1.
Dalton MA, McCormick C, DeLuca F, Clark IA, Maguire EA. Functional connectivity along the anterior–posterior axis of hippocampal subfields in the ageing human brain. Hippocampus. 2019;29(11):1049–1062. https://doi.org/10.1002/hipo.23097
Metcalfe J, Brezler JC, McNamara J, Maletta G, Vuorre M. Memory, stress, and the hippocampal hypothesis: Firefighters’ recollections of the fireground. Hippocampus. 2019;29(12):1141–1149. https://doi.org/10.1002/hipo.23128
Sugar J, Moser M‐B. Episodic memory: Neuronal codes for what, where, and when. Hippocampus. 2019;29(12):1190–1205. https://doi.org/10.1002/hipo.23132
Phillmore LS, Klein RM. The puzzle of spontaneous alternation and inhibition of return: How they might fit together. Hippocampus. 2019;29(8):762–770. https://doi.org/10.1002/hipo.23102
Nilssen ES, Doan TP, Nigro MJ, Ohara S, Witter MP. Neurons and networks in the entorhinal cortex: A reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways. Hippocampus. 2019;29(12):1238–1254. https://doi.org/10.1002/hipo.23145
Migues PV, Wong J, Lyu J, Hardt O. NMDA receptor activity bidirectionally controls active decay of long‐term spatial memory in the dorsal hippocampus. Hippocampus. 2019;29(9):883–888. https://doi.org/10.1002/hipo.23096
The articles listed above, intended for publication in Hippocampus volume 30, number 8, were erroneously published in Hippocampus, volume 29, number 8, 9, 11, or 12. The articles will remain as part of their prior issues, but appear in full on the following pages. These articles should be cited as listed above. The Publisher apologizes for this error.
2. Functional connectivity along the anterior–posterior axis of hippocampal subfields in the ageing human brain
Marshall A. Dalton Cornelia McCormick Flavia De Luca Ian A. Clark Eleanor A. Maguire
e.maguire@ucl.ac.uk
Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology
University College London
London
UK
ageing
functional connectivity
hippocampal subfields
perirhinal cortex
subiculum
tau
Wellcome
203147/Z/16/Z
210567/Z/18/Z
Table S1 Structural MRI volumes (mm3).
Table S2. Functional MRI voxels.
Abstract
While age‐related volumetric changes in human hippocampal subfields have been reported, little is known about patterns of subfield functional connectivity (FC) in the context of healthy ageing. Here we investigated age‐related changes in patterns of FC down the anterior–posterior axis of each subfield. Using high resolution structural MRI we delineated the dentate gyrus (DG), CA fields (including separating DG from CA3), the subiculum, pre/parasubiculum, and the uncus in healthy young and older adults. We then used high resolution resting state functional MRI to measure FC in each group and to directly compare them. We first examined the FC of each subfield in its entirety, in terms of FC with other subfields and with neighboring cortical regions, namely, entorhinal, perirhinal, posterior parahippocampal, and retrosplenial cortices. Next, we analyzed subfield to subfield FC within different portions along the hippocampal anterior–posterior axis, and FC of each subfield portion with the neighboring cortical regions of interest. In general, the FC of the older adults was similar to that observed in the younger adults. We found that, as in the young group, the older group displayed intrinsic FC between the subfields that aligned with the tri‐synaptic circuit but also extended beyond it, and that FC between the subfields and neighboring cortical areas differed markedly along the anterior–posterior axis of each subfield. We observed only one significant difference between the young and older groups. Compared to the young group, the older participants had significantly reduced FC between the anterior CA1‐subiculum transition region and the transentorhinal cortex, two brain regions known to be disproportionately affected during the early stages of age‐related tau accumulation. Overall, these results contribute to ongoing efforts to characterize human hippocampal subfield connectivity, with implications for understanding hippocampal function and its modulation in the ageing brain.
Authors Cornelia McCormick and Flavia De Luca made equal contributions.
Funding information Wellcome, Grant/Award Numbers: 203147/Z/16/Z, 210567/Z/18/Z
Data Availability: Requests for the data can be sent to e.maguire@ucl.ac.uk.
Abbreviations
A, anterior (of the hippocampus)
AB, anterior body (of the hippocampus)
CA 1–4, cornu ammonis 1–4
DG, dentate gyrus
ENT, entorhinal cortex
FC, functional connectivity
PB, posterior body (of the hippocampus)
PHC, posterior parahippocampal cortex
PRC, perirhinal cortex
RSC, retrosplenial cortex
rsfMRI, resting state functional magnetic resonance imaging
T, tail (of the hippocampus)
3. INTRODUCTION
Lynn Nadel has had an immense influence on cognitive and memory neuroscience as is clearly evident in this special issue. His work, not only in the realm of spatial representations (O'Keefe & Nadel, 1978), but also autobiographical memory (Ryan et al., 2001), memory consolidation (Nadel & Moscovitch, 1997) and sleep (Payne & Nadel, 2004), has had a wide reach, including being influential on this article's senior author. Indeed, his 1991 article in Hippocampus (Nadel, 1991) appeared at the start of her PhD and was instrumental in directing her to ideas about cognitive maps and to a career seeking an understanding of hippocampal function. Given Nadel's unwavering curiosity coupled with an enviable knowledge of the literature, his prowess as a theoretician and his mentorship that so many of us have enjoyed, his high standing in the field is justly deserved.
Another feature of Nadel's work is its prescience. Many key ideas and concepts which went on to prove important in the field are contained in his classic book with John O'Keefe (O'Keefe & Nadel, 1978). One in particular is the focus of the current study and, in fact, was held by Nadel to be of such relevance for understanding the hippocampus that it was the subject of his PhD—Behavioral effects of dorsal and ventral hippocampal lesions in the rat (Nadel, 1967; see also Nadel, 1968). Nadel astutely realised (see also Kimura, 1958; Nauta, 1956) that the dorsal (posterior in humans) and ventral (anterior in humans) hippocampus likely facilitate different functions. At that point he was unable to derive a full explanation for this disparity.
In the five decades since his PhD, many others have gone on to note this anterior–posterior distinction adding further to the picture, including that the dorsal hippocampus in rats is more associated with spatial processing compared to the ventral (Moser & Moser, 1998), that place fields in the dorsal hippocampus of rats are smaller than those in the ventral hippocampus (Kjelstrup et al., 2008), that the posterior hippocampus in London taxi drivers is enlarged while the anterior hippocampus is decreased in volume (Maguire et al., 2000), and that the anterior human hippocampus seems to be heavily involved in constructing scene imagery (Zeidman & Maguire, 2016). Despite these insights, however, we still lack a clear understanding of why there is this anterior–posterior distinction in hippocampal function. This is likely due in no small part to the issue being more complex than merely a categorical difference. This becomes clear when considering hippocampal anatomy.
The primary input to the hippocampus is via the entorhinal cortex (ENT), the source of the canonical tri‐synaptic pathway. The ENT primarily innervates the dentate gyrus (DG) and, from here, intrahippocampal connectivity is generally acknowledged to follow a unidirectional pathway through the CA regions to the subiculum, the primary region of efferent projection from the hippocampus (Aggleton & Christiansen, 2015; Duvernoy, Cattin, & Risold, 2013). While this canonical circuitry is not in question, noncanonical feedback connections from CA3 to DG, and from subiculum to CA1, have been noted in rodents (Sik, Ylinen, Penttonen, & Buzsaki, 1994; Xu, Sun, Holmes, & López, 2016). Anatomical evidence from nonhuman primates has also shown that extra‐hippocampal regions including the ENT, perirhinal (PRC), posterior parahippocampal (PHC), and retrosplenial (RSC) cortices interact directly with specific hippocampal subfields, bypassing the canonical hippocampal pathway (Aggleton, 2012; Agster & Burwell, 2013; Kobayashi & Amaral, 2007; Leonard, Amaral, Squire, & Zola‐Morgan, 1995; Witter & Amaral, 1991). Moreover, tract tracing studies in nonhuman primates have revealed intrasubfield gradients of connectivity along the anterior–posterior axis of the hippocampus (Insausti & Muñoz, 2001). This suggests that different portions of hippocampal subfields may preferentially interact with other brain regions. This resonates with the known gradual genetic, anatomical, and functional differentiations along the long axis of the hippocampus that have also emerged over recent decades (see Fanselow & Dong, 2010; Poppenk, Evensmoen, Moscovitch, & Nadel, 2013; Strange, Witter, Lein, & Moser, 2014 for reviews).
Until recently, in vivo examination of the connectivity between different subfields, and different portions of subfields, in humans has been beyond the scope of direct scrutiny. However, high resolution magnetic resonance imaging (MRI) now makes these investigations tractable. Specifically, we have the spatial resolution to delineate individual subfields (Dalton, Zeidman, Barry, Williams, & Maguire, 2017; Yushkevich et al., 2015) in order to assess their functions and connectivity, although their connectivity has received much less attention, despite likely being of significant importance in driving anterior–posterior hippocampal differences.
One way to examine subfield connectivity is to characterize patterns of functional connectivity (FC) using resting state functional MRI (rsfMRI). While rsfMRI FC often reflects anatomical pathways, its statistical dependencies are not limited to the underlying anatomy (Honey et al., 2009; Honey, Thivierge, & Sporns, 2010). Thus, rsfMRI FC has the additional benefit of reflecting potential functional relationships between brain regions. In a recent study we used high resolution rsfMRI to interrogate FC in healthy young adults (Dalton, McCormick, & Maguire, 2019). We first analyzed the FC of each hippocampal subfield in its entirety, in terms of FC with other subfields and with neighboring regions, namely ENT, PRC, PHC, and RSC. We also analyzed FC for different portions of each hippocampal subfield along its anterior–posterior axis, in terms of FC between different parts of a subfield, FC with other subfield portions, and FC of each subfield portion with the neighboring cortical regions of interest (ROI). We found that intrinsic FC between the subfields aligned generally with the tri‐synaptic circuit but also extended beyond it. Our findings also revealed that patterns of FC between the subfields and neighboring cortical areas differed markedly along the anterior–posterior axis of each hippocampal subfield.
While these patterns were characterized in healthy young adults, it is widely acknowledged that there are changes in hippocampal structure and function during healthy ageing. Given the ageing population of the western world, understanding the course and correlates of hippocampal ageing assumes increasing significance. To date, the majority of studies that have investigated human hippocampal subfields in the context of healthy ageing have utilized structural imaging and volumetric analysis techniques. Taken together, these studies consistently show age‐related volume reductions in the subiculum (Chetelat et al., 2008; La Joie et al., 2010; Wang et al., 2003; Yang, Goh, Chen, & Qiu, 2013; Ziegler et al., 2012) and CA1 (de Flores et al., 2015; Frisoni et al., 2008; Mueller et al., 2007) although volume reductions have also been noted in other subfields (Pereira et al., 2014). This is interesting in light of post mortem examinations that showed the subiculum and CA1 were the first hippocampal subfields to be affected by age‐related processes (Lace et al., 2009) and neuron loss (Simic, Kostovic, Winblad, & Bogdanovic, 1997; West, Coleman, Flood, & Troncoso, 1994). Of particular note is that, while normally associated with forms of dementia such as Alzheimer's disease, tau protein accumulation is commonly observed in examinations of post mortem brain tissue from individuals who were clinically healthy at death (Davis, Schmitt, Wekstein, & Markesbery, 1999; Knopman et al., 2003). These lines of evidence suggest that the subiculum and CA1 may be particularly vulnerable to age‐related changes even in those who are cognitively healthy.
While some studies have used task‐based fMRI to investigate age‐related differences in hippocampal subfield function (Maass et al., 2014; Suthana et al., 2010; Yassa et al., 2010), recent studies have successfully utilized rsfMRI to examine FC. However, most rsfMRI investigations of age‐related changes in hippocampal FC used seed regions that were not specific to hippocampal subfields. Rather, some utilized larger seed regions that incorporated multiple subfields within a single ROI (Das et al., 2013) or smaller seed regions that likely encompassed portions of different subfields, or were unclear as to whether they were restricted or not to a specific subfield (Damoiseaux, Viviano, Yuan, & Raz, 2016). Only a few ageing studies have used hippocampal subfields as seed regions in FC analyses (Bai et al., 2011; de Flores et al., 2017; Wang et al., 2015). In most cases, the focus was on disease‐related changes in hippocampal FC. To the best of our knowledge, no study has systematically investigated differences in FC along the anterior–posterior axis of hippocampal subfields in the context of healthy ageing.
The aim of the current study was to conduct such an investigation. Taking into consideration the results of previous investigations of age effects on subfield volume and hippocampal pathology noted above, we predicted that, compared to a group of healthy young adults, healthy older participants would show reduced patterns of rsfMRI FC involving the subiculum and also CA1.
4. MATERIALS AND METHODS
4.1. Participants
Fifteen young and fifteen older right handed participants took part in the study (young: 6 females, mean age 23.8 years, SD 3.1; older: 6 females, mean age 69.6 years, SD 4.3). We defined individuals as “older” in this study if they were aged 65 years or above, given that this is the age at which a person can claim the state pension on retirement in the UK. All gave written informed consent to participate in accordance with the University College London research ethics committee. Note that the young adult participants were a completely separate group to that reported by Dalton et al. (2019). The participants were free from any significant health issues and were not taking any medication. They completed the matrix reasoning subtest of the Wechsler Adult Intelligence Scale (WAIS‐IV; Wechsler, 2008) as a measure of general intellectual ability and the Beck Depression Inventory (BDI‐II; Beck, Steer, & Brown, 1996) in order to screen for depression. Results of independent samples t‐tests showed that there were no significant differences between the two participant groups on either measure (matrix reasoning t[28] = 1.115, p = .274; BDI t[28] = .734, p = .469). We also conducted analyses to examine whether there were any group differences in grey matter volume in any of our ROIs. Analyses (in mm3) adjusted for intracranial volume revealed no statistically significant group differences in the volume of any whole subfield, portion of a subfield along the anterior–posterior axis or extra‐hippocampal cortical ROI. The young and older adults were, therefore, well matched. Two subfield ROIs did, however, come close to reaching significance—anterior CA1 (t[28] = 1.948, p = .057) and the whole uncus (t[28] = 1.809, p = .081), with reduced volume in the older participant group. We return to this point in Section 4.
4.2. Data acquisition and preprocessing
Structural and functional MRI data were acquired using a 3T Siemens Trio scanner (Siemens, Erlangen, Germany) with a 32‐channel head coil within a partial volume centered on the temporal lobe that included the entire extent of the temporal lobes and our other ROIs.
Structural images were collected using a single‐slab 3D T2‐weighted turbo spin echo sequence with variable flip angles (SPACE; Mugler 3rd. et al., 2000) in combination with parallel imaging, to simultaneously achieve a high image resolution of ∼500 μm, high sampling efficiency and short scan time while maintaining a sufficient signal‐to‐noise ratio (SNR). After excitation of a single axial slab the image was read out with the following parameters: resolution = 0.52 × 0.52 × 0.5 mm3, matrix = 384 × 328, partitions = 104, partition thickness = 0.5 mm, partition oversampling = 15.4%, field of view = 200 × 171 mm2, TE = 353 ms, TR = 3,200 ms, GRAPPA x 2 in phase‐encoding (PE) direction, bandwidth = 434 Hz/pixel, echo spacing = 4.98 ms, turbo factor in PE direction = 177, echo train duration = 881, averages = 1.9, plane of acquisition = sagittal. For reduction of signal bias due to, for example, spatial variation in coil sensitivity profiles, the images were normalized using a prescan, and a weak intensity filter was applied as implemented by the scanner's manufacturer. Each scan lasted 12 min. To improve the SNR of the anatomical image, three scans were acquired for each participant, coregistered and averaged. Each structural scan was visually inspected for quality. Where scan quality was compromised due to movement artifacts, it was discarded. We considered participants with two high quality structural scans a minimum requirement for inclusion in the study. Additionally, a whole brain 3D FLASH structural scan was acquired with a resolution of 1 × 1 × 1 mm.
Functional data were acquired using a 3D echo planar imaging (EPI) sequence which has been demonstrated to yield improved BOLD sensitivity compared to 2D EPI acquisitions (Lutti, Thomas, Hutton, & Weiskopf, 2013). Image resolution was 1.5 × 1.5 × 1.5 mm3 and the field‐of‐view was 192 mm2 in‐plane. Forty slices were acquired with 20% oversampling to avoid wrap‐around artifacts due to the imperfect slab excitation profile. The echo time (TE) was 37.30 ms and the volume repetition time (TR) was 3.65 s. Parallel imaging with GRAPPA image reconstruction (Griswold et al., 2002) acceleration factor 2 along the phase‐encoding direction was used to minimize image distortions and yield optimal BOLD sensitivity. The dummy volumes necessary to reach steady state and the GRAPPA reconstruction kernel were acquired prior to the acquisition of the image data as described in Lutti et al. (2013). Correction of the distortions in the EPI images was implemented using B0‐field maps obtained from double‐echo FLASH acquisitions (matrix size 64 × 64; 64 slices; spatial resolution 3 × 3 × 3 mm3; short TE = 10 ms; long TE = 12.46 ms; TR = 1,020 ms) and processed using the FieldMap toolbox in SPM (Hutton et al., 2002). Two hundred and five volumes were acquired with the scan lasting just under 13 min.
Preprocessing of structural and rsfMRI data was conducted using SPM12 (www.fil.ion.ac.uk/spm). All images were first bias‐corrected, to compensate for image inhomogeneity associated with the 32 channel head coil (van Leemput, Maes, Vandermeulen, & Suetens, 1999). Fieldmaps were collected and used to generate voxel displacement maps. EPIs were then realigned to the first image and unwrapped using the voxel displacement maps calculated above. The two/three high‐resolution structural images were averaged to reduce noise, and co‐registered to the whole brain structural FLASH scan. EPIs were also co‐registered to the whole brain structural scan. In order to keep the EPI signal within each hippocampal subfield mask as pure as possible no spatial smoothing was applied for these analyses.
4.3. Segmentation of hippocampal subfields
For each participant, we first manually delineated hippocampal subfields, bilaterally, on native space high resolution structural images according to the methodology described by Dalton et al. (2017) using the ITK Snap software version 3.2.0 (Yushkevich et al., 2006). Masks were created for the following subregions: DG/CA4, CA3/2, CA1, subiculum, pre/parasubiculum, and uncus (Figure 1a). Subfield segmentations were conducted by three researchers (M.A.D., C.M., and F.D.L.). To assess inter‐rater reliability, each researcher independently segmented the hippocampi of the same five participants and analyses for each subfield were conducted using the Dice overlap metric (Dice, 1945) to produce a score between 0 (no overlap) and 1 (perfect overlap). Inter‐rater reliability was 0.84 for DG/CA4, 0.67 for CA3/2, 0.76 for CA1, 0.75 for subiculum, 0.69 for pre/parasubiculum and 0.82 for the uncus. These values are equivalent to those reported in the extant literature (e.g., Bonnici et al., 2012; Palombo et al., 2013). Following this, to allow investigation of FC for different portions of each subfield along the longitudinal axis of the hippocampus, we divided each subfield either into 4 (for CA1, subiculum and pre/parasubiculum), into 3 (for DG/CA4 and CA3/2) or into 2 (for the uncus) separate sections along its longitudinal axis (anterior (A), anterior body (AB), posterior body (PB), and tail (T); Figure 1b) according to the methodology described by Dalton et al. (2019).
Figure 1.
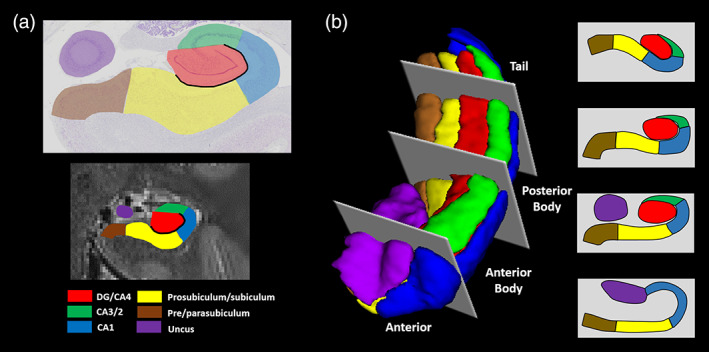
Subregions of the human hippocampus. (a) Top panel: a section of postmortem human hippocampus stained with cresyl violet to visualise cell bodies and overlaid with hippocampal subregion masks. Bottom panel: a T2‐weighted structural MRI scan of the human hippocampus overlaid with hippocampal subregion masks. (b) Left panel: a 3D model of hippocampal subregion masks with representative examples of demarcation points for anterior, anterior body, posterior body and tail portions of the subfields. Right panel: schematic representation of the subfields present in each portion of the hippocampus [Color figure can be viewed at wileyonlinelibrary.com]
To summarize, the often‐used method of using the final slice of the uncus as a demarcation point for anterior and posterior hippocampus (Zeidman, Lutti, et al., 2015; Poppenk et al., 2013), while anatomically useful, may be problematic from a functional perspective. We have consistently observed a functional cluster in the medial hippocampus which extends across this demarcation point in tasks relating to scene‐based cognition (Dalton, Zeidman, McCormick, & Maguire, 2018; Zeidman, Lutti, et al., 2015; Zeidman, Mullally, et al., 2015; Zeidman & Maguire, 2016). Hence, we believe that this portion of the hippocampus may represent a functional module which, when utilizing the uncus‐based anatomical demarcation point, would potentially be split between two separate ROIs. We, therefore, developed a method which allowed us to sample broad portions of each subfield while ensuring this region was kept intact. For the A masks, the anterior boundary was the first slice of the hippocampus and the posterior boundary was the slice preceding the first slice of the DG. This resulted in a mean of 14.4 (SD 3.1) slices in the A mask for the older participants and 15.9 (SD 3.3) slices for the younger participants. The T mask encompassed the posterior most 15 slices of the hippocampus. We had initially planned to use the crus of the fornix as the anterior demarcation for the T masks but found that, due to individual variability in hippocampal morphology and flexure of the posterior hippocampus, this resulted in some participants having very few slices within the T mask. In order to ensure that the T mask contained an equivalent number of slices across participants we set the anterior most slice of the posterior portion to 15 slices anterior to and including the final slice of the hippocampus. The remaining slices were summed and split in half to create the AB and PB masks. This resulted in a mean of 24.1 (SD 3.2) and 23.1 (SD 1.9) slices in the AB for older and younger participants respectively, and a mean of 23.7 (SD 3.2) and 22.4 (SD 2.1) slices in the PB for older and younger participants, respectively. Importantly, results of independent samples t‐tests showed that there were no significant differences between the two participant groups in the number of slices in the A (t[28] = 1.295, p = .206), AB (t[28] = 1.011, p = .321), or PB (t[28] = 1.324, p = .196) portions of the hippocampus. Structural volumes (in mm3) for each hippocampal subfield portion for each participant group are provided in Supporting Information Table S1.
4.4. Segmentation of extra‐hippocampal ROIs
The ENT, PRC, and PHC were segmented using the guidelines laid out by Augustinack et al. (2013), Fischl et al. (2009) and Berron et al. (2017). The anterior portions of ENT and PRC were generally prone to signal dropout on the fMRI scans. We, therefore, only included posterior portions of these subfields in our analyses. To segment the RSC, we used the cytological investigation of the human RSC by Vogt, Vogt, Perl, and Hof (2001) and the Allen Brain Atlas http://atlas.brain-map.org to gain insights into the likely location of the RSC in the human brain. Of note, this mask only encompassed the thin strip of RSC lying posterior to the corpus callosum and did not include the posterior cingulate cortex, which is commonly conflated with the RSC in neuroimaging investigations. Only ventral portions of the RSC were included owing to the partial volume.
4.5. Data analysis
All analyses were performed using the CONN toolbox version 14 for rsfMRI (http://www.nitrc.org/projects/conn). The data were temporally bandpass filtered (0.01–0.1 Hz) and corrected for white matter and ventricular signal. To create FC matrices, time series of voxels within each of the ROIs were averaged and correlated with the averaged time series of all other ROIs resulting in correlation coefficients which were then transformed using Fisher's z calculation. Rather than using simple bivariate correlations, we used semi‐partial correlations which allowed us to identify the “unique” contribution of a given source on a target area. Of note, semi‐partial correlations are computed between unmodified and other residualised variables, essentially regressing out or controlling contributions of additional variables, including the activity in all other ROIs in the analysis. Therefore, for each seed analysis in turn, slightly different values were regressed out, resulting in test statistics that vary marginally in their magnitude. That is, the semi‐partial correlations between source Region A and target Region B might be slightly different from the semi‐partial correlation between source Region B and target Region A. The resulting semi‐partial ROI‐to‐ROI correlation matrices from the native space first‐level analyses were further averaged at the second level in order to examine group effects. Importantly, this ROI‐to‐ROI approach allowed us to test hypotheses regarding FC between each ROI and all other ROIs using minimally preprocessed data (i.e., unsmoothed and not normalized). This approach minimized the mixing of BOLD signal between adjacent subfields. For all analyses, ROI‐to‐ROI results were corrected for multiple comparisons and reported when significant at a level of p < .05 false discovery rate (FDR) corrected (Chumbley, Worsley, Flandin, & Friston, 2010). The mean number of functional voxels for each hippocampal subfield portion for each participant group is provided in Supporting Information Table S2.
Note that in all cases analyses were based on bilateral masks. We did not investigate laterality differences in the current study as we did not have specific predictions regarding age‐related changes in left/right hippocampal subfield function in this task‐free FC analysis. This would be interesting to examine in the context of future task‐based FC studies.
5. RESULTS
5.1. Whole subfield rsfMRI analyses
We first analyzed the FC of each hippocampal subfield in its entirety in terms of FC with other subfields and with the cortical ROIs using 10 bilateral ROIs (DG/CA4, CA3/2, CA1, subiculum, pre/parasubiculum, uncus, ENT, PRC, PHC, and RSC). We initially examined each group (young and older) separately, and then conducted direct between‐group comparisons to investigate age‐related differences in FC. The results of these whole subfield analyses are summarized in Figure 2 and Tables 1 and 2, which also include the statistically significant results of the analyses.
Figure 2.
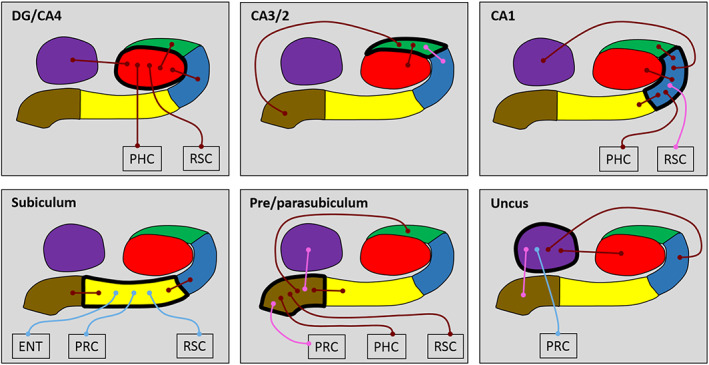
Results of the whole subfield analyses for the young and older participant groups. The relevant subfield in each panel is outlined in a thick black line. The thin lines with circular termini represent significant correlations of activity with the activity in other hippocampal subfields and/or extra‐hippocampal ROIs. Dark red lines represent significant correlations common to both young and old groups. Light blue lines represent significant correlations present only in the young group. Pink lines represent significant correlations present only in the older group. DG/CA4 (red), CA3/2 (green), CA1 (blue), subiculum (yellow), pre/parasubiculum (brown), uncus (purple); ENT, entorhinal cortex; PHC, posterior parahippocampal cortex; PRC, perirhinal cortex; RSC, retrosplenial cortex [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Statistically significant results of the whole subfield analyses: young participants
| Seed ROI | Significant target ROIs | T—statistic t(28) | p—FDR corrected |
|---|---|---|---|
| DG/CA4 | CA3/2 | 9.01 | <.0001 |
| CA1 | 11.12 | <.0001 | |
| Uncus | 4.74 | <.0001 | |
| Perirhinal cortex | −4.03 | .0122 | |
| Parahippocampal cortex | 3.46 | .0306 | |
| Retrosplenial cortex | 2.24 | .0499 | |
| CA3/2 | DG/CA4 | 8.81 | <.0001 |
| Pre/parasubiculum | 3.88 | .0017 | |
| Uncus | −5.57 | <.0001 | |
| CA1 | DG/CA4 | 10.31 | <.0001 |
| CA3/2 | 2.24 | .0493 | |
| Subiculum | 8.42 | <.0001 | |
| Pre/parasubiculum | −2.59 | .0273 | |
| Uncus | 3.41 | .0045 | |
| Parahippocampal cortex | 3.41 | .0045 | |
| Subiculum | CA1 | 8.21 | <.0001 |
| Pre/parasubiculum | 7.16 | <.0001 | |
| Entorhinal cortex | 2.54 | .0380 | |
| Perirhinal cortex | 4.58 | .0003 | |
| Retrosplenial cortex | 2.33 | .0489 | |
| Pre/parasubiculum | CA3/2 | 3.95 | .0011 |
| CA1 | −2.61 | .0257 | |
| Subiculum | 7.05 | <.0001 | |
| Parahippocampal cortex | 7.50 | <.0001 | |
| Retrosplenial cortex | 4.75 | .0002 | |
| Uncus | DG/CA4 | 4.77 | .0002 |
| CA3/2 | −5.65 | <.0001 | |
| CA1 | 3.25 | .0068 | |
| Entorhinal cortex | −3.71 | .0027 | |
| Perirhinal cortex | 2.98 | .0106 |
Negative correlations are shown in italics.
Table 2.
Statistically significant results of the whole subfield analyses: older participants
| Seed ROI | Significant target ROIs | T—statistic t(28) | p—FDR corrected |
|---|---|---|---|
| DG/CA4 | CA3/2 | 9.88 | <.0001 |
| CA1 | 8.37 | <.0001 | |
| Uncus | 3.73 | .0019 | |
| Perirhinal cortex | −3.53 | .0026 | |
| Parahippocampal cortex | 4.06 | .0011 | |
| Retrosplenial cortex | 2.36 | .0380 | |
| CA3/2 | DG/CA4 | 9.95 | <.0001 |
| CA1 | 3.03 | .0117 | |
| Pre/parasubiculum | 3.29 | .0080 | |
| Uncus | −4.31 | .0008 | |
| CA1 | DG/CA4 | 7.75 | <.0001 |
| CA3/2 | 2.89 | .0109 | |
| Subiculum | 8.49 | <.0001 | |
| Pre/parasubiculum | −5.01 | <.0001 | |
| Uncus | 6.03 | <.0001 | |
| Parahippocampal cortex | 4.37 | .0003 | |
| Retrosplenial cortex | 2.22 | .0449 | |
| Subiculum | CA1 | 8.59 | <.0001 |
| Pre/parasubiculum | 7.68 | <.0001 | |
| Pre/parasubiculum | CA3/2 | 3.42 | .0035 |
| CA1 | −4.96 | <.0001 | |
| Subiculum | 7.31 | <.0001 | |
| Uncus | 2.58 | .0232 | |
| Perirhinal cortex | 2.31 | .0367 | |
| Parahippocampal cortex | 5.56 | <.0001 | |
| Retrosplenial cortex | 4.92 | <.0001 | |
| Uncus | DG/CA4 | 3.56 | .0040 |
| CA3/2 | −4.27 | .0009 | |
| CA1 | 5.49 | <.0001 | |
| Pre/parasubiculum | 2.47 | .0450 |
Negative correlations are shown in italics.
In young participants, DG/CA4 was significantly correlated with CA3/2, CA1, uncus, PHC and RSC. This pattern was identical in the older participants.
In young participants, CA3/2 was correlated with DG/CA4 and the pre/parasubiculum. This pattern was consistent in the older participants with the addition of a correlation with CA1.
In young participants, CA1 was correlated with DG/CA4, CA3/2, subiculum, uncus, and PHC. This pattern was consistent in the older participants with the addition of a correlation with RSC.
In young participants, subiculum was correlated with CA1, pre/parasubiculum, ENT, PRC, and RSC. While intrahippocampal correlations were consistent in the older participants, correlations with extra‐hippocampal ROI's were markedly different to those observed in young participants with no correlation between subiculum and ENT, PRC or RSC in the older group.
In young participants, pre/parasubiculum was correlated with the CA3/2, subiculum, PHC, and RSC. This pattern was consistent in the older participants with the addition of a correlation with the uncus and PRC.
In young participants, the uncus was correlated with DG/CA4, CA1, and PRC. This pattern was consistent in the older participants with the exception of the correlation with PRC and the addition of a correlation with pre/parasubiculum.
Direct between‐group analyses revealed no significant differences in patterns of FC between young and older participants for any whole subfield or cortical ROI.
These whole subfield results suggest that each hippocampal subfield had a unique pattern of FC with other hippocampal subfields and cortical ROIs. These patterns largely align with our previous report in a separate group of young adult participants (Dalton et al., 2019). Notably, patterns of FC did not differ significantly between the young and older participant groups, although there was a suggestion of less FC between the subiculum and the cortical ROIs in the older participants, which we explored next with more fine‐grained analyses.
5.2. Longitudinal axis rsfMRI analyses
We next analyzed subfield to subfield FC within different portions of the hippocampus along its anterior–posterior axis, and FC of each subfield portion with the cortical ROIs. We examined this first in the young and older participant groups separately, and then conducted direct between‐group comparisons to investigate age‐related differences in FC. To do this, we performed separate analyses for each portion of the hippocampus: A (8 bilateral ROIs; A CA1, A subiculum, A pre/parasubiculum, A uncus, ENT, PRC, PHC, RSC), AB (10 bilateral ROI's; AB DG/CA4, AB CA3/2, AB CA1, AB subiculum, AB pre/parasubiculum, AB uncus, ENT, PRC, PHC, RSC), PB (9 bilateral ROIs; PB DG/CA4, PB CA3/2, PB CA1, PB subiculum, PB pre/parasubiculum, ENT, PRC, PHC, RSC) and T (9 bilateral ROIs: T DG/CA4, T CA3/2, T CA1, T subiculum, T pre/parasubiculum, ENT, PRC, PHC, RSC). The results are summarized in Figure 3 and Tables 3 and 4, which also includes the statistically significant results of the analyses.
Figure 3.
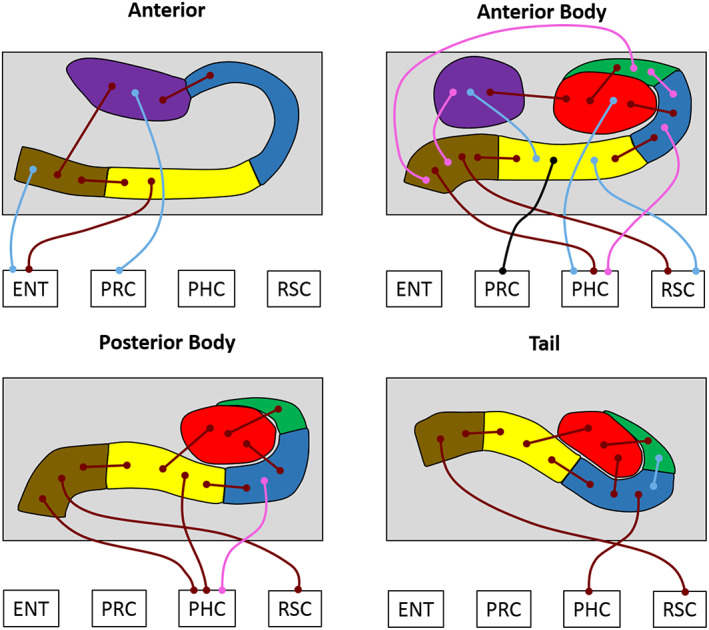
Results of the longitudinal subfield analyses for the young and older participant groups. The thin lines with circular termini represent significant correlations of activity with the activity in other hippocampal subfields and/or extra‐hippocampal ROIs. Dark red lines represent significant correlations common to both young and old groups. Light blue lines represent significant correlations present only in the young group. Pink lines represent significant correlations present only in the older group. The black line represents a significant increase in FC for young compared to older participants. DG/CA4 (red), CA3/2 (green), CA1 (blue), subiculum (yellow), pre/parasubiculum (brown), uncus (purple); ENT, entorhinal cortex; PHC, posterior parahippocampal cortex; PRC, perirhinal cortex; RSC, retrosplenial cortex [Color figure can be viewed at wileyonlinelibrary.com]
Table 3.
Statistically significant results of the longitudinal axis subfield analyses: young participants
| Seed ROI | Significant target ROIs | T—statistic t(28) | p—FDR corrected |
|---|---|---|---|
| Anterior | |||
| CA1 | Uncus | 4.54 | .0007 |
| Pre/parasubiculum | −3.42 | .0068 | |
| Subiculum | Pre/parasubiculum | 7.36 | <.0001 |
| Entorhinal cortex | 3.33 | .0085 | |
| Pre/parasubiculum | CA1 | −3.45 | .0031 |
| Subiculum | 7.05 | <.0001 | |
| Uncus | 3.92 | .0018 | |
| Entorhinal cortex | 3.60 | .0029 | |
| Uncus | CA1 | 4.77 | .0004 |
| Pre/parasubiculum | 4.17 | .0009 | |
| Entorhinal cortex | −3.92 | .0012 | |
| Perirhinal cortex | 3.59 | .0022 | |
| Anterior body | |||
| DG/CA4 | CA3/2 | 5.26 | <.0001 |
| CA1 | 13.49 | <.0001 | |
| Uncus | 8.38 | <.0001 | |
| Parahippocampal cortex | 3.98 | .0010 | |
| CA3/2 | DG/CA4 | 4.62 | .0007 |
| CA1 | DG/CA4 | 10.94 | <.0001 |
| Subiculum | 4.44 | .0006 | |
| Subiculum | CA1 | 4.25 | .0006 |
| Pre/parasubiculum | 5.69 | <.0001 | |
| Uncus | 3.73 | .0019 | |
| Perirhinal cortex | 5.49 | <.0001 | |
| Retrosplenial cortex | 3.56 | .0024 | |
| Pre/parasubiculum | Subiculum | 5.46 | <.0001 |
| Parahippocampal cortex | 7.78 | <.0001 | |
| Retrosplenial cortex | 3.92 | .0016 | |
| Uncus | DG/CA4 | 8.31 | <.0001 |
| Subiculum | 3.65 | .0048 | |
| Posterior body | |||
| DG/CA4 | CA3/2 | 8.69 | <.0001 |
| CA1 | 8.85 | <.0001 | |
| Subiculum | 6.84 | <.0001 | |
| CA3/2 | DG/CA4 | 8.42 | <.0001 |
| CA1 | DG/CA4 | 9.23 | <.0001 |
| Subiculum | 10.21 | <.0001 | |
| Pre/parasubiculum | −5.73 | <.0001 | |
| Subiculum | DG/CA4 | 6.63 | <.0001 |
| CA1 | 10.23 | <.0001 | |
| Pre/parasubiculum | 12.64 | <.0001 | |
| Parahippocampal cortex | 4.19 | .0005 | |
| Pre/parasubiculum | CA1 | −5.93 | <.0001 |
| Subiculum | 12.18 | <.0001 | |
| Parahippocampal cortex | 4.42 | .0004 | |
| Retrosplenial cortex | 3.65 | .0022 | |
| Tail | |||
| DG/CA4 | CA3/2 | 9.97 | <.0001 |
| CA1 | 8.71 | <.0001 | |
| Subiculum | 4.53 | .0003 | |
| CA3/2 | DG/CA4 | 9.58 | <.0001 |
| CA1 | 3.90 | .0022 | |
| CA1 | DG/CA4 | 9.31 | <.0001 |
| CA3/2 | 4.22 | .0005 | |
| Subiculum | 7.75 | <.0001 | |
| Parahippocampal cortex | 4.70 | <.0001 | |
| Subiculum | DG/CA4 | 4.58 | .0002 |
| CA1 | 8.14 | <.0001 | |
| Pre/parasubiculum | 7.19 | <.0001 | |
| Pre/parasubiculum | Subiculum | 7.33 | <.0001 |
| Retrosplenial cortex | 4.91 | <.0001 | |
Negative correlations are shown in italics.
Table 4.
Statistically significant results of the longitudinal axis subfield analyses: older participants
| Seed ROI | Significant target ROIs | T—statistic t(28) | p—FDR corrected |
|---|---|---|---|
| Anterior | |||
| CA1 | Uncus | 7.46 | <.0001 |
| Subiculum | Pre/parasubiculum | 6.08 | <.0001 |
| Entorhinal cortex | 3.50 | .0056 | |
| Pre/parasubiculum | Subiculum | 5.57 | <.0001 |
| Uncus | 4.47 | .0004 | |
| Uncus | CA1 | 7.55 | <.0001 |
| Pre/parasubiculum | 4.78 | .0002 | |
| Anterior body | |||
| DG/CA4 | CA3/2 | 5.56 | <.0001 |
| CA1 | 10.61 | <.0001 | |
| Uncus | 7.60 | <.0001 | |
| CA3/2 | DG/CA4 | 5.38 | <.0001 |
| CA1 | 3.70 | .0028 | |
| Pre/parasubiculum | 3.72 | .0028 | |
| CA1 | DG/CA4 | 10.08 | <.0001 |
| CA3/2 | 3.68 | .0018 | |
| Subiculum | 3.78 | .0018 | |
| Pre/parasubiculum | −3.70 | .0018 | |
| Parahippocampal cortex | 5.99 | <.0001 | |
| Subiculum | CA1 | 3.41 | .0090 |
| Pre/parasubiculum | 6.52 | <.0001 | |
| Pre/parasubiculum | CA3/2 | 3.80 | .0016 |
| CA1 | −3.12 | .0062 | |
| Subiculum | 6.92 | <.0001 | |
| Uncus | 4.16 | .0008 | |
| Parahippocampal cortex | 3.55 | .0025 | |
| Retrosplenial cortex | 4.34 | .0008 | |
| Uncus | DG/CA4 | 8.31 | <.0001 |
| Posterior body | |||
| DG/CA4 | CA3/2 | 8.65 | <.0001 |
| CA1 | 8.36 | <.0001 | |
| Subiculum | 6.19 | <.0001 | |
| CA3/2 | DG/CA4 | 8.50 | <.0001 |
| CA1 | DG/CA4 | 8.82 | <.0001 |
| Subiculum | 9.87 | <.0001 | |
| Pre/parasubiculum | −4.95 | <.0001 | |
| Parahippocampal cortex | 4.53 | .0002 | |
| Subiculum | DG/CA4 | 6.34 | <.0001 |
| CA1 | 9.68 | <.0001 | |
| Pre/parasubiculum | 11.37 | <.0001 | |
| Parahippocampal cortex | 4.24 | .0004 | |
| Pre/parasubiculum | CA1 | −5.08 | <.0001 |
| Subiculum | 11.66 | <.0001 | |
| Parahippocampal cortex | 3.60 | .0024 | |
| Retrosplenial cortex | 4.26 | .0006 | |
| Tail | |||
| DG/CA4 | CA3/2 | 12.41 | <.0001 |
| CA1 | 8.92 | <.0001 | |
| Subiculum | 3.40 | .0054 | |
| CA3/2 | DG/CA4 | 12.15 | <.0001 |
| CA1 | DG/CA4 | 9.31 | <.0001 |
| Subiculum | 7.79 | <.0001 | |
| Parahippocampal cortex | 4.71 | .0002 | |
| Subiculum | DG/CA4 | 3.28 | <.0001 |
| CA1 | 8.28 | .0074 | |
| Pre/parasubiculum | 8.57 | <.0001 | |
| Pre/parasubiculum | Subiculum | 9.25 | <.0001 |
| Retrosplenial cortex | 3.72 | .0035 | |
Negative correlations are shown in italics.
5.2.1. Anterior
In young participants, activity in CA1 was significantly correlated with the uncus. Subiculum was correlated with pre/parasubiculum and ENT. Pre/parasubiculum was correlated with subiculum, uncus and ENT. The uncus was correlated with CA1, pre/parasubiculum and PRC. These patterns were consistent with those in the older participants, with the exception of the correlations between pre/parasubiculum‐ENT and uncus‐PRC, which were not significant in the older participants. No additional correlations were observed in the older group.
No statistically significant between‐group differences were observed.
5.2.2. Anterior body
In young participants, activity in DG/CA4 was significantly correlated with CA3/2, CA1, uncus, and PHC. CA3/2 was correlated with DG/CA4. CA1 was correlated with DG/CA4 and subiculum. Subiculum was correlated with CA1, pre/parasubiculum, uncus, PRC, and RSC. Pre/parasubiculum was correlated with subiculum, PHC, and RSC. The uncus was correlated with DG/CA4 and subiculum. These patterns were consistent in the older participants with the exception of the correlations between DG/CA4‐PHC, subiculum‐uncus, subiculum‐PRC, subiculum‐RSC which did not reach significance in the older participants. By contrast, significant correlations between CA3/2‐CA1, CA3/2‐pre/parasubiculum, CA1‐PHC, and pre/parasubiculum‐uncus were evident which were not observed in the younger group.
There was one significant between‐groups difference—compared to the young participants, older participants had significantly less FC between the subiculum and PRC (t[28] = 3.02, p = .048 FDR corrected; Figure 3 and Figure 4a).
Figure 4.
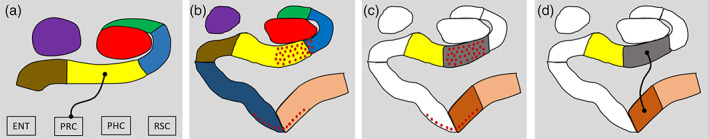
Exploratory analysis. (a) Results of the contrast of the young > older group for the AB hippocampus revealing the subiculum had reduced FC with the PRC in the older participants (thin black line with circular termini). DG/CA4 (red), CA3/2 (green), CA1 (blue), subiculum (yellow), pre/parasubiculum (brown), uncus (purple); ENT, entorhinal cortex; PHC, posterior parahippocampal cortex; PRC, perirhinal cortex; RSC, retrosplenial cortex. (b) Representation of our original segmentation scheme overlaid with red dots representing areas implicated in early (Stage 1) tau accumulation (adapted from Lace et al., 2009). Note the pattern of tau accumulation is largely restricted to the CA1‐subiculum transition region (predominantly within our subiculum mask) and the transentorhinal cortex (predominantly within our perirhinal cortex mask) during these early stages. (c) Representation of our amended segmentation scheme to create ROIs for the putatively tau‐affected CA1‐subiculum transition zone (grey) and transentorhinal cortex (rust). Amended ROIs for the medial subiculum (yellow) and lateral perirhinal cortex (coral) are also displayed. (d) Results for the contrast of the young > older group revealed the CA1‐subiculum transition region had reduced FC with the transentorhinal cortex in the older participants (thin black line with circular termini) [Color figure can be viewed at wileyonlinelibrary.com]
5.2.3. Posterior body
In young participants, activity in DG/CA4 was significantly correlated with CA3/2, CA1, and subiculum. Activity in CA3/2 was correlated with DG/CA4. CA1 was correlated with DG/CA4 and subiculum. Subiculum was correlated with DG/CA4, CA1, pre/parasubiculum and PHC. Pre/parasubiculum was correlated with subiculum, PHC, and RSC. These patterns were consistent in the older participants, with one additional correlation observed in this group between CA1 and PHC.
No statistically significant between‐group differences were observed.
5.2.4. Tail
In young participants, activity in DG/CA4 was significantly correlated with CA3/2, CA1, and subiculum. CA3/2 was correlated with DG/CA4 and CA1. CA1 was correlated with DG/CA4, CA3/2, subiculum, and PHC. Subiculum was correlated with DG/CA4, CA1, and pre/parasubiculum. Pre/parasubiculum was correlated with subiculum and RSC. These patterns were consistent in the older participants with the exception of the correlation between CA3/2‐CA1 which did not reach significance in this group.
No statistically significant between‐group differences were observed.
Overall, these patterns largely align with those reported in our recent investigation of FC along the anterior–posterior axis of hippocampal subfields in a separate group of young adult participants (Dalton et al., 2019). Our results support the idea that different portions of hippocampal subfields along the anterior–posterior axis of the hippocampus have unique patterns of connectivity with other subfields and extra‐hippocampal cortical ROIs. One difference emerged when the young and older groups were directly compared in the AB portion of the subiculum. Specifically, compared to the young group, the older group showed weaker FC between the AB subiculum and PRC.
Of note, there are numerous ways in which these data could be analyzed. Here we focused our analyses within each portion of the hippocampus, as this was the most efficient way to consider the data and the direct between‐group comparisons. We also conducted additional analyses to investigate differences in FC along the longitudinal axis of each subfield between the young and the older subjects. For each subfield, we included the anterior–posterior portions of that subfield (i.e., A, AB, PB, and T) and ENT, PRC, PHC, and RSC. As with the results reported above, the only significant between‐group difference was for the AB subiculum and PRC (t[28] = 3.02, p = .041 FDR corrected).
5.3. Further exploratory analysis
This observation of decreased FC between the AB subiculum and PRC in the older group is interesting in light of investigations of brain changes in healthy ageing. Most individuals over the age of 65 express tau pathology in the medial temporal lobes, and the earliest affected regions of tau accumulation during normal ageing are a region encompassing the CA1‐subiculum border and the transentorhinal cortex (TEC) (Lace et al., 2009). In the current study, the CA1‐subiculum border region and the TEC were incorporated predominantly in our subiculum and PRC ROIs, respectively. Taking this into consideration, we wondered whether our observation of decreased FC between the AB subiculum and PRC in older participants may be more strongly associated with the CA1‐subiculum border area and TEC, putatively as a consequence of normal age‐related tau accumulation.
To test this, and guided by the report of Lace et al. (2009), we created four new ROIs encompassing the CA1‐subiculum border (the cortical strip comprising CA1 and the subiculum that lies ventral to the DG/CA4), the adjacent medial portion of the subiculum, and we split the PRC mask into a medial TEC portion and a lateral PRC portion (see Figure 4b,c). We ran additional exploratory analyses within these ROIs. This allowed us to probe whether decreased FC between the AB subiculum and PRC was more specifically associated with any of these subregions. Considering the rationale outlined above, we predicted that the older group would show less FC than the younger participants, specifically between the CA1‐subiculum border and TEC. The only significant between‐group difference was, as predicted, less FC between the CA1‐subiculum border region and TEC in the older participants (t(28) = 2.89; p = .022 FDR corrected; Figure 4d). FC between the medial subiculum and lateral PRC was not significantly different between the groups (t(28) = 0.42, p = .74).
6. DISCUSSION
Understanding subfield connectivity down the long axis of the human hippocampus may be central to helping address the long‐standing question, highlighted by Nadel and others (Kimura, 1958; Nadel, 1968; Nauta, 1956) more than 50 years ago, as to why the anterior and posterior hippocampus seem to perform different functions. Having demonstrated our ability to study subfield rsfMRI FC previously in healthy young adults (Dalton et al., 2019), here we extended this work by examining the effects of healthy ageing. Specifically, we found no between‐group differences in patterns of FC between young and older participants when considering each subfield in its entirely. However, when a more fine‐grained approach was deployed that involved separately examining the A, AB, PB, and T portions of each hippocampal subfield, a group difference emerged. We observed age‐related reductions in FC specifically in the AB portion of the hippocampus, where the older group had reduced FC between the AB subiculum and PRC compared to the younger participants. Additional exploratory analyses revealed that reduced FC between the AB subiculum and PRC may be predominantly associated with decreased FC between the CA1‐subiculum transition region and the TEC, two brain regions known to be disproportionately affected during the early stages of age‐related tau accumulation.
Considering first how the current findings relate to those from our previous investigation of rsfMRI FC in hippocampal subfields in healthy young adults (Dalton et al., 2019), the two sets of results were similar. In this new group of young adults we found, as did Dalton et al. (2019), that intrinsic FC between the subfields aligned generally with the tri‐synaptic circuit but also extended beyond it. Patterns of FC between the subfields and neighboring cortical areas differed markedly along the anterior–posterior axis of each hippocampal subfield. The consistency of findings across two studies shows these effects are replicable and robust.
It is also notable that for both the whole subfield and longitudinal axis analyses, patterns of hippocampal subfield FC in the older participant group generally mirrored the patterns observed in the young participants. This suggests that the dynamics of hippocampal subfield rsfMRI FC may not differ greatly in the context of healthy ageing. This is perhaps not surprising given that our young and older groups were well‐matched on a range of factors that could have affected the FC findings. For example, all participants were healthy and medication‐free, of similar intellectual ability and, while perhaps surprising, there were no volume differences between the groups for any of the ROIs, hence FC differences could not be attributed to partial volume effects. In a sense, this study with its high functioning healthy agers is perhaps the best case scenario in terms of finding minimal effects of age on subfield FC. Nevertheless, even within this context, a significant reduction in AB subiculum connectivity with PRC was apparent.
While the specific functions of the subiculum remain a matter of debate, it is well characterized as the primary output structure of the hippocampus (Duvernoy et al., 2013). Some suggest it may be the heart of the extended hippocampal system (Aggleton & Christiansen, 2015). Our observation of reduced subicular FC in the older participant group aligns with a general consensus that the subiculum may be specifically prone to healthy age‐related changes. Post mortem investigations show that the subiculum and CA1 regions suffer a linear loss of neuron numbers as a function of ageing (Simic et al., 1997; West et al., 1994), and volumetric analyses of structural MRI scans have consistently confirmed age‐related volume reductions in the subiculum and CA1 (Chetelat et al., 2008; de Flores et al., 2015; Frisoni et al., 2008; La Joie et al., 2010; Mueller et al., 2007; Wang et al., 2003; Yang et al., 2013; Ziegler et al., 2012). The subiculum, therefore, appears to be particularly sensitive to the effects of ageing.
It was surprising, therefore, that we did not observe statistically significant between‐group differences in CA1 or subiculum volume in the present study. While not reaching significance, the A CA1 and whole uncus ROIs did show a trend for volume reduction in the older participant group. Our novel method of separating the uncus from the typical hippocampus may offer an explanation for why the expected patterns of age‐related atrophy to CA1 and the subiculum did not reach significance. Extant hippocampal segmentation schemes generally extend hippocampal subfield ROIs into the uncus to include both ‘typical’ and “uncal” portions of a subfield (see Adler et al., 2014; Iglesias et al., 2015; Wisse et al., 2012). In contrast, and in line with Dalton et al. (2017), we created a separate ROI for the uncus, thereby splitting the “uncal” and “typical” portions of CA1 and subiculum between different ROIs. We believe this is a better reflection of the underlying cytoarchitecture. As more researchers adopt this segmentation protocol, it will be interesting to see if, and how, this affects reports of volume differences in ageing. Of note, our goal here was to investigate functional rather than structural differences. Grey matter volume is not always a good proxy for function, given that there are patient cases where volume is reduced yet function is preserved (e.g., Maguire, Kumaran, Hassabis, & Kopelman, 2010), and vice‐versa. Volume and function, therefore, are not necessarily in a linear relationship.
In addition to cell loss and volume reduction, the subiculum is affected by another age‐related process. In the context of the current study, this provides a potential explanatory mechanism for our observation of an age‐related reduction of FC specifically between the AB subiculum and PRC. While commonly linked with Alzheimer's disease, tau protein accumulation also occurs in normal ageing. The slow accumulation of the tau protein results in progressive cell death and subsequent degradation of neuronal communication between affected brain regions. Within the medial temporal lobe, tau accumulation begins in the TEC and spreads, potentially through direct anatomical connections, to the CA1‐subiculum transition area (Lace et al., 2009). These two regions, therefore, are affected during the earliest stages of age‐related tau accumulation. The age‐related reduction in synchronicity between the CA1‐subiculum transition area and the TEC that we have observed here dovetails with this known progression of tau pathology (Lace et al., 2009) and another recent report showing that the subiculum was the only subfield to show reduced FC in patients diagnosed with mild cognitive impairment (de Flores et al., 2017). However, whether the weakening of FC between the AB CA1‐subiculum transition area and TEC is definitively a result of age‐related tau in these regions remains speculative and should be probed further in future investigations.
Our findings also highlight another issue that has relevance for future studies. Researchers using spherical seed based techniques to investigate putative functional differences down the hippocampal long axis should ensure that seeds are placed within the same subfield in the anterior and posterior hippocampus. Moreover, in the light of growing evidence, including that presented by us previously (Dalton et al., 2019; see also Plachti et al., 2019) and in the current study, that different regions of hippocampal subfields may have different functional connections, seed‐based methods should endeavor to specify which subfields are encompassed within the seed regions and discuss the results in the context of these subfields. On a related note, the current findings suggest that, in some contexts, it may be advantageous to eschew classical concepts of hippocampal subfields. Given that the CA1‐subiculum transition area appears to be a “hotspot” of anatomical connectivity across mammalian species (Insausti & Muñoz, 2001; Kondo, Saleem, & Price, 2005; Vogt & Pandya, 1987) and is implicated in the early spread of tau pathology before other regions of the hippocampus (Lace et al., 2009), it may be beneficial to investigate this region as a distinct entity.
In conclusion, while we investigated FC of broad portions of each subfield, we do not suggest that FC is segregated in such a coarse manner. Rather, the gradient nature of connectivity along hippocampal subfields is well documented (reviewed in Strange et al., 2014; Poppenk et al., 2013). Our rationale here was that, in line with this gradient, different portions of each subfield would have a greater proportion of neurons functionally interacting with, for example, the cortical ROIs, and this would be reflected in a stronger correlation between their rsfMRI activity. Overall, we suggest that investigating portions of hippocampal subfields may help to achieve a greater understanding of functional differentiation down the long axis of the hippocampus. In addition, this type of approach could potentially be leveraged to identify biomarkers that might facilitate early diagnosis of hippocampal dysfunction inherent to a range of neurodegenerative disorders. In the fifty years since Lynn Nadel first started contemplating the differences between the dorsal and ventral hippocampus, the huge complexity of this issue has become increasingly apparent. Nevertheless, the hope is that with ever‐more sophisticated techniques for examining the brains of humans and nonhumans, the hippocampus will eventually yield its secrets.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
7.
8. REFERENCES
Adler, D. H., Pluta, J., Kadivar, S., Craige, C., Gee, J. C., Avants, B. B., & Yushkevich, P. A. (2014). Histology‐derived volumetric annotation of the human hippocampal subfields in postmortem MRI. NeuroImage, 84, 505–523. https://doi.org/10.1016/j.neuroimage.2013.08.067
Aggleton, J. P. (2012). Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neuroscience and Biobehavioral Reviews, 36(7), 1579–1596. https://doi.org/10.1016/j.neubiorev.2011.09.005
Aggleton, J. P., & Christiansen, K. (2015). The subiculum: The heart of the extended hippocampal system. Progress in Brain Research, 219, 65–82. https://doi.org/10.1016/bs.pbr.2015.03.003
Agster, K. L., & Burwell, R. D. (2013). Hippocampal and subicular efferents and afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Behavioral Brain Research, 254, 50–64. https://doi.org/10.1016/j.bbr.2013.07.005
Augustinack, J. C., Huber, K. E., Stevens, A. A., Roy, M., Frosch, M. P., van der Kouwe, A. J., … the Alzheimer's Disease Neuroimaging Initiative. (2013). Predicting the location of human perirhinal cortex, Brodmann's area 35, from MRI. NeuroImage, 64, 32–42. https://doi.org/10.1016/j.neuroimage.2012.08.071
Bai, F., Xie, C., Watson, D. R., Shi, Y., Yuan, Y., Wang, Y., … Zhang, Z. (2011). Aberrant hippocampal subregion networks associated with the classifications of aMCI subjects: A longitudinal resting‐state study. PLoS ONE, 6(12), e29288. https://doi.org/10.1371/journal.pone.0029288
Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the beck depression inventory‐II. San Antonio, TX: Psychological Corporation.
Berron, D., Vieweg, P., Hochkeppler, A., Pluta, J. B., Ding, S. L., Maass, A., … Wisse, L. E. M. (2017). A protocol for manual segmentation of medial temporal lobe subregions in 7Tesla MRI. NeuroImage: Clinical, 15, 466–482. https://doi.org/10.1016/j.nicl.2017.05.022
Bonnici, H. M., Chadwick, M. J., Kumaran, D., Hassabis, D., Weiskopf, N., & Maguire, E. A. (2012). Multi‐voxel pattern analysis in human hippocampal subfields. Frontiers in Human Neuroscience, 6, 290. https://doi.org/10.3389/fnhum.2012.00290
Chetelat, G., Fouquet, M., Kalpouzos, G., Denghien, I., De la Sayette, V., Viader, F., … Desgranges, B. (2008). Three‐dimensional surface mapping of hippocampal atrophy progression from MCI to AD and over normal aging as assessed using voxel‐based morphometry. Neuropsychologia, 46(6), 1721–1731. https://doi.org/10.1016/j.neuropsychologia.2007.11.037
Chumbley, J., Worsley, K., Flandin, G., & Friston, K. (2010). Topological FDR for neuroimaging. NeuroImage, 49(4), 3057–3064. https://doi.org/10.1016/j.neuroimage.2009.10.090
Dalton, M. A., Zeidman, P., Barry, D. N., Williams, E., & Maguire, E. A. (2017). Segmenting subregions of the human hippocampus on structural MRI scans: an illustrated tutorial. Brain and Neuroscience Advances, 1:2398212817701448. https://doi.org/10.1177/2398212817701448
Dalton, M. A., Zeidman, P., McCormick, C., & Maguire, E. A. (2018). Differentiable processing of objects, associations and scenes within the hippocampus. Journal of Neuroscience, 38(38), 8146–8159. https://doi.org/10.1523/JNEUROSCI.0263‐18.2018
Dalton, M. A., McCormick, C., & Maguire, E. A. (2019). Differences in functional connectivity along the anterior‐posterior axis of human hippocampal subfields. NeuroImage, 192, 38–51. https://dx.doi.org/10.1016/j.neuroimage.2019.02.066
Damoiseaux, J. S., Viviano, R. P., Yuan, P., & Raz, N. (2016). Differential effect of age on posterior and anterior hippocampal functional connectivity. NeuroImage, 133, 468–476. https://doi.org/10.1016/j.neuroimage.2016.03.047
Das, S. R., Pluta, J., Mancuso, L., Kliot, D., Orozco, S., Dickerson, B. C., … Wolk, D. A. (2013). Increased functional connectivity within medial temporal lobe in mild cognitive impairment. Hippocampus, 23(1), 1–6. https://doi.org/10.1002/hipo.22051
Davis, D. G., Schmitt, F. A., Wekstein, D. R., & Markesbery, W. R. (1999). Alzheimer neuropathologic alterations in aged cognitively normal subjects. Journal of Neuropathology and Experimental Neurology, 58(4), 376–388. https://doi.org/10.1097/00005072‐199904000‐00008
de Flores, R., La Joie, R., Landeau, B., Perrotin, A., Mezenge, F., de La Sayette, V., … Chetelat, G. (2015). Effects of age and Alzheimer's disease on hippocampal subfields: comparison between manual and FreeSurfer volumetry. Human Brain Mapping, 36(2), 463–474. https://doi.org/10.1002/hbm.22640
de Flores, R., Mutlu, J., Bejanin, A., Gonneaud, J., Landeau, B., Tomadesso, C., … Chételat, G. (2017). Intrinsic connectivity of hippocampal subfields in normal elderly and mild cognitive impairment patients. Human Brain Mapping, 38(10), 4922–4932. https://doi.org/10.1002/hbm.23704
Dice, L. R. (1945). Measures of the amount of ecologic association between species. Ecology, 26(3), 297–302. https://doi.org/10.2307/1932409
Duvernoy, H. M., Cattin, F., & Risold, P. Y. (2013). The Human Hippocampus (4th ed.). New York: Springer‐Verlag Berlin Heidelberg.
Fanselow, M. S., & Dong, H. W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65(1), 7–19. https://doi.org/10.1016/j.neuron.2009.11.031
Fischl, B., Stevens, A. A., Rajendran, N., Yeo, B. T., Greve, D. N., Van Leemput, K., … Augustinack, J. C. (2009). Predicting the location of entorhinal cortex from MRI. NeuroImage, 47(1), 8–17. https://doi.org/10.1016/j.neuroimage.2009.04.033
Frisoni, G. B., Ganzola, R., Canu, E., Rub, U., Pizzini, F. B., Alessandrini, F., … Thompson, P. M. (2008). Mapping local hippocampal changes in Alzheimer's disease and normal ageing with MRI at 3 Tesla. Brain, 131(12), 3266–3276. https://doi.org/10.1093/brain/awn280
Griswold, M. A., Jakob, P. M., Heidemann, R. M., Nittka, M., Jellus, V., Wang, J., … Haase, A. (2002). Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Medicine, 47(6), 1202–1210. https://doi.org/10.1002/mrm.10171
Honey, C. J., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., & Hagmann, P. (2009). Predicting human resting‐state functional connectivity from structural connectivity. Proceedings of the National Academy of Science USA, 106(6), 2035–2040 https://doi.org/10.1073/pnas.0811168106
Honey, C. J., Thivierge, J. P., & Sporns, O. (2010). Can structure predict function in the human brain? NeuroImage, 52(3), 766–776. https://doi.org/10.1016/j.neuroimage.2010.01.071
Hutton, C., Bork, A., Josephs, O., Deichmann, R., Ashburner, J., & Turner, R. (2002). Image distortion correction in fMRI: A quantitative evaluation. NeuroImage, 16(1), 217–240 doi: http://dx.doi/org/10.1006/nimg.2001.1054
Iglesias, J. E., Augustinack, J. C., Nguyen, K., Player, C. M., Player, A., Wright, M., … Alzheimer's Disease Neuroimaging Initiative. (2015). A computational atlas of the hippocampal formation using ex vivo, ultra‐high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage, 115, 117–137 doi: http://dx.doi/org/10.1016/j.neuroimage.2015.04.042
Insausti, R., & Muñoz, M. (2001). Cortical projections of the non‐entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis). European Journal of Neuroscience, 14(3), 435–451 doi: https://doi.org/10.1046/j.0953‐816x.2001.01662.x
Kimura, D. (1958). Effects of selective hippocampal damage on avoidance behavior in the rat. Canadian Journal of Psychology, 12(4), 213–218 doi: https://doi.org/10.1037/h0083740
Kjelstrup, K. B., Solstad, T., Brun, V. H., Hafting, T., Leutgeb, S., Witter, M. P., … Moser, M. B. (2008). Finite scale of spatial representation in the hippocampus. Science, 321(5885), 140–143 doi: https://doi.org/10.1126/science.1157086
Knopman, D. S., Parisi, J. E., Salviati, A., Floriach‐Robert, M., Boeve, B. F., Ivnik, R. J., … Peterson, R. C. (2003). Neuropathology of cognitively normal elderly. Journal of Neuropathology and Experimental Neurology, 62(11), 1087–1095 doi: https://doi.org/10.1093/jnen/62.11.1087
Kobayashi, Y., & Amaral, D. G. (2007). Macaque monkey retrosplenial cortex: III. Cortical efferents. Journal of Comparative Neurology, 502(5), 810–833 doi: https://doi.org/10.1002/cne.21346
Kondo, H., Saleem, K. S., & Price, J. L. (2005). Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology, 493(4), 479–509 doi: https://doi.org/10.1002/cne.20796
La Joie, R., Fouquet, M., Mezenge, F., Landeau, B., Villain, N., Mevel, K., … Chetelat, G. (2010). Differential effect of age on hippocampal subfields assessed using a new high‐resolution 3T MR sequence. NeuroImage, 53(2), 506–514 doi: https://doi.org/10.1016/j.neuroimage.2010.06.024
Lace, G., Savva, G. M., Forster, G., de Silva, R., Brayne, C., Matthews, F. E., … Mrc, C. (2009). Hippocampal tau pathology is related to neuroanatomical connections: an ageing population‐based study. Brain, 132(5), 1324–1334 doi: https://doi.org/10.1093/brain/awp059
Leonard, B. W., Amaral, D. G., Squire, L. R., & Zola‐Morgan, S. (1995). Transient memory impairment in monkeys with bilateral lesions of the entorhinal cortex. Journal of Neuroscience, 15(8), 5637–5659 doi: https://doi.org/10.1523/JNEUROSCI.15‐08‐05637.1995
Lutti, A., Thomas, D. L., Hutton, C., & Weiskopf, N. (2013). High‐resolution functional MRI at 3 T: 3D/2D echo‐planar imaging with optimized physiological noise correction. Magnetic Resonance in Medicine, 69(6), 1657–1664 doi: https://doi.org/10.1002/mrm.24398
Maass, A., Schutze, H., Speck, O., Yonelinas, A., Tempelmann, C., Heinze, H. J., … Duzel, E. (2014). Laminar activity in the hippocampus and entorhinal cortex related to novelty and episodic encoding. Nature Communications, 5, 5547 doi: https://doi.org/10.1038/ncomms6547
Maguire, E. A., Gadian, D. G., Johnsrude, I. S., Good, C. D., Ashburner, J., Frackowiak, R. S. J., & Frith, C. D. (2000). Navigation‐related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences USA, 97(8), 4398–4403 doi: https://doi.org/10.1073/pnas.070039597
Maguire, E. A., Kumaran, D., Hassabis, D., & Kopelman, M. D. (2010). Autobiographical memory in semantic dementia: a longitudinal fmri study. Neuropsychologia, 48, 123–136 doi: https://doi.org/10.1016/j.neuropsychologia.2009.08.020
Moser, M. B., & Moser, E. I. (1998). Functional differentiation in the hippocampus. Hippocampus, 8(6), 608–619.
Mueller, S. G., Stables, L., Du, A. T., Schuff, N., Truran, D., Cashdollar, N., & Weiner, M. W. (2007). Measurement of hippocampal subfields and age‐related changes with high resolution MRI at 4T. Neurobiology of Aging, 28(5), 719–726 doi: https://doi.org/10.1016/j.neurobiolaging.2006.03.007
Mugler, J. P., 3rd., Bao, S., Mulkern, R. V., Guttmann, C. R., Robertson, R. L., Jolesz, F. A., & Brookeman, J. R. (2000). Optimized single‐slab three‐dimensional spin‐echo MR imaging of the brain. Radiology, 216(3), 891–899 doi: https://doi.org/10.1148/radiology.216.3.r00au46891
Nadel, L. (1967). Behavioral effects of dorsal ad ventral hippocampal lesions in the rat. (PhD thesis). McGill University.
Nadel, L. (1968). Dorsal and ventral hippocampal lesions and behavior. Physiology and Behavior, 3(6), 891–900 doi: https://doi.org/10.1016/0031‐9384(68)90174‐1
Nadel, L. (1991). The hippocampus and space revisited. Hippocampus, 1(3), 221–229 doi: https://doi.org/10.1002/hipo.450010302
Nadel, L., & Moscovitch, M. (1997). Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology, 7(2), 217–227 doi: https://doi.org/10.1016/S0959‐4388(97)80010‐4
Nauta, W. J. H. (1956). An experimental study of the fornix in the rat. Journal of Comparative Neurology, 104(2), 247–272 doi: https://doi.org/10.1002/cne.901040205
O'Keefe, J., & Nadel, L. (1978). The Hippocampus as a Cognitive Map. New York: Oxford University Press.
Palombo, D. J., Amaral, R. S., Olsen, R. K., Muller, D. J., Todd, R. M., Anderson, A. K., & Levine, B. (2013). KIBRA polymorphism is associated with individual differences in hippocampal subregions: evidence from anatomical segmentation using high‐resolution MRI. Journal of Neuroscience, 33(32), 13088–13093 doi: https://doi.org/10.1523/JNEUROSCI.1406‐13.2013
Payne, J. D., & Nadel, L. (2004). Sleep, dreams, and memory consolidation: the role of the stress hormone cortisol. Learning and Memory, 11(6), 671–678 doi: https://doi.org/10.1101/lm.77104
Pereira, J. B., Valls‐Pedret, C., Ros, E., Palacios, E., Falcon, C., Bargallo, N., … Junque, C. (2014). Regional vulnerability of hippocampal subfields to aging measured by structural and diffusion MRI. Hippocampus, 24(4), 403–414 doi: https://doi.org/10.1002/hipo.22234
Plachti, A., Eickhoff, S. B., Hoffstaedter, F., Patil, K. R., Laird, A. R., Fox, P. T., … Genon, S. (2019). Multimodal parcellations and extensive behavioral profiling tackling the hippocampus gradient. Cerebral Cortex doi: 10.1093/cercor/bhy336.
Poppenk, J., Evensmoen, H. R., Moscovitch, M., & Nadel, L. (2013). Long‐axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17(5), 230–240 doi: http://dx.doi.org/10.1016/j.tics.2013.03.005.
Ryan, L., Nadel, L., Keil, K., Putnam, K., Schnyer, D., Trouard, T., & Moscovitch, M. (2001). Hippocampal complex and retrieval of recent and very remote autobiographical memories: evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus, 11(6), 707–714 doi: https://doi.org/10.1002/hipo.1086
Sik, A., Ylinen, A., Penttonen, M., & Buzsaki, G. (1994). Inhibitory CA1‐CA3‐hilar region feedback in the hippocampus. Science, 265(5179), 1722–1724 doi: https://doi.org/10.1126/science.8085161
Simic, G., Kostovic, I., Winblad, B., & Bogdanovic, N. (1997). Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. Journal of Comparative Neurology, 379(4), 482–494.
Strange, B. A., Witter, M. P., Lein, E. S., & Moser, E. I. (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience, 15(10), 655–669 doi: https://doi.org/10.1038/nrn3785
Suthana, N. A., Krupa, A., Donix, M., Burggren, A., Ekstrom, A. D., Jones, M., … Bookheimer, S. Y. (2010). Reduced hippocampal CA2, CA3, and dentate gyrus activity in asymptomatic people at genetic risk for Alzheimer's disease. NeuroImage, 53(3), 1077–1084 doi: https://doi.org/10.1016/j.neuroimage.2009.12.014
van Leemput, K., Maes, F., Vandermeulen, D., & Suetens, P. (1999). Automated model‐based bias field correction of MR images of the brain. IEEE Transactions on Medical Imaging, 18(10), 885–896 doi: https://doi.org/10.1109/42.811268
Vogt, B. A., Vogt, L. J., Perl, D. P., & Hof, P. R. (2001). Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. Journal of Comparative Neurology, 438(3), 353–376 doi: https://doi.org/10.1002/cne.1320
Vogt, B. A., & Pandya, D. N. (1987). Cingulate cortex of the rhesus monkey: II. Cortical afferents. Journal of Compartive Neurology, 262(2), 271–289 doi: https://doi.org/10.1002/cne.902620208
Wang, L., Swank, J. S., Glick, I. E., Gado, M. H., Miller, M. I., Morris, J. C., & Csernansky, J. G. (2003). Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. NeuroImage, 20(2), 667–682 doi: https://doi.org/10.1016/S1053‐8119(03)00361‐6
Wang, Z., Yuan, Y., Bai, F., Shu, H., You, J., Li, L., & Zhang, Z. (2015). Altered functional connectivity networks of hippocampal subregions in remitted late‐onset depression: a longitudinal resting‐state study. Neuroscience Bulletin, 31(1), 13 doi: https://doi.org/10.1007/s12264‐014‐1489‐1
Wechsler, D. (2008). Wechsler adult intelligence scale–Fourth edition (WAIS–IV). San Antonio, TX: NCS Pearson.
West, M. J., Coleman, P. D., Flood, D. G., & Troncoso, J. C. (1994). Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. The Lancet, 344(8925), 769–772 doi: https://doi.org/10.1016/S0140‐6736(94)92338‐8
Wisse, L. E., Gerritsen, L., Zwanenburg, J. J., Kuijf, H. J., Luijten, P. R., Biessels, G. J., & Geerlings, M. I. (2012). Subfields of the hippocampal formation at 7 T MRI: in vivo volumetric assessment. NeuroImage, 61, 1043–1049. https://doi.org/10.1016/j.neuroimage.2012.03.023
Witter, M. P., & Amaral, D. G. (1991). Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. Journal of Comparative Neurology, 307(3), 437–459 doi: https://doi.org/10.1002/cne.903070308
Xu, X., Sun, Y., Holmes, T. C., & López, A. J. (2016). Noncanonical connections between the subiculum and hippocampal CA1. Journal of Comparative Neurology, 524(17), 3666–3673.
Yang, X., Goh, A., Chen, S. H., & Qiu, A. (2013). Evolution of hippocampal shapes across the human lifespan. Human Brain Mapping, 34(11), 3075–3085 doi: https://doi.org/10.1002/hbm.22125
Yassa, M. A., Stark, S. M., Bakker, A., Albert, M. S., Gallagher, M., & Stark, C. E. (2010). High‐resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. NeuroImage, 51(3), 1242–1252 doi: https://doi.org/10.1016/j.neuroimage.2010.03.040
Yushkevich, P. A., Piven, J., Hazlett, H. C., Smith, R. G., Ho, S., Gee, J. C., & Gerig, G. (2006). User‐guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage, 31(3), 1116–1128 doi: https://doi.org/10.1016/j.neuroimage.2006.01.015
Yushkevich, P. A., & the Hippocampal Subfields Group. (2015). Quantitative comparison of 21 protocols for labelling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. NeuroImage, 111, 526–541 doi: https://doi.org/10.1016/j.neuroimage.2015.01.004
Zeidman, P., Mullally, S., & Maguire, E. A. (2015). Constructing, perceiving, and maintaining scenes: hippocampal activity and connectivity. Cerebral Cortex, 25(10), 3836–3855 doi: https://doi.org/10.1093/cercor/bhu266
Zeidman, P., Lutti, A., & Maguire, E. A. (2015). Investigating the functions of subregions within anterior hippocampus. Cortex, 73, 240–256 doi: https://doi.org/10.1016/j.cortex.2015.09.002
Zeidman, P., & Maguire, E. A. (2016). Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nature Reviews Neuroscience, 17(3), 173–182 doi: https://doi.org/10.1038/nrn.2015.24
Ziegler, G., Dahnke, R., Jancke, L., Yotter, R. A., May, A., & Gaser, C. (2012). Brain structural trajectories over the adult lifespan. Human Brain Mapping, 33(10), 2377–2389 doi: https://doi.org/10.1002/hbm.21374
Memory, stress, and the hippocampal hypothesis: Firefighters' recollections of the fireground
Janet Metcalfe1| Jason C. Brezler2| James McNamara2| Gabriel Maletta1| Matti Vuorre1
1Department of Psychology, Columbia University, New York, New York
2FDNY Mental Performance Initiative, New York, New York
Correspondence
Janet Metcalfe, Department of Psychology,
Columbia University, New York, New York,
10027.
Email: jm348@columbia.edu
Abstract
Nadel, Jacobs, and colleagues have postulated that human memory under conditions of extremely high stress is “special.” In particular, episodic memories are thought to be susceptible to impairment, and possibly fragmentation, attributable to hormonally based dysfunction occurring selectively in the hippocampal system. While memory for highly salient and self‐relevant events should be better than the memory for less central events, an overall nonmonotonic decrease in spatio/temporal episodic memory as stress approaches traumatic levels is posited. Testing human memory at extremely high levels of stress, however, is difficult and reports are rare. Firefighting is the most stressful civilian occupation in our society. In the present study, we asked New York City firefighters to recall everything that they could upon returning from fires they had just fought. Communications during all fires were recorded, allowing verification of actual events. Our results confirmed that recall was, indeed, impaired with increasing stress. A nonmonotonic relation was observed consistent with the posited inverted u‐shaped memory‐stress function. Central details about emergency situations were better recalled than were more schematic events, but both kinds of events showed the memory decrement with high stress. There was no evidence of fragmentation. Self‐relevant events were recalled nearly five times better than events that were not self‐relevant. These results provide confirmation that memories encoded under conditions of extremely high stress are, indeed, special and are impaired in a manner that is consistent with the Nadel/Jacobs hippocampal hypothesis.
extreme stress, FDNY, human memory
9. INTRODUCTION
Despite intense interest in understanding how extremely high stress impacts human memory, scientific investigation of this issue remains problematic. Indeed, there is scarcely any area of psychological research that is more fraught with difficulties. The problem at issue is understanding what happens and why: Are special mental processes engaged under conditions of traumatic stress? Is the impact of stress selective to particular kinds of memories—perhaps those involving conscious recollection—or are all memories affected equally? Is encoding different for salient, threatening, or emotional events? Is memory altered by personal involvement? Is there anything in the memory systems of humans that could account for the disturbing and fragmented phenomenology reported by people who have undergone traumatic stress? There have been a number of conjectures on these issues, of course. Nadel and Jacobs (1998) laid out their position in an article entitled: “Traumatic memory is special.” As will be outlined below, they and their colleagues have proposed what we will here call the “hippocampal hypothesis” (Jacobs & Nadel, 1985; Nadel & Jacobs, 1996; Payne et al., 2006; Payne, Nadel, Allen, Thomas, & Jacobs, 2002), some predictions of which are investigated here. We will begin by contrasting this hypothesis with the default concerning the effects of traumatic stress on memory—the Freudian “repression” construct.
9.1. Historical overview
On the assumption that traumatic memory is, in fact, special, the best‐known specialized mechanism is repression. Repression is thought to be a defense mechanism that is recruited when an individual experiences stress at the level of trauma. Under these circumstances, the mind is conjectured to defensively push the memory down to a deep and inaccessible level (consciously and intentionally, in early writings by Freud, see Brueur & Freud, 1957, but unconsciously according to later accounts). Such a memory cannot be consciously accessed, but it can wreak havoc on the person's mental and emotional life, causing neuroses. Repression is not mere forgetting. Nor is it a lack of encoding. The trace, instead, is thought to be encoded in a detailed manner but actively pushed out of mind by a special process that may be engaged if the situation is sufficiently traumatic. The memory—which is susceptible to later recovery—is like an in‐focus, high contrast photograph that is buried and protected. It is inaccessible so long as the defense mechanism pushing it down is working, but even though it is consciously inaccessible it is nevertheless thought to be perfectly intact, preserved in a tightly sealed container awaiting recovery. Only under special circumstances, such as therapy, can repressed memories be recovered.
The notion of repression, as well as the purported recovery of repressed memories has come under fire. First, insofar as there is forgetting over time (Murdock, 1974) which can apply even to important events, a person's inability to retrieve a memory does not mean that some special mechanism is pushing it out of consciousness. Second, hypermnesia is characteristic of normal memory: sometimes memories that are not retrievable at time t can be retrieved later at time t + x (Roediger & Wheeler, 1993). Thus, recovery of the so‐called repressed memories is not the only pathway to bring a hitherto forgotten memory into consciousness. Third, people's judgments about the truth of their memories are fallible. They can believe that they are remembering events that never occurred. Examples of such false memories range from remembering that one was lost in a shopping mall as a child (Loftus, 1999), to remembering visitations from space aliens (McNally, 2012). Thus, good faith “recollections” of atypical events (as might occur for traumatic situations) does not guarantee that a traumatic event actually happened and was repressed. Fourth, people routinely smooth and rationalize fragmentary inconsistencies into a narratively coherent whole (Johnson, Foley, Suengas, & Raye, 1989). Thus, detailed narrative “recall” is no assurance of veridicality. In addition, in court cases in which recovered repressed memories have been evoked, secondary financial gain has sometimes been associated with the alleged recovered memories. These arguments undermine the notion of a special mechanism of repression. Indeed, the validity of the construct has risen to the level of a legal debate, and a number of courts have ruled that there is insufficient scientific evidence to support the construct for use in trials (see, Howe & Knott, 2015).
Outside of the courtroom, several investigators have studied whether there might be a psychological mechanism that is even remotely akin to repression. Bjork and colleagues have shown that people are sometimes able to selectively forget (e.g., Geiselman, Bjork, & Fishman, 1983), and they postulate an active process. Anderson and colleagues have shown that it is possible to selectively “not think” about particular items, and that this voluntary inhibition can make later recall less likely (Anderson & Green, 2001; c.f. Bulovitch, Roediger, Balota & Butler, 2006). While the idea of inhibitory processes in memory is gaining traction, it is not clear exactly how these postulated mechanisms relate to Freudian repression which specifically implicates stress as causal. Neither the selective forgetting mechanism nor the inhibition/repression mechanism has been proposed to be stress dependent. Indeed, some studies suggest that selective inhibition may be adaptive, and the failure to inhibit a marker of dysfunction (Eich, Razlighi, & Stern, 2017). Thus, the laboratory studies on selective forgetting and inhibition leave unresolved the question of whether there is a special memorial process associated with stress.
9.2. Ethical problems with testing memory under extreme stress in humans
Although there is considerable interest in understanding the effects of extremely high stress on memory, stressing human subjects experimentally in the laboratory is problematic due to ethical considerations. Accordingly, the stressors that have been used are benign enough to pass the scrutiny of ethics panels. At the most extreme, people might have done the Trier task, in which they are told they have to give a public presentation that will be evaluated and which is touted as indicating their intelligence. Alternatively, they might have had the unpleasant, painful (but not life threatening) experience of submerging a hand in icy cold water for 3 min. More usually, they read or hear a story, read words (some of which may be taboo), watch a movie, or see slides that are thought to be emotional, disturbing, or stressful. The results from such studies are mixed (Mather, 2007). Some show enhanced memory (MacKay & Ahmetzanov, 2005; Mather & Nesmith, 2008); a few show impaired memory (Kuhlmann, Piel, & Wolf, 2005; Sharot & Phelps, 2004). But it could be debated whether any of these experiments provide insight into the question of what happens to memory under extremely high stress because in none of them were the levels of stress extremely high.
9.3. The hippocampal hypothesis
Understanding of hippocampal function, which is at the center of an emerging story about the effects of extremely high stress on human memory, was spearheaded by O'Keefe and Nadel's (1978) breakthrough investigations and discoveries on the hippocampus as a spatial map—a system that they dubbed the “locale” system. The postulated interaction with stress was soon delineated in a seminal paper by Jacobs and Nadel (1985). This article set the stage for a number of subsequent elaborations (Jacobs & Nadel, 1998; Metcalfe & Jacobs, 1996; 1998; 2000; Metcalfe & Mischel, 1999; Nadel & Jacobs, 1996, 1998; Payne et al., 2006). Basically, Jacobs and Nadel (1985) puzzled over clinical findings indicating that many adult patients described symptoms of childhood phobias re‐emerging when they underwent extreme stressors in later life that typically had nothing to do with the distressing childhood event. These observations seemed nothing like uncovered repressed memories. They were often fragmentary and piecemeal. And, because of the lack of relation between the stressor and the original event, the re‐emergent phobias could also not be attributed to spontaneous recovery of conditioned responses. Jacobs and Nadel (1985) presciently proposed an alternative possibility. They postulated that under extreme stress, the hippocampal (locale) system shuts down, leaving exposed conditioned learning from childhood (in a different, taxon, system) that had been suppressed when the hippocampal system was fully functioning. These two systems (taxon and locale) morphed into the “hot” and “cool” systems of Metcalfe and Jacobs (1996), (1998), (2000) and of Metcalfe and Mischel (1998). Over the ensuing decades, the notion that extremely high stress can have a selective impairing effect on the hippocampus and frontal lobes has gained currency, and considerable research has been directed at the purportedly inverted u‐shaped function relating stress to memory and the hippocampal system. The argument, which is based almost exclusively on animal research, is that the complex, contextual, spatial, and temporal memory system does not behave in a “business‐as‐usual” manner when the stress level is extremely high.
The characterization of the hippocampal system was elaborated by the discovery of hippocampal time cells and the relational integration or binding postulate of Eichenbaum (2014, 2017). Taken together, these spatial/temporal/binding characteristics of the hippocampal system in animals seem to provide the scaffolding needed for an episodic memory system such as that which underpins human mental time travel, a mental capability that Tulving (2005) has referred to as autonoetic consciousness. Autonoetic consciousness is a type of awareness that involves recollection of personal contextually bound episodic memories, and mental time travel into both the past and future. The status of this kind of memory has been bolstered by extensive studies of patients with hippocampal damage who appear to lack such an ability while retaining other kinds of learning and memory abilities. The hippocampal hypothesis, then, proposes that under conditions of extremely high stress hippocampal functioning is impaired, and this results in a kind of acute amnesia specific to the episodic/autonoetic system. High‐ and low‐affinity glucocorticoid receptors that give rise to a u‐shaped stress response curve in hippocampus (Reul & DeKloet, 1985) substantiate this view. Such stress sensitive receptors, and their relation to hippocampal functioning, were pinned down in animal research. And, because of the ethical considerations in humans, most of the behavioral work concerning extreme stress that provides the basis for the hippocampal hypothesis, has been most rigorously and systematically investigated in animals.
The idea that under conditions of extremely high stress something special happens to memories is common to both the Freudian perspective and to the “hippocampal” hypothesis. But what happens is different. As noted earlier, by the Freudian view, the traumatic memory is pristine and replete but buried—awaiting recovery. By the hippocampal view, when the hippocampus becomes dysfunctional because of the influx of glucocorticoids associated with extremely high stress, episodic memories are likely to become fragmented (because of a binding failure) or may fail entirely to be recorded. If a spatially and temporally coherent representation is not encoded, then, by the encoding specificity principle (Tulving & Thomson, 1973), even the best retrieval cue will not provide access to it. Thus, there is no possibility of uncovering of a true coherent narrative episodic memory trace. The trace laid down under conditions of extreme stress is something like a photograph taken by an extremely jittery hand and projected onto overexposed film—blurry, fragmentary, and incomprehensible at the outset and subject to forgetting, overwriting, interference, and distortion induced by subsequent events.
Such loss of memory and fragmentation of what little memory persists is sometimes reflected in the recollections of traumatic events, of people experiencing PTSD. It is also reported by firefighters: “When we respond to a call, we always have to suppress our emotions and use our logic and our past experiences to perform our job. If they're very strong emotions, sometimes we never get to process them. So we wind up with fragments of an incident left over,” Captain Jacques Roy, Firefighter of 25 years (Ushery, Stulberger, Wagner, Bott, & Manney, 2018).
If there is a direct mapping between the kind of locale (hippocampal/spatial/temporal/relational binding) system that has been extensively explored in rats and the human episodic memory system, then we may be justified in using the extensive animal literature to compensate for the non‐existent human experimental literature on memory and trauma and to draw conclusions about the effect of stress on humans.
9.4. A cautionary note concerning overreliance on animal research
Despite the appeal of generalizing to humans from the findings in the animal literature, such a generalization is particularly precarious in the present case because the kind of memory (explicit/episodic memory, which is associated with mental time travel and autonoetic consciousness) that is of central interest in understanding the effects of extreme stress in humans, may not even exist in animals. Indeed, the literature on true episodic memory in animals provides scant assurance that any animals other than humans have this capability (Templer & Hampton, 2013). There are some examples of behavior resembling episodic‐like memory, but they are few and far between. Menzel (1999) showed that a lexigram‐trained chimpanzee could point to the lexigram of a food hidden several hours ago and direct a human caretaker to its location. Schwartz (2009) showed that King, a circus gorilla, was able, after extensive training, to select a token representing a food that he had eaten several hours ago along with a token representing the keeper who had given him this food, but at only slightly above chance levels. Scrub jays (Clayton & Dickinson, 1998) have been able to discriminate different foods, their locations, and how long ago they were cached. These are the only examples of episodic‐like memory in animals, and they are very minimal at best. Whether they are “real” episodic memory, as exhibited by people, is debatable. No such capabilities have been demonstrated in rodents—the animals on which most memory and stress‐related experiments have been conducted. Furthermore, no studies have yet indicated that these episodic‐like capabilities, in the animals that have them, break down under conditions of stress. It may be that stress impacts a delicate human system that is, at best, nascent in other animals, at worst, nonexistent. Thus, while taking the animal literature as a source of inspiration, it is essential that it be augmented by findings in human beings.
9.5. Studies with glucocorticoids
The responsiveness of the hippocampus to the stress hormone cortisol has been implicated in memory effects, both positive and negative, in both humans and animals (De Quervain, Aerni, Schelling, & Roozendaal, 2009). At low levels of glucocorticoids, memory is enhanced, whereas inhibition appears to occur at very high levels. This result that has been ascribed to the consequences of high‐ and low‐affinity glucocorticoid receptors in the hippocampus as has been illustrated in animal studies (Reul & DeKloet, 1985). The effects also appear to obtain in humans. Andreano and Cahill (2006), in a study using the cold‐pressor stress task in which the hand is submerged in ice water, were the first to have shown a quadratic relation between memory performance and endogenous glucocorticoid release in response to the stressor in humans. A number of other studies have revealed a relation between cortisol and memory when glucocorticoids have been administered exogenously. Frequently, high doses result in impaired memory, while low doses have a facilitative effect or no effect (Het, Ramlow, & Wolf, 2005).
Furthermore, chronic corticosteroid therapy to control inflammation such as in the treatment of arthritis or autoimmune conditions is associated with decreased hippocampal volume and poor memory (Sapolsky, 1996). Similarly, high levels of glucocorticoids resultant because of chronic stress, such as occurs with jet lag, depression, and PTSD, are also associated with decreased hippocampal volume and memory (Brown et al., 2004; McEwen, 1999). It is interesting that feelings of stress are not consistently reported with the administration of drugs such as prednisone or hydrocortisone (although psychiatric symptoms are sometimes observed, Henns, Poon, de los Angeles, & Koran, 2011). The phenomenology of stress and the memorial effects of corticosteroids—while unquestionably related—appears to be complex.
9.6. Quasi experimental studies of high stress in humans
The final approach that provides a window, albeit not a perfect window, on the effects of severe stress on human memory has involved tapping into extremely high stress experiences that people voluntarily engage in. For instance, Eich and Metcalfe (2009) contrasted explicit and implicit memory under stressful and unstressful conditions by testing participants either at the “bib parties” where runners obtained their numbers for the New York City or Boston marathons (the unstressful condition), or immediately after the runners had completed the 26.2 mile course (the stressful condition). They used the same tasks that—with amnesic hippocampal patients—had illustrated a selective impairment of the episodic/explicit memory system due to hippocampal lesions. The marathon study revealed a similar impairment for the runners in the stress condition, providing support for the hippocampal hypothesis of stress.
Yonelinas, Parks, Koen, Jorgenson, and Mendoza (2011) investigated the effect of the extremely high stress that occurred post encoding—during the retention interval—by interposing a parachute jump between encoding and retrieval. In this particular situation, stress improved the memory (of males but not females). At first blush, this finding might seem to go against the hippocampal hypothesis. However, the stressor did not occur during encoding or the retrieval of the to‐be‐remembered events. If high stress impairs the memory for events that occur while the stress is being experienced, then the events that had occurred during the jump may have been impacted (but were not tested) but those events that occurred prior to the jump may have been spared or might even have been subjected to less interference from the jump events—accounting for the pattern observed. Thompson, Williams, L'Esperance, and Cornelius (2001) had participants listen to word lists either while they were on the ground or while they were in the air, skydiving, and then recall, 8 min later, either while on the ground or in the air. They found impaired recall for encoding, retrieval, or both, in the air, and an interaction such that people who encoded, unstressed, on the ground and recalled, unstressed, on the ground revealed the best memory. Notably, these studies did not ask people to remember things that were relevant to the stressful event itself.
Several studies, conducted in a military context by Morgan and his colleagues, have done so, although. They investigated memory for events experienced during a mock prisoner of war training situation. Glucocorticoid levels during this training indicated that the participants were experiencing extreme, even traumatic, stress (Morgan et al., 2000). Many also experienced dissociative symptomology (Morgan et al., 2001). In one such study, Morgan et al. (2004) compared participants' recognition memory for a non‐threatening interrogator as compared to a highly threatening interrogator. Interestingly, the high stress, threatening, interrogator was consistently remembered less well, suggesting a decrease in memory with increasing stress in real‐life traumatic situations.
The research that will be detailed below is in the same tradition as those naturalistic studies outlined previously. We did not manipulate stress, experimentally. Instead, we asked our participants—New York City firefighters—for their memories of events they had recently experienced while voluntarily being exposed to what were sometimes extremely high levels of stress. We evaluated the degree of stress both by asking our subjects and an experienced firefighter not involved in the particular fire for stressfulness ratings, allowing us to begin to systematically relate the amount of stress experienced to memory.
9.7. Stress on the fireground
Firefighting is widely acknowledged to be the single most stressful, non‐military, occupation in our society. Firefighters are frequently exposed to risks such as structural collapse, structure fires, electrocutions, asphyxiation, burns, heat stress, physical injuries, noise exposure, hazardous materials, contaminants, and medical emergencies, such as are unknown in other occupations (Hard et al., 2018). Firefighters take on, and train for, such risks in the service of the community. Compounding factors include the fact that firefighters routinely work 10 or 11, 24 hr shifts per month and take on extra 24 hr shifts as needed. Sleep while on shift is frequently interrupted by emergencies. A firefighter may be sedentary or even sleeping and, within 2 min, has to be fully geared up (with gear weighing well in excess of 50 pounds), on the truck, and ready for a dire emergency with almost no information about what will be encountered next. As might be expected, there is a high rate of hypertension (Choi, Schnall & Dobson, 2016) and of PTSD (Kristin, Klimley, Van Hassel, & Stripling, 2018) among firefighters. Such reactions are buffered by protective factors that include a strong sense of belongingness and social support from co‐workers and family. Adverse reactions are also modulated by intense training, high levels of resilience, and a light hearted, supportive sense of humor among fire fighters. Following September 11, there have been several programs designed to address the human factors involved in firefighting, including the FDNY Mental Performance Initiative. The present study was conducted under the auspices of this initiative. To the best of our knowledge, it is the first study to investigate episodic memory for fires, among firefighters who were actively engaged in the incidents.
10. METHOD
10.1. Participants
The participants were 54 New York City firefighters. Eighteen participants provided reports from more than one incident (there were 21 incidents in total), for a total of 92 incident‐person reports. The mean age was 36 years, and all participants were male. This research was approved by the Uniformed Firefighters Association, the Uniformed Fire Officers Association, and the Columbia University IRB under protocol AAAR7542. Participation was entirely voluntary.
10.2. Procedure
In this study, we investigated the recall of incidents in which firefighters had very recently participated. Although firefighters often respond to calls that are not emergencies, because we were interested in memory under conditions of stress, only incidents that were fires were included in our study. The stress level at all fires is high. Upon returning to the firehouse, fire fighters provided their written free recall of the fire that they had recently fought. Because of the voluntary nature of the study, the exact amount of time between the end of the fire and recall was provided could vary. Typically, although, the recall was written down within 5 hr of returning to the firehouse.
One difficulty that occurs in nearly all cases of people's recall of naturalistic incidents is that it is impossible to know ground truth—there is usually no objective recording of what happened. This poses an obvious problem for evaluating the accuracy of recall. We did not have this problem in the present study. Firefighters co‐ordinate their actions at a fire by communicating with each other using Handy Talkies. All transmissions made on the Handy Talkies are recorded and were available to our team after each of the fires. These provided a detailed recording of all communications during the fire and hence of what happened. Scoring of the protocols was conducted by two officers at the FDNY (with consent of the participants and of the Firefighters' Union). The Columbia University team members were blind to the identity of the participants as well as to the location and public characteristics of the fires. They were provided only with de‐identified numerical data spreadsheets. Participants were simply asked to recall everything that they could about the fire from which they had just returned. They were given as much time as they needed to do so.
After recalling, the participants answered several standard questions. Most importantly, they were asked for their rating of the stressfulness of the fire, on a scale of 1–5, where 1 was “little to no stress” and 5 was “severe amount of stress.” They were asked how many years they had been a firefighter. They were asked to record the time and date of the recall session, to allow calculation of the retention interval. Forty‐three participants also provided a time estimation of how long the fire had lasted. The coders who scored the recall and who listened to the Handy Talkie recordings (and who were both experienced firefighters) independently rated the severity of each fire.
10.3. Scoring
The Handy Talkie protocols were categorized into two kinds of items: Schematic and Emergency. Concerning the former, just as in other events that recur (such as going to a restaurant) in which there are a set of events that are standard for that event (the hostess seats the person at a table; the diner looks at a menu; the diner orders; the waiter brings the food, etc.) so, too, are there standard events that occur in every fire. These Schematic items occurred in nearly all fires and were:
Start a Line: This is the first major communication that the action has started. According to firefighters' reports it feels like going to a fight—the heart rate is through the roof, and adrenaline is very high.
Start Water: This has a similar salience to [1], with high adrenaline and heart rate.
All visible fire is knocked down: This communication indicates significant progress, and is accompanied by an increasing level of relief and reduced stress.
Primary Searches are positive or negative: This communication conveys the results of the initial quick sweep of the structure.
Secondary searches on the floor(s) above and/or below the fire are positive or negative: This communication conveys the results of slower methodical searches, once the fire has been knocked down. If all is well then this is very reassuring.
The second kind of events that were isolated in the Handy Talkie protocols were Emergency items. These were extremely stressful events pointing to a dire situation. These communications did not occur in all fires. If they did occur in the Handy Talkies, although, they were noted, and the participants' recall protocols were scored for whether they had remembered each of these. They were
A “10–45” transmission: This transmission indicates that a firefighter has found a body. The person could be either alive or dead. This is a tremendously stressful event.
Lost water/back‐out: This communication means that there is enormous trouble with the fire hose. It is a huge, potentially catastrophic issue.
A “10–70” transmission: This code indicates that there is a problem with the water pumper. It is of similar urgency to 2, above.
“May‐Day”: This indicates that collapse may be about to happen, or that a firefighter is down. This is the single most severe radio transmission. It is as bad as it gets.
“Urgent”: This communication is used to clear the radio line for a must hear message. It gets everyone else to cease talking over the Handy Talkies immediately. It is only used in extreme circumstances.
The recall protocols were scored both in terms of whether the event had been recalled or not (0 or 1), and if it had been recalled, in how much detail (on a scale from 1 to 3). They were also scored for whether the communication was either personally relevant (made by the participant or directed at the participant) or not.
11. RESULTS
The mean time between the end of the fire and recall was 13.25 hr; the median time was about 5 hr. There was no effect of either the retention interval or of whether the subjects had slept before recalling, on recall or on ratings of the stressfulness of the fire. Time estimations of the duration of the fire were examined, but none of the effects relating time estimation and the stressfulness of the fire were significant (perhaps because of too few observations). Therefore, we did not further consider these variables in the analyses that follow.
Recall scores were lower for the Schematic (mean proportion recalled = 0.19, SE = 0.02) than for the Emergency items (0.69, SE = 0.08). The relation between stress and memory for these two categories of items was analyzed using a multilevel logistic regression model. The predictors of the binary recall scores were (a) item category (Schematic and Emergency) and, (b) stressfulness of the incident, as given by each firefighter for each incident. To allow for variation across participants and items, random intercepts were allowed for participants and items. Both standardized linear and quadratic predictors were used to allow for detection of the theoretical possibility of an inverted U‐shape relation between stress and memory.2
The results are shown in Figure 5, and the parameters of the model are provided in Table 5. There was a negative linear relation between recall and stress: recall was worse with increasing stress. The inverted U‐shaped relation between stress and memory that is postulated by the hippocampal hypothesis was also significant in the model outcomes; there was a quadratic relation between stress and memory. As another test of the importance of the inverted U‐shaped relation, we compared the model with the quadratic term against a model without the quadratic term using the model comparison metric WAIC (Watanabe, 2010): The former model (WAIC = 340.2) outperformed the latter (WAIC = 343.7). Thus, both the significance of the parameter and a model comparison approach supported the idea of an inverted U‐shaped relation between stress and memory. The model also showed that Emergency items were recalled better than were Schematic items.
Figure 5.
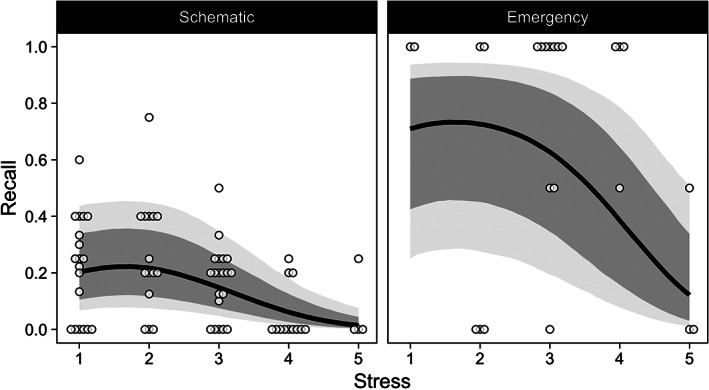
The relation between stress and recall for Schematic and Emergency questions. Points are proportions of recalled items for individual subjects at each level of stress (horizontal noise was added to display overlapping subjects). Lines are fitted recall probabilities (with 95% and 80% CIs as grey shades) from multilevel logistic regression model
Table 5.
Estimated parameters of model of recall scores
| Parameter | β estimate | SE | 95% CI | Post. Prob |
|---|---|---|---|---|
| Stress (linear) | −.55 | 0.18 | [−0.91, −0.21] | .999 |
| Stress (quadratic) | −.40 | 0.17 | [−0.75, −0.07] | .992 |
| Emergency vs. Schematic | 2.27 | 1.08 | [0.12, 4.30] | .021 |
| Intercept | −1.47 | 0.59 | [−2.67, −0.33] | .992 |
Note: Estimates are posterior means from multilevel logistic regression model, and as such indicate effects on the log‐odds scale (SE indicates the posterior standard deviation). “Post. Prob” is the posterior probability that the parameter was negative.
The literature on chronic medicinal administration of glucocorticoids suggests that exposure to glucocorticoids/stress over long periods of time might adversely impact the individual's reaction to stress and their memory. It was therefore hypothesized that those firefighters who had been on the job for longer might be both more stress prone and have worse memories that those firefighters with a shorter history of chronic stress. To investigate this possible detrimental effect of chronic stress, the relation among participants' stress ratings, their years on job, and the coder's ratings of incident severity were modeled. To allow for multiple measures on some individuals, intercepts and effects of severity were modeled as random across participants. All predictors were mean centered. As is shown in Figure 6, stress ratings were strongly positively associated with the event severity ratings (β = .54, SE = 0.13, 95% CI = [0.28, 0.78]). People who had been on the job longer, though, had lower stress ratings (β = −.36, SE = 0.14, 95% CI = [−0.64, −0.1]). The interaction between severity ratings and years on job was not different from zero (β = −.08, SE = 0.13, 95% CI = [−0.34, 0.17]). Additionally, the model's intercept was not different from zero, indicating that participant's stress ratings were, on average, in accordance with the fire chief's event severity rating (β = −.03, SE = 0.13, 95% CI = [−0.29, 0.23]).
Figure 6.
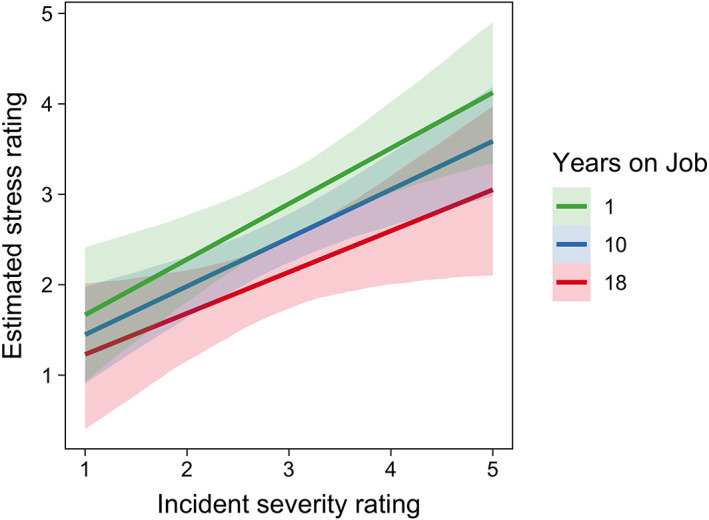
The relation between participants' stress ratings (y‐axis), the coder's rating of event severity rating (x‐axis) and years on job. Lines and shades indicate regression lines and 95% CIs, respectively, from multilevel regression model [Color figure can be viewed at wileyonlinelibrary.com]
To investigate possible fragmentation of memory as a function of stress, a conditional analysis was conducted to examine how much detail was given to each of the recalled events. Recall of each of the events had been coded as being either a 0 (not recalled), a 1 (indicating that the event was merely mentioned), a 2 (indicating a moderate level of detail) or a 3 (indicating that a considerable amount of detailed information was reported). The analysis shown in Figure 5 was based on binary scores: was the event recalled or not? For the present analysis, we looked only at those events that were recalled to determine how much detail was given for each item. If increasing stress resulted in fragmentary recall, then, we expected to see more detailed recall at lower levels of stress and less detail at higher levels. This prediction was not confirmed by the results, however. In a multilevel regression model of recall detail scores (1, 2, or 3) with random intercepts for participants, neither the linear (β = .10, SE = 0.10, p = .320) nor the quadratic effect (β = −.13, SE = 0.10, p = .201) of stress was statistically significant. However, there were only 85 recalled items, and thus the power to detect these effects was small. Furthermore, very few events, at any level of detail, were recalled at extremely high levels of stress, as can be seen from Figure 5. Finally, the events themselves that were recalled under extremely high stress were often highly salient—a fact that might attenuate fragmentation.
Finally, the hippocampal system in humans is thought to bear a relation to the self. As such, it seemed plausible to conjecture that events that were self‐relevant would be recalled better than events that were not self‐relevant. To investigate this possibility, memory for transmissions that were either made by the participant or were directed at the participant (i.e., were self‐relevant) were considered self‐relevant and were compared to memory for other transmissions. For the non‐self‐relevant items, overall recall averaged 0.19. Recall was much higher—0.94—for the self‐relevant items. However, it should be noted that only 17 of the total 383 (Schematic and Emergency items that had occurred in the Handy Talkie transmissions and were scored as having been recalled or not) were self‐relevant. Sixteen of these 17 were recalled. We modeled recall as a function of self‐relevance with a multilevel logistic regression model, with random intercepts for participants. The effect of self‐relevance was statistically significant (β = 4.25, SE = 1.05, p < .001).
12. DISCUSSION
Many years ago, Nadel, Jacobs, and colleagues, after careful study of their own experimental research and that of others, and factoring in their clinical observations, generated a hypothesis about the effects of extreme stress on memory. The core notion was that the hippocampal spatial/temporal memory system—that is primarily responsible for episodic memory in humans—can become impaired to the point of shutting down when a person is extremely stressed. They proposed an inverted u‐shaped curve relating hippocampal memory and stress. In the intervening years, most of the experiments and the supporting constructs about the characterization of this system have been conducted in non‐human animals. Some utilize human participants but typically only at low levels of stress. But although the research that originally generated the hippocampal hypothesis began with single cell recordings in rats and has been followed up most extensively in non‐humans, the thrust has always been toward contributing to an explanation of the experiences of people responding to and remembering highly stressful, traumatic, events. The problem comes in testing with humans. Studies with humans are rare. Firefighters, though, experience such events routinely. Because of their participation, we were able to observe their memory for events that they had experienced under conditions of extremely high stress.
The results of the study presented here based on the recall of firefighters in the FDNY for events that occurred while they were fighting fires while sometimes under extreme stress, lend substance to the hippocampal hypothesis. Memory was better for the threatening, emergency events than for more standard, schematic, events. Memory, both for emergency and for schematic events, was impaired by increasing stress. There was a quadratic (inverted u‐shaped) memory stress function. These results indicate that memory under stress is, indeed, special, and in a way specified by the hippocampal hypothesis.
The one thing that goes against the hippocampal hypothesis in our data is the lack of fragmentation. However, the data may not have been either powerful enough or specifically directed at the possibility of fragmentation to allow us to examine the hypothesis at this level of detail. One way in which future research might investigate this question—which would have both theoretical and practical implications—would be to specifically examine firefighters' memory for the spatial layout of the fires. Spatial memory is of enormous importance for firefighters. Knowing whether it is unreliable under extremely stressful conditions—as the hipppocampal hypothesis predicts—may, quite literally, be information that will save lives. If it is unreliable, as we posit here, then precautionary measures and training to offset this human fallibility can be implemented. If the hippocampus is indeed, a spatial and temporal map, then finding ways to compensate for a breakdown of those functions that are specific to this system, when the system is itself vulnerable, can be prioritized. Such knowledge and the specific resultant training may be of practical as well a theoretical importance.
These results indicate that memory under extreme stress is, indeed, special. In broad outlines, the patterns that we observed in this study in firefighters remembering events from real‐world highly stressful situations in which they were participants, were consistent with the Nadel/Jacobs hippocampal hypothesis.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Endnote
We used a t(7, .25, .5) prior on the standard deviation of the item‐specific intercepts, because they could not be identified on the data alone (i.e., there were responses to only three emergency items). We also fitted two additional models, one with an interaction between item category and stress ratings, and one with the firefighter's years on job as a predictor. None of these predictors turned out important, and the models had worse fits then the one presented in the main text.
REFERENCES
Anderson, M. C., & Green, C. (2001). Suppressing unwanted memories by executive control. Nature, 410, 366–369.
Andreano, J. M., & Cahill, L. (2006). Glucocorticoid release and memory consolidation in man and women. Psychological Science, 17, 466–470.
Brown, E. S., Woolston, D., Erol, A., Bobadilla, L., Khan, D. A., Hanczyc, M., … Cullum, C. M. (2004). Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biological Psychiatry, 55, 538–545.
Brueur, J., & Freud, S. (1957). Studies on Hysteria. NY: Basic Books, Inc.
Bulevich, J. B., Roediger, H. L., III, Balota, D. A., & Butler, A. B. (2006). Failures to find suppression of episodic memories in the think/no‐think paradigm. Memory & Cognition, 34, 1569–1577.
Choi, B., Schnall, P., & Dobson, M. (2016). Twenty‐four‐hour work shifts, increased job demands, and elevated blood pressure in professional fire fighters. International Archive of Occupational Environmental Health, 89, 1111–1125.
Clayton, N. S., & Dickinson, A. (1998). Episodic‐like memory during cache recovery by scrub jays. Nature, 395, 272–274.
De Quervain, D. J.‐F., Aerni, A., Schelling, G., & Roozendaal, B. (2009). Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology, 30, 358–370.
Eich, T. S., & Metcalfe, J. (2009). Effects of the stress of marathon running on implicit and explicit memory. Psychonomic Bulletin and Review, 16, 475–479.
Eich, T. S., Razlighi, O. R., & Stern, Y. (2017). Perceptual and memory inhibition deficits in clincially healthy older adults are associated with region‐specific doubly dissociable patterns of cortical thinning. Behavioral Neuroscience, 131, 220–225.
Eichenbaum, H. (2014). Time cells in the hippocampus: A new dimension for mapping memories. Nature Reviews Neuroscience, 15, 732–744.
Eichenbaum, H. (2017). On the integration of space, time, and memory. Neuron, 95, 1007–1019.
Geiselman, R. E., Bjork, R. A., & Fishman, E. L. (1983). Disrupted retrieval in directed forgetting: A link with posthypnotic amnesia. Journal of Experimental Psychology: General, 112, 58–72.
Hard, D. L., Marsh, S. M., Merinar, T. R., Bowyer, M. E., Miles, S. T., Loflin, M. E., & Moore, P. H. (2018). Summary of recommendations from the National Institute for Occupational Safety and Health fire fighter fatality investigation and prevention program, 2006–2014. Journal of Safety Research, 68, 21–25.
Henns, H. A., Poon, A. W., de los Angeles, C. P., & Koran, L. M. (2011). Psychiatric complications of treatment with corticosteroids: Review with case report. Psychiatric Clinical Neuroscience, 65, 549–560.
Het, S., Ramlow, G., & Wolf, O. T. (2005). A meta‐analytic review of the effects of acute cortisol administration on human memory. Psycholneuroendocrinology, 30, 771–784.
Howe, M. L., & Knott, L. M. (2015). The fallibility of memory in judicial processes: Lessons from the past and their modern consequences. Memory, 23, 633–656.
Jacobs, W. J., & Nadel, L. (1985). Stress‐induced recovery of fears and phobias. Psychological Review, 92, 512–531.
Jacobs, W. J., & Nadel, L. (1998). Neurobiology of reconstructed memory. Psychology, Public Policy, and Law, 4, 1110–1134.
Johnson, M. J., Foley, M. A., Suengas, A., & Raye, C. L. (1989). Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology: General, 117, 371–376.
Kristin, E., Klimley, K. E., Van Hassel, V. B., & Stripling, A. M. (2018). Posttraumatic stress disorder in police, firefighters, and emergency dispatchers. Aggression and Violent Behavior, 43, 33–44.
Kuhlmann, S., Piel, M., & Wolf, O. T. (2005). Cognitive impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience, 25, 2977–2982.
Loftus, E. F. (1999). Lost in the mall: Misrepresentations and misunderstandings. Ethics & Behavior, 9, 51–60.
MacKay, D. G., & Ahmetzanov, M. V. (2005). Emotion, memory, and attention in the taboo Stroop paradigm: An experimental analogue of flashbulb memories. Psychological Science, 16, 25–32.
Mather, M. (2007). Emotional arousal and memory binding: An object‐based framework. Perspectives on Psychological Science, 2, 33–52.
Mather, M., & Nesmith, K. (2008). Arousal‐enhanced location memory for pictures. Journal of Memory and Language, 58, 449–462.
McEwen, B. S. (1999). Stress and hippocampal plasticity. Annual Review of Neuroscience, 22, 105–122.
McNally, R. (2012). Explaining “memories” of space alien abduction and past lives: An experimental psychopathology approach. Journal of Experimental Psychopathology, 3, 2–16.
Menzel, C. R. (1999). Unprompted recall and reporting of hidden objects by a chimpanzee (Pan troglodytes) after extended delays. Journal of Comparative Psychology, 113, 426–434.
Metcalfe, J., & Jacobs, W. J. (1996). A "hot‐system/cool‐system" view of memory under stress. PTSD Research Quarterly, 7, 1–8.
Metcalfe, J., & Jacobs, W. J. (1998). Emotional memory: Effects of stress on 'Cool' and 'Hot' memory systems. The Psychology of Learning & Motivation, 38, 187–221.
Metcalfe, J., & Jacobs, W. J. (2000). 'Hot' emotions in human recollection: Towards a model of traumatic memory. In E. Tulving (Ed.), Memory, consciousness, and the brain: The Tallinn conference (pp. 228–242). Philadelphia: Psychology Press.
Metcalfe, J., & Mischel, W. (1999). A hot/cool system analysis of delay of gratification: Dynamics of willpower. Psychological Review, 106, 3–26.
Morgan, C. A., Hazlett, G., Doran, A., Garrett, S., Hoyt, G., Thomas, P., … Southwick, S. M. (2004). Accuracy of eyewitness memory for persons encountered during exposure to highly intense stress. International Journal of Law and Psychiatry, 27, 265–279.
Morgan, C. A., Hazlett, G., Wang, S., Richardson, G., Schnurr, P., & Southwick, S. M. (2001). Symptoms of dissociation in humans experiencing acute uncontrollable stress: A prospective investigation. American Journal of Psychiatry, 158, 1239–1247.
Morgan, C. A., Wang, S., Mason, J., Hazlett, G., Fox, P., Southwick, S. M., … Greenfield, G. (2000). Hormone profiles in humans experiencing military survival training. Biological Psychiatry, 47, 891–901.
Murdock, B. B. (1974). Human memory: Theory and data. NJ: Erlbaum.
Nadel, L., & Jacobs, W. J. (1996). The role of the hippocampus in PTSD, panic and phobia. In N. Kato (Ed.), Hippocampus: Functions and clinical relevance. Amsterdam: Elsevier Science B.V.
Nadel, L., & Jacobs, W. J. (1998). Traumatic memory is special. Current Directions in Psychological Science, 10, 154–157.
O'Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. New York, NY: Oxford University Press.
Payne, J. D., Jackson, E. D., Ryan, L., Hoscheidt, S., Jacobs, W. J., & Nadel, L. (2006). The impact of stress on neutral and emotional aspects of episodic memory. Memory, 14, 1–16.
Payne, J. D., Nadel, L., Allen, J. B., Thomas, K. G. F., & Jacobs, W. J. (2002). The effects of experimentally induced stress on false recognition. Memory, 10, 1–6.
Reul, J. M., & DeKloet, E. R. (1985). Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology, 117, 2505–2511.
Roediger, H., & Wheeler, M. (1993). Hypermnesia in episodic and semantic memory: Response to Bahrick and hall. Psychological Science, 4, 207–208.
Sapolsky, R. M. (1996). Why stress is bad for your brain. Science, 273, 749–750.
Schwartz, B. L. (2005). Do animals have episodic memory? In H. S. Terrace & J. Metcalfe (Eds.), The missing link in cognition: Origins of self‐reflective consciousness (pp. 225–241). New York: Oxford University Press.
Sharot, T., & Phelps, E. A. (2004). How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience, 4, 294–306.
Templer, V. L., & Hampton, R. R. (2013). Episodic memory in nonhuman animals. Current Biology, 23, R801–R806.
Thompson, L. A., Williams, K. L., L'Esperance, P. R., & Cornelius, J. (2001). Context‐dependent memory under stressful conditions: The case of skydiving. Human Factors, 43, 611–619.
Tulving, E. (2005). Episodic memory and autonoesis: Uniquely human? In H. Terrace & J. Metcalfe (Eds.), The missing link in cognition: Origins of self‐reflective consciousness (pp. 3–56). NY: Oxford University Press.
Tulving, E., & Thomson, D. M. (1973). Encoding specificity and retrieval processes in episodic memory. Psychological Review, 80, 352–373.
Ushery, D., Stulberger, E., Wagner, L., Bott, M. & Manney, D. (2018). I‐team: National Data Shows Firefighters' mental, emotional health not getting enough attention. NBC 4 New York. https://www.nbcnewyork.com/news/local/Firefighters‐Mental‐HealthSurvey‐PTSD‐474859323.html
Watanabe, S. (2010). Asymptotic equivalence of Bayes cross validation and widely applicable information criterion in singular learning theory. The Journal of Machine Learning Research, 11, 3571–3594.
Yonelinas, A. P., Parks, C. M., Koen, J. D., Jorgenson, J., & Mendoza, S. P. (2011). The effects of post‐encoding stress on recognition memory: Examining the impact of skydiving in young men and women. Stress, 14, 136–144.
13. Episodic memory: Neuronal codes for what, where, and when
Jørgen Sugar | May‐Britt Moser
Centre for Neural Computation, Egil and Pauline Braathen and Fred Kavli Center for Cortical Microcircuits, Kavli Institute for Systems Neuroscience, Norwegian University for Science and Technology (NTNU), rondheim, Norway
Correspondence
Jørgen Sugar and May‐Britt Moser, Centre for Neural Computation, Egil and Pauline Braathen and Fred Kavli Center for Cortical Microcircuits, Kavli Institute for Systems Neuroscience, Norwegian University for Science and Technology (NTNU), Trondheim, Norway.
Email: jorgen.sugar@ntnu.no, may-britt.moser@ntnu.no
allocentric, cognitive map, egocentric, pattern completion, pattern separation, place cell, time cell
Centre of Excellence scheme of the Research Council of Norway, Grant/Award Number: 223262; Egil and Pauline Braathen and Fred Kavli Centre for Cortical Microcircuits; the Research Council; Kavli Foundation
13.1. Abstract
Episodic memory is defined as the ability to recall events in a spatiotemporal context. Formation of such memories is critically dependent on the hippocampal formation and its inputs from the entorhinal cortex. To be able to support the formation of episodic memories, entorhinal cortex and hippocampal formation should contain a neuronal code that follows several requirements. First, the code should include information about position of the agent (“where”), sequence of events (“when”), and the content of the experience itself (“what”). Second, the code should arise instantly thereby being able to support memory formation of one‐shot experiences. For successful encoding and to avoid interference between memories during recall, variations in location, time, or in content of experience should result in unique ensemble activity. Finally, the code should capture several different resolutions of experience so that the necessary details relevant for future memory‐based predictions will be stored. We review how neuronal codes in entorhinal cortex and hippocampus follow these requirements and argue that during formation of episodic memories entorhinal cortex provides hippocampus with instant information about ongoing experience. Such information originates from (a) spatially modulated neurons in medial entorhinal cortex, including grid cells, which provide a stable and universal positional metric of the environment; (b) a continuously varying signal in lateral entorhinal cortex providing a code for the temporal progression of events; and (c) entorhinal neurons coding the content of experiences exemplified by object‐coding and odor‐selective neurons. During formation of episodic memories, information from these systems are thought to be encoded as unique sequential ensemble activity in hippocampus, thereby encoding associations between the content of an event and its spatial and temporal contexts. Upon exposure to parts of the encoded stimuli, activity in these ensembles can be reinstated, leading to reactivation of the encoded activity pattern and memory recollection.
Funding information Centre of Excellence scheme of the Research Council of Norway, Grant/Award Number: 223262; Egil and Pauline Braathen and Fred Kavli Centre for Cortical Microcircuits; the Research Council; Kavli Foundation
13.2. INTRODUCTION
In December 2017, a group of neuroscientists gathered in Tucson to celebrate one of the giants in neuroscience, Lynn Nadel (Figure 7). We celebrated his 75th birthday and his achievements, and the 40 years anniversary of his book “The Cognitive Map” (O'Keefe & Nadel, 1978). The ability to form episodic memories of such occasions is critically dependent on a set of interconnected brain areas including hippocampus and surrounding parahippocampal areas (Aggleton, 2014; Nadel & Moscovitch, 1997; Nadel & Peterson, 2013; Ranganath & Ritchey, 2012; Rugg & Vilberg, 2013). In humans, lesions to the parahippocampal areas result in episodic memory deficits (Annese et al., 2014; Scoville & Milner, 1957; Zola‐Morgan & Squire, 1990) and hippocampal and parahippocampal areas show increased activity during encoding and retrieval of such memories (Burgess, Maguire, & O'Keefe, 2002; Hayes, Ryan, Schnyer, & Nadel, 2004; Nadel, Campbell, & Ryan, 2007; Squire et al., 1992). Even though the subjective attributes of episodic memories are not accessible in animals, there are now several experimental models where episodic‐like memory can be studied in nonhumans. For example, Clayton and Dickinson (1998) showed that food caching scrub jays were able to preferentially search for fresh perishable food before searching for nonperishable food. Thus, the jays demonstrated that they knew “what” was stored “where” and “when,” meeting the criteria for episodic‐like memory as described by Tulving (1983). Likewise are rats able to integrate “what” and “where” information to retrieve the order of events (“when”), an ability which is lost after lesions to hippocampus, thus mimicking the amnestic syndrome first reported by Scoville and Milner in 1957 (Ergorul & Eichenbaum, 2004; Fortin, Wright, & Eichenbaum, 2004).
Figure 7.
Photo of four happy neuroscientists taken in December 2017 at Lynn Nadel's Festschrift meeting. From left; Lynn Nadel, May‐Britt Moser, John O'Keefe, Richard Morris, far left: Neil Burgess. Photo taken by Tor Grønbech [Color figure can be viewed at wileyonlinelibrary.com]
Scoville and Milner's reports on anterograde amnesia after removal of the hippocampal and surrounding cortical areas bolstered an interest in revealing the neural mechanisms underlying such functions. One major advance in understanding these memory circuits came with the discovery of hippocampal cells with spatial correlates and the idea that these neurons called “place‐cells” were suggested to be parts of a cognitive map of space (O'Keefe & Dostrovsky, 1971; O'Keefe & Nadel, 1978). The key idea was that place cells with neighboring place fields were suggested to be linked together so that an ensemble of place cells constitute a memory system for related locations. Extensions of the cognitive map theory have been suggested to serve the basis for several other higher mental functions. One of these was the addition of a temporal dimension to the spatial codes which would result in a memory system for episodes. Such spatiotemporal maps would not only represent spatial locations (i.e., Location A is next to Location B), but also of temporal relationships (i.e., I was at Location A first, then I moved to Location B). Thus, it could be suggested that hippocampus would provide a spatiotemporal scaffold, or a coordinate system, onto which experience could be mapped and thus serve as a neural substrate for episodic memories. Implicit in these ideas and extended by others was the suggestion that episodic memories are encoded into unique activity patterns in hippocampal ensembles and/or in unique sets of neurons (Hebb, 1949; Josselyn, Kohler, & Frankland, 2015; Leutgeb et al., 2005).
As these ideas were published, several steps have been made toward a more complete insight of how episodic memories are formed and retrieved. One of these steps is the acknowledgment that a key to understanding hippocampal functions is to understand the inputs it receives from entorhinal cortex, where the majority of the cortical inputs to the system originates (Cappaert, Van Strien, & Witter, 2015). A milestone in the understanding of the functional correlates of single cells in entorhinal cortex was the remarkable discovery of grid cells and the following findings of a conglomerate of spatially modulated neurons in the medial entorhinal cortex (MEC) (Moser, Moser, & McNaughton, 2017). These different functional cell types suggested that hippocampal place cells interacted with a spatial system in MEC and that an understanding of entorhinal–hippocampal interactions is a key to the understanding of hippocampal functions.
By now, it is still not fully understood how entorhinal–hippocampal circuits contribute to encoding and recollection of episodic memories and how these memories are coded by neural activity in these circuits. However, several requirements for what an episodic memory code should look like can be made; first, the code should include not only spatial information (“where”), but also information about sequences of episodes (“when”) and the content of the experience itself (“what”), so that these elements can be associated and integrated and recollected as a whole when the system is presented to a partial retrieval cue. Second, to avoid interference between similar episodes the circuits should be able to produce unique activity patterns for unique episodes. Experiences that vary in the spatial and/or temporal context in which they were acquired should result in activity patterns with minimal overlap so that similar memories are not interfering with each other during encoding or recall. Third, the code should arise instantly, supporting memory formation of one‐shot experiences. Finally, the code should be able to capture relevant scales of experience, that is, different resolutions of experience should be included in the code. For instance, the memory of a single event can be used differently; in some settings it might be useful to retrieve unique and detailed features of past experiences. In other situations, it could be more useful to retrieve commonalities between memories of the past and ongoing experience. To achieve such flexibility in the retrieval process, important details and coarse‐scale features of the experience should be encoded into memory in parallel.
In this paper, we review electrophysiological data recorded in rodents, shedding light on how the neural code in the entorhinal–hippocampal circuit fulfills these requirements of an episodic memory system.
13.3. IMMEDIATE AND UNIVERSAL MAPS OF SPACE IN ENTORHINAL CORTEX AND UNIQUE MAPS IN HIPPOCAMPUS
Entorhinal cortex is one of the key areas in the brain contributing to spatial computations. The discovery of the grid cell paved the way for this new insight and initiated a range of new discoveries of other functional cell types contributing to the positional system of the rodent brain (Hafting, Fyhn, Molden, Moser, & Moser, 2005; Kropff & Treves, 2008; Sargolini et al., 2006; Solstad, Boccara, Kropff, Moser, & Moser, 2008) and of related activity patterns in other species such as humans (Doeller, Barry, & Burgess, 2010; Jacobs, Kahana, Ekstrom, Mollison, & Fried, 2010; Reagh & Yassa, 2014). Let us briefly review electrophysiological recordings of each specific cell type below, to illustrate the diversity of functions provided by the MEC.
First, the grid cell is characterized by activity fields distributed in a pattern of hexagons in which the smallest unit formed by the vertices are triangles tiling the entire environment (Figure 8a). Each grid cell has a slight offset, a different phase, from other grid cells so that a small population of grid cells covers all positions within an environment (Hafting et al., 2005). The activity fields are stably anchored to the environment so that grid cells constitute a metric and a coordinate system of space. How can a biological system provide such a precise coordinate system while the animal is freely moving? Models of grid cell formation suggest that grid cells are organized in continuous attractor networks where information about both the heading direction of the animal and fine‐tuned speed information are needed to continuously update the locus of activity while the animal moves (Burak & Fiete, 2009; Couey et al., 2013; Fuhs & Touretzky, 2006; McNaughton, Battaglia, Jensen, Moser, & Moser, 2006). Indeed, MEC was found to contain cells signaling the head direction of the animal similar to the ones discovered by Ranck and Taube in the dorsal presubiculum over a decade earlier (Sargolini et al., 2006; Taube, Muller, & Ranck, 1990). Later, grid cells and head direction cells were found to be weakly correlated to the speed of the animal (Hinman, Brandon, Climer, Chapman, & Hasselmo, 2016; Wills, Barry, & Cacucci, 2012). In addition, a population of cells with a linear relationship to the speed of the animal was discovered (Kropff, Carmichael, Moser, & Moser, 2015). In the latter population, firing rate increases neatly in parallel to an increase in the animal's speed. A substantial proportion of these speed cells are fast‐spiking, likely parvalbumin‐positive interneurons (Ye, Witter, Moser, & Moser, 2018). Accordingly, the precise firing of both grid cells and speed cells is lost when parvalbumin interneuron activity is blocked (whereas other functional cell types such as the head direction cells are intact) (Miao, Cao, Moser, & Moser, 2017). Likewise, the grid signal is impaired when head‐direction signals are disrupted (Winter, Clark, & Taube, 2015). These findings support the idea that the precise location of the activity fields of grid cells is a result of integration of head‐direction signals and continuous information of the speed of the animal (McNaughton et al., 2006). A positional system based on path integration mechanisms is likely to accumulate errors over time and therefore needs to be reanchored at regular intervals (Hafting et al., 2005; McNaughton et al., 2006), for instance, when the animal is perceiving familiar environmental cues such as borders or landmarks (Campbell et al., 2018; Hardcastle, Ganguli, & Giocomo, 2015). In line with these ideas, border cells signaling positions close to boundaries such as walls have been identified in MEC and likely play a role for anchoring the activity of spatially modulated cells to the boundaries of an environment (Solstad et al., 2008). Hence, grid cells are likely a result of integration of self‐motion signals continuously corrected for by environmental cues. Accordingly, when an animal is placed in an environment, grid cells instantly display their characteristic tessellating grid pattern and are thus meeting the requirement for a spatial code supporting memory formation of one‐shot episodes.
Figure 8.
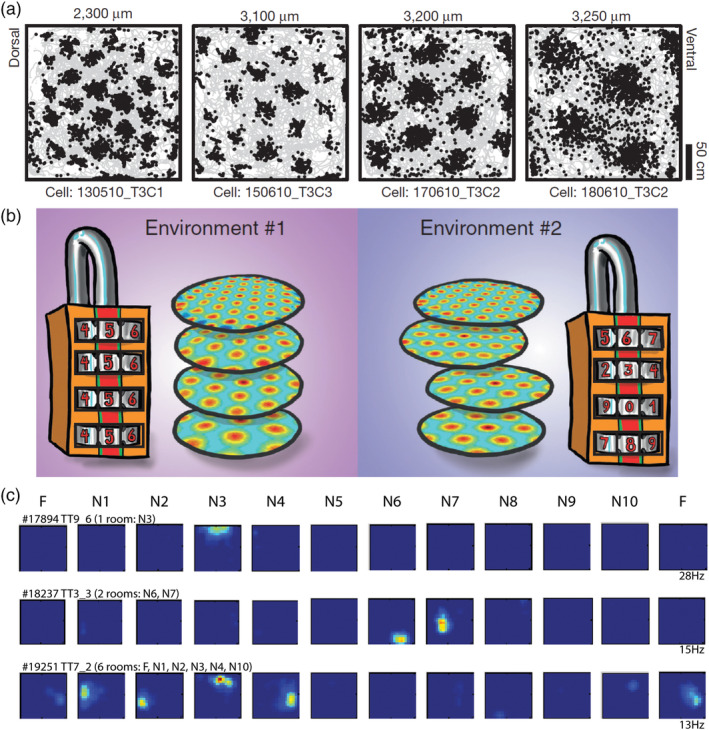
Unique place cell ensemble activity in hippocampus could be generated from multiple grid cell maps in medial entorhinal cortex. (a) Four example grids with distinct inter‐field spacing. Neuronal spikes (black dots) overlaid on the trajectory of the rat (grey). Dorsoventral location from the brain surface is indicated. Note the increasing inter‐field spacing at consecutively more ventrally recorded grid cells. Adapted from Stensola et al. (2012). (b) Suggested mechanism for the formation of unique place cell maps in hippocampus. In different environments, grid cells within one module shift and rotate together, whereas grid cells in different modules could shift and rotate independently. In this way, a small number of grid modules can encode a vast number of environments by the vast number of combinations of shifts and rotations, like the number combinations on a locker. Source: Drawing by Håkon Fyhn. Adapted from Rowland and Moser (2014). (c) Color‐coded rate maps from three example place cells showing distribution of firing rate between 11 environments (blue, no firing; red, peak firing). Each row comprises ratemaps from one neuron recorded in a familiar room (F, first and last column) and 10 novel rooms (N1–N10). Note that a population of place cells form unique ensemble activity in each room, with cells being active in one room and silent in another. If active, the position of the fields in two different rooms do not correlate, thus, an example of global remapping. Adapted from Alme et al. (2014) [Color figure can be viewed at wileyonlinelibrary.com]
The different groups of spatially modulated neurons are universal across environments. In navigating rats, a grid cell in one environment is always a grid cell in other environments. However, grid cells rotate and shift the grid location, that is, reanchor their x–y coordinates and orientation between environments. Grid cells with similar spacing between their grid fields maintain coherent spatial relationships to each other in all environments, so that two cells will keep their spatial offset between their firing fields in all environments (Fyhn, Hafting, Treves, Moser, & Moser, 2007; Hafting et al., 2005; Stensola et al., 2012). Similar coherencies also hold for head direction cells and border cells, so that if the environment is rotated 90°, all cells will show similar rotation of their head direction or border preference (Boccara et al., 2010; Sargolini et al., 2006; Solstad et al., 2008). Next, speed cells recorded in one environment can be used to decode the speed of the animal in another environment, thus speed cells maintain their linear firing relationship to speed across environments (Kropff et al., 2015). Accordingly, the networks of spatially modulated neurons in MEC provide an immediate, universal, and robust code for all environments.
13.3.1. Entorhinal cortex and hippocampus represent space at multiple resolutions
Spatial cells in entorhinal cortex and hippocampus display different sizes of their place fields. These differently “sized” spatial‐selective cells are distributed topographically throughout the long axis of entorhinal cortex and hippocampus; cells with smaller fields located most dorsally, whereas more broadly tuned cells are located in more ventral parts. In essence, grid cells, head direction cells and place cells with wider tuning curves are located in more ventral portions of entorhinal cortex and hippocampus (Barry, Hayman, Burgess, & Jeffery, 2007; Brun et al., 2008; Giocomo et al., 2014; Hafting et al., 2005; Killian, Jutras, & Buffalo, 2012; Kjelstrup et al., 2008; Stensola et al., 2012). Compared to dorsally recorded neurons, more ventrally recorded grid cells display larger field widths and a near 10‐fold increase in interpeak distance and ventrally recorded place cells in hippocampus display a field width up to 10 m when recorded in large environments. Intriguingly, these data are paralleled with data obtained from humans; increased activity in anterior hippocampus is related to processing and retrieval of large‐scale locations, whereas activity in posterior hippocampus is related to processing and retrieval of fine‐grained details of the environment and associated cues (Evensmoen et al., 2013; Evensmoen et al., 2015; Nadel, Hoscheidt, & Ryan, 2013). Thus, the spatial signal in MEC and hippocampus show a clear topography. This observation indicates that the more dorsal/posterior parts are more involved in processing details of space, whereas ventral/anterior parts are more involved in representing global spatial layouts. In the context of episodic memory, such a parallel multi‐scale representation of an environment is important mainly because the spatial code captures several relevant scales which episodes could be associated to (Erdem & Hasselmo, 2014; Marchette, Ryan, & Epstein, 2017). Dorsal/posterior portions of rodent/primate hippocampus contain a narrow‐meshed coordinate system that captures details of an environment, whereas ventral/anterior hippocampus contains a wide‐meshed coordinate system that captures larger spaces such as whole environments (Komorowski et al., 2013; Poppenk, Evensmoen, Moscovitch, & Nadel, 2013). Thus, the memory of an event can be generalized to large spaces and whole environments due to the large‐sized place fields that covers large areas. On the other hand, events happening at two nearby locations can be separated due to neurons with small‐sized place fields.
13.3.2. Unique maps in hippocampus
MEC contains spatially modulated cells providing coherent and always active universal maps. These maps are different from those formed by hippocampal place cells (O'Keefe & Conway, 1978; O'Keefe & Dostrovsky, 1971; O'Keefe & Nadel, 1978). Place cells turn on/off in different environments or if active in multiple environments the place fields are not located in comparable locations (Figure 8c). Thus, place cells provide statistically independent ensemble activity in separate environments. This phenomenon, commonly referred to as global remapping, has been suggested to reduce the risk for memory interference (Alme et al., 2014; Leutgeb et al., 2005; Muller & Kubie, 1987), as each environment has its distinct signature map which is recollected within a theta cycle (Jezek, Henriksen, Treves, Moser, & Moser, 2011). Thus, the environment‐specific ensemble activity in hippocampus provides a unique internal map of space and therefore offers a neural substrate which could associate events to a unique location.
How can a universal coherent map, including grid cells with repetitive and symmetrical activity patterns contribute to the formation of unique ensemble activity patterns of place cells in the downstream structure hippocampus? It turns out that grid cells are organized in distinct modules (Figure 8a), where each module contains grid cells with similar spacing between their firing fields and a similar orientation of the grid pattern relative to the environment (Gu et al., 2018; Stensola et al., 2012). If these modules orient and anchor independently to landmarks, a linear summation of grid cells from different modules would, in different environments, create unique ensemble activity in the hippocampus (Fyhn et al., 2007; McNaughton et al., 2006; Solstad, Moser, & Einevoll, 2006; Stensola et al., 2012). Just as each of the wheels on a combination lock can be turned independently from each other and thereby make thousands of unique combinations, a few differently sized grid modules, each with independent anchoring to the environment would be sufficient to create unique ensemble activity for a large number of environments in the downstream structure hippocampus (Figure 8b). Converging inputs from multiple independent grid modules provide a potential mechanism for remapping of hippocampal ensemble activity, thus forming unique ensemble activity of place cells in each environment (global remapping) (Monaco & Abbott, 2011; Solstad et al., 2006; Sreenivasan & Fiete, 2011; Stemmler, Mathis, & Herz, 2015). It should also be noted that place cells receive inputs from all classes of spatially modulated neurons in MEC (Ye et al., 2018; Zhang et al., 2013) and place cells have been reported to be responsive to environmental cues such as borders or objects (Deshmukh & Knierim, 2013; O'Keefe & Burgess, 1996). These observations clearly indicate that also other functionally defined cell groups may contribute to the generation of unique place cell ensemble activity in hippocampus. It has been suggested that the formation of unique ensemble activity is the neural substrate of episodic memories and that the formation of such ensembles is dependent on long‐term potentiation. This idea is supported by experiments where plasticity in hippocampal synapses were saturated by high‐frequency stimulations (Brun, Ytterbo, Morris, Moser, & Moser, 2001); in well‐trained rats such stimulations deteriorated performance in a spatial memory task, suggesting that memory retrieval is dependent on the pattern of synaptic weights in these ensembles.
Taken together, entorhinal–hippocampal circuitry fulfills the requirements of a system supporting the spatial component of episodic memories. First, the spatial code in entorhinal cortex and hippocampus appears immediately after introduction to a novel environment and covers relevant scales of space, thereby providing an instant, universal metric which could be used for one‐shot encoding of episodes. Next, the spatial code in hippocampus is unique in different environments, thus providing environment‐specific maps which events can be associated with. The unique hippocampal maps are possibly achieved by combining multiple independent scales of space in MEC. Thus, MEC constitutes a perfect system for generating hippocampal cognitive maps in which objects, landmarks, and events can be mapped into.
13.4. POPULATION ACTIVITY IN ENTORHINAL CORTEX AND HIPPOCAMPUS VARY WITH TIME
Episodic memories are organized not only in space but also in time. Encoding “when” an experience happened relative to other events is essential for successful retrieval. In humans, this ability is dependent on an intact hippocampus (Dede, Frascino, Wixted, & Squire, 2016; Mayes et al., 2001) which shows increased activity when subjects recall the temporal order of events (Kalm, Davis, & Norris, 2013; Lehn et al., 2009). Hippocampal‐lesioned rats are similarly impaired in determining the duration of an event or the sequence of events (Fortin, Agster, & Eichenbaum, 2002; Jacobs, Allen, Nguyen, & Fortin, 2013). The introduction of a temporal gap between a conditioned and unconditioned stimulus or within a spatial working memory task changes the task from being insensitive to become sensitive to hippocampal lesions (Ainge, van der Meer, Langston, & Wood, 2007; Jacobs et al., 2013; McEchron, Bouwmeester, Tseng, Weiss, & Disterhoft, 1998). Likewise, inactivation of entorhinal neurons in rodents also disturbs time perception (Robinson et al., 2017; Suh, Rivest, Nakashiba, Tominaga, & Tonegawa, 2011). These observations suggest an important role for the hippocampus and entorhinal cortex in sorting and tagging events which are separated in time.
How does hippocampus code the temporal relationship of events? Electrophysiological recordings have revealed that hippocampal neurons are organized into ensembles where the activity of individual cells can be temporally organized. For instance, sequences of place cells are compressed within theta cycles so that place cells coding passed or upcoming locations are active during the same theta cycle. Importantly, the order of active cells during each of these cycles reflects the order each of the place fields are visited (O'Keefe & Recce, 1993; Skaggs, McNaughton, Wilson, & Barnes, 1996). This phenomenon commonly referred to as “phase precession” is also present in cells representing nonspatial features of experience (Lenck‐Santini, Fenton, & Muller, 2008; Pastalkova, Itskov, Amarasingham, & Buzsaki, 2008; Terada, Sakurai, Nakahara, & Fujisawa, 2017) suggesting that chunking neuronal activity into temporally ordered ensembles is a fundamental organization principle of hippocampus (Jensen & Lisman, 1996; Skaggs & McNaughton, 1996).
In addition to organizing neural ensembles into sequences within a theta cycle, hippocampal neurons also code for temporal progression within episodes. “Time cells” have receptive fields for a specific duration from an event (ranging from 0 to 20 s in experiments; Figure 9a). The activity of time cells tile an interval such as time elapsed during running on a linear track, in a running wheel or time of a delay within a working memory task (Gill, Mizumori, & Smith, 2011; Itskov, Curto, Pastalkova, & Buzsaki, 2011; Kraus et al., 2013; MacDonald, Carrow, Place, & Eichenbaum, 2013; Pastalkova et al., 2008; Redish, Rosenzweig, Bohanick, McNaughton, & Barnes, 2000; Salz et al., 2016), and is suggested to code for the temporal organization of an episode. Importantly, these time cell sequences develop and stabilize in parallel with learning, suggesting a link between the formation of time cell ensembles and memory (Modi, Dhawale, & Bhalla, 2014).
Figure 9.
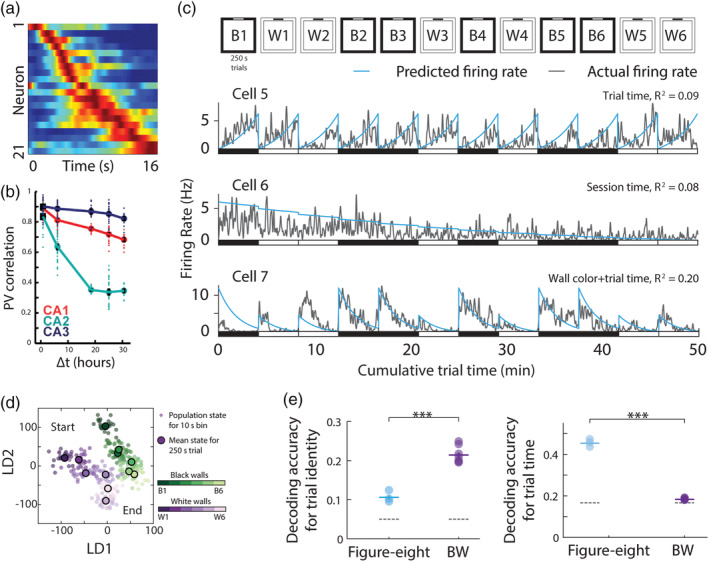
Time codes in hippocampus and entorhinal cortex. (a) Time cells in hippocampus tile a time interval within an episode when a rat runs on a treadmill. Each row represents the normalized firing rate of one neuron (blue, no firing; red, peak firing of that neuron) and the neurons are sorted by their peak firing time. Adapted from Kraus, Robinson, White, Eichenbaum, and Hasselmo (2013). (b) Stability of location specific activity of place cells in CA1 (red), CA2 (green), and CA3 (blue) was measured as population vector correlations between pairs of recordings. The black error bars report the mean ± SEM for pair‐wise comparisons at each time interval. Over time the population activity of CA2 (green) and CA1 neurons (red) decrease as a function of elapsed time between recording sessions. Adapted from Mankin, Diehl, Sparks, Leutgeb, and Leutgeb (2015). (c) Temporal codes in lateral entorhinal cortex. Top: experimental design; Animals ran 12 times 250 s trials in boxes with either black or white walls. Bottom: example general linear model fits for cells with selectivity for trial time (Cells 5 and 7) or session time (Cell 6), with the observed firing rate shown in grey, and predicted firing rate in blue, suggesting that the passage of time is encoded in firing rates of individual cells. (d) Two‐dimensional projections of neural population responses during the experiment depicted in “a”. Axes correspond to the first two linear discriminants (LD1 and LD2; arbitrary units). The wall color of each trial is indicated by a shade of green (black walls) or purple (white walls) with progression of shade from dark to light indicating the progression of trials. Population responses showed a progression corresponding to the temporal order of the experiment. (e) Left: comparison of decoding accuracy for trial identity when the rat is either engaged in alternating left/right laps on a figure eight maze or during the 12 black/white trials‐experiment (BW) depicted in “c”. Decoding accuracy for trial identity is higher during free foraging in BW than in the figure eight maze (p < .0001). Right: same as left, but for time epochs within a trial. Decoding of trial time is higher in the figure eight maze than in BW (p < 10−10). Grey‐dotted lines indicate chance levels. c–e adapted from (Tsao et al., 2018) [Color figure can be viewed at wileyonlinelibrary.com]
Intriguingly, time cells share many features with place cells. Activity of time cells, like activity of place cells, differentiates memory‐based decisions by modulating their rate (Gill et al., 2011; Pastalkova et al., 2008). Like place cells, in which the place fields are reorganized after changes in the spatial layout, the receptive fields of time cells are reorganized if the duration of the mapped interval is changed (Kraus et al., 2013; MacDonald, Lepage, Eden, & Eichenbaum, 2011). Next, different sequences of time cells are responsive to the nature of the mapped interval; depending on the event or condition which initiated the delay period, different ensembles of time cells are enrolled (Gill et al., 2011; MacDonald et al., 2011; MacDonald et al., 2013; Pastalkova et al., 2008). Thus, as distinct place cell ensembles are active during exploration of different environments (“remapping”), distinct ensembles of time cells are active during delay periods initiated by different conditions (“retiming”). These findings are paralleled by experiments in humans; during recall of sequences, hippocampus display activity patterns that are specific to unique sequences, thus resembling the retiming phenomena of time cells in rodents (Hsieh, Gruber, Jenkins, & Ranganath, 2014; Thavabalasingam, O'Neil, Tay, Nestor, & Lee, 2019). Importantly, retiming in both humans and rodents develops with acquisition and supports the role of hippocampus in separating different memories also in time (Gill et al., 2011; Kalm et al., 2013).
As grid cells in MEC are thought to contribute to place cell formation in hippocampus, sequence‐related activity in the form of time cells in hippocampus has been shown to rely on MEC activity. Inactivation of the latter neurons result in a degradation of time cells in hippocampus (Robinson et al., 2017). Accordingly, a large set of MEC neurons, including grid cells, have been shown to code for time similarly to time cells in hippocampus (Heys & Dombeck, 2018; Kraus et al., 2015). Taken together, these findings suggest that features of time cell activity in hippocampus and MEC share many features with place and grid cell activity, suggesting shared mechanisms for the generation of place and time cell codes in hippocampus.
While time cells in hippocampus keep track of the temporal progression within an event, hippocampal ensembles also keep track of time at longer timescales by a slow drift of their population activity over time (Figure 9b) (Folkerts, Rutishauser, & Howard, 2018; Howard, Viskontas, Shankar, & Fried, 2012; Manns, Howard, & Eichenbaum, 2007; Mau et al., 2018; Ziv et al., 2013). This phenomenon is particularly prominent in Cornu Ammonis (CA)2, and to some extent in other hippocampal and parahippocampal areas such as CA1 and MEC (Diehl, Hon, Leutgeb, & Leutgeb, 2019; Mankin et al., 2015). In these areas, the population of neurons display a variable degree of stability; some neurons maintain their receptive field and firing rate whereas other neurons vary their firing rates and/or shift their receptive field. This mix of stable and unstable codes results in population activity that slowly drifts over hours, so that two events happening close in time are represented by more similar ensemble activity compared to two events happening at distant time points. This phenomenon is in accordance with the hypothesis that temporal relationships of episodes are encoded by gradually changing representations in CA2 and CA1 so that the duration of an episode or durations between episodes can be read out by the dissimilarity of ensemble activity (Figure 9b) (Mankin et al., 2015). These ideas are supported by experimental evidence; if rats or humans sample a sequence of stimuli and afterwards have to decide which of two of the stimuli were sampled most recently, there is a significant difference between error and success trials; during correct trials the change of population activity is larger compared to in error trials (Jenkins & Ranganath, 2016; Manns et al., 2007). Next, functional magnetic resonance imaging (fMRI) patterns of activity is more similar for pairs of stimuli which is remembered as being close in time (Ezzyat & Davachi, 2014). Together, these studies suggest that continuously changing population activity is involved in coding passage of time.
An unstable and drifting signal might seem counterintuitive in the context of a system used for providing cognitive maps. A cognitive map should remain stable across the same exposure to sensory stimuli, which is partly not the case for place cells in some regions of the hippocampus (Mankin et al., 2012; Mankin et al., 2015). In an episodic memory context, a mix of stable and unstable activity patterns might be necessary to map temporal relations of experiences. Continuously varying ensemble activity would implicate that memorable events experienced at two different time points are allocated to partly different population activity, where time‐dependent unique activity patterns provide a temporal context for specific time points (Cai et al., 2016; Howard & Kahana, 2002). The idea is that neurons that stably represent prominent features of the experience are associated with neurons providing a temporal tag for that experience and together they form a unique representation of a particular experience. These ideas are supported from experiments in rodents. It is now established that episodic memories, such as the memory of being foot‐shocked, are partly allocated to hippocampal neurons that transiently express c‐fos during encoding, and that memory retrieval can be elicited by experimentally inducing activity in these cells (Liu et al., 2012). Interestingly, the same neurons display greater drift compared to c‐fos negative cells (Tanaka et al., 2018), suggesting a link between drifting cells and their role in providing unique ensemble activity during encoding of one‐shot episodic memories. How could such a system function during natural memory recall? When the subject is exposed to a retrieval cue, cells which stably represent features common to the retrieval cue and the encoded experience would lead to pattern completion processes in hippocampus which would reinstate the activity pattern at encoding, including the associated neuronal temporal context (Howard, Fotedar, Datey, & Hasselmo, 2005). Data from single‐cell recordings and fMRI in humans suggest that this is the case; during successful recall, hippocampal ensemble activity reinstates the activity pattern that was present during encoding (Folkerts et al., 2018). Next activity patterns for two events that are remembered as close in time are more similar compared to two events that are remembered to be separated by a longer time interval on timescales ranging from minutes to months, suggesting that the temporal context had drifted between encoding of the two events and that the temporal context is retrieved during recall (Deuker, Bellmund, Navarro Schroder, & Doeller, 2016; Nielson, Smith, Sreekumar, Dennis, & Sederberg, 2015). Thus, two similar episodes experienced at the same place could be separated by a temporally varying signal and encoded onto distinct ensemble activity (Figure 11). Together these studies suggest that signals varying over time are essential for allocating memories of one‐shot experiences into unique activity patterns.
Figure 11.
Schematic overview of spatial and temporal codes in entorhinal cortex and hippocampus. (a) Agent moves and have four experiences (two of them in the same position). Black dashed line indicates path of the animal. (b) Information concerning internal states of the animal, sensory inputs, and self‐motion signals reach entorhinal cortex. (c) Neuronal population activity (green line) in lateral entorhinal cortex moves with the experience of the agent. (d) Neuronal population activity (green line) in medial entorhinal cortex moves with the position of the animal. (e) Neuronal population activity (green line) in CA2 and CA1 in hippocampus moves with the position and experience of the animal. Episodes are mapped in a spatiotemporal framework conveyed from entorhinal cortex. Neural activity is more similar (but not identical) during two visits to the same location [Color figure can be viewed at wileyonlinelibrary.com]
Sequential firing of different cells during learned time intervals and continuously drifting population representations are pronounced temporal signals in the hippocampus. However, both of these temporal representations may only partly fulfill the requirements for a temporal code supporting the encoding of episodic memories. Time‐cell sequences develop with learning and therefore does not fulfill the requirement of an instantaneous time signal needed to capture one‐shot episodes. The drifting representations of CA2 and CA1 arise spontaneously as required; however, the temporal drift occurs on the scales of hours that is not necessarily sufficient to capture the details of a typical episode. Recent electrophysiological studies in rodents and fMRI studies in humans suggest that a likely source of temporal information is found in the lateral entorhinal cortex (LEC) (Bellmund, Deuker, & Doeller, 2018; Montchal, Reagh, & Yassa, 2019; Tsao et al., 2018). We recorded LEC neurons while rats were freely foraging in an arena for 12 trials each lasting about 4 min (Figure 9c). In such a setup, we hypothesized that the rat would treat each of the 12 visits to the arena as distinct episodes and would serve as a reference frame for putative temporal representations. Interestingly, about 20% of the recorded neurons in LEC displayed ramping activity through the experiment (Figure 9c). As a new trial was initiated, neurons started out with a certain firing rate and from then on displayed a tendency to either increase or decrease their firing rates as time passed by. These ramping cells displayed two important features. First, the change in firing rates displayed a wide range of time constants as some cells ramped up/down faster than others. Such a feature has been proposed to be sufficient for the formation of cells responsive at certain time intervals, like time cells observed in hippocampus and MEC (Howard et al., 2014; Kraus et al., 2015; MacDonald et al., 2011). Secondly, ramping cells were reset by different “landmark” events. Some cells reset when the animal was moved from the holding pot and into the arena, whereas others did not reset to events related to our experimental design. These observations suggest that different cells had different triggers for resetting of their ramping activity. Population activity of LEC cells could thereby provide a unique tag for any time point in the experiment. Time epochs ranging from seconds, minutes, and possible also hours could be decoded from LEC population activity (Figure 9d).
Intriguingly, the temporal signal in LEC is dependent on ongoing experience. While rats were running on a figure eight maze, where the nature of the task result in repetitive behavior, neural activity could be used to decode time across the experiment (i.e., different laps) far less accuratey, whereas the ability to decode time within each of the laps improved (Figure 9e). Consequently, activity from the recorded cells could not be used to differentiate early laps from late laps, but could instead be used to differentiate events at very short time scales. Thus, the temporal signal in LEC changed when the animal was engaged in different tasks. During free exploration, LEC population activity continuously changed, whereas activity was anchored to temporal landmarks when engaged in the structured and repetitive tasks. This difference is presumably linked to changes in inputs from higher order sensory areas, areas devoted represent behavioral and internal states of the animal, and/or inputs from hippocampus which likely is more structured and repetitive during the memory task (Tsao, 2017; Tsao et al., 2018). These observations imply that LEC derives time directly from the structure of ongoing experience. Just as the spatially modulated cells in MEC are continuously updated by self‐motion signals and reset by environmental cues, the flow of experience moves and resets population activity in LEC, suggesting a strong link between “what” a subject experiences and the encoding of temporal information (“when”).
These features make the temporal signal in LEC particularly well suited for coding “episodic time”; the order of events within experience. It arises spontaneously and covers the temporal granularities expected of a code supporting encoding of episodic memories. These findings are paralleled by reports from human fMRI studies reporting that activity patterns in entorhinal cortex, including its lateral part, continuously change during encoding of an experience and that the amounts of change during encoding is related to later recalled duration of the same experience (Bellmund et al., 2018; Lositsky et al., 2016). Even though it is currently not known how the temporal signals in the entorhinal–hippocampal circuits are related, it is tempting to suggest that both the formation of time cell sequences and the drifting activity in hippocampus could be driven by the temporal signals in LEC which covers both of these scales. Thus, it could be hypothesized that the temporal code in LEC, driven by the continuous flow of experience, could elicit sequential activity patterns in hippocampus covering multiple temporal scales.
13.5. ENTORHINAL CORTEX FILLS MEMORIES WITH CONTENT
Our memories are populated with content such as sensory cues, objects, and emotions. In experimental settings, content of an experience is often operationalized by presenting objects or sensory stimuli while relating neuronal responses to these stimuli. How does neuronal activity in entorhinal cortex represent such stimuli? Cells in the entorhinal cortex of monkeys and in rat and human LEC are preferably active when the animal encounters objects or cues (Deshmukh & Knierim, 2011; Kreiman, Koch, & Fried, 2000; Neunuebel, Yoganarasimha, Rao, & Knierim, 2013; Quiroga, Reddy, Kreiman, Koch, & Fried, 2005; Reagh et al., 2018; Reagh & Yassa, 2014; Suzuki, Miller, & Desimone, 1997). More specifically, a population of rat LEC neurons is active when the animal is in close vicinity of objects (Deshmukh & Knierim, 2011) and a small proportion of LEC neurons signals where objects have previously been localized (Tsao, Moser, & Moser, 2013). The presence of these “trace cells” suggest that LEC codes object information within a spatial framework and that object–place associations exist already at the level of LEC.
There exists even more complex object‐related activity patterns in entorhinal cortex. Although a subset of LEC cells code the direction to landmarks referenced to the animals head (egocentric) (Wang et al., 2018), object‐vector cells in MEC signal the direction and distance to the object referenced to a global environmental bound axis irrespective of the head direction of the animal (allocentric) (Høydal, Skytøen, Andersson, Moser, & Moser, 2019). Thus, as an object is moved, the firing field moves with the object so that the vectorial relationship between the object and the firing field is maintained. Intriguingly, the activity pattern of object‐vector cells is instant and universal; similar to the other spatially modulated cells in MEC. Object‐vector cells appear immediately after the animal is introduced to the environment and show generalized responses to all objects independent of their identity. Thus object‐vector cells conjunctively signal the presence of objects and the position of the animal relative to objects. In more realistic environments filled with multiple objects, the combined activity of multiple object‐vector cells with different directional and distance preferences could potentially signal the spatial arrangement of objects in an environment. In an environment with multiple objects there will, for each spatial constellation of objects, be a unique combination of active object‐vector cells in any position the animal occupies, likely supporting the ability to use object constellations to find hidden food (Collett, Cartwright, & Smith, 1986). Thus, single object‐vector cells signal the position of the animal relative to any object whereas an ensemble of object‐vector cells may signal the position of the animal relative to a specific spatial configuration of objects. These findings are likely related to findings in humans; a subset of cells in human entorhinal cortex display selectivity to specific scenes filled with landmarks (Mormann et al., 2017).
In parallel to these findings in entorhinal cortex, a subset of hippocampal cells has been shown to signal location close to objects (Battaglia, Sutherland, & McNaughton, 2004) or distance to objects (landmark‐vector cells) (Deshmukh & Knierim, 2013). Even though a limited number of such object‐landmark cells have been described, the data available suggest that such responses develop over time after an object is introduced to the environment and only a minority of the objects elicit a response. The landmark‐vector cells in hippocampus, in contrast to object‐vector cells in MEC, seem therefore to be dependent on experience and signal the position of the animal relative to unique sets of objects. These findings suggest that the object‐vector cells in MEC are likely entraining landmark‐vector cells in hippocampus. Moreover, they suggest that an immediate representation of the position of an animal relative to objects is likely combined with information of object identity in hippocampus so that spatial relationships to unique landmarks can be encoded, and thus providing a possible mechanism for how events are mapped relative to identified landmarks. Together, these observations of object‐responsive cells in entorhinal cortex and hippocampus could underlie the ability to use positions relative to objects, for instance to localize a hidden food storage (Collett et al., 1986).
Information from any sensory system is directed to the entorhinal–hippocampal system (Burwell & Amaral, 1998), and memory retrieval can be cued by all types of sensory stimuli. As described in Proust's (1913) novel, the taste and smell of a Madeleine cake dipped in tea sent the main character back to his childhood where he had dipped the cake in tea at visits at his aunt's house. How do entorhinal cortex and hippocampus interact during encoding and retrieval of such memories? In Section 4.1, we use odor processing in these areas as an example for how nonspatiotemporal variables are encoded and associated with the spatial scaffold provided by the entorhinal–hippocampal circuit. We tested the example in Proust's novel in the lab—how can odors be encoded and used as retrieval cues for memories? Igarashi, Lu, Colgin, Moser, and Moser (2014) trained animals to use odors as retrieval cues for reward‐locations while simultaneously recording cells in LEC and in the hippocampus. In these experiments, Igarashi et al. observed that the proportion of odor‐selective neurons in LEC increased in parallel to the acquisition of odor–reward location associations (Figure 10). A similar development was also seen in hippocampal neurons; however, the increase of odor‐selective neurons in hippocampus lagged behind the development in LEC (Figure 10b,c). In addition, Igarashi et al. observed changes in the local field potential; in naïve animals, LEC and hippocampal local field potentials were uncoupled. However, in parallel to learning and in parallel to the increasing number of odor‐selective LEC neurons, hippocampus and LEC developed coherent oscillatory activity in the gamma band (20–40 Hz). Thus, the proportion of odor‐selective neurons first increased in LEC, whereas the number of hippocampal odor‐selective neurons increased during the emergence of gamma coupling between the two regions (Figure 10c). These findings propose that stimulus associations can occur in LEC before the hippocampus, suggesting that LEC entrains hippocampus to obtain odor–place associations, somewhat similar to the suggested relationship between object‐vector cells in MEC and landmark‐vector cells in hippocampus. Moreover, Igarashi et al. proposed that gamma coupling is important for such encoding likely because coherent activity in this frequency range provides presynaptic and postsynaptic activity within a time window that allows synaptic strengthening to occur (Bi & Poo, 1998). Next, they also suggest that gamma coupling is important for recollection; when coherent activity was disrupted, odor maps in both entorhinal cortex and hippocampus vanished and animals searched at the wrong site for the reward.
Figure 10.
Olfactory coding in lateral entorhinal cortex (LEC) and hippocampus. Adapted from Igarashi et al. (2014). (a) Rats were trained to associate two odors with two different reward locations. Responses are shown for cells with significant activity at the cue port during training of naïve animals (T1) until reaching asymptotic performance (85% correct, T5). Right column contains error trials during T5. Each row shows data for one cell around the time of odor sampling (starting from white dashed line). Top: distal CA1 cells; bottom: LEC cells. Selectivity is color coded (−1 and + 1 indicate complete selectivity for banana [red] and chocolate [green], respectively). (b) Population odor selectivity was measured by correlating population activity obtained during sampling of the two different odors. Higher values indicate more odor‐selective population coding. Red lines indicate 95th percentiles from shuffled distributions. Odor selectivity develops in both LEC and hippocampus in parallel to improved performance. Odor selectivity decreases during error trials (T5e) compared to during a similar number of correct trials (T5d). (c) Development of task performance, gamma coherence and selectivity in CA1 and LEC. Variables are normalized onto a scale from 0 (T1) to 1 (T5) (mean ± SEM). Odor selectivity increases faster in LEC compared to CA1 [Color figure can be viewed at wileyonlinelibrary.com]
Taken together, the odor‐based retrieval of a spatial memory and the entorhinal and hippocampal codes for objects illustrate how content of experiences are represented and integrated into memory. Entorhinal cortex provides codes for sensory cues and associations between these cues. Such associations can first be observed in LEC followed by hippocampal neurons. The observation that entorhinal neurons (Aronov, Nevers, & Tank, 2017; Keene et al., 2016; Young, Otto, Fox, & Eichenbaum, 1997) and hippocampal neurons (Ho et al., 2011; Komorowski, Manns, & Eichenbaum, 2009; Leutgeb et al., 2005) can acquire selectivity to nonspatial features of experience suggests that the findings described above can likely be generalized to how information from any sensory system is coded into episodic memory. It should still be emphasized that our knowledge of how these types of representations are generated and organized in entorhinal–hippocampal circuits are still at a nascent state compared to our knowledge and ideas of how spatial codes (and to some extent temporal codes) are generated and organized in the same circuits.
13.5.1. What, where, and when signals are entangled and form unique ensembles
During an experience, the brain is constantly bombarded by massive amounts of external sensory information which potentially could be encoded and stored into memory. The binding of sensory stimuli into a cohesive and unique episodic memory likely depends on neuronal activity in entorhinal cortex that signals temporal relationships (“when”), a spatial universal metric (“where”), and the experience itself (“what”). The spatial and temporal signals arise spontaneously as a result of changes in ongoing experience; the spatial signal is likely driven by self‐motion signals and updated by environmental cues along the route (such as interactions with known landmarks). Similarly, the temporal signal found in LEC depends on the content and structure of the experience, thus suggesting that the sense of time and space are subjective and depends on how the agent is experiencing and perceiving the world.
Population activity in entorhinal cortex, including the spatial signal, the temporal varying signal and signals representing sensory aspects of an experience are conveyed to hippocampus where they are stored into unique ensemble activity (Figure 11). This is likely achieved due to hippocampus' ability to orthogonalize the pattern of activity against already encoded patterns (pattern separation) (Leutgeb, Leutgeb, Treves, Moser, & Moser, 2004), in addition to synaptic modifications due to spike‐time‐dependent plasticity and due to hippocampal ability to consolidate memories by its capacity to replay activity (Bi & Poo, 1998; McNaughton & Morris, 1987). The result of these processes is unique ensembles of place cell maps and time cell sequences which have been suggested to conjunctively represent a memory trace of space, time, and other aspects of experience (Hasselmo, 2009). When hippocampus is exposed to a partial or degraded input of the encoded memory trace, the hippocampal system can recollect the full memory trace through the process of pattern completion (McNaughton & Morris, 1987). Subiculum and CA1 provide outputs to the rest of cortex and have therefore been suggested to serve as indices for cortical reactivation during recall (Teyler & DiScenna, 1986). Population activity of hippocampal ensembles is therefore likely carriers of high‐dimensional aspects of episodic memories that are encoded in a spatiotemporal framework.
Both the spatial and temporal codes capture multiple levels of details. Spatial codes in MEC and hippocampus display both a fine‐ and coarse‐grained resolution of space. Comparably can the sequence of events be inferred with a precision ranging from seconds to hours. The temporal code in LEC covers these time scales. Thus, the different spatiotemporal codes are particularly suited to support formation of episodic memories; fine‐grained representations could link events to the details of the local environment and to the detailed sequences of events, whereas coarse‐grained representations could link events to environments and to longer episodes. These observations are paralleled with findings in humans where spatiotemporal codes are organized at multiple levels of granularity with a corresponding anatomically organization as in rodents (Marchette et al., 2017; Nadel et al., 2013; Nielson et al., 2015). Such an organization could for instance support both the ability to remember the exact location of hidden food storages and the sequence these storages were collected. Likewise, we might speculate that low spatial and temporal resolutions might lead to high interference and generalization of memories which could occur for instance during the development of phobias.
Given the entorhinal and hippocampal signals that are correlated with features of ongoing experience, what is the evidence that the same neurons actually contribute to the formation and retrieval of episodic memories? There are several lines of reports suggesting that this is the case. First of all, activity patterns elicited during sharp wave ripples during sleep and rest are similar to those that can be recorded during encoding (Jensen & Lisman, 1996; Skaggs & McNaughton, 1996; Wilson & McNaughton, 1994). In essence, previous experiences are replayed in hippocampus, a feature that has been suggested to be an important mechanism for memory retrieval and consolidation in both hippocampus and connected extra‐hippocampal structures (Ego‐Stengel & Wilson, 2010; Girardeau, Benchenane, Wiener, Buzsaki, & Zugaro, 2009; Jadhav, Kemere, German, & Frank, 2012; Karlsson & Frank, 2009). Next, the same neuronal signals as was present during encoding of an experience are reinstated when humans recollect the same experience from memory (Gelbard‐Sagiv, Mukamel, Harel, Malach, & Fried, 2008; Mack & Preston, 2016; Miller et al., 2013; Vaz, Inati, Brunel, & Zaghloul, 2019). Somehow comparably are sequences of neurons activated when rodents make memory‐based decisions on how to get to a future goal location, as if possible paths are recruited from memory and evaluated before a decision is made (Johnson & Redish, 2007; Pfeiffer & Foster, 2013). These findings suggest that functional correlates present during encoding are actually necessary for retrieval of the same memory.
Our understanding of encoding and retrieval of episodic memories has made considerable progress the last decades. Much of this progress is anchored in the pioneering and thought‐provoking book of O'Keefe and Nadel (1978). The idea that there exists a memory system involved in forming cognitive maps of the environments we encounter is still the foundation stone for understanding how activity in entorhinal–hippocampal circuits can underlie higher mental functions, such as episodic memories. As suggested in the book, the spatial cognitive maps could be extended to also provide cognitive “maps” for distinct episodes; spatiotemporal scaffolds in which different aspects of an event can be registered. We endorse this idea, but we would like to emphasize the role of the entorhinal cortex in this process. Here, we have reviewed data showing that entorhinal cortex provides a spatial (“where”) and temporal scaffold (“when”) of ongoing experience. In addition, we would like to emphasize that associations between sensory stimuli and space are already formed in LEC. We suggest that hippocampus maps these associations on top of the spatiotemporal scaffolds. Thus, we can imagine a process where entorhinal cortex presents a “movie” of ongoing experience to the hippocampus that acts as an editor of this continuous flow of information. In essence, hippocampus is able to extract and tag memorable moments of ongoing experience and consolidate them into memory. In this way, entorhinal cortex and hippocampus could contain the neural coding mechanisms that underlie our ability to form episodic memories.
CONFLICT OF INTERESTS
The authors declare no potential conflict of interest.
REFERENCES
Aggleton, J. P. (2014). Looking beyond the hippocampus: Old and new neurological targets for understanding memory disorders. Proceedings of the Biological Sciences, 281(1786), 20140565. https://doi.org/10.1098/rspb.2014.0565
Ainge, J. A., van der Meer, M. A., Langston, R. F., & Wood, E. R. (2007). Exploring the role of context‐dependent hippocampal activity in spatial alternation behavior. Hippocampus, 17(10), 988–1002. https://doi.org/10.1002/hipo.20301
Alme, C. B., Miao, C., Jezek, K., Treves, A., Moser, E. I., & Moser, M. B. (2014). Place cells in the hippocampus: Eleven maps for eleven rooms. Proceedings of the National Academy of Sciences of the United States of America, 111(52), 18428–18435. https://doi.org/10.1073/pnas.1421056111
Annese, J., Schenker‐Ahmed, N. M., Bartsch, H., Maechler, P., Sheh, C., Thomas, N., … Corkin, S. (2014). Postmortem examination of patient H.M.'s brain based on histological sectioning and digital 3D reconstruction. Nature Communications, 5, 3122. https://doi.org/10.1038/ncomms4122
Aronov, D., Nevers, R., & Tank, D. W. (2017). Mapping of a non‐spatial dimension by the hippocampal‐entorhinal circuit. Nature, 543(7647), 719–722. https://doi.org/10.1038/nature21692
Barry, C., Hayman, R., Burgess, N., & Jeffery, K. J. (2007). Experience‐dependent rescaling of entorhinal grids. Nature Neuroscience, 10(6), 682–684. https://doi.org/10.1038/nn1905
Battaglia, F. P., Sutherland, G. R., & McNaughton, B. L. (2004). Local sensory cues and place cell directionality: Additional evidence of prospective coding in the hippocampus. The Journal of Neuroscience, 24(19), 4541–4550. https://doi.org/10.1523/jneurosci.4896‐03.2004
Bellmund, J. L. S., Deuker, L., & Doeller, C. F. (2018). Structuring time in human lateral entorhinal cortex. bioRxiv. https://doi.org/10.1101/458133
Bi, G. Q., & Poo, M. M. (1998). Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. The Journal of Neuroscience, 18(24), 10464–10472.
Boccara, C. N., Sargolini, F., Thoresen, V. H., Solstad, T., Witter, M. P., Moser, E. I., & Moser, M. B. (2010). Grid cells in pre‐ and parasubiculum. Nature Neuroscience, 13(8), 987–994. https://doi.org/10.1038/nn.2602
Brun, V. H., Solstad, T., Kjelstrup, K. B., Fyhn, M., Witter, M. P., Moser, E. I., & Moser, M. B. (2008). Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus, 18(12), 1200–1212. https://doi.org/10.1002/hipo.20504
Brun, V. H., Ytterbo, K., Morris, R. G., Moser, M. B., & Moser, E. I. (2001). Retrograde amnesia for spatial memory induced by NMDA receptor‐mediated long‐term potentiation. The Journal of Neuroscience, 21(1), 356–362.
Burak, Y., & Fiete, I. R. (2009). Accurate path integration in continuous attractor network models of grid cells. PLoS Computational Biology, 5(2), e1000291. https://doi.org/10.1371/journal.pcbi.1000291
Burgess, N., Maguire, E. A., & O'Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron, 35(4), 625–641.
Burwell, R. D., & Amaral, D. G. (1998). Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. The Journal of Comparative Neurology, 398(2), 179–205.
Cai, D. J., Aharoni, D., Shuman, T., Shobe, J., Biane, J., Song, W., … Silva, A. J. (2016). A shared neural ensemble links distinct contextual memories encoded close in time. Nature, 534(7605), 115–118. https://doi.org/10.1038/nature17955
Campbell, M. G., Ocko, S. A., Mallory, C. S., Low, I. I. C., Ganguli, S., & Giocomo, L. M. (2018). Principles governing the integration of landmark and self‐motion cues in entorhinal cortical codes for navigation. Nature Neuroscience, 21(8), 1096–1106. https://doi.org/10.1038/s41593‐018‐0189‐y
Cappaert, N. L. M., Van Strien, N. M., & Witter, M. P. (2015). Chapter 20—Hippocampal formation. In G. Paxinos (Ed.), The rat nervous system (4th ed., pp. 511–573). San Diego, CA: Academic Press.
Clayton, N. S., & Dickinson, A. (1998). Episodic‐like memory during cache recovery by scrub jays. Nature, 395(6699), 272–274. https://doi.org/10.1038/26216
Collett, T. S., Cartwright, B. A., & Smith, B. A. (1986). Landmark learning and visuo‐spatial memories in gerbils. Journal of Comparative Physiology. A, 158(6), 835–851.
Couey, J. J., Witoelar, A., Zhang, S. J., Zheng, K., Ye, J., Dunn, B., … Witter, M. P. (2013). Recurrent inhibitory circuitry as a mechanism for grid formation. Nature Neuroscience, 16(3), 318–324. https://doi.org/10.1038/nn.3310
Dede, A. J., Frascino, J. C., Wixted, J. T., & Squire, L. R. (2016). Learning and remembering real‐world events after medial temporal lobe damage. Proceedings of the National Academy of Sciences of the United States of America, 113(47), 13480–13485. https://doi.org/10.1073/pnas.1617025113
Deshmukh, S. S., & Knierim, J. J. (2011). Representation of non‐spatial and spatial information in the lateral entorhinal cortex. Frontiers in Behavioral Neuroscience, 5, 69. https://doi.org/10.3389/fnbeh.2011.00069
Deshmukh, S. S., & Knierim, J. J. (2013). Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus, 23(4), 253–267. https://doi.org/10.1002/hipo.22101
Deuker, L., Bellmund, J. L., Navarro Schroder, T., & Doeller, C. F. (2016). An event map of memory space in the hippocampus. eLife, 5, e16534. https://doi.org/10.7554/eLife.16534
Diehl, G. W., Hon, O. J., Leutgeb, S., & Leutgeb, J. K. (2019). Stability of medial entorhinal cortex representations over time. Hippocampus, 29(3), 284–302. https://doi.org/10.1002/hipo.23017
Doeller, C. F., Barry, C., & Burgess, N. (2010). Evidence for grid cells in a human memory network. Nature, 463(7281), 657–661. https://doi.org/10.1038/nature08704
Ego‐Stengel, V., & Wilson, M. A. (2010). Disruption of ripple‐associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus, 20(1), 1–10. https://doi.org/10.1002/hipo.20707
Erdem, U. M., & Hasselmo, M. E. (2014). A biologically inspired hierarchical goal directed navigation model. Journal of Physiology, Paris, 108(1), 28–37. https://doi.org/10.1016/j.jphysparis.2013.07.002
Ergorul, C., & Eichenbaum, H. (2004). The hippocampus and memory for "what," "where," and "when". Learning & Memory, 11(4), 397–405. https://doi.org/10.1101/lm.73304
Evensmoen, H. R., Ladstein, J., Hansen, T. I., Moller, J. A., Witter, M. P., Nadel, L., & Haberg, A. K. (2015). From details to large scale: The representation of environmental positions follows a granularity gradient along the human hippocampal and entorhinal anterior‐posterior axis. Hippocampus, 25(1), 119–135. https://doi.org/10.1002/hipo.22357
Evensmoen, H. R., Lehn, H., Xu, J., Witter, M. P., Nadel, L., & Haberg, A. K. (2013). The anterior hippocampus supports a coarse, global environmental representation and the posterior hippocampus supports fine‐grained, local environmental representations. Journal of Cognitive Neuroscience, 25(11), 1908–1925. https://doi.org/10.1162/jocn_a_00436
Ezzyat, Y., & Davachi, L. (2014). Similarity breeds proximity: Pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron, 81(5), 1179–1189. https://doi.org/10.1016/j.neuron.2014.01.042
Folkerts, S., Rutishauser, U., & Howard, M. W. (2018). Human episodic memory retrieval is accompanied by a neural contiguity effect. The Journal of Neuroscience, 38(17), 4200–4211. https://doi.org/10.1523/JNEUROSCI.2312‐17.2018
Fortin, N. J., Agster, K. L., & Eichenbaum, H. B. (2002). Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience, 5(5), 458–462. https://doi.org/10.1038/nn834
Fortin, N. J., Wright, S. P., & Eichenbaum, H. (2004). Recollection‐like memory retrieval in rats is dependent on the hippocampus. Nature, 431(7005), 188–191. https://doi.org/10.1038/nature02853
Fuhs, M. C., & Touretzky, D. S. (2006). A spin glass model of path integration in rat medial entorhinal cortex. The Journal of Neuroscience, 26(16), 4266–4276. https://doi.org/10.1523/JNEUROSCI.4353‐05.2006
Fyhn, M., Hafting, T., Treves, A., Moser, M. B., & Moser, E. I. (2007). Hippocampal remapping and grid realignment in entorhinal cortex. Nature, 446(7132), 190–194. https://doi.org/10.1038/nature05601
Gelbard‐Sagiv, H., Mukamel, R., Harel, M., Malach, R., & Fried, I. (2008). Internally generated reactivation of single neurons in human hippocampus during free recall. Science, 322(5898), 96–101. https://doi.org/10.1126/science.1164685
Gill, P. R., Mizumori, S. J., & Smith, D. M. (2011). Hippocampal episode fields develop with learning. Hippocampus, 21(11), 1240–1249. https://doi.org/10.1002/hipo.20832
Giocomo, L. M., Stensola, T., Bonnevie, T., Van Cauter, T., Moser, M. B., & Moser, E. I. (2014). Topography of head direction cells in medial entorhinal cortex. Current Biology, 24(3), 252–262. https://doi.org/10.1016/j.cub.2013.12.002
Girardeau, G., Benchenane, K., Wiener, S. I., Buzsaki, G., & Zugaro, M. B. (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nature Neuroscience, 12(10), 1222–1223. https://doi.org/10.1038/nn.2384
Gu, Y., Lewallen, S., Kinkhabwala, A. A., Domnisoru, C., Yoon, K., Gauthier, J. L., … Tank, D. W. (2018). A map‐like micro‐organization of grid cells in the medial entorhinal cortex. Cell, 175(3), 736–750 e730. https://doi.org/10.1016/j.cell.2018.08.066
Hafting, T., Fyhn, M., Molden, S., Moser, M. B., & Moser, E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature, 436(7052), 801–806. https://doi.org/10.1038/nature03721
Hardcastle, K., Ganguli, S., & Giocomo, L. M. (2015). Environmental boundaries as an error correction mechanism for grid cells. Neuron, 86(3), 827–839. https://doi.org/10.1016/j.neuron.2015.03.039
Hasselmo, M. E. (2009). A model of episodic memory: Mental time travel along encoded trajectories using grid cells. Neurobiology of Learning and Memory, 92(4), 559–573. https://doi.org/10.1016/j.nlm.2009.07.005
Hayes, S. M., Ryan, L., Schnyer, D. M., & Nadel, L. (2004). An fMRI study of episodic memory: Retrieval of object, spatial, and temporal information. Behavioral Neuroscience, 118(5), 885–896. https://doi.org/10.1037/0735‐7044.118.5.885
Hebb, D. O. (1949). The organization of behavior; a neuropsychological theory. Oxford, England: Wiley.
Heys, J. G., & Dombeck, D. A. (2018). Evidence for a subcircuit in medial entorhinal cortex representing elapsed time during immobility. Nature Neuroscience, 21(11), 1574–1582. https://doi.org/10.1038/s41593‐018‐0252‐8
Hinman, J. R., Brandon, M. P., Climer, J. R., Chapman, G. W., & Hasselmo, M. E. (2016). Multiple running speed signals in medial entorhinal cortex. Neuron, 91(3), 666–679. https://doi.org/10.1016/j.neuron.2016.06.027
Ho, A. S., Hori, E., Nguyen, P. H., Urakawa, S., Kondoh, T., Torii, K., … Nishijo, H. (2011). Hippocampal neuronal responses during signaled licking of gustatory stimuli in different contexts. Hippocampus, 21(5), 502–519. https://doi.org/10.1002/hipo.20766
Howard, M. W., Fotedar, M. S., Datey, A. V., & Hasselmo, M. E. (2005). The temporal context model in spatial navigation and relational learning: Toward a common explanation of medial temporal lobe function across domains. Psychological Review, 112(1), 75–116. https://doi.org/10.1037/0033‐295X.112.1.75
Howard, M. W., & Kahana, M. J. (2002). A distributed representation of temporal context. Journal of Mathematical Psychology, 46(3), 269–299. https://doi.org/10.1006/jmps.2001.1388
Howard, M. W., MacDonald, C. J., Tiganj, Z., Shankar, K. H., Du, Q., Hasselmo, M. E., & Eichenbaum, H. (2014). A unified mathematical framework for coding time, space, and sequences in the hippocampal region. The Journal of Neuroscience, 34(13), 4692–4707. https://doi.org/10.1523/JNEUROSCI.5808‐12.2014
Howard, M. W., Viskontas, I. V., Shankar, K. H., & Fried, I. (2012). Ensembles of human MTL neurons “jump back in time” in response to a repeated stimulus. Hippocampus, 22(9), 1833–1847. https://doi.org/10.1002/hipo.22018
Høydal, O. A., Skytøen, E. R., Andersson, S. O., Moser, M. B., & Moser, E. I. (2019). Object‐vector coding in the medial entorhinal cortex. Nature, 568(7752), 400–404. https://doi.org/10.1038/s41586‐019‐1077‐7
Hsieh, L. T., Gruber, M. J., Jenkins, L. J., & Ranganath, C. (2014). Hippocampal activity patterns carry information about objects in temporal context. Neuron, 81(5), 1165–1178. https://doi.org/10.1016/j.neuron.2014.01.015
Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M. B., & Moser, E. I. (2014). Coordination of entorhinal–hippocampal ensemble activity during associative learning. Nature, 510(7503), 143–147. https://doi.org/10.1038/nature13162
Itskov, V., Curto, C., Pastalkova, E., & Buzsaki, G. (2011). Cell assembly sequences arising from spike threshold adaptation keep track of time in the hippocampus. The Journal of Neuroscience, 31(8), 2828–2834. https://doi.org/10.1523/JNEUROSCI.3773‐10.2011
Jacobs, J., Kahana, M. J., Ekstrom, A. D., Mollison, M. V., & Fried, I. (2010). A sense of direction in human entorhinal cortex. Proceedings of the National Academy of Sciences of the United States of America, 107(14), 6487–6492. https://doi.org/10.1073/pnas.0911213107
Jacobs, N. S., Allen, T. A., Nguyen, N., & Fortin, N. J. (2013). Critical role of the hippocampus in memory for elapsed time. The Journal of Neuroscience, 33(34), 13888–13893. https://doi.org/10.1523/JNEUROSCI.1733‐13.2013
Jadhav, S. P., Kemere, C., German, P. W., & Frank, L. M. (2012). Awake hippocampal sharp‐wave ripples support spatial memory. Science, 336(6087), 1454–1458. https://doi.org/10.1126/science.1217230
Jenkins, L. J., & Ranganath, C. (2016). Distinct neural mechanisms for remembering when an event occurred. Hippocampus, 26(5), 554–559. https://doi.org/10.1002/hipo.22571
Jensen, O., & Lisman, J. E. (1996). Hippocampal CA3 region predicts memory sequences: Accounting for the phase precession of place cells. Learning & Memory, 3(2–3), 279–287.
Jezek, K., Henriksen, E. J., Treves, A., Moser, E. I., & Moser, M. B. (2011). Theta‐paced flickering between place‐cell maps in the hippocampus. Nature, 478(7368), 246–249. https://doi.org/10.1038/nature10439
Johnson, A., & Redish, A. D. (2007). Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. The Journal of Neuroscience, 27(45), 12176–12189. https://doi.org/10.1523/JNEUROSCI.3761‐07.2007
Josselyn, S. A., Kohler, S., & Frankland, P. W. (2015). Finding the engram. Nature Reviews. Neuroscience, 16(9), 521–534. https://doi.org/10.1038/nrn4000
Kalm, K., Davis, M. H., & Norris, D. (2013). Individual sequence representations in the medial temporal lobe. Journal of Cognitive Neuroscience, 25(7), 1111–1121. https://doi.org/10.1162/jocn_a_00378
Karlsson, M. P., & Frank, L. M. (2009). Awake replay of remote experiences in the hippocampus. Nature Neuroscience, 12(7), 913–918. https://doi.org/10.1038/nn.2344
Keene, C. S., Bladon, J., McKenzie, S., Liu, C. D., O'Keefe, J., & Eichenbaum, H. (2016). Complementary functional organization of neuronal activity patterns in the perirhinal, lateral entorhinal, and medial entorhinal cortices. The Journal of Neuroscience, 36(13), 3660–3675. https://doi.org/10.1523/JNEUROSCI.4368‐15.2016
Killian, N. J., Jutras, M. J., & Buffalo, E. A. (2012). A map of visual space in the primate entorhinal cortex. Nature, 491(7426), 761–764. https://doi.org/10.1038/nature11587
Kjelstrup, K. B., Solstad, T., Brun, V. H., Hafting, T., Leutgeb, S., Witter, M. P., … Moser, M. B. (2008). Finite scale of spatial representation in the hippocampus. Science, 321(5885), 140–143. https://doi.org/10.1126/science.1157086
Komorowski, R. W., Garcia, C. G., Wilson, A., Hattori, S., Howard, M. W., & Eichenbaum, H. (2013). Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. The Journal of Neuroscience, 33(18), 8079–8087. https://doi.org/10.1523/JNEUROSCI.5458‐12.2013
Komorowski, R. W., Manns, J. R., & Eichenbaum, H. (2009). Robust conjunctive item‐place coding by hippocampal neurons parallels learning what happens where. The Journal of Neuroscience, 29(31), 9918–9929. https://doi.org/10.1523/JNEUROSCI.1378‐09.2009
Kraus, B. J., Brandon, M. P., Robinson, R. J., 2nd, Connerney, M. A., Hasselmo, M. E., & Eichenbaum, H. (2015). During running in place, grid cells integrate elapsed time and distance run. Neuron, 88(3), 578–589. https://doi.org/10.1016/j.neuron.2015.09.031
Kraus, B. J., Robinson, R. J., 2nd, White, J. A., Eichenbaum, H., & Hasselmo, M. E. (2013). Hippocampal “time cells”: Time versus path integration. Neuron, 78(6), 1090–1101. https://doi.org/10.1016/j.neuron.2013.04.015
Kreiman, G., Koch, C., & Fried, I. (2000). Category‐specific visual responses of single neurons in the human medial temporal lobe. Nature Neuroscience, 3(9), 946–953. https://doi.org/10.1038/78868
Kropff, E., Carmichael, J. E., Moser, M. B., & Moser, E. I. (2015). Speed cells in the medial entorhinal cortex. Nature, 523(7561), 419–424. https://doi.org/10.1038/nature14622
Kropff, E., & Treves, A. (2008). The emergence of grid cells: Intelligent design or just adaptation? Hippocampus, 18(12), 1256–1269. https://doi.org/10.1002/hipo.20520
Lehn, H., Steffenach, H. A., van Strien, N. M., Veltman, D. J., Witter, M. P., & Haberg, A. K. (2009). A specific role of the human hippocampus in recall of temporal sequences. The Journal of Neuroscience, 29(11), 3475–3484. https://doi.org/10.1523/JNEUROSCI.5370‐08.2009
Lenck‐Santini, P. P., Fenton, A. A., & Muller, R. U. (2008). Discharge properties of hippocampal neurons during performance of a jump avoidance task. The Journal of Neuroscience, 28(27), 6773–6786. https://doi.org/10.1523/JNEUROSCI.5329‐07.2008
Leutgeb, S., Leutgeb, J. K., Barnes, C. A., Moser, E. I., McNaughton, B. L., & Moser, M. B. (2005). Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science, 309(5734), 619–623. https://doi.org/10.1126/science.1114037
Leutgeb, S., Leutgeb, J. K., Treves, A., Moser, M. B., & Moser, E. I. (2004). Distinct ensemble codes in hippocampal areas CA3 and CA1. Science, 305(5688), 1295–1298. https://doi.org/10.1126/science.1100265
Liu, X., Ramirez, S., Pang, P. T., Puryear, C. B., Govindarajan, A., Deisseroth, K., & Tonegawa, S. (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature, 484(7394), 381–385. https://doi.org/10.1038/nature11028
Lositsky, O., Chen, J., Toker, D., Honey, C. J., Shvartsman, M., Poppenk, J. L., … Norman, K. A. (2016). Neural pattern change during encoding of a narrative predicts retrospective duration estimates. eLife, 5, pii:e16070. https://doi.org/10.7554/eLife.16070
MacDonald, C. J., Carrow, S., Place, R., & Eichenbaum, H. (2013). Distinct hippocampal time cell sequences represent odor memories in immobilized rats. The Journal of Neuroscience, 33(36), 14607–14616. https://doi.org/10.1523/JNEUROSCI.1537‐13.2013
MacDonald, C. J., Lepage, K. Q., Eden, U. T., & Eichenbaum, H. (2011). Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron, 71(4), 737–749. https://doi.org/10.1016/j.neuron.2011.07.012
Mack, M. L., & Preston, A. R. (2016). Decisions about the past are guided by reinstatement of specific memories in the hippocampus and perirhinal cortex. NeuroImage, 127, 144–157. https://doi.org/10.1016/j.neuroimage.2015.12.015
Mankin, E. A., Diehl, G. W., Sparks, F. T., Leutgeb, S., & Leutgeb, J. K. (2015). Hippocampal CA2 activity patterns change over time to a larger extent than between spatial contexts. Neuron, 85(1), 190–201. https://doi.org/10.1016/j.neuron.2014.12.001
Mankin, E. A., Sparks, F. T., Slayyeh, B., Sutherland, R. J., Leutgeb, S., & Leutgeb, J. K. (2012). Neuronal code for extended time in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 109(47), 19462–19467. https://doi.org/10.1073/pnas.1214107109
Manns, J. R., Howard, M. W., & Eichenbaum, H. (2007). Gradual changes in hippocampal activity support remembering the order of events. Neuron, 56(3), 530–540. https://doi.org/10.1016/j.neuron.2007.08.017
Marchette, S. A., Ryan, J., & Epstein, R. A. (2017). Schematic representations of local environmental space guide goal‐directed navigation. Cognition, 158, 68–80. https://doi.org/10.1016/j.cognition.2016.10.005
Mau, W., Sullivan, D. W., Kinsky, N. R., Hasselmo, M. E., Howard, M. W., & Eichenbaum, H. (2018). The same hippocampal CA1 population simultaneously codes temporal information over multiple timescales. Current Biology, 28(10), 1499–1508.e1494. https://doi.org/10.1016/j.cub.2018.03.051
Mayes, A. R., Isaac, C. L., Holdstock, J. S., Hunkin, N. M., Montaldi, D., Downes, J. J., … Roberts, J. N. (2001). Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cognitive Neuropsychology, 18(2), 97–123. https://doi.org/10.1080/02643290125897
McEchron, M. D., Bouwmeester, H., Tseng, W., Weiss, C., & Disterhoft, J. F. (1998). Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus, 8(6), 638–646. https://doi.org/10.1002/(SICI)1098‐1063(1998)8:6<638::AID‐HIPO6>3.0.CO;2‐Q
McNaughton, B. L., Battaglia, F. P., Jensen, O., Moser, E. I., & Moser, M. B. (2006). Path integration and the neural basis of the “cognitive map”. Nature Reviews. Neuroscience, 7(8), 663–678. https://doi.org/10.1038/nrn1932
McNaughton, B. L., & Morris, R. G. M. (1987). Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences, 10(10), 408–415. https://doi.org/10.1016/0166‐2236(87)90011‐7
Miao, C., Cao, Q., Moser, M. B., & Moser, E. I. (2017). Parvalbumin and somatostatin interneurons control different space‐coding networks in the medial entorhinal cortex. Cell, 171(3), 507–521 e517. https://doi.org/10.1016/j.cell.2017.08.050
Miller, J. F., Neufang, M., Solway, A., Brandt, A., Trippel, M., Mader, I., … Schulze‐Bonhage, A. (2013). Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science, 342(6162), 1111–1114. https://doi.org/10.1126/science.1244056
Modi, M. N., Dhawale, A. K., & Bhalla, U. S. (2014). CA1 cell activity sequences emerge after reorganization of network correlation structure during associative learning. eLife, 3, e01982. https://doi.org/10.7554/eLife.01982
Monaco, J. D., & Abbott, L. F. (2011). Modular realignment of entorhinal grid cell activity as a basis for hippocampal remapping. The Journal of Neuroscience, 31(25), 9414–9425. https://doi.org/10.1523/JNEUROSCI.1433‐11.2011
Montchal, M. E., Reagh, Z. M., & Yassa, M. A. (2019). Precise temporal memories are supported by the lateral entorhinal cortex in humans. Nature Neuroscience, 22, 284–288. https://doi.org/10.1038/s41593‐018‐0303‐1
Mormann, F., Kornblith, S., Cerf, M., Ison, M. J., Kraskov, A., Tran, M., … Fried, I. (2017). Scene‐selective coding by single neurons in the human parahippocampal cortex. Proceedings of the National Academy of Sciences of the United States of America, 114(5), 1153–1158. https://doi.org/10.1073/pnas.1608159113
Moser, E. I., Moser, M. B., & McNaughton, B. L. (2017). Spatial representation in the hippocampal formation: A history. Nature Neuroscience, 20(11), 1448–1464. https://doi.org/10.1038/nn.4653
Muller, R. U., & Kubie, J. L. (1987). The effects of changes in the environment on the spatial firing of hippocampal complex‐spike cells. The Journal of Neuroscience, 7(7), 1951–1968.
Nadel, L., Campbell, J., & Ryan, L. (2007). Autobiographical memory retrieval and hippocampal activation as a function of repetition and the passage of time. Neural Plasticity, 2007, 90472. https://doi.org/10.1155/2007/90472
Nadel, L., Hoscheidt, S., & Ryan, L. R. (2013). Spatial cognition and the hippocampus: The anterior‐posterior axis. Journal of Cognitive Neuroscience, 25(1), 22–28. https://doi.org/10.1162/jocn_a_00313
Nadel, L., & Moscovitch, M. (1997). Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology, 7(2), 217–227.
Nadel, L., & Peterson, M. A. (2013). The hippocampus: Part of an interactive posterior representational system spanning perceptual and memorial systems. Journal of Experimental Psychology. General, 142(4), 1242–1254. https://doi.org/10.1037/a0033690
Neunuebel, J. P., Yoganarasimha, D., Rao, G., & Knierim, J. J. (2013). Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. The Journal of Neuroscience, 33(22), 9246–9258. https://doi.org/10.1523/JNEUROSCI.0946‐13.2013
Nielson, D. M., Smith, T. A., Sreekumar, V., Dennis, S., & Sederberg, P. B. (2015). Human hippocampus represents space and time during retrieval of real‐world memories. Proceedings of the National Academy of Sciences of the United States of America, 112(35), 11078–11083. https://doi.org/10.1073/pnas.1507104112
O'Keefe, J., & Burgess, N. (1996). Geometric determinants of the place fields of hippocampal neurons. Nature, 381(6581), 425–428. https://doi.org/10.1038/381425a0
O'Keefe, J., & Conway, D. H. (1978). Hippocampal place units in the freely moving rat: Why they fire where they fire. Experimental Brain Research, 31(4), 573–590.
O'Keefe, J., & Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely‐moving rat. Brain Research, 34(1), 171–175.
O'Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford, England: Oxford University Press.
O'Keefe, J., & Recce, M. L. (1993). Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus, 3(3), 317–330. https://doi.org/10.1002/hipo.450030307
Pastalkova, E., Itskov, V., Amarasingham, A., & Buzsaki, G. (2008). Internally generated cell assembly sequences in the rat hippocampus. Science, 321(5894), 1322–1327. https://doi.org/10.1126/science.1159775
Pfeiffer, B. E., & Foster, D. J. (2013). Hippocampal place‐cell sequences depict future paths to remembered goals. Nature, 497(7447), 74–79. https://doi.org/10.1038/nature12112
Poppenk, J., Evensmoen, H. R., Moscovitch, M., & Nadel, L. (2013). Long‐axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17(5), 230–240. https://doi.org/10.1016/j.tics.2013.03.005
Proust, M. (1913). A la recherche du temps perdu. Paris: Gallimard.
Quiroga, R. Q., Reddy, L., Kreiman, G., Koch, C., & Fried, I. (2005). Invariant visual representation by single neurons in the human brain. Nature, 435(7045), 1102–1107. https://doi.org/10.1038/nature03687
Ranganath, C., & Ritchey, M. (2012). Two cortical systems for memory‐guided behaviour. Nature Reviews. Neuroscience, 13(10), 713–726. https://doi.org/10.1038/nrn3338
Reagh, Z. M., Noche, J. A., Tustison, N. J., Delisle, D., Murray, E. A., & Yassa, M. A. (2018). Functional imbalance of anterolateral entorhinal cortex and hippocampal dentate/CA3 underlies age‐related object pattern separation deficits. Neuron, 97(5), 1187–1198.e1184. https://doi.org/10.1016/j.neuron.2018.01.039
Reagh, Z. M., & Yassa, M. A. (2014). Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proceedings of the National Academy of Sciences of the United States of America, 111(40), E4264–E4273. https://doi.org/10.1073/pnas.1411250111
Redish, A. D., Rosenzweig, E. S., Bohanick, J. D., McNaughton, B. L., & Barnes, C. A. (2000). Dynamics of hippocampal ensemble activity realignment: Time versus space. The Journal of Neuroscience, 20(24), 9298–9309.
Robinson, N. T. M., Priestley, J. B., Rueckemann, J. W., Garcia, A. D., Smeglin, V. A., Marino, F. A., & Eichenbaum, H. (2017). Medial entorhinal cortex selectively supports temporal coding by hippocampal neurons. Neuron, 94(3), 677–688 e676. https://doi.org/10.1016/j.neuron.2017.04.003
Rowland, D. C., & Moser, M. B. (2014). From cortical modules to memories. Current Opinion in Neurobiology, 24(1), 22–27. https://doi.org/10.1016/j.conb.2013.08.012
Rugg, M. D., & Vilberg, K. L. (2013). Brain networks underlying episodic memory retrieval. Current Opinion in Neurobiology, 23(2), 255–260. https://doi.org/10.1016/j.conb.2012.11.005
Salz, D. M., Tiganj, Z., Khasnabish, S., Kohley, A., Sheehan, D., Howard, M. W., & Eichenbaum, H. (2016). Time cells in hippocampal area CA3. The Journal of Neuroscience, 36(28), 7476–7484. https://doi.org/10.1523/JNEUROSCI.0087‐16.2016
Sargolini, F., Fyhn, M., Hafting, T., McNaughton, B. L., Witter, M. P., Moser, M. B., & Moser, E. I. (2006). Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science, 312(5774), 758–762. https://doi.org/10.1126/science.1125572
Scoville, W. B., & Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20(1), 11–21.
Skaggs, W. E., & McNaughton, B. L. (1996). Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science, 271(5257), 1870–1873.
Skaggs, W. E., McNaughton, B. L., Wilson, M. A., & Barnes, C. A. (1996). Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus, 6(2), 149–172. https://doi.org/10.1002/(SICI)1098‐1063(1996)6:2<149::AID‐HIPO6>3.0.CO;2‐K
Solstad, T., Boccara, C. N., Kropff, E., Moser, M. B., & Moser, E. I. (2008). Representation of geometric borders in the entorhinal cortex. Science, 322(5909), 1865–1868. https://doi.org/10.1126/science.1166466
Solstad, T., Moser, E. I., & Einevoll, G. T. (2006). From grid cells to place cells: A mathematical model. Hippocampus, 16(12), 1026–1031. https://doi.org/10.1002/hipo.20244
Squire, L. R., Ojemann, J. G., Miezin, F. M., Petersen, S. E., Videen, T. O., & Raichle, M. E. (1992). Activation of the hippocampus in normal humans: A functional anatomical study of memory. Proceedings of the National Academy of Sciences of the United States of America, 89(5), 1837–1841. https://doi.org/10.1073/pnas.89.5.1837
Sreenivasan, S., & Fiete, I. (2011). Grid cells generate an analog error‐correcting code for singularly precise neural computation. Nature Neuroscience, 14(10), 1330–1337. https://doi.org/10.1038/nn.2901
Stemmler, M., Mathis, A., & Herz, A. V. (2015). Connecting multiple spatial scales to decode the population activity of grid cells. Science Advances, 1(11), e1500816. https://doi.org/10.1126/science.1500816
Stensola, H., Stensola, T., Solstad, T., Froland, K., Moser, M. B., & Moser, E. I. (2012). The entorhinal grid map is discretized. Nature, 492(7427), 72–78. https://doi.org/10.1038/nature11649
Suh, J., Rivest, A. J., Nakashiba, T., Tominaga, T., & Tonegawa, S. (2011). Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science, 334(6061), 1415–1420. https://doi.org/10.1126/science.1210125
Suzuki, W. A., Miller, E. K., & Desimone, R. (1997). Object and place memory in the macaque entorhinal cortex. Journal of Neurophysiology, 78(2), 1062–1081. https://doi.org/10.1152/jn.1997.78.2.1062
Tanaka, K. Z., He, H., Tomar, A., Niisato, K., Huang, A. J. Y., & McHugh, T. J. (2018). The hippocampal engram maps experience but not place. Science, 361(6400), 392–397. https://doi.org/10.1126/science.aat5397
Taube, J. S., Muller, R. U., & Ranck, J. B., Jr. (1990). Head‐direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. Journal of Neuroscience, 10(2), 420–435.
Terada, S., Sakurai, Y., Nakahara, H., & Fujisawa, S. (2017). Temporal and rate coding for discrete event sequences in the hippocampus. Neuron, 94(6), 1248–1262 e1244. https://doi.org/10.1016/j.neuron.2017.05.024
Teyler, T. J., & DiScenna, P. (1986). The hippocampal memory indexing theory. Behavioral Neuroscience, 100(2), 147–154.
Thavabalasingam, S., O'Neil, E. B., Tay, J., Nestor, A., & Lee, A. C. H. (2019). Evidence for the incorporation of temporal duration information in human hippocampal long‐term memory sequence representations. Proceedings of the National Academy of Sciences of the United States of America, 116, 6407–6414. https://doi.org/10.1073/pnas.1819993116
Tsao, A. (2017). Revising the parallel‐pathways hypothesis with time. Frontiers in Systems Neuroscience, 11, 59. https://doi.org/10.3389/fnsys.2017.00059
Tsao, A., Moser, M. B., & Moser, E. I. (2013). Traces of experience in the lateral entorhinal cortex. Current Biology, 23(5), 399–405. https://doi.org/10.1016/j.cub.2013.01.036
Tsao, A., Sugar, J., Lu, L., Wang, C., Knierim, J. J., Moser, M. B., & Moser, E. I. (2018). Integrating time from experience in the lateral entorhinal cortex. Nature, 561, 57–62. https://doi.org/10.1038/s41586‐018‐0459‐6
Tulving, E. (1983). Elements of episodic memory. In Elements of episodic memory. Oxford, England: Clarendon Press.
Vaz, A. P., Inati, S. K., Brunel, N., & Zaghloul, K. A. (2019). Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science, 363(6430), 975–978. https://doi.org/10.1126/science.aau8956
Wang, C., Chen, X., Lee, H., Deshmukh, S. S., Yoganarasimha, D., Savelli, F., & Knierim, J. J. (2018). Egocentric coding of external items in the lateral entorhinal cortex. Science, 362(6417), 945–949. https://doi.org/10.1126/science.aau4940
Wills, T. J., Barry, C., & Cacucci, F. (2012). The abrupt development of adult‐like grid cell firing in the medial entorhinal cortex. Frontiers in Neural Circuits, 6, 21. https://doi.org/10.3389/fncir.2012.00021
Wilson, M. A., & McNaughton, B. L. (1994). Reactivation of hippocampal ensemble memories during sleep. Science, 265(5172), 676–679.
Winter, S. S., Clark, B. J., & Taube, J. S. (2015). Spatial navigation. Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science, 347(6224), 870–874. https://doi.org/10.1126/science.1259591
Ye, J., Witter, M. P., Moser, M. B., & Moser, E. I. (2018). Entorhinal fast‐spiking speed cells project to the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 115(7), E1627–E1636. https://doi.org/10.1073/pnas.1720855115
Young, B. J., Otto, T., Fox, G. D., & Eichenbaum, H. (1997). Memory representation within the parahippocampal region. The Journal of Neuroscience, 17(13), 5183–5195.
Zhang, S. J., Ye, J., Miao, C., Tsao, A., Cerniauskas, I., Ledergerber, D., … Moser, E. I. (2013). Optogenetic dissection of entorhinal–hippocampal functional connectivity. Science, 340(6128), 1232627. https://doi.org/10.1126/science.1232627
Ziv, Y., Burns, L. D., Cocker, E. D., Hamel, E. O., Ghosh, K. K., Kitch, L. J., … Schnitzer, M. J. (2013). Long‐term dynamics of CA1 hippocampal place codes. Nature Neuroscience, 16(3), 264–266. https://doi.org/10.1038/nn.3329
Zola‐Morgan, S. M., & Squire, L. R. (1990). The primate hippocampal formation: Evidence for a time‐limited role in memory storage. Science, 250(4978), 288–290.
The puzzle of spontaneous alternation and inhibition of return: How they might fit together
Leslie S. Phillmore | Raymond M. Klein
Department of Psychology and Neuroscience, Dalhousie University, Halifax, Nova Scotia, Canada
Correspondence Raymond M. Klein, Department of Psychology and Neuroscience, Dalhousie University, Halifax, NS, Canada. Email: ray.klein@dal.ca
Funding information Natural Sciences and Engineering Research Council of Canada, Discovery Grants to both authors
1. Abstract
Two isolated spatial phenomena share a similar “been there; done that” effect on spatial behavior. Originally discovered in rodent learning experiments, spontaneous alternation is a tendency for the organism to visit a different arm in a T‐maze on subsequent trials. Originally discovered in human studies of attention, inhibition of return is a tendency for the organism to orient away from a previously attended location. Whereas spontaneous alternation was identified by O'Keefe & Nadel as dependent on an intact hippocampus, inhibition of return is dependent on neural structures that participate in oculomotor control (the superior colliculus, parietal and frontal cortex). Despite the isolated literatures, each phenomenon has been assumed to reflect a basic novelty‐seeking process, avoiding places previously visited or locations attended. In this commentary, we explore and compare the behavioral manifestations and neural underpinnings of these two phenomena, and suggest what is still needed to determine whether they operate in parallel or serial.
KEYWORDS
foraging, hippocampus, inhibition of return, spatial behavior, spontaneous alternation
Funding information Natural Sciences and Engineering Research Council of Canada, Discovery Grants to both authors
2. INTRODUCTION
Whether foraging for food, seeking shelter, attending to offspring, or engaging in search for a particular target object, animals must make choices among spatial locations of various scales. These decisions are hypothesized to rely upon a neural representation of the space within which different exploratory behaviors, from eye gaze and attention to locomotion of the whole body, take place. In many of these situations, a novelty seeking mechanism would be adaptive to minimize the probability of returning to locations already explored. Such a novelty seeking functionality has been attributed to two seemingly disparate phenomena that describe how an organism interacts with its environment when exploring: spontaneous alternation and inhibition of return. In this paper, we will explore whether these two behaviors are indeed as disparate as they seem, or whether they share common features, ontogeny, mechanisms, and neurobiology—including whether the hippocampus is important to both.
Spontaneous alternation is typically associated with behavior of animals (usually rodents) in an unbaited T‐maze (e.g., Dennis, 1939; Fowler, Blond, & Dember, 1959; LaLonde, 2002) and was first described by Tolman (1925). In a T‐maze trial, animals are placed in a start box at the long end of the T and either allowed to choose freely between either arm of the T at the opposite end, or forced to choose one arm over the other (in a forced‐choice procedure). In both types of T‐maze procedures, animals are given free choice in subsequent trials. In these trials, there is a tendency for mice and rats to explore rather than perseverate: that is, after visiting one arm the probability that they will visit the other arm on their next exposure to the same maze is significantly greater than chance (50%). The degree of spontaneous alternation is often used as a measure of spatial memory, because without some memory of their previous behavior an animal's probability of selecting a particular arm of the T‐maze (relative to their last choice) should not depart from chance (for a review, see LaLonde, 2002). In their landmark book, The Hippocampus as a Cognitive Map, O'Keefe and Nadel (1978) incorporate the literature on spontaneous alternation into their framework, proposing that
“The hippocampal animal…is bereft of cognitive maps and any tendency to explore novelty. It would thus be predicted that these animals would not alternate (pp. 260–261)”.
Their review of the literature on spontaneous alternation following hippocampal lesions supported this prediction and led O'Keefe and Nadel to conclude that after lesions to the hippocampus animals “lack those mechanisms driving exploratory behaviour in normal animals.” However, lesions to the hippocampus do not produce simply a general inhibition of alternation as alternating lever pressing is not affected in animals with hippocampal lesions, but seems to be more specific to spatial tasks, spontaneous alternation included (Lalonde, 2002).
Inhibition of return (inhibition of return) was discovered, characterized, and named by Posner & colleagues in the mid 1980's (Posner & Cohen, 1984; Posner, Rafal, Choate, & Vaughan, 1985). In the prototypical experiment, reaction time to a target preceded by a peripheral event that was uninformative about the location of the target is slower when the target repeats the location of the cue than when it is presented at a new location. In these early studies, it was suggested that the inhibitory effect was a novelty seeking mechanism that might operate in visual search. Confirming this suggestion, Klein (1988) demonstrated that inhibition of return was present at the locations that attention had presumably visited during a search episode to determine that items (distractors) were not the target. And, later, using eye movement monitoring and a “Where's Waldo”‐type (Wally in the UK) task, Klein and MacInnes (1999) discovered that eye movements were inhibited from returning to recently visited locations. With minor qualifications, both of these findings have been confirmed by other investigators (for a review, see Wang & Klein, 2010). At the end of his 1988 paper, Klein speculated: “…it is interesting to consider that a similar inhibitory tagging system may operate within neuro‐cognitive maps that mediate foraging behavior in other species (O'Keefe & Nadel, 1978; Olton, Handelman, Walker, Kamil, & Sargent, 1981)” and the 1999 paper was titled by its conclusion: “Inhibition of return is a foraging facilitator in visual search.” Behaviorally, then, both spontaneous alternation and inhibition of return may be described as a tendency to avoid a location just visited in physical space, whether an arm of a maze with the whole body (spontaneous alternation) or a location on a screen with eye‐gaze (inhibition of return).
3. PRIOR POINTERS TO THIS POSSIBLE PARALLEL
It is not surprising that a few scholars have pointed to the functional similarity these two phenomena share. In an early developmental study of inhibition of return in infants, Clohessy, Posner, Rothbart, and Vecera (1991) said this:
“Our basic idea is that inhibition of return is related to a general tendency to avoid repeating a motor program1 that has just been executed. This general tendency has been observed throughout the animal kingdom in the form of spontaneous alternation of responses (Dember, 1989; Vecera, Rothbart, & Posner, 1991). Inhibition of return can be seen as a special case of this tendency that relates to programs to move the eyes. Studies in our laboratory suggest that the eye movement system shows inhibition of return at an earlier age than one sees spontaneous alternation of reaching in infants (Vecera et al., 1991). Thus, inhibition of return may be the earliest of a number of separate mechanisms that eventually involve all motor activity.”
In a contemporaneous paper, Vecera et al. (1991) explored both phenomena in human infants. In their introduction, they noted the similarity and some differences between them:
“Inhibition of return and spontaneous alternation are similar phenomena in that they both index preference for a novel location in space, but inhibition of return involves either covert orienting of attention or overt eye movements and a time course of 2‐3 sec, whereas spontaneous alternation involves skeletal motor activity and a longer time course. In addition, inhibition of return has been related to functioning of the superior colliculus and surrounding midbrain areas (Posner & Petersen, 1990), whereas spontaneous alternation has been most closely linked to hippocampal functioning (Douglas, 1972, 1989).”
To measure spontaneous alternation, Vecera et al. (1991) used a reaching task in which infants seated at a table were given experience with a toy at one location and then presented simultaneously with two of the same toys as the first toy, but at both the original and a different location. For inhibition of return, eye gaze was quantified in seated infants presented with visual stimuli on monitors central and peripheral to the infant. While 6‐month old infants showed inhibition of return, but not alternation in reaching for toys, 18‐month old infants showed both phenomena. However, spontaneous alternation in these 18‐month olds was negatively correlated to inhibition of return.
We think it was 15 years before this functional parallelism was pointed to again, this time by Ivanoff and Klein (2006) who asserted that one effect of inhibition of return:
“…resembles an expectancy whereby, in the absence of task‐relevant target information, the cued location is disfavored over novel uncued locations. Perhaps this finding is not unlike the spontaneous alternation behavior observed in some species (e.g., see Lalonde, 2002, for a review). In a T maze, rats (and a variety of other species) tend to avoid the arm of the T maze that they had entered on a previous trial. Note that, in a T maze, the rat initially has no information regarding the whereabouts of the reward. Spontaneous alternation is thought to promote exploration. Likewise, inhibition of return has been argued to facilitate visual exploration (Klein & MacInnes, 1999). (p. 917)”
4. EXPLORING SIMILARITIES AND DIFFERENCES
It is revealing to begin this exercise with brief comparison of the early research into these two phenomena. Early studies of spontaneous alternation used an animal learning paradigm (e.g., Wingfield & Dennis, 1934) whereas inhibition of return was discovered in a paradigm developed by Posner to explore human visual attention (Posner & Cohen, 1984). Early studies of both phenomena were concerned with characterizing their nature by manipulating situational variables such as timing and context (for reviews see Lalonde, 2002, and Klein, 2000). Organismic variables such as age and species were also explored, and a variety of neuroscientific manipulations have been applied to both phenomena. Not surprisingly, the research trajectories for both phenomena have been strongly influenced by the paradigms employed in their discovery. Most studies of inhibition of return have been conducted with human participants using Posner's cueing paradigm, or a variant, in which a spatially uninformative peripheral cue precedes a target requiring a response of some sort. In contrast, most studies of spontaneous alternation have been conducted with rodent species using T‐mazes (and also Y‐mazes for continuous spontaneous alternation paradigms). Most neuroscientific studies of inhibition of return have used noninvasive neuro‐imaging techniques like EEG and fMRI or have studied how the phenomenon is affected by naturally occurring brain damage and disease. In contrast, the most common neuroscientific manipulations that have been applied to spontaneous alternation have been experimentally induced lesions, neurochemical agonists or antagonists, or genetic modifications in rodent species.
As noted in the introduction, animals with impaired spatial memory do not tend to show spontaneous alternation. Consequently, measurement of spontaneous alternation has become a common component of test batteries used to assess this type of memory in the face of various manipulations, such as lesions, developmental adversity, or pharmacological administration (Hughes, 2004). Indeed, there are no recent studies focused on the fundamentals of spontaneous alternation, that is, studies aimed at seeking to understand it better. Rather, spontaneous alternation has become a memory assay used to assess deficits (although a failure to show spontaneous alternation is not necessarily due to a memory deficit2). Inhibition of return has occasionally been used in a similar way (as an assay of inhibition or attention); however, the majority of studies of inhibition of return have been concerned with revealing its nature and neural implementation.
4.1. Situational variables
4.1.1. Context and frame of reference
Glanzer (1953) interprets Dennis's (1939) suggestion that alternation is avoidance of an area or location already experienced to mean that spontaneous alternation is a stimulus‐driven phenomenon, and then provides a response‐based alternative account—that spontaneous alternation is based on the response an animal has just made—that is, a left turn because they had previously made a right turn. Glanzer goes on to refute these response‐based explanations, presenting evidence from several early studies. For example, spontaneous alternation in an altered T‐maze where the choice arms were situated at a minimal angle from each other meant there was little difference in the actual behavioral response (i.e., a left turn and a right turn were practically identical in response appearance) did not reduce alternation (Jackson, 1941). Glanzer then presents a theory explaining spontaneous alternation as a stimulus‐driven behavior, stating that “with continued exposure to an environment—to the same stimuli—the organism becomes less active in that environment”. Key evidence supporting this theory comes from Glanzer's (1953) study showing that in a two‐choice situation where cues in the maze are shifted between trials, animals will alternate according to where the stimulus is, rather than which direction they turned on the previous trial. Other studies support these findings: Ellen and DeLoache (1968) alternated spatial and brightness cues while rotating a maze between trials, and found animals alternated according to the cues, rather than the previously made response. In addition, Lennartz (2008) showed that spontaneous alternation on the first day of trials in a plus maze was greater in a stimulus‐rich environment (with lots of external cues) than in a dark or stimulus‐impoverished environment (light on, no cues). However, after 4 days spontaneous alternation was higher in the impoverished and dark environment compared to the stimulus‐rich maze.
Related to the idea that spontaneous alternation is driven by response to external stimuli in the maze, rather than the intrinsic response itself, is that if the context of the cues changes, alternation is affected. Dennis (1939) examined whether spontaneous alternation would occur when the animal was run in two consecutive T‐mazes, rather than running two consecutive trials on the same maze. Rats did not show spontaneous alternation: in fact, choices on the second maze were clearly independent of the first.
In the earliest studies in the inhibition of return literature (Maylor & Hockey, 1985; Posner & Cohen, 1984) the environment was also shown to be critical. This was demonstrated by interposing one (Maylor & Hockey) or more (Posner & Cohen) eye movements between the initial cue and the subsequent target. Responses to the target were slower when it appeared at the originally cued location in space and not (or not so much, see Satel, Wang, Hilchey, & Klein, 2012) when it was presented at the same location on the retina as the original cue. From this finding, it was concluded that inhibition of return is primarily coded in an environmental, rather than retinotopic (or oculocentric), frame of reference.
By cueing an object before it moved predictably in space, Tipper, Driver, and Weaver (1991) discovered that inhibition of return could be tagged an object (coded in object‐centered coordinates). It was later demonstrated that this object‐based inhibition of return effect survived occlusion of the cued object (Yi, Kim, & Chun, 2003) and was observed when the objects in the scene moved in random and unpredictable directions (Ogawa, Takeda, & Yagi, 2002). Importantly, in studies exploring inhibition of return in the aftermath of a visual search task it has been demonstrated (see Wang & Klein, 2010, for a review) that the inhibitory tags depend on the presence of the scene (a finding that has also been observed in the standard cue‐target paradigm, Redden, Klages, & Klein, 2017). Thus, if the inhibitory tags are coded into the representation of the scene they are removed when the scene is removed.
4.1.2. Timecourse: How long does it last?
Two time factors play a role in spontaneous alternation behavior. First, the duration of exposure to the arm after a choice is made, either through keeping the animal in the arm or through repeated exposures to the same arm before the second choice. If a rat is kept in the chosen arm of the T, spontaneous alternation will occur at a higher rate the longer the animal is kept in that arm (Glanzer, 1953). Second, the duration of the interval between the first arm choice and the second: when the interval between successive trials to the same T‐maze was varied, the tendency to alternate decreased monotonically out to about 120 s after which this tendency was absent (Heathers, 1940). Glanzer attributes both these phenomena to effects of satiation: longer exposure to an arm increases satiation to that arm's cues, resulting in stronger spontaneous alternation tendencies, while longer delays decrease satiation, resulting in weaker, or non‐existent spontaneous alternation behavior. Interestingly, the rate of decay (forgetting?) was substantially decreased by making the two arms of the T‐maze more distinctive from each other (Walker, as cited in Glanzer, 1953b), a manipulation that extended the tendency to alternate to at least an hour.
Time is also a factor in inhibition of return—with investigators interested in when it begins after presentation of a cue and how long it lasts. In a typical cueing task, immediately following an uninformative peripheral cue there is often facilitation at the cued location, facilitation that is attributed to the capture of attention by the cue. The inhibitory after‐effect, which has been called inhibition of return, appears some time (50–500 ms, depending on methods) later. Although some investigators have interpreted this appearance as the onset time of the inhibitory effect, the alternative, that the inhibition begins with the cue and is simply overshadowed by facilitation until attention is disengaged from the cued location (Posner & Cohen, 1984), cannot be ruled out and has been endorsed by many scholars. When the interval between such a spatially uninformative peripheral cue and target is extended to explore the duration of the inhibitory aftereffect, inhibition of return has been shown to last up to at least 3 s (for a review, see Samuel & Kat, 2003) and sometimes longer.
4.2. Organismic variables
4.2.1. Comparative (phylogeny)
As we pointed out in the introduction, research on spontaneous alternation began with, and continues primarily in, rodents. This is likely related to how spontaneous alternation has evolved into a standard test for memory in transgenic rodent models of human conditions (e.g., O'Leary, Hussin, Gunn, & Brown, 2018; Snider & Obrietan, 2018) or evaluating effects of pharmacological agents (Hughes, 2004). However, spontaneous alternation has been observed in several other nonhuman organisms, including larval zebrafish (Bögli & Huang, 2017) and black molly fish (Creson, Woodruff, Ferslew, Rasch, & Monaco, 2003), ants (Czaczkes, Koch, Fröber, & Dreisbach, 2018), fruit flies (Drosophila melanogaster; Lewis, Negelspach, Kaladchibachi, Cowen, & Fernandez, 2017), paramecium (Harvey & Bovell, 2006), marmosets (Izumi, Tsuchida, & Yamaguchi, 2013), and in some species of crab (but not others—Balcı, Ramey‐Balcı, & Ruamps, 2014; Ramey, Teichman, Oleksiak, & Balci, 2009). In other species, evidence for spontaneous alternation behavior is debatable (lemurs, Dal‐Pan et al., 2011; chicks, Hayes & Warren, 1963; hens, Haskell, Forkman & Waddington, 1998) or contrary (e.g., pigeons, Hughes, 1989). More recent studies in non‐rodent models seem to be returning to a curiosity‐driven motive for studying spontaneous alternation, using a comparative approach to understand its basic mechanisms, but may also be establishing spontaneous alternation as a memory assay to use in newer animal models of human conditions.
Spontaneous alternation has also been studied in humans, both children (see next section) and adults. Wingfield (1943) first used a card‐picking task in which adults were asked to pick “the highest card” out of two, but only alternated about 50% of the time. Using a different response paradigm, alternation was about 67% in the first choice after a button press when keys were both white lights, and increased to 87% when one key was blue and one was red—an interesting parallel with rodent research showing that arm choice alternation increases with increased discriminability of the arms. However, these tasks were not similar to the rodent T‐maze in that subjects were reaching for an object rather than navigating to a position in physical space. A study by Denny and Allen (as cited in Schultz, 1964) is perhaps closer to a T‐maze task—subjects were asked to trace a path in an L shape on a piece of paper repeatedly, and then given a T shape to trace a path. Using this method, adults showed 90% alternation after no delay, which decreased over increasing intervals to 50% at 72 hr. However, Manning and Artman (1973) also found significant rates of spontaneous alternation in humans using a method similar to Wingfield, but adjusted calculation of alternation to account for chance levels of alternation among individuals. Schultz summarized four factors that facilitate spontaneous alternation in humans: no knowledge of a “correct” response or reinforcement, distinct stimuli, greater practice with one direction, and a short inter‐trial interval—all similar to rodent alternation.
Research on inhibition of return, on the other hand, was established and has been tested extensively in humans. Fewer nonhuman animals have been tested for inhibition of return than for spontaneous alternation. There is evidence for inhibition of return in both nonhuman primates (rhesus monkeys, Dorris, Taylor, Klein, & Munoz, 1999; Dorris, Klein, Everling, & Munoz, 2002; Mirpour, Arcizet, Ong, & Bisley, 2009; Torbaghan, Yazdi, Mirpour, & Bisley, 2012) and archer fish (Gabay, Leibovich, Ben‐Simon, Henik, & Segev, 2013). However, perhaps surprisingly, neither rats (Wagner, Baker, & Rostron, 2014) nor pigeons (Gibson, Juricevic, Shettleworth, Pratt, & Klein, 2005) show inhibition of return, although whether this is related to method or a true lack of inhibition of return in these species is unclear.
4.2.2. Development (ontogeny)
The point in development at which spontaneous alternation and inhibition of return appear in an organism could lend clues as to whether they are possibly driven by the same mechanism. The difficulty is that spontaneous alternation and inhibition of return have not been studied much in the same organisms—let alone the same subjects/participants. In fact, only one study we know of has done this: as mentioned above, Vecera et al. (1991) examined a non‐maze version of spontaneous alternation (alternating reach) and inhibition of return in 6‐ and 18‐month old infants. While inhibition of return was present at both ages, spontaneous alternation was only evident in the older infants. This appears to support the idea that inhibition of return appears before spontaneous alternation in humans, a conclusion that fits logically with the developmental capabilities of human infants—control over eye gaze, so important for connecting visually as a babe‐in‐arms, is needed before ability to control reach and grasp for food and other objects, and develops in that order (Law, Lee, Hulse, & Tomassetti, 2011). However, spontaneous alternation and inhibition of return were negatively correlated in 18‐month‐old infants—meaning that higher levels of spontaneous alternation was associated with lower levels of inhibition of return—evidence contrary to these two behaviors having the same mechanism. In addition, it is important to consider that the spontaneous alternation task used did not require the infant to navigate a maze with their whole body, but rather only move their arms from a seated position, and therefore may not be a true measure of spontaneous alternation. Wertlieb and Rose (1979) tested 3‐, 4‐, and 5‐year‐old children in a 10′ × 10′ full‐body sized maze with several correct paths to a goal box, and found that younger children tended to perseverate in choosing a path that did not lead to reward, while older children tended to change their path after an error, meaning that a task such as spontaneous alternation, which requires lack of perseveration, would be more likely in 5‐year‐olds than in 3‐year‐olds.
As spontaneous alternation has become a standard test in animal models of human conditions, there are many studies testing for spontaneous alternation in rodents over development. An early study (Kirkby, 1967) compared spontaneous alternation in a T‐maze among 20, 40, 60, and 80‐day old rats. The two younger groups did not alternate above chance (53.3 and 65.4%, respectively) but the older groups did (74.7 and 86.7%); unlike human infants, rate of alternation was positively correlated with age. More recent studies also trace development of spontaneous alternation behavior: for example, it is not present in rats 17–19 days of age but is present at 22–24 days (Blair et al., 2013), and there is no difference in rates of spontaneous alternation between adolescent (28 days) and adult rats (56–70 days of age; Sakakibara et al., 2014). Many studies examining emergence of spontaneous alternation over development examine the behavior in conjunction with various aspects of neural development, including that of the hippocampus (see Albani, McHail, & Dumas, 2014). Spontaneous alternation has also been tested in older animals: for example, 18‐ and 23‐month old mice decreased alternation to chance levels at much shorter ITIs (90s and 30s, respectively) than did younger mice (180s), perhaps reflecting a decline in hippocampal memory for spontaneous alternation in aging mice (Vandesquille, Krazem, Louis, Lestage, & Béracochéa, 2011; see also Gold & Korol, 2014).
4.3. Neuroscientific manipulations
Because most recent and common use of spontaneous alternation has been as a behavioral assay to evaluate neural deficits in genetically altered animals or those administered pharmacological agents, there is a surfeit of studies testing involvement of neural regions (see Lalonde, 2002) and neurotransmitters (see Myhrer, 2003). Many of these have been guided by the spatial nature of the task, and gross motor movements required to navigate it. Lesions to the anterior thalamic nuclei (Aggleton & Nelson, 2015), fornix (Dumont, Amin, Wright, Dillingham, & Aggleton, 2015) and the prelimbic region of the medial prefrontal cortex (Delatour & Gisquet‐Verrier, 1996), as well as disruption to the cerebellum via genetic mutation (Lalonde, Joyal, Cote, & Botez, 1993) impair many aspects of spatial learning (and nonspatial learning)—including those measured by spontaneous alternation. As noted by O'Keefe and Nadel (1978), the region most readily associated with spatial tasks, the hippocampus, is critical for spontaneous alternation behavior: lesions to the hippocampus eliminate spontaneous alternation (e.g., Stoneham et al., 2017), and promotion of neuroplasticity or signalling within the hippocampus increases spontaneous alternation (e.g., Cao et al., 2018).
Research into the neural underpinnings of inhibition of return have focused more on structures associated with vision. Evidence that the superior colliculus (SC, a subcortical structure responsible for saccadic eye movements) might play an important role in generating inhibition of return was first provided by Posner et al. (1985). They tested patients with progressive supranuclear palsy (PSP), a degenerative condition affecting mid‐brain structures including the SC. These patients have difficulty generating saccades, particularly along the vertical axis. Suggesting a role for the SC in generating inhibition of return, it was found that inhibition of return was absent in PSP patients for vertically oriented stimuli while it was normal for control participants. The demonstration of inhibition of return in newborn infants (Simion, Valenza, Umiltà, & Dalla Barba, 1995; Valenza, Simion, & Umiltà, 1994) provides one source of converging evidence because, whereas the cortex is still developing in the newborn human infant, the SC is relatively fully operational (Johnson, 1990). More direct evidence was provided from patients with more localized damage to the SC than is present in PSP (Sapir, Soroker, Berger, & Henik, 1999; Sereno, Briand, Amador, & Szapiel, 2006). Although the SC plays a critical role in the generation of inhibition of return, circuits in this structure do not appear to be inhibited when inhibition of return is generated (Dorris et al., 2002).
The SC, a subcortical structure that controls gaze direction in retinotopic coordinates (Robinson, 1972), cannot be the whole story behind inhibition of return. That the presence of inhibition of return is modulated by task demands (e.g., Dodd, Van der Stigchel, & Hollingworth, 2009) is one behavioral finding that points to the involvement of brain mechanisms beyond the SC. Two findings described earlier, that inhibition of return can be mapped in environmental (Maylor & Hockey, 1985) and object coordinates (Tipper et al., 1991), also imply that higher cortical structures must also be involved in producing inhibition of return. Tipper et al. (1997) provided one source of evidence for this by demonstrating that object‐based inhibition of return does not cross the vertical midline in patients whose hemispheres had been separated by callosotomy to control for intractible epilepsy. Sapir, Hayes, Henik, Danziger, and Rafal (2004) later demonstrated that environmental (spatiotopic) coding of inhibition of return was eliminated in patients with lesions to the parietal lobe, and using trans‐cranial magnetic stimulation to temporarily disrupt the right intraparietal sulcus, van Koningsbruggen, Gabay, Sapir, Henik, and Rafal (2010) provided converging evidence for this finding. Recording from single neurons in the parietal lobe of monkeys while they searched an array of targets and distractors, Mirpour et al. (2009), found evidence for an inhibitory tagging system in the lateral intraparietal area. Thus, while it is clear that the SC plays a substantial role in generating inhibition of return, it is almost certainly operating in concert with other brain regions. Moreover, evidence from both TMS (Ro, Farnè, & Chang, 2003) and single unit recording (Bichot & Schall, 2002) implicates the frontal eye fields in the manifestation of inhibition of return.
5. HOW MIGHT SPONTANEOUS ALTERNATION AND INHIBITION OF RETURN FIT TOGETHER?
Our idea that spontaneous alternation and inhibition of return might be more similar than they first appear is rooted in the fact that they share a novelty‐seeking function in the spatial domain implemented by an inhibitory mechanism. Spontaneous alternation is the tendency to avoid going to a previously visited location and inhibition of return is the tendency to avoid orienting toward a previously attended location. It is true that the temporal and spatial scales on which they are observed are quite different. Inhibition of return lasts seconds, is tested while the observer is generally stationary, and takes place in peripersonal space. Spontaneous alternation, on the other hand, lasts for seconds if not minutes, and typically entails the subjects navigating their entire body through space. Despite the spatial and temporal scale differences, both phenomena seem to be influenced by similar factors in that they are both sensitive to the context in which they occur; that is to external cues that signal locations or objects in the behaving organism's environment.
So then, how could spontaneous alternation and inhibition of return fit together in the day‐to‐day behavior and brain of an organism? If we think about time scale, we could ask whether they are related sequentially, with attention (inhibition of return) preceding action (spontaneous alternation). If we think about spatial scales, the principles governing spontaneous alternation could be in play when navigating to a particular location, while inhibition of return might be recruited to control orienting once there. Alternatively, spontaneous alternation and inhibition of return might simply be examples of functionally similar but somewhat independent phenomena. Whether functionally linked or independent from a behavioral perspective, it is still possible there is overlap in the neural systems that mediate these two phenomena.
If the same neural regions or pathways that are important to spontaneous alternation were also involved in inhibition of return, this would provide a foundation for generating convincing evidence that the two phenomena are governed by the same inhibitory processes. However, to our knowledge, spontaneous alternation and inhibition of return have not been explored in the same organism while disrupting a particular region potentially critical to both. This gap in research is likely for several reasons. In the rodent, the experimental subject which most easily lends itself to lesion or pharmacological disruption studies, inhibition of return has yet to be observed. Wagner et al. (2014), for example, suggested several reasons why they might not have observed inhibition of return in rats using their methods, including the low visual acuity in rodents. Given that inhibition of return in humans is multimodal (it has been observed between all pairings of vision, audition and touch, Spence, Lloyd, McGlone, Nicholls, & Driver, 2000), perhaps a task using a different sense modality more relevant to rodent spatial behavior, such as smell or sound, should be explored. In humans, the subjects in which inhibition of return has most extensively been tested, truly parallel tests of spontaneous alternation (as might be generated using virtual reality mazes, e.g., Shore, Stanford, MacInnes, Brown, & Klein, 2001), have not been tried in recent literature. And there is no human data pointing to which brain regions are active (e.g., via fMRI) or required (e.g., via TMS or brain damage) for spontaneous alternation. Thus, we must use indirect observations to speculate whether the two phenomena are related in this way.
One strategy to address this gap would be to look at the regions known to be critical to inhibition of return in humans and determine whether these are also important to spontaneous alternation in animals. One possible candidate is the superior colliculus: rodent SC is involved in orienting head and body movements (used in spontaneous alternation), and primate SC in saccadic eye movements (used in inhibition of return; Lee, Tai, Zador, & Wilbrecht, 2015). Conversely, inhibition of return researchers could make a concerted effort to examine whether the hippocampus, clearly important for spontaneous alternation, is involved in inhibition of return in humans. The contextual sensitivity of inhibition of return that is implied by the finding that inhibitory tags are removed when the scene or array of stimuli in which the inhibition of return was generated is removed (see Wang & Klein, 2010, for a review), would be consistent with such an involvement, as would the coding of inhibition of return in a spatio‐topic frame of reference. In line with this, Meister and Buffalo (2016) discuss the possibility that the hippocampus has a role in directing eye movements in allocentric (spatio‐topic) coordinates, and specifically in inhibition of return. Recent studies showing that there are polysynaptic pathways linking hippocampal and oculomotor circuitry (Ryan et al., 2019; Shen, Bezgin, Selvam, McIntosh, & Ryan, 2016) provide a concrete neuroanatomical basis for hippocampal involvement in inhibition of return. Studies of people with medial temporal lobe damage that have revealed the importance of human hippocampal circuitry for some attention‐related phenomena (e.g., Chun & Phelps, 1999; Cosman & Vecera, 2013) provide one methodological model for how such an involvement might be explored.
Although the hippocampus plays a role in spontaneous alternation in the rat, spontaneous alternation can be seen in crabs and drosophila (which lack this structure). How is that possible? The much simpler nervous systems in these species may not have a hippocampus per se, but do have a structure that is roughly and functionally homologous with the hippocampus in mammals (ellipsoid body, e.g., Ofstad, Zuker, & Reiser, 2011). In addition, we believe that the sophisticated methods demonstrating contextual sensitivity of spontaneous alternation in rodents have not been, and ought to be, implemented more extensively in studies of spontaneous alternation with these simpler organisms—for example, the spatial task used with drosophila described by Ofstad et al. (2011) where fruit flies had to use external visual cues to locate a preferred cool spot in a warm arena. Given that the hippocampus mediates spontaneous alternation's sensitivity to context, we might predict that if these methods were applied to studies of spontaneous alternation in organisms like the crab and drosophila, the spontaneous alternation observed would also be sensitive to context. Thus, comparative work testing for spontaneous alternation behavior in non‐rodent organisms that show inhibition of return, such as archerfish, or indeed testing for inhibition of return in those that show spontaneous alternation, such as drosophila, might give a better idea of whether these two phenomena are related.
6. CONCLUSION
We have described two putative novelty‐seeking phenomena that have, for the most part, been studied independently. However, the parallels between these behaviors we have noted suggest that inhibition of return and spontaneous alternation could function similarly and, despite the evidence to date, might depend on overlapping neural circuitry. On the other hand, the differences between the two suggest phenomena that are quite independent—perhaps active in separate contexts or needs of the organisms in its environment. Our title reflects this situation and asks: “How might these two phenomena fit together.” We do not know the answer, and suggest several directions of study that might produce data that would solve the puzzle. We hope that readers will find the question an interesting one and that our commentary will stimulate the kind of research that will provide evidence and answers.
7. End Notes
This is clearly wrong for both spontaneous alternation and inhibition of return given that they are about locations in space when there is context: see section on “frame of reference.”
An organism that shows spontaneous alternation is demonstrating use of memory, however, animal that does not might in fact remember, but other factors might be operating to reduce or even overcome whatever is responsible for the tendency to alternate (e.g., see Hughes, 2004).
8. REFERENCES
Aggleton, J. P., & Nelson, A. J. (2015). Why do lesions in the rodent anterior thalamic nuclei cause such severe spatial deficits? Neuroscience & Biobehavioral Reviews, 54, 131–144.
Albani, S. H., McHail, D. G., & Dumas, T. C. (2014). Developmental studies of the hippocampus and hippocampal‐dependent behaviors: Insights from interdisciplinary studies and tips for new investigators. Neuroscience Biobehavioral Reviews, 43, 183–190.
Balcı, F., Ramey‐Balcı, P. A., & Ruamps, P. (2014). Spontaneous alternation and locomotor activity in three species of marine crabs: Green crab (Carcinus maenas), blue crab (Callinectes sapidus), and fiddler crab (Uca pugnax). Journal of Comparative Psychology, 128(1), 65–73.
Bichot, N. P., & Schall, J. D. (2002). Priming in macaque frontal cortex during popout visual search: Feature‐based facilitation and location‐based inhibition of return. Journal of Neuroscience, 22(11), 4675–4685.
Blair, M. G., Nguyen, N. N. Q., Albani, S. H., Matthew, M. L., Andrawis, M. M., Owen, L. M., … Stoneham, E. T. (2013). Developmental changes in structural and functional properties of hippocampal AMPARs parallels the emergence of deliberative spatial navigation in juvenile rats. Journal of Neuroscience, 33(30), 12218–12228.
Bögli, S. Y., & Huang, M. Y. Y. (2017). Spontaneous alternation behavior in larval zebrafish. Journal of Experimental Biology, 220(2), 171–173.
Cao, Z., Dai, D., Wei, P., Han, Y., Guan, Y., Li, H., … Li, C. (2018). Effects of cordycepin on spontaneous alternation behavior and adenosine receptors expression in hippocampus. Physiology and Behavior, 184, 135–142.
Chun, M. M., & Phelps, E. A. (1999). Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neuroscience, 2(9), 844–847.
Clohessy, A. B., Posner, M. I., Rothbart, M. K., & Vecera, S. P. (1991). The development of inhibition of return in early infancy. Journal of Cognitive Neuroscience, 3(4), 345–350.
Cosman, J. D., & Vecera, S. P. (2013). Learned control over distraction is disrupted in amnesia. Psychological Science, 24(8), 1585–1590.
Creson, T. K., Woodruff, M. L., Ferslew, K. E., Rasch, E. M., & Monaco, P. J. (2003). Dose–response effects of chronic lithium regimens on spatial memory in the black molly fish. Pharmacology Biochemistry and Behavior, 75(1), 35–47.
Czaczkes, T. J., Koch, A., Fröber, K., & Dreisbach, G. (2018). Voluntary switching in an invertebrate: The effect of cue and reward change. Journal of Experimental Psychology: Animal Learning and Cognition, 44(3), 247–257.
Dal‐Pan, A., Pifferi, F., Marchal, J., Picq, J. L., Aujard, F., & RESTRIKAL Consortium. (2011). Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One, 6(1), e16581. https://doi.org/10.1371/journal.pone.0016581
Delatour, B., & Gisquet‐Verrier, P. (1996). Prelimbic cortex specific lesions disrupt delayed‐variable response tasks in the rat. Behavioral Neuroscience, 110, 1282–1298.
Dember, W. N. (1989). Spontaneous alternation. New York: Springer‐Verlag.
Dennis, W. (1939). Spontaneous alternation in rats as an ndicator of the persistence of stimulus effects. Journal of Comparative Psychology, 28(2), 305–312.
Dodd, M. D., Van der Stigchel, S., & Hollingworth, A. (2009). Novelty is not always the best policy: Inhibition of return and facilitation of return as a function of visual task. Psychological Science, 20(3), 333–339.
Dorris, M. C., Klein, R. M., Everling, S., & Munoz, D. P. (2002). Contribution of the primate superior colliculus to inhibition of return. Journal of Cognitive Neuroscience, 14(8), 1256–1263.
Dorris, M. C., Taylor, T. L., Klein, R. M., & Munoz, D. P. (1999). Influence of previous visual stimulus or saccade on saccadic reaction times in monkey. Journal of Neurophysiology, 81(5), 2429–2436.
Douglas, R. J. (1972). Pavlovian conditioning and the brain. In R. A. Boakes & M. E. Halliday (Eds.), Inhibition and learning (pp. 529–553). New York: Academic Press.
Douglas, R. J. (1989). Spontaneous alternation behavior and the brain. In W. N. Dember & C. L. Richman (Eds.), Spontaneous alternation behavior (pp. 73–108). New York: Springer‐Verlag.
Dumont, J. R., Amin, E., Wright, N. F., Dillingham, C. M., & Aggleton, J. P. (2015). The impact of fornix lesions in rats on spatial learning tasks sensitive to anterior thalamic and hippocampal damage. Behavioural Brain Research., 278, 360–374.
Ellen, P., & Deloache, J. (1968). Hippocampal lesions and spontaneous alternation behavior in the rat. Physiology & Behavior, 3(6), 857–860.
Fowler, H., Blond, J., & Dember, W. N. (1959). Alternation behavior and learning: The influence of reinforcement magnitude, number, and contingency. Journal of Comparative and Physiological Psychology, 52(5), 609–614.
Gabay, S., Leibovich, T., Ben‐Simon, A., Henik, A., & Segev, R. (2013). Inhibition of return in the archer fish. Nature Communications, 4, 1657.
Gibson, B. M., Juricevic, I., Shettleworth, S. J., Pratt, J., & Klein, R. M. (2005). Looking for inhibition of return in pigeons. Learning & Behavior, 33(3), 296–308.
Glanzer, M. (1953a). The role of stimulus satiation in spontaneous alternation. Journal of Experimental Psychology, 45(6), 387–393.
Glanzer, M. (1953b). Stimulus satiation: An explanation of spontaneous alternation and related phenomena. Psychological Review, 60(4), 257–268.
Gold, P. E., & Korol, D. L. (2014). Forgetfulness during aging: An integrated biology. Neurobiology of Learning and Memory, 112, 130–138.
Harvey, A. W., & Bovell, N. K. (2006). Spontaneous alternation behavior in paramecium. Learning & Behavior, 34(4), 361–365.
Haskell, M. J., Forkman, B., & Waddington, D. (1998). An investigation into the occurrence of spontaneous alternation behaviour in the domestic hen. Behavioural Processes, 43(1), 43–51.
Hayes, W. N., & Warren, J. M. (1963). Failure to find spontaneous alternation in chicks. Journal of Comparative and Physiological Psychology, 56(3), 575–577.
Heathers, G. L. (1940). The avoidance of repetition of a maze reaction in the rat as a function of the time interval between trials. The Journal of Psychology, 10(2), 359–380.
Hughes, R. N. (1989). Lack of spontaneous alternation in favor of perseveration in domestic fowls and pigeons. Behavioural Processes, 20(1–3), 85–92.
Hughes, R. N. (2004). The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neuroscience & Biobehavioral Reviews, 28(5), 497–505.
Ivanoff, J., & Klein, R. M. (2006). A speed‐accuracy analysis of inhibition of return in go/no‐go and choice‐RT tasks. Journal of Experimental Psychology: Human Perception and Performance, 32, 908–919.
Izumi, A., Tsuchida, J., & Yamaguchi, C. (2013). Spontaneous alternation behavior in common marmosets (Callithrix jacchus). Journal of Comparative Psychology, 127(1), 76–81.
Jackson, M. M. (1941). Reaction tendencies of the white rat in running and jumping situations. Journal of Comparative Psychology, 31(2), 255–262.
Johnson, M. H. (1990). Cortical maturation and the development of visual attention in early infancy. Journal of Cognitive Neuroscience, 2(2), 81–95.
Kirkby, R. J. (1967). A maturation factor in spontaneous alternation. Nature, 215(5102), 784.
Klein, R. M. (1988). Inhibitory tagging system facilitates visual search. Nature, 334(6181), 430–431.
Klein, R. M. (2000). Inhibition of return. Trends in Cognitive Sciences, 4(4), 138–147.
Klein, R. M., & MacInnes, W. J. (1999). Inhibition of return is a foraging facilitator in visual search. Psychological Science, 10(4), 346–352.
Lalonde, R. (2002). The neurobiological basis of spontaneous alternation. Neuroscience & Biobehavioral Reviews, 26(1), 91–104.
Lalonde, R., Joyal, C. C., Cote, C., & Botez, M. I. (1993). Delayed spontaneous alternation in lurcher mutant mice. Psychobiology, 21(2), 139–141.
Law, J., Lee, M., Hulse, M., & Tomassetti, A. (2011). The infant development timeline and its application to robot shaping. Adaptive Behaviour, 19(5), 335–358.
Lee, A. M., Tai, L., Zador, A., & Wilbrecht, L. (2015). Between the primate and ‘reptilian’ brain: Rodent models demonstrate the role of corticostriatal circuits in decision making. Neuroscience, 296, 66–74.
Lennartz, R. C. (2008). The role of extramaze cues in spontaneous alternation in a plus‐maze. Learning & Behavior, 36(2), 138–144.
Lewis, S. A., Negelspach, D. C., Kaladchibachi, S., Cowen, S. L., & Fernandez, F. (2017). Spontaneous alternation: A potential gateway to spatial working memory in drosophila. Neurobiology of Learning and Memory, 142, 230–235.
Manning, S. K., & Artman, J. A. (1973). New procedure to test some factors in human spontaneous alternation. Journal of Experimental Psychology, 97(2), 274–277.
Maylor, E. A., & Hockey, R. (1985). Inhibitory component of externally controlled covert orienting in visual space. Journal of Experimental Psychology: Human Perception and Performance, 11(6), 777–787.
Meister, M. L., & Buffalo, E. A. (2016). Getting directions from the hippocampus: The neural connection between looking and memory. Neurobiology of Learning and Memory, 134, 135–144.
Mirpour, K., Arcizet, F., Ong, W. S., & Bisley, J. W. (2009). Been there, seen that: A neural mechanism for performing efficient visual search. Journal of Neurophysiology, 102(6), 3481–3491.
Myhrer, T. (2003). Neurotransmitter systems involved in learning and memory in the rat: A meta‐analysis based on studies of four behavioral tasks. Brain Research Reviews, 41(2–3), 268–287.
Ofstad, T. A., Zuker, C. S., & Reiser, M. B. (2011). Visual place learning in Drosophila melanogaster. Nature, 474, 204–209.
Ogawa, H., Takeda, Y., & Yagi, A. (2002). Inhibitory tagging on randomly moving objects. Psychological Science, 13(2), 125–129.
O'Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford: Clarendon Press.
O'Leary, T. P., Hussin, A. T., Gunn, R. K., & Brown, R. E. (2018). Locomotor activity, emotionality, sensori‐motor gating, learning and memory in the APPswe/PS1dE9 mouse model of Alzheimer's disease. Brain Research Bulletin, 140, 347–354.
Olton, D. S., Handelman, G. E., Walker, J. A., Kamil, A. C., & Sargent, T. D. (1981). Spatial memory and food searching strategies. In A. C. Kamil & T. D. Sargent (Eds.), Foraging behavior: Ecological, ethological, and psychological approaches (pp. 333–54). New York: Garland STPM.
Posner, M. I., & Cohen, Y. (1984). Components of visual orienting. In H. Bouma & D. G. Bouwhuis (Eds.), Attention and performance X: Control of language processes (Vol. 32, pp. 531–556). Hove: Lawrence Erlbaum Associates Ltd.
Posner, M. I., & Petersen, S. E. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13(1), 25–42.
Posner, M. I., Rafal, R. D., Choate, L. S., & Vaughan, J. (1985). Inhibition of return: Neural basis and function. Cognitive Neuropsychology, 2, 211–228.
Ramey, P. A., Teichman, E., Oleksiak, J., & Balci, F. (2009). Spontaneous alternation in marine crabs: Invasive versus native species. Behavioural Processes, 82(1), 51–55.
Redden, R. S., Klages, J., & Klein, R. M. (2017). The effect of scene removal on inhibition of return in a cue target task. Attention Perception and Psychophysics, 79(1), 78–84.
Ro, T., Farnè, A., & Chang, E. (2003). Inhibition of return and the human frontal eye fields. Experimental Brain Research, 150(3), 290–296.
Robinson, D. A. (1972). Eye movements evoked by collicular stimulation in the alert monkey. Vision Research, 12(11), 1795–1808.
Ryan, J. D., Shen, K., Kacollja, A., Tian, H., Griffiths, J., Bezgin, G., & McIntosh, A. R. (2019). The functional reach of the hippocampal memory system to the oculomotor system. bioRxiv. https://doi.org/10.1101/303511
Sakakibara, Y., Kasahara, Y., Hall, F. S., Lesch, K‐P., Murphy, D. L., Uhl, G. R., & Sora, I. (2014). Developmental alterations in anxiety and cognitive behavior inserotonin transporter mutant mice. Psychopharmacology, 231, 4119–4133.
Samuel, A. G., & Kat, D. (2003). Inhibition of return: A graphical meta‐analysis of its time course and an empirical test of its temporal and spatial properties. Psychonomic Bulletin & Review, 10(4), 897–906.
Sapir, A., Hayes, A., Henik, A., Danziger, S., & Rafal, R. (2004). Parietal lobe lesions disrupt saccadic remapping of inhibitory location tagging. Journal of Cognitive Neuroscience, 16(4), 503–509.
Sapir, A., Soroker, N., Berger, A., & Henik, A. (1999). Inhibition of return in spatial attention: Direct evidence for collicular generation. Nature Neuroscience, 2(12), 1053–1054.
Satel, J., Wang, Z., Hilchey, M. D., & Klein, R. M. (2012). Examining the dissociation of retinotopic and spatiotopic inhibition of return with event‐related potentials. Neuroscience Letters, 524(1), 40–44.
Schultz, D. P. (1964). Spontaneous alternation behavior in humans: Implications for psychological research. Psychological Bulletin, 62(6), 394–400.
Sereno, A. B., Briand, K. A., Amador, S. C., & Szapiel, S. V. (2006). Disruption of reflexive attention and eye movements in an individual with a collicular lesion. Journal of Clinical and Experimental Neuropsychology, 28(1), 145–166.
Shen, K., Bezgin, G., Selvam, R., McIntosh, A. R., & Ryan, J. D. (2016). An anatomical Interface between memory and oculomotor systems. Journal of Cognitive Neuroscience, 28, 1772–1783.
Shore, D. I., Stanford, L., MacInnes, W. J., Brown, R. E., & Klein, R. M. (2001). Of mice and men: Virtual Hebb—Williams mazes permit comparison of spatial learning across species. Cognitive, Affective, & Behavioral Neuroscience, 1(1), 83–89.
Simion, F., Valenza, E., Umiltà, C., & Dalla Barba, B. (1995). Inhibition of return in newborns is temporo‐nasal asymmetrical. Infant Behavior and Development, 18(2), 189–194.
Snider, K. H., & Obrietan, K. (2018). Modulation of learning and memory by the genetic disruption of circadian oscillator populations. Physiology & Behavior, 194, 387–393.
Spence, C., Lloyd, D., McGlone, F., Nicholls, M. E. R., & Driver, J. (2000). Inhibition of return is supramodal: A demonstration between all possible pairings of vision, touch, and audition. Experimental Brain Research, 134(1), 42–48. http://doi.org/10.1007/s002210000442
Stoneham, E. T., McHail, D. G., Boggs, K. N., Albani, S. H., Carty, J. A., Evans, R. C., … Dumas, T. C. (2017). Functional perturbation of forebrain principal neurons reveals differntial effects in novel and well‐learned tasks. Brain Research, 1671, 1–13.
Tipper, S. P., Driver, J., & Weaver, B. (1991). Object‐centred inhibition of return of visual attention. The Quarterly Journal of Experimental Psychology Section A, 43(2), 289–298.
Tipper, S. P., Rafal, R., Reuter‐Lorenz, P. A., Starrveldt, Y., Ro, T., Egly, R., … Weaver, B. (1997). Object‐based facilitation and inhibition from visual orienting in the human split‐brain. Journal of Experimental Psychology: Human Perception and Performance, 23(5), 1522–1532.
Tolman, E. C. (1925). Purpose and cognition: The determiners of animal learning. Psychological Review, 32(4), 285–297.
Torbaghan, S. S., Yazdi, D., Mirpour, K., & Bisley, J. W. (2012). Inhibition of return in a visual foraging task in non‐human subjects. Vision Research, 74, 2–9.
Valenza, E., Simion, F., & Umiltà, C. (1994). Inhibition of return in newborn infants. Infant Behavior and Development, 17(3), 293–302.
van Koningsbruggen, M. G., Gabay, S., Sapir, A., Henik, A., & Rafal, R. D. (2010). Hemispheric asymmetry in the remapping and maintenance of visual saliency maps: A TMS study. Journal of Cognitive Neuroscience, 22(8), 1730–1738.
Vandesquille, M., Krazem, A., Louis, C., Lestage, P., & Béracochéa, D. (2011). S 18986 reverses spatial working memory impairments in aged mice: Comparison with memantine. Psychopharmacology, 215(4), 709–720.
Vecera, S. P., Rothbart, M. K., & Posner, M. I. (1991). Development of spontaneous alternation in infancy. Journal of Cognitive Neuroscience, 3(4), 351–354.
Wagner, U., Baker, L., & Rostron, C. (2014). Searching for inhibition of return in the rat using the covert orienting of attention task. Animal Cognition, 17, 1121–1135.
Wang, Z., & Klein, R. M. (2010). Searching for inhibition of return in visual search: A review. Vision Research, 50, 220–228.
Wertlieb, D., & Rose, D. (1979). Maturation of maze behavior in preschool children. Developmental Psychology, 15(4), 478–479.
Wingfield, R. C. (1943). Some factors influencing spontaneous alternation in human subjects. Journal of Comparative Psychology, 35(3), 237–243.
Wingfield, R. C., & Dennis, W. (1934). The dependence of the rat's choice of pathways upon the length of the daily trial series. Journal of Comparative Psychology, 18(1), 135–147.
Yi, D. J., Kim, M. S., & Chun, M. M. (2003). Inhibition of return to occluded objects. Perception & Psychophysics, 65(8), 1222–1230.
14. Neurons and networks in the entorhinal cortex: A reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways
Eirik S. Nilssen1 | Thanh P. Doan1 | Maximiliano J. Nigro1 | Shinya Ohara1,2 | Menno P. Witter1
1Kavli Institute for Systems Neuroscience, Centre for Neural Computation, Egil and Pauline Braathen and Fred Kavli Centre for Cortical Microcircuits, NTNU Norwegian University of Science and Technology, Trondheim, Norway
2Laboratory of Systems Neuroscience, Tohoku University Graduate School of Life Sciences, Sendai, Japan
Correspondence
Menno P. Witter, Kavli Institute for Systems Neuroscience, MTFS, The Faculty of Medicine and Health Sciences, NTNU, PO Box 8905, 7491 Trondheim, Norway. Email: menno.witter@ntnu.no
Funding information Kavli Foundation; Norges Forskningsråd, Grant/Award Numbers: 197467, 223262, 227769
14.1. INTRODUCTION
Memory is an important capacity of the brain and has intrigued scientists ever since they started to study the brain. The ability to store and recall information comes of use in a variety of daily behaviors, and the likely most important role is for us to make predictions based on previous experiences. Previous experiences with a high similarity become eventually stored as generalized concepts or schemes, which are being updated with new experiences. The efficacy of our memory system to make accurate predictions about future events depends on the relative robustness of our stored memories. This same robustness, however, provides a potential threat in that memories might become harder to change and thus our behavior may become guided by concepts that are no longer an adequate representation of the current situation. Research on memory suffers from a comparable threat in that well‐established theories might become difficult to adjust to encompass new insights.
The focus on the medial temporal lobe as being critically involved in episodic memory was essentially initiated by the influential paper on patient HM, reporting the devastating anterograde amnesia as the result of bilateral resections of the antero‐medial portions of the temporal lobe. The lesions included a substantial part of the hippocampal formation (HF), the amygdala and the parahippocampal region (PHR), in particular the entorhinal cortex (EC) and perirhinal cortex (PER) (Annese et al., 2014; Augustinack et al., 2014; Scoville & Milner, 1957). Irrespective of the fact that the lesions included several different brain structures aside HF bilaterally, the field quickly zoomed in on HF as the likely most critical structure underlying episodic memory (Milner, Squire, & Kandel, 1998). This emphasis on HF was strengthened by a large body of existing data reporting the beautiful morphological simplicity of HF and its intrinsic organization (Blackstad, 1956, 1958; Haug, 1976; Hjorth‐Simonsen, 1971; Hjorth‐Simonsen & Jeune, 1972; Lorente de Nó, 1934; Ramón y Cajal, 1893), the first description of the spatially modulated “place cell” (O'Keefe, 1976; O'Keefe & Dostrovsky, 1971), the phenomenon of long‐term potentiation (Bliss & Lømo, 1973), all culminating in the very influential book in which O'Keefe and Nadel proposed the theory of the hippocampus as a cognitive map (O'Keefe & Nadel, 1978). These authors managed to integrate all these seemingly disparate observations into a coherent theoretical framework organized around the concept of place cells as the cellular basis for representation of space as well as events and experiences associated with space. Although clearly unintended by these two authors at that time, the appealing experimental simplicity of the navigational focus set the scene for a hippocampal‐centric hierarchical view of the medial temporal lobe memory system. The latter includes the amygdala and the PHR. Although the amygdala does affect memory functions through influencing consolidation of emotional stimuli (Adolphs, Cahill, Schul, & Babinsky, 1997; Sutherland & McDonald, 1990; Zola‐Morgan, Squire, Alvarez‐Royo, & Clower, 1991), restricted lesions to the amygdala do not produce appreciable memory impairments (Mishkin, 1978; Sutherland & McDonald, 1990; Zola‐Morgan, Squire, & Amaral, 1989). In contrast, PHR with the entorhinal cortex (EC) as a nodal point, eventually became recognized as a player of substance. The latter structure was positioned to mediate the overall reciprocal connections of HF with the cortex (Buzsaki, 1996; Eichenbaum, 2000; Kosel, Van Hoesen, & Rosene, 1982; Squire, Stark, & Clark, 2004).
Ramon y Cajal drew attention to EC or the “sphenoidal cortex”/“angular ganglion” as he initially referred to it (Ramón y Cajal, 1902), describing the massive bundle of entorhinal fibers, perforating the subiculum on its way to HF. This led him to suggest that the functional significance of EC had to be related to that of HF. Subsequent anatomical studies showed that EC provides a main input to HF (Witter, Groenewegen, Lopes da Silva, & Lohman, 1989). A second seminal observation was that in the monkey, HF distributes a main output to deep layers of EC, which in turn originates major projections to adjacent parts of PHR as well as to frontal cortical domains (Kosel et al., 1982; Van Hoesen & Pandya, 1975; G. Van Hoesen, Pandya, & Butters, 1975; Van Hoesen & Pandya, 1975). This was later corroborated and further detailed in the monkey (Munoz & Insausti, 2005) and in a number of other species, including rodents (Witter, et al., 1989). Although in subsequent years anatomical studies detailed the connectional organization of PHR, and EC in particular, the role of EC was not really appreciated; the functional attributes of EC remained in the shadow, only to achieve recognition more recently, resulting in a still ongoing explosion of rich and surprising new details. One initial finding contributing to this recognition was that damage to EC results in strong functional impairments in episodic memory (Buckmaster, Eichenbaum, Amaral, Suzuki, & Rapp, 2004; Leonard, Amaral, Squire, & Zola‐Morgan, 1995; Meunier, Bachevalier, Mishkin, & Murray, 1993). In addition, the discovery of place fields in area CA1 of HF initiated a discussion on whether these functional properties were the result of internal HF computations or depended on inputs from outside HF. A recent comprehensive review (Moser, Moser, & McNaughton, 2017) summarized this debate in detail and introduced the subsequent discovery of spatially modulated grid cells in the most dorsal part of the medial entorhinal cortex (MEC) in rodents. This and subsequent reports on many functional cell types, all relevant to path‐integration‐based representation of self‐location in MEC, contributed to the current strong interest in the functional attributes of MEC. The discovery of the grid cell further led to a substantial number of studies aiming to describe or model the neuronal networks underlying their specific firing properties (Moser et al., 2017).
The focus on MEC as the location of the myriad of functional cell types relevant for spatial navigation and spatial memory has enhanced our understanding of the entorhinal‐hippocampal interplay and led to an interaction between computational and experimental neuroscience, aiming to identify and study generic circuit motifs underlying spatial perception and navigation. Although very productive, this focus distracted from the fact that there is a nonspatial side to episodic memory. For example, although partial or even complete lesions of MEC do impact the precision and long‐term stability of place cells in HF (Brun et al., 2008; Hales et al., 2018), they do not abolish them. Such lesions do impair performance in the water maze of rats, similar to HF‐lesions, but do not affect other HF‐dependent tasks such as memory for object‐location and context (Hales et al., 2018). For an episodic memory, one needs not only to store where the event took place and the position of the observer/participant in an allocentric parametric space, but also what happened and when it happened. This final convergence likely takes place in HF (Eichenbaum, 2017). Anatomical and functional data in rodents, monkeys, and humans suggest that the “What” is represented in the lateral entorhinal cortex (LEC) (Ritchey, Libby, & Ranganath, 2015; M. P. Witter et al., 2000), whereas time was suggested to be mediated through MEC (Eichenbaum, 2017) though more recent data indicated a role for LEC as well (Montchal, Reagh, & Yassa, 2019; Tsao et al., 2018).
It was particularly the knowledge about cortical connectivity that led to the notion of two functionally different portions in EC. The concept of LEC and MEC as entorhinal areas belonging to two parallel input–output streams mediating the encoding and storage of respectively what and where information is currently well accepted (Eichenbaum, Yonelinas, & Ranganath, 2007; Ritchey et al., 2015). An important component of this concept hinges on the claim that a major component of their cortical connections is with the PER cortex and postrhinal (POR) in rodents, or parahippocampal cortex (PHC) in primates. In this scenario, LEC and PER are connectionally associated and POR and MEC are likewise partners. However, already in the early anatomical studies, there are indications that this connectional dissociation is not as evident as generally portrayed (Burwell & Amaral, 1998, 1998; Insausti & Amaral, 2008; Suzuki & Amaral, 1994). Moreover, several authors emphasized that both PER and POR as well as LEC and MEC are interconnected (Burwell & Amaral, 1998; Dolorfo & Amaral, 1998; Köhler, 1986, 1988; Lavenex, Suzuki, & Amaral, 2004). Although these interconnections have been included by some authors (Burke et al., 2018; Knierim, Neunuebel, & Deshmukh, 2014; Lisman, 2007; Ranganath & Ritchey, 2012), they have not really surfaced as relevant components in the appraisal of the potential functional roles of LEC and MEC and likewise PER and POR/PHC (cf. Furtak, Ahmed, & Burwell, 2012). So, a reappraisal of the parallel model is considered relevant (Figure 12) to prevent the field from consolidating on an incomplete model of the functional relevance of PHR.
Figure 12.
Schematic representation of the proposed updated version of the wiring scheme of the parahippocampal‐hippocampal system. The lateral and medial entorhinal cortex mediate parallel input streams, conveying integrated representations of two complementary sets of cortical inputs to the hippocampus. The lateral entorhinal cortex (LEC) receives strong inputs from perirhinal (PER), orbitofrontal, medial prefrontal and insular cortices (PFC), and olfactory structures (OLF) including the olfactory bulb and the olfactory or piriform cortex. In contrast, MEC receives main inputs from presubiculum (PRS), parasubiculum (PAS), and retrosplenial cortex (RSC). The postrhinal/parahippocampal cortex (POR/PHC) provides inputs to both MEC and LEC as well as to PER. Dashed dividers in boxes imply that incoming projections distribute to both components of the box. CA3, CA2, CA1, subfields of the hippocampus proper; DG, dentate gyrus; dist, distal part; prox, proximal part [Color figure can be viewed at wileyonlinelibrary.com]
We further need to consider that although the multitude of functionally specialized cell types in MEC is remarkable, many of them express more than one type of information. Such conjunctive neurons are particularly abundant in deeper Layers III and V of MEC, whereas pure grid cells are predominant in Layer II. In the deeper layers of MEC, a majority of the not so numerous grid cells fire conjunctively for position and head direction or speed, and many border cells are direction‐selective (Hardcastle, Maheswaranathan, Ganguli, & Giocomo, 2017; Kropff, Carmichael, Moser, & Moser, 2015; Sargolini et al., 2006; Solstad, Boccara, Kropff, Moser, & Moser, 2008). Until recently, very little was known about the local intra—and interlaminar networks in MEC, except for the local network in Layer II, associated with the grid cell phenotype, briefly mentioned earlier. The emergent functional properties of the deeper cells are thus still poorly understood in terms of local architecture and its interactions with input/output connectivity. Even less is known about LEC. Based on the striking difference in functional cell types in LEC and MEC (Deshmukh & Knierim, 2011; Hargreaves, Rao, Lee, & Knierim, 2005; Neunuebel, Yoganarasimha, Rao, & Knierim, 2013; Tsao et al., 2018; Tsao, Moser, & Moser, 2013; Wang et al., 2018) expectations were that local circuits might differ between the two EC subdivisions. This has only recently been studied in detail, and these recent results indicate that the local circuits in LEC and MEC might not be all that different (Fuchs et al., 2016; Leitner et al., 2016; Nilssen et al., 2018; Ohara et al., 2018).
In this review, we aim to relate the organization of local networks to what is known about cortical inputs and their postsynaptic targets, with a focus on the concept of parallel cortical connectivity streams. Although this review is dominated by rodent data, we aim to integrate relevant primate data. We will argue that EC, changed from being insignificant into possibly one of the most important characters in the tale of the medial temporal lobe. Moreover, instead of considering LEC and MEC as mediating segregated parallel input pathways to HF, the network structure emphasizes the potential of substantial integration of cortical information through interactions between LEC and MEC. Integration is likely reflected in the complex conjunctive properties of neurons seen throughout EC, and more in particular in LEC (Deshmukh & Knierim, 2011; Naya & Suzuki, 2011; Suzuki, Miller, & Desimone, 1997; Tsao et al., 2013; Wang et al., 2018). We therefore feel the need to revise the current parallel model where the medial and lateral entorhinal cortex provide parallel input streams to HF into one where EC is considered as an area allowing for integration of two or even more parallel cortical streams (Yoo & Lee, 2017), providing HF with high‐order complex representations of the external environment, its stability, as well as apparent changes either as an inherent feature of a biological environment or as the result of navigating the environment.
14.1.1. The entorhinal cortex comprises two subdivisions
EC can be best defined based on its projections to the hippocampus, which target neurons in all main hippocampal subdivisions. Although EC projections to some of the HF fields, particularly those to CA1 and subiculum are paralleled by projections from PER and POR, the EC projections to DG are currently considered a unique projection, identifying EC (Cappaert, Van Strien, & Witter, 2014; Witter, Doan, Jacobsen, Nilssen, & Ohara, 2017). EC is associated with the rhinal sulcus and in many if not all mammalian species, EC is characterized by a regular six‐layered structure with a neuron‐sparse superficial Layer I and a similarly neuron‐sparse Layer IV in the center, sandwiched between Layer III and Layer V. In the posteromedially positioned MEC, all layers are clearly demarcated and show a relatively homogeneous distribution of neurons. The opposite, anterolateral part, LEC, has a less stringent laminar structure, and the overall distribution of neurons is less homogeneous. Note that there is generally an area in between these extremes and in particular this intermediate area has been subdivided differently in various species. It is a common observation that the cytoarchitectonically based subdivision of this intermediate area is increasingly complex in primates (Amaral, Insausti, & Cowan, 1987; Insausti, Tunon, Sobreviela, Insausti, & Gonzalo, 1995; Krimer, Hyde, Herman, & Saunders, 1997). A detailed description and comparison of all subdivisional schemes that have been proposed is beyond the scope of the review but has been covered in several papers in detail (Insausti, Munoz‐Lopez, Insausti, & Artacho‐Perula, 2017; Witter, Groenewegen, et al., 1989). For this review we will use LEC and MEC as indications for two areas, irrespective of species, for which most functional data are available, including in humans (Maass, Berron, Libby, Ranganath, & Duzel, 2015; Montchal et al., 2019; Navarro Schroder, Haak, Zaragoza Jimenez, Beckmann, & Doeller, 2015). Moreover, in a recent comparative review on the distribution of chemically defined neurons and neuropil, we have argued that these are best described as a gradient related to the distance from the rhinal/collateral sulcus and not related to any of the traditional cytorarchitectural subdivisions (Kobro‐Flatmoen & Witter, 2019).
In most if not all studied nonprimate species, the organization of the EC projection to DG, originating from reelin expressing neurons in Layer II of both LEC and MEC, supports the subdivision of EC into two subareas, whereby LEC targets dendritic compartments located distally to those targeted by MEC fibers (Hjorth‐Simonsen, 1972; Hjorth‐Simonsen & Jeune, 1972; Witter, 2007; Witter et al., 2017). Whereas axons from LEC terminate in the outer one‐third of the DG molecular layer, those from MEC terminate in the middle one‐third. This spatial segregation is less evident in the monkey (Witter & Amaral, 1991; Witter, Van Hoesen, & Amaral, 1989). Irrespective of these anatomical differences, it is likely that in all species the projections from all parts of EC, irrespective of the number of subdivisions recognized by various authors, converge onto single neurons in DG and likely this holds true for CA3 and CA2 as well. In rodents and monkeys, entorhinal Layer III projections to CA1 and subiculum show a strikingly different organization from those arising from Layer II in that axons from LEC target neuronal populations different from those targeted by projections from MEC (Naber, Lopes da Silva, & Witter, 2001; van Groen, Miettinen, & Kadish, 2003; Witter & Amaral, 1991). Fibers from LEC innervate a part of CA1 close to the subiculum and the directly adjacent portion of the subiculum, whereas fibers from MEC terminate in the CA1 part adjacent to CA2 and in the subicular part adjacent to the presubiculum. The return projections to Layer V of EC from CA1 and subiculum follow a similar topographical organization, thus creating segregated anatomical connectivity loops between LEC and MEC on the one hand and discrete portions of CA1 and subiculum on the other hand (Tamamaki & Nojyo, 1995; Witter, 1993).
Further data in support of a dissociation between the two EC subdivisions come from recent gene expression studies. Embryonic gene expression patterns in mice indicate that the two subdivisions of EC originate from two different pallial structures. Whereas MEC originates in close association with HF, LEC has its origin in a specific dorsoposterior part of the cortical anlage. Interestingly, these genetically defined subdivisions of EC were also recognized in birds and reptiles (Medina, Abellan, & Desfilis, 2017). In line with this is a report that LEC and MEC in adult mice show strikingly different enhancer‐expression profiles (Blankvoort, Witter, Noonan, Cotney & Kentros, 2018).
14.1.2. Emergent functional cell types
14.1.2.1. MEC
In MEC, most if not all of the functionally defined neuron types seem to relate to coding aspects of space or navigation relevant to path‐integration‐based representation of self‐location. One finds at least two types of spatially modulated cells types, grid cells, which have multiple equidistant firing fields organized in a hexagonal pattern (Fyhn, Molden, Witter, Moser, & Moser, 2004; Hafting, Fyhn, Molden, Moser, & Moser, 2005), as well as spatially modulated nongrid cells (Miao, Cao, Moser, & Moser, 2017; Rowland et al., 2018). Grid cells have been reported in rats (Hafting et al., 2005), mice (Fyhn, Hafting, Witter, Moser, & Moser, 2008), bats (Yartsev, Witter, & Ulanovsky, 2011), and nonhuman primates (Killian, Jutras, & Buffalo, 2012). Periodic, grid‐like signals have been identified also in the human EC (Doeller, Barry, & Burgess, 2010; J. Jacobs & Lee, 2016). Grid cells coexist in MEC with other functionally defined cell types that code for the heading of the animal (head‐direction cells), for speed (speed cells), environmental borders (border cells), or the distance and angle to objects (object‐vector cells) (Høydal, Skytøen, Moser, & Moser, 2018; Kropff et al., 2015; Sargolini et al., 2006; Solstad et al., 2008). MEC is thus best considered as a cortical structure capable of computations underpinning path integration, an idiothetic navigation strategy in which the animal uses self‐motion cues to track its current position relative to an arbitrary reference location (Buzsaki & Moser, 2013; Moser et al., 2017).
The complement of cortical relationships of MEC seems to match this overall presence of functional neuron‐types. Main inputs to MEC originate from presubiculum and parasubiculum (Caballero‐Bleda & Witter, 1993; Köhler, 1985; Room & Groenewegen, 1986; Shipley, 1975; van Groen & Wyss, 1990, 1990). Likewise, in rodents, cats, and monkeys, the retrosplenial cortex projects densely to MEC (Burwell & Amaral, 1998; Jones & Witter, 2007; Kobayashi & Amaral, 2007; Room & Groenewegen, 1986). Additional inputs to MEC originate in visual association areas of the occipital cortex in the rat (Burwell & Amaral, 1998; Kerr, Agster, Furtak, & Burwell, 2007), whereas these areas in monkeys primarily target PHC (Van Hoesen, 1982; Van Hoesen, Pandya, & Butters, 1972), and might thus influence MEC activity only indirectly. Projections from parietal cortex to MEC are weak to absent in all species studied; likely parietal cortex projects to PER and POR/PHC instead (Burwell & Amaral, 1998; Kerr et al., 2007; Olsen, Ohara, Iijima, & Witter, 2017).
A final input that was historically specifically associated with MEC, a notion refuted in this paper, originates in POR in rodents and the cat or PHC as the likely homologous area in the monkey is referred to (Burwell, Witter, & Amaral, 1995). This notion of POR/PHC preferred connectivity with MEC seems in line with recent resting state connectional studies in humans (Maass et al., 2015; Navarro Schroder et al., 2015). However, a reanalysis of the available data has made us to reconsider this notion (Doan, Donate Lagartos, Nilssen, & Witter, 2018). As it turns out, in the monkey, the largest subdivision of PHC (area TF) sends projections that cover almost the entire AP axis of EC, showing an oblique distribution from caudomedial to rostrolateral, thus interacting with neurons in both MEC and LEC. Interestingly, the TF projections show an increasing density more rostrally in close association with the collateral sulcus (Insausti & Amaral, 2008; Suzuki & Amaral, 1994). A reanalysis of the three main rodent studies (Burwell & Amaral, 1998, 1998; Naber, Caballero‐Bleda, Jorritsma‐Byham, & Witter, 1997) and analysis of own additional anterograde tracing material in mice and rats led to a comparable conclusion that POR in the rat projects to both LEC and MEC. These analyses indicate that, in rodents at least, these projections do not differ much in anatomical strength, in line with quantitative retrograde data indicating that POR provide 7% of cortical input to MEC and 5% to LEC (Burwell & Amaral, 1998). Like in the monkey, the projections from POR in the rat preferentially target more lateral and central parts of EC (Doan et al., 2018).
14.1.2.2. Lateral entorhinal cortex
Functional descriptions of neurons in LEC are unfortunately less detailed and less numerous. It is clear that space does not represent a main correlate. In the rodent, grid cells have not been recorded in LEC and spatially modulated cells are scarce (Hargreaves et al., 2005; Yoganarasimha, Rao, & Knierim, 2011). Across cortical layers, LEC contains a low number of neurons that show emerging spatially confined firing fields, resembling hippocampal place fields, following the exposure to objects. These neurons signal either the current or previous locations of the introduced objects, that is, some represent a memory for object location or show spatial firing not associated to current or past object presence, but these cells seem to require objects present in the environment (Deshmukh & Knierim, 2011; Tsao et al., 2013). Similar physiological responses have been reported in upstream connected areas, including PER (Burke et al., 2012; Deshmukh, Johnson, & Knierim, 2012). Likewise, in the monkey EC, cells that responded specifically to the visual presentation of objects or their spatial location have been reported. Furthermore, a number of cells displayed sustained activity after the removal of the visual stimulus, indicating that object features, or locations were maintained in memory (Suzuki et al., 1997), thus strongly resembling neurons in LEC in the rat (Tsao et al., 2013). Whereas such object‐in‐place neurons are found preferentially in the anterior parts of EC, likely thus in LEC, place‐selective neurons were more equally distributed along the anteroposterior extent of EC, thus likely such cells are common to both LEC and MEC (Suzuki et al., 1997).
Neurons in LEC are also involved in olfactory processing, as witnessed by the modulation of LEC neuronal activity by olfactory stimuli in rats (Leitner et al., 2016; Xu & Wilson, 2012; Young, Otto, Fox, & Eichenbaum, 1997). Such a role of LEC is in line with data from studies in rats, guinea pigs, and cats demonstrating that olfactory information to HF is mediated by way of LEC (Biella & de Curtis, 2000; Boeijinga & van Groen, 1984; Habets, Lopes da Silva, & Mollevanger, 1980; Schwerdtfeger, Buhl, & Germroth, 1990; Van Groen, Lopes da Silva, & Wadman, 1987; R. C. Wilson & Steward, 1978). The importance of the LEC in olfactory memory processes is indicated by observations of altered behavior in olfactory‐dependent tasks following electrolytic damage of the LEC. Such interventions in rats have been shown to result in olfactory anterograde amnesia (Staubli, Fraser, Kessler, & Lynch, 1986; Staubli, Ivy, & Lynch, 1984), but also facilitation of olfactory recognition abilities (Otto, Schottler, Staubli, Eichenbaum, & Lynch, 1991; Wirth, Ferry, & Di Scala, 1998). These effects are in line with the important role of LEC in olfactory associate learning (Ferry, Ferreira, Traissard, & Majchrzak, 2006; Igarashi, Lu, Colgin, Moser, & Moser, 2014). For example, coherence in the slow gamma range (20–40 Hz) between LEC and distal CA1 has been demonstrated during successful odor–place associations in an associative learning task. This coherence suggests a state of synchronized activity likely mediating information transfer between LEC and the HF during odor learning or facilitating the use of retrieved olfactory memory from HF to fine‐tune olfactory discrimination (Colgin, 2016). Interestingly, for similar trials, such coherence was not observed between MEC and CA1 (Igarashi et al., 2014). Note that during spatial navigation MEC and CA1 showed coherence in the high gamma range (Colgin & Moser, 2010).
Like for MEC, also for LEC the accompaniment of cortical relationships seems to match this overall presence of functional neuron‐types. Evoked odor responses in LEC are in agreement with extensive axonal projections to LEC from the piriform cortex and the olfactory bulb, reported in several species including mice, rat, cat, and monkey (Boeijinga & van Groen, 1984; Burwell & Amaral, 1998; Haberly & Price, 1977; Insausti, Amaral, & Cowan, 1987; Kerr et al., 2007; Kosel, Van Hoesen, & West, 1981; Room, Groenewegen, & Lohman, 1984; Shipley & Adamek, 1984; G. W. Van Hoesen et al., 1972; Wouterlood, Mugnaini, & Nederlof, 1985; Wouterlood & Nederlof, 1983). Note that the projection from the olfactory bulb in monkeys is restricted to more rostral areas of LEC (Insausti et al., 1987).
Representation of objects likely reflect LEC's prominent input from PER, which only provide weak input to MEC (Burwell & Amaral, 1998; Suzuki & Amaral, 1994). PER is involved in discrimination between novel and familiar objects both in rodents and primates, and its activity reflects the integration of multimodal sensory aspects of objects, items, or events (Brown, 2008; Buckley & Gaffan, 2006; Bussey & Saksida, 2005, 2007; Bussey, Saksida, & Murray, 2006; Kealy & Commins, 2011; Naya, 2016; Taylor, Moss, Stamatakis, & Tyler, 2006).
14.1.3. Neurons and networks in MEC and LEC are remarkably similar
The EC comprises six cortical layers, four of which contain the main populations of neurons, Layers II, III, V, and VI. The molecular Layer I contains only a low number of interneurons, and Layer IV or the lamina dissecans as it is often referred to, also contains very low numbers of neurons. Here we focus on the networks of Layers II, III, and V, because for the remaining layers, detailed connectional data for both entorhinal subdivisions are lacking.
14.1.3.1. Layer II
Principal cells in Layer II of LEC and MEC come in at least two chemical types, calbindin‐ and reelin‐expressing cells. In MEC, stellate cells make up most of the principal neurons and they are typically reelin‐positive and calbindin‐negative. The main counterparts in Layer II of MEC are the calbindin‐positive pyramidal neurons. In LEC, a comparable subdivision has been reported with fan and multipolar neurons forming a substantial part of the reelin‐positive principal cells and pyramidal neurons corresponding largely to calbindin‐positive neurons (for review see Kobro‐Flatmoen & Witter, 2019; Witter et al., 2017). In MEC, these two main principal cell types can also be distinguished based on their electrophysiological profiles. Stellate cells have a prominent sag potential, resonance, and membrane oscillations, whereas in the pyramidal neurons these properties are absent (Canto & Witter, 2012; Fuchs et al., 2016). Note that the typical stellate properties are most pronounced in medially located neurons and become less apparent in more laterally positioned neurons. This gradient continues into LEC, such that in LEC medially positioned stellate/multipolar neurons share some of these properties with adjacent MEC stellate cells (Canto & Witter, 2012). In lateral LEC, more subtle electrophysiological differences between the two chemically and morphologically defined neuron classes have been reported (Leitner et al., 2016; Tahvildari & Alonso, 2005) though this is not supported by others (Canto & Witter, 2012; Desikan, Koser, Neitz, & Monyer, 2018).
Reelin‐positive neurons in Layer II of both LEC and MEC give rise to the projections to DG, and likely also to CA3 and CA2. Likewise, calbindin‐positive neurons show connectional motifs in both LEC and MEC that are very similar, in that they contribute to a wide range of extrinsic projections including hippocampal field CA1, many if not all of EC extrahippocampal target areas as well as commissural projections (Fuchs et al., 2016; Kitamura et al., 2014; Leitner et al., 2016; Varga, Lee, & Soltesz, 2010). Interestingly, recent data in rodents show that almost 50% of Layer II calbindin‐positive neurons originate local excitatory projections, with MEC neurons projecting within MEC and sending projections to LEC, whereas the local LEC calbindin‐positive projections predominantly distribute within LEC (Ohara et al., 2016; Figure 13).
Figure 13.
Summary of shared neuron types and local circuit motifs of the lateral and medial entorhinal cortex. Because very little to nothing is known concerning Layer VI, no neurons and circuits are indicated. In Layer II, we show the two types of principal neurons, reelin (RE) and calbindin (CB) positive, and their specific local connectivity to parvalbumin (PV) and 5HT3a‐receptor (5H) expressing interneurons, respectively. Also shown are the main projections to hippocampal fields and intrinsic and commissural projections. Not included is the observation that these two populations of principal cells do communicate through a separate class of pyramidal neurons. In Layer III, about 40% of the neurons projecting to CA1 and subiculum do give rise to commissural collaterals. Pyramidal cells in Layer III show a relatively strong developed local excitatory network (not indicated). In Layer V, we indicate that VB neurons project to Va as well as to Layers II and III. Note that although data indicate that the superficially projecting Layer Vb neurons also project to Laver Va, conclusive evidence for that is still lacking, so we have depicted as if these respective projections originate from different principal neurons. Inputs to layers and identified neurons therein are not indicated since they differ between LEC and MEC. CA3, CA2, CA1 subfields of the hippocampus proper; CB, calbindin‐positive neuron; DG, dentate gyrus; EC, entorhinal cortex; LD, lamina dissecans; RE, reelin‐positive neuron [Color figure can be viewed at wileyonlinelibrary.com]
The local circuits of principal cells in Layer II of MEC have been probed extensively and all data indicate that individual stellate reelin‐positive cells lack monosynaptic connections with other principal cells, and the same is the common connectivity pattern between pyramidal calbindin‐positive neurons. However, pyramidal neurons do have a relatively strong connection with stellate neurons (Fuchs et al., 2016; Winterer et al., 2017). Communication among neurons of the same class occurs through an intermediate inhibitory interneuron, in a mechanism by which activation of one or more principal neurons evokes disynaptic inhibitory currents in neighboring principal neurons (Couey et al., 2013; Fuchs et al., 2016; Pastoll, Solanka, van Rossum, & Nolan, 2013). The functional disynaptic link that illustrates the core principle of the stellate reelin‐positive microcircuit is mediated by a single type of inhibitory neuron, the PV positive fast spiking cell (Armstrong, Szabadics, Tamas, & Soltesz, 2011; Fuchs et al., 2016; Varga et al., 2010) and in case of grid cells in Layer II the same has been reported (Buetfering, Allen, & Monyer, 2014). In case of calbindin‐positive pyramidal cells, the interneuron in between belongs to the heterogeneous 5HT3A expressing population of interneurons (Fuchs et al., 2016). In a recent study, the Layer II network in LEC was analyzed, showing that very similar connectivity motifs are present. Like in MEC, principal neurons in LEC lack monosynaptic connectivity among members of their own class, showing a preferred disynaptic connectivity mediated by interneurons (Nilssen et al., 2018). Note that the prevalent types of interneurons mediating disynaptic inhibitory connectivity between principal neurons in LEC are partially different from those in MEC. A detailed analysis of the diverse population of interneurons in EC is not yet available and the relevance of these interneuronal differences is not yet fully understood.
14.1.3.2. Layer III
Layer III in both LEC and MEC comprises a homogenous population of spiny excitatory pyramidal neurons, multipolar neurons, and interneurons (Germroth, Schwerdtfeger, & Buhl, 1989; Gloveli, Schmitz, Empson, Dugladze, & Heinemann, 1997; Köhler & Chan‐Palay, 1983; Wouterlood & Pothuizen, 2000; Wouterlood, van Denderen, van Haeften, & Witter, 2000). The pyramidal and multipolar neurons are the source of the projections to CA1 and subiculum (Canto & Witter, 2012, 2012; Germroth et al., 1989; Tahvildari & Alonso, 2005; Tang et al., 2015). Layer III neurons also project contralaterally to the hippocampus and EC (Steward & Scoville, 1976), with about 40% of the Layer III hippocampal projecting cells in MEC sending collaterals to the contralateral MEC (Tang et al., 2015).
The microcircuits of Layer III seem markedly different from those seen in Layer II, showing higher connection probability between principal neurons (Dhillon & Jones, 2000; Kloosterman, Van Haeften, Witter, & Lopes Da Silva, 2003; Tang et al., 2015; van der Linden & Lopes da Silva, 1998). Neurons in Layer III, like those in Layer II, are main recipients of the local deep‐to‐superficial projections, which predominantly originate from neurons in Layer V (Kloosterman et al., 2003; Ohara et al., 2018; van Haeften, Baks‐Te Bulte, Goede, Wouterlood, & Witter, 2003). Currently, no correlations have been reported between morphology, connectional profile, and electrophysiological in vitro and in vivo properties (Canto & Witter, 2012, 2012; Tang et al., 2015) (Figure 13).
14.1.3.3. Layer V
Layer V is commonly subdivided into a Layer Va and Vb (Amaral et al., 1987; Boccara et al., 2015; Canto & Witter, 2012, 2012; Hamam, Amaral, & Alonso, 2002; Hamam, Kennedy, Alonso, & Amaral, 2000). In mice and rats, the expression pattern of the transcription factors Etv1 and Ctip2 provides for the differentiation between the two sublayers Va and Vb, respectively. This organization prevails across the whole mediolateral and dorsoventral extent of EC. In both MEC and LEC, Layer Va cells are the major output neurons projecting to diverse cortical and subcortical structures (Kosel et al., 1982; Ohara et al., 2018; Ramsden, Surmeli, McDonagh, & Nolan, 2015; Surmeli et al., 2015; Swanson & Köhler, 1986; G. W. van Hoesen, 1982). Surprisingly, Layer Vb cells are selectively targeted by the outputs from the hippocampus, originating in CA1 and subiculum (Surmeli et al., 2015), though this is apparently only true for projections originating from dorsal levels of subiculum and CA1; increasingly more ventral levels apparently innervate neurons in both Layer Va and Vb (Egorov, Lorenz, Rozov, & Draguhn, 2017; Ohara and Witter, unpublished data). Layer Vb neurons in both LEC and MEC innervate Layer Va as well as Layers II and III (Ohara et al., 2018), corroborating older data that neurons in Layer Vb issue superficially directed axon collaterals (Canto & Witter, 2012, 2012; Hamam et al., 2000; Hamam et al., 2002). Preliminary in vitro single cell recordings indicate that the effective connectivity to Layer III neurons is higher than the connectivity to Layer II (Ohara and Witter, unpublished data). Layer Vb neurons, but not Layer Va neurons, are also targeted by projections originating from reelin neurons in Layer II of MEC (Surmeli et al., 2015). Layer V is also innervated by cortical projections from frontal and cingular domains, including the anterior cingular cortex (Area 24) in case of LEC and retrosplenial cortex (Area 29 and 30) in case of MEC. Projections from the retrosplenial cortex target, among others, spiny pyramidal neurons that issue axons to superficial layers (Czajkowski et al., 2013).
In conclusion, neuron types, local circuit motifs, and the laminar origin and termination of outputs and inputs respectively, in MEC and LEC are strikingly similar (Figure 13). This seems somewhat counterintuitive to the striking functional differences described earlier, and reports that LEC and MEC develop from different parts of the pallium (Medina et al., 2017). As concisely and eloquently reviewed by Desfilis and colleagues (Desfilis, Abellan, Sentandreu, & Medina, 2018), MEC shares its embryological pallial origin with HF, whereas LEC shares its origin with PER, orbitofrontal, and insular domains of the cortex. The latter are cortical structures with which LEC selectively is connected and that are also strongly interconnected as argued earlier. Data on the origin of POR are currently lacking. Both LEC and MEC share an input from the olfactory or piriform cortex, but the connections with the olfactory bulb are almost exclusive with LEC. Comparable patterns can be found in case of the presumed homologous regions in lizards and chicken (Desfilis et al., 2018). Interestingly, LEC and MEC also differ with respect to the sequential developmental origin of the different layers, in that LEC follows the “neocortical” inside‐out pattern, whereas in MEC, like in HF, the developmental gradient is such that outside layers, that is, Layer II in case of MEC, develop first. This latter observation is supported by developmental data recently reported in the mouse (Donato, Jacobsen, Moser, & Moser, 2017). The latter authors not only reported that neurons in MEC Layer II are the first to mature, but that interfering with the maturation of these early developing Layer II MEC neurons postpones the subsequent maturation of all neurons in LEC. This suggests that MEC layer II neurons already in early stages of development directly influence the development of LEC.
One way for such a developmental influence to take place is through the presence of projections from MEC to LEC. Though long‐ranging intrinsic connections may already be partially present in the postnatally developing brain (O'Reilly et al., 2015), they are quite extensive in adults; note that in the monkey the long‐range extent does not cover the total AP axis of EC but seems to indicate that the connectional hub is formed by the central portion of EC (Chrobak & Amaral, 2007; Dolorfo & Amaral, 1998; Köhler, 1986, 1988; Witter, Room, Groenewegen, & Lohman, 1986). It would be of interest to know whether similar connections exist in the reptilian brain. Comparable long‐range projections exist between PER and POR in rats (Burwell & Amaral, 1998) and PER and PHC in monkeys (Lavenex et al., 2004). Similar to what was noted above for intrinsic EC connections in the monkey, the caudal part of PER, located centrally along the AP axis of PER/PHC is the main hub for these long‐range connections. The overall patterns of origin and terminal distributions of these projections supports the conjecture that POR/PHC projections to PER are of the feedforward type whereas the reverse projections fit more the patterns of feedback projections (Barbas & Rempel‐Clower, 1997).
These observations, taken together with the data described above that the projections of POR/PHC are not restricted to MEC but also target LEC, makes it relevant to ask the question what these posterior parts of PHR contribute functionally to PHR and thus to HF. To address this question, it is worthwhile to summarize the cortical input patterns described earlier by emphasizing that the widespread projections from POR/PHC to both MEC and LEC is the exception to the rule because most cortical afferents to LEC and MEC, like those to PER and POR/PHC, are selective for one or the other.
14.1.4. Connectional and functional position of POR/PHC
Inputs from POR/PHC and PER in monkeys give rise to 60% of the cortical input to the EC (Insausti et al., 1987; Insausti & Amaral, 2008). This percentage includes the temporal polar cortex, which is considered part of the perirhinal cortex, likely specific for primates (Insausti et al., 1987). Within the primate PHC, there are two main subdivisions, TH and TF, where TF is further subdivided into lateral and medial components. Whereas area TH receives mainly auditory input from the superior temporal gyrus but weak or no direct visual input, both subdivisions of area TF receives strong visual inputs from areas TEO and V4, as well as from the retrosplenial cortex and the dorsal bank of the superior temporal sulcus. The lateral part of TF receives additional inputs from posterior parietal areas (Suzuki & Amaral, 1994). In the rat, approximately 13% of the total cortical inputs to EC originate in PER and POR (Kerr et al., 2007). In case of POR, 40% of its cortical inputs originate in visual areas, 7% in posterior parietal cortex, and 16% in temporal association cortex; inputs from auditory, somatosensory, olfactory as well as frontal areas including insular, orbitofrontal, and medial prefrontal areas are negligible (Burwell & Amaral, 1998; Furtak, Wei, Agster, & Burwell, 2007). Note that these input patterns are very different from those reported for PER (see later). In line with these prominent cortical inputs, which are largely reciprocated, POR is typically portrayed as providing visuospatial information to EC. This is supported by reports in humans that the PHC supports spatial perception in real time (Epstein, Parker, & Feiler, 2007), though there are also strong data both in rats and primates that POR/PHC is particularly relevant in relation to processing contextual associations (Aminoff, Gronau, & Bar, 2007; Furtak et al., 2012). In many instances the data relate to object‐in‐space/location or object‐in‐context associations (Bohbot et al., 1998; Hayes, Nadel, & Ryan, 2007; Maguire, Frith, Burgess, Donnett, & O'Keefe, 1998). Data in rats are sparser, but single cells responses of neurons in POR indicate that around 30% of POR cells showed object‐location conjunctive encoding (Furtak et al., 2012). The latter authors suggested that POR combines object and pattern information from PER with incoming contextual and spatial information from retrosplenial and posterior parietal cortices to represent specific environmental contexts. This is in line with results of lesion studies in rats, showing that POR processes information about objects in relation to place or context (Gaffan, Healey, & Eacott, 2004; Norman & Eacott, 2005). Furtak and colleagues (Furtak et al., 2012) further reported that neuronal responses in POR show evidence of reflecting changes in context, or responses that relate to egocentric coding, which they relate to inputs from parietal cortex, as well as. The latter is reminiscent of recent reports of egocentric coding in LEC (Wang et al., 2018; see also below) whereas the former is suggested to be associated with the strong connectivity from PER to POR. Based on these additional neuronal properties, they suggest that POR monitors the context for changes and updates the representation of the context accordingly. This updated representation would be a subsequent input to downstream areas, such as PER, EC, and HF. This suggestion seems to conflict somewhat with data indicating that PHC in humans is more active in response to stationary, spatially defining objects than to spatially ambiguous objects (Mullally & Maguire, 2011).
An alternative proposal, which we prefer to entertain, might be that changes in object/contextual or spatial relationships are perceived in downstream areas, such as PER, and fed back to the POR network to allow for an update of the contextual representation as to secure stability. This fits with the laminar pattern of projections between PER and POR/PHC (Lavenex et al., 2004). Interestingly, the proposition that POR plays a critical role in providing a stable representation of object‐place associations is in line with very recent data showing that POR receives information from the superior colliculus, via its connections to LP (Beltramo & Scanziani, 2019; Bennett et al., 2019), which might provide an unconscious representation of self‐movement related changes in the perceived position of objects. It further fits with the recent suggestion that both LEC and MEC may process visual context information, likely thus derived from POR, but that both use this information in a completely different functional way, related to the appropriate motor output (Yoo & Lee, 2017). Based on this notion one could predict that silencing of visual inputs to POR or silencing POR itself might change the representation of the context and thus will induce place cell remapping the hippocampus.
14.1.5. Connectional and functional position of the PER/LEC interface
Neurons in LEC are responsive to objects‐in‐position associations, likely without discriminating between the nature of the object (Deshmukh & Knierim, 2011; Tsao et al., 2013). However, neurons and networks in LEC code beyond this by incorporating representations of context, because LEC is critically involved in complex object‐context associations binding together information relating to objects, places, and contexts (Scaplen, Ramesh, Nadvar, Ahmed, & Burwell, 2017; Wilson et al., 2013; Wilson, Watanabe, Milner, & Ainge, 2013). Recent electrophysiological studies provide data suggesting that distinct contextual features of experiences are represented in LEC both at the single‐cell and population level (Pilkiw et al., 2017; Tsao et al., 2018). Further analysis of LEC ensemble activity indicated a shift of population states according to the temporal progression of the experimental event. These data suggest that the activity of LEC populations carries a representation of time, brought about by the encoding of sequences of ongoing events. Although comparable data have been obtained in the anterolateral parts of the monkey and human EC (Montchal et al., 2019; Naya & Suzuki, 2011), the representation of incremental timing information, based on the sequence of ongoing events is weaker in EC compared to that in PER and HF (Naya & Suzuki, 2011). Likewise, although LEC neurons can integrate item and time information (Naya & Suzuki, 2011) conjunctive item neurons seem to be a more prevalent type in monkey LEC (Naya, Chen, Yang, & Suzuki, 2017).
Neurons and networks in the lateral part of LEC may embed other features to these already complex representations, including olfactory and salience percepts. The proposition that LEC neurons code for high order associations is in line with recent observations, indicating that individual principal cells in Layer II of LEC receive convergent inputs from PER, POR, MEC, olfactory piriform cortex, and from contralateral LEC Doan, Nilssen, & Witter, 2016). It is worth reiterating that the connectivity motif in Layer II in LEC is comparable to that of Layer II in MEC (Nilssen et al., 2018). We thus proposed that neurons in Layer II of LEC may show hexagonal, or at least regularly repeating, firing patterns along dimensions defined by their inputs (Nilssen et al., 2018). In contrast to the pure spatial representation observed in MEC, periodic patterns might arise in LEC to represent complex features of the context as part of a particular episode (Bellmund, Gardenfors, Moser, & Doeller, 2018; Constantinescu, O'Reilly, & Behrens, 2016). In this view, the inputs from POR and MEC provide LEC with relevant information to act as an integrative hub between what has been referred to as an egocentric representation of a context with the allocentric representation of self‐position in that context (Wang et al., 2018; Yoo & Lee, 2017).
Here we emphasize the relevance of the PER/LEC interface. As argued earlier, multimodal representations of objects depend on perirhinal networks and PER also plays a relevant role in novelty‐familiarity discriminations. Such functions likely reflect the variety of inputs targeting PER. Interestingly, PER shares most of these inputs with the strongly reciprocally connected directly adjacent lateral parts of LEC. These inputs include dense inputs from insular, orbitofrontal cortex, anterior cingulate cortex, temporal association cortex, as well as from the lateral and basal amygdala. In rats, additional inputs originate from the medial prefrontal prelimbic and infralimbic cortex, although these projections do target MEC and POR as well, be it with a lesser density of termination (Burwell & Amaral, 1998; Insausti et al., 1987; Jones & Witter, 2007; Kerr et al., 2007; Kondo & Witter, 2014; Krettek & Price, 1977; Mathiasen, Hansen, & Witter, 2015; Mohedano‐Moriano et al., 2007; Pitkanen, Kelly, & Amaral, 2002; Room & Groenewegen, 1986; Stefanacci & Amaral, 2000; Suzuki & Amaral, 1994; Van Hoesen & Pandya, 1975; Van Hoesen et al., 1975; Van Hoesen, 1982; Van Hoesen et al., 1972; Vaudano, Legg, & Glickstein, 1991; Vertes, 2004). Many of these forebrain areas play a role in coding of information concerning the salience or the reinforcing value of a particular context or elements in that context (Dixon, Thiruchselvam, Todd, & Christoff, 2017; Ritchey, Wang, Yonelinas, & Ranganath, 2018; Wallis, 2007). This would enable the PER/LEC interface to evaluate sensory cues not only as part of a particular context or episode but add information about the current emotional value of individual elements of the context or the context as a whole. Note that the frontal cortical inputs mainly, though not exclusively, target deeper layers of the PER/LEC interface and thus are in a potential position to influence the main cortical output stream, mediated by the deep EC layers. Irrespective, we argued that deep entorhinal circuits also influence superficial circuits, so likely these frontal inputs have a role to play in modifying sensory representations in the superficial input network of PER/LEC as well. Of course, the lateral amygdala input might be the most relevant, because it terminates densely in superficial layers and it shares this superficial termination with olfactory inputs and those from higher order temporal sensory association cortex (Pitkanen et al., 2002; Pitkanen, Pikkarainen, Nurminen, & Ylinen, 2000).
In line with this shared input, we propose that it is the PER/LEC interface that provides the optimal substrate to detect changes in the context. This proposition is strengthened by an additional unique transmission property in this network. Connectivity from PER to adjacent LEC is governed by a striking inhibitory gating (de Curtis & Pare, 2004). This “wall of inhibition” is overcome by the convergence in time and space of at least two coincident inputs (Samarth, Ball, Unal, Pare, & Nair, 2017). These could be coincident inputs from temporal cortex or PER with lateral amygdala (Kajiwara, Takashima, Mimura, Witter, & Iijima, 2003; R. Paz, Pelletier, Bauer, & Pare, 2006; Pelletier, Apergis‐Schoute, & Pare, 2005), mPFC and PER (Rony Paz, Bauer, & Pare, 2007), or insular cortex and amygdala (Willems, Wadman, & Cappaert, 2016). Coincident changes in sensory and saliency inputs would thus allow activation of LEC where neurons are capable of coding such changes over time. As already proposed, POR/PHC inputs would provide a stable representation of the current context, allowing the PER/LEC interface to detect relevant changes in the context over time, in line with the aforedescribed sequence coding that apparently occurs in the network. Subsequent transmission of salient changes in these contextual features would then result in updating HF representations of an episode. At the same time PER projections to POR and LEC projections to MEC would provide feedback information allowing these networks to incorporate these changes into their updated stable representations.
14.1.6. Conclusions and future perspectives
We started this review with the concept of parallel pathways connecting HF with the cortical mantle and that there might be subdivisions of EC mediating such parallel pathways, because EC forms a major cortical input and output hub for HF. A key element in the development of this notion was the conceived preferred connectivity of PER with LEC and PHC/POR with MEC. Of likely similar influence was the notion of a hierarchical organization of the parallel streams culminating in the final convergence at the level of the networks in HF. We have argued that this conceived preferred connectivity in case of POR/PHC is incorrect. POR/PHC contributes to both pathways, providing both MEC and PER/LEC with what we propose is a continuously stable representation of context.
We support earlier suggestions that convergence takes place at multiple levels in the EC‐HF memory system and provide new evidence, integrated in already existing data that this happens predominantly in LEC. In addition, we conclude that the connectional differences between LEC and MEC strongly support the concept of functional differences. Whereas the PER/LEC interface provides the hippocampus with a highly integrated, multidimensional representation of sensory information, including changes over time, constituting the content of an episodic memory, MEC provides the position of the subject, coded in an allocentric space (Eichenbaum et al., 2007; Lisman, 2007).
Contrary to previous expectations, all data concerning the intrinsic network motifs of LEC and MEC point to a striking overall similarity, notwithstanding that subtle differences in interneuron contributions may exist. The delicate role, undoubtedly played by interneurons, will be important to refine our understanding of information coding in the two subdivisions of EC. Our current knowledge leads to the intriguing conclusion that two embryologically different parts of the cortex, that even follow different developmental schemes, inside‐out, versus outside‐in, eventually result in two similar and strongly interconnected areas, which independently cannot fully support hippocampal functions. The shared network motifs of LEC and MEC suggest that HF requires an input that uses a particular “language” that originates from these network motifs. The developmental dependence of LEC on MEC input (Donato et al., 2017) supports the notion that the hippocampal anlage shapes its LEC input system to represent evolutionary new, more complex sensory and higher order stimuli, and communicates with HF using the same network dependent language to communicate with HF as the developmentally HF‐associated‐MEC system. It would be of interest to study this conjecture experimentally. One approach might be to use the reptilian brain as a simple model comparing olfactory and spatial representations in the likely homologues of LEC and MEC, which, like in the mammalian brain, project to all subdivisions of HF (Desfilis et al., 2018). Understanding this coding principle might be relevant, because olfaction has been proposed as a universal system among the sensory systems to mediate navigation and memory formation (L. F. Jacobs, Arter, Cook, & Sulloway, 2015). A second, very relevant and promising approach would be to pursue computational modeling of the output of such a network motif and study how HF responses depend on this input by systematically perturbing the input language. A similar argument can be made for the functional relevance of the hippocampal output network mediated by EC deep layers, which is still grossly understudied.
CONFLICT OF INTERESTS
The authors declare no potential conflict of interest.
REFERENCES
Adolphs, R., Cahill, L., Schul, R., & Babinsky, R. (1997). Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learning and Memory, 4(3), 291–300.
Amaral, D. G., Insausti, R., & Cowan, W. M. (1987). The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. Journal of Comparative Neurology, 264(3), 326–355. https://doi.org/10.1002/cne.902640305
Aminoff, E., Gronau, N., & Bar, M. (2007). The parahippocampal cortex mediates spatial and nonspatial associations. Cerebral Cortex, 17(7), 1493–1503. https://doi.org/10.1093/cercor/bhl078
Annese, J., Schenker‐Ahmed, N. M., Bartsch, H., Maechler, P., Sheh, C., Thomas, N., … Corkin, S. (2014). Postmortem examination of patient H.M.'s brain based on histological sectioning and digital 3D reconstruction. Nature Communications, 5, 3122. https://doi.org/10.1038/ncomms4122
Armstrong, C., Szabadics, J., Tamas, G., & Soltesz, I. (2011). Neurogliaform cells in the molecular layer of the dentate gyrus as feed‐forward gamma‐aminobutyric acidergic modulators of entorhinal‐hippocampal interplay. Journal of Comparative Neurology, 519(8), 1476–1491. https://doi.org/10.1002/cne.22577
Augustinack, J. C., van der Kouwe, A. J., Salat, D. H., Benner, T., Stevens, A. A., Annese, J., … Corkin, S. (2014). H.M.'s contributions to neuroscience: A review and autopsy studies. Hippocampus, 24(11), 1267–1286. https://doi.org/10.1002/hipo.22354
Barbas, H., & Rempel‐Clower, N. (1997). Cortical structure predicts the pattern of corticocortical connections. Cerebral Cortex, 7(7), 635–646.
Bellmund, J. L. S., Gardenfors, P., Moser, E. I., & Doeller, C. F. (2018). Navigating cognition: Spatial codes for human thinking. Science, 362(6415), eaat6766. https://doi.org/10.1126/science.aat6766
Beltramo, R., & Scanziani, M. (2019). A collicular visual cortex: Neocortical space for an ancient midbrain visual structure. Science, 363(6422), 64–69. https://doi.org/10.1126/science.aau7052
Bennett, C., Gale, S. D., Garrett, M. E., Newton, M. L., Callaway, E. M., Murphy, G. J., & Olsen, S. R. (2019). Higher‐order thalamic circuits channel parallel streams of visual information in mice. Neuron, 102(2), 477–492.e475. https://doi.org/10.1016/j.neuron.2019.02.010
Biella, G., & de Curtis, M. (2000). Olfactory inputs activate the medial entorhinal cortex via the hippocampus. Journal of Neurophysiology, 83(4), 1924–1931.
Blackstad, T. W. (1956). Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. Journal of Comparative Neurology, 105(3), 417–537.
Blackstad, T. W. (1958). On the termination of some afferents to the hippocampus and fascia dentata. An experimental study in the rat. Acta Anatomica, 35, 202–214.
Blankvoort, S., Witter, M. P., Noonan, J., Cotney, J., & Kentros, C. (2018). Marked diversity of unique cortical enhancers enables neuron‐specific tools by enhancer‐driven gene expression. Current Biology, 28(13), 2103–2114 e2105. https://doi.org/10.1016/j.cub.2018.05.015
Bliss, T. V., & Lømo, T. (1973). Long‐lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of Physiology, 232(2):331–56.
Boccara, C. N., Kjonigsen, L. J., Hammer, I. M., Bjaalie, J. G., Leergaard, T. B., & Witter, M. P. (2015). A three‐plane architectonic atlas of the rat hippocampal region. Hippocampus, 25(7), 838–857. https://doi.org/10.1002/hipo.22407
Boeijinga, P. H., & van Groen, T. (1984). Inputs from the olfactory bulb and olfactory cortex to the entorhinal cortex in the cat. II. Physiological studies. Experimental Brain Research, 57(1), 40–48.
Bohbot, V. D., Kalina, M., Stepankova, K., Spackova, N., Petrides, M., & Nadel, L. (1998). Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia, 36(11), 1217–1238.
Brown, M. W. (2008). Hippocampal and perirhinal functions in recognition memory. Nature Reviews. Neuroscience, 9(5), 405. doi:nrn2154‐c1 [pii]. https://doi.org/10.1038/nrn2154‐c1
Brun, V. H., Leutgeb, S., Wu, H. Q., Schwarcz, R., Witter, M. P., Moser, E. I., & Moser, M. B. (2008). Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron, 57(2), 290–302.
Buckley, M. J., & Gaffan, D. (2006). Perirhinal cortical contributions to object perception. Trends in Cognitive Sciences, 10(3), 100–107.
Buckmaster, C. A., Eichenbaum, H., Amaral, D. G., Suzuki, W. A., & Rapp, P. R. (2004). Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. Journal of Neuroscience, 24(44), 9811–9825.
Buetfering, C., Allen, K., & Monyer, H. (2014). Parvalbumin interneurons provide grid cell‐driven recurrent inhibition in the medial entorhinal cortex. Nature Neuroscience, 17(5), 710–718. https://doi.org/10.1038/nn.3696
Burke, S. N., Gaynor, L. S., Barnes, C. A., Bauer, R. M., Bizon, J. L., Roberson, E. D., & Ryan, L. (2018). Shared functions of perirhinal and parahippocampal cortices: Implications for cognitive aging. Trends in Neurosciences, 41, 349–359. https://doi.org/10.1016/j.tins.2018.03.001
Burke, S. N., Maurer, A. P., Hartzell, A. L., Nematollahi, S., Uprety, A., Wallace, J. L., & Barnes, C. A. (2012). Representation of three‐dimensional objects by the rat perirhinal cortex. Hippocampus, 22(10), 2032–2044. https://doi.org/10.1002/hipo.22060
Burwell, R. D., & Amaral, D. G. (1998a). Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Journal of Comparative Neurology, 398(2), 179–205.
Burwell, R. D., & Amaral, D. G. (1998b). Perirhinal and postrhinal cortices of the rat: Interconnectivity and connections with the entorhinal cortex. Journal of Comparative Neurology, 391(3), 293–321.
Burwell, R. D., Witter, M. P., & Amaral, D. G. (1995). Perirhinal and postrhinal cortices of the rat: A review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus, 5(5), 390–408. https://doi.org/10.1002/hipo.450050503
Bussey, T. J., & Saksida, L. M. (2005). Object memory and perception in the medial temporal lobe: An alternative approach. Current Opinion in Neurobiology, 15(6), 730–737.
Bussey, T. J., & Saksida, L. M. (2007). Memory, perception, and the ventral visual‐perirhinal‐hippocampal stream: Thinking outside of the boxes. Hippocampus, 17(9), 898–908.
Bussey, T. J., Saksida, L. M., & Murray, E. A. (2006). Perirhinal cortex and feature‐ambiguous discriminations. Learning and Memory, 13(2), 103–105.
Buzsaki, G. (1996). The hippocampo‐neocortical dialogue. Cerebral Cortex, 6(2), 81–92. https://doi.org/10.1093/cercor/6.2.81
Buzsaki, G., & Moser, E. I. (2013). Memory, navigation and theta rhythm in the hippocampal‐entorhinal system. Nature Neuroscience, 16(2), 130–138. https://doi.org/10.1038/nn.3304 nn.3304 [pii].
Caballero‐Bleda, M., & Witter, M. P. (1993). Regional and laminar organization of projections from the presubiculum and parasubiculum to the entorhinal cortex: An anterograde tracing study in the rat. Journal of Comparative Neurology, 328(1), 115–129. https://doi.org/10.1002/cne.903280109
Canto, C. B., & Witter, M. P. (2012a). Cellular properties of principal neurons in the rat entorhinal cortex. I. The lateral entorhinal cortex. Hippocampus, 22(6), 1256–1276. https://doi.org/10.1002/hipo.20997
Canto, C. B., & Witter, M. P. (2012b). Cellular properties of principal neurons in the rat entorhinal cortex. II. The medial entorhinal cortex. Hippocampus, 22(6), 1277–1299. https://doi.org/10.1002/hipo.20993
Cappaert, N. L. M., Van Strien, N. M., & Witter, M. P. (2014). Hippocampal formation. In G. Paxinos (Ed.), The rat nervous system (4th ed.). San Diego: Academic Press.
Chrobak, J. J., & Amaral, D. G. (2007). Entorhinal cortex of the monkey: VII. Intrinsic connections. Journal of Comparative Neurology, 500(4), 612–633. https://doi.org/10.1002/cne.21200
Colgin, L. L. (2016). Rhythms of the hippocampal network. Nature Reviews. Neuroscience, 17(4), 239–249. https://doi.org/10.1038/nrn.2016.21
Colgin, L. L., & Moser, E. I. (2010). Gamma oscillations in the hippocampus. Physiology (Bethesda), 25(5), 319–329. doi:25/5/319 [pii]. https://doi.org/10.1152/physiol.00021.2010
Constantinescu, A. O., O'Reilly, J. X., & Behrens, T. E. J. (2016). Organizing conceptual knowledge in humans with a gridlike code. Science, 352(6292), 1464–1468. https://doi.org/10.1126/science.aaf0941
Couey, J. J., Witoelar, A., Zhang, S. J., Zheng, K., Ye, J., Dunn, B., … Witter, M. P. (2013). Recurrent inhibitory circuitry as a mechanism for grid formation. Nature Neuroscience, 16(3), 318–324. https://doi.org/10.1038/nn.3310 nn.3310 [pii].
Czajkowski, R., Sugar, J., Zhang, S. J., Couey, J. J., Ye, J., & Witter, M. P. (2013). Superficially projecting principal neurons in layer V of medial entorhinal cortex in the rat receive excitatory retrosplenial input. Journal of Neuroscience, 33(40), 15779–15792. https://doi.org/10.1523/JNEUROSCI.2646‐13.2013
de Curtis, M., & Pare, D. (2004). The rhinal cortices: A wall of inhibition between the neocortex and the hippocampus. Progress in Neurobiology, 74(2), 101–110.
Desfilis, E., Abellan, A., Sentandreu, V., & Medina, L. (2018). Expression of regulatory genes in the embryonic brain of a lizard and implications for understanding pallial organization and evolution. Journal of Comparative Neurology, 526(1), 166–202. https://doi.org/10.1002/cne.24329
Deshmukh, S. S., Johnson, J. L., & Knierim, J. J. (2012). Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: Comparison with lateral entorhinal cortex. Hippocampus, 22(10), 2045–2058. https://doi.org/10.1002/hipo.22046
Deshmukh, S. S., & Knierim, J. J. (2011). Representation of non‐spatial and spatial information in the lateral entorhinal cortex. Frontiers in Behavioral Neuroscience, 5, 69. https://doi.org/10.3389/fnbeh.2011.00069
Desikan, S., Koser, D. E., Neitz, A., & Monyer, H. (2018). Target selectivity of septal cholinergic neurons in the medial and lateral entorhinal cortex. Proceedings of the National Academy of Sciences of the United States of America, 115(11), E2644–E2652. https://doi.org/10.1073/pnas.1716531115
Dhillon, A., & Jones, R. S. (2000). Laminar differences in recurrent excitatory transmission in the rat entorhinal cortex in vitro. Neuroscience, 99(3), 413–422.
Dixon, M. L., Thiruchselvam, R., Todd, R., & Christoff, K. (2017). Emotion and the prefrontal cortex: An integrative review. Psychological Bulletin, 143(10), 1033–1081. https://doi.org/10.1037/bul0000096
Doan, T. P., Donate Lagartos, M. J., Nilssen, E. S., & Witter, M. P. (2018). Challenging the concept of parellel parahippocampal input streams to the hippocampus. FENS Abstract, F18, 2143.
Doan, T. P., Nilssen, E. S., & Witter, M. P. (2016). Postsynaptic targets of inputs to the lateral entorhinal cortex. SFN Abstracts, 16(16), 183.
Doeller, C. F., Barry, C., & Burgess, N. (2010). Evidence for grid cells in a human memory network. Nature, 463(7281), 657–661. https://doi.org/10.1038/nature08704
Dolorfo, C. L., & Amaral, D. G. (1998). Entorhinal cortex of the rat: Organization of intrinsic connections. Journal of Comparative Neurology, 398(1), 49–82.
Donato, F., Jacobsen, R. I., Moser, M. B., & Moser, E. I. (2017). Stellate cells drive maturation of the entorhinal‐hippocampal circuit. Science, 355(6330), eaai8178. https://doi.org/10.1126/science.aai8178
Egorov, A. V., Lorenz, F. S., Rozov, A., & Draguhn, A. (2017). Local circuitry in the medial entorhinal cortex layer V. SFN Abstracts, 474, 09.
Eichenbaum, H. (2000). A cortical‐hippocampal system for declarative memory. Nature Reviews Neuroscience, 1(1), 41–50. https://doi.org/10.1038/35036213
Eichenbaum, H. (2017). On the integration of space, time, and memory. Neuron, 95(5), 1007–1018. https://doi.org/10.1016/j.neuron.2017.06.036
Eichenbaum, H., Yonelinas, A. P., & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30, 123–152. https://doi.org/10.1146/annurev.neuro.30.051606.094328
Epstein, R. A., Parker, W. E., & Feiler, A. M. (2007). Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. Journal of Neuroscience, 27(23), 6141–6149.
Ferry, B., Ferreira, G., Traissard, N., & Majchrzak, M. (2006). Selective involvement of the lateral entorhinal cortex in the control of the olfactory memory trace during conditioned odor aversion in the rat. Behavioral Neuroscience, 120(5), 1180–1186.
Fuchs, E. C., Neitz, A., Pinna, R., Melzer, S., Caputi, A., & Monyer, H. (2016). Local and distant input controlling excitation in layer II of the medial entorhinal cortex. Neuron, 89(1), 194–208. https://doi.org/10.1016/j.neuron.2015.11.029
Furtak, S. C., Ahmed, O. J., & Burwell, R. D. (2012). Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination. Neuron, 76(5), 976–988. https://doi.org/10.1016/j.neuron.2012.10.039
Furtak, S. C., Wei, S. M., Agster, K. L., & Burwell, R. D. (2007). Functional neuroanatomy of the parahippocampal region in the rat: The perirhinal and postrhinal cortices. Hippocampus, 17(9), 709–722. https://doi.org/10.1002/hipo.20314
Fyhn, M., Hafting, T., Witter, M. P., Moser, E. I., & Moser, M. B. (2008). Grid cells in mice. Hippocampus, 18(12), 1230–1238. https://doi.org/10.1002/hipo.20472
Fyhn, M., Molden, S., Witter, M. P., Moser, E. I., & Moser, M. B. (2004). Spatial representation in the entorhinal cortex. Science, 305(5688), 1258–1264. https://doi.org/10.1126/science.1099901
Gaffan, E. A., Healey, A. N., & Eacott, M. J. (2004). Objects and positions in visual scenes: Effects of perirhinal and postrhinal cortex lesions in the rat. Behavioral Neuroscience, 118(5), 992–1010.
Germroth, P., Schwerdtfeger, W. K., & Buhl, E. H. (1989). Morphology of identified entorhinal neurons projecting to the hippocampus. A light microscopical study combining retrograde tracing and intracellular injection. Neuroscience, 30(3), 683–691.
Gloveli, T., Schmitz, D., Empson, R. M., Dugladze, T., & Heinemann, U. (1997). Morphological and electrophysiological characterization of layer III cells of the medial entorhinal cortex of the rat. Neuroscience, 77(3), 629–648.
Haberly, L. B., & Price, J. L. (1977). The axonal projection patterns of the mitral and tufted cells of the olfactory bulb in the rat. Brain Research, 129(1), 152–157 doi:0006‐8993(77)90978‐7 [pii].
Habets, A. M., Lopes da Silva, F. H., & Mollevanger, W. J. (1980). An olfactory input to the hippocampus of the cat: Field potential analysis. Brain Research, 182(1), 47–64.
Hafting, T., Fyhn, M., Molden, S., Moser, M. B., & Moser, E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature, 436(7052), 801–806.
Hales, J. B., Vincze, J. L., Reitz, N. T., Ocampo, A. C., Leutgeb, S., & Clark, R. E. (2018). Recent and remote retrograde memory deficit in rats with medial entorhinal cortex lesions. Neurobiology of Learning and Memory, 155, 157–163. https://doi.org/10.1016/j.nlm.2018.07.013
Hamam, B. N., Amaral, D. G., & Alonso, A. A. (2002). Morphological and electrophysiological characteristics of layer V neurons of the rat lateral entorhinal cortex. Journal of Comparative Neurology, 451(1), 45–61. https://doi.org/10.1002/cne.10335
Hamam, B. N., Kennedy, T. E., Alonso, A., & Amaral, D. G. (2000). Morphological and electrophysiological characteristics of layer V neurons of the rat medial entorhinal cortex. Journal of Comparative Neurology, 418(4), 457–472.
Hardcastle, K., Maheswaranathan, N., Ganguli, S., & Giocomo, L. M. (2017). A multiplexed, heterogeneous, and adaptive code for navigation in medial entorhinal cortex. Neuron, 94(2), 375–387 e377. https://doi.org/10.1016/j.neuron.2017.03.025
Hargreaves, E. L., Rao, G., Lee, I., & Knierim, J. J. (2005). Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science, 308(5729), 1792–1794.
Haug, F. M. (1976). Sulphide silver pattern and cytoarchitectonics of parahippocampal areas in the rat. Special reference to the subdivision of area entorhinalis (area 28) and its demarcation from the pyriform cortex. Advances in Anatomy, Embryology and Cell Biology, 52(4), 3–73.
Hayes, S. M., Nadel, L., & Ryan, L. (2007). The effect of scene context on episodic object recognition: Parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus, 17(9), 873–889.
Hjorth‐Simonsen, A. (1971). Hippocampal efferents to the ipsilateral entorhinal area: An experimental study in the rat. Journal of Comparative Neurology, 142(4), 417–437. https://doi.org/10.1002/cne.901420403
Hjorth‐Simonsen, A. (1972). Projection of the lateral part of the entorhinal area to the hippocampus and fascia dentata. Journal of Comparative Neurology, 146(2), 219–232. https://doi.org/10.1002/cne.901460206
Hjorth‐Simonsen, A., & Jeune, B. (1972). Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. Journal of Comparative Neurology, 144(2), 215–232. https://doi.org/10.1002/cne.901440206
Høydal, O. A., Skytøen, E. R., Moser, M.‐B., & Moser, E. I. (2018). Object‐vector coding in the medial entorhinal cotex. bioRxiv. 568(7752):400–404. https://doi.org/10.1038/s41586‐019‐1077‐7.
Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M. B., & Moser, E. I. (2014). Coordination of entorhinal‐hippocampal ensemble activity during associative learning. Nature, 510(7503), 143–147. https://doi.org/10.1038/nature13162
Insausti, R., & Amaral, D. G. (2008). Entorhinal cortex of the monkey: IV. Topographical and laminar organization of cortical afferents. Journal of Comparative Neurology, 509(6), 608–641. https://doi.org/10.1002/cne.21753
Insausti, R., Amaral, D. G., & Cowan, W. M. (1987). The entorhinal cortex of the monkey: II. Cortical afferents. Journal of Comparative Neurology, 264(3), 356–395. https://doi.org/10.1002/cne.902640306
Insausti, R., Munoz‐Lopez, M., Insausti, A. M., & Artacho‐Perula, E. (2017). The human periallocortex: Layer pattern in presubiculum, parasubiculum and entorhinal cortex. A review. Frontiers in Neuroanatomy, 11, 84. https://doi.org/10.3389/fnana.2017.00084
Insausti, R., Tunon, T., Sobreviela, T., Insausti, A. M., & Gonzalo, L. M. (1995). The human entorhinal cortex: A cytoarchitectonic analysis. Journal of Comparative Neurology, 355(2), 171–198. https://doi.org/10.1002/cne.903550203
Jacobs, J., & Lee, S. A. (2016). Spatial cognition: Grid cells support imagined navigation. Current Biology, 26(7), R277–R279. https://doi.org/10.1016/j.cub.2016.02.032
Jacobs, L. F., Arter, J., Cook, A., & Sulloway, F. J. (2015). Olfactory orientation and navigation in humans. PLoS One, 10(6), e0129387. https://doi.org/10.1371/journal.pone.0129387
Jones, B. F., & Witter, M. P. (2007). Cingulate cortex projections to the parahippocampal region and hippocampal formation in the rat. Hippocampus, 17(10), 957–976. https://doi.org/10.1002/hipo.20330
Kajiwara, R., Takashima, I., Mimura, Y., Witter, M. P., & Iijima, T. (2003). Amygdala input promotes spread of excitatory neural activity from perirhinal cortex to the entorhinal‐hippocampal circuit. Journal of Neurophysiology, 89(4), 2176–2184. https://doi.org/10.1152/jn.01033.2002
Kealy, J., & Commins, S. (2011). The rat perirhinal cortex: A review of anatomy, physiology, plasticity, and function. Progress in Neurobiology, 93(4), 522–548. https://doi.org/10.1016/j.pneurobio.2011.03.002
Kerr, K. M., Agster, K. L., Furtak, S. C., & Burwell, R. D. (2007). Functional neuroanatomy of the parahippocampal region: The lateral and medial entorhinal areas. Hippocampus, 17(9), 697–708. https://doi.org/10.1002/hipo.20315
Killian, N. J., Jutras, M. J., & Buffalo, E. A. (2012). A map of visual space in the primate entorhinal cortex. Nature, 491(7426), 761–764. https://doi.org/10.1038/nature11587
Kitamura, T., Pignatelli, M., Suh, J., Kohara, K., Yoshiki, A., Abe, K., & Tonegawa, S. (2014). Island cells control temporal association memory. Science, 343(6173), 896–901. https://doi.org/10.1126/science.1244634
Kloosterman, F., Van Haeften, T., Witter, M. P., & Lopes Da Silva, F. H. (2003). Electrophysiological characterization of interlaminar entorhinal connections: An essential link for re‐entrance in the hippocampal‐entorhinal system. European Journal of Neuroscience, 18(11), 3037–3052.
Knierim, J. J., Neunuebel, J. P., & Deshmukh, S. S. (2014). Functional correlates of the lateral and medial entorhinal cortex: Objects, path integration and local‐global reference frames. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 369(1635), 20130369. https://doi.org/10.1098/rstb.2013.0369
Kobayashi, Y., & Amaral, D. G. (2007). Macaque monkey retrosplenial cortex: III. Cortical efferents. Journal of Comparative Neurology, 502(5), 810–833. https://doi.org/10.1002/cne.21346
Kobro‐Flatmoen, A., & Witter, M. P. (2019). Neuronal chemo‐architecture of the entorhinal cortex: A comparative review. European Journal of Neuroscience in press. https://doi.org/10.1111/ejn.14511
Köhler, C. (1985). Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. Journal of Comparative Neurology, 236(4), 504–522.
Köhler, C. (1986). Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. Journal of Comparative Neurology, 246(2), 149–169.
Köhler, C. (1988). Intrinsic connections of the retrohippocampal region in the rat brain: III. The lateral entorhinal area. Journal of Comparative Neurology, 271(2), 208–228. https://doi.org/10.1002/cne.902710204
Köhler, C., & Chan‐Palay, V. (1983). Gamma‐aminobutyric acid interneurons in the rat hippocampal region studied by retrograde transport of glutamic acid decarboxylase antibody after in vivo injections. Anatomy and Embryology, 166(1), 53–66.
Kondo, H., & Witter, M. P. (2014). Topographic organization of orbitofrontal projections to the parahippocampal region in rats. Journal of Comparative Neurology, 522(4), 772–793. https://doi.org/10.1002/cne.23442
Kosel, K. C., Van Hoesen, G. W., & Rosene, D. L. (1982). Non‐hippocampal cortical projections from the entorhinal cortex in the rat and rhesus monkey. Brain Research, 244(2), 201–213.
Kosel, K. C., Van Hoesen, G. W., & West, J. R. (1981). Olfactory bulb projections to the parahippocampal area of the rat. The Journal of Comparative Neurology, 198(3), 467–482.
Krettek, J. E., & Price, J. L. (1977). Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. The Journal of Comparative Neurology, 172(4), 723–752.
Krimer, L. S., Hyde, T. M., Herman, M. M., & Saunders, R. C. (1997). The entorhinal cortex: An examination of cyto‐ and myeloarchitectonic organization in humans. Cerebral Cortex, 7(8), 722–731.
Kropff, E., Carmichael, J. E., Moser, M. B., & Moser, E. I. (2015). Speed cells in the medial entorhinal cortex. Nature, 523(7561), 419–424. https://doi.org/10.1038/nature14622
Lavenex, P., Suzuki, W. A., & Amaral, D. G. (2004). Perirhinal and parahippocampal cortices of the macaque monkey: Intrinsic projections and interconnections. Journal of Comparative Neurology, 472(3), 371–394. https://doi.org/10.1002/cne.20079
Leitner, F. C., Melzer, S., Lutcke, H., Pinna, R., Seeburg, P. H., Helmchen, F., & Monyer, H. (2016). Spatially segregated feedforward and feedback neurons support differential odor processing in the lateral entorhinal cortex. Nature Neuroscience, 19(7), 935–944. https://doi.org/10.1038/nn.4303
Leonard, B. W., Amaral, D. G., Squire, L. R., & Zola‐Morgan, S. M. (1995). Transient memory impairment in monkeys with bilateral lesions of the entorhinal cortex. Journal of Neuroscience, 15(8), 5637–5659.
Lisman, J. E. (2007). Role of the dual entorhinal inputs to hippocampus: A hypothesis based on cue/action (non‐self/self) couplets. Progress in Brain Research, 163, 615–818.
Lorente de Nó, R. (1934). Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. Journal für Psychologie Und Neurologie, 46, 113–177.
Maass, A., Berron, D., Libby, L. A., Ranganath, C., & Duzel, E. (2015). Functional subregions of the human entorhinal cortex. eLife, 4. https://doi.org/10.7554/eLife.06426
Maguire, E. A., Frith, C. D., Burgess, N., Donnett, J. G., & O'Keefe, J. (1998). Knowing where things are parahippocampal involvement in encoding object locations in virtual large‐scale space. Journal of Cognitive Neuroscience, 10(1), 61–76.
Mathiasen, M. L., Hansen, L., & Witter, M. P. (2015). Insular projections to the parahippocampal region in the rat. Journal of Comparative Neurology, 523(9), 1379–1398. https://doi.org/10.1002/cne.23742
Medina, L., Abellan, A., & Desfilis, E. (2017). Contribution of genoarchitecture to understanding hippocampal evolution and development. Brain, Behavior and Evolution, 90(1), 25–40. https://doi.org/10.1159/000477558
Meunier, M., Bachevalier, J., Mishkin, M., & Murray, E. A. (1993). Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience, 13(12), 5418–5432.
Miao, C., Cao, Q., Moser, M. B., & Moser, E. I. (2017). Parvalbumin and somatostatin interneurons control different space‐coding networks in the medial entorhinal cortex. Cell, 171(3), 507–521.e517. https://doi.org/10.1016/j.cell.2017.08.050
Milner, B., Squire, L. R., & Kandel, E. R. (1998). Cognitive neuroscience and the study of memory. Neuron, 20(3), 445–468.
Mishkin, M. (1978). Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature, 273(5660), 297–298.
Mohedano‐Moriano, A., Pro‐Sistiaga, P., Arroyo‐Jimenez, M. M., Artacho‐Perula, E., Insausti, A. M., Marcos, P., … Insausti, R. (2007). Topographical and laminar distribution of cortical input to the monkey entorhinal cortex. Journal of Anatomy, 211(2), 250–260. https://doi.org/10.1111/j.1469‐7580.2007.00764.x
Montchal, M. E., Reagh, Z. M., & Yassa, M. A. (2019). Precise temporal memories are supported by the lateral entorhinal cortex in humans. Nature Neuroscience., 22, 284–288. https://doi.org/10.1038/s41593‐018‐0303‐1
Moser, E. I., Moser, M. B., & McNaughton, B. L. (2017). Spatial representation in the hippocampal formation: A history. Nature Neuroscience, 20(11), 1448–1464. https://doi.org/10.1038/nn.4653
Mullally, S. L., & Maguire, E. A. (2011). A new role for the parahippocampal cortex in representing space. Journal of Neuroscience, 31(20), 7441–7449. https://doi.org/10.1523/jneurosci.0267‐11.2011
Munoz, M., & Insausti, R. (2005). Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis). European Journal of Neuroscience, 22(6), 1368–1388. https://doi.org/10.1111/j.1460‐9568.2005.04299.x
Naber, P. A., Caballero‐Bleda, M., Jorritsma‐Byham, B., & Witter, M. P. (1997). Parallel input to the hippocampal memory system through peri‐ and postrhinal cortices. Neuroreport, 8(11), 2617–2621.
Naber, P. A., Lopes da Silva, F. H., & Witter, M. P. (2001). Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus, 11(2), 99–104.
Navarro Schroder, T., Haak, K. V., Zaragoza Jimenez, N. I., Beckmann, C. F., & Doeller, C. F. (2015). Functional topography of the human entorhinal cortex. eLife, 4. https://doi.org/10.7554/eLife.06738
Naya, Y. (2016). Declarative association in the perirhinal cortex. Neuroscience Research, 113, 12–18. https://doi.org/10.1016/j.neures.2016.07.001
Naya, Y., Chen, H., Yang, C., & Suzuki, W. A. (2017). Contributions of primate prefrontal cortex and medial temporal lobe to temporal‐order memory. Proceedings of the National Academy of Sciences of the United States of America, 114(51), 13555–13560. https://doi.org/10.1073/pnas.1712711114
Naya, Y., & Suzuki, W. A. (2011). Integrating what and when across the primate medial temporal lobe. Science, 333(6043), 773–776. https://doi.org/10.1126/science.1206773
Neunuebel, J. P., Yoganarasimha, D., Rao, G., & Knierim, J. J. (2013). Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. Journal of Neuroscience, 33(22), 9246–9258. https://doi.org/10.1523/JNEUROSCI.0946‐13.2013
Nilssen, E. S., Jacobsen, B., Fjeld, G., Nair, R. R., Blankvoort, S., Kentros, C., & Witter, M. P. (2018). Inhibitory connectivity dominates the fan cell network in layer II of lateral entorhinal cortex. Journal of Neuroscience, 38(45), 9712–9727. https://doi.org/10.1523/JNEUROSCI.1290‐18.2018
Norman, G., & Eacott, M. J. (2005). Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behavioral Neuroscience, 119(2), 557–566. doi:2005‐03585‐020 [pii]. https://doi.org/10.1037/0735‐7044.119.2.557
Ohara, S., Itou, K., Shiraishi, M., Gianatti, M., Sota, Y., Kabashima S., … Iijima T (2016) Efferent projections of the calbindin‐positiveentorhinal neurons in the rat: Connectional differences between the medial and lateral entorhinal cortex. SFN Abstr 84.13.96.
Ohara, S., Onodera, M., Simonsen, O. W., Yoshino, R., Hioki, H., Iijima, T., … Witter, M. P. (2018). Intrinsic projections of layer Vb neurons to layers Va, III, and II in the lateral and medial entorhinal cortex of the rat. Cell Reports, 24(1), 107–116. https://doi.org/10.1016/j.celrep.2018.06.014
O'Keefe, J. (1976). Place units in the hippocampus of the freely moving rat. Experimental Neurology, 51(1), 78–109.
O'Keefe, J., & Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely‐moving rat. Brain Research, 34(1), 171–175.
O'Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford: Clarendon Press.
Olsen, G. M., Ohara, S., Iijima, T., & Witter, M. P. (2017). Parahippocampal and retrosplenial connections of rat posterior parietal cortex. Hippocampus, 27(4), 335–358. https://doi.org/10.1002/hipo.22701
O'Reilly, K. C., Flatberg, A., Islam, S., Olsen, L. C., Kruge, I. U., & Witter, M. P. (2015). Identification of dorsal‐ventral hippocampal differentiation in neonatal rats. Brain Structure & Function, 220(5), 2873–2893. https://doi.org/10.1007/s00429‐014‐0831‐8
Otto, T., Schottler, F., Staubli, U., Eichenbaum, H., & Lynch, G. (1991). Hippocampus and olfactory discrimination learning: Effects of entorhinal cortex lesions on olfactory learning and memory in a successive‐cue, go‐no‐go task. Behavioral Neuroscience, 105(1), 111–119.
Pastoll, H., Solanka, L., van Rossum, M. C., & Nolan, M. F. (2013). Feedback inhibition enables theta‐nested gamma oscillations and grid firing fields. Neuron, 77(1), 141–154. https://doi.org/10.1016/j.neuron.2012.11.032 S0896‐6273(12)01119‐1 [pii].
Paz, R., Bauer, E. P., & Pare, D. (2007). Learning‐related facilitation of rhinal interactions by medial prefrontal inputs. Journal of Neuroscience, 27(24), 6542–6551.
Paz, R., Pelletier, J. G., Bauer, E. P., & Pare, D. (2006). Emotional enhancement of memory via amygdala‐driven facilitation of rhinal interactions. Nature Neuroscience, 9(10), 1321–1329.
Pelletier, J. G., Apergis‐Schoute, J., & Pare, D. (2005). Interaction between amygdala and neocortical inputs in the perirhinal cortex. Journal of Neurophysiology, 94(3), 1837–1848.
Pilkiw, M., Insel, N., Cui, Y., Finney, C., Morrissey, M. D., & Takehara‐Nishiuchi, K. (2017). Phasic and tonic neuron ensemble codes for stimulus‐environment conjunctions in the lateral entorhinal cortex. eLife, 6. https://doi.org/10.7554/eLife.28611
Pitkanen, A., Kelly, J. L., & Amaral, D. G. (2002). Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus, 12(2), 186–205. https://doi.org/10.1002/hipo.1099
Pitkanen, A., Pikkarainen, M., Nurminen, N., & Ylinen, A. (2000). Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann.N.Y.Acad.Sci, 911, 369–391.
Ramón y Cajal, S. (1893). Estructura del asta de Ammon y fascia dentata. Ann Soc Esp his Nat, 22, 53–114.
Ramón y Cajal, S. (1902). Sobre un ganglio especial de la corteza esfeno‐occipital. Trabajos del Laboratorio de Investigaciones Biologicas de la Universidad de Madrid, 1, 189–206.
Ramsden, H. L., Surmeli, G., McDonagh, S. G., & Nolan, M. F. (2015). Laminar and dorsoventral molecular organization of the medial entorhinal cortex revealed by large‐scale anatomical analysis of gene expression. PLoS Computational Biology, 11(1), e1004032. https://doi.org/10.1371/journal.pcbi.1004032
Ranganath, C., & Ritchey, M. (2012). Two cortical systems for memory‐guided behaviour. Nature Reviews Neuroscience, 13(10), 713–726. https://doi.org/10.1038/nrn3338 nrn3338 [pii].
Ritchey, M., Libby, L. A., & Ranganath, C. (2015). Cortico‐hippocampal systems involved in memory and cognition: The PMAT framework. Progress in Brain Research, 219, 45–64. https://doi.org/10.1016/bs.pbr.2015.04.001
Ritchey, M., Wang, S. F., Yonelinas, A. P., & Ranganath, C. (2018). Dissociable medial temporal pathways for encoding emotional item and context information. Neuropsychologia, 124, 66–78. https://doi.org/10.1016/j.neuropsychologia.2018.12.015
Room, P., & Groenewegen, H. J. (1986). Connections of the parahippocampal cortex. I. Cortical afferents. Journal of Comparative Neurology, 251(4), 415–450.
Room, P., Groenewegen, H. J., & Lohman, A. H. (1984). Inputs from the olfactory bulb and olfactory cortex to the entorhinal cortex in the cat. I. Anatomical observations. Experimental Brain Research, 56(3), 488–496.
Rowland, D. C., Obenhaus, H. A., Skytoen, E. R., Zhang, Q., Kentros, C. G., Moser, E. I., & Moser, M. B. (2018). Functional properties of stellate cells in medial entorhinal cortex layer II. eLife, 7. https://doi.org/10.7554/eLife.36664
Samarth, P., Ball, J. M., Unal, G., Pare, D., & Nair, S. S. (2017). Mechanisms of memory storage in a model perirhinal network. Brain Structure & Function, 222(1), 183–200. https://doi.org/10.1007/s00429‐016‐1210‐4
Sargolini, F., Fyhn, M., Hafting, T., McNaughton, B. L., Witter, M. P., Moser, M. B., & Moser, E. I. (2006). Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science, 312(5774), 758–762. https://doi.org/10.1126/science.1125572
Scaplen, K. M., Ramesh, R. N., Nadvar, N., Ahmed, O. J., & Burwell, R. D. (2017). Inactivation of the lateral entorhinal area increases the influence of visual cues on hippocampal place cell activity. Frontiers in Systems Neuroscience, 11, 40. https://doi.org/10.3389/fnsys.2017.00040
Schwerdtfeger, W. K., Buhl, E. H., & Germroth, P. (1990). Disynaptic olfactory input to the hippocampus mediated by stellate cells in the entorhinal cortex. Journal of Comparative Neurology, 292(2), 163–177.
Scoville, W. B., & Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry, 20(1), 11–21.
Shipley, M. T. (1975). The topographical and laminar organization of the presubiculum's projection to the ipsi‐ and contralateral entorhinal cortex in the guinea pig. Journal of Comparative Neurology, 160(1), 127–145.
Shipley, M. T., & Adamek, G. D. (1984). The connections of the mouse olfactory bulb: A study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Research Bulletin, 12(6), 669–688.
Solstad, T., Boccara, C. N., Kropff, E., Moser, M. B., & Moser, E. I. (2008). Representation of geometric borders in the entorhinal cortex. Science, 322(5909), 1865–1868. doi:322/5909/1865 [pii]. https://doi.org/10.1126/science.1166466
Squire, L. R., Stark, C. E., & Clark, R. E. (2004). The medial temporal lobe. Annual Review of Neuroscience, 27, 279–306. https://doi.org/10.1146/annurev.neuro.27.070203.144130
Staubli, U., Fraser, D., Kessler, M., & Lynch, G. (1986). Studies on retrograde and anterograde amnesia of olfactory memory after denervation of the hippocampus by entorhinal cortex lesions. Behavioral and Neural Biology, 46(3), 432–444.
Staubli, U., Ivy, G., & Lynch, G. (1984). Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proceedings of the National Academy of Sciences of the United States of America, 81(18), 5885–5887.
Stefanacci, L., & Amaral, D. G. (2000). Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: A retrograde tracing study. Journal of Comparative Neurology, 421(1), 52–79.
Steward, O., & Scoville, S. A. (1976). Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. Journal of Comparative Neurology, 169(3), 347–370. https://doi.org/10.1002/cne.901690306
Surmeli, G., Marcu, D. C., McClure, C., Garden, D. L., Pastoll, H., & Nolan, M. F. (2015). Molecularly defined circuitry reveals input‐output segregation in deep layers of the medial entorhinal cortex. Neuron, 88(5), 1040–1053. https://doi.org/10.1016/j.neuron.2015.10.041
Sutherland, R. J., & McDonald, R. J. (1990). Hippocampus, amygdala, and memory deficits in rats. Behavioural Brain Research, 37(1), 57–79.
Suzuki, W. A., & Amaral, D. G. (1994a). Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. Journal of Comparative Neurology, 350(4), 497–533. https://doi.org/10.1002/cne.903500402
Suzuki, W. A., & Amaral, D. G. (1994b). Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. Journal of Neuroscience, 14(3 Pt 2), 1856–1877.
Suzuki, W. A., Miller, E. K., & Desimone, R. (1997). Object and place memory in the macaque entorhinal cortex. Journal of Neurophysiology, 78(2), 1062–1081.
Swanson, L. W., & Köhler, C. (1986). Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. Journal of Neuroscience, 6(10), 3010–3023.
Tahvildari, B., & Alonso, A. (2005). Morphological and electrophysiological properties of lateral entorhinal cortex layers II and III principal neurons. Journal of Comparative Neurology, 491(2), 123–140. https://doi.org/10.1002/cne.20706
Tamamaki, N., & Nojyo, Y. (1995). Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. Journal of Comparative Neurology, 353(3), 379–390. https://doi.org/10.1002/cne.903530306
Tang, Q., Ebbesen, C. L., Sanguinetti‐Scheck, J. I., Preston‐Ferrer, P., Gundlfinger, A., Winterer, J., … Burgalossi, A. (2015). Anatomical organization and spatiotemporal firing patterns of layer 3 neurons in the rat medial entorhinal cortex. Journal of Neuroscience, 35(36), 12346–12354. https://doi.org/10.1523/JNEUROSCI.0696‐15.2015
Taylor, K. I., Moss, H. E., Stamatakis, E. A., & Tyler, L. K. (2006). Binding crossmodal object features in perirhinal cortex. Proceedings of the National Academy of Sciences of the United States of America, 103(21), 8239–8244. https://doi.org/10.1073/pnas.0509704103
Tsao, A., Moser, M. B., & Moser, E. I. (2013). Traces of experience in the lateral entorhinal cortex. Current Biology, 23(5), 399–405. https://doi.org/10.1016/j.cub.2013.01.036
Tsao, A., Sugar, J., Lu, L., Wang, C., Knierim, J. J., Moser, M. B., & Moser, E. I. (2018). Integrating time from experience in the lateral entorhinal cortex. Nature, 561, 57–62. https://doi.org/10.1038/s41586‐018‐0459‐6
van der Linden, S., & Lopes da Silva, F. H. (1998). Comparison of the electrophysiology and morphology of layers III and II neurons of the rat medial entorhinal cortex in vitro. European Journal of Neuroscience, 10(4), 1479–1489.
Van Groen, T., Lopes da Silva, F. H., & Wadman, W. J. (1987). Synaptic organization of olfactory inputs and local circuits in the entorhinal cortex: A current source density analysis in the cat. Experimental Brain Research, 67(3), 615–622.
van Groen, T., Miettinen, P., & Kadish, I. (2003). The entorhinal cortex of the mouse: Organization of the projection to the hippocampal formation. Hippocampus, 13(1), 133–149.
van Groen, T., & Wyss, J. M. (1990a). The connections of presubiculum and parasubiculum in the rat. Brain Research, 518(1–2), 227–243.
van Groen, T., & Wyss, J. M. (1990b). The postsubicular cortex in the rat: Characterization of the fourth region of the subicular cortex and its connections. Brain Research, 529(1–2), 165–177.
van Haeften, T., Baks‐Te Bulte, L. T., Goede, P. H., Wouterlood, F. G., & Witter, M. P. (2003). Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus, 13(8), 943–952.
Van Hoesen, G., & Pandya, D. N. (1975a). Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Research, 95(1), 1–24.
Van Hoesen, G., Pandya, D. N., & Butters, N. (1975). Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Research, 95(1), 25–38.
van Hoesen, G. W. (1982). The parahippocampal gyrus: New observations regarding its cortical connections in the monkey. Trends in Neurosciences, 5, 345–350.
Van Hoesen, G. W., & Pandya, D. N. (1975b). Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Research, 95(1), 39–59.
Van Hoesen, G. W., Pandya, D. N., & Butters, N. (1972). Cortical afferents to the entorhinal cortex of the rhesus monkey. Science, 175(4029), 1471–1473.
Varga, C., Lee, S. Y., & Soltesz, I. (2010). Target‐selective GABAergic control of entorhinal cortex output. Nature Neuroscience, 13(7), 822–824. https://doi.org/10.1038/nn.2570
Vaudano, E., Legg, C. R., & Glickstein, M. (1991). Afferent and efferent connections of temporal association cortex in the rat: A horseradish peroxidase study. European Journal of Neuroscience, 3(4), 317–330.
Vertes, R. P. (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51(1), 32–58.
Wallis, J. D. (2007). Orbitofrontal cortex and its contribution to decision‐making. Annual Review of Neuroscience, 30, 31–56. https://doi.org/10.1146/annurev.neuro.30.051606.094334
Wang, C., Chen, X., Lee, H., Deshmukh, S. S., Yoganarasimha, D., Savelli, F., & Knierim, J. J. (2018). Egocentric coding of external items in the lateral entorhinal cortex. Science, 362(6417), 945–949. https://doi.org/10.1126/science.aau4940
Willems, J. G., Wadman, W. J., & Cappaert, N. L. (2016). Distinct spatiotemporal activation patterns of the perirhinal‐entorhinal network in response to cortical and amygdala input. Front Neural Circuits, 10, 44. https://doi.org/10.3389/fncir.2016.00044
Wilson, D. I., Langston, R. F., Schlesiger, M. I., Wagner, M., Watanabe, S., & Ainge, J. A. (2013). Lateral entorhinal cortex is critical for novel object‐context recognition. Hippocampus, 23(5), 352–366. https://doi.org/10.1002/hipo.22095
Wilson, D. I., Watanabe, S., Milner, H., & Ainge, J. A. (2013). Lateral entorhinal cortex is necessary for associative but not nonassociative recognition memory. Hippocampus, 23(12), 1280–1290. https://doi.org/10.1002/hipo.22165
Wilson, R. C., & Steward, O. (1978). Polysynaptic activation of the dentate gyrus of the hippocampal formation: An olfactory input via the lateral entorhinal cortex. Experimental Brain Research, 33, 523–534.
Winterer, J., Maier, N., Wozny, C., Beed, P., Breustedt, J., Evangelista, R., … Schmitz, D. (2017). Excitatory microcircuits within superficial layers of the medial entorhinal cortex. Cell Reports, 19(6), 1110–1116. https://doi.org/10.1016/j.celrep.2017.04.041
Wirth, S., Ferry, B., & Di Scala, G. (1998). Facilitation of olfactory recognition by lateral entorhinal cortex lesion in rats. Behavioural Brain Research, 91(1–2), 49–59.
Witter, M. P. (1993). Organization of the entorhinal hippocampal system ‐ a review of current anatomical data. Hippocampus, 3, 33–44.
Witter, M. P. (2007). The perforant path: Projections from the entorhinal cortex to the dentate gyrus. Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications, 163, 43–61. https://doi.org/10.1016/s0079‐6123(07)63003‐9
Witter, M. P., & Amaral, D. G. (1991). Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. Journal of Comparative Neurology, 307(3), 437–459. https://doi.org/10.1002/cne.903070308
Witter, M. P., Doan, T. P., Jacobsen, B., Nilssen, E. S., & Ohara, S. (2017). Architecture of the entorhinal cortex: A review of entorhinal anatomy in rodents with some comparative notes. Frontiers in Systems Neuroscience, 11, 46. https://doi.org/10.3389/fnsys.2017.00046
Witter, M. P., Groenewegen, H. J., Lopes da Silva, F. H., & Lohman, A. H. (1989). Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Progress in Neurobiology, 33(3), 161–253.
Witter, M. P., Naber, P. A., van Haeften, T., Machielsen, W. C., Rombouts, S. A., Barkhof, F., … Lopes da Silva, F. H. (2000). Cortico‐hippocampal communication by way of parallel parahippocampal‐subicular pathways. Hippocampus, 10(4), 398–410. https://doi.org/10.1002/1098‐1063(2000)10:4<398::AID‐HIPO6>3.0.CO;2‐K
Witter, M. P., Room, P., Groenewegen, H. J., & Lohman, A. H. (1986). Connections of the parahippocampal cortex in the cat. V. Intrinsic connections; comments on input/output connections with the hippocampus. Journal of Comparative Neurology, 252(1), 78–94.
Witter, M. P., Van Hoesen, G. W., & Amaral, D. G. (1989). Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. Journal of Neuroscience, 9(1), 216–228.
Wouterlood, F. G., Mugnaini, E., & Nederlof, J. (1985). Projection of olfactory bulb efferents to layer I GABAergic neurons in the entorhinal area. Combination of anterograde degeneration and immunoelectron microscopy in rat. Brain Research, 343(2), 283–296.
Wouterlood, F. G., & Nederlof, J. (1983). Terminations of olfactory afferents on layer II and III neurons in the entorhinal area: Degeneration‐Golgi‐electron microscopic study in the rat. Neuroscience Letters, 36(2), 105–110.
Wouterlood, F. G., & Pothuizen, H. (2000). Sparse colocalization of somatostatin‐ and GABA‐immunoreactivity in the entorhinal cortex of the rat. Hippocampus, 10(1), 77–86. https://doi.org/10.1002/(SICI)1098‐1063(2000)10:1<77::AID‐HIPO8>3.0.CO;2‐P
Wouterlood, F. G., van Denderen, J. C., van Haeften, T., & Witter, M. P. (2000). Calretinin in the entorhinal cortex of the rat: Distribution, morphology, ultrastructure of neurons, and co‐localization with gamma‐aminobutyric acid and parvalbumin. Journal of Comparative Neurology, 425(2), 177–192.
Xu, W., & Wilson, D. A. (2012). Odor‐evoked activity in the mouse lateral entorhinal cortex. Neuroscience, 223, 12–20. https://doi.org/10.1016/j.neuroscience.2012.07.067
Yartsev, M. M., Witter, M. P., & Ulanovsky, N. (2011). Grid cells without theta oscillations in the entorhinal cortex of bats. Nature, 479(7371), 103–107. https://doi.org/10.1038/nature10583
Yoganarasimha, D., Rao, G., & Knierim, J. J. (2011). Lateral entorhinal neurons are not spatially selective in cue‐rich environments. Hippocampus, 21(12), 1363–1374. https://doi.org/10.1002/hipo.20839
Yoo, S. W., & Lee, I. (2017). Functional double dissociation within the entorhinal cortex for visual scene‐dependent choice behavior. eLife, 6. https://doi.org/10.7554/eLife.21543
Young, B. J., Otto, T., Fox, G. D., & Eichenbaum, H. (1997). Memory representation within the parahippocampal region. Journal of Neuroscience, 17(13), 5183–5195.
Zola‐Morgan, S. M., Squire, L. R., Alvarez‐Royo, P., & Clower, R. P. (1991). Independence of memory functions and emotional behavior: Separate contributions of the hippocampal formation and the amygdala. Hippocampus, 1(2), 207–220.
Zola‐Morgan, S. M., Squire, L. R., & Amaral, D. G. (1989). Lesions of the amygdala that spare adjacent cortical regions do not impair memory or exacerbate the impairment following lesions of the hippocampal formation. Journal of Neuroscience, 9(6), 1922–1936.
15. NMDA receptor activity bidirectionally controls active decay of long‐term spatial memory in the dorsal hippocampus
Paola Virginia Migues1 | Jacinda Wong1 | Jeongho Lyu1 | Oliver Hardt1,2
1Department of Psychology, Behavioural Neuroscience, McGill University, Montréal, Québec, Canada
2Centre for Discovery Brain Sciences, Simons Initiative for the Developing Brain, The University of Edinburgh, Scotland, UK
Correspondence
Oliver Hardt, McGill University, Department of Psychology, 1205, Dr Penfield AveMontréal, QC H3A 1B1Canada. Email: oliver.hardt@mcgill.ca
15.1. Abstract
The time‐dependent forgetting of long‐term spatial memories involves activation of NMDA receptors (NMDARs) in the hippocampus. Here, we tested whether NMDARs regulate memory persistence bidirectionally, decreasing or increasing the rate of forgetting. We found that blocking NMDAR activation with AP5 or the GluN2B‐selective antagonist Ro25‐6981 in the dorsal hippocampus (dHPC) prevented the natural forgetting of long‐term memory for the locations of objects in an open field arena. In contrast, while enhancing NMDAR function with the partial agonist D‐Cycloserine did not affect the speed of forgetting for these types of memories, infusing the NMDAR co‐agonist D‐Serine significantly shortened their persistence. These results suggest that NMDAR activity can modulate the speed of constitutive long‐term memory decay in the dHPC and that regulating NMDAR expression and D‐Serine availability could provide a mechanism to control the duration of long‐term memory.
KEYWORDS forgetting, active decay, long‐term memory, NMDA receptor
15.2. INTRODUCTION
In the beginning of the scientific research of memory the issue of forgetting featured prominently in a series of seminal reports published around the same time. In 1882, Ribot noted that the head trauma patients he studied experienced a peculiar, temporally selective memory loss, with recent memories being more likely forgotten than remote ones (Ribot, 1882). Foreshadowing the modern concept of synaptic, and in some sense, systems consolidation, he speculated that this gradient—now bearing his name—suggested the existence of a transient metabolic process, which newly formed memories require to be retained in the long term. Shortly thereafter in 1885, Ebbinghaus published his now famed forgetting curve, illustrating that seemingly stable memories progressively fade over time, with the most substantial loss occurring shortly after acquisition (Ebbinghaus, 1885). Then, around the turn of the century, Müller and Pilzecker observed that newly learned material requires a period of recurrent reactivation, lest it will be forgotten, leading them to propose that long‐term memory formation requires a perseveration‐consolidation process following learning. In the wake of these puzzling observations, Burnham aptly concluded in 1903 that “Not memory, but forgetting is the mystery” (Burnham, 1903). It is fair to say that these original questions still dominate memory research. Indeed, in the years that followed, consolidation failure, interference, retrieval error, inhibition, and trace decay have been intensively explored as possible causes of these and other forms of forgetting, yet we still know little about these phenomena and their neurobiological mechanisms (Hardt, Nader, & Nadel, 2013; Wixted, 2004).
About a century after these early reports, while developing a new hypothesis on memory consolidation, Lynn Nadel and colleagues also contemplated how established memories might be lost—an aspect in the life of memories that consolidation accounts sometimes leave untouched. Taking a more neurobiological perspective, they suggested that “[..] forgetting involves an actual loss of connectivity among the neuronal elements participating in a representation, i.e., disappearance of at least some of the changes in synaptic connectivity that originally embodied the information. […] Loss of connectivity among elements due to forgetting is accompanied by, causes, or results from a process of reorganization of that which remains” (Squire, Cohen, & Nadel, 1984). In recent years, Nadel revisited these ideas: it turned out that much of these initial speculations were presciently accurate, describing processes we now believe to underpin at least one particular form of forgetting—active decay (Hardt et al., 2013).
Exploring the neurobiological mechanisms involved in active decay, we have found that it involves the activity‐dependent removal of GluA2‐containing AMPA receptors (GluA2/AMPARs) from post‐synaptic sites (Migues et al., 2016). Since learning and memory formation, as well as the expression of long‐term potentiation (LTP), are associated with increased levels of GluA2/AMPARs at post‐synaptic densities, this particular forgetting process thus resembles the reversal of enhanced connectivity Nadel and colleagues had envisioned in 1984. Our findings have recently been replicated and extended by others who identified the calcium sensor synaptotagmin‐3 as a key element in reducing the expression of AMPARs during the active decay of long‐term memories (Awasthi et al., 2019). Thus, the current evidence strongly suggests that long‐term memories are lost by a constitutive process that progressively disassembles the morphological changes to synapses that emerged during learning and memory formation, and that this process involves calcium signaling. These findings raise the question of how this endogenous decay process may be regulated to control the speed of memory loss, so that some memories can last longer than others.
During certain forms of synaptic plasticity, such as LTP, long‐term depression (LTD), and depotentiation, NMDA receptor (NMDAR) activation and the subsequent influx of Ca2+ generally initiates activity‐dependent AMPAR trafficking, promoting their increased or decreased expression at synapses. Thus, active decay, which involves the synaptotagmin‐mediated synaptic removal of AMPARs, might involve NMDAR signaling as well. Indeed, some findings have shown that administering compounds during the memory retention interval that block the activity of NDMARs can curb certain forms of forgetting (Shinohara & Hata, 2014; Villarreal, Do, Haddad, & Derrick, 2001). Here, we therefore explored whether NMDARs might regulate the persistence of long‐term memories in a behavioral paradigm capable of measuring active decay of long‐term memories in the rat (Migues et al., 2016).
NMDARs are heterotetramers consisting of two obligatory GluN1 subunits, and GluN2 or GluN3 subunits (Traynelis et al., 2010). In the hippocampus, the vast majority of NMDARs comprises GluN2A or GluN2B subunits (Monyer, Burnashev, Laurie, Sakmann, & Seeburg, 1994). Channel opening requires glutamate binding, co‐agonist binding (D‐Serine or glycine) on GluN1, as well as membrane depolarization to remove the Mg2+ ion blocking the receptor channel. The relatively slower decay kinetics of GluN2B‐containing NMDARs leads to a greater Ca2+ influx following receptor activation than for receptors composed of GluN2A subunits (Sheng, Cummings, Roldan, Jan, & Jan, 1994). NMDARs that contain GluN2A subunits are activated and deactivated faster, leading to a lower increase in intracellular Ca2+ than observed following activation of GluN2B/NMDARs (Shipton & Paulsen, 2014). Thus, the relative expression of GluN2A‐ to GluN2B‐containing NMDARs at post‐synaptic sites can affect spontaneous synaptic transmission (Erreger, Dravid, Banke, Wyllie, & Traynelis, 2005), as well as (activity dependent) synaptic plasticity and its direction.
These subunit‐specific differences in Ca2+ influx suggest that subunit composition may underpin the involvement of NMDAR activation in both strengthening of synaptic connections during LTP as well as weakening them during LTD and depotentiation (Hardt, Nader, & Wang, 2014). Thus, NMDARs might modulate the speed of decay of long‐term memories (Hardt et al., 2013), such that higher levels of NMDAR activation during active decay phases will accelerate the rate of long‐term memory loss, while lower levels of NMDAR activation will slow it down (Hardt et al., 2014). As a first step to test these predictions, we here explored whether attenuating NMDAR activity in the dorsal hippocampus (dHPC) during the memory retention interval will prevent the loss of long‐term object location memories, while enhancing NMDAR function will accelerate it.
15.3. METHODS
We bilaterally implanted male Long‐Evans rats, weighing 300–350−g at the time of surgery, with stainless steel cannulas (Plastics One, 26 gauge), aiming at CA1 in the dHPC (AP −3.60−mm, ML ±3.10 mm, DV −2.40−mm), as we have described in detail elsewhere (Migues et al., 2016). After 1 week, during which rats were handled daily, the animals participated in an object location novelty recognition task, following a protocol we have used before (Migues et al., 2016). We used an open field (600−mm−×−600−mm−×−600−mm), walls and floors made of laminated medium‐density fiberboard, in which the floor was covered with sawdust bedding also used in the home cages of the rats. All experiments reported here used the same basic procedure, which provides a reliable method for measuring active decay of long‐term object location memories using a location novelty recognition task (Migues et al., 2016) The protocol consists of (a) habituation phase, (b) sampling phase, followed by (c) daily intracranial drug infusions during the memory retention interval, and (d) a single probe trial. (a) Habituation: We placed rats singly into the empty open field for 10 min during each of the four consecutive days of habituation and allowed them to explore the testing arena unfettered. No objects were present during these trials. (b) Sampling: Twenty‐four hours after the last habituation trial, sampling started. For seven consecutive days, we put rats each day for 10 min into the open field, where two identical copies of a junk object were located, at two opposing corners, at positions that remained unaltered for individual rats throughout sampling. (c) Retention interval: Daily drug infusions started 24 h after the last sampling trial. We used this basic behavioral protocol with different groups of animals in a series of experiments to explore the role of NMDAR signaling in active decay: For either 6 or 13 days, animals received either AP5 (Exp 1), Ro25‐6981 (Exp 2), D‐cycloserine (DCS) (Exp 3), D‐Serine (Exp 4), or vehicle (Tris‐saline; final pH 7.2 for all drugs) directly into the dHPC, in their home colony. (d) Probe: Twenty‐four hours after the last infusion, animals were again placed into the open field for 3 min, where we had now moved one of the two known objects to a novel location, while the other one remained at its familiar place. The object positions during sampling and probe were counterbalanced.
We measured the time animals explored the object at the familiar and the object at the novel location during the first minute of the probe trial. Because rats naturally prefer exploring novel compared to familiar stimuli, their bias to examine the moved object reflects that they are expressing memory for its former position (Ennaceur & Delacour, 1988). We quantified the preference for exploring the location novelty using the discrimination index d = [(time exploring novelty) − (time exploring familiarity)]/(total time spent exploring objects). We used one‐sample t‐tests to determine whether d was significantly different from zero, that is, whether animals preferred to explore the moved object, thus expressing object location memory. Treatment groups were compared with unpaired t‐tests. All tests were two‐tailed, and effects were considered significant when p−<−.05.
15.4. RESULTS
For all experiments discussed below, exploratory activity during the sampling phase was not different between the groups (for all effects, F−<−1; data not shown). We found that rats decreased their overall exploratory activity from the first to the last sampling session, suggesting that they acquired knowledge about the stimuli in the test arena during sampling. Both objects were explored for the same amount of time, demonstrating the absence of preference for a location at the beginning and at the end of sampling, which represents the typical observations in this protocol (Migues et al., 2016).
We have established previously that cannulated rats handled and trained as described here prefer to explore the displaced object for as long as 7–9 days after training (Migues et al., 2016). Based on these findings, we tested now whether blocking NMDARs with AP5 during the retention interval prevented this natural decay of consolidated, long‐term object location memories. Twenty‐four hours after the last sampling trial animals received the first of 26 infusions, two per day (AM and PM, 5 hr apart from each other). One group received AP5 into the dHPC to block NMDAR activation, the other one received vehicle (Figure 14a). In the probe trial, 14−days after the last sampling session, only those animals that had received AP5 during the retention interval expressed a significant preference for the object that had been moved to the novel location, t(11) = 3.2, p−<−.01, which was significantly different from the VEH group, t(22) = −2.2, p = .04, in which rats explored both objects equally, t−<−1 (Figure 14b). Both groups spent the same amount of time exploring objects during the Probe trial, t−<−1 (Figure 14c), indicating that the difference in novelty preference expression did not reflect differences in motility or motivation. This was the case for all experiments we describe here (Figures 14f and 15f).
Figure 14.
Blocking NMDAR activity in the dorsal hippocampus during the retention interval prevents the decay of long‐term object location memories. (a) During sampling, animals were exposed to two copies of a junk objects for 10 min a day for the seven consecutive days that remained at the same locations. This protocol leads to long‐term object location memories lasting for about 8 days (Migues et al., 2016). Twenty‐four hours after the last sampling session, animals were infused with AP5 or Veh into the dorsal hippocampus twice daily for 13−days. The following day, 14−days after the end of sampling, animals were returned to the open field for the probe trial, where one of the original objects was moved to a novel location. (b) Animals infused with AP5 preferred to explore the relocated object, thus expressing memory for the original object locations, while animals infused with vehicle explored both objects the same. (c) Both groups expressed the same overall exploratory activity during the probe trial. (d) Using the same behavioral protocol, animals were infused with Ro25‐6981 during the memory retention interval. (e) Only animals that received the GluN2B‐selective antagonist Ro25‐6981 preferred exploring the object at the novel location. (f) There were no differences in overall exploratory activity. *p−<−.05, **p−<−.01
Figure 15.
Enhancing NMDAR activity in the dorsal hippocampus with D‐Serine, but not D‐Cyloserine accelerates the natural forgetting of long‐term object location memories. (a) We used the same protocol as in Figure 14 with the difference that the retention interval was only 7 days. Animals were infused with the partial NMDAR agonist D‐Cycloserine during each day of this 6‐day memory retention interval. Animals were tested 7 days after the end of sampling, at a time when they naturally express long‐term object location memory in this paradigm (Migues et al., 2016). (b) At all doses tested, animals infused with D‐Cycloserine preferred to explore the object at the novel location, just like animals that had received Veh. Group differences were absent. (c) Overall exploratory activity during the probe trial was the same for all groups. (d) Instead of D‐Cycloserine, animals were infused with the NMDAR co‐agonist D‐Serine during the 6‐day memory retention interval. (e) Animals that had received D‐Serine showed no object location preference during the probe trial, while animals infused with Veh preferred to explore the moved object. (f) There were no differences in overall exploratory activity. *p−<−.05, **p−<−.01
The results from the first experiment add to findings demonstrating that reducing the activity of NMDARs during the memory retention interval can preserve spatial memory (Shinohara & Hata, 2014; Villarreal et al., 2001). Considering the critical role for GluN2B‐containing NMDARs in synaptic plasticity, we next explored whether selectively blocking them during the retention interval would be sufficient to prevent memory decay. We used the polyamine antagonist Ro25‐6981, which is highly selective for GluN2B‐containing NMDARs, unlike AP5, which binds to both GluN2A‐ and GluN2B‐containing NMDARs. We trained, infused, and tested animals as described above (Figure 14d), and obtained similar results. Only the group of rats that received Ro25‐6981 during the retention interval explored the object moved to the novel location more so than the object that had remained in place, t(6) = 8.7, p−<−.01, while the animals that had received vehicle during the retention interval explored both objects the same, t−<−1 (Figure 14e). The difference in exploratory preference between the groups was significant, t(13) = −4.3, p−<−.01. Thus, blocking GluN2B‐containing NMDARs during the retention interval was sufficient to preserve the ability to express object location memory in the location novelty recognition test.
Taken together, the results confirm that blocking NMDAR activity can preserve memory expression, as predicted in active decay theory (Hardt et al., 2013). We reasoned that memory loss was prevented in our experiments because we reduced Ca2+ currents activating pathways involved in weakening synaptic connections, such as those involved in LTD and depotentiation, which both required NMDAR activity. This would suggest that promoting NMDAR activity during the retention interval could increase the rate of long‐term memory decay. To test this hypothesis, we first used the partial NMDAR agonist D‐Cycloserine, which has been shown to facilitate the formation of various forms of memory that require NMDAR activation (Thompson, Moskal, & Disterhoft, 1992; Vervliet, 2008), including spatial memory (Land & Riccio, 1999; Lelong, Dauphin, & Boulouard, 2001; Quartermain, Mower, Rafferty, Herting, & Lanthorn, 1994). We trained rats as in the previous two experiments and infused the drug once daily into the dHPC during the memory retention interval. Unlike in the previous experiments, we tested for the expression of object location recognition memory 7 days after the last day of sampling, at a time when rats normally still express novelty recognition in this paradigm (fig. 14 in Migues et al., 2016). No concentration of DCS that we tested (3, 15, 30−μg) promoted memory loss, and rats in the DCS and vehicle groups alike preferred to explore the moved object (3 μg: Veh t(6) = 2.9, p = .03; DCS t(6) = 2.6, p = .04; 15−μg: Veh t(5) = 3.14, p = .03; DCS t(7) = 6.6, p−<−.01; 30−μg: Veh t(7) = 5.2, p−<−.01; DCS t(8) = 9.03, p−<−.01). Group differences were absent (F−<−1 for main effects and interaction). These results suggest that modulating NMDAR function in the dHPC with the partial agonist DCS does not accelerate active decay of long‐term object location recognition memory.
We considered that DCS might had been ineffective because the decay “signal” itself that activates NMDARs might be relatively weak such that the partial agonist DCS was not potent enough to amplify NMDAR function sufficiently to enhance active decay. Unlike DCS, D‐Serine is a NMDAR co‐agonist required for receptor opening, acting as the endogenous ligand of the glycine site. Thus, infusing D‐Serine during the retention interval might provide sufficient levels of the essential co‐agonist to permit receptor activation when active decay processes unfold. We therefore replicated the previous experiment, infusing D‐Serine during the 6 days between the end of sampling and the probe trial (Figure 15d). During the probe test, animals that had received D‐Serine explored both objects the same, t(7) = −1.0, p = .35, while the group of rats that had received vehicle infusions preferred to explore the object that had been moved to the novel location, t(6) = 2.9, p = .03 (Figure 15e). The difference in novelty exploration between the two groups was significant, t(13) = −2.9, p = .01. These results suggest that promoting NMDAR activation during the memory retention interval with D‐Serine can accelerate the natural loss of long‐term memory in the hippocampus.
15.5. DISCUSSION
This series of experiments shows that drugs known to modulate NMDAR function in the hippocampus can affect the persistence of long‐term object location recognition memory. Our results support a prediction of active decay theory that forgetting of long‐term memory requires NMDARs (Hardt et al., 2013), in that attenuating their activation can decrease and enhancing it can accelerate the decay of long‐term memory. Thus, NMDARs may contribute to signaling involved in active decay, supporting the idea that regulating the expression of GluN2B/NMDARs could provide a possible mechanism to control the persistence of memories.
The calcium‐sensing protein synaptotagmin has been shown to participate in regulating active decay of long‐term object location recognition memory in mice (Awasthi et al., 2019). In conjunction with the findings we report here, and considering the role of activity‐dependent internalization of GluA2/AMPARs we described earlier (Migues et al., 2016), these results thus support our basic metaplastic model in which GluN2B/NMDAR signaling could play a pivotal role in determining the levels of Ca2+ supplied to the signaling pathways involved in reducing the expression of GluA2/AMPARs, thereby erasing long‐term memories (Hardt et al., 2014).
According to this model, the ratio of GluN2A to GluN2B levels could represent a metaplastic parameter determining the direction of synaptic plasticity and thus memory persistence (Abraham & Bear, 1996; Hardt et al., 2014). For example, it has been shown that higher expression of GluN2B during learning could facilitate long‐term memory formation, while memory retention deficits are associated with relatively higher levels of GluN2A‐containing receptors (Brim et al., 2013; Cao et al., 2007; Cui et al., 2013; Plattner et al., 2014). Similarly, the persistence of established long‐term memories correlates with GluN2B expression in the brain areas supporting these memories, such that unusually stable memories are associated with lower levels of GluN2B. For example, GluN2B expression in the brain circuit critical for imprinting memories, comprising visual Wulst and intermediate medial mesopallium, is elevated in chicks during the critical time period during which imprinting memories can be readily and efficiently acquired. Outside this developmental phase, more GluN2A than GluN2B are expressed (Nakamori, Maekawa, Sato, Tanaka, & Ohki‐Hamazaki, 2013), which might also contribute to the extraordinary stability of these memories (Hardt et al., 2014). In rats, auditory fear memories acquired with strong training (i.e., increased number of tone‐shock pairings during conditioning) results in lower GluN2B expression in the amygdala than weaker training (i.e., less pairings); importantly, the former, unlike the latter memories do not enter a phase of enhanced plasticity upon activation (Wang, de Oliveira Alvares, & Nader, 2009), such that interventions that normally impair memory reconsolidation are ineffective. Yet, one month after training, reactivation can again promote plasticity in these memories: comparing GluN2B levels at recent and remote time points revealed that receptor expression in the amygdala was significant lower when assessed shortly after strong training than after one month. Put differently, both in chicks and in rats higher levels of GluN2B‐containing NMDARs contribute to diminished memory stability, while lower levels help to preserve it. Thus, regulating the expression of GluN2B/NMDARs could directly determine how long memories may persist.
There is an important potential alternative explanation for the involvement of NMDAR in memory loss as envisioned here. It is widely accepted that NMDARs are involved in certain forms of LTP as well as learning and memory (Lüscher & Malenka, 2012; Shipton & Paulsen, 2014). Therefore, it is possible that blocking NMDARs during the retention interval might have prevented new learning in our experiments, while enhancing NMDARs with D‐Serine might have promoted it. In the former case, this could have reduced memory interference arising from new learning, while in the latter memory interference might have been increased. Thus, instead of regulating active decay, modulating NMDAR function might have affected forgetting because it had an effect on new learning during the retention interval. This possible explanation seems unlikely for several reasons. First, as we have previously established, new spatial learning during the retention interval neither causes proactive nor retroactive interference in this paradigm (Migues et al., 2016), which supports the generally held view that interference predominantly affects memories shortly after learning or retrieval, that is, during memory consolidation or reconsolidation, but not long‐term memories we have targeted in our experiments (Gisquet‐Verrier & Riccio, 2018). Second, unlike D‐Serine, infusing DCS did not increase memory loss, although DCS has been characterized as a potent cognitive enhancer that can effectively promote new learning and memory formation at the dosages we used here in the hippocampus (Ren et al., 2013). Thus, if new learning and interference would have caused forgetting in our experiments, DCS should have decreased expression of recognition memory at least to some extent. Taken together, it seems unlikely that modulating NMDARs in our experiments regulated memory interference and thereby affected forgetting.
While enhancing NMDAR function with the partial agonist D‐Cycloserine did not affect memory retention in our experiments, infusing the NMDAR co‐agonist D‐Serine resulted in accelerated decay of long‐term memory. This could imply that the endogenous signal involved in active decay may be relatively weak and thus the partial agonist will not suffice to noticeably enhance it. D‐Serine, such as glycine, is an endogenous co‐agonist necessary for synaptic plasticity, and, although not fully resolved, astrocytes seem to present the main source delivering it to synapses. Indeed, astrocyte signaling has been implicated in synaptic plasticity, and in the hippocampus, astroglial CB1 receptors modulate D‐Serine release from astrocytes, regulating, for example, LTP induction as well as memory formation (Robin et al., 2018). Although it is unclear how pharmacologically supplying D‐Serine compares to these constitutive processes, the ability of D‐Serine to promote endogenous forgetting in our experiments suggest the interesting possibility that astrocytes participate in the regulating of memory persistence.
Taken together, our findings lend support to an active decay model in which GluN2B/NMDAR activity determines the speed of constitutive memory loss, controlling the rate at which AMPARs are removed from post‐synaptic sites. Considering the findings implicating reduced GluN2B expression for very strong memories, it is possible that during memory acquisition neuromodulators, such as dopamine, norepinephrine, or stress hormones, affect memory retention by altering the relative expression of GluN2A and GluN2B/NMDARs. Such a mechanism could provide the brain with means to regulate which of the many memories formed during the day may be worth retaining (Bethus, Tse, & Morris, 2010), thereby controlling what remains of decay.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
DATA SHARING
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Abraham, W. C., & Bear, M. F. (1996). Metaplasticity: The plasticity of synaptic plasticity. Trends in Neurosciences, 19, 126–130.
Awasthi, A., Ramachandran, B., Ahmed, S., Benito, E., Shinoda, Y., Nitzan, N., … Dean, C. (2019). Synaptotagmin‐3 drives AMPA receptor endocytosis, depression of synapse strength, and forgetting. Science, 363, eaav1483–eaav1416 Available from: http://www.sciencemag.org/lookup/doi/10.1126/science.aav1483
Bethus, I., Tse, D., & Morris, R. G. M. (2010). Dopamine and memory: Modulation of the persistence of memory for novel hippocampal NMDA receptor‐dependent paired associates. Journal of Neuroscience, 30, 1610–1618.
Brim, B. L., Haskell, R., Awedikian, R., Ellinwood, N. M., Jin, L., Kumar, A., … Magnusson, K. R. (2013). Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behavioural Brain Research, 238, 211–226.
Burnham, W. (1903). Retroactive amnesia: Illustrative cases and a tentative explanation. American Journal of Psychology, 14, 382–396.
Cao, X., Cui, Z., Feng, R., Tang, Y.‐P., Qin, Z., Mei, B., & Tsien, J. Z. (2007). Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. European Journal of Neuroscience, 25, 1815–1822.
Cui, Z., Feng, R., Jacobs, S., Duan, Y., Wang, H., Cao, X., & Tsien, J. Z. (2013). Increased NR2A:NR2B ratio compresses long‐term depression range and constrains long‐term memory. Scientific Reports, 3, 1036.
Ebbinghaus, H. (1885). über Das Gedächtnis. Leipzig, Germany: von Duncker & Humblot.
Ennaceur, A., & Delacour, J. (1988). A new one‐trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research, 31, 47–59.
Erreger, K., Dravid, S. M., Banke, T. G., Wyllie, D. J. A., & Traynelis, S. F. (2005). Subunit‐specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. Journal of Physiology (London), 563, 345–358.
Gisquet‐Verrier, P., & Riccio, D. C. (2018). Memory integration: An alternative to the consolidation/reconsolidation hypothesis. Progress in Neurobiology, 171, 15–31.
Hardt, O., Nader, K., & Nadel, L. (2013). Decay happens: The role of active forgetting in memory. Trends in Cognitive Sciences, 17, 111–120.
Hardt, O., Nader, K., & Wang, Y. T. (2014). GluA2‐dependent AMPA receptor endocytosis and the decay of early and late long‐term potentiation: Possible mechanisms for forgetting of short‐ and long‐term memories. Philosophical Transactions of the Royal Society B: Biological Sciences, 369, 20130141.
Land, C., & Riccio, D. C. (1999). d‐Cycloserine: Effects on long‐term retention of a conditioned response and on memory for contextual attributes. Neurobiology of Learning and Memory, 72, 158–168.
Lelong, V., Dauphin, F., & Boulouard, M. (2001). RS 67333 and D‐cycloserine accelerate learning acquisition in the rat. Neuropharmacology, 41, 517–522.
Lüscher, C., & Malenka, R. C. (2012). NMDA receptor‐dependent long‐term potentiation and long‐term depression (LTP/LTD). Cold Spring Harbor Perspectives in Biology, 4, 1–15.
Migues, P. V., Liu, L., Archbold, G. E. B., Einarsson, E. Ö., Wong, J., Bonasia, K., … Hardt, O. (2016). Blocking synaptic removal of GluA2‐containing AMPA receptors prevents the natural forgetting of long‐term memories. Journal of Neuroscience, 36, 3481–3494.
Monyer, H., Burnashev, N., Laurie, D. J., Sakmann, B., & Seeburg, P. H. (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron, 12, 529–540.
Nakamori, T., Maekawa, F., Sato, K., Tanaka, K., & Ohki‐Hamazaki, H. (2013). Neural basis of imprinting behavior in chicks. Development, Growth & Differentiation, 55, 198–206.
Plattner, F., Hernández, A., Kistler, T. M., Pozo, K., Zhong, P., Yuen, E. Y., … Bibb, J. A. (2014). Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron, 81, 1070–1083.
Quartermain, D., Mower, J., Rafferty, M. F., Herting, R. L., & Lanthorn, T. H. (1994). Acute but not chronic activation of the NMDA‐coupled glycine receptor with D‐cycloserine facilitates learning and retention. European Journal of Pharmacology, 257, 7–12.
Ren, J., Li, X., Zhang, X., Li, M., Wang, Y., & Ma, Y. (2013). The effects of intra‐hippocampal microinfusion of D‐cycloserine on fear extinction, and the expression of NMDA receptor subunit NR2B and neurogenesis in the hippocampus in rats. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 44, 257–264.
Ribot, T. (1882). Diseases of memory. New York, NY: D. Appleton and Company.
Robin, L. M., da Cruz, J. F. O., Langlais, V. C., Martin‐Fernandez, M., Metna‐Laurent, M., Busquets‐Garcia, A., … Marsicano, G. (2018). Astroglial CB1 receptors determine synaptic D‐serine availability to enable recognition memory. Neuron, 98, 935–944.e5.
Sheng, M., Cummings, J., Roldan, L. A., Jan, Y. N., & Jan, L. Y. (1994). Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature, 368, 144–147.
Shinohara, K., & Hata, T. (2014). Post‐acquisition hippocampal NMDA receptor blockade sustains retention of spatial reference memory in Morris water maze. Behavioural Brain Research, 259, 261–267.
Shipton, O. A., & Paulsen, O. (2014). GluN2A and GluN2B subunit‐containing NMDA receptors in hippocampal plasticity. Philosophical Transactions of the Royal Society B: Biological Sciences, 369, 20130163.
Squire, L. R., Cohen, N. J., & Nadel, L. (1984). In H. Weingartner & E. S. Parker (Eds.), Memory consolidation: Psychobiology of cognition. Hillsdale, NJ: Lawrence Earlbaum Associates.
Thompson, L. T., Moskal, J. R., & Disterhoft, J. F. (1992). Hippocampus‐dependent learning facilitated by a monoclonal antibody or D‐cycloserine. Nature, 359, 638–641.
Traynelis, S. F., Wollmuth, L. P., McBain, C. J., Menniti, F. S., Vance, K. M., Ogden, K. K., … Dingledine, R. (2010). Glutamate receptor ion channels: Structure, regulation, and function. Pharmacological Reviews, 62, 405–496.
Vervliet, B. (2008). Learning and memory in conditioned fear extinction: Effects of d‐cycloserine. Acta Psychologica, 127, 601–613.
Villarreal, D. M., Do, V., Haddad, E., & Derrick, B. E. (2001). NMDA receptor antagonists sustain LTP and spatial memory: Active processes mediate LTP decay. Nature Neuroscience, 5, 48–52.
Wang, S.‐H., de Oliveira Alvares, L., & Nader, K. (2009). Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nature Neuroscience, 12, 905–912.
Wixted, J. T. (2004). The psychology and neuroscience of forgetting. Annual Review of Psychology, 55, 235–269.
ACKNOWLEDGMENTS
This work was supported by a Wellcome Principal Research Fellowship to E.A.M. (210567/Z/18/Z) and a Centre Award from Wellcome (203147/Z/16/Z).
ACKNOWLEDGMENTS
We are grateful for the support from the Kavli Foundation, the Centre of Excellence scheme of the Research Council of Norway (Centre for Neural Computation, grant number 223262) and the Egil and Pauline Braathen and Fred Kavli Centre for Cortical Microcircuits. We also wish to thank Edvard Moser, Emilie Ranheim Skytøen, Matthias Nau, and Albert Tsao for constructive feedback on the manuscript.
ACKNOWLEDGEMENTS
This study has been supported by grant number 227769 of the Research Council of Norway, the Centre of Excellence scheme of the Research Council of Norway—Centre for Neural Computation, grant number 223262, the National Infrastructure scheme of the Research Council of Norway—NORBRAIN, grant number 197467, and the Kavli Foundation. We thank Bente Jacobsen for stimulating discussions and comments on the semi‐final version of the manuscript.
ACKNOWLEDGMENTS
Funding provided by CIHR (Operating Grant 133444) to OH.
DATA AVAILABILITY
Requests for the data can be sent to e.maguire@ucl.ac.uk.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for the data can be sent to e.maguire@ucl.ac.uk.


