Abstract
Background:
Guidelines for clinicians treating military concussion recommend exertional testing before return-to-duty, yet there is currently no standardized task or inclusion of an objective physiological measure like heart rate variability (HRV).
Methodology & results:
We pilot-tested two clinically feasible exertional tasks that include HRV measures and examined reliability of a commercially available heart rate monitor. Testing healthy participants confirmed that the 6-min step test and 2-min pushup test evoked the targeted physiological response, and the Polar H10 was reliable to the gold-standard electrocardiogram.
Conclusion:
Both tasks are brief assessments that can be implemented into primary care setting including the Polar H10 as an affordable way to access HRV. Additional research utilizing these tasks to evaluate concussion recovery can validate standardized exertional tasks for clinical use.
Keywords: : autonomic dysfunction, exercise intolerance, exertional tasks, heart rate variability, military concussion, primary care, return to activity
Concussion or mild traumatic brain injury (mTBI) is a prevalent injury in civilian, athletic and military populations. mTBI results in a variety of symptoms that limit activity. Both early return to normal activity and prolonged rest have been shown to increase symptom duration [1–3]. Clinicians commonly determine military duty readiness based on self-reported absence of symptoms and return to ‘normal’ performance on clinical assessments that may have ceiling effects for this population [4].
The Traumatic Brain Injury Center of Excellence (TBI CoE) has developed clinical recommendations for military primary care providers (PCP) for management of mTBI that outline a five-stage activity progression, similar to sports concussion consensus return-to-play recommendations [5–7]. TBI CoE guidelines recommend an exertional test before resumption of activity and again before return to duty. However, implementation of exertion testing is inconsistent, and there are no standardized exertional assessments that are feasible and validated for primary care.
Symptom self-report at rest serves as the primary measure that clinicians use to recommend return-to-activity [8], but physiological deficits may persist beyond symptom resolution [9,10]. Although concussive symptoms may have multiple causes, one contributor to impairment is autonomic nervous system (ANS) dysfunction [11,12]. The ANS drives communication between the brain and circulatory regulation that may be disrupted after mTBI [13,14]. Sympathetic and parasympathetic function is reflected in heart rate variability (HRV), which is the variation in time between successive heartbeats. HRV can serve as a proxy for ‘top-down’ integration of mechanisms that regulate peripheral physiology and can provide insight on stress and overall health [15]. One critical HRV component, respiratory sinus arrhythmia (RSA), or high frequency (HF), represents parasympathetic activity or vagal tone [15,16]. After concussion, it may be necessary to induce physical stress to observe subtle ANS and cardiovascular dysfunction [17].
The current gold standard for HRV measurement requires electrocardiogram (ECG) measurement [18] because the sequence of times between R-peaks provides a noninvasive measure of the neural regulation of the heart, but ECG is not always feasible. High-quality heart rate monitors (HRM) like the Polar H10 (Polar Electro Oy, Kempele, Finland) are a less expensive alternative with adequate reliability compared to ECG in a resting state, but lower reliability under higher exertion levels has been reported [19–21].
Exertional testing is a reasonable clinical approach to identify possible ANS dysfunction after concussion [22]. Standardized and validated exertional tasks for mTBI in acute and prolonged recovery are the Buffalo Concussion Treadmill Task (BCTT) [23] and a similar bike test [24], but time and equipment requirements make them more appropriate for rehabilitation settings. Military service members with concussion are typically followed by primary care for the first month postinjury. Although exertional testing [25] is encouraged to guide return to activity and duty, the required time, space, and equipment [26,27] are not available for most primary care providers. A field expedient test of exertion for PCPs guided by objective physiological measures could improve clinical feasibility and implementation.
The goals of the present study are to pilot test clinically feasible exertional tasks developed based on minimal time, space, equipment requirements and determine whether the objective physiological measure of HRV could be implemented during the testing protocols. We tested healthy adults, a majority of whom were service members (SMs), in two brief exertional tests: a stepping (STEP) and a pushup (PU) task that were easy to administer using readily available equipment and typical PCP exam room space [4,27]. We used a modified 6-min Chester Step Test, a graded step test validated for emergency service providers to quantify occupational aerobic capacity [28,29]. The step task progressively increases speed every 2 min, and similar to the BCTT [30] and HRV has been successfully collected during a stepping task [31]. The second exertional task was performance of pushups for 2 min. Pushups are part of the current Army Physical Fitness Test and an important training component for all military branches [32] with clear functional health relevance [33,34], but they have not been studied to test exertion after concussion. We hypothesized that HRV measurement before, during and after each task using clinically available equipment (Polar H10) would be reliable compared with the gold standard ECG (Faros 180, Mega Electronics Ltd., Pioneerinkatu, Finland) measurement. We also hypothesized that both tasks would be feasible for participants to complete and achieve targeted exertion levels and physiological responses.
Methods
Participants
All participants were healthy adults between the ages of 18 and 45 years who were active, exercising at least three times a week to be representative of the active duty military population. A majority of participants were affiliated with the US Marine Corps, serving in eastern North Carolina at a recruiting station. Exclusion criteria were any medical condition or injury that limited ability to perform a physical training session or moderate exertion of stepping or pushups for 10 min, history of moderate to severe TBI or self-reported concussion in the past year. Screening occurred during in-person briefings via review of inclusion/exclusion criteria and study procedures; individuals interested in participating could contact researchers.
Testing procedures
Participants were seen for a single test session lasting approximately 45–60 min. We continuously recorded heart rate (HR) and interbeat intervals (IBIs) with two HRM (Polar H10 and Faros 180), during baseline, STEP and PU tasks (counterbalanced order) and recovery periods after each task (Figure 1). During exertion HR was monitored in real time via Bluetooth, and exertion level and concussive symptoms were surveyed each minute.
Figure 1. . Layout of testing session.
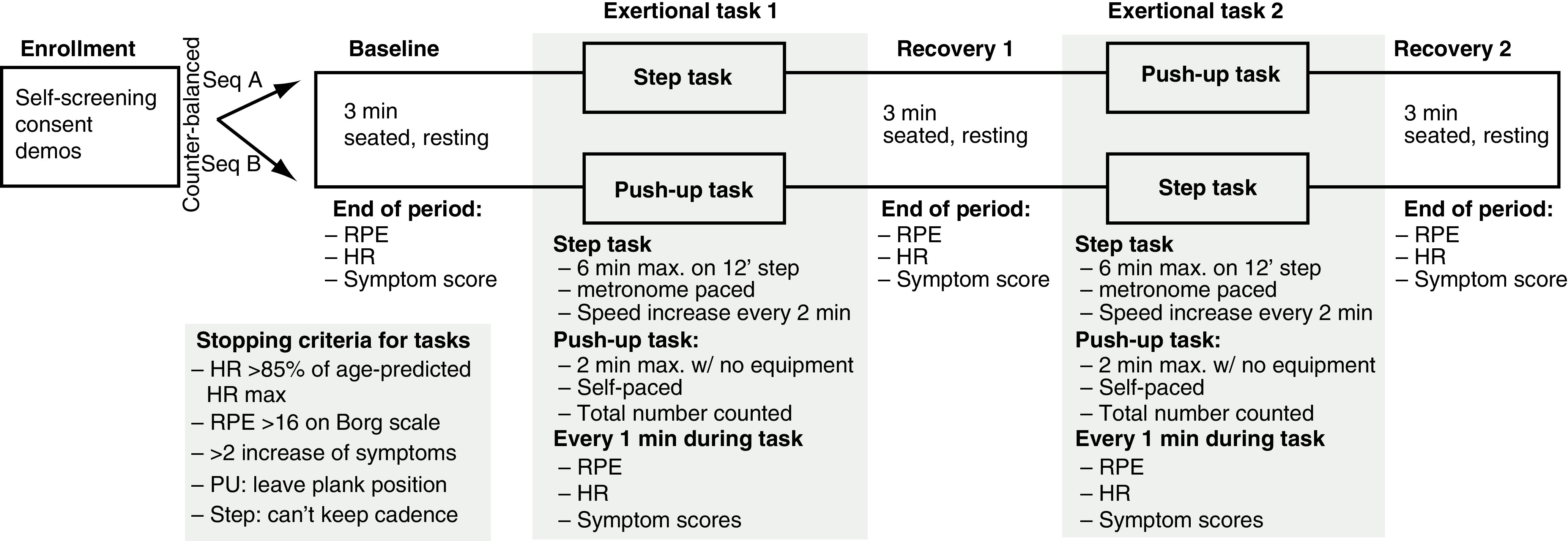
HR: Heart rate; PU: Pushup; RPE: Rate of perceived exertion.
Self-report measures
Participants completed a demographic questionnaire including questions on self-reported concussion history, military experience, current pain (0–10 scale), sleep (number of hours) and caffeine (number of drinks) within the past 24 h. Rate of perceived exertion (RPE) using the Borg Scale, a 6–20 scale reflecting subjective measure of workload, was used to document self-perceived exertion during exercise [35]. The presence or increase of symptoms was assessed using a 0–10 Likert scale focusing on headache, dizziness, nausea, light/sound sensitivity and fogginess, similar to the approach used in other exertional tests for concussion [23,36]. Throughout the session, we recorded verbal RPE and concussive symptom scores at the end of baseline (BL), rest following first test (R1), rest following second test (R2) and each minute during each exertional task, not expecting testing to cause symptom exacerbation.
Heart rate recording
To examine the reliability of HRV measurement we employed the Faros 180 ECG monitor (Mega Electronics Ltd.) and the more clinically feasible Polar H10 monitor (Polar Electro Oy). Both monitors recorded HR and IBIs. Participants wore the Polar H10 around their chest and the Faros 180 was connected by three lead electrodes (right and left collarbone, left ribcage). The target range for heart rate (60–85% of predicted HRmax) was determined from the Fox and Haskell's equation (HRmax =220 -age[years]) [37]. This equation is a simple, commonly used part of the BCTT protocol [30] and has been recommended for use in military populations for graded exercise tests [38].
Stopping criteria
We used BCTT guidelines for safety in our protocol to halt either test based on signs of excessive participant stress [39], including HR >85% of predicted HRmax, RPE >16, a reported increase >2 on the symptom scale over baseline values or the examiner perceived that testing was unsafe. Participants were also instructed that they could discontinue testing at any time if they deemed it necessary.
STEP task
The step task was a maximum of 6 min in duration and required a 12-inch step and a smartphone metronome app. Every 2 min the stepping pace increased as a participant stepped up and down a 12-inch step (using preferred lead and trail legs) beginning at 80 bpm (20 steps/min), then 100 bpm (25 steps/min), and finally 120 bpm (30 steps/min). The test was discontinued based on safety stopping criteria or if the participant was unable to maintain the metronome pace.
PU task
The PU task was a maximum of 2 min long. Participants were instructed to complete as many pushups as possible during the time duration without resting. This is especially relevant for military populations [33]. The test was discontinued for the stopping criteria or if the participant released from plank position to rest at any point. The total number of pushups was measured with a handheld counter by the examiner.
Data processing & reduction
The Faros 180 recorded a complete ECG waveform at 1 kHz. IBIs, the time between consecutive heartbeats expressed in milliseconds, were derived from detected R peaks in ECG using the Cardio Peak-Valley Detector (CPVD) [40] to create the IBI event series. Polar H10 monitor automatically reduced the heart rate electrical signal to IBIs. Before analysis, both sequences of IBIs were synchronized automatically, then visually inspected to ensure proper alignment. Each aligned sequence was then transformed into a 2-Hz equally sampled time series by linear interpolation, extracting of HRV parameters while preventing the two series from becoming decoupled. The unedited IBI file was visually inspected and edited offline with CardioEdit software (developed in the Porges laboratory and implemented by researchers trained in the Porges laboratory).
HRV frequency components were calculated with CardioBatch software (Brain-Body Center, University of Illinois at Chicago), which implements the Porges–Bohrer method [41]. This method is neither moderated by respiration nor influenced by nonstationarity and reliably generates stronger effect sizes than other commonly used metrics of RSA [41]. Variables included average heart rate (i.e., normalized mean IBIs every 60 s), RSA defined by the frequencies of spontaneous breathing (0.12–0.4 Hz), low frequency (LF) HRV occurring within the frequencies of spontaneous vasomotor and blood pressure oscillations (0.06–0.10 Hz) and heart period (i.e., total HRV, mean IBIs) (Appendix 1).
Data analysis
Means, standard deviations, medians, interquartile ranges and 95% CIs were calculated for all demographic and questionnaire data. Alpha was set a priori at α <0.05 for all statistical analyses. Normality was assessed for all dependent variables using the Shapiro–Wilk test. Only participants with complete data for both sensors were included in reliability analyses.
Reliability and accuracy of the Polar H10 IBIs compared with the Faros 180 IBIs were analyzed using Bland–Altman (B-A) plots and generalized estimating equations (GEE). Comparison of independent measurements was facilitated by visualizing the distribution between the mean measurement and the difference [42]. B-A plots, examined with SPSS statistical software (IBM SPSS Statistics, Version 26.0 [IBM Corp., NY, USA]), show agreement between two sensors, by plotting the mean between pair of measurements against its difference. Visual inspection of the B-A plots is used to identify systematic biases and possible outliers. Paired t-tests evaluated whether the differences between the signals were biased (i.e., one signal source generating longer or shorter values). B-A plots and the t-test were performed on IBIs collected from all participants during all tasks. Scatter plot and linear regression analyses were used to visualize and calculate the level of convergence between the Polar H10 and Faros 180. A strong correlation of threshold of R2 ≥0.9 of IBI time series was determined as a target representing strong agreement [43]. For each HRV component measure a GEE model was conducted using PROC GENMOD in SAS 9.5 (SAS Institute, NC, USA) to estimate group mean differences (95% CIs) for HR monitor methods (Polar H10 vs Faros) and session (BL, R1, R2, STEP, PU). This allowed evaluation of the effects of method of HR measurement on HRV components.
Clinical feasibility was assessed by participant completion of the tasks and the ability to record HRV at baseline, during exertion and throughout recovery. Physiological responses were assessed using HR and RPE measurements. HR was measured during both tasks with the exertional range (60–85% of age-predicted HRmax) as the primary target for a successful exertional task. Self-reported RPE between 12 and 16 (moderate exertion range) during the tasks was considered an appropriate physiological response.
Results
Participants
Fifteen healthy adults completed our testing protocol, 13 were active reservists for the US Marine Corps. Four of the Marine participants had a history of concussion, and nine had been deployed serving an average of 2.8 deployments (SD = 0.8). Full demographics are presented in Table 1.
Table 1. . Demographic characteristics of all participants: military and health history for service members.
| Demographic characteristic | n = 15 |
|---|---|
| Age (years) | 29.33 (6.36) |
| Sex Women Men |
2 (13.3%) 13 (86.7%) |
| Race/ethnicity Caucasian Hispanic/Latino African–American Native American |
7 (46.7%) 4 (26.7%) 2 (13.3%) 2 (13.3%) |
| Education High school Trade school Some college/associate's degree Bachelor's degree Advanced degree |
1 (6.7%) 1 (6.7%) 8 (53.3%) 4 (26.7%) 1 (6.7%) |
| Military affiliation US Marine Corps None |
13 (86.7%) 2 (13.3%) |
| Military & health history | n = 13 |
| Time serving (years) | 10.0 (5.5) |
| Military rank/pay grade E1–E5 E6–E9 O1–O3 |
4 (30.8%) 6 (46.1%) 3 (23.1%) |
| Deployment history Yes No |
9 (69.2%) 4 (30.8%) |
| Concussion history Yes No |
4 (30.8%) 9 (69.2%) |
| Behavioral health history Combat stress Posttraumatic stress Anxiety Depression |
1 (7.7%) 2 (15.3%) 2 (15.3%) 1 (7.7%) |
| Caffeine (drinks/supplements in past 24 h) | 1.9 (2.0) |
| Sleep (hours in past 24 h) | 5.7 (1.2) |
Values are n (%) or mean (SD).
Reliability and accuracy of Polar H10
A subset of 10 participants had complete data for both the Polar H10 and the Faros 180. The Faros 180 was less able to detect HR peaks during pushups than the Polar H10, requiring more than 5% editing of total IBIs, beyond the recommended editing standard from HRV Task Force guidelines [18]. Therefore, the reliability analysis is based on data collected during STEP. Visual inspection of the B-A plot (Figure 2A) indicated excellent agreement and minimal bias between the sequential IBIs measured with Polar H10 and Faros 180. The mean of the differences between sensors was -0.0231 ms (SD = 3.197; t(23352) = -1.105 [95% CI: -0.064 to 0.017; p = 0.27]) with limits of agreement of -6.28 to 6.24 and no significant proportional bias (B = 0.0; t = 1.07; p = 0.28). The t-test results confirm that the pairs of sensors were measuring the same parameter. The mean of the differences was not significantly different from zero, indicating that there was no fixed sensor bias. The B-A plots suggested that error magnitude was driven by few participants and the IBI differences were closer to zero with longer IBIs (lower exertion). A scatterplot with regression analyses contrasting the sensor pair with linear regression of IBIs provide excellent fit to the IBI data with R2 of 1.00 driven by large amount of IBIs for the model of y = 0.03+x (Figure 2B).
Figure 2. . Bland-Altman and scatter plot for inter-beat interval from the Faros 180 electrocardiogram and Polar H10.
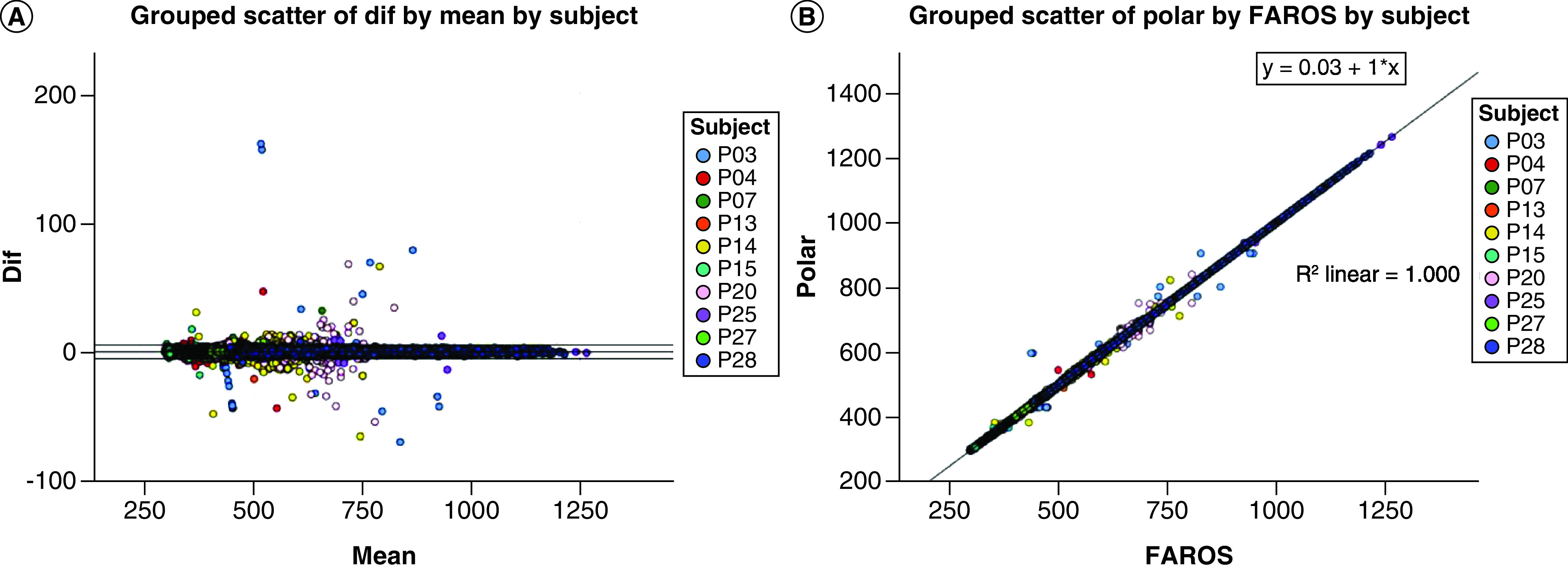
(A) Plot of the IBI differences versus the means for the Faros 180 and Polar H10. Outer lines indicate the 95% confidence interval. (B) Scatter plot of the Faros versus Polar H10 IBIs with regression and R2.
IBI: Inter-beat interval.
After HRV analyses were completed for the IBIs from both sensors, a scatterplot with regression analysis contrasting the derived HRV components from Polar H10 and Faros 180 confirmed excellent fit with R2 >0.95 (Figure 3A–C). GEE was used to demonstrate the sensitivity of both sensors regarding the change across time points in each HRV parameter (Table 2). For each HRV component (RSA, LFHRV, HP), sensor type was not a significant predictor, indicating that methods of HR recording were comparable (Figure 3D–F). STEP compared with other time points (BL, R1, R2) was a significant predictor of lower RSA, LFHRV and HP (Figure 3D–F).
Figure 3. . First row: Scatterplots between sensors for heart rate variability components, color-coded by time point with regression and R2.
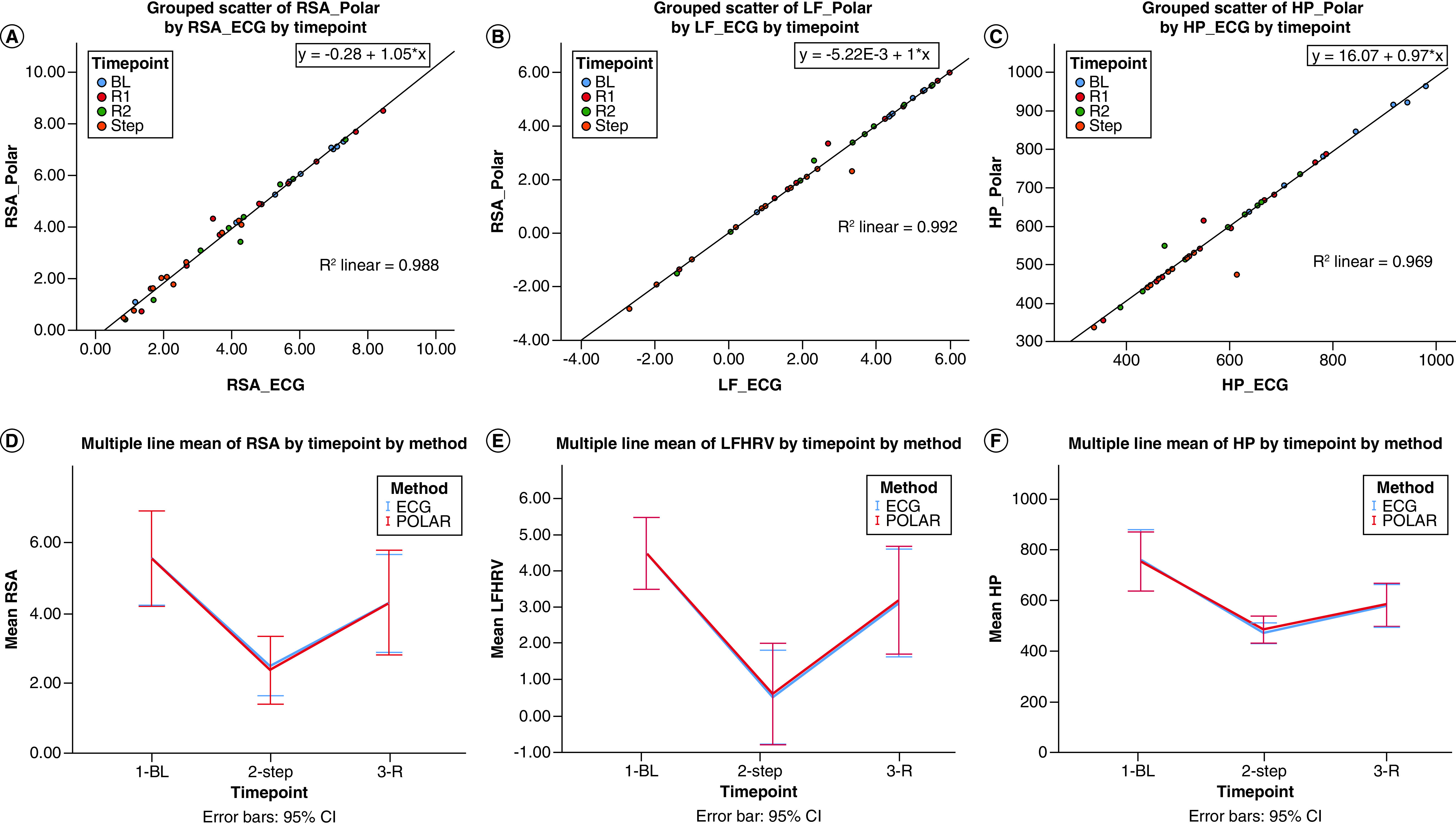
(A) RSA. (B) LF. (C) HP. D–F: Mean of HRV measures at baseline, exertion, and rest after exertion for Faros 180 electrocardiogram and Polar H10. (D) RSA. (E) LF. (F) HP.
BL: Baseline; ECG: Faros 180; HP: Heart period; HRV: Heart rate variability; LF: Low-frequency; POLAR: Polar H10; R: Recovery period after STEP; RSA: Respiratory sinus arrhythmia; STEP: Step task.
*Statistically significant at p < 0.05.
Table 2. . Generalized estimating equations analyses for the Polar H10 sensor compared with ECG and for each resting time point compared with exertion (STEP).
| HRV component | Parameter | β | SE | 95% CI | Z | Pr > |Z| | ||
|---|---|---|---|---|---|---|---|---|
| RSA | Sensor | ECG | 0.086 | 0.048 | -0.007 | 0.180 | 1.81 | 0.071 |
| POLAR | 0.000 | 0.000 | 0.000 | 0.000 | – | – | ||
| Time point | BL | 0.976 | 0.490 | 0.016 | 1.936 | 1.99 | 0.046* | |
| R1 | 2.048 | 0.428 | 1.208 | 2.887 | 4.78 | <0.0001* | ||
| R2 | 1.235 | 0.402 | 0.447 | 2.023 | 3.07 | 0.002* | ||
| STEP | 0.000 | 0.000 | 0.000 | 0.000 | – | – | ||
| LFHRV | Sensor | ECG | 0.026 | 0.025 | -0.022 | 0.074 | 1.05 | 0.295 |
| POLAR | 0.000 | 0.000 | 0.000 | 0.000 | – | – | ||
| Time point | BL | 2.355 | 0.310 | 1.748 | 2.962 | 7.61 | <0.0001* | |
| R1 | 2.347 | 0.356 | 1.649 | 3.044 | 6.59 | <0.0001* | ||
| R2 | 1.764 | 0.341 | 1.096 | 2.432 | 5.18 | <0.0001* | ||
| STEP | 0.000 | 0.000 | 0.000 | 0.000 | – | – | ||
| HP | Sensor | ECG | 1.427 | 0.748 | -0.039 | 2.892 | 1.91 | 0.063 |
| POLAR | 0.000 | 0.000 | 0.000 | 0.000 | – | – | ||
| Time point | BL | 284.089 | 36.562 | 212.430 | 355.749 | 7.77 | <0.0001* | |
| R1 | 123.924 | 28.618 | 67.835 | 180.013 | 4.33 | <0.0001* | ||
| R2 | 103.123 | 28.129 | 47.991 | 158.256 | 3.67 | 0.0002* | ||
| STEP | 0.000 | 0.000 | 0.000 | 0.000 | – | – | ||
BL: Baseline; HP: Heart period; ECG: Faros 180; LFHRV: Low frequency heart rate variability; POLAR: Polar H10; R: Recovery period following step task; RSA: Respiratory sinus arrhythmia; STEP: Step task.
*Statistically significant at p < 0.05.
Clinical & physiological feasibility
All 15 participants were able to complete both tasks as instructed without the examiner having to stop based on safety criteria. None of the participants reported symptom exacerbation during either task. HRV analysis was feasible for all of the phases (BL, ST, R1, PU, R2) based on IBI recordings from the commonly available Polar H10.
Both STEP and PU tasks evoked appropriate exertional physiological responses. All participants reached the exertional range (60–85% of age-predicted HRmax) during the 6-min step test and the 2-min push-ups. During STEP all participants reported a RPE between 12 and 16 at least once during the task. Fourteen of 15 participants reported a RPE in the exertional range (12–16) for PU.
Discussion
The development of clinically feasible, standardized exertional tasks for PCPs to administer to SMs after an acute mTBI is an important step in the treatment and management of mTBI in accordance with TBI CoE recommendations. Our two exertional tasks were ecologically valid for SMs because they build on familiar tasks and are in use to test aerobic capacity and strength [29,33]. Our exertional task protocols appear feasible, induce adequate physiological responses and can be used to characterize HR recovery with an affordable HRM.
We found the Polar H10 recordings of beat-to-beat HR data for the exertional protocol collected through Bluetooth to be accurate and reliable compared with ECG recordings during the STEP, suggesting that it is a reasonable alternative for clinical use. Hernado et al. found HRM and ECG methods to be interchangeable when analyzing HRV at rest [19], but we also found excellent reliability and agreement indices of the HRV components between sensors under exertion. The use of clinically available HRM allows for straightforward administration and may increase utility for PCP.
Both the STEP and PU were feasible to perform during a PCP appointment, requiring less than 10 min, space consistent with a standard exam room and minimal or easily accessible equipment. Besides the Polar H10 HRM, the STEP requires a 12-inch step and metronome app, while the PU only requires a hand counter. Both tasks were easily conducted by one tester and could be completed in our largely military population without stopping for safety reasons. Previous studies have tested mTBI targeted assessments in healthy individuals before completing testing in a clinical cohort [44,45].
All participants demonstrated an appropriate HR exertion range during each task, indicating that these tasks were sufficiently challenging to cause the targeted physiological stress. RPE ratings also supported the use of the tasks, with only one participant rating below 12 on the RPE scale for pushups (participant stopped pushups at 30 s). Both exertional tasks are of greater difficulty than current commonly used concussion balance assessments, which may reduce test ceiling effects [4].
Exploratory analyses showed there were significant differences in HRV for the stepping task compared with the baseline and recovery time periods, indicating that HR monitors could sufficiently detect changes in all three HRV components induced by brief exertion. Similar to previous studies, we found a decrease in RSA when under exertion, consistent with the parasympathetic withdrawal that occurs upon initiation of exercise [46,47]. We also found a decrease in the LFHRV and HP [48,49].
As with any research, this study had limitations. First, we tested exertional tasks in a healthy population, therefore, future studies need to investigate tolerance of these exertional tasks for individuals who have sustained a concussion to characterize possible HRV impairments compared with healthy controls. However, our majority military study cohort supports the feasibility of tasks and physiological response in our target population. Second, comparisons between Polar H10 and Faros 180 did not include the pushup task because of concerns with peak detection in the Faros 180 leading to overediting. This finding further supports the use of the Polar H10 because it may be more reliable at recording valid IBI data during pushups and similar exercises, as well as being commercially available. In addition, only ten participants were used in reliability calculations due to initial Bluetooth technical difficulties leading to missing data. Yet with more than 15 min of IBI data for each participant, the sample size reflects previous studies, and the total number of IBIs supports adequate power in reliability analyses [40]. Furthermore, inclusion of a commercial Bluetooth dongle resolved connectivity dropout with Polar H10 and improvement to our data platform allows for continual saving to minimize any data loss due to technical issues.
Feasibility testing in a healthy, largely military population allowed improvements to the protocol for future studies. For instance, the duration of the baseline and recovery time periods was increased from 3 to 5 min. Although 3-min recovery between tasks was sufficient for these healthy participants to return to RPE of 6, we expect that individuals with concussion may need longer to recover. Additionally, we will add measures of medicine and alcohol intake, which can also influence HRV values.
Conclusion
The implementation of standardized exertional tasks that includes an objective physiological measure may improve the standard of care for military mTBI. Monitoring symptoms, RPE, and HR during exertional tasks assesses physiological recovery and informs activity recommendations [35]. The treatment and management of concussion remains a priority for TBI CoE and the armed forces. Further research is needed to determine the utility of such measures with acute concussion in order to facilitate clinical implementation of exertional testing by PCPs.
Future perspective
Research about concussion and the ability to begin activity after 24–48 h of rest after injury is increasing. Military PCPs are in a position to offer guidance about progressive activity by considering more than self-reported symptoms if they have validated performance-based tests that are feasible in the office setting. Wearable sensors are increasingly used by civilian and military populations and may provide additional evidence for activity progression after concussion.
Summary points.
The Department of Defense Traumatic Brain Injury Center of Excellence guidelines for Primary Care Providers treating concussion recommends brief exertional testing before return to duty, yet there is currently no standardized task validated for that purpose.
Heart rate variability (HRV) is an objective measure of autonomic nervous system activity and may be useful in assessing physiological impairments after concussion.
Comparing reliability of an affordable commercially available heart rate monitor (Polar H10) to the gold standard electrocardiogram (ECG) under exertional conditions and verifying the sensitivity of HRV changes with new test protocols was a necessary step toward development of new clinical tests.
With input from military medical providers, two brief exertion tests were developed that could be easily administered in a primary care office environment requiring minimal space, equipment and time: a 6-min metronome-paced step test and a 2-min pushup test.
A sample of largely military healthy participants successfully completed both tasks, with a 3-min baseline and a 3-min rest after each task with the order counterbalanced.
Both tasks evoked the targeted physiological response of 60–85% of predicted heart rate maximum and moderate rate of perceived exertion.
The reliability of the Polar H10 was better than the ECG during the pushup task and comparable during the step task, favoring the use of the Polar H10 as an affordable and easier to use device to capture HRV.
Both the step and pushup task are brief, clinically feasible assessments that could be used in primary care practice as measures of recovery.
A standardized test incorporating HRV measurement could be used with self-report of symptoms to aid clinicians in prescribing activity and managing recovery.
Acknowledgments
JH Prim acknowledges Captain Jared Hollis for his recruiting assistance at US Marine Corps Recruiting Office, NC, USA; K Adams for assistance during data collection; A Cecchini for assistance with task development; and UNC Doctor of Physical Therapy students who assisted with pilot testing.
Footnotes
Financial & competing interests disclosure
This research was supported by the Carolina Digital Health Research Initiative (CaDHRI) and Research Electronic Data Capture (REDCap). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All testing procedures were approved by UNC Chapel Hill Institutional Review Board #17-0429. Participants provided written consent before any data collection and were free to opt out at any time during testing.
Disclaimer
The views expressed are solely those of the authors and do not reflect the official policy or position of the US Army, US Navy, US Air Force, the Department of Defense or the US government. The study protocol and data can be made available in response to written request to the corresponding author (JHP).
References
Papers of special note have been highlighted as: • of interest
- 1.Silverberg ND, Iverson GL. Is rest after concussion “the best medicine?”: recommendations for activity resumption following concussion in athletes, civilians, and military service members. J. Head Trauma Rehabil. 28(4), 250–259 (2013). [DOI] [PubMed] [Google Scholar]
- 2.McCrea M, Guskiewicz K, Randolph C et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery 65(5), 876–882 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Giza CC, Choe MC, Barlow KM. Determining if rest is best after concussion. JAMA Neurol. 75(4), 399–400 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Scherer MR, Weightman MM, Radomski MV et al. Returning service members to duty following mild traumatic brain injury: exploring the use of dual-task and multitask assessment methods. Phys. Ther. 93(9), 1254–1267 (2013). [DOI] [PubMed] [Google Scholar]
- 5.McCulloch KL, Goldman S, Lowe L et al. Development of clinical recommendations for progressive return to activity after military mild traumatic brain injury: guidance for rehabilitation providers. J. Head Trauma Rehabil. 30(1), 56–67 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Management of Concussion/mTBI Working Group. Va/dod clinical practice guideline for management of concussion/mild traumatic brain injury. J. Rehabil. Res. Dev. 46(6), CP1–CP68 (2009). [PubMed] [Google Scholar]
- 7.McCrory P, Meeuwisse W, Dvořák J et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 51(11), 838–847 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Makdissi M, Davis G, McCrory P. Updated guidelines for the management of sports-related concussion in general practice. Aust. Fam. Physician. 43(3), 94–99 (2014). [PubMed] [Google Scholar]
- 9.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery 75(Suppl. 4), S24–S33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamins J, Bigler E, Covassin T et al. What is the physiological time to recovery after concussion? A systematic review. Br. J. Sports Med. 51(12), 935–940 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Pertab JL, Merkley TL, Cramond AJ et al. Concussion and the autonomic nervous system: an introduction to the field and the results of a systematic review. Neurorehabilitation 42(4), 397–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leddy JJ, Baker JG, Willer B. Active rehabilitation of concussion and post-concussion syndrome. Phys. Med. Rehabil. Clin. N. Am. 27(2), 437–454 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Conder RL, Conder AA. Heart rate variability interventions for concussion and rehabilitation. Front. Psychol. 5, 890 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop SA, Dech RT, Guzik P, Neary JP. Heart rate variability and implication for sport concussion. Clin. Physiol. Funct. Imaging. 38(5), 733–742 (2018). [DOI] [PubMed] [Google Scholar]; • This article proports that autonomic impairments exist after concussion and that exertional tasks can magnify them.
- 15.Porges S. The polyvagal perspective. Biol. Psychol. 74(2), 116–143 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol. Psychol. 74(2), 286–294 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Len TK, Neary JP, Asmundson GJG et al. Cerebrovascular reactivity impairment after sport-induced concussion. Med. Sci. Sports Exerc. 43(12), 2241–2248 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93(5), 1043–1065 (1996). [PubMed] [Google Scholar]
- 19.Hernando D, Garatachea N, Almeida R et al. Validation of heart rate monitor polar RS800 for heart rate variability analysis during exercise. J. Strength Cond. Res. 32(3), 716–725 (2018). [DOI] [PubMed] [Google Scholar]; • This article compares reliability between a commercial heart rate monitor and ECG, finding that as heart rate gets closer to maximal, reliability decreases, presenting the need for reliability analyses during our exertional tasks.
- 20.Weippert M, Kumar M, Kreuzfeld S et al. Comparison of three mobile devices for measuring R-R intervals and heart rate variability: Polar S810i, Suunto t6 and an ambulatory ECG system. Eur. J. Appl. Physiol. 109(4), 779–786 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Tsitoglou KI, Koutedakis Y, Dinas PC. Validation of the Polar RS800CX for assessing heart rate variability during rest, moderate cycling and post-exercise recovery [version 1; peer review: 2 approved with reservations]. F1000Res 7, 1501 (2018). [Google Scholar]
- 22.Blake TA, McKay CD, Meeuwisse WH, Emery CA. The impact of concussion on cardiac autonomic function: a systematic review. Brain Inj. 30(2), 132–145 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Curr. Sports Med. Rep. 12(6), 370–376 (2013). [DOI] [PubMed] [Google Scholar]; • The article is about testing the utility of the Buffalo Concussion Treadmill Test, which was the model we used in developing our step task.
- 24.Haider MN, Johnson SL, Mannix R et al. The Buffalo Concussion Bike Test for concussion assessment in adolescents. Sports Health 11(6), 492–497 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Defense and Veterans Brain Injury Center. Progressive Return to Activity Following Acute Concussion/Mild TBI Clinical Suite. https://dvbic.dcoe.mil/material/progressive-return-activity-following-acute-concussionmild-tbi-clinical-suite ; • Current Defense and Veterans Brain Injury Center guidelines for primary care providers treating acute concussion.
- 26.Iacobucci G. GP appointments last less than five minutes for half the world's population. BMJ 359, j5172 (2017). [Google Scholar]
- 27.Konrad TR, Link CL, Shackelton RJ et al. It's about time: physicians' perceptions of time constraints in primary care medical practice in three national healthcare systems. Med. Care 48(2), 95–100 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley JP, Sim J, Eston RG et al. Reliability and validity of measures taken during the Chester Step Test to predict aerobic power and to prescribe aerobic exercise. Br. J. Sports Med. 38(2), 197–205 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sykes K, Roberts A. The Chester Step Test – a simple yet effective tool for the prediction of aerobic capacity. Physiotherapy 90(4), 183–188 (2004). [Google Scholar]
- 30.Leddy JJ, Kozlowski K, Donnelly JP et al. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin. J. Sport Med. 20(1), 21–27 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Javorka M, Zila I, Balhárek T, Javorka K. Heart rate recovery after exercise: relations to heart rate variability and complexity. Braz. J. Med. Biol. Res. 35(8), 991–1000 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Marines. Marine Physical Fitness Test & Training Requirements. https://www.marines.com/being-a-marine/life-in-the-corps/physical-fitness.html
- 33.Knapik J. The army physical fitness test (APFT): a review of the literature. Mil. Med. 154(6), 326–329 (1989). [PubMed] [Google Scholar]
- 34.Yang J, Christophi CA, Farioli A et al. Association between push-up exercise capacity and future cardiovascular events among active adult men. JAMA Netw. Open 2(2), e188341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quatman-Yates C, Bailes A, Constand S et al. Exertional tolerance assessments after mild traumatic brain injury: a systematic review. Arch. Phys. Med. Rehabil. 99(5), 994–1010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leddy JJ, Hinds AL, Miecznikowski J et al. Safety and prognostic utility of provocative exercise testing in acutely concussed adolescents: a randomized trial. Clin. J. Sport Med. 28(1), 13–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox SM, Haskell WL, Eliakim M, Neufeld HN. The exercise stress test: needs for standardization. Cardiol. Curr Topics Prog. (1970). [Google Scholar]
- 38.Sporis G, Vucetic V, Jukic I et al. How reliable are the equations for predicting maximal heart rate values in military personnel? Mil. Med. 176(3), 347–351 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Leddy JJ, Baker JG, Kozlowski K et al. Reliability of a graded exercise test for assessing recovery from concussion. Clin. J. Sport Med. 21(2), 89–94 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Davila MI, Lewis GF, Porges SW. The PhysioCam: a novel non-contact sensor to measure heart rate variability in clinical and field applications. Front Public Health 5, 300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis GF, Furman SA, McCool MF, Porges SW. Statistical strategies to quantify respiratory sinus arrhythmia: are commonly used metrics equivalent? Biol. Psychol. 89(2), 349–364 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476), 307–310 (1986). [PubMed] [Google Scholar]
- 43.Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 24(3), 69–71 (2012). [PMC free article] [PubMed] [Google Scholar]
- 44.Weightman MM, McCulloch KL, Radomski MV et al. Further development of the assessment of military multitasking performance: iterative reliability testing. PLoS One 12(1), e0169104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prim JH, Favorov OV, Cecchini AS et al. Clinical utility and analysis of the run-roll-aim task: informing return-to-duty readiness decisions in active-duty service members. Mil. Med. 184(5-6), e268–e277 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Michael S, Graham KS, Davis GM. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals – a review. Front. Physiol. 8, 301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh N, Moneghetti KJ, Christle JW et al. Heart rate variability: an old metric with new meaning in the era of using mHealth technologies for health and exercise training guidance. Part one: physiology and methods. Arrhythm. Electrophysiol. Rev. 7(3), 193–198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiraishi Y, Katsumata Y, Sadahiro T et al. Real-time analysis of the heart rate variability during incremental exercise for the detection of the ventilatory threshold. J. Am. Heart Assoc. 7(1), e006612 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Draghici AE, Taylor JA. The physiological basis and measurement of heart rate variability in humans. J. Physiol. Anthropol. 35(1), 22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


