The objectives of this study were to evaluate the population pharmacokinetics of prophylactic cefazolin (CFZ) from its serum and hip joint capsule concentrations in patients undergoing total hip arthroplasty and to establish the pharmacodynamic target concentration exceeding the MIC for designing an effective dosing regimen for serum and the hip joint capsule. We analyzed 249 serum samples and 125 hip joint capsule samples from 125 individuals using a nonlinear mixed-effects model.
KEYWORDS: cefazolin, hip joint capsule, population pharmacokinetic
ABSTRACT
The objectives of this study were to evaluate the population pharmacokinetics of prophylactic cefazolin (CFZ) from its serum and hip joint capsule concentrations in patients undergoing total hip arthroplasty and to establish the pharmacodynamic target concentration exceeding the MIC for designing an effective dosing regimen for serum and the hip joint capsule. We analyzed 249 serum samples and 125 hip joint capsule samples from 125 individuals using a nonlinear mixed-effects model. The pharmacodynamic index target value obtained from our results indicates the probability of maintaining CFZ trough and hip joint capsule concentrations exceeding the MIC of 1 mg/liter to account for methicillin-susceptible S. aureus (MSSA). We estimated the population pharmacokinetics using a two-compartment model. The estimated population pharmacokinetic parameters were as follows: clearance (CL) (liters/h) = 1.46 × (creatinine clearance [CLcr] [ml/min]/77)0.891, volume of distribution of the central compartment (Vc) (liters) = 7.5, central-hip joint capsule compartment clearance (Q) (liters/h) = 3.38, and volume of distribution in the hip joint capsule compartment (VJC) (liters) = 36.1. The probability of achieving concentrations exceeding the MIC90 for MSSA was approximately 100% for serum and 100% for the hip joint capsule at 3 h after the initial dose. Our findings suggest that population-based parameters are useful for evaluating CFZ pharmacokinetics and that individual dosages should be determined based on the dosage regimen that achieves and maintains adequate tissue CFZ concentration.
TEXT
Cefazolin (CFZ), a first-generation cephalosporin, shows good activity against Gram-positive cocci. It is widely used for prophylaxis in several surgical procedures. Indeed, owing to its broad-spectrum activity, low toxicity, and cost (1, 2), CFZ is recommended as an ideal prophylactic antibiotic for perioperative administration. To prevent surgical site infections (SSIs), a prophylactic antibiotic dosing schedule that achieves and maintains adequate tissue concentrations of antibiotics near the surgical site is critical (3). To prevent infections, prophylactic antibiotic concentrations that exceed the MIC of the targeted pathogen for at least the duration between incision and surgical wound closure are required (4). Moreover, an antibiotic should achieve these optimal concentrations in not only the serum but also the target site (5). Therefore, intraoperative redosing is needed when the duration of the procedure exceeds two half-lives of the drug. The American Society of Health-System Pharmacists (ASHP) guidelines recommend cefazolin redosing at 4 h (1). However, data concerning prophylactic antibiotic concentration in the tissue site are frequently insufficient.
The aim of this study was to describe the population pharmacokinetics of CFZ in both serum and hip joint capsule and to estimate the pharmacodynamic target concentration exceeding the MIC for designing an effective dosing regimen.
RESULTS
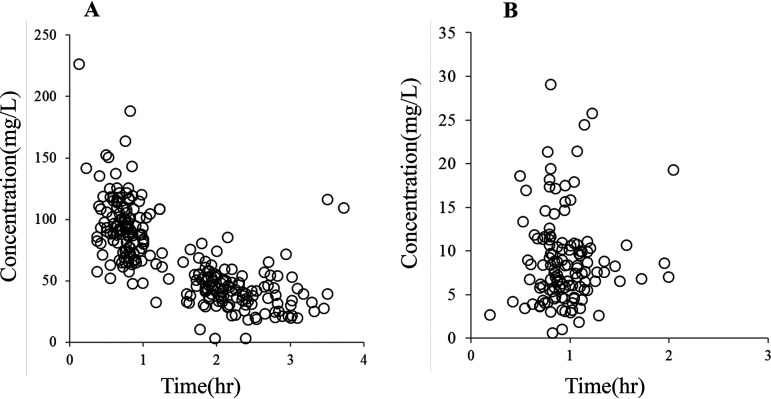
The CFZ concentrations in 26 men and 99 women were evaluated in this study. Patient characteristics are shown in Table 1. The individual observed CFZ concentrations in the serum and hip joint capsule at various time points are shown in Fig. 1. The two-compartment model fit the data better than the three-compartment model based on our discrimination criteria. Each covariate model is shown in Table 2. The final population parameters are presented in Table 2. The only covariate observed was CFZ clearance, and CLcr was normalized to 77 ml/min. The final model was as follows:
where the volume of distribution of the central compartment (Vc) (liters) = 7.5, the central-hip joint capsule compartment clearance (Q (liters/h) = 3.38, and the volume of distribution in the hip joint capsule compartment (VJC) (liters) = 36.1. The coefficients of variation for the interindividual variability (ω2) of clearance (CL), Vc, Q, Vjc, and residual variability (σ2) were 58.0%, 15.9%, 21.7%, 57.0%, and 16.6%, respectively.
TABLE 1.
Characteristics of the patients
| Characteristic | No. | Median (IQR) | Range |
|---|---|---|---|
| No. of patients (male/female) | 26/99 | ||
| Serum concn (mg/liter) | 249 | 65.0 (42.1–93.4) | 3.3–226.7 |
| Hip joint capsule concn (mg/liter) | 125 | 8.0 (5.6–10.8) | 0.6–29.1 |
| Age (yrs) | 64 (56–72) | 30–95 | |
| Wt (kg) | 56.0 (49.7–69.1) | 32.9–99.4 | |
| Creatinine clearancea | 77 (61–103) | 20–180 |
Estimated using the Cockcroft-Gault equation.
FIG 1.
Observed cefazolin concentrations. (A and B) Observed individual concentrations of cefazolin in the serum (A) and hip joint capsule (B) at various times after dosing.
TABLE 2.
Summary of population pharmacokinetic analysis for cefazolin
| Models | OBJa | −2.l.l.db | P value |
|---|---|---|---|
| Basic modelc (2-compartment model)d | 2165.442 | ||
| CL | |||
| θ1 × (CLcr/CLcr median)θ5 | 2,150.072 | 15.37 | <0.01 |
| θ1 (age > 65), θ5 (age ≦ 65) | 2,165.094 | 0.348 | NS |
| θ1 (sex = male), θ5 (sex = female) | 2,165.374 | 0.068 | NS |
| Vc | |||
| θ1 + θ5 × BW | 2,155.392 | 10.05 | <0.01 |
| Q | |||
| θ1 × (CLcr/CLcr median)θ5 | 2,163.986 | 1.456 | NS |
| θ1 (age > 65), θ5 (age ≦ 65) | 2,164.271 | 1.171 | NS |
| θ1 (sex = male), θ5 (sex = female) | 2,165.339 | 0.103 | NS |
| VJC | |||
| θ1 + θ5 × BW | 2,150.933 | 14.449 | <0.01 |
OBJ, objective function.
–2 log-likelihood.
CL, clearance; Vc, volume of distribution of the central compartment; Q, central hip joint capsule compartment clearance; VJC, volume of distribution of the hip joint capsule compartment; NS, not significant.
CL (liters/h) = θ1, Vc (liters) = θ2, Q (liters/h) = θ3, VJC (liters) = θ4.
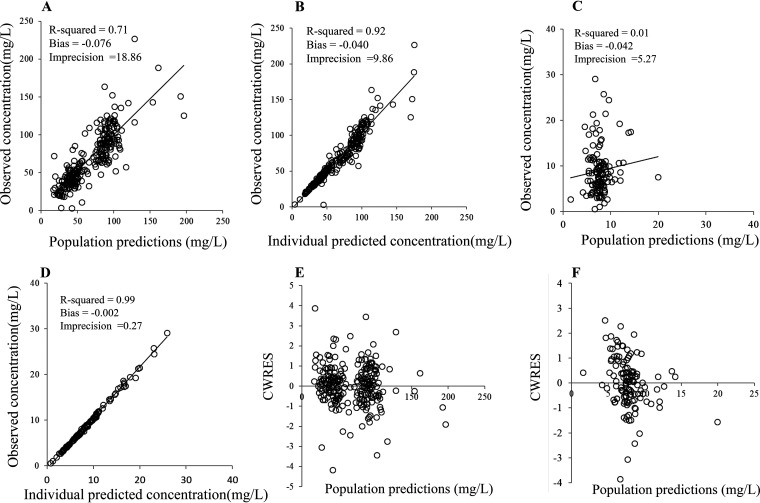
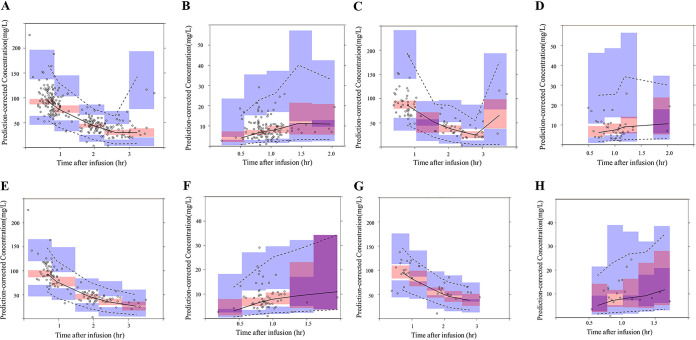
The assessment results of the predictive performance of the final model are presented in scatterplots of the observed versus population-predicted concentrations (Fig. 2) and of the individual-predicted concentrations of CFZ (Fig. 2). The weighted-residual concentration versus the population-predicted concentration is presented in Fig. 2. These plots were symmetrically distributed around the line of identity, indicating that the model adequately described the serum and hip joint capsule CFZ concentration. A visual predictive check (VPC) is shown in Fig. 3. The median and percentile intervals of the predicted values are relatively close to the observed values. In the bootstrap analysis of the final model, 802 of 1,000 bootstraps showed successful results. The values of the parameters used in the final model were close to the value in the bootstrap samples, and the relative standard errors were acceptably small. In addition, the parameters from the bootstrap analysis followed a normal distribution and comprised all parameter estimates from the final population model (Table 3).
FIG 2.
Diagnostic plots for the final covariate model. (A and C) Observed versus population predicted concentrations in the serum (A) and hip joint capsule (C). (B and D) Observed versus individual predicted concentrations in the serum (B) and hip joint capsule (D). (E and F) Conditional weighted residuals (CWRES) versus population predictions in the serum (E) and hip joint capsule (F).
FIG 3.
(A and B) Visual predictive check based on the final population pharmacokinetic of the serum (A) and hip joint capsule (B). (C and D) In subjects with normal renal function (CLcr ≥ 90 ml/min), serum (C) and hip joint capsule (D). (E and F) In subjects with moderate renal impairment (CLcr 50 to 90 ml/min), serum (E) and hip joint capsule (F). (G and H) In subjects with severe renal impairment (CLcr < 50 ml/min), serum (G) and hip joint capsule (H). The solid and dotted lines are median profiles and 90% predicted intervals (PIs).
TABLE 3.
Parameter estimates of the population pharmacokinetic model
| Characteristic | Parameter | Estimate | Final model |
Final bootstrap estimates (n = 1,000) |
|||
|---|---|---|---|---|---|---|---|
| RSEb (%) | SHRc (%) | Avg | Lower 2.5% | Upper 97.5% | |||
| Structural model parametersa | |||||||
| CL (liters/h) = θ1 × (Ccr/77)θ2 | θ1 | 1.46 | 16.3 | 1.49 | 0.71 | 2.54 | |
| θ2 | 0.891 | 25.1 | 0.941 | 0.556 | 1.507 | ||
| Vc (liters) = θ3 | θ3 | 7.50 | 2.6 | 7.49 | 7.07 | 8.01 | |
| Q (liters/h) = θ4 | θ4 | 3.38 | 10.2 | 3.32 | 2.13 | 4.28 | |
| VJC (liters) = θ5 | θ5 | 36.1 | 25.1 | 35.5 | 22.3 | 47.1 | |
| Between-subject variability | |||||||
| Clearance | CL (% CV) | 58.0 | 23.6 | 25.8 | 58.4 | 22.7 | 95.2 |
| Vol of distribution of the central compartment | Vc (% CV) | 15.9 | 32.3 | 44.2 | 18.3 | 6.56 | 27.2 |
| Central skeletal muscle compartment clearance | Q (% CV) | 21.7 | 25.9 | 46.9 | 23.3 | 10.9 | 34.5 |
| Vol of distribution of the tensor fasciae latae compartment | VJC (% CV) | 57.0 | 11.3 | 9.3 | 56.5 | 43.6 | 69.5 |
| Between-subject variability | RUVPROP CFZ | 16.6 | 20.3 | 37.8 | 15.6 | 6.1 | 23.0 |
CL, clearance; Vc, volume of distribution of the central compartment; Q, central-hip joint capsule compartment clearance; VJC, volume of distribution of the hip joint capsule compartment; CV, coefficient variation.
Relative standard error.
Shrinkage.
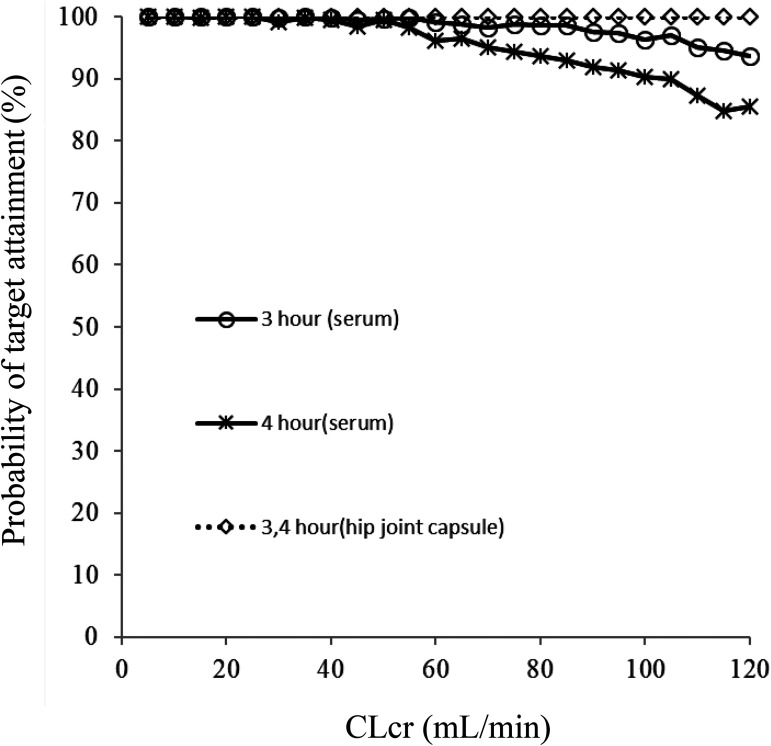
A simulation (n = 240,000) was performed using the final model to determine the optimal dosing regimen in patients with renal impairment. The CFZ trough concentration in both free serum and hip joint capsule was simulated for every 10,000 patients, with 24 levels of CLcr ranging from 5 to 120 ml/min every 5 ml/min. The target was the probability of maintaining the CFZ trough concentration above 1 mg/liter in free serum and hip joint capsule (Fig. 4).
FIG 4.
Monte Carlo simulations. The probability of target attainment of serum trough and hip joint capsule concentrations with a 1-g dose for CLcr 5 ml/min through CLcr 120 ml/min at 3- and 4-h points postdosing.
DISCUSSION
We characterized the pharmacokinetics and pharmacodynamics of CFZ in the serum and hip joint capsule of patients with total hip arthroplasty (THA) and calculated the CFZ dosing regimen based on the renal function to achieve the serum and hip joint capsule CFZ concentrations above the MIC of 1 mg/liter. Our data revealed that the concentration of CFZ in the hip joint capsule was approximately 10% of the corresponding serum concentration. Schurman et al. reported that when 1 g of CFZ was intravenously administered over a 5-min period before the operation (6), the concentration of CFZ in the serum and synovial fluid ranged from 56.0 to 135.0 mg/liter and from 7.1 to 63.0 mg/ml, respectively. Thus, CFZ in the serum did not penetrate the hip joint capsule, largely because CFZ is a water-soluble antibiotic with a strong protein-binding characteristic.
Our population pharmacokinetic data showed CLcr as a significant covariant for the central compartment clearance of CFZ. CFZ is considered to be eliminated by the kidneys. Previous studies have shown that CLcr is an affective factor (7, 8). Sharareh et al. reported that trabecular bone CFZ concentration did not affect body weight. Therefore, in our final model, weight did not have an effect on the Vjc (9).
The results of the present study revealed that population means are a good predictor of performance. The weighted residuals were acceptable to within three standard deviations, and this is generally recognized as the criterion for no selection bias. Therefore, we conclude that the final model has a good predictive capability.
Generally, the ASHP guidelines recommend that intraoperative redosing is needed to maintain adequate serum and tissue concentrations of the antimicrobial if the duration of the procedure exceeds two half-lives of the drug. Therefore, a CFZ redosing interval of 4 h is suggested (1). In contrast, Ohge et al. recommend that a second dose of CFZ be administered 3 h after the first administration to maintain adequate antibiotic activity (10). Similarly, based on our data, we recommend a CFZ redosing interval of 3 h to maintain adequate serum and hip joint capsule CFZ concentrations, regardless of the value of renal function. In contrast, for a CFZ redosing interval of 4 h, as shown in Table 2, the probability of target attainment in the serum was below 90% under normal renal function (≥90 ml/min). Therefore, our results suggest that an additional dose of CFZ should be considered every 3 h, rather than every 4 h, under normal renal function. Additionally, the CFZ redosing interval should be longer than 4 h for renal failure because the half-life of CFZ in the serum is considerably prolonged in patients with reduced renal function.
There were some limitations to this study. First, we utilized a small number of sample points; serum and tissue points were derived from only two sample points. Additionally, our observations did not extend longer than 4 h because THA is a short operation. The serum and hip joint capsule CFZ concentrations after 4 h were simulated from the population pharmacokinetic data. Therefore, the limited information might be insufficient for accurate predictions at 4 h.
Second, the ASHP guidelines recommend the prophylactic use of CFZ at 2- and 3-g dosages to patients weighing <120 and ≥120 kg, respectively (1). However, the mean body weight of patients in our study was lower (59 kg), and most patients received only 1 g CFZ. Only four patients received 2 g CFZ, and these were individuals with a body weight of ≥80 kg. Therefore, our dosing regimen is not adapted for individuals with a body weight of ≥80 kg. Additional studies, including model validation with external data, are required, and this is our next objective. Notwithstanding the limitations of our study, the optimal dosing regimen reported should be helpful in determining intraoperative CFZ redosing intervals.
In conclusion, we performed CFZ population pharmacokinetics to determine the serum and hip joint capsule CFZ concentrations and to evaluate the influence of CLcr on the pharmacokinetics of CFZ. Additionally, based on our population pharmacokinetics, we developed an optimal dosing regimen for intraoperative redosing (which exceeds the MIC90 for MSSA). A CFZ redosing interval of 3 h is recommended assuming normal renal function.
MATERIALS AND METHODS
Patients.
Patients undergoing THA from February 2017 to January 2019 in Kitasato University Hospital, Kangawa, Japan, were enrolled. After the induction of anesthesia (and within 60 min before the surgical incision), all patients received 1 or 2 g of intravenous CFZ for 10 min. All patients provided written informed consent before the procedure. The study was performed in accordance with the Helsinki Declaration after approval by the Ethical Review Board of our hospital (approval number B19-156).
Sample collection.
Blood samples were collected at the time of initial incision for hip joint capsule resection. Hip joint capsule samples were collected at the time of hip joint capsule resection. The blood samples were centrifuged immediately after collection, and the resulting serum samples were stored frozen at −80°C until further analysis. The hip joint capsule samples were rinsed with phosphate buffer solution and stored at −80°C until further analysis.
Measurement of CFZ concentration in the serum and hip joint capsule.
CFZ concentration in the serum and hip joint capsule was measured by high-performance liquid chromatography as previously described (11). Briefly, the hip joint capsule samples were pulverized in liquid nitrogen using CRYO-PRESS to yield a powder (Microtec Nition, Chiba, Japan). The powdered samples were then homogenized in phosphate buffer solution (300 μl). Subsequently, the homogenate was centrifuged and the supernatant was collected for further processing. The tissue supernatant (100 μl) and serum samples (200 μl) were mixed with 1.5 times methanol (vol/vol), and centrifuged at 10,000 g for 10 min. The sample solution (50 μl) was then injected into a C18 column at 10°C. The samples were separated in the mobile phase (see below) at a flow rate of 1.0 ml/min, and the eluate was monitored at 254 nm using a UV absorption detector. The mobile phase consisted of 85% 0.01 M sodium acetate (pH 5.2) and 15% acetonitrile (96%)/methanol (4%) solution. The lower limit of detection of CFZ was 0.5 mg/ml with interday and intraday coefficients of variation of <5%.
Population pharmacokinetic analysis.
Population pharmacokinetic modeling was performed using NONMEM software version 7.3.0 (ICON Development Solution, Ellicott City, MD, USA). The first-order conditional estimation with interaction (FOCE-I) method was used for the analysis. All serum and hip joint capsule concentrations were simultaneously fit to a two-compartment model or a three-compartment model. Multiple models were evaluated and discriminated using the Akaike information criterion (AIC) (12).
Interindividual variability of the parameters was assessed using an exponential error model:
where Pi represents individual values, TV(Pi) is the population value for the parameters described in the equation, and ηi is the random deviation of Pi from TV(Pi). The value of ηi was assumed to be independently and normally distributed with a mean of 0 and a variance of ω2. The residual (intraindividual) variability of the parameters was assessed using a proportional error model:
where Cobs,ij and Cpred,ij denote the jth observed and predicted concentrations for the ith subject, respectively; ε is the random intraindividual error that is normally distributed with a mean of 0 and variance σ2.
Age, body weight (BW), serum creatinine, sex, and CLcr (estimated using the Cockcroft-Gault formula) were selected as candidates for pharmacokinetic covariates. Covariance showing a correlation with the pharmacokinetic parameters was introduced into the model. The significance of influence of the covariates was evaluated by a change of –2-log likelihood (the minimum value of the objective function, OBJ). An OBJ decrease of more than 6.63 from the basic structural model (χ2; degree of freedom, 1; P < 0.01) was considered statistically significant during the forward inclusion process. The full model was structured by incorporating the significant covariates, and the final model was developed using the backward elimination method. When one covariate factor was excluded from the full model, an OBJ increase of more than 6.63 from the full model (χ2; degree of freedom, 1; P < 0.01) was considered statistically significant.
The adequacy of fitting was assessed by plotting the predicted versus the observed concentrations of CFZ, the individual predicted concentration after each Bayesian step versus the observed concentration, and the weighted residual concentration versus the predicted concentration. To assess the predictive performance, a prediction-corrected VPC was performed. The VPC was evaluated by comparing the observed concentrations with a 90% predictable interval simulated from the final parameters (13). A nonparametric bootstrap analysis was performed using Perl-speak-NONMEM software to assess the reliability and stability of the estimated parameter (14). The final model was fit repeatedly to 1,000 additional bootstrap data sets. The average, standard deviation (SD), relative standard error (%RSE), and 95% confidence interval (CI) were calculated from the empirical bootstrap distribution and compared with estimates from the original data set.
Pharmacodynamic Monte Carlo simulation.
A Monte Carlo simulation was conducted to simulate CFZ trough concentrations in both free serum and hip joint capsule at 3 and 4 h after CFZ bolus infusion (1 g). The free CFZ concentration in the serum was corrected using a protein-binding rate of 86% (15). Virtual patients were randomly generated using uniform random numbers based on the population pharmacokinetic model yielding mean estimates (θ) and interindividual variances (ω). The pharmacodynamic index target value was the probability of maintaining the CFZ concentration above 1 mg/liter to account for methicillin-susceptible Staphylococcus aureus (MSSA) for which the MIC90 is 1 mg/liter (16).
ACKNOWLEDGMENTS
We thank all staff members of Kitasato University Hospital for their involvement in this study.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We have no conflicts of interest to declare.
REFERENCES
- 1.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA, American Society of Health-System Pharmacists, Infectious Disease Society of America, Surgical Infection Society, Society for Healthcare Epidemiology of America . 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 70:195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 2.Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR, Society of Thoracic Surgeons . 2006. The Society of Thoracic Surgeons Practice Guideline Series: antibiotic prophylaxis in cardiac surgery. Part I. Duration. Ann Thorac Surg 81:397–404. doi: 10.1016/j.athoracsur.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Burke JF. 1961. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 50:161–168. [PubMed] [Google Scholar]
- 4.Kirby JP, Mazuski JE. 2009. Prevention of surgical site infection. Surg Clin North Am 89:365–389, viii. doi: 10.1016/j.suc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Muller M, Dela Pena A, Derendorf H. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob Agents Chemother 48:1441–1453. doi: 10.1128/aac.48.5.1441-1453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurman DJ, Hirshman HP, Kajiyama G, Moser K, Burton DS. 1978. Cefazolin concentrations in bone and synovial fluid. J Bone Joint Surg Am 60:359–362. doi: 10.2106/00004623-197860030-00016. [DOI] [PubMed] [Google Scholar]
- 7.Bellouard R, Deschanvres C, Deslandes G, Dailly É, Asseray N, Jolliet P, Boutoille D, Gaborit B, Grégoire M. 2019. Population pharmacokinetic study of cefazolin dosage adaptation in bacteremia and infective endocarditis based on a nomogram. Antimicrob Agents Chemother 63:e00806-19. doi: 10.1128/AAC.00806-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts JA, Udy AA, Jarrett P, Wallis SC, Hope WW, Sharma R, Kirkpatrick CM, Kruger PS, Roberts MS, Lipman J. 2015. Plasma and target-site subcutaneous tissue population pharmacokinetics and dosing simulations of cefazolin in post-trauma critically ill patients. J Antimicrob Chemother 70:1495–1502. doi: 10.1093/jac/dku564. [DOI] [PubMed] [Google Scholar]
- 9.Sharareh B, Sutherland C, Pourmand D, Molina N, Nicolau DP, Schwarzkopf R. 2016. Effect of body weight on cefazolin and vancomycin trabecular bone concentrations in patients undergoing total joint arthroplasty. Surg Infect (Larchmt) 17:71–77. doi: 10.1089/sur.2015.067. [DOI] [PubMed] [Google Scholar]
- 10.Ohge H, Takesue Y, Yokoyama T, Murakami Y, Hiyama E, Yokoyama Y, Kanehiro T, Itaha H, Matsuura Y. 1999. An additional dose of cefazolin for intraoperative prophylaxis. Surg Today 29:1233–1236. doi: 10.1007/BF02482213. [DOI] [PubMed] [Google Scholar]
- 11.Tchaick RM, Sa M, Figueira FRM, Paz KC, Ferraz AAB, Moraes FRN. 2017. Cefazolin concentration in the mediastinal adipose tissue of patients undergoing cardiac surgery. Braz J Cardiovasc Surg 32:239–244. doi: 10.21470/1678-9741-2016-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaoka K, Nakagawa T, Uno T. 1978. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- 13.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindbom L, Pihlgren P, Jonsson EN, Jonsson N. 2005. PsN-Toolkit: a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Kirby WM, Regamey C. 1973. Pharmacokinetics of cefazolin compared with four other cephalosporins. J Infect Dis 128:(Suppl):S341–S346. doi: 10.1093/infdis/128.supplement_2.s341. [DOI] [PubMed] [Google Scholar]
- 16.Yanagihara K, Watanabe A, Aoki N, Matsumoto T, Yoshida M, Sato J, Wakamura T, Sunakawa K, Kadota J, Kiyota H, Iwata S, Kaku M, Hanaki H, Ohsaki Y, Fujiuchi S, Takahashi M, Takeuchi K, Takeda H, Ikeda H, Miki M, Nakanowatari S, Takahashi H, Utagawa M, Nishiya H, Kawakami S, Morino E, Takasaki J, Mezaki K, Chonabayashi N, Tanaka C, Sugiura H, Goto H, Saraya T, Kurai D, Katono Y, Inose R, Niki Y, Takuma T, Kudo M, Ehara S, Sato Y, Tsukada H, Watabe N, Honma Y, Mikamo H, Yamagishi Y, Nakamura A, Ohashi M, Seki M, Hamaguchi S, et al. 2017. Nationwide surveillance of bacterial respiratory pathogens conducted by the surveillance committee of Japanese Society of Chemotherapy, the Japanese Association for Infectious Diseases, and the Japanese Society for Clinical Microbiology in 2012: general view of the pathogens’ antibacterial susceptibility. J Infect Chemother 23:587–597. doi: 10.1016/j.jiac.2017.05.010. [DOI] [PubMed] [Google Scholar]