Key Points
Question
Among patients with chronic immune-mediated inflammatory diseases initiating treatment with infliximab, does proactive therapeutic drug monitoring (TDM) improve clinical remission rates compared with standard therapy?
Findings
In this randomized clinical trial that included 411 patients, the proportion of patients who experienced disease remission after 30 weeks was 50.5% in the TDM group and 53.0% in the standard therapy group, a difference that was not statistically significant.
Meaning
These findings do not support routine use of proactive TDM during infliximab induction for improving disease remission rates.
Abstract
Importance
Proactive therapeutic drug monitoring (TDM), defined as individualized drug dosing based on scheduled monitoring of serum drug levels, has been proposed as an alternative to standard therapy to maximize efficacy and safety of infliximab and other biological drugs. However, whether proactive TDM improves clinical outcomes when implemented at the time of drug initiation, compared with standard therapy, remains unclear.
Objective
To assess whether TDM during initiation of infliximab therapy improves treatment efficacy compared with standard infliximab therapy without TDM.
Design, Setting, and Participants
Randomized, parallel-group, open-label clinical trial of 411 adults with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, ulcerative colitis, Crohn disease, or psoriasis initiating infliximab therapy in 21 hospitals in Norway. Patients were recruited from March 1, 2017, to January 10, 2019. Final follow-up occurred on November 5, 2019.
Interventions
Patients were randomized 1:1 to receive proactive TDM with dose and interval adjustments based on scheduled monitoring of serum drug levels and antidrug antibodies (TDM group; n = 207) or standard infliximab therapy without drug and antibody level monitoring (standard therapy group; n = 204).
Main Outcomes and Measures
The primary end point was clinical remission at week 30.
Results
Among 411 randomized patients (mean age, 44.7 [SD, 14.9] years; 209 women [51%]), 398 (198 in the TDM group and 200 in the standard therapy group) received their randomized intervention and were included in the full analysis set. Clinical remission at week 30 was achieved in 100 (50.5%) of 198 and 106 (53.0%) of 200 patients in the TDM and standard therapy groups, respectively (adjusted difference, 1.5%; 95% CI, −8.2% to 11.1%; P = .78). Adverse events were reported in 135 patients (68%) and 139 patients (70%) in the TDM and standard therapy groups, respectively.
Conclusions and Relevance
Among patients with immune-mediated inflammatory diseases initiating treatment with infliximab, proactive therapeutic drug monitoring, compared with standard therapy, did not significantly improve clinical remission rates over 30 weeks. These findings do not support routine use of therapeutic drug monitoring during infliximab induction for improving disease remission rates.
Trial Registration
ClinicalTrials.gov Identifier: NCT03074656
This randomized trial assesses the effect of therapeutic drug monitoring vs standard therapy on clinical remission at 30 weeks among patients with immune-mediated inflammatory diseases (rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, ulcerative colitis, Crohn disease, or psoriasis) initiating infliximab therapy.
Introduction
Infliximab and other tumor necrosis factor (TNF) inhibitors can improve outcomes for patients with common chronic immune-mediated inflammatory diseases, such as rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, ulcerative colitis, Crohn disease, and psoriasis. However, approximately 20% to 55% of patients do not respond to these therapies,1,2,3,4 resulting in reduced quality of life and risk of irreversible organ damage and disability. Strategies to optimize TNF inhibitor treatment are needed.
Lack of response to TNF inhibitors has been attributed to immunogenicity, defined as an immune response against the drug, leading to formation of antidrug antibodies. Antibodies against TNF inhibitors and other biologic drugs reduce serum drug levels and are associated with adverse effects such as infusion reactions.5,6 Infliximab, a chimeric antibody, is more immunogenic than other TNF inhibitors, and antibody formation is particularly common during initiation of infliximab.5,6
Substantial interindividual variation exists for serum drug levels for infliximab and other TNF inhibitors. Furthermore, higher serum drug levels are associated with greater efficacy.7,8,9,10 Therapeutic drug monitoring (TDM) has been proposed as a method to maximize efficacy, safety, and cost-effectiveness of TNF inhibitor therapy.10,11,12 Proactive TDM, an individualized treatment strategy in which drug doses and timing of administered doses are adjusted based on scheduled measurements of serum drug levels, has been adopted by some clinicians.13 However, clinical trial data supporting proactive TDM of TNF inhibitor therapy for improving clinical outcomes, compared with standard therapy, is lacking. Guidelines are inconsistent regarding recommendations for proactive TDM, largely because of lack of evidence regarding TDM benefits during the induction phase of treatment.14,15,16,17
The Norwegian Drug Monitoring Trial (NOR-DRUM) Part A was a randomized clinical trial designed to assess the effect of proactive TDM during induction of infliximab therapy for improving disease remission rates in patients with the 6 diagnoses for which infliximab is indicated and approved.
Methods
Study Design and Participants
In this 38-week randomized, open-label, parallel-group, phase 4 multicenter superiority study, patients were recruited and followed up at 21 Norwegian hospitals (eTable 1 in Supplement 1). The trial was designed to determine whether TDM was more effective than standard therapy for the primary outcome of disease remission. The trial was conducted in accordance with the principles of the Declaration of Helsinki18 and International Conference on Harmonization guidelines for Good Clinical Practice. The trial protocol and the consent form were approved by an independent ethics committee (Regional Committees for Medical and Health Research Ethics South East). A steering group (eAppendix 1 in Supplement 1), including researchers and clinicians representing all relevant specialties, biostatisticians, and patient representatives, planned and conducted the study. All patients provided written informed consent. The study was conducted and analyzed according to the trial protocol and statistical analysis plan (Supplement 2). This report does not include the prespecified noninferiority comparison that was planned if superiority was not established. The noninferiority comparison is not presented because of lack of sufficient rationale for the noninferiority comparison and because of lack of sufficient justification for the prespecified noninferiority margin.
All Norwegian hospitals using infliximab were invited to participate. Patients were recruited by their treating physician and enrolled by the site investigators. Adult patients diagnosed as having rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, ulcerative colitis, Crohn disease, or psoriasis with a clinical indication to start treatment with infliximab were potential participants. Patients in remission according to diagnosis-specific disease activity scores, those who had received infliximab within the past 6 months, and those unable to provide written informed consent were excluded. All eligibility criteria are described in Supplement 2.
Randomization
Patients were randomized 1:1 to either infliximab therapy according to TDM or to standard infliximab therapy. A computer-generated random block randomization (block sizes of 4, 6, and 8), stratified by diagnosis, was integrated in the electronic data capture software solution (Viedoc, version 4). Randomized group assignments were available to study personnel and participants only after eligibility was confirmed and patients were randomized.
Interventions
Patients in the standard therapy group received infliximab therapy according to standard clinical practice. Following the summary of product characteristics, infliximab was administered intravenously in doses of 5 mg/kg (3 mg/kg for rheumatoid arthritis) at 0, 2, and 6 weeks and every eighth week thereafter. Adjustments in doses and intervals according to clinical parameters were considered standard clinical practice. Investigators did not have access to information on levels of infliximab or antidrug antibodies and no algorithm was provided to guide dosing adjustments.
In the TDM group, infliximab administration was adjusted according to an algorithm designed to maintain infliximab levels within the therapeutic range (eFigure 1 in Supplement 1).19 At each infusion, serum trough levels of infliximab and antidrug antibodies were measured and results entered in the interactive web-based electronic case report form, which provided the investigator with the recommended infliximab dose and interval. During the induction phase, the dose was adjusted by decreasing the time between infusions if serum infliximab was low (<20 mg/L at the second infusion, <15 mg/L at the third infusion, and <3 mg/L at subsequent infusion[s] up to week 14). During weeks 1 to 14, drug doses were not decreased and time between infusions was not increased. After week 14, the infliximab dose or interval could be either increased or decreased to reach the therapeutic range of 3 to 8 mg/L. If a patient developed clinically significant levels of antidrug antibodies (defined as >50 μg/L), the algorithm recommended switching to another therapy.
Concomitant immunosuppressive treatment initiated prior to study inclusion was maintained in both groups. Patients who discontinued infliximab initiated other medication at the discretion of treating physicians (eTable 2 in Supplement 1). Patients who discontinued infliximab were followed according to their randomized group with visits according to the prior infusion schedule.
Clinical assessments, blood samples, and patient-reported outcome measures were collected at each visit. The primary efficacy parameters were (1) for rheumatoid arthritis and psoriatic arthritis, the Disease Activity Score in 28 Joints (range, 0-9.4; higher scores indicate worse disease activity; minimum clinically important difference, 1.2)20,21; (2) for spondyloarthritis, the Ankylosing Spondylitis Disease Activity Score (range, 0.6-7.6; higher scores indicate worse disease activity; minimum clinically important difference, 1.1)22,23; (3) for ulcerative colitis, the Partial Mayo Score (range, 0-9; higher scores indicate worse disease; no minimum clinically important difference defined)24; (4) for Crohn disease, the Harvey-Bradshaw Index (minimum of 0 with no upper limit; higher values indicate worse outcome; no minimum clinically important difference defined)25,26; and (5) for psoriasis, the Psoriasis Area and Severity Index (range, 0-72; higher scores indicate worse disease; no minimum clinically important difference defined).27 The Box herein and eTable 3 in Supplement 1 provide more details on the disease-specific assessment tools. Levels of infliximab and antidrug antibodies were measured at the Department of Medical Biochemistry, Oslo University Hospital, Radiumhospitalet, using in-house assays automated on the AutoDELFIA immunoassay platform (PerkinElmer).28
Box. Disease-Specific Assessment Toolsa.
Spondyloarthritis
The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) includes 6 questions pertaining to the 5 major symptoms of ankylosing spondylitis: fatigue, spinal pain, joint pain/swelling, areas of localized tenderness, morning stiffness duration, and morning stiffness severity. Each question is scored on a numeric rating scale of 0 to 10. The 2 morning stiffness scores are averaged and added to the average of the other scores, forming a total score in the range of 0 to 10, with higher values indicating worse disease. Components of the BASDAI are included in the Ankylosing Spondylitis Disease Activity Score.
The Ankylosing Spondylitis Disease Activity Score (ASDAS) includes total back pain and patient global assessment of disease activity on visual analog scales of 0 to 100, peripheral pain and swelling and duration of morning stiffness on numeric rating scales of 0 to 10, and C-reactive protein (CRP) measured in milligrams per liter. The ASDAS is calculated as: ASDAS − CRP = 0.121 × total back pain + 0.0110 × patient global + 0.073 × peripheral pain/swelling + 0.058 × duration of morning stiffness + 0.579 × ln(CRP + 1). The ASDAS range is 0.6 to 7.6; higher values indicate worse disease.
Ulcerative Colitis
The Partial Mayo Score is a disease activity score used for ulcerative colitis and consists of 3 components (rectal bleeding, stool frequency, and physician assessment of disease activity) scored from 0 to 3 and summed for a total score that ranges from 0 to 3. The range is 0 to 9; higher scores indicate worse disease.
Rheumatoid Arthritis and Psoriatic Arthritis
The Disease Activity Score in 28 Joints (DAS28) is used for rheumatoid arthritis and psoriatic arthritis. It includes the 28 tender and swollen joint counts (SJC28 and TJC28), the erythrocyte sedimentation rate (ESR), and a patient global assessment (PGA). The DAS28 is calculated as follows: DAS28 = 0.56 × square root of TJC28 + 0.28 × square root of SJC28 + 0.70 × ln(ESR) + 0.014 × PGA. The DAS28 range is 0 to 9.4; higher values indicate worse disease.
Crohn Disease
The Harvey-Bradshaw Index (HBI) is a disease activity score used for Crohn disease and consists of 5 domains: general well-being (range, 0-4), abdominal pain (range, 0-3), number of liquid/soft stools per day, abdominal mass (range, 0-3), and number of predefined complications. The scores of each subdomain are summed to compute the HBI. The HBI minimum score is 0, with no upper limit; higher values indicate worse disease.
Psoriasis
The Psoriasis Area and Severity Index (PASI) is a disease activity score used for psoriasis and includes measures of redness, thickness, and scaliness of lesions (each scored from 0 to 4), weighted by the area and location of involvement. The PASI score ranges from 0 to 72; higher scores indicate worse disease.
Outcome Measures
The primary end point was clinical remission at week 30. Clinical remission was defined by disease-specific composite scores: Disease Activity Score in 28 Joints lower than 2.6 in patients with rheumatoid arthritis and psoriatic arthritis, Ankylosing Spondylitis Disease Activity Score lower than 1.3 in patients with spondyloarthritis, Harvey-Bradshaw Index of 4 or lower in Crohn disease, Partial Mayo Score of 2 or lower with no subscores greater than 1 in patients with ulcerative colitis, and Psoriasis Area and Severity Index of 4 or lower in patients with psoriasis.21,23,26,28,29,30 Seventy-one secondary efficacy outcomes (listed in eTable 4 in Supplement 1) assessed disease-specific disease activity scores, patient’s and physician’s global assessments of disease activity, biochemical parameters of disease activity (erythrocyte sedimentation rate and C-reactive protein), time to remission, time to sustained remission (defined as presence of remission at all visits following initial remission), clinical remission at week 14, and patient-reported outcome measures. Details regarding secondary efficacy end points are given in eTable 3 in Supplement 1. Development of antidrug antibodies, remission rate restricted to patients developing antidrug antibodies, infliximab dose, and drug discontinuation were predefined exploratory outcomes.
Adverse events were coded according to the Medical Dictionary for Regulatory Activities, version 21, 1E.
Statistical Analyses
The sample size was calculated with 80% power to detect a between-group difference in the primary end point of 15% (40% vs 55%). The choice of 15% as a clinically meaningful difference was based on a combination of statistical reasoning and clinical judgement after thorough discussions between the clinicians and biostatisticians in the steering group and was consistent with previous infliximab trials.28,31 Using a 2-sided test and a significance level of α = .05, it was necessary to have 358 patients completing the trial. To account for dropout, we aimed to randomize 400 patients.
The primary end point was analyzed by mixed-effects logistic regression. The analysis included outcomes at all visits up to and including the week 30 visit, used patient-level random intercepts, and included time, treatment group, diagnosis (the stratification factor), and time × treatment interaction as fixed categorical variables. The risk difference in remission at week 30 was estimated using the average marginal effect at that time point. The delta method was used to estimate the standard error of this estimator, and inference was based on a normal approximation. This model yields consistent estimates when missing outcomes are missing at random, provided no other misspecification.
Secondary and exploratory outcomes were analyzed in a similar manner, using mixed-effects logistic/linear regression for binary/continuous outcomes. Time-to-event end points were analyzed by Cox proportional hazards regression, adjusting for diagnosis. The proportional hazards assumption (Schoenfeld test) was met. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be considered exploratory.
As specified in the trial protocol, patients exposed to the randomized intervention (patients having received the second infusion with a recorded treatment decision for the third infusion) comprised participants in the primary analyses. Prespecified sensitivity analyses of the primary end point included alternative approaches to deal with missing values (complete case analysis, last observation carried forward, and worst/best case imputation); an analysis restricted to patients with high adherence to the protocol (defined as patients fulfilling all of the following criteria: no withdrawal from the study prior to the week 30 visit, no deviations from eligibility criteria, no intervals between infusions >12 weeks, and no deviations from the TDM strategy); and an analysis that adjusted for baseline parameters (age, sex, prednisolone use, number of prior TNF inhibitors, immunosuppressive comedication, and disease activity). Post hoc sensitivity analyses included adjustment for center (as fixed and random effect), analyses of all patients receiving at least 1 infliximab infusion, and a test of treatment × diagnosis interaction. We used 2-sided statistical tests at an α = .05 significance level with corresponding 95% CIs for the treatment effect estimates. All analyses, unless termed post hoc, were prespecified in the statistical analysis plan (Supplement 2) and carried out using Stata version 16 (StataCorp) and R version 3.4.4 (R Foundation).
Results
Study Participants
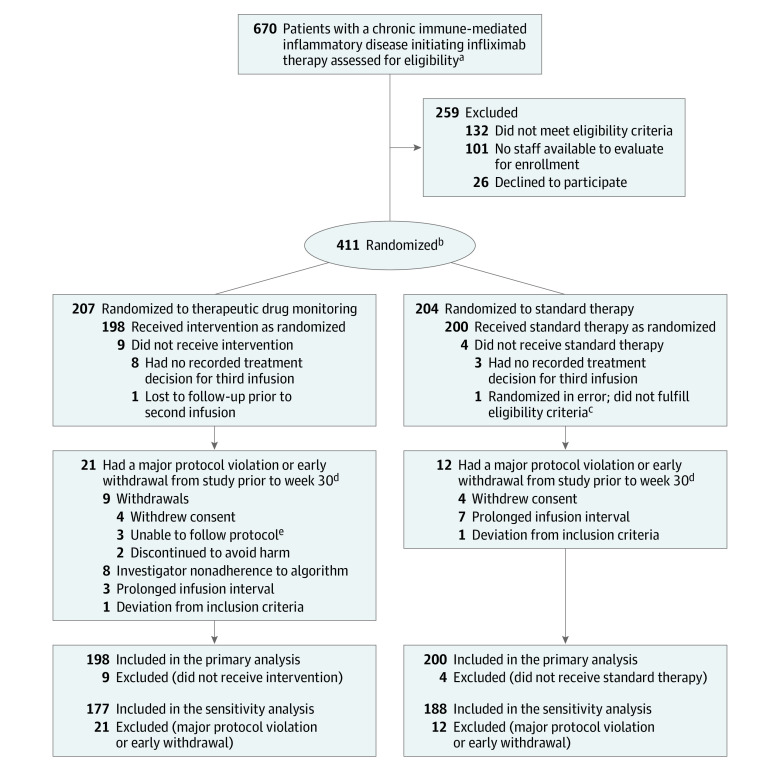
From March 1, 2017, to January 10, 2019, a total of 411 patients with rheumatoid arthritis (n = 84), psoriatic arthritis (n = 45), spondyloarthritis (n = 119), ulcerative colitis (n = 83), Crohn disease (n = 58), or psoriasis (n = 22) initiating infliximab therapy were randomized. Of these patients, 398 (198 in the TDM group and 200 in the standard therapy group) received at least 2 doses of study drug with a recorded treatment decision for the third dose and were included in the primary analyses (Figure 1). All patients received the same infliximab biosimilar (CT-P13, infliximab-dyyb). Of the included patients, 189 (91%) in the TDM group and 196 (96%) in the standard therapy group completed the trial (Figure 1). The 2 groups were balanced regarding baseline demographic, clinical, and treatment characteristics (Table 1; eTable 5, A-F, in Supplement 1).
Figure 1. Flow of Participants Through the Norwegian Drug Monitoring Trial Part A.
aChronic immune-mediated inflammatory disease included rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, ulcerative colitis, Crohn disease, and psoriasis.
bRandomization was stratified by diagnosis.
cPatient had a colostomy, which was one of the defined exclusion criteria (outcome measure includes stool frequency).
dMajor protocol violations were prespecified in the statistical analysis plan as follows: deviations to inclusion and/or exclusion criteria or delay in scheduled infusion with an infusion interval of more than 12 weeks, or investigator nonadherence to study algorithm, defined as discrepancies between recommended and actual dose/interval at more than 1 visit.
ePatients for various reasons were not able to adhere to the study routine (eg, did not arrive for scheduled infusions).
Table 1. Baseline Participant Characteristics (Full Analysis Set).
| Characteristics | Therapeutic drug monitoring (n = 198) | Standard therapy (n = 200) |
|---|---|---|
| Age, median (IQR), y | 44 (35-57) | 44 (30-55) |
| Sex, No. (%) | ||
| Women | 110 (56) | 93 (47) |
| Men | 88 (44) | 107 (53) |
| Disease duration, median (IQR), y | 3.5 (0.8-15.0) | 3.8 (0.8-12.3) |
| Diagnosis, No. (%) | ||
| Spondyloarthritis | 59 (30) | 58 (29) |
| Ulcerative colitis | 39 (20) | 41 (21) |
| Rheumatoid arthritis | 38 (19) | 42 (21) |
| Crohn disease | 29 (15) | 28 (14) |
| Psoriatic arthritis | 20 (10) | 22 (11) |
| Psoriasis | 13 (7) | 9 (5) |
| Therapy, No. (%) | ||
| Prior biologic therapy | 47 (24) | 45 (22) |
| Prior use of 1 TNF inhibitora | 30 (15) | 29 (15) |
| Prior use of ≥2 TNF inhibitorsa | 15 (8) | 15 (8) |
| Prior use of other biologic therapyb | 8 (4) | 9 (5) |
| Concomitant immunosuppressive therapyc | 112 (57) | 109 (55) |
| Concomitant use of glucocorticoids | 40 (21) | 31 (16) |
| Erythrocyte sedimentation rate, median (IQR), mm/hd | 13 (6-25) | 14 (6-25) |
| C-reactive protein, median (IQR), mg/Le | 5.0 (2.0-14.0) | 5.0 (1.0-15.0) |
| Patient global assessment of disease activity, mean (SD)f | 59.6 (23.0) | 56.8 (22.3) |
| Physician global assessment of disease activity, mean (SD)f | 46.6 (21.1) | 46.4 (21.6) |
| Disease-specific characteristics | ||
| Spondyloarthritis | ||
| HLA-B27 positive, No./total (%) | 41/53 (77) | 42/55 (76) |
| Bath Ankylosing Spondylitis Disease Activity Index, mean (SD)g | 5.1 (1.7) [n = 59] | 5.3 (1.5) [n = 58] |
| Ankylosing Spondylitis Disease Activity Score, mean (SD)g | 3.1 (1.0) [n = 59] | 3.1 (0.9) [n = 58] |
| Ulcerative colitis: Partial Mayo Score, median (IQR)g | 6 (5-7) [n = 39] | 6 (4-7) [n = 41] |
| Rheumatoid arthritis | ||
| Anti–citrullinated protein antibody positive, No./total (%) | 26/38 (68) | 28/42 (67) |
| Rheumatoid factor positive, No./total (%) | 26/37 (70) | 27/42 (64) |
| Disease Activity Score in 28 Joints, mean (SD)g | 4.6 (1.1) [n = 38] | 4.4 (1.2) [n = 42] |
| Crohn disease: Harvey-Bradshaw Index, median (IQR)g | 8 (6-10) [n = 29] | 8 (5-9.5) [n = 28] |
| Psoriatic arthritis: Disease Activity Score in 28 Joints, mean (SD)g | 4.3 (1.0) [n = 20] | 4.8 (1.3) [n = 22] |
| Psoriasis: Psoriasis Area and Severity Index, mean (SD)g | 10.1 (4.8) [n = 13] | 9.7 (4.1) [n = 9] |
Abbreviations: HLA, human leukocyte antigen; IQR, interquartile range; TNF, tumor necrosis factor.
Prior TNF inhibitor use includes etanercept, adalimumab, certolizumab pegol, golimumab, and infliximab.
Other biologic therapy includes abatacept, rituximab, secukinumab, tocilizumab, ustekinumab, and vedolizumab.
Concomitant immunosuppressive therapy includes methotrexate, leflunomide, sulfasalazine, and azathioprine.
For erythrocyte sedimentation rate, the normal range is 0 to 12 mm/h for men and 0 to 17 mm/h for women.
For C-reactive protein, the normal range is 0 to 4 mg/L.
Global assessment of disease activity range, 0 to 100 on a visual analog scale, with 0 indicating no disease activity and 100 indicating the highest possible disease activity.
See the Box and eTable 3 in Supplement 1 for detailed descriptions of the Bath Ankylosing Spondylitis Disease Activity Index, Ankylosing Spondylitis Disease Activity Score, Partial Mayo Score, Disease Activity Score in 28 Joints, Harvey-Bradshaw Index, and Psoriasis Area and Severity Index.
Primary End Point
The primary end point of clinical remission at week 30 was reached in 100 patients (50.5%) and 106 patients (53.0%) in the TDM and standard therapy groups, respectively, with an adjusted difference of 1.5% (95% CI, −8.2% to 11.1%) (Figure 2). The between-group difference was not statistically significant (P = .78). Analyses of the primary end point by disease subgroups did not show significant differences between the groups (Figure 2).
Figure 2. Clinical Remission at 30 Weeks (Primary Outcome).
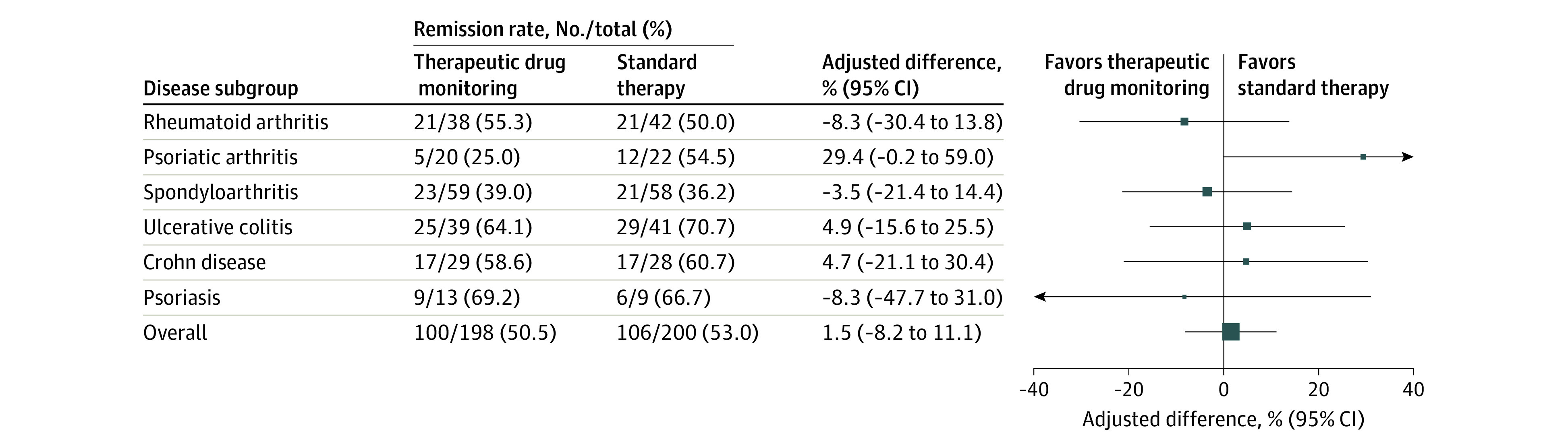
Adjusted difference in clinical remission rate at 30 weeks overall (the primary end point) and by disease subgroup. The adjusted difference in remission rate was assessed by mixed-effects logistic regression using data from all patients exposed to the randomized intervention (patients having received the second infusion with a recorded treatment decision for the third infusion). Size of data markers is proportional to the number of patients in the group. Clinical remission was defined by disease-specific composite scores: a Disease Activity Score in 28 Joints lower than 2.6 in patients with rheumatoid arthritis and psoriatic arthritis, an Ankylosing Spondylitis Disease Activity Score lower than 1.3 in patients with spondyloarthritis, a Partial Mayo Score of 2 or lower with no subscores greater than 1 in patients with ulcerative colitis, a Harvey-Bradshaw Index of 4 or lower in patients with Crohn disease, and a Psoriasis Area and Severity Index of 4 or lower in patients with psoriasis. See the Box and eTable 3 in Supplement 1 for detailed descriptions of the Disease Activity Score in 28 Joints, Ankylosing Spondylitis Disease Activity Score, Partial Mayo Score, Harvey-Bradshaw Index, and Psoriasis Area and Severity Index.
Prespecified Sensitivity Analyses
Prespecified sensitivity analyses of the primary end point (adjustments for baseline variables, different methods for handling missing data, and analyses restricted to patients with high adherence to the protocol [defined as not withdrawn from the study prior to week 30 and having no major protocol violations]) yielded consistent results (eTable 6A in Supplement 1).
Secondary Outcomes
At week 14, 91 patients (49.1%) in the TDM group and 104 patients (54.7%) in the standard therapy group had attained clinical remission (adjusted difference, 6%; 95% CI, −3.7% to 15.6%). The median time to remission was 56 days in the TDM group and 46 days in the standard therapy group. There were no significant differences between groups in time to remission or time to sustained remission (hazard ratios were 1.21 [95% CI, 0.97-1.52] and 1.25 [95% CI, 0.89-1.75], respectively). The change from baseline to week 30 was not significantly different between groups for any of the assessed secondary efficacy outcomes (Figure 3; eTable 7, eTable 8, and eFigure 3 in Supplement 1).
Figure 3. Disease Activity by Disease Subgroup.
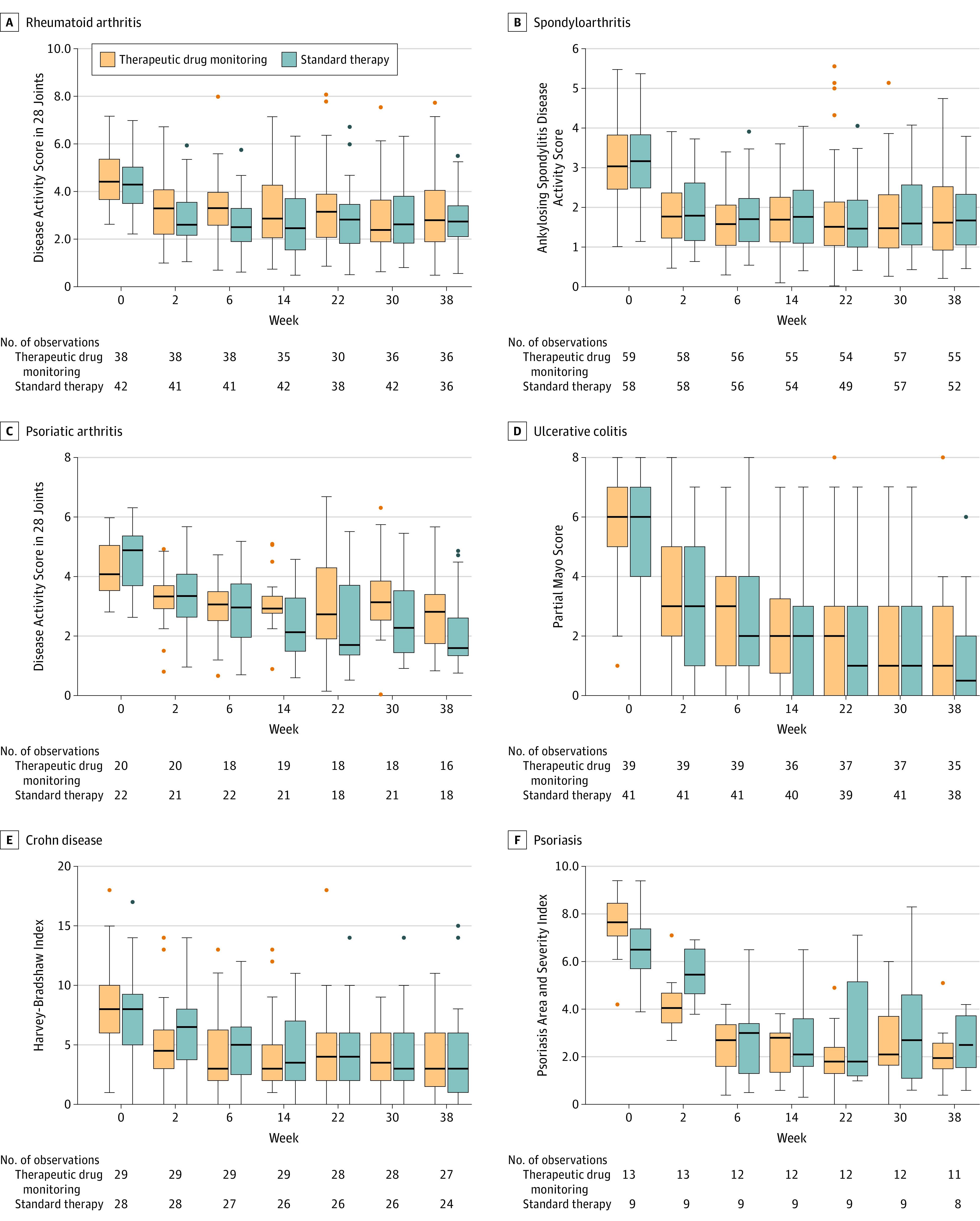
Orange indicates therapeutic drug monitoring; blue, standard therapy. Box tops and bottoms indicate interquartile range; horizontal bars inside boxes, median; whiskers, highest and lowest values within 1.5 × the interquartile range. Dots indicate individual patient outliers. See the Box and eTable 3 in Supplement 1 for detailed descriptions of the Disease Activity Score in 28 Joints, Ankylosing Spondylitis Disease Activity Score, Partial Mayo Score, Harvey-Bradshaw Index, and Psoriasis Area and Severity Index.
Exploratory Outcomes
Infliximab was discontinued in 59 patients (30%) in the TDM group and 43 patients (22%) in the standard therapy group, with a between-group difference of −9.5% (95% CI, −18% to 1%). Nineteen patients in the TDM group discontinued because of formation of antidrug antibodies (see eTable 9 in Supplement 1 for reasons for drug discontinuation). New medications started in patients discontinuing infliximab and the numbers of patients taking immunosuppressive comedications during the study are described in eTable 2 in Supplement 1.
The median serum level of infliximab (week 14 to week 38) was 6.9 mg/L in both groups (interquartile ranges, 3.8-10.7 mg/L and 3.1-12.1 mg/L in the TDM and standard therapy groups, respectively) (eFigure 2 in Supplement 1). Mean infliximab doses received during the trial were 4.9 mg/kg (SD, 1.1 mg/kg) and 4.8 mg/kg (SD, 1.2 mg/kg) in the TDM and standard therapy groups, respectively, with a between-group difference of −0.01 mg/kg (95% CI, −0.17 to 0.15 mg/kg).
Thirty-six patients (18%) in the TDM group and 34 patients (17%) in the standard therapy group developed antidrug antibodies (≥15 μg/L) during infliximab treatment (between-group difference, −1.2%; 95% CI, −8.7% to 1.8%). Twenty patients (10%) in the TDM group and 30 patients (15%) in the standard therapy group had antidrug antibodies above the threshold for discontinuation (≥50 μg/L) defined in the treatment algorithm (between-group difference, 4.9%; 95% CI, −1.6% to 11.4%). The remission rate at week 30 in patients developing antidrug antibodies was 20 (56%) of 36 patients and 12 (35%) of 34 patients in the TDM and standard therapy groups, respectively (between-group difference, −23%; 95% CI, −43% to −2%).
Adjustments to infliximab administration (relative to the standard schedule) were made at 297 (25%) of the visits in the TDM group (194 doses increased, 103 doses decreased) of which 264 adjustments (89%) were according to algorithm recommendations. In the standard therapy group, adjustments were made based on clinical assessment at 99 (9%) of the visits (76 doses increased, 23 doses decreased). Investigator adherence to the treatment algorithm was high. Deviations from the predefined TDM strategy occurred in only 16% of patients.
Adverse Events
Adverse events were reported for 135 (68%) and 139 (70%) patients in the TDM and standard therapy groups, respectively (Table 2). The most frequent adverse events were related to infections. Five patients (2.5%) in the TDM group compared with 16 patients (8%) in the standard therapy group experienced an infusion-related reaction (Table 2).
Table 2. Treatment-Emergent Adverse Events in the Full Analysis Set.
| Adverse eventsa | No. (%) of participants | |
|---|---|---|
| Therapeutic drug monitoring (n = 198) | Standard therapy (n = 200) | |
| Total adverse events | 135 (68) | 139 (70) |
| Serious adverse eventsb | 21 (11) | 20 (10) |
| Most frequent adverse eventsc | ||
| Upper respiratory tract infection | 39 (20) | 40 (20) |
| Elevated liver enzymesd | 19 (10) | 12 (6) |
| Headache | 9 (5) | 3 (2) |
| Influenza-like illness | 8 (4) | 8 (4) |
| Lower urinary tract infection | 8 (4) | 3 (2) |
| Pneumonia | 7 (4) | 4 (2) |
| Tonsillitis | 6 (3) | 7 (4) |
| Infusion-related reaction | 5 (3) | 16 (8) |
| Abdominal pain | 4 (2) | 11 (6) |
| Most frequent serious adverse eventse | ||
| Abdominal pain | 0 | 6 (3) |
| Pneumonia | 3 (2) | 2 (1) |
| Pancreatitis | 2 (1) | 1 (<1) |
Adverse events were defined as any unfavorable and unintended sign, symptom, or disease temporally associated with the use of the study drug and were assessed continuously throughout the study.
Serious adverse events were adverse events resulting in death, a life-threatening condition, hospitalization (initial or prolonged), disability, or permanent damage and were assessed continuously throughout the study.
Most frequent adverse events were defined as those occurring in more than 5% of the study participants.
Deemed as a clinically important elevation by site investigators.
Most frequent serious adverse events were defined as those occurring in 3 or more patients. Serious adverse events occurring in fewer than 3 patients were acute myocardial infarction (n = 2), chest pain (n = 2), headache (n = 2), and abdominal hernia (n = 1).
Post Hoc Analyses
Post hoc sensitivity analyses of the primary end point (adjustment for center and analyses of all patients receiving at least 1 dose of infliximab) yielded consistent results (eTable 6B in Supplement 1). A likelihood ratio test for a treatment × diagnosis interaction was not significant (P = .12).
Discussion
In this randomized clinical trial of patients with immune-mediated inflammatory diseases initiating infliximab, proactive TDM, compared with standard therapy, did not significantly improve remission rates or any secondary efficacy outcomes.
The study evaluated the effect of proactive TDM compared with standard therapy during the induction phase of TNF inhibitor therapy, a period of high incidence of immunogenicity.6 Two prior clinical trials addressing proactive TDM of TNF inhibitors included only patients with inflammatory bowel disease and focused on the maintenance phase of infliximab treatment.32,33 These 2 studies, with sample sizes of 12232 and 263,33 both concluded that proactive TDM was not superior to standard therapy during the maintenance period.
Observational data suggested that low drug levels in the induction phase were associated with low remission rates.34,35,36,37,38,39 These observations led to the hypothesis that TDM during induction of infliximab might improve remission rates. There are several potential explanations for the findings reported herein that TDM was not better than standard therapy. First, observational data are subject to confounding and selection bias. Second, it is possible that proactive TDM is beneficial only in patients who develop antidrug antibodies, in which case the population studied in the current trial did not include a sufficient number of people with antidrug antibodies to show benefit. Third, standard of care in Norway allows for liberal dose increases in infliximab at the discretion of physicians. This may have helped the standard of care group attain a high rate of efficacy, minimizing differences from the TDM group. Fourth, it is possible that the process of antidrug antibody formation is irreversible and results in resistance to dose modifications.
Further study is needed to determine whether TDM might be associated with fewer infusion reactions than standard therapy. Future research should identify risk factors for immunogenicity as well as the value of TDM in patients receiving maintenance infliximab treatment. It remains unclear whether patients who do not respond or who lose efficacy to treatment may benefit from targeted drug monitoring (reactive TDM).
Strengths of this study include the randomized clinical trial design, the relatively large number of included patients, the high retention rate, and high adherence to the algorithm.
Limitations
This study has several limitations. First, the trial was open label. Bias due to lack of double-blinding is possible. However, objective measures including acute-phase reactants were incorporated as part of the primary outcome measure. Second, the trial did not have statistical power to test hypotheses within each disease subgroup. Third, as in the NOR-SWITCH study,28 the primary end point was designed to evaluate the occurrence of clinical remission across the disease groups. Results were consistent across diagnoses of included patients. Definitions of clinical remission in each diagnosis were based on well-established measures of disease activity with predefined cutoff points.20,21,22,23,24,25,26,27,29,30,40 Fourth, randomized patients who withdrew before receiving their randomized treatment strategy were excluded. A more rigorous design would have included all randomized participants, regardless of receipt of their randomized therapy. However, a post hoc sensitivity analysis of a less strict exclusion of patients (all patients receiving at least 1 dose of infliximab) showed consistent results. Fifth, minimum clinically important differences were not available for all outcomes. Sixth, the proactive TDM strategy used for this trial was based on available literature and clinical experience within the investigative team. The therapeutic range defined for serum infliximab was consistent with prior studies32,33 and clinical guidelines. However, it is possible that some patients might benefit from higher drug levels than prescribed in this study. Seventh, whereas infliximab levels are comparable between assays, antidrug antibody levels are not always comparable between assays.41
Conclusions
Among patients with immune-mediated inflammatory diseases initiating treatment with infliximab, proactive therapeutic drug monitoring, compared with standard therapy, did not significantly improve clinical remission rates over 30 weeks. The findings do not support routine use of therapeutic drug monitoring during infliximab induction for improving disease remission rates.
eAppendix 1. The NOR-DRUM Steering Group
eAppendix 2. Principal Investigators From Each Study Center
eTable 1. Number of Randomized Patients by Study Hospital
eTable 2. Changes in Medication During the Trial
eTable 3. Details of Study Endpoints
eTable 4. Prespecified Secondary Outcomes
eTable 5. Demographic and Baseline Characteristics in Disease Subgroups
eTable 6. Sensitivity Analyses of the Primary Endpoint
eTable 7. Results Secondary Endpoints
eTable 8. Secondary Efficacy Endpoints (by Disease Subgroup)
eTable 9. Infliximab Discontinuation
eFigure 1. Treatment Algorithm in the Therapeutic Drug Monitoring Group
eFigure 2. Serum Infliximab Level
eFigure 3. Secondary Efficacy Outcomes (Box and Whiskers Plots)
eReferences
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement
Footnotes
More details of these assessments are given in eTable 3 in Supplement 1.
References
- 1.Maini R, St Clair EW, Breedveld F, et al. ; ATTRACT Study Group . Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999;354(9194):1932-1939. doi: 10.1016/S0140-6736(99)05246-0 [DOI] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462-2476. doi: 10.1056/NEJMoa050516 [DOI] [PubMed] [Google Scholar]
- 3.Hanauer SB, Feagan BG, Lichtenstein GR, et al. ; ACCENT I Study Group . Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541-1549. doi: 10.1016/S0140-6736(02)08512-4 [DOI] [PubMed] [Google Scholar]
- 4.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357(9271):1842-1847. doi: 10.1016/S0140-6736(00)04954-0 [DOI] [PubMed] [Google Scholar]
- 5.Thomas SS, Borazan N, Barroso N, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. a systematic review and meta-analysis. BioDrugs. 2015;29(4):241-258. doi: 10.1007/s40259-015-0134-5 [DOI] [PubMed] [Google Scholar]
- 6.Nencini F, Vultaggio A, Pratesi S, et al. The kinetics of antidrug antibodies, drug levels, and clinical outcomes in infliximab-exposed patients with immune-mediated disorders. J Allergy Clin Immunol Pract. 2018;6(6):2065-2072. doi: 10.1016/j.jaip.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 7.St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(6):1451-1459. doi: 10.1002/art.10302 [DOI] [PubMed] [Google Scholar]
- 8.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147(6):1296-1307. doi: 10.1053/j.gastro.2014.08.035 [DOI] [PubMed] [Google Scholar]
- 9.Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2Gut. 2015;64(10):1539-1545. doi: 10.1136/gutjnl-2014-307883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papamichael K, Vogelzang EH, Lambert J, Wolbink G, Cheifetz AS. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev Clin Immunol. 2019;15(8):837-848. doi: 10.1080/1744666X.2019.1630273 [DOI] [PubMed] [Google Scholar]
- 11.Medina F, Plasencia C, Goupille P, Ternant D, Balsa A, Mulleman D. Current practice for therapeutic drug monitoring of biopharmaceuticals in rheumatoid arthritis. Ther Drug Monit. 2017;39(4):364-369. doi: 10.1097/FTD.0000000000000421 [DOI] [PubMed] [Google Scholar]
- 12.Ma C, Battat R, Jairath V, Vande Casteele N. Advances in therapeutic drug monitoring for small-molecule and biologic therapies in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2019;17(1):127-145. doi: 10.1007/s11938-019-00222-9 [DOI] [PubMed] [Google Scholar]
- 13.Grossberg LB, Papamichael K, Feuerstein JD, Siegel CA, Ullman TA, Cheifetz AS. A survey study of gastroenterologists’ attitudes and barriers toward therapeutic drug monitoring of anti-TNF therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2017;24(1):191-197. doi: 10.1093/ibd/izx023 [DOI] [PubMed] [Google Scholar]
- 14.Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960-977. doi: 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 15.Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017;153(3):835-857. doi: 10.1053/j.gastro.2017.07.031 [DOI] [PubMed] [Google Scholar]
- 16.Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee . American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153(3):827-834. doi: 10.1053/j.gastro.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 17.Ricciuto A, Dhaliwal J, Walters TD, Griffiths AM, Church PC. Clinical outcomes with therapeutic drug monitoring in inflammatory bowel disease: a systematic review with meta-analysis. J Crohns Colitis. 2018;12(11):1302-1315. doi: 10.1093/ecco-jcc/jjy109 [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 19.Syversen SW, Goll GL, Jørgensen KK, et al. Therapeutic drug monitoring of infliximab compared to standard clinical treatment with infliximab: study protocol for a randomised, controlled, open, parallel-group, phase IV study (the NOR-DRUM study). Trials. 2020;21(1):13. doi: 10.1186/s13063-019-3734-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44-48. doi: 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 21.England BR, Tiong BK, Bergman MJ, et al. 2019 update of the American College of Rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care Res (Hoboken). 2019;71(12):1540-1555. doi: 10.1002/acr.24042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukas C, Landewé R, Sieper J, et al. ; Assessment of Spondyloarthritis International Society . Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(1):18-24. doi: 10.1136/ard.2008.094870 [DOI] [PubMed] [Google Scholar]
- 23.Machado P, Landewé R, Lie E, et al. ; Assessment of Spondyloarthritis International Society . Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70(1):47-53. doi: 10.1136/ard.2010.138594 [DOI] [PubMed] [Google Scholar]
- 24.Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14(12):1660-1666. doi: 10.1002/ibd.20520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1(8167):514. doi: 10.1016/S0140-6736(80)92767-1 [DOI] [PubMed] [Google Scholar]
- 26.Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8(4):357-363. doi: 10.1016/j.cgh.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 27.Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157(4):238-244. doi: 10.1159/000250839 [DOI] [PubMed] [Google Scholar]
- 28.Jørgensen KK, Olsen IC, Goll GL, et al. ; NOR-SWITCH Study Group . Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304-2316. doi: 10.1016/S0140-6736(17)30068-5 [DOI] [PubMed] [Google Scholar]
- 29.D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132(2):763-786. doi: 10.1053/j.gastro.2006.12.038 [DOI] [PubMed] [Google Scholar]
- 30.Berth-Jones J, Grotzinger K, Rainville C, et al. A study examining inter- and intrarater reliability of three scales for measuring severity of psoriasis: Psoriasis Area and Severity Index, Physician’s Global Assessment and Lattice System Physician’s Global Assessment. Br J Dermatol. 2006;155(4):707-713. doi: 10.1111/j.1365-2133.2006.07389.x [DOI] [PubMed] [Google Scholar]
- 31.Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613-1620. doi: 10.1136/annrheumdis-2012-203090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Haens G, Vermeire S, Lambrecht G, et al. ; GETAID . Increasing infliximab dose based on symptoms, biomarkers, and serum drug concentrations does not increase clinical, endoscopic, and corticosteroid-free remission in patients with active luminal Crohn’s disease. Gastroenterology. 2018;154(5):1343-1351. doi: 10.1053/j.gastro.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 33.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320-1329. doi: 10.1053/j.gastro.2015.02.031 [DOI] [PubMed] [Google Scholar]
- 34.Teresa J, Chamaida PR, Ana MF, et al. Predictive value of serum infliximab levels at induction phase in rheumatoid arthritis patients. Open Rheumatol J. 2017;11:75-87. doi: 10.2174/1874312901711010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi T, Suzuki Y, Motoya S, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis—results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol. 2016;51(3):241-251. doi: 10.1007/s00535-015-1102-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63(11):1721-1727. doi: 10.1136/gutjnl-2012-304094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14(4):543-549. doi: 10.1016/j.cgh.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 38.Verstockt B, Moors G, Bian S, et al. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther. 2018;48(7):731-739. doi: 10.1111/apt.14943 [DOI] [PubMed] [Google Scholar]
- 39.Kennedy NA, Heap GA, Green HD, et al. ; UK Inflammatory Bowel Disease Pharmacogenetics Study Group . Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341-353. doi: 10.1016/S2468-1253(19)30012-3 [DOI] [PubMed] [Google Scholar]
- 40.Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1-10. doi: 10.1007/s00403-010-1080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steenholdt C, Bendtzen K, Brynskov J, Thomsen OØ, Ainsworth MA. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol. 2014;109(7):1055-1064. doi: 10.1038/ajg.2014.106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. The NOR-DRUM Steering Group
eAppendix 2. Principal Investigators From Each Study Center
eTable 1. Number of Randomized Patients by Study Hospital
eTable 2. Changes in Medication During the Trial
eTable 3. Details of Study Endpoints
eTable 4. Prespecified Secondary Outcomes
eTable 5. Demographic and Baseline Characteristics in Disease Subgroups
eTable 6. Sensitivity Analyses of the Primary Endpoint
eTable 7. Results Secondary Endpoints
eTable 8. Secondary Efficacy Endpoints (by Disease Subgroup)
eTable 9. Infliximab Discontinuation
eFigure 1. Treatment Algorithm in the Therapeutic Drug Monitoring Group
eFigure 2. Serum Infliximab Level
eFigure 3. Secondary Efficacy Outcomes (Box and Whiskers Plots)
eReferences
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement