Abstract
Although overall donation and transplantation activity is higher in Europe than on other continents, differences between European countries in almost every aspect of transplantation activity (for example, in the number of transplantations, the number of people with a functioning graft, in rates of living versus deceased donation, and in the use of expanded criteria donors) suggest that there is ample room for improvement. Herein we review the policy and clinical measures that should be considered to increase access to transplantation and improve post-transplantation outcomes. This Roadmap, generated by a group of major European stakeholders collaborating within a Thematic Network, presents an outline of the challenges to increasing transplantation rates and proposes 12 key areas along with specific measures that should be considered to promote transplantation. This framework can be adopted by countries and institutions that are interested in advancing transplantation, both within and outside the European Union. Within this framework, a priority ranking of initiatives is suggested that could serve as the basis for a new European Union Action Plan on Organ Donation and Transplantation.
Subject terms: Renal replacement therapy, Social sciences
Organ transplantation improves patient survival and quality of life and has a major beneficial impact on public health and the socio-economic burden of organ failure. This Roadmap presents an outline of the challenges to increasing transplantation rates and proposes 12 key areas along with specific measures that should be considered to promote transplantation.
Key points
Differences in the frequency of transplantation between countries in the European Union suggest that there is room for improvement, wherein countries with low transplantation rates could learn from the experience of countries that are doing well.
Efforts to increase transplantation rates require a variety of strategies, including approaches to increasing living and deceased donation, improving coordination of the donation and intensive care unit processes, increasing graft quality and optimizing expanded donation criteria.
Education should cover the complete spectrum of society (the general population, patients and medical professionals) with specific outreach methods to under-represented communities and individuals who are health illiterate.
Infrastructural and financial barriers to transplantation should be banned.
Introduction
Organ transplantation improves patient survival and quality of life and has a major beneficial impact on public health and the socio-economic burden of organ failure. In the European Union (EU), a relatively coherent and structured approach to transplantation exists, with well-developed national programmes, international schemes to facilitate organ sharing and well-defined exchange policies1, making Europe a leader in the field. Between 2009 and 2015, the EU operated a successful Action Plan to promote organ donation and transplantation2. However, transplantation rates today differ markedly between EU countries, suggesting that there remains room for improvement. To address the differences, the European Commission convened a Thematic Network coordinated by the European Kidney Health Alliance (EKHA), tasked with providing guidance to increase organ donation and transplantation and presenting key action points that would increase the prevalence of patients living with a functioning transplant throughout Europe. This thematic network culminated in the publication of a joint statement that recommends strategies to promote transplantation and donation in the EU and, by extension, throughout Europe3. Although the focus of this statement is on adult and paediatric transplantation of solid organs, many recommendations are also applicable to tissue transplantation (for example, cornea).
This Roadmap summarizes and builds on the Joint Statement and the experience gained from implementation of the earlier Action Plan to recommend strategies through which transplantation activities and the number of individuals living with a functioning transplant in Europe can be enhanced. We outline the challenges posed by the development and implementation of a EU-wide transplantation strategy and propose 12 key areas in which specific measures should be considered to promote transplantation, providing an overall framework that can be adopted by countries and institutions to improve rates of donation and transplantation (Fig. 1). These areas were selected and defined by a group of experts, including members of professional organizations, and authorities from national health-care bodies. As the Joint Statement is a product of a European Commission initiative, most of the recommendations herein are aimed at improving the current status of transplantation within the EU, but importantly these recommendations are also relevant to the 17 EU-associated countries and to regions elsewhere in the world, with some adaptations to local conditions if required.
Fig. 1. Key areas in which specific measures should be considered to promote transplantation.
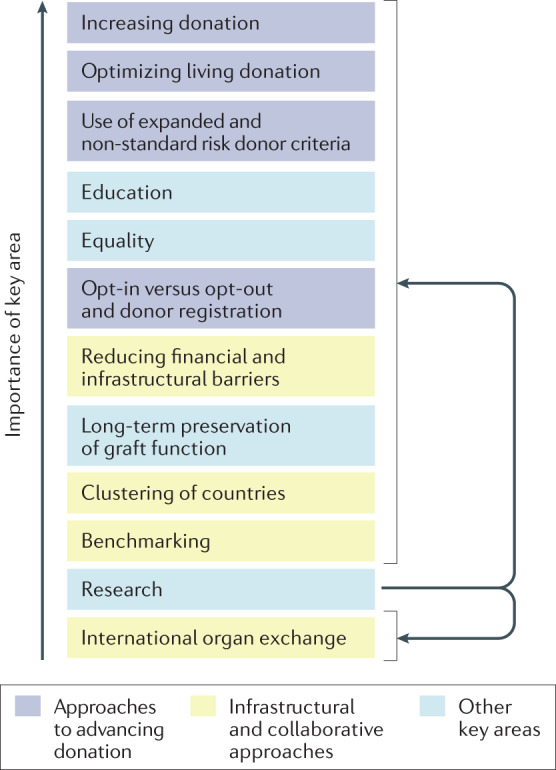
These topics have been ranked in order of priority; however, this ranking should be considered with caution as it represents a subjective judgement by the authors of this Roadmap, possibly biased by confounding factors such as region of residence, precise involvement and responsibility in transplantation activity, personal opinion and extent of solid evidence base. Furthermore, given the variations discussed in the Roadmap, the ranking of these topics may vary between different countries or stakeholders. In addition, the topics are highly interdependent (as illustrated by colour coding) and cannot be considered in isolation. Research is linked to all topics.
Current status of transplantation in Europe
Non-communicable (chronic) diseases (NCDs) impose a substantial burden on health-care systems, economies, quality of life, employment status and social activities. In Europe, NCDs are responsible for 77% of the disease burden and 86% of deaths4, many of which are in young individuals5. Changes in population demographics and the growing prevalence of risk factors have contributed to an increase in the demand for organ replacement therapies. Artificial organ support is an option in some instances, but is only available on a large scale for kidney failure in the form of dialysis. Hence, transplantation is for many patients the only solution to restoring organ function and preventing premature death. The WHO has urged countries to progress towards self-sufficiency in transplantation, first by preventing NCDs and their progression to end-stage organ failure, but also through the provision of sufficient numbers of life-saving transplants to match their need6,7. The WHO further emphasizes that deceased donation should be developed to its maximum therapeutic potential.
More than 34,285 solid organ transplantations were performed in the EU in 2019, 85% of which were kidney (21,235) and liver (7,900) transplants. Cardiothoracic transplantation represented 13% of activity with 2,269 hearts and 2,136 lungs transplanted, whereas pancreas (2%), small bowel and multi-visceral transplants represented only a small fraction (Table 1; Fig. 2). Although the total number of annual transplantations rose by 4,540 between 2010 and 2017, the number of annual transplantations from 2017 to 2019 increased by only 161, indicative of a stagnation in transplantation activity, possibly related to the end of the EU Action Plan in 2015 (Fig. 2).
Table 1.
Organ transplantation activity in the EU from 2009 to 2019
| Number of transplantations in 2010 (PMP) | Number of transplantations in 2019 (PMP) | % change (PMP) | |
|---|---|---|---|
| Kidney | 18,490 (36.8) | 21,235 (41.6) | +14.8 |
| Liver | 6,767 (13.5) | 7,900 (15.5) | +16.7 |
| Heart | 2,020 (4.0) | 2,269 (4.4) | +12.3 |
| Lung | 1,505 (3.0) | 2,136 (4.2) | +41.9 |
| Pancreas | 752 (1.5) | 710 (1.4) | −5.6 |
| Small bowel | 50 (0.1) | 35 (0.1) | −30.0 |
| TOTAL | 29,584 (58.9) | 34,285 (67.2) | +15.9 |
Transplantation activity data for the EU in 2010 (including data for Croatia, which was not at the time part of the EU) and 2019. Data were calculated based on the databases that are used for the production of the Transplant Newsletter135. PMP, per million population.
Fig. 2. Number of organ transplantations performed in the EU between 2010 and 2019.
As Croatia was not part of the EU in 2010–2011, the data for transplantations performed in Croatia were added to the data for the 27 EU member states for those years. The absolute total number of transplantations for each year is provided. The corresponding number per million population increased from 59 (2010) to 67 (2019). The EU Action Plan on Organ Donation and Transplantation was operational between 2009 and 2015. The marked rise in yearly transplantation rate observed particularly in the last years of the EU Action Plan on Organ Donation and Transplantation (2012–2015) seems to have levelled off in the years 2016–2019. Data were calculated based on data from the Transplant Newsletter135.
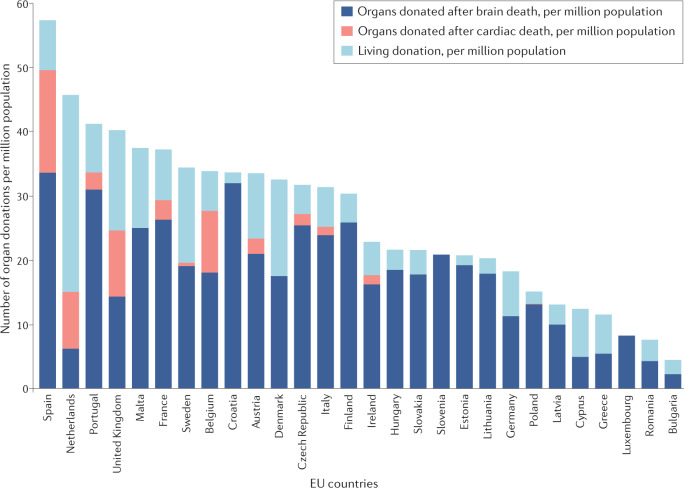
Transplantation of organs from deceased donors remains the most prevalent form of transplantation throughout the EU. Although deceased donor transplantation occurs most frequently from donors declared dead by neurological criteria (donation after brain death; DBD), donation after death declared by circulatory criteria (donation after circulatory death; DCD) contributes to transplant activity in a number of countries8 (Table 2; Fig. 3). However, substantial variability exists in the use of DCD transplantation between EU member states. DCD is not permitted in a number of European countries because of legislative and ethical obstacles9,10, and practiced in only a few cases in many other countries8. In 2019, 28 of 35 European countries had an active DCD programme compared with just 10 in 2011 (ref.10), but in several of these countries the DCD activity was marginal (Fig. 3). In 2019, DCD contributed to 17.8% of deceased donation transplantations in the EU. Living donation (almost exclusively kidney and liver), which is particularly beneficial to paediatric recipients, represents a considerable proportion of transplant activity in some but not all European countries8 (Tables 1,2; Figs 3,4).
Table 2.
Organ donation activity in the EU from 2009 to 2019
| 2010 N (PMP) | 2019 N (PMP) | % change (PMP) | |
|---|---|---|---|
| DBD | 8,626 (17.2) | 9,443 (18.5) | +9.5 |
| DCD | 715 (1.4) | 2,049 (4.0) | +186.6 |
| Actual DD | 9,341 (18.6) | 11,492 (22.5) | +23.0 |
| LD | 3,879 (7.7) | 4,259 (8.3) | +9.8 |
| TOTAL DONATIONS | 13,220 (26.3) | 15,751 (30.8) | +19.1 |
Organ donation activity data for the EU in 2010 (including data for Croatia, which was not at the time part of the EU) and 2019. Data were calculated based on data from the Transplant Newsletter135. DBD, donation after brain death; DCD, donation after circulatory death; DD, deceased donations; LD, living donations.
Fig. 3. Number of organ donations per million population in 2019.
Substantial differences exist between EU countries in terms of their overall rates of donation but also in terms of the specific types of donation. Only 11 of the 28 countries (39%) use notable quantities of organs donated after cardiac death. Although all EU countries perform transplantation after living donation, substantial differences in the rates of living donor transplantation exist between countries. Data were calculated based on data from the Transplant Newsletter135.
Fig. 4. Number of organ transplantations per million population in 2010 and 2019.
Of 28 countries, 12 (43%) showed an increase in transplantation activity of >20% (asterisks). Although most countries tended to increase or stagnate their rates of transplantation, a few show a decrease. Also shown are the absolute numbers of transplants (from deceased plus living donors) in 2019. Data were calculated based on data from the Transplant Newsletter135.
The number of organ transplant procedures for the EU as a whole was 67.2 per million population in 2019, with marked differences between countries8,11 (Fig. 3), reflecting differences in local health-care processes, efforts to develop living and deceased organ donation, available infrastructure and expertise, and economic factors12. Most EU member states have seen an upward trend in transplantation rates over the past decade, but some countries have seen a substantial decrease (Fig. 4). These decreases are in some cases influenced by external factors, such as public mistrust13, and have negative consequences on patient outcomes8,11.
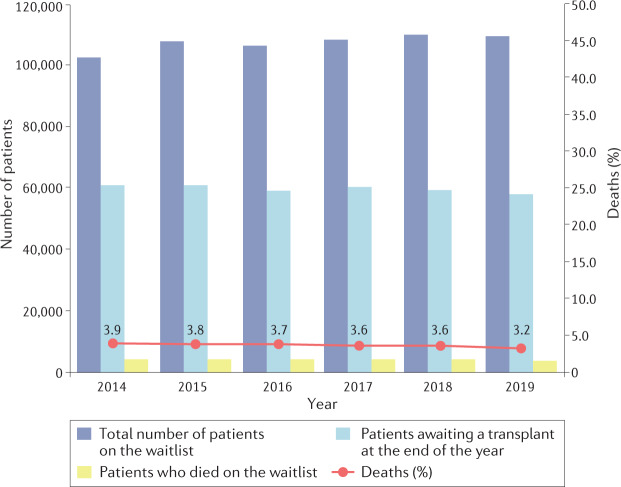
For most vital organs (liver, heart, lungs), transplantation is the only life-saving therapy. For patients with kidney failure (also known as end-stage kidney disease), which is rapidly rising in the ranked order of fatal diseases14, kidney transplantation offers not only a better survival and quality of life than dialysis15–17, but can be life-saving when vascular access options are lost. Yet, by the end of 2019, more than 58,000 patients were waiting for an organ transplant in the EU (Fig. 5). Yearly, 3–4% of those on the waiting list die before being transplanted, representing 10–11 patient deaths daily8. This figure is probably an underestimate, owing to incomplete data reporting in some countries. The mismatch between the need for transplants and donor supply, which excludes patients from lifesaving treatment, is exacerbated by the rising prevalence of health problems, such as diabetes mellitus and obesity, which reduces the donor pool; the presence of major public health challenges, such as the current COVID-19 pandemic; and improvements in critical care processes or car safety, which prevent deaths but also reduce the pool of deceased organ donors. The problems associated with access to donor organs are further illustrated by the small proportion of patients who receive a pre-emptive kidney transplant, which in most countries represents <10% of patients starting kidney replacement therapy18, necessitating a variable period on dialysis with a negative impact on survival and high associated costs19.
Fig. 5. Number of waitlisted patients and patients dying on the waiting list in the EU from 2014 to 2019.
The percentage of deaths (red line) is calculated as the ratio of those who died that year while waitlisted to the total number active on the waiting list that year multiplied by 100. There was a 7% increase in the number waitlisted over this 5-year period. The percentage of deaths remained relatively stable between 3–4%. Data were calculated based on data from the Transplant Newsletter135.
Of note, NCDs also present a considerable health economic burden through a life-long need for consultations, medication, surgery, imaging, interventions and hospitalization. It is difficult to quantify the economic impact of organ transplantation in the absence of large-scale artificial organ treatment as an alternative option. However, for kidney failure, for which dialysis consumes at least 2% of health expenditure for only 0.1–0.2 % of the general population1, transplantation is by far the most cost-effective kidney replacement option, particularly from the second year post-transplantation20,21. Economic evaluations for other solid organ transplants are less straightforward. Costs associated with liver transplantation can be substantial, particularly in the context of biliary complications that can increase the duration of hospitalization and the need for diagnostic studies and further therapeutics22. Nevertheless, liver transplantation has been reported to be cost effective23 in comparison with the rapidly rising costs of non-transplanted liver disease (including costs of medication, radiological procedures, and repeated and prolonged hospital admissions)24. Heart failure is a leading cause of morbidity and mortality worldwide and places a huge burden on health-care systems; available data suggest that heart transplantation is also cost-effective in eligible adult and paediatric recipients25,26.
Unemployment among patients with chronic NCDs generates pressure not only on social security but also on productivity and buying power27. Transplantation can interrupt this vicious circle, although pro-active mechanisms are needed to promote socio-economic (re)integration of individuals following transplantation, as 40–80% of transplanted patients remain unemployed or permanently disabled28–30.
Finally, patients with NCDs also experience a heavy burden of polypharmacy, diet restrictions, comorbidities, and time spent in hospital and travelling to medical appointments. Transplantation restores not only organ function but also quality of life20,31. For children, transplantation also leads to improvement in development, growth, education and mental health in the recipient and in quality of life for the carer32.
The benefits of transplantation prompted the EU to launch the Action Plan on Organ Donation and Transplantation, which aimed to increase organ availability, enhance efficiency and accessibility of transplant procedures, and improve the quality and safety of organs intended for transplantation. It was implemented from 2009 to 2015 (refs2,33). At the end of 2019, a 16% overall increase in transplantation rate was observed compared with 20108 (Table 1 and Fig. 2). This increase varied for different types of transplants (for example, 42% increase for lung transplantation, 17% for liver and 15% for kidney transplantation) and was primarily a result of a substantial increase in DCD8, suggesting that DBD and living donation may also benefit from further stimulatory interventions. Moreover, the initial rise in transplantation rate observed after 2012 seems to have somewhat levelled off in the past few years (Fig. 2), suggesting that the effect of the EU Action Plan has lost some momentum and that a new plan may be needed.
Data specific to kidney transplantation show that implementation of the Action Plan was associated with a rise in the total number of kidney transplantations and in the percentage of patients living with a functioning kidney graft in the EU (Supplementary Table 1). However, marked differences between countries are evident, underscoring the need for further action to boost transplantation rates in some regions.
Topics for action
Variations in transplantation practices and policies between European countries have led to differences in access to transplantation; in some instances, patients who may benefit from transplantation are not considered eligible (for example, owing to age or the presence of comorbidities or mental health issues)34. The optimal approach to increasing transplantation rates is to set well defined, ambitious goals, such as an aggregated increase in the number of EU transplantations by 10% in 10 years, complemented by specific development plans that detail the elements required to support individual countries or groups of countries according to the local conditions. This strategy should be followed by an implementation plan at a national level with internal and external auditing. Several organizations, such as the Council of Europe, have previously formulated recommendations and resolutions to increase transplantation at the institutional level (summarized in Supplementary Table 2). This Roadmap is complementary to those efforts and extends these initiatives by outlining a comprehensive multinational policy approach33,35,36. Barriers to transplantation37,38, which are often psychological and practical in nature, may be avoided through appropriate education and regulation2 (Box 1).
Lessons can also be learned from countries that are performing well. For example, measures taken by Spain to increase rates of deceased organ donation39–41 over recent decades have had remarkable success8 (Supplementary Figure 1). These measures have included a strong emphasis on coordinating the donor process, use of a pyramidal structure to coordinate processes from local to regional and national offices, engagement of the critical care community, benchmarking, provision of guidance and continuous professional training, and the increased use of living donation, expanded donor criteria and DCD organs39–41. Regions such as Croatia, Northern Italy, the UK and France, which adapted the Spanish model to their local circumstances, also saw an increase in transplantation rates42, enabling these regions to focus on equality of access and approaches to optimizing outcomes and education. Other countries, such as the Netherlands, have also increased their transplantation rates substantially, largely through increasing rates of living donation (Supplementary Table 1).
Herein we summarize 12 key domains that informed the Joint Statement commissioned by the European Commission3 and in which action could enable further increases in the number of donations, transplantations and patients living with a functioning transplant. These topics form the basis of a Roadmap that is intended for use by the EU, EU health-care authorities, patient associations and professional societies to guide the implementation of measures to stimulate organ donation and transplantation. Beyond increasing rates of organ donation and transplantation, the involved communities should do their utmost to maximize the longevity of transplanted organs, which is an absolute priority for the recipients43,44. To guide implementation of strategies that address each of these areas, we have ranked the 12 key topics in order of importance (Fig. 1). Of note, however: this ranking should be considered with caution given that it is largely opinion based, given the variation in the extent to which each of these areas may need to be addressed differently in different countries, and the interdependent nature of the areas, such that they can only be considered and implemented together. The highly integrated nature of these areas renders it near impossible to disaggregate and quantify the potential impact of individual interventions within a topic. However, all examples provided within this Roadmap refer to countries with a high transplantation rate (>60 per million population per year).
Box 1 Non-medical barriers to transplantation.
Barriers at the patient level
Attitude, role perception, motivation
Distrust of health-care professionals
Lack of knowledge
- Fears and concerns
- Fear of rejection or graft failure
- Fear of surgery
- Fear of medication or adverse effects
- Previous negative experiences (self or others)
- Fear for the living donor’s health
Sociocultural background
Religious reasons that oppose transplantation
Unsuitable living circumstances
Costs
Shortcomings in patient efforts or investments
Reluctance to ask potential living donors
Lack of social support
Lack of adherence or hygiene
Barriers at the level of the health-care professional
Attitude, role perception, motivation
Lack of knowledge and expertise
Fears and concerns
Difficulty in selecting patients
Lack of communication skills
Barriers at the level of the health-care system
Financial barriers
Lack of support staff
Competition with other treatment modalities
Patient doing well on other treatment modalities
Adapted with permission from refs2,136, the European Kidney Health Alliance.
Increasing donation
Increasing the number and quality of donated organs is a key element in increasing donation rates. Several strategies exist to facilitate this donation process.
Maximizing the role of donor coordinators
Proper coordination of the donation process is a key element in increasing donations and optimizing outcomes. The European models that have been most successful centre around the involvement of efficient donor coordinators, who are independent of the transplantation team, and are based in each hospital that has potential for deceased donation. These coordinators have key roles in the steps leading to the traditional model of deceased donation — a process that involves potential donor selection, maintenance of the haemodynamic status of the donor and organ perfusion, diagnosis of death and communication with the family. These individuals are trained in recognizing donation opportunities in end-of-life care pathways and in providing grieving families with the psychological support required to make the often difficult decision to agree to donation. Critical to the overall success of these programmes has been the appointment of professionals who develop a proactive programme for the identification of possible donors, in close cooperation with the critical care community. Donor coordinators should receive continuous training, with special attention given to the skills required to communicate with grieving relatives and organize organ handling with minimal delay. Local networks should be supported by national and regional cells that focus more on policy and technical aspects39. Regular internal and external audits should be used to identify areas for improvement39.
Optimizing the role of intensive care professionals
Engagement with intensive care professionals is particularly important to ensure that deceased donation is always considered as an option for patients receiving end-of-life care, provided that it is appropriate and consistent with the potential donor’s wishes and values45,46. Optimizing the role of intensive care professionals in the donation process requires a number of steps, including the identification of intensivists who will champion donation in their unit and in their hospital or region as a whole as well as lead education efforts; training of all intensive care staff in approaches to identifying possible organ donors on the basis of simple triggers (that is, the identification of individuals who have died or are likely to die imminently in a condition compatible with organ donation); maintenance of organ viability until donation; ensuring timely referral to donor coordinators; and ensuring an appropriate and consistent approach to the families of potential donors46.
Minimizing the duration of the donation process
The time between consideration of donation opportunities and initiation of the actual donation procedure can vary considerably and can exceed 24 h sometimes substantially47. Hesitation in donor identification and donor handling by medical staff as well as indecision of families owing to socio-cultural, religious or educational barriers48 all affect the duration of the donation process, as do organizational factors such as the length of the process before an organ is offered to a potential recipient, the need for additional tests and lack of timely access to surgical theatres. These delays may have adverse effects on donation by increasing the risk of donor organ deterioration or withdrawal of family consent, leading to the loss of otherwise transplantable organs. Therefore, national programmes should focus on approaches to facilitating the identification of all possible donors, the early notification of donor coordinators and work with families to reduce the rate of donation refusal49, thus avoiding donor loss.
Optimizing living donation
Transplantation of a kidney from a living donor offers markedly better chances for graft and patient survival than transplantation of a kidney from a deceased donor50, whereas living donor liver transplantation involves a similar hospital stay and survival rates to deceased donor transplantation51. Although practiced in almost every EU country, living donation has a variable contribution to overall transplantation activity. It is markedly low in many countries, even in those with well-established transplantation programmes, with the Netherlands and Iceland as notable exceptions (Figs 3,4, Supplementary Table 1). Living donation remains the method of choice for infants and children, in particular for those with kidney failure, because it enables pre-emptive transplantation and avoids the need for dialysis. In the Netherlands, kidney transplantation is largely driven by living donation, making it a country with one of the highest proportion of patients on kidney replacement therapy living with a functioning transplant in the EU (Supplementary Table 1).
Donor safety remains paramount and should be the primary focus of any living donation programme. However, it is equally important to demystify the risks of living donation, through a uniform process of information (for both the donor and recipient) and evaluation (via an online approach if convenient) to ensure that all essential information is conveyed and understood52 (see below and Supplementary Box 1). This approach will encourage expansion of living donation programmes, increasing access to transplants for patients from ethnic minorities and economically challenged backgrounds who are often disadvantaged overall in transplantation programmes (see below). Living kidney donors may have a low but increased risk of developing hypertension53 or kidney failure54, and therefore robust living donor programmes should carefully select donors, include an adequate follow-up, and if needed, preventive treatment. Moreover, and in contrast to the experience of many organ donors55, the donation process should be financially neutral. Processes therefore need to be in place to ensure that living donors do not face out-of-pocket costs and lost wages, or difficulty securing health or life insurance56.
Approaches to broadening donor and recipient criteria, including the involvement of emotionally related and unrelated altruistic donors and organ-sharing schemes, enable the expansion of living donation programmes. Although sharing schemes exist in a number of EU countries such as in the Netherlands and Spain, they remain non-existent or very limited in others8,57,58. Of note, a number of European and cross-border organ-sharing initiatives have been implemented to redress this situation59,60.
The implementation of initiatives to encourage living donation raises organizational and ethical questions, which have been addressed in a reference toolkit developed by the European Commission and National Agencies61. Any common ethical framework for unrelated living donation should be regulated at an EU level to alleviate any concern that pressure may be exerted on the candidate donor to benefit an irreversibly sick person and alleviate the associated societal costs62.
Use of expanded donor criteria
The term ‘expanded criteria donor’ is commonly applied to donors whose clinical–demographic characteristics would have an impact on the quality of the organ and its expected longevity. The traditional definition of an expanded criteria kidney donor includes age >60 years or age >50 years with at least two of the following: a history of hypertension, serum creatinine level >1.5 mg/dl (132.6 µmol/l) or death from cerebrovascular accident63. However, this dichotomous definition has increasingly been replaced by risk scores to guide the categorization and use of all organs (liver, kidney, pancreas)64–66. The increased use of expanded criteria donor organs and the changing profile of the potential donor pool has led to the increased use of organs from donors with a high comorbidity burden (for example, donors with diabetes mellitus). For patients with kidney failure, these organs can improve survival compared with remaining on dialysis67. Dual kidney transplantation (whereby both kidneys from a donor are transplanted into a recipient) can also allow the use of organs from marginal (for example, older) donors68.
DCD organs have in the past been considered to yield inferior post-transplant results compared with those achieved with DBD donor organs. However, increased experience has led to the attainment of appropriate post-transplantation outcomes with DCD organs69,70. Efforts can be required to overcome the legal and ethical barriers to DCD transplantation, such as the absence of a legal framework regulating the cessation of therapy, and to increase the confidence of transplantation professionals in the outcomes obtained with the use of DCD organs. Advances in organ perfusion protocols may be required to better preserve DCD organ quality and prevent the unnecessary discarding of suitable organs71–73; however, the role of in situ and ex situ preservation strategies and the type of organs that require these interventions require further study73.
Non-standard risk donors are defined as those with specific conditions or diseases (for example, infections or malignancies) that can potentially affect the safety of the transplant recipient. Transplantation of these organs can be appropriate provided that an individualized risk-assessment is performed and that recipients are properly selected74. Examples of this scenario include the use of organs with unusual anatomy but appropriate functionality (Supplementary Box 2), transplantation of HIV-infected grafts into recipients with HIV75, or transplantation of hepatitis C virus (HCV)-infected grafts into HCV-negative recipients — a process that is now possible with the use of direct-acting antiviral agents73,76. For heart transplantation alone, full use of all available organs from HCV-positive donors would increase the transplantation rate by about 3%77. Thus, the combined use of all available donor expansion measures could increase transplantation rates substantially.
EU countries that provide a legal framework for euthanasia are the Netherlands, Luxembourg, Belgium and as of June 2021, Spain. Organ donation after euthanasia in those countries is medically possible and thus represents a further option to provide these patients with the opportunity of organ donation and to expand the donor pool78,79.
Despite satisfactory outcomes10,69,70, many expanded and non-standard criteria donor organs remain underused in European countries8,10,80,81. An analysis from the USA demonstrated that the transplanted counterparts of >15% of unilaterally discarded donor kidneys showed a death-censored 5-year survival >85%82. Thus, discarded organs have the potential to contribute substantially to the donor organ pool and their use should be supported through the provision of information to health professionals about the benefit of using them and to the potential recipient, highlighting the benefits and risks of alternative choices. The rate of organ discard in Europe might be reduced by the application of risk score systems to guide the identification of suitable organs and appropriate recipients83.
Education
Several countries have implemented educational tools to promote organ donation and transplantation (Supplementary Box 1). A more harmonized approach across the EU could result in further structural improvements.
Improving communication skills of health-care professionals
Communication training should in particular focus on professionals involved in the early stages of the deceased donation process — such as emergency and intensive care physicians and donor coordinators45,84. Communication training should cover both sides of the donation and transplantation process; donor coordinators and professionals in intensive care should be trained in approaches to communicating with the families of possible donors, whereas transplantation professionals should be trained in approaches to communicating with potential recipients in an informative and efficient way. Information on the benefits and practical aspects of transplantation should be embedded in the curricula of all health practitioners, from medical students to postgraduate teaching of specialists and general practitioners (Supplementary Box 1). Specific involvement of health-care professionals trained in patient education as part of the treating team is extremely helpful.
Education of the public
Insufficient public awareness of organ donation and transplantation85,86, including the concept of brain death87, necessitates continuous education. A highly efficient strategy involves use of mass and social media, and requires the building of active partnerships with journalists41. Education activities in schools and use of flyers or web-based tools may help to increase awareness. In Croatia, information pamphlets with answers to frequently asked questions are made available to the public, whereas in Finland, an online educational tool is provided to educate patients and the general public about kidney transplantation (Supplementary Box 1). The EKHA “Gift of life” campaign88 offers advice to policy makers and citizens on approaches to promoting organ donation. This campaign stresses the need for a coordinated European approach based on appropriate legal and structural frameworks to allow individuals and professional nephrology and patient societies to promote kidney transplantation at the national policy level in an equitable way throughout Europe with respect to the local cultural background88. Such initiatives that focus on organ donation as a whole would further public education about the benefits and processes of transplantation.
Additional barriers that exist in economically or socially disadvantaged groups, including those with a low level of educational attainment, refugees, migrants and under-represented communities, should be specifically addressed with the help of patients, patient organizations, and minority communities, to understand attitudes and develop strategies and ensure equitable access to transplantation (see later)39. This aspect is particularly important as patients from these groups are generally over-represented on the transplant waiting list and under-represented in the donor population. Limited willingness to donate exacerbates the challenge of finding suitable HLA matches for kidney transplant candidates, prolonging the wait-list time, increasing the risk of mismatch, and jeopardizing long-term outcomes89.
Patient education and information
Limited health literacy (discussed below) and patient disinformation also deter transplantation90. The information provided by physicians and nurses to candidate organ recipients should discuss all treatment options, especially for kidney transplant candidates, for whom dialysis is a readily available but in many cases a less desirable alternative. Information about deceased and living donation2 should be provided, as often no information is offered about the two options (Supplementary Table 3). Education about organ replacement options should be provided as patients approach organ failure and be delivered in a tailored way either in hospital, in outpatient clinics or at home (Supplementary Box 1). Patient records should include an explicit statement on the suitability of the patient for transplantation, including the views of the patient and, particularly in the case of living donation, the views of their next of kin. To improve long-term patient outcomes91,92, education should include lifestyle advice, particularly approaches to addressing excess weight, smoking, excessive alcohol intake and hypertension and to promoting a healthy diet and exercise.
In 2017, EKHA distributed a questionnaire to determine the satisfaction level of patients from six EU countries with the information that had been provided to them about the different types of kidney replacement therapy in the period preceding the start of those therapies2 (Supplementary Table 3). Patient dissatisfaction with the quality of information provided about transplantation ranged from 11% (the Netherlands) to 45% (Greece). These data confirmed findings from a previous analysis93 of data collected from 2010 to 2011, suggesting little change since then, and underscoring the need for streamlined European education for all transplant candidates. A centralized quality check on information delivery and patient satisfaction might encourage excellence. Not surprisingly, dissatisfaction about the information provided coincides with low application of a given practice, suggesting a self-fulfilling circle (for example, in the Netherlands, which has a high transplantation rate, patient satisfaction is also very high; however, proportionally more patients received only information on living donation, which very likely occurred because that is the preferred mode of donation in that country).
Equality
Achieving equality in transplantation requires that all suitable candidates — irrespective of their ethnicity, race, sex, education, socio-economic status, religion, health literacy, or language barriers — have an equal probability of receiving a transplant. However, inequalities are rife in medical practice94 and deserve specific attention. Approaches to removing barriers to transplantation can in many instances be adapted to the specific needs of minority patient groups, as exemplified by the implementation of measures to increase access to transplantation for Jehovah’s Witnesses in Croatia (Supplementary Box 3). However, despite the existence of legal frameworks designed to prevent discrimination and ensure equitable access to health care and transplantation, in practice, access to transplantation remains extremely problematic for certain populations, especially for minority groups and immigrants, including undocumented migrants. These individuals face considerable barriers in access to health care, in particular to chronic therapies, including transplantation services. These barriers can arise from willing or unwilling institutional discrimination, the bias and prejudices of health professionals, as well as from non-familiarity of migrants with the medical model of the host country94,95. Educational approaches developed for the general public may not be appropriate for these communities, and specific efforts are required to ensure that these approaches reach affected individuals and are developed with input from the relevant populations. Comorbidities, such as diabetes, can be more prevalent in some ethnic minority populations, and negatively affect donation and transplantation rates96. Inequities among migrant populations are also closely linked to socio-economic status, exemplified by the well-documented associations between socio-economic status, waitlist placement and receipt of a transplant97. In the USA, African Americans, Hispanics and individuals of Asian ancestry are less likely than white Americans to receive a deceased donor kidney transplant89. In the UK, individuals of Asian and African Caribbean ancestry comprise 8% of the general population but 23% of the kidney transplant waitlist98,99. In the USA, the fact that the proportion of waitlisted patients vastly exceeds the number of available organs from donors of the same ethnicity has prompted adaptations of the kidney transplant allocation system, for example, by calculating the wait time from the start of dialysis instead of start of waitlisting, and by prioritizing the most sensitized patients89. These measures have reduced disparities in access to transplantation by increasing the proportion of actively waitlisted patients from under-represented communities and by decreasing inactive waitlisting of these individuals. These observations indicate that international and national authorities as well as professional organizations should provide regulations or recommendations to avoid discrimination in the selection process of donors and recipients for transplantation.
Disparities in access to transplantation among under-represented communities can also arise from a lower awareness of donation and transplantation processes, religious or cultural distrust of local medical professionals, fear of racism, linguistic obstacles, a lack of awareness of service availability, financial constraints, and a lack of perception of mainly asymptomatic chronic illnesses, such as kidney failure95,100. Given the importance of ethnicity as a determinant of tissue compatibility between donor and recipient, educational programmes aimed at increasing outreach to under-represented populations and at overcoming cultural or linguistic barriers are of critical importance. Although tissue matching is less important a determinant for liver or cardiothoracic transplantation than for kidney transplantation, the cultural and societal concerns are also evident in this setting.
Other subgroups, such as infants and highly sensitized individuals, also experience barriers to transplantation. Dedicated transplantation programmes, such as those that focus on identifying donor organs of appropriate size or on detecting appropriate donors using specific cross-matching methods, are needed101.
Finally, women are more likely to become a living donor than to receive a living organ donation102. Moreover, transplant recipients — irrespective of whether the organ is from a living or deceased donor — are predominantly male, especially for kidney transplants103. Although this inequality in access to transplantation might reflect a sex bias in the incidence of pathologies necessitating transplantation104, psychological and socio-economic factors also contribute to this disparity105, and could be prevented by addressing aspects of the transplantation system, such as inequalities in selection for waitlisting or unbalanced prioritization scores, that disadvantage women105.
Opt-in versus opt-out and donor registration
Considerable variation in legal frameworks for donation exists across the EU. Several EU and EU-associated countries, including Ireland, Germany, Denmark, Estonia and Switzerland, use opt-in legislation whereby consent for donation needs to be specifically sought from the donors and/or their families. Other countries apply an opt-out system (that is, consent for donation is presumed, unless the potential donors have officially registered their refusal). The Netherlands has in the past few years transitioned to an opt-out system, whereas the German government was unsuccessful in making this step. Most European countries that use an opt-out system apply a ‘soft approach’, allowing for objections by family members but supported by moral and legal leverage provided by the policy acceptance of the opt-out approach.
Compared with opt-in systems, opt-out systems are associated with higher donor rates ranging from 23.3% to 61.5% according to some studies106,107. However, a 2019 study of 35 countries found no difference in transplantation rates between opt-out and opt-in systems108. Multivariate analyses performed as part of that study showed that opt-out systems were independently predictive of lower rates of living donation. The divergent findings of studies that have compared opt-in and opt-out policies may be attributable to residual confounding resulting from differences in definitions, the selection of countries analysed, the period of analysis, or the choice of adjustment factors. Although there seems to be a gradual shift towards the use of opt-out systems, available data suggest that this approach as such is not sufficient to increase transplantation rates, and thus the adoption of opt-out legislation should be accompanied by other measures109, such as all those outlined in this Roadmap. In addition, simplified donor registration procedures such as those applied in Italy (Supplementary Box 4) offer an approach to encouraging donation without imposing judicial pressure110.
Financial and infrastructural barriers
Clinical activity related to transplantation should be subject to fair remuneration. Insufficient reimbursement to hospitals for deceased donation and organ retrieval is a major issue in some countries and may adversely affect donation rates. In many countries, the reimbursement for different kidney replacement therapies is disproportionate, such that dialysis is financially more rewarding than transplantation for care providers.
In the USA, for example, patients who receive dialysis at units managed by for-profit organizations have a lower chance of undergoing transplantation than patients treated at not-for-profit units111,112. Although difficult to extrapolate these findings to the EU, this suggests that the current imbalance in financial yield between different kidney replacement therapies can jeopardize transplantation rates, but also that differences in economic models governing health care should be considered by health-care administrations to incentivize transplantation over other approaches. For example, additional reimbursement could be given to units that have achieved high rates of transplantation among their population of patients with end-stage organ failure (Supplementary Box 5).
Furthermore, expansion of transplantation programmes should be supported by investment in adequate infrastructure. Recommendations for optimal infrastructure requirements and staffing of transplantation and intensive care units — including optimal numbers of surgeons, operating theatres, intensive care facilities, appropriate hospitalization and outpatient follow-up facilities, and well-trained nursing and medical staff — are all urgently needed.
Long-term preservation of graft function
Long-term preservation of graft function is the most important outcome for transplant recipients43. Maintenance of graft function entails avoidance of damage by rejection, medication, complications, comorbidities, or damage to other organs (for example, avoiding kidney damage in heart or liver transplant recipients due to immunosuppressive medication), but also requires specific attention to fatal outcomes or complications that might jeopardize future transplantation procedures (such as opportunistic infections, malignancy, cardiovascular disease, post-transplantation diabetes mellitus)50,113. In the first 10 years after transplantation mortality is substantially higher than that of the general population, at around 40%114, with a similar percentage of fatalities over the subsequent 10 years50,115. In addition, at least 15% of survivors lose function of the transplanted organ per decade116. Of note, despite a consistent improvement in kidney graft survival in the first 5 years post-transplant between 1986 and 2015, graft survival after the fifth year of transplantation has not substantially changed over time116.
Several aspects of the transplantation process should be addressed to maximize the likelihood of transplant survival. Cold ischaemia time is an important modifiable risk factor for poor transplant outcomes117–119, and it is imperative that transplantation logistics are constantly reviewed and improved to keep cold ischaemia time as short as possible. Controlling organ fibrosis may be one of the few solutions to preventing long-term graft loss, but therapeutic solutions to tackle this problem are scarce120. For recipients of kidney transplants experiencing graft loss, timely and uncomplicated transition onto dialysis is essential, as mortality is high in the period of dialysis (re-)initiation121. Non-adherence to medication is a major contributor to graft loss122,123 and interventions that augment adherence increase graft survival124. Monitoring markers of immunosuppression can also help to individualize immunosuppressive therapy to maximize drug efficacy and minimize toxicity125,126.
A critical consideration for paediatric transplant recipients is their transition to adult transplantation clinics. This transition period can be associated with reduced compliance related to the change in environment, as differences in the approach and philosophy of adult transplantation clinics may be perceived as inhospitable by adolescents who are often psychologically and socially vulnerable127.
A significant number of graft-survival years are lost when young donor kidneys are transplanted into older recipients and vice versa128. Matching the life expectancy of the intended recipient with the projected life span of the transplant is likely to maximize graft survival and cost savings128. Complex algorithms are required to ensure optimal matching and account for differences in population demographics. Other factors that affect long-term organ function, such as the presence of low-level preformed donor-specific HLA antibodies require further study129. The role of lifestyle factors and the possible role of tailored medication also deserve further consideration.
Clustering of countries
Some countries have a strong track record in living donation and others in deceased donation, but few do both well. Similarly, some may be more successful than others at transplanting specific organs. Several countries could benefit from improving their donor coordination and recruitment processes and/or from adopting or improving expanded donation criteria. Specific scenarios should be developed according to the areas that require improvement, with countries grouped according to these characteristics. Even countries that perform well overall have room for further improvement, as exemplified by Spain, which manages to improve every year upon already high transplantation rates (Supplementary Figure 1).
Clustering of countries with similar needs and characteristics can streamline the development of action plans that enable different strategies for each cluster. These action plans should also account for country-specific measures and include in-depth consultation with the local transplant communities, including National Competent Authorities, transplant physicians, coordinators, regulators and authorities with representatives of other countries and the EU, enabling rapid dissemination and implementation of good clinical practice. This clustering approach could group countries with specific characteristics, for example, those needing to increase living donation compared with rates of deceased donation, or those where expanded donation criteria or donation overall could be enhanced.
Benchmarking
The optimization of transplantation programmes necessitates continuous assessment with external audits and comparison of their efficiency with peer programmes130. A uniform registration process and quality control system for organ donation and transplantation throughout Europe is necessary to enable this benchmarking. Transparency of hospitals in reporting their performance for access to and outcomes of transplantation is essential112. It is imperative that pan-European transplant registries are established for each organ, to enable benchmarking and ensure that comparable results are achieved across the EU. These comparisons would inform the specific areas for development and address local factors to ensure equitable access to transplantation and to optimize outcomes across Europe. Initiatives that enable comparisons of organ donation and transplantation rates between countries, similar to that developed by the Council of Europe Committee on Organ Transplantation, can help to stimulate countries that are seeking to achieve best practices131. Studying the approaches of the best performers will identify a number of critical factors for success, which can then be implemented elsewhere132.
Specific frameworks that promote and guide appropriate evidence-based decision making in the context of transplantation should be facilitated and supported. Recommendations might include but should not be limited to criteria for acceptance of patients on the waiting list; adequate follow-up post-transplantation; criteria for DCD transplantation; standards for transplant centres to achieve a well-functioning programme supported by adequate infrastructure; and optimal conditions for donor organ recovery. The application of European recommendations should be based on a continuously audited pan-European platform but allow adaptations according to the situation of individual countries. In Spain, benchmarking of different elements of the transplantation process is one of the cornerstones of its success39.
Research
Despite substantial national and trans-national progress over the past five decades, several fundamental questions in the field of transplantation remain unsolved. These range from the basic patho-physiology, immunology and molecular biology of the transplantation process and the response of the host to the donor organ, to clinical aspects and those relating to organizational, societal, psychological and quality of life issues. Transplantation research deserves specific attention for funding and support. Patients, medical professionals and society as a whole will benefit from research streamlined to focus on a number of areas and topics of prime interest (Box 2), with the aim of better shaping future priorities, developments and policy actions in the field.
Box 2 Suggested research topics.
Approaches to improving organ quality and assessment, and increasing organ availability
Studies on novel preservation methods and new technologies for testing organ quality
Establishing pan-European registries that include follow-up data
Exploring alternative sources of organs (hybrids, xenotransplantation)
Studies of factors that affect outcomes of expanded donation criteria and donation after circulatory death
Comparison of strategies for increasing donor availability
Identification and prevention of factors leading to delayed graft function
Studies on non-HLA incompatibility
Development of strategies to combat acute and chronic rejection
Development and assessment of methods to improve transplantation rates in children, elderly individuals and highly sensitized patients
Studies of barriers to transplantation and measures to correct those; comparisons between countries
Socio-economic and societal impact of transplantation
Health-economic comparison of transplantation programmes in different EU countries
Identification of approaches to decrease the societal cost of transplantation
Studies of the ecological footprint of kidney transplantation compared with dialysis
Studies of factors preventing reemployment after transplantation
Extending the life of the transplant and reducing graft loss
Defining surrogate end points for post-transplantation outcomes
Identification of biomarkers of acute and chronic rejection, graft failure and negative outcomes at large
Detection of mechanisms causing graft dysfunction via development of fibrosis and ways of preventing this evolution
Prevention of post-transplantation malignancy and cardiovascular disease
Prevention and adequate treatment of infections after transplantation
Strategies to improve outcomes for patients with a failing kidney transplant who are transitioning to dialysis
Benchmarking, professionalism and governance
Comparisons of different types of machine perfusion related to different organs for preservation, especially donation after circulatory death
Certification of skills of professionals and professional regulation
Benchmarking of transplantation outcomes (based on registry data)
Study of barriers to transplantation in different countries
Patient priorities
Studies on patient-reported outcomes
Studies of mechanisms that determine treatment choice (transplantation compared with alternatives such as dialysis) and suggestions as to how valid patients can be encouraged to undergo transplantation
Comparative studies of educational programmes (for the general population, patients, students, professionals) and development of best practices
Adapted with permission from ref.136, the European Kidney Health Alliance.
International organ exchange
Several EU countries do not have an efficient system to enable the internal offering and sharing of donated organs, nor do they collaborate in wider, usually transnational programmes for deceased donor organ exchange (such as Eurotransplant, Scandiatransplant or the South Alliance for Transplantation). At a minimum, each country should have a national sharing scheme between the local transplant centres whereby an optimal match between donor and recipient and rapid organ removal and transfer to the receiving centre are assured. Furthermore, gradual incorporation into one of the existing international exchange systems should be encouraged as an approach to boost transplantation activity42, as exemplified by Croatia in the early 2000s, or more recently by Hungary133. Such programmes enable expansion of the donor pool and provide a transparent, equitable and defensible method with which to match the most appropriate donor–recipient pairs as rapidly as possible and thereby improve donor outcomes. In Switzerland, which in essence operates an individual national procurement and offering system (Swisstransplant), close to 6% of the heart transplants and 3% of the lung transplants originate from cross-border organ sharing134.
In 2012 the FOEDUS-EOEO platform was launched. FOEDUS-EOEO is an IT-based system that allows European countries to connect with allocation offices ensuring that the organs that cannot be matched within the national or collaborative supra-national systems are available internationally. This type of broad international collaboration is especially beneficial for children and adolescents11 but also for other vulnerable recipients within a small donor pool (for example, highly sensitized patients).
Conclusions
Despite a good overall track record in the field of transplantation, disparities in transplantation rates between EU countries suggest that there remains ample room for improvement. The Action Plan launched by the EU in 2009 increased organ donation and transplantation and was in place until 2015, but further action is now needed to boost activity. Given the substantial differences between countries in transplantation practices (for example, in the overall transplantation rate, ratio of deceased compared with living donation, application of expanded donation and transplantation of specific organs), an in-depth analysis of discrepancies in transplantation rates is required to inform future improvements across the EU. Optimization and coordination of the donation process is indispensable for a successful transplantation programme. Education of patients, professionals and the general population as well as the provision of appropriate legal and financial frameworks is also necessary. This Roadmap, formulated from a thematic network of European organizations, gives a number of recommendations3 that provide a framework for further action with which to better cope with the growing transplant waiting lists, reduce the number of patients dying on waiting lists, improve equality in access to transplantation, and improve the outcomes of transplanted organs, inside, as well as outside, the EU.
In this Roadmap, we assume that the primary element needed to increase the number of patients with a functioning graft is increased organ donation, which requires investment in processes to coordinate the donation process, approaches to encouraging living donation and consideration of expanded donation criteria. However, donation cannot be enhanced without a parallel investment in infrastructure, the implementation of approaches to overcoming financial barriers, and educational efforts. The remaining factors, such as the need for benchmarking, registration, research and efforts to abolish inequities, might not directly affect the total number of transplants, but their consideration remains essential for ethical reasons and because they support the other strategies. The Joint Statement on which this Roadmap is based3 outlines a number of key areas along which policy makers could streamline such a plan (Supplementary Box 6). The development of such a plan should aim to stimulate increases in transplantation rates similar to those achieved following implementation of the original EU Action Plan on Organ Donation and Transplantation (2009–2015), and should involve yearly assessments and adjustments per country or group of countries, with successful countries serving as examples for the others.
Supplementary information
Acknowledgements
Each year the European Union commissions a number of Joint Statements regarding health issues, which bring together several stakeholder groups to make recommendations to the European Commission for the coming years. The coordination of the joint statement informing the present Roadmap was allocated to the European Kidney Health Alliance (EKHA). The EKHA represents a common effort by all European key stakeholders in kidney care (patients, nurses, physicians, foundations) to propose solutions for the challenges of kidney disease in Europe through the development of effective prevention and more efficient care pathways. In addition to the EKHA, the organizations involved in this publication and the European Commission Joint Statement are either pan-European Organizations with a focus on transplantation or transplantable organs or the National Transplant Organizations of Croatia and Spain. Specifically, these organizations are the European Society of Organ Transplantation (ESOT); the European Renal Association–European Dialysis and Transplant Association (ERA–EDTA); the ERA-EDTA Registry; United European Gastroenterology (UEG); the European Association for the Study of the Liver (EASL) and the National Competent Authorities for Organ Donation and Transplantation of Croatia and Spain. The Joint Statement was endorsed by 54 Organizations and Institutions and by 19 Members of the European Parliament. The authors are indebted to Marine Faure and Sophie Bruno (Senior Consultants, Interel European Affairs) and Nazli Gül (Consultant, Interel European Affairs) for their assistance in the preparation of the Joint Statement that is the basis for this publication.
Glossary
- European Kidney Health Alliance
(EKHA). A non-governmental advocacy organization promoting the cause of kidney patients at the level of the European Union and the European Union member states.
- Pre-emptive transplantation
A situation in which a kidney transplant candidate receives a donor kidney before starting on dialysis.
- Organ-sharing schemes
Programmes that enable sharing of organs between donor–recipient pairs who cannot exchange a donor organ directly with each other.
- Inactive waitlisting
A category of patients on the transplant waitlist who are temporarily ineligible for transplantation because of medical, social or personal reasons.
Author contributions
R.V. contributed to conceptualizing and designing the Roadmap, data analyses, the literature search and writing of the manuscript. B.D.-G. contributed to designing the Roadmap, performing the literature search, data collection and writing. G.C.O. contributed to designing the Roadmap, performing the literature search, writing and editing the manuscript before submission. M.B., H.C.-P. and J.C.C. contributed to writing of the manuscript. K.J.J. contributed to writing and data collection. B.M. contributed to data collection. V.S.S. and M.O.V. contributed to the literature search, writing and data collection.
Competing interests
R.V. reports unrestricted grants to the European Kidney Health Alliance from Baxter Health Care, Astellas, Amgen, BBraun, CSL Behring, Hansa BioPharma and Viforpharma, for the organization of the annual EKHA Kidney Forum at the European Parliament. H.C.-P. reports personal fees from Intercept, Gilead, Promethera and Genfit, outside of the submitted work. K.J.J. reports grants from ERA-EDTA during the conduct of the study, and personal fees from Fresenius Medical Care, outside of the submitted work. The other authors declare no conflicts of interest.
Footnotes
Peer review information
Nature Reviews Nephrology thanks L. Hilbrands, who co-reviewed with M. Baas, A. Rahmel, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
European Kidney Health Alliance: http://ekha.eu/
FOEDUS-EOEO: https://www.foedus-eoeo.eu/#/public
Supplementary information
The online version contains supplementary material available at 10.1038/s41581-021-00425-3.
References
- 1.Vanholder R, et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat. Rev. Nephrol. 2017;13:393–409. doi: 10.1038/nrneph.2017.63. [DOI] [PubMed] [Google Scholar]
- 2.Vanholder R, et al. How to increase kidney transplant activity throughout Europe — an advocacy review by the European Kidney Health Alliance. Nephrol. Dial. Transplant. 2019;34:1254–1261. doi: 10.1093/ndt/gfy390. [DOI] [PubMed] [Google Scholar]
- 3.European Kidney Health Alliance. Joint Statement. Thematic Network on Improving Organ Donation and Transplantation in the EU 2019http://ekha.eu/wp-content/uploads/FINAL_Joint-Statement-of-the-Thematic-Network-on-Organ-Donation-and-Transplantation.pdf (2019).
- 4.World Health Organization. Noncommunicable diseases. WHOhttp://www.euro.who.int/en/health-topics/noncommunicable-diseases/noncommunicable-diseases (2017).
- 5.NCD Countdown Collaborators. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet. 2018;392:1072–1088. doi: 10.1016/S0140-6736(18)31992-5. [DOI] [PubMed] [Google Scholar]
- 6.Delmonico FL, Dominguez-Gil B, Matesanz R, Noel L. A call for government accountability to achieve national self-sufficiency in organ donation and transplantation. Lancet. 2011;378:1414–1418. doi: 10.1016/S0140-6736(11)61486-4. [DOI] [PubMed] [Google Scholar]
- 7.WHO, Transplantation Society, Organization Nacional de Transplantes Third WHO Global Consultation on Organ Donation and Transplantation: striving to achieve self-sufficiency, March 23-25, 2010, Madrid, Spain. Transplantation. 2011;91(Suppl. 11):S27–S28. doi: 10.1097/TP.0b013e3182190b29. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez-Gil, B. International figures on donation and transplantation 2019. Newsletter Transplant. European Parliamenthttps://www.europarl.europa.eu/EPRS/Newsletter_Transplant_2019.pdf (2020).
- 9.Haase B, et al. Ethical, legal, and societal issues and recommendations for controlled and uncontrolled DCD. Transpl. Int. 2016;29:771–779. doi: 10.1111/tri.12720. [DOI] [PubMed] [Google Scholar]
- 10.Lomero M, et al. Donation after circulatory death today: an updated overview of the European landscape. Transpl. Int. 2020;33:76–88. doi: 10.1111/tri.13506. [DOI] [PubMed] [Google Scholar]
- 11.Bouwman, R. et al. Study on the uptake and impact of the EU Action Plan on Organ Donation and Transplantation (2009-2015) in the EU Member States. European Commissionhttps://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/2017_euactionplan_2009-2015_impact_exe_en.pdf (2017).
- 12.Zoccali C, Kramer A, Jager K. The databases: renal replacement therapy since 1989 — the European Renal Association and European Dialysis and Transplant Association (ERA-EDTA) Clin. J. Am. Soc. Nephrol. 2009;4(Suppl. 1):18–22. doi: 10.2215/CJN.05210709. [DOI] [PubMed] [Google Scholar]
- 13.No Authors Listed. Organ Transplant Scandal Shocks Germany. Spiegelhttp://www.spiegel.de/international/germany/organ-transplant-scandal-shocks-germany-a-848016.html (2012).
- 14.Foreman KJ, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonelli M, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 2011;11:2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 16.Haller MC, Kainz A, Baer H, Oberbauer R. Dialysis vintage and outcomes after kidney transplantation: a retrospective cohort study. Clin. J. Am. Soc. Nephrol. 2017;12:122–130. doi: 10.2215/CJN.04120416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong G, et al. Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co-morbidities. PLoS ONE. 2012;7:e29591. doi: 10.1371/journal.pone.0029591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Renal Association – European Dialysis and Transplant Association. ERA-EDTA Registry: ERA-EDTA Registry Annual Report 2017. ERA-EDTAhttps://era-edta-reg.org/files/annualreports/AnnRep2017.pdf (2017).
- 19.Cassuto JR, et al. Wait list death and survival benefit of kidney transplantation among nonrenal transplant recipients. Am. J. Transplant. 2010;10:2502–2511. doi: 10.1111/j.1600-6143.2010.03292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haller M, Gutjahr G, Kramar R, Harnoncourt F, Oberbauer R. Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol. Dial. Transplant. 2011;26:2988–2995. doi: 10.1093/ndt/gfq780. [DOI] [PubMed] [Google Scholar]
- 21.Mohnen SM, et al. Healthcare costs of patients on different renal replacement modalities - Analysis of Dutch health insurance claims data. PLoS ONE. 2019;14:e0220800. doi: 10.1371/journal.pone.0220800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhutiani N, et al. A cost analysis of early biliary strictures following orthotopic liver transplantation in the United States. Clin. Transplant. 2018;32:e13396. doi: 10.1111/ctr.13396. [DOI] [PubMed] [Google Scholar]
- 23.Dageforde LA, Feurer ID, Pinson CW, Moore DE. Is liver transplantation using organs donated after cardiac death cost-effective or does it decrease waitlist death by increasing recipient death? HPB. 2013;15:182–189. doi: 10.1111/j.1477-2574.2012.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams R, et al. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2018;391:1097–1107. doi: 10.1016/S0140-6736(17)32866-0. [DOI] [PubMed] [Google Scholar]
- 25.Long EF, Swain GW, Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ. Heart Fail. 2014;7:470–478. doi: 10.1161/CIRCHEARTFAILURE.113.000807. [DOI] [PubMed] [Google Scholar]
- 26.Ye XT, Parker A, Brink J, Weintraub RG, Konstantinov IE. Cost-effectiveness of the National Pediatric Heart Transplantation Program in Australia. J. Thorac. Cardiovasc. Surg. 2018;157:1158–1166. doi: 10.1016/j.jtcvs.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 27.OECD & European Union. Health at a Glance: Europe 2016 – State of Health in the EU Cycle. OECD iLibrary10.1787/9789264265592-en (2016).
- 28.Danuser B, et al. Employment 12 months after kidney transplantation: An in-depth bio-psycho-social analysis of the Swiss Transplant Cohort. PLoS ONE. 2017;12:e0175161. doi: 10.1371/journal.pone.0175161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dukic MZ, Zibar L. Employment in patients with renal replacement therapy. SEEMED J. 2019;3:11–20. [Google Scholar]
- 30.Julian Mauro JC, Molinuevo Tobalina JA, Sanchez Gonzalez JC. Employment in the patient with chronic kidney disease related to renal replacement therapy. Nefrologia. 2012;32:439–445. doi: 10.3265/Nefrologia.pre2012.Apr.11366. [DOI] [PubMed] [Google Scholar]
- 31.Liem YS, Bosch JL, Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2008;11:733–741. doi: 10.1111/j.1524-4733.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 32.Manificat S, et al. Quality of life of children and adolescents after kidney or liver transplantation: child, parents and caregiver’s point of view. Pediatr. Transplant. 2003;7:228–235. doi: 10.1034/j.1399-3046.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 33.Commission of the European Communities. Action plan on Organ Donation and Transplantation (2009-2015): Strengthened Cooperation between Member. States. European Commissionhttps://ec.europa.eu/health/ph_threats/human_substance/oc_organs/docs/organs_action_en.pdf (2008).
- 34.Wu DA, et al. Barriers to living donor kidney transplantation in the United Kingdom: a national observational study. Nephrol. Dial. Transplant. 2017;32:890–900. doi: 10.1093/ndt/gfx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Council of Europe. Legal Framework. https://www.edqm.eu/en/legal-framework (2021).
- 36.No Authors Listed The Madrid resolution on organ donation and transplantation: national responsibility in meeting the needs of patients, guided by the WHO principles. Transplantation. 2011;91(Suppl. 11):S29–S31. doi: 10.1097/01.tp.0000399131.74618.a5. [DOI] [PubMed] [Google Scholar]
- 37.Hanson CS, et al. Nephrologists’ perspectives on recipient eligibility and access to living kidney donor transplant. Transplantation. 2016;100:943–953. doi: 10.1097/TP.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 38.de Jong RW, et al. Non-medical barriers reported by nephrologists when providing renal replacement therapy or comprehensive conservative management to end-stage kidney disease patients: a systematic review. Nephrol. Dial. Transplant. 2020 doi: 10.1093/ndt/gfz271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matesanz R, Dominguez-Gil B, Coll E, de la Rosa G, Marazuela R. Spanish experience as a leading country: what kind of measures were taken? Transplant. Int. 2011;24:333–343. doi: 10.1111/j.1432-2277.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- 40.Matesan ZR, Marazuela R, Coll E, Mahillo B, Dominguez-Gil B. About the opt-out system, live transplantation, and information to the public on organ donation in Spain… Y ole! Am. J. Transpl. 2017;17:1695–1696. doi: 10.1111/ajt.14296. [DOI] [PubMed] [Google Scholar]
- 41.Dominguez-Gil B. End-of-life practices in patients with devastating brain injury in Spain: implications for organ donation. Med. Intensiva. 2017;41:162–173. doi: 10.1016/j.medin.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Zivcic-Cosic S, et al. Development of the Croatian model of organ donation and transplantation. Croat. Med. J. 2013;54:65–70. doi: 10.3325/cmj.2013.54.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.European Kidney Health Alliance. European Kidney Forum 2019 “Organ Donation and Transplantation in Europe – Are We Meeting the Needs of Patients?”. EKHAhttp://ekha.eu/blog/european-kidney-forum-2019-organ-donation-and-transplantation-in-europe-are-we-meeting-the-needs-of-patients/ (2019).
- 44.Sautenet B, et al. Developing consensus-based priority outcome domains for trials in kidney transplantation: a multinational Delphi survey with patients, caregivers, and health professionals. Transplantation. 2017;101:1875–1886. doi: 10.1097/TP.0000000000001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominguez-Gil B, Murphy P, Procaccio F. Ten changes that could improve organ donation in the intensive care unit. Intensive Care Med. 2016;42:264–267. doi: 10.1007/s00134-015-3833-y. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Delgado MC, et al. Summary of Spanish recommendations on intensive care to facilitate organ donation. Am. J. Transplant. 2019;19:1782–1791. doi: 10.1111/ajt.15253. [DOI] [PubMed] [Google Scholar]
- 47.Shrestha S, et al. Logistical factors influencing cold ischemia times in deceased donor kidney transplants. Transplantation. 2016;100:422–428. doi: 10.1097/TP.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 48.Miller C, Breakwell R. What factors influence a family’s decision to agree to organ donation? A critical literature review. London J. Prim. Care. 2018;10:103–107. doi: 10.1080/17571472.2018.1459226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dominguez-Gil B, et al. The critical pathway for deceased donation: reportable uniformity in the approach to deceased donation. Transplant. Int. 2011;24:373–378. doi: 10.1111/j.1432-2277.2011.01243.x. [DOI] [PubMed] [Google Scholar]
- 50.Traynor C, et al. Twenty-year survivors of kidney transplantation. Am. J. Transplant. 2012;12:3289–3295. doi: 10.1111/j.1600-6143.2012.04236.x. [DOI] [PubMed] [Google Scholar]
- 51.Reichman TW, et al. Living donor versus deceased donor liver transplantation: a surgeon-matched comparison of recipient morbidity and outcomes. Transpl. Int. 2013;26:780–787. doi: 10.1111/tri.12127. [DOI] [PubMed] [Google Scholar]
- 52.Mandelbrot DA, Pavlakis M. Living donor practices in the United States. Adv. Chron. Kidney Dis. 2012;19:212–219. doi: 10.1053/j.ackd.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holscher CM, et al. Self-reported incident hypertension and long-term kidney function in living kidney donors compared with healthy nondonors. Clin. J. Am. Soc. Nephrol. 2019;14:1493–1499. doi: 10.2215/CJN.04020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kestenbaum BR, Seliger SL. Commentary on risks of living kidney donation: current state of knowledge on core outcomes important to donors. Clin. J. Am. Soc. Nephrol. 2019;14:609–610. doi: 10.2215/CJN.01650219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lennerling A, et al. Living organ donation practices in Europe — results from an online survey. Transpl. Int. 2013;26:145–153. doi: 10.1111/tri.12012. [DOI] [PubMed] [Google Scholar]
- 56.Lentine KL, Lam NN, Segev DL. Risks of living kidney donation: current state of knowledge on outcomes important to donors. Clin. J. Am. Soc. Nephrol. 2019;14:597–608. doi: 10.2215/CJN.11220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrari P, Weimar W, Johnson RJ, Lim WH, Tinckam KJ. Kidney paired donation: principles, protocols and programs. Nephrol. Dial. Transplant. 2015;30:1276–1285. doi: 10.1093/ndt/gfu309. [DOI] [PubMed] [Google Scholar]
- 58.Biro P, et al. Building kidney exchange programmes in europe-an overview of exchange practice and activities. Transplantation. 2019;103:1514–1522. doi: 10.1097/TP.0000000000002432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valentin MO, et al. International cooperation for kidney exchange success. Transplantation. 2019;103:e180–e181. doi: 10.1097/TP.0000000000002664. [DOI] [PubMed] [Google Scholar]
- 60.Bohmig GA, et al. Czech-Austrian kidney paired donation: first European cross-border living donor kidney exchange. Transplant. Int. 2017;30:638–639. doi: 10.1111/tri.12945. [DOI] [PubMed] [Google Scholar]
- 61.Working Group on Living Donation. Toolbox Living Kidney Donation. European Commissionhttps://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/eutoolbox_living_kidney_donation_en.pdf (2016).
- 62.Truog RD. The ethics of organ donation by living donors. N. Engl. J. Med. 2005;353:444–446. doi: 10.1056/NEJMp058155. [DOI] [PubMed] [Google Scholar]
- 63.Port FK, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–1286. doi: 10.1097/00007890-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 64.Watson CJ, Johnson RJ, Birch R, Collett D, Bradley JA. A simplified donor risk index for predicting outcome after deceased donor kidney transplantation. Transplantation. 2012;93:314–318. doi: 10.1097/TP.0b013e31823f14d4. [DOI] [PubMed] [Google Scholar]
- 65.Collett D, Friend PJ, Watson CJ. Factors associated with short- and long-term liver graft survival in the United Kingdom: development of a UK donor liver index. Transplantation. 2017;101:786–792. doi: 10.1097/TP.0000000000001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dahmen M, et al. Validation of the Kidney Donor Profile Index (KDPI) to assess a deceased donor’s kidneys’ outcome in a European cohort. Sci. Rep. 2019;9:11234. doi: 10.1038/s41598-019-47772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen JB, et al. Survival benefit of transplantation with a deceased diabetic donot kidney compared with remaining on the waitlist. Clin. J. Am. Soc. Nephrol. 2017;12:974–982. doi: 10.2215/CJN.10280916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibrahim M, et al. Utilization and outcomes of single and dual kidney transplants from older deceased donors in the United Kingdom. Clin. J. Am. Soc. Nephrol. 2020;15:1320–1329. doi: 10.2215/CJN.02060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaapherder A, et al. Equivalent long-term transplantation outcomes for kidneys donated after brain death and cardiac death: conclusions from a nationwide evaluation. EClinicalMedicine. 2018;4:25–31. doi: 10.1016/j.eclinm.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Summers DM, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet. 2010;376:1303–1311. doi: 10.1016/S0140-6736(10)60827-6. [DOI] [PubMed] [Google Scholar]
- 71.Smith M, Dominguez-Gil B, Greer DM, Manara AR, Souter MJ. Organ donation after circulatory death: current status and future potential. Intensive Care Med. 2019;45:310–321. doi: 10.1007/s00134-019-05533-0. [DOI] [PubMed] [Google Scholar]
- 72.Minambres E, Rubio JJ, Coll E, Dominguez-Gil B. Donation after circulatory death and its expansion in Spain. Curr. Opin. Organ. Transplant. 2018;23:120–129. doi: 10.1097/MOT.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 73.Trapero-Marugan M, Little EC, Berenguer M. Stretching the boundaries for liver transplant in the 21st century. Lancet Gastroenterol. Hepatol. 2018;3:803–811. doi: 10.1016/S2468-1253(18)30213-9. [DOI] [PubMed] [Google Scholar]
- 74.No Authors Listed. NEW release: 7th edition Guide to the quality and safety of Organs for transplantation. Council of Europehttps://www.edqm.eu/en/news/new-release-7th-edition-guide-quality-and-safety-organs-transplantation (2018).
- 75.Kumar RN, Stosor V. Organ transplantation in persons with HIV. AIDS. 2020;34:1107–1116. doi: 10.1097/QAD.0000000000002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Potluri VS, et al. National trends in utilization and 1-year outcomes with transplantation of HCV-viremic kidneys. J. Am. Soc. Nephrol. 2019;30:1939–1951. doi: 10.1681/ASN.2019050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Givertz MM, Woolley AE, Baden LR. Expanding the donor pool with use of hepatitis C infected hearts. Circ. Heart Fail. 2018;11:e005656. doi: 10.1161/CIRCHEARTFAILURE.118.005656. [DOI] [PubMed] [Google Scholar]
- 78.Ysebaert D, et al. Organ procurement after euthanasia: Belgian experience. Transplant. Proc. 2009;41:585–586. doi: 10.1016/j.transproceed.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 79.Wilkinson D, Savulescu J. Should we allow organ donation euthanasia? Alternatives for maximizing the number and quality of organs for transplantation. Bioethics. 2012;26:32–48. doi: 10.1111/j.1467-8519.2010.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bouwman, R., Wiegers, T., van Schoten, S., Coppen, R. & Friele, R. Study on the uptake and impact of the EU Action Plan on Organ Donation and Transplantation (2009-2015) in the EU Member States. European Commissionhttps://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/2017_euactionplan_2009-2015_impact_exe_en.pdf (2017).
- 81.Dominguez-Gil B, et al. Current situation of donation after circulatory death in European countries. Transplant. Int. 2011;24:676–686. doi: 10.1111/j.1432-2277.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 82.Mohan S, et al. Factors leading to the discard of deceased donor kidneys in the United States. Kidney Int. 2018;94:187–198. doi: 10.1016/j.kint.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stallone G, Grandaliano G. To discard or not to discard: transplantation and the art of scoring. Clin. Kidney J. 2019;12:564–568. doi: 10.1093/ckj/sfz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manara A, Procaccio F, Dominguez-Gil B. Expanding the pool of deceased organ donors: the ICU and beyond. Intensive Care Med. 2019;45:357–360. doi: 10.1007/s00134-019-05546-9. [DOI] [PubMed] [Google Scholar]
- 85.Scandroglio B, et al. Analysis of the attitudes and motivations of the Spanish population towards organ donation after death. Transpl. Int. 2011;24:158–166. doi: 10.1111/j.1432-2277.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 86.Byrne M, et al. School education to increase organ donation and awareness of issues in transplantation in the UK. Pediatr. Transplant. 2019;23:e13492. doi: 10.1111/petr.13492. [DOI] [PubMed] [Google Scholar]
- 87.Febrero B, et al. Knowledge of the brain death concept among older people. Transplant. Proc. 2020;52:506–508. doi: 10.1016/j.transproceed.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 88.No Authors Listed. Gift of Life. European Kidney Health Alliancehttp://ekha.eu/gift-of-life/ (2018).
- 89.Zhang X, et al. Racial/ethnic disparities in waitlisting for deceased donor kidney transplantation 1 year after implementation of the new national kidney allocation system. Am. J. Transplant. 2018;18:1936–1946. doi: 10.1111/ajt.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garg AX. Helping more patients receive a living donor kidney transplant. Clin. J. Am. Soc. Nephrol. 2018;13:1918–1923. doi: 10.2215/CJN.00760118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoogeveen EK, et al. Effect of obesity on the outcome of kidney transplantation: a 20-year follow-up. Transplantation. 2011;91:869–874. doi: 10.1097/TP.0b013e3182100f3a. [DOI] [PubMed] [Google Scholar]
- 92.Oste MC, et al. Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res. Care. 2017;5:e000283. doi: 10.1136/bmjdrc-2016-000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Biesen W, van der Veer SN, Murphey M, Loblova O, Davies S. Patients’ perceptions of information and education for renal replacement therapy: an independent survey by the European Kidney Patients’ Federation on information and support on renal replacement therapy. PLoS ONE. 2014;9:e103914. doi: 10.1371/journal.pone.0103914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J. Am. Soc. Nephrol. 2008;19:1261–1270. doi: 10.1681/ASN.2008030276. [DOI] [PubMed] [Google Scholar]
- 95.Poulakou G, Len O, Akova M. Immigrants as donors and transplant recipients: specific considerations. Intensive Care Med. 2019;45:401–403. doi: 10.1007/s00134-019-05534-z. [DOI] [PubMed] [Google Scholar]
- 96.Ikram UZ, Kunst AE, Lamkaddem M, Stronks K. The disease burden across different ethnic groups in Amsterdam, the Netherlands, 2011–2030. Eur. J. Publ. Health. 2013;24:600–605. doi: 10.1093/eurpub/ckt136. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y, Gerdtham UG, Rydell H, Jarl J. Socioeconomic inequalities in the kidney transplantation process: a registry-based study in Sweden. Transplant. Direct. 2018;4:e346. doi: 10.1097/TXD.0000000000000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Department of Health. Organs for Transplants A report from the Organ Donation Taskforce. NHShttps://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/4245/organsfortransplantstheorgandonortaskforce1streport.pdf.
- 99.Tiessen, J. et al. Improving Organ Donation and Transplantation in the European Union. Assessing the Impacts of European Action. Rand Europehttps://www.rand.org/content/dam/rand/pubs/technical_reports/2008/RAND_TR602.pdf (2008).
- 100.Bratton C, Chavin K, Baliga P. Racial disparities in organ donation and why. Curr. Opin. Organ. Transpl. 2011;16:243–249. doi: 10.1097/MOT.0b013e3283447b1c. [DOI] [PubMed] [Google Scholar]
- 101.Valentin MO, et al. Implementation of a national priority allocation system for hypersensitized patients in spain, based on virtual crossmatch: initial results. Transplant. Proc. 2016;48:2871–2875. doi: 10.1016/j.transproceed.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 102.Puoti F, et al. Organ transplantation and gender differences: a paradigmatic example of intertwining between biological and sociocultural determinants. Biol. Sex. Differ. 2016;7:35. doi: 10.1186/s13293-016-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Antlanger M, et al. Sex differences in kidney replacement therapy initiation and maintenance. Clin. J. Am. Soc. Nephrol. 2019;14:1616–1625. doi: 10.2215/CJN.04400419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018;14:151–164. doi: 10.1038/nrneph.2017.181. [DOI] [PubMed] [Google Scholar]
- 105.Melk A, et al. Equally interchangeable? How sex and gender affect transplant. Transplantation. 2019;103:1094–1110. doi: 10.1097/TP.0000000000002655. [DOI] [PubMed] [Google Scholar]
- 106.Shepherd L, O’Carroll RE, Ferguson E. An international comparison of deceased and living organ donation/transplant rates in opt-in and opt-out systems: a panel study. BMC Med. 2014;12:131. doi: 10.1186/s12916-014-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abadie A, Gay S. The impact of presumed consent legislation on cadaveric organ donation: a cross-country study. J. Health Econ. 2006;25:599–620. doi: 10.1016/j.jhealeco.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 108.Arshad A, Anderson B, Sharif A. Comparison of organ donation and transplantation rates between opt-out and opt-in systems. Kidney Int. 2019;95:1453–1460. doi: 10.1016/j.kint.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 109.Matesanz R, Dominguez-Gil B. Opt-out legislations: the mysterious viability of the false. Kidney Int. 2019;95:1301–1303. doi: 10.1016/j.kint.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 110.Howard K, et al. Preferences for policy options for deceased organ donation for transplantation: a discrete choice experiment. Transplantation. 2016;100:1136–1148. doi: 10.1097/TP.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 111.Gander JC, et al. Association between dialysis facility ownership and access to kidney transplantation. JAMA. 2019;322:957–973. doi: 10.1001/jama.2019.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boulware LE, Wang V, Powe NR. Improving access to kidney transplantation: business as usual or new ways of doing business? JAMA. 2019;322:931–933. doi: 10.1001/jama.2019.12784. [DOI] [PubMed] [Google Scholar]
- 113.Heemann U, Abramowicz D, Spasovski G, Vanholder R, European Renal Best Practice Work Group on Kidney Transplantation Endorsement of the kidney disease improving global outcomes (KDIGO) guidelines on kidney transplantation: a European Renal Best Practice (ERBP) position statement. Nephrol. Dial. Transplant. 2011;26:2099–2106. doi: 10.1093/ndt/gfr169. [DOI] [PubMed] [Google Scholar]
- 114.Pippias M, et al. The European Renal Association — European Dialysis and Transplant Association Registry Annual Report 2014: a summary. Clin. Kidney J. 2017;10:154–169. doi: 10.1093/ckj/sfw135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khush KK, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult heart transplantation report-2018; focus theme: multiorgan transplantation. J. Heart Lung Transplant. 2018;37:1155–1168. doi: 10.1016/j.healun.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 116.Coemans M, et al. Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int. 2018;94:964–973. doi: 10.1016/j.kint.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 117.Schroppel B, Legendre C. Delayed kidney graft function: from mechanism to translation. Kidney Int. 2014;86:251–258. doi: 10.1038/ki.2014.18. [DOI] [PubMed] [Google Scholar]
- 118.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–1827. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- 119.Debout A, et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015;87:343–349. doi: 10.1038/ki.2014.304. [DOI] [PubMed] [Google Scholar]
- 120.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Discov. 2016;15:568–588. doi: 10.1038/nrd.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gill JS, Rose C, Pereira BJ, Tonelli M. The importance of transitions between dialysis and transplantation in the care of end-stage renal disease patients. Kidney Int. 2007;71:442–447. doi: 10.1038/sj.ki.5002072. [DOI] [PubMed] [Google Scholar]
- 122.Prendergast MB, Gaston RS. Optimizing medication adherence: an ongoing opportunity to improve outcomes after kidney transplantation. Clin. J. Am. Soc. 2010;5:1305–1311. doi: 10.2215/CJN.07241009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dobbels F, Van Damme-Lombaert R, Vanhaecke J, De Geest S. Growing pains: non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr. Transplant. 2005;9:381–390. doi: 10.1111/j.1399-3046.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 124.Russell CL, et al. Improving medication adherence and outcomes in adult kidney transplant patients using a personal systems approach: SystemCHANGE results of the MAGIC randomized clinical trial. Am. J. Transplant. 2020;20:125–136. doi: 10.1111/ajt.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuypers DR. Immunosuppressive drug monitoring — what to use in clinical practice today to improve renal graft outcome. Transpl. Int. 2005;18:140–150. doi: 10.1111/j.1432-2277.2004.00041.x. [DOI] [PubMed] [Google Scholar]
- 126.Wieland E, et al. Biomarkers as a tool for management of immunosuppression in transplant patients. Ther. Drug Monit. 2010;32:560–572. doi: 10.1097/FTD.0b013e3181efb3d2. [DOI] [PubMed] [Google Scholar]
- 127.Bell LE, et al. Adolescent transition to adult care in solid organ transplantation: a consensus conference report. Am. J. Transplant. 2008;8:2230–2242. doi: 10.1111/j.1600-6143.2008.02415.x. [DOI] [PubMed] [Google Scholar]
- 128.Meier-Kriesche HU, Schold JD, Gaston RS, Wadstrom J, Kaplan B. Kidneys from deceased donors: maximizing the value of a scarce resource. Am. J. Transplant. 2005;5:1725–1730. doi: 10.1111/j.1600-6143.2005.00923.x. [DOI] [PubMed] [Google Scholar]
- 129.Huang E, Jordan SC. Clinical and public policy implications of pre-formed DSA and transplant outcomes. Clin. J. Am. Soc. Nephrol. 2019;14:972–974. doi: 10.2215/CJN.05950519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de la Rosa G, et al. Continuously evaluating performance in deceased donation: the Spanish quality assurance program. Am. J. Transplant. 2012;12:2507–2513. doi: 10.1111/j.1600-6143.2012.04138.x. [DOI] [PubMed] [Google Scholar]
- 131.Weiss J, et al. Evolution of deceased organ donation activity versus efficiency over a 15-year period: an international comparison. Transplantation. 2018;102:1768–1778. doi: 10.1097/TP.0000000000002226. [DOI] [PubMed] [Google Scholar]
- 132.Matesanz R, et al. Benchmarking in the process of donation after brain death: a methodology to identify best performer hospitals. Am. J. Transplant. 2012;12:2498–2506. doi: 10.1111/j.1600-6143.2012.04128.x. [DOI] [PubMed] [Google Scholar]
- 133.No Authors Listed. Eurotransplant region. Eurotransplanthttps://www.eurotransplant.org/about-eurotransplant/region/ (2021).
- 134.Schneider S, Schreiner P, Weiss J, Immer FF. Assessing the potential of international organ exchange–the Swiss experience. Eur. J. Cardiothorac. Surg. 2011;40:1368–1373. doi: 10.1016/j.ejcts.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 135.Organizacion Nacional De Trasplantes. Publicaciones ONThttp://www.ont.es/publicaciones/Paginas/Publicaciones.aspx (2021).
- 136.European Kidney Health Alliance. Thematic Network on Improving Organ Donation and Transplantation in the EU 2019. EKHAhttp://ekha.eu/wp-content/uploads/FINAL_14.01.2020_Joint-Statement-of-the-Thematic-Network-on-Organ-Donation-and-Transplantation.pdf (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.