Cell wall proteins with sialidase activity are involved in carbohydrate assimilation, adhesion to mucosal surfaces, and biofilm formation. Gardnerella spp. inhabit the human vaginal microbiome and encode up to three sialidase enzymes, two of which are suspected to be cell wall associated. Here, we demonstrate that the gene encoding extracellular sialidase NanH3 is found almost exclusively in Gardnerella piotii and the closely related species Gardnerella genome sp. 3, and its presence correlates with a sialidase-positive phenotype in a collection of 112 Gardnerella isolates.
KEYWORDS: Gardnerella, phase variation, sialidase, vaginosis
ABSTRACT
Cell wall proteins with sialidase activity are involved in carbohydrate assimilation, adhesion to mucosal surfaces, and biofilm formation. Gardnerella spp. inhabit the human vaginal microbiome and encode up to three sialidase enzymes, two of which are suspected to be cell wall associated. Here, we demonstrate that the gene encoding extracellular sialidase NanH3 is found almost exclusively in Gardnerella piotii and the closely related species Gardnerella genome sp. 3, and its presence correlates with a sialidase-positive phenotype in a collection of 112 Gardnerella isolates. The nanH3 gene sequence includes a homopolymeric repeat of cytosines that varies in length within cell populations, indicating that this gene is subject to slipped-strand mispairing, a mechanism of phase variation in bacteria. Variation in the length of the homopolymer sequence results in production of either the full-length sialidase protein or truncated peptides lacking the sialidase domain due to introduction of reading-frame shifts and premature stop codons. Phase variation in NanH3 may be involved in immune evasion or modulation of adhesion to host epithelial cells and formation of biofilms characteristic of the vaginal dysbiosis known as bacterial vaginosis.
INTRODUCTION
Bacterial vaginosis (BV) is a condition characterized by altered composition of the vaginal microbiota that occurs when the healthy microbiota is replaced by an overgrowth of mixed aerobic and anaerobic species, including Gardnerella spp. (1, 2). An abundance of Gardnerella spp. is often found in cases of symptomatic BV, although the bacteria are also found in healthy women with no clinical signs or symptoms of BV (3). Gardnerella spp. can be resolved into four subgroups based on cpn60 barcode sequencing (4) or whole-genome sequencing (5). Recently, the description of Gardnerella vaginalis was amended and three new species were defined within the genus Gardnerella: Gardnerella leopoldii, Gardnerella swidsinskii, and Gardnerella piotii (6). G. piotii and G. vaginalis correspond to cpn60 subgroup B and C, respectively. G. leopoldii and G. swidsinskii were previously grouped together as subgroup A based on cpn60 sequences of the isolates available at that time. Isolates belonging to cpn60 subgroup D corresponded to three distinct genome species: genome species 8, 9, and 10 (7).
In addition to the characteristic changes in microbiota and elevated pH, sialidase activity in vaginal fluid is a diagnostic marker of BV (8, 9). Sialidase enzymes cleave the glycosidic linkages of sialic acids from terminal glycans, including vaginal mucins, immunoglobulin A molecules, and epithelial cell surface glycoproteins (10, 11). Activity on the latter may be related to the adhesion of bacteria to epithelial cells, which is the initial step in biofilm formation (12). Previously, we have demonstrated that sialidase activity is almost exclusively confined to G. piotii and Gardnerella genome species 3 (cpn60 subgroup B) (13).
A putative sialidase gene, nanH1 (sialidase A), was identified in Gardnerella spp. and was initially thought to be the gene responsible for sialidase activity (14). Although nanH1 appears to be present in all sialidase-positive strains, it is also found in sialidase-negative strains (13). This observation, combined with the lack of signal peptide on the NanH1 protein, suggests that this protein is an intracellular enzyme, likely involved in manipulation of nutritional substrates as is the case in many bacteria (15). The discrepancy between nanH1 presence and enzyme activity led to the recognition in some Gardnerella isolates of two additional sialidase genes, nanH2 and nanH3 (16), which are unevenly distributed among Gardnerella spp. (17). Based on biochemical studies, Robinson et al. (16) concluded that NanH2 and NanH3 are the proteins responsible for sialidase activity detected in vaginal fluid. The presence of signal peptides and/or C-terminal transmembrane alpha-helices in some of the identified sequences further suggested that they are secreted and tethered to the cell, although some proportion may subsequently be released into the extracellular environment due to proteolytic activity (16). Cell-wall-anchored proteins with sialidase activity in other species have been found to be involved in adhesion to mucosal surfaces and biofilm initiation, as well as carbohydrate assimilation (18–21).
Interestingly, in our examination of genome sequences of our collection of Gardnerella isolates, we noted that nanH3 contains a homopolymeric tract of cytosine residues. Genomic regions that contain short, homogenous or heterogenous repeats are susceptible to slipped-strand mispairing in which the length of the repeat region can change with each replication (22). The result of this modulation is phase variation, a reversible process in which the expression of the encoded protein can be rapidly switched on and off (23). Phase variation in cell surface proteins can result in immune evasion and alteration of biofilm phenotypes (24, 25).
Here, we determined the distribution of genes encoding sialidases NanH2 and NanH3 in the context of newly reclassified Gardnerella spp. and demonstrated that nanH3 is subject to slipped-strand mispairing.
RESULTS
Distribution of extracellular sialidases in Gardnerella spp. isolates.
Robinson et al. (16) had previously reported that sialidase activity was associated with the presence of either nanH2 or nanH3 in a collection of 34 Gardnerella isolates but did not report the species or subgroup affiliation. In order to confirm this observation and to reconcile the distribution of genes and activities with the new Gardnerella taxonomic framework (6), we queried an unrelated set of 36 isolates in our culture collection for which whole-genome sequence data and sialidase activity data were available (Table S1 in the supplemental material). The presence of the genes was assessed by aligning nanH2 of G. piotii JCP8151B (ATJH01000056) and nanH3 of Gardnerella genome sp. 3 strain W11 to the genome sequences using BLASTn. BLASTn results were dichotomous, with either one clear match per genome (E = 0.0, bitscore 1275 to 5049, percent identity 95 to 99%) or no significant hits reported. Sialidase activity was reported in 13/36 isolates (Table 1), including 12 G. piotii or genome sp. 3 (cpn60 subgroup B) isolates and one G. vaginalis isolate. The nanH3 gene was found in 12/13 sialidase-activity-positive isolates, while nanH2 was present in only 7/13 isolates. Six isolates possessed both nanH2 and nanH3 genes in their genomes. VN002 was sialidase activity positive but only nanH2 was identified in the genome sequence. This isolate subsequently screened PCR positive for nanH3 (see next section), suggesting that the gene was not included in the shotgun assembly of the genome that consisted of multiple gap-containing scaffolds. None of the G. swidsinskii, G. leopoldii, or subgroup D isolates were sialidase positive and none contained nanH2 or nanH3.
TABLE 1.
Distribution of nanH2 and nanH3 genes in Gardnerella spp. whole-genome sequences
| cpn60 subgroup | Isolate | Species | Sialidase activitya | nanH2 | nanH3 | |
|---|---|---|---|---|---|---|
| B | GH007 | G. piotii | + | + | + | |
| GH019 | G. piotii | + | + | + | ||
| GH020 | G. piotii | + | + | + | ||
| GH022 | G. piotii | + | + | + | ||
| N170 | Genome species 3 | + | − | + | ||
| NR026 | Genome species 3 | + | − | + | ||
| VN002 | G. piotii | + | + | −b | ||
| N144 | Genome species 3 | + | − | + | ||
| W11 | Genome species 3 | + | − | + | ||
| N101 | Genome species 3 | + | + | + | ||
| N153 | Genome species 3 | + | + | + | ||
| N95 | Genome species 3 | + | − | + | ||
| A | GH005 | G. leopoldii | − | − | − | |
| NR015 | Unknown | − | − | − | ||
| NR016 | G. swidsinskii | − | − | − | ||
| NR017 | G. leopoldii | − | − | − | ||
| NR019 | G. leopoldii | − | − | − | ||
| NR020 | G. swidsinskii | − | − | − | ||
| NR021 | G. swidsinskii | − | − | − | ||
| VN003 | G. leopoldii | − | − | − | ||
| WP021 | G. swidsinskii | − | − | − | ||
| WP022 | G. swidsinskii | − | − | − | ||
| NR010 | G. leopoldii | − | − | − | ||
| N072 | G. swidsinskii | − | − | − | ||
| C | GH021 | G. vaginalis | − | − | − | |
| NR001 | G. vaginalis | − | − | − | ||
| NR037 | G. vaginalis | − | − | − | ||
| NR038 | G. vaginalis | − | − | − | ||
| NR039 | G. vaginalis | − | − | − | ||
| WP023 | G. vaginalis | − | − | − | ||
| N165 | G. vaginalis | − | − | − | ||
| GH015 | G. vaginalis | + | − | + | ||
| D | NR003 | Genome sp. 8 | − | − | − | |
| NR047 | Unknown | − | − | − | ||
| WP012 | Genome sp. 9 | − | − | − | ||
| N160 | Genome sp. 10 | − | − | − |
Previously determined (13).
PCR-positive for nanH3.
Interestingly, the NanH3 of G. piotii strain JCP8151B investigated by Robinson et al. was reported to lack a signal peptide (16). When we examined the sequence upstream of nanH3 in the JCP8151B sequence (GenBank accession ATJH01000033), we found there is an alternative start codon, and also a cytosine homopolymer. The frameshift that resulted in the annotation of the gene without the signal peptide-encoding N terminus is caused by the homopolymer. This was also the case for isolates GH020, GH022, N170, and NR026, where the homopolymer created a frameshift that resulted in a protein annotation lacking the signal peptide encoded by sequence immediately upstream of the homopolymer. In N144, W11, N101, N153, and N95, the homopolymer length did not create a frameshift and the annotated NanH3 protein sequence includes a signal peptide. In GH007, GH019, and GH015, we were unable to determine if a signal-peptide-encoding sequence was present due to assembly gaps immediately upstream of the homopolymer in these genomes (Table S1).
Signal peptides were detected in 4/7 predicted NanH2 sequences (GH019, GH020, N101, and N153). In the other three cases where a nanH2 sequence was identified, we could not conclude if a signal peptide was included in the protein sequence provided in the GenBank annotation since assembly gaps in those regions of the genomes affected the annotation of these proteins.
Correlation of nanH3 with sialidase activity.
In order to examine further the relationship of nanH3 to sialidase activity, 112 Gardnerella spp. isolates for which sialidase activity data were available (13) were screened for the presence of nanH3 using PCR primers JH0684/JH0685 (Fig. 1). Sialidase activity was detected in 32/33 G. piotii/genome sp. 3 isolates and 3/35 G. vaginalis isolates. All sialidase-activity-positive isolates were positive for nanH3 by PCR with the exception of one G. piotii isolate, WP027, which was PCR negative. Unfortunately, genome sequence data were not available for WP027.
FIG 1.
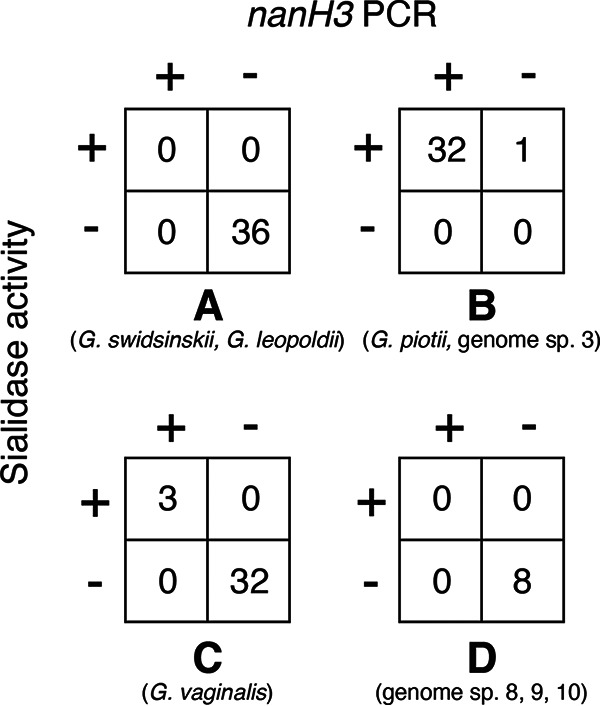
Correlation of nanH3 and sialidase activity in 112 Gardnerella isolates. Numbers of isolates positive or negative for sialidase activity and nanH3 by PCR are given in the boxes, with the cpn60 subgroup affiliation and species indicated below each crosstab. Sialidase results were determined by Schellenberg et al. (13).
Characterization of a poly(C) homopolymer in nanH3.
The protein sequences encoded by all nine nanH3 sequences without assembly gaps were predicted to encode a signal peptide (amino acids [aa] 1 to 32), a sialidase domain (amino acids 183 to 547 in strain W11), and a C-terminal transmembrane domain (amino acids 789 to 807 in W11) (Fig. 2A). We observed a polycytosine homopolymer (8 to 14 cytosines) in all nanH3 genes identified in the whole-genome sequences, approximately 100 bases from the start of the open reading frame. The homopolymer occurred immediately following the region of the sequence predicted to encode the signal peptide (amino acids 1 to 32) (Fig. 2B). When Sanger sequencing was performed on PCR products corresponding to nucleotides 2 to 310 of nanH3 amplified from genomic DNA extracted from broth cultures of strain W11, the results suggested variable lengths of the homopolymer. Specifically, clean data were obtained upstream of the poly(C) tract, but sequence 3′ to that region was indicative of a mixed template (Fig. S1). Since it has been demonstrated that simple repeats such as homopolymers can be subject to slipped strand mispairing, we set out to determine if the homopolymer region of nanH3 varied in length within and between strains of G. piotii/genome species 3.
FIG 2.
(A) Predicted locations of signal peptide, sialidase domain, and transmembrane domain of NanH3 from strain W11 based on InterProScan analysis. (B) Location of predicted signal peptide, cleavage site, and adjacent homopolymer in the nanH3 gene of strain W11. Analysis was conducted with SignalP-5.0.
PCR product libraries were made from two colonies each of isolates W11, VN014, VN015, and NR032 (8 clone libraries total). Plasmids were purified from 10 colonies from each of the 8 clone libraries and sequenced. High-quality sequence data were obtained from 15, 14, 18, and 16 clones from the W11, VN014, VN015, and NR032 libraries, respectively. Example results are shown in Fig. S2. The length of the homopolymer varied from 8 to 14 among all strains (Table 2), and within each strain the sequence flanking the homopolymer was identical. In silico translation of the encoded polypeptides showed that most homopolymer lengths resulted in a truncated peptide (37 to 44 amino acids), while full-length protein (812 aa) would result when there were 9 or 12 cytosines in the homopolymer region. Truncated peptides would include the signal peptide (amino acids 1 to 32) but no part of the predicted sialidase domain (amino acids 183 to 547) (Fig. 2). Interestingly, the most common length of the poly(C) region varied among strains. C10 was most common in VN014 and VN015, while C9 and C12 were most frequently observed in NR032 and W11, respectively. VN015 had the highest number of homopolymer variants (Fig. 3). A second open reading frame within the nanH3 open reading frame was also identified, corresponding to amino acids Met397 to Tyr812 of the NanH3 protein. This 415-aa polypeptide encompasses part of the predicted sialidase domain and the C-terminal transmembrane domain, but lacks a signal peptide.
TABLE 2.
Homopolymer length variants in Gardnerella piotii and genome sp. 3 isolates
| Isolate | Homopolymer sequence | Predicted peptide length (amino acids)a |
|---|---|---|
| W11 | CAACTA-C11-ATGAACAAA | 43 |
| CAACTA-C12-ATGAACAAA | 812 | |
| CAACTA-C13-ATGAACAAA | 38 | |
| CAACTA-C14-ATGAACAAA | 44 | |
| VN014 | CAACTA-C9-TCGAACAAA | 812 |
| CAACTA-C10-TCGAACAAA | 37 | |
| CAACTA-C11-TCGAACAAA | 43 | |
| VN015 | CAACTA-C9-TCGAACAAA | 812 |
| CAACTA-C10-TCGAACAAA | 37 | |
| CAACTA-C11-TCGAACAAA | 43 | |
| CAACTA-C12-TCGAACAAA | 812 | |
| CAACTA-C13-GAACAAA | 43 | |
| NR032 | CAACTA-C8-ATGAACAAA | 42 |
| CAACTA-C9-ATGAACAAA | 812 |
Full-length NanH3 is 812 amino acids.
FIG 3.

Frequencies of the homopolymer length variants in isolates W11, VN014, VN015, and NR032.
DISCUSSION
The genus Gardnerella is known as a hallmark of BV and the abundance of these species is used as a criterion for the laboratory diagnosis of the condition (1). In addition to participating in biofilms that coat epithelial cells in BV, Gardnerella spp. produce enzymes and a cholesterol-dependent cytolysin (vaginolysin) that contribute to degrading the protective barriers of the vaginal mucosa (26). Sialidase activity can provide nutrients by releasing sialic acid moieties from vaginal sialylated mucins (11), altering the physical properties of vaginal mucus. Removal of sialic acid residues from epithelial cell surface glycans can facilitate bacterial adhesion and initiation of biofilm formation (12, 18, 20, 21).
The two putative sialidase genes (nanH2 and nanH3) in Gardnerella spp. have been demonstrated to encode proteins with high enzymatic potency (16). Taken together, the results of the previous study and our current study show that nanH3 is more common and that nanH2 is virtually never found without nanH3. It is now clear that extracellular sialidase activity is a property of G. piotii and the closely related Gardnerella genome sp. 3. We found only three occurrences of sialidase-positive, nanH3-positive G. vaginalis isolates (3/35 isolates screened), and Vaneechoutte et al. (6) reported sialidase activity in 1/4 G. vaginalis isolates used in the amendment of the genus. This infrequent prevalence of nanH3 in G. vaginalis could be the result of lateral gene transfer from G. piotii, since these species coexist in the same microbiome and women are usually colonized by more than one species (7, 27, 28).
The nanH3 sequences we examined were predicted to encode a signal peptide, a sialidase domain, and a C-terminal membrane domain, suggesting a cell-wall-tethered protein, similar to SiaBb2 of Bifidobacterium bifidum, which enhances adhesion to intestinal mucosal surfaces and contributes to carbohydrate assimilation (21). The presence of the homopolymer immediately downstream of the signal peptide-encoding region presents a challenge to automated annotation of this sequence. Sequencing and assembly methods commonly used in bacterial whole-genome sequencing struggle with homopolymeric sequences, which can result in frame-shifts such as we observed in the nanH3 sequence of JCP8151B.
Genomic regions that contain homogenous or heterogenous repeats are prone to changes in length of the repeat at each replication due to slipped-strand mispairing (22). This can lead to consequent changes in the transcription or translation product of a gene, depending on whether the slipped strand mispairing occurs within the open reading frame or in extragenic regions such as promoter sequences. Phase variation results when slipped-strand mispairing creates “on” and “off” states of expression of a protein. Phase variation of cell surface proteins has been documented in many bacterial species, including Neisseria spp. (29–31), Salmonella spp. (32–34), Treponema pallidum (35, 36), and Helicobacter pylori (25). It was first described in the opa genes that encode opacity surface proteins of Neisseria spp. (37). The opa genes contain CTCTT pentamer repeats within the signal peptide-encoding region and the expression of the Opa protein is regulated by slipped-strand mispairing. With 6, 9, or 12 CTCTT repeats, the initiation codon is in frame with the remaining opa gene translating the Opa protein. However, 4 or 8 coding repeats makes the initiation codon out of frame with rest of the opa codons. The phase “on” state allows them to adhere to specific surface receptors on host cells (38).
Our results clearly show variation of the homopolymer length among G. piotii and Gardnerella genome sp. 3 within colonies growing on agar, with anywhere from 8 to 14 C residues observed. The effect of this change would be a mixture of cells with translation of NanH3 on or off. Why would G. piotii have a sialidase subject to phase variation? Two possible reasons are immune evasion and cell adhesion (biofilm initiation and dispersal).
Although BV is often referred to as a noninflammatory condition because of the lack of typical clinical signs of inflammation (leukocyte infiltration, pain, redness, or swelling), there is evidence of a host immune response to bacteria associated with BV, including Gardnerella spp. IgA specific for Gardnerella-produced vaginolysin, has been detected in women with clinical signs of BV (39), and IgA levels correlate with interleukin 8 (IL-8) expression in vaginal secretions (40). Gardnerella has also been shown to stimulate proinflammatory cytokines in vitro (41). Balancing this inflammatory response are the actions of bacterial enzymes like prolidases and sialidases that can degrade the effectors of inflammation. Whether there is a specific host response to NanH3 remains to be determined.
The initial step in biofilm formation is adhesion, which in the case of BV-associated biofilms is to epithelial cells (2). Streptococcus pneumoniae surface proteins with sialidase activity have been shown to reveal carbohydrate ligands for bacterial adhesion to host cells (18). Similarly, Soong et al. (19) showed that a cell-wall-associated sialidase of Pseudomonas aeruginosa plays a critical role in facilitating respiratory mucosa infection through biofilm formation. Specific antibodies against B. bifidum SiaBb2, a cell-wall-tethered sialidase, inhibit adhesion to cells (21). The involvement of Gardnerella sialidases in adhesion has not been demonstrated directly, although it has been reported that inhibition of sialidase activity in Gardnerella strain JCP8066 by an antiviral drug (zanamavir) resulted in reduction of adherence to vaginal epithelial cells in vitro (12). Gardnerella spp. can form multispecies biofilms in vitro (42) and, although “Gardnerella vaginalis” has been shown to participate in multispecies biofilms with other BV-associated bacteria in vivo (43, 44) and in vitro (45), it is not yet known how each of the Gardnerella spp. participate in this process. Interestingly, G. piotii and Gardnerella genome sp. 3 (cpn60 subgroup B) have been previously associated with “intermediate” grades of vaginal dysbiosis (7, 27, 46, 47), and may also contribute to transition from eubiosis to dysbiosis and enhance colonization by other BV-associated anaerobes. Phase variation in a cell surface-associated sialidase enzyme might be critical for turning on or off the initial adhesion of Gardnerella to epithelial cells and thus the cascade of events following that result in the biofilm-covered “clue cells” typical of BV (48).
It seems likely that some of the NanH3 protein produced by G. piotii does not remain tethered to the cell surface, as is the case with S. pneumoniae (49), since the sequence upstream of the C-terminal transmembrane domain may be susceptible to proteolytic cleavage, and heterologously expressed NanH3 lacking the transmembrane domain is active (16). Taken together, the activity of NanH3 while anchored to the cell surface or released into the extracellular environment, and the likelihood that it is subject to phase variation through slipped-strand mispairing, make this protein an intriguing puzzle for future study. Development of genetic tools to create mutants where NanH3 production is locked “on” or “off” would be a significant step toward understanding the role of this protein in the initiation and maintenance of vaginal dysbiosis.
MATERIALS AND METHODS
Protein domain identification and sequence alignment.
DNA and protein sequence alignments were performed with Clustal Omega (EMBL_EBI) and NCBI BLAST (basic local alignment search tool). InterproScan (https://www.ebi.ac.uk/interpro/) and SignalP were used to predict the location of functional domains and signal peptides (50).
Bacterial strains and culture conditions.
Gardnerella strains (n = 112) from a previously described culture collection were used in the study (13). Sialidase activity for all isolates had been determined previously (13) using a quantitative assay and fluorogenic substrate 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid sodium salt hydrate (11), and whole-genome sequences for 36 of these isolates were previously determined (51). Complete strain information and sequence accessions are provided in Table S1 in the supplemental material.
Gardnerella isolates were grown on Columbia sheep blood agar plates (BBL, Becton, Dickinson and Company, Sparks, MD, USA) at 37°C for 48 h with anaerobic BD GasPak EZ (Becton, Dickinson and Company, Sparks, MD, USA). For broth cultures, a few colonies from the plate were collected with a 10-μl inoculation loop and used to inoculate NYC III (ATCC 1685 medium; per liter: 2.4 g HEPES, 15 g proteose peptone, 3.8 g yeast extract, 5 g NaCl, 5 g glucose). Broth cultures were incubated at 37°C for 48 h in anaerobic conditions.
PCR screen for nanH3.
Genomic DNA was purified from broth cultures using a modified salting-out procedure (52). All DNA extracts were initially tested by PCR for the universal cpn60 barcode to confirm the quality of the DNA.
To screen isolates for the presence of nanH3, PCR primers were designed based on multiple sequence alignments of 15 nanH3 sequences obtained from the Integrated Microbial Genomes database (https://img.jgi.doe.gov/). Degenerate primers were designed to account for sequence variability within the gene sequence and to amplify a product of 375 bp in length (JH0684, 5′-GTT GTA GAR CTT TCT GAT GG-3′; JH0685, 5′-YRY TAT TAT CGC CCT CAT ATA-3′). PCR reaction mixtures contained 1× PCR buffer (0.2 M Tris-HCl [pH 8.4], 0.5 M KCl), 2.5 μM MgCl2, 0.40 μM dNTP, 0.20 μM forward primer, 0.20 μM reverse primer, 2 U Taq DNA polymerase, ultrapure water, and 2 μl of template DNA in a final volume of 50 μl. PCRs were conducted using the following thermocycling parameters in a Mastercycler Pro 6321 (Eppendorf AG, Hamburg, Germany): 94°C for 3 min; 40 cycles of 94°C for 30 s/55°C for 30 s/72°C for 30 s; 72°C for 1 min, hold at 20°C. PCR products were visualized under UV light on a 1.0% (wt/vol) agarose gel containing ethidium bromide.
Homopolymer PCR, cloning and sequencing.
To determine the length of the homopolymer region of nanH3, four strains (W11, VN014, VN015, and NR032) were cultured on Columbia agar plates with 5% (vol/vol) sheep blood. Primers JH0780 (5′-ATG ATT GGA ACA GCG CAT AAA G-3′) and JH0781 (5′-GAT TTC TCC ACC TAC AGT TAC C-3′) were designed to PCR amplify a region, including base pairs 2 to 310 of the open reading frame of nanH3.
The DNA sequence (308 bp) was amplified by PCR in a Mastercycler Pro 6321 (Eppendorf AG, Hamburg, Germany). The components of the PCR mix (50 μl per reaction) were added to achieve final concentrations of 1× high fidelity PCR buffer (60 mM Tris-SO4 [pH 8.9], 18 mM [NH4]2SO4), 0.2 mM dNTP mix, 2 mM MgSO4, and 1 U platinum high fidelity (Hi-Fi) proof reading Taq polymerase (Invitrogen, Carlsbad, CA, USA). Two colonies each of G. piotii or genome sp. 3 strain were randomly picked and added to separate PCR mixtures using sterile toothpicks. Thermocycling conditions included 35 cycles of denaturation at 94°C for 15 s, annealing at 55°C for 30 s, extension at 68°C for 1 min, and final extension at 68°C for 5 min. PCR products were visualized on a 1% (wt/vol) agarose gel. PCR products were purified using QiaQuick PCR purification kit (Qiagen, Hilden, Germany) and purified PCR products were sequenced using the amplification primers (JH0780 and JH0781).
To clarify the exact length of the homopolymer region, amplicons generated from the two colonies of each of the four strains were A-tailed in 10-μl reaction mixtures containing 1× platinum PCR buffer (20 mM Tris HCl [pH 8.4], 50 mM KCl), 2 mM MgCl2, 0.5 mM dATP, 5 U platinum Taq polymerase (Invitrogen, Carlsbad, CA, USA), and less than 500 ng of the purified PCR product. The reaction mixture was incubated at 72°C for 20 min in Mastercycler Pro 6321 (Eppendorf AG, Hamburg, Germany). End-modified PCR products were ligated into pGEM-T easy vector (Promega, Madison, WI, USA). The vector-insert construct was used to transform chemically competent DH5α cells or OneShot Top 10 Escherichia coli (Invitrogen, Carlsbad, CA, USA) and plated on LB/AMP/X-gal agar medium. Ten white colonies were randomly selected from the transformants of each Gardnerella strain and transferred into LB + ampicillin broth. Cultures were grown overnight at 37°C. Plasmid DNA was isolated using QiaPrep Spin miniprep kit (Qiagen, Hilden, Germany). Plasmids were sequenced using vector primers T7 and SP6.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by a Natural Sciences and Engineering Research Council of Canada Discovery Grant to J.E.H.
The authors are grateful to Champika Fernando for excellent technical assistance, and to all our colleagues in the Hill Lab for helpful discussions and feedback.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29:297–301. doi: 10.1128/JCM.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosca AS, Castro J, Sousa LGV, Cerca N. 2020. Gardnerella and vaginal health: the truth is out there. FEMS Microbiol Rev 44:73–105. doi: 10.1093/femsre/fuz027. [DOI] [PubMed] [Google Scholar]
- 3.van de Wijgert JH, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, Jespers V. 2014. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One 9:e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paramel Jayaprakash T, Schellenberg JJ, Hill JE. 2012. Resolution and characterization of distinct cpn60-based subgroups of Gardnerella vaginalis in the vaginal microbiota. PLoS One 7:e43009. doi: 10.1371/journal.pone.0043009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed A, Earl J, Retchless A, Hillier SL, Rabe LK, Cherpes TL, Powell E, Janto B, Eutsey R, Hiller NL, Boissy R, Dahlgren ME, Hall BG, Costerton JW, Post JC, Hu FZ, Ehrlich GD. 2012. Comparative genomic analyses of 17 clinical isolates of Gardnerella vaginalis provide evidence of multiple genetically isolated clades consistent with subspeciation into genovars. J Bacteriol 194:3922–3937. doi: 10.1128/JB.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaneechoutte M, Guschin A, Van Simaey L, Gansemans Y, Van Nieuwerburgh F, Cools P. 2019. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int J Syst Evol Microbiol 69:679–687. doi: 10.1099/ijsem.0.003200. [DOI] [PubMed] [Google Scholar]
- 7.Hill JE, Albert AYK. 2019. Resolution and cooccurrence patterns of Gardnerella leopoldii, Gardnerella swidsinskii, Gardnerella piotii and Gardnerella vaginalis within the vaginal microbiome. Infect Immun 87:e00532-19. doi: 10.1128/IAI.00532-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGregor JA, French JI, Jones W, Milligan K, McKinney PJ, Patterson E, Parker R. 1994. Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: results of a controlled trial of topical clindamycin cream. Am J Obstet Gynecol 170:1048–1059. doi: 10.1016/S0002-9378(94)70098-2. [DOI] [PubMed] [Google Scholar]
- 9.Myziuk L, Romanowski B, Johnson SC. 2003. BVBlue test for diagnosis of bacterial vaginosis. J Clin Microbiol 41:1925–1928. doi: 10.1128/JCM.41.5.1925-1928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briselden AM, Moncla BJ, Stevens CE, Hillier SL. 1992. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J Clin Microbiol 30:663–666. doi: 10.1128/JCM.30.3.663-666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL. 2013. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem 288:12067–12079. doi: 10.1074/jbc.M113.453654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govinden G, Parker JL, Naylor KL, Frey AM, Anumba DOC, Stafford GP. 2018. Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella vaginalis. Arch Microbiol 200:1129–1133. doi: 10.1007/s00203-018-1520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schellenberg JJ, Paramel Jayaprakash T, Withana Gamage N, Patterson MH, Vaneechoutte M, Hill JE. 2016. Gardnerella vaginalis subgroups defined by cpn60 sequencing and sialidase activity in isolates from Canada, Belgium and Kenya. PLoS One 11:e0146510. doi: 10.1371/journal.pone.0146510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santiago GL, Deschaght P, El Aila N, Kiama TN, Verstraelen H, Jefferson KK, Temmerman M, Vaneechoutte M. 2011. Gardnerella vaginalis comprises three distinct genotypes of which only two produce sialidase. Am J Obstet Gynecol 204:450.e1–e7. doi: 10.1016/j.ajog.2010.12.061. [DOI] [PubMed] [Google Scholar]
- 15.Corfield T. 1992. Bacterial sialidases—roles in pathogenicity and nutrition. Glycobiology 2:509–521. doi: 10.1093/glycob/2.6.509. [DOI] [PubMed] [Google Scholar]
- 16.Robinson LS, Schwebke J, Lewis WG, Lewis AL. 2019. Identification and characterization of NanH2 and NanH3, enzymes responsible for sialidase activity in the vaginal bacterium Gardnerella vaginalis. J Biol Chem 294:5230–5245. doi: 10.1074/jbc.RA118.006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter RF, Burnham C-AD, Dantas G. 2019. In silico analysis of Gardnerella genomospecies detected in the setting of bacterial vaginosis. Clin Chem 65:1375–1387. doi: 10.1373/clinchem.2019.305474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarre WW, Schneewind O. 1999. Surface proteins of Gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev 63:174–229. doi: 10.1128/MMBR.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soong G, Muir A, Gomez MI, Waks J, Reddy B, Planet P, Singh PK, Kaneko Y, Kanetko Y, Wolfgang MC, Hsiao Y-S, Tong L, Prince A. 2006. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J Clin Invest 116:2297–2305. doi: 10.1172/JCI27920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker D, Soong G, Planet P, Brower J, Ratner AJ, Prince A. 2009. The NanA neuraminidase of Streptococcus pneumoniae is involved in biofilm formation. Infect Immun 77:3722–3730. doi: 10.1128/IAI.00228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishiyama K, Yamamoto Y, Sugiyama M, Takaki T, Urashima T, Fukiya S, Yokota A, Okada N, Mukai T. 2017. Bifidobacterium bifidum extracellular sialidase enhances adhesion to the mucosal surface and supports carbohydrate assimilation. mBio 8:e00928-17. doi: 10.1128/mBio.00928-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Woude MW, Bäumler AJ. 2004. Phase and antigenic variation in bacteria. Clin Microbiol Rev 17:581–611. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips ZN, Tram G, Seib KL, Atack JM. 2019. Phase-variable bacterial loci: how bacteria gamble to maximise fitness in changing environments. Biochem Soc Trans 47:1131–1141. doi: 10.1042/BST20180633. [DOI] [PubMed] [Google Scholar]
- 24.Seib KL, Jen FE-C, Scott AL, Tan A, Jennings MP. 2017. Phase variation of DNA methyltransferases and the regulation of virulence and immune evasion in the pathogenic Neisseria. Pathog Dis 75. doi: 10.1093/femspd/ftx080. [DOI] [PubMed] [Google Scholar]
- 25.Bergman M, Del Prete G, van Kooyk Y, Appelmelk B. 2006. Helicobacter pylori phase variation, immune modulation and gastric autoimmunity. Nat Rev Microbiol 4:151–159. doi: 10.1038/nrmicro1344. [DOI] [PubMed] [Google Scholar]
- 26.Schellenberg JJ, Patterson MH, Hill JE. 2017. Gardnerella vaginalis diversity and ecology in relation to vaginal symptoms. Res Microbiol 168:837–844. doi: 10.1016/j.resmic.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Balashov SV, Mordechai E, Adelson ME, Gygax SE. 2014. Identification, quantification and subtyping of Gardnerella vaginalis in noncultured clinical vaginal samples by quantitative PCR. J Med Microbiol 63:162–175. doi: 10.1099/jmm.0.066407-0. [DOI] [PubMed] [Google Scholar]
- 28.Albert AY, Chaban B, Wagner EC, Schellenberg JJ, Links MG, van Schalkwyk J, Reid G, Hemmingsen SM, Hill JE, Money D, VOGUE Research Group. 2015. A study of the vaginal microbiome in healthy Canadian women utilizing cpn60-based molecular profiling reveals distinct Gardnerella subgroup community state types. PLoS One 10:e0135620. doi: 10.1371/journal.pone.0135620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayliss CD, Hoe JC, Makepeace K, Martin P, Hood DW, Moxon ER. 2008. Neisseria meningitidis escape from the bactericidal activity of a monoclonal antibody is mediated by phase variation of lgtG and enhanced by a mutator phenotype. Infect Immun 76:5038–5048. doi: 10.1128/IAI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apicella MA, Shero M, Jarvis GA, Griffiss JM, Mandrell RE, Schneider H. 1987. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect Immun 55:1755–1761. doi: 10.1128/IAI.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Putten JP. 1993. Phase variation of lipopolysaccharide directs interconversion of invasive and immuno-resistant phenotypes of Neisseria gonorrhoeae. EMBO J 12:4043–4051. doi: 10.1002/j.1460-2075.1993.tb06088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lederberg J, Iino T. 1956. Phase variation in Salmonella. Genetics 41:743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverman M, Zieg J, Hilmen M, Simon M. 1979. Phase variation in Salmonella: genetic analysis of a recombinational switch. Proc Natl Acad Sci U S A 76:391–395. doi: 10.1073/pnas.76.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonifield HR, Hughes KT. 2003. Flagellar phase variation in Salmonella enterica is mediated by a posttranscriptional control mechanism. J Bacteriol 185:3567–3574. doi: 10.1128/jb.185.12.3567-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, Gwinn M, Hickey EK, Clayton R, Ketchum KA, Sodergren E, Hardham JM, McLeod MP, Salzberg S, Peterson J, Khalak H, Richardson D, Howell JK, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton MD, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith HO, Venter JC. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 36.Pinto M, Borges V, Antelo M, Pinheiro M, Nunes A, Azevedo J, Borrego MJ, Mendonça J, Carpinteiro D, Vieira L, Gomes JP. 2017. Genome-scale analysis of the non-cultivable Treponema pallidum reveals extensive within-patient genetic variation. Nat Microbiol 2:16190. doi: 10.1038/nmicrobiol.2016.190. [DOI] [PubMed] [Google Scholar]
- 37.Stern A, Brown M, Nickel P, Meyer TF. 1986. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47:61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- 38.Stern A, Nickel P, Meyer TF, So M. 1984. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell 37:447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- 39.Cauci S, Scrimin F, Driussi S, Ceccone S, Monte R, Fant L, Quadrifoglio F. 1996. Specific immune response against Gardnerella vaginalis hemolysin in patients with bacterial vaginosis. Am J Obstet Gynecol 175:1601–1605. doi: 10.1016/s0002-9378(96)70112-6. [DOI] [PubMed] [Google Scholar]
- 40.Cauci S, Driussi S, Guaschino S, Isola M, Quadrifoglio F. 2002. Correlation of local interleukin-1beta levels with specific IgA response against Gardnerella vaginalis cytolysin in women with bacterial vaginosis. Am J Reprod Immunol 47:257–264. doi: 10.1034/j.1600-0897.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- 41.Eade CR, Diaz C, Wood MP, Anastos K, Patterson BK, Gupta P, Cole AL, Cole AM. 2012. Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PLoS One 7:e50106. doi: 10.1371/journal.pone.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan S, Voordouw MJ, Hill JE. 2019. Competition among Gardnerella subgroups from the human vaginal microbiome. Front Cell Infect Microbiol 9:374. doi: 10.3389/fcimb.2019.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machado A, Cerca N. 2015. Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis 212:1856–1861. doi: 10.1093/infdis/jiv338. [DOI] [PubMed] [Google Scholar]
- 44.Machado D, Castro J, Palmeira-de-Oliveira A, Martinez-de-Oliveira J, Cerca N. 2015. Bacterial vaginosis biofilms: challenges to current therapies and emerging solutions. Front Microbiol 6:1528. doi: 10.3389/fmicb.2015.01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro J, Machado D, Cerca N. 2019. Unveiling the role of Gardnerella vaginalis in polymicrobial Bacterial Vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. ISME J 13:1306–1317. doi: 10.1038/s41396-018-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hilbert DW, Schuyler JA, Adelson ME, Mordechai E, Sobel JD, Gygax SE. 2017. Gardnerella vaginalis population dynamics in bacterial vaginosis. Eur J Clin Microbiol Infect Dis 36:1269–1278. doi: 10.1007/s10096-017-2933-8. [DOI] [PubMed] [Google Scholar]
- 47.Shipitsyna E, Krysanova A, Khayrullina G, Shalepo K, Savicheva A, Guschin A, Unemo M. 2019. Quantitation of all four Gardnerella vaginalis clades detects abnormal vaginal microbiota characteristic of bacterial vaginosis more accurately than putative G. vaginalis sialidase A gene count. Mol Diagn Ther 23:139–147. doi: 10.1007/s40291-019-00382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simoes JA, Discacciati MG, Brolazo EM, Portugal PM, Dini DV, Dantas MC. 2006. Clinical diagnosis of bacterial vaginosis. Int J Gynaecol Obstet 94:28–32. doi: 10.1016/j.ijgo.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Lock RA, Paton JC, Hansman D. 1988. Purification and immunological characterization of neuraminidase produced by Streptococcus pneumoniae. Microb Pathog 4:33–43. doi: 10.1016/0882-4010(88)90046-0. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen H, Tsirigos KD, Brunak S, von Heijne G. 2019. A brief history of protein sorting prediction. Protein J 38:200–216. doi: 10.1007/s10930-019-09838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan S, Vancuren SJ, Hill JE. 2020. A generalist lifestyle allows rare Gardnerella spp. to persist at low levels in the vaginal microbiome. Microb Ecol doi: 10.1007/s00248-020-01643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin-Platero AM, Valdivia E, Maqueda M, Martinez-Bueno M. 2007. Fast, convenient, and economical method for isolating genomic DNA from lactic acid bacteria using a modification of the protein “salting-out” procedure. Anal Biochem 366:102–104. doi: 10.1016/j.ab.2007.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



