Abstract
The aim of the present study was to investigate the effects of gallic acid (GA) on the proliferation and apoptosis of colon cancer cells and to further clarify the mechanism of GA function associated with SRC and EGFR phosphorylation. HCT116 and HT29 cells were treated with different concentrations of GA for 24 h. Cell proliferation and apoptosis were analyzed using plate clone formation and flow cytometry assays, respectively. In addition, the expression of apoptosis-related proteins was examined by western blotting. Furthermore, the level of STAT3, AKT, SRC and EGFR phosphorylation was analyzed by western blotting and immunofluorescence. Subsequently, the SRC inhibitor PP2 and the EGFR inhibitor gefitinib were used to analyze the GA-associated mechanisms. In addition, a xenograft tumor model was established to confirm the effects of GA in vivo. The results indicated that GA inhibited cell proliferation and promoted cell apoptosis by upregulating the ratio of cleaved caspase-3/pro-caspase-3 and cleaved caspase-9/pro-caspase-9. Concurrently, GA decreased the level of phosphorylated (p)-SRC, p-EGFR, p-AKT and p-STAT3. Following treatment with PP2 and gefitinib in both cancer cell lines and animal model, GA was demonstrated to inhibit EGFR and SRC phosphorylation to downregulate STAT3 and AKT phosphorylation. In vivo, GA prevented tumor growth, promoted tumor apoptosis and decreased the level of p-SRC, p-EGFR, p-STAT3 and p-AKT. In conclusion, GA was indicated to suppress colon cancer proliferation by inhibiting SRC and EGFR phosphorylation.
Keywords: gallic acid, proto-oncogene tyrosine-protein kinase Src, epidermal growth factor receptor, signal transducer and activator of transcription 3, protein kinase B/AKT, colon cancer
Introduction
Colon cancer is a malignant tumor and is the third most common cancer worldwide which is expected to increase by 60% to >2.2 million new cases and 1.1 million deaths by 2030(1). Colon cancer is a serious threat to public health due to its difficulty to operate on and its ability to easily metastasize (1,2). A number of factors may cause colon cancer, such as environment, lifestyle (including lack of exercise, which can lead to obesity) and eating habits (excessive intake of saturated fats and low vegetable and fruit intake) (3,4). The current treatment of colon cancer mainly involves surgery, supplemented by chemotherapy and improved diet. Early diagnosis by endoscopy is a primary goal in diagnosing and treating colon cancer (5). Therefore, chemotherapy serves an important role in the clinical treatment of colon cancer (5).
Current commonly used chemotherapeutic drugs for colon cancer include 5-fluorouracil (5-FU) (6), oxaliplatin (6), capecitabine (7) and irinotecan (8). These drugs are cytotoxic, often due to insufficient selectivity of the drug to the site of colon cancer, resulting in a series of adverse reactions, such as neutropenia, anemia, diarrhea, gastrointestinal toxicity, mucositis, nausea and vomiting, as well as blood system diseases and liver toxicity (6-8). In addition, drug resistance may also develop, which seriously affects the quality of life and lifespan of patients (6-8). Compared with traditional chemotherapeutics, several natural biological compounds (9), including artemisinin, celastrol and resveratrol, present unique advantages, such as less toxicity, fewer side effects and pain relief for patients with tumors. Therefore, researchers have focused on discovering novel antitumor drugs from natural sources.
Plant extracts have demonstrated unique advantages in preventing colon cancer. For example, a previous study (10) revealed that piperine can affect HT29 cells, which is a colon cancer cell line, and inhibit tumor cell proliferation. Moreover, piperine has been indicated to increase the production of reactive oxygen species, activate endoplasmic reticulum emergency-associated proteins, inhibit the phosphorylation of AKT and JNK and activate p38 MAPK, which can eventually induce cells to undergo caspase-mediated mitochondrial apoptosis (10). Other studies have also demonstrated that anthocyanins are non-toxic to normal intestinal cell lines in vitro (11,12), and can inhibit human colon cancer cell growth and induce apoptosis in a dose- and time-dependent manner (13).
Gallic acid (GA) is a type of natural polyphenol compound that exists in nature, and it is widely found in several Chinese medicines, such as green tea, Ganoderma lucidum and other plants (14). GA has been indicated to exert important anti-inflammatory (15) and antiviral (16) effects. Of note, GA can inhibit the proliferation of tumor cells. For instance, several studies have indicated that GA exhibited an inhibitory effect on lung cancer and esophageal cancer cells (17,18). However, to date, there are few systematic and comprehensive studies on GA in colon cancer, and the underlying mechanism needs to be further elucidated.
Therefore, the present study was designed to investigate whether GA could be used to inhibit the proliferation of colon cancer cells and determine the potential mechanisms via in vivo and in vitro experiments.
Materials and methods
Cells and grouping
The human colon cancer cell lines HCT116 (cat. no. BNCC337692) and HT29 (cat. no. BNCC100164) were purchased from BeNa Culture Collection, Beijing Beina Chuanglian Institute of Biotechnology. Cells were cultured in RPMI-1640 medium (Beijing Solarbio Science & Technology Co., Ltd.) supplemented with 10% FBS (Beijing Solarbio Science & Technology Co., Ltd.) and 1% penicillin-streptomycin at 37˚C with 5% CO2. Cell growth was observed under a light microscope, and when cells reached 80% confluence, they were digested and passaged.
The aforementioned cells were grouped as follows: i) Control group, no treatment; ii) GA high-dose group (GA-H), cells stimulated with 60 µM GA (cat no. G131992; Shanghai Aladdin Biochemical Technology Co., Ltd.) dissolved in PBS for 24 h; iii) GA medium-dose group (GA-M), cells stimulated with 30 µM GA for 24 h; iv) GA low-dose group (GA-L), cells stimulated with 15 µM GA for 24 h (19); and v) positive drug control group (5-FU), cells stimulated with 10 µM 5-FU (cat. no. F0123.0005; Duchefa Biochemie) for 24 h (20). The experiments were performed in three parallel groups per condition.
Subsequently, to verify whether GA could inhibit SRC and EGFR phosphorylation to reduce colon cancer proliferation, cells were divided into five groups as follows: i) Control group; ii) GA-H group; iii) PP2 group, cells stimulated with 5 µM SRC inhibitor PP2 (cat. no. P125361; Shanghai Aladdin Biochemical Technology Co., Ltd.) for 24 h (21); iv) gefinitib group, cells stimulated with 10 µM EGFR antagonist gefinitib (Nanjing Duly Biotechnology Co. Ltd.) for 24 h (22); and v) GA-H + PP2 group, cells simultaneously stimulated with 60 µM GA and 5 µM PP2 for 24 h.
Plate clone formation assay
When HCT116 and HT29 cells reached the logarithmic growth phase, they were uniformly seeded in a six-well plate (500 cells/well) and cultured at 37˚C with 5% CO2 for 2-3 weeks. When the number of cells reached 50-150, the formed colony is visible. Once formed colonies were visible, cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Cells were subsequently stained with Giemsa (cat. no. D010-1-2; Nanjing Jiancheng Bioengineering Institute) for 25 min at room temperature. Following washing by PBS, the colonies were dried at room temperature for 72 h. And image of each well was captured under x100 magnification using a light microscopy (Olympus BX51; Olympus Corporation). Finally, ImageJ v6.0 software (National Institutes of Health) was used to analyze the colony number.
Flow cytometry
After HCT116 and HT29 cells were cultured for 24 h in a 5% CO2 incubator at 37˚C, the supernatant was collected and washed with PBS. A total of 2x105 cells were collected by trypsin digestion. Cells were then resuspended in 100 µl binding buffer, followed by the addition of 400 µl PBS. The assay was performed according to the manufacturer's instructions (cat. no. G003-1-2; Nanjing Jiancheng Bioengineering Institute). A total of 5 µl PI staining solution and 10 µl Annexin V-FITC were added to each tube, and cells were incubated in the dark for 15 min at room temperature to detect apoptosis by Coulter@ EPICS® XLTM flow cytometry (Beckman Coulter, Inc.). The results were analyzed using CytExpert (version 2.0; Beckman Coulter, Inc.) software.
Western blotting
Total protein from each group of HCT116 and HT29 cells or tumor tissues was extracted using RIPA buffer containing protein inhibitors (cat. no. W062-1-1; Nanjing Jiancheng Bioengineering Institute). Following centrifugation at 12,000 x g for 10 mins at 4˚C, the protein concentration of the supernatant was quantified using a bicinchoninic acid protein assay kit (Nanjing Jiancheng Bioengineering Institute). In total, 50 µg protein samples were separated by 10% SDS-PAGE and then transferred to PVDF membranes for 2 h. After blocking with 5% non-fat dry milk at room temperature for 2 h, primary antibodies against cleaved caspase-9 (cat. no. orb227889; 1:1,000; Biorbyt Ltd.), pro-caspase-9 (ab138412; 1:800), cleaved caspase-3 (cat. no. ab49822; 1:500), pro-caspase-3 (ab32150; 1:1,000; all from Abcam), Ki-67 (cat. no. orb475270; 1:200; Biorbyt Ltd.), rabbit anti-human phosphorylated (p)-EGFR (cat. no. ab32578; 1:1,000), EGFR (cat. no. ab52894; 1:1,000), p-AKT (cat. no. ab38449; 1:1,000), AKT (cat. no. ab8805; 1:500), p-SRC (cat. no. ab32078; 1:5,000), SRC (cat. no. ab109381; 1:10,000), p-STAT3 (cat. no. ab76315; 1:2,000), STAT3 (cat. no. ab68153; 1:1,000) and β-actin (cat. no. ab8227; 1:1,000; all from Abcam) were added to the membranes and incubated overnight at 4˚C. The membranes were washed with 1X TBST (containing 0.1% Tween-20) and incubated with goat anti-rabbit horseradish peroxidase conjugated IgG secondary antibody (cat. no. ab6721; 1:2,000; Abcam) for 1 h at room temperature. The membranes were washed again with 1X TBS-T and protein bands were exposed using an ECL kit (cat. no. W028-2-1; Nanjing Jiancheng Bioengineering Institute). The relative expression of target proteins was analyzed by Image J v6.0 software (National Institutes of Health) and calculated based on β-actin as the internal reference.
Immunofluorescence
HCT116 and HT29 cells were fixed with 4% paraformaldehyde for 20 min at room temperature. The cells were then permeabilized with 0.5% Triton X-100 for 20 min and blocked with 5% BSA (Wuhan Boster Biological Technology, Ltd.) for 40 min, both at room temperature. Subsequently, the cells were incubated with p-STAT3 (cat. no. ab76315; 1:500), p-SRC (cat. no. ab32078; 1:50), p-EGFR (cat. no. ab32578; 1:25; all from Abcam) and p-AKT (cat. no. orb312153; 1:100; Biorbyt Ltd.) antibodies overnight at 4˚C. After washing with PBS, the cells were incubated with goat anti-rabbit IgG H&L (Alexa Fluor® 488; cat. no. ab150077; 1:200; Abcam) at 37˚C for 1.5 h. Following three washes with PBS, the nuclei were stained with 5 µg/ml DAPI for 5 min at room temperature. Immunofluorescence was observed by confocal microscopy (Nikon Corporation) with the magnification of x400 and the mean fluorescence intensity were quantified using Image J v6.0 software (National Institutes of Health).
Animals
A total of 48 specific-pathogen-free BALB/c female nude mice (16-18 g; 4 weeks old) were purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd. [SCXK (Beijing) 20160006]. Operations were performed after 7 days of adaptive feeding. Animal experiments in the present study were agreed and approved by the Animal Protection and Use Committee of Yantai University (Yantai, China). The animals were raised at 26-28˚C, 40-60% humidity, light/dark cycle for 12-h and free access to water and food. The food, drinking water and bedding materials were sterilized. Animal experiments followed the National Institutes of Health guidelines (NIH publication no. 85-23, revised 1996).
Xenograft tumor model
HCT116 and HT29 cells were suspended with RPMI-1640 medium without 10% FBS. A total of 0.2 ml HCT116 and HT29 cell suspended with PBS (5x107 cells/ml) were inoculated subcutaneously into the left backs of nude mice in the corresponding group. If a rice-sized tumor (~50 mm3) appeared at the inoculation site on the 7th day, the inoculation was considered to be successful. Nude mice were randomly divided into four groups (n=6): i) Model, mice inoculated with cancer cells; ii) GA group, following inoculation of cancer cells, mice were injected daily with 80 mg/kg/day GA (23); iii) PP2 group, following inoculation of cancer cells, mice were injected daily with 10 mg/kg/day PP2(24); iv) gefitinib group, following inoculation of cancer cells, mice were injected daily with 50 mg/kg/day gefitinib (25); and v) GA + PP2 group, following inoculation of cancer cells, mice were injected daily with 80 mg/kg/day GA and 10 mg/kg/day PP2. Continuous administration was performed for 28 days. Tumor volumes were recorded every 7 days. At the end of the experiment, the mice were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (40 mg/kg) and sacrificed by cervical dislocation. Death was confirmed by cardiac arrest. Tumors were excised, photographed and weighed.
TUNEL assay
Tumor tissues were fixed with 4% paraformaldehyde for 24 h at room temperature, dehydrated and embedded in paraffin. The slices (5 µm) were heated at 60-70˚C for 2 h, dewaxed with xylene and then rehydrated using a descending ethanol gradient. The tissue slices were incubated in 3% H2O2-methanol for 10 min at room temperature and rinsed with PBS three times (3 min each time). Subsequently, the slices were stained with TUNEL solution (cat. no. G002-2-2; Nanjing Jiancheng Bioengineering Institute). A sealing film was used to perform the reaction in a dark wet box for 1 h at 37˚C. After TUNEL-staining, DAB solution was added for 5 min at room temperature. After washing with PBS for three times, the slices were re-stained using hematoxylin at room temperature for 60 sec, then dehydrated in ascending series of ethanol and cleared in xylene. Finally, the slices were sealed with neutral gum and observed under a light microscope (magnification, x400; Olympus BX51; Olympus Corporation) from five randomly selected fields. The number of positive-stained cells was analyzed by ImageJ v6.0 software (National Institutes of Health). Cell apoptosis rate was calculated using the following formula: Apoptosis rate = (number of apoptotic cells/total number of cells) x100%.
Statistical analysis
All data were analyzed with SPSS v19.0 (IBM Corp.). Numerical data are presented as the mean ± SD. Differences among multiple groups were evaluated by one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
GA promotes apoptosis and inhibits colon cancer cell proliferation
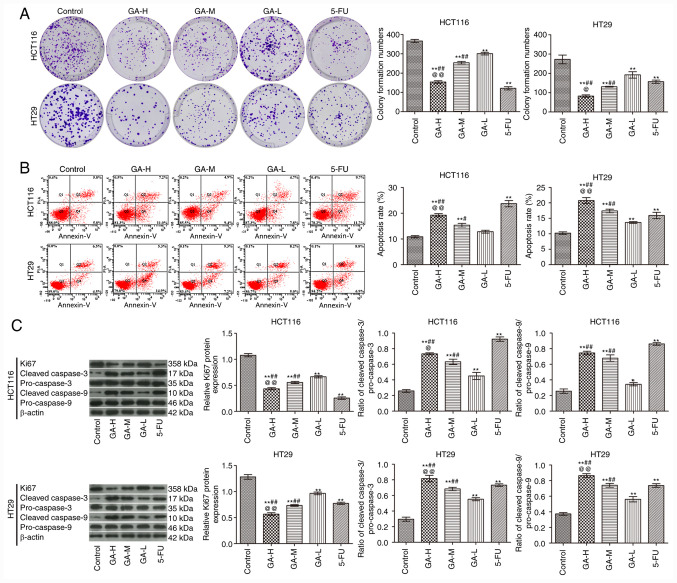
Compared with the control group, GA inhibited the proliferation (Fig. 1A) and promoted the apoptosis (Fig. 1B) of HCT116 and HT29 cells in a concentration-dependent manner. In addition, GA increased the ratio of cleaved caspase-3/pro-caspase-3 and cleaved caspase-9/pro-caspase-9 and inhibited Ki-67 expression when compared with the control group (Fig. 1C). Notably, the effects of GA-H treatment were similar with those observed with 5-FU treatment. These data demonstrated that GA administration promoted apoptosis and inhibited proliferation in colon cancer cells.
Figure 1.
GA affects the apoptosis and proliferation of HCT116 and HT29 colon cancer cells. (A) Plate clone formation assay was used to examine cell proliferation. (B) Flow cytometry was used to examine apoptosis. (C) Western blotting was used to analyze the expression of proliferation-related protein Ki-67 and apoptosis-related proteins, cleaved caspase-3, pro-caspase-3, cleaved caspase-9 and pro-caspase-9 (n=3). *P<0.05 and **P<0.01 vs. the control group; #P<0.05 and ##P<0.01 vs. the GA-L group; @P<0.05 and @@P<0.01 vs. the GA-M group. GA, gallic acid; L, low dose; M, medium dose; H, high dose; 5-FU, 5-fluorouracil.
GA inhibits the phosphorylation of SRC in colon cancer cells
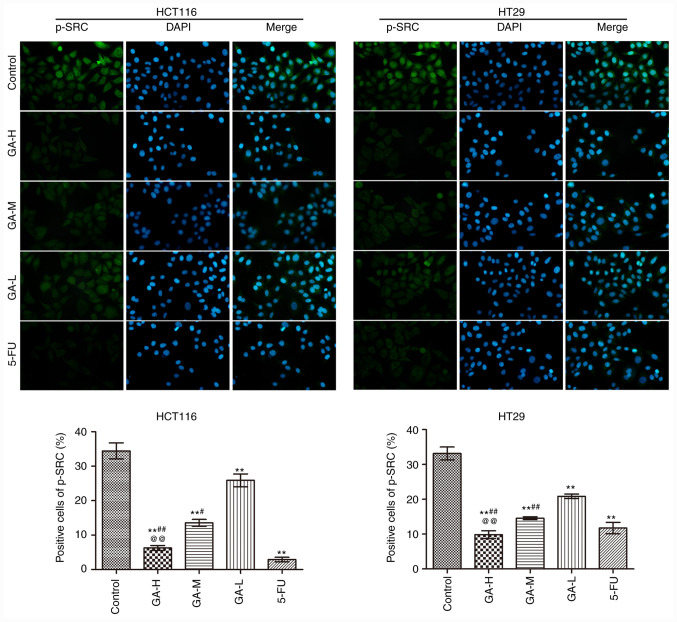
The effects of GA on SRC phosphorylation in HCT116 and HT29 cells were analyzed using immunofluorescence (Fig. 2). GA and 5-FU intervention significantly reduced the positive p-SRC staining in HCT116 and HT29 cells compared with the control group, especially in 5-FU. Moreover, high GA concentrations exerted the strongest inhibitory effect on SRC phosphorylation in HCT116 cells and HT29 cells. These data suggested that GA suppressed SRC phosphorylation.
Figure 2.
GA inhibits p-SRC expression in HCT116 and HT29 cells by Immunofluorescence (n=3; magnification, x400). **P<0.01 vs. the control group; #P<0.05 and ##P<0.01 vs. the GA-L group; @@P<0.01 vs. the GA-M group. GA, gallic acid; L, low dose; M, medium dose; H, high dose; 5-FU, 5-fluorouracil; p, phosphorylated.
GA decreases STAT3 and AKT phosphorylation level via inhibiting SRC and EGFR phosphorylation
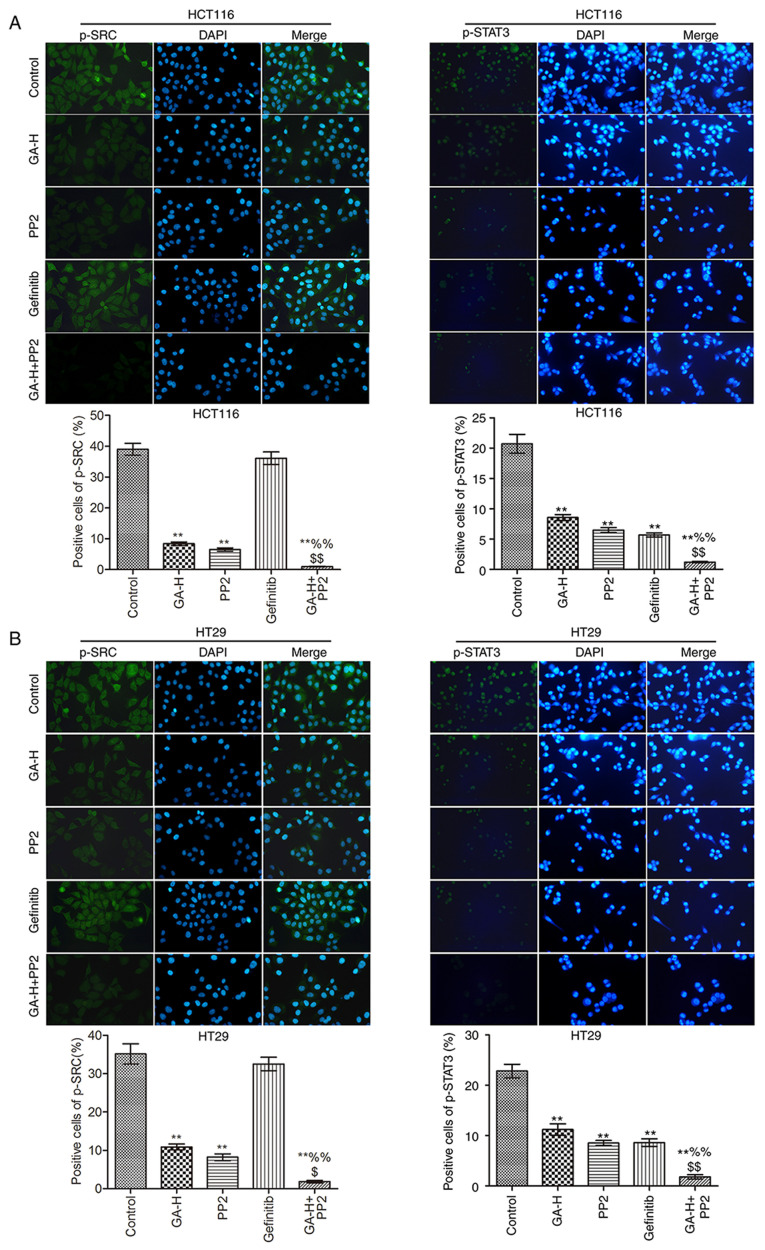
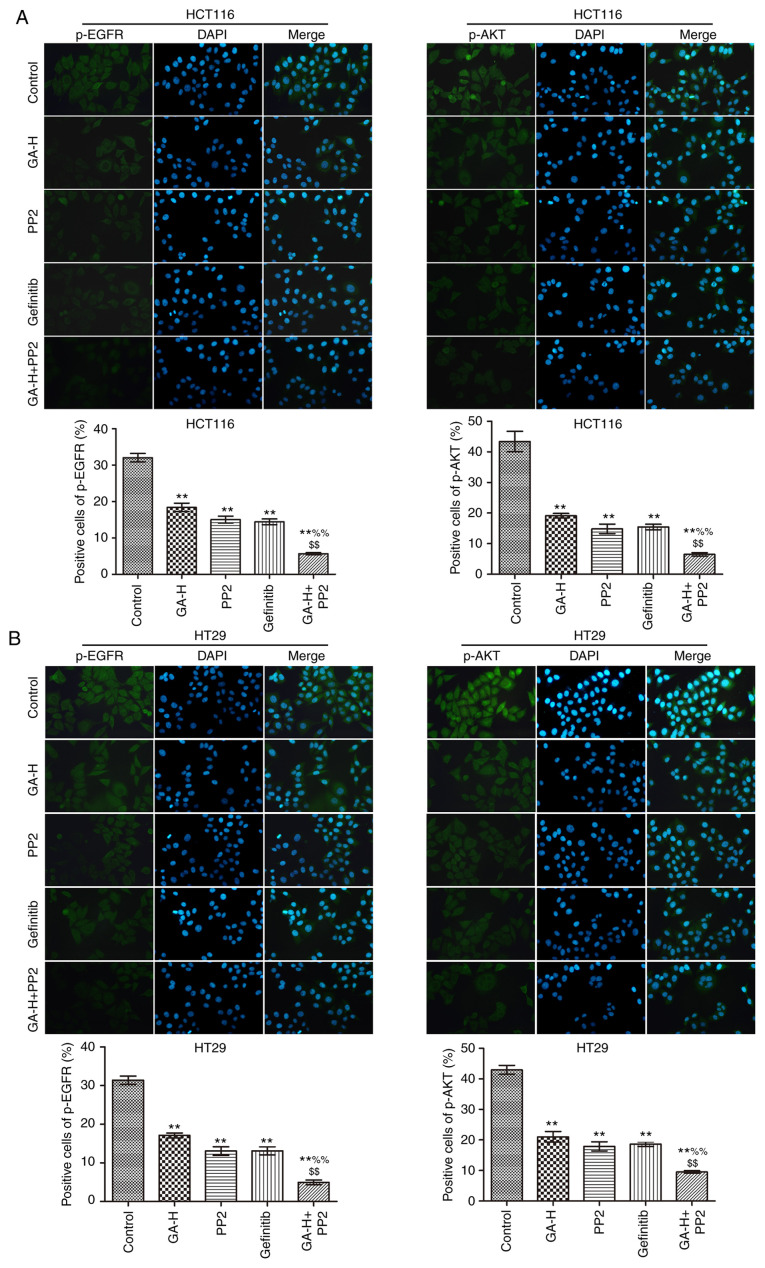
The levels of p-SRC, p-STAT3 (Fig. 3), p-EGFR and p-AKT (Fig. 4) were examined using immunofluorescence. Compared with the control group, GA-H and PP2 markedly inhibited the level of p-STAT3, p-EGFR, p-AKT and p-SRC, and gefitinib significantly reduced the level of p-STAT3, p-EGFR and p-AKT, but had no effect on p-SRC level. When GA-H and PP2 were used in combination, the phosphorylation level of the aforementioned proteins was further decreased compared with the GA-H or PP2 groups alone. These results indicated that GA could decrease SRC and EGFR phosphorylation level to inhibit STAT3 and AKT phosphorylation.
Figure 3.
Effects of GA on p-SRC and p-STAT3 level. The effects of GA in (A) HCT116 and (B) HT29 colon cancer cells were analyzed by immunofluorescence. n=3. Magnification, x400. **P<0.01 vs. the control group; %%P<0.01 vs. the GA-H group; $P<0.05 and $$P<0.01 vs. the PP2 group. GA-H, gallic acid-high dose; p, phosphorylated.
Figure 4.
Effects of GA on p-EGFR and p-AKT level. The effects of GA in (A) HCT116 and (B) HT29 colon cancer cells were analyzed by immunofluorescence. n=3. Magnification, x400. **P<0.01 vs. the control group; %%P<0.01 vs. the GA-H group; $$P<0.01 vs. the PP2 group. GA-H, gallic acid-high dose; p, phosphorylated.
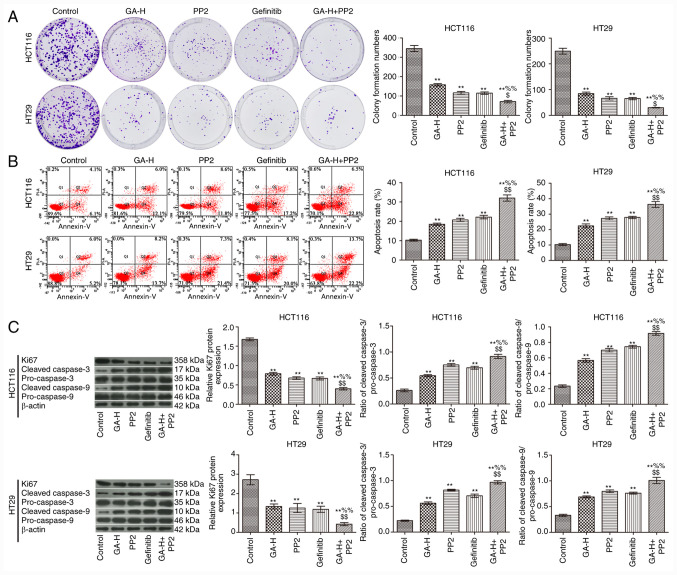
GA decreases SRC and EGFR phosphorylation level to promote colon cancer cell apoptosis and inhibit proliferation
To verify whether GA could inhibit SRC and EGFR phosphorylation to reduce colon cancer cell proliferation, cells were also treated with PP2 and gefitinib. As indicated in Fig. 5, GA-H, PP2 and gefitinib significantly inhibited cell proliferation (Fig. 5A) and promoted cell apoptosis (Fig. 5B) compared with the control group. The combined administration of GA-H and PP2 further promoted cell apoptosis and inhibited proliferation compared with the GA-H or PP2 groups. In addition, western blot analysis demonstrated that GA-H, PP2 and gefitinib increased the ratio of cleaved caspase-3/pro-caspase-3 and cleaved caspase-9/pro-caspase-9, and decreased Ki-67 protein expression level compared with the control group. The combined administration of GA-H and PP2 further enhanced the ratio of cleaved caspase-3/pro-caspase-3 and cleaved caspase-9/pro-caspase-9, and reduced Ki-67 protein expression level compared with the GA-H or PP2 groups (Fig. 5C). These results revealed that GA promoted colon cancer cell apoptosis and inhibited proliferation via inhibiting SRC and EGFR phosphorylation.
Figure 5.
GA decreases SRC and EGFR phosphorylation to promote apoptosis and inhibit proliferation in HCT116 and HT29 cells. (A) Plate clone formation assay was used to examine cell proliferation. (B) Flow cytometry was used to examine apoptosis. (C) Western blotting was used to analyze the expression of proliferation-related protein Ki-67 and apoptosis-related proteins, cleaved caspase-3, pro-caspase-3, cleaved caspase-9 and pro-caspase-9. n=3. **P<0.01 vs. the control group; %%P<0.01 vs. the GA-H group; $P<0.05 and $$P<0.01 vs. the PP2 group. GA-H, gallic acid-high dose.
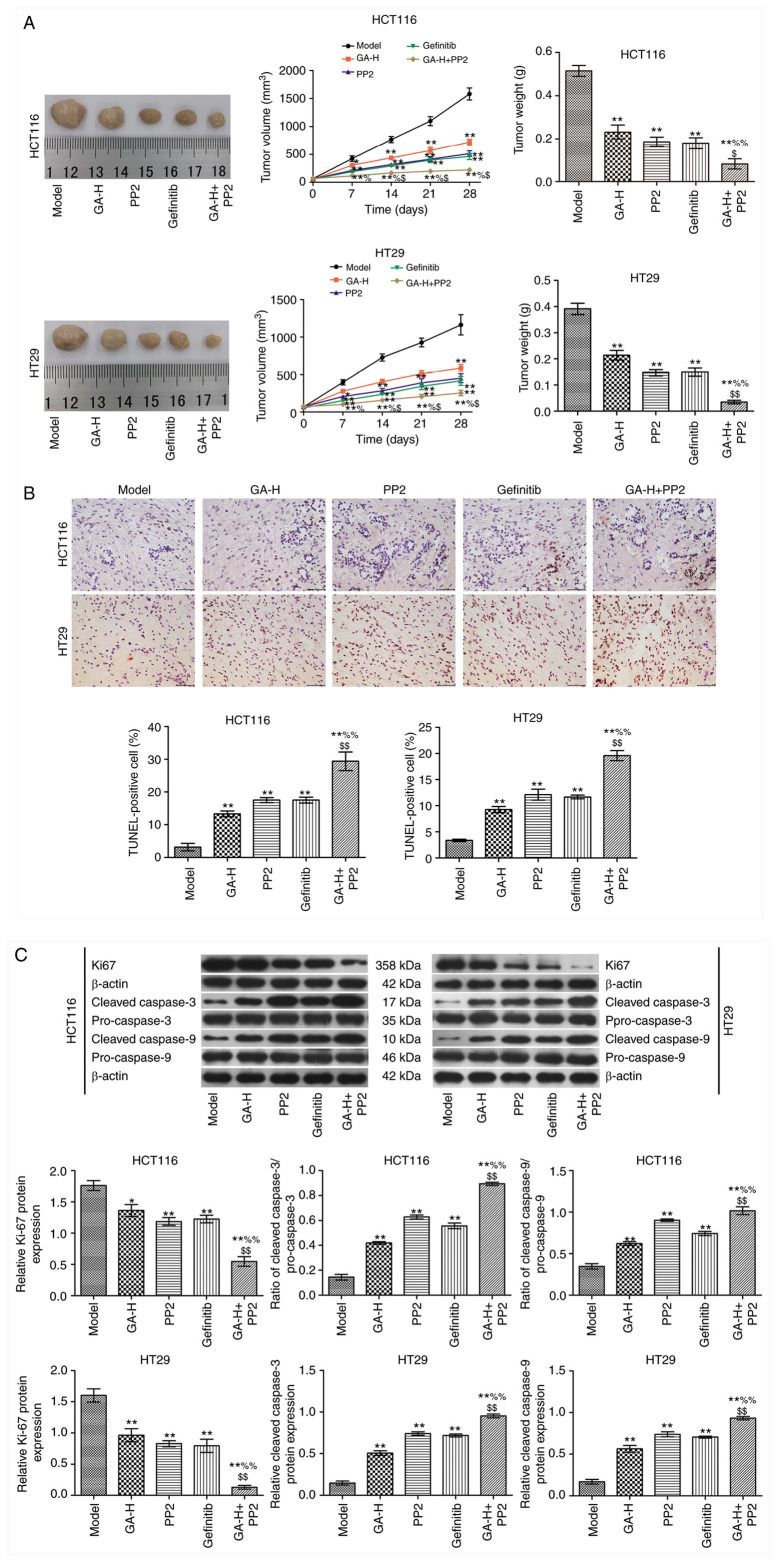
GA decreases SRC and EGFR phosphorylation level to inhibit tumor growth
In the present study, the maximum tumor diameter was 1.56 cm, and the maximum tumor volume was 1,900.4 mm3. GA-H, PP2 and gefitinib inhibited tumor growth compared with the model group (Fig. 6A). When GA-H and PP2 were administered in combination, tumor growth was significantly reduced compared with the GA or PP2 groups alone. The results in Fig. 6B demonstrated that GA-H, PP2 and gefitinib significantly increased the apoptosis rate of tumor cells in vivo compared with the model group. This effect was enhanced in the GA-H + PP2 group compared with the GA or PP2 groups. The expression level of Ki67 and the ratio of cleaved caspase-3/pro-caspase-3 and cleaved caspase-9/pro-caspase-9 are presented in Fig. 6C. The results revealed that GA-H, PP2 and gefitinib decreased Ki-67 expression level and increased the ratio of cleaved caspase-3/pro-caspase-3 and cleaved caspase-9/pro-caspase-9 compared with the model group. These effects were further enhanced when GA-H and PP2 were administered concurrently.
Figure 6.
GA decreases SRC and EGFR phosphorylation level to inhibit tumor growth. (A) Tumor images, volume and weight. (B) TUNEL staining (scale bar=50 µm). (C) Western blot analysis. n=6. *P<0.05 and **P<0.01 vs. the model group; %P<0.05 and %%P<0.01 vs. the GA-H group; $P<0.05 and $$P<0.01 vs. the PP2 group. GA-H, gallic acid-high dose.
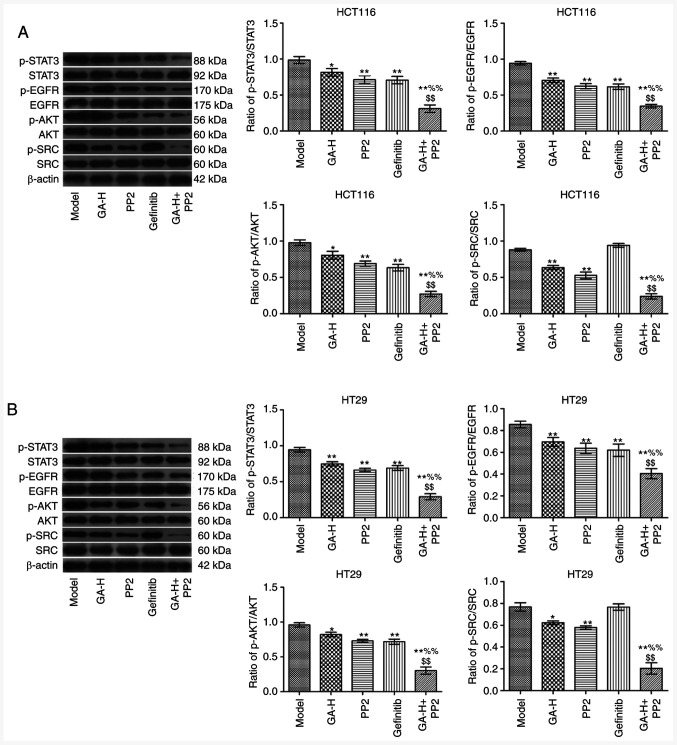
GA inhibits SRC and EGFR phosphorylation to regulate STAT3 and AKT in vivo
The relative levels of p-STAT3/STAT3, p-EGFR/EGFR, p-AKT/AKT and p-SRC/SRC in tumor tissues was analyzed by western blotting (Fig. 7). The results indicated that the administration of GA-H and PP2 significantly decreased the ratio of p-STAT3/STAT3, p-EGFR/EGFR, p-AKT/AKT and p-SRC/SRC in tumor tissues compared with the model group (Fig. 7). Gefitinib significantly reduced the level of p-STAT3/STAT3, p-EGFR/EGFR and p-AKT/AKT, but did not affect p-SRC level compared with the model group. Furthermore, following the combined treatment of GA-H and PP2, the phosphorylation level of the aforementioned four proteins was significantly lower compared with the GA-H or PP2 groups.
Figure 7.
GA decreases SRC and EGFR phosphorylation level to inhibit STAT3 and AKT phosphorylation. (A) The proteins expression in HCT116 tumor tissues. (B) The proteins expression in HT29 tumor tissues. The level of p-SRC/SRC, p-EGFR/EGFR, p-STAT3/STAT3 and p-AKT/AKT in tumor tissues were analyzed by western blotting. β-actin was used as the internal reference gene. n=6. *P<0.05 and **P<0.01 vs. the control group; %%P<0.01 vs. the GA-H group; $$P<0.01 vs. the PP2 group. GA-H, gallic acid-high dose; p, phosphorylated.
Discussion
The present study demonstrated that GA significantly suppressed the proliferation of HCT116 and HT29 colon cancer cells and accelerated their apoptotic process, which was consistent with the results of previous studies. In previous studies, GA has been indicated to inhibit cell proliferation and invasion of different tumors, such as non-small cell lung cancer (NSCLC) (26,27) and bladder cancer (28). The occurrence of colon cancer is closely associated with the abnormal expression of multiple genes (29). Therefore, regulating the expression of certain key genes, such as SRC, during tumorigenesis to inhibit malignant transformation is an effective means to control tumor growth.
The SRC protein is encoded by the proto-oncogene Src and is a member of the non-receptor tyrosine protein kinase family, which can activate multiple signaling pathways, such as PI3K/AKT, MAPK and STAT3(30). SRC activity is enhanced in 80% of patients with colon cancer, and is significantly associated with the occurrence and development of colon cancer (31,32). In the present study, GA was administered to HCT116 and HT29 colon cancer cells and colon cancer animal models. It was revealed that GA treatment significantly inhibited the proliferation and promoted the apoptosis of colon cancer cells, and this effect was associated with p-SRC level.
The EGFR signal transduction pathway is also widely considered to serve a key role in tumor formation and development and is one of the most important targets for numerous cancers (33,34). It often exhibits abnormal activation during cancer progression and is closely associated with poor prognosis (33). Nam et al (34) demonstrated that GA induced apoptosis in EGFR-mutant NSCLC via acceleration of EGFR turnover, which suggested that GA-mediated inhibition of cancer growth may be associated with EGFR signaling. Previously, SRC was indicated to activate EGFR via different pathways to promote tumor proliferation and metastasis (35). In addition, STAT3 has been also revealed to be continuously activated in tumors, which serves crucial roles in regulating the proliferation, migration and survival of cells and can be also regulated by SRC (36). In present study, GA was indicated to inhibit SRC and EGFR phosphorylation resulting in a decrease in STAT3 and AKT phosphorylation level. When the SRC inhibitor PP2 and the EGFR inhibitor gefitinib were administered in vitro or in vivo, these results were further confirmed. However, Chen et al (37) demonstrated that constitutively active AKT and MEK-1 or SRC overexpression reversed the inhibitory effect of GA on the EGF-induced MMP-9 upregulation in breast cancer cells. It is possible that the reported effects are the results of the effect of GA on different, independent pathways and not part of the mechanism mediating the effect on tumor growth.
Few studies have investigated the mechanism of GA in colon cancer in vivo and in vitro. The present study demonstrated that GA inhibited colon cancer cell proliferation and induced apoptosis via inhibiting SRC and EGFR phosphorylation. The results of the present study may provide novel ideas, strategies and treatment options for the prevention and targeted therapy of colon cancer.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XL, LH and PL conceived the study and performed literature research, data analysis, experiments, manuscript writing and review; GW and YG designed the experiments and were involved in the data acquisition and statistical analysis; TW was involved in data acquisition, manuscript preparation and data analysis; KC performed study design, literature research, experiments and manuscript editing. XL and GW confirmed the authenticity of the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Animal experiments followed the NIH guidelines (NIH publication no. 85-23, revised 1996) and were approved by the Animal Protection and Use Committee of Yantai University (Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016;68:7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 3.Giftson JS, Jayanthi S, Nalini N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1,2-dimethyl hydrazine induced rat colon carcinogenesis. Invest New Drugs. 2010;28:251–259. doi: 10.1007/s10637-009-9241-9. [DOI] [PubMed] [Google Scholar]
- 4.Carole M, Livine D, Daniel K, Järvinen JR, Annelise L, Muller CD. Impact of procyanidins from different berries on caspase 8 activation in colon cancer. Oxid Med Cell Longev. 2015;2015(154164) doi: 10.1155/2015/154164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grothey A, Venook AP. Optimizing adjuvant therapy for localized colon cancer and treatment selection in advanced colorectal cancer. J Natl Compr Canc Netw. 2018;16:611–615. doi: 10.6004/jnccn.2018.0038. [DOI] [PubMed] [Google Scholar]
- 6. doi: 10.1016/j.ctrv.2004.09.002. No authors listed: Adjuvant chemotherapy with oxaliplatin, in combination with fluorouracil plus leucovorin prolongs disease-free survival, but causes more adverse event cancer Abstracted from: Andre T, Boni C, Mounedji-Boudiaf L, et al. Multicenter international study of oxaliplatin/5-fluorouracil/leucovorin in the adjuvant treatment of colon cancer (MOSAIC) investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350, 2343-2351, 2004. Cancer Treat Rev 30, 711-713, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Schmoll HJ, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K, Haller DG. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: Final results of the NO16968 randomized controlled phase III trial. J Clin Oncol. 2015;33:3733–3740. doi: 10.1200/JCO.2015.60.9107. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Jiang J, Chan R, Ji Y, Lu J, Liao YP, Okene M, Lin J, Lin P, Chang CH, et al. Improved efficacy and reduced toxicity using a custom-designed irinotecan-delivering silicasome for orthotopic colon cancer. ACS Nano. 2019;13:38–53. doi: 10.1021/acsnano.8b06164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng S, Shanmugam MK, Kumar AP, Yap CT, Sethi G, Bishayee A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 2019;125:1228–1246. doi: 10.1002/cncr.31978. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe PB, Power Coombs MR, Doucette CD, Walsh M, Hoskin DW. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog. 2015;54:1070–1085. doi: 10.1002/mc.22176. [DOI] [PubMed] [Google Scholar]
- 11.Venancio VP, Cipriano PA, Kim H, Antunes LM, Talcott ST, Mertens-Talcott SU. Cocoplum (Chrysobalanus icaco L.) anthocyanins exert anti-inflammatory activity in human colon cancer and non-malignant colon cells. Food Funct. 2017;8:307–314. doi: 10.1039/c6fo01498d. [DOI] [PubMed] [Google Scholar]
- 12.Kubow S, Iskandar MM, Melgar-Bermudez E, Sleno L, Sabally K, Azadi B, How E, Prakash S, Burgos G, Felde TZ. Effects of simulated human gastrointestinal digestion of two purple-fleshed potato cultivars on anthocyanin composition and cytotoxicity in colonic cancer and non-tumorigenic cells. Nutrients. 2017;9(953) doi: 10.3390/nu9090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazewski C, Liang K, de Mejia EG. Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J Funct Foods. 2017;34:254–265. [Google Scholar]
- 14.Niemetz R, Gross GG. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry. 2005;66:2001–2011. doi: 10.1016/j.phytochem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Kroes BH, van den Berg AJ, Quarles van Ufford HC, van Dijk H, Labadie RP. Anti-inflammatory activity of gallic acid. Planta Med. 1992;58:499–504. doi: 10.1055/s-2006-961535. [DOI] [PubMed] [Google Scholar]
- 16.Choi HJ, Song JH, Bhatt LR, Baek SH. Anti-human rhinovirus activity of gallic acid possessing antioxidant capacity. Phytother Res. 2010;24:1292–1296. doi: 10.1002/ptr.3101. [DOI] [PubMed] [Google Scholar]
- 17.You BR, Park WH. Gallic acid-induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicol In Vitro. 2010;24:1356–1362. doi: 10.1016/j.tiv.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Sun GL, Wang D. Gallic acid from terminalia chebula inhibited the growth of esophageal carcinoma cells by suppressing the Hippo signal pathway. Iran J Basic Med Sci. 2020;23:1401–1408. doi: 10.22038/ijbms.2020.42283.9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HL, Lin CS, Kao SH, Chou MC. Gallic acid induces G1 phase arrest and apoptosis of triple-negative breast cancer cell MDA-MB-231 via p38 mitogen-activated protein kinase/p21/p27 axis. Anticancer Drugs. 2017;28:1150–1156. doi: 10.1097/CAD.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Hou N, Faried A, Tsutsumi S, Takeuchi T, Kuwano H. Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells. Ann Surg Oncol. 2009;16:761–771. doi: 10.1245/s10434-008-0260-0. [DOI] [PubMed] [Google Scholar]
- 21.Kundu J, Choi BY, Jeong CH, Kundu JK, Chun KS. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and Src-mediated phosphorylation of EGF receptor tyrosine kinase. Oncol Rep. 2014;32:821–828. doi: 10.3892/or.2014.3223. [DOI] [PubMed] [Google Scholar]
- 22.Agelaki S, Spiliotaki M, Markomanolaki H, Kallergi G, Mavroudis D, Georgoulias V, Stournaras C. Caveolin-1 regulates EGFR signaling in MCF-7 breast cancer cells and enhances gefitinib-induced tumor cell inhibition. Cancer Biol Ther. 2009;8:1470–1477. doi: 10.4161/cbt.8.15.8939. [DOI] [PubMed] [Google Scholar]
- 23.Li ZJ, Liu M, Dawuti G, Dou Q, Ma Y, Liu HG, Aibai S. Antifungal activity of gallic acid in vitro and in vivo. Phytother Res. 2017;31:1039–1045. doi: 10.1002/ptr.5823. [DOI] [PubMed] [Google Scholar]
- 24.Lou L, Yu Z, Wang Y, Wang S, Zhao Y. c-Src inhibitor selectively inhibits triple-negative breast cancer overexpressed vimentin in vitro and in vivo. Cancer. 2018;109:1648–1659. doi: 10.1111/cas.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruzzese F, Di Gennaro E, Avallone A, Pepe S, Arra C, Caraglia M, Tagliaferri P, Budillon A. Synergistic antitumor activity of epidermal growth factor receptor tyrosine kinase inhibitor gefitinib and IFN-alpha in head and neck cancer cells in vitro and in vivo. Clin Cancer Res. 2006;12:617–625. doi: 10.1158/1078-0432.CCR-05-1671. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Ma L, Wu P, Li W, Li T, Gu R, Dan X, Li Z, Fan X, Xiao Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol Rep. 2019;41:1779–1788. doi: 10.3892/or.2019.6976. [DOI] [PubMed] [Google Scholar]
- 27.Phan AN, Hua TN, Kim MK, Vo VT, Choi JW, Kim HW, Rho JK, Kim KW, Jeong Y. Gallic acid inhibition of Src-Stat3 signaling overcomes acquired resistance to EGF receptor tyrosine kinase inhibitors in advanced non-small cell lung cancer. Oncotarget. 2016;7:54702–54713. doi: 10.18632/oncotarget.10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao CC, Chen SC, Huang HP, Wang CJ. Gallic acid inhibits bladder cancer cell proliferation and migration via regulating fatty acid synthase (FAS) J Food Drug Anal. 2018;26:620–627. doi: 10.1016/j.jfda.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H, Wu J, Zhang J, Yang Z, Jin W, Li Y, Jin L, Yin L, Liu H, Wang Z. Integrated bioinformatics analysis of key genes involved in progress of colon cancer. Mol Genet Genomic Med. 2019;7(e00588) doi: 10.1002/mgg3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Elfiky A, Han M, Chen C, Saif MW. The role of Src in colon cancer and its therapeutic implications. Clin Colorectal Cancer. 2014;13:5–13. doi: 10.1016/j.clcc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 32.Xie G, Peng Z, Raufman JP. Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1006–G1015. doi: 10.1152/ajpgi.00427.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atmaca A, Werner D, Pauligk C, Steinmetz K, Wirtz R, Altmannsberger HM, Jäger E, Al-Batran SE. The prognostic impact of epidermal growth factor receptor in patients with metastatic gastric cancer. BMC Cancer. 2012;12(524) doi: 10.1186/1471-2407-12-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam B, Rho JK, Shin DM, Son J. Gallic acid induces apoptosis in EGFR-mutant non-small cell lung cancers by accelerating EGFR turnover. Bioorg Med Chem Lett. 2016;26:4571–4575. doi: 10.1016/j.bmcl.2016.08.083. [DOI] [PubMed] [Google Scholar]
- 35.Liu ZM, Huang HS. As2O3-induced c-Src/EGFR/ERK signaling is via Sp1 binding sites to stimulate p21WAF1/CIP1 expression in human epidermoid carcinoma A431 cells. Cell Signal. 2006;18:244–255. doi: 10.1016/j.cellsig.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res. 2009;69:1958–1965. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen YJ, Lin KN, Jhang LM, Huang CH, Lee YC, Chang LS. Gallic acid abolishes the EGFR/Src/Akt/Erk-mediated expression of matrix metalloproteinase-9 in MCF-7 breast cancer cells. Chem Biol Interact. 2016;252:131–140. doi: 10.1016/j.cbi.2016.04.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.