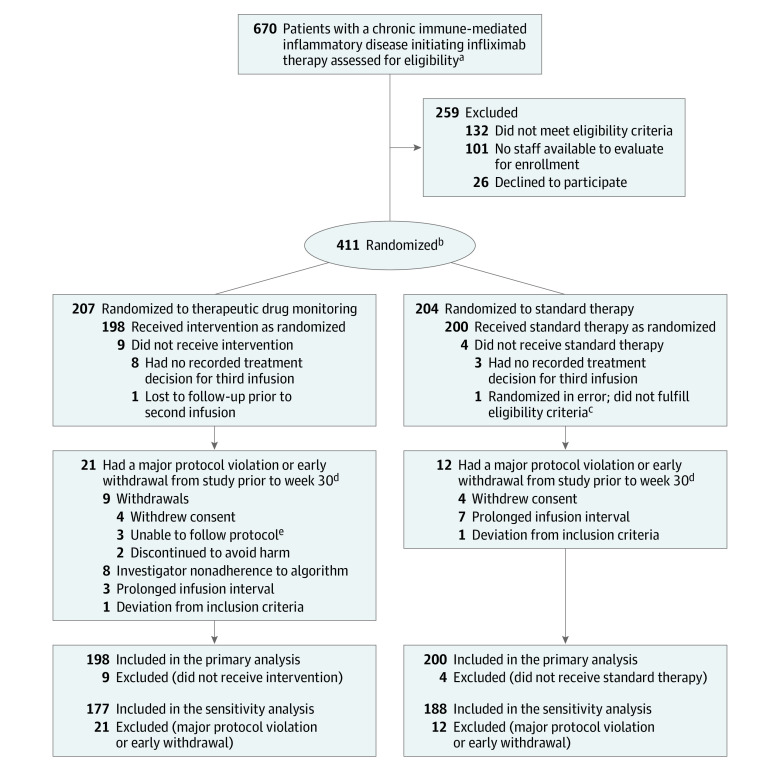
Figure 1. Flow of Participants Through the Norwegian Drug Monitoring Trial Part A.
aChronic immune-mediated inflammatory disease included rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, ulcerative colitis, Crohn disease, and psoriasis.
bRandomization was stratified by diagnosis.
cPatient had a colostomy, which was one of the defined exclusion criteria (outcome measure includes stool frequency).
dMajor protocol violations were prespecified in the statistical analysis plan as follows: deviations to inclusion and/or exclusion criteria or delay in scheduled infusion with an infusion interval of more than 12 weeks, or investigator nonadherence to study algorithm, defined as discrepancies between recommended and actual dose/interval at more than 1 visit.
ePatients for various reasons were not able to adhere to the study routine (eg, did not arrive for scheduled infusions).