In the article by Deitelzweig et al,1 corrections are needed.
Due to an error in the underlying data cut received by the authors for the CMS Medicare database, a proportion of Medicare patients who should have been included in the analysis were inadvertently excluded. Specifically, those excluded patients were newly diagnosed with atrial fibrillation and initiated anticoagulation therapy in the same calendar year (2014 or 2015).
The authors have corrected the analyses by adding back those inadvertently excluded patients. Results sections in the abstract and main body of the article, Figures 1, 2, 3, Tables 1, 2, and Supplemental Tables S2‐S15 and Figures S1‐S4 have been changed to reflect the corrected analyses. These corrections have increased the sample size from 103,525 to 161,369 patients.
Figure 1.
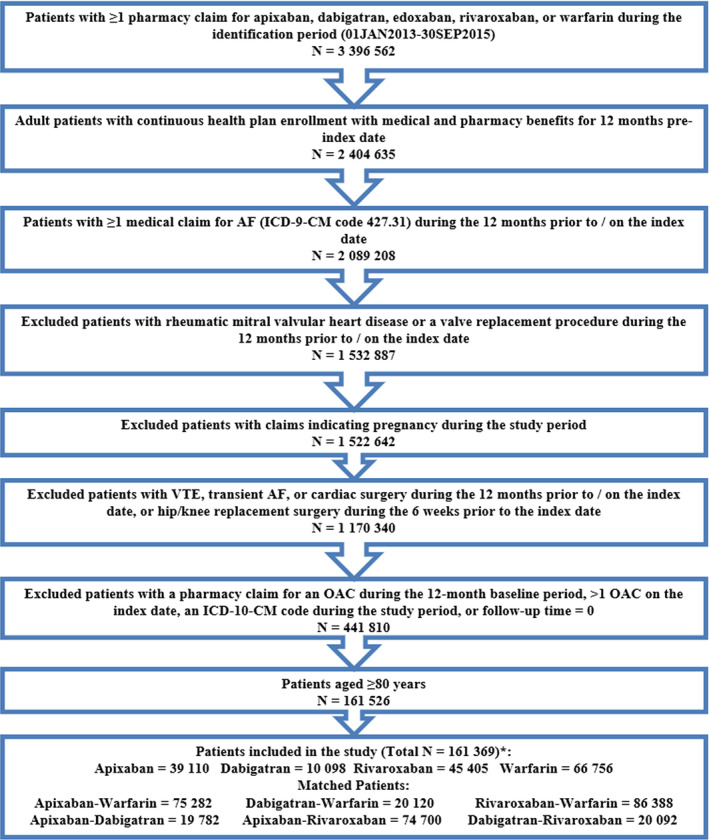
Patient selection criteria. AF, atrial fibrillation; ICD‐9/10‐CM, International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification; OAC, oral anticoagulant; VTE, venous thromboembolism. *Edoxaban was not included in the study given the recent Food and Drug Administration approval in 2015, and hence the small sample size (N = 157).
Figure 2.
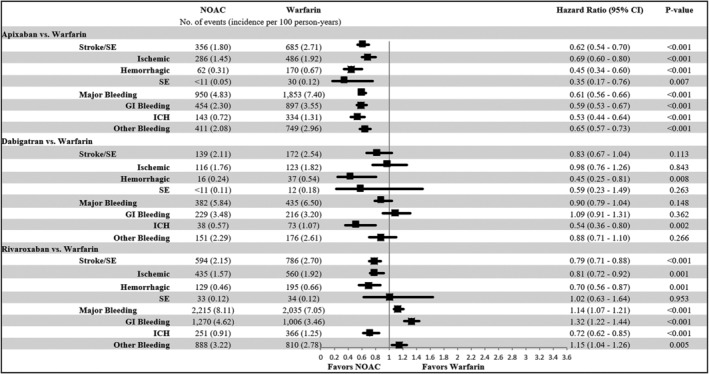
Propensity score matched incidence rates and hazard ratios of stroke/systemic embolism (SE) and major bleeding for non–vitamin K antagonist oral anticoagulants (NOACs) vs warfarin. CI, confidence interval; GI, gastrointestinal; ICH, intracranial hemorrhage; SE, systemic embolism.
Note: Cells with 10 or fewer observations were redacted. The components of stroke/SE and major bleeding are not mutually exclusive, and therefore the component values do not add up to the overall number of stroke/SE and major bleeding events.
Figure 3.
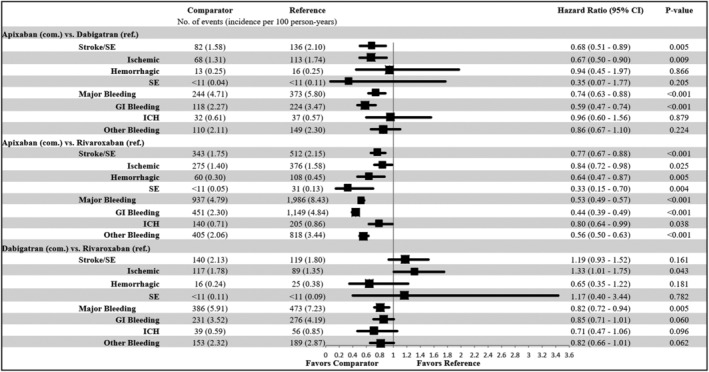
Propensity score matched incidence rates and hazard ratios of stroke/systemic embolism (SE) and major bleeding for non–vitamin K antagonist oral anticoagulants (NOACs) vs NOACs. CI, confidence interval; GI, gastrointestinal; ICH, intracranial hemorrhage; SE, systemic embolism.
Note: Cells with 10 or fewer observations were redacted. The components of stroke/SE and major bleeding are not mutually exclusive, and therefore the component values do not add up to the overall number of stroke/SE and major bleeding events.
Table 1.
PSM Baseline Characteristics for Apixaban vs Warfarin, Dabigatran vs Warfarin, and Rivaroxaban vs Warfarin.
| Apixaban (N = 37,641) | Warfarin (N = 37,641) | Dabigatran (N = 10,060) | Warfarin (N = 10,060) | Rivaroxaban (N = 43,194) | Warfarin (N = 43,194) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | |
| Age a | 85.4 | 4.1 | 85.4 | 4.1 |
84.8 |
3.9 | 85.1 | 4.1 |
85.1 |
4.0 | 85.2 | 4.1 |
| 80–83 | 14,518 | 38.6% | 14,440 | 38.4% |
4,428 |
44.0% | 4,275 | 42.5% |
17,901 |
41.4% | 17,854 | 41.3% |
| ≥84 | 23,123 | 61.4% | 23,201 | 61.6% |
5,632 |
56.0% | 5,785 | 57.5% |
25,293 |
58.6% | 25,340 | 58.7% |
| Sex | ||||||||||||
| Male | 15,265 | 40.6% | 15,217 | 40.4% |
4,235 |
42.1% | 4,283 | 42.6% |
17,892 |
41.4% | 17,875 | 41.4% |
| Female | 22,376 | 59.4% | 22,424 | 59.6% |
5,825 |
57.9% | 5,777 | 57.4% |
25,302 |
58.6% | 25,319 | 58.6% |
| Baseline Comorbidity | ||||||||||||
| Deyo‐Charlson Comorbidity Index | 3.4 | 2.7 | 3.4 | 2.7 | 3.1 | 2.6 | 3.2 | 2.7 | 3.2 | 2.6 | 3.2 | 2.7 |
| CHA 2 DS 2 ‐VASc Score | 4.7 | 1.4 | 4.7 | 1.4 | 4.6 | 1.4 | 4.6 | 1.4 | 4.6 | 1.4 | 4.6 | 1.4 |
| HAS‐BLED Score b | 3.4 | 1.3 | 3.3 | 1.3 | 3.2 | 1.2 | 3.2 | 1.2 | 3.3 | 1.2 | 3.3 | 1.3 |
| Index Prescription Dose | ||||||||||||
| Standard Dose c | 18,224 | 48.4% |
6,403 |
63.6% |
21,271 |
49.2% | ||||||
| Lower Dose d | 19,417 | 51.6% |
3,657 |
36.4% |
21,923 |
50.8% | ||||||
| Follow‐up Time, Days | 189.7 | 169.6 | 243.7 | 221.6 |
237.3 |
234.7 | 243.4 | 221.6 |
231.3 |
218.9 | 244.6 | 222.2 |
| Median | 129 | 159 |
133 |
158 |
145 |
160 | ||||||
PSM, propensity score matching; SD, standard deviation; SE, systemic embolism.
The maximum age in PharMetrics is 84; the maximum age in Optum and Humana is 89 years. Patients older than these thresholds are set to the maximum age due to privacy concerns.
Because the international normalized ratio value is not available in the databases, a modified HAS‐BLED score was calculated with a range of 0 to 8.
Standard dose: 5 mg Apixaban, 150 mg Dabigatran, 20 mg Rivaroxaban.
Lower dose: 2.5 mg Apixaban, 75 mg Dabigatran, 10 or 15 mg Rivaroxaban. A total of 3,370 patients in rivaroxaban‐warfarin cohort received 10 mg rivaroxaban.
Table 2.
PSM Baseline Characteristics for Apixaban vs Dabigatran, Apixaban vs Rivaroxaban, and Dabigatran vs Rivaroxaban.
| Apixaban (N = 9,891) | Dabigatran (N = 9,891) | Apixaban (N = 37,350) | Rivaroxaban (N = 37,350) | Dabigatran (N = 10,046) | Rivaroxaban (N = 10,046) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | |
| Age a | 85.2 | 4.1 | 84.9 |
3.9 |
85.4 | 4.1 | 85.3 |
4.0 |
84.8 | 3.9 | 84.8 | 3.9 |
| 80–83 | 4,135 | 41.8% | 4,274 |
43.2% |
14,631 | 39.2% | 14,482 |
38.8% |
4,421 | 44.0% | 4,620 | 46.0% |
| ≥84 | 5,756 | 58.2% | 5,617 |
56.8% |
22,719 | 60.8% | 22,868 |
61.2% |
5,625 | 56.0% | 5,426 | 54.0% |
| Sex | ||||||||||||
| Male | 4,129 | 41.7% | 4,135 |
41.8% |
15,018 | 40.2% | 14,969 |
40.1% |
4,227 | 42.1% | 4,291 | 42.7% |
| Female | 5,762 | 58.3% | 5,756 |
58.2% |
22,332 | 59.8% | 22,381 |
59.9% |
5,819 | 57.9% | 5,755 | 57.3% |
|
Baseline Comorbidity | ||||||||||||
| Deyo‐Charlson Comorbidity Index | 3.2 | 2.6 | 3.1 | 2.6 | 3.3 | 2.7 | 3.3 | 2.7 | 3.1 | 2.6 | 3.0 | 2.5 |
| CHA 2 DS 2 ‐VASc Score | 4.6 | 1.4 | 4.6 | 1.4 | 4.7 | 1.4 | 4.7 | 1.4 | 4.6 | 1.4 | 4.7 | 1.3 |
| HAS‐BLED Score b | 3.3 | 1.2 | 3.2 | 1.2 | 3.3 | 1.2 | 3.3 | 1.2 | 3.2 | 1.2 | 3.2 | 1.2 |
| Index Prescription Dose | ||||||||||||
| Standard Dose c | 4,981 | 50.4% | 6,284 |
63.5% |
18,228 | 48.8% | 17,897 |
47.9% |
6,403 | 63.7% | 5,068 | 50.4% |
| Lower Dose d | 4,910 | 49.6% | 3,607 |
36.5% |
19,122 | 51.2% | 19,453 |
52.1% |
3,643 | 36.3% | 4,978 | 49.6% |
| Follow‐up Time, Days | 190.1 | 170.1 | 237.1 |
234.6 |
190.2 | 170.0 | 230.9 |
219.0 |
237.2 | 234.7 | 237.6 | 224.4 |
| Median | 128 | 132 | 130 | 144 | 132 | 148 | ||||||
PSM, propensity score matching; SD, standard deviation; SE, systemic embolism.
The maximum age in PharMetrics is 84; the maximum age in Optum and Humana is 89 years. Patients older than these thresholds are set to the maximum age due to privacy concerns.
Because the international normalized ratio value is not available in the databases, a modified HAS‐BLED score was calculated with a range of 0 to 8.
Standard dose: 5 mg Apixaban, 150 mg Dabigatran, 20 mg Rivaroxaban.
Lower dose: 2.5 mg Apixaban, 75 mg Dabigatran, 10 or 15 mg Rivaroxaban. A total of 2,932 and 741 patients in the apixaban‐rivaroxaban and dabigatran‐rivaroxaban cohorts, respectively, received 10 mg rivaroxaban.
Overall, the updated results were consistent with the original analyses. One change in statistical significance was observed for dabigatran vs warfarin: while directionally consistent with the original analysis, this updated analysis shows no significant difference in risk of stroke/SE for dabigatran compared with warfarin. No other changes were observed in conclusions for either the direction of the comparative results or the statistical significance for the NOAC vs warfarin and NOAC vs NOAC comparisons.
The corrected materials include the Abstract, the Results and Discussion sections, and all tables and figures. The supplemental material has also been updated to reflect the accurate data.
1. CORRECTED ABSTRACT
OBJECTIVES: Older adult patients are underrepresented in clinical trials comparing non‐vitamin K antagonist oral anticoagulants (NOACs) and warfarin. This subgroup analysis of the ARISTOPHANES study used multiple data sources to compare the risk of stroke/systemic embolism (SE) and major bleeding (MB) among very old patients with nonvalvular atrial fibrillation (NVAF) prescribed NOACs or warfarin.
DESIGN: Retrospective observational study.
SETTING: The Centers for Medicare & Medicaid Services and three US commercial claims databases.
PARTICIPANTS: A total of 149,761 very old (aged ≥80 y) NVAF patients newly initiating apixaban, dabigatran, rivaroxaban, or warfarin from January 1, 2013, to September 30, 2015.
MEASUREMENTS: In each database, six 1:1 propensity score matched (PSM) cohorts were created for each drug comparison. Patient cohorts were pooled from all four databases after PSM. Cox proportional hazards models were used to estimate hazard ratios (HRs) of stroke/SE and MB.
RESULTS: The patients in the six matched cohorts had a mean follow‐up time of 6 to 8 months. Compared with warfarin, apixaban (HR = .62; 95% confidence interval [CI] = .54–.70) and rivaroxaban (HR = .79; 95% CI = .71–.88) were associated with lower risks and dabigatran (HR = .83; 95% CI = .67–1.04) was associated with similar risk of stroke/SE. For MB, apixaban (HR = .61; 95% CI = .56–.66) was associated with a lower risk; dabigatran (HR = .90; 95% CI = .79–1.04) was associated with a similar risk, and rivaroxaban (HR = 1.14; 95% CI = 1.07–1.21) was associated with a higher risk compared with warfarin. Apixaban was associated with a lower risk of stroke/SE and MB compared with dabigatran (stroke/SE: HR = .68; 95% CI = .51–.89; MB: HR = .74; 95% CI = .63–.88) and rivaroxaban (stroke/SE: HR = .77; 95% CI = .67–.88; MB: HR = .53; 95% CI = .49–.57). Dabigatran was associated with a lower risk of MB (HR = .82; 95% CI = .72–.94) compared with rivaroxaban.
CONCLUSION: Among very old NVAF patients, NOACs were associated with lower or similar rates of stroke/SE and varying rates of MB compared with warfarin.
2. CORRECTED RESULTS
0.02w?>After applying the selection criteria, a total of 161,369 NVAF patients 80 years or older were identified, including 39,110 (24.2%) apixaban, 10,098 (6.3%) dabigatran, 45,405 (28.1%) rivaroxaban, and 66,756 (41.4%) warfarin patients (Figure 1). Around 80% of the patients had CHA2DS2‐VASc of 4 or higher and around 70% had HAS‐BLED 3 or higher. For apixaban, dabigatran, and rivaroxaban patients, 52% (2.5 mg), 36% (75 mg), and 51% (43% on 15 mg and 8% on 10 mg) had lower dosage regimens, respectively (Table S2).
The unadjusted incidence rate of stroke/SE was 1.8 (apixaban), 2.1 (dabigatran), 2.1 (rivaroxaban), and 2.7 (warfarin) per 100 person‐years. The unadjusted incidence rate of MB was 4.9 (apixaban), 5.9 (dabigatran), 8.1 (rivaroxaban), and 7.3 (warfarin) per 100 person‐years (Table S2).
After PSM, 149,761 unique patients were included, with 37,641 apixaban‐warfarin, 10,060 dabigatran‐warfarin, 43,194 rivaroxaban‐warfarin, 9,891 apixaban‐dabigatran, 37,350 apixaban‐rivaroxaban, and 10,046 dabigatran‐rivaroxaban PSM pairs. The median follow‐up time was 4 to 5 months for the matched cohorts. Select baseline characteristics of the matched populations are shown in Tables 1 and 2. The complete baseline characteristics can be found in Tables S3 and S4.
The pre‐ and post‐PSM baseline characteristics in the very old CMS population meeting all eligibility criteria are shown in Tables S5 and S7. The CMS patient population was older, but other baseline characteristics generally had a similar trend compared with the pooled population.
3. NOAC‐Warfarin Comparisons
The Kaplan–Meier curves for cumulative incidence rates of stroke/SE and MB in the matched populations are shown in Figures S1A and S1B.
In the comparisons with warfarin, apixaban and rivaroxaban were associated with a lower risk of stroke/SE: apixaban (hazard ratio [HR] = .62; 95% confidence interval [CI] = .54–.70) and rivaroxaban (HR = .79; 95% CI = .71–.88). Dabigatran (HR = .83; 95% CI = .67–1.04) was associated with similar risk of stroke/SE compared with warfarin. Ischemic stroke was the most prevalent type of stroke/SE, of which the risk was lower in apixaban and rivaroxaban patients compared with warfarin patients. All NOACs were associated with a lower risk of hemorrhagic stroke vs warfarin.
Apixaban (HR: .61, 95% CI: .56–.66) was associated with a lower risk of MB compared with warfarin. Dabigatran (HR: .90, 95% CI: .79–1.04) was associated with a similar risk and rivaroxaban (HR: 1.14, 95% CI: 1.07–1.21) was associated with a higher risk of MB compared with warfarin. GI bleeding was most prevalent, which showed the same trend as the overall MB. All NOACs were associated with a lower risk of ICH vs warfarin (Figure 2).
4. NOAC‐NOAC Comparisons
In the comparisons between NOACs, apixaban was associated with a lower risk of stroke/SE and MB compared with dabigatran (stroke/SE: HR = .68; 95% CI = .51–.89; MB: HR = .74; 95% CI = .63–.88) and rivaroxaban (stroke/SE: HR = .77; 95% CI = .67–.88; MB: HR = .53; 95% CI = .49–.57). Dabigatran was associated with a similar risk of stroke/SE (HR = 1.19; 95% CI = .93–1.52), and lower risk of MB (HR = .82; 95% CI = .72–.94), compared with rivaroxaban (Figure 3).
5. All‐Cause Mortality
In the CMS population, compared with warfarin, all NOACs were associated with a lower risk of all‐cause mortality: apixaban (HR: .68, 95% CI: .64–.72), dabigatran (HR: .71, 95% CI: .64–.80), and rivaroxaban (HR: .80, 95% CI: .76–.84). Apixaban was associated with a similar risk of all‐cause mortality compared with dabigatran (HR = .96; 95% CI = .85–1.10) and a lower risk of all‐cause mortality compared with rivaroxaban (HR: .84, 95% CI: .79–.89). Dabigatran was associated with a similar risk of all‐cause mortality (HR: .98, 95% CI: .87–1.10) compared with rivaroxaban (Figure S2).
5.1. Subgroup and Sensitivity Analyses
In the dose subgroup analysis among the pooled population, the pre‐ and post‐PSM baseline characteristics are shown in Tables S8 to S13. After PSM, both lower and standard dose patients showed broadly consistent results to the main analysis (Figure S3).
In the age subgroup analysis among the CMS population, the results for stroke/SE, MB, and all‐cause mortality were generally consistent with the main analysis. Several significant interactions were found for all‐cause mortality comparisons for apixaban or rivaroxaban vs warfarin across age subgroups, with lower HRs observed in patients who were relatively younger (Figure S4).
The two sensitivity analyses showed generally consistent results as the main analysis that supported the robustness of the findings for comparative risk of stroke/SE and MB (Tables S14 and S15).
6. CORRECTED DISCUSSION
This comparative effectiveness and safety analysis among patients aged 80 years or older in the ARISTOPHANES study showed that very old NVAF patients who initiated apixaban or rivaroxaban were associated with lower rates and dabigatran was associated with a similar rate of stroke/SE compared with very old patients who initiated warfarin, and the safety results varied across NOACs. In the very old CMS Medicare population, all NOACs were associated with a lower risk of all‐cause mortality compared with warfarin.
Very old subjects were underrepresented in the pivotal phase III NOAC RCTs. Subgroup analyses by age in the RCTs showed that older patients with NVAF who were treated with OACs could have a distinct effectiveness and safety profiles compared with younger patients.23,24,25 For example, the analysis of the RE‐LY trial showed a significant interaction between MB and age among NVAF patients treated with dabigatran and warfarin: while 110 mg and 150 mg twice/day dabigatran were associated with a lower risk of MB among patients younger than 75 years, they were associated with a similar risk in older patients (≥75 y).23 The 110 mg and 150 mg twice/day dabigatran were associated with a lower risk of stroke/SE compared with warfarin for both young and older NVAF patients.23 Similar trends were observed in our analysis of patients aged 80 years or older; dabigatran was associated with a similar risk of stroke/SE and MB compared with warfarin. In the ARISTOTLE and ROCKET AF trials, no interactions between stroke/SE or MB and age were found for patients younger than 75 years and older (≥75) patients.23,24 In the ROCKET AF trial, 20 mg and 15 mg once/day rivaroxaban showed similar risk of stroke/SE and MB compared with warfarin in both age cohorts.24 In the ARISTOTLE trial, patients prescribed apixaban had a lower risk of stroke/SE and MB in both younger and older patients.25
In addition to RCTs, very few real‐world studies have been conducted to compare the safety and effectiveness between OACs focusing on very old NVAF patients.13,26,27,28 Using different age categories and real‐world data, these studies provide supplementary information on the comparative efficacy and safety between NOACs and warfarin in clinical practice. A population‐based analysis on linked claims data among patients aged 80 years or older in north‐eastern Italy found numerically lower risks of ischemic stroke and MB among NOACs (dabigatran, rivaroxaban, or apixaban) compared with warfarin users.25 Similarly, a study among patients aged 90 years or older using the National Health Insurance Research Database in Taiwan found that NOACs were associated with a lower risk of ICH with no difference in ischemic stroke.27 A retrospective claims study using US MarketScan data comparing rivaroxaban and warfarin found that, among NVAF patients aged 80 years or older, rivaroxaban was associated with a lower risk of stroke/SE and a similar risk of MB compared with warfarin.13 This is also evident in a meta‐analysis including both real‐world studies and RCTs, where Bai et al. concluded that among patients aged 65 years or older, NOACs were associated with a decrease in risk of MB and stroke/SE compared with warfarin.29
Several real‐world studies were conducted with subgroup analysis by age, including age 80 years or older or 85 years, as subcategories.30,31,32 Consistent with previous real‐world studies, our study shows generally more favorable outcomes for NOACs vs warfarin in very old patients.
This study is by far the largest retrospective observational study examining the comparative effectiveness and safety between OACs with the focus of very old NVAF patients. In addition to the comparisons between NOACs and warfarin, which would supplement the results of the RCTs for each NOAC, comparisons between each NOAC were also conducted. Moreover, the CMS Medicare data were also used individually for the analysis of all‐cause mortality and the age subgroup analysis. By pooling four data sets and including a comprehensive comparison of the OACs, this study was able to add supplementary information to the literature in assisting the decision of treatment selection for stroke prevention among very old NVAF patients.
6.1. Limitations
As with many real‐world studies, our study has several limitations. This study was designed to examine the associations between clinical outcomes and OAC treatment, so causal relationships cannot be evaluated. As is the nature with retrospective observational studies, our study was subject to confounders. Although PSM with a comprehensive list of covariates was used, this study remains bound by the limitation of claims data; variables such as over‐the‐counter use of aspirin, serum creatinine/creatinine clearance, and laboratory values are unavailable and thus were not controlled for in the model. International Classification of Diseases, Ninth Revision, Clinical Modification codes were used to identify baseline characteristics and outcomes that may lack clinical accuracy. Moreover, age is top‐coded in several data sets that may have caused the underestimation of the mean age. Additionally, we are unable to determine time in therapeutic range for patients prescribed warfarin. The functional characteristics of patients are also unknown. Nevertheless, by analyzing the real‐world data, our study reflects the quality of anticoagulation experienced by patients in clinical practice. For example, given that very old patients are likely to have poorer measurement for the international normalized ratio in real‐world clinical practice, this may in part explain the higher risk of stroke/SE for warfarin users in our study. Due to the lack of data on renal function and body weight, it is not clear whether patients used lower dose of NOACs appropriately. In addition, at the time of the study, no reversal agents were available on the market for NOACs for patients with life‐threatening bleeding or requiring urgent surgery, which may have impacted the choice of OAC treatment and the safety results. Lastly, although the main and the additional subgroup analyses added healthcare outcome evidence related to the very old NVAF patient population who were newly prescribed OACs, limited generalizability of the results to a different population, such as an institutionalized older NVAF population, may be expected.
0.01w?>In conclusion, this retrospective observational study among very old (≥80 y) NVAF patients newly initiated on OACs showed that, compared with warfarin, NOACs were associated with lower or similar risks of stroke/SE and all‐cause mortality, and various comparative risks of MB. This study adds to the growing body of evidence in a population that is vulnerable and also at high risk of NVAF‐related stroke.
Supporting information
Appendix S1: Supporting information
REFERENCES
- 1. Deitelzweig S, Keshishian A, Li X, et al. Comparisons between oral anticoagulants among older non‐valvular atrial fibrillation patients. J Am Geriatr Soc. 2019;67:1662–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


