Abstract
Inactivating variants as well as a missense variant in the centrosomal CEP78 gene have been identified in autosomal recessive cone-rod dystrophy with hearing loss (CRDHL), a rare syndromic inherited retinal disease distinct from Usher syndrome. Apart from this, a complex structural variant (SV) implicating CEP78 has been reported in CRDHL. Here we aimed to expand the genetic architecture of typical CRDHL by the identification of complex SVs of the CEP78 region and characterization of their underlying mechanisms. Approaches used for the identification of the SVs are shallow whole-genome sequencing (sWGS) combined with quantitative polymerase chain reaction (PCR) and long-range PCR, or ExomeDepth analysis on whole-exome sequencing (WES) data. Targeted or whole-genome nanopore long-read sequencing (LRS) was used to delineate breakpoint junctions at the nucleotide level. For all SVs cases, the effect of the SVs on CEP78 expression was assessed using quantitative PCR on patient-derived RNA. Apart from two novel canonical CEP78 splice variants and a frameshifting single-nucleotide variant (SNV), two SVs affecting CEP78 were identified in three unrelated individuals with CRDHL: a heterozygous total gene deletion of 235 kb and a partial gene deletion of 15 kb in a heterozygous and homozygous state, respectively. Assessment of the molecular consequences of the SVs on patient’s materials displayed a loss-of-function effect. Delineation and characterization of the 15-kb deletion using targeted LRS revealed the previously described complex CEP78 SV, suggestive of a recurrent genomic rearrangement. A founder haplotype was demonstrated for the latter SV in cases of Belgian and British origin, respectively. The novel 235-kb deletion was delineated using whole-genome LRS. Breakpoint analysis showed microhomology and pointed to a replication-based underlying mechanism. Moreover, data mining of bulk and single-cell human and mouse transcriptional datasets, together with CEP78 immunostaining on human retina, linked the CEP78 expression domain with its phenotypic manifestations. Overall, this study supports that the CEP78 locus is prone to distinct SVs and that SV analysis should be considered in a genetic workup of CRDHL. Finally, it demonstrated the power of sWGS and both targeted and whole-genome LRS in identifying and characterizing complex SVs in patients with ocular diseases.
Keywords: CEP78, inherited retinal disease, cone-rod dystrophy with hearing loss, long-read sequencing, structural variants, single-cell gene expression analysis
Introduction
During the last years, next-generation sequencing (NGS) techniques mostly relying on short-read sequencing (SRS) have accelerated molecular diagnoses in individuals with inherited retinal diseases (IRDs) (Jespersgaard et al., 2019). IRD is characterized by a tremendous genetic heterogeneity with variants identified in more than 270 genes [RetNet (Retinal Information Network)1 ]. Different types of variants can give rise to IRD, both single-nucleotide variants (SNVs) as well as structural variants (SVs), of which copy number variants (CNVs) have been most frequently reported (Khateb et al., 2016; Ellingford et al., 2018; Van Schil et al., 2018; Daiger et al., 2019; Zampaglione et al., 2020). The latter are estimated to contribute to at least 7%–10% of pathogenic alleles in IRD (Khateb et al., 2016; Ellingford et al., 2018; Daiger et al., 2019; Zampaglione et al., 2020). Interestingly, genomic features such as gene size have been shown to correlate with CNV occurrence in IRD genes (Van Schil et al., 2018).
Structural variant detection based on standard molecular karyotyping and on sequencing depth algorithms is capable of detecting only large CNVs, such as deletions and duplications greater than 50 kb in size, whereas cryptic SVs, such as smaller CNVs, copy neutral, and complex rearrangements, may be missed (Lindstrand et al., 2019). Nowadays, it is possible to detect approximately 27,000 SVs (>50 bp) per human genome only using a combination of technologies, where the majority of these SVs is located in the non-coding part of the genome and missed using exome-based approaches (Belkadi et al., 2015; Chaisson et al., 2019; Mahmoud et al., 2019). Whole-genome sequencing (WGS) proved to be particularly powerful to detect SVs, variants in GC-rich regions, and variants in non-coding regulatory regions (Nishiguchi et al., 2013; Ellingford et al., 2016; Carss et al., 2017; de Bruijn et al., 2020). While predictions are indicating that at least 48% of deletions and 83% of insertions are routinely missed by short-read-calling algorithms (Eichler, 2019), long-read sequencing (LRS) is particularly valuable for detecting SVs, as the long reads provide the necessary context to call and resolve SVs, regardless of their sequence composition (De Coster and Van Broeckhoven, 2019).
One of the more recently identified disease genes that contribute to the genetic heterogeneity of IRD is the centrosomal gene CEP78 (MIM# 617110), in which several types of variants, inactivating sequence variants as well as a unique missense variant have been found in autosomal recessive cone-rod dystrophy with hearing loss (CRDHL; MIM# 617236), a recognizable phenotype distinct from Usher syndrome (Fu et al., 2016; Namburi et al., 2016; Nikopoulos et al., 2016; Ascari et al., 2020). Apart from CRDHL, sperm abnormalities causing infertility have been reported in two unrelated affected males (Ascari et al., 2020). Functional studies pointed to a loss-of-function effect with decreased amounts of protein, normal subcellular localization, and elongated primary cilia in patients’ cells (Namburi et al., 2016; Nikopoulos et al., 2016; Ascari et al., 2020). Interestingly, a complex SV implicating CEP78 has been reported in one individual with CRDHL, being a homozygous deletion–inversion–deletion overlapping CEP78 (Sanchis-Juan et al., 2018). CEP78 localizes to the mature centrioles (Brunk et al., 2016), which are the main components of the centrosomes, key microtubule-organizing hubs in eukaryotic cells, with the mother centriole acting as the basal body during cilia formation (Gönczy and Hatzopoulos, 2019). Centrioles duplicate once per cell cycle, and irregularities in their structure, or number, are associated with several diseases including cancer or ciliopathies (Nigg and Raff, 2009; Gönczy, 2015).
Here, we report CEP78 SVs and SNVs in four unrelated CRDHL families of Belgian, Danish, and Turkish origin. Interestingly, two distinct SVs were identified in three of the four CRDHL families using a combination of NGS technologies. Targeted or whole-genome nanopore LRS was used to delineate breakpoint junctions at the nucleotide level. For the smallest SV, a founder effect was shown. Overall, this study supports that the CEP78 locus is prone to distinct SVs and emphasizes the importance of SV analysis in the genetic workup of CRDHL, leveraging the power of shallow whole-genome sequencing (sWGS) and both targeted and whole-genome LRS. Furthermore, data mining of bulk and single-cell (sc) transcriptional datasets, in combination with CEP78 immunostaining, displayed a CEP78 expression domain in agreement with the phenotypic manifestations of CRDHL.
Materials and Methods
Ethics Statement
This study followed the tenets of the Declaration of Helsinki, and ethical approval was given by the local ethics committee (Ghent University Hospital, EC UZG 2017/1540). All individuals involved gave their informed consent prior to inclusion in this study.
Phenotypic Evaluation
Affected individuals were subjected to ophthalmologic evaluation including best-corrected visual acuity measurement, funduscopy, visual field assessment, infrared and blue light reflectance and autofluorescence imaging, spectral-domain optical coherence tomography, and electroretinography, when possible. In addition, an audiological assessment was performed before or after the molecular diagnosis.
Whole-Exome Sequencing and Variant Validation
Genomic DNA (gDNA) was extracted from leukocytes according the manufacturer’s guidelines. Whole-exome sequencing (WES) was performed using SureSelectXT Human All Exon V6 enrichment (Agilent Technologies) and HiSeq 3000 sequencing (Illumina) (F1 and F4), or SureSelectXT Low Input Human All Exon V7 kit (Agilent Technologies) and NovaSeq 6000 sequencing (Illumina) (F3). Reads were aligned to the human hg38 reference genome with BWA (v0.7.15) (Li and Durbin, 2009). SNVs and small insertions and deletions were detected with the GATK HaplotypeCaller (v3.82). The VCF (variant call format) files were annotated with the Ensembl Variant Effect Predictor (release 95) and the dbNSFP (v3.4a) and dbscSNV (v1.1) databases. Variants were scored heterozygous or homozygous and were assessed with our in-house variant filtering and visualization tool. A selection of 272 and 275 RetNet genes was assessed, respectively (versions 3 and 4 of the RetNet panel). Nucleotide numbering was done following HGVS guidelines3 with nucleotide “A” of the ATG as “c.1.” Classification of variants was based on the ACMG and ACGS guidelines with adaptations (Richards et al., 2015; Nykamp et al., 2017; Abou Tayoun et al., 2018; Ellard et al., 2019). CNVs were assessed using ExomeDepth (Plagnol et al., 2012) (v1.1.10) and Pindel (Ye et al., 2009). Pathogenic (likely) variants were confirmed via Sanger sequencing. Sanger sequencing was performed using an ABI 3730xl DNA Analyzer (Applied Biosystems) with the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems).
For F2, II:1 gene panel sequencing of 13 genes [ABHD12, ADGRV1 (GPR98), CDH23, CIB2, CLRN1, DFNB31 (WHRN), HARS1, MYO7A, PCDH15, PDZD7, USH1C, USH1G, and USH2A] associated with Usher syndrome was performed using a HaloPlex custom design created by use of Agilent SureDesign and included exons and 50 bp of the intron at each intron–exon boundary. The enrichment library from patient DNA was sequenced on a MiSeq sequencer (Illumina). FASTQ files were analyzed using SureCall v.3.0.1.4 (Agilent Technologies) using default settings. Whole-exome trio sequencing was performed for F2, II:1 and her parents. Library preparation was done using the Ion AmpliSeq exome kit (Thermo Fisher), and libraries were sequenced using the IonProton system (Thermo Fisher). Base calling, read alignment, and variant calling were performed using the Torrent Suite including the Torrent Variant Caller (Thermo Fisher). VarSeq (GoldenHelix) was used for annotation and filtering of the variants. Confirmation of the identified CEP78 variant was performed via Sanger sequencing using NM_001098802.2 as reference sequence.
Shallow WGS
Shallow whole-genome sequencing was performed using the Hiseq3000 (Illumina), starting from 200 ng of gDNA. For library construction the NEXTflex Rapid DNA Sequencing kit (Bio Scientific) was used. Pipetting steps were automated using a Hamilton Star robot (Hamilton). Library concentrations were measured by the Qubit High-Sensitivity kit (Thermo Fisher Scientific), and equimolar concentrations were pooled before sequencing. The minimal number of mapped reads was set at 50 million. Copy number analysis was performed with WisecondorX and further visualized with the ViVar platform (Sante et al., 2014; Raman et al., 2019).
Quantitative and Long-Range Polymerase Chain Reaction for Deletion Confirmation and Delineation
Copy number variants were confirmed by quantitative polymerase chain reaction (qPCR), and primers were designed in coding exons or intronic regions as previously described (D’haene et al., 2010). Assays were prepared using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories) and run on LightCycler 480 System (Roche). Data were analyzed with qbase + software (Biogazelle). ZNF80 and GPR15 were used as reference genes. Subsequent to iterative qPCRs (long-range), PCR was performed to obtain junction deletion products using Phusion High-Fidelity PCR kit (New England Biolabs) and visualized on 1% UltraPure Agarose (Thermo Fisher Scientific) gels. Primer design was done using Primer3Plus. Primers sequences are listed in Supplementary Table 1.
Targeted LRS
Amplified (long-range) PCR product was quality checked using DropSense (Trinean), Qubit (ThermoFisher), and Fragment Analyzer (Agilent), using DNF-492 Large Fragment analysis kit (Agilent). A library was constructed according to the Ligation sequencing protocol (SQK-LSK109, Oxford Nanopore Technologies, ONT; GDE_9063_v109_revU_14Aug2019) with minor adaptations. DNA repair and end-prep using ONT consumables (SQK-LSK109) and NEBNext FFPE DNA repair mix and NEBNext Ultra II End repair/dA tailing Module (M6630, E7546 both from New England Biolabs) started with 100 fmol of the PCR amplicon, with extended enzymatic incubation times, 30 min at 20°C and 30 min at 65°C. Repaired and end-prepped amplicon was Ampure XP (Beckman Coulter) cleaned up for increased adaptor ligation efficiency, using a ratio of 1:1 (vol/vol) and extended incubation (10 min on Hulamixer). After bead cleanup, the pellet was eluted in 32 μL Nuclease Free Water, of which 30 μL was used for adapter ligation. Adapter ligation using SQK-LSK109, ONT, and NEBNext Quick Ligation Module (E6056, New England Biolabs) was performed according to the protocol with extension of the ligation incubation to 30 min at room temperature. The ligation reaction was cleaned up using Ampure XP (Beckman Coulter) in vol/vol ratio of 0.4 with extended incubation (10 min on Hulamixer). A purified pellet was eluted in 10 μL of MilliQ water with the final yield of library calculated using concentration and size information (F1, II:1 yield = 508.2 ng/72.6 fmol and F3, II:1 yield = 462 ng/61 fmol). Ten fmol (64 ng for F1, II:1) and 20 fmol (151.5 ng for F3, II:1) amount of the library was loaded and sequenced on MinION using Flongle Flowcell (both Oxford Nanopore Technologies) with, respectively, 85 (F1, II:1) and 47 (F3, II:1) number of pores sequencing after loading of the library. Sequencing was complete in 24 h and generated in total 1.08 Gb (F1, II:1) and 827.9 Mb (F3, II:1) of data, equaling 172.75 K (F1, II:1) and 140.99 K (F3, II:1) reads with an estimated N50 of 10.55 kb (F1, II:1) and 10.7 kb (F3, II:1).
Whole-Genome LRS
Extracted DNA was checked for concentration, purity, and integrity using DropSense (Trinean), Qubit (ThermoFisher), and Fragment Analyzer (Agilent), using 464 High Sensitivity Large Fragment 50-Kb kit (Agilent). The sample was sheared using Mega3 (Diagenode) to the final average size of the peak 21,015 bp (smear analysis 25,230 bp). Short fragments were eliminated using SRE XS (Circulomics). Sheared and size-selected DNA sample was used in library prep following the protocol gDNA by Ligation (SQK-LSK109, GDE_9063_v109_revU_14Aug2019, Oxford Nanopore Technologies) with minor adaptations. DNA repair and end-prep using ONT consumables (SQK-LSK109) and NEBNext FFPE DNA repair mix and NEBNext Ultra II End repair/dA tailing Module (M6630, E7546 both New England Biolabs) started with 182 fmol of the PCR amplicon, with extended enzymatic incubation times, 30 min at 20°C and 30 min at 65°C. Repaired and end-prepped amplicon was Ampure XP (Beckman Coulter) cleaned up for increased adaptor ligation efficiency, using a ratio of 1:1 (vol/vol) and extended incubation (10 min on Hulamixer). After bead cleanup, the pellet was eluted in 63 μL nuclease-free water, of which 60 μL was used for adapter ligation. Adapter ligation using SQK-LSK109, ONT, and NEBNext Quick Ligation Module (E6056, New England Biolabs) was performed according to the protocol with extension of the ligation incubation to 30 min at room temperature. The ligation reaction was cleaned up using Ampure XP (Beckman Coulter) in vol/vol ratio of 0.4 with extended incubation (10 min on Hulamixer). A purified pellet was eluted in 40 μL of MilliQ water with final yield of library calculated using concentration and size information (yield = 1,627.4 ng/105.6 fmol). Thirty femtomoles of the final library prep was loaded onto PromethION Flow cells (Oxford Nanopore Technologies). In order to generate enough data, the sample was loaded on three flow cells (FCs). Runs started with 6,844 (FC1), 5,835 (FC2), and 6,314 (FC3) sequenceable pores and ran for 72 h, generating in total 32.55 Gb (FC1), 36.18 Gb (FC2), and 40.99 Gb (FC3) of data, equaling 2.12 M (FC1), 2.39 M (FC2), and 2.68 M (FC3) reads with an estimated N50 of 27.86 kb (FC1), 28.76 kb (FC2), and 28.96 kb (FC3).
Data Analysis LRS
Base calling of the Nanopore data was performed using the Guppy base caller (v4.0.9 + 92ae093). Further analysis was performed using a pipeline integrated in GenomeComb (0.101.0) (Reumers et al., 2012). Reads were aligned to the hg38 genome reference (Schneider et al., 2017) using minimap2 (2.17-r941) (Li, 2018), and the resulting SAM file sorted and converted to BAM using Samtools (1.10) (Li et al., 2009). SVs were called using Sniffles (1.0.11) (Sedlazeck et al., 2018). The region of interest was filtered out and scanned for well-supported SVs using GenomeComb (Reumers et al., 2012).
Expression Analysis on Patient’s Material and Splicing Assessment
For quantitative reverse transcription–PCR (RT-PCR), total RNA was extracted from short-term cultured lymphocytes or fibroblasts using MagCore according the manufacturer’s guidelines. cDNA was synthesized with the iScript cDNA Synthesis Kit (Bio-Rad Laboratories). For each cDNA sample, assays were prepared using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories) and run on LightCycler 480 System (Roche). Data were analyzed with qbase + and normalized to the YWHAZ and HMBS or SDHA genes. Primers were designed using Primer3plus, and sequences are listed in Supplementary Table 1. For non-quantitative RT-PCR, cDNA was synthesized using SuperScript IV Reverse Transcriptase kit (Thermo Fisher) and underwent standard PCR, loaded on 2% UltraPure Agarose gel, and Sanger sequenced.
Haplotype Analysis
In total, 18 single-nucleotide polymorphisms (SNPs) (dbSNP, build 151) were selected for haplotype reconstruction in the upstream and downstream areas flanking CEP78. SNPs were Sanger sequenced according to the standard procedures (see above). PCR primers were designed with Primer3Plus, and sequences can be found in Supplementary Table 2.
CEP78 Expression in Mouse and Human Retina and Inner Ear
Data Mining in Single-Cell Retinal and Cochlear Transcriptional Datasets
Human adult retinal and murine P1 cochlear sc transcriptional datasets were mined for evaluating CEP78 expression at the sc level. Expression matrices derived from pooling three donor neural retinal (Cowan et al., 2020) and four cochlear samples (Kolla et al., 2020) were retrieved and processed separately using SCANPY (v1.4.6) (Wolf et al., 2018). Preprocessing and quality control were conducted to remove outlier cells. Briefly, we considered only genes with counts of at least three cells and filtered out cells that had unique feature counts <200 or >2,500 and/or that expressed >5% mitochondrial counts. The data were then total-count normalized, logarithmized, filtered for highly variable features, and scaled to unit variance. After quality control preprocessing, a total of 19,768 retinal and 11,332 cochlear cells were kept for subsequent dimensionality reduction, embedding, and clustering. Markers associated with major neural retina and cochlear cell populations were used to assess CEP78 expression at the sc level.
Data Mining in Bulk Retinal and Cochlear Transcriptional Datasets
Retina— Expression levels by transcripts per million (TPMs) were retrieved from postmortem retina samples characterized in Ratnapriya et al. (2019). From 453 samples that passed quality control, we only considered donor retinas that showed no age-related macular degeneration progression (n = 102) to avoid confounding variables in downstream analyses. To remove potential noise, 20% of genes with the lowest mean expression across all samples were filtered out. TPM values were then filtered for a set of candidate genes, which included all genes reported to cause IRD (RetNet) and ciliary genes (SCGSv1) (van Dam et al., 2013). A total of 519 genes were eventually considered. Before evaluating correlations in the expression of the candidate genes, the set was subjected to a variance-stabilizing transformation to correct for mean-variance dependency (Zwiener et al., 2014). We then examined the expression of CEP78 and several ciliary genes listed in the top 10 of the Human Gene Connectome (HGC) (Itan et al., 2013) (SCLT1, MKS1, CEP57, CEP76, CEP135, CEP152, CEP63, CEP164, OFD1, and CEP250). Spearman correlations were computed along with pairwise p values adjusted for multiple comparisons (Holm method). A correlogram was then generated for visualization.
Cochlea— We retrieved paired-end FASTQ files (GSE111348) derived from adult (P28-P32) mouse inner (IHC) and outer (OHC) cochlear hair cells (∼1,000 cells per sample; n = 4 and 6 for IHC and OHC, respectively) (Li et al., 2018). Transcripts were quantified through pseudoalignment by Kallisto (v.0.46.1), for which default parameters were used for both index build and transcript quantification (Bray et al., 2016). Additionally, data generated by Schrauwen et al. (2016) were used to retrieve expression values of CEP78 in adult human cochlea and components of the vestibular labyrinth.
CEP78 Immunostaining on Human Retina
Human retina used for immunohistochemistry was fixed in 10% neutral buffered formaldehyde and embedded in paraffin. Staining for CEP78 was performed on 3-μm-thick sections using an automatic immunostainer (BenchMark Ultra, Ventana Medical Systems, Tucson, AZ, United States). The rabbit polyclonal antibody anti-CEP78 (1:100; LN2004459, LabNed) was used, and visualization was achieved with the OptiView Amplification Kit (Ventana Medical Systems). Heat-induced epitope retrieval was performed using Cell Conditioning 2 (Ventana Medical Systems).
Results
The CRDHL families included in this study are of Belgian (F1 and F3), Danish (F2), and Turkish origin (F4). Consanguinity was reported for F4. Pedigrees are represented in Figure 1.
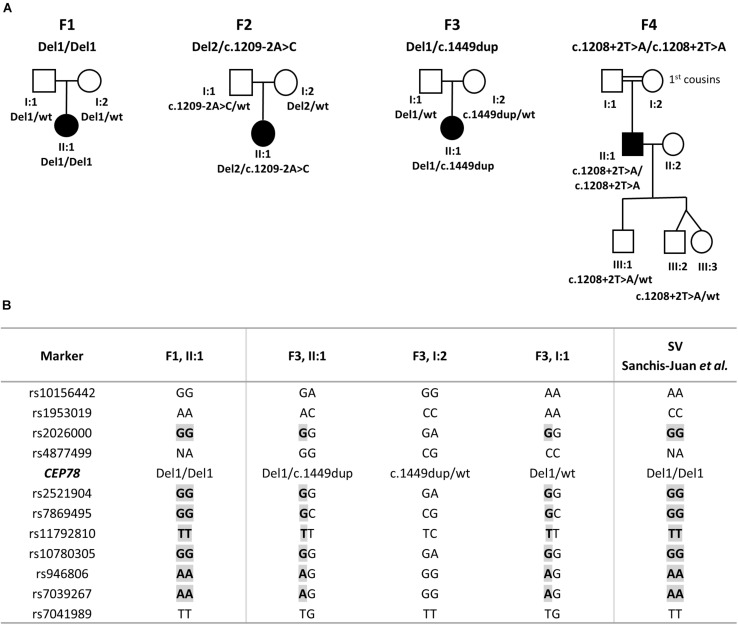
FIGURE 1.
Pedigrees segregating the CEP78 structural and sequence variants and haplotype reconstruction for the shared SV. (A) F1, II:1 is homozygous for a complex deletion–inversion–deletion involving exons 1–5 of CEP78. F2, II:1 is compound heterozygous for a deletion of 235 kb overlapping CEP78 and PSAT1 and for splice site variant c.1209-2A>C. F3, II:1 is compound heterozygous for the same deletion–inversion–deletion of F1, II:1 and the c.1449dup variant in CEP78. F4, II:1 is homozygous for splice site variant c.1208+2T>A. Extended F4 pedigree available in Supplementary Clinical Data 2. (B) Genotyping of 18 flanking single-nucleotide polymorphisms (SNPs) revealed a common haplotype of 1.9 Mb, between the individuals carrying the deletion–inversion–deletion [F1, F3 and the case originally described by Sanchis-Juan et al. (2018)]. Extended version available in Supplementary Table 5. Abbreviations: Del, deletion (or deletion–inversion–deletion); wt, wild type.
CNV Analysis on WES Data, sWGS, Long-Range PCR, and Targeted LRS in F1
For F1, II:1 CNV analysis on WES data using ExomeDepth revealed a potential homozygous deletion overlapping CEP78 [initial coordinates (hg38) chr9: g.78236351–78243636]. Subsequent sWGS confirmed the homozygous deletion spanning exons 1–5 of CEP78 [new coordinates (hg38) chr9:g.78230001–78245000], covering a region of 15–20 kb. The sWGS output is available in Supplementary Table 3. The deletion could be refined up to ∼12 kb using iterative qPCRs, and a junction product could be obtained via long-range PCR (Supplementary Figure 1). Targeted LRS on the long-range PCR amplicon allowed final delineation, identifying a complex deletion–inversion–deletion (Figure 2). The left breakpoint is located at (hg38) chr9:78228782, whereas the right breakpoint at chr9:78244762. The inverted segment spans chr9:78234546–78234844 [nearby an L1ME3Cz repetitive element (chr9:78234521–78234902)]. The SV overlaps with the one previously described in a British patient by Sanchis-Juan et al. (2018), delineated using microarray and Sanger sequencing (Sanchis-Juan et al., 2018). A smaller deletion, overlapping exons 2 and 3 in CEP78, is reported in gnomAD SV (DEL_9_103019; gnomAD SVs v2.1). Segregation analysis was performed via qPCR and confirmed both parents as heterozygous carriers of the deletion. Expression analysis of CEP78 mRNA on available lymphocytes (F1, II:1) and three controls showed complete loss of CEP78 expression due to the homozygous deletion (Supplementary Figure 2).
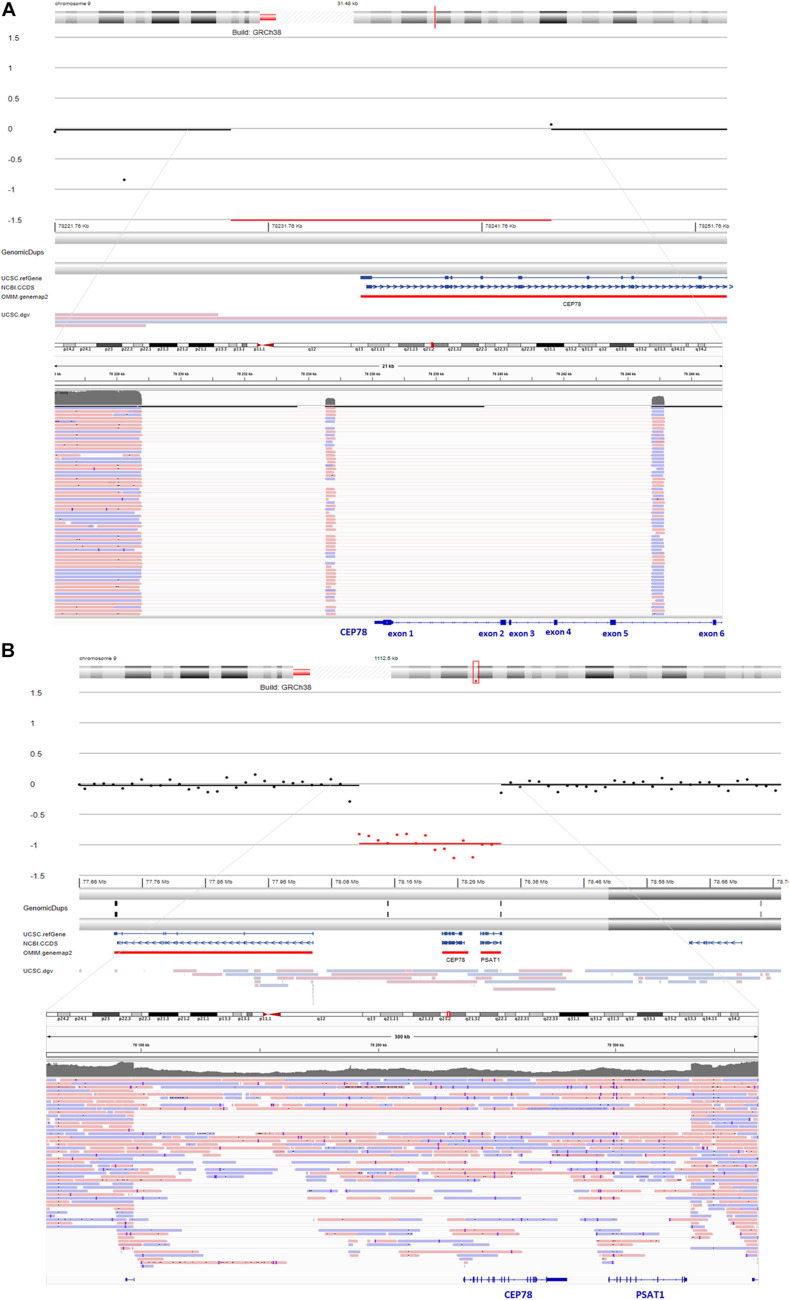
FIGURE 2.
sWGS output and integrative genomics viewer (IGV) view of the structural variants (SVs) detected via long-read sequencing (LRS) in F1 and F2. (A) sWGS in F1, II:1 revealed a homozygous deletion of the region spanning exons 1–5 of CEP78 (top). Subsequent delineation of the SV via targeted LRS of the junction product identified a complex deletion–inversion–deletion (bottom). The left breakpoint is located at (hg38) chr9:78228782, whereas the right breakpoint is located at chr9:78244762. The inverted segment spans chr9:78234546–78234844 [nearby an L1ME3Cz repetitive element (chr9:78234521–78234902)]. The SV overlaps with a previously described SV affecting CEP78 (Sanchis-Juan et al., 2018). (B) sWGS in F2, II:1 identified a heterozygous deletion spanning the entire CEP78 and PSAT1 genes (top). Final delineation was obtained via whole-genome LRS, coordinates are crossing chr9: 78096930–78331887 and cover 235 kb (bottom).
Segregation Analysis, sWGS, and Whole-Genome LRS in F2
For F2, II:1, initial targeted gene panel sequencing was negative, as it was carried out before the CEP78 gene had been associated with CRDHL. Thus, trio WES was performed for the proband and revealed a novel heterozygous splice variant c.1209-2A>C p.(?) (NM_001098802.2) in CEP78. The c.1209-2A>C variant in CEP78 was confirmed via Sanger sequencing, where the proband appeared homozygous, the father heterozygous and the mother wild-type (Supplementary Figure 3). No additional family members have been tested. The splice variant is located in the acceptor splice site of intron 9, reported in dbSNP (rs778035330) with a frequency of 0.00002628 (gnomAD), and predictions suggest a skip of exon 10. It is predicted to disrupt the consensus splice site and likely results in an absent or disrupted protein product and loss of function. Subsequent RT-PCR confirmed the skip of exon 10 (Supplementary Figure 3). Population frequencies and in silico predictions of the CEP78 splice variant are listed in Supplementary Table 4 and Supplementary Figure 4. To our knowledge, this variant has not been reported in literature, but is reported as likely pathogenic in one case in ClinVar (ID 851351). Segregation analysis suggested a heterozygous genomic deletion of the CEP78 region in F2, II:1, in trans with the splice variant. The heterozygous deletion was confirmed by sWGS, showing an interval of 225–240 kb spanning the entire CEP78 and PSAT1 genes (chr9: g.78105001–78330000). The sWGS output is available in Supplementary Table 3. The final delineation was obtained via whole-genome LRS allowing the identification of a heterozygous (13 reference sequence reads, 21 deletion reads) deletion spanning the region chr9:78096930–78331887 and covering 235 kb (Figure 2). Breakpoint junction analysis highlighted microhomology (Supplementary Figure 5). Expression analysis of CEP78 mRNA on available fibroblasts (F2, II:1) and two controls showed loss of CEP78 expression due to the CEP78 genotype (Supplementary Figure 2).
CNV Analysis on WES Data, Long-Range PCR, and Targeted LRS in F3
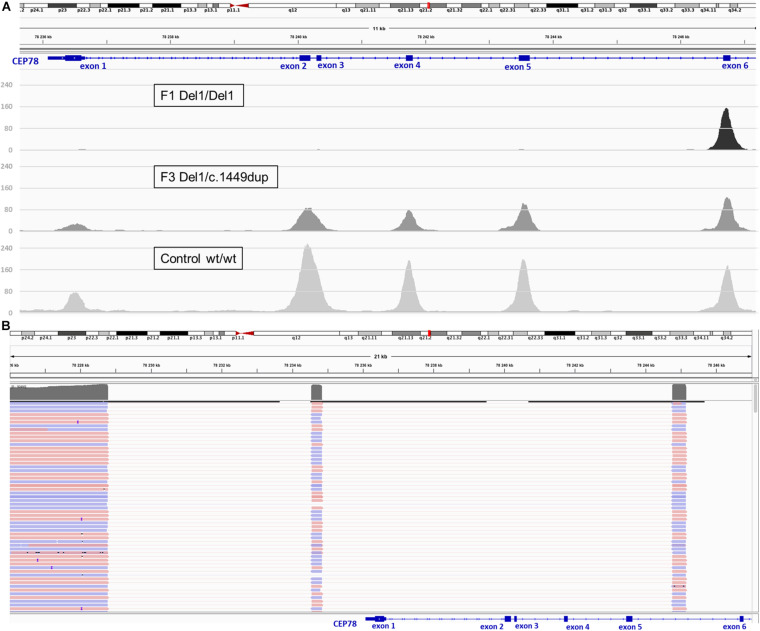
For F3, II:1 CNV analysis on WES data using ExomeDepth revealed a heterozygous deletion overlapping CEP78 with the same coordinates of the deletion in F1, II:1 (Figure 3) in combination with c.1449dup [p.(Arg484Thrfs∗4)]. The latter is a novel 1-bp duplication in exon 12, creating a frameshift starting at codon Arg484. The new reading frame ends in a stop codon at position 4 (Supplementary Table 4). Segregation analysis confirmed the presence of c.1449dup on the maternal allele, while the deletion has paternal origin (Supplementary Figure 6). A junction product could be obtained via long-range PCR using the same primers used for F1, II:1. Targeted LRS on the long-range PCR amplicon allowed a final delineation, identifying once more the same complex deletion–inversion–deletion as found in F1, II:1 (Figure 3). Expression analysis of CEP78 mRNA on available lymphocytes (F3 I:1, I:2, and II:1) and three controls showed extremely reduced CEP78 expression due to the variants in the index case (Supplementary Figure 2).
FIGURE 3.
Deletion coverage plots and IGV view of the complex SV detected via targeted LRS in F3, II:1. (A) ExomeDepth coverage plots for homozygous deletion (F1, II:1, top) and heterozygous deletion (F3, II:1, middle) compared to control (bottom). Coverage suggested for F3, II:1 the same deletion described for F1, II:1 but in heterozygous state. (B) Delineation of the SV via targeted LRS of the junction product obtained in F3, II:1 identified the same complex deletion–inversion–deletion found previously in F1, II:1.
Haplotype Reconstruction for Recurrent Deletion–Inversion–Deletion of CEP78 Identified in F1 and F3
The same deletion–inversion–deletion, originally described by Sanchis-Juan et al. (2018), has been identified here in F1 (homozygous) and F3 (heterozygous, with paternal inheritance); therefore, haplotype reconstruction using genotyping of 18 SNPs was performed in the proband of F1 (II:1) and in the proband and parents of F3 (II:1, I:1, and I:2). In addition, the haplotype of the homozygous CEP78 SV reported by Sanchis-Juan et al. (2018), was reconstructed here on the basis of available WGS data. A common shared haplotype of at least 1.9 Mb was identified (Supplementary Table 5).
Phenotypic Expression of CEP78 Disease Caused by SVs and SNVs
In F4, II:1 WES filtering revealed a novel homozygous splice variant c.1208+2T>A [p.(?)] (NM_001098802.2), located in the donor splice site of intron 9 and predicted to cause skipping of exon 9. The variant involves the same intron as the one affected by c.1209-2A>C in F2, II:1 and is not reported in gnomAD. Segregation analysis in two of the obligate carrier children confirmed they are heterozygous carriers. Population frequencies, in silico predictions, and variant classification according to ACMG and ACGS criteria are listed in Supplementary Table 4 and Supplementary Figure 4. All patients included received an initial (F1, F2, and F3) or post hoc, genotype-driven (F4) clinical diagnosis compatible with CRDHL. In agreement with previous studies, the IRD phenotype displays a more pronounced cone dysfunction at the onset. The age at onset of the IRD ranged from the second to third decade and, of the hearing loss, from congenital to the first decade of life. More detailed clinical features are provided in Figure 4, Table 1, and Supplementary Clinical Data 1, 2. Affected individuals from F1 to F3 are females, whereas the affected individual from F4 is a male, without signs of subfertility/infertility. Apart from CRDHL, no additional clinical features were reported except for a balance disorder in F1. Overall, no genotype–phenotype correlation could be observed for the IRD phenotype in probands with biallelic CEP78 SVs, SNVs, or a combination of both.
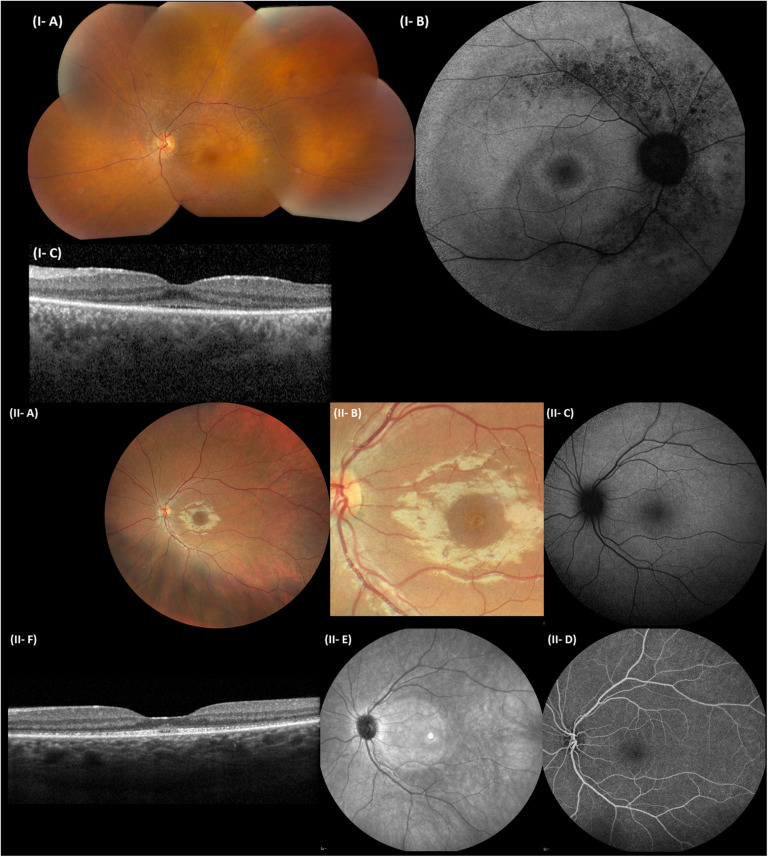
FIGURE 4.
Representative ophthalmological pictures of individuals from F1 and F3 carrying CEP78 structural variants. (I) F1, II:1. (I-A) Color fundus photograph of LE with normal optic disk, minimal narrowing of retinal arterioles, RPE mottling around the temporal vascular arcades, no bone spiculae. (I-B) BAF of the RE with hyperautofluorescent ring surrounding the fovea and granular hypo-autofluorescence around the temporal vascular arcades. (I-C) Spectral-domain OCT of the LE depicting loss of perifoveal photoreceptor layer and better foveal quality with loss, however, of integrity of photoreceptor outer segments. (II) F3, II:1. (II-A) Eye fundus of the left eye with perifoveal fine, granular, yellowish material upon close inspection. Normal aspect of optic disk, peripheral retina, and retinal vessels. (II-B) Close-up of the macular area, demonstrating the fine, granular, yellowish material encircling the fovea. (II-C) BAF of the LE without obvious abnormalities. (II-D) Fluorescein angiography of the LE with normal fluorescence. (II-E) Near-infrared imaging of the LE (hyperintense circular area is an artifact). (II-F) Spectral-domain OCT of the LE, revealing a mottled aspect of the ellipsoid zone and subfoveal collection of fluffy material. Enlarged and flat foveal depression. BAF, blue-light autofluorescence imaging; OCT, spectral-domain optical coherence tomography; RE, right eye; LE, left eye; RPE, retinal pigment epithelium.
TABLE 1.
Overview of clinical findings in CEP78-associated CRDHL.
| Age/sex | Origin | Age at onset | BCVA (RE/LE) | Goldmann visual fields | Fundus imaging | BAF | OCT | ERG | Hearing impairment | Other findings reported | |
| F1 II:1 | 45 y/F | Belgian | 22 y | 0.1/0.1 | Small central island of less than 5°, surrounded by large absolute (mid)peripheral scotoma (100° horizontal, 70° vertical) and normal peripheral limits | Normal optic disk, normal veins, narrowing of the arteries, perimacular grayish mottling around the vascular arcades and midperiphery, absence of bone spiculae | Mottled hypofluo rescence around the large vascular arcades, perifoveal hyperfluo rescent ring | Loss of outer retinal layers with sparing of subfoveal region with foveal swelling and blending | Maximum tendency toward electronegativity, scotopic function flat, cone function: both 30-Hz flicker and single flash present but delayed and reduced | Present since the age of 12 y, hearing aids | No fertility problems, balance disorder |
| F2 II:1 | 52 y/F | Danish | 30 y | 0.25/0.2 (49 y) | Paracentral scotomas, normal outer borders (49 y) | Normal optic disk, attenuated vessels, absence of hyperpigme ntations (49 y) | Mottled appearance midperiphery (49 y) | Attenuation of outer retinal layers (49 y) | Reduced cone and rod responses (39 y) | Congenital progressive hearing loss, hearing aids | None |
| F3 II:1 | 25 y/F | Belgian | 24 y | 0.3/0.2 | Pericentral concentric scotoma | Discrete macular mottling | Unremarkable | Wide foveal depression | Lowered cone and rod responses, but worse for cones | High frequencies | Subtle mottling in central macula on infrared |
| F4 II:1 | 57 y/M | Turkish | NA | 0.08/0.08 | Small central island less than 5°, currently stable | Pale optic disk, narrow blood vessels, grayish discoloration, some bone spiculae in midperiphery | Mottled hypoautoflu orescence around the large vascular arcades and nasally, perifoveal hyperflu orescent ring | Attenuation of outer retina | Extinguished responses of cones and rods | Present since childhood, not progressive | None |
BAF, blue-light autofluorescence imaging; BCVA, best-corrected visual acuity; CF, counting fingers; ERG, full-field flash electroretinography; F, female; HM, hand movements; M, male; NA, not available; OCT, spectral-domain optical coherence tomography; RE, right eye; LE, left eye.
CEP78 Expression in Human and Mouse Tissues Affected in CRDHL
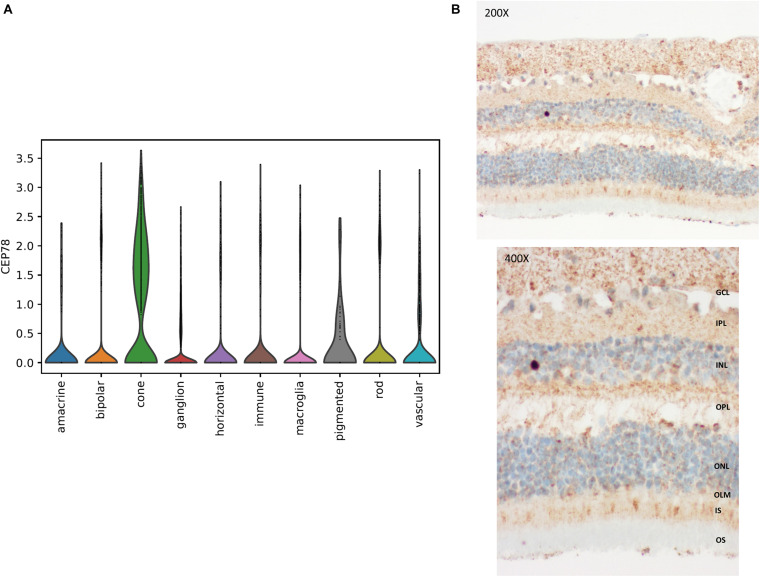
In line with the main systems affected in CRDHL, i.e., neural retina and the inner ear, we examined CEP78 expression in sc transcriptional datasets of human neural retina together with mouse and human cochlear or inner ear cells. This showed that CEP78 is predominantly expressed in cone photoreceptor and in hair cell clusters, respectively (Figure 5 and Supplementary Figures 7, 8). Next, the expression patterns of CEP78 and other ciliary genes listed in the top 10 of the HGC (Itan et al., 2013), SCLT1, MKS1, CEP57, CEP76, CEP135, CEP152, CEP63, CEP164, OFD1, and CEP250, in human adult bulk retinal transcriptional datasets were assessed for coordinated correlation, reasoning that this could offer insight into possible regulatory interactions. Interestingly, a matrix correlation plot (Supplementary Figure 9) showed CEP78 expression to be correlated with SCLT1 (ρ = 0.63, p < 0.001) and to be anticorrelated with CEP250 (ρ = −0.53, p = 0.0007), both of them already associated with IRD (Khateb et al., 2014; Tonda et al., 2016; Kubota et al., 2018). Immunohistochemistry analysis of human retina shows predominant cytoplasmic expression of CEP78 in both cones and rods, with strong staining at the base of the inner segments, concordant with previous findings (Figure 5; Namburi et al., 2016; Nikopoulos et al., 2016).
FIGURE 5.
Single-cell transcriptional data in human retina and immunohistochemistry of CEP78 in adult human retina. (A) Violin plot for visualization of scaled and corrected expression of CEP78 in 19,768 human neural retinal cells. Cells belonging to the cone cell population exhibit the highest expression. (B) Immunostaining of the CEP78 protein is visible in light brown. Light microscopy at 200 × (top) and 400 × (bottom). Predominant cytoplasmic expression of CEP78 can be seen in both cone and rod photoreceptors with strong staining at the base of the inner segments. Antibody: LN2004459 (1:100, rabbit, LabNed). GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; OLM, outer limiting membrane; IS, inner segments; OS, outer segments.
Discussion
In this study, we focused on CEP78-associated IRD, of which several studies have linked CEP78 variants with a presumed loss-of-function effect to CRDHL. Interestingly, a homozygous complex SV affecting CEP78, i.e., a deletion–inversion–deletion, was recently described in a case of CRDHL (Sanchis-Juan et al., 2018). We assessed the role of SVs in CRDHL cases and found two distinct SVs affecting the CEP78 region, emphasizing their role in CEP78 disease. We showed the efficacy of CNV analysis of WES data to identify subtle CNVs; more specifically, we identified a deletion spanning exons 1–5 of CEP78, in both homozygous and heterozygous states, leading to loss of function. Furthermore, we showed the power of combined sWGS, qPCR, and targeted or whole-genome LRS to delineate and characterize SVs at the nucleotide level. This approach revealed a complex 15-kb deletion–inversion–deletion, which proved to be the same as the previously reported CEP78 SV that was found and characterized by combined molecular karyotyping and Sanger sequencing. As this deletion was identified in the two families of Belgian origin and in a previously published case of a British patient, haplotype reconstruction was performed and suggested a founder effect. Apart from this, a distinct larger SV, being a 235-kb heterozygous deletion, was identified using molecular karyotyping and characterized using whole-genome LRS. This deletion encompasses the CEP78 and PSAT1 (MIM #610936) genes, the latter of which has so far only been implicated in recessive phosphoserine aminotransferase deficiency (MIM # 610992) and Neu-Laxova syndrome 2 (MIM # 616038) (Hart et al., 2007; Acuna-Hidalgo et al., 2014). Overall, for all cases with CEP78 SVs identified by us, loss of CEP78 expression was confirmed on patient-derived material. The latter is in line with the molecular effects of the previously reported CEP78 sequence variants.
Sanchis-Juan et al. (2018) reported that the sequences surrounding all the breakpoints of the described SV in CEP78 present high similarity to long interspersed nuclear elements (LINEs). It was hypothesized that repetitive elements enable replication-based SV formation, providing the necessary microhomology islands or increasing the vulnerability of the region to the formation of secondary DNA structures, which can lead to replication fork collapse (Sanchis-Juan et al., 2018). We performed a similar extensive in silico analysis of all breakpoints and junctions of the SVs identified in this study (Supplementary Figures 5, 10 and Supplementary Tables 6, 7). Interestingly, all breakpoint junctions, including the 235-kb CEP78-PSAT1 deletion, showed microhomology, supporting repetitive element-mediated replication-based SV formation underlying CEP78 SVs/complex SVs. Our findings underscore that SVs contribute to the genetic diversity of the human genome and are of high relevance for the molecular pathogenesis of rare diseases. Very recently, SVs were mapped and characterized in 17,795 deeply sequenced human genomes (Abel et al., 2020). It is estimated that SVs account for 17.2% of rare alleles genome-wide and that approximately 90% of such SVs are non-coding deletions. The number of complex SVs as a cause of genetic diseases is emerging. This is illustrated by de novo interspersed repeat insertions found in 124 cases with a genetic disease, 76 of which are caused by Alu short interspersed elements (SINEs), and 30 can be attributed to LINE-1 insertions (Payer et al., 2017). Two remarkable examples of complex SVs as a cause of IRD are SVA retrotransposon insertions in MFSD8- and BBS1-associated disease, of which the first SV served as a target for antisense oligonucleotide treatment, and the second appears to be a frequent cause of Bardet–Biedl syndrome (Kim et al., 2019; Delvallée et al., 2020). Another recent example is the identification of 33 pathogenic SVs in a cohort of 722 patients with autosomal dominant retinitis pigmentosa (adRP). Indeed, eight distinct complex non-coding SVs were found as the underlying mechanism of RP17-linked adRP in 22 affected families with >300 affected individuals, clearly emphasizing the importance of the non-coding genome as target for SVs in IRD (de Bruijn et al., 2020).
Given a high contribution of CNVs to ∼7%–10% of the pathogenic alleles in IRD, the fact that the majority of the SVs in our genome are non-coding and the emerging number of non-coding or complex SVs causing IRD (Abel et al., 2020; Zampaglione et al., 2020), it can be expected that SVs represent an important part of missing heritability of IRD. Hence, there is a need to assess the impact and frequency of SVs in IRD cohorts more systematically. Here, we showed how CNV calling on WES data, sWGS, and targeted or whole-genome LRS led to a confirmed molecular diagnosis in CEP78-associated IRD cases. CNV assessment using NGS-based algorithms has been already described as a reliable method to enhance the diagnostic rate of IRD (Khateb et al., 2016; Ellingford et al., 2018). Furthermore, this study supports the superiority of LRS for fast characterization of complex SVs. Massively parallel sequencing is currently dominated by second-generation sequencing technology, mainly relying on SRS, having its limitations for the identification of cryptic SVs, sequencing repetitive regions, phasing of alleles, and distinguishing highly homologous genomic regions, mainly due to its short-read lengths. Third-generation or LRS technologies offer improvements in the characterization of genetic variation and regions that are difficult to assess with the current SRS approaches. The main advantage of LRS compared to SRS technologies comes from the use of long reads (>10 kb on average), originating from single-DNA molecules. Apart from this, the sequencing occurs in real time without the need of PCR amplification, therefore being mostly free from PCR-related bias. The power of LRS approaches to overcome specific limitations of second generation–based analyses will further become clear when LRS will be implemented in routine genetic testing workflows.
Apart from the CEP78 SVs, the two novel splice variants found here expand the spectrum of CEP78 (likely) pathogenic SNVs: a novel canonical acceptor and donor splice variant of intron 9, respectively, c.1209-2A>C (p.?), predicted to lead to an exon 10 skip, and c.1208 + 2T > A (p.?), predicted to cause an exon 9 skip. Interestingly, skipping of the 46-bp exon 10 was previously reported for c.1254 + 5G>A (CEP78, NM_032171) (Fu et al., 2016). When comparing the phenotypes found in CRDHL cases with SNV and SVs, no apparent genotype–phenotype correlation could be demonstrated that would allow discriminating between the two classes of variants, emphasizing the need for a systematic SV assessment in the genetic workup of CRDHL cases, in which a CEP78 genotype is suspected or in “atypical” Usher syndrome cases.
Using data mining of bulk or sc transcriptional datasets from human retina, mouse cochlea, and human inner ear, we showed expression in cone photoreceptors and in hair cell clusters. These expression domains are in agreement with the main systems affected in CRDHL, i.e., neural retina and the inner ear. Apart from this, a correlation study of CEP78 expression strengthened the importance of CEP78 in the ciliary machinery.
To conclude, this study supports that the CEP78 locus is prone to microhomology-mediated, replication-based SV formation and that (complex) SV analysis should be included in molecular genetic testing of CRDHL or “atypical” Usher syndrome. Finally, it demonstrates the power of WES-based CNV assessment, sWGS, and whole or targeted LRS in identifying and characterizing suspected complex SVs in patients with CEP78-associated IRD. Systematic SV assessment in IRD will certainly close a diagnostic gap and will contribute to precision medicine in IRD.
Data Availability Statement
The variants presented in this study can be found in online repositories. The name of the repository and accession numbers can be found below: Leiden Open Variation Database (LOVD), https://databases.lovd.nl/shared/individuals/00332412; https://databases.lovd.nl/shared/individuals/00332413; and https://databases.lovd.nl/shared/individuals/00332414.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee Ghent University Hospital, Ghent University Hospital, Ghent. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
GAs and EDB designed the study and wrote the overall manuscript. GAs, NR, MDB, MVH, DC, JVD, TVL, TDP, AR, and EDB performed the research. JDZ, MVL, JJ, MBe, and LT clinically evaluated and described the families included in the study. GAs, NR, MDB, MBa, MVH, GAr, DC, JVD, TR, TDP, PDR, WDC, BM, AR, MS, and EDB analyzed and described the data. All authors reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Lone Sandbjerg Hindbaek is thanked for excellent technical help with RT-PCR studies. Sarah De Jaegere and Valerie Baumont are thanked for their assistance. We are also grateful to the families for their participation in the study.
Funding. This work was supported by grants from Ghent University Special Research Fund (BOF15/GOA/011; BOF20/GOA/023) to EDB and BM; Ghent University Hospital Innovation Fund NucleUZ to EDB; and European Union’s Horizon 2020 research and innovation program Marie Sklodowska-Curie Innovative Training Networks (ITN) StarT (grant no. 813490) to EDB. EDB is a Senior Clinical Investigator of the Research Foundation-Flanders (FWO) (1802215N; 1802220N); GAs is a Ph.D. fellow of the FWO (11B4818N). AR is Early Starting Researcher of StarT (grant no. 813490). EDB is member of the ERN-EYE consortium which is co-funded by the Health Program of the European Union under the Framework Partnership Agreement No 739534-ERN-EYE.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.664317/full#supplementary-material
References
- Abel H. J., Larson D. E., Regier A. A., Chiang C., Das I., Kanchi K. L., et al. (2020). Mapping and characterization of structural variation in 17,795 human genomes. Nature 583 83–89. 10.1038/s41586-020-2371-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Tayoun A. N., Pesaran T., DiStefano M. T., Oza A., Rehm H. L., Biesecker L. G., et al. (2018). Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum. Mutat. 39 1517–1524. 10.1002/humu.23626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna-Hidalgo R., Schanze D., Kariminejad A., Nordgren A., Kariminejad M. H., Conner P., et al. (2014). Neu-laxova syndrome is a heterogeneous metabolic disorder caused by defects in enzymes of the l-serine biosynthesis pathway. Am. J. Hum. Genet. 95 285–93. 10.1016/j.ajhg.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascari G., Peelman F., Farinelli P., Rosseel T., Lambrechts N., Wunderlich K. A., et al. (2020). Functional characterization of the first missense variant in CEP78, a founder allele associated with cone-rod dystrophy, hearing loss and reduced male fertility. Hum. Mutat. 41 998–1011. 10.1002/humu.23993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkadi A., Bolze A., Itan Y., Cobat A., Vincent Q. B., Antipenko A., et al. (2015). Whole-genome sequencing is more powerful than whole-exome sequencing for detecting exome variants. Proc. Natl. Acad. Sci. U.S.A. 112 5473–5478. 10.1073/pnas.1418631112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray N. L., Pimentel H., Melsted P., Pachter L. (2016). Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34 525–527. 10.1038/nbt.3519 [DOI] [PubMed] [Google Scholar]
- Brunk K., Zhu M., Bärenz F., Kratz A., Haselmann-Weiss U., Antony C., et al. (2016). Cep78 is a new centriolar protein involved in Plk4-induced centriole overduplication. J. Cell Sci. 129 2713–2718. 10.1242/jcs.184093 [DOI] [PubMed] [Google Scholar]
- Carss K., Arno G., Erwood M., Stephens J., Sanchis-Juan A., Hull S., et al. (2017). Comprehensive rare variant analysis via whole-genome sequencing to determine the molecular pathology of inherited retinal disease. Am. J. Hum. Genet. 100 75–90. 10.1016/j.ajhg.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisson M. J. P., Sanders A. D., Zhao X., Malhotra A., Porubsky D., Rausch T., et al. (2019). Multi-platform discovery of haplotype-resolved structural variation in human genomes. Nat. Commun. 10:1784. 10.1038/s41467-018-08148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C. S., Renner M., De Gennaro M., Gross-Scherf B., Goldblum D., Hou Y., et al. (2020). Cell types of the human retina and its organoids at single-cell resolution. Cell 182 1623–1640.e34. 10.1016/j.cell.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’haene B., Vandesompele J., Hellemans J. (2010). Accurate and objective copy number profiling using real-time quantitative PCR. Methods 50 262–270. 10.1016/j.ymeth.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Daiger S. P., Sullivan L. S., Bowne S. J., Cadena E. D., Koboldt D., Bujakowska K. M., et al. (2019). Detection of large structural variants causing inherited retinal diseases. Adv. Exp. Med. Biol. 1185 197–202. 10.1007/978-3-030-27378-1_32 [DOI] [PubMed] [Google Scholar]
- de Bruijn S. E., Fiorentino A., Ottaviani D., Fanucchi S., Melo U. S., Corral-Serrano J. C., et al. (2020). Structural variants create new topological-associated domains and ectopic retinal enhancer-gene contact in dominant retinitis pigmentosa. Am. J. Hum. Genet. 107 802–814. 10.1016/j.ajhg.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster W., Van Broeckhoven C. (2019). Newest methods for detecting structural variations. Trends Biotechnol. 37 973–982. 10.1016/j.tibtech.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Delvallée C., Nicaise S., Antin M., Leuvrey A., Nourisson E., Leitch C. C., et al. (2020). A BBS1 SVA Fretrotransposon insertion is a frequent cause of Bardet-Biedl syndrome. Clin. Genet. 99 318–324. 10.1111/cge.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler E. E. (2019). Genetic variation, comparative genomics, and the diagnosis of disease. N. Engl. J. Med. 381 64–74. 10.1056/nejmra1809315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard S., Baple E. L., Berry I., Forrester N., Turnbull C., Owens M., et al. (2019). ACGS best practice guidelines for variant classification 2019. Assoc. Clin. Genet. Sci. 1–32. [Google Scholar]
- Ellingford J. M., Barton S., Bhaskar S., Williams S. G., Sergouniotis P. I., O’Sullivan J., et al. (2016). Whole genome sequencing increases molecular diagnostic yield compared with current diagnostic testing for inherited retinal disease. Ophthalmology 123 1143–1150. 10.1016/j.ophtha.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingford J. M., Horn B., Campbell C., Arno G., Barton S., Tate C., et al. (2018). Assessment of the incorporation of CNV surveillance into gene panel next-generation sequencing testing for inherited retinal diseases. J. Med. Genet. 55 114–121. 10.1136/jmedgenet-2017-104791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Xu M., Chen X., Sheng X., Yuan Z., Liu Y., et al. (2016). CEP78 is mutated in a distinct type of Usher syndrome. J. Med. Genet. 54 190–195. 10.1136/jmedgenet-2016-104166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy P. (2015). Centrosomes and cancer: revisiting a long-standing relationship. Nat. Rev. Cancer 15 639–652. 10.1038/nrc3995 [DOI] [PubMed] [Google Scholar]
- Gönczy P., Hatzopoulos G. N. (2019). Centriole assembly at a glance. J. Cell Sci. 132:jcs228833. 10.1242/jcs.228833 [DOI] [PubMed] [Google Scholar]
- Hart C. E., Race V., Achouri Y., Wiame E., Sharrard M., Olpin S. E., et al. (2007). Phosphoserine aminotransferase deficiency: a novel disorder of the serine biosynthesis pathway. Am. J. Hum. Genet. 80 931–937. 10.1086/517888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itan Y., Zhang S.-Y., Vogt G., Abhyankar A., Herman M., Nitschke P., et al. (2013). The human gene connectome as a map of short cuts for morbid allele discovery. Proc. Natl. Acad. Sci. U.S.A. 110 5558–5563. 10.1073/pnas.1218167110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersgaard C., Fang M., Bertelsen M., Dang X., Jensen H., Chen Y., et al. (2019). Molecular genetic analysis using targeted NGS analysis of 677 individuals with retinal dystrophy. Sci. Rep. 9:1219. 10.1038/s41598-018-38007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khateb S., Hanany M., Khalaileh A., Beryozkin A., Meyer S., Abu-Diab A., et al. (2016). Identification of genomic deletions causing inherited retinal degenerations by coverage analysis of whole exome sequencing data. J. Med. Genet. 53 600–607. 10.1136/jmedgenet-2016-103825 [DOI] [PubMed] [Google Scholar]
- Khateb S., Zelinger L., Mizrahi-Meissonnier L., Ayuso C., Koenekoop R. K., Laxer U., et al. (2014). A homozygous nonsense CEP250 mutation combined with a heterozygous nonsense C2orf71 mutation is associated with atypical Usher syndrome. J. Med. Genet. 51 460–469. 10.1136/jmedgenet-2014-102287 [DOI] [PubMed] [Google Scholar]
- Kim J., Hu C., Moufawad El Achkar C., Black L. E., Douville J., Larson A., et al. (2019). Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 381 1644–1652. 10.1056/nejmoa1813279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla L., Kelly M. C., Mann Z. F., Anaya-Rocha A., Ellis K., Lemons A., et al. (2020). Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat. Commun. 11:2389. 10.1038/s41467-020-16113-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota D., Gocho K., Kikuchi S., Akeo K., Miura M., Yamaki K., et al. (2018). CEP250 mutations associated with mild cone-rod dystrophy and sensorineural hearing loss in a Japanese family. Ophthalmic Genet. 39 500–507. 10.1080/13816810.2018.1466338 [DOI] [PubMed] [Google Scholar]
- Li H. (2018). Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34 3094–3100. 10.1093/bioinformatics/bty191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., et al. (2009). The sequence alignment / map (SAM) Format and SAMtools 1000 Genome project data processing subgroup. Bioinformatics 25 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu H., Giffen K. P., Chen L., Beisel K. W., He D. Z. Z. (2018). Transcriptomes of cochlear inner and outer hair cells from adult mice. Sci. Data 5:180199. 10.1038/sdata.2018.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrand A., Eisfeldt J., Pettersson M., Carvalho C. M. B., Kvarnung M., Grigelioniene G., et al. (2019). From cytogenetics to cytogenomics: whole-genome sequencing as a first-line test comprehensively captures the diverse spectrum of disease-causing genetic variation underlying intellectual disability. Genome Med. 11:68. 10.1186/s13073-019-0675-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M., Gobet N., Cruz-Dávalos D. I., Mounier N., Dessimoz C., Sedlazeck F. J. (2019). Structural variant calling: the long and the short of it. Genome Biol. 20:246. 10.1186/s13059-019-1828-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namburi P., Ratnapriya R., Khateb S., Lazar C. H., Kinarty Y., Obolensky A., et al. (2016). Bi-allelic truncating mutations in CEP78, encoding centrosomal protein 78, cause cone-rod degeneration with sensorineural hearing loss. Am. J. Hum. Genet. 99 777–784. 10.1016/j.ajhg.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Raff J. W. (2009). Centrioles, centrosomes, and cilia in health and disease. Cell 139 663–678. 10.1016/j.cell.2009.10.036 [DOI] [PubMed] [Google Scholar]
- Nikopoulos K., Farinelli P., Giangreco B., Tsika C., Royer-Bertrand B., Mbefo M. K., et al. (2016). Mutations in CEP78 cause cone-rod dystrophy and hearing loss associated with primary-cilia defects. Am. J. Hum. Genet. 99 770–776. 10.1016/j.ajhg.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi K. M., Tearle R. G., Liu Y. P., Oh E. C., Miyake N., Benaglio P., et al. (2013). Whole genome sequencing in patients with retinitis pigmentosa reveals pathogenic DNA structural changes and NEK2 as a new disease gene. Proc. Natl. Acad. Sci. U.S.A. 110 16139–16144. 10.1073/pnas.1308243110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykamp K., Anderson M., Powers M., Garcia J., Herrera B., Ho Y. Y., et al. (2017). Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet. Med. 19 1105–1117. 10.1038/gim.2017.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer L. M., Steranka J. P., Yang W. R., Kryatova M., Medabalimi S., Ardeljan D., et al. (2017). Structural variants caused by Alu insertions are associated with risks for many human diseases. Proc. Natl. Acad. Sci. U.S.A. 114 E3984–E3992. 10.1073/pnas.1704117114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagnol V., Curtis J., Epstein M., Mok K. Y., Stebbings E., Grigoriadou S., et al. (2012). A robust model for read count data in exome sequencing experiments and implications for copy number variant calling. Bioinformatics 28 2747–2754. 10.1093/bioinformatics/bts526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman L., Dheedene A., De Smet M., Van Dorpe J., Menten B. (2019). WisecondorX: improved copy number detection for routine shallow whole-genome sequencing. Nucleic Acids Res. 47 1605–1614. 10.1093/nar/gky1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnapriya R., Sosina O. A., Starostik M. R., Kwicklis M., Kapphahn R. J., Fritsche L. G., et al. (2019). Retinal transcriptome and eQTL analyses identify genes associated with age-related macular degeneration. Nat. Genet. 51 606–610. 10.1038/s41588-019-0351-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumers J., De Rijk P., Zhao H., Liekens A., Smeets D., Cleary J., et al. (2012). Optimized filtering reduces the error rate in detecting genomic variants by short-read sequencing. Nat. Biotechnol. 30 61–68. 10.1038/nbt.2053 [DOI] [PubMed] [Google Scholar]
- Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet. Med. 17 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Juan A., Stephens J., French C. E., Gleadall N., Mégy K., Penkett C., et al. (2018). Complex structural variants in mendelian disorders: identification and breakpoint resolution using short- and long-read genome sequencing. Genome Med. 10:95. 10.1186/s13073-018-0606-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sante T., Vergult S., Volders P. J., Kloosterman W. P., Trooskens G., De Preter K., et al. (2014). ViVar: a comprehensive platform for the analysis and visualization of structural genomic variation. PLoS One 9:e113800. 10.1371/journal.pone.0113800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider V. A., Graves-Lindsay T., Howe K., Bouk N., Chen H. C., Kitts P. A., et al. (2017). Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome Res. 27 849-864. 10.1101/gr.213611.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen I., Hasin-Brumshtein Y., Corneveaux J. J., Ohmen J., White C., Allen A. N., et al. (2016). A comprehensive catalogue of the coding and non-coding transcripts of the human inner ear. Hear. Res. 333 266–274. 10.1016/j.heares.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlazeck F. J., Rescheneder P., Smolka M., Fang H., Nattestad M., Von Haeseler A., et al. (2018). Accurate detection of complex structural variations using single-molecule sequencing. Nat. Methods 15 461–468. 10.1038/s41592-018-0001-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonda R., Escudero-ferruz P., Andre R., Castro-miro M., De Ciccioli M., Hidalgo D. A., et al. (2016). Novel candidate genes and a wide spectrum of structural and point mutations responsible for inherited retinal dystrophies revealed by exome sequencing. PLoS One 11:e0168966. 10.1371/journal.pone.0168966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam T. J., Wheway G., Slaats G. G., Huynen M. A., Giles R. H. (2013). The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia 2:7. 10.1186/2046-2530-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schil K., Naessens S., Van de Sompele S., Carron M., Aslanidis A., Van Cauwenbergh C., et al. (2018). Mapping the genomic landscape of inherited retinal disease genes prioritizes genes prone to coding and noncoding copy-number variations. Genet. Med. 20 202–213. 10.1038/gim.2017.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf F. A., Angerer P., Theis F. J. (2018). SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19:15. 10.1186/s13059-017-1382-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K., Schulz M. H., Long Q., Apweiler R., Ning Z. (2009). Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 25 2865–2871. 10.1093/bioinformatics/btp394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampaglione E., Kinde B., Place E. M., Navarro-Gomez D., Maher M., Jamshidi F., et al. (2020). Copy-number variation contributes 9% of pathogenicity in the inherited retinal degenerations. Genet. Med. 22 1079–1087. 10.1038/s41436-020-0759-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiener I., Frisch B., Binder H. (2014). Transforming RNA-Seq data to improve the performance of prognostic gene signatures. PLoS One 9:e85150. 10.1371/journal.pone.0085150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The variants presented in this study can be found in online repositories. The name of the repository and accession numbers can be found below: Leiden Open Variation Database (LOVD), https://databases.lovd.nl/shared/individuals/00332412; https://databases.lovd.nl/shared/individuals/00332413; and https://databases.lovd.nl/shared/individuals/00332414.