Abstract
Changes in composition of the intestinal microbiota are linked to the development of obesity and can lead to endothelial cell (EC) dysfunction. It is unknown whether EC can directly influence the microbiota. Insulin‐like growth factor‐1 (IGF‐1) and its receptor (IGF‐1R) are critical for coupling nutritional status and cellular growth; IGF‐1R is expressed in multiple cell types including EC. The role of ECIGF‐1R in the response to nutritional obesity is unexplored. To examine this, we use gene‐modified mice with EC‐specific overexpression of human IGF‐1R (hIGFREO) and their wild‐type littermates. After high‐fat feeding, hIGFREO weigh less, have reduced adiposity and have improved glucose tolerance. hIGFREO show an altered gene expression and altered microbial diversity in the gut, including a relative increase in the beneficial genus Akkermansia. The depletion of gut microbiota with broad‐spectrum antibiotics induces a loss of the favourable metabolic differences seen in hIGFREO mice. We show that IGF‐1R facilitates crosstalk between the EC and the gut wall; this crosstalk protects against diet‐induced obesity, as a result of an altered gut microbiota.
Keywords: endothelium, IGF‐1R, microbiota, obesity
Subject Categories: Metabolism; Microbiology, Virology & Host Pathogen Interaction; Signal Transduction
It remained unclear if gut endothelial cells can directly influence the microbiota. Here, endothelial specific over‐expression of IGF‐1R is shown to promote advantageous remodelling of the gut microbiota upon high fat diet, which protects against the development of obesity.
Introduction
In the intestine are trillions of microorganisms which are collectively described as the gut microbiota. The traditional dogma that the gut microbiota is pathogenic has evolved with an appreciation of its important role in the maintenance of human health (Lynch & Pedersen, 2016). Recent studies indicate that the gut microbiota is important in the metabolic response to changes in dietary composition (Backhed et al, 2004; Turnbaugh et al, 2006; Vrieze et al, 2012). Obesity secondary to excess calorie intake is a major risk factor for the development of a range of common disorders of human health including the following: type 2 diabetes (Guariguata et al, 2013), fatty liver (Yki‐Järvinen, 2014) and a number of cancers (Gallagher & Leroith, 2015). While our understanding of the mechanisms underlying the development and complications of obesity remains incomplete, a role for adverse remodelling of the gut microbiota has recently emerged as an important factor in the unfavourable effects of the disorder in a range of tissues and organs (Backhed et al, 2004; Turnbaugh et al, 2006; Khan et al, 2016; Patterson et al, 2016; Castaner et al, 2018) including the vascular endothelium (Koren et al, 2011; Karlsson et al, 2012; Catry et al, 2018; Leslie & Annex, 2018; Amedei & Morbidelli, 2019). The endothelium, previously thought to be an inert monolayer, has emerged as a complex paracrine/autocrine organ, important in the regulation of a range of homeostatic processes (Lee et al, 2007; Ding et al, 2010; Kivelä et al, 2019; Tang et al, 2020). It is currently unknown whether the endothelium can influence the composition of the intestinal microbiota.
The insulin‐like growth factors (IGF‐I and IGF‐II) are evolutionally conserved peptide hormones that couple nutrient intake to cellular growth (Jones & Clemmons, 1995). The effects of IGF‐I are predominantly mediated by the activation of its plasma membrane receptor—IGF‐1R (Adams et al, 2000). During calorie excess, the expression of IGF‐1R changes in a range of tissues, including the endothelium, where we have shown it to decline (Mughal et al, 2019). The IGF‐1R has also been shown to modulate the intestinal barrier (Dong et al, 2014), and conversely, the microbiome has been shown to modulate IGF‐1R signalling in muscle (Schieber et al, 2015) and bone formation (Yan et al, 2016). Therefore, to explore the effects of endothelial IGF‐1R on metabolic responses to obesity and the microbiome, we fed mice with endothelial cell overexpression of human IGF‐1R (hIGFREO) (Imrie et al, 2012) an obesogenic high‐fat high‐calorie diet. Feeding hIGFREO an obesogenic diet revealed a hitherto unrecognised mode of communication between the endothelium and the gut wall leading to favourable remodelling of the gut microbiota which protects against the development of diet‐induced obesity and its adverse metabolic sequelae.
Results and Discussion
Endothelial IGF‐1R overexpression prevents high‐fat diet‐associated weight gain
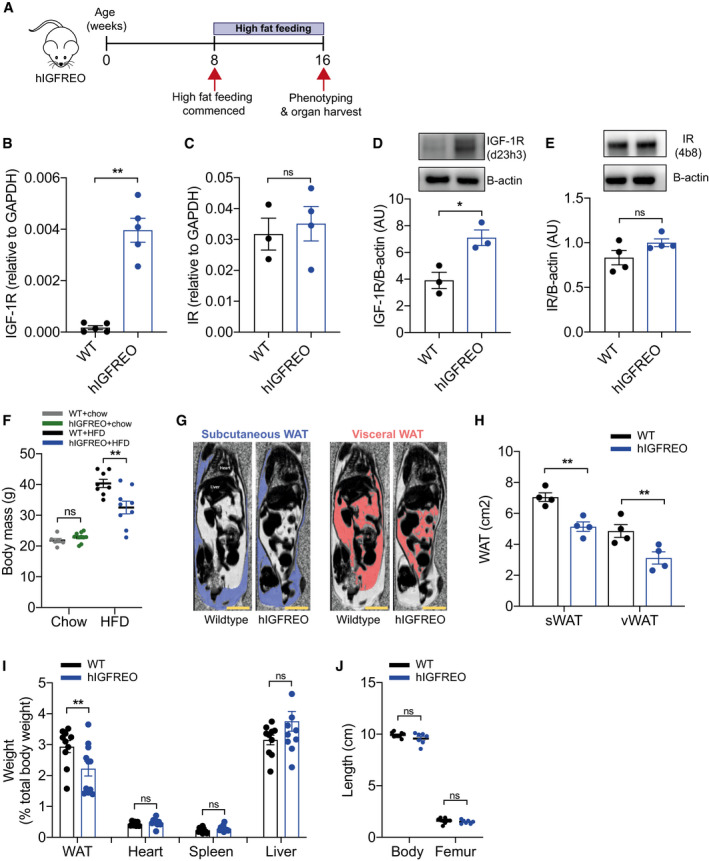
To explore the role of IGF‐1R in the endothelium under circumstances recapitulating diet‐induced obesity, we fed hIGFREO and wild‐type littermates (WT) a 60% high‐fat diet (HFD) for 8 weeks (Fig 1A). Endothelial overexpression of hIGF‐1R was confirmed using qPCR (Fig 1B); endothelial insulin receptor expression was similar in hIGFREO and WT (Fig 1C); this expression pattern was recapitulated at the protein level (Fig 1D and E). Protein markers of vascular function (eNOS and AKT) in the aorta were unchanged between the genotypes (Fig EV1A and B). On chow diet, hIGFREO had similar weight to WT, as we have previously reported (Imrie et al, 2012); however, on HFD, hIGFREO did not gain as much weight as WT mice (Fig 1F). MRI was used to assess whole‐body adiposity; hIGFREO had significantly less subcutaneous and visceral adipose tissue compared with WT on HFD (Fig 1G and H). Wet organ weight confirmed that hIGFREO had smaller white epididymal adipose depots than WT on HFD, with no difference in heart, spleen or liver weight (Fig 1I). The IGF‐1R is known to be an important regulator of foetal and postnatal growth (Woods et al, 1996; Garcia et al, 2014; Fujimoto et al, 2015; Juanes et al, 2015), and hIGFREO and WT mice had similar body and femur length (Fig 1J), demonstrating that endothelial IGF‐1R overexpression did not cause growth retardation.
Figure 1. Endothelial IGF‐1R overexpression prevents high‐fat diet (HFD)‐induced weight gain.
-
ASchematic representation of feeding time course.
-
B, CIn primary endothelial cells isolated from human IGF‐1 receptor endothelial overexpressing mice (hIGFREO) and wild‐type littermates (WT), quantitative polymerase chain reaction (qPCR) shows that hIGFREO have increased expression of human IGF‐1R but similar levels of murine insulin receptor (IR) gene expression as WT (n = 3–5 mice per group).
-
D, EIn primary endothelial cells isolated from WT and hIGFREO, immunoblotting shows that hIGFREO have increased expression of IGF‐1R but similar levels of IR protein expression (n = 3–4 mice per group).
-
FChow‐fed hIGFREO had similar body mass to WT; however, hIGFREO did not gain as much weight as WT after 8 weeks of HFD (n = 6–10 mice per group).
-
GRepresentative images of difference in fat and water distribution shown by magnetic resonance (MR) imaging in hIGFREO and WT. Scale bar = 1 cm.
-
HSubcutaneous white adipose tissue (sWAT) and visceral white adipose tissue (vWAT) volumes were reduced in hIGFREO (n = 4 per genotype).
-
IhIGFREO had reduced white epididymal adipose depot weight compared with WT; there was no difference in heart, spleen or liver weight (n = 7–11 mice per group).
-
JhIGFREO had similar whole‐body and femur length as WT (n = 7–9 mice per group).
Data information: Data shown as mean ± SEM, individual mice are shown as data points, P < 0.05 taken as being statistically significant using Student’s t‐test and denoted as * (** denotes P ≤ 0.01, ns denotes not significant).
Source data are available online for this figure.
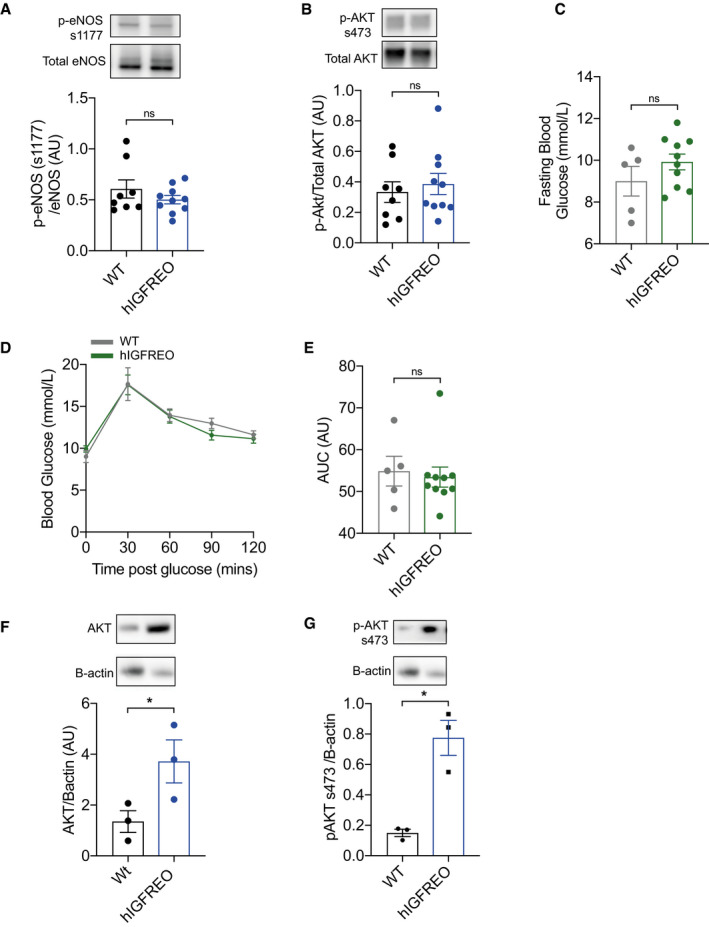
Figure EV1. Endothelial IGF‐1R overexpression has no effect on glucose tolerance in chow‐fed mice.
-
A, BIn aorta from human IGF‐1R endothelial overexpressing mice (hIGFREO) and wild‐type littermates (WT) after 8 weeks HFD, immunoblotting shows that hIGFREO have similar protein expression of phospho‐eNOS at Serine 1177 and phospho‐AKT at Serine 473 (n = 8–10 mice per group).
-
ChIGFREO had comparable glucose fasting blood glucose levels compared with WT on chow diet (n = 5–10 mice per group).
-
D, EhIGFREO had comparable glucose intolerance, compared to WT on chow diet (as measured by glucose tolerance test and area under the curve (AUC)) (5–10 mice per group).
-
F, GIn muscle from WT and hIGFREO after 8 weeks of HFD, immunoblotting shows that hIGFREO have increased protein expression of total and phospho‐AKT at Serine 473 (n = 3 per genotype).
Data information: Data shown as mean ± SEM and individual mice are shown as data points, P < 0.05 taken as being statistically significant using Student’s t‐test. ns denotes not significant.
Source data are available online for this figure.
Overexpression of endothelial IGF‐1R prevents obesity‐associated glucose intolerance
Chow‐fed hIGFREO had similar glucose tolerance as WT (Fig EV1, EV2, EV3, EV4, EV5). However, when challenged by a HFD, hIGFREO had significantly lower fasting blood glucose compared with WT (Fig 2A) and were also protected from the glucose intolerance seen in WT (Fig 2B and C). hIGFREO on HFD were also more insulin sensitive as shown using the homeostatic model assessment of insulin resistance (HOMA‐IR) analysis (Fig 2D), which was associated with an increase in the expression of AKT and phosphorylation of AKT at serine 437 in skeletal muscle of hIGFREO (Fig EV1F and G). hIGFREO and WT had similar fasting plasma concentrations of IGF‐I and insulin (Fig 2E and F). HFD‐fed hIGFREO handled olive oil gavage more effectively over a 3‐hr period postgavage with a significantly smaller increment in plasma triglycerides than WT (Fig 2G and H).
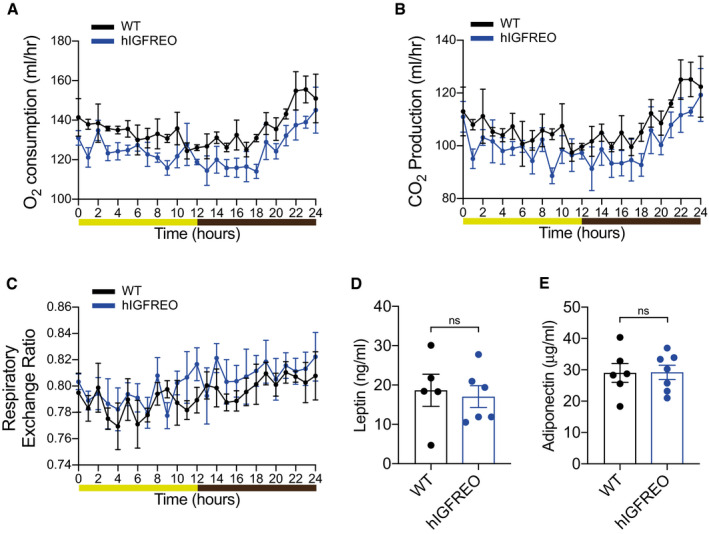
Figure EV2. Protection from high‐fat diet (HFD)‐induced weight gain is not due to altered energy expenditure in mice overexpressing human IGF‐1R in the endothelium (hIGFREO).
-
A–ChIGFREO exhibit no difference in oxygen consumption, CO2 production or respiratory exchange ratio using indirect calorimeter assessment after HFD when compared to WT after 8 weeks of HFD (n = 4 per genotype).
-
D, EhIGFREO had comparable concentrations of fasting plasma leptin and adiponectin to WT after 8 weeks of HFD (n = 5–7 mice per group).
Data information: Data shown as mean ± SEM and individual mice are shown as data points, P < 0.05 taken as being statistically significant using Student’s t‐test and denoted as * and ns denotes not significant. For indirect calorimetry, ANOVA testing was performed using mass as a co‐variant (ANCOVA testing) using calrapp.org.
Figure EV3. Adipose remodelling was no different in human IGF‐1 receptor endothelial overexpressing mice (hIGFREO) in the setting of high‐fat diet (HFD)‐induced obesity.
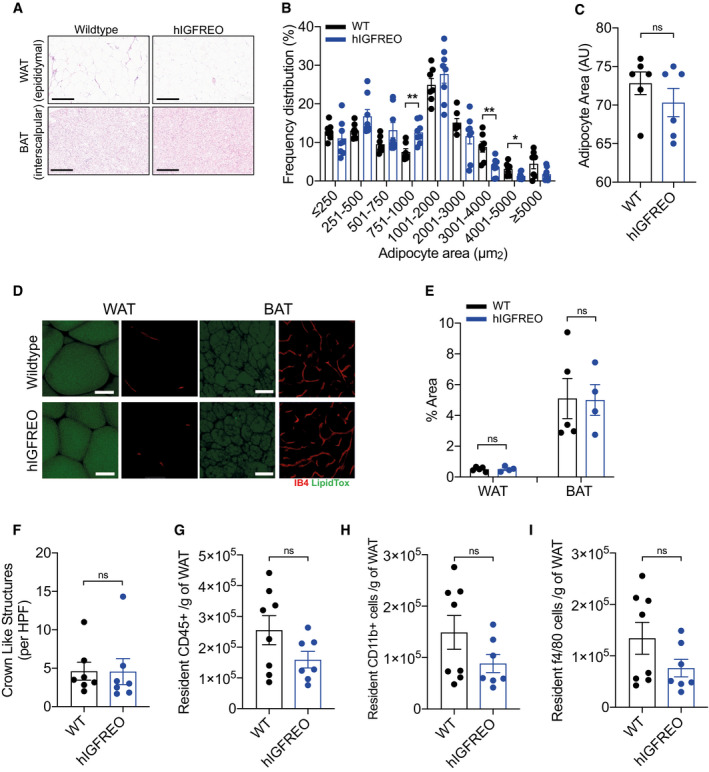
-
A–C(A), Histological examination of brown and white adipose tissue in hIGFREO compared with WT after 8 weeks of HFD. Scale bar = 300 µm. (B), hIGFREO mice have increased abundance of smaller adipocytes than WT. (C), There is no difference in average size of adipocytes in brown adipose tissue of hIGFREO compared with WT using haematoxylin and eosin stain (n = 6 per genotype).
-
DRepresentative confocal microscopy images of whole mount white and brown adipose tissue. Adipocytes stained with LipidTOX (green) and endothelial cells stained with Isolectin B4 (IB4‐647) (red). Scale bar = 50 µm (n = 4–5 mice per group).
-
EThere is no difference in vascular density in white or brown adipose tissue from hIGFREO compared with WT after 8 weeks of HFD (7 per genotype).
-
FMacrophage infiltration, shown by crown‐like structure (CLS) analysis, was similar in hIGFREO and WT after 8 weeks of HFD (n = 7–8 mice per group).
-
G–IThere is also no difference in resident adipose CD45+ cells, CD11b+ cells or F4/80 in hIGFREO compared with WT after 8 weeks of HFD (n = 7–8 mice per group).
Data information: Data shown as mean ± SEM and individual mice are shown as data points, P < 0.05 taken as statistically significant using Student’s t‐test and denoted as * and ** for P < 0.01. ns denotes not significant.
Figure EV4. There was no difference in hepatic steatosis in human IGF‐1 receptor endothelial overexpressing mice (hIGFREO) in the setting of high‐fat diet‐induced obesity.
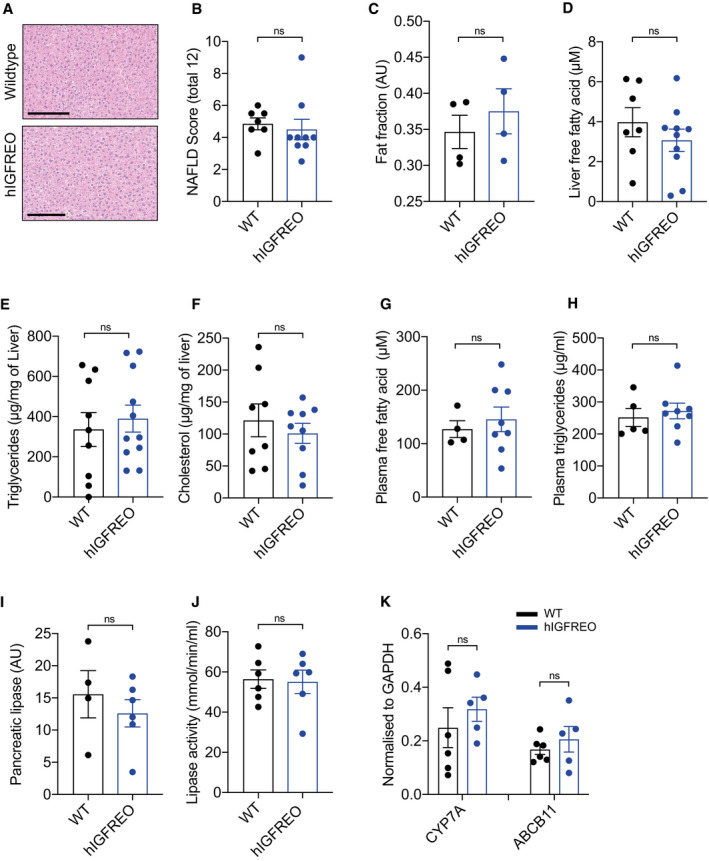
-
ARepresentative images of haematoxylin‐ and eosin‐stained liver from hIGFREO compared with wild‐type littermates (WT). Scale bar = 200 µm.
-
BNo difference in extent of hepatic fibrosis in hIGFREO compared with WT after 8 weeks of HFD (n = 7–9 mice per group).
-
CThis was confirmed using MR images showing that there was no difference in hepatic fat content when comparing hIGFREO to WT after 8 weeks of HFD (n = 4 per genotype).
-
D–FHepatic levels of free fatty acids, triglycerides and cholesterol are similar when comparing hIGFREO to WT after 8 weeks of HFD (n = 7–11 mice per group).
-
G, HFasted plasma concentration of free fatty acids and triglycerides is also similar in hIGFREO and WT (n = 4–8 mice per group).
-
I, J(I) Pancreatic lipase levels and lipase activity (J) were similar in hIGFREO and WT after 8 weeks of HFD (n = 4–6 mice per group).
-
KHepatic expression of CYP7A and ABCB11 is also similar in hIGFREO and WT after 8 weeks of HFD (n = 5–6 mice per group).
Data information: Data shown as mean ± SEM and individual mice are shown as data points, P < 0.05 taken as being statistically significant using Student’s t‐test. ns denotes not significant.
Figure EV5. Endothelial cells from human IGF‐1 receptor endothelial overexpressing mice (hIGFREO) can communicate with the gut wall.
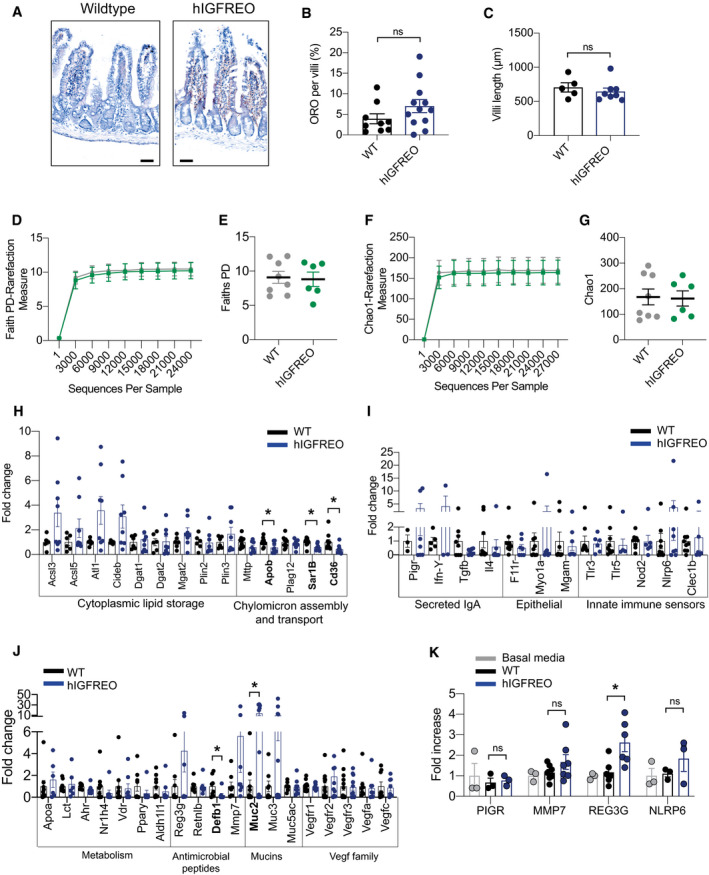
-
A–C(A), Representative images of haematoxylin‐ and eosin‐stained villi from hIGFREO compared with WT after 8 weeks of HFD. No difference in villi lipid content (B) or villi length (C) in hIGFREO compared with WT (n = 5–12 mice per group).
-
D, EFaith’s phylogenetic diversity (PD) was used to measure the faecal microbial diversity and demonstrates no difference between hIGFREO mice and WT on chow diet (n = 5‐8 mice per group).
-
F, GChao‐1 analysis was used to measure the faecal microbial diversity and abundance and again demonstrates no difference between hIGFREO and WT mice on chow diet (n = 5–8 mice per group).
-
H–JTargeted gene expression of the small intestine from hIGFREO normalised to WT after 8 weeks of high‐fat diet demonstrating a significant increment in Muc2 and decrement in Cd36, Sar1b, Apob and Defb1 (n = 6–8 mice per group).
-
KGene expression of Caco‐2 cells treated with conditioned media from hIGFREO endothelial cells showed a significant increase in Reg3g expression (n = 3–6 mice per group).
Data information: Data shown as mean ± SEM and individual mice are shown as data points, P < 0.05 taken as being statistically significant using Student’s t‐test and denoted as *. Ns denotes not significant. Diversity analyses were run on the resulting OTU/feature.biom tables to provide both phylogenetic and non‐phylogenetic metrics of alpha and beta diversity. Additional data analysis (PLS‐DA) and statistics were performed with R.
Figure 2. Endothelial IGF‐1R overexpression prevents high‐fat diet (HFD)‐induced glucose intolerance.
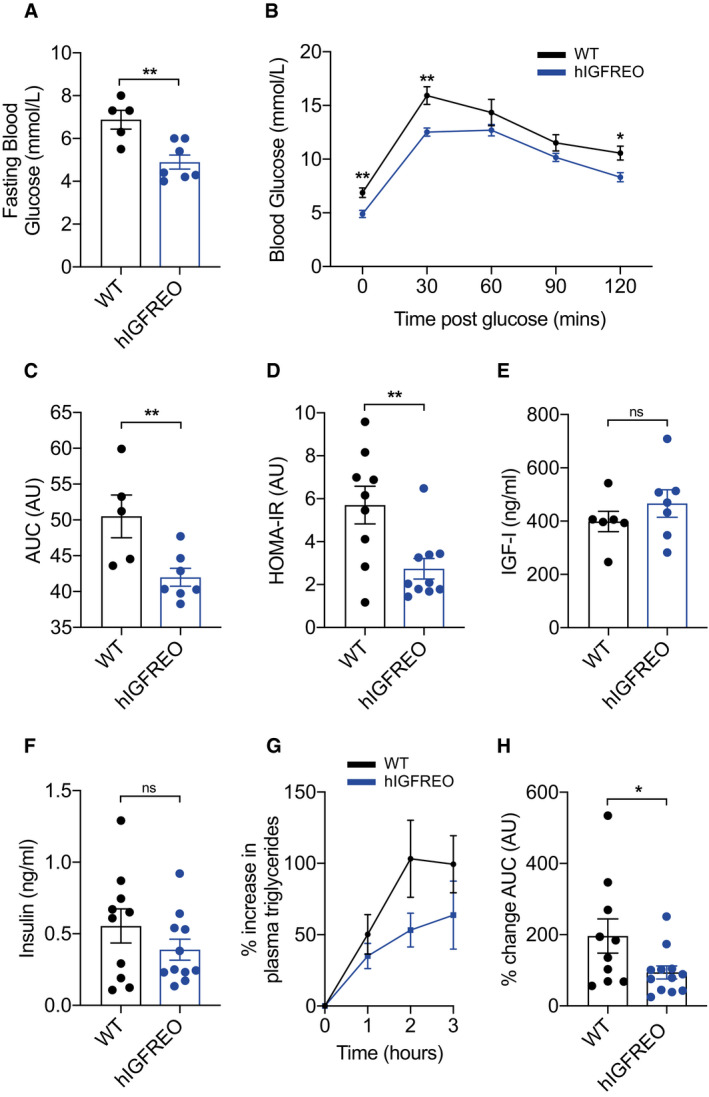
-
AHuman IGF‐1R endothelial overexpressing mice (hIGFREO) had significantly lower fasting blood glucose compared with wild‐type littermates (WT) after HFD (n = 5–7 mice per group).
-
B, ChIGFREO had reduced glucose intolerance compared with WT (as measured by glucose tolerance test and area under the curve (AUC)) (n = 5–7 mice per group).
-
DhIGFREO had improved insulin sensitivity compared with WT as shown by lower HOMA‐IR score (n = 9–10 mice per group).
-
E, FhIGFREO and WT had similar fasting plasma IGF‐1 and insulin concentrations (n = 6–12 mice per group).
-
G, HPercentage change in plasma levels of triglycerides after an olive oil oral gavage was reduced over the 3‐h period postgavage in hIGFREO compared with WT and shown as area under the curve (n = 10–12 mice per group).
Data information: Data shown as mean ± SEM and individual mice are shown as data points, P < 0.05 taken as being statistically significant using Student’s t‐test and denoted as * (** denotes P ≤ 0.01, ns denotes not significant).
Endothelial IGF‐1R overexpression does not lead to changes in activity, food intake or energy expenditure
To further probe the mechanisms underpinning the anti‐obesity and anti‐diabetic effect of endothelial IGF‐1R, metabolic cages were used to perform measurement of multiple metabolic parameters. hIGFREO on HFD showed no difference in activity levels (Fig 3A), food consumption (Fig 3B), oxygen consumption (Fig EV2A), carbon dioxide production (Fig EV2B), energy expenditure (Fig 3C) or respiratory exchange ratio (Fig EV2C) compared with WT on HFD. IGF‐1R are thought to contribute to temperature homeostasis and may contribute to regulation of energy homeostasis during calorie restriction (Cintron‐colon et al, 2017). Going against this possibility adipose tissue expression of browning markers (Fig 3D) and body temperature (Fig 3E) were all unchanged in hIGFREO compared to WT. Plasma leptin and adiponectin were also no different (Fig EV2D and E). There was also no difference in adipose tissue remodelling, shown by similar adipocyte size (Fig EV3, EV4, EV5), adipose tissue vascularity (Fig EV3D and E) and adipose tissue inflammatory markers, in hIGFREO and WT on HFD (Fig EV3, EV4, EV5). There was no difference in hepatic steatosis (Fig EV4, EV5), pancreatic lipase or gene expression of cholesterol 7alpha‐hydroxylase (Cyp7a) and ATP Binding Cassette Subfamily B Member 11(Abcb11) in liver when comparing hIGFREO to WT (Fig EV4, EV5K). There was no difference in small intestine length (Fig 3F), villi histology (Fig EV5A–C) or gut transit time (Fig 3G).
Figure 3. Protection from high‐fat diet (HFD)‐induced weight gain in human IGF‐1R endothelial overexpressing mice (hIGFREO) is not due to changes in activity, food intake, energy expenditure, adipose browning or gut transit time.
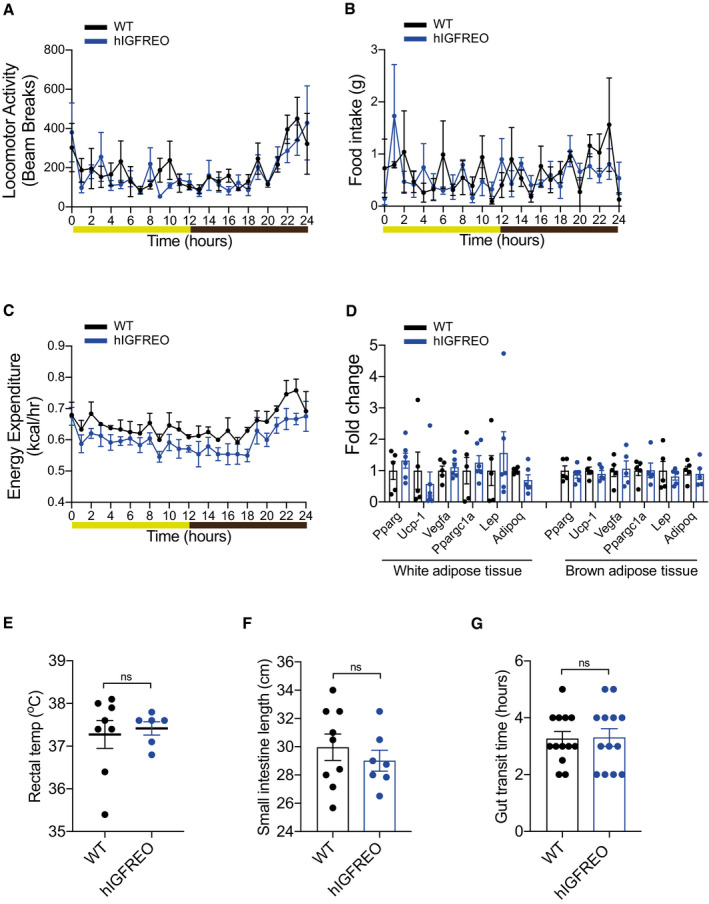
-
A–ChIGFREO exhibit no difference in activity levels, food consumption or energy expenditure using indirect calorimeter assessment after HFD compared with wild‐type littermates (WT) after HFD. (n = 4 per genotype).
-
DAdipose expression of browning markers is also no different in white epididymal adipose tissue and brown adipose tissue compared with WT (n = 6 per genotype).
-
ECore body temperature is no different in hIGFREO compared with WT (n = 6–8 mice per group).
-
F, GGut transit time is also unaltered in hIGFREO compared with WT as shown by no change in small intestine length (F) (n = 7–9 mice per group), or total gut transit time after a carmine red gavage (G) (n = 12–13 mice per group).
Data information: The light/dark cycle for graphs A–C is shown as follows: light in yellow and dark in brown. Data shown as mean ± SEM and individual mice are shown as data points. For indirect calorimetry, ANOVA testing was performed using mass as a co‐variant (ANCOVA testing) using calrapp.org. ns denotes not significant.
Endothelial IGF‐1R overexpression alters the gut microbiota and augments the abundance of the beneficial genus Akkermansia
We then asked whether IGF‐1R facilitated endothelial communication with the gut wall to influence the microbiota. Faith’s phylogenetic diversity (PD), a measure of faecal microbial diversity, was significantly different in hIGFREO compared with WT after HFD (Fig 4A and B). Chao‐1 analysis, a complementary measure of faecal microbial diversity and abundance, was also significantly different (Fig 4C and D). To further investigate these changes to the microbiota and assess the contribution of each genus to the difference between hIGFREO and WT, partial least squares discriminant analysis (PLS‐DA) modelling and the variable importance in projection (VIP) score were performed. This demonstrated that hIGFREO mice on HFD have increased abundance of Escherichia Shigella, Coriobacteriaceae UCG‐002, Faecalibaculum, Peptococcus, Akkermansia and Dehalobacerium. hIGFREO mice on a HFD are depleted in Enterococcus, Barnesiella, Helicobacter, Streptococcs, Tyzzerella, Lachnospiraceae NK4A136 and Bilophila, as well as several genera from the Ruminococcaceae family (Fig 4E and F).
Figure 4. Endothelial IGF‐1R overexpression alters the gut microbiota and augments the abundance of the beneficial genus Akkermansia .
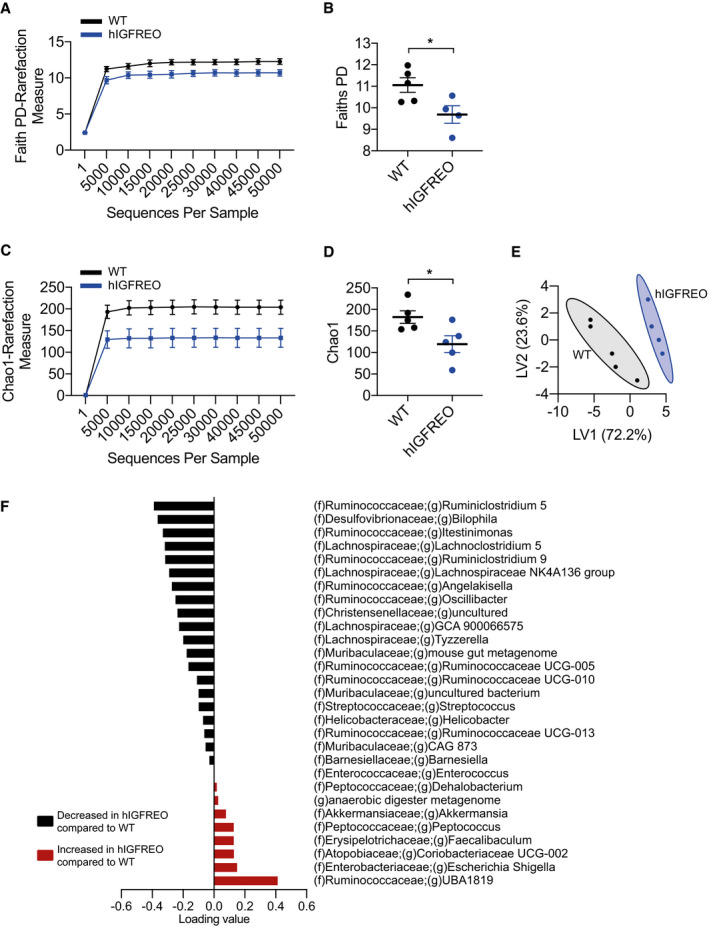
-
A, BFaith’s phylogenetic diversity (PD) was used to measure the faecal microbial diversity and demonstrates a significant difference between human IGF‐1R endothelial overexpressing mice (hIGFREO) mice and wild‐type littermates (WT) mice after high‐fat diet feeding (n = 4–5 mice per group).
-
C, DChao‐1 analysis was used to measure the faecal microbial diversity and abundance and demonstrates a significant difference between hIGFREO and WT (n = 4–5 mice per group).
-
E, FPartial least squares discriminant analysis (PLS‐DA) model and used the variable importance in projection (VIP) score was used to assess the contribution of each genus, shown as a scores plot in (E), and a loading plot of PLS‐DA of genus abundances in (F). VIP score cut‐off of 1 (n = 4–5 mice per group).
Data information: Data shown as mean ± SEM and individual mice are shown as data points. Diversity analyses were run on the resulting OTU/feature.biom tables to provide both phylogenetic and non‐phylogenetic metrics of alpha and beta diversity. Additional data analysis (PLS‐DA) and statistics were performed with R. P < 0.05 taken as being statistically significant using Student’s t‐test and denoted as *.
Of particular relevance to our findings was the increase in relative abundance of the genus Akkermansia (Derrien, 2004) seen in high‐fat‐fed hIGFREO. Akkermansia is thought to have anti‐obesity and anti‐diabetic effects in both humans and rodents (Everard et al, 2013; Cani & de Vos, 2017; Plovier et al, 2017; Depommier et al, 2019). Specifically, Akkermansia muciniphila reduces diet‐induced weight gain, fat mass development, fasting hyperglycaemia and improves glucose tolerance without affecting food intake in mice (Everard et al, 2013), the same phenotype observed in hIGFREO. Increased levels of Akkermansia muciniphila are also associated with better clinical outcomes, such as insulin sensitivity, after a calorie restricted diet in overweight/obese adults (Dao et al, 2016). More recently, a proof‐of‐concept clinical trial in obese humans demonstrated that supplementation with Akkermansia muciniphila was a safe, well‐tolerated intervention which improved several metabolic parameters (Depommier et al, 2019). However, it is also noteworthy that Bilophila was depleted in high‐fat‐fed hIGFREO; Bilophila has previously been shown to contribute to HFD‐induced metabolic dysfunction (Natividad et al, 2018). Dehalobacerium was enhanced in high‐fat‐fed hIGFREO mice and has previously been shown to be protective against atherosclerosis and reduced cholesterol (Chan et al, 2016). It is difficult to speculate further about the contribution of these other genera as little more is known about their role in obesity and metabolic disease; further studies would be of interest. Interestingly, when hIGFREO mice were unchallenged on a chow diet, there was no difference in microbial diversity compared with WT (Fig EV5D–G).
To dissect potential mechanisms underpinning the altered microbial diversity, we examined the expression of genes known to modulate the microbiota (Chang & Kao, 2019). We saw several changes in gene expression in the gut wall (Fig EV5H–J), raising the possibility that crosstalk between endothelial cells and the gut wall can influence gene expression. It is well established that endothelial cells can act in a paracrine/autocrine fashion (Lee et al, 2007; Ding et al, 2010; Kivelä et al, 2019) and equally well established that enterocytes respond to microbial metabolites (Nuenen et al, 2005; Garrett, 2020). To examine a role for secreted factors from endothelial cells in the altered gene expression seen in hIGFREO small intestine, we used primary endothelial cells from hIGFREO to condition culture media to treat Caco‐2 cells, as a model of the intestinal epithelial barrier. Caco‐2 cells treated with conditioned media from hIGFREO showed a significant increase in regenerating islet‐derived III‐γ (REG3G) compared with WT gene expression (Fig EV5K). REG3G belongs to the family of C‐type lectins and is one of several antimicrobial peptides produced by Paneth cells and enterocytes (Chang & Kao, 2019; Shin & Seeley, 2019). REG3G destroys gram‐positive bacteria by binding to the peptidoglycan layer, exerting bactericidal activity by oligomerising to form hexameric transmembrane pores (Shin & Seeley, 2019), thus providing one explanation as to why hIGFREO display reduced microbiota diversity and possibly providing an explanation as to why relative levels of Akkermansia, a gram‐negative bacteria, are enhanced. This raises the intriguing possibility that endothelial cell IGF‐1R could be a nutrient sensor responding to nutritional cues to influence the architecture of the intestinal microbiome (Bettedi & Foukas, 2017).
Antibiotic administration in the setting of obesity prevents the anti‐obesity and anti‐diabetic actions of endothelial IGF‐1R overexpression
To investigate the contribution of the altered microbiota to the anti‐obesity and anti‐diabetic effects of endothelial IGF‐1R overexpression, hIGFREO and WT were given broad‐spectrum antibiotics in their drinking water (Rodrigues et al, 2017) for the duration of HFD (Fig 5A). The addition of antibiotic treatment alongside HFD abolished the difference in weight gain seen between hIGFREO and WT (Fig 5Bi). However, WT on HFD and antibiotic treatment did not gain as much weight as WT on HFD alone. On chow diet hIGFREO and WT did not tolerate prolonged antibiotic treatment and for welfare reasons had to be culled, thus suggesting that the mice did not completely tolerate antibiotic treatment. Nevertheless, both WT and hIGFREO gained significantly more weight than mice on chow diet (Fig 5Bii). Antibiotic treatment also prevented the difference in glucose intolerance, seen between the genotypes when on HFD alone (Fig 5C–E). Wet organ weights were comparable between hIGFREO and WT (Fig 5F). Chao‐1 analysis was no different between hIGFREO and WT after HFD and antibiotic treatment (Fig 5G). Alpha diversity in hIGFREO and WT on HFD treated with antibiotics was also similar demonstrating no difference in microbial diversity using a range of approaches (Fig 5H and Table EV1). Taken together, these data confirm a causal role for the microbiota in the favourable changes seen in hIGFREO.
Figure 5. Antibiotic administration in the setting of high‐fat diet (HFD) eliminates the anti‐obesity and anti‐diabetic actions of endothelial IGF‐1R overexpression.
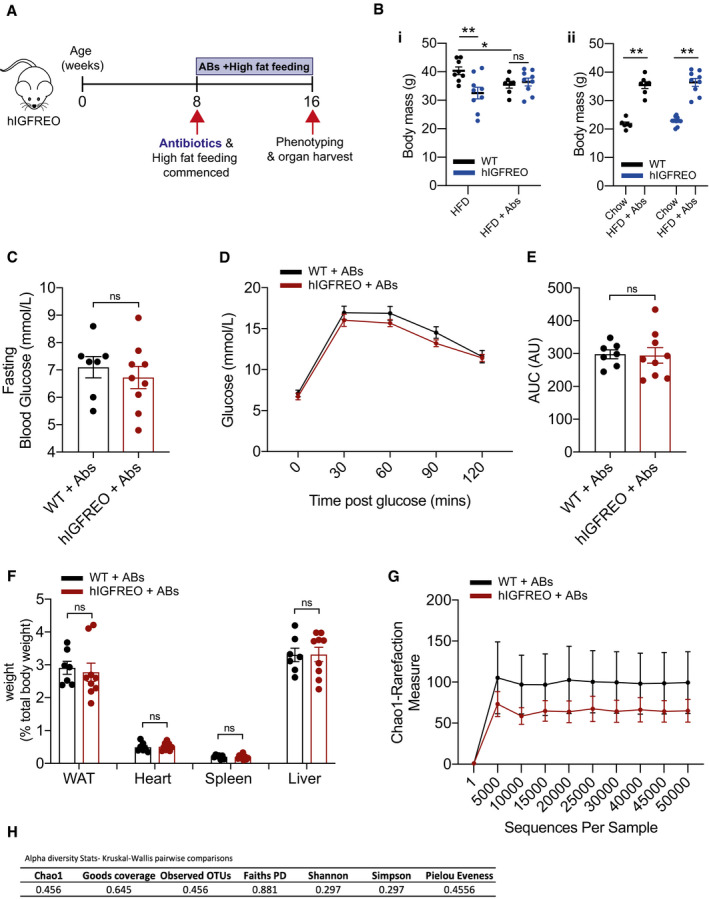
-
ASchematic representation of antibiotic dosing and feeding time course.
-
B(Bi), Human IGF‐1R endothelial overexpressing mice (hIGFREO) had comparable weight gain as wild‐type littermates (WT) after 8 weeks of HFD + antibiotics (ABs) when compared to WT. (Bii), Both hIGFREO and WT gained significant weight compared with chow‐fed mice (n = 7–9 mice per group).
-
CThere was no difference in fasting blood glucose in hIGFREO compared with WT (n = 7–9 mice per group).
-
D, EThere was no difference in hIGFREO and WT glucose tolerance (as measured by glucose tolerance test and area under the curve (AUC)) (n = 7–9 mice per group).
-
FWet organ weights were similar in hIGFREO and WT (n = 7–9 mice per group).
-
GChao‐1 analysis was used to measure the faecal microbial diversity and abundance and demonstrates no difference between hIGFREO and WT after HFD + antibiotic treatment (n = 3–5 mice per group).
-
HAlpha diversity P values using Kruskal–Wallis pairwise comparisons show there is no difference in microbial diversity.
Data information: Data shown as mean ± SEM and individual mice are shown as data points, P < 0.05 taken as being statistically significant using Student’s t‐test and denoted as * or ** for P P < 0.01 and NS denotes not significant. Diversity analyses were run on the resulting OTU/feature.biom tables to provide both phylogenetic and non‐phylogenetic metrics of alpha and beta diversity. Additional data analysis (PLS‐DA) and statistics were performed with R.
Conclusion
To our knowledge, this is the first report to demonstrate communication between the endothelium and the gut wall, which in turn can modulate the gut microbiota. We report a novel role for endothelial cell IGF‐1R in this crosstalk, which protects against diet‐induced obesity and its associated adverse metabolic sequelae, by potentially remodelling the architecture of the microbiota.
Materials and Methods
Animal husbandry
hIGFREO mice with endothelial cell‐specific overexpression of the IGF‐1 receptor (previously described Imrie et al, 2012) and their wild‐type control littermates (WT) were bred in house. Experiments were carried out under the authority of UK Home Office project licence P144DD0D6. Mice were group housed in cages of up to five, which contained a mix of genotypes. Researchers were blinded to genotype until the data analysis stage. Cages were maintained in humidity and temperature‐controlled conditions (humidity 55% at 22°C) with a 12‐h light–dark cycle. All interventions were performed within the light cycle. Only male mice were used for experimental procedures to prevent variability associated with the oestrous cycle on adiposity and metabolic readouts (Stubbins et al, 2012; Griffin et al, 2016). Genotyping was carried out by Transnetyx commercial genotyping using ear biopsies.
To induce obesity, mice received high‐fat diet (HFD) ad libitum from 8 weeks of age for a further 8 weeks (60% of energy from fat) (F1850, Bioserve) with the following composition: protein 20.5, fat 36% and carbohydrate 36.2% (5.51 kcal/g).
Antibiotics were administered in a cocktail with the following concentrations: ampicillin (1/gl), metronidazole (1/gl), neomycin trisulfate (1/gl) and vancomycin (0.5/gl) in drinking water (Rodrigues et al, 2017), from the age of 8 weeks old for the duration of high‐fat feeding (further 8 weeks).
Metabolic phenotyping
Mice were fasted overnight prior to glucose tolerance or for 2hr prior to insulin tolerance tests. Blood glucose was measured using a handheld Glucose Meter (Accu‐Chek Aviva). An intra‐peritoneal injection of glucose (1 mg/g) or recombinant human insulin (Actrapid; Novo Nordisk) (0.75 IU/kg) was given and glucose concentration measured at 30‐min intervals for 2 h from the point of glucose/insulin administration. Mice were not restrained between measurements (Haywood et al, 2017).
Fasting plasma samples were collected from the lateral saphenous vein (EDTA collection tubes Sarstedt 16.444). Samples were then spun at 12,300 g for 10 min in a bench top centrifuge. Fasting plasma insulin (90080, Crystal Chem), IGF‐I (MG100, R and D systems), leptin (EZML‐82K, Merck Millipore) and adiponectin (EZMADP‐60K, Merck Millipore) were measured as per manufacturer’s instructions.
Core body temperature was measured using an Indus rectal temperature probe (Vevo2100 (VisualSonics, FujiFilm).
After 8 weeks of HFD, metabolic parameters were measured by indirect calorimetry using Comprehensive Lab Animal Monitoring Systems (CLAMS) (Columbus Instruments). In brief, mice were individually housed for 5 days and measurement of oxygen consumption, carbon dioxide production, food intake and locomotor activity were continuously recorded. For each mouse, a full 24‐h period, taking into account sleep and wake cycles, was analysed after an acclimatisation period (Roberts et al, 2014).
After 8 weeks of HFD (or at 8 weeks old for chow control mice), all mice were sacrificed using terminal anaesthesia and organ weights measured using a standard laboratory balance.
Lipid absorption
Mice were fasted overnight and blood samples collected from the lateral saphenous vein (EDTA collection tubes Sarstedt 16.444). Mice underwent oral gavage with 200 µl olive oil, and blood was taken from the saphenous vein every hour for a further 3 h (Zhang et al, 2018). Plasma triglycerides were measured using a commercially available kit (ab65336, Abcam).
Intestinal transit time
Mice were fasted overnight before oral gavage with 300 µl of Carmine solution (6% Carmine red (C1022, Sigma) in 0.5% methyl cellulose (M7140, Sigma‐Aldrich)). Mice were then individually caged and monitored until the appearance of the first red faecal pellet (Li et al, 2011).
Magnetic resonance imaging (MRI)
Anaesthesia was induced using 5% isoflurane in 100% oxygen and then maintained using 1.5–3% isoflurane at 2 l/min oxygen flow. Animals were positioned prone on a dedicated mouse cradle. Body temperature was maintained with a custom resistive blanket placed on the back of the animal. Cardiac and respiratory signals were continuously monitored (BIOPAC Systems, Inc., Goleta, USA). Mice were imaged on a 7T preclinical MRI scanner with a 660 mT/m shielded gradient system and a quadrature‐driven transmit/receive volume coil with inner diameter of 72 mm (Bruker BioSpin MRI GmbH, Ettlingen, Germany). A 2D cardiac‐triggered and respiratory‐gated 3‐point Dixon spoiled gradient‐echo sequence was used: TR = 5.65 ms, TE = 2.42/2.75/3.09 ms, Matrix = 256 × 128, field‐of‐view = 80 × 30 mm, number of slices = 28 in sagittal orientation, slice thickness = 1 mm, number of signal averages = 8, total scan time ~30 min. The data were analysed in MATLAB (MathWorks, Natick, USA) using the hierarchical iterative decomposition of water and fat with echo asymmetry and least squares estimation (IDEAL) method(Tsao & Jiang, 2013). The proton density fat fraction (PDFF, the amount of lipid signal over total signal) was used to segment adipose tissue depots. Subcutaneous and visceral adipose depots were segmented separately using Osirix Lite v11.0.2 (Bernex, Switzerland) 2D threshold region growing algorithm tool with segmentation parameters set to a lower threshold of 80% PDFF.
Gene expression
RNA was isolated from cells and tissue samples using the monarch total RNA mini kit (NEB, T2010S). The concentration of RNA in each sample (ng/µl) was measured using a NanoDrop. cDNA was reverse transcribed (NEB, E3010S). Quantitative PCR (qPCR) was performed using a Roche LightCycler 480 Instrument II, using SYBR Green PCR Master Mix (Bio‐Rad, 1725270) and relevant primers (See Table 1). The “cycles to threshold” (cT) was measured for each well, the average of triplicate readings for each sample taken, normalised to GAPDH, and finally, the differential expression of each gene was calculated for each sample.
Table 1.
Primer details for qPCR.
| Gene | Assay ID | |
|---|---|---|
| IGF1R | Insulin‐like growth factor‐1 receptor | qHsaCED0044963 |
| PIGR | Polymeric immunoglobulin receptor | qHsaCID0021506 |
| MMP7 | Matrix metallopeptidase 7 | qHsaCED0044775 |
| REG3G | Regenerating family member 3 gamma | qHsaCED0004912 |
| NLRP6 | NLR family pyrin domain containing 6 | qHsaCED0004389 |
| GAPDH | Glyceraldehyde‐3‐phosphate dehydrogenase | qHsaCED0038674 |
| Insr | Insulin receptor | qMmuCID0018034 |
| Ucp1 | Uncoupling protein 1 | qMmuCID0005832 |
| Pparg | Peroxisome proliferator‐activated receptor gamma | qMmuCID0018821 |
| Ppargc1a | Peroxisome proliferative‐activated receptor, gamma, coactivator 1 alpha | qMmuCID0006032 |
| Vegfa | Vascular endothelial growth factor A | qMmuCED0040260 |
| Adipoq | Adiponectin | qMmuCID0023242 |
| Lep | Leptin | qMmuCID0040177 |
| Cyp7a1 | Cytochrome P450, family 7, subfamily a, polypeptide 1 | qMmuCED0046994 |
| Abcb11 | ATP‐binding cassette, subfamily B (MDR/TAP), member 11 | qMmuCID0015514 |
| Mttp | Microsomal triglyceride transfer protein | qMmuCED0047210 |
| Apob | Apolipoprotein B | qMmuCED0044141 |
| Plagl2 | Pleiomorphic adenoma gene‐like 2 | qMmuCED0037791 |
| Sar1b | Secretion‐associated Ras‐related GTPase 1B | qMmuCED0044630 |
| Acsl3 | Acyl‐CoA synthetase long‐chain family member 3 | qMmuCED0046845 |
| Acsl5 | Acyl‐CoA synthetase long‐chain family member 5 | qMmuCED0045475 |
| Atl1 | Atlastin GTPase 1 | qMmuCED0044156 |
| Cideb | Cell death‐inducing DFFA‐like effector b | qMmuCED0046272 |
| Dgat1 | Diacylglycerol O‐acyltransferase 1 | qMmuCID0021210 |
| Dgat2 | Diacylglycerol O‐acyltransferase 2 | qMmuCID0012338 |
| Mgat2 | Mannoside acetylglucosaminyltransferase 2 | qMmuCED0049876 |
| Plin2 | Perilipin 2 | qMmuCID0016776 |
| Plin3 | Perilipin 3 | qMmuCID0005622 |
| Aqp7 | Aquaporin 7 | qMmuCID0025269 |
| Tlr3 | Toll‐like receptor 3 | qMmuCID0005723 |
| Tlr5 | Toll‐like receptor 5 | qMmuCID0005789 |
| Nod2 | Nucleotide‐binding oligomerisation domain containing 2 | qMmuCED0049905 |
| Nlrp6 | NLR family pyrin domain containing 6 | qMmuCED0048619 |
| Clec1b | C‐type lectin domain family 1, member b | qMmuCED0047632 |
| Lct | Lactase | qMmuCED0045800 |
| Ahr | Aryl‐hydrocarbon receptor | qMmuCED0044800 |
| Nr1h4 | Nuclear receptor subfamily 1, group H, member 4 | qMmuCID0014006 |
| Vdr | Vitamin D receptor | qMmuCID0006555 |
| Aldh1l1 | qMmuCID0021991 | qMmuCID0021991 |
| Reg3g | Regenerating family member 3 gamma | qMmuCED0040314 |
| Retnlb | Resistin‐like beta | qMmuCED0001569 |
| Defb1 | Defensin beta 1 | qMmuCID0008786 |
| Mmp7 | Matrix metallopeptidase 7 | qMmuCID0022398 |
| F11r | F11 receptor | qMmuCID0006275 |
| Myo1a | Myosin IA | qMmuCID0022137 |
| Mgam | Maltase‐glucoamylase | qMmuCID0022182 |
| Pigr | Polymeric immunoglobulin receptor | qMmuCID0009049 |
| Ifng | Interferon gamma | qMmuCID0006268 |
| Il4 | Interleukin 4 | qMmuCID0006552 |
| Muc2 | Mucin 2 | qMmuCID0019583 |
| Muc3 | Mucin 3 | qMmuCID0023019 |
| Flt1 | FMS‐like tyrosine kinase 1 | qMmuCID0016762 |
| Kdr | Kinase insert domain protein receptor | qMmuCID0005890 |
| Flt4 | FMS‐like tyrosine kinase 4 | qMmuCID0021117 |
| Vegfc | Vascular endothelial growth factor C | qMmuCID0017182 |
| Cd36 | CD36 antigen | qMmuCID0014852 |
| Gapdh | Glyceraldehyde‐3‐phosphate dehydrogenase | qMmuCED0027497 |
Quantification of protein expression
Cells were lysed or tissue mechanically homogenised in lysis buffer (Extraction buffer, FNN0011) and protein content quantified using a BCA assay (Sigma‐Aldrich, St. Louis, MO). Twenty micrograms of protein was resolved on a 4–12% Bis‐Tris gel (Bio‐Rad, Hertfordshire, UK) and transferred to nitrocellulose membranes. Membranes were probed with antibodies diluted in 5% BSA as per Table 2, before incubation with appropriate secondary horseradish peroxidase‐conjugated antibody. Blots were visualised with Immobilon Western Chemiluminescence HRP Substrate (Merck Millipore, Hertfordshire, UK) and imaged with Syngene chemiluminescence imaging system (SynGene, Cambridge, UK). Densitometry was performed in ImageJ (Haywood et al, 2017).
Table 2.
Antibody details for protein expression.
| Protein | Supplier | Code | Secondary antibody |
|---|---|---|---|
| IGF1R (D23H3) | Cell Signaling | #9750 | Anti Rabbit |
| IR (4B8) | Cell Signaling | #3025 | Anti Rabbit |
| AKT | Cell Signaling | #9272 | Anti Rabbit |
| p‐AKT Ser473 | Cell Signaling | #9271 | Anti Rabbit |
| eNOS | Cell Signaling | #9572 | Anti Rabbit |
| p‐eNOS Ser 1177 | Cell Signaling | #9570 | Anti Rabbit |
Primary endothelial cell isolation
Primary endothelial cells (PECs) were isolated from lungs, as previously reported (Abbas et al, 2011; Watt et al, 2017). Briefly, lungs were harvested, washed, finely minced and digested in Hanks’ balanced salt solution containing 0.18 units/ml collagenase (10 mg/ml; Roche) for 45 min at 37°C. The digested tissue was filtered through a 70‐μm cell strainer and centrifuged at 400 g for 10 min. The cell pellet was washed with PBS/0.5%BSA, centrifuged, re‐suspended in 1 ml PBS/0.5% and incubated with 1 × 106 CD146 antibody‐coated beads (Miltenyi Biotec, 130‐092‐007) at 4°C for 30 min. Bead‐bound endothelial cells were separated from non‐bead‐bound cells using a magnet.
Microbiome analysis
Microbiome analysis was performed by UC Davis MMPC. Briefly, frozen faecal samples were shipped on dry ice to UC Davis MMPC and Host Microbe Systems Biology Core. Total DNA was extracted using Mo‐Bio (now Qiagen) Power Fecal Kit. Sample libraries were prepared and analysed by barcoded amplicon sequencing(Anderson, 2001; Price et al, 2010; Lozupone et al, 2011; Quast et al, 2012; Katoh & Standley, 2013; Mandal et al, 2015; Callahan et al, 2016; Bolyen et al, 2019).
Quantification of white and brown adipose tissue vascularity
White adipose tissue (WAT) and brown adipose tissue (BAT) (< 0.5 g) were harvested into cold 1% paraformaldehyde (PFA) and allowed to fix for 2hrs at room temperature. Samples were incubated overnight with Isolectin B4 Alexa Fluor 647 (I32450, Thermo Fisher Scientific) and diluted 1:100 in 5% BSA in phosphate‐buffered saline (PBS) at 4°C. After washing with PBS, they were incubated with HCS LipidTOX (H34475, Thermo Fisher Scientific) diluted 1:200 in PBS for 20mins at room temperature. Whole tissue was then mounted onto slides beneath coverslips using a silicone spacer (Grace bio‐labs, 664113), with Prolong Gold (P36930, Thermo Fisher Scientific). Slides were then imaged using laser scanning confocal microscopy (LSM880, Zeiss), with 8 areas of each sample imaged. Vascular density (the proportion of each image stained with IB4) was measured using thresholding in ImageJ.
Histological assessment of adipocyte size, non‐alcoholic fatty liver disease and villi structure
Samples for histology were fixed in 4% PFA for at least 24 h and then processed into paraffin blocks. 5‐µm sections were taken and collected onto 3‐triethoxysilylpropylamine (TESPA) coated slides. After drying, slides were stained with haematoxylin and eosin to assess gross morphology ± oil red o (ORO) for lipid staining. Slides were imaged using an Olympus BX41 microscope at 10× and 20× magnification.
For assessment of adipocyte size, three separate fields of view for each sample were assessed. For each one, the average of 20 randomly selected independent cells measured using ImageJ.
For assessment of non‐alcoholic fatty liver disease (NAFLD) in sections of murine liver, a validated rodent NAFLD scoring system was used (Liang et al, 2014), which takes into account micro‐ and macro‐steatosis, inflammation and hypertrophy. Each sample was assessed by at least two blinded independent verifiers (NH, KB or NW) and the average score per sample taken.
Flow cytometry
To isolate the stromal vascular fraction, epididymal fat pads were harvested, washed, finely minced and digested in Hanks’ balanced salt solution containing collagenase (1 mg/ml; Roche) for 45 min at 37°C. The digested tissue was agitated using a cannula and centrifuged at 1,000 rpm for 10 min. The upper lipid phase was removed and the aqueous phase with pellet was filtered through a 70‐µM cell strainer and centrifuged at 1,000 rpm for 7 min. The pellet was re‐suspended in PBS containing 0.5% BSA (Sigma‐Aldrich) and 2 mM EDTA (Sigma‐Aldrich) and was filter through a 30‐µM cell strainer and further centrifuged at 1,000 rpm for 7 min.
Cells from the stromal vascular fraction were washed and re‐suspended in PBS containing 0.5% BSA (Sigma‐Aldrich) and 2 mM EDTA (Sigma‐Aldrich). Fc receptors were blocked with a CD16/32 antibody (Miltenyi Biotec, 130‐092‐575) for 10 min at 4°C. Samples were then incubated with anti‐CD45‐VioBlue (Miltenyi Biotec, 130‐110‐802), anti‐CD11b‐FITC (Miltenyi Biotec, 130‐081‐201), anti‐Ly6G‐PE (Miltenyi Biotec, 130‐107‐913), anti‐Ly6C‐APC (eBioscience, 17‐5932‐82) or anti‐F4/80‐APC (Miltenyi Biotec, 130‐102‐379) for 10mins at 4°C, according to the manufacturer’s protocol. Stained cells were washed in PBS containing 0.5% BSA and 2 mM EDTA. Samples were analysed by flow cytometry (CytoFLEX S, Beckman Coulter). Leukocytes were identified based on typical light scatter properties, with further gating to define: CD45+ leukocytes, CD45+CD11b+ myeloid cells, CD45+CD11b+Ly6G−Ly6Chi inflammatory monocytes, CD45+CD11b+Ly6G−Ly6Clow reparative monocytes, CD45+CD11b+Ly6GhiLy6Chi neutrophils and CD45+CD11b+F4/80+ macrophages. Data were scaled to cells/ml of blood or weight of fat pad.
Pancreatic lipase activity
Tissue was harvested under terminal anaesthesia. 40 mg of pancreas was homogenised and used in a lipase activity assay (Abcam, ab102524).
Liver and plasma lipid measurements
100mg of tissue was weighed and homogenised in 1 ml of 5% Igepal (I8896, Sigma) and heated to 80°C for 5 min, cooled and re‐heated again before centrifuging for 2 min. The supernatant was used to measure, triglycerides, free fatty acids and cholesterol (Abcam, ab65336, ab65341 and ab65359, respectively).
Conditioning media
Conditioned media experiments require a large number of EC, and pulmonary EC provides an appropriate yield of cells to perform these experiments. Therefore, when PECs reached confluency, supplemented growth media was removed and replaced with basal endothelial growth medium‐MV2 for 24 h. Conditioned media was then removed and used in further experiments as described.
Caco‐2 cells
Caco‐2 cells were purchased from Public Health England Culture Collections (86010202). Pre‐differentiated Caco‐2 cells were maintained in 20% (v/v) FBS/MEME containing non‐essential amino acids, 0.292 g/l l‐glutamine, 2.2 g/l sodium bicarbonate (Sigma‐Aldrich #M4655), supplemented with 1XAntibiotic Antimycotic Solution (Sigma‐Aldrich #A5955) and incubated at 37°C in 5% CO2. Upon confluency, Caco‐2 cell differentiation was initiated by seeding on Transwell inserts. Differentiated Caco‐2 cells were subjected to boiled (5 min at 95°C) conditioned media stimulation at the basal side for 24 h at 37°C in 5% CO2.
Data analysis
All data are shown as mean ± standard error of mean (SEM) unless stated, with individual mice presented as data points. All image analysis was performed in ImageJ unless stated. Student unpaired t‐test was used for statistical analyses and performed with GraphPad Prism software version 8 unless stated. For plasma concentration–time profile experiments, area under the curve analyse was used and performed with GraphPad Prims. For metabolic parameters measured by indirect calorimetry, ANOVA testing was performed using mass as a co‐variant (ANCOVA testing) using calrapp.org. P < 0.05 taken as statistically significant.
Author contributions
NJH, KIB, NM, AS, NYY performed in vivo experiments. NJH, CL, KIB, CHO, NW, MD, CGW, NTW performed ex vivo experiments. JK‐P, IT, JHB, SS, JES, AM performed in vivo imaging experiments. NJH, CL, performed cell culture experiments. NJH and MTK wrote the manuscript. NJH, CL, KIB, NM, AS, CHO, NW, MD, CGW, NTW, JKP, IT, JHB, SS, JES, NYY, LDR, DJB, PS, SBW, RMC, MTK reviewed the manuscript. MTK, RMC, SBW, NYY, LDR, DJB, PS obtained funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Acknowledgements
NJH was funded by British Heart Foundation Project grant (PG/18/82/34120). CL was funded by a British Heart Foundation studentship (FS/19/59/34896). MD was funded by British Heart Foundation Clinical Research Training Fellowship (FS/18/44/33792). LDR was funded by a Diabetes UK RD Lawrence Fellowship (16/0005382). RMC was funded by a British Heart Foundation Clinical Intermediate Fellowship (FS/12/80/29821). MTK holds a British Heart Foundation Chair in Cardiovascular and Diabetes Research (RG/15/7/31521). NTW was funded by a British Heart Foundation project grant (PG/14/54/30939). The Experimental and Preclinical Imaging Centre was co‐funded by the BHF (SI/14/1/30718). We would like to acknowledge the histology service from the Division of Pathology and Data Analytics, Colorectal Pathology Trials, University of Leeds, for sectioning and staining adipose and liver samples. We would also like to acknowledge the Bio‐imaging and Flow Cytometry Facility, Faculty of Biological Sciences, University of Leeds, for acquiring images from histology slides. We would also like to acknowledge the UC Davis MMPC services, whose research was supported by NIH grant U24‐DK092993 (MMPC‐University of California Davis Microbiome and Host Response Core, RRID:SCR_015361). MTK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
EMBO reports (2021) 22: e50767.
See also: Z Bouman Chen & N Kaur Malhi (May 2021)
Data availability
No primary data sets have been generated and deposited.
References
- Abbas A, Imrie H, Viswambharan H, Sukumar P, Rajwani A, Cubbon Rm, Gage M, Smith J, Galloway S, Yuldeshava N et al (2011) The insulin‐like growth factor‐1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes 60: 2169–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams TE, Epa VC, Garrett TPJ, Ward CW (2000) Structure and function of the type 1 insulin‐like growth. Cell Mol Life Sci 57: 1050–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedei A, Morbidelli L (2019) Circulating metabolites originating from gut microbiota control endothelial cell function. Molecules 24: 3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ (2001) A new method for non‐parametric multivariate analysis of variance. Austral Ecol 26: 32–46 [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci 101: 15718–15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettedi L, Foukas LC (2017) Growth factor, energy and nutrient sensing signalling pathways in metabolic ageing. Biogerontology 18: 913–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al‐Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37: 852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High‐resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, de Vos WM (2017) Next‐generation beneficial microbes: the case of Akkermansia muciniphila . Front Microbiol 8: 1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaner O, Goday A, Park Y‐M, Lee S‐H, Magkos F, Shiow S‐ATE, Schröder H (2018) The gut microbiome profile in obesity: a systematic review. Int J Endocrinol 2018: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catry E, Bindels LB, Tailleux A, Lestavel S, Neyrinck AM, Goossens J‐F, Lobysheva I, Plovier H, Essaghir A, Demoulin J‐B et al (2018) Targeting the gut microbiota with inulin‐type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 67: 271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YK, Brar MS, Kirjavainen PV, Chen Y, Peng J, Li D, Leung FC‐C, El‐Nezami H (2016) High fat diet induced atherosclerosis is accompanied with low colonic bacterial diversity and altered abundances that correlates with plaque size, plasma A‐FABP and cholesterol: a pilot study of high fat diet and its intervention with Lactobacillus rhamno . BMC Microbiol 16: 264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C‐S, Kao C‐Y (2019) Current understanding of the gut microbiota shaping mechanisms. J Biomed Sci 26: 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintron‐Colon R, Sanchez‐Alavez M, Nguyen W, Mori S, Gonzalez‐Rivera R, Lien T, Bartfai T, Aïd S, François J‐C, Holzenberger M et al (2017) Insulin‐like growth factor 1 receptor regulates hypothermia during calorie restriction. Proc Natl Acad Sci 114: 9731–9736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao MC, Everard A, Aron‐Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L et al (2016) Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65: 426–436 [DOI] [PubMed] [Google Scholar]
- Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira‐Silva S, Falony G, Raes J, Maiter D, Delzenne NM et al (2019) Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof‐of‐concept exploratory study. Nat Med 25: 1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M, Vaughan EE, Plugge CM, de Vos WM (2004) Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin‐degrading bacterium. Int J Syst Evol Microbiol 54: 1469–1476 [DOI] [PubMed] [Google Scholar]
- Ding B‐S, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D et al (2010) Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468: 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CX, Zhao W, Solomon C, Rowland KJ, Ackerley C, Robine S, Holzenberger M, Gonska T, Brubaker PL (2014) The intestinal epithelial insulin‐like growth factor‐1 receptor links glucagon‐like peptide‐2 action to gut barrier function. Endocrinology 155: 370–379 [DOI] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk Jp, Druart C, Bindels Lb, Guiot Y, Derrien M, Muccioli Gg, Delzenne Nm et al (2013) Cross‐talk between Akkermansia muciniphila and intestinal epithelium controls diet‐induced obesity. Proc Natl Acad Sci 110: 9066–9071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Kawashima Sonoyama Y, Hamajima N, Hamajima T, Kumura Y, Miyahara N, Nishimura R, Adachi K, Nanba E, Hanaki K et al (2015) Heterozygous nonsense mutations near the C‐terminal region of IGF1R in two patients with small‐for‐gestational‐age‐related short stature. Clin Endocrinol 83: 834–841 [DOI] [PubMed] [Google Scholar]
- Gallagher EJ, Leroith D (2015) Obesity and diabetes: the increased risk of cancer and cancer related mortality epidemiology. Physiol Rev 95: 727–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Kakarieka E, Johnson MC, Jose J (2014) IGF‐IR signal transduction protein content and its activation by IGF‐I in human placentas: relationship with gestational age and birth weight. PLoS One 9: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS (2020) Immune recognition of microbial metabolites. Nat Rev Immunol 20: 91–92 [DOI] [PubMed] [Google Scholar]
- Griffin C, Lanzetta N, Eter L, Singer K (2016) Sexually dimorphic myeloid inflammatory and metabolic responses to diet‐induced obesity. Am J Physiol Integr Comp Physiol 311: R211–R216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariguata L, Whiting Dr, Hambleton I, Beagley J, Linnenkamp U, Shaw Je (2013) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103: 137–149 [DOI] [PubMed] [Google Scholar]
- Haywood NJ, Cordell PA, Tang KY, Makova N, Yuldasheva NY, Imrie H, Viswambharan H, Bruns AF, Cubbon RM, Kearney MT et al (2017) Insulin‐like growth factor binding protein 1 could improve glucose regulation and insulin sensitivity through its RGD domain. Diabetes 66: 287–299 [DOI] [PubMed] [Google Scholar]
- Imrie H, Viswambharan H, Sukumar P, Abbas A, Cubbon Rm, Yuldasheva N, Gage M, Smith J, Galloway S, Skromna A et al (2012) Novel role of the IGF‐1 receptor in endothelial function and repair: studies in endothelium‐targeted IGF‐1 receptor transgenic mice. Diabetes 61: 2359–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR (1995) Insulin‐like growth factors and their binding proteins: biological actions. Endocr Rev 16: 3–34 [DOI] [PubMed] [Google Scholar]
- Juanes M, Guercio G, Marino R, Berensztein E, Diana M, Ciaccio M, Gil S, Bailez M, Rivarola MA, Belgorosky A (2015) Three novel IGF1R mutations in microcephalic patients with prenatal and postnatal growth impairment. Clin Endocrinol 82: 704–711 [DOI] [PubMed] [Google Scholar]
- Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J (2012) Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3: 1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG (2016) Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes 2016: 1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelä R, Hemanthakumar KA, Vaparanta K, Robciuc M, Izumiya Y, Kidoya H, Takakura N, Peng X, Sawyer DB, Elenius K et al (2019) Endothelial cells regulate physiological cardiomyocyte growth via VEGFR2‐mediated paracrine signaling. Circulation 139: 2570–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, Behre Cj, Knight R, Fagerberg B, Ley Re et al (2011) Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci 108: 4592–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela‐Arispe ML (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell 130: 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie JL, Annex BH (2018) The microbiome and endothelial function. Circ Res 123: 1015–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chalazonitis A, Huang Y‐y, Mann Jj, Margolis Kg, Yang Qm, Kim Do, Cote F, Mallet J, Gershon Md (2011) Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 31: 8998–9009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Menke AL, Driessen A, Koek GH, Lindeman JH, Stoop R, Havekes LM, Kleemann R, van den Hoek AM (2014) Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One 9: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2011) UniFrac: an effective distance metric for microbial community comparison. ISME J 5: 169–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SV, Pedersen O (2016) The human intestinal microbiome in health and disease. N Engl J Med 375: 2369–2379 [DOI] [PubMed] [Google Scholar]
- Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD (2015) Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Heal Dis 26: 27663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughal RS, Bridge K, Buza I, Slaaby R, Worm J, Klitgaard‐Povlsen G, Hvid H, Schiødt M, Cubbon R, Yuldasheva N et al (2019) Effects of obesity on insulin: insulin‐like growth factor 1 hybrid receptor expression and Akt phosphorylation in conduit and resistance arteries. Diabetes Vasc Dis Res 16: 160–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad JM, Lamas B, Pham HP, Michel M‐L, Rainteau D, Bridonneau C, da Costa G, van Hylckama Vlieg J, Sovran B, Chamignon C et al (2018) Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun 9: 2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuenen MHMC, Ligt RAF, Doornbos RP, Woude JCJ, Kuipers EJ, Venema K (2005) The influence of microbial metabolites on human intestinal epithelial cells and macrophages in vitro. FEMS Immunol Med Microbiol 45: 183–189 [DOI] [PubMed] [Google Scholar]
- Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, Stanton C (2016) Gut microbiota, obesity and diabetes. Postgrad Med J 92: 286–300 [DOI] [PubMed] [Google Scholar]
- Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L et al (2017) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23: 107–113 [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP (2010) FastTree 2 – approximately maximum‐likelihood trees for large alignments. PLoS One 5: e9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web‐based tools. Nucleic Acids Res 41: D590–D596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L, Boström P, O’Sullivan J, Schinzel R, Lewis G, Dejam A, Lee Y‐K, Palma M, Calhoun S, Georgiadi A et al (2014) β‐aminoisobutyric acid induces browning of white fat and hepatic β‐oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19: 96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues RR, Greer RL, Dong X, DSouza KN, Gurung M, Wu JY, Morgun A, Shulzhenko N (2017) Antibiotic‐induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol 8: 2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber AMP, Lee YM, Chang MW, Leblanc M, Collins B, Downes M, Evans RM, Ayres JS (2015) Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF‐1 signaling. Science 350: 558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Seeley RJ (2019) Reg3 proteins as gut hormones? Endocrinology 160: 1506–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbins RE, Holcomb VB, Hong J, Núñez NP (2012) Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr 51: 861–870 [DOI] [PubMed] [Google Scholar]
- Tang X, Miao Y, Luo Y, Sriram K, Qi Z, Lin F‐M, Gu Y, Lai C‐H, Hsu C‐Y, Peterson KL et al (2020) Suppression of endothelial AGO1 promotes adipose tissue browning and improves metabolic dysfunction. Circulation 142: 365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao J, Jiang Y (2013) Hierarchical IDEAL: fast, robust, and multiresolution separation of multiple chemical species from multiple echo times. Hierarchical IDEAL Fast, Robust, Multiresolution Sep Mult Chem Species from Mult Echo Times 159: 155–159 [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031 [DOI] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga–Thie GM, Ackermans MT, Serlie MJ, Oozeer R et al (2012) Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143: 913–916.e7 [DOI] [PubMed] [Google Scholar]
- Watt NT, Gage MC, Patel PA, Viswambharan H, Sukumar P, Galloway S, Yuldasheva NY, Imrie H, Walker AMN, Griffin KJ et al (2017) Endothelial SHIP2 suppresses Nox2 NADPH oxidase‐dependent vascular oxidative stress, endothelial dysfunction, and systemic insulin resistance. Diabetes 66: 2808–2821 [DOI] [PubMed] [Google Scholar]
- Woods K, Camacho‐Hubner C, Savage M, Clark A (1996) Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin ‐ like growth factor I gene. N Engl J Med 335: 1363–1367 [DOI] [PubMed] [Google Scholar]
- Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF (2016) Gut microbiota induce IGF‐1 and promote bone formation and growth. Proc Natl Acad Sci 113: E7554–E7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki‐Järvinen H (2014) Non‐alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabet Endocrinol 2: 901–910 [DOI] [PubMed] [Google Scholar]
- Zhang F, Zarkada G, Han J, Li J, Dubrac A, Ola R, Genet G, Boyé K, Michon P, Künzel SE et al (2018) Lacteal junction zippering protects against diet‐induced obesity. Science 361: 599–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Table EV1
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Data Availability Statement
No primary data sets have been generated and deposited.


