997–2006 and 2007–2016, respectively, compared with 1987–1996; p-trend=0.01). Furthermore, the incidence of kidney cancer increased significantly over time in the unadjusted analysis (IRR [95%CI] 1.81 [1.01–3.22] for 1997–2006 and 2.47 [1.36–4.49] for 2007–2016 compared with 1987–1996; p-trend<0.01). This trend was attenuated and no longer significant after adjustment (p=0.18).
Table 2:
Incidence rate ratios for cancer among US kidney transplant recipients, by calendar decade of transplant
| Number of Cases | Unadjusted IRR (95%CI)a | Adjusted IRR (95%CI)a, b | P-Value for Trend | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1987–1996 | 1997–2006 | 2007–2016 | 1997–2006 | 2007–2016 | 1997–2006 | 2007–2016 | Unadjusted | Adjustedb | |
| All Cancer | 560 | 1848 | 970 | 1.13 (0.97, 1.32) | 1.13 (0.95, 1.33) | 0.98 (0.76, 1.26) | 0.89 (0.67, 1.17) | 0.158 | 0.398 |
| Colorectum | 41 | 97 | 38 | 0.81 (0.49, 1.36) | 0.60 (0.32, 1.13) | 0.79 (0.37, 1.70) | 0.50 (0.20, 1.24) | 0.112 | 0.137 |
| Lung | 75 | 216 | 93 | 0.99 (0.67, 1.46) | 0.81 (0.51, 1.27) | 0.74 (0.43, 1.26) | 0.54 (0.29, 1.01) | 0.354 | 0.054 |
| Melanoma | 29 | 85 | 53 | 1.01 (0.53, 1.92) | 1.19 (0.59, 2.39) | 0.68 (0.25, 1.83) | 0.67 (0.22, 2.03) | 0.624 | 0.475 |
| Breastc | 37 | 105 | 53 | 0.98 (0.56, 1.73) | 0.89 (0.47, 1.68) | 0.68 (0.29, 1.62) | 0.62 (0.23, 1.67) | 0.709 | 0.343 |
| Prostatec | 76 | 261 | 105 | 1.20 (0.82, 1.75) | 0.91 (0.58, 1.41) | 0.78 (0.46, 1.31) | 0.48 (0.27, 0.86) | 0.655 | 0.014 |
| Kidney | 39 | 205 | 148 | 1.81 (1.01, 3.22) | 2.47 (1.36, 4.49) | 1.37 (0.57, 3.24) | 1.88 (0.74, 4.77) | 0.003 | 0.182 |
| NHL | 75 | 188 | 122 | 0.86 (0.52, 1.42) | 1.06 (0.62, 1.81) | 0.85 (0.38, 1.90) | 1.01 (0.41, 2.50) | 0.834 | 0.984 |
Abbreviations: IRR incidence rate ratio, CI = confidence interval, NHL = non-Hodgkin lymphoma
Reference group is decade 1987–1996.
IRRs are adjusted for gender, age, race/ethnicity, body mass index, cause of ESKD, waiting time for transplant, kidney donor status (living vs. deceased), and induction and maintenance immunosuppression.
Breast cancer analyses are restricted to females, and prostate cancer analyses are restricted to males.
Bold text indicates results that are statistically significant (p < 0.05).
Table 3 presents standardized incidence ratios (SIRs) comparing cancer risk in KTRs to the general population. The SIR for overall cancer was elevated but declined over time, from 1.57 (95%CI 1.44–1.70) in 1987–1996 to 1.28 (1.20–1.36) in 2007–2016 (unadjusted p-trend=0.019), and similar significant declines in SIRs were observed specifically for colorectal and prostate cancers. However, only the trend for prostate cancer was significant in the adjusted analysis (p-trend=0.014).
Table 3:
Standardized incidence ratios for cancer among US kidney transplant recipients, by calendar decade of transplant
| Standardized incidence ratio (95%CI) | P-Value for Trend | ||||
|---|---|---|---|---|---|
| 1987–1996 | 1997–2006 | 2007–2016 | Unadjusted | Adjusteda | |
| All Cancer | 1.57 (1.44–1.70) | 1.31 (1.26–1.38) | 1.28 (1.20–1.36) | 0.019 | 0.844 |
| Colorectum | 1.13 (0.81–1.53) | 0.70 (0.57–0.86) | 0.55 (0.39–0.76) | 0.004 | 0.223 |
| Lung | 1.42 (1.12–1.78) | 1.15 (1.00–1.31) | 0.99 (0.80–1.21) | 0.180 | 0.375 |
| Melanoma | 3.08 (2.06–4.43) | 2.23 (1.78–2.76) | 2.43 (1.82–3.18) | 0.420 | 0.228 |
| Breastb | 0.86 (0.60–1.18) | 0.67 (0.55–0.81) | 0.62 (0.46–0.80) | 0.101 | 0.253 |
| Prostateb | 1.06 (0.83–1.32) | 0.82 (0.73–0.93) | 0.68 (0.55–0.82) | 0.002 | 0.014 |
| Kidney | 4.21 (2.99–5.76) | 4.33 (3.76–4.97) | 4.94 (4.17–5.80) | 0.578 | 0.237 |
| NHL | 4.51 (3.55–5.65) | 2.92 (2.52–3.37) | 3.38 (2.81–4.04) | 0.387 | 0.751 |
Abbreviations: CI = confidence interval, NHL = non-Hodgkin lymphoma
Trends are adjusted for gender, age, race/ethnicity, body mass index, cause of ESKD, waiting time for transplant, kidney donor status (living vs. deceased), and induction and maintenance immunosuppression.
Breast cancer analyses are restricted to females, and prostate cancer analyses are restricted to males. Bold text indicates trend tests that are statistically significant (p < 0.05).
Associations of cancer with death-censored graft failure and death with functioning graft among KTRs
For each decade of transplant, we next assessed the associations of cancer with subsequent risk for graft failure or death, capturing the earlier of these two outcomes. These outcomes are referred to as death-censored graft failure (DCGF, i.e., graft failure treating death as a censoring event) and death with functioning graft (DWFG, i.e., death treating graft failure as a censoring event); we also assessed the combined event of graft failure or death. For all cancers combined, there were persistently elevated risks in all three decades for DCGF (adjusted hazard ratio [HR] range of 1.85–2.30), DWFG (range 9.74–10.97), and the combined outcomes (range 5.38–6.12), without a significant change over the three decades (Table 4). However, for NHL in particular, the associations with these outcomes significantly diminished in magnitude across the calendar periods, with adjusted HR (95%CI) in 1987–1996 and 2007–2016, respectively, of 6.32 (3.86–10.34) and 2.31 (0.60–8.85) for DCGF (p=0.048 for heterogeneity across periods), 19.71 (14.01–27.74) and 11.72 (5.22–26.30) for DWFG (p=0.007), and 11.68 (8.84–15.45) and 7.14 (3.61–14.10) for the combined outcomes (p=0.002). For the non-NHL cancers combined, there were no significant changes in the associations across the calendar periods for DCGF (adjusted HRs 1.54–2.29), DWFG (9.30–10.17), or the combined outcome (4.69–5.80).
Table 4:
Associations of cancer with DCGF, DWFG, and the combined outcomes across calendar periods, by cancer diagnosis date lymphoma
| DCGF HR (95%C) | P-value | DWFG HR (95%CI) | P-value | Combined Outcomes HR (95%CI) | P-value | |
|---|---|---|---|---|---|---|
| All Cancer | 0.346 | 0.257 | 0.258 | |||
| 1987–1996 | 1.97 (1.51, 2.55) | 10.64 (9.19, 12.33) | 5.38 (4.76, 6.10) | |||
| 1997–2006 | 1.85 (1.04, 3.27) | 9.74 (7.12, 13.33) | 5.55 (4.25, 7.24) | |||
| 2007–2016 | 2.30 (1.24, 4.27) | 10.97 (7.78, 15.47) | 6.12 (4.57, 8.19) | |||
| NHL | 0.048 | 0.007 | 0.002 | |||
| 1987–1996 | 6.32 (3.86, 10.34) | 19.71 (14.01, 27.74) | 11.68 (8.84, 15.45) | |||
| 1997–2006 | 3.45 (1.15, 10.36) | 9.78 (4.57, 20.95) | 6.10 (3.27, 11.38) | |||
| 007–2016 | 2.31 (0.60, 8.85) | 11.72 (5.22, 26.30) | 7.14 (3.61, 14.10) | |||
| Non-NHL Cancer | 0.080 | 0.570 | 0.068 | |||
| 1987–1996 | 1.54 (1.14, 2.10) | 9.30 (7.94, 10.90) | 4.69 (4.09, 5.38) | |||
| 1997–2006 | 1.63 (0.84, 3.16) | 9.33 (6.66, 13.06) | 5.36 (4.00, 7.17) | |||
| 2007–2016 | 2.29 (1.13, 4.62) | 10.17 (7.03, 14.71) | 5.80 (4.22, 7.98) |
Abbreviations: DCGF death-censored graft failure, DWFG death with functioning graft, NHL non-Hodgkin lymphoma
Hazard ratio estimates (95% CIs) compare KTRs with cancer to those without cancer and are adjusted for age, gender, race/ethnicity (white, black, Hispanic, Asian), body mass index, kidney donor status (living vs. deceased), waiting time for transplant, cause of ESKD, and induction and maintenance immunosuppression medications.
P-values are for tests of interaction of cancer by calendar period, and bold text indicates interactions that are statistically significant (p < 0.05).
Absolute risks of DWFG and graft failure among KTRs with cancer
Finally, we estimated absolute risks for DWFG and graft failure over the 4-year period following cancer diagnosis for individuals transplanted in 2007–2016; unlike in the Cox models that produced the HRs above, these analyses treat graft failure and DWFG as competing rather than censoring events for each other, so we refer to this outcome as “graft failure” rather than “DCGF.” Overall, 38% of KTRs had a DWFG event and 14% had a graft failure event within 4 years of a cancer diagnosis (Figure 1). The absolute risk of DWFG was very high for KTRs with lung cancer (78%), NHL (38%), melanoma (35%), or colorectal cancer (49%). Absolute risk of DWFG was 20% or higher for KTRs with kidney or prostate cancer, and was noticeably lower only for breast cancer (7%). The absolute risk of graft failure was highest in KTRs with breast cancer (24%), kidney cancer (23%), or prostate cancer (23%). The absolute risk of the combined outcomes within 4 years after cancer diagnosis ranged from 31% for breast cancer to 87% for lung cancer.
Figure 1. Cumulative incidence of DWFG and graft failure following a cancer diagnosis among KTRs transplanted in 2007–2016.
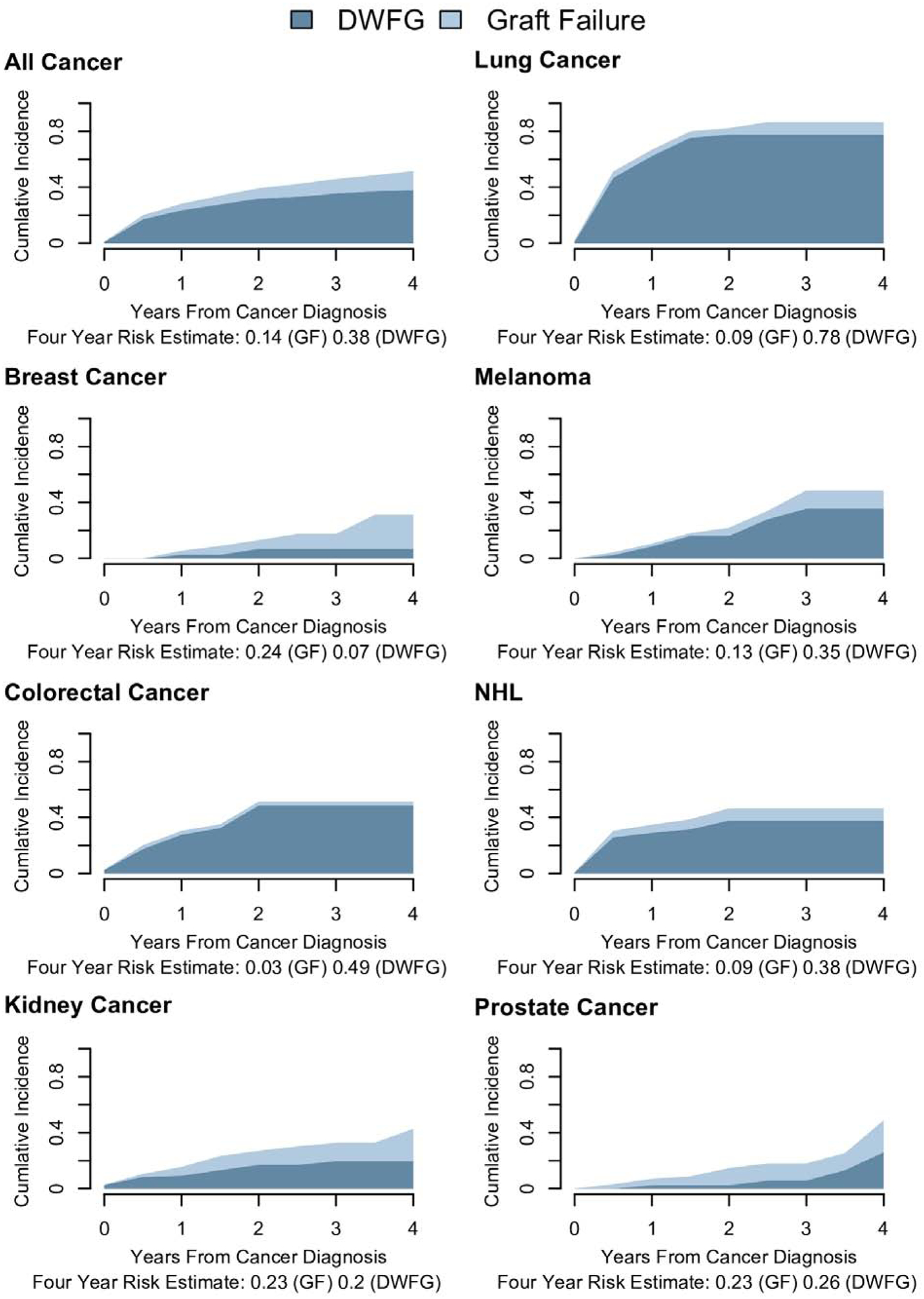
Cumulative incidence was estimated using competing risk methods. Results are shown for graft failure and death with functioning graft (DWFG) as stacked regions. The proportion of KTRs with each event at four years after transplantation are presented below each panel.
Abbreviations: DWFG death with functioning graft, GF graft failure, NHL non-Hodgkin lymphoma.
Discussion
Cancer continues to be a major cause of morbidity and mortality among KTRs. In the present study, there was no overall change in cancer incidence across a 30-year period in the US. KTRs with NHL experienced improved outcomes over time, with declines in the relative risk of DWFG and DCGF, but there was no change in these associations for other cancers combined. Indeed, the absolute risks of DWFG and graft failure following a cancer diagnosis remained high for the most recent KTRs who were transplanted in 2007–2016.
The KTR population has become increasingly older, more diverse and with a higher BMI over time, while experiencing longer waiting times and relatively more living donor kidney transplants. Diabetes mellitus and hypertension caused a greater percentage of ESKD while glomerular diseases contributed a decreasing amount. Greater use of polyclonal and interleukin-2 receptor antibodies for induction, and tacrolimus and mycophenolate-based therapies as maintenance immunosuppression, was also noted. While maintenance corticosteroid use has decreased, the combination of corticosteroids and polyclonal or IL-2 receptor antibody induction has been a common approach over the last two decades and may have longer-term impact on the risk of cancer. Because age, other demographic and transplant characteristics, and individual immunosuppressive medications are associated with cancer incidence among KTRs, we adjusted for these factors in our multivariate analyses of cancer incidence (Table 2).3,5,21–25 These changes in the transplant population also led to trends in the cancer risk relative to the general population (Table 3); most SIR trends were not significant after multivariate adjustment.
We did not find a significant change over time in the overall incidence of cancer, but there was an increase in the incidence of kidney cancer in the unadjusted analysis. Most kidney cancers are renal cell carcinomas and among KTRs occur in the native kidneys in association with acquired cystic kidney disease.6,26 The rise in kidney cancer incidence over time could partly reflect the increasing age at transplantation, prior waitlist time, and body mass index in the KTR population, since all are risk factors for renal cell carcinoma.6,27,28 The trend in kidney cancer incidence was attenuated after multivariate adjustment that included these factors (Table 2), and we also did not observe a trend in risk relative to the general population (Table 3).
In contrast, there was a strong decline in prostate cancer incidence that was apparent in our adjusted analyses (Table 2). Male transplant recipients appear to have a reduced risk of prostate cancer compared to the general population.3 Among KTRs, this deficit has become more pronounced over time and was strongest in the most recent period (i.e., SIR of 0.68 in 2007–2016). The reasons for this pattern are unclear. Prostate cancer is often detected by prostate-specific antigen screening when it is asymptomatic, and there has been a decrease in prostate cancer screening in the US general population since 2012 reflecting changing guidelines.29,30 Two possible explanations for the deficit of prostate cancer among KTRs both relate to screening: either candidates for kidney transplantation are screened more frequently than men in the general population, so that there are fewer subsequent prostate cancers remaining to be diagnosed after transplantation, or KTRs are screened less frequently following transplantation than men in the general population, so that some cancers are missed. For both explanations, growing differences in screening between KTRs and the general population could then explain the decreasing calendar trend in prostate cancer incidence. It is not possible to determine which of these explanations, or whether another explanation, underlies the prostate cancer trend without more data.
Importantly, cancer among KTRs was associated with a substantially elevated risk of DCGF, DWFG and the combined outcomes. In the absence of strong unmeasured confounding, and given the magnitude of the HRs for DWFG and (for NHL) for DCGF, most of these adverse outcomes in cancer patients can be attributed to their cancer or its treatment. Specifically, the attributable fraction is given by the formula (HR-1)/HR.31 As an example, 91% of DWFG events among KTRs with cancer who were transplanted in 2007–2016 were attributable to their cancer or its treatment (based on the HR of 10.97 in Table 4).
For cancers other than NHL, there was no significant change in the risk of these adverse outcomes over time. These findings highlight the need for improved management of cancer in KTRs, with the coordinated aim of directly targeting the malignancy and maintaining a functioning kidney graft. Of note, the 2009 KDIGO Guidelines for the Care of Kidney Transplant Recipients advise weaning immunosuppression as tolerated in the setting of cancer.20 Although a reduction in immunosuppression may improve immunologic function to control cancer, not all cancers are immunoresponsive, and there are higher risks of graft failure when immunosuppression is decreased or withdrawn.32–34
For KTRs with NHL, we found significant declines in the relative risks for DCGF and DWFG over the 3 decades. The likely reasons for these improvements include greater understanding of the role of Epstein-Barr virus, which has facilitated surveillance of high-risk KTRs and early diagnosis of NHL and other forms of post-transplant lymphoproliferative disorder.35,36 Moreover, the advent of rituximab therapy in 1997 and its addition to chemotherapy regimens may also have contributed to improved outcomes.37–39 Despite these improvements over time, KTRs with NHL still had elevated risks of DCGF and DWFG compared with KTRs without cancer in the most recent calendar period.
Overall, KTRs with cancer experienced a very high risk of DWFG or graft failure in the four years after cancer diagnosis. The risk of graft failure in KTRs was higher with breast, kidney and prostate cancers, while the risk of death was especially high with lung and colorectal cancers, melanoma, and NHL. In light of relatively strong screening and treatment options for colorectal cancer and melanoma, the risk of early death in KTRs with a new diagnosis of either cancer was higher than expected. These cancers can be present and undiagnosed prior to transplant, and immunosuppressive therapy may increase their aggressiveness.5 Additionally, the risk of adverse outcomes in KTRs with kidney cancer and prostate cancer was high and points to the need for improved diagnostic and therapeutic options. Kidney transplant guidelines advise implementing a patient-centered cancer screening plan based on individual history and risks.20 Updated general population guidelines include annual low-dose computed tomography of the chest to screen for lung cancer in high risk individuals.40 Since mortality from lung cancer is relatively high, performing targeted lung cancer screening in KTRs appears appropriate.
Many changes in cancer management, including advanced diagnostics plus molecular and immune-based therapies, have markedly improved cancer outcomes in the general population, and they offer new opportunities for treatment in KTRs.14,41,42 However, there are unique risks in the KTR population that must be considered, including acute kidney injury from multiple therapeutic-related mechanisms (e.g., ischemic acute tubular necrosis, autoimmune-related direct or indirect injury, rejection).21,37,43 Use of immunotherapies has been attempted with variable success in organ transplant recipients, including achievement of cancer cure in some cases, but there is also a high risk of graft rejection, graft loss, and death.15,44 Further research in this area is thus required to understand and mitigate the risks.
This study provides an extensive analysis of incidence and outcomes of cancer after kidney transplant. Our study population is large and, due to selection based only on state of residence, representative of the US KTR population. In addition, we achieved near complete ascertainment of post-transplant cancers through linkage with cancer registries. A limitation, however, is that we lacked data on cancer screening, some major cancer risk factors (e.g., smoking, Epstein-Barr virus infection), and cancer treatment, so we could not assess how temporal changes in these factors might explain the trends we observed. Small numbers for specific cancers limited our analyses of incidence and outcomes. Non-melanoma skin cancers (specifically, squamous cell and basal cell carcinomas) are not collected in the participating cancer registries, so could not be evaluated. Although these cancers are common, they are not likely a major cause of graft loss or mortality.
In conclusion, with improvements in post-transplant management, KTRs are experiencing longer graft and overall survival.1 Unfortunately, there has been no major change in cancer incidence among KTRs in the US over the last 3 decades, and only KTRs with NHL have experienced declines in the relative risks for death and graft failure over time. Moreover, absolute risks of DWFG and graft failure remain high for KTRs with cancer, including those with NHL. These findings highlight the need for research on cancer screening, diagnostics, and therapeutics to enable more timely and accurate diagnoses and improve curative treatments in kidney transplant recipients.
Methods
The Transplant Cancer Match (TCM) Study (https://www.transplantmatch.cancer.gov) is a cohort study linking data on US solid organ transplant recipients from the Scientific Registry of Transplant Recipients (SRTR) with population-based cancer registries.3 At the time of this study, the TCM Study included data for 1987–2016 from 18 state and metropolitan area cancer registries (see Table 1 footnote) covering approximately half of the US transplant population based on state of residence. This study was approved by human subjects review committees at the National Cancer Institute and, as required, participating cancer registries.
For this study, we included all first kidney transplants that were performed in participating TCM areas during years with cancer registry coverage (see Table 1 notes). From the initial N=151,915 potentially eligible KTRs, we excluded those with less than 90 days of follow-up or a cancer diagnosis before or up to 90 days after transplant (N=6062), HIV infection (N=305), age < 18 years at transplantation (N=8345), or missing data on body mass index or waitlist time (N=36,189). Thus, our cohort included 101,014 KTRs. The SRTR provided information on KTR demographic and transplant characteristics as well as baseline immunosuppressive medications.
Cancer diagnoses in KTRs were identified from the linked cancer registries. In addition to cancer overall, information was analyzed for seven common cancers that were specified a priori: colorectal, lung, female breast, prostate, and kidney cancers, melanoma, and non-Hodgkin lymphoma. Squamous and basal cell skin cancers are not reported to cancer registries and were not included in this study. Only first cancers were considered.
Deaths were identified from the SRTR and cancer registries, and graft failure (i.e., chronic dialysis re-initiation or retransplantation) was identified from the SRTR. KTRs with death and graft failure recorded on the same day were classified as dying with a functioning graft. We limited study outcomes to the first five years after transplant to allow KTRs in all three calendar periods to have equivalent follow-up. Thus, follow-up began 90 days post-transplant and ended at the earliest of death, graft failure, transplantation of another organ, loss to follow-up, end of cancer registry coverage, or five years post-transplant.
KTRs were classified into three groups based on the decade in which they received their transplant: 1987–1996, 1997–2006, and 2007–2016. We used Poisson regression to compare cancer incidence rates across decades, with IRRs across decades calculated separately for cancer overall and each of the seven cancer types. In addition, a multivariate model was fitted adjusting for age (as a continuous variable), gender, race/ethnicity (white, black, Hispanic, Asian), body mass index, kidney donor status (living vs. deceased), waiting time for transplant (as a continuous variable, based on SRTR patient data), cause of ESKD, and reported induction and initial maintenance immunosuppression medications. Primary cause of ESKD was categorized as diabetes mellitus, glomerular diseases, hypertensive nephrosclerosis, polycystic kidney disease, tubular/interstitial diseases, vascular diseases, congenital/rare familial/metabolic disorders, or other/unknown conditions. Induction medications included alemtuzumab, interleukin-2 receptor antagonists, muromonab-CD3, polyclonal antibodies, and corticosteroids. Maintenance therapies included azathioprine, cyclosporine, mTOR inhibitors, mycophenolate mofetil/mycophenolic acid, tacrolimus, and corticosteroids. Induction and maintenance therapies were included as individual binary variables. For maintenance therapies, statistical interactions were included for azathioprine and cyclosporine, cyclosporine and mycophenolate mofetil/mycophenolic acid, as well as tacrolimus and mycophenolate mofetil/mycophenolic acid. For each model, p-values for linear trend across decade of transplant were calculated.
We calculated SIRs to compare cancer risk in KTRs to the general population. These SIRs incorporate general population expected rates stratified by age, sex, race/ethnicity, calendar year, and cancer registry region. SIR analyses exclude 62 Hispanic KTRs followed before 1992 because expected rates were unavailable. We used Poisson regression to assess calendar trends in SIRs, with the same adjustments as in the models for incidence.
To assess associations of cancer as a risk factor for DCGF, DWFG, and the combined event of graft failure or death within each period, we used Cox regression treating cancer as a time-dependent risk factor and included an interaction between decade of transplantation and cancer. The analyses of DCGF and DWFG each treat the complementary outcome (i.e., DWFG and DCGF) as a censoring event. We present HRs from unadjusted models and multivariate models with adjustment for the additional factors listed above. To assess changes in the associations over time, p-values from likelihood ratio tests were calculated to evaluate the significance of the interaction terms. Models were fitted for cancer overall, NHL, and all non-NHL cancers grouped together, because the number of DWFG and DCGF outcomes was too small for some decades to examine most cancers separately.
For the final decade of KTRs (transplanted in 2007–2016), absolute risks for graft failure and DWFG were estimated for the 4-year period following cancer diagnosis by constructing cumulative incidence curves using competing risk time-to-event methods. The estimates correspond to the cumulative incidence of each as the first event to occur. These curves were derived for cancer overall and each of the seven individual cancers. This manuscript satisfies all relevant components of the STROBE checklist for observational studies.
Acknowledgements
This research was supported in part by the Intramural Research Program of the National Cancer Institute. The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California (Tina Clarke), Colorado (Jack Finch), Connecticut (Lou Gonsalves), Florida (Brad Wohler), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa (Charles Lynch), Illinois (Lori Koch), Kentucky (Jaclyn Nee), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Ohio (Roberta Slocumb), Pennsylvania (Jim Rubertone), Texas (Leticia Nogueria), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank Kelly Yu at the National Cancer Institute for study management, and analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors.
The SRTR is currently operated under contract number HHSH250201500009C (Health Resources and Services Administration) by the Hennepin Healthcare Research Institute, Minneapolis, MN. Previously the SRTR was managed under contracts HHSH250201000018C and HHSH234200537009C. The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201800002I), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201300021I, N01-PC-2013-00021), New York (75N91018D00005 [Task Order 75N91018F00001]), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN2612013000171). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5U58DP003883-03), Maryland (U58DP12-1205 3919-03), Michigan (5U58DP003921-03), New Jersey (NU58DP003931-05-00), New York (5NU58DP006309), North Carolina (U58DP003933) and Texas (5U58DP000824-04). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, Massachusetts (Massachusetts Cancer Prevention and Control Cooperative Agreement 5458DP003920), New Jersey, New York (including the Cancer Surveillance Improvement Initiative), Texas, Utah, and Washington, as well as the University of Utah and Fred Hutchinson Cancer Research Center in Seattle, WA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr Blosser has received honoraria from CareDx and Natera.
References
- 1.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am J Transplant 2019;19 Suppl 2:19–123. [DOI] [PubMed] [Google Scholar]
- 2.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59–67. [DOI] [PubMed] [Google Scholar]
- 3.Engels EA, Pfeiffer RM, Fraumeni JF Jr., et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 2004;77:1777–82. [DOI] [PubMed] [Google Scholar]
- 5.Robbins HA, Clarke CA, Arron ST, et al. Melanoma Risk and Survival among Organ Transplant Recipients. J Invest Dermatol 2015;135:2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karami S, Yanik EL, Moore LE, et al. Risk of Renal Cell Carcinoma Among Kidney Transplant Recipients in the United States. Am J Transplant 2016;16:3479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 2005;294:2726–33. [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Maclean JR, Snyder JJ. Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol 2006;17:900–7. [DOI] [PubMed] [Google Scholar]
- 9.Foster BJ, Dahhou M, Zhang X, Platt RW, Hanley JA. Change in mortality risk over time in young kidney transplant recipients. Am J Transplant 2011;11:2432–42. [DOI] [PubMed] [Google Scholar]
- 10.Hall EC, Pfeiffer RM, Segev DL, Engels EA. Cumulative incidence of cancer after solid organ transplantation. Cancer 2013;119:2300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao Y, Everly JJ, Gross TG, et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation 2009;87:1347–59. [DOI] [PubMed] [Google Scholar]
- 12.van de Wetering J, Roodnat JI, Hemke AC, Hoitsma AJ, Weimar W. Patient survival after the diagnosis of cancer in renal transplant recipients: a nested case-control study. Transplantation 2010;90:1542–6. [DOI] [PubMed] [Google Scholar]
- 13.Dharnidharka VR, Naik AS, Axelrod D, et al. Clinical and Economic Consequences of Early Cancer After Kidney Transplantation in Contemporary Practice. Transplantation 2017;101:858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiberstis PA, Travis J. Stocking oncology’s medicine cabinet. Science 2017;355:1142–3. [DOI] [PubMed] [Google Scholar]
- 15.Alhamad T, Venkatachalam K, Linette GP, Brennan DC. Checkpoint Inhibitors in Kidney Transplant Recipients and the Potential Risk of Rejection. Am J Transplant 2016;16:1332–3. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2002;2:970–4. [DOI] [PubMed] [Google Scholar]
- 17.Bunthof KLW, Hazzan M, Hilbrands LB. Review: Management of patients with kidney allograft failure. Transplant Rev (Orlando) 2018;32:178–86. [DOI] [PubMed] [Google Scholar]
- 18.Kerr S, Johnson E, Pandian K, Gillingham K, Matas A. Psychological impact of a failed kidney transplant. Transplant Proc 1997;29:1573. [DOI] [PubMed] [Google Scholar]
- 19.Griva K, Davenport A, Harrison M, Newman SP. The impact of treatment transitions between dialysis and transplantation on illness cognitions and quality of life - a prospective study. Br J Health Psychol 2012;17:812–27. [DOI] [PubMed] [Google Scholar]
- 20.Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int 2010;77:299–311. [DOI] [PubMed] [Google Scholar]
- 21.Jhaveri KD, Shah HH, Patel C, Kadiyala A, Stokes MB, Radhakrishnan J. Glomerular diseases associated with cancer, chemotherapy, and hematopoietic stem cell transplantation. Adv Chronic Kidney Dis 2014;21:48–55. [DOI] [PubMed] [Google Scholar]
- 22.Hall EC, Engels EA, Pfeiffer RM, Segev DL. Association of antibody induction immunosuppression with cancer after kidney transplantation. Transplantation 2015;99:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Leeuwen MT, Grulich AE, Webster AC, et al. Immunosuppression and other risk factors for early and late non-Hodgkin lymphoma after kidney transplantation. Blood 2009;114:630–7. [DOI] [PubMed] [Google Scholar]
- 24.Safaeian M, Robbins HA, Berndt SI, Lynch CF, Fraumeni JF Jr., Engels EA. Risk of Colorectal Cancer After Solid Organ Transplantation in the United States. Am J Transplant 2016;16:960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall EC, Segev DL, Engels EA. Racial/ethnic differences in cancer risk after kidney transplantation. Am J Transplant 2013;13:714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scandling JD. Acquired cystic kidney disease and renal cell cancer after transplantation: time to rethink screening? Clin J Am Soc Nephrol 2007;2:621–2. [DOI] [PubMed] [Google Scholar]
- 27.Freisling H, Arnold M, Soerjomataram I, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer 2017;116:1486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weir HK, Thompson TD, Soman A, Moller B, Leadbetter S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer 2015;121:1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnani CJ, Li K, Seto T, et al. PSA Testing Use and Prostate Cancer Diagnostic Stage After the 2012 U.S. Preventive Services Task Force Guideline Changes. J Natl Compr Canc Netw 2019;17:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welch HG, Kramer BS, Black WC. Epidemiologic Signatures in Cancer. N Engl J Med 2019;381:1378–86. [DOI] [PubMed] [Google Scholar]
- 31.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. American journal of public health 1998;88:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae S, Garonzik Wang JM, Massie AB, et al. Early Steroid Withdrawal in Deceased-Donor Kidney Transplant Recipients with Delayed Graft Function. J Am Soc Nephrol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haller MC, Royuela A, Nagler EV, Pascual J, Webster AC. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev 2016:CD005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karpe KM, Talaulikar GS, Walters GD. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst Rev 2017;7:CD006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dharnidharka VR. Peripheral Blood Epstein-Barr Viral Nucleic Acid Surveillance as a Marker for Posttransplant Cancer Risk. Am J Transplant 2017;17:611–6. [DOI] [PubMed] [Google Scholar]
- 36.Dharnidharka VR. Comprehensive review of post-organ transplant hematologic cancers. Am J Transplant 2018;18:537–49. [DOI] [PubMed] [Google Scholar]
- 37.Trappe R, Oertel S, Leblond V, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol 2012;13:196–206. [DOI] [PubMed] [Google Scholar]
- 38.Ganne V, Siddiqi N, Kamaplath B, et al. Humanized anti-CD20 monoclonal antibody (Rituximab) treatment for post-transplant lymphoproliferative disorder. Clin Transplant 2003;17:417–22. [DOI] [PubMed] [Google Scholar]
- 39.Ghobrial IM, Habermann TM, Ristow KM, et al. Prognostic factors in patients with post-transplant lymphoproliferative disorders (PTLD) in the rituximab era. Leuk Lymphoma 2005;46:191–6. [DOI] [PubMed] [Google Scholar]
- 40.Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nixon AB, Schalper KA, Jacobs I, Potluri S, Wang IM, Fleener C. Peripheral immune-based biomarkers in cancer immunotherapy: can we realize their predictive potential? J Immunother Cancer 2019;7:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 43.Perazella MA, Shirali AC. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int 2019. [DOI] [PubMed] [Google Scholar]
- 44.Lipson EJ, Bagnasco SM, Moore J Jr., et al. Tumor Regression and Allograft Rejection after Administration of Anti-PD-1. N Engl J Med 2016;374:896–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


