Abstract
Radiation-induced lung injury (RILI) encompasses any lung toxicity induced by radiation therapy (RT) and manifests acutely as radiation pneumonitis and chronically as radiation pulmonary fibrosis. Because most patients with thoracic and breast malignancies are expected to undergo RT in their lifetime, many with curative intent, the population at risk is significant. Furthermore, indications for thoracic RT are expanding given the advent of stereotactic body radiation therapy (SBRT) or stereotactic ablative radiotherapy (SABR) for early-stage lung cancer in nonsurgical candidates as well as oligometastatic pulmonary disease from any solid tumor. Fortunately, the incidence of serious pulmonary complications from RT has decreased secondary to advances in radiation delivery techniques. Understanding the temporal relationship between RT and injury as well as the patient, disease, and radiation factors that help distinguish RILI from other etiologies is necessary to prevent misdiagnosis. Although treatment of acute pneumonitis is dependent on clinical severity and typically responds completely to corticosteroids, accurately diagnosing and identifying patients who may progress to fibrosis is challenging. Current research advances include high-precision radiation techniques, an improved understanding of the molecular basis of RILI, the development of small and large animal models, and the identification of candidate drugs for prevention and treatment.
Key Words: cancer, fibrosis, lung injury, pneumonitis, radiation, thoracic
Abbreviations: HRF, high-risk radiologic feature; IMRT, intensity-modulated radiation therapy; RILI, radiation-induced lung injury; RP, radiation pneumonitis; RPF, radiation pulmonary fibrosis; RT, radiation therapy; SABR, stereotactic ablative radiotherapy; SBRT, stereotactic body radiation therapy; TGF-β, transforming growth factor beta
First discovered as treatment for breast tumors at the beginning of the 1900s, radiation therapy (RT) remains a cornerstone of treatment in both the definitive (ie, curative) and palliative settings for many malignancies. Patients undergoing definitive RT typically have locally advanced, surgically unresectable, or medically inoperable malignancies. For patients in whom surgery or chemotherapy are the definitive treatment modalities, RT is commonly used in the adjuvant/consolidative setting. The goal of RT is to eliminate or reduce tumor growth/burden while sparing normal tissues, a feat facilitated by improvements in imaging techniques, stereotactic RT, and treatment planning. However, the benefits of RT are occasionally complicated by off-target adverse effects.
Radiation-induced lung injury (RILI) is an important dose-limiting factor in radiation directed to the thorax and may primarily affect patients treated for lung cancer, breast cancer, lymphoma, or those who receive total body irradiation as part of a bone marrow transplant.1, 2 In lung cancer, about 200,000 new cases were reported in the US alone in 2017 and up to 80% of these patients had unresectable disease.3, 4 For early-stage disease, stereotactic body radiation therapy (SBRT) or stereotactic ablative radiotherapy (SABR) has emerged as a standard treatment regimen for nonsurgical candidates.5 In patients with locally advanced disease, thoracic radiotherapy with concurrent chemotherapy remains the standard of care for definitive management.6 Furthermore, > 50% of patients with breast or other thoracic malignancies receive RT, and improvements in systemic therapy are likely to only increase the number of patients receiving thoracic RT in the palliative or oligometastatic setting.7, 8 Thus, the overall number of patients at risk of RILI is significant and not limited to lung cancer.
The estimated incidence of RILI varies widely across studies, likely due to varying definitions of clinically significant lung injury and the citation of data prior to modern radiotherapy planning (two-dimensional era). More recent data suggest RILI incidence is highest for lung cancer (5%-25%) followed by mediastinal lymphoma (5%-10%) and breast cancer (1%-5%).9, 10, 11, 12, 13, 14 It is imperative for clinicians to recognize and distinguish RILI from other lung pathologies when evaluating respiratory symptoms in a patient with cancer and a history of thoracic RT. This distinction proves particularly difficult in patients with cancer, whose clinical presentation is often complicated by the presence of other underlying conditions, such as the tumor itself (atelectasis, obstruction, and lymphangitis), drugs (ie, chemotherapy), or infection. In clinical practice, clinicians are often not familiar with the modality of radiation, the differences in dose, delivery technique, and the associated biological effect of RT, factors which may directly correlate with toxicity.
The present review offers current knowledge about the risk factors associated with RILI, outlines the molecular and cellular mechanisms of RILI, and highlights clinically relevant perspectives on the approach to its diagnosis and management.
Pathophysiology
The effect of radiation on the lung was originally described in 1925 by Evans and Leucutia,15 who categorized RILI into an acute injury stage, radiation pneumonitis (RP), and the ensuing chronic injury stage, radiation pulmonary fibrosis (RPF). Although the two stages are interdependent, they can be clearly separated in time: RP occurs within 6 months of therapy (most often within 12 weeks), whereas RPF occurs > 1 year following therapy.2, 16, 17 A temporal biological phenomenon exists, with events proceeding at a molecular level until the development of histopathological abnormalities that define lung injury. The majority of patients will never reach the level of clinically significant RPF; however, fibrosis is believed to be the end point of a continuous progression of events, whereby each phase contributes to the eventual development of irreversible damage.10, 18
The pathophysiology of RP can be described as two distinct mechanisms, “classic” or “sporadic.” Classic RP is well described and historically has been used to describe damage following radiation to large lung volumes.19 The injury occurs “in-field” (eg, within the treatment volume), likely due to the direct cytotoxic action of ionizing radiation on lung cells and can progress to pulmonary fibrosis. A proposed sequence of histologic changes leading to classic pneumonitis was first described in 1968 by Rubin and Casarett20 and later detailed by others.21, 22, 23 This sequence divides the clinical picture into three main phases of radiation response: an early phase, an intermediate phase (acute pneumonitis), and a late phase (pulmonary fibrosis). Immediately following radiation exposure, increased capillary permeability contributes to pulmonary edema. Damage to type I and II pneumocytes leads to loss of surfactant and transudation of serum proteins into the alveoli.
These changes are not visible by light microscopy, and there are no radiographic or clinical signs of damage; thus, the first sequence is known as the “latent phase.” However, electron microscopy has allowed visualization of the degenerative changes to pneumocytes, thickened secretions of mucus from goblet cells, basement membrane swelling, and endothelial cell changes that occur during this phase. Cytokines released from damaged lung cells (eg, tumor necrosis factor alpha) attract inflammatory cells to the alveoli and pulmonary interstitium, inducing the acute-phase pneumonitis.19 Injury in the acute phase seems to be radiation dose-dependent in that higher doses are associated with more severe pneumonitis.24, 25, 26 The late phase constitutes pulmonary fibrosis, which stems from pathological repair following classical RP. Cytokines, growth factors, and reactive oxygen species released by macrophages and other resident lung cells stimulate collagen production by fibroblasts, leading to reductions in lung elasticity and scarring seen on imaging studies. Transforming growth factor beta (TGF-β) is particularly important in stimulating collagen synthesis,27 and thoracic irradiation has been associated with persistently elevated TGF-β levels upon therapy completion.28
Simply, the summarized sequence of classic RP is as follows: cellular injury leads to cytokine release, cytokine recruitment of the inflammatory infiltrate causes acute pneumonitis, and the body’s attempt to repair the injury results in pulmonary fibrosis (Fig 1). However, several questions about the progression of the disease remain, as some investigators note many cases of RP cannot be explained entirely by this process. For example, if there is a dose-dependent response to radiation in the lungs, why is the occurrence and onset of RP unpredictable? Furthermore, in many cases, the severity of dyspnea or radiologically evident lung damage appears out of proportion to the radiation dose or lung volume irradiated. Finally, it is unclear why most patients have complete resolution of symptoms without progression to pulmonary fibrosis. The mechanism for “sporadic” RP was developed to account for cases unexplained through the “classic” mechanism. The most accepted model, proposed by Morgan et al,29 suggests that “sporadic” RP mimics hypersensitivity pneumonitis. BAL from such patients receiving unilateral radiotherapy showed significant lymphocytosis in both lungs composed primarily of CD4+ T cells.30, 31 This effect is more pronounced in patients who develop clinically significant RP. This model of pneumonitis, comparable to hypersensitivity pneumonitis, helps explain cases that fall outside the classic presentation. The recognition of sporadic RP can be particularly difficult for clinicians because it is rare (approximately 10% of cases29), and patients often present with severe dyspnea and/or “out-of-field” radiographic findings that may raise the possibility of other disease processes.
Figure 1.
The pathobiology of radiation pneumonitis and radiation-induced lung injury. Ionizing radiation induces free radicals and DNA damage to promote oxidative stress, vascular damage, and inflammation that manifest during radiation pneumonitis. Persistent inflammation sustains alveolar epithelial and vascular endothelial cell damage and contributes to pathologic changes, including immune cell infiltration, capillary permeability, and pulmonary edema. Prolonged alveolar and vascular damage leads to EMT and/or EndoMT and eventually culminates in fibrotic changes. α-SMA = alpha smooth muscle actin; CTGF = connective tissue growth factor; ECM = extracellular matrix; EMT = epithelial-to-mesenchymal transition; EndoMT = endothelial-to-mesenchymal transition; FGF = fibroblast growth factor; ILs = interleukins; ROS = reactive oxygen species; RNS = reactive nitrogen species; TGF-β = transforming growth factor beta; TNF-α = tumor necrosis factor alpha; VEGF = vascular endothelial growth factor.
Risk Factors
Although most patients receiving thoracic irradiation are at risk for RILI, the presence or absence of several factors may modify their risk (Table 1). For all patients, history of smoking, COPD, and interstitial lung disease are all associated with increased risk.32, 33, 34, 35, 36, 37, 38 In patients with breast cancer, concurrent use of chemotherapy or tamoxifen, older age, chest wall irradiation with electrons, and supraclavicular field treatment are correlated with increased risk.39, 40 In patients with lymphoma, risk of pneumonitis is increased in those treated for relapsed or refractory disease vs those who received consolidation therapy.14 Both induction and concurrent chemotherapy increase the risk of RILI, particularly in chemotherapeutic agents known to cause lung injury.41 In addition, systemic agents, including chemotherapy, immunotherapy, and targeted therapies, have all been implicated in cases of radiation recall pneumonitis. Radiation recall is a poorly understood, unpredictable, acute inflammatory phenomenon in the irradiated field, and in the case of thoracic RT, the patient develops or redevelops RP at a time interval significantly later than expected and secondary to drug administration.42, 43, 44 Historically, doxorubicin, docetaxel/paclitaxel, gemcitabine, and capecitabine are known offenders.45
Table 1.
Risk Factors for Radiation-Induced Lung Injury
| Radiation risk factors |
| % Total lung volume receiving ≥ 20 Gy (V20), ≥ 30% |
| % Total lung volume receiving ≥ 5 Gy (V5), ≥ 65% |
| Mean lung dose, > 20 Gy |
| Absolute volume lung spared >5 Gy (AVS5), < 500 cc |
| Target location, lower lobe |
| Disease risk factors |
| Refractory or relapsed disease (lymphoma) |
| Supraclavicular field (breast cancer) |
| Bulky disease |
| Chemotherapy |
| Re-irradiation |
| Host risk factors |
| Age ≥ 50 y |
| Autoimmune disease |
| Interstitial lung disease |
| Former or current smoker |
| COPD |
In general (but particularly for patients with lung cancer), radiation oncologists attempt to limit RILI by minimizing the volume of lung receiving ≥ 20 Gy or 30 Gy (V20 and V30, respectively), as a higher V20 in particular has been shown to be predictive of RP.9, 46, 47, 48, 49 Higher mean lung doses and irradiation of the lower lung fields also correlate with increased incidence of pneumonitis.24, 48, 50 Given this understanding, the radiation oncologist must balance dose between toxicity and target, the latter of which, if compromised, can yield inferior local tumor control and overall survival in thoracic malignancies.51 Recently, even low doses of radiation, given to a large volume of lung, have been associated with increased risk of acute lung toxicity. One increasingly used metric is the volume of lung receiving > 5 Gy (V5).52 Inversely, the absolute volume of lung spared 5 Gy is labeled AVS5, and higher AVS5 values have been associated with reduced incidence of RILI.46, 53 As radiation dose constraints are defined from clinical end points and the dosimetric data of every patient are maintained by their radiation oncologist, it would be prudent to inquire on these when assessing pretest probability for RILI.
More sophisticated techniques of conformal RT technologies such as intensity-modulated RT (IMRT), volumetric arc radiotherapy (VMAT), SBRT, and stereotactic radiosurgery (SRS) are associated with a lower incidence of RILI compared with standard, three-dimensional conformal RT.54, 55, 56, 57, 58, 59 This finding is likely due to increased precision of radiation delivery to a tumor while sparing surrounding normal tissue (Fig 2). In addition, the versatility of these techniques helps account for patients’ anatomy, breathing pattern, and organ motion to guide the beams’ intensity and direction. All of these modalities are expected to lower the volume and dose of lung irradiated (both associated with risk of RILI9, 24, 60, 61, 62, 63) while maintaining millimeter range accuracy. Furthermore, many centers use daily-image guidance as well as deep inspiratory breath hold or respiratory-gating. In patients with early-stage lung cancer undergoing SBRT, fiducial-based tracking (ie, CyberKnife; Accuray Incorporated) of the tumor may be used to reduce the total treatment volume given increased precision. In theory, charged particle therapy (ie, protons) provides superior dose distributions vs photon therapy, as energy is primarily deposited over a specific depth with essentially no exit dose (Fig 3).64 However, a significant clinical benefit in reducing RP was not seen in a recent clinical trial compared with IMRT.65
Figure 2.
Stereotactic body radiation therapy (SBRT) for a mediastinal lymph node. SBRT allows for high doses of radiation to small volumes of disease. The example presented demonstrates the use of SBRT to treat a site of oligometastatic disease in the mediastinum of a patient with otherwise controlled metastatic lung cancer. A dose-volume histogram (DVH) is also presented that shows the volume by dose of the target and organ at risk (in this case, the lungs). When radiation oncologists evaluate a DVH, the target should span to the upper right corner. Ideally, organs at risk will be to the bottom left corner. A pulmonologist can request the DVH or treatment plan for any patient from the radiation oncologist. SBRT is often used for early-stage lung cancer in patients medically ineligible for surgery and can spare normal tissue by distributing dose to numerous individual beamlets, which converge to an ablative dose at a specified target. The most common mode of delivery is volumetric modulated arc therapy, in which there exists a coplanar arc-beam arrangement. Essentially, as the gantry head rotates around the patient, beams from a complete spectrum of angles and with varying intensity deliver highly conformal dose to the target.
Figure 3.
Conformal radiation techniques (3DCRT vs IMRT vs PSPT). Three cases are demonstrated with respective dose (color wash) utilizing the differing techniques. 3DCRT was the initial form of conformal radiotherapy, which requires beams to be manually arranged with custom blocks using multileaf collimators. IMRT is currently the most utilized technique for modern treatment planning. IMRT allows for an inverse planning computer optimization algorithm to select the optimum configuration of beam arrangements and multileaf collimator positions to produce ideal target dose and organ-sparing based on preset dosimetric goals. PSPT represents the most commonly used form of proton therapy and takes advantage of the lack of exit dose in a proton beam. Of note, IMRT tends to limit higher doses of radiation to normal structures vs 3DCRT, although it can increase low-dose spillage to a high volume of lung if V5 (percent lung volume receiving ≥ 5 Gy) constraint is not accounted for. PSPT in certain cases can completely spare contralateral lung. 3DCRT = three-dimensional conformal radiotherapy; IMRT = intensity-modulated radiation therapy; PSPT = passively scattered proton therapy. (Reprinted from Roelofs et al.64 Copyright [2012], with permission from Elsevier.)
Clinical Assessment
The severity of RP varies from radiographic findings with no clinical symptoms to life-threatening disease requiring hospitalization.18 Two widely used radiation toxicity grading systems, the Radiation Therapy Oncology Group and the Common Terminology Criteria for Adverse Events, categorize patients based on the severity of their pneumonitis (Table 2).66 The most common symptoms are dyspnea, which can be mild to severe, and a dry, nonproductive cough. Low-grade fevers present in < 10% of cases, but high fevers can occur in some patients. Hemoptysis is also a rare symptom and is sometimes reflective of other disease. The physical examination of a patient with RP may be normal; in other cases, evidence of consolidation, a pleural rub, or adventitious lung sounds may indicate disease progression. Dullness to percussion may be detected in patients who develop pleural effusions, but these are typically small, often cause no symptoms, and are distinguishable from malignant effusions as they remain stable in size over time. Clinically significant radiation-induced pulmonary fibrosis typically occurs months to years following therapy and is described as progressive dyspnea associated with lung scarring. Tachypnea and cyanosis are both signs of advanced disease. In both RP and PF, chronic pulmonary insufficiency can occur if the volume of lung damaged is large enough. This insufficiency can result in pulmonary hypertension and may progress to cor pulmonale.18
Table 2.
Radiation Pneumonitis Grading Systems
| Grading System | RTOG | CTCAE |
|---|---|---|
| Grade 1 | Asymptomatic or mild symptoms (dry cough). Slight radiographic appearances | Mild; asymptomatic. Clinical and diagnostic observations only. Intervention not indicated |
| Grade 2 | Moderate symptomatic fibrosis or pneumonitis (severe cough). Low-grade fever. Patchy radiographic appearances | Moderate. Minimal, local, or noninvasive intervention indicated. Limiting age-appropriate instrumental ADL |
| Grade 3 | Severe symptomatic fibrosis or pneumonitis. Dense radiographic changes | Severe or medically significant but not immediately life-threatening. Hospitalization or prolongation of hospitalization indicated. Disabling. Limiting self-care ADL |
| Grade 4 | Severe respiratory insufficiency/continuous oxygen/assisted ventilation | Life-threatening consequences. Urgent intervention indicated |
| Grade 5 | Death related to adverse events | Death related to adverse events |
ADL = activities of daily living; CTCAE = Common Terminology Criteria for Adverse Events; RTOG = Radiation Therapy Oncology Group.
The differential for the vague symptoms presented here is broad and includes infection, sequela from malignancy, COPD, chemotherapy-induced pneumonitis, disease recurrence in the lung, and cardiac disease.67 However, if a patient presents with these symptoms weeks or months following completion of RT, physicians should also be suspicious for RILI and promptly begin a diagnostic evaluation (Fig 4). Generally, RP develops at 4 weeks following conventionally fractionated therapy. Signs of pulmonary infection include a unilateral or bilateral lung opacity appearing prior to completion of radiation, tree-in-bud opacities, and cavitation. RT-related necrosis and local recurrence can also manifest as cavitation; however, these generally occur at a later interval following completion of therapy.68
Figure 4.
Clinical algorithm outlining the assessment and management of RILI. Suspicion of RILI should be initiated when a patient’s physical examination findings correlate temporally (typically within 3 months) with completion of thoracic radiation. RILI = radiation-induced lung injury.
No commonly used laboratory or imaging tests can definitively identify RP, as it is a clinical diagnosis. Some patients may have an elevated WBC count, erythrocyte sedimentation rate, or C-reactive protein, but these findings are nonspecific. A complete blood count with differential is used to evaluate most patients, whereas further laboratory studies may be obtained as needed to evaluate for alternate etiologies. Chest radiograph may be normal in the latent phase of RP, but ground-glass opacities or consolidations in the radiation treatment field reflect later stages of lung injury (Figure 5, Figure 6). Generally, pulmonary opacities develop in areas receiving > 40 Gy and thus correlation can be made with the patient’s radiation treatment plan.68 As the disease progresses to RPF, the chest radiograph may show a defined area of volume loss, scarring, and consolidation.69
Figure 5.
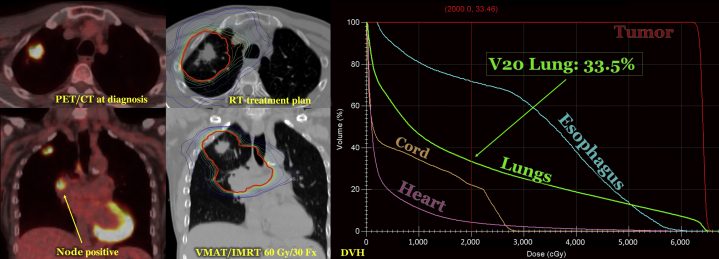
Locally advanced lung cancer treated with definitive chemoradiation. We present a case of locally advanced non-small cell lung cancer in a patient who underwent chemoradiation. The patient had node-positive disease as illustrated by staging PET/CT scan. He received 60 Gy/30 Fx utilizing VMAT/IMRT with daily image guidance. The RT plan with isodose lines (IDLs) is displayed. IDLs are generated to describe where the radiation dose is distributed. The bolded, red IDL represents the prescription line (6,000 cGy). The patient’s DVH is also displayed, which identifies organs-at-risk by displaying dose vs organ volume. In this patient’s case, the lung V20 or volume of lung receiving ≥ 20 Gy is above the 30% constraint. DVH = dose volume histogram; RT = radiation therapy; VMAT = volumetric modulated arc therapy. See Figure 3 legend for expansion of other abbreviation.
Figure 6.
Radiographic appearance of RILI. The previously described patient (Fig 5) developed clinically significant radiation pneumonitis in the form of cough and required a short course of steroids at 6 weeks from the end of radiation (Grade 2). The radiographic findings of radiation pneumonitis at 6 weeks are illustrated on CT imaging (homogeneous ground-glass attenuation) and chest radiograph (linear, reticular stranding). The patient improved clinically following initiation of steroids, with stable radiographic findings. On follow-up, the patient’s mass initially decreased in size (24 months); however, at 48 months, there was significant concern for local recurrence given enlarging soft tissue density arising from the nodule on CT imaging. Results of biopsy and PET/CT imaging were negative for pathologic or metabolic evidence of malignancy. Ultimately, following continued observation, the lung findings improved, and the patient remains without evidence of disease at 5 years. Radiographic findings of progressive fibrosis are displayed over time on CT imaging (right upper lobe traction bronchiectasis, volume loss, and thickened interstitium). A chest radiograph represents radiographic fibrosis (linear fibrosis, scarring, and volume loss). Of note, the radiographic findings in this patient correspond anatomically to the original radiation fields, which is a key finding when making a diagnosis of RILI in the clinically symptomatic patient. At last follow-up, this patient was doing well and has no clinically significant fibrosis. See Figure 4 legend for expansion of abbreviation.
In general, chest CT imaging is the preferred imaging technique over chest radiography, as it is more sensitive in detecting RILI. Homogeneous ground-glass attenuation representing early RP can be detected on CT imaging a few weeks following completion of therapy, even when there are no findings on chest radiograph.25, 70, 71 The appearance of RP on CT imaging often correlates with the phase of lung injury, progressing from ground-glass attenuation in the initial phase, patchy areas of consolidation in later phases, and linear scarring with consolidation and volume loss as pulmonary fibrosis develops.69 For both radiographic modalities, opacities on imaging frequently conform to the radiation treatment field, although this finding is not universal. Additional diagnostic techniques such as bronchoscopy, thoracentesis, and lung biopsy allow differentiation of RP from underlying tumor or infectious pneumonitis and are occasionally necessary to make a more accurate diagnosis of RP. Pulmonary function tests are often used to distinguish RILI from other lung diseases such as COPD. Lung dysfunction from RILI is typically associated with a restrictive pattern with decreased lung volumes, compliance, FVC, and diffusing capacity.72, 73, 74 Bronchoscopy is typically used to evaluate for spread of malignancy, infection, bleeding, or drug hypersensitivity. As described earlier, bronchoscopy with BAL can reveal nonspecific findings such as increased leukocytes and primary CD4+ lymphocytes.75
Evolving postradiation treatment changes may also be mistaken for local disease recurrence or progression months or years later. In fact, it is possible to observe a reduction in the size of a lung nodule/mass followed by radiographic stability, only for it to later enlarge radiographically, secondary to postradiation change. This phenomenon is demonstrated by the case in Figure 6 in which a suspicious enlarging opacity at 48 months was biopsied and revealed notable fibrotic and degenerative lung tissue with chronic focal inflammation but no evidence of malignancy. In these situations, PET/CT imaging is helpful in evaluating for metabolic activity in the treated field prior to biopsy and yields a sensitivity of 98% and specificity of 82% for identifying disease recurrence.76 An endobronchial ultrasound approach is appropriate for central and mediastinal lesions, whereas peripheral lesions generally require CT scan-guided biopsy.77 PET/CT imaging for surveillance and/or biopsy sooner than 6 weeks following definitive radiotherapy may be difficult to interpret because patients may continue to have treatment response in this period.
SBRT/SABR provides a nonsurgical treatment option with excellent local control (approximately 90% at 3 years) and has become standard for patients who are not candidates for or refuse surgery for early-stage lung cancer.5 Clinicians managing these patients should be aware of unique radiographic scar properties that generally form after 3 months and do not necessarily suggest recurrence. This scenario varies from conventionally fractionated radiation in which RT-related radiographic findings generally appear after 4 weeks.68 Several studies have defined these high-risk radiologic features (HRFs) (Table 3), including one recent study evaluating 88 patients without local recurrence for a minimum of 2 years.78 The investigators found that one HRF developed in > 50% and ≥ 3 HRFs developed in nearly 25% of patients undergoing SBRT/SABR. Furthermore, a diagnosis of clinically significant RILI should be avoided in patients with only a radiographic appearance of an SBRT/SABR scar, specifically when clinical correlation to patients’ pulmonary symptoms cannot be established anatomically or temporally. Ultimately, these high-precision techniques target a small volume to an ablative dose while generally sparing the majority of lung tissue from significant radiation; thus, the incidence of symptomatic RILI is low (approximately 10%).79
Table 3.
SBRT/SABR High-Risk Radiologic Features and Frequency in Patients Without Local Recurrence
| SBRT/SABR High-Risk Radiologic Features | % Observeda |
|---|---|
| Enlarging opacity at primary site | 65 |
| Sequential enlarging opacity | 50 |
| Enlarging opacity after 12 mo | 14 |
| Loss of air bronchograms | 4 |
| Loss of linear margins | 2 |
| Cranio-caudal growth | 2 |
| Bulging margin | 2 |
SABR = stereotactic ablative radiotherapy; SBRT = stereotactic body radiation therapy.
Frequency of high-risk radiologic features observed in a retrospective study of 88 patients undergoing SBRT/SABR without local recurrence.78
Diagnosis, ultimately, is based on history of RT with consideration of target, dose, and technique as well as a combination of symptoms, timing, imaging findings, laboratory studies, and exclusion of other causes of lung disease. Evaluation should be started immediately to appropriately treat any underlying pathology responsible for the patient’s symptoms.
Management Strategies
No controlled studies have been conducted to evaluate the role of various therapies in treating RILI in humans. For very mild symptoms, clinical observation can be considered. However, most experts recommend systemic glucocorticoids to treat significantly symptomatic RP,72 provided that lung infection has been ruled out. For example, a treatment course of 1 mg/kg per day of prednisone can be given for 2 to 4 weeks, followed by a slow tapering of the medication for an additional 6 to 12 weeks. Patients may experience marked symptomatic relief, with reduction in cough, chest tightness, dyspnea, and fever, along with resolution of radiographic changes. However, relapse is possible following response to steroids. Single case studies have also reported effectiveness for both azathioprine and cyclosporine.80, 81 However, the only two drugs that have shown efficacy in reducing rates of pneumonitis in humans are amifostine and pentoxifylline in combination with tocopherol. Amifostine, a free radical scavenger, was shown to significantly reduce rates of grade 2 or higher pneumonitis in patients receiving radiation for advanced-stage lung cancer.82 Furthermore, amifostine is currently the only radioprotective agent approved by the US Food and Drug Administration for clinical use. Unfortunately, due to its significant side-effect profile (ie, hypotension and severe nausea) and poor tolerability (especially when administered intravenously), its use is limited in modern practice. Studies involving the subcutaneous use of amifostine in patients with head and neck cancer suggest feasibility and less hypotension, although nausea remains problematic.83 Pentoxifylline inhibits proinflammatory molecules such as tumor necrosis factor alpha and leukotriene and has been shown to significantly prevent pneumonitis in both lung and breast cancer patients.84, 85
There are no established guidelines for the treatment of RPF. The mechanism of pulmonary fibrosis (primarily driven by TGF-β and collagen overproduction, as described earlier) is different from pneumonitis and does not necessarily derive from inflammation; therefore, glucocorticoids and other antiinflammatory agents are not effective and should be avoided to reduce unwanted side effects. For example, clinical studies that evaluated the efficacy of “triple therapy,” which combined the antiinflammatory drugs prednisone and azathioprine, found that these drugs increased the risk of hospitalization and death in patients with established lung fibrosis.86 Unfortunately, there is no effective therapy for established lung fibrosis, and treatment is primarily supportive: supplemental oxygen should be provided for symptomatic relief, and patients should be referred to a pulmonologist with experience treating restrictive lung disease. Several agents are hypothesized to be effective in treating radiation fibrosis but have not been tested in prospective randomized controlled clinical trials. In preclinical studies, angiotensin-converting enzyme inhibitors were shown to reduce fibrosis in irradiated rat lungs,87 and drugs that inhibit collagen synthesis, such as colchicine and interferon-γ, may have efficacy in preventing lung fibrosis or reducing its progression rather than reversing it. Antiinflammatory agents such as corticosteroids and azithromycin have failed to show efficacy clinically in the prevention of RILI despite encouraging outcomes in animal models.88, 89 Recently, interest has surfaced for the multi-kinase inhibitor nintedanib, which targets vascular endothelial growth factor among other growth factors and has been identified as a possible treatment to prevent RP and reduce the incidence of lung fibrosis. Preclinical results have been promising; however, several phase II trials are ongoing.90, 91, 92 Pirfenidone, which downregulates procollagens and growth factors, is another emerging medication, and an early pilot study has suggested subjective improvement in RPF; however, this requires validation in a large, randomized trial.93 Evidence continues to emerge on the efficacy of new chemical entities to prevent and/or treat RP and RPF.94
Future Directions
Irradiated volumes continue to decrease as external beam radiation planning becomes increasingly conformal and spares healthy tissue while maximizing dose delivery to target. In addition, emerging clinical, serologic, and radiographic predictors of lung injury may lead to further personalized and risk-adaptive radiation planning.11 Several promising immunomodulating agents targeting interleukins such as IL-1β, IL-13, and IL-17α and the transcription factor STAT3 have shown promising efficacy in suppressing TGF-β-mediated fibrosis in animal models.95, 96, 97, 98 Other agents such as nebulized synthetic lamellar lipids have also shown promise in preventing RILI in large animal models.99
Well-designed, randomized controlled trials are needed, as RILI remains a significant toxicity in patients undergoing thoracic irradiation in the setting of significant risk factors. Development of objective measures is also needed to properly quantify RILI, as opposed to relying on physician- and patient-reported outcomes. In this regard, several studies have evaluated the use of advanced imaging techniques, which may assist in determining the efficacy of pharmacotherapy in future clinical trials.100, 101, 102 Ideally, radioprotective agents developed for RILI should carry minimal adverse effects and not impair the ability of radiation to kill underlying cancer cells. In addition, such therapeutic agents should be easy to administer (eg, aerosolized or subcutaneous delivery) in a period immediately preceding radiation treatment.
Conclusions
Although it is important to recognize RILI as a possible etiology in the appropriate patient, it should only be considered after other possibilities have been fully exhausted. With modern treatment techniques, the incidence of adverse effects from radiation have declined immensely,103 and it is important to realize that the vast majority of patients receiving thoracic RT may not have significant pulmonary toxicity requiring medical intervention. Furthermore, patients undergoing RT outside the thorax are not at risk for RILI. Benefits of consultation with the treating radiation oncologist to obtain precise details regarding lung volumes irradiated and perceived clinical risk cannot be understated.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
References
- 1.Bracci S., Valeriani M., Agolli L., De Sanctis V., Maurizi Enrici R., Osti M.F. Renin-angiotensin system inhibitors might help to reduce the development of symptomatic radiation pneumonitis after stereotactic body radiotherapy for lung cancer. Clin Lung Cancer. 2016;17(3):189–197. doi: 10.1016/j.cllc.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Bradley J., Movsas B. Radiation Toxicity: A Practical Guide. Springer US; Boston, MA: 2008. Radiation pneumonitis and esophagitis in thoracic irradiation; pp. 43–64. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Schaake-Koning C., van den Bogaert W., Dalesio O. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992;326(8):524–530. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]
- 5.Chang J.Y., Senan S., Paul M.A. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ettinger D.S., Wood D.E., Aisner D.L. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 7.Madan R., Benson R., Sharma D.N., Julka P.K., Rath G.K. Radiation induced heart disease: pathogenesis, management and review literature. J Egypt Natl Canc Inst. 2015;27(4):187–193. doi: 10.1016/j.jnci.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Palma D.A., Olson R.A., Harrow S. Stereotactic Ablative Radiation Therapy for the Comprehensive Treatment of Oligometastatic Tumors (SABR-COMET): results of a randomized trial. Int J Radiat Oncol. 2018;102(3):S3–S4. [Google Scholar]
- 9.Marks L.B., Bentzen S.M., Deasy J.O. Radiation dose-volume effects in the lung. Int J Radiat Oncol. 2010;76(3):S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsoutsou P.G., Koukourakis M.I. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol. 2006;66(5):1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 11.Ebert N., Baumann M., Troost E.G.C. Radiation-induced lung damage—clinical risk profiles and predictive imaging on their way to risk-adapted individualized treatment planning? Radiother Oncol. 2015;117(1):1–3. doi: 10.1016/j.radonc.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Marks L., Yu X., Vujaskovic Z., Smalljr W., Folz R., Anscher M. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13(3):333–345. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 13.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol. 2005;63(1):5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Pinnix C.C., Smith G.L., Milgrom S. Predictors of radiation pneumonitis in patients receiving intensity modulated radiation therapy for Hodgkin and non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2015;92(1):175–182. doi: 10.1016/j.ijrobp.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans W.A., Leucutia T. Intrathoracic changes induced by heavy radiation. Am J Roentgenol. 1925;13:203–220. [Google Scholar]
- 16.Yarnold J., Vozenin Brotons M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97(1):149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Bernchou U., Schytte T., Bertelsen A., Bentzen S.M., Hansen O., Brink C. Time evolution of regional CT density changes in normal lung after IMRT for NSCLC. Radiother Oncol. 2013;109(1):89–94. doi: 10.1016/j.radonc.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Graves P.R., Siddiqui F., Anscher M.S., Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20(3):201–207. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Abratt R.P., Morgan G.W., Silvestri G., Willcox P. Pulmonary complications of radiation therapy. Clin Chest Med. 2004;25:167–177. doi: 10.1016/S0272-5231(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 20.Rubin P., Casarett G.W. Clinical radiation pathology as applied to curative radiotherapy. Cancer. 1968;22(4):767–778. doi: 10.1002/1097-0142(196810)22:4<767::aid-cncr2820220412>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Coggle J.E., Lambert B.E., Moores S.R. Radiation effects in the lung. Environ Health Perspect. 1986;70:261. doi: 10.1289/ehp.8670261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross N.J. Pulmonary effects of radiation therapy. Ann Intern Med. 1977;86(1):81–92. doi: 10.7326/0003-4819-86-1-81. [DOI] [PubMed] [Google Scholar]
- 23.Gross N.J. The pathogenesis of radiation-induced lung damage. Lung. 1981;159(3):115–125. doi: 10.1007/BF02713907. [DOI] [PubMed] [Google Scholar]
- 24.Kwa S.L., Lebesque J.V., Theuws J.C. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42(1):1–9. doi: 10.1016/s0360-3016(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 25.Mah K., Van Dyk J., Keane T., Poon P.Y. Acute radiation-induced pulmonary damage: a clinical study on the response to fractionated radiation therapy. Int J Radiat Oncol Biol Phys. 1987;13(2):179–188. doi: 10.1016/0360-3016(87)90125-8. [DOI] [PubMed] [Google Scholar]
- 26.Hernando M.L., Marks L.B., Bentel G.C. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):650–659. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 27.Fine A., Goldstein R.H. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987;262(8):3897–3902. [PubMed] [Google Scholar]
- 28.Anscher M.S., Kong F.M., Andrews K. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1998;41(5):1029–1035. doi: 10.1016/s0360-3016(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 29.Morgan G.W., Pharm B., Breit S.N. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol. 1995;31(2):361–369. doi: 10.1016/0360-3016(94)00477-3. [DOI] [PubMed] [Google Scholar]
- 30.Roberts C.M., Foulcher E., Zaunders J.J. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med. 1993;118(9):696–700. doi: 10.7326/0003-4819-118-9-199305010-00006. [DOI] [PubMed] [Google Scholar]
- 31.Molls M., van Beuningen D. Springer; Berlin, Heidelberg, Germany: 1991. Radiation Injury of the Lung: Experimental Studies, Observations After Radiotherapy and Total Body Irradiation Prior to Bone Marrow Transplantation; pp. 369–404. [Google Scholar]
- 32.Johansson S., Bjermer L., Franzen L., Henriksson R. Effects of ongoing smoking on the development of radiation-induced pneumonitis in breast cancer and oesophagus cancer patients. Radiother Oncol. 1998;49(1):41–47. doi: 10.1016/s0167-8140(98)00064-4. [DOI] [PubMed] [Google Scholar]
- 33.Takeda A., Kunieda E., Ohashi T. Severe COPD is correlated with mild radiation pneumonitis following stereotactic body radiotherapy. Chest. 2012;141(4):858–866. doi: 10.1378/chest.11-1193. [DOI] [PubMed] [Google Scholar]
- 34.Yirmibesoglu E., Higginson D.S., Fayda M. Challenges scoring radiation pneumonitis in patients irradiated for lung cancer. Lung Cancer. 2012;76(3):350–353. doi: 10.1016/j.lungcan.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palma D., Lagerwaard F., Rodrigues G., Haasbeek C., Senan S. Curative treatment of stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys. 2012;82(3):1149–1156. doi: 10.1016/j.ijrobp.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Ueki N., Matsuo Y., Togashi Y. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol. 2015;10(1):116–125. doi: 10.1097/JTO.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 37.Chen H., Senan S., Nossent E.J. Treatment-related toxicity in patients with early-stage non-small cell lung cancer and coexisting interstitial lung disease: a systematic review. Int J Radiat Oncol Biol Phys. 2017;98(3):622–631. doi: 10.1016/j.ijrobp.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Bahig H., Filion E., Vu T. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Pract Radiat Oncol. 2016;6(5):367174. doi: 10.1016/j.prro.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Jeba J., Isiah R., Subhashini J., Backianathan S., Thangakunam B., Christopher D.J. Radiation pneumonitis after conventional radiotherapy for breast cancer: a prospective study. J Clin Diagn Res. 2015;9(7):XC01–XC05. doi: 10.7860/JCDR/2015/13969.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bentzen S., Skoczylas J., Overgaard M., Overgaard J. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst. 1996;88(13):918–922. doi: 10.1093/jnci/88.13.918. [DOI] [PubMed] [Google Scholar]
- 41.Vogelius I.R., Bentzen S.M. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51(8):975–983. doi: 10.3109/0284186X.2012.718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibaki R., Akamatsu H., Fujimoto M., Koh Y., Yamamoto N. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann Oncol. 2017;28(6):1404–1405. doi: 10.1093/annonc/mdx115. [DOI] [PubMed] [Google Scholar]
- 43.Faiz S.A., Balachandran D.D., Bashoura L., Shannon V.R. Pulmonary radiation recall induced by gemcitabine. Am J Respir Crit Care Med. 2016;194(7):909–910. doi: 10.1164/rccm.201606-1235IM. [DOI] [PubMed] [Google Scholar]
- 44.Togashi Y., Masago K., Mishima M., Fukudo M., Inui K. A case of radiation recall pneumonitis induced by erlotinib, which can be related to high plasma concentration. J Thorac Oncol. 2010;5(6):924–925. doi: 10.1097/JTO.0b013e3181dab0dd. [DOI] [PubMed] [Google Scholar]
- 45.Burris H.A., Hurtig J., Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15(11):1227–1237. doi: 10.1634/theoncologist.2009-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsujino K., Hashimoto T., Shimada T. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9(7):983–990. doi: 10.1097/JTO.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 47.Ramella S., Trodella L., Mineo T.C. Adding ipsilateral V20 and V30 to conventional dosimetric constraints predicts radiation pneumonitis in stage IIIA-B NSCLC treated with combined-modality therapy. Int J Radiat Oncol. 2010;76(1):110–115. doi: 10.1016/j.ijrobp.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 48.Palma D.A., Senan S., Tsujino K. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85(2):444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen G., Tan Y.T., Lan X.W. New clinical features and dosimetric predictor identification for symptomatic radiation pneumonitis after tangential irradiation in breast cancer patients. J Cancer. 2017;8(18):3795–3802. doi: 10.7150/jca.21158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks L.B., Bentzen S.M., Deasy J.O. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2013;76(suppl 3):S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aupérin A., Péchoux C Le, Rolland E. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 52.Bernard M.E., Glaser S.M., Gill B.S. Results of a single institution experience with dose-escalated chemoradiation for locally advanced unresectable non-small cell lung cancer. Front Oncol. 2017;7:1. doi: 10.3389/fonc.2017.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J., Hong J., Zou X. Association between absolute volumes of lung spared from low-dose irradiation and radiation-induced lung injury after intensity-modulated radiotherapy in lung cancer: a retrospective analysis. J Radiat Res. 2015;56(6):rrv057. doi: 10.1093/jrr/rrv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chun S.G., Hu C., Choy H. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pang Q., Wei Q., Xu T. Functional promoter variant rs2868371 of HSPB1 is associated with risk of radiation pneumonitis after chemoradiation for non-small cell lung cancer. Int J Radiat Oncol. 2013;85(5):1332–1339. doi: 10.1016/j.ijrobp.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Okubo K., Gotoh M., Asako M. Efficacy and safety of bilastine in Japanese patients with perennial allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase III study. Allergol Int. 2017;66(1):97–105. doi: 10.1016/j.alit.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Verma V., Shostrom V.K., Zhen W. Influence of fractionation scheme and tumor location on toxicities after stereotactic body radiation therapy for large (≥5 cm) non-small cell lung cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;97(4):778–785. doi: 10.1016/j.ijrobp.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verma V., Simone C.B., Allen P.K. Multi-institutional experience of stereotactic ablative radiation therapy for stage I small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97(2):362–371. doi: 10.1016/j.ijrobp.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao J., Yorke E.D., Li L. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: a pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys. 2016;95(5):1357–1366. doi: 10.1016/j.ijrobp.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marks L.B., Yorke E.D., Jackson A. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(suppl 3):S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim T.H., Cho K.H., Pyo H.R. Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology. 2005;235(1):208–215. doi: 10.1148/radiol.2351040248. [DOI] [PubMed] [Google Scholar]
- 62.Carruthers S.A., Wallington M.M. Total body irradiation and pneumonitis risk: a review of outcomes. Br J Cancer. 2004;90(11):2080–2084. doi: 10.1038/sj.bjc.6601751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fay M., Tan A., Fisher R., Manus M Mac, Wirth A., Ball D. Dose-volume histogram analysis as predictor of radiation pneumonitis in primary lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61(5):1355–1363. doi: 10.1016/j.ijrobp.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 64.Roelofs E., Engelsman M., Rasch C. Results of a multicentric in silico clinical trial (ROCOCO): comparing radiotherapy with photons and protons for non-small cell lung cancer. J Thorac Oncol. 2012;7(1):165–176. doi: 10.1097/JTO.0b013e31823529fc. [DOI] [PubMed] [Google Scholar]
- 65.Liao Z., Lee J.J., Komaki R. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(18):1813–1822. doi: 10.1200/JCO.2017.74.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang Y., Yuan L., Wu Q., Yin F., Ge Y. Normal tissue toxicity criteria in radiation therapy. Int J Radiat Oncol. 2013;87(2):S621–S622. [Google Scholar]
- 67.Kocak Z., Evans E.S., Zhou S.M. Challenges in defining radiation pneumonitis in patients with lung cancer. Int J Radiat Oncol. 2005;62(3):635–638. doi: 10.1016/j.ijrobp.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 68.Benveniste M.F., Gomez D., Viswanathan C. Lung cancer: posttreatment imaging: radiation therapy and imaging findings. J Thorac Imaging. 2017;32(5):288–299. doi: 10.1097/RTI.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 69.Choi Y.W., Munden R.F., Erasmus J.J. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. Radiographics. 2004;24(4):985–997. doi: 10.1148/rg.244035160. [DOI] [PubMed] [Google Scholar]
- 70.Bell J., McGivern D., Bullimore J., Hill J., Davies E.R., Goddard P. Diagnostic imaging of post-irradiation changes in the chest. Clin Radiol. 1988;39(2):109–119. doi: 10.1016/s0009-9260(88)80003-5. [DOI] [PubMed] [Google Scholar]
- 71.Libshitz H.I., Southard M.E. Complications of radiation therapy: the thorax. Semin Roentgenol. 1974;9(1):41–49. doi: 10.1016/0037-198x(74)90008-x. [DOI] [PubMed] [Google Scholar]
- 72.Bledsoe T.J., Nath S.K., Decker R.H. Radiation pneumonitis. Clin Chest Med. 2017;38(2):201–208. doi: 10.1016/j.ccm.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Borst G.R., Jaeger K De, Belderbos J.S.A., Burgers S.A., Lebesque J.V. Pulmonary function changes after radiotherapy in non-small-cell lung cancer patients with long-term disease-free survival. Int J Radiat Oncol Biol Phys. 2005;62(3):639–644. doi: 10.1016/j.ijrobp.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 74.Stone B., Mangona V.S., Johnson M.D., Ye H., Grills I.S. Changes in pulmonary function following image-guided stereotactic lung radiotherapy: neither lower baseline nor post-SBRT pulmonary function are associated with worse overall survival. J Thorac Oncol. 2015;10(12):1762–1769. doi: 10.1097/JTO.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 75.Morgan G. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research: in regard to Tsoutsou and Koukourkis (Int J Radiat Oncol Biol Phys 2006;66:1281–1293) Int J Radiat Oncol. 2007;68(5):1581. doi: 10.1016/j.ijrobp.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 76.Volpi S., Ali J.M., Tasker A., Peryt A., Aresu G., Coonar A.S. The role of positron emission tomography in the diagnosis, staging and response assessment of non-small cell lung cancer. Ann Transl Med. 2018;6(5):95. doi: 10.21037/atm.2018.01.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tuzi A., Bolzacchini E., Suter M.B. Biopsy and re-biopsy in lung cancer: the oncologist requests and the role of endobronchial ultrasounds transbronchial needle aspiration. J Thorac Dis. 2017;9(suppl 5):S405–S409. doi: 10.21037/jtd.2017.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ronden M.I., van Sörnsen de Koste J.R., Johnson C. Incidence of high-risk radiologic features in patients without local recurrence after stereotactic ablative radiation therapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2018;100(1):115–121. doi: 10.1016/j.ijrobp.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 79.Baker R., Han G., Sarangkasiri S. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol. 2013;85(1):190–195. doi: 10.1016/j.ijrobp.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 80.McCarty M.J., Lillis P., Vukelja S.J. Azathioprine as a steroid-sparing agent in radiation pneumonitis. Chest. 1996;109(5):1397–1400. doi: 10.1378/chest.109.5.1397. [DOI] [PubMed] [Google Scholar]
- 81.Muraoka T., Bandoh S., Fujita J. Corticosteroid refractory radiation pneumonitis that remarkably responded to cyclosporin A. Intern Med. 2002;41(9):730–733. doi: 10.2169/internalmedicine.41.730. [DOI] [PubMed] [Google Scholar]
- 82.Antonadou D., Coliarakis N., Synodinou M. Randomized phase III trial of radiation treatment ± amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol. 2001;51(4):915–922. doi: 10.1016/s0360-3016(01)01713-8. [DOI] [PubMed] [Google Scholar]
- 83.Ozsahin M., Betz M., Matzinger O. Feasibility and efficacy of subcutaneous amifostine therapy in patients with head and neck cancer treated with curative accelerated concomitant-boost radiation therapy. Arch Otolaryngol Neck Surg. 2006;132(2):141. doi: 10.1001/archotol.132.2.141. [DOI] [PubMed] [Google Scholar]
- 84.Ozturk B., Egehan I., Atavci S., Kitapci M. Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int J Radiat Oncol Biol Phys. 2004;58(1):213–219. doi: 10.1016/s0360-3016(03)01444-5. [DOI] [PubMed] [Google Scholar]
- 85.Delanian S., Porcher R., Balla-Mekias S., Lefaix J.L. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21(13):2545–2550. doi: 10.1200/JCO.2003.06.064. [DOI] [PubMed] [Google Scholar]
- 86.Idiopathic Pulmonary Fibrosis Clinical Research Network. Raghu G., Anstrom K.J., King T.E., Lasky J.A., Martinez F.J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ward W.F., Kim Y.T., Molteni A., Solliday N.H. Radiation-induced pulmonary endothelial dysfunction in rats: modification by an inhibitor of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys. 1988;15(1):135–140. doi: 10.1016/0360-3016(88)90357-4. [DOI] [PubMed] [Google Scholar]
- 88.Kwok E., Chan C.K. Corticosteroids and azathioprine do not prevent radiation-induced lung injury. Can Respir J. 1998;5(3):211–214. doi: 10.1155/1998/896131. [DOI] [PubMed] [Google Scholar]
- 89.Tang F., Li R., Xue J. Azithromycin attenuates acute radiation-induced lung injury in mice. Oncol Lett. 2017;14(5):5211–5220. doi: 10.3892/ol.2017.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Ruysscher D., Granton P.V., Lieuwes N.G. Nintedanib reduces radiation-induced microscopic lung fibrosis but this cannot be monitored by CT imaging: a preclinical study with a high precision image-guided irradiator. Radiother Oncol. 2017;124(3):482–487. doi: 10.1016/j.radonc.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 91.ClinicalTrials.gov. NCT02452463. Nintedanib Compared With Placebo in Treating Against Radiation-Induced Pneumonitis in Patients With Non-small Cell Lung Cancer That Cannot Be Removed by Surgery and Are Undergoing Chemoradiation Therapy, https://clinicaltrials.gov/ct2/show/NCT02452463, 05/22/2015, 3/10/2019.
- 92.ClinicalTrials.gov. NCT02496585. Study to Evaluate the Efficacy and Safety of Nintedanib (BIBF 1120) + Prednisone Taper in Patients With Radiation Pneumonitis, https://clinicaltrials.gov/ct2/show/NCT02496585, 7/14/2015, 3/10/2019.
- 93.Simone N.L., Soule B.P., Gerber L. Oral pirfenidone in patients with chronic fibrosis resulting from radiotherapy: a pilot study. Radiat Oncol. 2007;2(1):19. doi: 10.1186/1748-717X-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kainthola A., Haritwal T., Tiwari M. Immunological aspect of radiation-induced pneumonitis, current treatment strategies, and future prospects. Front Immunol. 2017;8:506. doi: 10.3389/fimmu.2017.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang C., Zeng W., Yao Y. Naringenin ameliorates radiation-induced lung injury by lowering IL-1 β level. J Pharmacol Exp Ther. 2018;366(2):341–348. doi: 10.1124/jpet.118.248807. [DOI] [PubMed] [Google Scholar]
- 96.Chung S.I., Horton J.A., Ramalingam T.R. IL-13 is a therapeutic target in radiation lung injury. Sci Rep. 2016;6(1):39714. doi: 10.1038/srep39714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang B.Z., Wang L.P., Han H. Interleukin-17A antagonist attenuates radiation-induced lung injuries in mice. Exp Lung Res. 2014;40(2):77–85. doi: 10.3109/01902148.2013.872210. [DOI] [PubMed] [Google Scholar]
- 98.Yu J., Yuan X., Liu Y. Delayed administration of WP1066, an STAT3 inhibitor, ameliorates radiation-induced lung injury in mice. Lung. 2016;194(1):67–74. doi: 10.1007/s00408-015-9821-8. [DOI] [PubMed] [Google Scholar]
- 99.Collie D., Murchison J.T., Wright S.H. Nebulisation of synthetic lamellar lipids mitigates radiation-induced lung injury in a large animal model. Sci Rep. 2018;8(1):13316. doi: 10.1038/s41598-018-31559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carter C.L., Jones J.W., Barrow K. A MALDI-MSI approach to the characterization of radiation-induced lung injury and medical countermeasure development. Health Phys. 2015;109(5):466–478. doi: 10.1097/HP.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Troost E.G.C., Thorwarth D., Oyen W.J.G. Imaging-based treatment adaptation in radiation oncology. J Nucl Med. 2015:1–26. doi: 10.2967/jnumed.115.162529. [DOI] [PubMed] [Google Scholar]
- 102.Ghobadi G., Wiegman E.M., Langendijk J.A., Widder J., Coppes R.P., Van Luijk P. A new CT-based method to quantify radiation-induced lung damage in patients. Radiother Oncol. 2015;117(1):4–8. doi: 10.1016/j.radonc.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 103.Toma C.L., Ciprut T., Bugarin S., Roşca D., Bogdan M.A. Radiation induced lung injuries secondary to radiotherapy for breast cancer [in Romanian] Pneumologia. 2011;60(1):40–46. [PubMed] [Google Scholar]