Vancomycin-resistant enterococci (VRE) represent a major public health threat that requires the development of new therapeutics. In the present study, acetazolamide (AZM) was evaluated against enterococci.
KEYWORDS: carbonic anhydrase inhibitors, acetazolamide, vancomycin-resistant enterococci (VRE), VRE bloodstream infections, VRE decolonization
ABSTRACT
Vancomycin-resistant enterococci (VRE) represent a major public health threat that requires the development of new therapeutics. In the present study, acetazolamide (AZM) was evaluated against enterococci. It inhibited different enterococcal strains tested at clinically achievable concentrations. Moreover, AZM outperformed linezolid, the drug of choice for VRE infections, in two in vivo VRE mouse models—murine colonization-reduction and VRE septicemia. Collectively, these results indicate that AZM warrants consideration as a promising treatment option for VRE infections.
INTRODUCTION
Vancomycin-resistant enterococci (VRE) are listed as high-priority pathogens which urgently require the development of new antibiotics (1). Currently, linezolid is the only FDA-approved drug for treating VRE infections. However, the treatment outcomes of linezolid are unsatisfactory, especially in bloodstream infections, with a high mortality rate that can reach as high as 30%, and it demonstrates little activity as a VRE decolonizing agent (2). Further compounding the VRE problem is the VRE’s growing resistance to other available treatment options, such as daptomycin, quinupristin/dalfopristin, and tigecycline (3–6). Consequently, there is a critical need for development of new anti-VRE therapeutics.
Repurposing FDA-approved drugs is an attractive strategy for drug discovery that reduces the cost, time, and risk associated with antimicrobial drug innovation (7–12). Utilizing this approach, we identified the FDA-approved carbonic anhydrase inhibitors (CAIs), acetazolamide (AZM), ethoxzolamide (EZM), and methazolamide (MZM), as a novel class of promising anti-VRE agents (8). Building upon our previous work, the aim of the current study is to evaluate the antimicrobial activity of these CAIs against enterococci and evaluate AZM’s in vivo efficacy in two VRE mouse models, VRE decolonization and VRE peritonitis.
Clinical enterococcal isolates (Table 1) were obtained from the BEI Resources and ATCC (Manassas, VA, USA). Drugs used in the study were purchased commercially as follows: AZM from Alfa Aesar (Ward Hill, MA, USA), EZM from Sigma-Aldrich (Saint Louis, MO, USA), MZM, LZD, and vancomycin (VAN) from Chem-Impex International (Wood Dale, IL, USA), and ampicillin from IBI Scientific (Peosta, IA, USA). Media and reagents were purchased from commercial vendors. Susceptibility determinations were performed (3 independent replicates) by broth microdilution following the CLSI guidelines (13).
TABLE 1.
MICs of CAIs and control drugs against clinical VRE isolatesa
| VRE strain | MIC for CAIs/control antibiotics (μg/ml) |
||||
|---|---|---|---|---|---|
| AZM | EZM | MZM | LZD | VAN | |
| E. faecalis NR-31971 | 2 | 1 | 2 | 1 | 64 |
| E. faecium NR-31914 | 1 | 1 | 4 | 1 | >128 |
| E. faecium HM-968 | 1 | 1 | 4 | 1 | >128 |
| E. faecalis NR-31972 | 2 | 1 | 2 | 1 | >128 |
| E. faecium NR-28978 | 1 | 2 | 8 | 1 | >128 |
| E. faecium NR-31903 | 2 | 1 | 8 | 16 | >128 |
| E. faecium NR-31909 | 1 | 1 | 4 | 1 | >128 |
| E. faecium NR-31912 | 1 | 1 | 4 | 0.5 | >128 |
| E. faecium NR-31915 | 1 | 2 | 8 | 1 | >128 |
| E. faecium NR-31916 | 1 | 2 | 8 | 0.5 | 128 |
| E. faecium NR-32052 | 2 | 1 | 8 | 0.5 | >128 |
| E. faecium NR-32053 | 1 | 1 | 8 | 0.5 | >128 |
| E. faecium NR-32054 | 1 | 1 | 8 | 0.5 | 128 |
| E. faecium NR-32065 | 1 | 1 | 1 | 0.25 | >128 |
| E. faecium NR-32094 | 1 | 1 | 8 | 0.5 | >128 |
| E. faecium HM-952 | 1 | 1 | 8 | 1 | >128 |
| E. faecium HM-965 | 1 | 2 | 4 | 0.5 | >128 |
| E. faecium ATCC 700221 | 1 | 1 | 4 | 0.5 | >128 |
| E. faecalis ATCC 51299 | 1 | 1 | 1 | 1 | 64 |
| E. faecalis HM-201 | 2 | 1 | 2 | 1 | >128 |
| E. faecalis HM-334 | 1 | 1 | 1 | 1 | >128 |
| E. faecalis HM-335 | 2 | 1 | 1 | 0.5 | >128 |
| E. faecalis HM-934 | 4 | 4 | 4 | 1 | >128 |
| E. faecium HM-970 | 1 | 1 | 2 | 1 | >128 |
| MIC50 | 1 | 1 | 4 | 1 | >128 |
| MIC90 | 2 | 2 | 8 | 1 | >128 |
CAIs, carbonic anhydrase inhibitors; AZM, acetazolamide; EZM, ethoxzolamide; MZM, methazolamide; LZD, linezolid; VAN, vancomycin.
The antibacterial activity of the CAIs (AZM, EZM, and MZM) was evaluated against a wide panel of clinical VRE strains. AZM and EZM exhibited the most potent activity against the tested isolates (MICs ranging from 1 to 4 μg/ml) (Table 1). They inhibited 50% (MIC50) and 90% (MIC90) of the tested isolates at a concentration of 1 μg/ml and 2 μg/ml, respectively. MZM inhibited the tested VRE strains at concentrations ranging from 1 to 8 μg/ml with MIC50 and MIC90 of 4 μg/ml and 8 μg/ml, respectively. Notably, the MICs of AZM are several-fold lower than its clinically achievable blood concentration, where AZM’s serum concentration reaches up to 100 μg/ml after a single oral dose (14).
Next, we investigated the activity of AZM and EZM against vancomycin-sensitive enterococci. Similarly, they maintained potent activity against vancomycin-sensitive enterococci (MICs, 1 to 4 μg/ml). Moreover, both were tested against other non-faecalis non-faecium enterococcal species. Diseases and mortality due to these Enterococcus strains are significantly increasing worldwide. E. gallinarum and E. casseliflavus infections are of special interest because of their intrinsic resistance to vancomycin (15, 16). These strains can also cause hospital-acquired infections, particularly bloodstream, urinary tract, and surgical wound infections (17). Interestingly, AZM and EZM maintained the same potency (or even better) against these strains. Advantageously, they were 2-fold more potent than linezolid (LZD) against E. saccharolyticus and E. durans. In addition, they were as effective as LZD against E. hirae and E. casseliflavus with the exception of EZM, which was 4 times more potent than LZD (MIC, 0.25 μg/ml) (Table 2).
TABLE 2.
MICs of AZM and EZM and control drugs against clinical vancomycin-sensitive Enterococcus faecium and Enterococcus faecalis isolates and other clinically important enterococcal speciesa
| Enterococcal strains | MICs for CAIs/control antibiotics (μg/ml) |
|||
|---|---|---|---|---|
| AZM | EZM | LZD | VAN | |
| E. faecium NR-31933 | 4 | 4 | 2 | 4 |
| E. faecium NR-31935 | 2 | 2 | 0.5 | 1 |
| E. faecium NR-31937 | 2 | 2 | 1 | 2 |
| E. faecium NR-31954 | 4 | 4 | 0.5 | 2 |
| E. faecalis NR-31975 | 2 | 1 | 1 | 1 |
| E. faecalis NR-31970 | 2 | 2 | 1 | 1 |
| E. gallinarum ATCC 49573 | 4 | 2 | 0.5 | 16 |
| E. saccharolyticus ATCC 43076 | 0.5 | 0.5 | 1 | 0.5 |
| E. casseliflavus ATCC 700327 | 1 | 0.25 | 1 | 16 |
| E. hirae ATCC 10541 | 2 | 2 | 1 | 1 |
| E. durans ATCC 11576 | 0.25 | 0.25 | 0.5 | 1 |
CAIs, carbonic anhydrase inhibitors; AZM, acetazolamide; EZM, ethoxzolamide; LZD, linezolid; VAN, vancomycin.
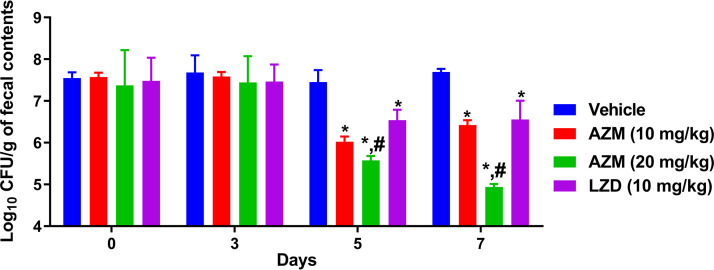
Dysbiosis caused by a patient’s exposure to broad-spectrum antibiotics can lead to VRE colonization, which serves as the origination point for VRE to spread in the body, leading to life-threatening infections (18). Thus, VRE decolonization from the gastrointestinal tract (GIT) is an important strategy to curb VRE infections, particularly in immunocompromised, organ transplant, and intensive-care unit patients (19, 20). Therefore, we evaluated AZM’s activity as a VRE decolonizer in a mouse model as reported before (20–22). Briefly, 8-week-old female C57BL/6 mice (Jackson Laboratories, Maine, USA) were exposed to drinking water containing ampicillin (0.5 g/liter) for 7 days before infection with 1.3 × 108 CFU/ml of E. faecium HM-952 via oral gavage. Then, mice were left for 7 days to colonize with VRE, after which treatments started. Treatment groups (n = 5) were as follows: one group for AZM (10 mg/kg), one group for AZM (20 mg/kg), one group treated with LZD (10 mg/kg), and one group treated with vehicle (10% DMSO in phosphate-buffered saline [PBS]) (negative control). Mice received treatments orally for 8 days. Mouse fecal pellets were aseptically collected on days 0, 3, 5, and 7. Following euthanasia, the ceca and ilea were aseptically collected. Fecal pellets and cecal and ileal contents were diluted and plated on Enterococcosel agar plates containing vancomycin (8 μg/ml) to determine the bacterial count present in each sample. The data of CFU count in fecal contents were analyzed via two-way analysis of variance (ANOVA) with a post hoc Dunnett’s test for multiple comparisons (P < 0.05). The cecal and ileal content data were analyzed via one-way ANOVA with a post hoc Dunnett’s test for multiple comparisons (P < 0.05). An asterisk (*) denotes a statistically significant difference between the results obtained for AZM or LZD in comparison to the those of the untreated group (vehicle). A pound sign (#) denotes a statistically significant difference between the results obtained for AZM in comparison to those for LZD. Although both AZM and LZD exhibit a bacteriostatic activity against VRE (23), AZM was found to be superior to LZD in decreasing the VRE burden in the intestinal organs of the colonized mice (Fig. 1 and 2). After 5 days of treatment, AZM (10 mg/kg) significantly reduced the VRE burden in mouse fecal samples by 1.43 log10 (96.3% reduction), while AZM (20 mg/kg) significantly reduced the burden by 1.88 log10 (98.7% reduction). LZD, in contrast, generated a 0.9-log10 reduction (87.4%) in VRE count. The VRE burden continued to significantly decrease with AZM (20 mg/kg) treatment, resulting in a 2.75-log10 (99.8%) reduction of VRE in fecal samples after 7 days of treatment. On the other hand, AZM (10 mg/kg) generated a similar reduction to that produced after 5 days of treatment. Conversely, LZD only generated a 1.14-log10 (92.6%) reduction in VRE CFU after 7 days of treatment (Fig. 1). (All animal housing and experiments were reviewed, approved, and performed under the guidelines of the Purdue University Animal Care and Use Committee and carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.)
FIG 1.
Burden of VRE (E. faecium HM-952) in the fecal contents of colonized mice. The CFU data were analyzed via a two-way ANOVA with post hoc Dunnett’s test for multiple comparisons. An asterisk (*) indicates a significant difference (P < 0.05) between mice treated with AZM or LZD compared with vehicle. A pound sign (#) indicates a significant difference (P < 0.05) between mice treated with AZM compared to LZD-treated mice.
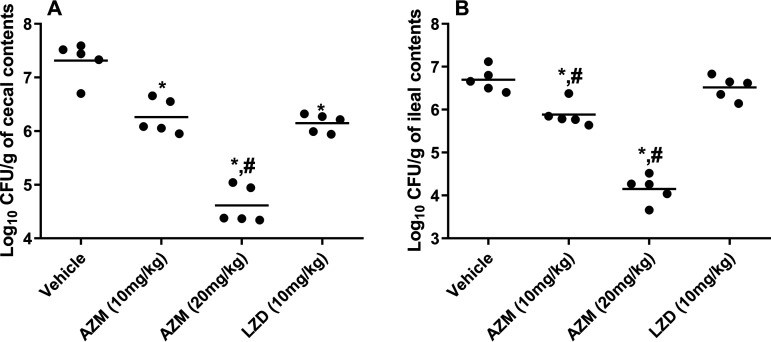
FIG 2.
(A and B) Burden of VRE (E. faecium HM-952) in (A) the cecal contents of colonized mice and (B) the ileal contents of colonized mice (collected at sacrifice on day 8). The CFU data were analyzed via a one-way ANOVA with post hoc Dunnett’s test for multiple comparisons. An asterisk (*) indicates a significant difference (P < 0.05) between mice treated with AZM or LZD compared with untreated mice (vehicle). A pound sign (#) indicates a significant difference (P < 0.05) between mice treated with AZM compared to LZD-treated mice.
Similar to the results from the fecal samples, AZM significantly reduced the VRE count in the mouse cecal and ileal contents (Fig. 2). In the cecal contents, AZM (10 mg/kg) significantly decreased the VRE burden by 1.06 log10 (91.2% reduction). This was similar to the reduction obtained with LZD, which decreased the VRE count in the cecal contents by 1.1 log10 (93.2% reduction). On the other hand, AZM (20 mg/kg) significantly outperformed LZD, generating a 2.71-log10 (99.8%) reduction in the cecal VRE burden. In the ileal contents, AZM (10 mg/kg and 20 mg/kg) significantly surpassed LZD in reducing the VRE burden (0.82-log10 [84.6%] reduction and 2.54-log10 [99.7%] reduction, respectively). LZD did not reduce the VRE count in the ileal contents (Fig. 2), in accordance with previous reports (20, 21). The lower activity of LZD in reducing the bacterial burden in the GIT could be attributed to its rapid absorption from the GIT (24), its low concentration in the stool (25), or its limited activity against a high bacterial inoculum (∼108 CFU) as in the case of VRE colonization (26).
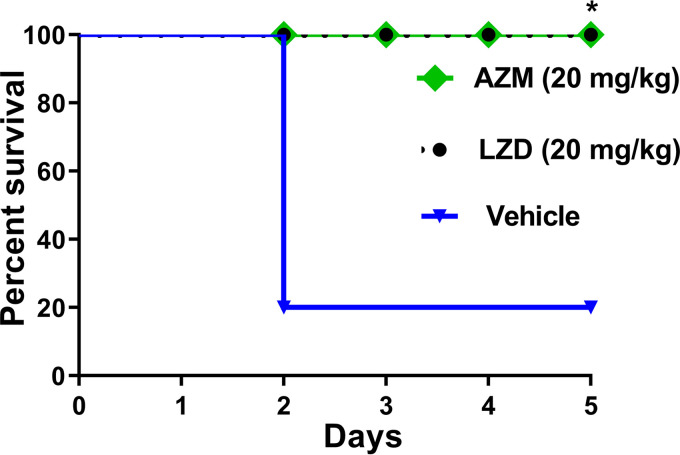
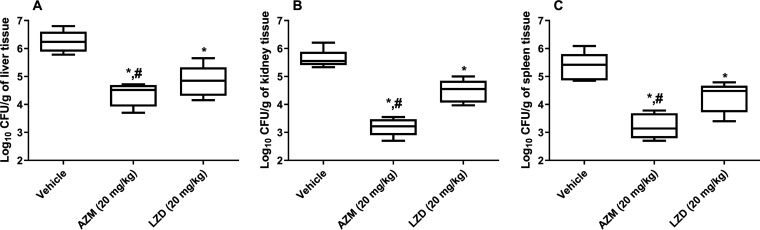
Finally, we aimed to investigate the activity of AZM in the murine VRE septicemic peritonitis model (9). Enterococci, mainly VRE, are a common cause of nosocomial bloodstream infections, and their incidence is continually rising. Management of VRE bloodstream infections is compromised by the enterococcal resistance to several antibiotics, especially cell wall inhibitors and aminoglycosides that are commonly used in combination for treatment of such infections (27). As a result, there is a critical need for new agents effective against systemic VRE infections. Thus, the efficacy of AZM in an in vivo VRE peritonitis murine model was evaluated. Female BALB/c mice (8 weeks old; Jackson Laboratories, Maine, USA) were infected intraperitoneally with E. faecium NR-31909 (3 × 107 CFU/ml) premixed with 20% sterile rat fecal extract (SRFE) (2:1). One hour later, mouse groups (n = 5) were treated orally with either AZM (20 mg/kg), LZD (20 mg/kg), or the vehicle (10% DMSO in PBS). Mice received treatments for 4 days before they were humanely euthanized. Afterward, mouse internal organs (livers, kidneys, and spleens) were aseptically removed. Bacteria were recovered from the internal organs, serially diluted, and plated on Enterococcosel agar containing vancomycin (8 μg/ml). The CFU data were analyzed via one-way ANOVA with a post hoc Dunnett’s test for multiple comparisons (P < 0.05). Asterisks (*) denote statistically significant differences between the results obtained for AZM or LZD in comparison to those of the negative-control group (P < 0.05). Pound signs (#) denote statistically significant differences between the results obtained for AZM in comparison to those for LZD. As presented in Fig. 3, AZM (20 mg/kg) and LZD (20 mg/kg) significantly protected 100% of the mice from a lethal dose of VRE. However, AZM showed a statistically significant decrease in the number of CFU/g of organ tissue compared to both vehicle control and LZD-treated animals (Fig. 4). AZM reduced the VRE burden in mouse liver, kidney, and spleen tissues by 1.9-log10 (98.7%), 2.43-log10 (99.6%), and 2.13-log10 (99.3%) reductions, respectively. The highest reduction observed with AZM was in the kidney tissue, which could be attributed to the fact that AZM is excreted unmetabolized through the kidneys and urine (28). LZD generated 1.42-log10 (96.1%), 1.14-log10 (92.8%), and 1.1-log10 (91.9%) reductions of the VRE in the livers, kidneys, and spleens of mice, respectively (Fig. 4), in agreement with a previous report (9).
FIG 3.
In vivo activity of AZM in the murine VRE peritonitis model after infection with E. faecium NR-31909. Kaplan-Meier survival curves were analyzed using a log-rank (Mantel-Cox) test. The asterisk (*) denotes a statistically significant difference (P < 0.05) between mice treated with either AZM or LZD in comparison with the vehicle-treated mice.
FIG 4.
(A to C) Burden of VRE (E. faecium NR-31909) in (A) liver, (B) kidneys, and (C) spleen of the infected mice (determined at the time of death for the vehicle-treated animals and at day 5 for all surviving animals). The CFU data were analyzed via a one-way ANOVA with post hoc Dunnett’s test for multiple comparisons. An asterisk (*) indicates a significant difference (P < 0.05) between mice treated with AZM or LZD compared with untreated mice (vehicle). A pound sign (#) indicates a significant difference (P < 0.05) between mice treated with AZM compared to LZD-treated mice.
In conclusion, the current study highlights AZM as a new potent antienterococcal agent. AZM demonstrated greater reductions in VRE CFU counts compared to LZD in mouse models of VRE colonization reduction and VRE systemic infection. In addition, AZM has a well-studied safety profile and can be administered in dosages up to 4 g/day to humans, and it possesses highly acceptable pharmacokinetic properties (29, 30). Thus, AZM represents a promising, novel treatment option for VRE infections.
ACKNOWLEDGMENTS
We declare no competing interests.
This work was supported by the National Institutes of Health (grant no. R01AI148523).
REFERENCES
- 1.Tacconelli E, Magrini N, Kahlmeter G, Singh N. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Britt NS, Potter EM, Patel N, Steed ME. 2017. Effect of continuous and sequential therapy among veterans receiving daptomycin or linezolid for vancomycin-resistant Enterococcus faecium bacteremia. Antimicrob Agents Chemother 61:e02216-16. doi: 10.1128/AAC.02216-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quinn JP. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. doi: 10.1016/S0140-6736(00)04376-2. [DOI] [PubMed] [Google Scholar]
- 4.Munoz-Price LS, Lolans K, Quinn JP. 2005. Emergence of resistance to daptomycin during treatment of vancomycin-resistant Enterococcus faecalis infection. Clin Infect Dis 41:565–566. doi: 10.1086/432121. [DOI] [PubMed] [Google Scholar]
- 5.Donabedian SM, Perri MB, Vager D, Hershberger E, Malani P, Simjee S, Chow J, Vergis EN, Muder RR, Gay K, Angulo FJ, Bartlett P, Zervos MJ. 2006. Quinupristin-dalfopristin resistance in Enterococcus faecium isolates from humans, farm animals, and grocery store meat in the United States. J Clin Microbiol 44:3361–3365. doi: 10.1128/JCM.02412-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiedler S, Bender J, Klare I, Halbedel S, Grohmann E, Szewzyk U, Werner G. 2016. Tigecycline resistance in clinical isolates of Enterococcus faecium is mediated by an upregulation of plasmid-encoded tetracycline determinants tet(L) and tet(M). J Antimicrob Chemother 71:871–881. doi: 10.1093/jac/dkv420. [DOI] [PubMed] [Google Scholar]
- 7.Brown D. 2015. Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void? Nat Rev Drug Discov 14:821–832. doi: 10.1038/nrd4675. [DOI] [PubMed] [Google Scholar]
- 8.Younis W, AbdelKhalek A, Mayhoub AS, Seleem MN. 2017. In vitro screening of an FDA-approved library against ESKAPE pathogens. Curr Pharm Des 23:2147–2157. doi: 10.2174/1381612823666170209154745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abutaleb NS, Seleem MN. 2020. Antivirulence activity of auranofin against vancomycin-resistant enterococci: in vitro and in vivo studies. Int J Antimicrob Agents 55:105828. doi: 10.1016/j.ijantimicag.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AbdelKhalek A, Abutaleb NS, Mohammad H, Seleem MN. 2019. Antibacterial and antivirulence activities of auranofin against Clostridium difficile. Int J Antimicrob Agents 53:54–62. doi: 10.1016/j.ijantimicag.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abutaleb NS, Seleem MN. 2020. Repurposing the antiamoebic drug diiodohydroxyquinoline for treatment of Clostridioides difficile infections. Antimicrob Agents Chemother 64:e02115-19. doi: 10.1128/AAC.02115-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abutaleb NS, Seleem MN. 2020. Auranofin, at clinically achievable dose, protects mice and prevents recurrence from Clostridioides difficile infection. Sci Rep 10:1–8. doi: 10.1038/s41598-020-64882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 9th ed. Approved standard; M07-A9, 32 no. 2. CLSI, Wayne, PA. [Google Scholar]
- 14.Dahl A, Russell D, Rootwelt K, Nyberg-Hansen R, Kerty E. 1995. Cerebral vasoreactivity assessed with transcranial Doppler and regional cerebral blood flow measurements: dose, serum concentration, and time course of the response to acetazolamide. Stroke 26:2302–2306. doi: 10.1161/01.str.26.12.2302. [DOI] [PubMed] [Google Scholar]
- 15.Hollenbeck BL, Rice LB. 2012. Intrinsic and acquired resistance mechanisms in Enterococcus. Virulence 3:421–569. doi: 10.4161/viru.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hershberger E, Donabedian S, Konstantinou K, Zervos MJ, Eliopoulos GM. 2004. Quinupristin-dalfopristin resistance in gram-positive bacteria: mechanism of resistance and epidemiology. Clin Infect Dis 38:92–98. doi: 10.1086/380125. [DOI] [PubMed] [Google Scholar]
- 17.Monticelli J, Knezevich A, Luzzati R, Di Bella S. 2018. Clinical management of non-faecium non-faecalis vancomycin-resistant enterococci infection. Focus on Enterococcus gallinarum and Enterococcus casseliflavus/flavescens. J Infect Chemother 24:237–246. doi: 10.1016/j.jiac.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MRM, Kamboj M, Pamer EG. 2010. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong MT, Kauffman CA, Standiford HC, Linden P, Fort G, Fuchs HJ, Porter SB, Wenzel RP, Ramoplanin VRE2 Clinical Study Group . 2001. Effective suppression of vancomycin-resistant Enterococcus species in asymptomatic gastrointestinal carriers by a novel glycolipodepsipeptide, ramoplanin. Clin Infect Dis 33:1476–1482. doi: 10.1086/322687. [DOI] [PubMed] [Google Scholar]
- 20.Mohammad H, AbdelKhalek A, Abutaleb NS, Seleem MN. 2018. Repurposing niclosamide for intestinal decolonization of vancomycin-resistant enterococci. Int J Antimicrob Agents 51:897–904. doi: 10.1016/j.ijantimicag.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AbdelKhalek A, Abutaleb NS, Elmagarmid KA, Seleem MN. 2018. Repurposing auranofin as an intestinal decolonizing agent for vancomycin-resistant enterococci. Sci Rep 8:8353. doi: 10.1038/s41598-018-26674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AbdelKhalek A, Abutaleb NS, Mohammad H, Seleem MN. 2018. Repurposing ebselen for decolonization of vancomycin-resistant enterococci (VRE). PLoS One 13:e0199710. doi: 10.1371/journal.pone.0199710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur J, Cao XF, Abutaleb NS, Elkashif A, Graboski AL, Krabill AD, AbdelKhalek AH, An WW, Bhardwaj A, Seleem MN, Flaherty DP. 2020. Optimization of acetazolamide-based scaffold as potent inhibitors of vancomycin-resistant Enterococcus. J Med Chem 63:9540–9562. doi: 10.1021/acs.jmedchem.0c00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beringer P, Nguyen M, Hoem N, Louie S, Gill M, Gurevitch M, Wong-Beringer A. 2005. Absolute bioavailability and pharmacokinetics of linezolid in hospitalized patients given enteral feedings. Antimicrob Agents Chemother 49:3676–3681. doi: 10.1128/AAC.49.9.3676-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lode H, Von der Höh N, Ziege S, Borner K, Nord CE. 2001. Ecological effects of linezolid versus amoxicillin/clavulanic acid on the normal intestinal microflora. Scand J Infect Dis 33:899–903. doi: 10.1080/00365540110076714. [DOI] [PubMed] [Google Scholar]
- 26.Pultz NJ, Stiefel U, Donskey CJ. 2005. Effects of daptomycin, linezolid, and vancomycin on establishment of intestinal colonization with vancomycin-resistant enterococci and extended-spectrum-β-lactamase-producing Klebsiella pneumoniae in mice. Antimicrob Agents Chemother 49:3513–3516. doi: 10.1128/AAC.49.8.3513-3516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mundy L, Sahm D, Gilmore M. 2000. Relationships between enterococcal virulence and antimicrobial resistance. Clin Microbiol Rev 13:513–522. doi: 10.1128/cmr.13.4.513-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yano I, Takayama A, Takano M, Inatani M, Tanihara H, Ogura Y, Honda Y, Inui K. 1998. Pharmacokinetics and pharmacodynamics of acetazolamide in patients with transient intraocular pressure elevation. Eur J Clin Pharmacol 54:63–68. doi: 10.1007/s002280050422. [DOI] [PubMed] [Google Scholar]
- 29.Ritschel WA, Paulos C, Arancibia A, Agrawal MA, Wetzelsberger KM, Lücker PW. 1998. Pharmacokinetics of acetazolamide in healthy volunteers after short‐and long‐term exposure to high altitude. J Clin Pharmacol 38:533–539. doi: 10.1002/j.1552-4604.1998.tb05791.x. [DOI] [PubMed] [Google Scholar]
- 30.ten Hove MW, Friedman DI, Patel AD, Irrcher I, Wall M, McDermott MP, NORDIC Idiopathic Intracranial Hypertension Study Group . 2016. Safety and tolerability of acetazolamide in the Idiopathic Intracranial Hypertension Treatment Trial. J Neuroophthalmol 36:13–19. doi: 10.1097/WNO.0000000000000322. [DOI] [PubMed] [Google Scholar]