Summary
Background
People who inject drugs (PWID) are at increased risk for HIV and hepatitis C virus (HCV) infection and also have high levels of homelessness and unstable housing. We assessed whether homelessness or unstable housing is associated with an increased risk of HIV or HCV acquisition among PWID compared with PWID who are not homeless or are stably housed.
Methods
In this systematic review and meta-analysis, we updated an existing database of HIV and HCV incidence studies published between Jan 1, 2000, and June 13, 2017. Using the same strategy as for this existing database, we searched MEDLINE, Embase, and PsycINFO for studies, including conference abstracts, published between June 13, 2017, and Sept 14, 2020, that estimated HIV or HCV incidence, or both, among community-recruited PWID. We only included studies reporting original results without restrictions to study design or language. We contacted authors of studies that reported HIV or HCV incidence, or both, but did not report on an association with homelessness or unstable housing, to request crude data and, where possible, adjusted effect estimates. We extracted effect estimates and pooled data using random-effects meta-analyses to quantify the associations between recent (current or within the past year) homelessness or unstable housing compared with not recent homelessness or unstable housing, and risk of HIV or HCV acquisition. We assessed risk of bias using the Newcastle-Ottawa Scale and between-study heterogeneity using the I2 statistic and p value for heterogeneity.
Findings
We identified 14 351 references in our database search, of which 392 were subjected to full-text review alongside 277 studies from our existing database. Of these studies, 55 studies met inclusion criteria. We contacted the authors of 227 studies that reported HIV or HCV incidence in PWID but did not report association with the exposure of interest and obtained 48 unpublished estimates from 21 studies. After removal of duplicate data, we included 37 studies with 70 estimates (26 for HIV; 44 for HCV). Studies originated from 16 countries including in North America, Europe, Australia, east Africa, and Asia. Pooling unadjusted estimates, recent homelessness or unstable housing was associated with an increased risk of acquiring HIV (crude relative risk [cRR] 1·55 [95% CI 1·23–1·95; p=0·0002]; I2= 62·7%; n=17) and HCV (1·65 [1·44–1·90; p<0·0001]; I2= 44·8%; n=28]) among PWID compared with those who were not homeless or were stably housed. Associations for both HIV and HCV persisted when pooling adjusted estimates (adjusted relative risk for HIV: 1·39 [95% CI 1·06–1·84; p=0·019]; I2= 65·5%; n=9; and for HCV: 1·64 [1·43–1·89; p<0·0001]; I2= 9·6%; n=14). For risk of HIV acquisition, the association for unstable housing (cRR 1·82 [1·13–2·95; p=0·014]; n=5) was higher than for homelessness (1·44 [1·13–1·83; p=0·0036]; n=12), whereas no difference was seen between these outcomes for risk of HCV acquisition (1·72 [1·48–1·99; p<0·0001] for unstable housing, 1·66 [1·37–2·00; p<0·0001] for homelessness).
Interpretation
Homelessness and unstable housing are associated with increased risk of HIV and HCV acquisition among PWID. Our findings support the development of interventions that simultaneously address homelessness and unstable housing and HIV and HCV transmission in this population.
Funding
National Institute for Health Research, National Institute on Drug Abuse, National Institute of Allergy and Infectious Diseases, and Commonwealth Scholarship Commission.
Introduction
Globally, HIV and viral hepatitis are leading causes of mortality,1, 2 with people who inject drugs (PWID) being highly susceptible to HIV and hepatitis C virus (HCV) infection.3, 4, 5, 6 Over 2018–30, an estimated 43% of global HCV transmission is projected to be attributed to unsafe injecting practices among PWID.6 Approximately 8% of new HIV infections globally and 20% outside sub-Saharan Africa occur among PWID.5 Although effective prevention and treatment interventions exist for reducing the transmission of HIV and HCV among PWID,7, 8, 9, 10, 11 coverage remains low globally.12 Additionally, PWID are usually exposed to multiple adverse environments (eg, incarceration and homelessness) that can increase their risk of HIV and HCV infection.13, 14, 15, 16 Homelessness, defined by the Institute of Global Homelessness as lacking access to adequate housing, and unstable housing, typically defined as being without fixed housing, are widely acknowledged as important risk factors for acquisition of HCV or HIV infection,13, 14, 15, 16 and have been implicated in a series of HIV outbreaks among PWID over the past 10 years.17
Research in context.
Evidence before this study
We searched PubMed, with no language restrictions, for publications since database inception up to July 9, 2020, using the terms (“HIV” OR “hepatitis C” OR “HCV”) AND (“homelessness” OR “unstable housing”) AND (“inject drugs” OR “injecting drug” OR “substance abuse” OR “intravenous/epidemiology [MeSH]” OR “substance-related disorders/epidemiology [MeSH]”). We identified studies that suggested that recent homelessness or unstable housing was negatively associated with initiation and adherence to opioid substitution treatment (OST; eight studies), use of needle-syringe programmes (two studies), initiation and adherence to HIV and hepatitis C virus (HCV) treatment and their outcomes (20 studies), cessation of injecting drug use (three studies), and access to primary care (one study). Conversely, recent homelessness or unstable housing was positively associated with prevalent HIV and HCV infection (18 studies), stimulant injecting (ten studies), increased injecting frequency (six studies), increased duration of injecting (one study), exposure to physical and sexual violence (nine studies), use of emergency services and admission to hospital (six studies), overdose (three studies), and mortality (three studies). Our search also identified studies that reported a positive association between homelessness or unstable housing and unemployment (two studies), food insecurity (one study), incarceration (three studies), high-risk sexual behaviours such as commercial sex (15 studies), and high-risk injecting practices such as receptive syringe sharing and public injecting (27 studies). We identified a systematic review on factors associated with injecting-related risk behaviours among people who inject drugs (PWID) that implicated homelessness and unstable housing as important determinants of risky injecting practices, such as increased injecting frequency, public injecting, syringe sharing, and stimulant injecting. Additionally, we found a systematic review of factors associated with HIV treatment adherence among PWID, which identified homelessness or unstable housing as a substantial structural barrier to adherence. We also found several studies linking homelessness or unstable housing with incident HIV and HCV infection or reinfection among PWID (21 studies). We also identified a study in which PWID who experienced increased housing stability over time had lower injecting frequency than those with decreasing housing stability. Furthermore, we identified a systematic review of outcomes associated with participation in Housing First programmes, which suggests that housing interventions are associated with reduced high-risk sexual behaviours and improved treatment outcomes among homeless populations with HIV.
Added value of this study
To our knowledge, this is the first systematic review and meta-analysis on the effect of recent homelessness or unstable housing on risk of HIV and HCV acquisition among PWID compared with PWID who are not homeless or who are stably housed. Our study also builds on previously published evidence through collating and pooling unpublished estimates, obtained by contacting authors of all identified studies of PWID that reported a measure of HIV or HCV incidence but not on our outcomes of interest. This resulted in an additional 21 studies being included in our review, nearly tripling the overall number of estimates included. We found that recent homelessness or unstable housing (current, or past 1–12 months) was associated with an increased risk of HIV and HCV acquisition and these associations mostly persisted in sensitivity analyses.
Implications of all the available evidence
Our review supports increasing evidence on the deleterious effect of housing instability on the health and social outcomes of PWID, specifically increased risk of HIV and HCV acquisition. Homeless and unstably housed PWID often face profound disadvantages and have multiple competing priorities. Therefore, a comprehensive approach that not only provides housing but also addresses many of the interlinked health and social concerns of this population is necessary to reduce HIV and HCV risk. Further research is needed to better understand how homelessness and unstable housing increases the risk of HIV and HCV acquisition, and what interventions could most effectively reduce this risk. This research would guide policies to help PWID attain and maintain housing stability.
Homelessness or unstable housing is a well established determinant of poor health outcomes and excess mortality,18, 19, 20, 21 affecting many PWID. Globally, an estimated 22% of PWID report experiencing homelessness or unstable housing in the past year.4 Relative to PWID living in stable housing, those who are homeless or unstably housed are more likely to engage in high-risk behaviours associated with HIV and HCV transmission, such as sex work, public injecting, and sharing of injection equipment.22, 23, 24 They also experience barriers to accessing drug addiction treatment25, 26 and HIV and HCV prevention and care.23, 27, 28 Studies exploring the perceptions of PWID around high-risk behaviours for HCV infection have further highlighted the detrimental effect of homelessness.29
Although multiple studies have reported associations between homelessness or unstable housing and incident HIV and HCV infection among PWID,30, 31, 32, 33, 34 to date, no systematic review has synthesised these data. We did a systematic review and meta-analysis to quantify the associations between homelessness or unstable housing and the risk of HIV and HCV acquisition among PWID.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we updated an existing database of HIV and HCV incidence studies published between Jan 1, 2000, and June 13, 2017. This reference database was compiled during a previous systematic review and meta-analysis.35 Using the same strategy developed previously,35 CA did a systematic literature search of MEDLINE, Embase, and PsycINFO for studies published between June 13, 2017, and Sept 14, 2020, including conference abstracts, without language restrictions. We used terms related to HIV infection, HCV infection, injecting drug use, and study designs that could be used to estimate incidence of HIV or HCV (a full list of search terms is in the appendix [pp 7–8]). We restricted our analysis to studies done among community-recruited (ie, not recruited in prisons) PWID (those with history of recent or ever injecting drug use) that assessed HIV or HCV incidence. We included studies if they had already assessed or were able to assess whether or not an association existed between recent homelessness or unstable housing and HIV or HCV incidence compared with PWID who were not recently homeless or were stably housed. We allowed for study-level differences in the definition of homelessness and unstable housing, and the timeframe definition of recent, which varied from currently to the past 1–12 months. We included studies that measured HIV or HCV incidence through longitudinal follow-up and testing or biological markers of recent infection (eg, anti-HCV avidity and BED assays for HCV and HIV).33, 36 We only included studies reporting original results without restrictions to design or language. We contacted authors of studies (including those published as conference abstracts) that reported estimates of HIV and HCV incidence, but did not report on the association with homelessness or unstable housing, to request data. We also contacted the lead investigators of other ongoing HIV and HCV incidence cohorts to request data. We requested crude and, where possible, adjusted effect estimates (preferably in the form of hazard ratios [HRs] and adjusting for opioid substitution therapy [OST] exposure, recent incarceration, and stimulant injecting), as done previously.7, 8, 35 Throughout this Article, we use the term unpublished estimates to refer to estimates that we calculated for this study from raw data obtained by contacting authors. Although these estimates of associations between homelessness or unstable housing and HIV or HCV acquisition had not been previously presented, the vast majority were calculated using raw data from studies with published estimates of HIV or HCV incidence. For these unpublished estimates, we cite the most recently published article that was based on the same cohort. Some of the cohorts included in this systematic review and meta-analysis extend over long periods of time. Estimates derived from the same cohort but based on different durations of follow-up were identified on the basis of matching study names, settings, or authors, or a combination of these. To avoid publication bias, we included only one estimate, retaining the one with the most person-years of follow-up overall.
We created an Endnote library (version X9) to catalogue the search results and to de-duplicate references. After removal of duplicates, CA screened the titles and abstracts of the studies to identify papers or reports that might contain relevant information. After finding discrepancies during an initial double-screening of a random 10% of studies, HF, JS, SB, AT, JGW, AGL, LM, JJO, and ZW double-screened all the titles and abstracts, with disagreements resolved by group discussion. CA screened the full texts of potentially relevant records to identify those that met the inclusion criteria. HF, JS, SB, AT, JGW, AGL, LM, JJO, and ZW double-screened these records with discrepancies resolved by group discussion. We used Google Translate to read non-English language papers.
This study is reported in accordance with PRISMA guidelines37 (appendix pp 1–2). Details of the methods were prespecified and documented in a protocol (appendix pp 3–6). No deviations from the protocol occurred.
Data analysis
We sought unadjusted and adjusted summary estimates for our main analysis, as well as summaries of participant-level and study-level characteristics for our subgroup analyses, meta-regression, and sensitivity analyses. CA extracted data from selected studies into Microsoft Excel using a pre-defined data extraction spreadsheet (appendix p 8). JS, HF, and AAA double-checked the extracted data, with discrepancies resolved by group discussion. We extracted one unadjusted and adjusted effect measure from published papers, prioritising HRs, incidence rate ratios, and relative risks (RRs) over odds ratios (ORs). When an effect estimate was not reported, we calculated one from the available data. Consistent with previously published methods,38 we transformed ORs and their 95% CIs into RRs when incidence was high (>10 per 100 person-years).
We assessed the risk of bias for each estimate of HIV or HCV incidence for each study using the Newcastle-Ottawa Scale.39 The scale allocates a maximum of 9 points indicating a low risk of bias depending on the selection of study participants, comparability of participants, and ascertainment of the outcome. To assess the comparability of participants, we examined whether the reported effect estimates were adjusted for three variables that we identified a priori as being potentially confounding factors: OST exposure, history of incarceration, and history of stimulant injecting. This decision was based on studies reporting a lower risk of HIV and HCV infection among PWID on OST,7, 8 and an inverse association between homelessness or unstable housing and OST exposure.30, 40 Conversely, some studies have indicated that PWID who have been recently incarcerated or who inject stimulants have a greater risk of HIV and HCV infection41, 42 and are more likely to be homeless or unstably housed.23, 43, 44 We gave 1 point to studies that adjusted for OST exposure and 1 point to those that adjusted for history of incarceration or stimulant injecting, or both. To assess the risk of bias in unpublished estimates, we consulted the corresponding published paper or papers. For all records, risk of bias was independently assessed by two authors (CA and JS or HF) with discrepancies resolved by discussion with AAA.
We combined study-specific effect estimates using random-effects meta-analysis, because we anticipated high between-study variation. We log-transformed effect measures and their SEs, with crude and adjusted estimates pooled separately. We plotted the unadjusted estimates onto a forest plot and assessed heterogeneity by inspection of these plots, and using the I2 statistic and p value for heterogeneity.45
We did separate sensitivity analyses to assess the robustness of pooled estimates by restricting the meta-analysis to: studies that reported HRs (most common effect measure), studies at low-to-moderate risk of bias (Newcastle-Ottawa Scale score of ≥6), longitudinal studies, adjusted estimates, and studies in which 90% or more of participants had injected recently. The confounding factors included in the adjusted analyses varied between studies. We used funnel plot and Egger's test for funnel plot asymmetry to assess the risk of bias due to missing results for each outcome.
We did subgroup and random-effects meta-regression analyses to investigate potential sources of heterogeneity, as prespecified in the protocol (appendix p 5). These potential sources of heterogeneity included the baseline characteristics of participants (proportion female, proportion ever incarcerated, proportion recently incarcerated, proportion prescribed OST, mean or median age, time since starting injecting drug use, and HIV or HCV prevalence), study characteristics (publication status, study design, years of study, study duration, effect measure, timeframe for defining recent homelessness or unstable housing), and geographical and economic region.
We did all analyses separately for HIV and HCV. We did all data analysis using Stata (version 15.1).
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Through our database searches of HIV and HCV incidence studies published between June 13, 2017, and Sept 14, 2020, we identified 14 351 potentially eligible studies, of which 5766 were duplicates (figure 1). Initial screening of titles and abstracts of the remaining 8585 studies resulted in 392 studies eligible for full-text review. We retrieved 277 additional studies from our existing database of HIV and HCV incidence studies (search from Jan 1, 2000, to June 13, 2017),35 which resulted in 669 references for full-text review. Of these, 55 studies (79 estimates) met the inclusion criteria. Additionally, we identified 227 studies that measured HIV or HCV incidence among PWID but did not report an association with the exposure of interest. We contacted the authors of these studies and known lead investigators of other ongoing HIV and HCV incidence cohorts to request data. 48 unpublished estimates (from 21 studies) were obtained from these sources; 43 were from published studies reporting HIV and HCV incidences, but not reporting the exposure of interest, four were updates of previously published estimates, and one was from an ongoing study that had not yet been published. Overall, we identified 76 studies with 127 estimates that met the inclusion criteria for our review. Of these, we excluded 39 studies with 57 estimates as duplicate data (details of excluded studies are shown in the appendix [pp 9–10]).
Figure 1.
Study selection
Unpublished estimates are those that have been calculated from raw data for this study and have not been presented in previously published studies. HCV=hepatitis C virus. PWID=people who inject drugs.
The characteristics of studies included in the final analysis are shown in table 1, which includes 37 studies, of which eight reported data for both HIV and HCV incidence, originating from 16 countries, giving 70 effect estimates (22 published and 48 unpublished estimates) done between 1986 and 2020. There were 29 314 participants across HIV studies and 21 842 participants across HCV studies. There were 32 longitudinal studies (15 for HIV; 25 for HCV) and five cross-sectional studies (two for HIV; three for HCV); total person-years of follow-up for HIV longitudinal studies was 51 977 person-years (data missing for three studies) and for HCV longitudinal studies was 22 370 person-years (data missing for four studies). Of included effect estimates, 49 measured the effect of recent homelessness (19 for HIV; 30 for HCV), while 21 measured the effect of recent unstable housing (seven for HIV; 14 for HCV), with the timeframe for recent defined as currently for 12 studies, 6 months or less for 19 studies, and less than 12 months for six studies. Although not all included studies provided an explicit definition for homelessness or unstable housing, homelessness was generally defined as living on the streets, in cars, and in abandoned houses,41, 67, 69, 75 whereas unstable housing was defined as being without fixed housing,75 including so-called sofa-surfing and living on the street, in hostels, in guesthouses, in shelters, in single-room occupancies, or in short-term rentals (typically motels, hotels, rooming or multitenant houses, or rented rooms).47, 48, 68, 75 These definitions were not mutually exclusive, such that unstable housing often incorporated homelessness. The overall proportion of female participants was 4127 (15·6%) of 26 384 participants for HIV studies (data missing for two studies) and 5332 (28·6%) of 18 628 for HCV studies (data missing for five studies). We saw large differences in baseline prevalence of HIV (0·0–43·6%) and HCV (11·5–85·0%). In total, there were 1224 incident HIV infections (data missing for two studies), with HIV incidence ranging from 0·9–20·0 per 100 person-years, and 1051 incident HCV infections (data missing for three studies), with HCV incidence ranging from 0·9–64·5 per 100 person-years. Further details on study characteristics are in the appendix (pp 20–28).
Table 1.
Study characteristics of 37 studies, of which eight report data for HIV and HCV incidence, included in systematic review and meta-analysis
| Study period | Location (city, country) | Study design | Effect measured and definition | HCV or HIV estimate | Sample size | Incidence (per 100 person-years) | Effect estimates | Confounding factors included in adjusted estimates | Newcastle-Ottawa Scale score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Alary (unpublished estimate) | 2003–18 | Province of Quebec and Ottawa, Canada | Retrospective cohort46 | Unstable housing in the past 6 months | HIV and HCV | HIV: 1930 HCV: 814 | HIV: 1·08 HCV: 20·2 | HIV: HR 1·26 (0·83–1·93);aHR 0·98 (0·62–1·53) HCV: HR 1·64 (1·34–2·00);aHR 1·44 (1·16–1·78) | Living in jail within the past 6 months, OST exposure, using syringes used by someone else, cocaine being most often injected drug, injecting drugs every day, age ≥25 years, male gender, prostitution, urban sites | HIV: 7 HCV: 7 |
| Artenie et al (2019)47 | 2004–17 | Montreal, QC, Canada | Prospective cohort | Unstable housing in the past month | HCV | 513 | 11·8 | HR 2·34 (1·72–3·17);aHR 2·14 (1·54–2·96) | OAT dose and perceived adequacy, sex, duration of injection drug use, cocaine injection in the past month, incarceration in the past 3 or 6 months, previous HCV infection | 8 |
| Bruneau et al (2011)48 | 1992–2008 | Montreal, QC, Canada | Prospective cohort | Unstable housing in the past 6 months | HIV | 2137 | 3·3 | HR 3·08 (2·22–4·28);aHR 2·07 (1·47–2·90) | Age ≥30 years, gender, cocaine use in the past month, heroin use in the past month, sharing syringes with a person known to be HIV positive, “booting”, having sex with a person known to be HIV positive, period of recruitment, NEP participation, obtaining 100% of syringes from a safe source | 7 |
| Craine et al (2009)49 | 2004–06 | Newport and Calidicot, Cardiff and Barry, Bridgend, Neath & Porth Talbot, Swansea, Merthyr Tydfil, Pontypridd, Rhydfelin, Treorchy, Aberdare, Abergavenny, UK | Prospective cohort | Homelessness in the past 12 months | HCV | 286 | 5·9 | IRR 4·41 (1·60–12·5);aIRR 2·9 (1·02–8·28) | In OST at follow-up, any equipment sharing in the past year, sharing needles and syringes in past year, population size of region | 7 |
| Debeck (unpublished estimate) | 2005–16 | Vancouver, BC, Canada | Prospective cohort50 | Homelessness in the past 6 months | HIV and HCV | HIV: 476 HCV: 405 | NA | HIV: HR 1·88 (0·27–13·03);aHR 1·96 (0·31–12·27) HCV: HR 1·69 (1·1–2·6);aHR 1·45 (0·92–2·28) | Recent incarceration (past 6 months), MMT coverage, crack injecting (past 6 months) | HIV: 8 HCV: 8 |
| Dumchev (unpublished estimate) | 2013–15 | Ukraine | Prospective cohort51 | Current homelessness | HIV and HCV | HIV: 2157 HCV: 2157 | HIV: 1·8 HCV: 21·5 | HIV: IRR 1·16 (0·07–18·86) HCV: HR 1·80 (0·80–4·07);aHR 1·57 (0·69–3·54) | Ever been in prison, age (continuous), injecting drug use duration (continuous) | HIV: 6 HCV: 6 |
| Hagan et al (2001)41 | 1994–97 | Seattle, WA, USA | Prospective cohort | Homelessness in the past 12 months | HCV | 317 | 16·7 | RR 1·08 (0·59–1·97) | NA | 5 |
| Hagan et al (2010)52 | 2002–04 | Baltimore, MA; Chicago, IL; Los Angeles, CA; New York, NY; and Seattle, WA USA | Prospective cohort | Homelessness in the past 6 months | HCV | 483 | 17·2 | HR 0·93 (0·68–2·29) | NA | 5 |
| Hayashi (unpublished estimate) | 1996–2016 | Vancouver, BC, Canada | Prospective cohort32 | Homelessness in the past 6 months | HIV and HCV | HIV: 1763 HCV: 387 | NA | HIV: HR 0·78 (0·54–1·14);aHR 0·73 (0·50–1·06);HCV: HR 1·57 (1·11–2·22);aHR 1·62 (1·14–2·29) | Recent incarceration (past 6 months), MMT coverage, crack injecting (past 6 months) | HIV: 8 HCV: 9 |
| Hope et al (2018)33 | 2011–13 | England, Wales, and Northern Ireland, UK | Cross-sectional | Homelessness in the past 12 months | HCV | 2816 | 12·3 | RR 1·40 (1·02–1·92) | NA | 3 |
| Hope (unpublished estimate) | 2006–09 | Birmingham, Bristol, Glasgow, and Leeds, UK | Cross-sectional53 | Homelessness in the past 12 months | HCV | 1247 | 16·9 | RR 1·85 (0·72–4·73);aRR 1·62 (0·55–4·56) | Recent incarceration (past 12 months), current OST status, cocaine use, duration of injecting | 6 |
| Judd (unpublished estimate) | 2001–03 | London and Brighton, UK | Prospective cohort54 | Unstable housing in the past 12 months | HIV and HCV | HIV: 263 HCV: 149 | HIV: 3·5 HCV: 39·7 | HIV: HR 0·94 (0·23–3·76) HCV: HR 1·53 (0·84–2·77) | NA | HIV: 5 HCV: 5 |
| Kåberg (unpublished estimate) | 1987–2020 | Stockholm, Sweden | Retrospective cohort55 | Homelessness in the past 3 months | HCV | 832 | 17·5 | HR 2·12 (1·62–2·78) | NA | 5 |
| Kral et al (2001)56 | 1986–98 | San Francisco, CA, USA | Case control | Current homelessness | HIV | 6115 | 1·2 | OR 1·24 (0·71–2·17) | NA | 4 |
| Kurth (unpublished estimate) | 2012–15 | Nairobi and Coastal region of Kenya | Retrospective cohort57 | Current homelessness | HIV | 978 | 2·6 | IRR 3·45 (1·48–7·62) | NA | 5 |
| Lucidarme et al (2004)58 | 1999–2001 | Northern and eastern France | Prospective cohort | Unstable housing in the past 3 months | HCV | 165 | 9·0 | IRR 2·20 (0·51–7·22) | NA | 5 |
| Maher (unpublished estimate) | 1999–2002 | New South Wales, Australia | Prospective cohort59 | Unstable housing in the past 6 months | HCV | 258 | 26·1 | HR 1·01 (0·31–3·23) | NA | 6 |
| Maher (unpublished estimate) | 2008–14 | Sydney, NSW, Australia | Prospective cohort60 | Unstable housing in the past 6 months | HCV | 169 | 6·6 | HR 1·17 (0·58–2·36) | NA | 7 |
| Mehta (unpublished estimate) | 1993–2019 | Baltimore, MD, USA | Prospective cohort61 | Homelessness in the past 6 months | HIV and HCV | HIV: 2456 HCV: 1731 | HIV: 1·1 HCV: 0·9 | HIV: IRR 1·58 (1·15–2·17);aIRR 1·16 (0·84–1·60);HCV: IRR 1·74 (1·11–2·73);aIRR 1·66 (1·01–2·74) | Injected cocaine in past 6 months; incarcerated in past 6 months; OST or MAT | HIV: 8 HCV:9 |
| Mehta (unpublished estimate) | 2013 | India | Cross-sectional62 | Current homelessness | HIV | 9440 | 5·2 | IRR 1·56 (0·90–2·70);aIRR 1·52 (0·88–2·63) | Injected stimulants in past 6 months, participated in OST programme in past 6 months, incarcerated in past 6 months | 6 |
| Morris (unpublished estimate) | 2000–19 | San Francisco, CA, USA | Prospective cohort63 | Homelessness in the past 3 months | HCV | 712 | 24·9 | HR 1·95 (1·44–2·64);aHR 1·65 (1·21–2·25) | Gender, age, injecting frequency, recent unsafe injecting behaviours, number of injecting partners | 5 |
| Niccolai et al (2011)64 | 2005–08 | St Petersburg, Russia | Cross-sectional | Homelessness in the past 12 months | HIV | 438 | Estimate 1: 18·7* estimate 2: 20·0* | RR 0·70 (0·33–1·52) | NA | 4 |
| Palmateer et al (2014)65 | 2008–12 | Scotland, UK | Cross-sectional | Homelessness in the past 6 months | HCV | 7951 | 10·0 | RR 3·80 (2·20–6·57) | NA | 5 |
| Sacks-Davis (unpublished estimate) | 2005–10 | Melbourne, VIC, Australia | Prospective cohort66 | Current unstable housing | HCV | 89 | 15·4 | HR 1·63 (0·72–3·70):aHR 1·58 (0·66–3·79) | OST (any pharmacotherapy in the past 3 months), type of infection (primary, reinfection), correlation within individuals | 6 |
| Samo et al (2013)67 | 2009–11 | Karachi, Pakistan | Prospective cohort | Current homelessness | HIV | 474 | 12·4 | IRR 1·70 (1·20–2·50);aIRR 1·70 (1·10–2·50) | Sharing of syringes, non-Muslim religion, daily frequency of injecting drugs, source of registration (registered with drop-in centres through outreach compared with other methods), physical disability, monthly income and sources of syringes or needles | 5 |
| Schulkind et al (2019)68 | 2012–16 | Dundee, UK | Prospective cohort | Current unstable housing | HCV | 94 | 21·5 | IRR: 0·42 (0·06–3·23) | NA | 6 |
| Spittal et al (2012)69 | 2003–09 | Vancouver, BC, Canada | Prospective cohort | Homelessness in the past 6 months | HCV | 148 | 11·6 | HR 1·26 (0·83–1·90) | NA | 5 |
| Strathdee (unpublished estimate) | 2006–10 | Tijuana, Mexico | Prospective cohort70 | Unstable housing in the past 6 months | HIV | 812 | 0·9 | HR 1·50 (0·55–4·07) | NA | 7 |
| Strathdee (unpublished estimate) | 2011–20 | Tijuana, Mexico | Prospective cohort71 | Unstable housing in the past 6 months | HIV | 472 | 2·5 | HR 2·10 (1·13–3·90) | NA | 5 |
| Sypsa et al (2017)31 | 2012–13 | Athens, Greece | Retrospective cohort | Current homelessness | HIV | 3320 | 4·5 | HR 1·75 (1·30–2·36);aHR 1·96 (0·98–3·85) | Age, sex, country of origin, history of any imprisonment, size of participant's network of PWID, currently on OST programme, main substance of use, injecting drug use in past 1 month, frequency of injecting drug use, sharing syringes, use of drugs divided with a syringe that someone else had already used for injection | 8 |
| Sypsa (unpublished estimate) | 2012–13 | Athens, Greece | Retrospective cohort31 | Current homelessness | HCV | 63 | 64·5 | IRR 2·31 (0·86–6·19) | NA | 5 |
| Thorpe et al (2002)72 | 1997–99 | Chicago, IL, USA | Prospective cohort | Homelessness in the past 6 months | HCV | 353 | 10·0 | HR 0·76 (0·31–1·86);aHR 0·63 (0·25–1·58) | Injection related risk exposures (sharing cookers, sharing cotton filters, sharing rinse water, sharing syringes), demographic covariates (high-school diploma, suburban residence), drug use covariates (daily injection in the past 6 months, cocaine injection in the past 6 months) | 6 |
| Todd (unpublished estimate) | 2007–09 | Kabul, Afghanistan | Prospective cohort73 | Homelessness in the past 6 months | HIV and HCV | HIV: 316 HCV: 191 | HIV: 1·5 HCV: 40·4 | HIV: HR 0·45 (0·05–3·91) HCV: HR 0·76 (0·45–1·29) | NA | HIV: 6 HCV: 6 |
| Valencia (unpublished estimate) | 2003–16 | Madrid, Spain | Prospective cohort74 | Current homelessness | HCV | 127 | 60·4 | HR 3·82 (0·80–16·9);aHR 4·90 (1·07–23·1) | Crack injecting, OST exposure | 7 |
| Vallejo et al (2015)75 | 2001–06 | Barcelona, Madrid, and Valencia, Spain | Prospective cohort | Unstable housing in the past 12 months | HCV | 513 | 39·8 | IRR 1·71 (0·9–3·25) | NA | 5 |
| Van Santen (unpublished estimate) | 1989–2014 | Amsterdam, Netherlands | Prospective cohort76 | Homelessness in the past 6 months | HIV and HCV | HIV: 690 HCV: 174 | HIV: 1·2 HCV:3·9 | HIV: HR 2·02 (1·01–4·02);aHR 2·02 (1·01–4·01) HCV: HR 2·95 (1·39–6·23);aHR 3·04 (1·42–6·52) | Methadone dosing (no methadone vs <60 mg/day vs ≥60 mg/day) | HIV: 6 HCV: 6 |
| Wijnand (unpublished estimate) | 2011–19 | Melbourne, VIC, Australia | Prospective cohort77 | Current homelessness or unstable housing | HCV | 125 | 5·7 | Homelessness: HR 1·18 (0·16–8·86);aHR 1·09 (0·14–8·59) Unstable housing: HR 1·19 (0·41–3·48);aHR 1·21 (0·40–3·68) | Methamphetamine (ice, crystal, or shabu) injected in the past month, OST | 8 |
Data in parentheses are 95% CIs. For unpublished estimates, studies are listed by the name of the investigator who provided the data or the unpublished estimate and we cited the most recently published article that was based on the same cohort. Unpublished estimates are those that have been calculated from raw data for this study and have not been presented in previously published papers. aHR=adjusted hazard ratio. aIRR=adjusted incidence rate ratio. aRR=adjusted relative risk. BED EIA=BED capture enzyme immunoassay. HCV=hepatitis C virus. HR=unadjusted hazard ratio. IRR=unadjusted incidence rate ratio. MAT=medication-associated treatment. MMT=methadone maintenance treatment. NA=not applicable. NEP=needle exchange programme. OAT=opioid agonist therapy. OST=opioid substitution treatment. RR=unadjusted relative risk.
Two estimates for HIV incidence were available because of use of two different formulas for incidence estimation to adjust for misclassification due to sensitivity and specificity characteristics of the BED EIA.
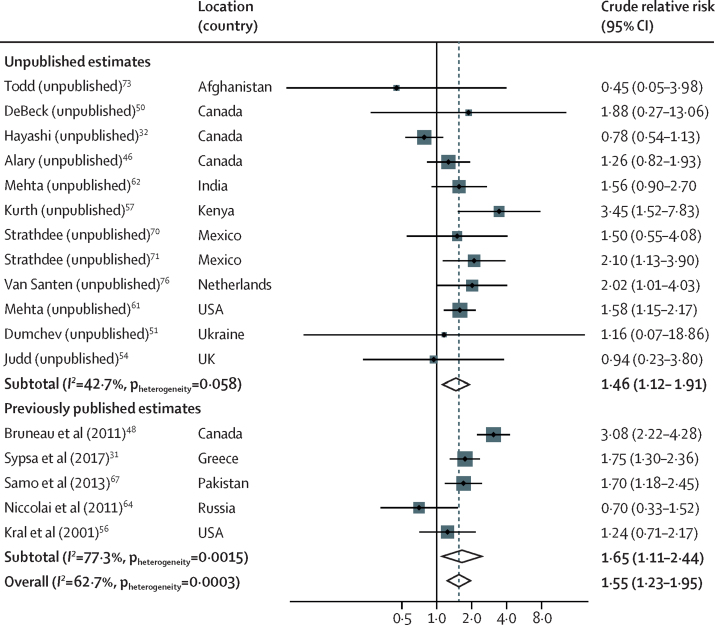
17 studies provided 26 estimates (of which 18 were unpublished estimates) for the association between recent homelessness or unstable housing and risk of HIV acquisition. 17 estimates were unadjusted estimates and nine were adjusted estimates (table 1). In the unadjusted analysis, compared with PWID who were not recently homeless or were stably housed, recent homelessness or unstable housing was associated with a crude RR (cRR) for HIV acquisition of 1·55 (95% CI 1·23–1·95; p=0·0002) with substantial between-study heterogeneity (I2=62·7%; p=0·0003; table 2; figure 2). In the sensitivity analyses, the pooled unadjusted estimate from published estimates was similar (cRR 1·65 [95% CI 1·11–2·44]; p=0·012) to the pooled unadjusted estimate of those calculated from raw data (1·46 [1·12–1·91]; p=0·0048; table 2; figure 2). The risk was reduced slightly when adjusted estimates were pooled (adjusted RR 1·39 [1·06–1·84]; p=0·019). The direction of association was consistent and similar in all other sensitivity analyses, except when considering the association between recent unstable housing and risk of HIV acquisition, for which the effect size was larger (cRR 1·82 [1·13–2·95]; p=0·014; table 2). Additionally, when pooling studies with at least 90% of participants with recent injecting the association was no longer significant. In the subgroup and meta-regression analyses, we found no evidence that the effect of recent homelessness or unstable housing on HIV acquisition risk differed by region, baseline characteristics of study participants, or study characteristics (appendix pp 11–12).
Table 2.
Sensitivity analysis of the effect of recent homelessness or unstable housing on risk of HIV or HCV acquisition in PWID compared with PWID who are not homeless or are stably housed
| Number of estimates | Effect size (95% CI) | p value | I2 | pheterogeneity | |
|---|---|---|---|---|---|
| Effect on risk of HIV acquisition | |||||
| Unadjusted effect estimates | 17 | 1·55 (1·23–1·95) | 0·0002 | 62·7 | 0·0003 |
| Adjusted effect estimates | 9 | 1·39 (1·06–1·84) | 0·019 | 65·5 | 0·0031 |
| Published unadjusted estimates | 5 | 1·65 (1·11–2·44) | 0·012 | 77·3 | 0·0015 |
| Unpublished unadjusted estimates* | 12 | 1·46 (1·12–1·91) | 0·0048 | 42·7 | 0·058 |
| Only longitudinal studies | 15 | 1·62 (1·27–2·07) | 0·0001 | 63·4 | 0·0005 |
| Only studies with hazard ratios | 10 | 1·56 (1·08–2·25) | 0·018 | 73·5 | <0·0001 |
| Only studies with at least 90% of participants injecting recently | 11 | 1·44 (0·97–2·13) | 0·068 | 75·4 | 0·0001 |
| Only studies at low-to-moderate risk of bias | 10 | 1·56 (1·14–2·12) | 0·0052 | 72·6 | 0·0001 |
| Estimate for homelessness | 12 | 1·44 (1·13–1·83) | 0·0036 | 52·7 | 0·016 |
| Estimate for unstable housing | 5 | 1·82 (1·13–2·95) | 0·014 | 67·8 | 0·014 |
| Effect on risk of HCV acquisition† | |||||
| Unadjusted effect estimates | 28 | 1·65 (1·44–1·89) | <0·0001 | 44·8 | 0·0060 |
| Adjusted effect estimates | 14 | 1·64 (1·43–1·89) | <0·0001 | 9·6 | 0·35 |
| Published unadjusted estimates | 11 | 1·61 (1·18–2·19) | 0·0029 | 66·9 | 0·0008 |
| Unpublished unadjusted estimates* | 17 | 1·69 (1·49–1·92) | <0·0001 | 14·2 | 0·29 |
| Only longitudinal studies | 25 | 1·61 (1·40–1·86) | <0·0001 | 38·6 | 0·027 |
| Only studies with hazard ratios | 18 | 1·59 (1·36–1·86) | <0·0001 | 45·6 | 0·019 |
| Only studies with at least 90% of participants injecting recently | 20 | 1·54 (1·32–1·80) | <0·0001 | 39·0 | 0·039 |
| Only studies at low-to-moderate risk of bias | 19 | 1·67 (1·37–2·03) | <0·0001 | 52·4 | 0·0042 |
| Estimate for homelessness | 19 | 1·66 (1·37–2·00) | <0·0001 | 55·3 | 0·0018 |
| Estimate for unstable housing | 10 | 1·72 (1·48–1·99) | <0·0001 | 0·0 | 0·47 |
HCV=hepatitis C virus. PWID=people who inject drugs.
Unpublished estimates are those that have been calculated from raw data for the current study and have not been presented in previously published papers.
One study (unpublished estimate, provided by Wijnand and colleagues) provided estimates for both unstable housing and homelessness. For all sensitivity analyses, the effect of unstable housing from this study was used, except when pooling estimates specifically for homelessness or unstable housing. Hence, the number of studies for these two sensitivity analyses add up to 29.
Figure 2.
Meta-analysis of studies showing the unadjusted effect of recent homelessness or unstable housing on risk of HIV acquisition in PWID compared with PWID who are not homeless or are stable housed, by publication status
For unpublished estimate studies, we listed the studies by the principal investigator who supplied the data and referenced the most recently published article that was based on the same cohort. Unpublished estimates are those that have been calculated from raw data for this study and have not been presented in previously published studies. PWID=people who inject drugs.
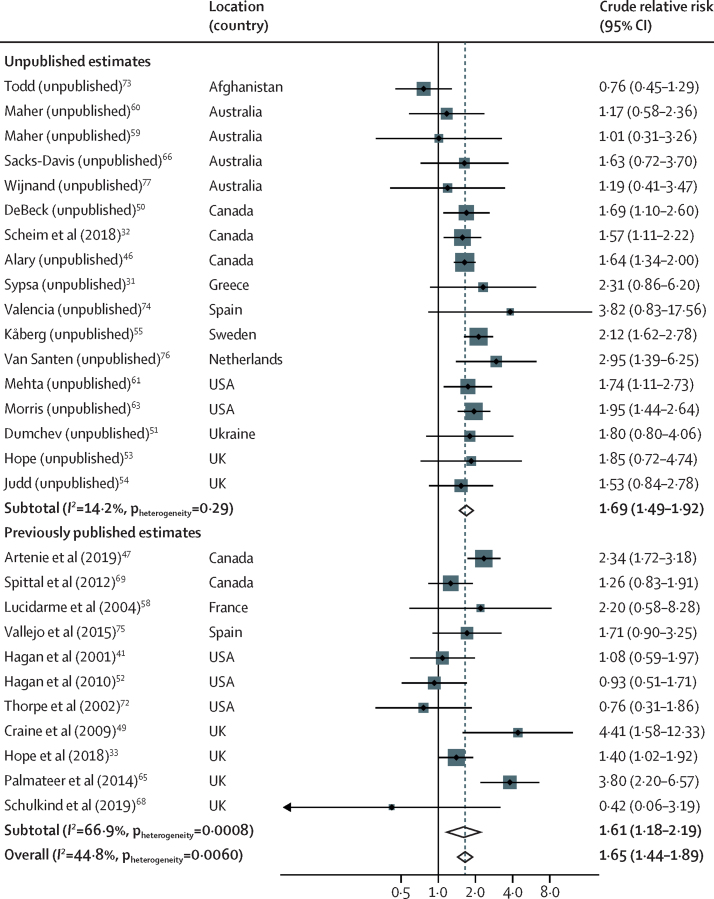
28 studies provided 44 estimates (of which 30 were unpublished) of the association between homelessness or unstable housing and risk of HCV acquisition. One study (unpublished estimate, provided by Wijnand and colleagues; table 1) provided unadjusted and adjusted effect estimates for both homelessness and unstable housing, with the definition of unstable housing encompassing homelessness. For all analyses, we used the estimate for unstable housing, except for the sensitivity analysis where effect estimates for homelessness and unstable housing were pooled separately. Therefore, in the main analyses, we included 28 unadjusted and 14 adjusted estimates (table 1). In the unadjusted analysis, compared with PWID who were not homeless or were stably housed, recent homelessness or unstable housing was associated with a cRR for HCV acquisition of 1·65 (95% CI 1·44–1·89; p<0·0001) with moderate between-study heterogeneity (I2= 44·8%, p=0·0060; table 2; figure 3). In sensitivity analyses, the pooled unadjusted estimate from published estimates was similar (cRR 1·61 [1·18–2·19; p=0·0029) to the pooled unadjusted estimate of those calculated from raw data (1·69 [1·49–1·92]; p<0·0001; table 2; figure 3). The risk was similar when adjusted estimates were pooled (adjusted RR 1·64 [1·43–1·89]; p<0·0001; table 2). The association was similar across all other sensitivity analyses (table 2). The subgroup and meta-regression analyses suggest that the association between recent homelessness or unstable housing and HCV acquisition risk was higher in studies done in Europe than elsewhere (North America, Australasia, and south and central Asia), higher in studies with greater baseline OST coverage, and higher in studies spanning longer time periods (appendix pp 12–14).
Figure 3.
Meta-analysis of studies showing unadjusted effect of recent homelessness or unstable housing on risk of HCV acquisition in PWID compared with PWID who are not homeless or are stably housed, by publication status
For unpublished estimate studies, we listed the studies by the principal investigator who supplied the data and referenced the most recently published article that was based on the same cohort. Unpublished estimates are those that have been calculated from raw data for this study and have not been presented in previously published studies. HCV=hepatitis C virus. PWID=people who inject drugs.
We found no evidence of asymmetry in funnel plots for either of the two summary measures by visual inspection or Egger's test (p=0·51 for both HIV and HCV; appendix pp 16–17). Overall, the risk of bias score based on the Newcastle-Ottawa Scale varied from 4 points (three studies) to 9 points (two studies), and the median score did not differ for estimates of HIV and HCV studies, both being 6, indicating a low-to-moderate risk of bias (table 1; appendix pp 29–31).
Discussion
We found strong evidence of an increased risk of HIV and HCV acquisition among PWID who are exposed to recent homelessness or unstable housing compared with PWID who are not homeless or are stably housed. Across all included studies, recent homelessness or unstable housing was associated with a 1·55 times greater risk of HIV acquisition and a 1·65 times greater risk of HCV acquisition. Although our estimates remained largely consistent in most sensitivity analyses, the pooled adjusted estimates indicated a slightly lower effect for HIV (RR 1·39) but not HCV (RR 1·64).
The association between recent homelessness or unstable housing and risk of HIV acquisition did not vary according to any of the factors considered, including participant and study characteristics and geographical or economic region. Conversely, the association with risk of HCV acquisition was greater in studies done in Europe, studies done in regions of higher OST coverage, and in longer studies. The reasons for these associations are unclear. Possibly, compared with North America and Australasia, PWID who are homeless or unstably housed in Europe are less likely to attend harm-reduction services, and thus are more likely to engage in high-risk behaviours. Alternatively, the definition of homelessness or unstable housing adopted in studies done in Europe might have been more likely to capture those at higher risk of HCV infection than studies done outside of Europe. Overall, although our findings could reflect true differences, they should be interpreted with caution. Some covariates had many missing values because they were not systematically reported in all studies, leading to sparse data across some categories. Also, some differences might be due to confounding, at least partly, given that the high number of missing values precluded multivariable meta-regression analyses.
Our finding of greater risk of HIV and HCV infection among PWID who experience housing instability than among PWID who are not homeless or who are stably housed can be attributed to increased injection and high-risk sexual behaviours in this population, as previously reported.22, 23, 44, 78, 79 These behaviours are probably the result of broader health and social factors and difficult socioeconomic circumstances experienced by homeless and unstably housed PWID, including inadequate access to harm-reduction services,25, 26, 30, 40, 80 mental health disorders,44 incarceration,23, 24, 44 unemployment,44 and food insecurity.81 Overall, our findings align with the syndemic framework for conceptualising health outcomes, which suggests that overlapping biosocial problems do more than just cluster together but exacerbate health inequalities.82 Both homelessness and drug dependence carry stigma, discrimination, and structural inequalities, and together can amplify susceptibility to adverse health outcomes. For example, in studies exploring the perceptions of homeless PWID, the stress surrounding their precarious living circumstances and the pervasiveness of drugs in the social environment were found to considerably amplify drug use and high-risk behaviours.79, 83, 84
Housing First85, 86, 87 is one initiative that aims to reduce homelessness that has been adopted in some high-income countries. This model centres on providing immediate housing to marginalised populations, and is not contingent on them being enrolled into drug treatment services or ceasing drug use. Additional support for those with mental health and substance use disorders is also offered. Although the effectiveness of this initiative on HIV and HCV transmission and injecting-related risk behaviours has yet to be determined, it has been shown to have a positive impact on mental health and substance use, social integration, housing stability, quality of life, and involvement with the criminal justice system.87, 88 Other housing interventions can also reduce high-risk sexual behaviours and improve treatment outcomes among homeless populations living with HIV,89 but few studies have considered homeless PWID.
Despite evidence of their benefit, housing initiatives are not well established in most high-income countries,87 and even less so in low-income and middle-income countries. However, during the ongoing COVID-19 pandemic, numerous countries (including the UK, France, Australia, and the USA) rapidly escalated their efforts to provide safe and secure housing to homeless or unstably housed individuals.90, 91, 92 In England, UK, these efforts have not only potentially reduced COVID-19 outbreaks in this group,93 but has also resulted in increased linkage to care with opportunities to address long-standing health issues, such as testing and treatment for tuberculosis, HIV, and HCV.94 Comprehensive efforts like these that provide housing alongside harm-reduction programmes and access to HIV and HCV treatment for those who are infected are needed to achieve meaningful reductions in transmission. Expanding access to treatment among homeless PWID should be prioritised given the unique barriers faced by this group,95, 96 despite the availability of effective and cost-effective HIV and HCV testing and treatment interventions.97, 98, 99
A strength of our study is that it includes 48 unpublished estimates, increasing the number of included estimates from 22 to 70, so minimising the risk of publication bias. Nonetheless, our study had several limitations. First, because our review involved observational studies, we cannot rule out unmeasured confounding. Although our estimates remained consistent after adjustment for OST exposure, history of incarceration, and stimulant injecting, and we found a low-to-moderate risk of bias across studies, other factors might have confounded the association (eg, ethnicity, severity of addiction, or mental health disorders, which are rarely measured in these studies). Second, selection bias is another limitation of longitudinal observational studies because PWID who are homeless or unstably housed might have increased attrition. If participants who were lost to follow-up had a higher risk of acquisition than those who remained in follow-up, we might have underestimated the true associations between homelessness or unstable housing and risk of HIV and HCV acquisition. Importantly, we assessed selection bias using the Newcastle-Ottawa Scale and our estimates remained consistent when pooling studies at low-to-moderate risk of bias. Third, differences across studies in the measurement of homelessness and unstable housing could have introduced bias in the pooled estimates. Our sensitivity and meta-regression analyses revealed some non-significant differences between estimates that defined exposure using different timeframes (eg, past year vs past 6 months or currently) or based on whether participants were homeless or unstably housed. Unfortunately, we did not have the statistical power to draw conclusions with regards to these differences. These limitations are likely to be amplified by the absence of a universal definition of homelessness or unstable housing that accounts for differences across sociocultural settings, making the monitoring of homelessness and its effect on health outcomes across different countries and regions difficult. We were also restricted in our ability to examine whether the association between homelessness and unstable housing and HIV or HCV risk varied as a function of other factors, such as coverage of needle and syringe provision or antiretroviral therapy. As mentioned, meta-regression analyses were underpowered to detect significant differences due to the high proportion of missing data. Fourth, data from low-income and middle-income countries was scarce, with only nine studies being available from such settings for this systematic review and meta-analysis (seven HIV studies and two HCV studies). Further studies are needed from low-income and middle-income countries to enable us to draw more generalisable conclusions. Finally, although details of the methods were prespecified and documented in a protocol, we were unable to register our protocol as intended due to changes in the PROSPERO eligibility criteria during the conduct of our study.
In summary, our study provides strong evidence that current or recent homelessness and unstable housing is associated with increased risk of HIV and HCV acquisition among PWID. These findings frame housing instability as an important driver of HIV and HCV transmission among PWID and call for intensified efforts to assess and implement housing initiatives and targeted prevention services that are tailored to the needs of this marginalised population. To help PWID attain and maintain housing stability, integrated strategies that address their competing health and social concerns are urgently needed. Great changes, albeit only the temporary provision of housing, achieved during the COVID-19 pandemic have shown that such strategies are possible when there is sufficient political will.
Data sharing
Extracted data sheets will be made available immediately after publication of this Article. These data sheets will be shared with researchers who provide a methodologically sound proposal approved by JS and PV. Proposals should be directed to jack.stone@bristol.ac.uk and Peter.vickerman@bristol.ac.uk; requesters will need to sign a data access agreement.
Declaration of interests
HF reports honoraria from MSD outside of the submitted work. JA reports grants from National Institutes of Health (NIH) during the conduct of the study. SSS reports grants from NIH during the conduct of the study, and grants from Gilead Sciences and Abbot Diagnostics outside of the submitted work. VS reports grants, personal fees, and non-financial support from Gilead Sciences and AbbVie and personal fees from Janssen outside of the submitted work. JGW reports grants from Gilead Sciences outside of the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
CA received funding from Commonwealth Scholarship Commission for his master's degree. PV, HF and JS acknowledge support from US National Institute for Drug Abuse (NIDA grant number, R01 DA033679, R01 DA037773, R21 DA046809 and R01 DA047952) and National Institute of Allergy and Infectious Diseases (NIAID)/NIDA (R01AI147490). PV, ZW, HF, and JS are supported by the National Institute for Health Research (NIHR) Health Protection Research Units (HPRUs) in Evaluation of Interventions and Behavioural Science at the University of Bristol in partnership with Public Health England. PV and HF also acknowledge support from the NIHR funded EPIToPe project. AAA is supported through postdoctoral fellowships through the Canadian Institute of Health Research, Fonds de recherche du Québec – Santé and Canadian Network on Hepatitis C. LM is supported by Australian National Health and Medical Research Council Research Fellowship (GNT1154839). JJO acknowledges support from Australian National Health and Medical Research Council (APP1104781). MA acknowledges funding from the Public Health Agency of Canada and the Ministère de la santé et des services sociaux du Québec. MM acknowledges funding from NIDA (K01DA037802). The views expressed in this Article are those of the authors and not necessarily those of the UK National Health Service, the NIHR, UK Department of Health and Social Care, Public Health England, NIDA, NIAID, or the NIH. Acknowledgements for other members of The Homelessness, HIV, and HCV Review Collaborative Group are in the appendix (p 32).
Contributors
PV and JS conceived the idea for the study, which was then designed by PV, JS, HF, and CA. CA did the literature search and data analyses, with the guidance of PV, JS, and HF. JS, HF, JJO, SB, AT, AGL, JGW, ZW, and LM contributed to the screening of studies. CA, JS, HF, and AAA were responsible for data extraction. PV contacted study authors to request unpublished data. MA, JA, JI, MM, RS-D, DKvS, SSS, VS, WVDB, and all members of the Homelessness, HIV, and HCV Review Collaborative Group contributed unpublished data for the study. SB, AT, JA, MM, LP, RS-D, DKvS, SSS, VS, JV, WVDB, and JGW did additional analyses on the unpublished data. CA, JS, PV, HF, and AAA wrote the first draft of the manuscript and all authors contributed to interpretation of data and critical revision of the article. CA, HF, AAA, and JS accessed and verified the data reported in the study. All authors had full access to the data reported in the study and agreed with the decision to submit for publication.
The Homelessness, HIV, and HCV Review Collaborative Group
Peter Cherutich, Kora Debeck, Paul Dietze, Kostyantyn Dumchev, Kanna Hayashi, Margaret Hellard, Matthew Hickman, Vivian Hope, Ali Judd, Martin Kåberg, Ann E Kurth, Pascale Leclerc, Lisa Maher, Shruti H Mehta, Kimberly A Page, Maria Prins, Steffanie A Strathdee, Catherine S Todd.
Contributor Information
Jack Stone, Email: jack.stone@bristol.ac.uk.
Homelessness, HIV, and HCV Review Collaborative Group:
Peter Cherutich, Kora Debeck, Paul Dietze, Kostyantyn Dumchev, Kanna Hayashi, Margaret Hellard, Matthew Hickman, Vivian Hope, Ali Judd, Martin Kåberg, Ann E. Kurth, Pascale Leclerc, Lisa Maher, Shruti H. Mehta, Kimberly A Page, Maria Prins, Catherine S. Todd, and Steffanie A. Strathdee
Supplementary Material
References
- 1.Stanaway JD, Flaxman AD, Naghavi M. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Abate D, Abate KH. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degenhardt L, Charlson F, Stanaway J. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:1385–1398. doi: 10.1016/S1473-3099(16)30325-5. [DOI] [PubMed] [Google Scholar]
- 4.Degenhardt L, Peacock A, Colledge S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS (UNAIDS) UNAIDS; 2017. UNAIDS Data 2017.https://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf [PubMed] [Google Scholar]
- 6.Trickey A, Fraser H, Lim AG. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol. 2019;4:435–444. doi: 10.1016/S2468-1253(19)30085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platt L, Minozzi S, Reed J. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9 doi: 10.1002/14651858.CD012021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacArthur GJ, Minozzi S, Martin N. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345 doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aspinall EJ, Nambiar D, Goldberg DJ. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:235–248. doi: 10.1093/ije/dyt243. [DOI] [PubMed] [Google Scholar]
- 10.Martin NK, Vickerman P, Grebely J. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598–1609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MS, Chen YQ, McCauley M. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larney S, Peacock A, Leung J. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health. 2017;5:e1208–e1220. doi: 10.1016/S2214-109X(17)30373-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strathdee SA, Hallett TB, Bobrova N. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376:268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altice FL, Azbel L, Stone J. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in eastern Europe and central Asia. Lancet. 2016;388:1228–1248. doi: 10.1016/S0140-6736(16)30856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes T. Risk environments and drug harms: a social science for harm reduction approach. Int J Drug Policy. 2009;20:193–201. doi: 10.1016/j.drugpo.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Galea S, Vlahov D. Social determinants and the health of drug users: socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135–S145. [PMC free article] [PubMed] [Google Scholar]
- 17.Des Jarlais DC, Sypsa V, Feelemyer J. HIV outbreaks among people who inject drugs in Europe, North America, and Israel. Lancet HIV. 2020;7:e434–e442. doi: 10.1016/S2352-3018(20)30082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldridge RW, Story A, Hwang SW. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. Lancet. 2018;391:241–250. doi: 10.1016/S0140-6736(17)31869-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aldridge RW, Hayward AC, Hemming S. High prevalence of latent tuberculosis and bloodborne virus infection in a homeless population. Thorax. 2018;73:557–564. doi: 10.1136/thoraxjnl-2016-209579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salhi BA, White MH, Pitts SR, Wright DW. Homelessness and emergency medicine: a review of the literature. Acad Emerg Med. 2018;25:577–593. doi: 10.1111/acem.13358. [DOI] [PubMed] [Google Scholar]
- 21.Zivanovic R, Milloy MJ, Hayashi K. Impact of unstable housing on all-cause mortality among persons who inject drugs. BMC Public Health. 2015;15:106. doi: 10.1186/s12889-015-1479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aidala A, Cross JE, Stall R, Harre D, Sumartojo E. Housing status and HIV risk behaviors: implications for prevention and policy. AIDS Behav. 2005;9:251–265. doi: 10.1007/s10461-005-9000-7. [DOI] [PubMed] [Google Scholar]
- 23.Topp L, Iversen J, Baldry E, Maher L. Housing instability among people who inject drugs: results from the Australian needle and syringe program survey. J Urban Health. 2013;90:699–716. doi: 10.1007/s11524-012-9730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linton SL, Celentano DD, Kirk GD, Mehta SH. The longitudinal association between homelessness, injection drug use, and injection-related risk behavior among persons with a history of injection drug use in Baltimore, MD. Drug Alcohol Depend. 2013;132:457–465. doi: 10.1016/j.drugalcdep.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appel PW, Ellison AA, Jansky HK, Oldak R. Barriers to enrollment in drug abuse treatment and suggestions for reducing them: opinions of drug injecting street outreach clients and other system stakeholders. Am J Drug Alcohol Abuse. 2004;30:129–153. doi: 10.1081/ada-120029870. [DOI] [PubMed] [Google Scholar]
- 26.Havens JR, Latkin CA, Pu M. Predictors of opiate agonist treatment retention among injection drug users referred from a needle exchange program. J Subst Abuse Treat. 2009;36:306–312. doi: 10.1016/j.jsat.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J. 2013;10:7. doi: 10.1186/1477-7517-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makarenko I, Artenie A, Hoj S. Transitioning from interferon-based to direct antiviral treatment options: a potential shift in barriers and facilitators of treatment initiation among people who use drugs? Int J Drug Policy. 2019;72:69–76. doi: 10.1016/j.drugpo.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes T, Treloar C. The social production of hepatitis C risk among injecting drug users: a qualitative synthesis. Addiction. 2008;103:1593–1603. doi: 10.1111/j.1360-0443.2008.02306.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim C, Kerr T, Li K. Unstable housing and hepatitis C incidence among injection drug users in a Canadian setting. BMC Public Health. 2009;9:270. doi: 10.1186/1471-2458-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sypsa V, Psichogiou M, Paraskevis D. Rapid decline in HIV incidence among persons who inject drugs during a fast-track combination prevention program after an HIV outbreak in Athens. J Infect Dis. 2017;215:1496–1505. doi: 10.1093/infdis/jix100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheim AI, Nosova E, Knight R, Hayashi K, Kerr T. HIV incidence among men who have sex with men and inject drugs in a Canadian setting. AIDS Behav. 2018;22:3957–3961. doi: 10.1007/s10461-018-2185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hope VD, Harris RJ, Vickerman P. A comparison of two biological markers of recent hepatitis C virus (HCV) infection: implications for the monitoring of interventions and strategies to reduce HCV transmission among people who inject drugs. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.47.1700635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spada E, Rezza G, Garbuglia AR. Incidence and risk factors for hepatitis C virus infection among illicit drug users in Italy. J Urban Health. 2018;95:99–110. doi: 10.1007/s11524-017-0207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone J, Fraser H, Lim AG. Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2018;18:1397–1409. doi: 10.1016/S1473-3099(18)30469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smoleń-Dzirba J, Wąsik TJ. Current and future assays for identifying recent HIV infections at the population level. Med Sci Monit. 2011;17:RA124–RA133. doi: 10.12659/MSM.881757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 39.Wells GA, Shea B, O'Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 40.Corneil TA, Kuyper LM, Shoveller J. Unstable housing, associated risk behaviour, and increased risk for HIV infection among injection drug users. Health Place. 2006;12:79–85. doi: 10.1016/j.healthplace.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91:42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tavitian-Exley I, Vickerman P, Bastos FI, Boily MC. Influence of different drugs on HIV risk in people who inject: systematic review and meta-analysis. Addiction. 2015;110:572–584. doi: 10.1111/add.12846. [DOI] [PubMed] [Google Scholar]
- 43.Lloyd-Smith E, Wood E, Li K, Montaner JS, Kerr T. Incidence and determinants of initiation into cocaine injection and correlates of frequent cocaine injectors. Drug Alcohol Depend. 2009;99:176–182. doi: 10.1016/j.drugalcdep.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whittaker E, Swift W, Roxburgh A. Multiply disadvantaged: Health and service utilisation factors faced by homeless injecting drug consumers in Australia. Drug Alcohol Rev. 2015;34:379–387. doi: 10.1111/dar.12257. [DOI] [PubMed] [Google Scholar]
- 45.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. John Wiley & Sons; Chichester: 2009. Introduction to meta-analysis; pp. 105–125. [Google Scholar]
- 46.Blouin K, Leclerc P, Morissette C. Sex work as an emerging risk factor for human immunodeficiency virus seroconversion among people who inject drugs in the SurvUDI network. Sex Transm Dis. 2016;43:648–655. doi: 10.1097/OLQ.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 47.Artenie AA, Minoyan N, Jacka B. Opioid agonist treatment dosage and patient-perceived dosage adequacy, and risk of hepatitis C infection among people who inject drugs. CMAJ. 2019;191:e462–e468. doi: 10.1503/cmaj.181506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruneau J, Daniel M, Abrahamowicz M, Zang G, Lamothe F, Vincelette J. Trends in human immunodeficiency virus incidence and risk behavior among injection drug users in montreal, Canada: a 16-year longitudinal study. Am J Epidemiol. 2011;173:1049–1058. doi: 10.1093/aje/kwq479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Craine N, Hickman M, Parry JV. Incidence of hepatitis C in drug injectors: the role of homelessness, opiate substitution treatment, equipment sharing, and community size. Epidemiol Infect. 2009;137:1255–1265. doi: 10.1017/S095026880900212X. [DOI] [PubMed] [Google Scholar]
- 50.Puri N, DeBeck K, Feng C, Kerr T, Rieb L, Wood E. Gender influences on hepatitis C incidence among street youth in a Canadian setting. J Adolesc Health. 2014;55:830–834. doi: 10.1016/j.jadohealth.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumchev K, Samko M, Barska J, Salyuk T. HIV testing and ART are associated with lower HIV incidence among PWID in Ukraine. 21th International AIDS Conference; Durban, South Africa; July 18–22, 2016 (poster LBPE015).
- 52.Hagan H, Pouget ER, Williams IT. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201:378–385. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 53.Turner KM, Hutchinson S, Vickerman P. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106:1978–1988. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 54.Judd A, Hickman M, Jones S. Incidence of hepatitis C virus and HIV among new injecting drug users in London: prospective cohort study. BMJ. 2005;330:24–25. doi: 10.1136/bmj.38286.841227.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kåberg M, Navér G, Hammarberg A, Weiland O. Incidence and spontaneous clearance of hepatitis C virus (HCV) in people who inject drugs at the Stockholm Needle Exchange-importance for HCV elimination. J Viral Hepat. 2018;25:1452–1461. doi: 10.1111/jvh.12969. [DOI] [PubMed] [Google Scholar]
- 56.Kral AH, Bluthenthal RN, Lorvick J, Gee L, Bacchetti P, Edlin BR. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. Lancet. 2001;357:1397–1401. doi: 10.1016/S0140-6736(00)04562-1. [DOI] [PubMed] [Google Scholar]
- 57.Kurth AE, Cleland CM, Des Jarlais DC. HIV prevalence, estimated incidence, and risk behaviours among people who inject drugs in Kenya. J Acquir Immune Defic Syndr. 2015;70:420–427. doi: 10.1097/QAI.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucidarme D, Bruandet A, Ilef D. Incidence and risk factors of HCV and HIV infections in a cohort of intravenous drug users in the north and east of France. Epidemiol Infect. 2004;132:699–708. doi: 10.1017/s095026880400247x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maher L, Jalaludin B, Chant KG. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101:1499–1508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 60.White B, Madden A, Prins M. Assessing the feasibility of hepatitis C virus vaccine trials: results from the Hepatitis C Incidence and Transmission Study-community (HITS-c) vaccine preparedness study. Vaccine. 2014;32:5460–5467. doi: 10.1016/j.vaccine.2014.07.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith DK, Pan Y, Rose CE. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226–232. doi: 10.1097/ADM.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lucas GM, Solomon SS, Srikrishnan AK. High HIV burden among people who inject drugs in 15 Indian cities. AIDS. 2015;29:619–628. doi: 10.1097/QAD.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris MD, Yen IH, Shiboski S, Evans JL, Page K. Housing stability and hepatitis C infection for young adults who inject drugs: examining the relationship of consistent and intermittent housing status on HCV infection risk. J Urban Health. 2020;97:831–844. doi: 10.1007/s11524-020-00445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niccolai LM, Verevochkin SV, Toussova OV. Estimates of HIV incidence among drug users in St. Petersburg, Russia: continued growth of a rapidly expanding epidemic. Eur J Public Health. 2011;21:613–619. doi: 10.1093/eurpub/ckq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmateer NE, Taylor A, Goldberg DJ. Rapid decline in HCV incidence among people who inject drugs associated with national scale-up in coverage of a combination of harm reduction interventions. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sacks-Davis R, Aitken CK, Higgs P. High rates of hepatitis C virus reinfection and spontaneous clearance of reinfection in people who inject drugs: a prospective cohort study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samo RN, Altaf A, Agha A. High HIV incidence among persons who inject drugs in Pakistan: greater risk with needle sharing and injecting frequently among the homeless. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schulkind J, Stephens B, Ahmad F. High response and re-infection rates among people who inject drugs treated for hepatitis C in a community needle and syringe programme. J Viral Hepat. 2019;26:519–528. doi: 10.1111/jvh.13035. [DOI] [PubMed] [Google Scholar]
- 69.Spittal PM, Pearce ME, Chavoshi N. The Cedar Project: high incidence of HCV infections in a longitudinal study of young Aboriginal people who use drugs in two Canadian cities. BMC Public Health. 2012;12:632. doi: 10.1186/1471-2458-12-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strathdee SA, Lozada R, Pollini RA. Individual, social, and environmental influences associated with HIV infection among injection drug users in Tijuana, Mexico. J Acquir Immune Defic Syndr. 2008;47:369–376. doi: 10.1097/QAI.0b013e318160d5ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robertson AM, Garfein RS, Wagner KD. Evaluating the impact of Mexico's drug policy reforms on people who inject drugs in Tijuana, B.C., Mexico, and San Diego, CA, United States: a binational mixed methods research agenda. Harm Reduct J. 2014;11:4. doi: 10.1186/1477-7517-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thorpe LE, Ouellet LJ, Hershow R. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645–653. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 73.Todd CS, Nasir A, Stanekzai MR. Hepatitis C and HIV incidence and harm reduction program use in a conflict setting: an observational cohort of injecting drug users in Kabul, Afghanistan. Harm Reduct J. 2015;12:22. doi: 10.1186/s12954-015-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.La Rosa JV, Ryan P, Alvaro-Meca A. HCV seroconversion in a cohort of people who use drugs followed in a mobile harm reduction unit in Madrid: breaking barriers for HCV elimination. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vallejo F, Barrio G, Brugal MT. High hepatitis C virus prevalence and incidence in a community cohort of young heroin injectors in a context of extensive harm reduction programmes. J Epidemiol Community Health. 2015;69:599–603. doi: 10.1136/jech-2014-205070. [DOI] [PubMed] [Google Scholar]
- 76.Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102:1454–1462. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horyniak D, Higgs P, Jenkinson R. Establishing the Melbourne Injecting Drug User Cohort Study (MIX): rationale, methods, and baseline and twelve-month follow-up results. Harm Reduct J. 2013;10:11. doi: 10.1186/1477-7517-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeBeck K, Small W, Wood E, Li K, Montaner J, Kerr T. Public injecting among a cohort of injecting drug users in Vancouver, Canada. J Epidemiol Community Health. 2009;63:81–86. doi: 10.1136/jech.2007.069013. [DOI] [PubMed] [Google Scholar]
- 79.Dickson-Gomez J, Hilario H, Convey M, Corbett AM, Weeks M, Martinez M. The relationship between housing status and HIV risk among active drug users: a qualitative analysis. Subst Use Misuse. 2009;44:139–162. doi: 10.1080/10826080802344823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah NG, Celentano DD, Vlahov D. Correlates of enrollment in methadone maintenance treatment programs differ by HIV-serostatus. AIDS. 2000;14:2035–2043. doi: 10.1097/00002030-200009080-00020. [DOI] [PubMed] [Google Scholar]
- 81.Schmitz J, Kral AH, Chu D, Wenger LD, Bluthenthal RN. Food insecurity among people who inject drugs in Los Angeles and San Francisco. Public Health Nutr. 2016;19:2204–2212. doi: 10.1017/S1368980016000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389:941–950. doi: 10.1016/S0140-6736(17)30003-X. [DOI] [PubMed] [Google Scholar]
- 83.Wright NM, Tompkins CN, Jones L. Exploring risk perception and behaviour of homeless injecting drug users diagnosed with hepatitis C. Health Soc Care Community. 2005;13:75–83. doi: 10.1111/j.1365-2524.2005.00552.x. [DOI] [PubMed] [Google Scholar]
- 84.Crowley D, Cullen W, Lambert JS, Van Hout MC. Competing priorities and second chances - a qualitative exploration of prisoners' journeys through the Hepatitis C continuum of care. PLoS One. 2019;14 doi: 10.1371/journal.pone.0222186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luchenski S, Maguire N, Aldridge RW. What works in inclusion health: overview of effective interventions for marginalised and excluded populations. Lancet. 2018;391:266–280. doi: 10.1016/S0140-6736(17)31959-1. [DOI] [PubMed] [Google Scholar]
- 86.Advisory Council on the Misuse of Drugs . UK Government; 2019. Drug-related harm in homeless population and how they can be reduced.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/810284/Drug-related_harms_in_homeless_populations.pdf [Google Scholar]
- 87.Bretherton B, Pleace N. Centre for Housing Policy, University of York; York: February, 2015. Housing First in England: an evaluation of nine services. [Google Scholar]
- 88.Woodhall-Melnik JR, Dunn JR. A systematic review of outcomes associated with participation in Housing First programs. Housing Stud. 2016;31:287–304. [Google Scholar]
- 89.Fitzpatrick-Lewis D, Ganann R, Krishnaratne S, Ciliska D, Kouyoumdjian F, Hwang SW. Effectiveness of interventions to improve the health and housing status of homeless people: a rapid systematic review. BMC Public Health. 2011;11:638. doi: 10.1186/1471-2458-11-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kirby T. Efforts escalates to protect homeless people from COVID-19 in UK. Lancet. 2020;8:447–449. doi: 10.1016/S2213-2600(20)30160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.US Centers for Disease Control and Prevention Interim guidance for homeless service providers to plan and respond to coronavrius disease 2019 (COVID-19) Feb 25, 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/homeless-shelters/plan-prepare-respond.html
- 92.Baggett TP, Racine MW, Lewis E. Addressing COVID-19 among people experiencing homelessness: description, adaptation, and early findings of a multiagency response in Boston. Public Health Rep. 2020;135:435–441. doi: 10.1177/0033354920936227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lewer D, Braithwaite I, Bullock M. COVID-19 among people experiencing homelessness in England: a modelling study. Lancet Respir Med. 2020;8:1181–1191. doi: 10.1016/S2213-2600(20)30396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.NHS England and Improvement, supported by Healthy London Partnership, Pathway and other partners Case study: homelessness health in London—the response to COVID-19. June 3, 2020. https://www.healthylondon.org/wp-content/uploads/2020/04/COVID-19-Homeless-Health-in-London-case-study-v1.pdf
- 95.Kumar S, Gupte HA, Isaakidis P, Mishra JK, Munjattu JF. “They don't like us….”: barriers to antiretroviral and opioid substitution therapy among homeless HIV positive people who inject drugs in Delhi: a mixed method study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Neale J. Homelessness, drug use and hepatitis C: a complex problem explored within the context of social exclusion. Int J Drug Policy. 2008;19:429–435. doi: 10.1016/j.drugpo.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 97.Ward Z, Campbell L, Surey J. The cost-effectiveness of an HCV outreach intervention for at-risk populations in London, UK. J Antimicrob Chemother. 2019;74(suppl 5):v5–16. doi: 10.1093/jac/dkz451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Croxford S, Tavoschi L, Sullivan AK. HIV testing strategies outside of health care settings in the European Union (EU)/European Economic Area (EEA): a systematic review to inform European Centre for Disease Prevention and Control guidance. HIV Med. 2020;21:142–162. doi: 10.1111/hiv.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imbert E, Hickey MD, Clemenzi-Allen A. POP-UP clinic: a multicomponent model of care for people living with HIV (PLHIV) who experience homelessness or unstable housing (HUH) J Int AIDS Soc. 2020;23:85. doi: 10.1097/QAD.0000000000002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Extracted data sheets will be made available immediately after publication of this Article. These data sheets will be shared with researchers who provide a methodologically sound proposal approved by JS and PV. Proposals should be directed to jack.stone@bristol.ac.uk and Peter.vickerman@bristol.ac.uk; requesters will need to sign a data access agreement.