Schistosomiasis is a parasitic helminth disease that can cause organ lesions leading to health damage. During a schistosome infection, schistosome eggs can flow into the liver along the portal vein.
KEYWORDS: S. japonicum, liver injury, STAT3, inflammation, oxidative stress, proliferation, apoptosis
ABSTRACT
Schistosomiasis is a parasitic helminth disease that can cause organ lesions leading to health damage. During a schistosome infection, schistosome eggs can flow into the liver along the portal vein. Numerous inflammatory cells gather around the eggs, causing granulomas and fibrosis in the liver. In this process, many molecules are involved in the initiation and regulation of the fibrous scar formation. However, the precise molecular mechanisms responsible for the progression of granuloma formation and fibrosis initiation caused by schistosome infection have not been extensively studied. In this study, C57BL/6 wild-type mice and Stat3flox/flox Alb-Cre mice were infected with cercariae of Schistosoma japonicum. Liver injury, effector molecule levels, and RNA transcriptome resequencing of liver tissue were detected at 4, 5, and 6 weeks postinfection. We investigated the role of STAT3 (signal transducer and activator of transcription 3) in Schistosoma-induced liver injury in mice. After 6 weeks postinfection, there was obvious liver fibrosis. A sustained pathological process (inflammation, oxidative stress, proliferation, and apoptosis) occurred in S. japonicum-induced liver fibrosis initiation. Meanwhile, we observed activation of the STAT3 pathway in hepatic injury during S. japonicum infection by RNA transcriptome resequencing. Liver deficiency of phospho-STAT3 alleviated infection-induced liver dysfunction, hepatic granuloma formation, and fibrosis initiation. It also promoted STAT3-dependent apoptosis and reduced liver inflammation, oxidative stress, and proliferation. Our results suggest that STAT3 signal pathway and its mediating inflammation, oxidative stress, proliferation, and apoptosis are involved in S. japonicum-induced liver injury and may be a new potential guideline for the treatment of schistosomiasis.
INTRODUCTION
Schistosomiasis is the second most prevalent parasitic diseases in the world that seriously harms human health. Two hundred million people worldwide have been affected by the disease, which causes severe clinical symptoms (1, 2). Three major species of schistosome infect humans worldwide: Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum. The species most closely associated with liver fibrosis and hepatocellular carcinoma (HCC) are S. mansoni and S. japonicum. The central causative agent of infection in Southeast Asia and China is S. japonicum. The main pathogenesis of S. japonicum is the formation of egg granulomas in the liver and intestine, which then progresses to liver fibrosis (3, 4). When the development of hepatic fibrosis cannot be completely reversed, it will progress to cirrhosis and portal hypertension, etc., which are the primary reason for morbidity and mortality in humans with schistosome infection. Therefore, reversing fibrosis progression is the key to treating schistosomiasis.
After schistosome cercariae infect humans and animals, they bloom into adult worms in the portal venous system. Female schistosomes lay eggs, most of which emerge in the liver in the bloodstream of the portal-vein mesenteric venous system. Soluble egg antigen (SEA) secreted by miracidia in eggs can continuously stimulate liver tissue, which sequentially recruits inflammatory and immune cells to the sites of infection and then causes the formation of periovular granulomas and eventually chronic fibrosis. It is characterized by the accumulation of T helper-2 cytokines (interleukin 4 [IL-4] and IL-13), eosinophils and/or neutrophils, and alternatively activated macrophages (5, 6). Cytokines and chemokines released by T lymphocytes and resident liver cells promote the formation and resolution of hepatic granulomas. Moreover, hepatic stellate cells (HSCs) can attract migrating immune cells to the site of egg deposition (7, 8).
When schistosome infection occurs, the quiescent HSCs are activated and then transformed into myofibroblasts, which secrete a large number of extracellular matrix (ECM) components, giving rise to extreme deposition of ECM. The accumulation of ECM leads to an abnormal wound healing response. In this process, many pathways and molecules are involved in the regulation of the fibrous scar formation.
STAT3 (signal transducer and activator of transcription 3) is a cytoplasmic signal transcription factor that is part of the Janus protein tyrosine kinase (JAK)-STAT pathway and plays a crucial role in the process of regulating liver injury. The JAK/STAT pathway in mammals is an important regulatory pathway for many cytokines and growth factors (9). Generally, upon combining with their receptors, cytokines induce receptor dimerization and then receptor-linked JAK dimerization. Activation of JAK triggers phosphorylation of specific tyrosine residue receptors, and phosphorylated tyrosine sites form a specific “docking site” with surrounding amino acid sequences. STAT proteins are recruited to this “docking site.” The STAT protein is immediately phosphorylated, and the activated STAT protein enters the nucleus as a dimer to bind to the target gene and regulates gene transcription (9, 10). More than 40 peptide hormones can activate STAT3. Cytokines of the IL-6 family are the best examined of them (11). However, the effect of the STAT3 pathway in liver fibrogenesis is disputed on account of the recorded hepatoprotective and proliferative functions of STAT3 (12, 13).
STAT3 improves cell survival and proliferation in many respects. However, when persistently activated, STAT3 has a harmful influence and gives rise to various pathological conditions (14, 15). Activation of STAT3 occurred in many fibrotic tissues. For example, in patients with Montreal B2 fibrostenotic Crohn’s disease, STAT3 phosphorylation at S727 of intestinal mesenchymal muscle cells promotes fibrogenesis. STAT3 can promote liver fibrosis by upregulating transforming growth factor β (TGF-β) expression, and suppressor of cytokine signaling 3 (SOCS3) inhibits this process (14, 16, 17). However, the molecular mechanisms of STAT3 in the initiation and progression of granuloma formation and fibrosis initiation in schistosome infection are still not fully understood.
The elements of schistosome SEA and culture supernatants of schistosome egg in the hamster model and cell culture experiments could activate STAT3 (18). Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that accelerate tumor progression by weakening the effect of T cells, especially CD8+ T cells. Yang et al. demonstrated that SEA and schistosome worm antigen enhance the accumulation of MDSCs by JAK/STAT3 signaling (19). Moreover, S. japonicum egg antigen p40 (Sjp40) could significantly inhibit activation of hepatic stellate LX-2 cells and trigger cell caducity by the STAT3/protein 53 (p53)/p21 signaling pathway (20). Thus, cellular senescence regulated by STAT3 could also play a vital suppressive role in liver fibrosis.
Schistosoma antigen can activate the STAT3 pathway, but the specific mechanism of STAT3 in Schistosoma infection has not been studied systematically. Here, we investigated the role and mechanism of STAT3 in liver injury after the infection of S. japonicum. We found that the STAT3 signal pathway is involved in the pathogenesis of Schistosoma and that liver deficiency in phospho-STAT3 (p-STAT3) could ameliorate S. japonicum-associated liver injury. Furthermore, liver p-STAT3 deficiency promoted apoptosis and suppressed the activation of inflammation, proliferation, and oxidative stress induced by S. japonicum infection.
RESULTS
Liver granulomas and fibrosis are evident at 6 weeks after S. japonicum infection.
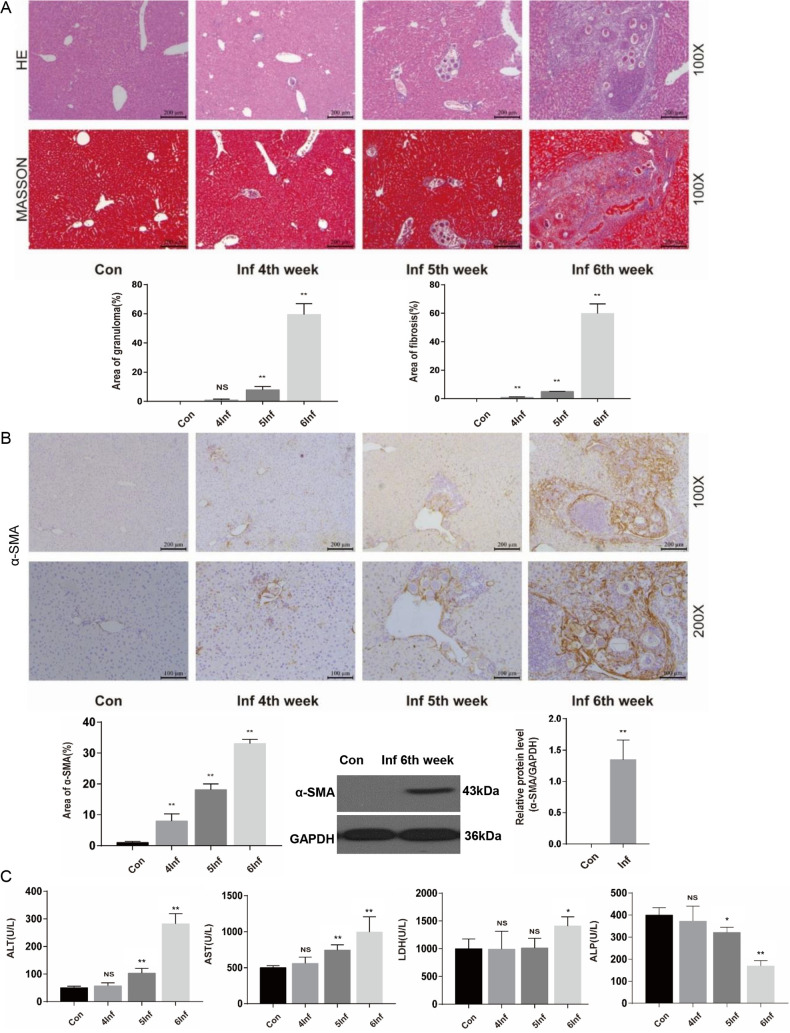
Hepatic periovular granulomas and chronic fibrosis have been characterized as the main pathological features of schistosome infection. At 4, 5, and 6 weeks postinfection, we detected pathological damage in the liver by hematoxylin-and-eosin (H&E) and Masson’s trichrome staining, immunohistochemistry, and biochemical analysis, etc. H&E staining showed that Schistosoma eggs were trapped in the liver from 5 weeks postinfection, and significant granulomas appeared at 6 weeks after S. japonicum infection (Fig. 1A). Meanwhile, at 6 weeks after S. japonicum infection, Masson’s trichrome staining showed that infected livers had obvious fibrosis (Fig. 1A). Moreover, mice infected with S. japonicum have marked expression of α-smooth muscle actin (α-SMA), an indicator of the activated HSCs at 6 weeks postinfection (Fig. 1B). Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) levels indicated that mice infected with S. japonicum have significant liver function injury at 6 weeks postinfection (Fig. 1C). These results showed that mice infected with S. japonicum have obvious hepatic pathological lesions at 6 weeks postinfection.
FIG 1.
Liver granulomas and fibrosis are evident at 6 weeks after S. japonicum infection. Wild-type mice were percutaneously treated with or without 30 ± 2 S. japonicum cercaria. At 4, 5, and 6 weeks postinfection, liver tissue and serum were collected for analysis. (A) H&E staining andMasson’s trichrome staining of liver sections. H&E staining shows the granuloma, and Masson’s trichrome staining shows collagen content and distribution. The granulomatous and fibrotic area as a percentage of total area was measured by computer-assisted morphometric analysis (n = 6). Magnification, ×100. Bar, 200 μm. (B) Representative immunohistochemistry images of α-SMA in liver tissue. Magnification, ×100 (top) and ×200 (bottom). Bars, 200 μm (top) and 100 μm (bottom). Analytical results show the percentage of α-SMA-positive staining in the liver. Western blot analysis of protein levels of α-SMA in liver and quantification (n = 5) are also shown. (C) Serum levels of ALT, AST, LDH, and ALP were measured by use of biochemical analyzer (n = 4). Data are means and SEM for 4 to 6 mice/group. Con, control group; Inf, infected group. * and **, P < 0.05 and P < 0.01 compared to control values. NS, no statistical significance.
STAT3 is involved in regulating host hepatic pathological lesions during S. japonicum infection.
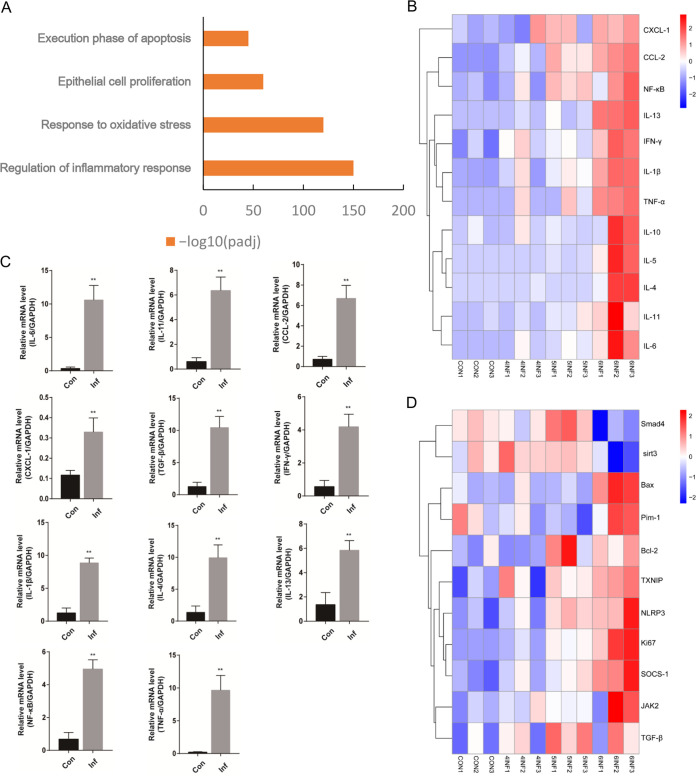
To elucidate the molecular mechanisms of hepatic pathological lesions during S. japonicum infection, we performed RNA transcriptome resequencing of liver tissue isolated from uninfected mice and mice infected with S. japonicum. We identified several biologic processes that were present in the liver through RNA sequencing and discovered that inflammation, oxidative stress, proliferation, and apoptosis were closely associated with liver fibrosis initiation caused by S. japonicum infection (Fig. 2A). Further analysis of differentially expressed genes in this cluster showed that S. japonicum infection upregulated the expression of key genes in inflammation (Fig. 2B). Real-time PCR (RT-PCR) results verified that the extensive hyper inflammatory response was associated with S. japonicum infection. The expression of mRNAs, including those for IL-6, IL-11, CCL-2, CXCL-1, TGF-β, interferon-γ (IFN-γ), IL-1β, IL-4, IL-13, nuclear factor kappa B (NF-κB), and tumor necrosis factor alpha (TNF-α), was elevated at 6 weeks after S. japonicum infection (Fig. 2C). Interestingly, we found that the upregulation of key genes in oxidative stress, proliferation, and apoptosis was closely related to STAT3. Compared with control mice, S. japonicum-infected mice had increased expression of the upstream and downstream genes of STAT3 in the liver (Fig. 2D). Therefore, we next identified and investigated the role and mechanism of the STAT3 pathway in hepatic injury during S. japonicum infection.
FIG 2.
Identification of STAT3 involved in regulating host hepatic pathological lesions during S. japonicum infection. Wild-type mice were percutaneously treated with or without 30 ± 2 S. japonicum cercariae. At 4, 5, and 6 weeks postinfection, liver tissue was collected for analysis. (A) GO analysis showing that S. japonicum infection induced the expression of genes involved in several biologic processes (inflammation, oxidative stress, proliferation, and apoptosis) (n = 3). (B) Heat map of the microarray results showing significant upregulation of the inflammatory genes in mice of S. japonicum infection after 4, 5, and 6 weeks compared with control mice. Red, upregulated; blue, downregulated; white, no change. Results are representative of 2 independent experiments (n = 3). (C) Expression of IL-6, IL-11, CCL-2, CXCL-1, TGF-β, IFN-γ, IL-1β, IL-4, IL-13, NF-κB, and TNF-α mRNAs in liver tissue after infection of S. japonicum (n = 4). (D) Heat map of the microarray results showing significant upregulation of the upstream and downstream related genes in mice of S. japonicum infection after 4, 5, and 6 weeks compared with control mice (n = 3). Red, upregulated; blue, downregulated; white, no change. Results are representative of 2 independent experiments. Data are means and SEM for 3 or 4 mice/group. Con, control group; Inf, infected group. * and **, P < 0.05 and P < 0.01 compared to control values.
The JAK2/STAT3 signal pathway is activated in the livers of mice with S. japonicum infection.
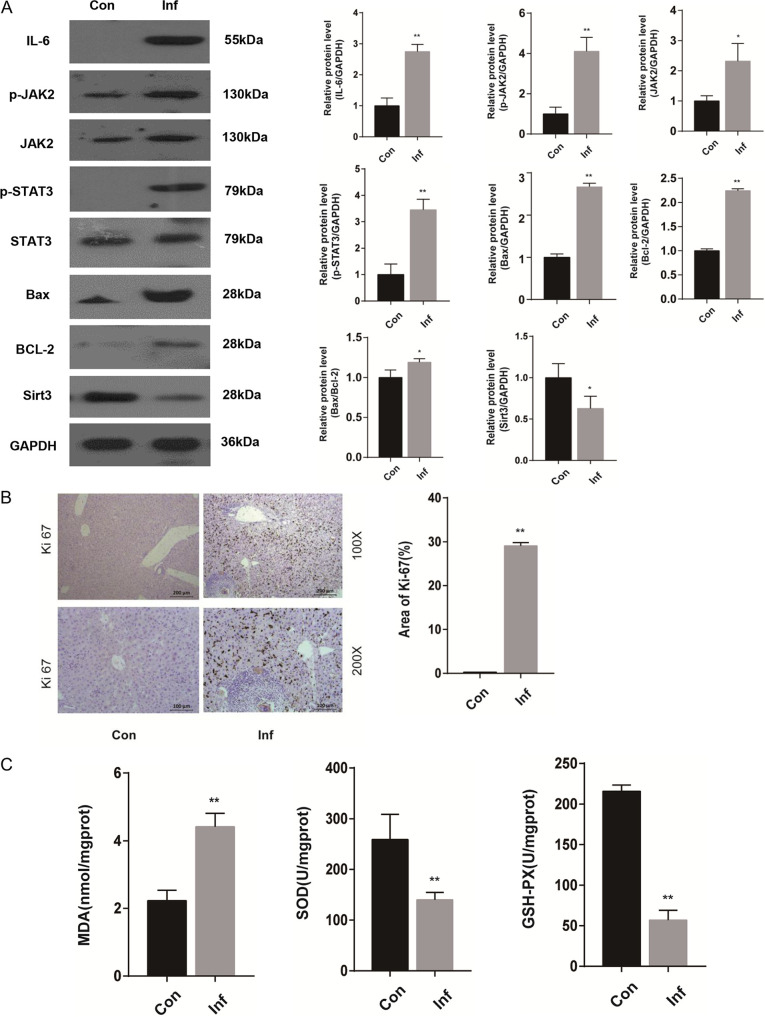
To investigate whether STAT3 is involved in S. japonicum-induced liver injury, we detected the STAT3-related signal pathway involved in oxidative stress, proliferation, and apoptosis. Protein levels of IL-6, p-JAK2, JAK2, STAT3, p-STAT3, B-cell lymphoma-2 (Bcl-2)-associated X protein (Bax), Bcl-2, and sirtuin3 (Sirt3) in the livers of mice with S. japonicum infection were examined by Western blot. The results showed that IL-6, p-JAK2, JAK2, p-STAT3, Bax, and Bcl-2 were significantly enhanced in infected livers compared with uninfected controls. Furthermore, the ratio of Bax to Bcl-2 was upregulated in the infected livers. However, Sirt3 was decreased in infected livers compared with uninfected controls (Fig. 3A). Consistent with the increased apoptosis phenotypes, immunohistochemical analysis of Ki-67 indicated enhanced proliferation in S. japonicum-infected mice compared with control mice (Fig. 3B). Oxidative stress is an initial and critical element in the development of hepatic injury. S. japonicum-infected mice showed upregulation of oxidative stress compared with control mice. S. japonicum infection increased the liver malonaldehyde (MDA) level, whereas it decreased the level of its contrary indicators, the activity of glutathione peroxidase (GSH-PX) and superoxide dismutase (SOD) (Fig. 3C).
FIG 3.
The JAK2/STAT3 signal pathway is activated in the livers of mice with S. japonicum infection after 6 weeks. Wild-type mice were percutaneously treated with or without 30 ± 2 S. japonicum cercariae. At 6 weeks postinfection, liver tissue was collected for analysis. (A) Western blot analysis and quantification of protein levels of IL-6, p-JAK2, JAK2, p-STAT3, STAT3, Bax, Bcl-2, and Sirt3 in liver. Data are meansand SEM (n = 3 to 6 in each group) from three independent experiments. (B) Representative immunohistochemistry images of Ki-67 in liver tissue. Magnifications, ×100 (top) and ×200 (bottom). Bars, 200 μm (top) and 100 μm (bottom) (n = 4). (C) Measurement of MDA, SOD, and GSH-PX in the liver using a colorimetric reaction with 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) (n = 6). Data are means and SEM for 3 to 6 mice/group. Con, control group; Inf, infected group. * and **, P < 0.05 and P < 0.01 compared to control values.
In addition, we analyzed p-STAT3 and STAT3 protein levels in liver tissue at 8, 10, and 12 weeks after S. japonicum infection. p-STAT3 was not enhanced in infected livers compared with controls. This result demonstrated that STAT3 was not activated in the livers of mice at 8, 10, and 12 weeks after S. japonicum infection (Fig. S1).
Taken together, these results demonstrated that JAK2/STAT3 signal pathway and related molecules, such as IL-6, could be activated in the initial stage of liver injury induced by S. japonicum infection. These molecules are closely related to oxidative stress, proliferation, and apoptosis.
Liver p-STAT3 deficiency attenuates hepatic injury caused by S. japonicum infection.
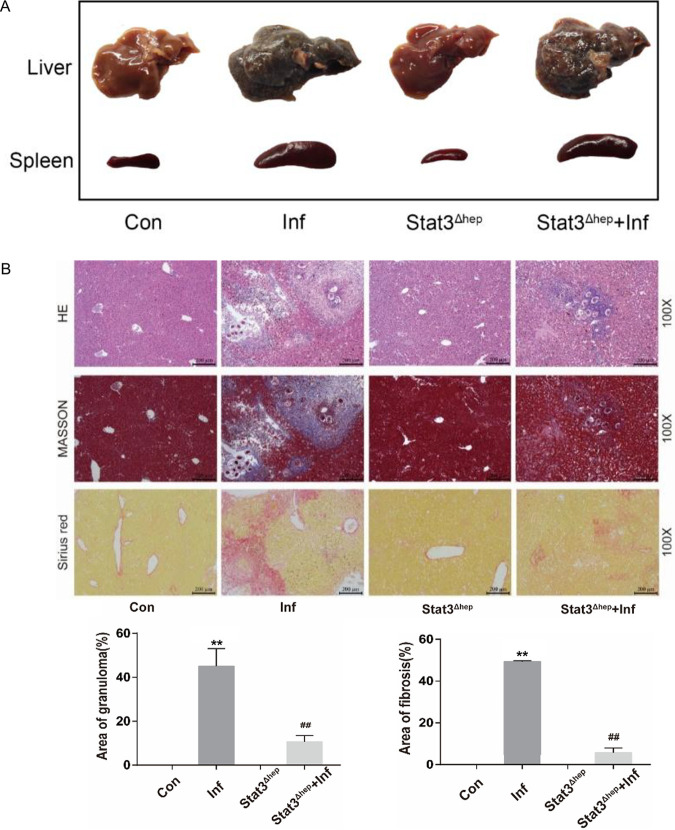
To further verify the effect of STAT3 in Schistosoma-induced liver injury, we infected mice that lack p-STAT3 in hepatocytes (Stat3flox/flox Alb-Cre mice, also called Stat3Δhep mice) and wild-type mice with S. japonicum. At 6 weeks postinfection, we detected pathological damage in the liver by biochemical studies, H&E and Sirius red staining, Masson’s trichrome staining, RT-PCR, etc. In terms of appearance and egg granuloma tubercles, livers were greatly improved in infected Stat3Δhep mice versus infected wild-type mice (Fig. 4A). The granulomatous area was lower in infected Stat3Δhep mice than infected wild-type mice (Fig. 4B). Moreover, liver p-STAT3 deficiency reduced the fibrosis area (Fig. 4B) and the expression of α-SMA, a hallmark of activated HSCs (Fig. 4C). In addition, ALT, AST, and LDH levels indicated that liver p-STAT3 deficiency alleviated liver function injury caused by schistosomiasis (Fig. 4D). Hence, liver p-STAT3 deficiency attenuated hepatic injury caused by S. japonicum infection.
FIG 4.
Liver p-STAT3 deficiency attenuates hepatic injury caused by S. japonicum infection. Wild-type and Stat3Δhep mice were percutaneously treated with or without 30 ± 2 S. japonicum cercariae. At 6 weeks postinfection, liver tissue and serum were collected for analysis. (A) Gross appearance of livers and spleens of control, infected, uninfected Stat3Δhep, and infected Stat3Δhep mice. (B) H&E, Masson’s trichrome, and Sirius red staining of liver sections. H&E staining showed the granuloma, and Masson’s trichrome and Sirius red staining showed collagen content and distribution. The granulomatous and fibrotic area (Masson’s trichrome staining) as a percentage of total area was measured by computer-assisted morphometric analysis (n = 6). Magnification, ×100. Bar, 200 μm. (C) Representative immunohistochemistry images of α-SMA in liver tissue. Magnification, ×100 (top) and ×200 (bottom). Bars, 200 μm (top) and 100 μm (bottom). Analytical results of α-SMA positive staining in the liver (n = 5) are also shown. (D) ALT, AST, LDH, and ALP were measured by use of a biochemical analyzer (n = 4). Data are means and SEM for 3 to 6 mice/group. Con, control group; Inf, infected group; Stat3Δhep: p-STAT3-deficient group; Stat3Δhep+Inf: p-STAT3-deficient infected group. * and **, P < 0.05 and P < 0.01 compared to control values; # and ##, P < 0.05 and P < 0.01 compared to infected-group values. NS, no statistical significance.
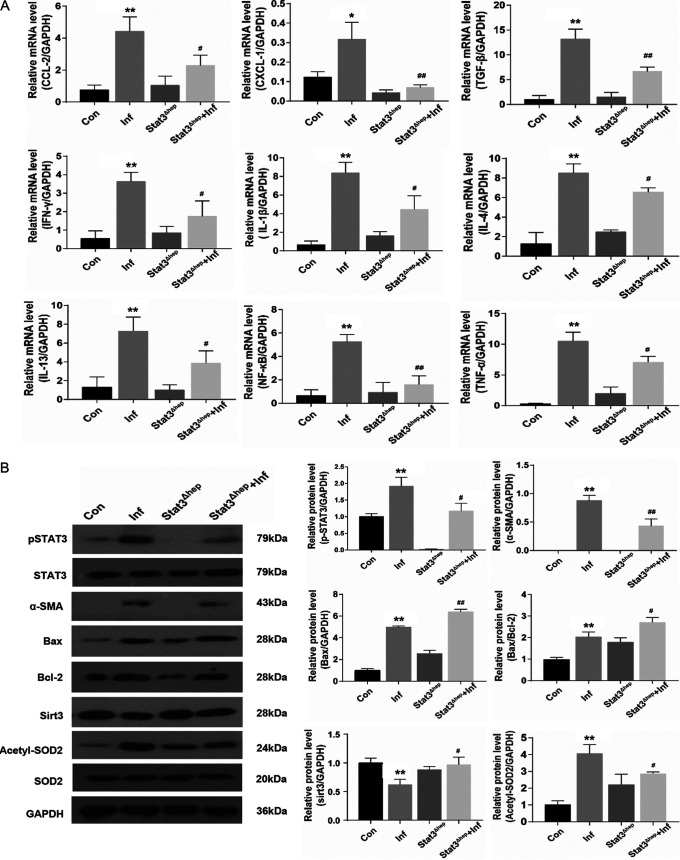
Liver p-STAT3 deficiency promotes apoptosis and blocks S. japonicum-induced hepatic inflammation, proliferation, and oxidative stress.
The inflammatory response is a typical phenotype of liver fibrosis. Proinflammatory chemokines and cytokines, including CCL-2, CXCL-1, TGF-β, IFN-γ, IL-1β, IL-4, IL-13, NF-κB, and TNF-α, were obviously higher in S. japonicum-infected than in noninfected mice, whereas liver p-STAT3 deficiency reversed this increase (Fig. 5A). Therefore, STAT3 plays a key role in accelerating liver inflammation. Moreover, liver p-STAT3 deficiency increased the expression of Bax; however, it did not signally affect the expression of Bcl-2; the ratio of Bax to Bcl-2 was therefore increased in infected Stat3Δhep mice (Fig. 5B). Furthermore, the level of Sirt3 was greatly increased and the protein level of acetylated SOD2 was greatly decreased in infected Stat3Δhep mice, but the expression of manganese SOD2 was not affected (Fig. 5B). Immunohistochemical analysis showed that Ki-67 was decreased in S. japonicum-infected mice because of liver p-STAT3 deficiency (Fig. 5C). In addition, in S. japonicum-infected mice, liver p-STAT3 deficiency downregulated the liver MDA level and upregulated the activity of GSH-PX and SOD (Fig. 5D). Altogether, liver p-STAT3 deficiency promotes apoptosis and blocks S. japonicum-induced hepatic inflammation, proliferation, and oxidative stress (Fig. 6).
FIG 5.
Liver p-STAT3 deficiency promotes apoptosis and blocks S. japonicum-induced hepatic inflammation, proliferation, and oxidative stress. Wild-type and Stat3Δhep mice were percutaneously treated with or without 30 ± 2 S. japonicum cercariae. At 6 weeks postinfection, liver tissues were analyzed. (A)Expression of CCL-2, CXCL-1, TGF-β, IFN-γ, IL-1β, IL-4, IL-13, NF-κB, and TNF-α mRNAs in liver tissue after infection of S. japonicum (n = 4). (B) Western blot analysis and quantification of protein levels of p-STAT3, STAT3, α-SMA, Bax, Bcl-2, Sirt3, acetyl-SOD2, and SOD2 in liver. Data are means and SEM (n = 3 to 6 in each group) from three independent experiments (n = 3). (C) Representative immunohistochemistry images of Ki-67 in liver tissue. Magnification, ×100 (top) and ×200 (bottom). Bars, 200 μm (top) and 100 μm (bottom) (n = 4). (D) Measurement of MDA, SOD, and GSH-PX in the liver using a colorimetric reaction with DNTB (n = 6). Data are means and SEM for 3 to 6 mice/group. Con, control group; Inf, infected group; Stat3Δhep, uninfected p-STAT3-deficient group; Stat3Δhep+Inf, infected p-STAT3-deficient group. * and **, P < 0.05 and P < 0.01 compared with control values; # and ##, P < 0.05 and P < 0.01 compared with infected-group values; NS, no statistical significance.
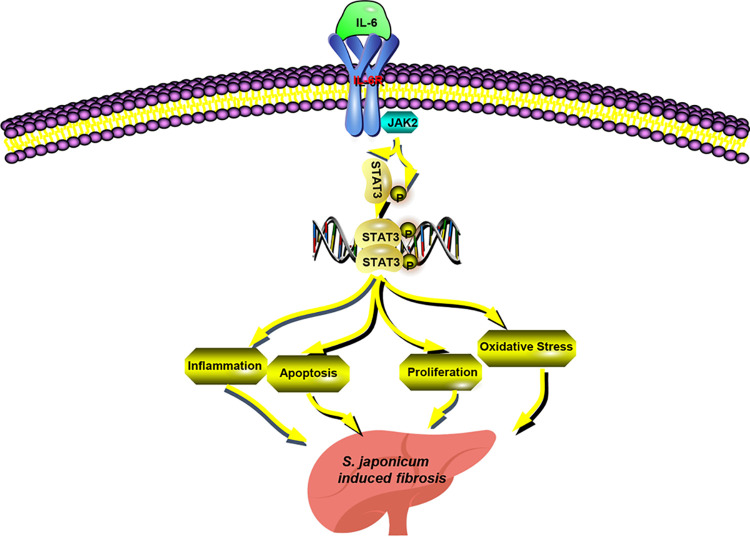
FIG 6.
Schematic overview of JAK2/STAT3-mediated effects on S. japonicum-induced liver fibrosis. The JAK2/STAT3 signal pathway and its related molecules, such as IL-6, could be activated in the livers of mice with S. japonicum infection. STAT3 constitutes a central node responsible for inflammation, apoptosis, proliferation, and oxidative stress by regulating various downstream targets that ultimately favor promotion of liver fibrosis.
DISCUSSION
Schistosomiasis-linked hepatic fibrosis is the main reason for morbidity and mortality in humans with schistosome infection. Alleviating schistosome egg-induced granuloma formation and fibrosis initiation can inhibit the progress of the illness. Therefore, it is important to identify key molecules that initiate and regulate hepatic granuloma and fibrosis during S. japonicum infection. In this research, we investigated the role of STAT3 in initial stage of hepatic injury during S. japonicum infection. We discovered that STAT3 was activated in the livers of mice infected with S. japonicum. Liver p-STAT3 deficiency could ameliorate S. japonicum infection-associated liver injury. Furthermore, liver p-STAT3 deficiency promoted apoptosis and suppressed the activation of inflammation, proliferation, and oxidative stress induced by S. japonicum infection.
A causal link between S. japonicum infection and the process of fibrosis has long been identified, and extensive investigation into the potential molecular mechanisms has been carried out in recent years. Eggs laid by female schistosomes are trapped in the liver via the portal venous system, inducing a granulomatous and fibrotic response. In our study, at 4, 5, and 6 weeks after S. japonicum infection, liver granuloma and fibrosis gradually appeared and worsened. By 6 weeks after infection, mice displayed obvious granulomatous and fibrotic lesions, causing significant liver function impairment. Therefore, our study focused on the effect of liver injury caused by 6 weeks of S. japonicum infection.
To elucidate the molecular mechanisms of hepatic pathological lesions during S. japonicum infection, we performed RNA transcriptome resequencing of liver. We found that the expression of upstream and downstream STAT3-related genes in mice with S. japonicum infection was upregulated compared with STAT3-related genes in control mice. Previous studies have shown that STAT3 was a proto-oncogene or transcription factor, the activation of which facilitates the occurrence and development of dysplasia during HCC tumorigenesis (18, 21). Mitsuhiko et al. discovered that STAT3 deficiency prevents hepatocarcinogenesis and promotes biliary proliferation in thioacetamide-induced liver injury (22). Roderfeld et al. indicated that STAT3 was activated by an extract from SEA and culture supernatants of live schistosome egg in hamster model and cell culture experiments (18). Yang et al. demonstrated that S. japonicum infection could activate JAK/STAT3 signaling (19). However, the molecular mechanisms of STAT3 in S. japonicum infection need further investigation. Therefore, we identified and investigated STAT3 as a novel research target with S. japonicum infection by RNA transcriptome resequencing.
Studies have found the effect of IL-6 in the liver was associated with IL-6 receptor 1 (IL-6R1) and gp130, which are highly expressed on hepatocytes. In general, upon the interaction between IL-6 and IL-6Rα, cytokines lead to homodimerization of gp130 and subsequent activation of the receptor-associated Janus kinases, known as JAK1, JAK2, and tyrosine kinase (Tyk2). This receptor kinase complex interacts with and activates the SH2-containing cytoplasmic STAT3 transcription factor and then translocates to the nucleus to activate the transcription of many target genes, such as those encoding c-Jun, c-Myc, JunB, cyclin D1, C/EBP, and p21WAF1/Cip1, and acute-phase genes (23). Treatment with IL-6 induces STAT3 activation in primary human hepatocytes, human hepatoma cells (24), and primary rat hepatocytes (23, 25). Consistent with these results, our studies showed that the JAK2/STAT3 signal pathway and its related molecules, such as IL-6, could be activated during S. japonicum-induced hepatic granuloma formation and fibrosis initiation. In addition, we discovered that STAT3 could not be activated in the livers of mice at 8, 10, and 12 weeks after S. japonicum infection. These results indicated that STAT3 signal pathway could not be activated in the late phase of S. japonicum infection.
Generally, increases in the activity of ALP in serum and other body fluids may reflect physiologic or pathological changes in the liver (26), whereas we discovered that the ALP level in infected mice is lower than that in normal mice. Several lines of indirect evidence indicate that ALP is downregulated in inflammatory and fibrosing cholangiopathies (for instance, primary biliary cirrhosis, primary sclerosing cholangitis and cystic fibrosis), because of the lack of sufficient alkalinity of bile or relocation from the canalicular domain (27). Therefore, the ALP level in infected mice is lower than that in normal mice, which indicates that the abnormal ALP level correlates with liver function injury. However, the specific significance of ALP in liver injury caused by S. japonicum infection is unclear and needs further research.
The initiation, development, and regression of hepatic granuloma and fibrosis are correlated with inflammation, oxidative stress, proliferation, and apoptosis. We found that S. japonicum infection induced liver inflammation, oxidative stress, proliferation, and apoptosis. Liver p-STAT3 deficiency promotes apoptosis and blocks S. japonicum-induced hepatic inflammation, proliferation, and oxidative stress. Recent research showed that liver inflammation was accompanied by development of liver fibrosis. It was characterized by a large number of inflammatory factors in liver fibrosis (28). We found increased levels of genes for the proinflammatory cytokines CCL-2, CXCL-1, TGF-β, IFN-γ, IL-1β, IL-4, IL-13, NF-κB, and TNF-α in the livers of S. japonicum-infected mice. Also, liver p-STAT3 deficiency inhibited the increase of proinflammatory genes.
The change of the redox state in the liver is closely related to the development of fibrosis. An excessive increase of reactive oxygen species (ROS) will interfere with the normal functions of cells in the liver, such as hepatocytes, HSCs, and Kupffer cells, and have a crucial effect on the pathogenesis of liver fibrosis (29). Previous studies reported that STAT3 was a crucial element of signaling pathways activated by oxidative stress in mitochondria (30). Astaxanthin could alleviate ethanol-induced liver inflammation, oxidative stress, and liver injury by reducing the activity of STAT3 (31). Yang et al. demonstrated that SEA and soluble adult worm antigen could enhance the accumulation of MDSCs in S. japonicum-infected mouse spleens by inducing ROS production via JAK/STAT3 signaling, which accelerates tumor progression (32). We found that in S. japonicum-infected mice, liver p-STAT3 deficiency downregulated the liver MDA level and upregulated the activity of GSH-PX and SOD. Therefore, liver p-STAT3 deficiency alleviated oxidative stress. Furthermore, we found that Sirt3 was greatly increased and acetylated SOD2 was greatly decreased in infected Stat3Δhep mice, but the expression of total SOD2 was not affected. Sirtuins are an evolutionarily conserved NAD+-dependent deacetylase family. Of the seven mammalian sirtuins, three (Sirt3, Sirt4, and Sirt5) are primarily localized to mitochondria. Sirt3 is the only sirtuin that has a powerful deacetylase activity in mitochondria and hepatocytes. Sirt3 is a crucial gatekeeper of redox status, epigenetic landscape, and lipid homeostasis (32, 33). A previous study found that Sirt3 deficiency led to reduced intracellular ATP level, accompanied by increased ROS levels (34). Assessment of mitochondrial respiration via measurement of the oxygen consumption rate (OCR) showed that Sirt3 knockout (KO) mice exhibited decreased OCR and exacerbated oxidative stress. In contrast, Sirt3 overexpression gives rise to increased OCR and reduced oxidative stress (35, 36). Several recent reports showed that Sirt3 directly activates SOD2 via protein/lysine deacetylation (37). Thus, STAT3 could activate SOD2 through Sirt3-mediated deacetylation. The upregulation of Sirt3 and downregulation of acetylated SOD2 also confirms that liver p-STAT3 deficiency inhibits S. japonicum-induced oxidative stress.
JAK/STAT proteins are ubiquitously expressed and are involved in the regulation and maintenance of many fundamental biological processes, including apoptosis, proliferation, immune response, and inflammation (10, 38). Apoptosis is a series of biochemical and molecular events that are induced by multiple stimuli. Cell shrinkage, chromatin condensation, and DNA and membrane fragmentation are the morphological alterations of apoptotic cells, which may lead to the phagocytosis of the affected cell, whereas pure apoptosis activation suppresses cell proliferation (39, 40). Zhu et al. discovered that blueberries and probiotics attenuate nonalcoholic fatty liver disease (NAFLD), partly by reducing the apoptosis of hepatocytes by inhibiting IL-22-mediated JAK1/STAT3/BAX signaling pathway, which dampens the development of liver fibrosis (41). We found an increased protein level of Ki-67 and an increased ratio of Bax to Bcl-2 in the livers of S. japonicum-infected mice. Also, p-STAT3 deficiency upregulated the ratio of Bax to Bcl-2 and the protein level of Bax and decreased the expression of Ki-67. Thus, S. japonicum-induced apoptosis and proliferation were at least partly dependent on activation of STAT3. Interestingly, Bcl-2 was significantly enhanced in the infected livers. However, p-STAT3 deficiency did not signally affect the expression of Bcl-2. Hence, the exact mechanism of apoptosis involved in STAT3 after S. japonicum infection needs further study.
In conclusion, our study revealed that S. japonicum infection triggers the JAK2/STAT3 signal pathway, which results in inflammation, oxidative stress, proliferation, and apoptosis. p-STAT3 deficiency could ameliorate S. japonicum infection-associated liver injury. These data provide a rationale for targeting STAT3 for the improvement of schistosomiasis.
MATERIALS AND METHODS
Animals, parasites, and infection.
Oncomelania hupensis snails infected with cercariae of S. japonicum (Chinese mainland strain) were from the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Shanghai, China). Male C57BL/6 mice, 20 ± 2 g, were from the Department of Laboratory Animal Science of Peking University Health Science Center (Beijing, China). Stat3flox/flox Alb-Cre mice (hepatic-STAT3-deficient mice) were from The Jackson Laboratory (USA).
All experimental protocols were conducted according to the Guide for the Care and Use of Laboratory Animals (42) and approved by the Animal Care Committee of Peking University Health Science Center (ethical approval number LA2020219). Mice were housed under specific-pathogen-free conditions.
S. japonicum cercariae were collected by shedding after exposing infected Oncomelania snails to a light for 6 h at 24 to 28°C. Anesthetized mice were percutaneously exposed to 30 ± 2 cercariae of S. japonicum freshly shed from snails to establish a mouse model of schistosomiasis. After 4, 5, 6, 8, 10, and 12 weeks of postinfection treatment, the mice were killed, and then liver tissue and serum samples were obtained for study.
Serum biochemical analysis.
Blood samples were rested overnight at 4°C to acquire serum samples. ALT, AST, ALP, and LDH were assessed with an Olympus AU5800 automatic biochemical analyzer (Olympus, Japan).
Histology and histopathology of liver sections.
Paraformaldehyde (4%; Sigma-Aldrich, St. Louis, MO, USA) was added to fix excised liver samples for 6 to 8 h, and then samples were treated with sucrose and embedded in paraffin. For histology, liver sections (5 μm) were obtained to assess liver granulomas with H&E staining. Liver sections were stained with Sirius red and Masson’s trichrome staining to assess the level of hepatic fibrosis. Egg granuloma and liver fibrosis areas were measured by using a Leica DMI 3000B fluorescence microscope. Then, we used computer-assisted morphometric software (Leica application suite 4.1) to analyze the liver granulomatous and fibrotic areas as a percentage of the total area.
For immunohistochemistry staining, paraffin sections (5 μm) from the livers of mice were blocked with 10% normal goat or rabbit serum for 1 h at 37°C, incubated at 4°C overnight with primary antibodies for α-SMA (1:200; Abcam) and Ki-67 (1:500; eBioscience), and then incubated with secondary antibodies for 1 h. Nuclei were stained with hematoxylin and then treated with diaminobenzidine (DAB). All secondary antibodies were from Santa Cruz Biotechnology. Image-Pro Plus v6.0 was used to quantify immunostaining data.
RNA preparation and transcriptome resequencing.
Fresh liver tissues were immediately obtained when mice were killed. The residual blood on the tissue surface was quickly rinsed with prechilled phosphate-buffered saline (PBS) solution (RNase free). Later, liver tissues were quickly placed in liquid nitrogen, kept frozen for 3 to 4 h, and then transferred to −80°C for long-term storage. Total RNA was extracted from liver tissues using TRIzol. RNA purity was checked using a NanoPhotometer spectrophotometer (Implen, CA, USA). The RNA Nano 6000 assay kit of the Bioanalyzer 2100 system (Agilent Technologies, CA, USA) was used to assess RNA integrity. Gene expression profiling was performed on 1 μg total RNA from each sample as input material. NEBNext Ultra RNA library prep kit for Illumina (NEB, USA) generated sequencing libraries by the manufacturer’s recommendations. Attribute sequences added index codes to each sample. The index-coded samples were clustered on a cBot cluster generation system using a TruSeq PE cluster kit, v3-cBot-HS (Illumina). Upon cluster generation, the DNA library preparations were sequenced with the 150-bp paired-end reads on an Illumina Novaseq platform. Differential gene expression analysis was estimated in DESeq2 R package (1.16.1). Gene Ontology (GO) enrichment analysis of the genes was performed with the cluster profiler R package, in which gene length bias was corrected. GO terms with corrected P values less than 0.05 were regarded as apparently enriched by differentially expressed genes.
RNA and quantitative real-time PCR.
Total RNA was extracted from liver tissue with the TRIzol reagent (Applygen Technologies, Beijing, China). In total, 1 μg RNA was isolated and reverse transcribed to cDNA by using Moloney murine leukemia virus (M-MLV) and an oligo(dT) primer (TaKaRa Biotechnology, Dalian, China). cDNA was amplified in triplicate with SYBR premix Ex Taq II (TaKaRa Biotechnology) on an ABI 7500 Fast real-time PCR system (Applied Biosystems, Fullerton, CA). The forward and reverse PCR primers were projected with the Primer-BLAST tool on the NCBI website. Relative mRNA quantification was performed according to the 2−ΔΔCT method, with the endogenous GAPDH level as a reference. The PCR primers are in Table 1.
TABLE 1.
Primers used for real-time RT-PCR assays
| Target | Orientation | Sequence |
|---|---|---|
| GAPDH | Sense | AAGAAGGTGGTGAAGCAG |
| Antisense | TCATACCAGGAAATGAGC | |
| IL-6 | Sense | TAGTCCTTCCTACCCCAATTTCC |
| Antisense | TTGGTCCTTAGCCACTCCTTC | |
| IL-11 | Sense | GCGCTGTTCTCCTAACCCG |
| Antisense | GAGTCCAGACTGTGATCTCCG | |
| CCL-2 | Sense | TAAAAACCTGGATCGGAACCAAA |
| Antisense | GCATTAGCTTCAGATTTACGGGT | |
| CXCL-1 | Sense | ACTGCACCCAAACCGAAGTC |
| Antisense | TGGGGACACCTTTTAGCATCTT | |
| TGF-β | Sense | TCTGCATTGCACTTATGCTGA |
| Antisense | AAAGGGCGATCTAGTGATGGA | |
| IFN-γ | Sense | GCCACGGCACAGTCATTGA |
| Antisense | TGCTGATGGCCTGATTGTCTT | |
| IL-1β | Sense | GAAATGCCACCTTTTGACAGTG |
| Antisense | TGGATGCTCTCATCAGGACAG | |
| IL-4 | Sense | GGTCTCAACCCCCAGCTAGT |
| Antisense | GCCGATGATCTCTCTCAAGTGAT | |
| IL-13 | Sense | CAGCCTCCCCGATACCAAAAT |
| Antisense | GCGAAACAGTTGCTTTGTGTAG | |
| NF-κB | Sense | ATGGCAGACGATGATCCCTAC |
| Antisense | CGGAATCGAAATCCCCTCTGTT | |
| TNF-α | Sense | CAGGCGGTGCCTATGTCTC |
| Antisense | CGATCACCCCGAAGTTCAGTAG |
Measurement of oxidative stress.
The activities of liver MDA, SOD, and GSH-PX were measured with an MDA assay kit, SOD assay kit, and GSH-PX assay kit (A005; NanJing JianCheng Bioengineering Institute, Nanjing, China) per the instructions of the manufacturer.
Western blot analysis.
Liver tissues were prepared by lysis buffer with a cocktail of protease inhibitors (Roche, Switzerland). The supernatant was obtained by centrifugation and the protein concentration in each sample was measured with the bicinchoninic acid (BCA) protein assay kit (Beyotime, China). Then protein was mixed with loading buffer and boiled for 15 min at 95°C. After dissolution in 10% or 12% SDS-PAGE, the total protein samples were transferred to nitrocellulose membranes for 2 h with transfer buffer and blocked with 5% nonfat dried milk, which was dissolved in TBST (20 mmol/liter Tris-HCl [pH 7.6], 150 mmol/liter NaCl, and 0.02% Tween 20) for 1 h.
Membranes were incubated with primary antibodies for GAPDH (no. 2118; Cell Signaling Technology), α-SMA (no. ab5694; Abcam), IL-6 (no. ab6672; Abcam), p-JAK2 (no. 3776; Cell Signaling Technology), JAK2 (no. 3230; Cell Signaling Technology), p-STAT3 (no. 9145; Cell Signaling Technology), STAT3 (no. 9139/4904; Cell Signaling Technology), Bax (no. SC493; Santa Cruz Biotechnology), Bcl-2 (no. SC492; Santa Cruz Biotechnology), Sirt3 (no. 5490s; Cell Signaling Technology), Acetylated SOD2 (K68) (no. ab137037; Abcam), SOD2 (no. ab13534; Abcam) overnight at 4°C. After three washes for 7 min each in TBST, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:2,000) for 1 h at room temperature. The proteins were detected by enhanced chemiluminescence (Applygen Technologies, Beijing, China). Protein expression was analyzed with NIH ImageJ and normalized to that of GAPDH. All experiments were repeated at least 3 times.
Statistical analysis.
Data are presented as means and standard errors of the means (SEM). Statistical analysis involved the use of GraphPad Prism v5.00 for Windows (GraphPad Software, San Diego, CA, USA). Student's t test was served as comparing the differences between the two groups. One-way analysis of variance (ANOVA) was used to detect the differences among multiple groups, followed by a Student-Newman-Keuls test. A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (no. 30901247).
We declare that we have no conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. 2014. Human schistosomiasis. Lancet 383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Zhang YR, Cai C, Ni XQ, Zhu Q, Ren JL, Chen Y, Zhang LS, Xue CD, Zhao J, Qi YF, Yu YR. 2019. Taurine alleviates Schistosoma-induced liver injury by inhibiting the TXNIP/NLRP3 inflammasome signal pathway and pyroptosis. Infect Immun 87:e00732-19. doi: 10.1128/IAI.00732-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan TZ, Ma HH, Jiang SQ, Zhong ZR, Wang XL, Li CX, Yu D, Liu L, Xu J, Xia CM. 2019. IL-9 blockage reduces early hepatic granuloma formation and fibrosis during Schistosoma japonicum infection in mice. Immunology 158:296–303. doi: 10.1111/imm.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barsoum RS, Esmat G, El-Baz T. 2013. Human schistosomiasis: clinical perspective: review. J Adv Res 4:433–444. doi: 10.1016/j.jare.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He X, Wang Y, Fan XB, Lei NH, Tian YN, Zhang DM, Pan WQ. 2020. A schistosome miRNA promotes host hepatic fibrosis by targeting transforming growth factor beta receptor III. J Hepatol 72:519–527. doi: 10.1016/j.jhep.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Pearce EJ, MacDonald AS. 2002. The immunobiology of schistosomiasis. Nat Rev Immunol 2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 7.Carson JP, Ramm GA, Robinson MW, McManus DP, Gobert GN. 2018. Schistosome-induced fibrotic disease: the role of hepatic stellate cells. Trends Parasitol 34:524–540. doi: 10.1016/j.pt.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Almadi MA, Aljebreen AM, Sanai FM, Marcus V, Almeghaiseeb ES, Ghosh S. 2011. New insights into gastrointestinal and hepatic granulomatous disorders. Nat Rev Gastroenterol Hepatol 8:455–466. doi: 10.1038/nrgastro.2011.115. [DOI] [PubMed] [Google Scholar]
- 9.Gao B, Wang H, Lafdil F, Feng D. 2012. STAT proteins - key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J Hepatol 57:430–441. doi: 10.1016/j.jhep.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodington DW, Desai HR, Woo M. 2018. JAK/STAT—emerging players in metabolism. Trends Endocrinol Metab 29:55–65. doi: 10.1016/j.tem.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Bataller R, Brenner DA. 2005. Liver fibrosis. J Clin Invest 115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Lafdil F, Kong X, Gao B. 2011. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci 7:536–550. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng YR, Ma HD, Tsuneyama K, Yang W, Wang YH, Lu FT, Liu CH, Liu P, He XS, Diehl AM, Gershwin ME, Lian ZX. 2013. STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis by the protein kinase inhibitor sorafenib. J Autoimmun 46:25–34. doi: 10.1016/j.jaut.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Kasembeli MM, Bharadwaj U, Robinson P, Tweardy DJ. 2018. Contribution of STAT3 to inflammatory and fibrotic diseases and prospects for its targeting for treatment. Int J Mol Sci 19:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy DE, Darnell JE, Jr. 2002. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 16.Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A. 2006. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene 25:2520–2530. doi: 10.1038/sj.onc.1209281. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Iness A, Yoon J, Grider JR, Murthy KS, Kellum JM, Kuemmerle JF. 2015. Noncanonical STAT3 activation regulates excess TGF-beta1 and collagen I expression in muscle of stricturing Crohn's disease. J Immunol 194:3422–3431. doi: 10.4049/jimmunol.1401779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roderfeld M, Padem S, Lichtenberger J, Quack T, Weiskirchen R, Longerich T, Schramm G, Churin Y, Irungbam K, Tschuschner A, Windhorst A, Grevelding CG, Roeb E. 2020. Schistosoma mansoni egg-secreted antigens activate hepatocellular carcinoma-associated transcription factors c-Jun and STAT3 in hamster and human hepatocytes. Hepatology 72:626–641. doi: 10.1002/hep.30192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q, Qiu H, Xie HY, Qi YW, Cha HF, Qu JL, Wang M, Feng YF, Ye X, Mu JB, Huang J. 2017. A Schistosoma japonicum infection promotes the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. J Immunol 198:4716–4727. doi: 10.4049/jimmunol.1601860. [DOI] [PubMed] [Google Scholar]
- 20.Chen JL, Xu TH, Zhu DD, Wang JX, Huang CQ, Lyu L, Hu B, Sun W, Duan YN. 2016. Egg antigen p40 of Schistosoma japonicum promotes senescence in activated hepatic stellate cells by activation of the STAT3/p53/p21 pathway. Cell Death Dis 7:e2315. doi: 10.1038/cddis.2016.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Tonsy MM, Hussein HM, Helal TE-S, Tawfik RA, Koriem KM, Hussein H. 2013. Schistosoma mansoni infection: is it a risk factor for development of hepatocellular carcinoma? Acta Trop 128:542–547. doi: 10.1016/j.actatropica.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Mitsuhiko A, Takafumi Y, Jun A, Yu I, Fumitaka W, Atsutaka M, Takahiko S, Toshimitsu T, Hideki I, Toru N, Michio S, Hironori K, Akihiko Y, Takuji T. 2017. STAT3 defciency prevents hepatocarcinogenesis and promotes biliary proliferation in thioacetamide-induced liver injury. World J Gastroenterol 23:6833–6844. doi: 10.3748/wjg.v23.i37.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao B. 2005. Cytokines, STATs and liver disease. Cell Mol Immunol 2:92–100. [PubMed] [Google Scholar]
- 24.Nguyen VA, Gao B. 1999. Cross-talk between alpha(1B)-adrenergic receptor (alpha(1B)AR) and interleukin-6 (IL-6) signaling pathways. Activation of alpha(1b)AR inhibits il-6-activated STAT3 in hepatic cells by a p42/44 mitogen-activated protein kinase-dependent mechanism. J Biol Chem 274:35492–35498. doi: 10.1074/jbc.274.50.35492. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Kunos G, Gao B. 1999. Ethanol rapidly inhibits IL-6-activated STAT3 and C/EBP mRNA expression in freshly isolated rat hepatocytes. FEBS Lett 457:162–168. doi: 10.1016/S0014-5793(99)01031-5. [DOI] [PubMed] [Google Scholar]
- 26.Nicole JF, Beverly AK. 2007. Liver fibrosis. Alkaline phosphatase: beyond the liver. Vet Clin Pathol 36:223–233. [DOI] [PubMed] [Google Scholar]
- 27.Raoul P. 2015. Liver alkaline phosphatase: a missing link between choleresis and biliary inflammation. Hepatology 61:2080–2090. [DOI] [PubMed] [Google Scholar]
- 28.Aydin MM, Akcali KC. 2018. Liver fibrosis. Turk J Gastroenterol 29:14–21. doi: 10.5152/tjg.2018.17330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luangmonkong T, Suriguga S, Mutsaers HAM, Groothuis GMM, Olinga P, Boersema M. 2018. Targeting oxidative stress for the treatment of liver fibrosis. Rev Physiol Biochem Pharmacol 175:71–102. doi: 10.1007/112_2018_10. [DOI] [PubMed] [Google Scholar]
- 30.Waris G, Huh KW, Siddiqui A. 2001. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol 21:7721–7730. doi: 10.1128/MCB.21.22.7721-7730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han JH, Ju JH, Lee YS, Park JH, Yeo IJ, Park MH, Roh YS, Han SB, Hong JT. 2018. Astaxanthin alleviated ethanol-induced liver injury by inhibition of oxidative stress and inflammatory responses via blocking of STAT3 activity. Sci Rep 8:14090. doi: 10.1038/s41598-018-32497-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng X, Yang J, Hu O, Huang J, Ran L, Chen M, Zhang Y, Zhou X, Zhu J, Zhang Q, Yi L, Mi M. 2019. Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid Redox Signal 30:163–183. doi: 10.1089/ars.2017.7172. [DOI] [PubMed] [Google Scholar]
- 33.Ahn BH, Kim HS, Song SW, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. 2008. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. 2013. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsström S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, Suomalainen A. 2014. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med 6:721–731. doi: 10.1002/emmm.201403943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang Q, Benavides GA, Vassilopoulos A, Gius D, Darley-Usmar V, Zhang JH. 2013. Bioenergetic and autophagic control by Sirt3 in response to nutrient deprivation in mouse embryonic fibroblasts. Biochem J 454:249–257. doi: 10.1042/BJ20130414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Lee YD, Kim HJ, Lee ZH, Kim HH. 2017. SOD2 and Sirt3 control osteoclastogenesis by regulating mitochondrial ROS. J Bone Miner Res 32:397–406. doi: 10.1002/jbmr.2974. [DOI] [PubMed] [Google Scholar]
- 38.O'Shea JJ, Plenge R. 2012. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva BDO, Ramos LF, Moraes KCM. 2017. Molecular interplays in hepatic stellate cells: apoptosis, senescence, and phenotype reversion as cellular connections that modulate liver fibrosis. Cell Biol Int 41:946–959. doi: 10.1002/cbin.10790. [DOI] [PubMed] [Google Scholar]
- 40.Wang K. 2015. Autophagy and apoptosis in liver injury. Cell Cycle 14:1631–1642. doi: 10.1080/15384101.2015.1038685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu J, Zhou M, Zhao X, Mu M, Cheng M. 2018. Blueberry, combined with probiotics, alleviates non-alcoholic fatty liver disease via IL-22-mediated JAK1/STAT3/BAX signaling. Food Funct 9:6298–6306. doi: 10.1039/c8fo01227j. [DOI] [PubMed] [Google Scholar]
- 42.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.