Abstract
Felidae as definitive hosts for Toxoplasma gondii play a major role in transmission to all warm-blooded animals trough oocysts dissemination. Therefore the current comprehensive study was performed to determine the global status of T. gondii infection in domestic and wild felids aiming to provide comprehensive data of interest for further intervention approaching the One Health perspective. Different databases were searched by utilizing particular key words for publications related to T. gondii infecting domestic and wild feline host species, worldwide, from 1970 to 2020. The review of 337 reports showed that the seroprevalence of T. gondii in domestic cats and wild felids was estimated in 37.5% (95% CI 34.7–40.3) (I2 = 98.3%, P < 0.001) and 64% (95% CI 60–67.9) (I2 = 88%, P < 0.0001), respectively. The global pooled prevalence of oocysts in the fecal examined specimens from domestic cats was estimated in 2.6% (95% CI 1.9–3.3) (I2 = 96.1%, P < 0.0001), and that in fecal samples from wild felids was estimated in 2.4% (95% CI 1.1–4.2) (I2 = 86.4%, P < 0.0001). In addition, from 13,252 examined soil samples in 14 reviewed studies, the pooled occurrence of T. gondii oocysts was determined in 16.2% (95% CI 7.66–27.03%). The observed high rates of anti-T. gondii antibodies seroprevalence levels and oocyst excretion frequency in the felids, along with soil (environmental) contamination with oocysts may constitute a potential threat to animal and public health, and data will result of interest in further prophylaxis programs.
Subject terms: Microbiology, Diseases
Introduction
Toxoplasma gondii is an opportunistic and successful coccidian parasite capable of infect virtually all homoeothermic vertebrates, including human beings1,2. Domestic cats and other Felidae constitute its specific definitive hosts3, and all non-feline animals are regarded as intermediate hosts; however, T. gondii can also undergo asexual reproduction in tissues of Felidae acting as intermediate hosts. First, tachyzoites have active multiplication in tissues, associated to rapid invasion causing harmful effects. Zoites present a special tropism to central nervous system and striated muscle, in which they remain latent confined in a cyst as bradyzoites, leading to a long-term chronic infection until another definitive host ingests the tissue. Then, released bradyzoites penetrate the epithelial cells of small intestine, giving rise to schizonts that will form gamonts and, finally, oocysts4. Felids excrete oocysts in their faeces, during a limited time lapse, contaminating soil and water5–8. In addition to the domestic cats, and under the view of the available literature, the role of wild Felidae in the epidemiology of T. gondii should not be neglected5,9. Therefore, felids constitute the key element in the epidemiology of T. gondii since an individual can shed millions of oocyts that can spread the infection to many other susceptible hosts10. Several important outbreaks of human toxoplasmosis were epidemiologically linked to oocyst contamination of drinking water11–13. By the way, oocysts were not detected in the samples collected from the water reservoir linked to a serious Canadian outbreak14, but viable oocysts were observed in contents of the intestine of a wild trapped cougar (Felis concolor vancouverensis) and in a fecal pile in close proximity to the reservoir15. It is important to highlight that the sporulated oocysts are very resistant and can remain viable and infective for more than 1 year in favourable conditions11,16–18. In this regard, a recent paper reviewed the environmental pathways by which T. gondii can infect animals and people mostly driven by water, soil or contaminated fresh produce or seafood19.
Toxoplasma gondii antibodies have been largely found in cats worldwide, and the seroprevalence degree increases with the age of the cat, suggesting postnatal transmission of T. gondii20. It is assumed that postnatal sero-conversion in cats is linked to oocysts excretion episodes. The life style of cats influences the occurrence of T. gondii infections since feral cats that hunt for their food will present higher rates than domestic cats with limited access to parasites21. Seroprevalence level varied among continents, countries and even cities, linked to many possible environmental factors influencing these variations. As an example, in an urban population of 301 domestic cats in Lyon, France20, the anti-T. gondii seroprevalence was only 18.6%, approximately half the prevalence in other surveys in Europe22,23. The control of rodents in the area and feeding of cats by people were considered as protective factors limiting infections. On the other hand, a low income and poor sanitation were not the determining factors for low seropositivity to T. gondii in cats in Durango, Mexico24. Since a high density of felines (specially domestic cats) increases the risk of infection and T. gondii prevalence in intermediate hosts, a gradient of prevalence rate of infection has been demonstrated depending on the anthropization degree of the environment25,26.
Nearly up to 30% of the world’s human population has had contact with the parasite evidenced by the presence of anti-T. gondii antibodies; while T. gondii infections are usually asymptomatic, they can lead to harmful effects, especially in congenital cases and immunocompromissed persons27,28. Humans become primarily infected mostly via oral ingestion of viable tissue cysts present in raw or undercooked meat and oocysts contaminating water or foodstuffs6,8,29. Nowadays, comprehensive local studies are still necessary to determine the source attribution of human infections; this constitutes an interesting challenge that should be approached under the One Health perspective.
To date, different surveys have been focused on domestic and wild felids in order to determine aspects as seroprevalence rates of anti-T. gondii antibodies, frequency of oocysts excretion and soil presence worldwide30–36, but with a certain degree of variance among studies. A systematic review recently assessed the seroprevalence of T. gondii in felids from 1967 to 2017 with a search strategy restricted to articles in English37. So that, the present investigation was aimed to determine the global frequency of T. gondii infections in domestic cats and wild felids, the occurrence of T. gondii-like oocysts shedding, and the frequency of oocysts in soil; such information will be useful to implement further measures aiming to reduce animal and human infections under a One Health perspective.
Methods
Search strategy
The review process exactly followed the protocol suggested by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Supplementary data: PRISMA/STROBE)38. We retrieved published studies from the databases of MEDLINE (via PubMed) (https://www.ncbi.nlm.nih.gov/pubmed/), Scopus (https://www.scopus.com/), Web of Science (https://www.webofknowledge.com/) and CAB Abstracts (https://www.cabi.org/AHPC) with no restriction on language from Jan 1, 1970, to Dec 31, 2019. Search terms included a combination of Medical Subject Heading terms (MeSH) and free-text words in titles, abstracts and full texts.
The systematic search for PubMed accomplished using several Medical Subject Heading terms (Table S1). In addition, Scopus, Web of Science and CAB Abstracts were searched using the same strategy (Supplementary DATA). The Google Scholar search engine was used for checking the search strategy. The reference lists of all included articles and relevant reviews were hand searched for potentially eligible literature. In addition, authors and experts in the field were consulted to aid in the identification of relevant conference abstracts related to Toxoplasma and toxoplasmosis. Sometimes, we have had to contact the authors for raw data collection39, especially in old literature.
Selection of studies
Initial screening by manuscript titles and abstracts was performed independently by two researchers (KHN and EA), that also assessed the full texts of all potentially relevant studies and applied inclusion criteria. Discrepancies when detected, were resolved after constructive discussion (AD, MZ and MTR).
The studies providing data on the seroprevalence of T. gondii in domestic or wild felids, frequency of oocyst excretion in felids, and those reporting soil contaminations with oocysts were included. On the other hand, studies meeting the epidemiology of T. gondii in non-feline hosts, studies where cat faecal samples were collected from the ground, and data from each animal was not independently retrievable, experimental studies, articles that only presented the final result and did not provide the raw data, or those without definite sample size, abstracts presented in congresses without full text, and case–control studies and clinical trials that could not report a correct estimate of prevalence were excluded. Any duplicated research was also excluded.
Quality assessment
The standard Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was used40. In present study, articles were evaluated as low quality: less than 16.5, moderate quality: 16.6–25.5, and high quality: 25.6–34; the articles included in the meta-analysis presented acceptable quality.
Data extraction
After comprehensive examination of selected articles, the following data were extracted: the first author’s last name, publication year, country, feline scientific names, keeping status of felids, sample size, source of soil samples, beginning and end date of study implementation, the number of the positive and negative cases, cut-off, age groups, as well as information about diagnostic tools. Data were extracted separately if two different populations had been studied. All extracted data from each study were entered into an Excel spreadsheet.
Meta-analysis
The collected data were entered into the StatsDirect statistical software package (version 2.7.2) (Stata Corporation, College Station, TX, USA) (http://www.statsdirect.com/). Statistical heterogeneity of the different years among studies was assessed using the Cochrane’s Q test and inconsistency I2 test. To determine whether there is a significant heterogeneity, a random effect model was used to estimate the pooled prevalence’s of cat infection41. In addition, potential publication bias was explored using Funnel plot and Egger’s test.
Results
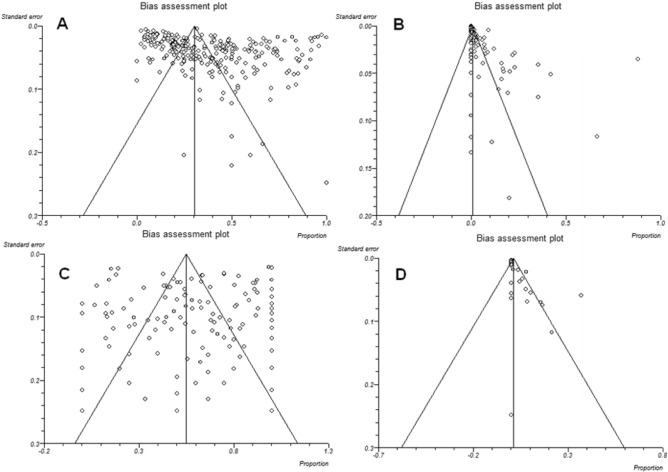
The initial database search retrieved 14,870 publications. First screening enabled us to exclude 14,441 studies not meeting the inclusion criteria. Altogether, 429 studies were retained for further investigation. In a secondary assessment, another 34 documents were excluded because of one of the following reasons: review articles, follow up studies or case series reports, and papers with insufficient data, or data from each animal were not independently retrievable. Eventually, 395 studies which met our eligibility criteria and evaluated soil contamination with T. gondii oocysts (n = 14), and T. gondii infection in domestic cats (serology, n = 268; oocyst in feces, n = 112) and wild felids (serology, n = 69; oocyst in feces, n = 15) during five decades were retained for analysis (Fig. 1). Potential publication bias in the conducted studies regarding the prevalence of T. gondii infection in domestic cats and wild felids, Toxoplasma-like oocysts shedding, and frequency of oocysts in soil are shown using Funnel plot and Egger’s test (Fig. 2).
Figure 1.
Flowchart of the study design process.
Figure 2.
Funnel plot of standard error by logit event rate to assess publication or other types of bias across prevalence studies. (A) Studies based on the seroprevalence of anti-Toxoplasma gondii antibodies in domestic cats, (B) studies based on detection of T. gondii-like oocyst and T. gondii oocyst DNA in domestic cat feces, (C) studies based on the seroprevalence of anti-Toxoplasma gondii antibodies in wild felids, (D) studies based on detection of T. gondii-like oocyst and T. gondii oocyst DNA in wild felids feces.
Prevalence of anti-T. gondii antibodies in blood/serum samples
The global pooled seroprevalence of T. gondii in domestic cats was estimated in 37.5% (95% CI 34.7–40.3) (I2 = 98.3%, P < 0.001) (Table 1). The highest rate was observed in Australia (66.6%, 95% CI 62.8–70.3) (I2 = 97.2%, P < 0.0001), followed by Africa (55.7%, 95% CI 35.6–74.8) (I2 = 98.9%, P < 0.0001), Europe (45.3%, 95% CI 41.1–49.6) (I2 = 96.7%, P < 0.0001), Central and South America (40.3%, 95% CI 34–49.6) (I2 = 97.7%, P < 0.0001) and North America (31.6%, 95% CI 27–36.4) (I2 = 96.8%, P < 0.0001). The lowest prevalence was observed in Asia (28.3%, 95% CI 24.1–32.6) (I2 = 98.1%, P < 0.0001) (Table 1). In the other hand, the worldwide pooled seroprevalence of T. gondii in wild felids (including lion, jaguarundi, jaguar, ocelot, cougar, leopard, tiger, geoffroy's cat, oncilla, margay, caracal, snow leopard, Eurasian lynx, bobcat, cheetah, Prionailurus cats, Iberian lynx, pampas cat, serval, pallas's cat, jungle cat, European wildcat, sand cat, Asian golden cat, Canadian lynx, clouded leopard, masked palm civet, and common genet) was estimated 64% (95% CI 60–67.9) (I2 = 88%, P < 0.0001) (Table 2). The highest and the lowest seroprevalence rates were related to Panthera leo (87.6%, 95% CI 79–94.3) and Leopardus colocolo (19.6%, 95% CI 6.1–38.3), respectively (Table 2). Heterogeneity was, however, very low I2 = 79.9%. Retrieved information of the eligible studies based on the of anti-Toxoplasma antibodies prevalence in domestic cats and wild felids are summarized in Tables S2 and S3. In total, 80,087 blood samples from domestic cats (n = 73,980) and wild felids (n = 6,107) from 337 eligible studies were examined for the presence of anti-T. gondii antibodies and/or T. gondii DNA, of which 26,903 subjects were diagnosed as positive (domestic cats n = 23,593; wild felids n = 3,310) (Tables S2, S3). Different diagnostic methods to evaluate anti-T. gondii antibodies in the cats have been identified in the studies: MAT (132 studies), IFAT (99 studies), and ELISA (92 studies) (Tables S2, S3). Amongst the reviewed studies, just one investigation applied PCR method for detection of T. gondii DNA in stray cats using blood samples42 (Table S2).
Table 1.
Pooled prevalence of Toxoplasma infection in domestic cats and subgroup analyses.
| Continent | No. of studies | Detection method | Prevalence % (95% CI) | Heterogeneity | Egger’s test | |||
|---|---|---|---|---|---|---|---|---|
| I2 | Q | P value | T | P value | ||||
| Africa | 6 | Stool exam | 9.8 (2.4–21.5) | 94.1 | 84.2 | < 0.0001 | 5.1 | 0.0434 |
| 16 | Serology | 55.7 (35.6–74.8) | 98.9 | 1408.4 | < 0.0001 | − 3.8 | 0.6571 | |
| Asia | 32 | Stool exam | 4 (1.9–6.9) | 96.9 | 1001.4 | < 0.0001 | 3.2 | 0.0195 |
| 90 | Serology | 28.3 (24.1–32.6) | 98.1 | 4730.5 | < 0.0001 | 7.7 | < 0.0001 | |
| Australia | 4 | Stool exam | 1.7 (0.2–4.5) | 79.1 | 14.3 | 0.0025 | 1.3 | 0.3488 |
| 6 | Serology | 66.6 (62.8–70.3) | 97.2 | 176.1 | < 0.0001 | − 9.66 | 0.1256 | |
| Europe | 55 | Stool exam | 1.21 (0.8–1.6) | 89.6 | 517.1 | < 0.0001 | 1.18 | < 0.0001 |
| 61 | Serology | 45.3 (41.1–49.6) | 96.7 | 1840.1 | < 0.0001 | 2.94 | 0.1269 | |
| North America | 16 | Stool exam | 0.9 (0.5–1.3) | 50.0 | 30.3 | 0.0108 | 0.94 | 0.0583 |
| 37 | Serology | 31.6 (27–36.4) | 96.8 | 1126.2 | < 0.0001 | 0.49 | 0.7212 | |
| Central/South America | 12 | Stool exam | 6.2 (1.8–1.3) | 97.1 | 374.2 | < 0.0001 | 3.4 | 0.0226 |
| 61 | Serology | 40.3 (34.0–46.8) | 97.7 | 2642.3 | < 0.0001 | 4.5 | 0.0467 | |
Table 2.
Pooled prevalence of Toxoplasma infection in wild felids and subgroup analyses.
| Host species | No. of studies | Detection method | Prevalence (95% CI) | Heterogeneity | Egger’s test | |||
|---|---|---|---|---|---|---|---|---|
| I2 | Q | P value | T | P value | ||||
| Asian golden cat (Catopuma temminckii) | 3 | Serology | 47.1 (8.9–87.4) | 72.7 | 7.3 | 0.0256 | – | – |
| Bobcat (Lynx rufus) | 18 | Serology | 60.5 (47.1–73.1) | 91.0 | 189.8 | < 0.0001 | 1.5 | 0.3255 |
| 5 | Stool exam | 4.1 (0.2–12.4) | 82.5 | 22.8 | 0.0001 | 1.0 | 0.1297 | |
| Canadian Lynx (Lynx canadiensis) | 3 | Serology | 36.4 (10.8–67.2) | 91.7 | 24.1 | < 0.0001 | – | – |
| Caracal (Caracal caracal) | 7 | Serology | 69.9 (49.6–86.8) | 0.0 | 5.1 | 0.5270 | − 0.3 | 0.9283 |
| Cheetah (Acinonyx jubatus) | 8 | Serology | 70.4 (48.1–88.5) | 81.0 | 36.7 | < 0.0001 | − 0.6 | 0.7904 |
| Clouded leopard (Neofelis nebulosa) | 4 | Serology | 36.3 (9.0–69.7) | 65.8 | 8.7 | 0.0325 | 4.2 | 0.2131 |
| Cougar (Puma concolor) | 24 | Serology | 56.1 (43.7–68.2) | 93.8 | 371.7 | < 0.0001 | 2.6 | 0.1155 |
| 6 | Stool exam | 4.7 (0.5–12.7) | 61.5 | 12.9 | 0.0236 | 0.8 | 0.0955 | |
| Eurasian lynx (Lynx lynx) | 6 | Serology | 42.1 (14.9–72.2) | 97.4 | 192.1 | < 0.0001 | 1.7 | 0.7428 |
| European wildcat (Felis silvestris) | 7 | Serology | 76.8 (62.6–88.5) | 46.0 | 11.1 | 0.0848 | 0.5 | 0.6714 |
| Geoffroy's cat (Leopardus geoffroyi) | 5 | Serology | 60.7 (39.9–79.6) | 60.8 | 10.1 | 0.0373 | 1.7 | 0.6131 |
| Iberian Lynx (Lynx pardinus) | 4 | Serology | 66.2 (50.1–80.5) | 81.9 | 16.5 | 0.0009 | 2.9 | 0.6198 |
| Jaguar (Panthera onca) | 9 | Serology | 74.4 (63.5–84) | 61.6 | 20.8 | 0.0076 | 1.7 | 0.1306 |
| 3 | Stool exam | 3.5 (1.3–13.7) | 66.9 | 6.0 | 0.0487 | – | – | |
| Jaguarundi (Herpailurus yagouaroundi) | 10 | Serology | 47.7 (41.6–53.9) | 0.0 | 8.5 | 0.4774 | 1.9 | 0.0053 |
| Jungle cat (Felis chaus) | 2 | Serology | 44.5 (7.5–97.1) | – | 7.9 | 0.0047 | – | – |
| Leopard (Panthera pardus) | 17 | Serology | 68.0 (46.5–86.1) | 72.5 | 58.1 | < 0.0001 | 5.1 | 0.0010 |
| 3 | Stool exam | 3.8 (0.1–17.6) | 84.9 | 13.2 | 0.0013 | – | – | |
| Lion (Panthera leo) | 20 | Serology | 87.6 (79–94.3) | 79.9 | 94.6 | < 0.0001 | − 1.6 | 0.0175 |
| 3 | Stool exam | 4.9 (0.3–23.8) | 85.6 | 13.8 | 1.0010 | – | – | |
| Margay (Leopardus wiedii) | 5 | Serology | 56.0 (46.4–65.4) | 29.0 | 5.6 | 0.2282 | 2.3 | 0.2958 |
| Ocelot (Leopardus pardalis) | 11 | Serology | 66.2 (58.1–73.8) | 45.6 | 18.3 | 0.0489 | 1.6 | 0.0921 |
| 3 | Stool exam | 15.9 (0.2–58.6) | 85.5 | 13.7 | 0.0010 | – | – | |
| Oncilla (Leopardus tigrinus) | 9 | Serology | 59.0 (49.7–68) | 47.9 | 15.3 | 0.0527 | 1.4 | 0.2027 |
| Pallas's cat (Otocolobus manul) | 10 | Serology | 70.6 (43.9–91.3) | 89.3 | 84.1 | < 0.0001 | − 2.4 | 0.4067 |
| Pampas cat (Leopardus colocolo) | 3 | Serology | 19.6 (6.1–38.3) | 0.0 | 0.7 | 0.6878 | – | – |
| Prionailurus cats (Prionailurus viverrinus) | 10 | Serology | 39.6 (24.3–56.1) | 60.0 | 22.4 | 0.0075 | 2.5 | 0.0606 |
| 5 | Stool exam | 4.0 (1.8–7.1) | 0.0 | 1.4 | 0.8433 | − 0.2 | 0.4709 | |
| Sand cat (Felis margarita) | 4 | Serology | 70.5 (49.9–87.5) | 66.8 | 9.0 | 0.0287 | 3.0 | 0.2817 |
| Serval (Leptailurus serval) | 4 | Serology | 64.3 (35 to88.6) | 8.8 | 3.2 | 0.3493 | -4.1 | 0.7970 |
| Snow leopard (Panthera uncial) | 2 | Serology | 52.6 (10.7–92.2) | – | 1.9 | 0.1607 | – | – |
| Tiger (Panthera tigris) | 16 | Serology | 66.2 (51.4–79.5) | 61.9 | 39.3 | 0.0006 | 1.3 | 0.3862 |
| 4 | Stool exam | 7.4 (0–27.4) | 80.2 | 15.1 | 0.0017 | 1.8 | 0.2827 | |
Occurrence of T. gondii oocysts in fecal samples
A total number of 137 eligible studies which examined 66,601 fecal samples from domestic cats (n = 63,458) and wild felids (n = 3,143), 1,330 were positive (domestic cats n = 1,254; wild felids n = 76) for T. gondii oocysts, T. gondii-like oocysts, and/or T. gondii DNA (Table S4, S5). The global pooled prevalence of oocysts in the fecal examined specimens from domestic cats was estimated in 2.6% (95% CI 1.9–3.3) (I2 = 96.1%, P < 0.0001) (Table 3). The highest and the lowest prevalence rates were detected in Africa (9.8%, 95% CI 2.4–21.5) (I2 = 94.1%, P < 0.0001), and North America (0.9%, 95% CI 0.5–1.3), respectively. Heterogeneity was, however, very low I2 = 50% (Tables 1, 3). The most used methodology for detection of oocysts was microscopy (99 studies) which was followed by molecular (16 studies) and mouse bioassay (10 studies) methods (Table S4). Some studies combined two techniques for detection of oocysts in feline feces. The highest prevalence was related to the molecular detection method (6.5%, 95% CI 3.7–10) (I2 = 92.1%, P < 0.0001), followed by bioassay (2.8%, 95% CI 0.6–6.4) (I2 = 95.1%, P < 0.0001), and microscopy (2.1%, 95% CI 1.4–2.8) (I2 = 96.3%, P < 0.0001) (Table 3).
Table 3.
The global pooled prevalence of Toxoplasma infection in feline hosts/felids.
| Group | Number of studies | Pooled prevalence (95% CI) | Heterogeneity | ||
|---|---|---|---|---|---|
| P value | I2 | Cochran Q | |||
| Domestic Cat | |||||
| Serology | 271 | 37.5 (34.7–40.3) | < 0.0010 | 98.3 | 15,984.3 |
| Stool exam | 125 | 2.6 (1.9–3.3) | < 0.0001 | 96.1 | 3164.3 |
| Microscopy | 99 | 2.1 (1.4–2.8) | < 0.0001 | 96.3 | 2664.6 |
| Bioassay | 10 | 2.8 (0.6– 6.4) | < 0.0001 | 95.1 | 182.7 |
| Molecular | 16 | 6.5 (3.7–10) | < 0.0001 | 92.1 | 189.3 |
| Wild Feline | |||||
| Serology | 223 | 64.0 (60–67.9) | < 0.0001 | 88 | 1854.9 |
| Stool exam | 12 | 2.4 (1.1–4.2) | < 0.0001 | 86.4 | 227.5 |
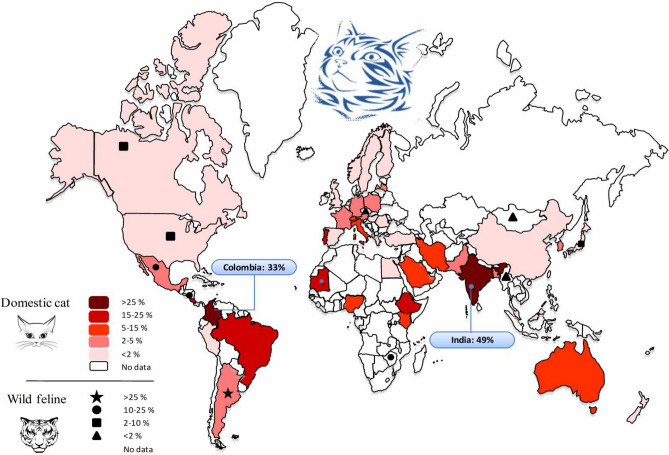
The worldwide pooled prevalence of T. gondii oocysts, T. gondii-like oocysts and T. gondii DNA in fecal specimens from wild felids was estimated in 2.4% (95% CI 1.1–4.2) (I2 = 86.4%, P < 0.0001) (Table 3). The highest and the lowest prevalence of oocysts in fecal specimens was related to Leopardus pardalis (15.89%, 95% CI 0.2–58.6) and Panthera onca (3.5%, 95% CI 1.3–13.7), respectively (Table S5). The prevalence of Toxoplasma-like oocysts detected in domestic and wild feline stool samples in different countries are shown in Fig. 3. As well India (49%) and Colombia (33%) had the highest prevalence.
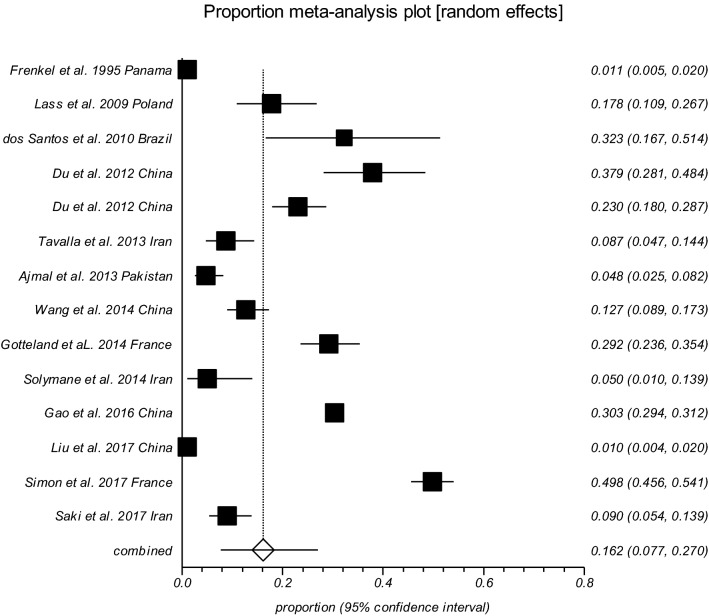
Figure 3.
Forest plot diagram of the present systematic review and meta-analysis based on studies focused on detection of soil contamination by Toxoplasma-like oocysts.
Occurrence of T. gondii oocysts in soil samples
Up to 14 studies reported the examination of 13,252 soil specimens resulting in 3,421 (25.8%) samples positive for T. gondii oocysts (or T. gondii-DNA) using mouse bioassay and different PCR procedures. Table S6 shows the conducted studies to detect T. gondii oocysts in soil samples; the pooled prevalence of T. gondii oocysts in those samples was estimated 16.2% (95% CI 7.66–27.03%) (Q = 1628.10, I2 = 99.2%, P < 0.0001) (Egger’s bias = 5.44, P = 0.3733) (Fig. 4).
Figure 4.
Pooled prevalence of Toxoplasma-like oocysts detected in domestic and wild feline stool samples in different countries (Map created by PowerPoint Microsoft office,
source of image: https://commons.wikimedia.org/wiki/File:BlankMap-World.svg).
Discussion
Felids as final host play an irreplaceable role for T. gondii life cycle that exclusively yield and excrete oocysts in their faeces, contaminating soil, water and food6–8,10. According to our findings, 37.5% of domestic cats showed exposure to T. gondii and 2.6% were actively shedding T. gondii or T. gondii-like oocysts. Similarly, the worldwide seroprevalence of Toxoplasma in domestic cats had been previously estimated at levels of 30–40%1,43.
Based on the results, the highest value of seroprevalence in domestic cats was observed in Australia, followed by Africa, Europe, Central/South America, North America and Asia; although the number of studies for Australia (n = 6) and Africa (n = 15) were relatively low, whereas only one study in USA included 12,628 animals. The lowest prevalence was observed in Asia (28.3%), nevertheless, most studies have been conducted in Asian countries (91 studies). The number of surveys was higher in USA (North America; 34 studies), followed by Brazil (South America; 30 studies). Our investigation identified a number of countries without any data on T. gondii infection in cats, emphasizing the need for further studies in this field. According to our findings, 64% of nondomestic cats showed evidence of exposure to T. gondii and 2.4% were actively shedding T. gondii oocysts. Accordingly, the highest sero-prevalence of T. gondii in different wild felids were in the following order: lion (Panthera leo), European wildcat (Felis silvestris), jaguar (Panthera onca), Pallas's cat (Otocolobus manul), sand cat (Felis margarita), cheetah (Acinonyx jubatus), caracal (Caracal caracal), leopard (Panthera pardus), Iberian lynx (Lynx pardinus), ocelot (Leopardus pardalis), tiger (Panthera tigris), serval (Leptailurus serval), Geoffroy's cat (Leopardus geoffroyi), bobcat (Lynx rufus), oncilla (Leopardus tigrinus), cougar (Puma concolor), margay (Leopardus wiedii), snow leopard (Panthera uncial), jaguarundi (Herpailurus yagouaroundi), Asian golden cat (Catopuma temminckii), Eurasian lynx (Lynx lynx), jungle cat (Felis chaus), Prionailurus cat (Prionailurus viverrinus), Canadian lynx (Lynx canadiensis), clouded leopard (Neofelis nebulosa), and Pampas cat (Leopardus colocolo). Although in general it should be considered that the number of studied nondomestic cats were lower compared to domestic cats, and keeping status of wilds cats, in captive (n = 2,492) or free ranging (n = 2,949), is probably associated with how they are fed and how they become infected. Albeit such findings also highlights the importance of serological and isolation studies on T. gondii infecting their prey (ungulates, birds, etc.) using validated methodologies for bias reducing.
The considerable gap between the prevalence of oocysts in feces and positive serum antibodies can be explained by the fact that infected felids can shed T. gondii oocysts for a short period (10–15 days), shortly after primoinfection, and then they become seropositive indefinitely. As, one important point, the activation of humoral immunity and antibodies production prevent from re-shedding of oocysts; new excretion episodes can occur when severe immunosuppression appears29,44. The short period of oocyst shedding and the low prevalence rate of felids which actively excrete oocysts, have led some authors to discuss that direct contact with felids should not be considered as a risk factor for human infection7,29,45. A systematic review in Iran showed that humans with history of close contact with cats presented a higher T. gondii seroprevalence rate compared to those without contact46. In the study conducted by Jones and colleagues29 in the USA, exposure to kittens was statistically linked to T. gondii infection. In the study conducted in different European centers45, infections in pregnant women were attributed to the consumption of undercooked or cured meat products and soil contact in the 30–63% and 6–17% of cases respectively, but contact with cats was not identified as a risk factor. Similarly, another study showed that contact with cats is not related to infections, while the ingestion of raw or undercooked meat highly increased the risk of infection47.
Based on the results, 16.2% of soil samples contained T. gondii oocysts (or T. gondii-DNA), those when sporulated can survive for several months under tough conditions and are resistant to common disinfectants48. Contaminated soil has been demonstrated as an important source for infection for humans and animals13,19. It has been shown that gardening and occupations in contact with soil increases the risk of T. gondii infection49, as previously seen50. In a follow-up study of the toxoplasmosis outbreak during 1977 in Georgia51, after 25 years, among 37 individual (exposure to an indoor horse arena), 14 equestrian were tested, that three (21%) were found to have toxoplasmic retinochoroiditis lesions. Based on the observations is possible that cat feces containing the organism were most likely stirred up when horses ran on the dirt floor, and were inhaled or ingested by riders and observers. Based upon number of studies conducted in different European centers, contact with soil or vegetables or fruit presumably contaminated with soil were highly associated to T. gondii infection in pregnant women45,52–54. Investigation on sentinels (i.e., molluscs) for environmental contamination55 and also the infection source attribution by using specific tests56 will be of great interest for integration with data compilation in definitive and intermediate susceptible hosts.
In the present investigation, the prevalence of soil contamination was highly variable in the selected studies, which might be influenced by the soil characteristics and the number of infected animals in the area57. The included studies also reported highly heterogeneous results regarding the prevalence of cat infections, which could be due to the different risk factors, to note: sex, age, climates, study periods, cat breeds, living conditions and diagnostic methods as well as other unrecognized confounding factors. Based on our results, T. gondii seroprevalence in cats (Felis domesticus) in different countries oscillated from less than 10% in Thailand, Taiwan and Angola to more than 70% in Qatar, and Ethiopia. This can partly be explained by the different environmental conditions among the countries58. It has been shown that cat infections present higher occurrence in warm, moist and low altitude regions, maybe linked to oocysts sporulation and survival of T. gondii oocysts in such latitudes34. Similarly, T. gondii seroprevalence in pigs was associated with lower geographical latitude and higher mean annual temperature59, fact that may suggest high environmental contamination with T. gondii sporulated oocysts. It seems to be clear that oocysts shedding by cats constitute the essential element for sustainment of the parasite in the environment, this was demonstrated when extremely low seroprevalence of T. gondii (0.9%) was detected in feral pigs from a remote island lacking cats in the USA60. Furthermore, the time period of study might influence the results, as the infection rate is higher in autumn, winter22, and rainy years20. One may consider breed as a variable factor for cat T. gondii infection. It has been shown that Toxoplasma seroprevalence is highly variable in different cat breeds from 18.8% in Burmese cats to 60% in Persian cats35. Even though, a high occurrence rate of T. gondii infection in cat may be attributed to some important factors including: uncontrolled food and access to contaminated sources, wandering outdoor, humid and temperate climate; and cat abundance. Furthermore, stray cats have been shown to have a higher seroprevalence compared to pet cats, which can be explained by more access to contaminated source and outdoor living31,34,61. Additionally, pet cats with an outdoor access are also at an increased risk of infection compared to those kept indoor32,35. It has been reported that rural cats show a higher seroprevalence rate of T. gondii compared to urban ones62.
Furthermore, the different diagnostic methods used to detect T. gondii antibodies and oocysts could influence the results. While the different techniques used for anti-T. gondii antibodies detection showed comparably good diagnostic performance, most of the studies aiming to detect T. gondii oocysts employed less reliable microscopic methods, which might result in false positives, as oocysts and sporocysts of some other coccidia (e.g. Hammondia hammondi, Besnoitia darlingi) may resemble those of T. gondii48. It shows the necessity of testing environmental and fecal samples by using specific-PCR aided with amplicon sequencing for identity confirmation63.
Felids as key elements in the epidemiology of toxoplasmosis should be considered as a potential threat to animal and public health, due potential oocysts contamination of the environment; such information is still missing in several worldwide locations, so further epidemiological investigations on final hosts would be of special interest for evaluating the status of T. gondii infection and risk assessment implementations. Further investigations based on QMRA approaches64,65 combining raw data in Felidae with those from the environmental side and those from susceptible hosts will complement the One Health puzzle in defined areas.
In present meta-analysis, it is shown that about one-third of domestic and non-domestic cats have been exposed to T. gondii, and globally about 1 in 50 cats are actively shedding T. gondii or T. gondii-like oocysts. In addition, 16.2% of the soil samples examined were contaminated with T. gondii-like oocysts informing on a broad environmental distribution. Felids are the only final host of T. gondii and play a major role in its life cycle, therefore measures aiming to reduce environmental contamination with T. gondii oocysts will be of major interest, and a One Health perspective covering human, animal and environmental health should be taken into account.
Supplementary Information
Acknowledgements
We are especially appreciative of Dr. Jitender P. Dubey and Dr. Solange M. Gennari for their kind help and constructive comments.
Author contributions
Conceptualization, K.H.N., R.C.B., and E.A.; methodology, A.S.P., K.H.N., M.Z., A.D., and M.T.R.; formal analysis, E.A., and M.T.R.,; investigation, R.C.B., A.S.P., and K.H.N.; data curation, E.A., and A.D.; writing—original draft preparation, A.S.P., M.T.R., K.H.N.;, and A.D.; writing—review and editing, E.A., R.C.B., and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The article was funded by Research Center for Infectious Diseases and Tropical Medicine, Tabriz University of Medical (Grant No. 63205). Rafael Calero-Bernal is part of the TOXOSOURCES consortium supported by the funding from the European Union’s Horizon 2020 Research and Innovation Programme under the grant agreement No 773830: One Health European Joint Programme.
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-89031-8.
References
- 1.Webster JP. Review of "toxoplasmosis of animals and humans" by JP Dubey. Parasit. Vectors. 2010;3:112. doi: 10.1186/1756-3305-3-112. [DOI] [Google Scholar]
- 2.Dubey JP. The history of Toxoplasma gondii—the first 100 years. J. Eukaryot. Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 3.Martorelli Di Genova B, Wilson SK, Dubey J, Knoll LJ. Intestinal delta-6-desaturase activity determines host range for Toxoplasma sexual reproduction. PLoS Biol. 2019;17:20. doi: 10.1371/journal.pbio.3000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calero-Bernal R, Gennari S. Clinical toxoplasmosis in dogs and cats: An update. Front. Vet. Sci. 2019;6:54. doi: 10.3389/fvets.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukesova D, Literák I. Shedding of Toxoplasma gondii oocysts by Felidae in zoos in the Czech Republic. Vet. Parasitol. 1998;74:1–7. doi: 10.1016/S0304-4017(97)00155-6. [DOI] [PubMed] [Google Scholar]
- 6.Dubey J. Toxoplasmosis—a waterborne zoonosis. Vet. Parasitol. 2004;126:57–72. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Dubey J, Jones J. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009;39:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva JCR, Ogassawara S, Marvulo MFV, Ferreira-Neto JS, Dubey J. Toxoplasma gondii antibodies in exotic wild felids from Brazilian zoos. J. Zoo Wildl. Med. 2001;32:349–351. doi: 10.1638/1042-7260(2001)032[0349:TGAIEW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Dubey J, et al. All about toxoplasmosis in cats: The last decade. Vet. Parasitol. 2020;20:109145. doi: 10.1016/j.vetpar.2020.109145. [DOI] [PubMed] [Google Scholar]
- 11.Bowie WR, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350:173–177. doi: 10.1016/S0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- 12.De Moura L, et al. Waterborne toxoplasmosis, Brazil, from field to gene. Emerg. Infect. Dis. 2006;12:326. doi: 10.3201/eid1202.041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto-Ferreira F, et al. Patterns of transmission and sources of infection in outbreaks of human toxoplasmosis. Emerg. Infect. Dis. 2019;25:2177. doi: 10.3201/eid2512.181565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaac-Renton J, et al. Detection of Toxoplasma gondii oocysts in drinking water. Appl. Environ. Microbiol. 1998;64:2278–2280. doi: 10.1128/AEM.64.6.2278-2280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aramini J, Stephen C, Dubey J. Toxoplasma gondii in Vancouver Island cougars (Felis concolor vancouverensis): Serology and oocyst shedding. J. Parasitol. 1998;20:438–440. doi: 10.2307/3284508. [DOI] [PubMed] [Google Scholar]
- 16.Mancianti F, et al. A retrospective molecular study of select intestinal protozoa in healthy pet cats from Italy. J. Feline Med. Surg. 2015;17:163–167. doi: 10.1177/1098612X14533549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatam-Nahavandi K, et al. Microscopic and molecular detection of Cryptosporidium andersoni and Cryptosporidium xiaoi in wastewater samples of Tehran Province, Iran. Iran. J. Parasitol. 2016;11:499. [PMC free article] [PubMed] [Google Scholar]
- 18.Nahavandi KH, et al. Molecular typing of Eimeria ahsata and E. crandallis isolated from slaughterhouse wastewater. Jundishapur J. Microbiol. 2016;9:20. doi: 10.5812/jjm.34140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro K, et al. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019;15:e00049. doi: 10.1016/j.fawpar.2019.e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afonso E, Thulliez P, Gilot-Fromont E. Transmission of Toxoplasma gondii in an urban population of domestic cats (Felis catus) Int. J. Parasitol. 2006;36:1373–1382. doi: 10.1016/j.ijpara.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 21.DeFeo ML, Dubey J, Mather TN, Rhodes RC., III Epidemiologic investigation of seroprevalence of antibodies to Toxoplasma gondii in cats and rodents. Am. J. Vet. Res. 2002;63:1714–1717. doi: 10.2460/ajvr.2002.63.1714. [DOI] [PubMed] [Google Scholar]
- 22.Simon JA, et al. A multi-event capture-recapture analysis of Toxoplasma gondii seroconversion dynamics in farm cats. Parasit. Vectors. 2018;11:339. doi: 10.1186/s13071-018-2834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veronesi F, et al. Detection of Toxoplasma gondii in faeces of privately owned cats using two PCR assays targeting the B1 gene and the 529-bp repetitive element. Parasitol. Res. 2017;116:1063–1069. doi: 10.1007/s00436-017-5388-z. [DOI] [PubMed] [Google Scholar]
- 24.Alvarado-Esquivel C, et al. Seroprevalence of Toxoplasma gondii antibodies in cats from Durango City, Mexico. J. Parasitol. 2007;93:1214–1216. doi: 10.1645/GE-1268R.1. [DOI] [PubMed] [Google Scholar]
- 25.Ballash GA, et al. Seroprevalence of Toxoplasma gondii in white-tailed deer (Odocoileus virginianus) and free-roaming cats (Felis catus) across a suburban to urban gradient in northeastern Ohio. EcoHealth. 2015;12:359–367. doi: 10.1007/s10393-014-0975-2. [DOI] [PubMed] [Google Scholar]
- 26.Ahlers A, et al. Survey of Toxoplasma gondii exposure in muskrats in a relatively pristine ecosystem. J. Parasitol. 2020;106:346–349. doi: 10.1645/19-126. [DOI] [PubMed] [Google Scholar]
- 27.Safarpour H, et al. Global status of Toxoplasma gondii infection and associated risk factors in people living with HIV. Aids (Lond., Engl.) 2020;34:469–474. doi: 10.1097/QAD.0000000000002424. [DOI] [PubMed] [Google Scholar]
- 28.Jafari-Modrek M, Hasanzadeh R, Azizi H, Hatam-Nahavandi K. A Seroprevalence Study of Toxoplasmosis in Female Students in Zahedan, South East of Iran. Iran. J. Public Health. 2019;48:988. [PMC free article] [PubMed] [Google Scholar]
- 29.Jones JL, et al. Risk factors for Toxoplasma gondii infection in the United States. Clin. Infect. Dis. 2009;49:878–884. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- 30.Aubert D, Villena I. Detection of Toxoplasma gondii oocysts in water: Proposition of a strategy and evaluation in Champagne-Ardenne Region, France. Mem. Inst. Oswaldo Cruz. 2009;104:290–295. doi: 10.1590/S0074-02762009000200023. [DOI] [PubMed] [Google Scholar]
- 31.Kulasena V, Rajapakse R, Dubey J, Dayawansa P, Premawansa S. Seroprevalence of Toxoplasma gondii in cats from Colombo, Sri Lanka. J. Parasitol. 2011;97:152–152. doi: 10.1645/GE-2640.1. [DOI] [PubMed] [Google Scholar]
- 32.Must K, Lassen B, Jokelainen P. Seroprevalence of and risk factors for Toxoplasma gondii infection in cats in Estonia. Vector Borne Zoonot. Dis. 2015;15:597–601. doi: 10.1089/vbz.2015.1809. [DOI] [PubMed] [Google Scholar]
- 33.Rahimi MT, et al. Cats and Toxoplasma gondii: A systematic review and meta-analysis in Iran. Onderstepoort J. Vet. Res. 2015;82:01–10. doi: 10.4102/ojvr.v82i1.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding H, Gao Y-M, Deng Y, Lamberton PH, Lu D-B. A systematic review and meta-analysis of the seroprevalence of Toxoplasma gondii in cats in mainland China. Parasit. Vectors. 2017;10:27. doi: 10.1186/s13071-017-1970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Must K, Hytönen MK, Orro T, Lohi H, Jokelainen P. Toxoplasma gondii seroprevalence varies by cat breed. PLoS ONE. 2017;12:20. doi: 10.1371/journal.pone.0184659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Rios A, et al. Toxoplasma gondii in captive wild felids of Mexico: Its frequency and capability to eliminate oocysts. Vector-Borne Zoonot. Dis. 2019;19:619–624. doi: 10.1089/vbz.2018.2385. [DOI] [PubMed] [Google Scholar]
- 37.Montazeri M, et al. The global serological prevalence of Toxoplasma gondii in felids during the last five decades (1967–2017): A systematic review and meta-analysis. Parasit. Vectors. 2020;13:1–10. doi: 10.1186/s13071-020-3954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 39.Bevins SN, et al. Three pathogens in sympatric populations of pumas, bobcats, and domestic cats: Implications for infectious disease transmission. PLoS One. 2012;7:20. doi: 10.1371/journal.pone.0031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Elm E, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 41.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Lee S, Lee E, Song K. Nested PCR-based detection of Toxoplasma gondii in German shepherd dogs and stray cats in South Korea. Res. Vet. Sci. 2008;85:125–127. doi: 10.1016/j.rvsc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Dubey JP, Beattie CP. Toxoplasmosis of Animals and Man. 2. CRC Press; 2010. [Google Scholar]
- 44.Dubey J. Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. J. Parasitol. 1995;20:410–415. doi: 10.2307/3283823. [DOI] [PubMed] [Google Scholar]
- 45.Cook A, et al. Sources of toxoplasma infection in pregnant women: European multicentre case–control study. Commentary: Congenital toxoplasmosis—further thought for food. BMJ. 2000;321:142–147. doi: 10.1136/bmj.321.7254.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daryani A, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: A systematic review and meta-analysis. Acta Trop. 2014;137:185–194. doi: 10.1016/j.actatropica.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Flegr J, Hrda S, Tachezy J. The role of psychological factors in questionnaire-based studies on routes of human toxoplasmosis transmission. Cent. Eur. J. Public Health. 1998;6:45–50. [PubMed] [Google Scholar]
- 48.Elmore SA, et al. Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26:190–196. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Flegr J. Predictors of Toxoplasma gondii infection in Czech and Slovak populations: The possible role of cat-related injuries and risky sexual behavior in the parasite transmission. Epidemiol. Infect. 2017;145:1351–1362. doi: 10.1017/S095026881700019X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coutinho SG, Lobo R, Dutra G. Isolation of Toxoplasma from the soil during an outbreak of toxoplasmosis in a rural area in Brazil. J. Parasitol. 1982;20:866–868. doi: 10.2307/3280995. [DOI] [PubMed] [Google Scholar]
- 51.Jones JL, et al. Follow-up of the 1977 Georgia outbreak of toxoplasmosis. Am. J. Trop. Med. Hyg. 2016;94:1299–1300. doi: 10.4269/ajtmh.15-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buffolano W, et al. Risk factors for recent Toxoplasma infection in pregnant women in Naples. Epidemiol. Infect. 1996;116:347–351. doi: 10.1017/S0950268800052675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapperud G, et al. Risk factors for Toxoplasma gondii infection in pregnancy: Results of a prospective case–control study in Norway. Am. J. Epidemiol. 1996;144:405–412. doi: 10.1093/oxfordjournals.aje.a008942. [DOI] [PubMed] [Google Scholar]
- 54.Baril L, Ancelle T, Thulliez P, Goulet V, Tirard V. Facteurs de risque d'acquisition de la toxoplasmose chez les femmes enceintes en 1995 (France) Bull. Épidémiol. Hebdomadaire. 1996;20:73–75. [Google Scholar]
- 55.Arkush KD, et al. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis) Int. J. Parasitol. 2003;33:1087–1097. doi: 10.1016/S0020-7519(03)00181-4. [DOI] [PubMed] [Google Scholar]
- 56.Liu X-Y, Wang Z-D, El-Ashram S, Liu Q. Toxoplasma gondii oocyst-driven infection in pigs, chickens and humans in northeastern China. BMC Vet. Res. 2019;15:1–7. doi: 10.1186/s12917-018-1758-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao X, Wang H, Wang H, Qin H, Xiao J. Land use and soil contamination with Toxoplasma gondii oocysts in urban areas. Sci. Total Environ. 2016;568:1086–1091. doi: 10.1016/j.scitotenv.2016.06.165. [DOI] [PubMed] [Google Scholar]
- 58.VanWormer E, Fritz H, Shapiro K, Mazet JA, Conrad PA. Molecules to modeling: Toxoplasma gondii oocysts at the human–animal–environment interface. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:217–231. doi: 10.1016/j.cimid.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foroutan M, et al. The global seroprevalence of Toxoplasma gondii in pigs: A systematic review and meta-analysis. Vet. Parasitol. 2019;269:42–52. doi: 10.1016/j.vetpar.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Dubey J, Rollor E, Smith K, Kwok O, Thulliez P. Low seroprevalence of Toxoplasma gondii in feral pigs from a remote island lacking cats. J. Parasitol. 1997;83:839–841. doi: 10.2307/3284277. [DOI] [PubMed] [Google Scholar]
- 61.Taggart PL, Caraguel CG, McAllister MM. Fractional seroprevalence rates in common prey species can cause more than half of feral cats to be exposed to Toxoplasma gondii annually. Vet. Parasitol. 2020;288:109306. doi: 10.1016/j.vetpar.2020.109306. [DOI] [PubMed] [Google Scholar]
- 62.Wang ZT, Verma SK, Dubey JP, Sibley LD. The aromatic amino acid hydroxylase genes AAH1 and AAH2 in Toxoplasma gondii contribute to transmission in the cat. PLoS Pathog. 2017;13:e1006272. doi: 10.1371/journal.ppat.1006272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robertson LJ, et al. Are molecular tools clarifying or confusing our understanding of the public health threat from zoonotic enteric protozoa in wildlife? Int. J. Parasitol. Parasit. Wildl. 2019;9:323–341. doi: 10.1016/j.ijppaw.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Opsteegh M, Prickaerts S, Frankena K, Evers EG. A quantitative microbial risk assessment for meatborne Toxoplasma gondii infection in The Netherlands. Int. J. Food Microbiol. 2011;150:103–114. doi: 10.1016/j.ijfoodmicro.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 65.Deng H, et al. Digging into Toxoplasma gondii infections via soil: A quantitative microbial risk assessment approach. Sci. Total Environ. 2020;20:143232. doi: 10.1016/j.scitotenv.2020.143232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.