Echinocandins have been used as primary therapy of invasive aspergillosis (IA), with suboptimal results at standard dosing. Here, we explored the efficacy of dose escalation in a validated in vitro pharmacokinetic/pharmacodynamic (PK/PD) model.
KEYWORDS: antifungal agents, aspergillosis, dose optimization, echinocandin, in vitro PK/PD model, pharmacodynamics
ABSTRACT
Echinocandins have been used as primary therapy of invasive aspergillosis (IA), with suboptimal results at standard dosing. Here, we explored the efficacy of dose escalation in a validated in vitro pharmacokinetic/pharmacodynamic (PK/PD) model. Six echinocandin wild-type (WT) and three non-WT A. fumigatus isolates were tested in an in vitro PK/PD model simulating anidulafungin, caspofungin, and micafungin exposures with a free drug maximum concentration (fCmax) of 0.01 to 16 mg/liter and a half-life (t1/2) of 8 to 22 h. The relationship between the area under the dosing interval time-free drug concentration curve (fAUC0–24)/minimum effective concentration (MEC) and % aberrant mycelium formation was analyzed. PK/PD indices associated with 50 to 99.99% maximal activity (EI50 to EI99.99) were correlated with the clinical outcome of a 50-mg/day standard dose of caspofungin. The probability of target attainment (PTA) was calculated for different dosing regimens of each echinocandin via Monte Carlo analysis. A sigmoidal PK/PD relationship was found for WT isolates with EI99 values of 766, 8.8, and 115 fAUC0–24/CLSI MEC for anidulafungin, caspofungin, and micafungin, respectively. No aberrant mycelia were observed for non-WT isolates, irrespective of their MEC and drug exposure. The EI99, EI99.9, and EI99.99 values corresponded to 2-, 3-, and 4-log10 formation of aberrant mycelia and correlated with survival, favorable, and complete response rates to caspofungin primary therapy in patients with IA. A very low PTA (<13%) was found for the standard doses of all echinocandins, whereas a PTA of ≥90% was found with 100 and 150 mg/day of caspofungin and 1,400 mg/day micafungin against WT isolates. For anidulafungin, the PTA for 1,500 mg/day was 10%. Among the three echinocandins, only caspofungin at 2 or 3 times the licensed dosing was associated with a high PTA. Caspofungin dose escalation might deserve clinical validation.
INTRODUCTION
Echinocandins are currently used as second-line agents for the treatment of invasive aspergillosis (IA), either alone or in combination therapy (1, 2). Among the three echinocandins, only caspofungin has a licensed indication for the treatment of IA in patients who are refractory to or intolerant of other therapies. Nevertheless, their use as an alternative chemotherapeutic option for the management of increasingly reported azole-resistant IA is increasing given their excellent tolerability, the rare occurrence of echinocandin resistance among Aspergillus fumigatus isolates, and the promising results of a randomized controled clinical trial of azole-echinocandin combination therapy against IA (3). It is of note, however, that caspofungin and micafungin have been used as primary therapy for IA with suboptimal results (∼50% favorable response) (4, 5), and breakthrough Aspergillus infections emerging during echinocandin treatment have been reported (6–9). These observations call into question the role of echinocandins at standard dosing for the treatment of IA. In a rabbit model of pulmonary aspergillosis, the pharmacokinetic/pharmacodynamic (PK/PD) index associated with the anidulafungin effect using residual fungal burden, lung weight pulmonary infarct score, and survival could not be clearly distinguished (10) whereas the anidulafungin area under the concentration-time curve from 0 to 24 h (AUC0–24)/minimum effective concentration (MEC) correlated better with survival in a nonneutropenic murine model of disseminated aspergillosis (11) and caspofungin drug maximum concentration (Cmax)/MEC was the driving PK/PD index in a neutropenic murine model of invasive pulmonary aspergillosis that used PCR to assess pulmonary fungal burden (12).
An integrated understanding of a drug’s PK/PD properties is the key of maximizing its therapeutic benefit. However, considering the unique mode of action of echinocandins against Aspergillus spp. (formation of aberrant mycelia without complete inhibition of growth), preclinical PD studies for describing exposure-effect relationships and dose optimization are hampered by the lack of a reliable endpoint to quantify antifungal activity. Indeed, animal models have showed conflicting results regarding the driving PK/PD index that best predicts their efficacy (10–13), highlighting the difficulty of in vivo PK/PD studies with echinocandins. We have recently described a new in vitro endpoint for the activity of echinocandins activity based on the property of Aspergillus mycelia to attach on a dialysis membrane (DM) tube after being exposed to echinocandins in an AUC-dependent manner (14). In particular, the proposed marker was easily quantified, reproducible, and amenable for PK/PD studies, while it correlated with in vivo survival in a mouse model of A. fumigatus infection. Here, we assessed the PD of anidulafungin, caspofungin and micafungin against A. fumigatus in an in vitro PK/PD model. For each echinocandin, the PK/PD relationship was described and the probability of target attainment (PTA) for different dosing regimens was calculated.
RESULTS
MECs/MICs.
The echinocandin MECs/MICs for the included isolates determined by each susceptibility testing methodology are shown in Table 1. All WT isolates had the same CLSI and EUCAST MECs for anidulafungin, caspofungin, and micafungin. For anidulafungin and caspofungin, the XTT [2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide] and MIC test strip (MTS) method revealed 1 and 1 to 2 2-fold-dilution differences in in vitro susceptibility, respectively. For micafungin, all isolates had the same XTT and MTS MICs. For non-WT isolates, CLSI and EUCAST MECs ranged from 2 to 16, 4 to 16, and 1 to 8 mg/liter for anidulafungin, caspofungin, and micafungin, whereas for XTT and MTS, the MIC ranges were 2 to 32, 2 to 16, and 0.5 to 8 mg/liter for anidulafungin, caspofungin, and micafungin.
TABLE 1.
Echinocandin MECs/MICs determined by different susceptibility testing methodsa of A. fumigatus isolates used in the present study
| Antifungal | WT status of isolate | MEC/MIC (mg/liter) by: |
|||||
|---|---|---|---|---|---|---|---|
| CLSI | EUCAST | XTT | MTS | ||||
| Anidulafungin | WT | ||||||
| AZN8196 | 0.016 | 0.03 | 0.004 | 0.004 | |||
| V52-35 | 0.016 | 0.03 | 0.008 | 0.008 | |||
| DPLRG101b | 0.016 | 0.03 | 0.004 | 0.004 | |||
| Non-WT | |||||||
| DPL1035-homo | 4 | 16 | 16 | 2 | |||
| DPL32458 | 2 | 16 | 32 | 8 | |||
| Caspofungin | WT | ||||||
| AZN8196 | 0.25 | 0.5 | 0.25 | 0.06 | |||
| NIH 4215 (ATCC no. MYA-3626) | 0.25 | 0.5 | 0.5 | 0.125 | |||
| AUH-25 | 0.25 | 0.5 | 0.5 | 0.25 | |||
| AUH-27 | 0.25 | 0.5 | 0.25 | 0.06 | |||
| AUH-29 | 0.25 | 0.5 | 0.25 | 0.06 | |||
| Non-WT | |||||||
| DPL1035-homo | 16 | 8 | 16 | 8 | |||
| DPL32458 | 16 | 4 | 8 | 2 | |||
| DPLRG101b | 8 | 4 | 8 | 2 | |||
| Micafungin | WT | ||||||
| AZN8196 | 0.016 | 0.03 | 0.008 | 0.004 | |||
| V52-35 | 0.016 | 0.03 | 0.008 | 0.004 | |||
| DPLRG101b | 0.016 | 0.03 | 0.008 | 0.004 | |||
| Non-WT | |||||||
| DPL1035-homo | 1 | 4 | 8 | 0.5 | |||
| DPL32458 | 8 | 8 | 8 | 1 | |||
Broth microdilution (CLSI, EUCAST, and XTT) and gradient concentration strips (MTS).
Demonstrated a non-WT phenotype only to caspofungin.
Pharmacokinetics.
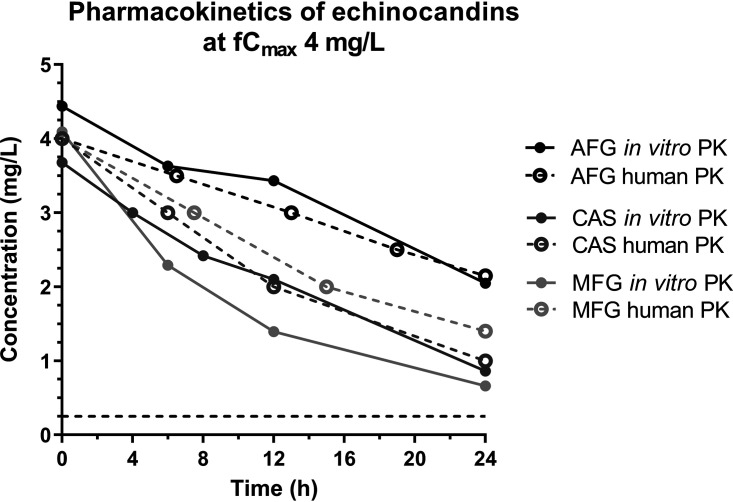
The simulated free drug maximum concentration (fCmax) values for all three echinocandins were within the target values (<20% deviation) with an average (95% confidence internal [CI]) half-life (t1/2) of 22.4 h (18.0 to 29.6 h) for anidulafungin, 13.0 h (9.8 to 19.2 h) for caspofungin, and 8.0 h (6.0 11.8 h) for micafungin for all dosing regimens. Representative time-concentration profiles for each echinocandin are depicted in Fig. 1. Anidulafungin, caspofungin, and micafungin simulated dosages resulted in area under the dosing interval time-free drug concentration curve (fAUC0–24) values of 1.39 to 278.2, 0.20 to 216.0, and 0.10 to 42.6 mg.h/liter, respectively.
FIG 1.
Representative time-concentration profiles for simulated human once-daily dosing regimens of anidulafungin (AFG), caspofungin (CAS), and micafungin (MFG) in an in vitro pharmacokinetic/pharmacodynamic (PK/PD) model with target maximum concentration (fCmax) of 4 mg/liter. Data represent drug levels in the internal compartment of the in vitro model (solid lines) and the respective target values observed in human serum (broken lines).
Pharmacodynamics.
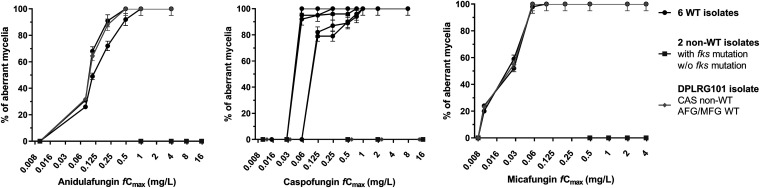
Regarding the WT isolates, the % of aberrant mycelia attached on DMs was progressively increased at higher drug exposures, with minimal variation among replicates (<9%) (Fig. 2). All non-WT isolates formed regular hyphae like those of the drug-free controls. In particular, no aberrant mycelia were observed for non-WT isolates, even at the highest concentrations tested (16 and 4 mg/liter for anidulafungin/caspofungin and micafungin, respectively), irrespective of the different corresponding MECs/MICs. The DPLRG101 demonstrated a non-WT phenotype only to caspofungin, with no formation of aberrant mycelia attached on DMs, in accordance with the elevated MEC compared to those for the other two echinocandins, where aberrant mycelia were observed in accordance with the WT MECs (Fig. 2).
FIG 2.
Percentage of Aspergillus fumigatus aberrant mycelia attached on dialysis membrane tubes after 48 h of incubation at different echinocandin concentrations. Error bars represent standard deviations (CV <9%). AFG, anidulafungin; CAS, caspofungin; MFG, micafungin.
For the WT isolates, near-maximal activity (≥80% aberrant mycelia) was observed at an anidulafungin fCmax of ≥0.25 and ≥0.5 mg/liter for isolates AZN8196/DPLRG101 and V52-35, respectively (Fig. 2). Those isolates had the same CLSI (0.016 mg/liter) and EUCAST (0.03 mg/liter) MECs, but different XTT (0.004/0.004 and 0.008 mg/liter) and MTS (0.004/0.004 and 0.008 mg/liter) MICs, respectively (Table 1). For caspofungin, near-maximal activity was observed at an fCmax of ≥0.06 and ≥0.125 mg/liter for isolates AZN8196/AUH-27/AUH-29 and NIH 4215/AUH-25, respectively (Fig. 2). Despite having identical CLSI (0.25 mg/liter) and EUCAST (0.5 mg/liter) MECs, those isolates exhibited different XTT (0.25/0.25/0.25 and 0.5/0.5 mg/liter) and MTS (0.064/0.064/0.064 and 0.125/0.25 mg/liter) MICs, respectively (Table 1). For micafungin, near-maximal activity was observed at an fCmax of ≥0.06 mg/liter for all isolates (Fig. 2). Notably, anidulafungin and micafungin effective fCmax values are 5 and 2 2-fold dilutions higher than the respective CLSI MECs, while caspofungin fCmax values are one to two 2-fold dilutions lower than the CLSI MECs, indicating sub-MEC antifungal effects only for caspofungin. At lower concentrations (<MEC of micafungin and anidulafungin and <0.25×MEC of caspofungin) healthy hyphae were formed similar to those in drug-free controls and non-WT isolates.
PK/PD relationship.
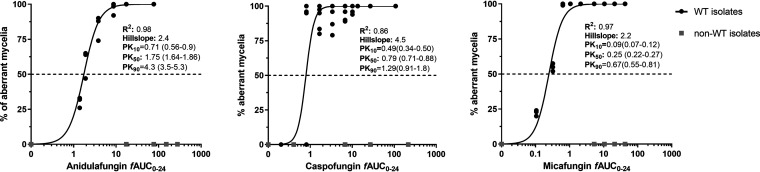
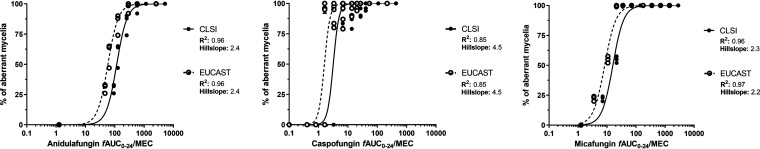
No effect was found even at high echinocandin exposures (fCmax ≥ 4 mg/liter) against non-WT isolates, independent of their MECs, indicating the absence of traditional exposure-response relationships against non-WT isolates. Therefore, non-WT isolates were excluded from PK/PD analysis. The in vitro exposure-effect relationship of the three echinocandins for the WT isolates followed sigmoid curves (R2 = 0.86 to 0.98; Hill slopes, 2.2 to 4.5) (Fig. 3). The in vitro PK/PD relationship of the three echinocandins also followed sigmoid curves (R2 = 0.85 to 0.97; Hill slopes, 2.2 to 4.5) (Fig. 4). Differences were found among the echinocandins, with caspofungin being the most potent and anidulafungin the least potent. The estimated exposure indices associated with 10 to 99.99% of maximal activity (EI10 to EI99.9) for CLSI and EUCAST endpoints are presented in Table 2.
FIG 3.
In vitro exposure-effect relationships of the three echinocandins against A. fumigatus isolates.
FIG 4.
In vitro PK/PD relationship of the three echinocandins against wild-type (WT) A. fumigatus isolates using the CLSI and EUCAST broth microdilution methodology for assessing the in vitro activity of each antifungal. See Table 2 for exposure indices associated with different effects, e.g., 50%, 80%, 90%, etc.
TABLE 2.
Exposure indices associated with 50 to 99.9% (EI50-EI99.99) of maximal activity estimated for each echinocandin and susceptibility testing method based on the Emax model
| Druga | Methodb | Mean (95% confidence interval) PK/PD index (fAUC0–24/MEC) associated with: |
|||||
|---|---|---|---|---|---|---|---|
| EI10 | EI50 | EI90 | EI99 | EI99.9 | EI99.99 | ||
| AFG | CLSI | 47 (37–60) | 116 (109–125) | 287 (232–354) | 766 (494–1,188) | 1,977 (1,020–3,832) | 5,083 (2,028–12,317) |
| EUCAST | 24 (19–30) | 58 (54–62) | 143 (116–177) | 383 (247–594) | 988 (510–1,916) | 2,541 (1,049–6,158) | |
| CAS | CLSI | 1.9 (1.3–2.8) | 3.2 (2.8–3.5) | 5.2 (3.6–7.4) | 8.8 (4.3–18.2) | 14.8 (5.1–43.6) | 24.8 (5.9–104) |
| EUCAST | 1.0 (0.7–1.4) | 1.6 (1.4–1.8) | 2.6 (1.8–3.7) | 4.4 (2.2–9.1) | 7.4 (2.5–21.8) | 12.4 (3.0–52.2) | |
| MFG | CLSI | 6.0 (4.5–8.4) | 15.8 (13.9–18.0) | 40.8 (33.2–50.2) | 115 (74.1–179) | 312 (158–616) | 843 (336–2,120) |
| EUCAST | 3.0 (2.4–4.0) | 8.2 (7.4–9.2) | 22.2 (18.4–26.7) | 65.3 (44.3–96.3) | 185 (102–335) | 521 (234–1,162) | |
AFG, anidulafungin; CAS, caspofungin; MFG, micafungin.
Broth microdilution.
Monte Carlo simulation analysis.
(i) Clinical correlation. Based on Monte Carlo simulation analysis, the caspofungin cumulative fraction of response (CFRs) for EI50, EI80, EI90, EI99, EI99.9, and EI99.99 were 99%, 98%, 97%, 89%, 57%, and 31%, respectively, using the in vitro CLSI PK/PD targets. The CFRs for EI99.9 (57%) and EI99.99 (31%) were comparable with the 56% (18/32) favorable (complete plus partial) and 38% (12/32) complete responses, respectively, at the end of treatment of immunocompromised hematological patients with proven/probable cases of pulmonary IA treated with 50 mg of caspofungin as primary therapy in a clinical trial (15). It is of note that the CFR for EI99 (89%) correlates with the overall treatment success of 78% (25/32) since the invasive fungal infections (IFI)-related mortality was 22% (8/32) (15).
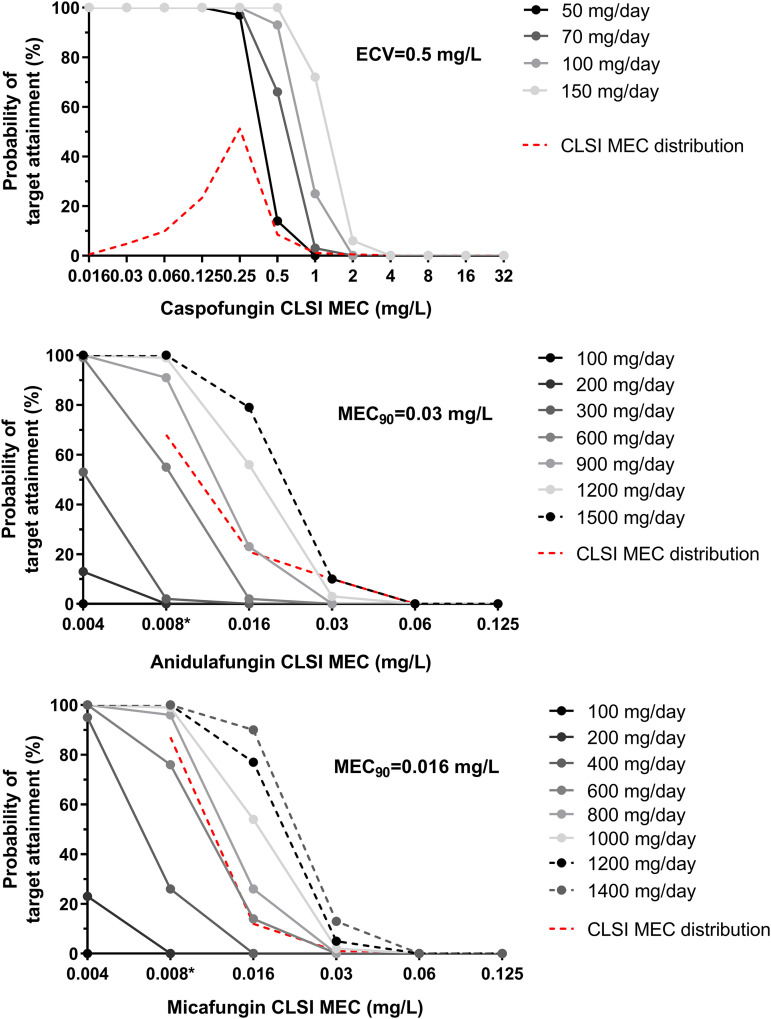
(ii) Probability of target attainment for standard doses of echinocandins. The proportions of patients attaining the corresponding EI99 targets of 766, 8.8, and 115 fAUC0–24/CLSI MEC for anidulafungin, caspofungin and micafungin, respectively, are shown in Fig. 5. A low PTA (13%) was found for A. fumigatus isolates at the CLSI caspofungin epidemiological cutoff value (ECV) of 0.5 mg/liter (16) for the standard dose of 50 mg, while the PTAs for isolates with micafungin and anidulafungin CLSI MEC90 values of 0.016 and 0.03 mg/liter, respectively (17), were 0% with the standard doses of 100 mg.
FIG 5.
Probabilities of EI99 target attainment for 5,000 simulated patients infected with A. fumigatus isolates with different CLSI echinocandin MECs and treated with various caspofungin, anidulafungin, and micafungin doses once daily. The CLSI epidemiological cutoff value (ECV) of caspofungin (16) and minimum effective concentration (MEC) values for 90% of isolates tested (MEC90) of anidulafungin and micafungin (17) against A. fumigatus are shown. The lowest anidulafungin and micafungin concentration tested for the determination of CLSI MEC distribution was 0.008 mg/liter (*) (17).
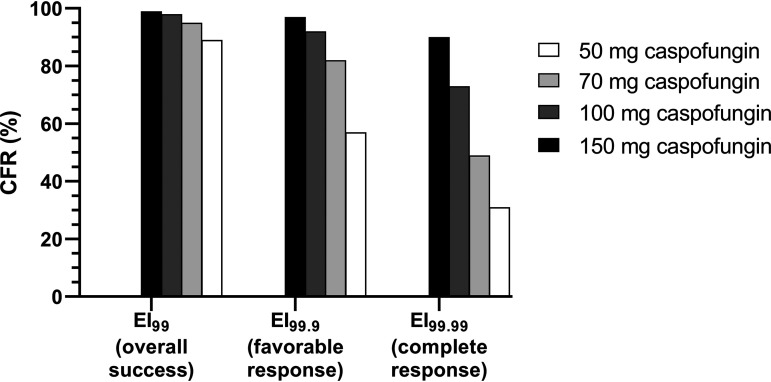
(iii) Dose optimization. The PTA for A. fumigatus isolates at the CLSI caspofungin ECV of 0.5 mg/liter increased significantly when different dosing regimens were explored. In particular, the PTA was 64%, 94%, and 100% for 70, 100, and 150 mg of caspofungin, respectively (Fig. 5). The CFRs based on EI99 increased slightly (<10%) with the alternative caspofungin doses (Fig. 6). On the other hand, the CFRs based on EI99.9 and EI99.99 reached 82%, 92%, and 97% for EI99.9 and 49%, 73%, and 90% for EI99.99 with the 70-, 100-, and 150-mg doses of caspofungin, respectively (Fig. 6). In contrast, for micafungin, based on EI99 a high PTA (90%) for isolates with a CLSI MEC90 of 0.016 mg/liter was found only with the highest dose, 1,400 mg, whereas for anidulafungin, the PTA for isolates with a CLSI MEC90 of 0.03 mg/liter was just 10% with the highest dose, 1,500 mg (Fig. 5).
FIG 6.
Cumulative fractional responses (CFRs) targeting different PK/PD indices for the standard (50 mg) and alternatives doses of caspofungin. The in vitro EI99, EI99.9, and EI99.99 values correlated with overall success, favorable (complete plus partial), and complete response, respectively, observed in a prospective phase II clinical trial where 50 mg of caspofungin was used as primary therapy for pulmonary invasive aspergillosis (IA) (15).
DISCUSSION
In the present study, a recently proposed in vitro endpoint was used to describe the echinocandins’ exposure-response relationships. Although a sigmoidal relationship was found for WT A. fumigatus isolates, all non-WT isolates behaved similarly in the in vitro model, with no formation of aberrant mycelia irrespective of their MEC and echinocandins’ exposure. For the WT strains, the in vitro PK/PD targets EI99 were 766, 8.8, and 115 fAUC0–24/CLSI MEC for anidulafungin, caspofungin and micafungin, respectively. Bridging those PK/PD targets with human PK of standard doses of echinocandins and MEC distribution of WT A. fumigatus isolates, a very low PTA (<13%) was found at the ECV and MIC90 of all three echinocandins. Simulating higher doses, a PTA of ≥90% was found for WT A. fumigatus strains with 100 and 150 mg/day of caspofungin and 1,400 mg/day of micafungin, in contrast to anidulafungin, where a PTA of 10% was found with a dose of 1,500 mg/day.
An interesting observation in the present study was that in addition to supra-MEC exposure-dependent effects observed for all three echinocandins, only caspofungin exhibited a sub-MEC antifungal effect at 0.5 to 0.25×MEC as well. The sub- and supra-MEC effects of caspofungin may be attributed to the dual-uptake model proposed for caspofungin transport into the cell, a high-affinity facilitated-diffusion carrier at low sub-MEC drug levels (≤1 μg/ml) and a nonspecific drug uptake through normal diffusion pathways across the bilayer of the plasma membrane at higher supra-MEC drug levels (18). In agreement with previous concentration-effect relationships (19), among the three echinocandins, caspofungin has the steepest and micafungin and anidulafungin the shallowest exposure-response curves. It is of note that micafungin and anidulafungin have similar structures in their aromatic side chains, whereas caspofungin has an aliphatic side chain. In agreement with our findings, where no aberrant mycelia were found for the non-WT isolates, when microcolonies of a caspofungin non-WT A. fumigatus isolate grown on porous aluminum oxide strips were stained with Syto9/propidium iodide, tip lysis was rarely detected at echinocandin concentrations sufficient to cause lysis in WT strains (20). No paradoxical phenomena were observed in the present study, in agreement with previous observations that such phenomena occur at a Cmax of >8 mg/liter on solid medium and in static liquid cultures (20–22).
The maximal efficacy (75 to 85% 4-day survival) of caspofungin in a temporarily neutropenic (cyclophosphamide IV 1 day before infection) murine model of pulmonary IA was found at plasma total AUC (tAUC) (fAUC) of 82.5 (2.5) mg · h/liter plasma, which corresponded to an AUC/MEC of 331 (fAUC/MEC of 9.9), in line with the effect in the present study (100%) (Fig. 4) (12). Similar findings were observed in a profoundly neutropenic (<100 granulocytes/μl for 10 days) rat model of pulmonary IA, in which tAUC (fAUC) values of 91.8 (2.8) and 20.1 (0.6) mg · h/liter were associated with 100% and 60% survival, respectively, as predicted from the exposure-response relationship in the present study (100% and ∼30%, respectively) (Fig. 3), indicating that the depth of neutropenia does not affect caspofungin efficacy (23). For micafungin, an AUC0–24 of 36.33 mg · h/liter (fAUC0–24 0.073 mg · h/liter) was associated with 20% 12-day survival in a model of persistently neutropenic (<100 granulocytes/μl with AraC every other day) rabbits infected intratracheally with A. fumigatus (13), whereas in a persistently neutropenic (cyclophosphamide every 3 days and cortisone on day 1) mouse model of pulmonary IA, 3 mg/kg/day (tAUC ∼ 84; fAUC ∼ 0.17 mg · h/liter [24]) of micafungin for 3 days resulted in 0% survival compared to that with placebo (25), effects that are close to the predicted effects by the exposure-effect relationship of micafungin in the present study (∼10% and ∼25%, respectively) (Fig. 3). Even in a nonneutropenic but glucocorticoid-suppressed (1 dose before infection) murine model of pulmonary IA, 0% survival was found with doses up to 10 mg/kg (26) (tAUC ∼ 260 mg · h/liter; fAUC = 0.52 mg · h/liter [24]). However, doses of 5 to 10 mg/kg (tAUC ∼ 138 to 260 mg · h/liter; fAUC = 0.28 to 0.52 mg · h/liter [24]) and as low as 1 mg/kg (tAUC ∼ 30 mg · h/liter; fAUC = 0.06 mg · h/liter [24]) in a temporarily neutropenic murine model of pulmonary IA resulted in 100% survival, indicating a profound effect of immune system and particularly of lung effector cells (e.g., alveolar macrophages and dendritic cells) on micafungin efficacy (27, 28). An anidulafungin fAUC0–24/CLSI MEC value of 43.3 was associated with 18% 23-day survival in transiently neutropenic rats with pulmonary IA (29) and 45% to 73% in a nonneutropenic murine model of disseminated IA with an anidulafungin fAUC0–24/EUCAST MEC of 108.7 (11), which are both within the predicted effects based on the exposure-effect relationship in the present study (∼10% and ∼75%, respectively) (Fig. 4). Thus, the in vitro model predicts the efficacy of caspofungin and anidulafungin in animal models of pulmonary IA, which seems not to be affected by underlying immunosuppression, where for micafungin, the in vitro model predicts the efficacy only in profoundly neutropenic animal models of pulmonary IA, since micafungin activity was enhanced by the local immune system, as previously found (30).
We have previously shown that the results of the in vitro PK/PD model were comparable to the in vivo outcome of anidulafungin therapy in a nonneutropenic model of experimental aspergillosis using the same A. fumigatus isolates, as the % of aberrant mycelia attached on DMs corresponded to 14-day survival in mice (14). In the present study, a clinical correlation of the % of aberrant mycelia was found for caspofungin when used as primary therapy against pulmonary IA in immunocompromised patients with hematological malignancies (15). Interestingly, we found that the fAUC0–24/MEC associated with 99% (EI99), 99.9% (EI99.9), and 99.99% (EI99.99) effects correlated with overall treatment success based on IFI-related mortality, favorable response (complete plus partial, where complete response is the resolution of all clinical signs and symptoms related to IFI with complete radiographic resolution, and partial response is the improvement or resolution of IFI clinical signs and symptom with improvement in radiological abnormalities of >50%), and complete response, respectively. Notably, the EC99, EC99.9, and EC99.99 values corresponded to 2-, 3-, and 4-log10 CFU reductions of unaffected conidia and a simultaneous increase of aberrant mycelia of the initial inoculum (as these two forms of conidia are supplementary, as shown previously [14]), giving a microbiological meaning of those endpoints as well. Since all calculations were made based on free drug levels in blood and because echinocandins are large molecules with small volumes of distribution, the 4-log10 effect based on blood levels may result in a 2-, 3-, or 4-log10 effect at the site of infection if tissue penetration is 1/100, 1/10, or 1/1, respectively. Furthermore, as PD effects were explored against conidia in the present study, the findings may be more applicable for early treatment, as in the settings of empirical therapy for or prophylaxis from fungal infections in febrile neutropenic patients where caspofungin is indicated.
Despite the fact that the efficacy of caspofungin as a first-line therapy of IA has not yet been evaluated in controlled randomized studies, a literature review paper has identified 7 studies where caspofungin was used as primary therapy against IA (4). Prospective phase II studies where caspofungin standard dose was used as primary therapy for IA reported a median 38% favorable response rate (31, 32), whereas in retrospective analyses and prospective observational registries, a wide range of favorable response rates (27 to 92%; median, 54%) was observed (4) that is similar to the CFR of 57% for the 3-log10 CFU reduction of unaffected conidia/aberrant mycelium formation endpoint found in the present study. Of note, voriconazole and liposomal amphotericin B, which are approved as primary therapies against IA, resulted in 53% (21% complete plus 32% partial) (33) and 50% (1% complete plus 49% partial) (34) favorable responses, respectively, which suggests that caspofungin has similar response rates. However, in both voriconazole and liposomal amphotericin B clinical trials, the 12-week survival rate was 71% (33, 34), which is higher than the 50% to 53% survival rates in the two multicenter prospective phase II studies of caspofungin (standard dose) primary therapy against IA in patients with hematological malignancies or autologous transplants (32) and in allogeneic transplants (31).
Nevertheless, various parameters, such as the underlying disease, neutropenia, and duration of treatment, could explain this difference. Indeed, subset analyses in the AmBiLoad study revealed significant negative associations for the baseline factors of allogeneic stem cell transplantation (12-week survival of 40% versus 71% for no transplantation) and uncontrolled hematological malignancy (12-week survival of 54% versus 81% for controlled malignancy) (34). In a voriconazole trial where the median (range) duration of treatment was 77 days (2 to 84 days), 52% of patients had hematological malignancies and 45% were neutropenic at some point during 2 weeks before the enrollment (33), whereas in the AmBiLoad trial, where the median (range) duration of treatment was 15 days (1 to 60 days), 93% of patients had hematological malignancies (64% uncontrolled) and 71% were neutropenic at baseline (34). In the two multicenter prospective phase II studies of caspofungin (standard dose) primary therapy for IA in patients with hematological malignancies or autologous transplants, the median duration of therapy was 27 days, 85% of patients were neutropenic at start of treatment, 75% had cancer not in remission, and 50% had poor clinical condition (Karnofsky score, <50) (32). In allogeneic transplants, the median duration of therapy was 24 days, and 50% of patients were neutropenic at baseline (31). It is of note that only patients with documented mycological criteria were considered to have IA and were enrolled in the latter studies, as opposed to the AmBiLoad trial (34) and, to a lesser extent, the voriconazole trial (33), in which a high proportion of high-risk patients with halo sign only and without mycological confirmation of IA were enrolled. Thus, in primary therapy studies, caspofungin was given to patients with mycologically confirmed IA for a shorter period than voriconazole (24 days versus 77 days) and in more neutropenic (85% versus 45%) with poor condition patients. Thus, caspofungin may indeed have a role in the management of IA, at least for a subset of patients with similar characteristics as those in the voriconazole clinical trial (33), provided that dose and treatment duration are optimized.
A higher dose of 150 mg/day of caspofungin resulted in an improved favorable response of 86%, which is in line with our finding supporting dose optimization (35). Importantly, no serious adverse events or dose-limiting toxicity were reported in most of the studies assessing caspofungin as first-line therapy for IA, even at higher doses (200 mg/day), classifying it in the category of antifungals with a good safety profile (4). In a dose-escalation study (70 to 200 mg/day of caspofungin) including 44 patients with immunocompromising condition associated with IFI and evidence of IA, the 12-week survival rate was 72% (median duration of therapy, 24.5 days), corroborating our findings (35). However, 28% of late relapses after a 12-week follow-up was observed, highlighting the need for longer treatment with caspofungin (35). Thus, notwithstanding the inevitable variation, the survival and response rates of caspofungin therapy are comparable with those of other antifungal agents licensed as primary therapy for IA. A randomized controlled clinical trial is needed in order to assess the efficacy of higher than the standard dose of 100 and 150 mg of caspofungin against IA.
The efficacy of micafungin monotherapy for the initial treatment of aspergillosis was assessed in a postmarketing survey showing a clinical response of 92/130 (70.8%) patients with micafungin (50 to 300 mg/day), although there was no information on the type of Aspergillus infection, and the diagnosis and clinical response were assessed based on the decision of the physician in charge rather than on quantifiable measures (36). A small Japanese multicenter study of 50 to 150 mg/day of micafungin up to 56 days reported an overall clinical response rate (improvements of radiologic invasive shadows and clinical symptoms) of 60% (6/10; 8 with leukemia/lymphoma, only 2 neutropenic) (37) whereas a prospective, open-label, noncomparative, multinational study with just 12 patients (2 neutropenic at baseline) with IA treated with 121 ± 64.9 mg/day for a mean duration of 61.3 days of micafungin as primary therapy showed no complete response and only 6/12 (50%) patients with partial response, with the remaining patients showing progression (4/12) and stabilization (2/12) (38). Similarly, in a noncomparative, phase IV open-label study with 42 patients with IA based on EORTC-MSG criteria treated with 50 to 300 mg/day of micafungin for up to 12 weeks, no complete and ∼50% partial responses were, found with the remaining showing stable disease (∼30%) or progression (39). Based on Monte Carlo simulation in the present study, the optimal dose (>90% PTA) of micafungin required to attain the clinically relevant in vitro EI99 which correlated with overall success is 1,400 mg every 24 h (q24h) for neutropenic patients. Using a stricter endpoint of EI99.9, which correlated with favorable response (complete plus partial), a much higher dose of micafungin would be required. Although micafungin has been safely administered in patients undergoing hematopoietic stem cell transplantation at repeated daily doses up to a mean (range) of 600 (442 to 896) mg/day for a median (range) of 18 days (8 to 28 days) with no evidence of dose-related toxicity (40), the safely of such extremely high doses is questionable. Since micafungin PD are greatly improved (>5 to 10 times) in animal models with an active local lung immune response, micafungin may have a role against pulmonary IA in patients recovering from neutropenia or against non-neutropenic patients like those with chronic pulmonary aspergillosis (41). Micafungin may be equally effective as caspofungin as empirical or prophylactic therapy for IFIs, including IA, in neutropenic patients (42, 43) which could be explained by the closer EI10 values, i.e., 1-log10 effect (if this is the clinically relevant PK/PD target for empirical or prophylactic therapy because fungal load is expected to be low), of the two compounds found in the present study.
Although a post hoc analysis of the randomized clinical trial of voriconazole-anidulafungin combination therapy demonstrated that 6-week all-cause mortality was significantly lower in a subset of patients with maximum galactomannan positivity and radiographic findings (−11.5% difference in favor of combination; P = 0.037) (44), data regarding anidulafungin monotherapy of IA are lacking. Monte Carlo analysis showed that anidulafungin was not prone to dose optimization, as the PTA was low (10%), even for the highest dose tested (1,500 mg/day).
In conclusion, the previously validated in vitro PK/PD model of echinocandins correlated with different clinical outcomes of caspofungin therapy in patients with IA, indicating that the present in vitro model’s predictions are within the clinical effect ranges. This model can be used to study the PD and optimize exposure of echinocandins against Aspergillus spp. This is of great importance given the lack of preclinical models for studying echinocandins and in light of new echinocandin drugs and glucan synthesis inhibitors under development. By no means does the correlation between in vitro endpoints and clinical endpoints/outcome indicate that the model can be used to make clinical predictions, but it can be used to generate hypotheses for further clinical investigation. Our results showed that among the three echinocandins, only caspofungin at higher than the standard doses of 100 and 150 mg/day may have a role in primary therapy against IA in neutropenic patients. This hypothesis warrants further clinical verification given the emergence of azole-resistance in A. fumigatus.
MATERIALS AND METHODS
Fungal isolates and antifungal susceptibility.
A total of six WT molecularly identified A. fumigatus clinical isolates with identical CLSI (45) anidulafungin, caspofungin, and micafungin MECs of 0.016, 0.25, and 0.016 mg/liter, respectively, were studied. In addition, the following three non-WT A. fumigatus isolates from D. Perlin’s laboratory (DPL) collection possessing elevated echinocandin MEC values were tested: the genetically engineered DPL1035-homo (46) isolate with S678P fks alteration and CLSI anidulafungin, caspofungin, and micafungin MECs of 4, 16, and 1 mg/liter; the clinical isolate DPL32458 (47) without known fks mutation and with CLSI anidulafungin, caspofungin, and micafungin MECs of 2, 16, and 8 mg/liter; and the spontaneous mutant DPLRG101 (47) after exposure to caspofungin without known fks alteration and with CLSI anidulafungin, caspofungin, and micafungin MECs of 0.016 (WT), 8 (non-WT), and 0.016 (WT) mg/liter, respectively. Antifungal susceptibility testing was also performed according to the EUCAST recommendations (48) and a recently described XTT [2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide] method based on 24 h 50% inhibition endpoint (49) and using gradient concentration MIC test strips (MTS; Liofilchem, Roseto degli Abruzzi, Italy) based on 24 h complete growth inhibition endpoint according to the manufacturer’s instructions. The isolates were stored in normal sterile saline with 10% glycerol at –70°C until use.
In vitro PK/PD model.
A previously optimized two-compartment dialysis/diffusion closed PK/PD model (14) was used with a 10-ml semipermeable cellulose DM tube with 20-kDa molecular weight cutoff [MWCO] pore sizes (Spectra/Por Float-A-Lyzer G2; Spectrum Laboratories, Inc., Breda, The Netherlands). The DM tube was inoculated with 103 CFU/ml conidial suspension in medium (RPMI 1640 [with l-glutamine and without bicarbonate] plus 0.165 M morpholinepropanesulfonic acid [MOPS] supplemented with 100 mg/liter chlorampenicol; AppliChem, Darmstadt, Germany) and placed inside a conical flask with medium that was diluted with fresh medium using a peristaltic pump at a rate equivalent to the drug’s elimination rate. The system was incubated on a magnetic heating stirrer at 37°C.
In vitro pharmacokinetics.
Different anidulafungin, caspofungin, and micafungin exposures with average beta elimination half-lives (t1/2) of 26 h (50), 12 h (51), and 15 h (52), respectively, were simulated in the in vitro PK/PD model targing a wide range of free drug maximum concentration (fCmax) (0.01 to 16 mg/liter of anifulafungin and caspofungin and 0.01 to 4 mg/liter of micafungin) in order to capture both noneffective and highly effective drug exposures. Anidulafungin (Pfizer, Inc., Groton, CT), caspofungin acetate (Merck & Co., Inc., Whitehouse, NJ) or micafungin (Astellas Pharma, Inc., Tokyo, Japan) solution prepared in dimethyl sulfoxide (DMSO; Chem-Lab NV, Zedelgem, Belgium) was injected at the corresponding fCmax values in both compartments of the model once daily for 48 h. Drug levels were determined at regular time intervals using microbiological agar diffusion assays for each echinocandin as previously described (53–55). A concentration-time curve was generated for each drug’s simulated dose and analyzed by nonlinear regression analysis using the equation Ct = C0 × e−kt, where Ct (dependent variable) is the concentration of drug at a given time t (independent variable), C0 is the initial concentration of the drug at t = 0 h, e is the physical constant 2.718, and k is the rate of drug removal. The t1/2 of each echinocandin was calculated using the equation t1/2 = 0.693/k and compared with the respective values observed in human serum. The area under the dosing interval time-free drug concentration curve (fAUC0–24) was calculated for each drug’s dosing regimen by applying the trapezoidal rule.
In vitro pharmacodynamics.
The antifungal effect of each echinocandin was determined using the % of aberrant mycelia attached on DMs, which was calculated based on the vertical height (cm) covered by aberrant mycelia (single isolated mycelia were not taken into account) on the DM after 48 h divided by the DM’s total height (12.3 cm), as recently described (14) (Fig. 7). We have previously shown that the number of aberrant mycelia affected by echinocandins attached on a DM was inversely related to the number of unaffected conidia floating inside the DM tube, while the 99% effect correlated with a 2-log10 CFU/ml reduction of unaffected floating conidia from the initial inoculum and increased formation of aberrant mycelia (14).
FIG 7.
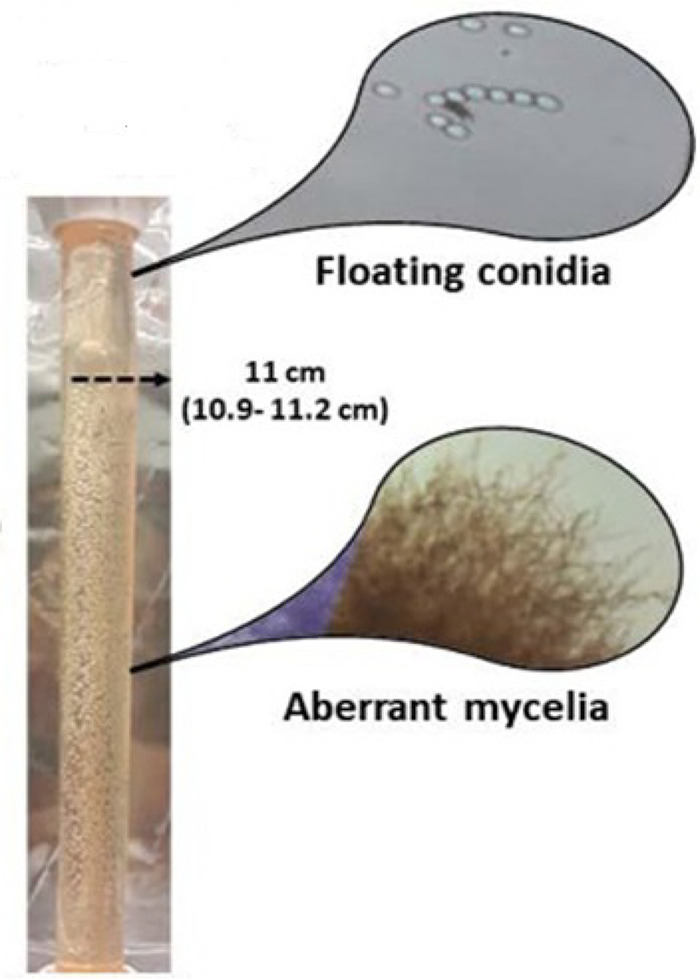
Aspergillus conidia after exposure to echincoandins forms, in a dose-dependent manner, aberrant mycelia that are attached to the dialysis membrane tubes (DM). The % of aberrant mycelia can be quantified using the height in the tube (height covered by aberrant mycelia/total height of DM). Unaffected conidia are floating inside the DM and can be quantified with CFU counts (14).
PK/PD modeling.
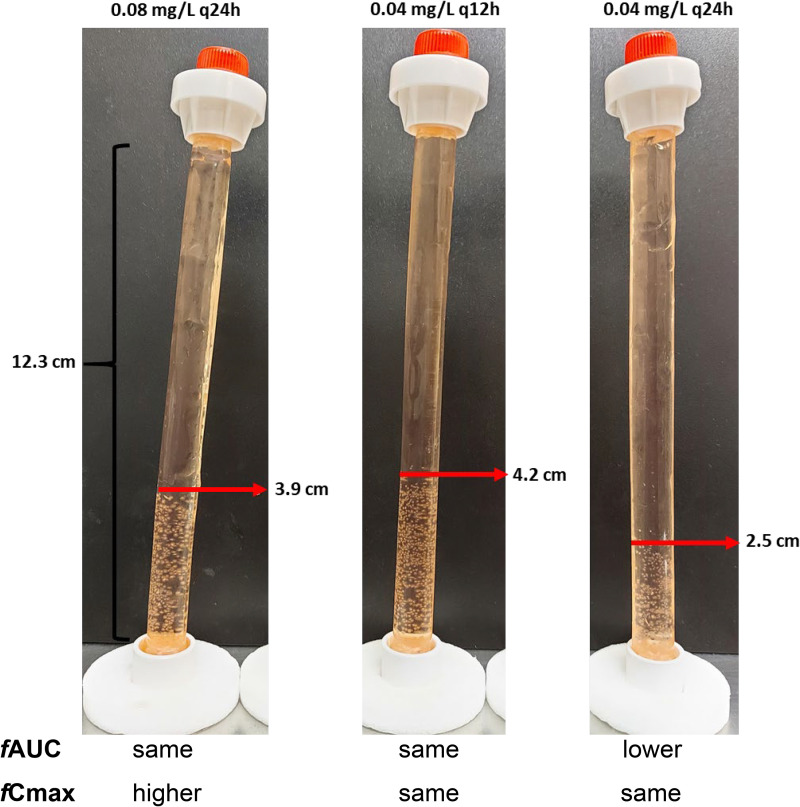
Previous dose fractionation studies with all three echinocandins showed that their activity is best described by fAUC rather than by fCmax (14). Since in the latter study, highly effective dosing regimens (>50% effect) were used, we conducted further dose fractionation studies using dosing regimens with <50% effect (0.08 mg/liter q24h, 0.04 mg/liter q12h, and 0.04 mg/liter q24h), and the same conclusion was found, i.e., 0.08 mg/liter q24h and 0.04 mg/liter q12h, which had the same AUC but a different Cmax, were equally and more effective than 0.04 mg/liter q24h (Fig. 8). The in vitro relationship of % aberrant mycelia versus fAUC0–24 after 48 h of treatment with each echinocandin was analyzed by nonlinear regression analysis using the sigmoidal Emax model with variable slope described by the equation E = (Emax − Emin) × PKn/(PKn + PK50n) + Emin, where Emax and Emin are the maximum (100%) and minimum (0%) effects, respectively, PK is the PK parameter fAUC0–24, PK50 is the PK parameter corresponding to 50% of Emax− Emin, and n is the Hill slope. The PK parameters associated with 10% (PK10) and 90% (PK90) of maximal activity was also determined. Furthermore, the in vitro exposure-response relationship of each echinocandin was explored by analyzing the in vitro relationship of % aberrant mycelia with fAUC0–24/MEC using the Emax model as described above. The goodness of fit of the Emax model was assessed by visual inspection of graphs, postrun tests, and R2. Different exposure indices associated with 10%, 50%, 90%, 99%, 99.9%, and 99.99% of maximal activity (EI10 to EI99.99) were also estimated for each echinocandin. It is of note that 99%, 99.9%, and 99.99% effect corresponds to a 2-, 3-, and 4-log10 CFU/ml reduction of unaffected floating conidia of initial inoculum and a proportional increase in aberrant mycelia attached on DMs (14).
FIG 8.
Dose fractionation experiment with suboptimal dosing regimens of anidulafungin (<50% effect) indicates that the fAUC0–24/MEC ratio is the driving PK/PD index. Drug-affected aberrant mycelia are attached on the dialysis membrane in an exposure-dependent manner (the higher the drug exposure, the more of the dialysis tube is covered by those aberrant mycelia). The regimen with 0.08 mg/liter every 24 h (q24h; left photo) exhibited an effect similar to that of a regimen of 0.04 mg/liter q12h (middle photo) (the two regimens had the same AUC but different Cmax values) and an effect lower than that of the regimen of 0.04 mg/liter q24h (right photo) (the latter regimen had a lower AUC than the other two and the same fCmax as the 0.04 q12h regimen).
Monte Carlo simulation.
To bridge the in vitro data with human PK, Monte Carlo simulation analysis was performed using the normal random number generator function of Excel spreadsheet (Microsoft Office 2010) for patients treated with the standard and alternative intravenous doses of each echinocandin.
(i) Caspofungin.
In order to find which is the most clinically relevant EI, the CFRs (expected population probability of target attainment for a certain drug dose and a specific population of microorganisms) were estimated for different EI10 to EI99.99 values for a previously published CLSI caspofungin MEC distribution of clinical A. fumigatus isolates, with a median (range) of 0.25 (0.016 to 32) mg/liter and ECV (MEC90) of 0.5 mg/liter (16), and for the standard caspofungin dose of 50 mg (1) previously used as first-line therapy for the treatment of pulmonary IA in a prospective phase II clinical trial (15). The PTA using the most clinically relevant EI was then calculated for A. fumigatus isolates, with CLSI caspofungin MECs of 0.016 to 4 mg/liter (16), using Monte Carlo analysis, for 5,000 hematology patients treated with 50, 70, 100, and 150 mg q24h and attaining steady-state mean ± standard deviation (SD) AUC0–24 values of 117 ± 28, 175 ± 56, 250 ± 80, and 375 ± 120 mg · h/liter (56, 57), respectively, taking into account the 97% protein binding (51).
(ii) Anidulafungin.
The PTA using the most clinically relevant EI was calculated for A. fumigatus isolates with CLSI anidulafungin MECs 0.004 to 0.125 mg/liter (17). Monte Carlo analysis was used to simulate 5,000 hematology patients treated with the licensed 100-mg daily dose regimen (1), corresponding to a steady-state mean ± SD AUC0–24 of 110.3 ± 37 mg · h/liter (58), and with 200, 300, 600, 900, 1,200, and 1,500 mg of anidulafungin q24h, attaining theoretically proportionally increased exposure and the same coefficient of variation with mean ± SD AUC0–24 values of 220.6 ± 74, 330.9 ± 111, 661.8 ± 222, 992.7 ± 333, 1,323.6 ± 444, and 1,654.5 ± 555 mg · h/liter, respectively, considering the 99% protein binding (50) and the linear PK in humans (59).
(iii) Micafungin.
The PTA using the most clinically relevant EI was calculated for A. fumigatus isolates with CLSI micafungin MECs of 0.004 to 0.125 mg/liter (17). Monte Carlo analysis was performed simulating 5,000 hematological patients treated with the standard 100-mg daily dose regimen (1), corresponding to a steady-state mean ± SD AUC0–24 of 97.1 ± 29 mg · h/liter (60), as well as with 200, 400, 600, 800, 1,000, 1,200, and 1,400 mg of micafungin q24h, attaining theoretically proportionally increased exposure and the same coefficient of variation with mean ± SD AUC0–24 values of 194.2 ± 58, 388.4 ± 116, 663 ± 212, 776.8 ± 232, 971 ± 290, 1,165.2 ± 348, and 1,359.4 ± 406 mg · h/liter, respectively, taking into account the 99.8% protein binding (52) and the linear PK in humans (61).
All data were analyzed using the statistics software package Prism version 7.0 for Windows (GraphPad Software, San Diego, CA). All experiments were carried out in duplicate and were independently performed on two different days with individually prepared inocula.
ACKNOWLEDGMENT
We declare that we have no conflicts of interest related to this study.
REFERENCES
- 1.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Flörl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Brüggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Löffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinkó J, Skiada A, Vehreschild MJGT, Viscoli C, Cornely OA. 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young J-AH, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aruanno M, Glampedakis E, Lamoth F. 2019. Echinocandins for the treatment of invasive aspergillosis: from laboratory to bedside. Antimicrob Agents Chemother 63:e00399-19. doi: 10.1128/AAC.00399-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinz WJ, Buchheidt D, Ullmann AJ. 2016. Clinical evidence for caspofungin monotherapy in the first-line and salvage therapy of invasive Aspergillus infections. Mycoses 59:480–493. doi: 10.1111/myc.12477. [DOI] [PubMed] [Google Scholar]
- 5.Enoch DA, Idris SF, Aliyu SH, Micallef C, Sule O, Karas JA. 2014. Micafungin for the treatment of invasive aspergillosis. J Infect 68:507–526. doi: 10.1016/j.jinf.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Pang K-AP, Godet C, Fekkar A, Scholler J, Nivoix Y, Letscher-Bru V, Massias L, Kauffmann-Lacroix C, Elsendoorn A, Uzunov M, Datry A, Herbrecht R. 2012. Breakthrough invasive mould infections in patients treated with caspofungin. J Infect 64:424–429. doi: 10.1016/j.jinf.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Arendrup MC, Garcia-Effron G, Buzina W, Mortensen KL, Reiter N, Lundin C, Jensen HE, Lass-Florl C, Perlin DS, Bruun B. 2009. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob Agents Chemother 53:1185–1193. doi: 10.1128/AAC.01292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madureira A, Bergeron A, Lacroix C, Robin M, Rocha V, de Latour RP, Ferry C, Devergie A, Lapalu J, Gluckmana E, Socié G, Ghannoum M, Ribaud P. 2007. Breakthrough invasive aspergillosis in allogeneic haematopoietic stem cell transplant recipients treated with caspofungin. Int J Antimicrob Agents 30:551–554. doi: 10.1016/j.ijantimicag.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Wetzstein GA, Green MR, Greene JN. 2007. Mould breakthrough in immunosuppressed adults receiving anidulafungin: a report of 2 cases. J Infect 55:e131–e133. doi: 10.1016/j.jinf.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Groll AH, Mickiene D, Petraitiene R, Petraitis V, Lyman CA, Bacher JS, Piscitelli SC, Walsh TJ. 2001. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob Agents Chemother 45:2845–2855. doi: 10.1128/AAC.45.10.2845-2855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seyedmousavi S, Bruggemann RJM, Melchers WJG, Verweij PE, Mouton JW. 2013. Pharmacodynamics of anidulafungin against clinical Aspergillus fumigatus isolates in a nonneutropenic murine model of disseminated aspergillosis. Antimicrob Agents Chemother 57:303–308. doi: 10.1128/AAC.01430-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J Infect Dis 190:1464–1471. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 13.Petraitis V, Petraitiene R, Groll AH, Roussillon K, Hemmings M, Lyman CA, Sein T, Bacher J, Bekersky I, Walsh TJ. 2002. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother 46:1857–1869. doi: 10.1128/AAC.46.6.1857-1869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meletiadis J, Siopi M, Tsakris A, Mouton JW, Pournaras S. 2018. A new marker of echinocandin activity in an in vitro pharmacokinetic/pharmacodynamic model correlates with an animal model of Aspergillus fumigatus infection. Antimicrob Agents Chemother 62:e02322-17. doi: 10.1128/AAC.02322-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candoni A, Mestroni R, Damiani D, Tiribelli M, Michelutti A, Silvestri F, Castelli M, Viale P, Fanin R. 2005. Caspofungin as first line therapy of pulmonary invasive fungal infections in 32 immunocompromised patients with hematologic malignancies. Eur J Haematol 75:227–233. doi: 10.1111/j.1600-0609.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 16.Espinel-Ingroff A, Fothergill A, Fuller J, Johnson E, Pelaez T, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for caspofungin and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob Agents Chemother 55:2855–2859. doi: 10.1128/AAC.01730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2009. In vitro susceptibility of clinical isolates of Aspergillus spp. to anidulafungin, caspofungin, and micafungin: a head-to-head comparison using the CLSI M38-A2 broth microdilution method. J Clin Microbiol 47:3323–3325. doi: 10.1128/JCM.01155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paderu P, Park S, Perlin DS. 2004. Caspofungin uptake is mediated by a high-affinity transporter in Candida albicans. Antimicrob Agents Chemother 48:3845–3849. doi: 10.1128/AAC.48.10.3845-3849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. 2008. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob Agents Chemother 52:321–328. doi: 10.1128/AAC.00699-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingham CJ, Schneeberger PM. 2012. Microcolony imaging of Aspergillus fumigatus treated with echinocandins reveals both fungistatic and fungicidal activities. PLoS One 7:e35478. doi: 10.1371/journal.pone.0035478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortwendel JR, Juvvadi PR, Perfect BZ, Rogg LE, Perfect JR, Steinbach WJ. 2010. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob Agents Chemother 54:1555–1563. doi: 10.1128/AAC.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiederhold NP. 2009. Paradoxical echinocandin activity: a limited in vitro phenomenon? Med Mycol 47:S369–S375. doi: 10.1080/13693780802428542. [DOI] [PubMed] [Google Scholar]
- 23.Van Vianen W, De Marie S, Ten Kate MT, Mathot RAA, Bakker-Woudenberg IAJM. 2006. Caspofungin: antifungal activity in vitro, pharmacokinetics, and effects on fungal load and animal survival in neutropenic rats with invasive pulmonary aspergillosis. J Antimicrob Chemother 57:732–740. doi: 10.1093/jac/dkl015. [DOI] [PubMed] [Google Scholar]
- 24.Andes DR, Diekema DJ, Pfaller MA, Marchillo K, Bohrmueller J. 2008. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 52:3497–3503. doi: 10.1128/AAC.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barchiesi F, Santinelli A, Biscotti T, Greganti G, Giannini D, Manso E. 2016. Delay of antifungal therapy influences the outcome of invasive aspergillosis in experimental models of infection. J Antimicrob Chemother 71:2230–2233. doi: 10.1093/jac/dkw111. [DOI] [PubMed] [Google Scholar]
- 26.Clemons KV, Stevens DA. 2006. Efficacy of micafungin alone or in combination against experimental pulmonary aspergillosis. Med Mycol 44:69–73. doi: 10.1080/13693780500148350. [DOI] [PubMed] [Google Scholar]
- 27.Warn PA, Morrissey G, Morrissey J, Denning DW. 2003. Activity of micafungin (FK463) against an itraconazole-resistant strain of Aspergillus fumigatus and a strain of Aspergillus terreus demonstrating in vivo resistance to amphotericin B. J Antimicrob Chemother 51:913–919. doi: 10.1093/jac/dkg185. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto S, Wakai Y, Nakai T, Hatano K, Ushitani T, Ikeda F, Tawara S, Goto T, Matsumoto F, Kuwahara S. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob Agents Chemother 44:619–621. doi: 10.1128/aac.44.3.619-621.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Sande WWJ, Mathot RAA, ten Kate MT, van Vianen W, Tavakol M, Rijnders BJA, Bakker-Woudenberg IAJM. 2009. Combination therapy of advanced invasive pulmonary aspergillosis in transiently neutropenic rats using human pharmacokinetic equivalent doses of voriconazole and anidulafungin. Antimicrob Agents Chemother 53:2005–2013. doi: 10.1128/AAC.01556-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretti S, Bozza S, Massi-Benedetti C, Prezioso L, Rossetti E, Romani L, Aversa F, Pitzurra L. 2014. An immunomodulatory activity of micafungin in preclinical aspergillosis. J Antimicrob Chemother 69:1065–1074. doi: 10.1093/jac/dkt457. [DOI] [PubMed] [Google Scholar]
- 31.Herbrecht R, Maertens J, Baila L, Aoun M, Heinz W, Martino R, Schwartz S, Ullmann AJ, Meert L, Paesmans M, Marchetti O, Akan H, Ameye L, Shivaprakash M, Viscoli C, for the Infectious Diseases Group of the EORTC. 2010. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant 45:1227–1233. doi: 10.1038/bmt.2009.334. [DOI] [PubMed] [Google Scholar]
- 32.Viscoli C, Herbrecht R, Akan H, Baila L, Sonet A, Gallamini A, Giagounidis A, Marchetti O, Martino R, Meert L, Paesmans M, Ameye L, Shivaprakash M, Ullmann AJ, Maertens J, Infectious Disease Group of the EORTC. 2009. An EORTC Phase II study of caspofungin as first-line therapy of invasive aspergillosis in haematological patients. J Antimicrob Chemother 64:1274–1281. doi: 10.1093/jac/dkp355. [DOI] [PubMed] [Google Scholar]
- 33.Aoul R, Erbrecht H, Enning AWD, Atterson HFP, Ohn J, Ennett EB, Eginald R, Reene EG, Estmann JÖ-W, Ern IVK, Arr IAM, Atricia P, Ibaud R, Ortholary LL, Ichard R, Ylvester S, Ubin OHR, Ingard RW, Aul P, Tark S, Hristine C, Urand D, Enis D, Aillot C, Ckhard E, Hiel T, Ranatharthi P, Handrasekar HC, Odges IRH, Chlamm ATS, Roke EFT. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415. [DOI] [PubMed] [Google Scholar]
- 34.Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, Heussel CP, Lortholary O, Rieger C, Boehme A, Aoun M, Horst H-A, Thiebaut A, Ruhnke M, Reichert D, Vianelli N, Krause SW, Olavarria E, Herbrecht R, AmBiLoad Trial Study Group. 2007. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis 44:1289–1297. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 35.Cornely OA, Vehreschild JJ, Vehreschild MJGT, Würthwein G, Arenz D, Schwartz S, Heussel CP, Silling G, Mahne M, Franklin J, Harnischmacher U, Wilkens A, Farowski F, Karthaus M, Lehrnbecher T, Ullmann AJ, Hallek M, Groll AH. 2011. Phase II dose escalation study of caspofungin for invasive aspergillosis. Antimicrob Agents Chemother 55:5798–5803. doi: 10.1128/AAC.05134-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanadate T, Wakasugi M, Sogabe K, Kobayashi T, Horita H, Kawamura I, Hori Y, Matsui K, Hoshino Y, Sou M. 2011. Evaluation of the safety and efficacy of micafungin in Japanese patients with deep mycosis: a post-marketing survey report. J Infect Chemother 17:622–632. doi: 10.1007/s10156-011-0219-0. [DOI] [PubMed] [Google Scholar]
- 37.Kohno S, Masaoka T, Yamaguchi H, Mori T, Urabe A, Ito A, Niki Y, Ikemoto H. 2004. A multicenter, open-label clinical study of micafungin (FK463) in the treatment of deep-seated mycosis in Japan. Scand J Infect Dis 36:372–379. doi: 10.1080/00365540410020406. [DOI] [PubMed] [Google Scholar]
- 38.Denning DW, Marr KA, Lau WM, Facklam DP, Ratanatharathorn V, Becker C, Ullmann AJ, Seibel NL, Flynn PM, Van Burik J-AH, Buell DN, Patterson TF. 2006. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect 53:337–349. doi: 10.1016/j.jinf.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji Y, Song Y, Zhou F, Liu T, Jiang M, Zhao X, Huang X. 2017. Efficacy and safety of micafungin for the treatment of patients with proven or probable invasive aspergillosis. Medicine (Baltimore) 96:e9443. doi: 10.1097/MD.0000000000009443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirohi B, Powles RL, Chopra R, Russell N, Byrne JL, Prentice HG, Potter M, Koblinger S. 2006. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant 38:47–51. doi: 10.1038/sj.bmt.1705398. [DOI] [PubMed] [Google Scholar]
- 41.Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C, on behalf of the European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. 2016. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 47:45–68. doi: 10.1183/13993003.00583-2015. [DOI] [PubMed] [Google Scholar]
- 42.Ziakas PD, Kourbeti IS, Mylonakis E. 2014. Systemic antifungal prophylaxis after hematopoietic stem cell transplantation: a meta-analysis. Clin Ther 36:292–306.e1. doi: 10.1016/j.clinthera.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi M, Kurokawa T, Ishiyama K, Aoki G, Ueda M, Matano S, Takami A, Yamazaki H, Sawazaki A, Yamauchi H, Yoshida T, Nakao S. 2011. Efficacy and safety of micafungin as an empirical therapy for invasive fungal infections in patients with hematologic disorders: a multicenter, prospective study. Ann Hematol 90:1209–1217. doi: 10.1007/s00277-011-1277-1. [DOI] [PubMed] [Google Scholar]
- 44.Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, Heinz WJ, Jagannatha S, Koh LP, Kontoyiannis DP, Lee D-G, Nucci M, Pappas PG, Slavin MA, Queiroz-Telles F, Selleslag D, Walsh TJ, Wingard JR, Maertens JA. 2015. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed. CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 46.Rocha EMF, Garcia-Effron G, Park S, Perlin DS. 2007. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob Agents Chemother 51:4174–4176. doi: 10.1128/AAC.00917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satish S, Jiménez-Ortigosa C, Zhao Y, Lee MH, Dolgov E, Krüger T, Park S, Denning DW, Kniemeyer O, Brakhage AA, Perlin DS. 2019. Stress-induced changes in the lipid microenvironment of β-(1,3)-d-glucan synthase cause clinically important echinocandin resistance in Aspergillus fumigatus. mBio 10:e00779-19. doi: 10.1128/mBio.00779-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J. 2017. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. EUCAST definitive document EDef 9.3.1. EUCAST. [DOI] [PubMed] [Google Scholar]
- 49.Meletiadis J, Siopi M, Kanioura L, Jørgensen KM, Perlin DS, Mouton JW, Arendrup MC. 2020. Multicentre study to optimize echinocandin susceptibility testing of Aspergillus species with the EUCAST methodology and a broth microdilution colorimetric method. J Antimicrob Chemother 75:1799–1806. doi: 10.1093/jac/dkaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfizer, Inc. 2016. Eraxis injection package insert.
- 51.Merck Sharpe & Dohme Corp. 2017. CancidasVR injection package insert.
- 52.Astellas Pharma US, Inc. 2016. Mycamine injection package insert.
- 53.Siopi M, Mirfendereski H, Mainas E, Apostolopoulou O, Sambatakou H, Dimopoulos G, Couet W, Meletiadis J. 2019. Development of a bioassay for determining caspofungin levels in human serum samples, abstr P2218. Abstr 29th Eur Congr Clin Microbiol Infect Dis (ECCMID), Amsterdam, Netherlands. [Google Scholar]
- 54.Siopi M, Mainas E, Apostolopoulou O, Apostolidi S, Neroutsos E, Dokoumetzidis A, Valsami G, Dimopoulos G, Sambatakou H, Meletiadis J. 2018. Development of a bioassay for measuring micafungin concentrations in human serum samples, abstr P0293. Abstr 28th Eur Congr Clin Microbiol Infect Dis (ECCMID), Madrid, Spain. [Google Scholar]
- 55.Siopi M, Siafakas N, Vourli S, Mouton JW, Zerva L, Meletiadis J. 2016. Dose optimization of voriconazole/anidulafungin combination against Aspergillus fumigatus using an in vitro pharmacokinetic/pharmacodynamic model and response surface analysis: clinical implications for azole-resistant aspergillosis. J Antimicrob Chemother 71:3135–3147. doi: 10.1093/jac/dkw276. [DOI] [PubMed] [Google Scholar]
- 56.Würthwein G, Cornely OA, Trame MN, Vehreschild JJ, Vehreschild MJGT, Farowski F, Müller C, Boos J, Hempel G, Hallek M, Groll AH. 2013. Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis. Antimicrob Agents Chemother 57:1664–1671. doi: 10.1128/AAC.01912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groll AH, Silling G, Young C, Schwerdtfeger R, Ostermann H, Heinz WJ, Gerss J, Kolve H, Lanvers-Kaminsky C, Pinheiro JPV, Gammelin S, Cornely OA, Wuerthwein G. 2010. Randomized comparison of safety and pharmacokinetics of caspofungin, liposomal amphotericin B, and the combination of both in allogeneic hematopoietic stem cell recipients. Antimicrob Agents Chemother 54:4143–4149. doi: 10.1128/AAC.00425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu P, Mould DR. 2014. Population pharmacokinetic analysis of voriconazole and anidulafungin in adult patients with invasive aspergillosis. Antimicrob Agents Chemother 58:4718–4726. doi: 10.1128/AAC.02808-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brüggemann RJM, Van Der Velden WJFM, Knibbe CAJ, Colbers A, Hol S, Burger DM, Donnelly JP, Blijlevens NMA. 2015. Rationale for reduced-frequency dosing of anidulafungin for antifungal prophylaxis in immunocompromised patients. J Antimicrob Chemother 70:1166–1174. doi: 10.1093/jac/dku477. [DOI] [PubMed] [Google Scholar]
- 60.Undre NA, Stevenson P, Kuse E-R, Demeyer I. 2012. Pharmacokinetics of micafungin in adult patients with invasive candidiasis and candidemia. OJMM 02:84–90. doi: 10.4236/ojmm.2012.23012. [DOI] [PubMed] [Google Scholar]
- 61.Hiemenz J, Cagnoni P, Simpson D, Devine S, Chao N, Keirns J, Lau W, Facklam D, Buell D. 2005. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob Agents Chemother 49:1331–1336. doi: 10.1128/AAC.49.4.1331-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]