There is an increasing need for novel drugs and new strategies for the therapy of invasive candidiasis. This study aimed to develop and characterize liposome-based nanoparticles of carvacrol, cinnamaldehyde, citral, and thymol with anti-Candida activities.
KEYWORDS: Candida, antifungal activity, phytocompounds, liposomes, carvacrol, cinnamaldehyde, citral, thymol, macrophages
ABSTRACT
There is an increasing need for novel drugs and new strategies for the therapy of invasive candidiasis. This study aimed to develop and characterize liposome-based nanoparticles of carvacrol, cinnamaldehyde, citral, and thymol with anti-Candida activities. Dioctadecyldimethylammonium bromide- and monoolein-based liposomes in a 1:2 molar ratio were prepared using a lipid-film hydration method. Liposomes were assembled with equal volumes of liposomal stock dispersion and stock solutions of carvacrol, cinnamaldehyde, citral, or thymol in dimethyl sulfoxide. Cytotoxicity was tested on RAW 264.7 macrophages. In vitro antifungal activity of liposomes with phytocompounds was evaluated according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) methodology using clinical isolates of Candida albicans, Candida auris, Candida dubliniensis, and Candida tropicalis. Finally, the ability of macrophage cells to kill Candida isolates after addition of phytocompounds and their nanoparticles was determined. Nanoparticles with 64 μg/ml of cinnamaldehyde, 256 μg/ml of citral, and 128 μg/ml of thymol had the best characteristics among the formulations tested. The highest encapsulation efficiencies were achieved with citral (78% to 83%) and carvacrol (66% to 71%) liposomes. Carvacrol and thymol in liposome-based nanoparticles were nontoxic regardless of the concentration. Moreover, carvacrol and thymol maintained their antifungal activity after encapsulation, and there was a significant reduction (∼41%) of yeast survival when macrophages were incubated with carvacrol or thymol liposomes. In conclusion, carvacrol and thymol liposomes possess high stability, low cytotoxicity, and antifungal activity that act synergistically with macrophages.
INTRODUCTION
Candida is a common commensal of human skin and mucosae that can cause superficial and invasive infections (1–4). Invasive candidiasis is an important public health problem because of its high mortality and morbidity (5–7). Candida albicans infection is the most frequent cause of candidiasis, but an increasing etiological relevance of other species of Candida, such as Candida parapsilosis, Candida glabrata, or Candida auris, is reported (8–10). The emergence of these species complicates the management of candidiasis due to their potential multidrug resistance (8, 11). Moreover, there are a limited number of antifungal drugs, many of them with moderate efficacy, which are not free from adverse effects and drug interactions. These facts highlight the need to search for alternative therapies or synergistic combinations of antimicrobial agents.
Many phytocompounds have antimicrobial activities (12–14), and some of them, such as carvacrol, cinnamaldehyde, citral, and thymol, have shown promising antifungal activities (13–15). Their incorporation into nanoparticles, like liposomes, can improve their therapeutic efficiency against candidiasis, decreasing allergic reactions or toxicity. Liposomes are lipid bilayer vesicles that have been employed to enhance the delivery of antifungal drugs, such as amphotericin B (16). The system dioctadecyldimethylammonium bromide (DODAB) and monoolein (MO), a cation liposome, which consists of lipid-multilayer vesicles with a positive charge, has been used for drug delivery with promising results (17–20). DODAB is a synthetic amphiphilic lipid, including a hydrophilic positively charged dimethyl ammonium group attached to two hydrophobic 18-carbon-long acryl chains. MO is used as a stabilizer because it confers fluidity to the DODAB system by favoring lipid chain mobility and improving the fusion of the liposomes with cell membranes (21).
In addition, application of the DODAB:MO system in the production of nanoparticles of phytocompounds as antifungal therapy represents a biodegradable and biocompatible novel alternative with respect to the most commonly used metallic or synthetic nanomaterials. The aim of this study was to develop and characterize DODAB:MO nanoparticles of carvacrol, cinnamaldehyde, citral, and thymol for drug delivery and to test their cytotoxicity on murine macrophages to identify the best formulation with anti-Candida activity.
RESULTS
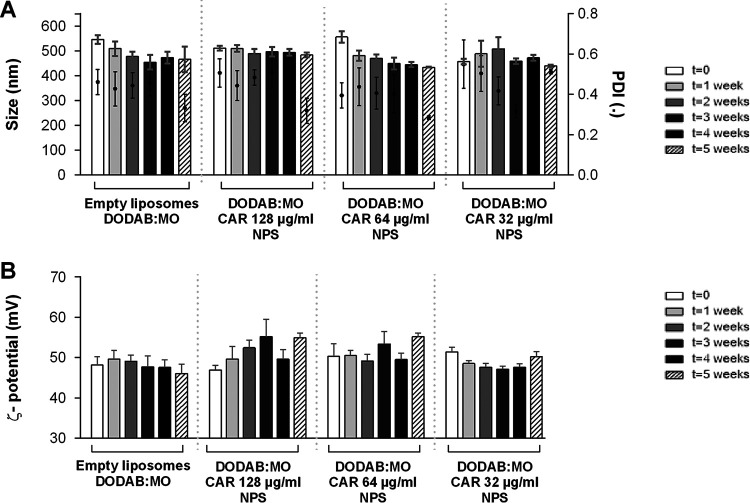
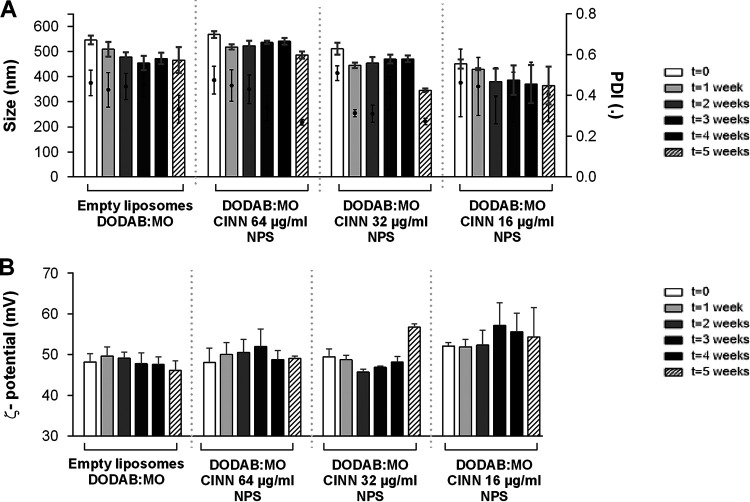
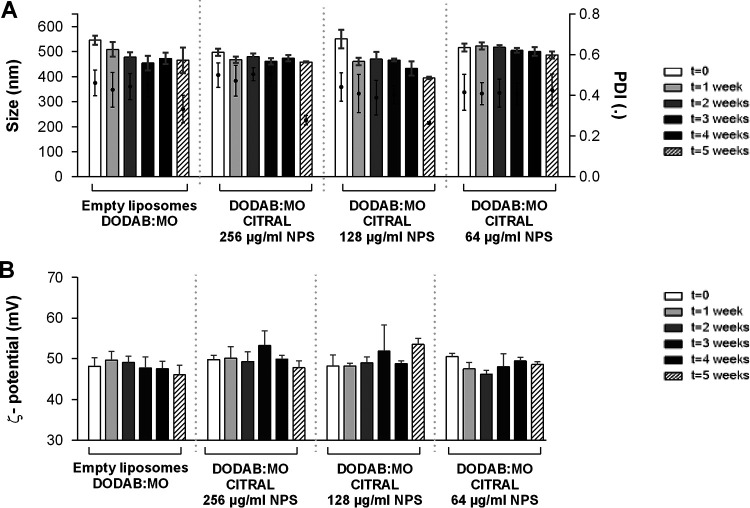
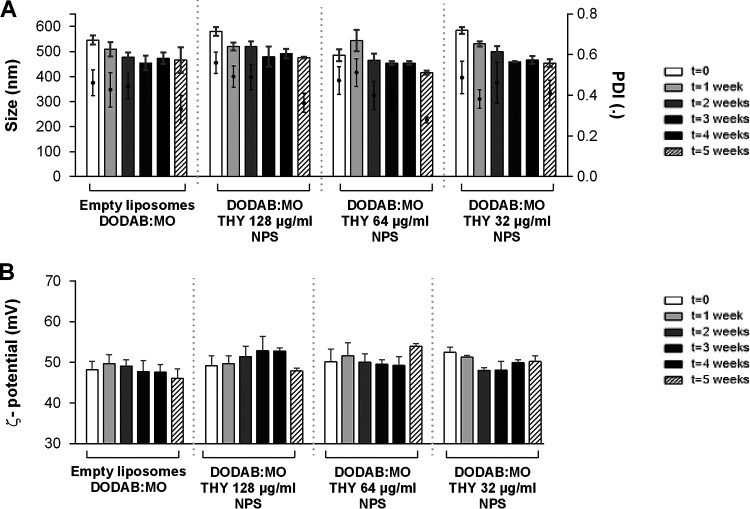
The characteristics of empty liposomes with 0.5% dimethyl sulfoxide (DMSO) DODAB:MO and the nanoparticles with three concentrations of carvacrol, cinnamaldehyde, citral, and thymol are presented in Fig. 1 to 4. DODAB:MO (1:2) empty liposomes had a mean size of 545 ± 17 nm on the first day of production, a ζ-potential of 48.2 ± 2.1 mV, and a polydispersity index (PDI) of 0.46 ± 0.06; with time, the ζ- potential remained similar, with no significant differences, but the mean size of the liposomes was reduced to 508 ± 29 nm right after the second week (P < 0.05) and remained stable thereafter. The PDI value remained similar during the first 4 weeks but was reduced in week 5. Empty liposomes seem to stabilize with time. Nanoparticles with carvacrol 128 μg/ml were more stable in terms of size than formulations with lower concentrations, but the ζ-potential was more unstable with time. Nanoparticles with carvacrol 32 μg/ml (lower concentration) were more variable in size over time (P < 0.0001) but more stable in terms of ζ-potential. This behavior was similar for nanoparticles of cinnamaldehyde, citral, and thymol; and the formulation with the greatest stability over time had the highest compound concentration. Thus, nanoparticles with 64 μg/ml of cinnamaldehyde, 256 μg/ml of citral, and 128 μg/ml of thymol had the best characteristics among the formulations tested, with thymol nanoparticles being the largest (580.3 ± 17.4 nm), followed by cinnamaldehyde (567.9 ± 13.9 nm), carvacrol (510 ± 9.6 nm), and citral (497.6 ± 13.8 nm) nanoparticles. Curiously, for all compounds at 5 weeks postproduction, the PDI was lower for the two highest concentrations, indicating size stabilization.
FIG 1.
Size and PDI (A) and ζ-potential (B) of carvacrol (CAR) nanoparticles (NPS) prepared with three different concentrations and analyzed weekly for 5 weeks. Empty liposomes and nanoparticles were prepared by hydration of DODAB:MO (1:2) 2-mM film with 0.5% DMSO in ultrapure water. Values are the results of three independent assays.
FIG 2.
Size and PDI (A) and ζ-potential (B) of cinnamaldehyde (CINN) nanoparticles (NPS) prepared with three different concentrations and analyzed weekly for 5 weeks. Empty liposomes and nanoparticles were prepared by hydration of DODAB:MO (1:2) 2-mM film with 0.5% DMSO in ultrapure water. Values are the results of three independent assays.
FIG 3.
Size and PDI (A) and ζ-potential (B) of citral nanoparticles (NPS) prepared with three different concentrations and analyzed weekly for 5 weeks. Empty liposomes and nanoparticles were prepared by hydration of DODAB:MO (1:2) 2-mM film with 0.5% DMSO in ultrapure water. Values are the results of three independent assays.
FIG 4.
Size and PDI (A) and ζ-potential (B) of thymol (THY) nanoparticles (NPS) prepared with three different concentrations and analyzed weekly for 5 weeks. Empty liposomes and nanoparticles were prepared by hydration of DODAB:MO (1:2) 2-mM film with 0.5% DMSO in ultrapure water. Values are the results of three independent assays.
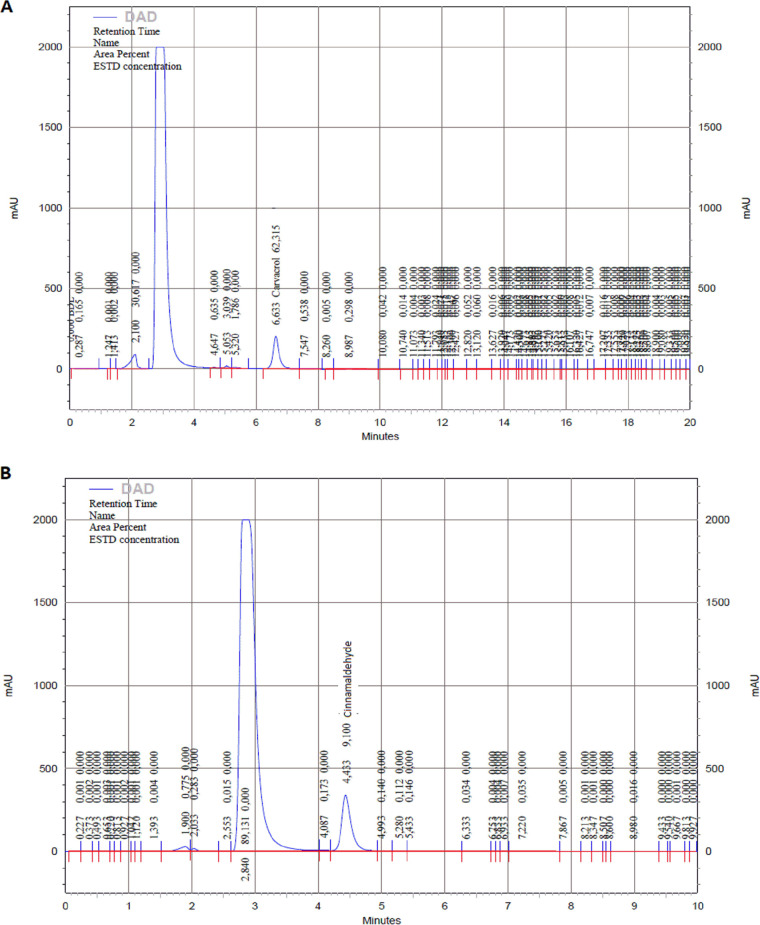
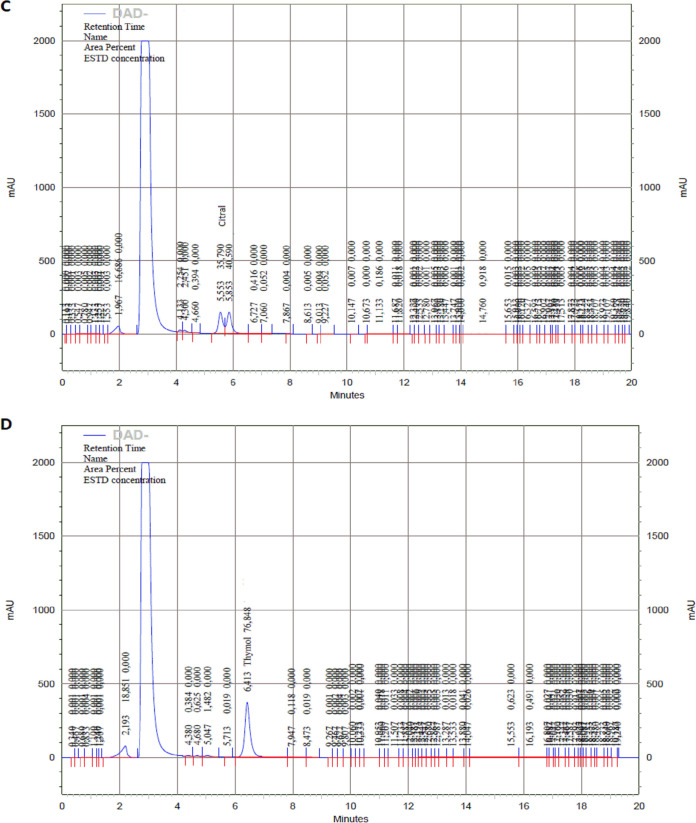
The parameters of high-performance liquid chromatography diode-array detection (HPLC-DAD) for each phytocompound are described in Table 1. In the case of citral, two peaks at 240 nm were observed due to the presence of isomers E (geranial or citral A) and Z (neral or citral B) (Fig. 5). Encapsulation was higher with the highest concentrations for all phytocompounds tested, with nanoparticles with 256 μg/ml of citral presenting the highest encapsulation efficiency (%EE) (83.2%), whereas all nanoparticles with cinnamaldehyde presented a lower %EE of 20.6% to 44.1% (Table 2).
TABLE 1.
HPLC-DAD parameters for the detection of phytocompounds
| Phytocompound | Wavelength detection (nm) | Coefficient (R2) | Linear range (μg/ml) | Time retention (min) |
|---|---|---|---|---|
| Carvacrol | 210 | 0.99 | 1–50 | 6.6 |
| Cinnamaldehyde | 290 | 0.98 | 1–50 | 4.4 |
| Citral | 240 | 0.99 | 1–50 | 5.5, 5.8 |
| Thymol | 210 | 0.99 | 1–50 | 6.4 |
FIG 5.
Chromatograms of carvacrol at 210nm (A), cinnamaldehyde at 290nm (B), citral at 240nm (C), and thymol at 210 (D) by HPLC-DAD of supernatants of correspondent nanoparticles after ultracentrifugation. In all cases, the highest peak corresponds to the DMSO content.
TABLE 2.
Encapsulation efficiency of phytocompound nanoparticles
| Phytocompound and concn (μg/ml) in nanoparticles | %EEa | SD |
|---|---|---|
| Carvacrol | ||
| 32 | 65.8 | 0.8 |
| 64 | 73.5 | 2.2 |
| 128 | 70.8 | 5.0 |
| Cinnamaldehyde | ||
| 16 | 20.6 | 0.1 |
| 32 | 30.3 | 1.5 |
| 64 | 44.1 | 2.7 |
| Citral | ||
| 64 | 77.9 | 0.1 |
| 128 | 79.4 | 0.1 |
| 256 | 83.2 | 1.2 |
| Thymol | ||
| 32 | 68.3 | 1.3 |
| 64 | 56.6 | 0.8 |
| 128 | 69.1 | 1.1 |
%EE, encapsulation efficiency.
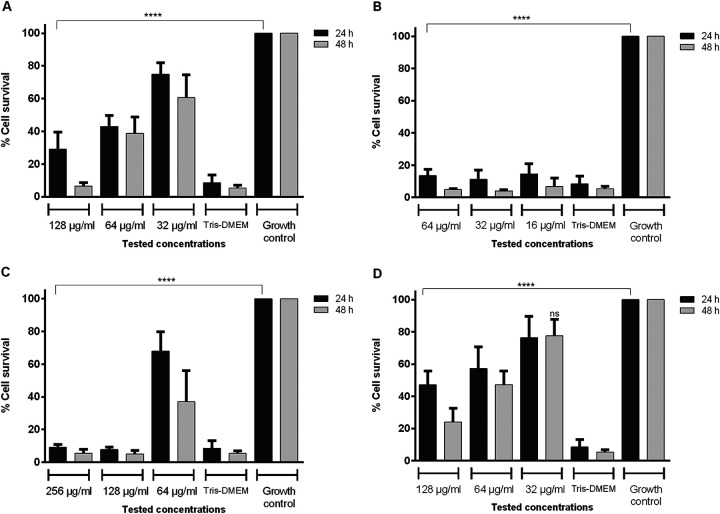
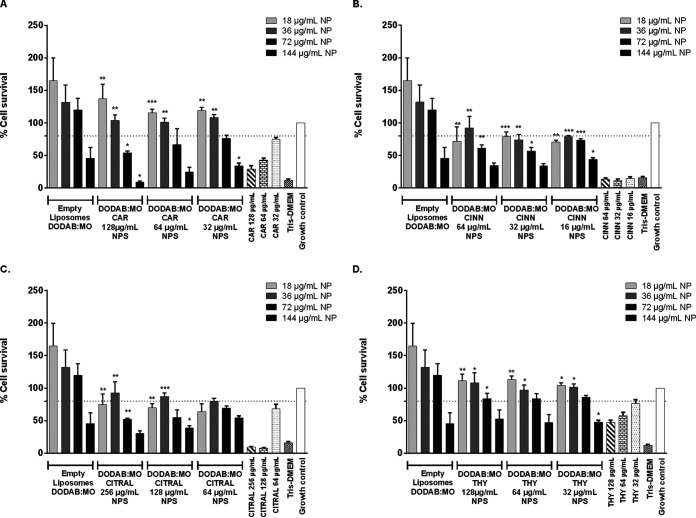
RAW 264.7 cell viability was significantly reduced (P < 0.05) after treatment with the highest concentrations of nonencapsulated phytocompounds (NEPs) tested, with survival at <80% the limit of accepted cytotoxicity. This cytotoxicity was dose dependent. Because no differences were found between 24 and 48 h, further studies were determined at 24 h (Fig. 6). Viability of cells incubated with the encapsulated phytocompounds was also dose dependent and overall significantly greater (Fig. 7). However, incubation with 144 μg/ml of lipids (DODAB:MO empty liposomes) reduced viability to <50%. At this lipid concentration, all nanoparticles with phytocompounds were cytotoxic due to both lipids and phytocompounds. DODAB:MO nanoparticles with 18 and 36 μg/ml of lipids, including carvacrol and thymol, were nontoxic, with > 80% macrophage survival. Furthermore, these nanoparticles were better tolerated by the cells than NEPs.
FIG 6.
Viability by MTT assay of RAW 264.7 cells after 24 and 48 h of incubation with different concentrations of nonencapsulated phytocompounds: carvacrol (A), cinnamaldehyde (B), citral (C), and thymol (D). ****, P < 0.0001. Differences were considered in comparison with the correspondent growth control at 24 or 48 h of incubation. Values are the results of three independent assays.
FIG 7.
Viability by MTT assay of RAW 264.7 cells after 24 h of incubation with nanoparticle formulations prepared with three different concentrations: carvacrol (A), cinnamaldehyde (B), citral (C), and thymol (D). Differences were considered in comparison with the correspondent concentration of nonencapsulated compound. Values are the results of three independent assays. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
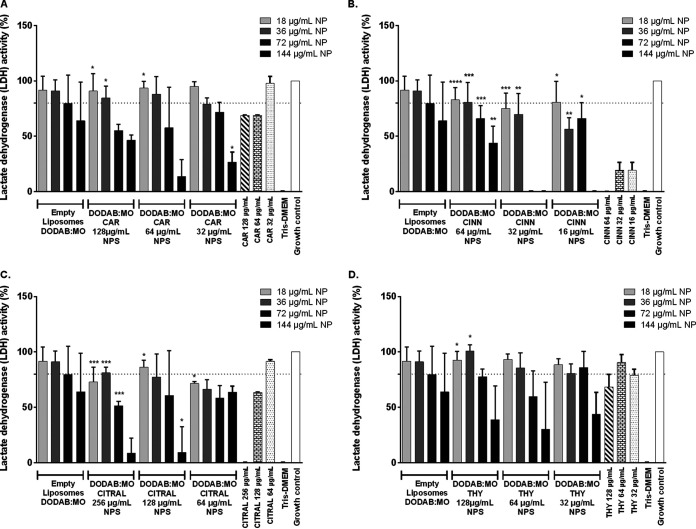
Analysis of cell viability by lactate dehydrogenase (LDH) of citral and cinnamaldehyde nanoparticles confirmed the results observed by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay that they are more cytotoxic (Fig. 8). Although encapsulation significantly enhanced cell viability, particularly for the highest concentrations of the phytocompounds, they are still considered toxic. For 64 μg/ml of citral, the corresponding nanoparticles were even more cytotoxic macrophages than the nonencapsulated compound. A similar result has been observed with the MTT assay for this formulation. All cinnamaldehyde nanoparticle formulations were significantly less toxic than the corresponding NEP. However, only nanoparticles with 64 μg/ml of cinnamaldehyde were considered noncytotoxic, in agreement with MTT results (92.3%) with 36 μg/ml of lipids (Fig. 7 and 8).
FIG 8.
Viability by LDH assay of RAW 264.7 cells after 24 h of incubation with nanoparticle formulations prepared with three different concentrations: carvacrol (A), cinnamaldehyde (B), citral (C), and thymol (D). Differences were considered in comparison with the correspondent concentration of nonencapsulated compound. Values are the results of three independent assays. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
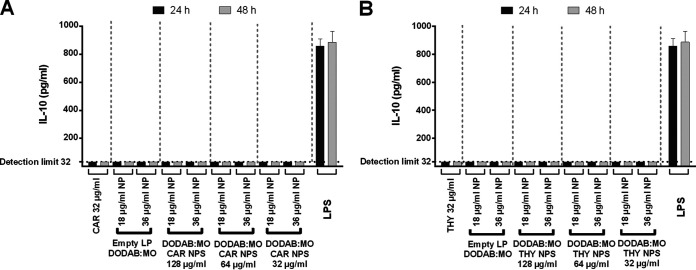
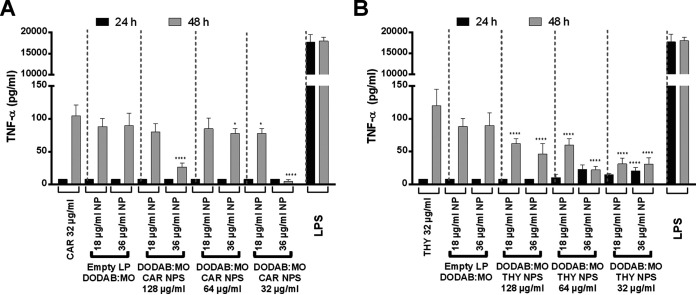
Cell survival rates after treatment with encapsulated phytocompounds and NEPs were taken into consideration in order to test cytokine production; IL-10, a known anti-inflammatory cytokine; and tumor necrosis factor-α (TNF-α), a known proinflammatory cytokine. Therefore, only conditions with >80% survival in both cell toxicity assays were analyzed, which corresponded only to nanoparticles with 32 μg/ml of carvacrol or thymol. Macrophages incubated with lipopolysaccharide (LPS) were considered positive controls and macrophages alone were negative controls. No compounds or nanoparticles were able to induce IL-10 production at both 2 and 48 h (Fig. 9). All quantifications were <32 pg/ml, which was the detection limit. TNF-α levels were also below the detection limit (8 pg/ml) for all conditions tested at 24 h; however, after 48 h of incubation with these compounds and nanoparticles, a slight increase in proinflammatory cytokine was observed (Fig. 10A). Carvacrol nanoparticles at 48 h induced a smaller amount of TNF-α production than the nonencapsulated carvacrol, particularly with liposomes at 36 μg/ml of lipid (P < 0.05) versus liposomes at 18 μg/ml of lipid. The most significant reduction in TNF-α production was observed with nanoparticles loaded with 32 and 128 μg/ml of carvacrol (P < 0.0001). With liposomes at 18 μg/ml, a small reduction in TNF-α production was observed only with 32-μg/ml carvacrol nanoparticles (P < 0.05) (Fig. 10A).
FIG 9.
Production of IL-10 by RAW 264.7 cells after incubation with carvacrol (CAR) (A) and thymol (THY) (B) for 24 and 48 h. LPS, 1 μg/ml. Results indicate the mean ± SEM of three measurements from three independent experiments. Differences were considered between nanoparticles and 32 μg/ml nonencapsulated phytocompound at 24 or 48 h of incubation, respectively.
FIG 10.
Production of TNF-α by RAW 264.7 cells after incubation with carvacrol (CAR) (A) and thymol (THY) (B) for 24 and 48 h. LPS, 1 μg/ml. Results indicate the mean ± SEM of three measurements from three independent experiments. *, P < 0.05, ****, P < 0.0001. Differences were considered between nanoparticles and 32 μg/ml nonencapsulated phytocompound at 24 or 48 h of incubation, respectively.
In the case of thymol nanoparticles, undetected levels of TNF-α were observed at 24 h with 128 μg/ml thymol regardless of the lipid concentration; however, a slight increase in TNF-α was observed at 64 and 32 μg/ml thymol encapsulated with 36 μg/ml of lipid (P < 0.0001) (Fig. 10B). At 48 h, the same pattern of TNF-α increase was observed as for carvacrol nanoparticles. Of interest, all thymol nanoparticles induced a much smaller amount of TNF-α production than NEPs or empty liposomes (P < 0.0001) (Fig. 10B).
Antifungal activities of encapsulated phytocompounds and NEPs are described in Table 3. NEPs were active against Candida isolates with mode inhibitory concentrations (IC) of 64 μg/ml for cinnamaldehyde, 128 μg/ml for carvacrol or thymol, and 256 μg/ml for citral. Nanoparticles were prepared with the same amount of phytocompounds: nanoparticles with 64 μg/ml of cinnamaldehyde were more active against planktonic cells of Candida, followed by 128 μg/ml thymol or carvacrol nanoparticles, as observed with NEPs. However, for some isolates, ICs were lower with phytocompound nanoparticles, particularly with thymol. Conversely, citral nanoparticle ICs for all strains analyzed were >256 μg/ml.
TABLE 3.
IC of encapsulated and nonencapsulated phytocompounds against Candida planktonic cells at 24 h
| Candida isolate | IC (μg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Carvacrol |
Cinnamaldehyde |
Citral |
Thymol |
|||||
| DODAB:MO liposomes | NEPa | DODAB:MO liposomes | NEP | DODAB:MO liposomes | NEP | DODAB:MO liposomes | NEP | |
| C. albicans UPV 05-007 | >128 | 128 | 64 | 64 | >256 | 256 | 128 | 128 |
| C. albicans UPV 05-013 | 128 | 128 | 64 | 64 | >256 | 512 | 32 | 32 |
| C. albicans UPV 11-342 | >128 | 128 | 64 | 64 | >256 | 256 | 128 | 128 |
| C. albicans UPV 11-345 | 128 | 128 | 64 | 64 | >256 | 256 | 64 | 128 |
| C. albicans UPV 12-298 | 128 | 128 | 64 | 64 | >256 | 256 | 128 | 128 |
| C. albicans UPV 15-101 | 128 | 128 | 64 | 64 | >256 | 256 | 32 | 128 |
| C. albicans UPV 15-106 | 32 | 128 | 64 | 64 | >256 | 256 | 64 | 128 |
| C. albicans UPV 15-157 | >128 | 128 | 64 | 64 | >256 | 256 | 128 | 128 |
| C. albicans SC5314 | 32 | 128 | 64 | 64 | >256 | 512 | 64 | 128 |
| Mode | 128 | 128 | 64 | 64 | >256 | 256 | 128 | 128 |
| IC range | 32 to >128 | 128 | 64 | 64 | >256 | 256–512 | 32 to >128 | 32–128 |
| IC geometric mean | 94.1 | 128 | 64 | 64 | 256 | 298.6 | 74.7 | 109.7 |
| C. auris UPV 18-029 | >128 | 128 | 64 | 64 | 256 | 128 | 32 | 128 |
| C. dubliniensis UPV 11-366 | 128 | 128 | 64 | 64 | >256 | 256 | >128 | 64 |
| C. tropicalis UPV 06-115 | 128 | 128 | 64 | 64 | >256 | 256 | 64 | 128 |
NEP, nonencapsulated phytocompounds.
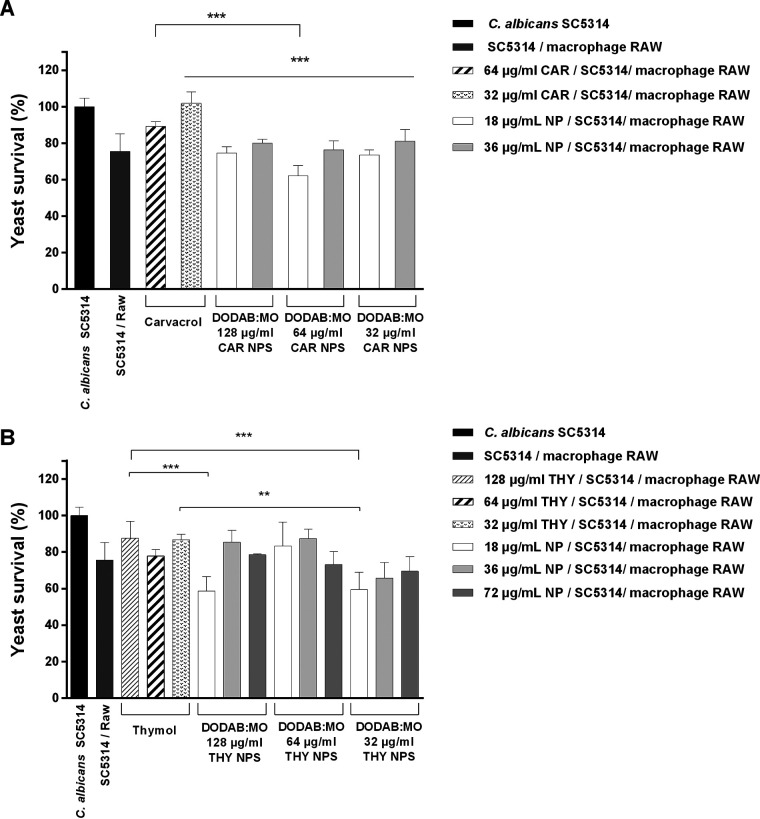
The ability of macrophages to kill Candida cells with the help of the phytocompounds or their corresponding nanoparticles was also evaluated. Only nanoparticles with 32 and 64 μg/ml of carvacrol or thymol, noncytotoxic, were tested. Although showing some cytotoxicity, the corresponding NEPs were tested for comparison. Macrophages treated with carvacrol nanoparticles reduced Candida survival compared with the corresponding nonencapsulated compound. The nanoparticles of 18 μg/ml of DODAB:MO and 64 μg/ml carvacrol induced the highest reduction. This formulation was able to reduce ∼38% of viable yeast cells compared with controls and ∼25% compared with NEPs (P < 0.001) (Fig. 11A). Thymol and carvacrol nanoparticles similarly enhanced the ability of macrophages to kill yeast cells. Nanoparticles composed of 18 μg/ml of DODAB:MO and 32 or 128 μg/ml of thymol had the greatest reduction (∼41%) in Candida survival (Fig. 11B).
FIG 11.
Results of macrophage killing assay with the carvacrol (CAR) (A) and thymol (THY) (B) nanoparticles (NPS) and corresponding nonencapsulated phytocompound. **, P < 0.01, ***, P < 0.001.
DISCUSSION
Different compounds with antimicrobial activity are obtained from plants: carvacrol and thymol from Lamiaceae, Verbenaceae, Scrophulariaceae, Ranunculaceae, and Apiaceae; cinnamaldehyde from Lauraceae; and citral from many citrus fruits and Cymbopogon citratus (13, 14, 22–24). Antifungal activities of these phytocompounds have been reported against Candida planktonic and sessile cells (25–27). However, their high volatilization, chemical instability, sparing water solubility, cytotoxicity, and irritant effects are some drawbacks for use in medical therapy (13, 24, 28, 29).
Encapsulation in liposomes has been used as a pharmaceutical drug delivery system to protect the bioactive compounds and to increase the permeation rate of drugs, increasing the mean retention time at the desired medical target (30). DODAB:MO (1:2) liposome formulation is very efficient in producing nanoparticles because of the high capacity of encapsulating compounds by forming homogeneous multilamellar vesicles (31). In the current study, the highest encapsulation efficiencies were achieved with citral (78% to 83%) and carvacrol (66% to 71%) liposomes. High encapsulation efficiency in soy phosphatidylcholine liposomes of carvacrol (∼98%) and citral (86%) has been described (32, 33). The same has been observed for thymol (79%) in lipoid S100 and cholesterol liposomes (34). Moreover, a %EE of <48% in cinnamaldehyde liposomes has been shown (35).
Encapsulation protects phytocompounds against thermal- and/or photodegradation; increases their stability; extends the final-product shelf life (36); and, according to our outcomes, significantly reduces the cytotoxicity in mammalian cells of carvacrol, cinnamaldehyde, and thymol prepared with DODAB:MO liposomes. Carvacrol and thymol nanoparticles prepared with 18 and 32 μg/ml of DODAB:MO liposomes were considered nontoxic regardless of the concentration of the phytocompound used.
In addition, carvacrol and thymol maintained and increased their antifungal activity after encapsulation, in agreement with previous studies (37–39). The improvement in antifungal activity was identified mainly when the liposome formulations of thymol were used against C. albicans, C. tropicalis, and C. auris isolates, with up to 4-fold reductions in ICs compared with those of nonencapsulated thymol; likewise, liposome formulations of carvacrol showed up to 4-fold reduction of the required concentration compared with NEPs against two C. albicans isolates. This fact can be related to positive ζ-potential of nanoparticles, since it is a relevant factor for the antifungal effect, enabling interaction with the negatively charged fungal surface (40). Cinnamaldehyde DODAB:MO liposomes also maintained their anti-Candida activity as well as multilamellar cinnamaldehyde liposomes (29).
Converse to the findings reported in other studies (32, 41), citral showed high encapsulation efficiency but very low antifungal activity in the current study. This suggests that citral in DODAB:MO liposomes does not easily get access to the extracellular medium to be active, in contrast to the nanoemulsion formulations and soy phosphatidylcholine liposomes.
Before testing whether the noncytotoxic formulations could help macrophages kill Candida cells, we tested whether they could be inflammatory. At 24 h of incubation, no significant TNF-α production was observed by macrophages treated with carvacrol or thymol. Encapsulation did not enhance TNF-α production; on the contrary, it significantly reduced this proinflammatory cytokine, particularly with nanoparticles of thymol. In all cases, TNF-α production was significantly less than that assessed in the presence of LPS. TNF-α is a potent pleiotropic and proinflammatory cytokine, one of the most abundant early mediators in inflamed tissue, produced mainly by cells of the monocyte lineage (42). Hence, slight TNF-α production from macrophages, such as that obtained for carvacrol and thymol liposomes, can lead to cell recruitment and antifungal action of macrophages and other inflammatory cells without severe deleterious inflammation (43). Reduction of yeast survival when macrophages were incubated with carvacrol and thymol liposomes was significant compared with that of the corresponding nonencapsulated compounds. This result indicates that even if the IC obtained with the encapsulated compounds is not significantly reduced or the encapsulation efficiency is not high, liposomes seem to be beneficial in host-pathogen interaction, helping macrophage killing of Candida cells.
The DODAB:MO system has been described as an efficient delivery system because of its high interaction with mammalian cells and their internalized formulations by endocytosis into fungal cells (19, 44). These features could be responsible for the improved bioavailability of these phytocompounds within the macrophage and the subsequent interaction with Candida cells, enabling their antifungal activity. However, antifungal mechanisms of these nanoparticles have not yet been defined.
In conclusion, our data confirm that carvacrol and thymol liposomes possess high stability, low cytotoxicity, and antifungal activity that act synergistically with macrophages. These phytocompounds can be promising therapeutic alternatives for candidiasis.
MATERIALS AND METHODS
Preparation and characterization of liposomes.
DODAB-based (Tokyo Kasei, Japan) and MO-based (Sigma-Aldrich) liposomes were prepared using a lipid-film hydration method (45). The liposomal stock dispersion was prepared at 4 mM total lipid concentration. Briefly, an MO molar fraction (χMO) of 0.330 (DOBAB:MO molar ratio of 1:2) was dissolved in ethanol (high spectral purity; Uvasol, UK) and mixed in a glass tube. The solvent was then removed by rotary evaporation using nitrogen gas. Liposomes were obtained after hydration of lipid film with ultrapure water at 60°C, vortexed for 2 min. Liposome-based nanoparticles were assembled with equal volumes of liposomal stock dispersion and the phytocompound solution to obtain formulations with 0.5% DMSO (Sigma-Aldrich, USA) and a final lipid concentration of 2 mM (888 μg/ml). Stock solutions of carvacrol (51,200 μg/ml), cinnamaldehyde (25,600 μg/ml), citral (102,400 μg/ml), and thymol (51,200 μg/ml) were prepared in DMSO. The final concentrations were 32, 64, and 128 μg/ml for carvacrol and thymol; 16, 32, and 64 μg/ml for cinnamaldehyde; and 64, 128, and 256 μg/ml for citral. These formulations were incubated for 45 min at 60°C to allow for compound absorption. Moreover, empty liposomes were developed and characterized at 2 mM with 0.5% DMSO.
Mean size, PDI, and error values of the nanoparticles conserved at 4°C were determined by dynamic light scattering at 25°C with a Malvern ZetaSizer Nano ZS particle analyzer every week from the first day of production until 5 weeks later. The charge of the liposome surface was measured indirectly by ζ-potential analysis, using electrophoretic light scattering at 25°C. Malvern dispersion technology software was used with multiple-narrow-mode (high-resolution) data processing for size and PDI, whereas monomodal data processing was used for average ζ-potential and error values. All characterization was performed in quintuplicate.
The prepared formulations were pelleted by ultracentrifugation (100,000 × g for 1 h at 4°C), after which the supernatant was removed and then filtered using 0.22-μm acetate cellulose filters (Millipore Merck, Germany). The concentration of NEP in the supernatant was determined by HPLC-DAD (Hitachi EZChrom Elite, Agilent Technologies, USA). The methodology was standardized for each phytocompound by preparing a standard curve for all. The analysis was carried out using the Vydac 218TP54 column (C18, 5 μm, 4.6 mm inside diameter × 250 mm), with acetonitrile and water (50:50) mobile phase in isocratic mode at 1 ml/min flow. A diode-array detector was set at 210 nm to detect carvacrol and thymol, 290 nm for cinnamaldehyde, and 240 nm for citral (46–48). The chromatographic runs were carried out at 30°C for 20 min after an injection of 90 μl of samples or controls in three independent experiments.
Cytotoxicity assay.
The murine macrophage-like cell line RAW 264.7 from strain ATCC TIB-71 (49) was cultured in cell-culture flasks with Dulbecco's modified Eagle's medium (DMEM) (Thermo Fisher Scientific, USA) supplemented with 10% heat-inactivated fetal bovine serum (Valbiotech, USA), 2 mM l-glutamine (1%), 1 mM sodium pyruvate (1%), and 10 mM HEPES buffer (1%) (Sigma-Aldrich, USA) in 5% CO2 at 37°C. After confluent growth, macrophage cells were recovered and washed with DMEM. Then, viable cells were determined with Trypan blue exclusion by counting in a hemocytometer, and a final concentration of 5 × 104 cells/ml in DMEM was prepared and 200 μl dispensed onto 96-well tissue culture plates (Thermo Fisher Scientific).
The culture plates with macrophages were incubated overnight at 37°C and 5% CO2; after that, the supernatant was removed, and empty liposomes, nanoparticles, and NEPs were added with DMEM in triplicate. Different concentrations of carvacrol, cinnamaldehyde, citral, thymol, their nanoparticles (DODAB:MO [1:2] 0.5% DMSO), and empty liposomes 2 mM (18, 36, 72, and 144 μg/ml) were evaluated.
Cell line RAW 264.7 metabolic activity was determined using MTT. The impact on membrane integrity was assessed by LDH assay after 24 and 48 h of incubation. Enzymatic activity over MTT was quantified after solubilization of MTT formazan by adding DMSO-ethanol (1:1) solution, and absorbance was measured at 570 nm (50). The control of viability (100%) was the untreated cells, and the control of cytotoxicity was the cells treated with Tris-HCl. Results of the percentage of viability were expressed as described previously (51), according to the following equation:
The LDH leakage assay was performed by measuring LDH activity in the extracellular medium at 30°C in a microplate reader (Spectra Max 340PC) at 340 nm, employing pyruvate 0.32 mM (in phosphate buffer, pH 7.4) as the substrate. Results were expressed as the percentage of viability compared with the control without treatment.
Cytokine production.
Proinflammatory cytokine TNF-α was quantified according to the manufacturer’s instructions (mouse TNF-α and IL-10 enzyme-linked immunosorbent assay kit, Thermo Scientific) from three independent assays on 96-well tissue culture plates with macrophages and empty liposomes, nanoparticles, and NEPs after 24 h of incubation, as described above. Macrophages incubated with 1 μg/ml LPS were used as positive controls, and macrophages alone were used as negative controls.
Antifungal activity of nanoparticles.
In vitro antifungal activity against Candida planktonic cells was evaluated according to EUCAST methodology (52, 53). Eight C. albicans, one C. dubliniensis, one C. tropicalis, and one C. auris clinical isolates and the high biofilm-producer strain C. albicans ATCC SC5314 were tested. C. albicans UPV 15–157, C. dubliniensis UPV 11–366, and C. auris UPV 18-029 were resistant to fluconazole (MIC, ≥64 μg/ml). To determine susceptibility, carvacrol, cinnamaldehyde, citral, and thymol were assayed at 2-fold serial concentrations ranging from 1 to 1,024 μg/ml. Liposomal formulations were tested in triplicate, including empty liposomes in RPMI 1640 (with l-glutamine and without bicarbonate; Sigma-Aldrich) supplemented with 2% glucose and buffered to pH 7.0 with 3-N-morpholinepropanesulfonic acid (Sigma-Aldrich). Briefly, a cell inoculum of 0.5 to 2.5 × 105 cells/ml from each Candida isolate cultured at 24 h and 37°C in Sabouraud dextrose agar (Difco, USA) was dispensed onto the previously prepared 96-well microplates with the phytocompounds and nanoparticles. Sterility and growth control wells were included in each microplate. Absorbance at 450 nm after 24 and 48 h of incubation at 37°C was measured with an iMark microplate absorbance reader (Bio-Rad, USA). ICs were calculated as the lowest phytochemical and nanoparticle concentrations inhibiting ≥50% growth after 24 h compared with controls.
Macrophage killing assay.
The ability of macrophage cells to kill Candida isolates after the addition of phytocompounds and their nanoparticles was determined according to a previously described protocol (54). Briefly, an overnight macrophage culture was disposed at a concentration of 1 × 104 cells/well of macrophages onto 96-well tissue culture plates and incubated at 37°C and 5% CO2 atmosphere. After 1 h of incubation, to allow macrophage adherence onto the tissue culture plate, the NEPs and the nanoparticles were added into the wells. At the same time, Candida cells were added to macrophages at a ratio of 5:1, using 100 μl of 5 × 105 cells/ml inoculum of C. albicans SC5314 in each well. After 1 h of incubation, the macrophages were lysed with 10% saponin solution (Sigma-Aldrich), and serial dilutions of the suspension were plated on yeast extract-peptone-dextrose agar (Sigma-Aldrich). CFUs were determined after 24 h of incubation at 37°C, including CFUs from macrophages incubated with Candida without treatment and Candida alone as control.
Statistical analysis.
Comparative analysis of different groups was made using analysis of variance followed by the Bonferroni test (GraphPad Prism 5.0, USA). Unless otherwise stated, results shown are from at least two independent experiments with three replicates. In all cases, P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by the Consejería de Educación, Universidades e Investigación (GIC15/78 IT-990-16) of Gobierno Vasco-Eusko Jaurlaritza, the Fundación ONCE Oportunidad al Talento, and the Fondo Social Europeo (to C.M.-A.), and the Federation of European Microbiological Societies (FEMS) by Research and Training grant FEMS-GO-2017-020 (to K.M.-C.).
We have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the current manuscript.
Footnotes
[This article was published on 18 March 2021 but required additional changes, now reflected in the Note Added after Publication on p. 15. The changes to the article were made on 28 April 2021.]
Note Added after Publication
In the original published version of this paper, incorrect files were used for Fig. 6 to 10. In addition, “P < 0.5” should be “P < 0.05” in the legends of Fig. 7, 8, and 10, and in the 5th paragraph of the Results section, “64” in the 2nd-to-last sentence should be “32” and “P < 0.5” in the last sentence should be “P < 0.05.” All of these corrections have been made in this version of the article.
REFERENCES
- 1.Pfaller MA, Pappas PG, Wingard JR. 2006. Invasive fungal pathogens: current epidemiological trends. Epidemiol Invasive Mycoses 43 (Suppl 1):S3–S14. doi: 10.1086/504490. [DOI] [Google Scholar]
- 2.De-La-Torre J, Quindós G, Marcos-Arias C, Marichalar-Mendia X, Gainza ML, Eraso E, Acha-Sagredo A, Aguirre-Urizar JM. 2018. Oral Candida colonization in patients with chronic periodontitis. Is there any relationship? Rev Iberoam Micol 35:134–139. doi: 10.1016/j.riam.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Gaitán A, Moret AM, Tasias-Pitarch M, Aleixandre-Lopez AI, Martinez-Morel H, Calabuig E, Salavert-Lleti M, Ramirez P, Lopez-Hontangas JL, Hagen F, Meis JF, Mollar-Maseres J, Peman J. 2018. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 61:498–505. doi: 10.1111/myc.12781. [DOI] [PubMed] [Google Scholar]
- 4.Kumamoto CA. 2011. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol 14:386–391. doi: 10.1016/j.mib.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pegorie M, Denning DW, Welfare W. 2017. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J Infect 74:60–71. doi: 10.1016/j.jinf.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Canela HMS, Cardoso B, Vitali LH, Coelho HC, Martinez R, Ferreira MEDS. 2018. Prevalence, virulence factors and antifungal susceptibility of Candida spp. isolated from bloodstream infections in a tertiary care hospital in Brazil. Mycoses 61:11–21. doi: 10.1111/myc.12695. [DOI] [PubMed] [Google Scholar]
- 8.Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. 2012. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev 36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- 9.Quindós G, Gil-Alonso S, Marcos-Arias C, Sevillano E, Mateo E, Jauregizar N, Eraso E. 2019. Therapeutic tools for oral candidiasis: current and new antifungal drugs. Med Oral 24:e172–e180. doi: 10.4317/medoral.22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quindós G, Marcos-Arias C, San-Millán R, Mateo E, Eraso E. 2018. The continuous changes in the aetiology and epidemiology of invasive candidiasis: from familiar Candida albicans to multiresistant Candida auris. Int Microbiol 21:107–117. doi: 10.1007/s10123-018-0014-1. [DOI] [PubMed] [Google Scholar]
- 11.Jeffery-Smith A, Taori SK, Schelenz S, Jeffery K, Johnson EM, Borman A, Manuel R, Browna CS. 2018. Candida auris: a review of the literature. Clin Microbiol Rev 31:1–18. doi: 10.1128/CMR.00029-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu M, Li T, Wan J, Li X, Yuan L, Sun S. 2017. Antifungal effects of phytocompounds on Candida species alone and in combination with fluconazole. Int J Antimicrob Agents 49:125–136. doi: 10.1016/j.ijantimicag.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Marchese A, Orhan IE, Daglia M, Barbieri R, Di Lorenzo A, Nabavi SF, Gortzi O, Izadi M, Nabavi SM. 2016. Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem 210:402–414. doi: 10.1016/j.foodchem.2016.04.111. [DOI] [PubMed] [Google Scholar]
- 14.Shreaz S, Wani WA, Behbehani JM, Raja V, Irshad M, Karched M, Ali I, Siddiqi WA, Hun LT. 2016. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 112:116–131. doi: 10.1016/j.fitote.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Doke SK, Raut JS, Dhawale S, Karuppayil SM. 2014. Sensitization of Candida albicans biofilms to fluconazole by terpenoids of plant origin. J Gen Appl Microbiol 60:163–168. doi: 10.2323/jgam.60.163. [DOI] [PubMed] [Google Scholar]
- 16.Allen TM, Cullis PR. 2013. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Carneiro C, Correia A, Lima T, Vilanova M, Pais C, Gomes AC, Real Oliveira MECD, Sampaio P. 2016. Protective effect of antigen delivery using monoolein-based liposomes in experimental hematogenously disseminated candidiasis. Acta Biomater 39:133–145. doi: 10.1016/j.actbio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Lopes I, Oliveira ACN, Sarria M, Neves Silva JP, Goncalves O, Gomes AC, Real Oliveira MECD. 2016. Monoolein-based nanocarriers for enhanced folate receptor-mediated RNA delivery to cancer cells. J Liposome Res 26:199–210. doi: 10.3109/08982104.2015.1076463. [DOI] [PubMed] [Google Scholar]
- 19.Silva JPN, Oliveira ACN, Casal MPPA, Gomes AC, Coutinho PJG, Coutinho OP, Oliveira M. 2011. DODAB:monoolein-based lipoplexes as non-viral vectors for transfection of mammalian cells. Biochim Biophys Acta 1808:2440–2449. doi: 10.1016/j.bbamem.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Voltan AR, Quindós G, Alarcón KPM, Fusco-Almeida AM, Mendes-Giannini MJS, Chorilli M. 2016. Fungal diseases: could nanostructured drug delivery systems be a novel paradigm for therapy? Int J Nanomedicine 11:3715–3730. doi: 10.2147/IJN.S93105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carneiro C, Correia A, Collins T, Vilanova M, Pais C, Gomes AC, Real Oliveira ME, Sampaio P. 2015. DODAB:monoolein liposomes containing Candida albicans cell wall surface proteins: a novel adjuvant and delivery system. Eur J Pharm Biopharm 89:190–200. doi: 10.1016/j.ejpb.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Leite MC, Bezerra AP, de Sousa JP, Guerra FQ, Lima EO. 2014. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid Based Complement Alternat Med 2014:378280. doi: 10.1155/2014/378280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad A, Molepo J, Patel M. 2016. Challenges in the development of antifungal agents against Candida: scope of phytochemical research. Curr Pharm Des 22:4135–4150. doi: 10.2174/1381612822666160607072748. [DOI] [PubMed] [Google Scholar]
- 24.Sharifi-Rad M, Varoni EM, Iriti M, Martorell M, Setzer WN, Del Mar Contreras M, Salehi B, Soltani-Nejad A, Rajabi S, Tajbakhsh M, Sharifi-Rad J. 2018. Carvacrol and human health: a comprehensive review. Phytother Res 32:1675–1687. doi: 10.1002/ptr.6103. [DOI] [PubMed] [Google Scholar]
- 25.Suntres ZE, Coccimiglio J, Alipour M. 2015. The bioactivity and toxicological actions of carvacrol. Crit Rev Food Sci Nutr 55:304–318. doi: 10.1080/10408398.2011.653458. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Feng K, Yang H, Zhang Z, Yuan Y, Yue T. 2018. Effect of cinnamaldehyde and citral combination on transcriptional profile, growth, oxidative damage and patulin biosynthesis of Penicillium expansum. Front Microbiol 9:1–14. doi: 10.3389/fmicb.2018.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatrath A, Gangwar R, Kumari P, Prasad R. 2019. In vitro anti-biofilm activities of citral and thymol against Candida tropicalis. J Fungi (Basel) 5:13. doi: 10.3390/jof5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhifi W, Bellili S, Jazi S, Bahloul N, Mnif W. 2016. Essential oils’ chemical characterization and investigation of some biological activities: a critical review. Medicines 3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan SN, Khan S, Iqbal J, Khan R, Khan AU. 2017. Enhanced killing and antibiofilm activity of encapsulated cinnamaldehyde against Candida albicans. Front Microbiol 8:1–15. doi: 10.3389/fmicb.2017.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zylberberg C, Matosevic S. 2016. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv 23:3319–3329. doi: 10.1080/10717544.2016.1177136. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira IM, Silva JP, Feitosa E, Marques EF, Castanheira EM, Real Oliveira ME. 2012. Aggregation behavior of aqueous dioctadecyldimethylammonium bromide/monoolein mixtures: a multitechnique investigation on the influence of composition and temperature. J Colloid Interface Sci 374:206–217. doi: 10.1016/j.jcis.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 32.Usach I, Margarucci E, Manca ML, Caddeo C, Aroffu M, Petretto GL, Manconi M, Peris J-E. 2020. Comparison between citral and pompia essential oil loaded in phospholipid vesicles for the treatment of skin and mucosal infections. Nanomaterials 10:286. doi: 10.3390/nano10020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayres Cacciatore F, Dalmás M, Maders C, Ataíde Isaía H, Brandelli A, da Silva Malheiros P. 2020. Carvacrol encapsulation into nanostructures: characterization and antimicrobial activity against foodborne pathogens adhered to stainless steel. Food Res Int 133:109143. doi: 10.1016/j.foodres.2020.109143. [DOI] [PubMed] [Google Scholar]
- 34.Hammoud Z, Gharib R, Fourmentin S, Elaissari A, Greige-Gerges H. 2019. New findings on the incorporation of essential oil components into liposomes composed of lipoid S100 and cholesterol. Int J Pharm 561:161–170. doi: 10.1016/j.ijpharm.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Liu L, Xia S, Muhoza B, Cai J, Zhang X, Duhoranimana E, Su J. 2019. Sodium carboxymethyl cellulose modulates the stability of cinnamaldehyde-loaded liposomes at high ionic strength. Food Hydrocoll 93:10–18. doi: 10.1016/j.foodhyd.2019.02.004. [DOI] [Google Scholar]
- 36.Yang FL, Li XG, Zhu F, Lei CL. 2009. Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Agric Food Chem 57:10156–10162. doi: 10.1021/jf9023118. [DOI] [PubMed] [Google Scholar]
- 37.Engel JB, Heckler C, Tondo EC, Daroit DJ, da Silva Malheiros P. 2017. Antimicrobial activity of free and liposome-encapsulated thymol and carvacrol against Salmonella and Staphylococcus aureus adhered to stainless steel. Int J Food Microbiol 252:18–23. doi: 10.1016/j.ijfoodmicro.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Manconi M, Petretto G, D'hallewin G, Escribano E, Milia E, Pinna R, Palmieri A, Firoznezhad M, Peris JE, Usach I, Fadda AM, Caddeo C, Manca ML. 2018. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf B Biointerfaces 171:115–122. doi: 10.1016/j.colsurfb.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 39.Coimbra M, Isacchi B, Van Bloois L, Torano JS, Ket A, Wu X, Broere F, Metselaar JM, Rijcken CJF, Storm G, Bilia R, Schiffelers RM. 2011. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int J Pharm 416:433–442. doi: 10.1016/j.ijpharm.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 40.Ing LY, Zin NM, Sarwar A, Katas H. 2012. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int J Biomater 2012:632698–632699. doi: 10.1155/2012/632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagan E, Berdejo D, Espina L, Garcia-Gonzalo D, Pagan R. 2018. Antimicrobial activity of suspensions and nanoemulsions of citral in combination with heat or pulsed electric fields. Lett Appl Microbiol 66:63–70. doi: 10.1111/lam.12815. [DOI] [PubMed] [Google Scholar]
- 42.Parameswaran N, Patial S. 2010. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr 20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue M, Arikawa T, Chen YH, Moriwaki Y, Price M, Brown M, Perfect JR, Shinohara ML. 2014. T cells down-regulate macrophage TNF production by IRAK1-mediated IL-10 expression and control innate hyperinflammation. Proc Natl Acad Sci U S A 111:5295–5300. doi: 10.1073/pnas.1321427111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbosa C, Santos-Pereira C, Soares I, Martins V, Terra-Matos J, Côrte-Real M, Lúcio M, Real Oliveira MECD, Gerós H. 2019. Resveratrol-loaded lipid nanocarriers are internalized by endocytosis in yeast. J Nat Prod 82:1240–1249. doi: 10.1021/acs.jnatprod.8b01003. [DOI] [PubMed] [Google Scholar]
- 45.Bangham AD, Standish MM, Watkins JC. 1965. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 46.Hajimehdipoor H, Shekarchi M, Khanavi M, Adib N, Amri M. 2010. A validated high performance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Pharmacogn Mag 6:154–158. doi: 10.4103/0973-1296.66927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gursale A, Dighe V, Parekh G. 2010. Simultaneous quantitative determination of cinnamaldehyde and methyl eugenol from stem bark of Cinnamomum zeylanicum Blume using RP-HPLC. J Chromatogr Sci 48:59–62. doi: 10.1093/chromsci/48.1.59. [DOI] [PubMed] [Google Scholar]
- 48.Gaonkar R, Yallappa S, Dhananjaya BL, Hegde G. 2016. Development and validation of reverse phase high performance liquid chromatography for citral analysis from essential oils. J Chromatogr Anal Technol Biomed Life Sci 1036–1037:50–56. doi: 10.1016/j.jchromb.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Raschke WC, Baird S, Ralph P, Nakoinz I. 1978. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell 15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 50.Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW. 2008. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm 364:272–280. doi: 10.1016/j.ijpharm.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 51.Carneiro C, Vaz C, Carvalho-Pereira J, Pais C, Sampaio P. 2014. A new method for yeast phagocytosis analysis by flow cytometry. J Microbiol Methods 101:56–62. doi: 10.1016/j.mimet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J, Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2020. EUCAST definitive document E.DEF 7.3.2. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf.
- 53.Van Dijck P, Sjollema J, Cammue BP, Lagrou K, Berman J, d'Enfert C, Andes DR, Arendrup MC, Brakhage AA, Calderone R, Cantón E, Coenye T, Cos P, Cowen LE, Edgerton M, Espinel-Ingroff A, Filler SG, Ghannoum M, Gow NAR, Haas H, Jabra-Rizk MA, Johnson EM, Lockhart SR, Lopez-Ribot JL, Maertens J, Munro CA, Nett JE, Nobile CJ, Pfaller MA, Ramage G, Sanglard D, Sanguinetti M, Spriet I, Verweij PE, Warris A, Wauters J, Yeaman MR, Zaat SAJ, Thevissen K. 2018. Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms. Microb Cell 5:300–326. doi: 10.15698/mic2018.07.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliveira-Pacheco J, Alves R, Costa-Barbosa A, Cerqueira-Rodrigues B, Pereira-Silva P, Paiva S, Silva S, Henriques M, Pais C, Sampaio P. 2018. The role of Candida albicans transcription factor RLM1 in response to carbon adaptation. Front Microbiol 9:1127. doi: 10.3389/fmicb.2018.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]