The risk of vancomycin (VAN)-associated acute kidney injury (AKI) may be altered with combination regimens. The specific AKI risk when VAN is combined with imipenem-cilastatin/relebactam (IMP-C/REL) or piperacillin/tazobactam (TZP) has not been clearly defined.
KEYWORDS: acute kidney injury, beta-lactams, carbapenems, glycopeptides, nephrotoxicity
ABSTRACT
The risk of vancomycin (VAN)-associated acute kidney injury (AKI) may be altered with combination regimens. The specific AKI risk when VAN is combined with imipenem-cilastatin/relebactam (IMP-C/REL) or piperacillin/tazobactam (TZP) has not been clearly defined. We sought to quantify the dose-AKI relationships of VAN alone and in combination with TZP or IMP-C/REL. Male C57BL/6J mice (Charles River Laboratory) aged 10 to 12 weeks were dosed with study drug regimens in three stages. Stage 1 consisted of a VAN dose-ranging design (0 to 600 mg/kg daily) over a 7-day period to identify the VAN monotherapy dose-AKI relationship in the murine model. Stage 2 evaluated the approximate VAN dose eliciting 50% AKI response in stage 1 in combination with the highest human equivalent doses (HEDs) used in preclinical murine models (2.5 and 320 mg/kg daily for TZP and IMP-C/REL, respectively). Stage 3 tested these combinations with fractionated doses of TZP or IMP-C/REL administered at 6- and 12-h intervals. In these studies, AKI was defined with biomarkers (serum creatinine [SCr], blood urea nitrogen [BUN]) and with histopathological assessment by a treatment-blinded pathologist. VAN doses of 300 to 500 mg/kg daily reproducibly led to development of AKI within 4 days of dosing. Mice treated with VAN alone had a near doubling of their baseline SCr and BUN levels compared with mice treated with control, IMP-C/REL alone, or TZP alone. Both VAN+IMP-C/REL and VAN+TZP had significantly (P < 0.05) lower SCr and BUN values than VAN alone when dosed once daily. This nephroprotective effect was retained with VAN+IMP-C/REL but not VAN+TZP when IMP-C/REL and TZP were administered every 6 h. Biomarker results were concordant with histopathological findings. The VAN dose-AKI relationship can be attenuated with single daily HEDs of TZP or IMP-C/REL in mice. IMP-C/REL, but not TZP, retained a nephroprotective effect compared with VAN monotherapy when administered as fractionated doses.
TEXT
Vancomycin (VAN) is a commonly used antibiotic against Gram-positive bacteria associated with acute kidney injury (AKI) (1). Several observational and retrospective clinical studies have documented an increased risk of AKI when VAN is combined with piperacillin/tazobactam (TZP) (2, 3). In a meta-analysis, the odds of AKI were 9.62 with VAN combined with TZP compared with 1.43 when VAN was combined with cefepime or carbapenem in critically ill patients (2). Although these findings are concerning, they are confounded by numerous patient-level and treatment factors that cannot be controlled in the real-world setting. Recent work in a rat model of VAN-induced AKI failed to replicate the observed synergistic nephrotoxicity of VAN and TZP in clinical settings, noting instead the nephroprotective potential of the combination (4). Understanding the factors that influence AKI risk is paramount because of the well-documented association between AKI and significant morbidity and mortality (5).
In contrast to observational clinical findings with TZP, retrospective data suggest that imipenem (IMP)-cilastatin in combination with VAN may lower the risk of AKI (6). These findings have biological plausibility because cilastatin, a dehydropeptidase I inhibitor, was specifically developed to improve the pharmacokinetic and safety profile of IMP by altering renal tubular transport and metabolism (7). Over time, these nephroprotective effects of cilastatin have been documented in several preclinical studies from multiple research groups against nephrotoxic drugs, including VAN, cyclosporine, tacrolimus, polymyxin B, and gentamicin (8, 9). Imipenem-cilastatin/relebactam (IMP-C/REL) was recently approved and contains the novel beta-lactamase inhibitor REL, which restores IMP activity against many IMP-nonsusceptible Gram-negative bacteria (10). IMP-C/REL has antimicrobial activity against multidrug-resistant Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae, including strains expressing Ambler class A or C beta-lactamases (10). The spectrum of IMP-C/REL activity does not limit its potential use in combination with VAN for empirical treatment of polymicrobial infections. This may be especially true among more severely ill or at-risk patient populations, such as those who require additional nephrotoxic medication therapy (e.g., cyclosporine, tacrolimus) posttransplantation. Notably, small clinical studies have previously demonstrated a nephroprotective effect of IMP-C among transplant patients receiving cyclosporine immunosuppression (8). The nephroprotective potential of cilastatin administered as IMP-C/REL has not been evaluated to date. The primary goal of this study was to quantify the dose-AKI relationships of VAN alone and in combination with TZP and IMP-C/REL in a murine model.
RESULTS
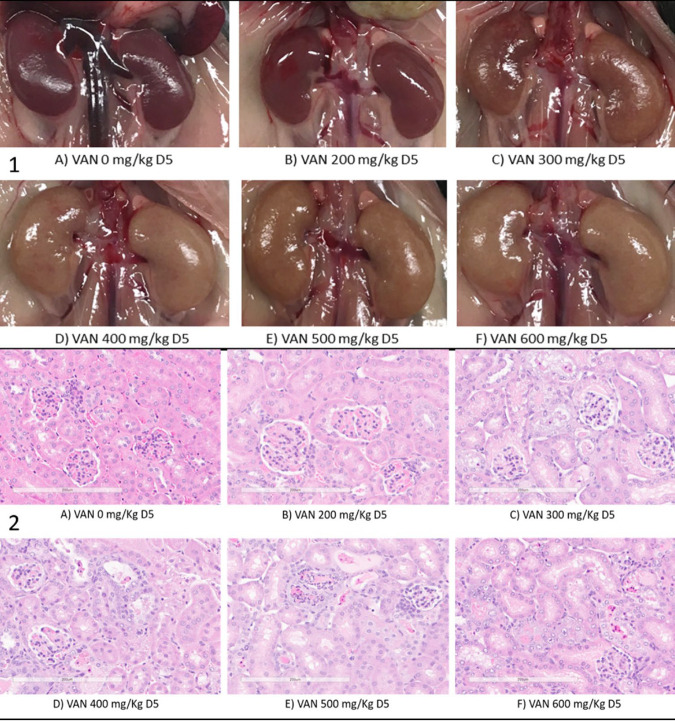
The dose-toxicity relationship of VAN was observable in animals. As illustrated in Fig. 1, serum creatinine (SCr) and blood urea nitrogen (BUN) concentrations increased with increasing VAN daily doses. For instance, a VAN dose-AKI relationship was noted with both SCr (Fig. 1A) and BUN (Fig. 1B), with near maximal values observed at a dose of 500 mg/kg daily. The profiles seen after the 4th and 7th doses of VAN were nearly superimposable, indicating that 4 doses were sufficient to induce AKI at the dose range 300 to 500 mg/kg daily. Treatment-blinded histology scores also paralleled these biomarkers across the tested dose range (Fig. 1C), with slightly higher tubular regeneration scores noted after dose 7 relative to dose 4. The development of AKI was grossly visible on necropsy, with the appearance of pale kidneys at doses of ≥300 mg/kg daily after the 4th dose (Fig. 2). Histologically, lesions ranged from focal and minimal to coalescing to widespread, based on the dose of VAN. Lesions of tubular degeneration/necrosis, regeneration, tubular ectasia, and tubular proteinosis were recorded as components of kidney injury (Fig. 2). Based on these findings, VAN doses of 300, 400, and 500 mg/kg daily were selected for assessment of the combination effect of IMP-C/REL and TZP. Table 1 provides a summary of the median histology scores by VAN dosage alone and in combination with IMP-C/REL 320 mg/kg daily every 24 h and TZP 2.5 g/kg daily every 24 h. These scores were numerically lower in the VAN+TZP group than in the VAN+IMP-C/REL group. However, as shown in Fig. 3, significantly lower SCr and BUN values were seen with the VAN combination arms than with VAN alone at the 300-mg/kg daily dose. Statistical significance was also observed with the difference in BUN when VAN (500 mg/kg daily) was combined with TZP administered once daily.
FIG 1.

Scatter and fitted plots of serum creatinine (A), blood urea nitrogen (B), and median histology scores (C) after the 4th and 7th doses of vancomycin.
FIG 2.
Gross pathology (1) visible during necropsy and hematoxylin and eosin-stained kidney tissue (2) illustrating renal tubular injury at ×20 magnification by dose on day 5 (D5, after 4th dose), based on increasing doses of vancomycin (VAN), where 0 mg/kg represents the control.
TABLE 1.
Median of severity grading of histological lesions of acute tubular injury associated with doses of VAN alone or VAN plus combination
| Drug and dose of VAN (mg/kg) | Severity grading (median)a |
|||
|---|---|---|---|---|
| Degeneration/necrosis | Regeneration | Tubular ectasia | Tubular proteinosis | |
| VAN | ||||
| 0 (saline) | 0 | 0 | 0 | 0 |
| 200 | 1 | 2 | 1 | 0 |
| 300 | 2 | 0 | 2 | 2 |
| 400 | 3 | 3 | 2 | 1 |
| 500 | 3 | 4 | 4 | 4 |
| 600 | 4 | 3 | 3 | 4 |
| VAN+IMP-C/REL | ||||
| 0 (IMP-C/REL alone) | 0 | 0 | 0 | 0 |
| 300 | 2 | 2 | 2 | 2 |
| 400 | 3 | 3 | 3 | 4 |
| 500 | 3 | 3 | 3 | 3 |
| VAN+TZP | ||||
| 0 (TZP alone) | 0 | 0 | 0 | 0 |
| 300 | 0 | 0 | 0 | 0 |
| 400 | 2 | 1 | 2 | 1 |
| 500 | 2 | 1 | 3 | 2 |
Severity scale based on percent involvement of kidney parenchyma in section: 0, no lesions; 1, rare focal lesions (<5%); 2, multifocal lesions (6% to 25%); 3, coalescing lesions (26% to 50%); 4, diffuse lesions (>50% of kidney parenchyma affected).
FIG 3.
Dose-toxicity relationship of VAN monotherapy compared with drug combinations of VAN plus IMP-C/REL and VAN plus TZP. Mean ± SD connected plots of renal biomarkers by drug (and combination). The 0 dose represents saline (VAN group), IMP-C/REL 320 mg/kg daily (VAN+IMP-C/REL group), or TZP 2.5 g/kg daily (VAN+TZP group). The x axis represents increasing doses of VAN when used alone and in combination. All groups treated with VAN+TZP received a single daily dose of TZP dosed at 2.5 g/kg daily of the piperacillin component. All groups treated with VAN+IMP-C/REL received a single daily dose of IMP-C/REL dosed at 320 mg/kg daily of the cilastatin component. Comparisons performed by VAN dose. *, Statistically significant.
Fractioned IMP-C/REL and TZP doses were tested in combination with VAN 300 mg/kg daily. Under dose-fractionation conditions, significantly lower SCr and BUN levels than those with VAN monotherapy were again noted, with 6-h fractionated doses of IMP-C/REL but not TZP (Fig. 4). These findings were consistent with the median histology scores for degeneration/necrosis, regeneration, tubular ectasia, and proteinosis, which were 1 point lower with the combination of VAN+IMP-C/REL than with VAN+TZP for the every-6-h dosing arm. No animal data were excluded in this analysis.
FIG 4.
Box-and-whisker plots of serum creatinine and blood urea nitrogen observed after the 4th day of VAN dosing alone and in combination with IMP-C/REL (A1, A2) or TZP (B1, B2), with the latter agents administered every 6, 12, or 24 h. *, Significant (P < 0.05) difference relative to the VAN treatment group.
DISCUSSION
Empiric management of infections in the acute care setting commonly relies on combination antibiotics with broad-spectrum Gram-positive and Gram-negative activity. The typical combination in several institutions includes the use of VAN and TZP until more definitive information about the causative pathogen and source can be defined. Antimicrobial stewardship efforts have been focused on limiting the duration of treatment with this combination to limit emergence of antimicrobial resistance, untoward side effects, and health care costs (11). Recently, epidemiological studies have suggested a higher rate of AKI with VAN+TZP than with VAN plus other beta-lactams (1–3, 12–14). Chertow et al. (5) demonstrated that even modest increases (≥0.5 mg/dl) in SCr are associated with a 6.5-fold (95% confidence interval, 5.0- to 8.5-fold) increase in the odds of death, a 3.5-day increase in length of stay, and nearly $7,500 in excess hospital costs, highlighting the clinical relevance of this potential antibiotic adverse effect.
Reducing the risk of VAN-associated AKI is of clear value. One potential approach to reducing the risk of this iatrogenic condition is the use of antioxidants to target mitochondrial reactive oxygen species and use of agents that reduce proximal tubule accumulation of VAN. Cilastatin and IMP-C have been shown to reduce this AKI risk; however, the combination of IMP-C/REL has not been tested to date (6–8). Using quartz crystal microbalance analysis, Hori et al. (7) demonstrated that cilastatin alters megalin binding of VAN. They also confirmed this pathway using a megalin-mosaic-knockout mouse model comprised of mice with proximal tubular cells replete of or deficient in megalin (7). These studies have shown time-dependent alterations in nephrotoxicity induced by 400 mg/kg daily of VAN in the presence of cilastatin that are observable at day 4 and reproducible at day 7 across studies (7). The nephroprotective effects of cilastatin have been demonstrated at doses of 100 mg/kg once daily in mice, which is equivalent to 8 mg/kg in humans (6–8). For comparison, the typical human dose is 1 to 2 g (12.5 to 25 mg/kg in an 80-kg adult) of cilastatin/day.
Given the prior findings, we quantified the VAN dose-AKI relationship alone and in combination with TZP and IMP-C/REL. Our working hypothesis was that TZP+VAN would increase AKI and IMP-C/REL+VAN would decrease AKI relative to VAN monotherapy based on the existing body of literature. Our findings suggested a more nuanced relationship with these combination regimens. We were able to demonstrate that IMP-C/REL lowers the risk of VAN-induced AKI at 300 mg/kg daily (HED, 25 to 30 mg/kg), and this effect remains when IMP-C/REL is administered in divided doses. Counter to clinical findings, TZP also decreased the risk of AKI when TZP was administered once daily but not when administered as a fractionated dose. Our findings provide independent confirmation of data reported recently by Pais et al. (4), who did not observe an increased risk for VAN-induced AKI when combined with TZP and suggested a potential nephroprotective effect. Note that we used high doses of TZP (2.5 g/kg daily) and were not able to demonstrate AKI with the use of this compound alone. For comparison, the TZP 50% lethal dose is 10 g/kg daily, and prior preclinical toxicological studies were based on 2.5 g/kg daily (15, 16).
Our study was not designed to establish toxicodynamic relationships through the measurement of serum drug concentrations or to determine mechanistic pathways leading to AKI. We did not control for potential confounders via randomization. We based our findings on established, clinically used biomarkers of kidney function and injury and histopathology. The histopathology data did not consistently match the biomarker data noted in Table 1 and highlighted the potential for subjective variability with this assessment tool. Although we did not measure drug concentrations, the finding that dose fractionation did not substantially reduce the effect of IMP-C/REL but may have impacted the effect of TZP suggests that maximum systemic concentration may be the driver for this nephroprotective effect. Additionally, loss of this nephroprotective effect at higher doses of VAN (≥400 mg/kg daily) implies that inhibition of the renal tubular uptake process is likely saturable.
In summary, a VAN dose-AKI relationship in a murine model was established using standard biomarkers and histopathology. Previous studies have shown that a dose of 400 mg/kg of VAN reliably induces AKI in mouse models but have not documented a clear dose-toxicity relationship (17, 18). We demonstrated that VAN nephrotoxicity can be mitigated with IMP-C/REL or TZP at VAN 300 mg/kg daily, which corresponds to a HED of ∼25 to 30 mg/kg daily. We confirmed that TZP monotherapy, even at very high doses, does not induce AKI, which is in line with recent preclinical findings. In conclusion, the dose-dependent AKI observed with VAN treatment can be attenuated with single daily doses of IMP-C/REL or TZP. IMP-C/REL maintains a nephroprotective effect compared with VAN monotherapy under dose-fractionation conditions in this model, whereas TZP does not.
MATERIALS AND METHODS
Animals.
Male C57BL/6J mice aged 10 to 12 weeks were acquired from Charles River Laboratory. Animals were maintained individually in vivaria with 12-h light/dark cycles and access to food and water ad libitum. Animals were allowed to acclimate for 3 to 5 days before conduct of experimental procedures. This staged series of experiments required 132 mice and was designed to reduce the number of animals used. Approval of the institutional animal care use committee was acquired before study commencement. Only healthy mice were used for the experiments.
Drugs.
Both VAN and TZP were purchased through the research pharmacy as a single lot from a single manufacturer for the entire study. The IMP-C/REL used in this study was the clinical grade drug product from a single lot and supplied in kind by Merck & Company.
Vancomycin Dose-Nephrotoxicity Relationship.
To characterize the VAN dose-AKI relationship, we performed a dose-ranging study. Six mice per group were dosed with 0 (saline), 200, 300, 400, 500, or 600 mg/kg of VAN by intraperitoneal (i.p.) injection once daily over a 7-day period (n = 36 total). Three mice were sacrificed per group 24 h after the 4th and 7th doses. The right kidney from each animal underwent histopathological semiquantitative morphometric analysis by a pathologist blinded to the treatment groups to grade acute tubular injury. Lesions were graded based on the distribution of each component of kidney injury. All components represent a continuum of acute tubular necrosis, tubular ectasia, and proteinosis and regenerative attempts to restore functional nephrons. Lesions were graded in a semiquantitative manner on a 0 to 4 severity scale based on the percent involvement of kidney parenchyma in section: 0, no lesions; 1, rare focal lesions (<5%); 2, multifocal lesions (6% to 25%); 3, coalescing lesions (26% to 50%); 4, diffuse lesions (>50% of kidney parenchyma affected). Scores were averaged based on 15 randomly selected viewing fields. Tubular degeneration/necrosis was characterized by cytoplasmic dissolution, fragmentation, vacuolation, and hypereosinophilia and shrinkage of tubular epithelial cells with nuclear fragmentation or pyknosis and by the absence of tubular epithelial cells or sloughing into tubular lumens. Regeneration was observed as clusters or regions of tubules with basophilic cytoplasm and large open-faced hyperchromatic nuclei with or without prominent nucleoli and increased mitotic rates. Tubular ectasia was characterized by variable expansion of tubule lumens lined by attenuated epithelium, and tubular proteinosis was reported when there was amorphous eosinophilic proteinaceous material present in tubular lumens, with or without the presence of hyaline casts. Blood was assayed for the renal function biomarkers SCr and BUN at the University of Michigan in vivo animal core diagnostic testing laboratory.
Vancomycin Combination Dose-Nephrotoxicity Relationship.
Three VAN dose levels were selected for evaluation in combination with TZP or IMP-C/REL based on the prior set of experiments. The doses selected were designed to be approximately equal to and plus or minus one dose level of the dose that elicited 50% of the maximal AKI response This approach was taken to minimize the number of animals studied while retaining sufficient power to detect shifts in the VAN-AKI dose response under combination conditions. VAN alone was given once daily i.p. at these doses, whereas TZP or IMP-C/REL was given once daily by subcutaneous (s.c.) injection. TZP was dosed at 2.5 g/kg s.c. daily of the piperacillin component, equivalent to the maximum total daily HED (15, 16). Similarly, IMP-C/REL was dosed at 320 mg/kg s.c. daily of the cilastatin component, representing the total daily HED and consistent with doses previously evaluated in developmental toxicology studies included in the product label. In addition to the combination arms, TZP, IMP-C/REL, and an equivalent volume of saline were administered as monotherapy control conditions. Three mice were sacrificed at each of the two time points outlined above and underwent histopathological scoring and renal biomarker assessment. All study drugs were administered as bolus doses, with administration of the second drug treatment within 15 min of prior drug administration. Six mice were used in each of the nine groups inclusive of controls (n = 54). Animals were evenly distributed between 4- and 7-day dosing schedules; sacrifice occurred 24 h after the last dose.
Vancomycin Combination Fractionated-Dose-Nephrotoxicity Relationship.
The aforementioned study procedures were repeated with VAN at the dose associated with the greatest combination effect on AKI compared with monotherapy. In these studies, VAN was administered once daily combined with TZP or IMP-C/REL administered as fractionated daily doses for 4 days. TZP was administered as 0.625 g/kg s.c. every 6 h and 1.25 g/kg s.c. every 12 h to match the total 2.5-g/kg daily HED based on the piperacillin component. Likewise, IMP-C/REL was administered as 80 mg/kg s.c. every 6 h and 160 mg/kg s.c. every 12 h to match the 320-mg/kg daily HED of the cilastatin component. This experiment required the use of three mice for each of the nine groups (n = 27). Based on the findings, the experiments with the every-6- and -12-h combination treatment-only dosing groups and saline control were repeated (n = 15).
Statistical analysis.
No specific randomization of treatment allocation was performed at dose initiation. Previous studies demonstrated that a dose of 400 mg/kg of VAN reliably induces nephrotoxicity in the mouse model but have not published a dose-toxicity curve relationship. A power analysis was difficult with the proposed experimental design that involved multiple treatment groups, so we relied on the triplicate sample principle for the VAN dose-ranging study. The resource equation method based on the law of diminishing returns was used to estimate the sample size. Nine groups with three animals per group minus the number of treatment groups was 27 – 9 = 18, which is between the acceptable degrees of freedom of 10 to 20. Between-group comparisons were performed using Dunn's test for stochastic dominance among multiple pairwise comparisons following a Kruskal-Wallis test of stochastic dominance among k groups. The Benjamini-Hochberg adjustment was used for multiple comparisons, with statistical significance based on an alpha level of 0.05 (Stata/SE 16.0, StataCorp LLC, College Station, TX, USA).
ACKNOWLEDGMENTS
This work was supported by an investigator-initiated grant from Merck (study code MK-7655A) and in part through start-up funds from the University of Michigan College of Pharmacy. Research reported in this publication was supported by the National Cancer Institute, NIH, under award number P30CA046592 by the use of the following Cancer Center Shared Resource(s): Pharmacokinetics Core.
We have no conflicts of interest to declare.
REFERENCES
- 1.Filippone EJ, Kraft WK, Farber JL. 2017. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 102:459–469. doi: 10.1002/cpt.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. 2018. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med 46:12–20. doi: 10.1097/CCM.0000000000002769. [DOI] [PubMed] [Google Scholar]
- 3.Watkins RR, Deresinski S. 2017. Increasing evidence of the nephrotoxicity of piperacillin/tazobactam and vancomycin combination therapy-what is the clinician to do? Clin Infect Dis 65:2137–2143. doi: 10.1093/cid/cix675. [DOI] [PubMed] [Google Scholar]
- 4.Pais GM, Liu J, Avedissian SN, Hiner D, Xanthos T, Chalkias A, d’Aloja E, Locci E, Gilchrist A, Prozialeck WC, Rhodes NJ, Lodise TP, Fitzgerald JC, Downes KJ, Zuppa AF, Scheetz MH. 2020. Lack of synergistic nephrotoxicity between vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother 75:1228–1236. doi: 10.1093/jac/dkz563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. 2005. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 6.Hakeam HA, AlAnazi L, Mansour R, AlFudail S, AlMarzouq F. 2019. Does nephrotoxicity develop less frequently when vancomycin is combined with imipenem-cilastatin than with meropenem? A comparative study. Infect Dis (Lond) 51:578–584. doi: 10.1080/23744235.2019.1619934. [DOI] [PubMed] [Google Scholar]
- 7.Hori Y, Aoki N, Kuwahara S, Hosojima M, Kaseda R, Goto S, Iida T, De S, Kabasawa H, Kaneko R, Aoki H, Tanabe Y, Kagamu H, Narita I, Kikuchi T, Saito A. 2017. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J Am Soc Nephrol 28:1783–1791. doi: 10.1681/ASN.2016060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruss E, Tomas JF, Bernis C, Rodriguez F, Traver JA, Fernandez-Ranada JM. 1996. Nephroprotective effect of cilastatin in allogeneic bone marrow transplantation. Results from a retrospective analysis. Bone Marrow Transplant 18:761–765. [PubMed] [Google Scholar]
- 9.Shayan M, Elyasi S. 2020. Cilastatin as a protective agent against drug-induced nephrotoxicity: a literature review. Expert Opin Drug Saf 19:999–1010. doi: 10.1080/14740338.2020.1796967. [DOI] [PubMed] [Google Scholar]
- 10.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, Lagace-Wiens PRS, Walkty A, Denisuik A, Golden A, Gin AS, Hoban DJ, Lynch JP, III, Karlowsky JA. 2018. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-beta-lactamase inhibitor combinations. Drugs 78:65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 11.Doron S, Davidson LE. 2011. Antimicrobial stewardship. Mayo Clin Proc 86:1113–1123. doi: 10.4065/mcp.2011.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess LD, Drew RH. 2014. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy 34:670–676. doi: 10.1002/phar.1442. [DOI] [PubMed] [Google Scholar]
- 13.Chen XY, Xu RX, Zhou X, Liu Y, Hu CY, Xie XF. 2018. Acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam administration: a systematic review and meta-analysis. Int Urol Nephrol 50:2019–2026. doi: 10.1007/s11255-018-1870-5. [DOI] [PubMed] [Google Scholar]
- 14.Navalkele B, Pogue JM, Karino S, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Koons J, Hussain T, Perry W, Evans R, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Kaye KS. 2017. Risk of acute kidney injury in patients on concomitant vancomycin and piperacillin-tazobactam compared to those on vancomycin and cefepime. Clin Infect Dis 64:116–123. doi: 10.1093/cid/ciw709. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Yada H, Anai M, Umano T, Kawazu K, Anai S, Kaziwara T, Yamasaki K. 1994. Single-dose toxicity studies of tazobactam/piperacillin and tazobactam. J Toxicol Sci 19 (Suppl 2):45–153. (In Japanese.) doi: 10.2131/jts.19.supplementii_145. [DOI] [PubMed] [Google Scholar]
- 16.Sato T, Lochry EA, Hoberman AM, Christian MS. 1994. Reproductive and developmental toxicity studies of tazobactam/piperacillin or tazobactam (2)–teratological study in rats with intravenously administration. J Toxicol Sci 19 (Suppl 2):215–232. (In Japanese.) doi: 10.2131/jts.19.supplementii_215. [DOI] [PubMed] [Google Scholar]
- 17.Dieterich C, Puey A, Lin S, Lyn S, Swezey R, Furimsky A, Fairchild D, Mirsalis JC, Ng HH. 2009. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol Sci 107:258–269. doi: 10.1093/toxsci/kfn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takigawa M, Masutomi H, Kishimoto Y, Shimazaki Y, Hamano Y, Kondo Y, Arai T, Lee J, Ishii T, Mori Y, Ishigami A. 2017. Time-dependent alterations of vancomycin-induced nephrotoxicity in mice. Biol Pharm Bull 40:975–983. doi: 10.1248/bpb.b16-00932. [DOI] [PubMed] [Google Scholar]