Abstract
Type 1 diabetes (T1D) is a medical condition that requires constant management, including monitoring of blood glucose levels and administration of insulin. Advancements in diabetes technology have offered methods to reduce the burden on people with T1D. Several hybrid closed-loop systems are commercially available or in clinical trials, each with unique features to improve care for patients with T1D. This article reviews the Omnipod® 5 Automated Glucose Control System Powered by Horizon™ and the safety and efficacy data to support its use in the management of T1D.
Keywords: : AP, artificial pancreas, automated insulin delivery, CGM, HCL, insulin pump, Omnipod® 5 System, T1D
Type 1 diabetes (T1D) is an autoimmune disorder resulting in the immune-mediated destruction of the pancreatic β-cells responsible for the production of insulin [1]. The destruction of these vital cells, results in insulin deficiency and inability of glucose to enter cells throughout the body. Glucose is necessary for energy production within cells to maintain normal function. In the absence of insulin, glucose remains in the blood stream resulting in hyperglycemia or high blood glucose. Prolonged exposure to hyperglycemia can result in a number of complications throughout the body, including retinal, renal, cardiac and neuropathic diseases [2].
The well-recognized Diabetes Control and Complications Trial (DCCT) was paramount in demonstrating the importance of intensive insulin therapy to reduce the risk of long-term diabetes complications for individuals with T1D [3]. The DCCT trial compared intense insulin therapy, consisting of three or more insulin injections per day and self-monitoring of blood glucose (SMBG) four or more times per day, with conventional diabetes management, consisting of one or two injections each day and once daily SMBG. This study showed that reduction in HbA1c values seen in the intensively managed group correlated with reduced incidence of long term complications; however, those with lower HbA1c levels also had increased risk of hypoglycemia or low blood glucose, an acute complication of T1D that patients deal with on a daily basis.
Since the DCCT, there have been tremendous advancements in therapies for patients with T1D. Insulin formulations have improved, with the availability of long acting insulin formulations (e.g., glargine) and rapid-acting insulin analogs (e.g., aspart, lispro). Multiple daily injection (MDI) regimens consist of an injection of long acting insulin once or twice daily and three or more doses of rapid acting insulin with meals. Current methods of administering subcutaneous insulin include an insulin syringe, an insulin pen or an insulin pump with continuous subcutaneous insulin infusion. Insulin pens are now commonly used and offer a benefit over a syringe as pens are more convenient to use, easier to carry around and more accurate to dose [4].
Despite the improvement in insulin analogs, glucose management remains a significant challenge. Insulin needs change daily and even vary throughout the day [5]. As such, a set daily dose of long-acting insulin may not provide correct insulin coverage at all times of the day. One challenge that is difficult to address with once daily basal insulin dosing is the dawn phenomenon, when counter regulatory hormones are elevated and lead to increased insulin resistance and therefore increased insulin needs [6]. In addition, the current rapid-acting insulins are not ‘rapid’ enough to match insulin with carbohydrate consumption and absorption. In fact, it is generally recommended to give the insulin 15–20 min before the meal to improve matching the pharmacokinetics of insulin with the glucose absorption from food [7,8]. Administering insulin prior to the meal is difficult for busy people or young children with unpredictable meal intake.
The development of insulin pumps presents an alternative to the MDI regimen and allows for fine-tuning of insulin dosing at different times of the day. Insulin pumps provide a continuous infusion of insulin and allow for easily bolusing insulin when needed (e.g., for meals, snacks or correction of high blood glucose levels) with a few button pushes. This feature can be used to help manage variations in insulin needs throughout the day and night, including the dawn phenomenon or other daily glucose excursions. In addition, basal insulin can be temporarily adjusted in response to acute stressors or exercise by either increasing, decreasing or suspending insulin delivery with a few button pushes [9,10]. In contrast, once a long-acting insulin dose is given by injection, it cannot be stopped or removed from the body. Insulin pumps eliminate the need for MDI. Since the infusion site is typically changed once every 2–3 days, the number of injections a person requires decreases significantly.
In addition to advancements in insulin administration, there have also been advancements in the ways people are able to monitor their glucose levels [11]. SMBG meters have improved in accuracy and have reduced the amount of blood needed from the finger poke. Further advances in glucose monitoring has led to the development of continuous glucose monitors (CGMs) that allow for monitoring of glucose levels every 1–5 min and the potential for real-time alerts for current hyper and hypoglycemia. CGMs consist of a sensor that is inserted subcutaneously, a transmitter that attaches to the sensor and sends the glucose data and a receiver that receives the signal from the transmitter and displays the glucose data for the user to see. The accuracy of CGM sensors continues to improve. The Dexcom G6® was the first CGM system to be approved by the US FDA for nonadjunctive use (glucose values can be used to determine insulin doses) and the system does not require calibrations [12,13]. These features are important for incorporation of these devices into advanced diabetes technologies.
Despite the evidence of the importance of reduced glycemic variability on long term complications, the advancements in the treatment methods and increased uptake of diabetes technology, [14] many patients with T1D are still not meeting the recommended HbA1c goals. In fact, between 2016 and 2018, only 17% of youth and 21% of adults were meeting the goals recommended by the American Diabetes Association (ADA) [14]. In an attempt to improve diabetes care, the newest diabetes technologies, called hybrid closed-loop (HCL) systems, incorporate an insulin pump and a CGM along with a closed-loop control algorithm that automatically adjust insulin doses based on the current glucose value and trends. These devices have been studied extensively in safety and efficacy trials and have been shown to significantly improve glycemic variability and reduce the incidence of hypoglycemia [15–17]. This article reviews one of these HCL systems, the Insulet Omnipod® 5 Automated Glucose Control System Powered by Horizon™, which is still undergoing Phase III clinical trials.
Review
Overview of the market
Treatment of T1D requires the administration of subcutaneous insulin to replace the insulin that can no longer be made in the pancreas. Current standard of care for patients with T1D includes bolus administration of rapid-acting insulin with each meal and continuous background basal insulin administered either as a long-acting insulin or as rapid-acting insulin delivered continuously via a continuous subcutaneous insulin infusion pump [18]. As of 2018, across the T1D exchange, insulin pump use increased to 63% of patients and CGM use increased to 30%, with the largest increases occurring in children [14].
There are two main types of insulin pumps available, the traditional ‘tubed’ insulin pump that connects the pump to the wearer by a tube and is often carried in a pocket and the patch (or tubeless) insulin pump which is attached to the skin and has a separate remote device for controlling insulin administration [10]. Insulin pumps allow for variations in basal insulin needs during different times of day. Built in software allows for easy calculation of the insulin dose by entering the carbohydrate content of meals or snacks and the blood glucose level. As a part of this feature, the pump reduces the risk for hypoglycemia due to ‘stacking’ of insulin by subtracting from the insulin to treat hyperglycemia based on the amount of ‘insulin on board’ from a bolus of insulin that was recently given. Another benefit is the ability to administer much smaller doses of insulin, something of interest to the pediatric population where insulin doses can be small. Pumps can typically deliver boluses of insulin down to 0.01 u, whereas an injection of insulin can only accurately go down to 0.5 u. These features take a significant amount of burden off the user compared with patients using MDI. Insulin pumps, however, still require the wearer to be aware of their glucose level, as infusion site failures are relatively common and can result in life-threatening diabetic ketoacidosis if glucose levels are left unmonitored [10].
CGMs offer users a method to monitor glucose levels throughout the day and night in order to recognize trends for determining insulin dosing adjustments and, in some cases, real-time alerts for high or low glucoses and pending high or low glucose. In general, a CGM system consists of a sensor that includes a filament that is inserted subcutaneously, a transmitter which send the glucose signal to a receiver and the receiver which interprets the signal to a glucose level and displays this result for the user to see [19]. Typically, CGMs read glucose levels every 1–5 min. Currently, there are several CGMs available on the market in the US. These include the Abbott FreeStyle Libre, Medtronic Enlite and Guardian Sensor 3™ and Dexcom G4, G5 and G6. Each CGM offers features unique to the device and may benefit users in different ways. The Freestyle Libre consists of a small, round sensor/transmitter combination. This system does not currently give real-time data; however, glucose information can be synced to a receiver/cell phone for current glucose data and a review of previous data for retrospective trend analysis. The Libre system does not require fingerstick blood glucose information for calibrations. The Libre 2 with values every 1 min, real-time hypo and hyperglycemia alerts and the ability to interface with an HCL system has recently been approved by the FDA. The Medtronic sensors can be paired with the Medtronic insulin pumps and currently require a minimum of two calibrations per day. While older Dexcom models are still available, the G6 is the current version used by most Dexcom users. The Dexcom G6 has approval for use for the dosing of insulin and does not require fingerstick glucose readings for calibrations. Senseonics, an implantable, 90-day sensor which includes a sensor rod that is implanted subcutaneously, a transmitter that is adhered to the skin over the sensor and a receiver app on a cell phone, was briefly on the market in the United States but has since been pulled pending a longer-lasting sensor.
Current advanced diabetes technology, called ‘artificial pancreas’ (AP) or ‘closed-loop’ devices, include systems that incorporate CGM values into an algorithm which then adjust the amount of insulin delivered to the wearer [19,20]. Currently available systems utilize tubed insulin pumps, leaving a gap in the market for closed-loop systems using tubeless systems for people who avoid pumps due to the tubing. Each system available or under development has unique algorithms. Currently, only HCL systems are available. These systems require the user to input carbohydrate intake for meals and glucose values for correction boluses. The goal is to develop fully automated control of insulin administration without user involvement. To continue to advance this field, it is important to have accurate and reliable CGM sensors, accurate and safe algorithms, fast insulin preparations and insulin pumps that work with each patient’s lifestyle.
Alternate devices & how the Omnipod 5 System fits in to the field
The first commercially available HCL system was the Medtronic 670G, FDA approved in September 2016. In 2019, the Tandem Control-IQ system was approved by the FDA and released for commercial use. Other devices continue to be in design and study phases. These include the BetaBionic’s iLet system, Tidepool’s Loop and Insulet’s Omnipod 5 System among others. These selected additional systems are described in more detail below. A comparison of the 670G, Control-IQ and Omnipod 5 systems are found in Table 1.
Table 1. . CARES Paradigm for Omnipod® 5 System versus Currently Available HCL Systems.
| Omnipod 5 System | MiniMed 670G | Tandem Control-IQ | |
|---|---|---|---|
| Calculation | HCL system • Automated basal doses will be based on an estimated TDD = (programmed total daily basal insulin) × 2 • After first pod change, automated basal will be based on the actual TDD • HCL set point can be programmed to 110, 120, 130, 140 and 150 mg/dl |
HCL system • Uses TDD calculated from last 2–6 days • Automated basal calculated by system every 5 min • HCL set point = 120 mg/dl • No automated correction doses. Manual correction doses based on HCL algorithm and not on programmed correction factors |
HCL system • Increases or decreases programmed basal rates based on CGM tracings and predicted glucose levels • Automated correction dose once/h of 60% calculated correction dose • Target range of 112.5 to 160 mg/dl • Correction bolus target of 110 mg/dl |
| Adjustment | User can modify in HCL: • I:C ratios (for meal boluses) • Correction factor (for high glucose correction boluses) • Active insulin time (for user-given correction boluses only, not for system delivered insulin) • Target glucose (for algorithm set point and correction boluses) • HypoProtect mode (for exercise) Users cannot modify in HCL: • Basal rates |
Users can modify in HCL: • I:C ratios • Active insulin time • Temp target of 150 mg/dl Users cannot modify in HCL: • Basal rates • Correction factor • HCL set point of 120 mg/dl (except when using temp target) |
User can modify in HCL: • I:C ratios • Basal rates • Correction factor • Exercise and sleep activities User cannot modify in HCL: • Active insulin time (set at 5 h) • Correction bolus target of 110 mg/dl |
| Revert | • Reverts to open loop automatically if loss of CGM data for 20 min. Resumes HCL mode automatically when CGM data returns. • Must turn off HCL to use temporary basal rates or combo boluses • Consider turning off for illness/ketones • Consider turning off for dramatic change in insulin sensitivity (e.g., steroid use) due to system taking days to adjust calculations |
• Reverts to open loop if persistent hyperglycemia, maximum or minimum delivery thresholds, loss of CGM data, sensor integrity concerns • Must turn off HCL to use temporary basal rates or combo boluses • Consider turning off for illnesses/ketones • Consider turning off for dramatic change in insulin sensitivity (e.g., steroid use) due to system taking days to adjust calculations |
• Reverts to open loop if loss of CGM data for 20 min. Resumes HCL mode automatically when CGM data returns. • Must turn off HCL to use temporary basal rates • Consider turning off for illnesses/ketones • Consider turning off for dramatic change in insulin sensitivity (e.g., steroid use) due to system taking days to adjust calculations |
| Education | • Consider treating hypoglycemia with less CHO (e.g., 5–10 g) as system will reduce/suspend insulin prebolus for optimal mealtime management • Cannot use temp basal or combo boluses in HCL mode • Use HypoProtect feature for exercise (provides temporary reduction in basal insulin delivery) |
• Consider treating hypoglycemia with less CHO (e.g., 5–10 g) as system will reduce/suspend insulin • Prebolus for optimal mealtime management • System may display ‘BG required’ for HCL functioning. This is different from a sensor calibration. • User should follow system prompts for ‘BG required’ • Change I:C ratios (10–25%) and active insulin time • Cannot use temp basal or combo boluses in HCL mode • Temp target will allow a temporary reduction in basal insulin delivery |
• Consider treating hypoglycemia with less CHO (e.g., 5–10 g) as system will reduce/suspend insulin • Prebolus for optimal mealtime management • Do not over-ride boluses. Extra insulin already on board from autocorrections may cause hypoglycemia. • Use exercise activity for temporary increase in target glucose to 140–160 mg/dl (results in decrease in insulin delivery) • Use sleep activity to change target glucose to 112.5–120 mg/dl. Automated correction boluses not administered in sleep activity. |
| Sensor | Dexcom G6 (future systems may incorporate alternative CGM devices) • Factory calibrated • 10-day sensor life • CGM glucose can be used for insulin doses without a fingerstick glucose |
MiniMed Guardian 3 • Requires 2–4 calibrations/day • 6–7 day sensor life • Perform fingerstick glucose for diabetes management decisions • Important to calibrate when glucose is stable (e.g., before meals, bedtime, or when no sensor trend arrows) to prevent calibration errors |
Dexcom G6 • Factory calibrated • 10-day sensor life • CGM glucose can be used for insulin doses without a fingerstick glucose |
The Medtronic MiniMed 670G was the first commercially available HCL system [15,16,21]. This system includes the Medtronic 670G insulin pump and the Guardian Sensor 3. The system functions in two modes, manual mode and auto mode. Manual mode allows the pump to function as a traditional insulin pump, however, when paired with the sensor it has the added benefit of predicted low glucose suspend (PLGS). Auto mode includes fully automated basal insulin delivery using a modified proportional-integral-derivative (PID) algorithm based on the total daily dose from the previous 6 days and the current sensor glucose value. Future versions of the Medtronic system are under development and in clinical trials.
In December 2019, the Tandem Control-IQ system was approved by the FDA for commercial use [17]. Prior to Control-IQ release, Basal IQ was made commercially available and included PLGS technology [22]. The Control-IQ system uses the Tandem t:slim X2 insulin pump and the Dexcom G6 CGM. This HCL algorithm is unique in that it will administer an automated correction bolus in addition to automated basal rate adjustments and hypoglycemia suspension. Clinical trials leading to FDA approval showed an increase in time in range (in particular during the night) and reduced time in hyperglycemia, without increasing hypoglycemia [17].
BetaBionic’s iLet system is designed to be used as both an insulin only closed-loop device [23] and as a dual hormone closed-loop system [24,25]. This system is unique in several ways. The iLet system does not require carbohydrate input for meals or correction doses. Instead, the user can enter a ‘size’ of meal to give the system notification of carbohydrate consumption and impending rise in glucose. The system then adjusts insulin based on the CGM glucose values. In addition, this device will be capable of using stable glucagon in a continuous and bolus fashion to reduce the frequency and duration of hypoglycemia.
The Do-It-Yourself (DIY) world of closed-loop technology is a movement that was started by lay engineers in the community who desired to enhance their insulin pump’s capabilities beyond what is commercially available [26]. The algorithms involved in DIY systems are typically created and built into an app that can be installed on a controller device, such as a cell phone. They then use an insulin pump and a CGM to drive the algorithm and administer insulin. There are different DIY algorithms that are published as open source code (e.g., Loop or Open APS), however none of them are currently approved for use by the FDA as they have not undergone rigorous testing to determine safety and efficacy. As such, there is typically little discussion about insulin management between DIY users and their diabetes healthcare providers as these providers are not legally allowed to recommend medical devices not evaluated by the FDA. Typically, patients using these devices will be fully responsible for their insulin management. Often this means users fall into a special category of patients who are technologically savvy. Tidepool Loop is currently undergoing clinical investigation in order to seek FDA approval for a DIY created closed loop algorithm.
The Omnipod 5 System offers a unique approach to insulin management beyond the systems currently available in that it is a fully on-body system. This means that there are no tubes connecting the infusion site to the HCL device and the HCL device is not removed to bathe or swim. The HCL algorithms are contained within the patch pump or Pod, therefore, if the personal diabetes manager (PDM) is misplaced or left behind, the HCL system will continue to function. The algorithm design also favors automated personalized tuning to limit patient and provider required adjustments. While tuning of meal doses are done by the provider/user, the personalized model predictive control (MPC) control which can be started on day 1 does not require manual tuning. The system has been designed and tested in patients 2 years and up, [27] emphasizing performance across the lifespan. While this device is still undergoing clinical trials to achieve approval by the FDA, the current data available from early clinical trials is promising.
Introduction to the device
The Omnipod Insulin Management System consists of two components: a disposable, tubeless insulin pump containing an insulin reservoir and indwelling infusion cannula, referred to as a ‘Pod’ and a wireless, handheld device to operate the Pod, referred to as a PDM. The Pod is waterproof (IP28 rating for up to 25 feet for 60 min) and worn directly on the body for up to 72 h (3 days) before being changed and adhered to the skin with adhesive. The PDM communicates wirelessly with each Pod and is used to program the basal rates and bolus settings (e.g., insulin to carb ratios, correction factors, insulin on board time and target glucose levels). It is also used to activate each new Pod and display information related to insulin delivery, insulin reservoir levels and Pod life and functioning. To activate a Pod, the user fills the Pod’s insulin reservoir with a minimum of 85 units and a maximum of 200 units of rapid-acting insulin (e.g., aspart, lispro, glulisine or Fiasp®), pairs the Pod with the PDM, removes the adhesive backing and adheres the Pod to the skin. The PDM is then used to activate the new Pod and insert and prime the infusion cannula. The Omnipod has a unique, automated, cannula insertion process where the user inserts and primes the infusion cannula with the press of a button on the PDM, without having to handle or even visualize the infusion cannula needle. During the auto-insertion process, the infusion cannula is inserted 6–7 mm deep, at a 50° angle, into the subcutaneous tissue. The insertion needle retracts back into the Pod, leaving the cannula under the skin. Once communication between the PDM and the Pod is established, the Pod will continue to deliver basal insulin without the PDM nearby. The user must use the PDM to program and command bolus insulin delivery, and therefore the PDM must be within 5 feet of the Pod to deliver bolus doses of insulin [28].
The current Omnipod 5 Automated Glucose Control System Powered by Horizon (Omnipod 5 System) is an HCL insulin delivery system consisting of the Pod, the Dexcom G6 CGM and the PDM (Figure 1). Future versions of the system will be able to incorporate other CGM devices (such as the FreeStyle Libre or Dexcom G7) and the use of personal mobile devices to replace the dedicated PDM, a feature that will be significant in reducing the need to carry an additional device. The Omnipod 5 System uses a MPC algorithm to calculate insulin microboluses, delivered every 5 min based on the sensor glucose data received from the Dexcom CGM, and the predicted glucose values across a 60-min prediction horizon. The MPC algorithm is housed on each Pod and the Dexcom CGM data is transmitted directly to the Pod and incorporated into the algorithm’s insulin dosing decisions. The MPC algorithm targets a set glucose value, programmed by the user between 110 and 150 mg/dl and aims to achieve and maintain the programmed target glucose value in its insulin dose calculations. The user is responsible for delivering bolus doses of insulin for meals using the bolus settings programmed into the PDM. The system has a bolus calculator built into the program that uses CGM values and trends in addition to programmed bolus settings to assist with bolus dose determination. In addition, the Omnipod 5 System includes HypoProtect, a feature that allows for temporary reduction in basal insulin delivery during exercise.
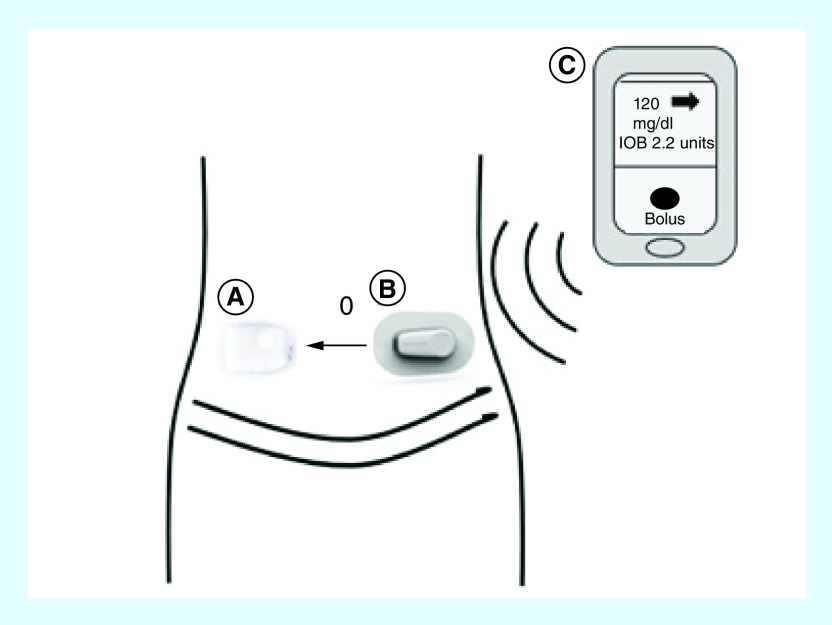
Figure 1. . Components of the Omnipod® 5 System.
The Omnipod 5 System consists of three components: (A) the pod with MPC algorithm which calculates micro-bolus insulin dose delivery every 5 min, (B) the Dexcom G6 CGM, which measures glucose values every 5 min in the interstitial fluid and sends the glucose information to the pod for use by the MPC algorithm, and (C) the Horizon PDM, which is used to program pump settings and deliver bolus insulin doses. The pod and the CGM communicate wirelessly with the PDM via the Horizon app and the Dexcom G6 app, respectively.
The Omnipod 5 System’s MPC algorithm is based on control strategies published by Doyle, Dassau, Gondhalekar and Lee [29–32]. The core model for the Horizon system incorporates a medically inspired personalization scheme to improve controller response in the face of inter and intra-individual variations in insulin sensitivity [30]. It utilizes asymmetric cost functions which account for the short-term consequences of hypoglycemia as being more significant than the short-term consequences of hyperglycemia. The algorithm also incorporates dynamic insulin-on-board calculations to minimize the likelihood of controller-induced hypoglycemia.
For clinical purposes, basal control from the algorithm is based on the patient’s total daily insulin dose (TDD) and requires no user tuning. The total daily dose is not entered directly but is rather estimated from the programmed basal rates at system start-up. The system assumes the user requires 50% of the TDD from basal insulin and 50% from bolus insulin. Thus, to estimate the TDD, the system calculates the total daily insulin delivery resulting from the programmed basal rates and then doubles this value. The basal rates are then modulated every 5 min based on this TDD. The system keeps track of the actual TDD delivered to the user and updates the TDD with each Pod change. The users programmed insulin-to-carb ratios and correction sensitivity are used for user-initiated meal and correction boluses. The insulin-on-board time is used to calculate decay for user administered insulin. The target glucose is used for both the control point for the system’s basal modulation as well as the correction point for correction boluses.
The Omnipod 5 System’s investigational PDM contains the Horizon application and the Dexcom G6 application. The Horizon application is used to: activate the Pod, pair the Dexcom transmitter to the Pod, program bolus settings and basal rates (basal rates are only used if system is operating in the conventional pump mode, referred to as manual mode), activate automated mode, program the target blood glucose used by the MPC algorithm, deliver meal boluses and high blood glucose correction boluses, display glucose data received from the Pod, respond to alerts and alarms and display information related to insulin delivery and Pod functionality. The Dexcom G6 app is used to: start and stop CGM sensor sessions, display sensor glucose information, program and receive alarms and alerts related to CGM glucose levels and CGM functionality, activate the Dexcom G6 remote data sharing feature (i.e., Share) and for troubleshooting.
All HCL systems vary in the ways they automate insulin delivery. In order to conceptualize the core differences between devices, it is helpful to understand 5 key aspects of each HCL: how each system CALCULATES insulin delivery, what parameters can be ADJUSTED, when to REVERT to open loop, key EDUCATION points and SENSOR characteristics [20,33]. The characteristics of the Omnipod 5 System are highlighted in Table 1.
Clinical profile
Phase I (safety & feasibility)
A series of studies have been completed using the Omnipod 5 System in various clinical conditions to assess the safety and performance of the algorithm (Table 2). The algorithm has been evaluated with meal bolus challenges in adults, with moderate intensity exercise challenge in adults and over 5 days under free-living conditions in adults, adolescents and children. These studies utilized a modified Omnipod insulin pump (or Pod), a modified PDM, the Dexcom G4 505 Share AP system and a Windows tablet configured with the personalized MPC algorithm.
Table 2. . Omnipod® 5 System clinical trials.
| Study setting | Device challenge | n | Age years (SD) |
% sensor time in range (70–180 mg/dl), (SD) |
% sensor time hypoglycemic (<70 mg/dl), (SD) |
% sensor time hyperglycemic | SAEs | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (>180 mg/dl), (SD) | ||||||||||||
| (≥250 mg/dl), (SD) | ||||||||||||
| Phase I | Overall | Night | Overall | Night | Overall | Night | ||||||
| Buckingham et al. (2018) | 36-h inpatient HCL use | Adults, 80% meal bolus | 24 | 37.1 (14.7) | 69.5 (14.4) | 87.8 (17.4) | 0.7 (1.7) | 0.7 (3.1) | 29.7 (14.4) | 11.4 (15.6) | None | [34] |
| 8.0 (7.5) | 1.0 (4.7) | |||||||||||
| Adults, 100% meal bolus | 10 | 41.7 (18.1) | 73.0 (15.0) | 90.8 (15.8) | 0.7 (1.2) | 0.7 (2.1) | 26.3 (14.4) | 8.6 (16.1) | ||||
| 3.6 (3.7) | 0.6 (1.9) | |||||||||||
| Adolescents, 100% meal bolus | 12 | 14.6 (1.5) | 72.6 (15.5) | 84.7 (25.1) | 2.0 (2.4) | 0.2 (0.6) | 25.4 (16.1) | 15.2 (25.3) | ||||
| 4.9 (6.3) | 1.5 (4.9) | |||||||||||
| Children, 100% meal bolus | 12 | 9.5 (1.7) | 70.1 (12.3) | 86.7 (17.7) | 2.0 (2.6) | 0.1 (0.3) | 27.9 (13.2) | 13.2 (17.8) | ||||
| 6.7 (5.6) | 2.2 (5.8) | |||||||||||
| Phase II | ||||||||||||
| Buckingham et al. (2018) | 54-h hotel/house-based | Meal bolus challenge: a) deliberate over bolus b) missed boluses c) extended boluses |
12 | 35.4 (14.1) | 76.1 (8.0) | 92.7 (13.3) | 0.6 (0.9) | 0.2 (0.6) | 23.3 (8.5) | 7.1 (13.4) | None | [35] |
| 4.5 (3.6) | 1.2 (4.1) | |||||||||||
| Forlenza et al. (2019) | 54-h hotel/house-based | Exercise challenge: a) temporary increase in glucose set point b) reduction in preprogrammed basal rate |
12 | 36.5 (14.4) | 85.1 (9.3) | 93.4 (14.2) | 1.4 (1.3) | 0.0 (0.0) | 13.5 (9.5) | 6.6 (14.2) | None | [36] |
| 1.8 (2.4) | 0.1 (0.3) | |||||||||||
| Sherr et al. (2020) | 96-h hotel/house-based | Free-living conditions: adults |
11 | 28.8 (7.9) | 73.7 (7.5) | 73.9 (21.0) | 1.9 (1.3) | 0.7 (1.1) | 24.5 (7.7) | 25.3 (21.2) | None | [37] |
| 4.5 (4.2) | 6.1 (10.9) | |||||||||||
| Adolescents | 10 | 14.3 (1.3) | 79.0 (12.6) | 85.4 (17.9) | 2.5 (2.0) | 1.3 (1.6) | 18.5 (13.5) | 13.3 (18.7) | ||||
| 3.5 (5.0) | 3.3 (7.0) | |||||||||||
| Children | 15 | 9.9 (1.0) | 69.2 (13.5) | 73.8 (19.3) | 2.2 (1.9) | 1.0 (1.9) | 28.7 (14.1) | 25.2 (19.7) | ||||
| 8.6 (8.8) | 8.6 (11.7) | |||||||||||
| Phase III | ||||||||||||
| 13-week outpatient HCL use | Free-living conditions: a) adults b) pediatrics |
≤240 | ≥6–≤70 | Primary effectiveness end point | Secondary end point | Secondary end point | ||||||
HCL: Hybrid closed-loop; SAE: Serious adverse event.
The initial Phase I safety study of the Omnipod 5 System was a hospital-based safety and feasibility study published by Buckingham and colleagues. The Omnipod 5 personalized MPC algorithm was tested in a multicentered, observational trial with 58 total participants [34]. This study consisted of a 1-week outpatient period in which the pump was in sensor augmented mode (or open-loop), followed by a 36-h inpatient phase in which the HCL system was activated. Participants were divided into four groups, adults who received 80% meal boluses (n = 24) and adults (n = 10), adolescents (n = 12) and children (n = 12) who received 100% meal boluses. Time with glucose <70 mg/dl ranged between 0.7 ± 1.7% in adults receiving 80% meal bolus and 2.0 ± 2.6% in pediatrics receiving 100% meal bolus. Time hyperglycemic >180 mg/dl ranged between 25.4 ± 16.1% and 29.7 ± 14.4% and time ≥250 mg/dl ranged between 3.6 ± 3.7% and 8 ± 7.5%, and the time in range (70–180 mg/dl) overall was between 69.5 ± 14.4% and 73.0 ± 15.0% and during the night was between 84.7 ± 25.1% and 90.8 ± 15.8%. There were no serious adverse events and at no time did participants meet criteria to stop the HCL system. The results of this study showed that the algorithm was safe for use during both the day and night with acceptable safety around a range of meal boluses allowing for progression to more real-world hotel studies.
Phase II (safety & efficacy)
Phase II safety, efficacy and performance testing occurred over a series of outpatient hotel/rental house-based studies (Table 2). Studies based in a hotel or rental house setting allow for semisupervised device use with activity and meal challenges similar to real-world conditions. These studies have been the bridge between hospital and at-home device use throughout AP system development. The first study was completed as a multicenter study using 12 adult subjects in a supervised hotel setting [35]. Participants wore their usual insulin pump and the Dexcom G4 for 7 days in the open-loop phase prior to the hotel phase of the study, where the HCL system was initiated. During the hotel phase, participants underwent several meal challenges in which the breakfast bolus was either 100% or deliberately overbolused at 130%, lunch was either a full bolus or a missed bolus and dinner was either a standard bolus or an extended bolus. Hypoglycemia (<70 mg/dl) occurred 0.0% (interquartile range [IQR] 0.0 to 1.2%) of the time during the entire 54-h HCL phase of the study and 0.0% (IQR 0.0–0.0%) overnight. Hyperglycemia (>180 mg/dl) occurred 23.3 ± 8.5% overall and 7.1 ± 13.4% at night and time ≥250 mg/dl occurred 4.5 ± 3.6% overall and 1.2 ± 4.1% overnight. In target range (70–180 mg/dl) was 76.1 ± 8.0% overall and 92.7 ± 13.3% overnight. A second portion of the Phase II testing evaluated the system during an exercise challenge [36]. In this study, participants completed a moderate intensity exercise session in the afternoon lasting more than 30 min. The algorithm was evaluated during the exercise with either a temporary increase in the glucose set point or reduction of preprogrammed basal rate. The time hypoglycemic (<70 mg/dl) overall was 1.4 ± 1.3% and overnight was 0.0 ± 0.0%. The time hyperglycemic (>180 mg/dl) was 13.5 ± 9.5% overall and 6.6 ± 14.2% at night and hyperglycemia ≥250 mg/dl was 1.8 ± 2.4% overall and 0.1 ± 0.3% overnight. The time in target range (70–180 mg/dl) was 85.1 ± 9.3% overall and 93.4 ± 14.2% overnight. In both studies, time in HCL was over 98 ± 1.8%. These clinical trials indicated the safety of the algorithm in conditions around mealtimes and exercise, indicating safety during carbohydrate consumption, missed or over-bolusing for meals and for moderate exercise.
Following the successful initial Phase II trials, further hotel/rental house-based safety and performance testing was conducted as a multicenter study including participants aged 6 to less than 65 years old (Table 2) [37]. This study consisted of a 7-day standard therapy (ST) period followed by a 96-h supervised hotel setting under free living conditions. Participants were able to eat meals and snacks on their own schedule and choosing. The participant or participant’s parent determined the carbohydrate content, entered the blood glucose for a correction bolus and decided the final amount of insulin to administer for the bolus. In addition, participants were encouraged to be active each day of the study. Mean time in range (70–180 mg/dl) was higher for all age groups during the HCL phase versus the ST phase (73.7 ± 7.5% vs 68.0 ± 15.6%, p = 0.08 for adults; 79.0 ± 12.6% vs 60.6 ± 13.4%, p = 0.01 for adolescents and 69.2 ± 13.5% vs 54.9 ± 12.9%, p = 0.003 for children). Adults had significantly less time hypoglycemic (<70 mg/dl) (1.9 ± 1.3% vs 5.1 ± 4.8%, p = 0.005) and less time hyperglycemic (≥250 mg/dl), but not statistically significant (4.5 ± 4.2% vs 7.4 ± 9.6%, p = 0.1). Adolescents and children had significantly less time spent hyperglycemic (≥250 mg/dl: adolescents 3.5 ± 5.0% vs 12.0 ± 6.5%, p = 0.02; children 8.6 ± 8.8% vs 17.5 ± 11.4%, p = 0.03 overall, and >180 mg/dl: adolescents 18.5 ± 13.5% vs 35.0 ± 16.2, p = 0.03; children 28.7 ± 14.1 vs 42.2 ± 14.7%, p = 0.007). The mean percentage of time in hypoglycemia was numerically lower during the HCL compared with ST for both adolescents and children, however, the difference was not found to be significant. There were no serious adverse events and no participants required early discontinuation. This study indicated that the Omnipod 5 System algorithm was safe in children and adults under free-living conditions. Longer and larger studies under free-living conditions need to be completed to fully assess the system’s use in the general T1D population.
Phase III (large scale safety & efficacy)
The large pivotal trial for the Omnipod 5 System is currently underway at the time of this manuscript (NCT04196140) (Table 2). This study is a single-arm, multicenter, prospective clinical study enrolling up to 240 subjects between the ages of 6 and 70 years old. This study includes two phases, the first being a 14-day ST outpatient phase and the second being a 13-week outpatient HCL phase with an optional 6-month extension. For this study, participants use the investigational Omnipod 5 System which includes the Pod with built in Horizon algorithm and the Omnipod 5 System PDM (a Samsung Android device that contains the Horizon App). The sensor used in this system is the Dexcom G6 CGM system, which does not require glucose calibrations and is approved for insulin dosing (nonadjunctive use). This study is unique compared with the previous studies as the algorithm is incorporated into the Pod with direct communication between the CGM and Pod for the first time where previous studies utilized a separate tablet which contained the algorithm. This build allows for closed loop control using only on-body devices. The primary safety objectives include incidence of severe hypoglycemia or diabetic ketoacidosis. The primary effectiveness end points include end of study HbA1c compared with baseline HbA1c and percentage of time in range (70–180 mg/dl) during HCL phase compared with ST. A number of secondary end points are included to assess additional glucose metrics and device usage. In addition, patient reported outcomes will be analyzed using questionnaires on general and disease-specific quality of life and device usability.
Conclusion
Advancements in diabetes technology aim to reduce the burden on patients and their families while improving health and reducing the risk for long term complications for those with T1D. While there are no fully automated closed-loop systems available to date, hybrid systems continue to advance. With multiple systems now available or available soon, patients now can pick a device that works best for them. The Omnipod 5 System, while not yet approved by the FDA for commercial use, has shown significant improvements in glycemic measures in both pediatric and adult patients during various challenges that would occur in daily life, such as meals and exercise. In addition, the system offers a unique design that includes the fully on-body insulin pump for patients who do not wish to wear a device with tubing.
Future perspective
Closed loop systems to manage diabetes have been a concept discussed since the mid-1970’s. The evolution of CGM technology paired with the handheld processing power of smartphones has driven this from theoretical concept to real-world use. The rapid evolution of this technology in the past 10 years has been matched only by the rapid expectations of persons with diabetes and providers. The gap between expectations and available technology has created some frustration and disappointment among those expecting more and more from emerging systems [38]. The Medtronic 670G has been truly groundbreaking in bringing automated insulin delivery to persons with diabetes for the first time. Despite this advancement, challenges with sensor usability, system alerts and user burden have limited the real-world benefits of this system [39–41]. The Omnipod 5 System will also break new ground as the first fully on-body HCL device. This will allow for automation in groups such as athletes and young children who would not be able to easily wear a traditional tubed HCL system or those who have chosen not to pursue an insulin pump due to the presence of tubing. Despite this advancement, continued challenges remain which must be addressed by future iterations of HCL technology.
Meal bolusing and associated ‘carbohydrate counting’ continue to remain a challenge and burden for persons with diabetes, even those on HCL systems. While basal rate modulation can compensate for some degree of inaccuracy in carbohydrate counting, as shown in the Omnipod 5 System studies discussed above, optimal glycemic control still appears to require meal announcement. One benefit of the Omnipod 5 System is the ability to link directly to a nutrition resource (CalorieKing) to search for foods and favorite meals allowing for quick, custom entries of meal carbohydrate content which can be saved to the system for future use. Research is ongoing to identify new approaches to improve automated insulin delivery in response to meals. As discussed above, the Damiano iLet system may be able to handle unannounced meals using an adaptive learning approach. Other meal detection and prediction algorithms have been tested in trials with various degrees of success [42]. Further refinement of meal detection algorithms will be essential to move from HCL control to fully closed-loop control.
Exercise also continues to present a significant hurdle for HCL systems. While existing devices and emerging devices like the Omnipod 5 System have modes for declared exercise, such features require significant pre-announcement and are infrequently used by most users. Exercise detection via accelerometry has been demonstrated to work in pilot studies [43]. The challenge of this technology in insulin-only HCL systems is that once exercise is detected it is often too late to prevent hypoglycemia due to insulin on board prior to exercise-associated insulin suspension. In this area, dual hormone systems (e.g., insulin and glucagon) may have an advantage as exercise detection could be paired with counter-regulatory hormone administration in addition to insulin suspension.
Current HCL systems have repeatedly shown significant increases in time in range and decreases in hypoglycemia during the night [15,17,36]. While sleeping, there is decreased variability due to lack of carbohydrate intake or activities, making it an ideal time to have tight glycemic target ranges. PLGS, automated insulin microboluses/basal adjustments and integrated glucagon administration allow closed-loop systems to target a tighter range of glucose and lower glucose levels than previously achievable. The ability to reach near-normal glucose levels and decreased glycemic variability for even a few additional hours per day is significant when considering the risk of long-term complications [44]. In addition, reduction in glycemic variability during the night may improve the duration and quality of sleep for people with T1D, an area that is recognized as an important component of routine diabetes care. Features such as Control-IQ’s Sleep mode and the ability to manually adjust glucose targets in the Omnipod 5 System, will be important components of future HCL systems.
As HCL systems continue to advance, device tuning will become a greater challenge. As discussed above, each existing and emerging system has different tuning parameters which play a different role in how each system functions. This interplay between settings and how they work in manual mode and with the algorithms in HCL modes will quickly become too complex for most healthcare providers to navigate. Potential solutions under development by various research groups involve cloud-based parameter tuning by decision support algorithms. Such automated systems can utilize large patient data sets to leverage machine learning and optimize device parameters for a given patient. In this way, HCL systems can continue to be optimized and tuned using higher level algorithms not required to run on on-body devices.
Executive summary.
Introduction
Type 1 diabetes (T1D) is an autoimmune condition resulting in loss of insulin production.
Treatment of T1D consists of exogenous insulin replacement with multiple daily injections or an insulin pump and frequent glucose monitoring.
Overview of the market
Diabetes management has advanced significantly and now includes the use of insulin pens, insulin pumps and continuous glucose monitors (CGMs) as a part of routine diabetes care.
The most advanced diabetes technology, the hybrid closed-loop systems (HCL), consist of an insulin pump and a CGM that utilize algorithms to make automated insulin adjustments.
To date, HCL systems have used tubed insulin pumps, leaving a gap in the market for those with diabetes who prefer a tubeless pump such as the Omnipod®.
Alternate devices & how the Omnipod® 5 System fits in to the field
Two other HCL systems (MiniMed 670G and Tandem Control-IQ) currently have US FDA approval.
Several other systems are in the research phase, including the Omnipod 5 System.
Each system offers unique features from the type of algorithm used to the glucose targets and the modes or activities available.
Introduction to the device
The Omnipod 5 System is an HCL system that includes the first tubeless pump to use closed-loop technology.
The Omnipod 5 System uses a model predictive control algorithm which is stored within the Pod.
The Omnipod 5 System uses a hand held device (personal diabetes manager) to activate the Pod, pair the CGM, program basal and bolus settings and administer meal or correction boluses, manage the HCL mode and display glucose and pump data and alarms.
Clinical profile
Multiple studies have been conducted to test the safety, efficacy and performance of the Omnipod 5 System in both adults and children.
These studies have shown significant improvements in HbA1c, time in range and reductions in time in hypoglycemia under exercise and meal challenge conditions.
There were no serious adverse events and o participants required early discontinuation.
The pivotal trial is currently under way.
Conclusion
The Omnipod 5 System is a unique design that offers a fully on-body insulin pump while offering the benefits of improved glycemic measures during various challenges that occur in daily life, such as meals and exercise.
Future perspective
Significant advancements have been made in the management of T1D.
Meal and exercise continue to be difficult times even for HCL systems and need to continue to be a focus of future system development.
Overnight has been the most effective time for improved glycemic variability with the use of HCL systems.
Future improvements in HCL systems may include the use of cloud-based parameter tuning by decision support algorithms.
Footnotes
Financial & competing interests disclosure
LH Messer has received speaking/consulting honoraria from Tandem Diabetes and DexCom, Inc. and also consults for Clinical Sensors and Capillary Biomedical. Her institution receives research grants from Medtronic, Tandem Diabetes, DexCom, Abbott and Insulet Corp. GP Forlenza conducts research sponsored by Medtronic, Dexcom, Abbott, Insulet, Tandem and Lilly and has served as a speaker/consultant for Medtronic, Dexcom, Insulet, Tandem and Lilly. Erin Cobry receives NIH funding: NIH K12DK094712. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Company review disclosure
In addition to the peer-review process, with the author’s consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made by the author at their discretion and based on scientific or editorial merit only. The author maintained full control over the manuscript, including content, wording and conclusions.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Pettitt DJ, Talton J, Dabelea D et al. Prevalence of Diabetes in U.S. Youth in 2009: the search for diabetes in youth study. Diabetes Care 37(2), 402–408 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faulds ER, Zappe J, Dungan KM. Real-world implications of hybrid close loop (HCL) insulin delivery system. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 25(5), 477–484 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group Nathan DM, Genuth S et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329(14), 977–986 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Phatak SR, Rao YS, Ramesh J, Sanyal D. Consensus on choice of insulin delivery devices in routine clinical practice. Diabetes Technol. Ther. (2020) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 5.Dovc K, Boughton C, Tauschmann M et al. Young children have higher variability of insulin requirements: observations during hybrid closed-loop insulin delivery. Diabetes Care 42(7), 1344–1347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauck MA, Lindmeyer AM, Mathieu C, Meier JJ. Twenty-four hour fasting (basal rate) tests to achieve custom-tailored, hour-by-hour basal insulin infusion rates in patients with Type 1 diabetes using insulin pumps (CSII). J. Diabetes Sci. Technol. (2019) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slattery D, Amiel SA, Choudhary P. Optimal prandial timing of bolus insulin in diabetes management: a review. Diabet. Med. J. Br. Diabet. Assoc. 35(3), 306–316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobry E, McFann K, Messer L et al. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with Type 1 diabetes. Diabetes Technol. Ther. 12(3), 173–177 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Berget C, Messer LH, Forlenza GP. A clinical overview of insulin pump therapy for the management of diabetes: past, present, and future of intensive therapy. Diabetes Spectr. Publ. Am. Diabetes Assoc. 32(3), 194–204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sora ND, Shashpal F, Bond EA, Jenkins AJ. Insulin pumps: review of technological advancement in diabetes management. Am. J. Med. Sci. 358(5), 326–331 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab. Syndr. 12(2), 181–187 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Wadwa RP, Laffel LM, Shah VN, Garg SK. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol. Ther. 20(6), 395–402 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol. Ther. 20(6), 428–433 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster NC, Beck RW, Miller KM et al. State of Type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol. Ther. 21(2), 66–72 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg SK, Weinzimer SA, Tamborlane WV et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with Type 1 diabetes. Diabetes Technol. Ther. 19(3), 155–163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Early in-home clinical trial showing improved glycemic outcomes using a hybrid closed-loop system.
- 16.Forlenza GP, Pinhas-Hamiel O, Liljenquist DR et al. Safety evaluation of the MiniMed 670G System in children 7–13 years of age with Type 1 diabetes. Diabetes Technol. Ther. 21(1), 11–19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SA, Kovatchev BP, Raghinaru D et al. Six-month randomized, multicenter trial of closed-loop control in Type 1 diabetes. N. Engl. J. Med. 381(18), 1707–1717 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Randomized controlled trial demonstrating successful in-home use of an advanced hybrid closed-loop system.
- 18.American Diabetes Association. Standards of Medical Care in Diabetes – 2020. Diabetes Care 43(S1), S1–S212 (2020).31862741 [Google Scholar]
- 19.Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res. Clin. Pract. 133, 178–192 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Messer LH, Berget C, Forlenza GP. A clinical guide to advanced diabetes devices and closed-loop systems using the CARES paradigm. Diabetes Technol. Ther. 21(8), 462–469 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders A, Messer LH, Forlenza GP. MiniMed 670G hybrid closed loop artificial pancreas system for the treatment of Type 1 diabetes mellitus: overview of its safety and efficacy. Expert Rev. Med. Devices. 16(10), 845–853 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forlenza GP, Li Z, Buckingham BA et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with Type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care 41(10), 2155–2161 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Ekhlaspour L, Nally LM, El-Khatib FH et al. Feasibility studies of an insulin-only bionic pancreas in a home-use setting. J. Diabetes Sci. Technol. 13(6), 1001–1007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Khatib FH, Balliro C, Hillard MA et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with Type 1 diabetes: a multicentre randomised crossover trial. Lancet Lond. Engl. 389(10067), 369–380 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell SJ, El-Khatib FH, Sinha M et al. Outpatient glycemic control with a bionic pancreas in Type 1 diabetes. N. Engl. J. Med. 371(4), 313–325 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis DM. Do-It-Yourself Artificial Pancreas System and the OpenAPS Movement. Endocrinol. Metab. Clin. North Am. 49(1), 203–213 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Buckingham Forlenza, Sherr Galderisi, Ekhlaspour Galderisi. Safety and performance of the omnipod hybrid closed-loop system in young children aged 2–6 years with Type 1 diabetes. Presented at: ADA CA, USA: (2019). [Google Scholar]
- 28.Ly TT, Layne JE, Huyett LM, Nazzaro D, O'Connor JB. Novel bluetooth-enabled tubeless insulin pump: innovating pump therapy for patients in the digital age. J. Diabetes Sci. Technol. 13(1), 20–26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dassau E, Zisser H, Cesar Palerm C, Buckingham Bruce A, Jovanovič L, Doyle J F III .Modular Artificial β-Cell System: a prototype for clinical research. J. Diabetes Sci. Technol. Online. 2(5), 863–872 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JB, Dassau E, Gondhalekar R, Seborg DE, Pinsker JE, Doyle FJ. Enhanced Model Predictive Control (eMPC) Strategy for automated glucose control. Ind. Eng. Chem. Res. 55(46), 11857–11868 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gondhalekar R, Dassau E, Doyle FJ. Periodic zone-MPC with asymmetric costs for outpatient-ready safety of an artificial pancreas to treat Type 1 diabetes. Autom. J. IFAC Int. Fed. Autom. Control. 71, 237–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JB, Dassau E, Seborg DE, Doyle FJ. Model-based personalization scheme of an artificial pancreas for Type 1 diabetes applications. Presented at: 2013 American Control Conference. Washington, DC, USA: (2013). [Google Scholar]
- 33.Messer LH, Forlenza GP, Wadwa RP et al. The dawn of automated insulin delivery: a new clinical framework to conceptualize insulin administration. Pediatr. Diabetes. 19(1), 14–17 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Buckingham BA, Forlenza GP, Pinsker JE et al. Safety and feasibility of the omnipod hybrid closed-loop system in adult, adolescent, and pediatric patients with Type 1 diabetes using a personalized model predictive control algorithm. Diabetes Technol. Ther. 20(4), 257–262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Early clinical trial evaluating the safety and feasibility of the Omnipod® 5 System in adult and pediatric patients.
- 35.Buckingham BA, Christiansen MP, Forlenza GP et al. Performance of the Omnipod Personalized Model Predictive Control Algorithm with Meal Bolus Challenges in adults with Type 1 diabetes. Diabetes Technol. Ther. 20(9), 585–595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clinical trial evaluating the Omnipod 5 System under meal bolus challenge conditions.
- 36.Forlenza GP, Buckingham BA, Christiansen MP et al. Performance of Omnipod Personalized Model Predictive Control Algorithm with Moderate Intensity Exercise in adults with Type 1 diabetes. Diabetes Technol. Ther. 21(5), 265–272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clinical trial evaluating the Omnipod 5 System under exercise challenge conditions.
- 37.Sherr JL, Buckingham BA, Forlenza GP et al. Safety and performance of the Omnipod Hybrid Closed-Loop System in adults, adolescents, and children with Type 1 diabetes over 5 days under free-living conditions. Diabetes Technol. Ther. 22(3), 174–184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Clinical trial evaluating the Omnipod 5 System in adult and pediatric patients in the home setting.
- 38.Messer LH. Why expectations will determine the future of artificial pancreas. Diabetes Technol. Ther. 20(S2), S2–S65 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Berget C, Messer LH, Vigers T et al. Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr. Diabetes 21(2), 310–318 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messer LH, Berget C, Vigers T et al. Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr. Diabetes 21(2), 319–327 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care 42(12), 2190–2196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forlenza GP, Cameron FM, Ly TT et al. Fully closed-loop multiple model probabilistic predictive controller artificial pancreas performance in adolescents and adults in a supervised hotel setting. Diabetes Technol. Ther. 20(5), 335–343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobs PG, Resalat N, El Youssef J et al. Incorporating an exercise detection, grading, and hormone dosing algorithm into the artificial pancreas using accelerometry and heart rate. J. Diabetes Sci. Technol. 9(6), 1175–1184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Battelino T, Danne T, Bergenstal RM et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on time in range. Diabetes Care 42(8), 1593–1603 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]