Abstract
Predators are often food limited in their habitat, and some are limited by specific macronutrients (protein, lipid or carbohydrate). It is unresolved, however, to what extent and in what way food and macronutrient limitation are connected. Using a carabid beetle (Nebria brevicollis), we compared macronutrient self-selection of the animals three times: immediately after collection in the field, after being fed to satiation and nutritional balance and after a subsequent period of starvation. Both sexes were food and females lipid limited in the field; after 7–21 days of starvation both sexes increased proportional carbohydrate intake significantly. Thus, starvation created a nutrient deficit that was different from what the animals had experienced in the field. We conclude that while macronutrient limitation in nature may be influenced by hunger due to food limitation, this is not its main determinant. A nutritional imbalance of available food may override this effect.
Keywords: Coleoptera, Carabidae, triple-test procedure, nutrition, macronutrient limitation
1. Introduction
Predators are often food limited in nature [1–3]. This means that food availability is too low to allow them to realize their full fitness potential. They may also be limited by one or more specific macronutrients [4,5]. In this case, their food has a proportional content of macronutrients that differ from the optimal (balanced) proportion that define their fundamental nutritional needs (the intake target [6]). If given the opportunity, the animals may compensate by selecting a diet that ‘mirrors' the previous deficient one, i.e. with an increased proportion of the nutrient(s) that the previous diet was short of [7]. Studies have shown predatory animals to be food but not macronutrient limited, or macronutrient but not food limited [5,8]. The first scenario may apply if food availability is low but the food present has a balanced composition for the predator. The second scenario may apply if food is plentiful but short of a particular nutrient [4,9]. Such findings may lead to the suggestion that food and macronutrient limitation are independent phenomena [5]. On the other hand, food deprivation may change physiological processes, e.g. lead to ‘protein sparing', reduced growth and production of gametes, but enhance other activities like foraging [10–12]. Compared with the satiated and nutritionally balanced state, this may increase the relative demand for lipid over protein and thus induce a preference for food with a higher lipid : protein ratio. In omnivorous predators that supplement their mostly carnivorous diet with plant-based foods [13,14], a starvation-induced energy deficit might also be relieved by increased intake of carbohydrate/sugar.
We here asked whether there is a causal relationship between food and macronutrient limitation: does starvation induce a specific macronutrient imbalance to which the animals respond by changing their macronutrient selection away from the optimal proportions, i.e. the proportions selected when satiated and in full nutritional balance? We used the double-test procedure [5] to test for food and macronutrient limitation in the field, then added a starvation treatment and tested for starvation-induced changes in macronutrient selection. This allowed us to compare macronutrient limitation in the field and the effects of starvation on the same individuals. The null hypothesis was that starvation does not change macronutrient selection, i.e. the animals become hungry but demand food with the same macronutrient composition as the target composition. If experimental starvation leads to a specific macronutrient deficit, this might also prevail among food limited animals in the field. Thus, if experimental starvation leads to a deficit of the same nutrient(s) that was/were found to be limiting in the field, then field macronutrient limitation may be a simple result of food limitation. If, however, experimental starvation leads to macronutrient deficiencies that differ from that experienced in the field, then field macronutrient limitation is determined by factors other than food limitation.
2. Material and methods
(a). Experimental animals
Nebria brevicollis is a eurytopic species found abundantly in forests as well as in agricultural fields. Adult beetles emerge in spring and build up large fat stores before the summer; then they enter a summer diapause that lasts for 5–6 weeks; reproduction occurs during the autumn months (September–December) [15–17]. Very few individuals overwinter and complete a second reproductive period the following year [18]. Our study took place in October at the peak of reproductive activity.
Beetles were collected at night while active on the ground in forest Risskov near Aarhus, Denmark (56°10' N, 10°13' E) on 5 October 2020. The animals were kept in a cool box until the following morning. Then, they were sexed and weighed and entered the first self-selection test.
Experimental procedures and preparation of foods follow, with a few modifications, those of previous studies [8,19]. Details are given in the electronic supplementary material. The main difference from previous studies is that the animals were subjected to three instead of two self-selection tests. Test 1 was run as soon as possible after the animals were collected in the field; consumption measures are supposed to reflect the animals' nutritional conditions in the habitat (named ‘compensation intake'). Test 2 was run after 4 days of ad libitum feeding, thus the animals were supposed to be satiated and in nutritional balance (balance intake). Test 3 was run after the beetles had been starving for 7, 14 or 21 days (starvation intake).
(b). Data analysis
We tested for food and macronutrient limitation in the field by comparing food and macronutrient intake in test 1 (compensation intake) and test 2 (balance intake) using repeated-measures (r-m) MANOVA. Food limitation is concluded if total consumption in tests 1 is significantly higher than in test 2. Macronutrient limitation is likewise concluded if consumption of a macronutrient is significantly higher in test 1 than in test 2. Effects of starvation were also analysed by r-m MANOVA by comparing results of test 2 and test 3 (starvation intake). As all carabid individuals were included in test 2 but then divided into the three starvation groups, r-m MANOVAs were run on each of the starvation groups separately. ANOVAs on data from each self-selection test were used to compare between the sexes and to compare the results of all starvation groups (including test 2 = 0 days starvation). Body mass was included in initial statistical tests but subsequently deleted. This was either because of non-significance (macronutrient selection) or because body size accounted for the difference between the sexes (total food consumption); here sex was retained. Statistical analyses were performed with JMP v. 14 (SAS Institute Inc., 1989–2019).
3. Results
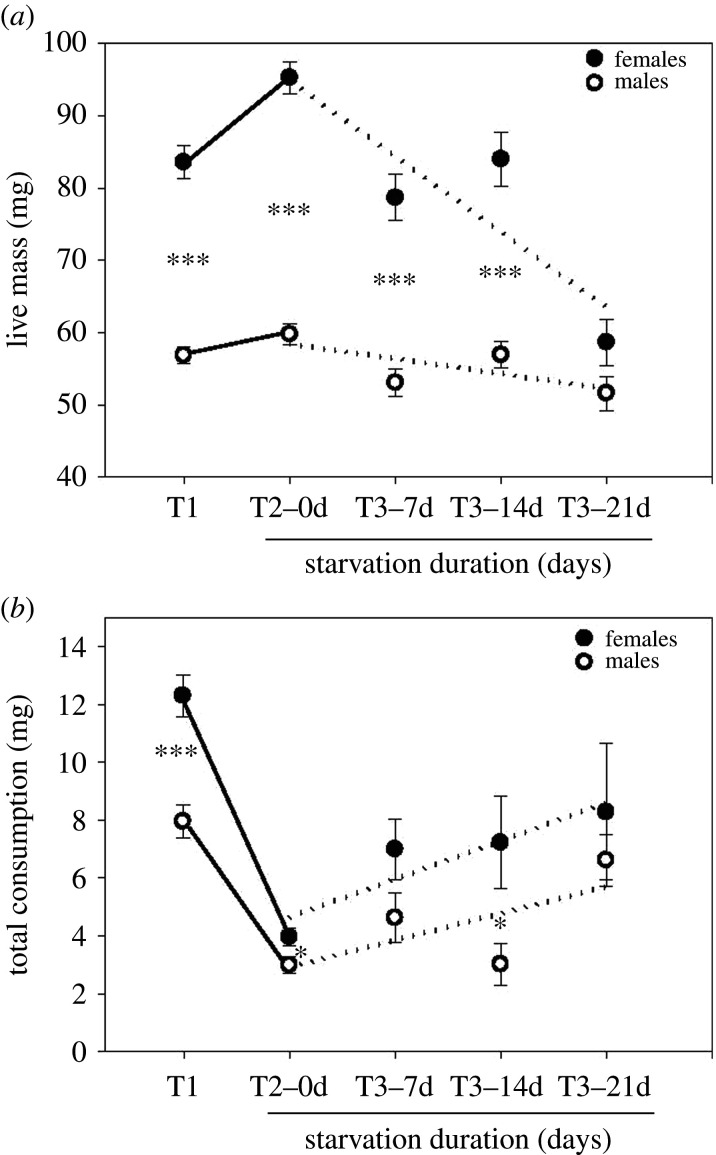
Live body weights were used to follow the course of mass change through the experiments. Both sexes increased their mass from test 1 to test 2 and then lost mass between test 2 and test 3 depending on the starvation period (figure 1a and table 1). Beetles starved for 21 days became lighter than they were in the field.
Figure 1.
Live body mass (a) and total consumption (b) of N. brevicollis during self-selection experiments. T1–T3: self-selection experiments 1–3 (compensation intake, balance intake and starvation intake, respectively). Notice that T1 and T2 include all individuals that were subsequently assigned to one of the three starvation period treatments (7, 14 and 21 days). Dotted lines are regression lines for the starvation part of the experiment: 0, 7, 14 and 21 days of starvation. Stars indicate significant differences between sexes (*p < 0.05, **p < 0.01 and ***p < 0.001). For analysis of changes between tests, see table 1.
Table 1.
Statistical analysis (repeated-measures MANOVA) of body mass and consumption data for the carabid beetle N. brevicollis measured through three self-selection tests. Factor ‘time' indicates the difference between two tests. Starv.dur. = starvation duration (7, 14 and 21 days). Significant p-values in italics.
| test 1 versus test 2 |
test 2 versus test 3 |
test 1 versus test 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | p | d.f. | F | p | d.f. | F | p | |
| (a) live body mass | |||||||||
| time | 1,43 | 90.17 | <0.0001 | 1,35 | 91.74 | <0.0001 | 1,35 | 20.80 | <0.0001 |
| time × sex | 1,43 | 31.78 | <0.0001 | 1,35 | 12.13 | 0.0014 | 1,35 | 0.42 | 0.5198 |
| time × starv.dur. | 2,35 | 42.83 | <0.0001 | 2,35 | 23.27 | <0.0001 | |||
| time × sex × starv.dur. | 2,35 | 4.09 | 0.0253 | 2,35 | 7.71 | 0.0017 | |||
| females–time | 1,20 | 88.78 | <0.0001 | 1,16 | 60.63 | <0.0001 | 1,16 | 7.63 | 0.0139 |
| time × starv.dur. | 1,16 | 22.93 | <0.0001 | 2,16 | 15.50 | 0.0002 | |||
| males–time | 1,23 | 9.75 | 0.0048 | 1,19 | 27.21 | <0.0001 | 1,19 | 18.68 | 0.0004 |
| time × starv.dur. | 2,19 | 20.98 | <0.0001 | 2,19 | 7.44 | 0.0041 | |||
| (b) total food consumption (mass) | |||||||||
| time | 1,43 | 201.62 | <0.0001 | 1,37 | 4.66 | 0.0374 | 1,39 | 50.94 | <0.0001 |
| time × sex | 1,43 | 12.68 | 0.0009 | 1,37 | 4.00 | 0.0530 | 1,39 | 1.24 | 0.2728 |
| time × starv.dur. | 2,37 | 1.88 | 0.1673 | ||||||
| females–time | 1,20 | 11.37 | <0.0001 | ||||||
| males–time | 1,23 | 82.44 | <0.0001 | ||||||
| (c) prop. protein intake (energy) | |||||||||
| time | 1,43 | 5.03 | 0.0301 | 1,37 | 7.14 | 0.0112 | |||
| time × sex | 1,43 | 11.96 | 0.0012 | 1,37 | 0.02 | 0.8816 | |||
| time × starv.dur. | 2,37 | 0.18 | 0.8359 | ||||||
| females–time | 1,20 | 18.42 | 0.0004 | ||||||
| males–time | 1,23 | 0.69 | 0.4152 | ||||||
| (d) prop. lipid intake (energy) | |||||||||
| time | 1,43 | 1.41 | 0.2418 | 1,37 | 0.04 | 0.8409 | |||
| time × sex | 1,43 | 8.35 | 0.0060 | 1,37 | 0.99 | 0.3265 | |||
| time × starv.dur. | 2,37 | 0.40 | 0.6763 | ||||||
| females–time | 1,20 | 4.87 | 0.0391 | ||||||
| males–time | 1,23 | 3.24 | 0.0849 | ||||||
| (e) prop. sugar intake (energy) | |||||||||
| time | 1,43 | 1.92 | 0.1734 | 1,37 | 4.82 | 0.0345 | |||
| time × sex | 1,43 | 1.24 | 0.2716 | 1,37 | 0.36 | 0.5512 | |||
| time × starv.dur. | 2,37 | 0.03 | 0.9739 | ||||||
Total food consumption dropped significantly between test 1 and test 2 in both sexes (figure 1b and table 1); thus, the population was food limited in the field at the time of collection. Both sexes were food limited, but females reduced food consumption relatively more than males. The animals increased consumption again between test 2 and test 3. The increase was independent of the starvation period in females, i.e. most of the increase had happened already at 7 days of starvation. In both sexes, consumption in test 3 was significantly less than in test 1. Thus, in the field, the animals suffered a hunger level equivalent to more than one week's starvation.
Proportional macronutrient consumption in the balance intake (test 2) was dominated by protein (56%), followed by lipid (25%) and sugar (19%) (table 2). Significant deviations from this were seen in the compensation intake (test 1) and in the starvation intake (test 3), but in different directions. In females, compensation intake had a lower proportion of protein and a higher proportion of lipid, while no change in sugar intake was seen (figure 2 and tables 1 and 2). The males showed no change in macronutrient selection between test 1 and test 2. These results show that females but not males were lipid limited when collected in the field. Proportional protein consumption dropped independently of sex in test 3, associated with an increase in sugar intake but not in lipid intake. Thus, starvation created a significantly increased demand for sugar in both sexes. All changes in macronutrient selection were independent of the duration of the starvation period.
Table 2.
Self-selected macronutrient targets (mean ± s.e.) in tests 1–3. Sexes distinguished if significantly different (t-tests). Test 1: compensation intake; test 2: balance intake; test 3: starvation intake.
| test 1 |
||||
|---|---|---|---|---|
| females | males | test 2 | test 3 | |
| prop. protein | 0.469 ± 0.018 | 0.567 ± 0.017 | 0.560 ± 0.014 | 0.455 ± 0.018 |
| prop. lipid | 0.306 ± 0.023 | 0.224 ± 0.017 | 0.246 ± 0.010 | 0.256 ± 0.017 |
| prop. sugar | 0.217 ± 0.012 | 0.194 ± 0.012 | 0.289 ± 0.021 | |
Figure 2.
Changes in proportional macronutrient selection of N. brevicollis during the three self-selection experiments. Test 1 (compensation intake); test 2 (balance intake); test 3 (starvation intake). Starvation treatments (7, 14 and 21 days) combined because the starvation period was a non-significant factor (table 1). Stars indicate significant differences between sexes (**p < 0.01 and ***p < 0.001). For analysis of changes between tests, see table 1.
4. Discussion
Both sexes of the beetles were strongly food limited when collected in the field, but only the females were macronutrient limited. This indicates that food limitation does not necessarily lead to macronutrient limitation as already concluded previously [5]. Macronutrient limitation in the field is assumed to be due to an unsatisfied demand for lipid. Lipid limitation was also prevailing among carabid species from agricultural fields at spring time, including N. brevicolllis [5]; the study was not fully conclusive on that point, however, since it did not test for carbohydrates. Starvation, by contrast, created a significantly increased proportion of sugar in the self-selected diet at the expense of protein, while lipid intake was unaffected. These results are evidence that (i) starvation does lead to changes in macronutrient selection compared with the optimal proportion and (ii) the macronutrient deficit after starvation may differ from the deficit the animals experience in the field. As expected, starvation leads to the enhanced selection of non-protein energy at the expense of protein, but the increased preference was for sugar, not lipid. In insects, sugar (mainly trehalose) is the main metabolic fuel in the early phases of starvation, but after a longer starvation period lipid metabolism may predominate [10,20]. A switch from sugar deficit at short starvation durations to lipid deficit at longer starvation durations might therefore be expected, but was not seen. Possibly, even three weeks of starvation are not a serious metabolic stress on these beetles, but something they are adapted to cope with. Thus, some carabid beetles show high starvation tolerance [21]. Quantification of food limitation in species from agricultural fields indicates hunger levels corresponding to two to three weeks of starvation [3]. We hypothesize that sugar was selected to support a starvation-induced increase in food searching activity [12] that ultimately aimed to provide protein and lipid for reproduction. It should be noticed also, that the three foods used in the present study were all relatively rich in protein and lipid as half of them consisted of pulverized grasshopper. Using the same food as here, sugar-rich food even better than the protein-rich and lipid-rich foods supported lean mass growth in post-hibernation carabid Anchomenus dorsalis [22]. The sugar-rich food thus provides protein and lipid that can be used for gamete production, but the increased preference for sugar indicates even greater importance of easily metabolizable sugar to fuel the synthesis of gamete materials. Further studies with animals of different feeding type and in different life cycle phases are required to determine whether starvation will always lead to sugar deficit. We hypothesize that this may depend on what resource-demanding activities continue during starvation (reproduction, preparing for hibernation, etc.) and the degree of omnivory.
Earlier studies have found that food limitation is more frequent than macronutrient limitation [5,8], i.e. members of a population are often food limited without being macronutrient limited, as the males in the present study. Lipid limitation, as seen in the females, indicates a protein-biased prey availability. At the height of reproduction, females of N. brevicollis have exhausted their body lipid stores [15]. This indicates that reproduction, which lasts far into the autumn, tolls heavily on their lipid stores. The reasons the males were not subject to lipid limitation may include their smaller size and lower nutritional requirements for reproductive products.
Food limitation differs from starvation because the animals may not be completely without food, but feed at a suboptimal rate. In addition, animals with fixed (e.g. strictly annual) life cycles as N. brevicollis cannot postpone reproductive activities until resources become more plentiful. Therefore, some starvation responses, such as reduced metabolism, are not possible. Food and macronutrient limitation may exist while the animals continue their normal life activities, including reproduction, only at a lower than maximal/optimal rates; expressed another way, food and macronutrient limitation develop because animals continue their life activities to the limit given by the resources available in the habitat.
Acknowledgement
We are grateful to two anonymous referees for their valuable comments.
Data accessibility
The dataset supporting this article has been deposited in Dryad: https://dx.doi.org/10.5061/dryad.dfn2z350v [23].
Authors' contributions
S.T. conceived the study, analysed data and wrote the manuscript draft. C.S.L. and L.K. participated in designing the study, performed the experiments, analysed data and wrote a preliminary report. All authors approved the final version and agree to be accountable for all aspects of the work.
Funding
There was no special funding for this study.
Competing interests
We declare we have no competing interests.
References
- 1.White TCR. 1978. The importance of a relative shortage of food in animal ecology. Oecologia 33, 71-86. ( 10.1007/BF00376997) [DOI] [PubMed] [Google Scholar]
- 2.Wise DH. 1993. Spiders in ecological webs. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Bilde T, Toft S. 1998. Quantifying food limitation of arthropod predators in the field. Oecologia 115, 54-58. ( 10.1007/s004420050490) [DOI] [PubMed] [Google Scholar]
- 4.Machovsky-Capuska GE, Senior AM, Zantis SP, Barna K, Cowieson AJ, Pandya S, Pavard C, Shiels M, Raubenheimer D. 2016. Dietary protein selection in a free-ranging urban population of common myna birds. Behav. Ecol. 27, 219-227. ( 10.1093/beheco/arv142) [DOI] [Google Scholar]
- 5.Toft S, Cuende E, Olesen AL, Mathiesen A, Larsen MM, Jensen K. 2019. Food and specific macronutrient limitation in an assemblage of predatory beetles. Oikos 128, 1467-1477. ( 10.1111/oik.06479) [DOI] [Google Scholar]
- 6.Simpson SJ, Raubenheimer D. 2012. The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ. 2005. Nutrient-specific foraging in invertebrate predators. Science 307, 111-113. ( 10.1126/science.1105493) [DOI] [PubMed] [Google Scholar]
- 8.Toft S, Pavón-Peláez C, Martínez-Villar M, Rengifo L, Arroyave A, Pompozzi G, Franco V, Albo MJ. In press. Contrasting patterns of food and macronutrient limitation in the field among co-existing omnivorous carnivores. Ecol. Entomol. ( 10.1002/EEN.13026) [DOI] [Google Scholar]
- 9.Meyrier E, Jenni L, Bötsch Y, Strebel S, Erne B, Tablado Z. 2017. Happy to breed in the city? Urban food resources limit reproductive output in western jackdaws. Ecol. Evol. 7, 1363-1374. ( 10.1002/ece3.2733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCue MD. 2010. Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. A 156, 1-18. ( 10.1016/j.cbpa.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 11.McCue MD, Guzman RM, Passement CA, Davidowitz G. 2015. How and when do insects rely on endogenous protein and lipid resources during lethal bouts of starvation? A new application for 13C-breath testing. PLoS ONE 10, e0140053. ( 10.1371/journal.pone.0140053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scharf I. 2016. The multifaceted effects of starvation on arthropod behaviour. Anim. Behav. 119, 37-48. ( 10.1016/j.anbehav.2016.06.019) [DOI] [Google Scholar]
- 13.Coll M, Guershon M. 2002. Omnivory in terrestrial arthropods: mixing plant and prey diets. Ann. Rev. Entomol. 47, 267-297. ( 10.1146/annurev.ento.47.091201.145209) [DOI] [PubMed] [Google Scholar]
- 14.Wäckers FL, van Rijn PCJ, Bruin J. 2005. Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Penney MM. 1969. Diapause and reproduction in Nebria brevicollis (F.) (Coleoptera: Carabidae). J. Anim. Ecol. 38, 219-233. ( 10.2307/2748) [DOI] [Google Scholar]
- 16.Manga N. 1972. Population metabolism of Nebria brevicollis (F.) (Coleoptera: Carabidae). Oecologia 10, 223-242. ( 10.1007/BF00368965) [DOI] [PubMed] [Google Scholar]
- 17.Jørum P. 1976. Life cycle and population density of Nebria brevicollis F. (Coleoptera, Carabidae) in a Danish beech forest. Vidensk. Medd. Dansk naturh. Foren. 139, 245-261. [Google Scholar]
- 18.Nelemans MNE, den Boer PJ, Spee A. 1989. Recruitment and summer diapause in the dynamics of a population of Nebria brevicollis (Coleoptera: Carabidae). Oikos 56, 157-169. ( 10.2307/3565331) [DOI] [Google Scholar]
- 19.Christensen J, Nielsen SMB, Toft S. 2020. The three-dimensional macronutrient niche of an invasive generalist predator. Ecol. Entomol. 45, 644-651. ( 10.1111/een.12840) [DOI] [Google Scholar]
- 20.Moreau R, Gourdoux L, Dutrieu J, Benkhay A. 1984. Hemolymph trehalose and carbohydrates in starved male adult Locusta migratoria: possibility of endocrine modification. Comp. Biochem. Phvsiol. A 78, 481-485. ( 10.1016/0300-9629(84)90582-6) [DOI] [Google Scholar]
- 21.Knapp M. 2016. Relative importance of sex, pre-starvation body mass and structural body size in the determination of exceptional starvation resistance of Anchomenus dorsalis (Coleoptera: Carabidae). PLoS ONE 11, e0151459. ( 10.1371/journal.pone.0151459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toft S, Nielsen SA. 2017. Diet-dependent heat emission reveals costs of post-diapause recovery from different nutritional sources in a carnivorous beetle. Sci. Nat. 104, 58. ( 10.1007/s00114-017-1481-5) [DOI] [PubMed] [Google Scholar]
- 23.Toft S, Lange CS, Kristensen L. 2021. Data from: Food limitation and starvation independently affect predator macronutrient selection. Dryad Digital Repository. ( 10.5061/dryad.dfn2z350v) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Toft S, Lange CS, Kristensen L. 2021. Data from: Food limitation and starvation independently affect predator macronutrient selection. Dryad Digital Repository. ( 10.5061/dryad.dfn2z350v) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The dataset supporting this article has been deposited in Dryad: https://dx.doi.org/10.5061/dryad.dfn2z350v [23].