Abstract
Background
Currently there is no agreement on the optimal time to treatment of breast cancer; however, given the considerable emphasis on early detection, one would expect a similar emphasis on early treatment. The purpose of our study was to assess the time interval to surgery from initiation of diagnosis among Quebec women with breast cancer and to examine the influence on waiting time of age, pattern of care and cancer stage.
Methods
Records of physician fee-for-service claims and of hospital admissions were obtained for all Quebec women who underwent an invasive procedure for the diagnosis or treatment of breast cancer between 1992 and 1998. Waiting time was calculated as the number of days between the first diagnostic procedure and surgical treatment.
Results
There were 29 606 episodes of breast cancer surgery among 28 100 women: 5922 mastectomies and 23 684 lumpectomies. The absolute number of episodes of breast cancer treated with surgery rose from 3626 in 1992 to 5162 in 1998. The overall median waiting time was 34 days (interquartile range [IQR] 19–62); 13.5% of the women waited longer than 90 days. The median waiting time rose from 29 days (IQR 15–54) in 1992 to 42 days (IQR 24–72) in 1998, representing a relative increase of 37% (95% confidence interval [CI] 32%–43%) after adjusting for age and cancer stage. The median waiting time increased with the number of diagnostic procedures, from 24 days (IQR 14–42) with 1 procedure to 48 days (IQR 27–84) with 3 procedures to 72 days (IQR 43–121) with 4 procedures, representing adjusted relative increases of 97% (95% CI 91%–103%) and 194% (95% CI 181%–208%), respectively. The proportion of women receiving 3 or more diagnostic procedures before surgery increased steadily over the study period, from 19.2% in 1992 to 33.0% in 1998. The median waiting time was shorter with more advanced stages of cancer: 53 days (IQR 30–86) for carcinoma in situ, 35 (IQR 20–62) for localized disease, 28 (IQR 16–49) for regional disease and 24 (IQR 11–52) for disseminated disease.
Interpretation
Waiting time between initial diagnosis and first surgery for breast cancer has increased substantially in Quebec between 1992 and 1998. Possible explanations include increased demand, decreased resources and changes in patterns of care.
Practice guidelines for breast cancer emphasize that the work-up of a lump in the breast should be completed as soon as possible after detection.1 Currently there is no agreement on what the optimal time to treatment should be, and decisions of both patients and health care providers influence the time from detection to treatment. Delays can arise if a women is reluctant to seek medical follow-up for a suspicious breast lesion or if health care providers are unable to evaluate and treat the lesion as quickly as they might wish. Evidence is lacking on the minimum delay that would have a negative impact on survival. Sainsbury and associates,2 in a retrospective analysis of data for 36 222 patients in Great Britain, found no evidence that delays of more than 90 days from family physician referral to treatment adversely affected survival. Indeed, they found that shorter delays were associated with poorer survival, likely reflecting more rapid treatment for women presenting with advanced disease. Nevertheless, Great Britain has recommended that all patients presenting with suspected breast cancer be seen within 14 days after referral. Australian authorities3 argue instead that arriving at an appropriate treatment decision is a more important influence than speed on the outcome of breast cancer.
A recent meta-analysis4 of data from 87 nonexperimental studies involving over 100 000 patients showed that women who delayed seeking medical attention for 3 months or more had a 12% lower 5-year survival rate than those who presented sooner (odds ratio 1.47; 95% confidence interval [CI] 1.42 to 1.53). The poorer survival was likely mediated through a mechanism that the authors referred to as “stage-drift,” whereby women presenting later have more advanced disease, which makes stage an intermediate variable between delay and outcome. Although only patient delay was examined, the authors' overall conclusion was that efforts should be made to keep delays by patients and health care providers to a minimum.
Given the considerable emphasis on screening and early detection of breast cancer, one would expect a similar emphasis on early treatment. In Great Britain in 1997, Spurgeon and associates5 reported that the median time from general practitioner referral to first definitive breast cancer treatment was 27 days for referrals classified as urgent and 35 days for those classified as less urgent. There are no Canadian data, but given the similarity in health care systems, one might expect similar waiting times.
The purpose of our study was to assess the time from initiation of diagnostic investigation to surgical treatment among women with breast cancer in Quebec from 1992 to 1998 and to examine the influence of age, choices of diagnostic investigations and treatment, cancer stage and year of surgery on waiting time.
Methods
The study was approved by the McGill University Institutional Review Board.
Data were extracted from administrative records for all women aged 20 years and over who underwent an invasive procedure for the diagnosis or treatment of breast cancer in the province of Quebec between 1992 and 1998. Data identifying procedures related to the breast were extracted from the database of physician fee-for-service claims maintained by the Régie de l'assurance maladie du Québec (RAMQ) and from Quebec's hospital discharge database (MedEcho).
Because these 2 databases use different coding systems, with varying levels of precision, the information was reconciled to produce a common classification for mammography, ultrasound, needle and surgical biopsies, lumpectomy and mastectomy. The 2 databases were reconciled using a unique encrypted identifier; patient age in 1992 was provided only in 5-year intervals in order to respect confidentiality requirements of RAMQ.
It was usual for women to have many breast-related procedures over the study period. In order to link procedures likely to be part of the same diagnostic work-up, consecutive procedures that were separated in time by 5 months or less were considered to be part of a single episode of care. Only procedures to the breast were included. The limit of 5 months was chosen because clinical follow-up is often routinely recommended at 6-month intervals, and we wanted to ensure that a routine 6-month follow-up would not be considered as a wait. No restriction was placed on the total duration of an episode (provided it did not contain a continuous period of 5 months or more of “inactivity”). Although the index period was from 1992 to 1998, prior data (1989–1991) and subsequent data (1999) were also used to avoid truncating episodes that spanned administrative periods.
Only episodes that involved surgery were retained for further analysis. We excluded episodes in which chemotherapy or radiotherapy was begun before surgery and those in which any procedure was performed outside Quebec.
Treatment was considered to be for breast cancer if there was a record of hospital admission or day surgery on or around the time of surgery with a diagnostic code indicating breast cancer. Topography and morphology codes listed in the hospital discharge database were used to estimate the stage of breast cancer as follows: localized (primary breast cancer with no reported lymph-node involvement, ICD-96 codes 174.0–174.9), regional (primary breast cancer with lymph-node involvement, ICD-9 codes 174.0–174.9 plus 196.0–196.9), disseminated (with metastases beyond lymph nodes, ICD-9 codes 174.0–174.9 plus 197.0–199.0), carcinoma in situ (ICD-9 code 233.0) and breast neoplasms of uncertain behaviour (ICD-9 code 238.3).
Waiting time was calculated as the number of days that elapsed between the first diagnostic procedure in the episode of care and the first definitive surgery for breast cancer. Episodes involving surgical treatment with no breast-related diagnostic procedures recorded during the preceding 5 months were counted but were not assigned a waiting time.
Percentiles of the distribution of waiting time in the study population and in various subgroups were obtained. To evaluate factors associated with waiting time, a linear regression model was used with the natural logarithm of waiting time as the dependent variable. The effect of each variable on log waiting time was evaluated, with adjustments for other relevant covariates. Exponentiating the parameter estimates produced with this model provide values that can be interpreted as representing adjusted relative change from the median. Interactions between pairs of variables were evaluated one at a time in the fully adjusted model and were found to have minimal impact.
Results
Over the 7-year study period, there were 28 100 women and 29 606 episodes that involved surgery for breast cancer (5922 mastectomies and 23 684 lumpectomies). Most (95.0%) of the women had only 1 episode of care, and only 0.3% had more than 2.
Table 1 presents a description of the study population overall and by year of surgery. Between 1992 and 1998, there was a steady increase in the absolute number of episodes of breast cancer treated with surgery, from 3626 in 1992 to 5162 to 1998.
Table 1
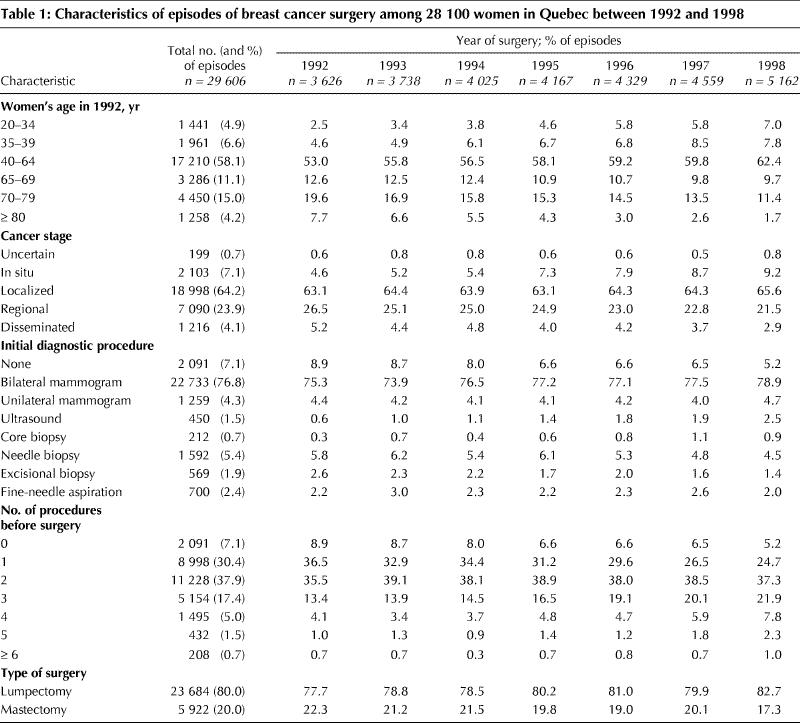
The proportion of older women decreased over the study period, as did the proportion of women with more advanced disease.
The initial diagnostic procedure did not vary greatly over time, with the majority of episodes (76.8%) beginning with a bilateral mammogram. The proportion of episodes in which the surgical treatment was lumpectomy rose from 77.7% in 1992 to 82.7% in 1998. Over the same period, the proportion of episodes in which women received 3 or more procedures to the breast before surgery increased steadily, from 19.2% to 33.0%. Overall, in 7.1% of the episodes there was no recorded diagnostic procedure within the 5 months before surgery; this proportion decreased from 8.9% in 1992 to 5.2% in 1998. Compared with women who had diagnostic procedures before surgery, those who did not were more likely to be 70 years of age or older (42.6% v. 17.5%) and to have disseminated disease (17.3% v. 3.1%).
Table 2 presents variations in time from the initial diagnostic procedure to surgery, both overall and in various subgroups. The overall median waiting time was 34 days (interquartile range [IQR] 19–62); in 13.5% of the episodes the women waited longer than 90 days. Variation in waiting time across categories of age, cancer stage, number and type of diagnostic procedures, and type of surgery is presented as the percent difference from the median for the reference category, after adjusting for relevant covariates.
Table 2
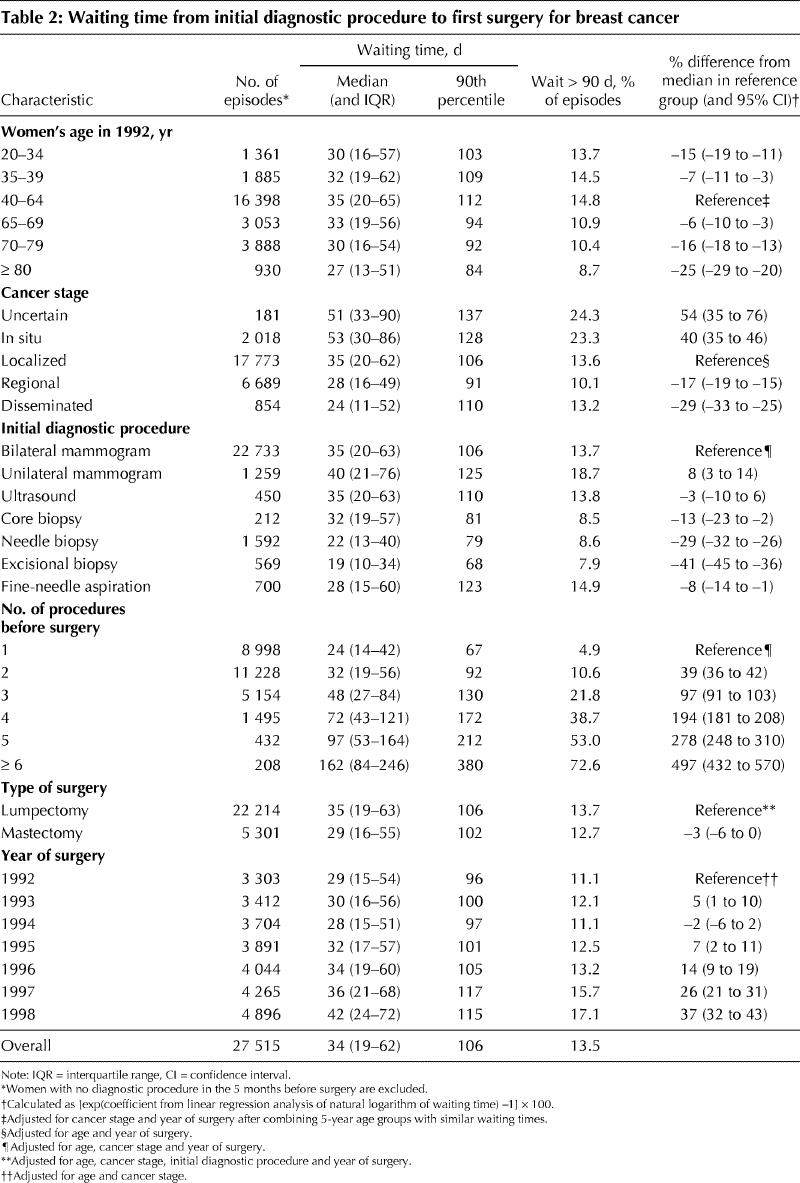
The percent difference for various age groups compared with the reference group of 40–64 years was always negative. This finding indicates that both younger and older women had shorter median waiting times than those in the reference group: the median waiting time was 15% shorter among women under 35 years of age and 25% shorter among those 80 and older.
Longer waiting times were observed among women with less advanced disease, those who began their episode of care with a mammogram, those with more than 1 diagnostic procedure preceding surgery and those treated by lumpectomy.
The median waiting time rose from 29 days in 1992 to 42 days in 1998, representing a relative increase of 37% after adjustment for age and cancer stage. When the number of diagnostic procedures is included as an adjustment variable, the percent increase (and 95% CI) over the baseline year (1992) was 4% (0% to 9%), –1% (–5% to 3%), 4% (0% to 8%), 10% (5% to 14%), 18% (14% to 23%) and 25% (20% to 30%) for 1993 to 1998, respectively. These increases, although smaller than those estimated with adjustment only for age and cancer stage, show the same pattern of statistical significance. The proportion of women waiting longer than 90 days also rose over the study period, from 11.1% in 1992 to 17.1% in 1998.
The increase in waiting time over the study period was seen among women whose initial diagnostic procedure was a bilateral mammogram and among those whose initial procedure was a needle or excisional biopsy. For the bilateral mammography group, the median waiting time rose from 30 days in 1992 to 43 days in 1998 (35% increase, 95% CI 30% to 41%, adjusted for age and cancer stage); for the biopsy group, the corresponding increase in median waiting time was from 18 days to 28 days (54% increase, 95% CI 32% to 79%).
Fig. 1 shows the distribution of overall time from initial diagnostic procedure to surgery, represented as the proportion of women still waiting for surgery at any point in time (dayk), by year of surgery. For purposes of presentation, the data for 1992–1994 and for 1995–1996 were combined. The 1992–1994 median indicates that 50% of the women were still waiting for surgery 29 days after starting their initial diagnostic procedure (day29). By 1998 the entire distribution had shifted such that the median had increased to 42 days. Because of the combined increase in waiting time and in the number of women with breast cancer, the number of women-days waiting almost doubled over the study period: from 142 695 in 1992 to 272 054 in 1998.
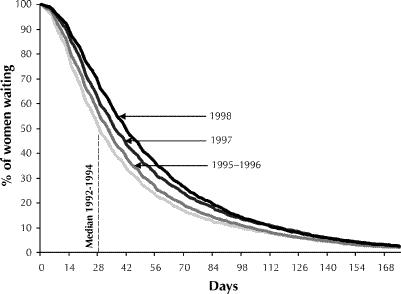
Fig. 1: Waiting times from initial diagnostic procedure to first surgery for breast cancer among women in Quebec between 1992 and 1998.
Interpretation
Waiting time for breast cancer surgery in Quebec increased over time, from a median of 29 days in 1992 to 42 days in 1998. In 1997 in Great Britain,5 the median time from general practitioner referral to first definitive breast cancer treatment was 27 days for urgent referrals and 35 days for less urgent referrals. For the same year, using a proxy indicator of urgency defined according to whether the initial diagnostic procedure was a biopsy or a mammography, we found median waiting times of 23 and 38 days, respectively. Although not vastly dissimilar to the waiting times in Great Britain, those in our study were calculated from the time of initial diagnostic procedure and not referral. Assuming that referral would follow the initial procedure, this suggests shorter waiting times in Great Britain. In both Great Britain and Quebec, it appears that practitioners are able to identify more serious cases of breast cancer early in the diagnostic process and act with haste.5,7
The number of diagnostic procedures before surgical treatment was the strongest factor contributing to waiting time. Women who were referred for surgery directly after their initial procedure had a median waiting time of 24 days, as compared with 32 and 48 days for women with 1 or 2 intervening diagnostic procedures, respectively. The only diagnostic procedures considered in our study were related to the breast. We did not include procedures such as bone and liver scans, which may have been used for cancer staging. The question is whether the increasing availability of complex diagnostic procedures adds important advantages that outweigh the disadvantages that might result from longer delays to definitive surgical procedures. In other words, are all of these additional diagnostic procedures necessary?
Clearly, diagnostic procedures and waiting time are related. When examining the impact of year of surgery on waiting time, it is not obvious that adjustment for this variable is appropriate, as it may be in the causal pathway.
Our data cannot be used to distinguish between system delays and patient delays. The strengths of our study lie in the fact that the entire population of women undergoing surgery for breast cancer in Quebec was captured and that the data are robust: physicians are paid on a fee-for-service basis, and completeness and accuracy of reporting have monetary incentives attached. Missing from these data would be procedures performed at private clinics; however, private medical care is the exception in Quebec. Procedures not billed for by physicians, because of an error in the procedure code or because the site was not identified (e.g., a biopsy to an unspecified site), would have been missed. This may have accounted for a portion of the women without a diagnostic procedure before surgery. However, women without a prior diagnostic procedure were a select group, tending to be elderly and to have advanced disease; they may very well have proceeded directly from physical examination to surgery. Another limitation of our study is that a window of time had to be assigned in order to define an episode of care. Some women may have had 2 distinct diagnostic encounters that were joined because they occurred within a 5-month period; on the other hand, this definition may have underestimated waiting times among women who actually had an interval between procedures of more than 5 months. In any case, as illustrated in Fig. 1, the proportion of women with waiting times greater than 150 days was minimal.
Also minimal was the number of women with more than 1 episode of care. Counting these as separate episodes might have affected the estimate of standard error, but only if the waiting times within women were more similar than those between women. Because the median time between the end of the first episode and the beginning of the subsequent episode was 712 days, this was unlikely to be the case; in fact, the correlation was 0.05. The rarity of the occurrence (95% of the women had only 1 episode) and the narrowness of the confidence intervals around the regression parameters suggest that the effect was negligible. Whether an episode is for treatment of a first breast cancer or a recurrence, the time a woman waits is the focus of concern.
There are a number of hypotheses that could be raised to explain the increase in waiting time over the study period. The incidence of breast cancer has been rising by about 1% annually over the past 20 years.8 This increase, combined with a growing older population, has resulted in more women requiring treatment for breast cancer. Concomitantly, there has been a reduction in available resources. In 1995, in response to a reduction in federal transfer payments,9 Quebec began to close hospitals and hospital beds.10 The number of beds was reduced from 21 680 in 1994 to 14 767 in 1998, a 32% reduction overall.9 The rate of reduction was 3% between 1994 and 1995 and 15% between 1996 and 1997. The reduction in inpatient and surgical resources associated with these cuts may have contributed to the increased waiting time to surgery.
The association between spending and resource use is ecological in nature, and causality cannot be inferred. However, the association between spending and outcome would be strengthened if waiting times improved as spending increased. Federal transfer payments are projected to return to 1995 levels by 2002.
There are no data to suggest what the waiting time should be. Clearly, treatment decisions involve major life-altering choices for women, and time is needed to make the best choice.3 However, there is no disputing the anxiety faced by women and their families while waiting for the results of tests and for surgery. What is of more concern is whether long waits also affect recurrence and survival rates. At this time, the data from our study provide information on expected delays and serve to warn us that rapid cuts in health care spending, if they are not accompanied by an effective planning process, may produce undesireable effects in service delivery.
Footnotes
This article has been peer reviewed.
Acknowledgements: We thank Hélène Marion, a breast cancer survivor, for providing insight into the issues of waiting. We also thank Claudette Corrigan for helping with the interpretation of the coding systems.
This project was funded by the Canadian Breast Cancer Research Initiative.
Competing interests: None declared.
Reprint requests to: Dr. Nancy E. Mayo, Division of Clinical Epidemiology, Rm. R4.29, Royal Victoria Hospital, 687 Pine Ave. W, Montreal QC H3A 1A1; fax 514 843-1493; nmayo@po-box.mcgill.ca
References
- 1.Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: 1. The palpable breast lump: information and recommendations to assist decision-making when a breast lump is detected. CMAJ 1998;158(3 Suppl):S3-8. Available: www.cma.ca/cmaj/vol-158/issue-3/breastcpg/0003.htm [PubMed]
- 2.Sainsbury R, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet 1999;353: 1132-5. [DOI] [PubMed]
- 3.Coates AS. Breast cancer: delays, dilemmas, and delusions [editorial]. Lancet 1999;353:1112-3. [DOI] [PubMed]
- 4.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 1999;353:1119-26. [DOI] [PubMed]
- 5.Spurgeon P, Barwell F, Kerr D. Waiting times for cancer patients in England after general practitioners' referrals: retrospective national survey. BMJ 2000; 320:838-9. [PMC free article] [PubMed]
- 6.World Health Organization. International classification of diseases. 9th rev. Geneva: WHO; 1978.
- 7.Afzelius P, Zedeler K, Sommer H, Mouridsen HT, Blichert-Toft M. Patient's and doctor's delay in primary breast cancer. Prognostic implications. Acta Oncol 1994;33(4):345-51. [DOI] [PubMed]
- 8.Canadian cancer statistics 2000. Toronto: National Cancer Institute of Canada. 2000. Available: www.cancer.ca/stats (accessed 2001 Mar 16).
- 9.Madore O. The Canada Health and Social Transfer: Operation and possible repercussions on the health care sector [Current Issues Review no 95-2E]. Ottawa: Parliamentary Research Branch, Library of Parliament; 1995 (revised Feb 2000). Available: dsp-psd.pwgsc.gc.ca/dsp-psd/Pilot/LoPBdP/CIR/952-e.htm (accessed 2001 Mar 16).
- 10.Raymond D, St-Pierre MA. Base de données STATÉVO, années respectives. Quebec: Service du développement de l'information, Direction de la gestion de l'information, Direction générale de la planification stratégique et de l'évaluation, Ministère de la Santé et des Services sociaux; 2000.


