Erpenbeck VJ, Vets E, Gheyle L, Osuntokun W, Larbig M, Neelakantham S, Sandham D, Dubois G, Elbast W, Goldsmith P, Weiss M. Pharmacokinetics, Safety, and Tolerability of Fevipiprant (QAW039), a Novel CRTh2 Receptor Antagonist: Results From 2 Randomized, Phase 1, Placebo‐Controlled Studies in Healthy Volunteers. Clin Pharmacol Drug Dev. 2016 Jul;5(4):306–13
Explanation of the corrections:
The purity/drug content of the reference standard for the acyl glucuronide metabolite was previously over‐estimated. Consequently, a correction factor of 0.76 was applied to revise these concentrations. These changes do not affect the conclusions or any other findings of the manuscript.
Content of the corrections (changed content in bold):
-
1.Methods
-
PK Assessments and Analysis (Pg 3, Line 32)
- LLOQ for AG‐metabolite was 1.52 ng/mL in plasma and 3.8 ng/mL in urine
-
-
2.Results
-
Pharmacokinetics (Pg 4, Line 19)
- The AUC molar ratios (AG‐metabolite/fevipiprant) close to 1 suggests a comparable exposure to the metabolite (Table 2)
-
-
3.Results
-
Food effect (Pg 4, Line 43)
-
The Ae0–120 molar ratio of metabolite to fevipiprant was0.6–0.9 in fed and fasted states, similar to the 0.5–0.7 ratio in the multiple dose part (Table 3) and confirming the importance of urinary excretion as a route of elimination for both fevipiprant and its glucuronide metabolite and that CLr is unaffected by food
-
-
-
4.Discussion (Pg 6, Line 23)
- Renal clearance was an important route of elimination, with up to one‐third of the dose excreted as fevipiprant in the urine; in addition, AG‐metabolite was found in urine
-
5.Table 2. Summary of Pharmacokinetic (PK) parameters of Fevipiprant (PK Analysis Set) for the Single‐ and Multiple‐Dose Studies (Pg 5)
- AG‐Metabolite/Fevipiprant (AUCtau molar ratiod) values for Fevipiprant Multiple‐Dose Study. These should be:
| |
|
|
|
|
|
|
|
|
AUCtau, AUC in the dosing interval; dAUCtau AG‐metabolite day 1 (μM.h)/AUCtau fevipiprant day 1 (μM.h)
-
6.Table 3. Urine Pharmacokinetic (PK) Parameters for Fevipiprant (PK Analysis Set)
- Molar Ratioa values for Fevipiprant Multiple‐Dose Study. These should be:
| Fevipiprant Multiple‐Dose Study | |
| Dose | Molar ratioa |
| 100 mg once daily | 0.6 (0.08) |
| 300 mg once daily | 0.5 (0.06) |
| 250 mg twice daily | 0.7 (0.09) |
aAetau AG‐metabolite (day 7)/Aetau fevipiprant (day 7)
-
7.
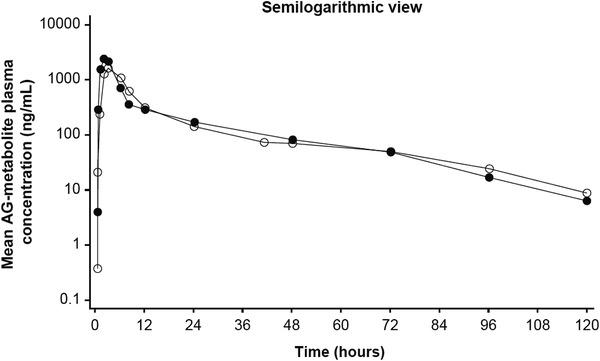
Figure 2. Mean plasma concentration–time profiles of (B) the acyl glucuronide metabolite (AG‐metabolite) on log‐linear scale in fed and fasted states.
Figure 2.