Abstract
Background
Investigations about the impact and consequences of the COVID-19 infection on the mental health of patients with chronic diseases and those with immunosuppressive conditions are growing. The current study aimed to systematically review and meta-analysis of studies that evaluated the level of depression and anxiety in cancer patients during the COVID-19 pandemic.
Methods
The PubMed, Scopus and Web of Sciences databases were searched to retrieve potential studies from January 2020 to 3 January 2021. Summary data on frequency and mean of depression and anxiety were extracted. Random-effect meta-analysis was conducted to estimate overall prevalence, mean and standardized mean difference.
Results
Thirty-four studies were included in the systematic review, of them 21 studies included in meta-analysis. Overall depression and anxiety were 0.37 (0.27, 0.47); I2 = 99.05%, P value < 0.001 and 0.38 (0.31, 0.46); I2 = 99.08%, P value < 0.001, respectively. Compared to controls, cancer patients had higher anxiety level [standard mean difference (SMD 0.25 (95% CI 0.08, 0.42)].
Conclusion
Overall, the findings of this study suggest that the prevalence of depression and anxiety among patients with cancer during the COVID-19 pandemic can reach considerable levels, although observed substantial heterogeneity should be considered when interpreting the results.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12029-021-00643-9.
Keywords: Depression, Anxiety, Cancer patients, Coronavirus, COVID-19, Systematic review and meta-analysis
Introduction
Since the start of the coronavirus disease 2019 (COVID-19) pandemic, over 93 million reported cases and over 2 million deaths reported through to 21 January [1]. Several prognostic factors can increase risk of COVID-19 infection and related death. For example, patients with pre-existing medical conditions and those with immunosuppressed status e.g. cancer patients have an increased risk of COVID-19 infection and related death [2–4].
One of the main challenges in patients with cancer during the COVID-19 pandemic is the trade-off between increased risk of COVID-19 infection acquisition when receiving treatment or reduced risk of COVID-19 infection acquisition with postponing treatment [5]. COVID-19 infection, access to cancer care, recurrence and progression of cancer due to delay in the treatment were most important concerns among patients with cancer during the COVID-19 pandemic [6–8]. These concerns can be along with mental health distress and psychological effects in patients with cancer. Given the vulnerability of the patients with cancer to mental disorders [9–11], the psychological effects of the COVID-19 infection in patients with cancer needs to be more attention by caregivers and cancer patient organizations.
In the line of rapid investigations on the all COVID-19 aspects, studies on mental disorders e.g. depression and anxiety among patients with cancer are growing [6–8]. To provide good evidence about the status of mental disorders among patients with cancer during the COVID-19 pandemic, the purpose of this work was to systematically review and meta-analyze depression and anxiety among patients with cancer during the COVID-19 pandemic.
Methods
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard [12].
Search Strategy
A systematic literature search of PubMed, Web of Sciences and Scopus was performed from January 2020 to Sunday–2021 03 January, with no language restriction. The following search strategy was performed for the PubMed; ((((((Depression[MeSH Terms]) OR (Depression[Title/Abstract])) OR ((Major depressive disorder[MeSH Terms]) OR (Major depressive disorder[Title/Abstract]))) OR ((Depressive disorder[MeSH Terms]) OR (Depressive disorder[Title/Abstract]))) OR ((anxiety[MeSH Terms]) OR (anxiety[Title/Abstract]))) AND (((Neoplasms[MeSH Terms]) OR (cancer[Title/Abstract])) OR (tumor[Title/Abstract]))) AND ((((COVID-19[MeSH Terms]) OR (COVID-19[Title/Abstract])) OR (SARS-CoV-2[MeSH Terms])) OR (SARS-CoV-2[Title/Abstract])). The developed search strategy for searching in the databases is available in the supplementary file (Tables S1–S3).
Eligibility Criteria
The observational studies were included into this systematic review that fulfilled the following criteria: a) depression and anxiety were evaluated using specific and validated instruments e.g. questionnaire or questions e.g. self-grading score among patients with cancer and b) original articles or research letters that provided data on proportion of depression and anxiety and published during the COVID-19 pandemic. Articles including review, editorial, commentary and case report were excluded.
Data Extraction
Following information was extracted from studies included in systematic review: first author, country, study highlights (design, patients and method of data collection on depression and anxiety), sample size, cancer type, age, sex, depression and anxiety scales, descriptive statistics about depression and anxiety and determinates of depression and anxiety from multivariable analysis. All study selection and data extraction was performed by one author (EA).
Statistical Analysis
The proportion (prevalence) of depression and anxiety among patients with cancer follows a binomial distribution. Meta-analyses of the proportions were conducted using the metaprop program [13]. Metaprop is developed for meta-analysis of proportions especially when the proportion is near the boundary of zero or 1, and the confidence interval can exceed these boundaries [13]. As expected, the studies included used different scales to measure depression and anxiety. The standardized mean difference (SMD) suggested by Cohen (Cohen’s d) [14] was used to compare the difference mean of anxiety between patients with cancer and the comparison group. For interpreting the magnitude of the SMD, the values of 0.2, 0.5 and 0.8 were considered as small, medium and large effect sizes, respectively [14]. The overall estimates were presented with 95% confidence interval (CI). Statistical heterogeneity was identified and quantified using the I2 statistic, proposed by Higgins et al. [15]. The I2 statistic quantifies the amount of dispersion across studies in which it is more than the chance. Higgins cut points for heterogeneity were suggested as the following: 25% (low), 50% (moderate) and 75% (high) [15].
When the measure of outcome in meta-analysis studies is prevalence, publication bias may not be a problem and there are no significant levels that may have biased publications. The reason for non-publication in these types of studies usually is small studies with poor methods [16]. Therefore, we did not assess publication bias in this study.
Summary proportions were estimated using random-effect meta-analysis. The study weighting was performed using the DerSimonian-Laird method [17]. Statistical analysis was performed using the STATA/SE 11.0 (StataCorp, College Station, TX, USA).
Results
Study Characteristics
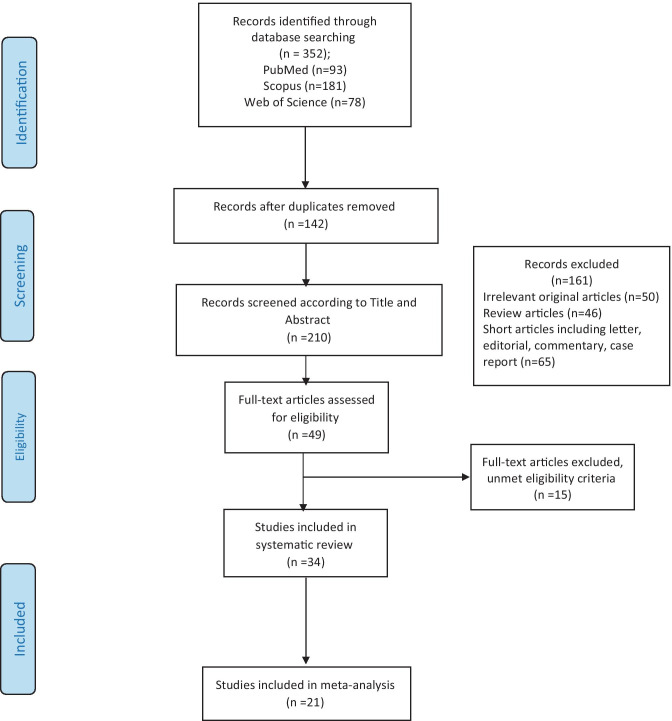
Figure 1 shows the flowchart describing the study selection process. A total of 352 records were retrieved from initial searches, of which 142 were duplicates, retaining 210 records for the next phase. After reviewing the title and abstract, 161 records were excluded because they were reviews, editorials, commentary and case report articles. Of the 49 potential eligible records, 15 were discarded. The majority of exclusion criteria were due to studies not providing sufficient data on depression and anxiety in cancer patients and did not use specific instruments. There were similar studies on the same dataset that we selected only one of them for the next phase. Finally, 34 articles [6–8, 18–47] were included in this systematic review and of them, 16 articles [6, 8, 20, 22, 26–29, 31, 35, 36, 39–42, 44–46] were considered for meta-analysis.
Fig. 1.
PRISMA flow diagram of the systematic review and meta-analyses selection process
Characteristics of studies included in systematic review are shown in Table S4. The majority of studies included had a cross-sectional design. Eleven of the studies included (32.3%) took place in China [8, 18–20, 27, 35, 40–42, 44, 45]. The sample size of the studies ranged from 31 [34] to 6213 [20] patients with cancer. Eighteen of 34 studies had specified that patients with breast cancer were one of cancer types under study [18, 19, 22, 24, 25, 28–30, 32, 33, 38, 40, 41, 43–47]. Hospital Anxiety and Depression Scale (HADS) was the most used questionnaire in the studies (n = 10, 29.4%) [6, 22, 26, 27, 29, 31, 36, 42, 46]. Generalized Anxiety Disorder 7-item (GAD-7) [20, 28, 30, 39, 40, 44, 48], Self-Rating Anxiety Scale (SAS) [8, 18, 19, 35, 45] and Patient Health Questionnaire (PHQ-9) [20, 40, 44, 48] were the other frequently used questionnaires. Three studies used specific anxiety related to the COVID-19 questionnaires [7, 24, 47].
Meta-Analysis of Depression and Anxiety
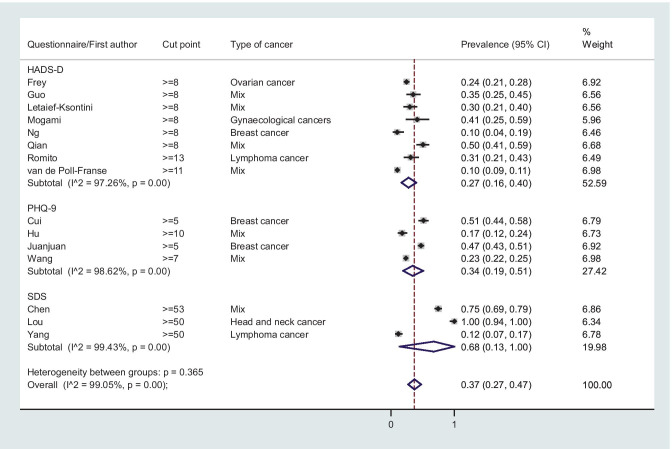
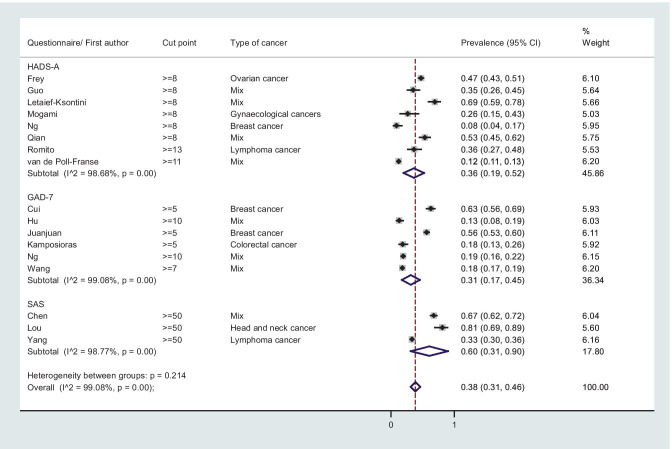
The overall prevalence (95% CI) of depression was 0.37 (0.27, 0.47); I2 = 99.05%, P-value < 0.001 (Fig. 2), and the corresponding figure for overall anxiety was 0.38 (0.31, 0.46); I2 = 99.08%, P-value < 0.001 (Fig. 3).
Fig. 2.
Forest plot of random meta-analysis for prevalence of depression among patients with cancer
Fig. 3.
Forest plot of random meta-analysis for prevalence of anxiety among patients with cancer
Subgroup Analysis According to Questionnaire
Hospital Anxiety and Depression Scale
The overall prevalence (95% CI) of depression among patients with cancer according to HADS-D 8 (mild, moderate and severe symptom) was 0.28 (0.18, 0.38); I2 = 96.60%, P-value < 0.001 (Fig. 2), while the corresponding figure for anxiety according to HADS-A 8 was 0.36 (0.19, 0.52); I2 = 98.68%, P-value < 0.001 (Fig. 3). The overall prevalence of moderate and severe depression and anxiety (HADS-D & HADS-A 11) were 0.10 (0.07, 14); I2 = 84.48, P-value < 0.001 and 0.18 (0.11, 0.25); I2 = 94.11%, P-value < 0.001, respectively (forest plots not shown). The overall mean (95% CI) of depression (HADS-D) and anxiety (HADS-A) was 6.08 (4.17, 7.98); I2 = 98.2%, P-value < 0.001 and 6.92 (4.44, 9.40); I2 = 98.8%, P-value < 0.001, respectively (Fig. S1).
Patient Health Questionnaire and Generalized Anxiety Disorder 7-item
The overall prevalence (95% CI) of depression among patients with cancer according to PHQ-9 5 (mild, moderate and severe symptom) was 0.34 (0.19, 0.49); I2 = 98.65, P-value < 0.001 (Fig. 2), while the overall prevalence of mild, moderate and severe anxiety (GAD-7 5) was 0.31 (0.17, 0.45); I2 = 99.08%, P-value < 0.001 (Fig. 3). The overall mean of anxiety (95% CI) according to GAD-7 was 5.84 (5.26, 6.41); I2 = 74.7, P-value = 0.02 (Fig. S2).
SDS & Self-Rating Anxiety Scale
The overall prevalence (95% CI) of mild, moderate and severe depression according to SDS 50 was 0.68 (0.13, 1.00); I2 = 99.43%, P-value < 0.001 (Fig. 2), and the overall mean (95% CI) of SDS was 54.14 (32.17, 76.10); I2 = 99.9%, P-value < 0.001 (Fig S3). The overall prevalence (95% CI) of mild, moderate and severe anxiety according to SAS ≥ 50 was 0.61 (0.31, 0.86); I2 = 98.77, P-value < 0.001 (Fig. 3), while the overall mean (95% CI) of SAS was 50.58 (40.18, 60.97); I2 = 99.8%, P-value < 0.001 (Fig. S3).
Mean Difference of Anxiety
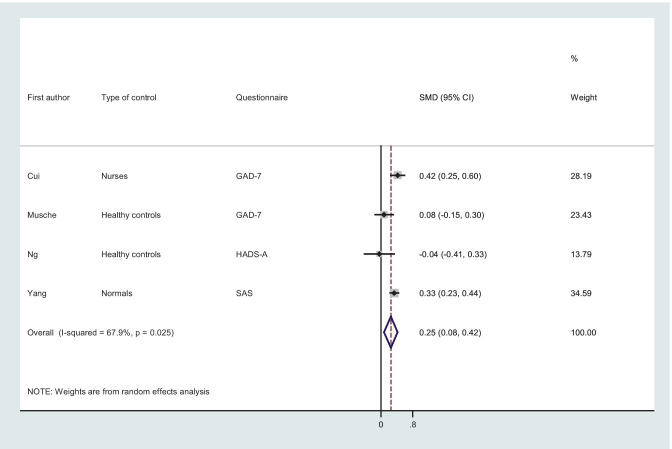
As shown in Fig. 4, the overall effect estimate suggested that patients with cancer had higher anxiety symptoms (SMD, 0.25; 95% CI, from 0.08 to 0.42; I2 = 67.9%, P-value = 0.02) than those in the control group.
Fig. 4.
Forest plot of random meta-analysis for mean difference of anxiety among patients with cancer; GAD-7; Generalized Anxiety Disorder Questionnaire, HADS-A; Hospital Anxiety and Depression Scale-depression, SAS; Self-Rating Anxiety Scale; SDM; standardized mean difference
Discussion
The present systematic review and meta-analysis aimed to assess the status of depression and anxiety among patients with cancer during the COVID-19 pandemic. Due to anticipation, patients with cancer may be concerned about COVID-19 and its effect on the treatment, recurrence and progression of cancer. This can make these patients more prone to anxiety and depression symptoms. In fact, after a conducted systematic review and meta-analysis, it was found that the people living with cancer have experienced the degree of anxiety and depression during COVID-19 so that prevalence of mild, moderate and severe depression and anxiety can exceed more than 30% and these patients experience more anxiety than healthy controls during the COVID-19 pandemic.
The previous meta-analyses of depression and anxiety in patients with cancers have shown that the overall estimates can be modified by several factors such as cancer types, time and method assessment of depression and anxiety, types (chemotherapy, radiotherapy, etc.) and time (pretreatment, on-treatment and post-treatment) of treatment, time from cancer diagnosis, consumption of time for assessment and status of disease (inpatients, outpatient, palliative care) [49–53]. For example, in a study by Walker et al. [50], the pooled prevalence of depression in outpatients ranged from 5 to 16% while it was 7 to 49% in palliative care. In our work, conducting more subgroup analysis was not possible due to a limited number of studies included in subgroups. We presented the overall estimates only according to method assessment of depression and anxiety. An analysis of the overall estimates in our meta-analysis demonstrated that the overall prevalence of depression and anxiety according to type of questionnaire was heterogeneous as a higher level of depression and anxiety estimated from SAS and SDS was observed compared to screening questionnaires including HADS, GAD-7 and PHQ-9. Our result is in line of previous meta-analysis on depression and anxiety in COVID‐19 patients [54] that showed higher depression and anxiety prevalence values from GAD-7 and SAS compared with HADS‐A, and they mentioned that it is due to lower cut-off values for PHQ‐9, GAD‐7, SAS and SDS for identifying depression and anxiety symptoms. Knowledge on comparing different method assessments in evaluating anxiety and depression in patients with cancer is still not well documented; however, some evidence suggested that HADS is a better option for identifying anxious and depressive states in cancer patients during clinical practice compared with other tools because of better compliance of patients, shorter consumption of time, good correlation with clinical features, good psychometric properties and specific of medical settings [55–58].
Our finding indicates that during the COVID-19 pandemic the level of depression and anxiety among patients with cancer can be reached to clinical importance level as the overall prevalence rates exceed more than 60% according to SDS and SAS. Moreover, cancer patients tend to experience higher levels of anxiety compared to healthy control. Other systematic reviews and meta-analyses showed a higher rate of both depression and anxiety during the COVID-19 crisis in some groups such as the general population [59], healthcare workers [60], COVID-19 patients [54] and pregnant women [61]. Such results send a clear message to the healthcare community about COVID-19 infection–related psychological effects and about targeting mental healthcare attention and resources for the needy at-risk group.
The present systematic review and meta-analysis has several limitations. First, we only included studies from three databases. Here, due to ignoring studies in the other databases and even gray literature, the risk of selection bias on the overall estimates should be considered. Second, due to observed substantial heterogeneity the results should be interpreted with caution and this heterogeneity needs to be explained via subgroup analysis and meta-regression analyses. However, the limited number of studies precludes conducting subgroup analysis e.g. overall prevalence of depression and anxiety in a given cancer type. Third, there were limited analytical studies that compared the depression and anxiety between patients with cancer and people without the disease (controls). Fourth, approximately one-third of studies included were from China, which can limit the generalizability of the finding.
Conclusion
In conclusion, the overall estimates of depression and anxiety among patients with cancer were considerable. Evidence from this systematic review and meta-analysis may highlight the importance of preventing, treating and identifying the most important determinants of depression and anxiety among patients with cancer during the COVID-19 pandemic.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
The Deputy of Research and Technology, Hamadan University of Medical Sciences, funded this study (Research ID:14000110149 Ethics code: IR.UMSHA.REC.1399.107).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/21/2021
The Funding statement of this article was updated from "The Deputy of Research and Technology, Hamadan University of Medical Sciences, funded this study." to "The Deputy of Research and Technology, Hamadan University of Medical Sciences, funded this study (Research ID:14000110149 Ethics code: IR.UMSHA.REC.1399.107).
References
- 1.Worldometer. COVID-19 Coronavirus Pandemic 2020 [updated January 19, 2021. Available from: https://www.worldometers.info/coronavirus/.
- 2.Deng G, Yin M, Chen X, Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Critical care (London, England) 2020;24(1):179. doi: 10.1186/s13054-020-02902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA oncology. 2020. [DOI] [PMC free article] [PubMed]
- 4.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21(5):629–630. doi: 10.1016/S1470-2045(20)30217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey MK, Ellis AE, Zeligs K, Chapman-Davis E, Thomas C, Christos PJ, et al. Impact of the coronavirus disease 2019 pandemic on the quality of life for women with ovarian cancer. Am J Obstet Gynecol. 2020;223(5):725.e1-.e9. [DOI] [PMC free article] [PubMed]
- 7.Rodler S, Apfelbeck M, Schulz GB, Ivanova T, Buchner A, Staehler M, et al. Telehealth in uro-oncology beyond the pandemic: toll or lifesaver? Eur Urol Focus. 2020;6(5):1097–1103. doi: 10.1016/j.euf.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, Dong D, Gu H, Gale RP, Ma J, Huang X. Impact of stopping therapy during the SARS-CoV-2 pandemic in persons with lymphoma. J Cancer Res Clin Oncol. 2020:1–11. [DOI] [PMC free article] [PubMed]
- 9.Akechi T, Nakano T, Okamura H, Ueda S, Akizuki N, Nakanishi T, et al. Psychiatric disorders in cancer patients: descriptive analysis of 1721 psychiatric referrals at two Japanese cancer center hospitals. Jpn J Clin Oncol. 2001;31(5):188–194. doi: 10.1093/jjco/hye039. [DOI] [PubMed] [Google Scholar]
- 10.Derogatis LR, Morrow GR, Fetting J, Penman D, Piasetsky S, Schmale AM, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751–757. doi: 10.1001/jama.1983.03330300035030. [DOI] [PubMed] [Google Scholar]
- 11.Singer S, Das-Munshi J, Brähler E. Prevalence of mental health conditions in cancer patients in acute care–a meta-analysis. Ann Oncol : official journal of the European Society for Medical Oncology. 2010;21(5):925–930. doi: 10.1093/annonc/mdp515. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systems Control Found Appl. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Archives of public health = Archives belges de sante publique. 2014;72(1):39. [DOI] [PMC free article] [PubMed]
- 14.Cohen J. Statistical power analysis for the behavioral sciences: Academic press; 2013.
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil. 2011;32(2):419–436. doi: 10.1016/j.ridd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Fu Z, Du L, He X, Li X, Chen J. Time to surgery in patients with breast cancer during the COVID-19 pandemic. Br J Surg. 2020;107(10):e419–e421. doi: 10.1002/bjs.11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang G, Xiao C, Li S, Yang N. The effect and mechanism of adverse childhood experience on suicide ideation in young cancer patients during coronavirus disease 2019 (COVID-19) pandemic. Risk management and healthcare policy. 2020;13:1293–1300. doi: 10.2147/RMHP.S266269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Duan Z, Ma Z, Mao Y, Li X, Wilson A, et al. Epidemiology of mental health problems among patients with cancer during COVID-19 pandemic. Transl Psychiatry. 2020;10(1):263. doi: 10.1038/s41398-020-00950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voisin MR, Oliver K, Farrimond S, Chee T, Arzbaecher J, Kruchko C, et al. Brain tumors and COVID-19: the patient and caregiver experience. Neuro-oncol adv. 2020;2(1):vdaa104. [DOI] [PMC free article] [PubMed]
- 22.van De Poll-Franse LV, De Rooij BH, Horevoorts NJE, May AM, Vink GR, Koopman M, et al. Perceived care and well-being of patients with cancer and matched norm participants in the COVID-19 crisis: results of a survey of participants in the Dutch PROFILES Registry. JAMA oncology. 2020. [DOI] [PMC free article] [PubMed]
- 23.Staehler M, Battle D, Pal SK, Bergerot CD. Counterbalancing COVID-19 with cancer surveillance and therapy: a survey of patients with renal cell carcinoma. Eur Urol Focus. 2020. [DOI] [PMC free article] [PubMed]
- 24.Sigorski D, Sobczuk P, Osmola M, Kuc K, Walerzak A, Wilk M, et al. Impact of COVID-19 on anxiety levels among patients with cancer actively treated with systemic therapy. Esmo Open. 2020;5(5). [DOI] [PMC free article] [PubMed]
- 25.Shinan-Altman S, Levkovich I, Tavori G. Healthcare utilization among breast cancer patients during the COVID-19 outbreak. Palliat Support Care. 2020;18(4):385–391. doi: 10.1017/S1478951520000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romito F, Dellino M, Loseto G, Opinto G, Silvestris E, Cormio C, et al. Psychological distress in outpatients with lymphoma during the COVID-19 pandemic. Front Oncol. 2020;10:1270. doi: 10.3389/fonc.2020.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Y, Wu K, Xu H, Bao D, Ran F, Wei W, et al. A survey on physical and mental distress among cancer patients during the COVID-19 epidemic in Wuhan. China J Palliative Med. 2020;23(7):888–889. doi: 10.1089/jpm.2020.0240. [DOI] [PubMed] [Google Scholar]
- 28.Ng KYY, Zhou S, Tan SH, Ishak NDB, Goh ZZS, Chua ZY, et al. Understanding the psychological impact of COVID-19 pandemic on patients with cancer, their caregivers, and health care workers in Singapore. JCO global oncology. 2020;6:1494–1509. doi: 10.1200/GO.20.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng DWL, Chan FHF, Barry TJ, Lam C, Chong CY, Kok HCS, et al. Psychological distress during the 2019 Coronavirus Disease (COVID-19) pandemic among cancer survivors and healthy controls. Psycho-oncology. 2020;29(9):1380–1383. doi: 10.1002/pon.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musche V, Bauerle A, Steinbach J, Schweda A, Hetkamp M, Weismuller B, et al. COVID-19-related fear and health-related safety behavior in oncological patients. Frontiers in Psychology. 2020;11. [DOI] [PMC free article] [PubMed]
- 31.Mogami T, Onuma E, Aoki M, Kamiya N, Sukegawa A, Miyagi E, et al. Increased anxiety and depression in patients with gynecologic cancers during the COVID-19 pandemic: a retrospective study from Japan. Int J Fed Gynaecol Obstet: the official organ of the Int Fed Gynaecol Obstet. 2020. [DOI] [PMC free article] [PubMed]
- 32.Mitra M, Basu M. A Study on challenges to health care delivery faced by cancer patients in India during the COVID-19 pandemic. J Prim Care Community Health. 2020;11:2150132720942705. doi: 10.1177/2150132720942705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miaskowski C, Paul SM, Snowberg K, Abbott M, Borno H, Chang S, et al. Stress and symptom burden in oncology patients during the COVID-19 pandemic. J Pain Symptom Manage. 2020;60(5):E25–E34. doi: 10.1016/j.jpainsymman.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahl C, Melo LRS, Almeida MHA, Carvalho CS, Santos LLS, Nunes PS, et al. Delay in head and neck cancer care during the COVID-19 pandemic and its impact on health outcomes. Braz Oral Res. 2020;34:e126. doi: 10.1590/1807-3107bor-2020.vol34.0126. [DOI] [PubMed] [Google Scholar]
- 35.Lou SC, Xu DJ, Li XD, Huan Y, Li J. Study of psychological state of cancer patients undergoing radiation therapy during novel coronavirus outbreak and effects of nursing intervention. Precis Med Sci. 2020;9(2).
- 36.Letaief-Ksontini F, Zenzri Y, Yahyaoui Y, Gabsi A, Mokrani A, Meddeb K, et al. Anxiety and depression in cancer patients during the COVID-19 pandemic: a single-centre study. Ann Oncol. 2020;31:S957–S958. doi: 10.1016/j.annonc.2020.08.2056. [DOI] [Google Scholar]
- 37.Kosir U, Loades M, Wild J, Wiedemann M, Krajnc A, Roskar S, et al. The impact of COVID-19 on the cancer care of adolescents and young adults and their well-being: results from an online survey conducted in the early stages of the pandemic. Cancer. 2020;126(19):4414–4422. doi: 10.1002/cncr.33098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karacin C, Bilgetekin I, B Basal F, Oksuzoglu OB. How does COVID-19 fear and anxiety affect chemotherapy adherence in patients with cancer. Future Oncol. 2020;16(29):2283–93. [DOI] [PMC free article] [PubMed]
- 39.Kamposioras K, Saunders M, Jonathan Lim KH, Marti K, Anderson D, Cutting M, et al. The impact of changes in service delivery in patients with colorectal cancer during the initial phase of the COVID-19 pandemic. Clin Colorectal Cancer. 2020. [DOI] [PMC free article] [PubMed]
- 40.Juanjuan L, Santa-Maria CA, Hongfang F, Lingcheng W, Pengcheng Z, Yuanbing X, et al. Patient-reported outcomes of patients with breast cancer during the COVID-19 outbreak in the epicenter of China: a cross-sectional survey study. Clin Breast Cancer. 2020;20(5):e651–e662. doi: 10.1016/j.clbc.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gultekin M, Ak S, Ayhan A, Strojna A, Pletnev A, Fagotti A, et al. Perspectives, fears and expectations of patients with gynaecological cancers during the COVID-19 pandemic: a Pan-European study of the European Network of Gynaecological Cancer Advocacy Groups (ENGAGe). Cancer med. 2020. [DOI] [PMC free article] [PubMed]
- 42.Guo Y, Cheng C, Zeng Y, Li Y, Zhu M, Yang W, et al. Mental health disorders and associated risk factors in quarantined adults during the COVID-19 outbreak in China: cross-sectional study. J Med Internet Res. 2020;22(8):e20328. doi: 10.2196/20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallagher S, Bennett KM, Roper L. Loneliness and depression in patients with cancer during COVID-19. J Psychosoc Oncol. [DOI] [PubMed]
- 44.Cui Q, Cai Z, Li J, Liu Z, Sun S, Chen C, et al. The psychological pressures of breast cancer patients during the COVID-19 outbreak in China-a comparison with frontline female nurses. Front Psych. 2020;11:559701. doi: 10.3389/fpsyt.2020.559701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G, Wu Q, Jiang H, Zhang H, Peng J, Hu J, et al. Fear of disease progression and psychological stress in cancer patients under the outbreak of COVID-19. Psycho-oncology. 2020;29(9):1395–1398. doi: 10.1002/pon.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chapman B, Swainston J, Grunfeld EA, Derakshan N. COVID-19 outbreak effects on job security and emotional functioning amongst women living with breast cancer. Front Psychol. 2020;11:582014. doi: 10.3389/fpsyg.2020.582014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahn MH, Lee J, Suh S, Lee S, Kim HJ, Shin YW, et al. Application of the Stress and Anxiety to Viral Epidemics-6 (SAVE-6) and Coronavirus Anxiety Scale (CAS) to measure anxiety in cancer patient in response to COVID-19. Front Psychol. 2020;11:604441. doi: 10.3389/fpsyg.2020.604441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu L, Tao H, Xu X, Chen H, Chang K, Pei X, et al. Factors for peripherally inserted central catheters care delay in cancer patients during the COVID-19 pandemic. Annals of palliative medicine. 2020;9(6):3818–3829. doi: 10.21037/apm-20-1887. [DOI] [PubMed] [Google Scholar]
- 49.Brandenbarg D, Maass S, Geerse OP, Stegmann ME, Handberg C, Schroevers MJ, et al. A systematic review on the prevalence of symptoms of depression, anxiety and distress in long-term cancer survivors: implications for primary care. Eur J Cancer Care. 2019;28(3):e13086. doi: 10.1111/ecc.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker J, Holm Hansen C, Martin P, Sawhney A, Thekkumpurath P, Beale C, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol: official journal of the European Society for Medical Oncology. 2013;24(4):895–900. doi: 10.1093/annonc/mds575. [DOI] [PubMed] [Google Scholar]
- 51.Watts S, Leydon G, Birch B, Prescott P, Lai L, Eardley S, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901. doi: 10.1136/bmjopen-2013-003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watts S, Prescott P, Mason J, McLeod N, Lewith G. Depression and anxiety in ovarian cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2015;5(11):e007618. doi: 10.1136/bmjopen-2015-007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang YL, Liu L, Wang Y, Wu H, Yang XS, Wang JN, et al. The prevalence of depression and anxiety among Chinese adults with cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13:393. doi: 10.1186/1471-2407-13-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng J, Zhou F, Hou W, Silver Z, Wong CY, Chang O, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2020. [DOI] [PMC free article] [PubMed]
- 55.Annunziata MA, Muzzatti B, Bidoli E, Flaiban C, Bomben F, Piccinin M, et al. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support Care Cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2020;28(8):3921–3926. doi: 10.1007/s00520-019-05244-8. [DOI] [PubMed] [Google Scholar]
- 56.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. [DOI] [PubMed]
- 57.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale–a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41. doi: 10.1016/S0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 58.Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101(21):1464–1488. doi: 10.1093/jnci/djp336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob Health. 2020;16(1):57. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav Immun. 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun F, Zhu J, Tao H, Ma Y, Jin W. A systematic review involving 11,187 participants evaluating the impact of COVID-19 on anxiety and depression in pregnant women. J Psychosom Obstet Gynaecol. 2020:1–9. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.