Abstract
Major depressive disorder (MDD) is characterized by pervasive mood disturbance as well as deficits in emotional processing, reactivity, and regulation. There is accumulating evidence that MDD is characterized by emotional patterns consistent with environmental disengagement, as reflected in attenuated positive and negative emotional reactivity, consistent with Emotion Context Insensitivity (ECI) theory. However, MDD individuals vary considerably in the extent to which they exhibit specific alterations in patterns of emotional responding. Emotions are complex, multicomponent processes that invoke responses across multiple functional domains and levels of analysis, including subjective experience, behavior, autonomic regulation, cognition, and neural processing. In this article, I review the current state of the literature on emotional responding and MDD from the lens of ECI. I focus on the importance of assessing emotional indices from multiple levels of analysis across development and contexts. I also discuss methodological and measurement issues that may contribute to inconsistent findings. In particular, I emphasize how psychophysiological measures can help elucidate emotional processes that underlie the pathophysiology of MDD as part of an integrated and contextualized approach.
Keywords: context, depression, development, ECI, emotion, flexibility, psychophysiology
1 |. INTRODUCTION
Alterations in mood, including persistent elevations in negative affect and deficient positive affect, are consistently identified as key features of Major Depressive Disorder (MDD) (APA, 2013). However, despite a rapidly growing scientific literature examining emotional processes in MDD, how exactly the mood alterations in MDD relate to specific emotional deficits remains elusive, both in terms of the phenomenology as well as associated mechanisms underlying these relationships (e.g., Rottenberg, 2017; Rottenberg & Bylsma, 2014). Given the high prevalence and chronicity of MDD, achieving a better understanding of the emotional processes that characterize this debilitating disorder is of paramount importance.
In this article, l review the current state of the literature on emotional reactivity and depression through the lens of Emotion Context Insensitivity (ECI). Although there is accumulating evidence for ECI in MDD, meta-analytic findings have also revealed significant heterogeneity of findings even within response systems (Bylsma et al., 2008). More recent daily life investigations of emotional reactivity in MDD have also observed a notable divergence in findings (Bylsma et al., 2011). These inconsistent results suggest that ECI may only hold in certain contexts (Rottenberg & Hindash, 2015) or that ECI may be the manifestation of multiple underlying mechanisms or subprocesses, such that depressed individuals may vary to the extent they show impairment on one or more subprocesses. Where associations between MDD and physiological alterations are observed, it is often unclear what specific mechanisms might underlie these relationships. Throughout this article, I elaborate on several key issues that contribute to heterogeneity in findings on emotional reactivity and related regulation processes in MDD, and I conclude with a path forward for future research. Figure 1 presents an integrated heuristic framework to guide assessments of emotional functioning in MDD, illustrating proposed relationships among emotional reactivity functional domains (positive and negative valence systems), emotion component outputs, regulatory processes, as well as the influence of context, developmental processes, and biological substrates.
FIGURE 1.
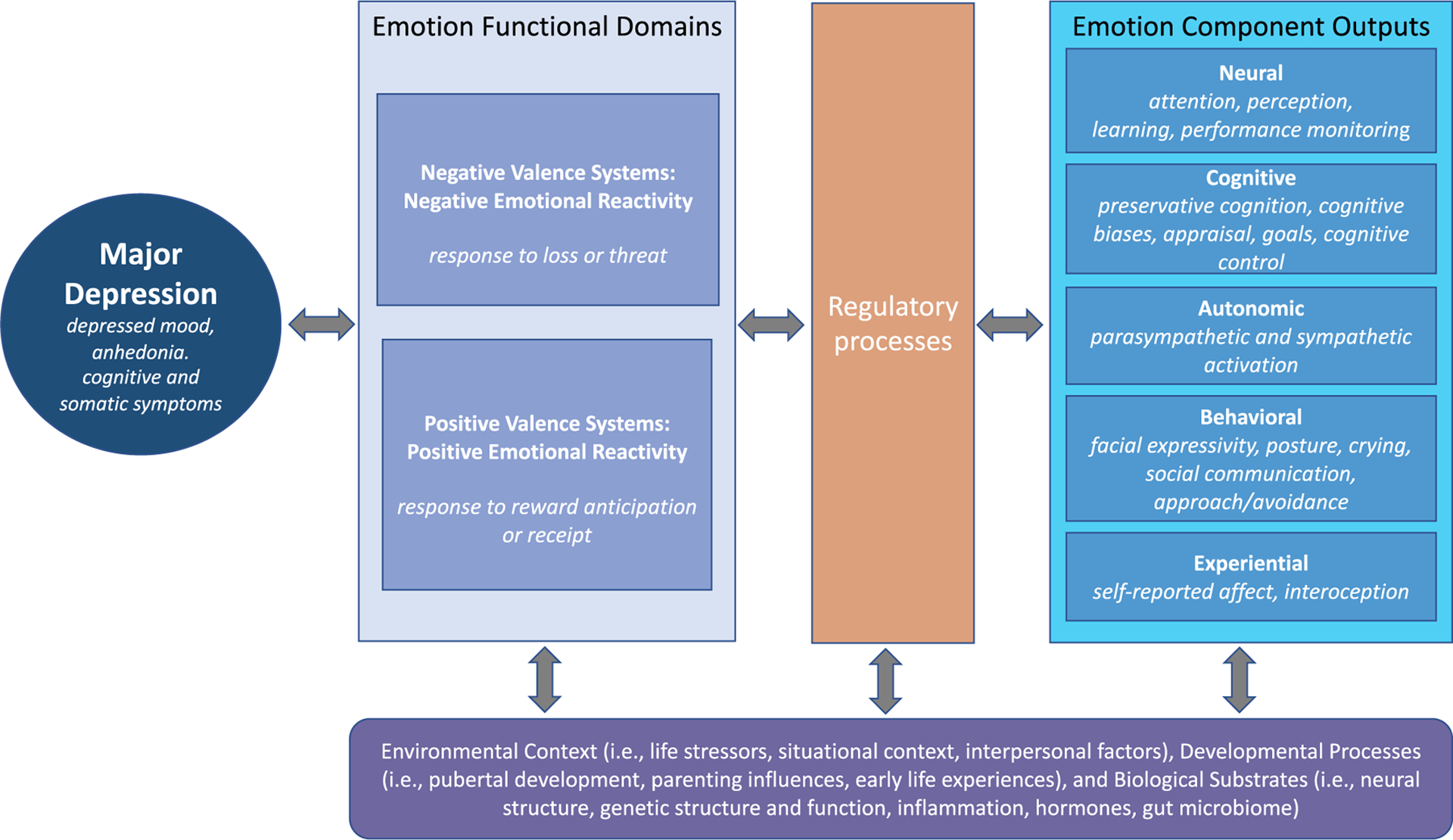
Proposed heuristic framework to guide research on emotional functioning in major depressive disorder (MDD) through the investigation of emotion reactivity across functional domains (i.e., positive and negative valence systems), emotion component outputs (i.e., units of analysis), and regulatory processes, while incorporating the influence of environmental context, developmental processes, and biological substrates. Most pathways are likely bidirectional and interactive. For example, a current MDD episode may result in alterations in functional domains, component outputs, and/or regulatory processes. Preexisting alterations may also contribute to risk for development of MDD with individual variation due to other moderating influences on these systems (i.e., environment, biology). Components of this framework are informed by the NIMH Research Domain Criteria (RDoC) framework (see Insel et al., 2010)
2 |. CURRENT EVIDENCE FOR ECI IN MDD
Consistent with ECI, accumulating evidence has suggested that MDD may be associated with an overall pattern of emotional disengagement to both positive and negative stimuli, reflected as blunted positive and negative emotional reactivity across physiological, behavioral and self-report response systems (Bylsma et al., 2008). These alterations in emotional reactions to positive and negative stimuli can be conceptualized in terms of positive and negative valence systems (from the National Institute of Mental Health Research Domain Criteria Framework, NIMH RDoC; Insel et al., 2010). In this section, I review some of the most robust evidence for ECI in MDD organized by positive and negative valence systems, with a focus on evidence from psychophysiological research. In subsequent sections, I will review examples of notable divergences in findings and possible contributions to heterogeneity in findings.
2.1 |. Positive valence systems
Much of the evidence for ECI in MDD has come from studies of neural response to positive or rewarding stimuli. It is well established that depression is linked with impaired reward functioning, including reduced positive affectivity, anhedonia, and reward insensitivity (see Forbes & Dahl, 2012). Some of the strongest evidence has been from studies of neural processing of reward, including neuroimaging and electrophysiology, which overall show evidence of reduced activation for reward anticipation and receipt in MDD (Keren et al., 2018).
The physiological assessment of neural activity from scalp recorded event-related potentials (ERPs) extracted from the electroencephalogram (EEG) may be particularly valuable in isolating-specific alterations in emotional processing in MDD. ERPs have increasingly been used to examine aspects of affective processing in a variety of populations, as ERPs have the advantage of a high degree of temporal resolution. Most prior ERP research on reward processing has focused on the Feedback Negativity (FN), which occurs approximately 250–350 ms following feedback of reward loss versus gains—also referred to as the Reward Positivity (RewP) when computed as response to gains versus loss (see Proudfit, 2015; Proudfit et al., 2015). The FN is associated with self-report and behavioral measures of reward sensitivity (Bress & Hajcak, 2013) and is generated primarily by the anterior cingulate cortex and the ventral striatum, key neural structures involved in reward processing (Carlson et al., 2011; Foti et al., 2014, 2015). Consistent with neuroimaging evidence that MDD is associated with reduced neural response to reward (e.g., Forbes, 2009; Forbes & Dahl, 2012), adults with current and remitted depression and youth with depressive symptoms all show a reduced FN amplitude following feedback of loss versus gains (e.g., Bress et al., 2012; Foti & Hajcak, 2009; Weinberg & Shankman, 2017).
Another relevant ERP component for examining emotional processing in depression is the Late Positive Potential (LPP). The LPP is thought to reflect facilitated processing of emotional or motivationally salient stimuli and is sensitive to instructed emotion regulation (e.g., Cuthbert et al., 2000; Hajcak et al., 2010; Schupp et al., 2000). The LPP is a sustained positive deflection in the ERP waveform over occipital and parietal electrodes that typically begins approximately 300 ms post stimulus onset and continues for the duration of stimulus presentation (i.e., typically emotional pictures), suggesting it may reflect sustained attention to emotional stimuli (Hajcak et al., 2010, for review). The LPP may be considered an index of emotional reactivity as it is reliability enhanced for high-arousing emotional (positive or negative) relative to neutral stimuli (Olofsson et al., 2008) and has been consistently associated with greater autonomic and self-reported arousal (Cuthbert et al., 2000). However, although the LPP reliably tracks emotional arousal, it does not differentiate valence (positive vs. negative). There is evidence that the LPP is generated in part, in occipital and parietal cortices, which both receive projections from the amygdala, a region critical for processing rewarding and emotionally salient information (Liu et al., 2012). The LPP is also sensitive to positive reward feedback (gains) in monetary guessing tasks (Broyd et al., 2012). Depressed adults (Klawohn et al., 2020) and adolescents (Webb et al., 2017) show a reduced LPP in response to reward relative to healthy individuals. Reductions in LPP amplitude in response to positive stimuli in depression are also not only specific to monetary reward tasks. For example, in an adult college student sample, depressive symptoms were associated with a reduced LPP in response to positive pictures (Hill et al., 2019). Further, in a clinical sample of MDD and controls, both the LPP and FN in response to positive pictures were independently associated with MDD, suggesting the importance of examining multiple components of reward processing in characterizing MDD (Klawohn et al., 2020). Overall, these findings provide support for a general insensitivity to reward and lack of engagement with positive stimuli in MDD, consistent with ECI as well as findings across other response systems, including self-report and expressive behavior (Bylsma, et al., 2008).
2.2 |. Negative valence systems
Evidence for ECI in negative valence systems is somewhat more mixed, with the most robust findings also based in physiological research. For example, a few studies have also investigated the LPP in response to negative stimuli in depressed or high-risk samples. For example, MDD adults show less of an increase in LPP amplitude to threatening faces (Foti et al., 2010). Further, Hill et al. (2019) found that depressive symptoms were associated with a reduced LPP to negative pictures more generally. Adults with current or remitted MDD also fail to show decreases in LPP following reappraisal relative to passive viewing of negative images (Bylsma, 2012). Similarly, reduced modulation of LPP following reappraisal has been associated with anxiety and depression symptoms among healthy children (Dennis & Hajcak, 2009). Overall, these results suggest that MDD individuals may have more difficulties with flexibly regulating their negative emotional responses appropriately to the context.
Some of the strongest evidence for ECI is found in alterations in autonomic functioning, including differences in autonomic indices at baseline and in response to negative emotional stimuli, such as laboratory stressors. Heart rate variability (HRV), also known as respiratory sinus arrhythmia (RSA), is an index of parasympathetic activity and indirect measure of the influence of the vagus nerve on the heart (Berntson et al., 1993) that is thought to index the integration of neural circuits that are important for guiding flexible control over behavior via autonomic regulation, as described by the neurovisceral integration model (Freidman, 2007; Pace-Schott et al., 2019; Thayer et al., 2012; Thayer & Lane, 2000). Along these lines, low resting HRV and blunted HRV reactivity have been increasingly considered to be reflective of poor self-regulation (Balzoretti et al., 2017; Pace-Schott et al., 2019). MDD has been repeatedly associated with low resting parasympathetic nervous system activity, as indexed by resting levels of RSA (Kemp et al., 2010; Koch et al., 2019; Rottenberg et al., 2007), although these findings have not always been consistent (e.g., Lehofer et al., 1997; Licht et al., 2008; Yeragani et al., 1991). Somewhat more consistent findings have been observed for RSA reactivity in response to laboratory tasks, with MDD adults exhibiting blunted RSA withdrawal to psychological stressors, such as a speech or mental arithmetic stressor task (Bylsma et al., 2014; Rottenberg et al., 2007; Schiweck et al., 2019, for review), as well as physical effort mobilization challenges (e.g., handgrip task, Nugent et al., 2011). To a somewhat lesser extent, MDD individuals also show abnormalities in sympathetic nervous system (SNS) activity. For example, Salomon et al. (2009) and Salomon et al. (2013) found blunted sympathetic nervous system reactivity (i.e., lengthened pre-ejection period, PEP), to a laboratory speech stressor task, although others have also found the opposite association (Light et al., 1998). Taken together, these findings suggest that MDD tends to be associated with less flexible autonomic reactions to stress, consistent with ECI, although the specific direction of the effects may vary across studies.
3 |. THE HETEROGENEITY OF MDD AS A DIAGNOSTIC CATEGORY
A first critical issue for understanding how emotional processes are altered in depression is the fact that MDD is a heterogeneous disorder where individuals can vary significantly in phenotype, including specific emotional deficits and symptom patterns, with over 1,000 unique symptom profiles identified (Fried & Nesse, 2015a). Even for the two gateway symptoms of depression (i.e., anhedonia and depressed mood), only the presence of one of the two symptoms is required to meet diagnostic criteria for MDD. It has been suggested that it may be more useful to focus on specific depressive symptoms rather than sum scores or diagnostic categories, as individual symptoms may have distinct underlying biology (Fried & Nesse, 2015b). For example, alterations in reward processing seen in MDD may be specific to those experiencing anhedonia. Indeed, there is evidence that within MDD individuals, reductions in neural response to reward (i.e., as indexed by the FN) are associated with severity of anhedonia, even after adjusting for overall depression severity (Liu et al., 2014). Further, evidence from unselected samples suggests that while anhedonia may predict both blunted positive and negative emotional reactivity, depressed mood may predict potentiated negative reactivity, although this research was based on self-report ratings of positive and negative images, which may reflects appraisals rather than true emotional reactivity (Saxena et al., 2017).
However, while there may be utility in investigations at the symptom level, the level of individual symptoms may not necessarily be the optimal level of analysis. Some depressive symptoms may be more strongly related than others and may reflect alterations in specific underlying biological processes or endophenotypes. Along these lines, specific clusters of symptoms may also show distinct physiological patterns. For example, cognitive symptoms such as feelings of guilt, worthlessness, suicidal thoughts, and concentration difficulties may all be related to patterns of perseverative negative thinking. Alternatively, difficulties with sleep, energy, and appetite could all reflect an underlying disruption in circadian systems. Thus, specific symptoms or clusters of symptoms may be more strongly related to neural or physiological markers rather than the diagnostic category itself and may have more utility in elucidating the nature of emotional dysfunction in depression. Some research has begun to focus on such clusters of related symptoms or deficits.
For example, we have found that much of the variance in RSA reactivity for MDD versus healthy controls could be explained by group differences in sleep quality, with poor sleep quality being associated with greater deficits in RSA reactivity (Bylsma et al., 2014). Others have found that low resting RSA moderates the relationship between sleep disturbance and depression, suggesting that individuals with lower resting RSA may be particularly vulnerable to the negative effects of sleep disturbance (Hamilton et al., 2019). Sleep quality has also been associated with subsequent alterations in daily life affect (Bower et al., 2010) and emotional reactivity (O’Leary, Small, et al., 2017) in MDD, and the relationship between sleep quality and daily life emotional functioning may be mediated by maladaptive emotion regulation (O’Leary, Bylsma, et al., 2017). There is also evidence that sleep may be disturbed in high familial risk adolescents (i.e., those with a parental history of depression) who have not yet experienced depression themselves, suggesting sleep disturbance may be a possible risk marker for depression that precedes first depression onset (Hamilton et al., 2020). We have also found that sleep disturbance among high familial risk youth may also be moderated by other environmental or behavioral factors, such as social media use (e.g., Hamilton, et al., 2020).
Adding further complexity to the heterogeneity of depression as a diagnostic category, MDD is also highly comorbid with other disorders, such as anxiety disorders. Some alterations seen in depression, such as alterations in autonomic functioning, may actually represent a transdiagnostic factor that cuts across multiple disorders (e.g., Friedman, 2007). For example, a reductions in baseline RSA is thought to reflect a deficit in emotion regulation capacity that may represent a transdiagnostic marker of psychopathology (Balzarotti et al., 2017; Beauchaine, 2015). Along these lines, research has started to focus on specific regulatory processes and behaviors that may be more reflective of underlying alterations in autonomic functioning, rather than diagnostic categories, consistent with the Research Domain Criteria (RDoC) initiative (Insel et al., 2010). For example, maladaptive emotion regulation processes involved in preservative cognition, such as worry and rumination (related to cognitive symptoms of depression), have been associated with reductions in RSA (Ottaviani et al., 2016, for review). Continuing to focus on overall group differences among diagnostic groupings and healthy controls has limited utility in improving our characterization of affective dysfunction as it relates to psychopathology.
4 |. EMOTIONS A DYNAMIC MULTILEVEL MULTICOMPONENT EMERGENT PHENOMENA
A second key issue in understanding how emotional reactivity is altered in MDD is that emotions are inherently multilevel multicomponent emergent phenomena. Indeed, emotions have long been viewed as multicomponent processes that help an individual adaptively and flexibly respond to features of the environment, including coordinated changes in experience, physiology, behavior, cognition, and neural activity (Lang & Bradley, 2010; Levenson, 2003). However, it has become increasingly clear that facets of emotion across response domains are only loosely coupled (Bylsma et al., 2016; Mauss et al., 2005).
Along these lines, there is increasing evidence that discrete emotions do not really exist in nature in an objective and measurable way (i.e., where emotions can be objectively defined in terms of biology rather than just psychological constructs), also referred to as the natural kinds theory of emotion (see Barrett, 2006, 2012; Barrett et al., 2009). Specifically, a natural kinds view suggests that emotions exist as neural circuits (or some other biological substrate) which drive the multicomponent emotional responses across domains, including experience, physiology, cognition, and behavior (Coan, 2010). According to such a latent variable model of emotion, all indicators of emotion from these varied response domains would be expected to be associated, such that a specific emotion reflects some consistency in responses that can be measured across multiple indicators.
However, no one-to-one relationships have been found between subjective emotional experience and any other biological or behavioral indicators. For example, physiological changes can occur in the absence of experiential changes, and vice versa (Kreibig, 2010). Meta-analytic findings have demonstrated that while several brain areas are sensitive to emotional stimuli (e.g., amygdala, dorsolateral prefrontal cortex), no brain areas have been linked invariably to specific discrete emotions (Lindquist et al., 2012). Similarly, while studies of the autonomic functioning have been successful at measuring components of emotion, such as arousal (Lang et al., 1998), these component measures have shown limited utility in distinguishing among various discrete emotions, such as happiness, sadness, anger, etc. (Cacioppo et al., 2000). For example, a meta-analysis of autonomic measures of emotion, only a small set of 37 autonomic indices showed any differentiation of discrete emotions, but even these findings were not consistent and may be explained by other processes, such as differences in arousal associated with specific emotions (i.e., anger tends to be a more highly arousing emotion than sadness) (Cacioppo et al., 2000). Other more recent reviews also observe a lack of consistency or autonomic specificity for specific discrete emotions (Kreibig, 2010).
Given the lack of consistency or coherence across emotion response systems, as well as evidence that coherence may vary depending on context (such as environmental or task demands), there has been increasing emphasis on emergent or psychologically constructed theories of emotion (Clore & Ortony, 2008; Coan, 2010; Gross & Feldman Barrett, 2011). According to these views, there may be a number of fundamental neural processes, or other component phenomena (e.g., arousal), that may be combined or coordinated in various ways to construct emotions contingent upon context (e.g., Wilson-Mendenhall et al., 2011). Along these lines, emotion categories are conceptualized as populations of highly variable instances of emotional features (i.e., components) that are tied to context-specific needs (Hoemann et al., 2020; Siegel et al., 2018).
Importantly, emergent or constructionist views of emotion do not predict that different indicators of emotion from multiple domains would covary either across situations within the same individual or across different individuals. Instead, demands of a given situation may determine which indicators covary and under what conditions (Coan, 2010). Relatedly, appraisal models of emotion (i.e., where emotions are presumed to depend on our evaluations of situations) also consider emotions to be loosely coordinated response tendencies that configure in a contextually sensitive manner. Along these lines, emotions may be characterized as dispositional tendencies to respond to features of the environment in a specific way, but there is no assumption of specific stereotypical outputs—rather, variability is expected across individuals and situations (Barrett et al., 2007; Gross & Barret, 2011). Therefore, it is insufficient to measure only a single emotion response system in a single context if one wants to understand the nature of emotional disturbance in MDD or other forms of psychopathology. Importantly, emergent or constructionist emotion theories are not inconsistent with ECI theoretical models of MDD, as more recent conceptualizations frame ECI as emotional responses that are flexibly sensitive to context (e.g., Kashdan & Rottenberg, 2010; Rottenberg, 2017; Rottenberg & Hindash, 2015). Along these lines, inflexibility across multiple affective domains (e.g., physiological, cognitive, and behavioral) has been viewed as a marker of depression vulnerability (Stange et al., 2017).
It is not yet clear what response domain or level of analysis (or combination thereof) will have the greatest utility in elucidating mechanisms underlying affective dysfunction in MDD. Even within the same functional domain and measurement level, it can be critical to examine multiple components of emotional processing. This is particularly well illustrated by reward processing, which is known to involve multiple components, including anticipation and consummation phases, as well as learning processes (Berridge et al., 2009; Glazer et al., 2018). Importantly, evidence suggests that while some reward components may overlap and show associations (e.g., reward learning can interface with multiple stages of processing), other components seem to be unrelated to each other and may represent discrete psychological processes with distinct neurobiological mechanisms (Zhang et al., 2013). Along these lines, it has been suggested that alterations in reward processing in MDD may differ by reward component (anticipation vs. receipt) and task context (type of reward, monetary vs. social) (Zhang et al., 2013). There is a critical need for research to examine different components of reward processing in MDD using multiple methods to better understand these findings.
5 |. CHARACTERIZATION OF WITHIN AND CROSS-LEVEL INTERACTIONS ACROSS EMOTION RESPONSE DOMAINS
A third key issue is that the vast majority of studies on emotional reactivity and MDD focus on only one or two different physiological indices or functional domains. However, there is evidence of complex with-in and cross-level interactions, as well as interactions across different functional domains, that remain largely unexplored in the context of depression. Further, the lack of coherence among emotion response domains highlights the importance of examining within and cross-level associations to gain a full picture of emotional functioning in MDD, as these complex interactions may also contribute to divergent findings. Although prior research has revealed only modest and inconsistent associations between physiology and self-reported emotion and affective behavior (Mauss et al., 2005; Mauss & Robinson, 2009), complex interactions may still be uncovered, and associations may change with context (e.g., Stone et al., 2020).
5.1 |. Within-level interactions: Autonomic regulation
One example of within-level interactions is in the domain of autonomic functioning. Research investigating autonomic functioning in MDD has focused almost exclusively on individual parasympathetic or sympathetic indices. However, consideration of the relationship between sympathetic and parasympathetic regulation is critically needed for a more complete understanding of alterations in autonomic functioning associated with depression (Berntson et al., 1991; Pace-Schott et al., 2019; Sunagawa et al., 1998). One useful (and relatively understudied) index for examining relative contributions of sympathetic and parasympathetic influences on stress or emotional reactivity is cardiac autonomic balance (CAB), defined as the difference between normalized sympathetic (e.g., PEP) and parasympathetic activity (e.g., RSA) (Berntson et al., 2008). Lower resting CAB has been observed in adults with MDD, reflecting greater sympathetic relative to parasympathetic dominance, although, as with the individual indices, there is notable individual variation (Brush et al., 2019). Further, we have examined changes in CAB in response to psychological challenges in youth with a history of juvenile onset depression in comparison to healthy controls and found that while controls showed an expected shift from sympathetic relative to parasympathetic activation in response to the laboratory stressors, youth with a depression history exhibited the opposite pattern, but examination of parasympathetic and sympathetic indices alone did not yield significant effects (Bylsma et al., 2015). Thus, indices like CAB may better reflect the ability to flexibly recruit autonomic systems to respond effectively to stressors, where a lack of context-appropriate changes may reflect evidence of ECI.
Even within the sympathetic nervous system there may still be divergence among indicators, suggesting that different sympathetic indices may confer complementary information regarding emotional processes in MDD. Typically, researchers interested in sympathetic activity assess either skin conductance (electrodermal activity, EDA), or PEP, as both are assumed to be relatively “pure” indices of sympathetic activity. However, recent work reveals that these indices may not even be significantly correlated (Stone et al., 2020) and may show differential relationships with parasympathetic activity and emotion regulation processes (Stone et al., 2020). This could be due to differences in temporal resolution in PEP and electrodermal measures (i.e., as electrodermal measures tend to have a slower onset and offset), or the fact that PEP is primarily driven by beta-adrenergic neurotransmission, while electrodermal measures reflect primarily cholinergic neurotransmission (Stone et al., 2020; Stone et al., 2020). Further, skin conductance reactivity has been more associated with threat of punishment, while PEP reactivity is more sensitive to reward receipt or anticipation (Obradović & Boyce, 2012), suggesting that the nature of the stimuli and context may be particularly relevant.
For parasympathetic activity, examining interactions between resting levels and reactivity has also shown utility in characterizing specific profiles associated with psychopathology (Yaroslavsky et al., 2013). For example, atypical patterns of resting RSA and RSA reactivity in response to a sad film have been associated with emotion regulation deficits (i.e., deficits in mood repair) in youths with remitted depression (Yaroslavsky et al., 2016) and predict internalizing symptoms (i.e., depression and anxiety) longitudinally in youth (Hinnant & El-Sheikh, 2009). Further, there is evidence that specific combinations of resting RSA and RSA reactivity moderate the relationship between maladaptive emotion regulation and depression. For example, high resting RSA in the context of context-appropriate RSA withdrawal to a sad film is associated with a reduction in maladaptive emotion regulation responses and mitigation of the depressogenic effect of maladaptive attempts to repair negative mood (Yaroslavsky et al., 2013). These findings underscore the importance of assessing multiple physiological indices of parasympathetic and sympathetic activity.
5.2 |. Cross-level interactions: Behavior
It is also important to consider cross-level interactions between psychophysiological indices and other response domains, such as behavior. Affective behavioral responses have historically received significant attention in the emotional reactivity literature, although interest has seemingly declined somewhat in recent years. Alterations in facial behavior in depression have been observed for some time, although results have not always been consistent and have shown poor correspondence with physiological measures of emotion (Mauss et al., 2005). There is some convergence in findings that MDD individuals exhibit decreased positive facial expressions, with less consistent findings for negative facial expressions (Davies et al., 2016, for meta-analytic review), although electromyography (EMG) findings demonstrate evidence of ECI in MDD individuals, as reflected by less corrugator muscle activity relative to controls in response to a sad film (Rottenberg et al., 2005). Much of the prior work has primarily used manual coding systems which are time and labor intensive, and EMG is limited to specific facial muscle groups. As a result, research in this area is relatively limited and sample sizes have been quite modest.
More recently, it has been shown that examining dynamic changes affective behavior over time, such as changes in intensity or variability of specific facial expressions, may prove valuable for uncovering more subtle alterations (Panaite, et al., 2019). Recent advances in automatic affective computing, may open new opportunities for examination of affective dynamics in depression. For example, Dibeklioğlu et al. (2015) were able to successfully classify depressed patients in three levels of depression severity using automatic detection of depression from multimodal behavioral indicators, including facial movement dynamics, head movement dynamics, and vocal prosody. Prior work has focused on identifying current depression, but computational approaches may be superior for detecting more subtle alterations that may appear prior to the onset of a depressive episode—or changes that may occur during an episode that may predict remission or response to treatment. It also remains to be examined how affective behavioral dynamics may track changes in other systems, such as psychophysiological responses. Affective behavior is also particularly well suited for examination of dyadic affective dynamics between individuals, such as couples, peers, or parent–child interactions that may be important for understanding interpersonal emotion regular processes or emotional development.
Crying is a relatively understudied affective behavioral response that has potential utility for increasing our understanding of emotional functioning in depression (e.g., Vingerhoets & Bylsma, 2016, for review). Notably, crying represents a well-defined, observable behavior with a relatively clear onset and offset. An increasing number of studies have already examined autonomic correlates of crying (see Bylsma et al., 2019).
Although prior research on crying has been primarily conducted in nonclinical samples, examination of normative emotional processes can lead to a better understanding of or alterations associated with depression and other forms of psychopathology. Evidence has converged to suggest that the period immediately preceding crying onset is characterized by increased sympathetic activity among criers, while parasympathetic activity increases just after crying onset (see Bylsma, Gračanin, & Vingerhoets, for review). Along these lines, there is evidence that crying may have a self-soothing regulatory function via physiological, cognitive, or social mechanisms (Gračanin et al., 2014). Only one study thus far has examined physiological correlates of crying in the context of MDD: Rottenberg et al. (2003) found that MDD individuals do not exhibit an increase in parasympathetic activity corresponding to the onset and resolution of crying, suggesting that the physiological self-regulatory mechanisms associated with crying may be compromised in depression. These results are also consistent with self-report findings that depressed individuals typically report experiencing less subjective mood benefit following crying (Rottenberg et al., 2008; Rottenberg et al., 2008; Vingerhoets & Bylsma, 2016), although mood benefits from crying may also vary by context and assessment method (Bylsma, et al., 2008; Bylsma et al., 2011). Depressed individuals also show alterations in their crying behavior, such as increases in crying frequency, although decreases in the ability to cry have also been observed at more severe levels of depression (Rottenberg et al., 2008). Crying has also been long considered an important focus of psychotherapy that may facilitate emotional processing, attachment, and interpersonal functioning (Bylsma, Gračanin, et al., 2020); however, little is known about how crying changes with the course of depression or in response to intervention.
5.3 |. Interactions across functional domains: Reward and stress
More research examining interactions across functional domains in the context of depression is also needed. One growing area of research that is highly relevant for depression is the consideration of interactions between reward and stress response systems. Much of this work has been done with nonclinical samples, but thus far results have revealed potential bidirectional relationships between reward and stress response, as well as interactive effects in predicting depressive symptoms longitudinally. For example, it is well known that stress is a robust predictor for MDD, and interpersonal stressors can play a central role in the onset of a major depressive episode (Auerbach et al., 2014; Tottenham & Galvan, 2016). Early life stress is also associated with alterations in autonomic stress regulation, including reduced RSA (e.g., Cyranowski et al., 2011; Miskovic et al., 2009; Stone et al., 2018) and less flexible patterns of parasympathetic activity, as indexed by less vagal withdrawal to a sad film and impaired physiological recovery during mood repair among adolescents with depression histories (Daches et al., 2017). It is also well-established that early life adversity, such as child maltreatment, can disrupt neural processing of reward (e.g., Auerbach et al., 2014; Novick et al., 2018).
Several researchers have posed a reward mediation model of depression, such that acute and chronic stress contribute to reward dysfunction, which, in turn, can lead to the development of depression (e.g., Auerbach et al., 2014). Stressful life events have also been shown to moderate the effect of neural response to reward on depression symptoms longitudinally, such that a blunted reward response (RewP) in combination with stressful life events predicted higher depression symptoms at follow-up (Goldstein et al., 2020). There is also evidence that these effects can operate on shorter time scales. For example, Foti et al. (2011) found that high familial risk adolescents were more sensitive to a negative mood induction, which predicted greater reductions in neural sensitivity to monetary gains versus losses (RewP). Potential bidirectional relationships have also been proposed, whereby reward dysfunction can lead to poorer stress regulation (Auerbach et al., 2014), and a blunted neural response to reward (RewP) also predicts greater generation of stressful life events (Mackin et al., 2019). However, the majority of prior research on stress and reward interactions has focused on the impact of early life or recent stressors on reward functioning and has not assessed physiological responses to stressors, which is critical for understanding how dysregulated stress responding may contribute to reward dysfunction.
5.4 |. Cross-system interactions: Interface with other neurobiological systems
Examinations of how other neurobiological systems may interface with emotional indices from other levels of analysis (i.e., autonomic, behavioral) may also reveal important insights about mechanisms underlying the pathogenesis of MDD and may ultimately lead to a better understanding of how various biological alterations observed in MDD are linked. For example, an interest in characterizing the gut microbiome in depression has been rapidly increasing in recent years. An accumulating body of research has demonstrated that depression is associated with reduced richness and diversity of gut microbiota (Kelly et al., 2016), and alterations in relative abundance of specific bacterial species (Cheung et al., 2019, for review). Preclinical studies with rodents suggest a causal role of gut microbiota on neural affective processing, including reward response and stress regulation (Cryan & Dinan, 2012; Foster et al., 2017; Rea et al., 2016; Sajdel-Sulkowska & Zabielski, 2013). Much of the work conducted thus far is based on preclinical studies in animal models, but research in human samples is beginning to emerge. A small number of studies in nonclinical human samples have demonstrated positive effects of probiotic consumptions on improving mood, lowering depression symptoms, reducing rumination (Benton et al., 2007; Messaoudi et al., 2011; Steenbergen et al., 2015), and altering neural emotional processing (Tillisch et al., 2013).
While the pathways are not yet fully understood, there is evidence of several mechanisms by which the gut microbiome can influence neural function, including via inflammatory processes (e.g., increasing or decreasing inflammatory cytokines) or generation of metabolites important for neurotransmitter synthesis (e.g., short-chain fatty acids), but the most direct link is via the vagus nerve, which extends from the brain through the heart, lungs, and gastrointestinal tract and contains both afferent (80%) and efferent (20%) fibers, suggesting bidirectional relationships (Cryan et al., 2019; Fülling et al., 2019). For example, Kelly et al. (2016) demonstrated that when researchers transplanted gut microbiota from depressed humans to rats, the rats subsequently showed behavioral and physiological characteristics (i.e., changes in HPA-axis function) consistent with depression (i.e., reduced reward seeking behavior for sucrose) and increased anxiety-related behaviors (i.e., decrease in visits to open arms of a maze). Importantly, the influence of gut microbiota on behavior in rodents ceases when the vagus is severed (i.e., vagectomy), suggesting a causal influence of the gut microbiome on behavior and brain function (e.g., Bravo et al., 2011). There is also evidence from preclinical studies that specific microbiota can directly contribute to vagal neural firing, which transmits information to the brain via afferent connections, including vagal protections to the paraventricular nucleus (PVN), an area critical for autonomic stress regulation (Perez-Burgos et al., 2013). Further, a neural circuit for gut microbiome influences on reward processing has recently been identified, where activation of gut-innervating vagal afferents is linked to increased neural activity in the striatum via dopamine release from the substantia nigra (Han et al., 2018).
The potential key role of the vagal pathway in the relationship between gut microbiome and depression is particularly intriguing considering the literature demonstrating alterations in vagal activity (i.e., RSA) associated with depression. Artificial stimulation of the vagus has also been shown to be efficacious for treatment-resistant depression (Rush et al., 2005) and inflammatory gastrointestinal disorders, such as irritable bowel disease (Bonaz et al., 2018), which has high comorbidity with depression, suggesting that this may also be an important pathway in humans. It may be possible for gut microbiota-induced vagal-activation to be indirectly observed via assessment of RSA. The relationship between gut microbiota and vagal activity has only been explored in one human study to date, where vagal activity (i.e., assessed using the pNN50 index) predicted significant variance in gut microbiome characteristics (including lower alpha diversity) among a nonclinical sample of adolescents, even after adjustment for other confounds (Michels et al., 2019). Thus, dysfunction of the gut microbiome may represent an important missing piece of the puzzle that can help elucidate mechanisms underlying other behavioral, physiological, and neurobiological alterations that have been observed in MDD. It may be that a healthy gut microbiome is critical for flexible regulation of emotional responding, including responses to stress and reward. However, there is a need for longitudinal investigations tracking gut microbiota changes over time in relation to depression symptom trajectories and a better characterization of the mechanistic pathways by which gut microbiota influence emotional processing in MDD.
6 |. THE INFLUENCE OF CONTEXT ON EMOTIONAL REACTIVITY AND REGULATION
A fourth critical issue that obscures our understanding of how emotions are altered in depression is the influence of context. Evidence points toward the importance of context in whether an emotion regulation strategy is effective (Aldao, 2013; Aldao et al., 2015). Indeed, situational demands have been shown to predict different patterns of autonomic responding in nonclinical samples (Laborde et al., 2018). Further, there is evidence that contextually appropriate autonomic responses facilitate effective emotion regulation, such that the effectiveness of specific emotion regulation strategy use may be moderated by specific patterns of parasympathetic and sympathetic activity (Stange et al., 2017). Along these lines, both blunted and potentiated reactivity to laboratory challenges have been associated with maladaptive emotional reactivity and regulation (see Obradović & Boyce, for review). Similarly, we have found that both maladaptive (i.e., dysphoric rumination) and adaptive (i.e., positive savoring) emotion regulation strategies are associated with increases in sympathetic activation among healthy individuals (Stone et al., 2020). The association between crying and corresponding emotional changes also is dependent upon context, particularly in relation to interpersonal factors (Bylsma, et al., 2008). These findings further underscore the importance of context and ability to flexibly respond in a way that is adaptive to the demands of a given situation, consistent with ECI (Kashdan & Rottenberg, 2010). However, examining context is challenging, as it is not always clear in a given scenario what is an adaptive or maladaptive response, and there is substantial variability in how individuals respond to different contexts. Laboratory studies are also limited in their ability to examine a rich range of ecologically valid contexts.
Some of the most compelling evidence of the importance of context comes from discrepancies between laboratory and daily life assessments of emotional reactivity. Laboratory findings of emotional reactivity in MDD samples in daily life have been notably discrepant from laboratory findings, providing further evidence for the importance of context (Bylsma, et al., 2016; Rottenberg, 2017). One of the key issues is that although there is evidence of ECI in laboratory studies (Bylsma, et al., 2008), with results particularly consistent with regard to positive emotional reactivity, ecological momentary assessment (EMA) studies of emotional reactivity in daily life in depressed individuals reveal a very different pattern of results. Most notably, a “mood brightening” effect has been observed, where depressed individuals are even more responsive to positive stimuli, even when controlling for baseline affect (Bylsma, et al., 2011; Khazanov et al., 2019; Panaite, Koval, et al., 2018; Peeters et al., 2003; Thompson et al., 2012). Further, only one study has observed evidence of blunted negative emotional reactivity in daily life in depression, also contrary to the predictions of ECI (Peeters et al., 2003). It is important to note that despite the increased reactivity, depressed individuals still have higher levels of negative affect relative to controls even following positive events, suggesting inflexibility (e.g., Bylsma, et al., 2011).
Several possible explanations have been suggested for these discrepant findings. For example, since the assessment of daily life emotional reactivity relies on individuals to self-identify positive and negative events, the sampled events may differ for MDD individuals versus control (in contrast to standardized laboratory stimuli). Along these lines, self-appraised positive events are relatively less frequent in depression, and thus, may elicit stronger reactions due to a contrast effect (Bylsma, et al., 2011). For negative reactivity, depressed individuals may be more responsive to events that are of personal relevance to them and which may be contributing to their depressed state, such as interpersonal stressors. It is plausible that depressed individuals preoccupied with personal stressors, may be less reactive to standardized negative emotional stimuli. Indeed, there is some evidence that depressed individuals show greater neurophysiological reactivity to personally relevant positive and negative stimuli, as indexed by the LPP (Benau et al., 2019). The differences in timescale between laboratory and daily life measures may also play a role, as laboratory measures typically assess changes on the level of seconds or minutes, while daily life measures assess over the course of minutes, hours, or days.
However, despite the surge in daily life research in recent years, studies combining laboratory and EMA methods are still notably lacking. Other environmental and social contextual features that are more controlled in the laboratory setting may also moderate the impact of depression on daily life emotional reactivity. Only one study to date has investigated emotional reactivity in MDD in both the lab and daily life, which also replicated blunted positive reactivity in response to an emotional film in the laboratory along with greater decreases in dysphoric affect in daily life in response to personal positive events (Panaite, Whittington, et al., 2018). These authors also observed that lower positive appraisals of the neutral laboratory film predicted a larger mood brightening effect in daily life. This further supports the idea of a contrast effect, as the MDD individuals may also experience their neutral daily life events as less positive. However, while emotional reactivity in both laboratory and daily life studies is typically assessed as emotional changes in response to emotional stimuli or events relative to neutral stimuli or events, differences in responses to neutral stimuli are rarely examined independently. It will be important for future research to address potential differences in responses to neutral reactivity that may have implications for interpretation of emotional reactivity findings.
Other lab-life work has also attempted to link similar constructs in daily life and laboratory measures, although this work is limited in MDD samples. For example, in nonclinical samples, lower levels of resting RSA predict affective instability Koval et al., 2013) and persistence of negative affect (inertia) in daily life (De Longis et al., 2020), suggesting that there may be links between experience and physiology on measures that assess flexibility in responding to the environment. Further supporting this idea, Ottaviani et al., (2015), also in a nonclinical sample, found associations between lower ambulatory HRV and concurrent self-reports of perseverative negative thought in daily life, which is associated with cognitive inflexibility. Resting RSA has also been found to mediate links between rumination and depression longitudinally in a nonclinical sample (Carnevali et al., 2018). However, further work is needed assessing whether MDD may moderate these relationships. Research linking laboratory and daily life measures have found evidence that autonomic functioning may impact daily life socioemotional functioning. Specifically, Hamilton and Alloy (2016) found in a remitted MDD young adult sample that blunted RSA reactivity predicted increased interpersonal stress generation in daily life, and interpersonal stress generation mediated the relationship between RSA reactivity and daily depression symptoms.
Some limited work has also found evidence of brain–behavior relationships, which are important for establishing the functional significance of neural indices of emotional processing. For example, Compton and colleagues () have found that a larger error-related negative (ERN, an index of self-monitoring associated with sensitivity to errors), is associated with reduced daily life stress reactivity among healthy adults as well as those with elevated depression symptoms. We have also observed preliminary associations between neural indices of reward and affective processing (i.e., FN, LPP) and daily life emotional functioning (e.g., affect, emotional reactivity, and regulation) among adolescents varying in familial depression risk (Bylsma et al., 2018), as well as associations among neural indices of performance monitoring (i.e., ERN), emotion processing (i.e., LPP), and daily life emotional functioning among youth with anxiety disorders (Bylsma, Tan, et al., 2020; Tan, Bylsma, et al., 2020). Further work is needed to improve characterization of how alterations in neural indices of emotional processes are reflected in daily life functioning in MDD and depression risk across development.
Ambulatory assessment technology incorporating passive sensing technology is rapidly increasing. It has become increasingly feasible to assess multiple indices of emotional functioning in daily life, including self-report, behavior, and physiology, although the richness of this intensively sampled multimodal data also leads to numerous methodological and analytic challenges (e.g., Stange et al., 2019; Wilhelm & Grossman, 2010; Wright & Zimmerman, 2019). Future work leveraging this developing technology along with advanced statistical methods that can manage large amounts of densely sampled data from multiple response systems will be critical in gaining a better understanding of emotional reactivity and regulation in the context of daily life. It is apparent that many contextual factors likely impact emotional processes, yet, these remain to be systematically described and are difficult to assess in a laboratory context. In line with emergent or constructionist views of emotion, examining self-reported emotional experience in combination with psychophysiology in daily life can help elucidate context-dependent patterns of physiological activity at both the between and within-person level (Hoemann et al., 2020). Further, combining laboratory and daily life assessments provides greater ecological validity and informs clinical significance of commonly used laboratory indices of emotional functioning.
7 |. THE INFLUENCE OF DEVELOPMENT AND EARLY ENVIRONMENTAL FACTORS ON EMOTION AND DEPRESSION RISK
The fifth issue concerns the need for consideration of developmental processes and contributions of the early environment (e.g., early life adversity, parenting) to emotional development and depression risk. Besides the challenge of integrating measures of emotion across multiple levels, systems, and functional domains, a further challenge is consideration of the environmental and developmental context in which these are embedded (Bylsma, et al., 2016). Measures of emotion dysregulation and the influence of environmental risk factors may dynamically change or interact in different ways across the life span (Beauchaine, 2001; Cicchetti & Rogosch, 1996; Rutter & Stroufe, 2000). For example, high resting RSA has been associated with negative emotionality in infants (Porges, 1994; Stifter & Fox, 1990), but by late childhood high resting RSA is associated with better emotion regulation abilities (Beauchaine, 2001; Blair & Peters, 2003; Fabes et al., 1993). Differences in emotional functioning or interactions across response systems or functional domains could lead to different symptom presentations and developmental psychopathology frameworks that could inform RDoC approaches that integrate across multiple systems (Franklin et al., 2015). Importantly, autonomic functioning continues to develop from infancy through adulthood with some evidence of sex-related differences, although longitudinal studies examining the same individuals over time are limited (e.g., Bar-Haim et al., 2000; Hamilton & Alloy, 2016; Koenig & Thayer, 2016; Obradović & Boyce, 2012).
The consideration of developmental processes on emotional functioning in depression is particularly important, as emotional systems continue to develop well throughout adolescence and young adulthood. For example, adolescence represents a key period of increasing flexibility and the development of neural systems supporting cognitive-affective processes (Crone & Dahl, 2012; Ladouceur, 2012). Neural substrates underpinning reward processing undergo significant maturational changes during adolescence, which is a developmental period characterized by increases in reward seeking behavior (Galvan, 2010). The onset of depressive disorders also rises sharply during this developmental stage, particularly in girls (Forbes et al., 2010; Kessler et al., 2001), suggesting that there may be possible alterations in the neurodevelopment of reward circuity that may confer risk (Forbes, 2009). Emotion regulation abilities, such as cognitive reappraisal skills, critical for the regulation of negative affect, also increase with age (McRae et al., 2012). Family interactions and parenting behaviors are particularly important in the development of emotion regulation skills (Bylsma, et al., 2016; Morris et al., 2007; Tan et al., 2020).
Few laboratory studies have examined measures of emotional reactivity in youth samples with depression, so it remains even less clear to what extent ECI may manifest in pediatric depression. However, consistent with ECI, there is some evidence of emotional blunting in behavioral and neuroimaging studies of emotional attention in depressed youth, which may be due to use of more avoidance and disengagement emotion regulation strategies (Jazbec et al., 2005; Silk et al., 2007; Thomas et al., 2001). Depressed adolescents also show reduced physiological reactivity in response to parent behavior (Allen et al., 2012) and to sad stimuli, although fearful stimuli tend to elicit increased reactivity in this population, which may be a result of comorbid anxiety (Somers et al., 2018). Similar to adults, low resting RSA is also observed in children and adolescents with depression (Koenig et al., 2016). However, a review of RSA reactivity in depressed children and adolescents revealed mixed findings, with some studies showing blunted RSA to be associated with depression in youth, while others finding the opposite pattern (Hamilton & Alloy, 2016). Other developmental studies tend to focus on dimensions of temperament or dimensional measures of internalizing or externalizing symptoms rather than diagnostic categories. For example, high trait behavioral inhibition, high levels of fearfulness, and internalizing symptoms are associated with potentiated sympathetic and parasympathetic reactivity. On the contrary, high levels of trait impulsivity, disinhibition, emotional lability, or externalizing symptoms are associated with low sympathetic reactivity, and, to some extent, low parasympathetic reactivity (Obradović & Broyce, 2012, for review).
Another important area of focus has been on understanding mechanisms conferring risk for depression in families and identifying vulnerability markers that may be present prior to the onset of depression. There is ample evidence that offspring of depressed parents, particularly mothers, are at significantly increased risk for developing depression themselves (Beardslee et al., 1988; Weissman et al., 1997; Williamson et al., 2004), although the specific mechanisms underlying these relationships remain elusive. There may be familial associations in emotion regulation repertoires, which may be one pathway conferring risk or resilience (Bylsma et al., 2015), but it is unclear whether there may be physiological or other biological mechanisms underlying these relationships. Youth at high familial risk for depression have shown alterations in both positive and negative valence systems. For example, high-risk female adolescents with a parental history of depression exhibit a reduced FN amplitude in response to monetary loss versus rewards (Foti et al., 2011), and a blunted FN has also been found to prospectively predict onset of major depression in adolescent girls (Bress et al., 2013). High-risk children also show a blunting of their LPP response to emotional faces, similar to findings for depressed adults (Kujawa et al., 2014). Similarly, we have found that youth with a history of early onset depression and their high-risk siblings also show blunted positive affect (Kovacs et al., 2016), dysregulated affective facial behavior in response to positive emotional stimuli (Panaite, et al., 2019), and deficits in positive autobiographical memory (Begovic et al., 2017), although in these samples we did not find physiological differences in response to rewarding laboratory stimuli. We have also found that youth with a history of juvenile onset depression show blunted autonomic responses to physical and psychological laboratory challenges, as indexed by changes in CAB (Bylsma et al., 2015). Further, reward processing deficits have been observed in remitted MDD and high-risk samples in neural and behavioral studies (Foti et al., 2011; Luking et al., 2016), suggesting that alterations in reward function may represent a vulnerability factor for depression (Auerbach et al., 2014; Gotlib et al., 2010). However, we have also found that deficits in reward learning behavior may only be apparent in youth experiencing current symptoms and not high familial risk youth more generally (Morris et al., 2015).
Some studies have examined interactions between environmental factors and neural or physiological indices in youth. For example, according to differential susceptibility models based on evolutionary biology perspectives, there may be specific endophenotypes (such as physiological reactivity patterns) that confer greater susceptibility to positive and/or negative environmental influences in some individuals (Belsky & Pluess, 2009). Along these lines, there is evidence that certain parental emotion socialization behaviors have a differential influence on youth emotional reactivity and regulation that is depending on functional and structural brain development, including functional connectivity of emotion circuits, highlighting the importance of cross-level interactions between socio-environmental influences in the development of neurobiological systems (Tan et al., 2020). In addition, bidirectional effects have been found in examinations of relationships between early life adversity and physiology, such that early life adversity is associated with reduced RSA, but parasympathetic or sympathetic physiological reactivity can also buffer or moderate the impact of early life adversity on emotional or behavioral difficulties (Orbradovic & Boyce, 2012, for review). Further, we have found that patterns of RSA at rest and in response to a sad film predicts emotion regulation effectiveness (both trait and state measures) among adolescents varying in depression history (Yaroslavsky et al., 2016). Additional research is needed to develop experimental paradigms and stimuli that elicit response in specific domains (rather than expecting comparable reactions across domains), as well as comprehensive investigation of contextual moderating factors that may explain conditions under which specific emotional domains may be activated.
There is some evidence that emotional functioning may predict the course of depression (see Morris et al., 2009, for review). Along these lines, we have also observed in separate studies that patterns of daily life emotional dynamics (Panaite et al., 2020) and autonomic responding (Panaite et al., 2016) predict the naturalistic course of depression in adults. Other work has found that baseline RSA predicts depression symptoms 1 year later among healthy emerging adults (Yaptangco et al., 2015). However, how these patterns of affective dynamics track physiological changes in emotional processing longitudinally across development and over the course of depression remains to be fully characterized. More research is needed to gain a better understanding of what affective alterations track the current depressed state versus which may represent a trait like marker that may confer vulnerability for depression, and how these markers may change across development and the course of the disorder.
8 |. RELIABILITY, VALIDITY, AND METHODOLOGICAL ISSUES IN EMOTION MEASUREMENT
A final key issue related to the multicomponent emergent nature of emotion is reliability of experimental paradigms to reliability elicit emotion, given that emotion is a multifaceted process with loosely corresponding components upon which a variety of regulation processes may have an influence at different temporal stages. Most standardization of emotional stimuli has focused on self-report measures of specific emotions or valence and arousal ratings, such as emotional films (Gross & Levenson, 1995; Rottenberg et al., 2007), positively or negatively valenced pictures (International Affective Picture System, IAPS; Lang & Bradley, 2007), or emotional faces (e.g., Egger et al., 2011). However, these stimuli are developed based on self-report ratings of discrete emotions (Samson et al., 2016), and are not typically able to produce reliable responses in psychophysiological or behavioral response domains (Carvalho et al., 2012; Fernandez et al., 2012; Gilman et al., 2017).
ERP components elicited in response to emotional stimuli, such as emotional pictures or reward, are an exception to this, as components such as the LPP or FN (RewP) can be reliably elicited using emotional pictures/faces (Olofsson et al., 2008) and reward stimuli (Ethridge & Weinberg, 2018; Proudfit, 2015). In particular, the FN has demonstrated good psychometric properties and high reliability longitudinally (Ethridge & Weinberg, 2018). This may be because stimuli used in ERP studies tend to be less complex, more static, and presented for very brief intervals (seconds), which allows for examining more specific component processes of emotion (such as sustained attention or reward processing). Laboratory stressor tasks, such as the Trier Social Stress Task (TSST), have also shown to be more reliable in terms of eliciting consistent physiological responses (e.g., physiological arousal and HPA activity) and self-reports of stress or negative affect (Kirschbaum et al., 1993), although there is a wide degree of variability in the strength of the responses, and changes in physiological arousal in response to stress are also only one specific component of emotional experience.
Although a growing number of studies have examined the influence of specific emotion regulation processes on an emotional response, such as reappraisal or suppression (e.g., Gross, 1998), research is only just beginning to unravel emotional reactions from regulatory processes. Emotion regulatory processes can operate on both the conscious (effortful) and unconscious (automatic) level at multiple stages of an emotional response, making it particularly difficult, if not impossible, to fully disentangle “pure” emotional reactivity from associated regulatory processes. However, research can continue to chip away at this issue in both healthy and clinical samples by investigating a broader array of regulatory processes in investigations of emotional reactivity across multiple contexts, levels of analysis, and functional domains.
Besides the challenge of differentiating emotional reactivity from regulation, it is also often quite challenging to differentiate emotional reactivity from appraisal processes. For example, if an individual provides valence and arousal self-ratings in response to emotional pictures, it is unclear if that truly reflects an emotional reaction or simply an appraisal of an emotional stimulus. Responses to emotional faces are also contingent upon the ability to perceive and process the nature of the facial affect being expressed and may reflect processing biases more than emotional reactivity (e.g., Suzuki et al., 2015), and there is also evidence that face stimuli may be less arousing in comparison to emotional images in healthy individuals (Britton et al., 2006). For daily life research, an inherent limitation is that the categorization of positive and negative events has to be based to some extent on the appraisals of the individual (e.g., Bylsma, et al., 2011). Even when objective coding of event descriptions are used by independent raters, the descriptions provided by the participants may still be biased in a way that may influence the objective ratings (i.e., a depressed person may describe a situation using more negative language than a healthy person).
Some attempts have been made to develop self-report scales that assess relevant components of emotional reactivity, which have potential utility in identifying the most relevant features of emotional reactivity that may be relevant for psychopathology in larger samples where laboratory or daily life assessments may be impractical (e.g., Emotion Reactivity Scale, Nock et al., 2008; Perth Emotional Reactivity Scale, Becerra et al., 2019). However, these scales are yet to be validated against physiological reactivity or other indicators of emotional reactivity, which will be important in determining how these components of emotional reactivity map on to emotional outputs across functional domains and units of analysis.
The lack of consistent results across emotion response domains in studies of emotional reactivity for discrete emotions is not surprising given the accumulating evidence for conceptualizing emotion as emergent phenomena, where coherence across emotional indicator is not expected (Gross & Barrett, 2011). Further, emotion regulation processes can be difficult (or even impossible) to disentangle from emotional reactivity, and, like emotional reactivity, can occur on many different levels as well as stages in the ongoing emotional process (Gross & Thompson, 2007). However, it is important to note that even if an emotional stimulus fails to consistently elicit a response in a particular domain (i.e., physiology, behavior, etc.), it does not necessarily mean that the experimental paradigm or measure is unreliable, as emotional changes occur in different response domains at multiple levels of analysis that are not expected to be coherent (except perhaps in some special circumstances). Along these lines, if a measure does not replicate across individuals or across time, it is not necessarily a reflection of poor reliability or validity, as there are many potential dynamic contextual moderators of emotional reactions, including internal states, external environmental features, individual differences, prior experiences, and developmental processes. Thus, it is critical to examine emotional processes across a variety of indicators, levels of analysis, temporal dimensions, and contexts. Any one measure alone is unlikely to provide an accurate picture of emotional functioning in depression, which makes it challenging to evaluating the theoretical predictions of ECI.
9 |. CONCLUSIONS
While it is clear that MDD involves alterations in emotional processing, reactivity, and regulation, the nature of these alterations across development and the course of the disorder remain to be fully characterized. Although there is converging evidence for ECI across multiple response systems and methodologies, there are also many examples of apparently divergent findings, particularly for daily life studies of emotion. These findings suggest the importance of context in predicting variation in patterns of emotional responding in MDD. It is possible that these seemingly discrepant findings may still be broadly consistent with ECI, as they may reflect a general pattern of insensitivity to changing contexts. For example, an emotional response pattern may be appropriate (adaptive) to one context, but inappropriate (maladaptive) for another. Discrepant findings highlight the need for a better understanding of specific proximal and distal contextual factors (including both internal state and external environment) that may moderate emotional responding in both healthy and depressed individuals, including the influence of individual differences, developmental processes, and early life experiences.
Given that emotions likely represent emergent phenomena where indicators are not expected to cohere, examination of emotional processes in MDD across multiple levels, response domains, and biological systems are needed to better understand the complex patterns of response and potential within-level and cross-level interactions, including studies that incorporate the complementary advantages of both laboratory and daily life measures. Assessment of emotional processes from different units of analyses and contexts is likely to provide unique variance that will increase our understanding of emotional deficits as they relate to current depression and depression risk. For example, creative integration of different emotion measures across multiple response systems at different levels of analysis can be used to complement the strengths and weaknesses of existing measures. For example, fNIRS and electrodermal activity have been combined to utilize the strengths of fNIRS in assessing in assessing cognitive control of emotion and electrodermal activity to index emotional arousal, as fNIRS is limited in assessing activity of deeper limbic structures (Bandara et al., 2016; Grabel, 2019).
Recent advances in data analytic and machine learning approaches facilitate the integration of large amounts of densely sampled data across different measures. Technological advances in ambulatory assessment that now allow for better integration of multiple response systems, including experience, behavior, and physiology, hold promise for a better understanding of the influence of contextual factors on emotional functioning in MDD. This will result in assessments that have greater ecological validity and clinical utility than standardized laboratory measures. Advances in these methodologies will support efforts to incorporate a multimodal, multimethod, developmentally informed contextual framework for understanding emotional functioning in MDD that will inform prevention and intervention efforts for this prevalent, disabling, and chronic condition.
Box 1. What is emotion context insensitivity (ECI)?
ECI posits that the pervasive mood disturbance in MDD influences ongoing emotional reactions in a way that leads to a general disengagement with the environment and a reduction in motivated activity (Rottenberg et al., 2005). This disengagement can be manifested in affective inflexibility and a lack of contextually appropriate responses in response to both positive and negative stimuli. ECI is derived from evolutionary perspectives on depression, where depression is viewed as an evolved defensive response to adverse environmental situations, such that continued action could be dangerous or wasteful (Nesse, 2000).
Box 2. Key challenges in depression emotion research.
The heterogeneity of MDD as a diagnostic category
Emotions are dynamic multilevel multicomponent emergent phenomena
Characterization of within and cross-level interactions across emotion response domains
The influence of context on emotional reactivity and regulation
The influence of development and early environmental factors on emotion and depression risk
Reliability, validity, and methodological issues in emotion measurement
ACKNOWLEDGMENTS
This article is based on a talk presented for receipt of an Award for Distinguished Early Career Contributions to Psychophysiological Research at the 59th Annual Meeting for the Society for Psychophysiological Research in Washington, DC in September 2019. I would like to acknowledge the guidance and support of all my mentors during my graduate and postdoctoral training, including Jonathan Rottenberg, Marika Kovacs, Cecile Ladouceur, and Jennifer Silk. I would also like to express my appreciation for all my informal mentors who helped contribute to my training incorporating psychophysiological methods in my research, including Kristen Salomon, Geoffrey Potts, Greg Hajcak, and J. Richard Jennings. I am also grateful for the guidance provided from Monica Fabiani (Editor-in-Chief, Psychophysiology) during the development of this manuscript, as well as those who provided feedback on earlier versions of this manuscript. Finally, I would like to give my appreciation to all my research collaborators, the staff, and students who have assisted with my research projects, the funding agencies that have supported my work, as well as the research participants and their families.
Funding information
Brain and Behavior Research Foundation: NARSAD Young Investigator Grant (26082), including support from Vital Projects Fund, Inc.; American Psychological Association of Graduate Students: Nancy B. Forest & L. Michael Honaker Master’s Scholarship for Research in Psychology; American Psychological Foundation: McGuigan Dissertation Award; National Institute of Mental Health, Grant/Award Number: MH018951, MH077669, MH080794, MH101750, MH104325 and MH118218; University of South Florida: Professor Charles D. and Carol Spielberger Endowed Fund
REFERENCES
- Aldao A (2013). The future of emotion regulation research: Capturing context. Perspectives on Psychological Science, 8(2), 155–172. 10.1177/1745691612459518 [DOI] [PubMed] [Google Scholar]
- Aldao A, Sheppes G, & Gross JJ (2015). Emotion regulation flexibility. Cognitive Therapy and Research, 39(3), 263–278. 10.1007/s10608-014-9662-4 [DOI] [Google Scholar]
- Allen NB, Kuppens P, & Sheeber LB (2012). Heart rate responses to parental behavior in depressed adolescents. Biological Psychology, 90(1), 80–87. 10.1016/j.biopsycho.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Author. [DOI] [PubMed]
- Auerbach RP, Admon R, & Pizzagalli DA (2014). Adolescent depression: Stress and reward dysfunction. Harvard Review of Psychiatry, 22(3), 139–148. 10.1097/HRP.0000000000000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarotti S, Biassoni F, Colombo B, & Ciceri MR (2017). Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biological Psychology, 130, 54–66. 10.1016/j.biopsycho.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Bandara D, Song S, Hirshfield L, & Velipasalar S (2016, July). A more complete picture of emotion using electrocardiogram and electrodermal activity to complement cognitive data. In International Conference on Augmented Cognition (pp. 287–298). Springer. [Google Scholar]
- Bar-Haim Y, Marshall PJ, & Fox NA (2000). Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 37(1), 44–56. ;2–7 [DOI] [PubMed] [Google Scholar]
- Barrett LF (2006). Are emotions natural kinds? Perspectives on Psychological Science, 1(1), 28–58. 10.1111/j.1745-6916.2006.00003.x [DOI] [PubMed] [Google Scholar]
- Barrett LF (2012). Emotions are real. Emotion, 12(3), 413–429. 10.1037/a0027555 [DOI] [PubMed] [Google Scholar]
- Barrett LF, Gendron M, & Huang YM (2009). Do discrete emotions exist? Philosophical Psychology, 22(4), 427–437. 10.1080/09515080903153634 [DOI] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, & Gross JJ (2007). The experience of emotion. Annual Review of Psychology, 58, 373–403. 10.1146/annurev.psych.58.110405.085709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardslee WR, Keller MB, Lavori PW, Klerman GK, Dorer DJ, & Samuelson H (1988). Psychiatric disorder in adolescent offspring of parents with affective disorder in a non-referred sample. Journal of Affective Disorders, 15(3), 313–322. 10.1016/0165-0327(88)90028-6 [DOI] [PubMed] [Google Scholar]
- Beauchaine T (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13(2), 183–214. 10.1017/S0954579401002012 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra R, Preece D, Campitelli G, & Scott-Pillow G (2019). The assessment of emotional reactivity across negative and positive emotions: Development and validation of the Perth Emotional Reactivity Scale (PERS). Assessment, 26(5), 867–879. 10.1177/1073191117694455 [DOI] [PubMed] [Google Scholar]
- Begovic E, Panaite V, Bylsma LM, George C, Kovacs M, Yaroslavsky I, Baji I, Benák I, Dochnal R, Kiss E, Vetró Á, Kapornai K, & Rottenberg J (2017). Positive autobiographical memory deficits in youth with depression histories and their never-depressed siblings. British Journal of Clinical Psychology, 56(3), 329–346. 10.1111/bjc.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, & Pluess M (2009). Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin, 135(6), 885–908. 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Benau EM, Hill KE, Atchley RA, O’Hare AJ, Gibson LJ, Hajcak G, Ilardi SS, & Foti D (2019). Increased neural sensitivity to self-relevant stimuli in major depressive disorder. Psychophysiology, 56(7), e13345. 10.1111/psyp.13345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D, Williams C, & Brown A (2007). Impact of consuming a milk drink containing a probiotic on mood and cognition. European Journal of Clinical Nutrition, 61(3), 355–361. 10.1038/sj.ejcn.1602546 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1991). Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review, 98(4), 459–487. 10.1037/0033-295X.98.4.459 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993). Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30(2), 183–196. 10.1111/j.1469-8986.1993.tb01731.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, & Cacioppo JT (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45(4), 643–652. 10.1111/j.1469-8986.2008.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW (2009). Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9(1), 65–73. 10.1016/j.coph.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Peters R (2003). Physiological and neurocognitive correlates of adaptive behavior in preschool among children in Head Start. Developmental Neuropsychology, 24(1), 479–497. 10.1207/S15326942DN2401_04 [DOI] [PubMed] [Google Scholar]
- Bonaz B, Bazin T, & Pellissier S (2018). The vagus nerve at the interface of the microbiota-gut-brain axis. Frontiers in Neuroscience, 12, 49. 10.3389/fnins.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower B, Bylsma LM, Morris BH, & Rottenberg J (2010). Poor reported sleep quality predicts low positive affect in daily life among healthy and mood-disordered persons. Journal of Sleep Research, 19(2), 323–332. 10.1111/j.1365-2869.2009.00816.x [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, & Cryan JF (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences, 108(38), 16050–16055. 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, & Hajcak G (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology, 50(1), 74–81. 10.1111/j.1469-8986.2012.01485.x [DOI] [PubMed] [Google Scholar]
- Bress JN, & Hajcak G (2013). Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology, 50(7), 610–616. 10.1111/psyp.12053 [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, & Hajcak G (2012). Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological Psychology, 89(1), 156–162. 10.1016/j.biopsycho.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, & Liberzon I (2006). Facial expressions and complex IAPS pictures: Common and differential networks. NeuroImage, 31, 906–919. 10.1016/j.neuroimage.2005.12.050 [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Richards HJ, Helps SK, Chronaki G, Bamford S, & Sonuga-Barke EJ (2012). An electrophysiological monetary incentive delay (e-MID) task: A way to decompose the different components of neural response to positive and negative monetary reinforcement. Journal of Neuroscience Methods, 209(1), 40–49. 10.1016/j.jneumeth.2012.05.015 [DOI] [PubMed] [Google Scholar]
- Brush CJ, Olson RL, Ehmann PJ, Bocchine AJ, Bates ME, Buckman JF, Leyro TM, & Alderman BL (2019). Lower resting cardiac autonomic balance in young adults with current major depression. Psychophysiology, 56(8), e13385. 10.1111/psyp.13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM (2012). Emotional reactivity and regulation in current and remitted depression: An event related potential study (graduate theses and dissertations). University of South Florida. http://scholarcommons.usf.edu/etd/4000 [Google Scholar]
- Bylsma LM, Croon MA, Vingerhoets AJ, & Rottenberg J (2011). When and for whom does crying improve mood? A daily diary study of 1004 crying episodes. Journal of Research in Personality, 45(4), 385–392. 10.1016/j.jrp.2011.04.007 [DOI] [Google Scholar]
- Bylsma LM, Gračanin A, & Vingerhoets AJ (2019). The neurobiology of human crying. Clinical Autonomic Research, 29(1), 63–73. 10.1007/s10286-018-0526-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Gračanin A, & Vingerhoets AJJM (2020). A clinical practice review of crying research. Psychotherapy. Advance online publication. 10.1037/pst0000342 [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Mauss IB, & Rottenberg J (2016). Is the divide a chasm?: Bridging affective science with clinical practice. Journal of Psychopathology and Behavioral Assessment, 38(1), 42–47. 10.1007/s10862-015-9525-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, & Rottenberg J (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review, 28(4), 676–691. 10.1016/j.cpr.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, & Rottenberg J (2014). RSA reactivity in current and remitted major depressive disorder. Psychosomatic Medicine, 76(1), 66. 10.1097/PSY.0000000000000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Silk JS, & Ladouceur CD (2018). Neural indices of emotion processing and associations with daily life emotional functioning in adolescents at high and low familial risk for depression. In Bylsma LM (chair), Multi-method approaches incorporating psychophysiological indices of emotional processing and regulation across development: Links with psychopathology and real world emotional functioning. Symposium presented at the Annual Conference of the Social for Psychophysiological Research in Quebec City, Canada. [Google Scholar]
- Bylsma LM, Tan PZ, Allen KB, Silk JS, Siegle GJ, Forbes EE, McMakin DL, Dahl R, Ryan ND, & Ladouceur CD (2020). Neural activity during affective picture processing is associated with daily life emotional functioning among anxious youth. Manuscript in preparation.
- Bylsma LM, Taylor-Clift A, & Rottenberg J (2011). Emotional reactivity to daily events in major and minor depression. Journal of Abnormal Psychology, 120(1), 155–167. 10.1037/a0021662 [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Vingerhoets AJ, & Rottenberg J (2008). When is crying cathartic? An international study. Journal of Social and Clinical Psychology, 27(10), 1165–1187. 10.1521/jscp.2008.27.10.1165sd [DOI] [Google Scholar]
- Bylsma LM, Yaroslavsky I, Rottenberg J, Jennings JR, George CJ, Kiss E, Kapornai K, Halas K, Dochnal R, Lefkovics E, Benák I, Baji I, Vetró Á, & Kovacs M (2015). Juvenile onset depression alters cardiac autonomic balance in response to psychological and physical challenges. Biological Psychology, 110, 167–174. 10.1016/j.biopsycho.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Yaroslavsky I, Rottenberg J, Kiss E, Kapornai K, Halas K, Dochnal R, Lefkovics E, Baji I, Vetrό Á, & Kovacs M (2016). Familiality of mood repair responses among youth with and without histories of depression. Cognition and Emotion, 30(4), 807–816. 10.1080/02699931.2015.1025707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, & Ito TA (2000). The psychophysiology of emotion. Handbook of Emotions, 2, 173–191. [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, & Hajcak G (2011). Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. NeuroImage, 57(4), 1608–1616. 10.1016/j.neuroimage.2011.05.037 [DOI] [PubMed] [Google Scholar]
- Carnevali L, Thayer JF, Brosschot JF, & Ottaviani C (2018). Heart rate variability mediates the link between rumination and depressive symptoms: A longitudinal study. International Journal of Psychophysiology, 131, 131–138. 10.1016/j.ijpsycho.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Carvalho S, Leite J, Galdo-Álvarez S, & Gonçalves OF (2012). The emotional movie database (EMDB): A self-report and psychophysiological study. Applied Psychophysiology and Biofeedback, 37(4), 279–294. 10.1007/s10484-012-9201-6 [DOI] [PubMed] [Google Scholar]
- Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, & Sublette ME (2019). Systematic review of gut microbiota and major depression. Frontiers in Psychiatry, 10, 34. 10.3389/fpsyt.2019.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8(4), 597–600. 10.1017/S0954579400007318 [DOI] [Google Scholar]
- Clore GL, & Ortony A (2008). Appraisal theories: How cognition shapes affect into emotion. In Lewis M, Haviland-Jones JM, & Barrett LF (), Handbook of emotions (pp. 628–642). The Guilford Press. [Google Scholar]
- Coan JA (2010). Emergent ghosts of the emotion machine. Emotion Review, 2(3), 274–285. 10.1177/1754073910361978 [DOI] [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A, & Robinson MD (2011). Neural and behavioral measures of error-related cognitive control predict daily coping with stress. Emotion, 11(2), 379–390. 10.1037/a0021776 [DOI] [PubMed] [Google Scholar]
- Compton RJ, Robinson MD, Ode S, Quandt LC, Fineman SL, & Carp J (2008). Error-monitoring ability predicts daily stress regulation. Psychological Science, 19(7), 702–708. 10.1111/j.1467-9280.2008.02145.x [DOI] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Cryan JF, & Dinan TG (2012). Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, 13(10), 701–712. 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, … Dinan TG (2019). The microbiota-gut-brain axis. Physiological Reviews, 99(4), 1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Swartz HA, Salomon K, & Gianaros PJ (2011). Cardiac vagal control in non-medicated depressed women and non-depressed controls: Impact of depression status, lifetime trauma history and respiratory factors. Psychosomatic Medicine, 73(4), 336–343. 10.1097/PSY.0b013e318213925d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daches S, Kovacs M, George CJ, Yaroslavsky I, Kiss E, Vetró Á, Dochnal R, Benák I, Baji I, Halas K, Makai A, Kapornai K, & Rottenberg J (2017). Childhood adversity predicts reduced physiological flexibility during the processing of negative affect among adolescents with major depression histories. International Journal of Psychophysiology, 121, 22–28. 10.1016/j.ijpsycho.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Wolz I, Leppanen J, Fernandez-Aranda F, Schmidt U, & Tchanturia K (2016). Facial expression to emotional stimuli in non-psychotic disorders: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 64, 252–271. 10.1016/j.neubiorev.2016.02.015 [DOI] [PubMed] [Google Scholar]
- De Longis E, Alessandri G, & Ottaviani C (2020). Inertia of emotions and inertia of the heart: Physiological processes underlying inertia of negative emotions at work. International Journal of Psychophysiology, 155, 210–218. 10.1016/j.ijpsycho.2020.06.007 [DOI] [PubMed] [Google Scholar]
- Dennis TA, & Hajcak G (2009). The late positive potential: A neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry, 50(11), 1373–1383. 10.1111/j.1469-7610.2009.02168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibeklioğlu H, Hammal Z, Yang Y, & Cohn JF (2015, November). Multimodal detection of depression in clinical interviews. In Proceedings of the 2015 ACM on international conference on multimodal interaction (pp. 307–310). 10.1145/2818346.2820776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Pine DS, Nelson E, Leibenluft E, Ernst M, Towbin KE, & Angold A (2011). The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): A new set of children’s facial emotion stimuli. International Journal of Methods in Psychiatric Research, 20(3), 145–156. 10.1002/mpr.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge P, & Weinberg A (2018). Psychometric properties of neural responses to monetary and social rewards across development. International Journal of Psychophysiology, 132, 311–322. 10.1016/j.ijpsycho.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, & Eisenbud L (1993). Behavioral and physiological correlates of children’s reactions to others in distress. Developmental Psychology, 29(4), 655–663. 10.1037/0012-1649.29.4.655 [DOI] [Google Scholar]
- Fernández C, Pascual JC, Soler J, Elices M, Portella MJ, & Fernández-Abascal E (2012). Physiological responses induced by emotion-eliciting films. Applied Psychophysiology and Biofeedback, 37(2), 73–79. 10.1007/s10484-012-9180-7 [DOI] [PubMed] [Google Scholar]
- Forbes EE (2009). Where’s the fun in that? Broadening the focus on reward function in depression. Biological Psychiatry, 66(3), 199–200. 10.1016/j.biopsych.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2012). Research review: Altered reward function in adolescent depression: What, when and how? Journal of Child Psychology and Psychiatry, 53(1), 3–15. 10.1111/j.1469-7610.2011.02477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, & Dahl RE (2010). Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 49(2), 162–172. 10.1016/j.jaac.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, Rinaman L, & Cryan JF (2017). Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress, 7, 124–136. 10.1016/j.ynstr.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Carlson JM, Sauder CL, & Proudfit GH (2014). Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage, 101, 50–58. 10.1016/j.neuroimage.2014.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2009). Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology, 81(1), 1–8. 10.1016/j.biopsycho.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Klein DN, & Hajcak G (2011). Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. Journal of Abnormal Child Psychology, 39(7), 913–924. 10.1007/s10802-011-9503-9 [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, & Hajcak G (2010). Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety, 27(9), 813–820. 10.1002/da.20712 [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Bernat EM, & Proudfit GH (2015). Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clinical Neurophysiology, 126(7), 1338–1347. 10.1016/j.clinph.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JC, Jamieson JP, Glenn CR, & Nock MK (2015). How developmental psychopathology theory and research can inform the research domain criteria (RDoC) project. Journal of Clinical Child & Adolescent Psychology, 44(2), 280–290. 10.1080/15374416.2013.873981 [DOI] [PubMed] [Google Scholar]
- Fried EI, & Nesse RM (2015a). Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR* D study. Journal of Affective Disorders, 172, 96–102. 10.1016/j.jad.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, & Nesse RM (2015b). Depression sum-scores don’t add up: Why analyzing specific depression symptoms is essential. BMC Medicine, 13(1), 1–11. 10.1186/s12916-015-0325-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH (2007). An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185–199. 10.1016/j.biopsycho.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Fülling C, Dinan TG, & Cryan JF (2019). Gut microbe to brain signaling: What happens in vagus…. Neuron, 101(6), 998–1002. 10.1016/j.neuron.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Galvan A (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience, 4, 6. 10.3389/neuro.09.006.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman TL, Shaheen R, Nylocks KM, Halachoff D, Chapman J, Flynn JJ, Matt LM, & Coifman KG (2017). A film set for the elicitation of emotion in research: A comprehensive catalog derived from four decades of investigation. Behavior Research Methods, 49(6), 2061–2082. 10.3758/s13428-016-0842-x [DOI] [PubMed] [Google Scholar]
- Glazer JE, Kelley NJ, Pornpattananangkul N, Mittal VA, & Nusslock R (2018). Beyond the FRN: Broadening the time-course of EEG and ERP components implicated in reward processing. International Journal of Psychophysiology, 132, 184–202. 10.1016/j.ijpsycho.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Goldstein BL, Kessel EM, Kujawa A, Finsaas MC, Davila J, Hajcak G, & Klein DN (2020). Stressful life events moderate the effect of neural reward responsiveness in childhood on depressive symptoms in adolescence. Psychological Medicine, 50(9), 1548–1555. 10.1017/S0033291719001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, & Joormann J (2010). Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry, 67(4), 380–387. 10.1001/archgenpsychiatry.2010.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabel A (2019). Using fNIRS and galvanic skin response as a novel approach to infer Limbic-Prefrontal processes in early childhood. Flash Talk presentation at the 2019 Flux Congress, New York, NY [Google Scholar]
- Gračanin A, Bylsma LM, & Vingerhoets AJ (2014). Is crying a self-soothing behavior? Frontiers in Psychology, 5, 502. 10.3389/fpsyg.2014.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (1998). Antecedent-and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74(1), 224–237. 10.1037/0022-3514.74.1.224 [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Feldman Barrett L (2011). Emotion generation and emotion regulation: One or two depends on your point of view. Emotion Review, 3(1), 8–16. 10.1177/1754073910380974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, & Levenson RW (1995). Emotion elicitation using films. Cognition & Emotion, 9(1), 87–108. 10.1080/02699939508408966 [DOI] [Google Scholar]
- Gross JJ, & Thompson RA (2007). Emotion regulation: Conceptual foundations. In Gross JJ (Ed.), Handbook of emotion regulation (pp. 3–24). Guilford. [Google Scholar]
- Hajcak G, MacNamara A, & Olvet DM (2010). Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology, 35(2), 129–155. 10.1080/87565640903526504 [DOI] [PubMed] [Google Scholar]
- Hamilton JL, & Alloy LB (2016). Atypical reactivity of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clinical Psychology Review, 50, 67–79. 10.1016/j.cpr.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Chand S, Reinhardt L, Ladouceur CD, Silk JS, Moreno M, Franzen PL, & Bylsma LM (2020). Social media use predicts later sleep timing and greater sleep variability: An ecological momentary assessment study of youth at high and low familial risk for depression. Journal of Adolescence, 83, 122–130. 10.1016/j.adolescence.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Ladouceur CD, Silk JS, Franzen PL, & Bylsma LM (2020). Higher rates of sleep disturbance among offspring of parents with recurrent depression compared to offspring of nondepressed parents. Journal of Pediatric Psychology, 45(1), 1–11. 10.1093/jpepsy/jsz079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Stange JP, Burke TA, Franzen PL, & Alloy LB (2019). Sleep disturbance and physiological regulation among young adults with prior depression. Journal of Psychiatric Research, 115, 75–81. 10.1016/j.jpsychires.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu Z-W, Gao X-B, Kaelberer MM, Bohórquez DV, Shammah-Lagnado SJ, de Lartigue G, & de Araujo IE (2018). A neural circuit for gut-induced reward. Cell, 175(3), 665–678. 10.1016/j.cell.2018.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KE, South SC, Egan RP, & Foti D (2019). Abnormal emotional reactivity in depression: Contrasting theoretical models using neurophysiological data. Biological Psychology, 141, 35–43. 10.1016/j.biopsycho.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Hinnant JB, & El-Sheikh M (2009). Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. Journal of Abnormal Child Psychology, 37(8), 1049–1061. 10.1007/s10802-009-9341-1 [DOI] [PubMed] [Google Scholar]
- Hoemann K, Khan Z, Feldman MJ, Nielson C, Devlin M, Dy J, Barrett LF, Wormwood JB, & Quigley KS (2020). Context-aware experience sampling reveals the scale of variation in affective experience. Scientific Reports, 10(1). 10.1038/s41598-020-69180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, & Wang P (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, & Ernst M (2005). Cognitive control under contingencies in anxious and depressed adolescents: An antisaccade task. Biological Psychiatry, 58(8), 632–639. 10.1016/j.biopsych.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Kashdan TB, & Rottenberg J (2010). Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review, 30(7), 865–878. 10.1016/j.cpr.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, & Dinan TG (2016). Transferring the blues: Depression-associated gut microbio ta induces neurobehavioural changes in the rat. Journal of Psychiatric Research, 82, 109–118. 10.1016/j.jpsychires.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, & Gatt JM (2010). Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry, 67(11), 1067–1074. 10.1016/j.biopsych.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, & Stringaris A (2018). Reward processing in depression: A conceptual and meta-analytic review across fMRI and EEG studies. American Journal of Psychiatry, 175(11), 1111–1120. 10.1176/appi.ajp.2018.17101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, & Merikangas KR (2001). Mood disorders in children and adolescents: An epidemiologic perspective. Biological Psychiatry, 49(12), 1002–1014. 10.1016/S0006-3223(01)01129-5 [DOI] [PubMed] [Google Scholar]
- Khazanov GK, Ruscio AM, & Swendsen J (2019). The “brightening” effect: Reactions to positive events in the daily lives of individuals with major depressive disorder and generalized anxiety disorder. Behavior Therapy, 50(2), 270–284. 10.1016/j.beth.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’–A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Klawohn J, Burani K, Bruchnak A, Santopetro N, & Hajcak G (2020). Reduced neural response to reward and pleasant pictures independently relate to depression. Psychological Medicine, 1–9. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Koch C, Wilhelm M, Salzmann S, Rief W, & Euteneuer F (2019). A meta-analysis of heart rate variability in major depression. Psychological Medicine, 49(12), 1948–1957. 10.1017/S0033291719001351 [DOI] [PubMed] [Google Scholar]
- Koenig J, Kemp AH, Beauchaine TP, Thayer JF, & Kaess M (2016). Depression and resting state heart rate variability in children and adolescents—A systematic review and meta-analysis. Clinical Psychology Review, 46, 136–150. 10.1016/j.cpr.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Koenig J, & Thayer JF (2016). Sex differences in healthy human heart rate variability: A meta-analysis. Neuroscience and Biobehavioral Reviews, 64, 288–310. 10.1016/j.neubiorev.2016.03.00 [DOI] [PubMed] [Google Scholar]
- Kovacs M, Bylsma LM, Yaroslavsky I, Rottenberg J, George CJ, Kiss E, Halas K, Benák I, Baji I, Vetró Á, & Kapornai K (2016). Positive affectivity is dampened in youths with histories of major depression and their never-depressed adolescent siblings. Clinical Psychological Science, 4(4), 661–674. 10.1177/2167702615607182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval P, Ogrinz B, Kuppens P, Van den Bergh O, Tuerlinckx F, & Sütterlin S (2013). Affective instability in daily life is predicted by resting heart rate variability. PLoS One, 8(11), e81536. 10.1371/journal.pone.0081536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. 10.1016/j.biopsycho.2010.03.010 [DOI] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, & Klein DN (2014). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology, 123(2), 287–297. 10.1037/a0036285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde S, Mosley E, & Mertgen A (2018). Vagal tank theory: The three Rs of cardiac vagal control functioning–Resting, reactivity, and recovery. Frontiers in Neuroscience, 12, 458. 10.3389/fnins.2018.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD (2012). Neural systems supporting cognitive-affective interactions in adolescence: The role of puberty and implications for affective disorders. Frontiers in Integrative Neuroscience, 6, 65. 10.3389/fnint.2012.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, & Bradley MM (2007). The International Affective Picture System (IAPS) in the study of emotion and attention. Handbook of Emotion Elicitation and Assessment, 29, 70–73. [Google Scholar]
- Lang PJ, & Bradley MM (2010). Emotion and the motivational brain. Biological Psychology, 84(3), 437–450. 10.1016/j.biopsycho.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1998). Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry, 44(12), 1248–1263. 10.1016/S0006-3223(98)00275-3 [DOI] [PubMed] [Google Scholar]
- Lehofer M, Moser M, Hoehn-Saric R, McLeod D, Liebmann P, Drnovsek B, Egner S, Hildebrandt G, & Zapotoczky H-G (1997). Major depression and cardiac autonomic control. Biological Psychiatry, 42(10), 914–919. 10.1016/S0006-3223(96)00494-5 [DOI] [PubMed] [Google Scholar]
- Levenson RW (2003). Blood, sweat, and fears: The autonomic architecture of emotion. Annals of the New York Academy of Sciences, 1000(1), 348–366. 10.1196/annals.1280.016 [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, & Penninx BW (2008). Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Archives of General Psychiatry, 65(12), 1358–1367. 10.1001/archpsyc.65.12.1358 [DOI] [PubMed] [Google Scholar]
- Light KC, Kothandapani RV, & Allen MT (1998). Enhanced cardiovascular and catecholamine responses in women with depressive symptoms. International Journal of Psychophysiology, 28(2), 157–166. 10.1016/S0167-8760(97)00093-7 [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, & Barrett LF (2012). The brain basis of emotion: A meta-analytic review. The Behavioral and Brain Sciences, 35(3), 121–143. 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, & Chan RC (2014). The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia, 53, 213–220. 10.1016/j.neuropsychologia.2013.11.023 [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, & Ding M (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience, 32(42), 14563–14572. 10.1523/JNEUROSCI.3109-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, & Barch DM (2016). Depression risk predicts blunted neural responses to gains and enhanced responses to losses in healthy children. Journal of the American Academy of Child & Adolescent Psychiatry, 55(4), 328–337. 10.1016/j.jaac.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin DM, Kotov R, Perlman G, Nelson BD, Goldstein BL, Hajcak G, & Klein DN (2019). Reward processing and future life stress: Stress generation pathway to depression. Journal of Abnormal Psychology, 128(4), 305–314. 10.1037/abn0000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion, 5(2), 175–190. 10.1037/1528-3542.5.2.175 [DOI] [PubMed] [Google Scholar]
- Mauss IB, & Robinson MD (2009). Measures of emotion: A review. Cognition and Emotion, 23(2), 209–237. 10.1080/02699930802204677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JDE, & Ochsner KN (2012). The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience, 7(1), 11–22. 10.1093/scan/nsr093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, & Cazaubiel JM (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal of Nutrition, 105(5), 755–764. 10.1017/S0007114510004319 [DOI] [PubMed] [Google Scholar]
- Michels N, Van de Wiele T, Fouhy F, O’Mahony S, Clarke G, & Keane J (2019). Gut microbiome patterns depending on children’s psychosocial stress: Reports versus biomarkers. Brain, Behavior, and Immunity, 80, 751–762. 10.1016/j.bbi.2019.05.024 [DOI] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA, Georgiades K, Boyle M, & MacMillan HL (2009). Stability of resting frontal electroencephalogram (EEG) asymmetry and cardiac vagal tone in adolescent females exposed to child maltreatment. Developmental Psychobiology, 51(6), 474–487. 10.1002/dev.20387 [DOI] [PubMed] [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, & Robinson LR (2007). The role of the family context in the development of emotion regulation. Social Development, 16(2), 361–388. 10.1111/j.1467-9507.2007.00389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BH, Bylsma LM, & Rottenberg J (2009). Does emotion predict the course of major depressive disorder? A review of prospective studies. British Journal of Clinical Psychology, 48(3), 255–273. 10.1080/02699931.2016.1247035 [DOI] [PubMed] [Google Scholar]
- Morris BH, Bylsma LM, Yaroslavsky I, Kovacs M, & Rottenberg J (2015). Reward learning in pediatric depression and anxiety: Preliminary findings in a high-risk sample. Depression and Anxiety, 32(5), 373–381. 10.1002/da.22358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM (2000). Is depression an adaptation? Archives of General Psychiatry, 57(1), 14–20. 10.1001/archpsyc.57.1.14 [DOI] [PubMed] [Google Scholar]
- Nock MK, Wedig MM, Holmberg EB, & Hooley JM (2008). The emotion reactivity scale: Development, evaluation, and relation to self-injurious thoughts and behaviors. Behavior Therapy, 39(2), 107–116. 10.1016/j.beth.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Novick AM, Levandowski ML, Laumann LE, Philip NS, Price LH, & Tyrka AR (2018). The effects of early life stress on reward processing. Journal of Psychiatric Research, 101, 80–103. 10.1016/j.jpsychires.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Bain EE, Thayer JF, Sollers JJ III, & Drevets WC (2011). Heart rate variability during motor and cognitive tasks in females with major depressive disorder. Psychiatry Research: Neuroimaging, 191(1), 1–8. 10.1016/j.pscychresns.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary K, Bylsma LM, & Rottenberg J (2017). Why might poor sleep quality lead to depression? A role for emotion regulation. Cognition and Emotion, 31(8), 1698–1706. 10.1080/02699931.2016.1247035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary K, Small BJ, Panaite V, Bylsma LM, & Rottenberg J (2017). Sleep quality in healthy and mood-disordered persons predicts daily life emotional reactivity. Cognition and Emotion, 31(3), 435–443. 10.1080/02699931.2015.1126554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović J, & Boyce WT (2012). Developmental psychophysiology of emotion processes. Monographs of the Society for Research in Child Development, 77(2), 120–128. 10.1111/j.1540-5834.2011.00670.x [DOI] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, & Polich J (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77(3), 247–265. 10.1016/j.biopsycho.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C, Medea B, Lonigro A, Tarvainen M, & Couyoumdjian A (2015). Cognitive rigidity is mirrored by autonomic inflexibility in daily life perseverative cognition. Biological Psychology, 107, 24–30. 10.1016/j.biopsycho.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, & Brosschot JF (2016). Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin, 142(3), 231–259. 10.1037/bul0000036 [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Amole MC, Aue T, Balconi M, Bylsma LM, Critchley H, Demaree HA, Friedman BH, Gooding AEK, Gosseries O, Jovanovic T, Kirby LAJ, Kozlowska K, Laureys S, Lowe L, Magee K, Marin M-F, Merner AR, Robinson JL, … VanElzakker MB (2019). Physiological feelings. Neuroscience & Biobehavioral Reviews, 103, 267–304. 10.1016/j.neubiorev.2019.05.002 [DOI] [PubMed] [Google Scholar]
- Panaite V, Bylsma LM, Kovacs M, O’Leary K, George CJ, Baji I, Benák I, Dochnal R, Kiss E, Vetró Á, Kapornai K, & Rottenberg J (2019). Dysregulated behavioral responses to hedonic probes among youth with depression histories and their high-risk siblings. Emotion, 19(1), 171–177. 10.1037/emo0000409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaite V, Hindash AC, Bylsma LM, Small BJ, Salomon K, & Rottenberg J (2016). Respiratory sinus arrhythmia reactivity to a sad film predicts depression symptom improvement and symptomatic trajectory. International Journal of Psychophysiology, 99, 108–113. 10.1016/j.ijpsycho.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaite V, Koval P, Dejonckheere E, & Kuppens P (2018). Emotion regulation and mood brightening in daily life vary with depressive symptom levels. Cognition and Emotion, 33(6), 1291–1301. 10.1080/02699931.2018.1543180 [DOI] [PubMed] [Google Scholar]
- Panaite V, Rottenberg J, & Bylsma LM (2020). Daily affective dynamics predict depression symptom trajectories among adults with major and minor depression. Affective Science, 1–13. 10.1177/0004867417700274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaite V, Whittington A, & Cowden Hindash A (2018). The role of appraisal in dysphoric affect reactivity to positive laboratory films and daily life events in depression. Cognition and Emotion, 32(6), 1362–1373. 10.1080/02699931.2017.1388216 [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J, Delespaul P, & deVries M (2003). Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology, 112(2), 203–211. 10.1037/0021-843X.112.2.203 [DOI] [PubMed] [Google Scholar]
- Perez-Burgos A, Wang B, Mao YK, Mistry B, Neufeld KAM, Bienenstock J, & Kunze W (2013). Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. American Journal of Physiology-Gastrointestinal and Liver Physiology, 304(2), G211–G220. 10.1152/ajpgi.00128.2012 [DOI] [PubMed] [Google Scholar]
- Porges SW (1994). Orienting in a defensive world: A poly-vagal theory of our evolutionary heritage. Presidential Address. Society for Psychophysiological Research. [DOI] [PubMed]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52(4), 449–459. 10.1111/psyp.12370 [DOI] [PubMed] [Google Scholar]
- Proudfit GH, Bress JN, Foti D, Kujawa A, & Klein DN (2015). Depression and event-related potentials: Emotional disengagement and reward insensitivity. Current Opinion in Psychology, 4, 110–113. 10.1016/j.copsyc.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea K, Dinan TG, & Cryan JF (2016). The microbiome: A key regulator of stress and neuroinflammation. Neurobiology of Stress, 4, 23–33. 10.1016/j.ynstr.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J (2017). Emotions in depression: What do we really know? Annual Review of Clinical Psychology, 13, 241–263. 10.1146/annurev-clinpsy-032816-045252 [DOI] [PubMed] [Google Scholar]
- Rottenberg J, & Bylsma LM (2014). Emotional functioning in depression. In Gotlib IH, & Hammen CL (Eds.), Handbook of depression (pp. 103–121). Guildford Press. [Google Scholar]
- Rottenberg J, Bylsma LM, & Vingerhoets AJ (2008). Is crying beneficial? Current Directions in Psychological Science, 17(6), 400–404. 10.1111/j.1467-8721.2008.00614.x [DOI] [Google Scholar]
- Rottenberg J, Bylsma LM, Wolvin V, & Vingerhoets AJ (2008). Tears of sorrow, tears of joy: An individual differences approach to crying in Dutch females. Personality and Individual Differences, 45(5), 367–372. 10.1016/j.paid.2008.05.006 [DOI] [Google Scholar]
- Rottenberg J, Cevaal A, & Vingerhoets AJ (2008). Do mood disorders alter crying? A Pilot Investigation. Depression and Anxiety, 25(5), E9–E15. 10.1002/da.20331 [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, & Gotlib IH (2005). Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology, 114(4), 627–639. 10.1037/0021-843X.114.4.627 [DOI] [PubMed] [Google Scholar]
- Rottenberg J, & Hindash AC (2015). Emerging evidence for emotion context insensitivity in depression. Current Opinion in Psychology, 4, 1–5. 10.1016/j.copsyc.2014.12.025 [DOI] [Google Scholar]
- Rottenberg J, Ray RD, & Gross JJ (2007). Emotion elicitation using films. Handbook of Emotion Elicitation and Assessment, 9–28. [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, & Gotlib IH (2003). Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology, 40(1), 1–6. 10.1111/1469-8986.00001 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, Lavori P, Howland R, Kling MA, Rittberg B, Carpenter L, Ninan P, Moreno F, Schwartz T, Conway C, Burke M, & Barry JJ (2005). Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: A naturalistic study. Biological Psychiatry, 58(5), 355–363. 10.1016/j.biopsych.2005.05.024 [DOI] [PubMed] [Google Scholar]
- Rutter M, & Sroufe LA (2000). Developmental psychopathology: Concepts and challenges. Development and Psychopathology, 12(3), 265–296. 10.1017/S0954579400003023 [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, & Zabielski R (2013). Gut microbiome and brain-gut axis in autism—Aberrant development of gut-brain communication and reward circuitry. In Recent advances in autism spectrum disorders-Volume I. IntechOpen. 10.5772/55425 [DOI] [Google Scholar]
- Salomon K, Bylsma LM, White KE, Panaite V, & Rottenberg J (2013). Is blunted cardiovascular reactivity in depression mood-state dependent? A comparison of major depressive disorder remitted depression and healthy controls. International Journal of Psychophysiology, 90(1), 50–57. 10.1016/j.ijpsycho.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon K, Clift A, Karlsdóttir M, & Rottenberg J (2009). Major depressive disorder is associated with attenuated cardiovascular reactivity and impaired recovery among those free of cardiovascular disease. Health Psychology, 28(2), 157–165. 10.1037/a0013001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson AC, Kreibig SD, Soderstrom B, Wade AA, & Gross JJ (2016). Eliciting positive, negative and mixed emotional states: A film library for affective scientists. Cognition and Emotion, 30(5), 827–856. 10.1080/02699931.2015.1031089 [DOI] [PubMed] [Google Scholar]
- Saxena A, Luking KR, Barch DM, & Pagliaccio D (2017). Individual differences in hedonic capacity, depressed mood, and affective states predict emotional reactivity. Motivation and Emotion, 41(3), 419–429. 10.1007/s11031-017-9610-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiweck C, Piette D, Berckmans D, Claes S, & Vrieze E (2019). Heart rate and high frequency heart rate variability during stress as biomarker for clinical depression. A systematic review. Psychological Medicine, 49(2), 200–211. 10.1017/S0033291718001988 [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, & Lang PJ (2000). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–261. 10.1111/1469-8986.3720257 [DOI] [PubMed] [Google Scholar]
- Siegel EH, Sands MK, Van den Noortgate W, Condon P, Chang Y, Dy J, Quigley KS, & Barrett LF (2018). Emotion fingerprints or emotion populations? A meta-analytic investigation of autonomic features of emotion categories. Psychological Bulletin, 144(4), 343–393. 10.1037/bul0000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Birmaher B, & Siegle GJ (2007). Pupillary reactivity to emotional information in child and adolescent depression: Links to clinical and ecological measures. American Journal of Psychiatry, 164(12), 1873–1880. 10.1176/appi.ajp.2007.06111816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers JA, Borelli JL, & Hilt LM (2018). Depressive symptoms, rumination, and emotion reactivity among youth: Moderation by gender. Journal of Clinical Child & Adolescent Psychology, 49(1), 106–117. 10.1080/15374416.2018.1466304 [DOI] [PubMed] [Google Scholar]
- Stange JP, Alloy LB, & Fresco DM (2017). Inflexibility as a vulnerability to depression: A systematic qualitative review. Clinical Psychology: Science and Practice, 24(3), 245–276. 10.1111/cpsp.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Hamilton JL, Fresco DM, & Alloy LB (2017). Flexible parasympathetic responses to sadness facilitate spontaneous affect regulation. Psychophysiology, 54(7), 1054–1069. 10.1111/psyp.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Kleiman EM, Mermelstein RJ, & Trull TJ (2019). Using ambulatory assessment to measure dynamic risk processes in affective disorders. Journal of Affective Disorders, 259, 325–336. 10.1016/j.jad.2019.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, van Hemert S, Bosch JA, & Colzato LS (2015). A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain, Behavior, and Immunity, 48, 258–264. 10.1016/j.bbi.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Stifter CA, & Fox NA (1990). Infant reactivity: Physiological correlates of newborn and 5-month temperament. Developmental Psychology, 26(4), 582–588. 10.1037/0012-1649.26.4.582 [DOI] [Google Scholar]
- Stone LB, Amole MC, Cyranowski JM, & Swartz HA (2018). History of childhood emotional abuse predicts lower resting-state high-frequency heart rate variability in depressed women. Psychiatry Research, 269, 681–687. 10.1016/j.psychres.2018.08.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LB, Lewis GM, & Bylsma LM (2020). The autonomic correlates of dysphoric rumination and post-rumination savoring. Physiology & Behavior, 224, 113027. 10.1016/j.physbeh.2020.113027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LB, McCormack CC, & Bylsma LM (2020). Cross system autonomic balance and regulation: Associations with depression and anxiety symptoms. Psychophysiology, 57(10), e13636. 10.1111/psyp.13636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagawa K, Kawada T, & Nakahara T (1998). Dynamic nonlinear vago-sympathetic interaction in regulating heart rate. Heart and Vessels, 13(4), 157–174. 10.1007/BF01745040 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Poon L, Kumari V, & Cleare AJ (2015). Fear biases in emotional face processing following childhood trauma as a marker of resilience and vulnerability to depression. Child Maltreatment, 20(4), 240–250. 10.1177/1077559515600781 [DOI] [PubMed] [Google Scholar]
- Tan PZ, Bylsma LM, Silk JS, Siegle GJ, Forbes EE, McMakin DL, Dahl R, Ryan ND, & Ladouceur CD (2020). Are neural substrates of self-monitoring associated with daily emotional functioning in anxious youth? An ERP and EMA study. Manuscript in preparation. [DOI] [PMC free article] [PubMed]
- Tan PZ, Oppenheimer CW, Ladouceur CD, Butterfield RD, & Silk JS (2020). A review of associations between parental emotion socialization behaviors and the neural substrates of emotional reactivity and regulation in youth. Developmental Psychology, 56(3), 516–527. 10.1037/dev0000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ III, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, & Casey BJ (2001). Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry, 58(11), 1057–1063. 10.1001/archpsyc.58.11.1057 [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Mata J, Jaeggi SM, Buschkuehl M, Jonides J, & Gotlib IH (2012). The everyday emotional experience of adults with major depressive disorder: Examining emotional instability, inertia, and reactivity. Journal of Abnormal Psychology, 121(4), 819–829. 10.1037/a0027978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain–Raspaud S, Trotin B, Naliboff B, & Mayer EA (2013). Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology, 144(7), 1394–1401. 10.1053/j.gastro.2013.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, & Galván A (2016). Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neuroscience & Biobehavioral Reviews, 70, 217–227. 10.1016/j.neubiorev.2016.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets AJ, & Bylsma LM (2016). The riddle of human emotional crying: A challenge for emotion researchers. Emotion Review, 8(3), 207–217. 10.1177/1754073915586226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CA, Auerbach RP, Bondy E, Stanton CH, Foti D, & Pizzagalli DA (2017). Abnormal neural responses to feedback in depressed adolescents. Journal of Abnormal Psychology, 126(1), 19–31. 10.1037/abn0000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, & Shankman SA (2017). Blunted reward processing in remitted melancholic depression. Clinical Psychological Science, 5(1), 14–25. 10.1177/2167702616633158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne P, Moreau D, & Olfson M (1997). Offspring of depressed parents: 10 years later. Archives of General Psychiatry, 54(10), 932–940. 10.1001/archpsyc.1997.01830220054009 [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, & Grossman P (2010). Emotions beyond the laboratory: Theoretical fundaments, study design, and analytic strategies for advanced ambulatory assessment. Biological Psychology, 84(3), 552–569. 10.1016/j.biopsycho.2010.01.017 [DOI] [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Axelson DA, Ryan ND, & Dahl RE (2004). First episode of depression in children at low and high familial risk for depression. Journal of the American Academy of Child & Adolescent Psychiatry, 43(3), 291–297. 10.1097/00004583-200403000-00010 [DOI] [PubMed] [Google Scholar]
- Wilson-Mendenhall CD, Barrett LF, Simmons WK, & Barsalou LW (2011). Grounding emotion in situated conceptualization. Neuropsychologia, 49(5), 1105–1127. 10.1016/j.neuropsychologia.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AG, & Zimmermann J (2019). Applied ambulatory assessment: Integrating idiographic and nomothetic principles of measurement. Psychological Assessment, 31(12), 1467–1480. 10.1037/pas0000685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaptangco M, Crowell SE, Baucom BR, Bride DL, & Hansen EJ (2015). Examining the relation between respiratory sinus arrhythmia and depressive symptoms in emerging adults: A longitudinal study. Biological Psychology, 110, 34–41. 10.1016/j.biopsycho.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Bylsma LM, Rottenberg J, & Kovacs M (2013). Combinations of resting RSA and RSA reactivity impact maladaptive mood repair and depression symptoms. Biological Psychology, 94(2), 272–281. 10.1016/j.biopsycho.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Bylsma LM, Jennings JR, George C, Baji I, Benák I, Dochnal R, Halas K, Kapornai K, Kiss E, Makai A, Varga H, Vetró Á, & Kovacs M (2016). Parasympathetic nervous system activity predicts mood repair use and its effectiveness among adolescents with and without histories of major depression. Journal of Abnormal Psychology, 125(3), 323–336. 10.1037/abn0000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, & Kovacs M (2013). The utility of combining RSA indices in depression prediction. Journal of Abnormal Psychology, 122(2), 314–321. 10.1037/a0032385 [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Balon R, Ramesh C, Glitz D, Jung I, & Sherwood P (1991). Heart rate variability in patients with major depression. Psychiatry Research, 37(1), 35–46. 10.1016/0165-1781(91)90104-W [DOI] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, & Wang J (2013). The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. Journal of Affective Disorders, 151(2), 531–539. 10.1016/j.jad.2013.06.039 [DOI] [PubMed] [Google Scholar]


