Abstract
Time-dependent diffusion behavior is probed over sub-millisecond timescales in a single shot using a nuclear magnetic resonance static gradient time-incremented echo train acquisition (SG-TIETA) framework. The method extends the Carr–Purcell–Meiboom–Gill cycle under a static field gradient by discretely incrementing the π-pulse spacings to simultaneously avoid off-resonance effects and probe a range of timescales (50–500 µs). Pulse spacings are optimized based on a derived ruleset. The remaining effects of pulse inaccuracy are examined and found to be consistent across pure liquids of different diffusivities: water, decane, and octanol-1. A pulse accuracy correction is developed. Instantaneous diffusivity, Dinst(t), curves (i.e., half of the time derivative of the mean-squared displacement in the gradient direction) are recovered from pulse accuracy-corrected SG-TIETA decays using a model-free log-linear least squares inversion method validated by Monte Carlo simulations. A signal-averaged 1-min experiment is described. A flat Dinst(t) is measured on pure dodecamethylcyclohexasiloxane, whereas decreasing Dinst(t) is measured on yeast suspensions, consistent with the expected short-time Dinst(t) behavior for confining microstructural barriers on the order of micrometers.
I. INTRODUCTION
As molecules diffuse, they interact with their surroundings and “[feel] the boundary,”1 causing their ensemble displacement behavior to be influenced by the morphology of the microenvironment. More specifically, long-range correlations such as confining barriers impart a nonlinear time-dependence to the ensemble-averaged net mean-squared displacement, (in ). This leads to a time-dependent diffusion coefficient,2,3
| (1) |
where is the causal velocity autocorrelation tensor, H(t) is the unit step function, and “tr” is the trace operation.
Microstructural features can be inferred from the behavior of D(t) at limiting short and long timescales (see Refs. 4 and 5 for review). At the short-time limit, D(t) exhibits universal behavior,2 which depends on the barrier surface-to-volume ratio, S/V,
| (2) |
where is the diffusion length scale and D0 ≡ D(t)|t=0 is the free diffusivity. As ℓD increases, the barrier permeability, κ, may begin to affect D(t).6–9 While ℓD remains short, barriers appear flat to the small fraction of nearby walkers that encounter them,8,10 introducing a linear κt term in Eq. (2),
| (3) |
where is the time to diffuse across the mean pore of size . Curvature8 and surface relaxivity3 may also affect D(t). Tortuosity principally affects the long-time D(t)11,12 and can be categorized into disorder classes with the structural exponent, p.10,13 The long-time D(t) follows a p-dependent power law,13
| (4) |
where D∞ = limt→∞D(t) and ϑ = (p + 3)/2.
Diffusion-weighted (DW) nuclear magnetic resonance (NMR) methods are highly sensitive to ⟨r2(t)⟩14–20 and provide a powerful means to probe rich D(t) behaviors and infer distinct microstructural features. DW-NMR experiments have been used to study the short- and long-time D(t) in porous media ranging from sedimentary rocks to skeletal muscles.5,12,13,21–25
The NMR spin echo dephasing, however, is not simply written in terms of D(t) itself. Instead, the echo dephasing is often expressed in terms of the real part, , of the Fourier transform of .26–28 From Eq. (1),28
| (5) |
High and low frequency behaviors reveal the short- and long-time D(t), respectively. The relationship between and the ensemble echo dephasing follows from a cumulant expansion of the phase expectation value and a Gaussian phase distribution approximation,29 yielding the normalized echo intensity,30,31
| (6) |
where F(ω) is the truncated Fourier transform of F(t) at the echo time, T [i.e., F(T) = 0],
| (7) |
where , G(t) is the gradient waveform, and γ is the gyromagnetic ratio. The assumed Gaussian phase approximation is valid for most relevant experimental cases (cf. Ref. 32).33,34
Equations (6) and (7) show that spectral tuning of |F(ω)|2 results in the narrow sampling of in the gradient direction, . G(t) can be periodically time-modulated35 [e.g., by using a sinusoidal G(t)24] so that the spectral density of |F(ω)|2 concentrates near some frequency, ωF. In this case, I(T)/I0 becomes well-approximated by , where16
| (8) |
Individual experiments become a point-wise sampling of . This “temporal diffusion spectroscopy”36 approach is robust but has limited time resolution because it individually probes ωF. Furthermore, the shortest probe-able timescale (i.e., largest ωF) is limited to about 10 ms by the pulsed gradient hardware.5,36 An alternative approach is needed for the real-time study of D(t) across timescales and to reach the information-rich short-time regime (≲1 ms) in biological systems. Static gradient (SG) hardware permits extremely fast cycling of the effective gradient direction using radiofrequency (RF) π-pulse trains and thereby provides access to these timescales.23,35 Here, we extend the classical Carr–Purcell–Meiboom–Gill (CPMG)37,38 experiment under an SG in the RF field to probe the time-varying sub-millisecond diffusivity in one shot.
II. THEORY
A. Time-dependent signal representation
To begin, an alternative signal representation is used. The echo attenuation is related to the stationary position autocorrelation tensor, —again assuming a Gaussian phase distribution30,31,39—by
| (9) |
Equation (9) can be rewritten according to Ning et al.39 by integrating along the level set of t − t′. For unidirectional encoding, i.e., G(t) = G(t) and ,
| (10) |
where Dinst(t) is the instantaneous diffusivity along the gradient direction ,
| (11) |
(dropping as implied) and is the cumulative integral of the γG(t) autocorrelation function,
| (12) |
schematized in Fig. 1. The attenuation becomes a sampling of Dinst(t) weighted by similar to how Eq. (6) describes a sampling of by |F(ω)|2.
FIG. 1.
Example calculation. (a) Radiofrequency (RF) pulses in a static gradient of amplitude g. (b) G(t), shifted G(t + s) (red, dashed), and F(t). (c) Corresponding . G(t) is assumed to be zero outside of t = [0, 2τ].
B. Recasting the problem
Equation (10) is advantageous compared to Eq. (6) because is simple for general non-periodic G(t). The ill-posed inverse problem of finding Dinst(t) from many trivial G(t) is tractable. For a train of N echoes refocusing at times Tn, can be evaluated for each inter-echo attenuation I(Tn)/I(Tn−1) (T0 = 0); that is, each pair of adjacent echoes can be treated as an independent spin echo diffusion measurement. The problem is then recast as a weighted and regularized log-linear least squares (LLS) inversion by discretizing the time domain into K bins of variable width, Δt(k),
| (13) |
with coefficients
where X consists of time-interval Dinst(t) averages,
and . The regularization matrix, Γ, is chosen to consist of first and second-order finite difference matrices, reflecting an a priori assumption of the smoothness and concavity of Dinst(t). The choice of N is dictated by the echo signal decay to the noise floor. The choice of K and Δt(k) is more arbitrary. As a preliminary heuristic, K should be similar in magnitude to N, and Δt(k) should be chosen such that (1) Dinst(t) does not vary greatly over any interval and (2) the integrals that comprise the entries of A are appreciable. Considering the behavior of Eqs. (3) and (4), Δt(k) should start out small and may gradually lengthen. The norm is weighted by a proportionality of the signal-to-noise ratio (SNR); since B consists of log ratios, the appropriate weights matrix, W, is an N × N matrix of signal differences, i.e., I(Tn−1) − I(Tn). In this way, Dinst(t) can be estimated from a single echo train with varied .
The motivating question of this Communication is as follows: Can a DW-NMR method probe the time-varying diffusivity in real-time? With Eq. (13) in mind, the question can be separated into two parts: (1) How can of various time sensitivities be produced in one echo train? (2) How can every echo be made accurate? The answer to the first part follows from a well-known DW-NMR protocol. As mentioned, the SG-CPMG experiment can produce rapid effective gradient oscillation characterized by a triangle wave F(t) with ωF = (π/2τ) rad/s, where 2τ is the spacing between π-pulses. This SG-CPMG ωF (up to tens of kilohertz23) exceeds that which is attainable with oscillating or pulsed field gradient (PFG) methods (up to ∼100 Hz). As a result, the SG-CPMG method is uniquely able to probe the short-time diffusion regime in small (m) structures.23,35,40–44 Stimulated echoes represent another candidate DW-NMR method but are ill-suited to single-shot multi-echo acquisitions due to the signal loss inherent to π/2-pulses.
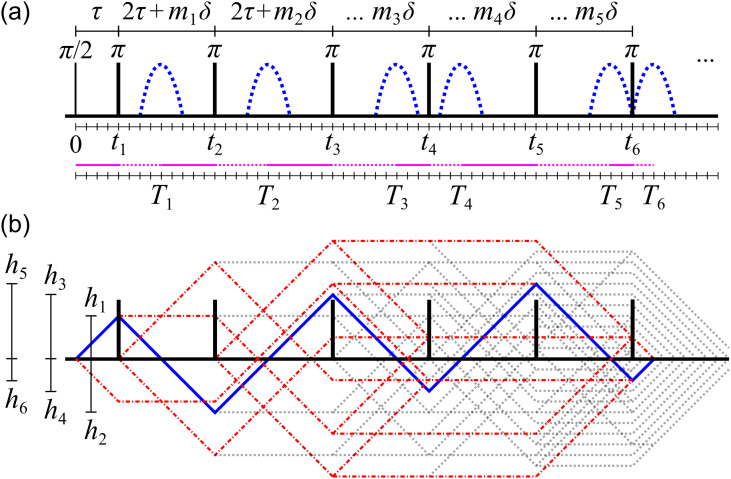
Varying the spacing of the π-pulses in an SG-CPMG styled acquisition is thus the preferred method to produce various in one shot. Others have explored the concept of modifying SG-CPMG pulse spacings to measure time-varying diffusion but ultimately retained a π-pulse train with repeated spacing.44 We extend such methods by modifying every π-pulse spacing. Spacings can be incremented to retain the signal. We choose the spacing between π-pulses to take the form 2τ + mjδ, where j indexes the π-pulse-to-pulse spacing, , and δ is a unit time increment. We term this discrete spacing method the SG time-incremented echo train acquisition (SG-TIETA), e.g., see Fig. 2. For SG-TIETA,
| (14) |
where hn is the nth peak of |F(t)|/γg. According to Eq. (14), the nth inter-echo interval probes Dinst(t) over the time interval [0, 2hn], with a peak at hn. With the core experimental method described, we turn toward the problem making each echo accurate. An initial step is to isolate the direct echo pathway.
FIG. 2.
Example SG-TIETA sequence. (a) Timings: mj = {1, 3, 1, 2, 1}, τ = 4δ, and δ = 14 µs = 1 dash. π-pulses occur at tn, and direct echoes (blue lines) form at Tn. Magenta line indicates timing behavior: Tn = tn + hn, where hn is the normalized |F(t)|/γg “height” at tn given by h1 = τ and hn = 2τ + mnδ − hn−1 for n > 1. (b) Direct echo F(t) and other coherence pathways that refocus (red dashed-dotted lines) or do not refocus (gray dotted lines). Relative values of hn are indicated.
C. Isolating the direct echo pathway in the time domain
Ignoring the effects of magnetic susceptibility, surface relaxivity,3 and spin–spin (T2) relaxation (for the meantime), the predominant source of extraneous signal behavior is off-resonance coherence transfer pathways (CTPs). When the bandwidth of Larmor precession frequencies spanned by an SG exceeds the bandwidth of π-pulses, every pulse is slice-selective and excites all CTPs.20 For SG-CPMG measurements, the number of refocused off-resonance CTPs grows exponentially with N (∼3N),45 resulting in significant deviations from the expected echo attenuation.45–48 Phase cycling remediation schemes require ∼2N steps49 and are thus infeasible. Unconventional approaches such as time-based avoidance of CTPs50,51 become necessary. An SG aids these time-based approaches by acting as an always-on crusher gradient. The minimum time separation to avoid the undesired signal, τsep, is shortened. Specifically, τsep is constrained by τsep ≥ τp, where τp = 2π/(γgΔz) is the length of the π-pulse and Δz is the resulting slice thickness. The echo width under an SG is of the order of τp52 such that this constraint can be understood as avoiding undesired echo overlap. For hard π-pulses, τp is on the order of ∼μs.
In the interest of acquiring an accurate direct echo CTP, we should ask the following question: What choice of mj, τ, and δ separates off-resonance CTPs from the direct echo CTP by ≥τp? Consider that CTPs are piecewise linear functions in F(t). The three magnetization states for a spin-1/2 nuclei (in shorthand: M ∈ {+, −, 0}48) correspond to +γg, −γg, and 0 slopes, respectively [see Fig. 2(b)]. Refocusing of the undesired signal occurs when the summed difference between an off-resonance F(t) and the direct echo F(t), ∑ΔF(t), equals 0. Rules for mj, τ, and δ are developed. Singly stimulated echoes (e.g., +0−) arise due to two hn matching. Thus,
-
(i)
absolute F(t) heights, or hn, may not be repeated.
Next, consider CTPs that see the initial π/2-pulse. These CTPs can alter ∑ΔF(t)/γg by one of . Accounting for ∑ΔF(t) = 0 with up to four non-zero terms:
-
(ii)
mj and mj±Δj with odd Δj may not be the same.
-
(iii)
Twice any mj may not equal the sum of mj+Δj and mj−Δj for even Δj.
-
(iv)
Any two mj with even j may not equal the sum of any two mj with odd j.
-
(v)
Twice any mj with even j may not equal the sum of any two mj with odd j and vice versa.
Another class of off-resonance CTPs is associated with the introduction of transverse magnetization from spins that incorrectly see π-pulses as initial π/2-pulses. These CTPs emerge at the time of π-pulses and thus invariably start with ∑ΔF(t) containing an odd multiple of τ (i.e., τ + 2jτ). Choosing τ and δ such that (τ mod δ) = δ/2 ensures that ∑ΔF(t) ≥ δ/2. Incorporating τp,
-
(vi)
δ and τ satisfy (τ mod δ) = δ/2 and δ > 2τp.
Note that (τ mod δ) = 0 results in the refocused CTPs in Fig. 2(b). The sequence in Fig. 2 does, in fact, satisfy rules (i)–(v). This limited ruleset [(i)–(vi)] may be sufficient to ostensibly avoid off-resonance effects, considering that singly stimulated echoes are known to be the most significant contributor to SG-CPMG off-resonance effects.45,48
The generation of mj that satisfies rules (i)–(v) is discussed in Sec. I of the supplementary material. The Python code is provided. A solution for τ, δ, and mj, used throughout, is
| (15) |
which gives time sensitivity over t ∼ 50–500 µs,
III. EXPERIMENTAL SETUP
NMR measurements were performed at B0 = 0.3239 T (proton ω0 = 13.79 MHz) using a PM-10 NMR MOUSE single-sided permanent magnet53 (Magritek, Aachen, Germany) and a Kea 2 spectrometer (Magritek, Wellington, New Zealand). The decay of the magnetic field with a distance from the magnet produces a strong SG of g = 15.3 T/m (650 kHz/mm). Measurements used a home-built test chamber and a 13 × 2 mm2 solenoid RF coil and RF circuit. Additional information concerning the experimental setup can be found in the work of Williamson et al.54 The SG-TIETA pulse program was written in Prospa V3.22 by modifying the standard CPMG sequence. See Sec. IV of the supplementary material for details.
For measurements on twice-distilled water, decane (Sigma-Aldrich, St. Louis, MO), 1-octanol (Sigma-Aldrich), and dodecamethylcyclohexasiloxane (D6) with kinematic viscosity = 6.6 cSt @ 25 °C (Gelest, Inc., Morrisville, PA), the liquids were transferred to 2 cm glass capillary sections (1.1 mm OD, Kwik-FilTM, World Precision Instruments, Inc., Sarasota, FL), and the capillaries were sealed with a hot glue gun.
For measurements on yeast (S. cerevisiae), 1.72 g of dry yeast was mixed in 10 ml of tap water and stored in a 50 ml tube with the lid screwed on loosely to allow the gas to escape. After three days (72 h), the yeast was re-suspended and samples were taken for NMR experiments and for cell density measurement. The density of the first sample (yeast 1) was measured to be 2.84 × 109 cells/ml using a hemocytometer. The remaining yeast was centrifuged, the pellet was rediluted to 2X the initial concentration, and a second sample (yeast 2) was taken for NMR experiments. Immediately prior to experiments, yeast was transferred to 2 cm KrosFlow® Implant Membrane sections (500 000 Da molecular weight cutoff, 1 mm outer diameter, SpectrumLabs, Waltham, MA, USA) and the membranes were sealed by pinching the membrane with heated forceps. Yeast was kept from drying out by performing measurements immediately upon filling and sealing the capillary and by lining the inside with wet tissue paper to increase humidity.
Experiments were performed at ambient temperature. The sample temperature was monitored with a fiber optic sensor (PicoM Opsens Solutions, Inc., Québec, Canada). The average temperatures of the samples during the course of the experiments were 25 °C, 22 °C, 22 °C, 23 °C, and 24 °C for the water, decane, octanol-1, D6, and yeast, respectively.
IV. RESULTS AND DISCUSSION
As an initial proof-of-principle, the LLS inversion described in Eq. (13) was performed on noisy SG-TIETA decays generated using the timings in Eq. (15) and curves from Monte Carlo simulations,55 as shown in Fig. 3. Further details and the MATLAB code are provided in Sec. II of the supplementary material. Inverted X values are shown to be accurate and robust to noise. No systematic errors other than potential over-regularization are observed.
FIG. 3.
LLS inversion on Monte Carlo simulated data. (a) Simulated (D0 = 2.15 μm2/ms) for free diffusion (black) and restricted geometries (see Fig. S1). (a.u.) for Eq. (15) is overlaid, omitting the first two echoes. (b) Echo decays simulated from same color curves in (a) for γg = 4.093 μm−1 ms−1. Insets show B with 2W1/2 for the black curve and A for Δt(k) = {50, 50, 50, 50, 75, 75, 125, 139} μs. (c) Dinst(t) from the gradient of curves in (a) (solid lines) compared to X inverted from decays in (b) with added Gaussian noise (SNR = 25). Error bars = ±1 SD from 100 replications. Initial X guesses were D0 for the first point and D∞ (dashed lines, {0.9, 1.45, 1.48} μm2/ms) for the remaining points. λ = 2 × 10−6 is selected manually. See Eq. (S1) for Γ.
Echo-to-echo accuracy remains experimentally difficult to achieve, however. Consider the signal decay due to T2 and pulse inaccuracy effects.48 Relaxation may be ignored if (2τ + mjδ) ≪ T2 ∀ j. Diffusion-weighted T2 values for each sample, as shown in Table I, indicate that this condition holds for all pure liquids studied here. However, the yeast is described by a distribution of T2 with a 5% water population with T2 similar to . The instantaneous diffusion measurements of yeast may be slightly weighted by T2 relaxation. See Sec. IV D of the supplementary material for relaxation measurement methods and a T2 distribution analysis for yeast 2. Unlike T2, pulse inaccuracy cannot be ignored. Inaccuracy effects may be described using an n-dependent pulse accuracy factor, Ap(n).48 The signal is then corrected as , assuming total avoidance of off-resonance CTPs via rules (i)–(vi).
TABLE I.
Relaxation times.
| Sample | T1 (ms) | T2 (ms) |
|---|---|---|
| Water | 3250 | 133 |
| Decane | 1340 | 166 |
| Octanol-1 | 440 | 217 |
| D6 | 788 | 292 |
| Yeast 1 (2.84B cells/ml) | 625 | 59 |
| Yeast 2 (5.68B cells/ml) | 319 | 41 |
Calibration Ap(n) values were approximated from decays of pure liquids, as shown in Fig. 4. Echo amplitudes were calculated as the sum of all real signal points within the echo window, which was set to 16 µs. All decays were normalized to the first echo, and each repetition consisted of 32 summed (i.e., signal-averaged) scans. To elucidate the expected Ap(n) behavior, we consider the spatial, i.e., slice effects. The bandwidth/slice excited by refocusing π-pulses has inconsistent frequency content56,57 such that Ap(n) < 1. With each pulse, spins that rotate by angles other than π do not refocus until only a stable central slice remains. In the time domain, this slice-thinning and loss of frequency content are expected to broaden the echo width, which we experimentally verify in Fig. 5. Based on the evolution of the echo shape, Ap(n) should sharply increase and then taper. This behavior is observed in Fig. 4(c) and is consistent across liquids of vastly different D0. Similar Ap(n) values were also obtained for τ = 77 µs (see Sec. III and Fig. S5 of the supplementary material), further supporting that Ap(n) is independent of the diffusion weighting.
FIG. 4.
SG-TIETA Ap(n) calibration using pure liquids. (a) Observed SG-TIETA decays compared to exp(−bD0). D0 is measured (see Sec. IV of the supplementary material) in the legend order as 2.22 ± 0.01, 1.27 ± 0.01, 0.121 ± 0.002 μm2/ms. Error bars = ±1 SD for 25, 38, and 70 repetitions, respectively. (b) Decay vs exp(−bD0) ratio truncated at n = 12, 15, 25. Inset shows cubic spline fits. (c) Ap(n) approximated using adjacent fitted ratios.
FIG. 5.
Comparison of the direct echo SG-TIETA (blue) and CPMG (orange) attenuation and echo shape for 1-octanol with 2τ = TE = 98 ms. Echo shapes show the real (solid lines) and imaginary (dotted lines) signals normalized by the area under the real signal curve in a 16 µs window. The CPMG echo width decreases with n and stabilizes around n = 3, consistent with the approach to the asymptotic behavior described by Hürlimann and Griffin.57 In contrast, the SG-TIETA echo width increases and stabilizes around n = 3, consistent with the direct echo CTP being preferential to the on-resonance signal (see Figs. S7 and S8 for all echo shapes and an exemplar echo decay, respectively)
Another pre-processing step is designed to mitigate the effects of early Ap(n) variability and to ensure the non-negativity of B and W entries. A piecewise linear fit of adjacent ln (I(Tn)/I0) points vs b is performed, specifying (1) an intercept with [b, ln (I(Tn)/I0)] = [0, 0], (2) that no slope exceeds D0, and (3) that the piecewise slopes decrease monotonically [i.e., Dinst(t) has non-negative concavity]. Altogether, SG-TIETA decays are analyzed in five steps: (1) summing 32×, (2) normalization to the first echo, (3) correction, (4) a constrained log b domain fit to the repetition(s), and, finally, (5) the LLS inversion. This pipeline was applied to SG-TIETA decays of D6 and yeast using the 1-octanol and water Ap(n) values obtained in Fig. 4(c), respectively.
Results are summarized in Fig. 6. The Dinst(t) for D6—with D0 = 0.114 ± 0.008 μm2/ms—is expectedly flat, thus validating the Ap(n) correction. The Dinst(t) for yeast are the key results of this Communication. For comparison, the short-time Dinst(t) predicted by Eq. (3) (i.e., ) is plotted for several S/V and κ values. Figure 6(b) indicates that m and that a doubling of the cell density approximately halves . For yeast’s ≈4 μm spherical diameter,58 is calculated as 42 and 21 μm at these cell densities, suggesting contributions from sub-cellular length scales. This ensemble estimate of ≃2 μm (≡V/S ≃ 3 μm) is within the range of estimates (m) reported in previous PFG and SG DW-NMR studies of similar yeast densities.58–62
FIG. 6.
SG-TIETA decays and inverted X for D6, yeast, and water. (a) Decays analyzed as described in the text. See Sec. II of the supplementary material for fitting procedures. Error bars = ±1 SD in the legend order for 38, 3, 4, and 25 repetitions truncated at N = 34, 17, 17, and 15, respectively. Note the erratic early Ap(n) behavior. (b) X solutions. Inversion parameters are identical to Fig. 3 other than Δt(k) for D6. Initial guesses of D0 = 2.22, D∞ = {0.9, 1.1} μm2/ms for yeast, and D0 = D∞ = 0.114 μm2/ms for D6 are provided. Zoomed-in plot compares short-time Dinst(t) plotted up to t < 0.08 × τD.
Several factors may contribute to the discrepancy between the experimental and the predicted short-time Dinst(t). On the numerical side, over-regularization may artificially flatten Dinst(t) at short times, as shown in Fig. 3. Values of X are also plotted inexactly at the midpoints of ∑kΔt(k). On the theoretical side, Eq. (3) does not include a term for the curvature, which may be significant at these timescales for sub-cellular water. Consider, also, the confounding effects of T2, surface relaxation, the Gaussian phase approximation, and the assumption of echo number translation invariance [i.e., that each Cn(t) is presumed to start from t = 0]. Echo number translation invariance does not hold for spatially heterogeneous microenvironments. If different water pools exhibit varying decay rates, the relative signal contributions will depend on the echo number, n. Indeed, spatial heterogeneity and the resulting weighting toward slowly decaying water pools at larger n may explain the convergence of the yeast X values. These yeast results are thus non-quantitative. Nonetheless, the sensitivity of SG-TIETA to apparent microstructural features is clear.
V. CONCLUSIONS
We have developed a real-time protocol to measure time-varying diffusion. Inversion for Dinst(t) from experimental SG-TIETA decays is demonstrated. An ∼1-min (32× repetition time of 2 s) experiment is described. In contrast to conventional temporal diffusion spectroscopy methods, the single-shot nature of SG-TIETA permits true signal averaging in order to improve the SNR. A post hoc Ap(n) correction is proposed to improve the quantitative accuracy of the method. To support the validity of this correction, we present preliminary evidence in the observed echo shape behavior and in the consistency of Ap(n) values across different diffusion weightings. Regarding potential applications, SG-TIETA at this g can probe the porous media microstructure on micrometer length scales, i.e., over sub-millisecond timescales. SG-TIETA can also be used to study the phenomena associated with other long-range correlations, e.g., polymer dynamics,63 the glass transition,64 and high Pèclet fluxes driven by flagella,65 which likewise exhibit time-dependence in this sub-millisecond range. The methods contained in this Communication may open new avenues of research within DW-NMR.
SUPPLEMENTARY MATERIAL
See the supplementary material for (I) the Python code to generate mj, (II) the representative MATLAB code for the Monte Carlo simulations, fitting procedures, and LLS inversion, (III) replication of the analysis in Figs. 4 and 6 using SG-TIETA decays for τ = 77 µs, and (IV) the additional NMR experimental methodology, which includes all echo shapes and a stitched echo decay for 1-octanol.
AUTHORS’ CONTRIBUTIONS
P.J.B., T.X.C., N.H.W., and V.J.W. conceptualized the work, T.X.C. developed the theory and performed computations, N.H.W., V.J.W., and R.R. designed and performed experiments, N.H.W. and T.X.C. analyzed the data, T.X.C. prepared this manuscript, and P.J.B. supervised the project. All authors edited this manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Dan Benjamini and Dr. Michal Komlosh for helpful discussions concerning numerical programming and time-based avoidance of unwanted coherence pathways, respectively.
T.X.C., V.J.W., R.R., and P.J.B. were supported by the IRP of the NICHD, NIH. T.X.C. is a graduate student in the NIH-Oxford-Cambridge Scholars Program. N.H.W. was funded by the NIGMS PRAT Fellowship (Award No. FI2GM133445-01).
Contributor Information
Teddy X. Cai, Email: .
Peter J. Basser, Email: .
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request. The SG-TIETA pulse program and macro can be downloaded from the GitHub repository: https://github.com/nathanwilliamson/SG-TIETA.
REFERENCES
- 1.Kac M., Am. Math. Mon. 73, 1 (1966). 10.1080/00029890.1966.11970915 [DOI] [Google Scholar]
- 2.Mitra P. P., Sen P. N., Schwartz L. M., and Le Doussal P., Phys. Rev. Lett. 68, 3555 (1992). 10.1103/physrevlett.68.3555 [DOI] [PubMed] [Google Scholar]
- 3.Mitra P. P., Sen P. N., and Schwartz L. M., Phys. Rev. B 47, 8565 (1993). 10.1103/physrevb.47.8565 [DOI] [PubMed] [Google Scholar]
- 4.Sen P. N., Concepts Magn. Reson., Part A 23A, 1 (2004). 10.1002/cmr.a.20017 [DOI] [Google Scholar]
- 5.Reynaud O., Front. Phys. 5, 58 (2017). 10.3389/fphy.2017.00058 [DOI] [Google Scholar]
- 6.Tanner J. E., J. Chem. Phys. 69, 1748 (1978). 10.1063/1.436751 [DOI] [Google Scholar]
- 7.Tanner J. E., Biophys. J. 28, 107 (1979). 10.1016/s0006-3495(79)85162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen P. N., J. Chem. Phys. 119, 9871 (2003). 10.1063/1.1611477 [DOI] [Google Scholar]
- 9.Sen P. N., J. Chem. Phys. 120, 11965 (2004). 10.1063/1.1756575 [DOI] [Google Scholar]
- 10.Novikov D. S., Fieremans E., Jensen J. H., and Helpern J. A., Nat. Phys. 7, 508 (2011). 10.1038/nphys1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mair R. W., Sen P. N., Hürlimann M. D., Patz S., Cory D. G., and Walsworth R. L., J. Magn. Reson. 156, 202 (2002). 10.1006/jmre.2002.2540 [DOI] [PubMed] [Google Scholar]
- 12.Latour L. L., Mitra P. P., Kleinberg R. L., and Sotak C. H., J. Magn. Reson. 101, 342 (1993). 10.1006/jmra.1993.1056 [DOI] [Google Scholar]
- 13.Novikov D. S., Jensen J. H., Helpern J. A., and Fieremans E., Proc. Natl. Acad. Sci. U. S. A. 111, 5088 (2014). 10.1073/pnas.1316944111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn E. L., Phys. Rev. 80, 580 (1950). 10.1103/physrev.80.580 [DOI] [Google Scholar]
- 15.Woessner D. E., J. Chem. Phys. 67, 1365 (1963). 10.1021/j100800a509 [DOI] [Google Scholar]
- 16.Stejskal E. O. and Tanner J. E., J. Chem. Phys. 42, 288 (1965). 10.1063/1.1695690 [DOI] [Google Scholar]
- 17.Neuman C. H., J. Chem. Phys. 60, 4508 (1974). 10.1063/1.1680931 [DOI] [Google Scholar]
- 18.Callaghan P. T., Coy A., MacGowan D., Packer K. J., and Zelaya F. O., Nature 351, 467 (1991). 10.1038/351467a0 [DOI] [Google Scholar]
- 19.Price W. S., NMR Studies of Translational Motion: Principles and Applications, Cambridge Molecular Science (Cambridge University Press, 2009). [Google Scholar]
- 20.Keeler J., Understanding NMR Spectroscopy (John Wiley and Sons, Chichester, UK, 2010). [Google Scholar]
- 21.Hurlimann M. D., Helmer K. G., Latour L. L., and Sotak C. H., J. Magn. Reson. 111, 169 (1994). 10.1006/jmra.1994.1243 [DOI] [PubMed] [Google Scholar]
- 22.Latour L. L., Svoboda K., Mitra P. P., and Sotak C. H., Proc. Natl. Acad. Sci. U. S. A. 91, 1229 (1994). 10.1073/pnas.91.4.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callaghan P. T. and Stepišnik J., J. Magn. Reson. 117, 118 (1995). 10.1006/jmra.1995.9959 [DOI] [Google Scholar]
- 24.Schachter M., Does M. D., Anderson A. W., and Gore J. C., J. Magn. Reson. 147, 232 (2000). 10.1006/jmre.2000.2203 [DOI] [PubMed] [Google Scholar]
- 25.Sigmund E. E., Novikov D. S., Sui D., Ukpebor O., Baete S., Babb J. S., Liu K., Feiweier T., Kwon J., McGorty K., Bencardino J., and Fieremans E., NMR Biomed. 27, 519 (2014). 10.1002/nbm.3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seymour J. D. and Callaghan P. T., AIChE J. 43, 2096 (1997). 10.1002/aic.690430817 [DOI] [Google Scholar]
- 27.Khrapitchev A. A. and Callaghan P. T., Phys. Fluids 15, 2649 (2003). 10.1063/1.1596914 [DOI] [Google Scholar]
- 28.Novikov D. S. and Kiselev V. G., J. Magn. Reson. 210, 141 (2011). 10.1016/j.jmr.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 29.Douglass D. C. and McCall D. W., J. Phys. Chem. 62, 1102 (1958). 10.1021/j150567a020 [DOI] [Google Scholar]
- 30.Stepišnik J., Physica B+C 104, 350 (1981). 10.1016/0378-4363(81)90182-0 [DOI] [Google Scholar]
- 31.Stepišnik J., Physica B 183, 343 (1993). 10.1016/0921-4526(93)90124-O [DOI] [Google Scholar]
- 32.Stepišnik J., Physica B 270, 110 (1999). 10.1016/S0921-4526(99)00160-X [DOI] [Google Scholar]
- 33.Axelrod S. and Sen P. N., J. Chem. Phys. 114, 6878 (2001). 10.1063/1.1356010 [DOI] [Google Scholar]
- 34.Sukstanskii A. L. and Yablonskiy D. A., J. Magn. Reson. 163, 236 (2003). 10.1016/s1090-7807(03)00131-9 [DOI] [PubMed] [Google Scholar]
- 35.Callaghan P. T. and Stepišnik J., in Advances in Magnetic and Optical Resonance, edited by Warren W. S. (Academic Press, 1996), Vol. 19, pp. 325–388. [Google Scholar]
- 36.Gore J. C., Xu J., Colvin D. C., Yankeelov T. E., Parsons E. C., and Does M. D., NMR Biomed. 23, 745 (2010). 10.1002/nbm.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carr H. Y. and Purcell E. M., Phys. Rev. 94, 630 (1954). 10.1103/physrev.94.630 [DOI] [Google Scholar]
- 38.Meiboom S. and Gill D., Rev. Sci. Instrum. 29, 688 (1958). 10.1063/1.1716296 [DOI] [Google Scholar]
- 39.Ning L., Setsompop K., Westin C.-F., and Rathi Y., Magn. Reson. Med. 78, 763 (2017). 10.1002/mrm.26403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zielinski L. J. and Hürlimann M. D., J. Magn. Reson. 172, 161 (2005). 10.1016/j.jmr.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 41.Lasič S., Stepišnik J., and Mohorič A., J. Magn. Reson. 182, 208 (2006). 10.1016/j.jmr.2006.06.030 [DOI] [PubMed] [Google Scholar]
- 42.Stepišnik J., Lasič S., Mohorič A., Serša I., and Sepe A., J. Magn. Reson. 182, 195 (2006). 10.1016/j.jmr.2006.06.023 [DOI] [PubMed] [Google Scholar]
- 43.Zielinski L. J. and Sen P. N., J. Chem. Phys. 119, 1093 (2003). 10.1063/1.1578615 [DOI] [Google Scholar]
- 44.Song Y.-Q., Hürlimann M. D., and Flaum C., J. Magn. Reson. 161, 222 (2003). 10.1016/s1090-7807(03)00009-0 [DOI] [PubMed] [Google Scholar]
- 45.Hürlimann M. D., J. Magn. Reson. 148, 367 (2001). 10.1006/jmre.2000.2263 [DOI] [PubMed] [Google Scholar]
- 46.Ross A., Czisch M., and King G. C., J. Magn. Reson. 124, 355 (1997). 10.1006/jmre.1996.1036 [DOI] [PubMed] [Google Scholar]
- 47.Goelman G. and Prammer M. G., J. Magn. Reson. 113, 11 (1995). 10.1006/jmra.1995.1050 [DOI] [Google Scholar]
- 48.Song Y.-Q., J. Magn. Reson. 157, 82 (2002). 10.1006/jmre.2002.2577 [DOI] [PubMed] [Google Scholar]
- 49.Baltisberger J. H., Walder B. J., Keeler E. G., Kaseman D. C., Sanders K. J., and Grandinetti P. J., J. Chem. Phys. 136, 211104 (2012). 10.1063/1.4728105 [DOI] [PubMed] [Google Scholar]
- 50.Song Y.-Q., Magn. Reson. Imaging 23, 301 (2005). 10.1016/j.mri.2004.11.033 [DOI] [PubMed] [Google Scholar]
- 51.Sigmund E. E., Cho H., and Song Y.-Q., Concepts Magn. Reson., Part A 30A, 358 (2007). 10.1002/cmr.a.20097 [DOI] [Google Scholar]
- 52.Casanova F., Blümich B., and Perlo J., Single-Sided NMR (Springer, Heidelberg, New York, 2011). [Google Scholar]
- 53.Eidmann G., Savelsberg R., Blümler P., and Blümich B., J. Magn. Reson. 122, 104 (1996). 10.1006/jmra.1996.0185 [DOI] [Google Scholar]
- 54.Williamson N. H., Ravin R., Benjamini D., Merkle H., Falgairolle M., O’Donovan M. J., Blivis D., Ide D., Cai T. X., Ghorashi N. S., Bai R., and Basser P. J., eLife 8, e51101 (2019). 10.7554/elife.51101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sousa D. N. and Ferreira H. A., J. Open Source Software 3, 966 (2018). 10.21105/joss.00966 [DOI] [Google Scholar]
- 56.Geil B., Concepts Magn. Reson. 10, 299 (1998). [DOI] [Google Scholar]
- 57.Hürlimann M. D. and Griffin D. D., J. Magn. Reson. 143, 120 (2000). 10.1006/jmre.1999.1967 [DOI] [PubMed] [Google Scholar]
- 58.Suh K.-J., Hong Y.-S., Skirda V. D., Volkov V. I., Lee C.-Y. J., and Lee C.-H., Biophys. Chem. 104, 121 (2003). 10.1016/s0301-4622(02)00361-7 [DOI] [PubMed] [Google Scholar]
- 59.Tanner J. E. and Stejskal E. O., J. Chem. Phys. 49, 1768 (1968). 10.1063/1.1670306 [DOI] [Google Scholar]
- 60.Åslund I. and Topgaard D., J. Magn. Reson. 201, 250 (2009). 10.1016/j.jmr.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 61.Mazur W. and Krzyżak A. T., Cells 9, 2124 (2020). 10.3390/cells9092124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karunanithy G., Wheeler R. J., Tear L. R., Farrer N. J., Faulkner S., and Baldwin A. J., J. Magn. Reson. 302, 1 (2019). 10.1016/j.jmr.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Callaghan P. T. and Coy A., Phys. Rev. Lett. 68, 3176 (1992). 10.1103/physrevlett.68.3176 [DOI] [PubMed] [Google Scholar]
- 64.Williamson N. H., Dower A. M., Codd S. L., Broadbent A. L., Gross D., and Seymour J. D., Phys. Rev. Lett. 122, 068001 (2019). 10.1103/physrevlett.122.068001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Short M. B., Solari C. A., Ganguly S., Powers T. R., Kessler J. O., and Goldstein R. E., Proc. Natl. Acad. Sci. U. S. A. 103, 8315 (2006). 10.1073/pnas.0600566103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See the supplementary material for (I) the Python code to generate mj, (II) the representative MATLAB code for the Monte Carlo simulations, fitting procedures, and LLS inversion, (III) replication of the analysis in Figs. 4 and 6 using SG-TIETA decays for τ = 77 µs, and (IV) the additional NMR experimental methodology, which includes all echo shapes and a stitched echo decay for 1-octanol.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The SG-TIETA pulse program and macro can be downloaded from the GitHub repository: https://github.com/nathanwilliamson/SG-TIETA.