Abstract
Background
Chimeric antigen-receptor T-cell and bispecific antibody therapies will likely necessitate a reconsideration of the role of autologous stem-cell transplantation (ASCT) in lymphoma. Patients who are likely to profit from ASCT need to be better identified.
Methods
Here, we investigated the value of positron emission tomography/computerized tomography (PET/CT) before ASCT. All 521 patients transplanted for lymphoma 1994–2019 at Karolinska (497 conditioned with BEAM) were included.
Results
Outcome improved over three calendar periods 1994–2004, 2005–2014, 2015–2019 (2-year overall survival [OS]: 66, 73, 83%; P = 0.018). Non-relapse mortality (NRM) at 100 days over the three periods were 9.8, 3.9, 2.9%, respectively. The OS improvement between 1994 and 2004 and 2005–2014 was due to lower NRM (P = 0.027), but the large OS advance from 2015 was not accompanied by a significant reduction in NRM (P = 0.6). The fraction of PET/CT as pre-ASCT assessment also increased over time: 1994–2004, 2%; 2005–2014, 24%; 2015–2019, 60% (P < 0.00005). Complete responses (PET/CT-CR) were observed in 77% and metabolically active partial responses (PET/CT-PR) in 23%. PET/CT-CR was a predictor for survival in the entire population (P = 0.0003), also in the subpopulations of aggressive B-cell (P = 0.004) and peripheral T-cell (P = 0.024) lymphomas. Two-year OS and progression-free survival (OS/PFS) for patients in PET/CT-CR were in relapsed/refractory aggressive B-cell lymphoma 87%/75% and peripheral T-cell lymphoma 91%/78%. The corresponding figures in PET/CT-PR were 43%/44 and 33%/33%. Patients with solitary PET/CT-positive lesions showed acceptable outcome with ASCT followed by local irradiation (2-year OS/PFS 80%/60%). CT was less discriminative: 2-year OS/PFS: CT-CR, 76%/66%; CT-PR, 62%/51%. Outcome was inferior after BEAC compared with BEAM conditioning.
Conclusions
We conclude that the improved outcome reflects better, PET/CT-informed, identification of patients who should proceed to ASCT. The excellent survival of patients in PET/CT-CR indicates that ASCT should remain part of standard therapy for lymphoma.
Keywords: Lymphoma, B cell, T cell, ASCT, Autologous stem-cell transplantation, PET/CT, Positron emission tomography/computerized tomography, PET
Background
Even though effective non-chemotherapeutic treatment options for lymphomas, such as chimeric antigen-receptor (CAR)-T cell and bispecific antibody therapies, are rapidly emerging, high-dose chemotherapy followed by autologous stem-cell transplantation (ASCT) remains the standard therapeutic option for many patients. Infections are the most common complication after ASCT and the largest contributor to non-relapse mortality (NRM) [1]. Another concern is the potential risk of developing secondary myeloid malignancies [1]. Careful patient selection prior to ASCT is essential to maximize patient benefit while keeping the rate of complications as low as possible. Additional tools are needed to further improve patient selection.
We have previously investigated stem-cell harvest yields and clinical characteristics which affect NRM, other toxicities, and the development of secondary myeloid disease, to help inform on timing and patient selection [2]. However, another important aspect of patient selection is the strength of the indication, which differ by diagnosis, primary or relapsed/refractory setting, and remission status.
Historically, computerized tomography (CT) has been used to assess patients’ remission status prior to ASCT. The combination of 18-fluorodeoxyglucose positron emission tomography and CT (PET/CT) enables radiologic mapping of metabolic activity and can be used for identifying and staging the lymphoid malignancies which have elevated glucose consumption. PET/CT is superior to CT in discriminating between malignant and non-malignant residual tissue, decreasing the risk of incorrectly identifying radiologic remnants post-chemotherapy as active lymphoma [3]. The utility of PET/CT before ASCT to predict outcome has been shown in several lymphoma entities [4–16], although negative series also exist [17, 18]. In these studies, several different conditioning regimens were used, and the timing of the PET/CT differed. We wanted to investigate whether outcome after ASCT had improved with the increasing use of pre-transplant PET/CT and to assess outcome in 521 consecutive lymphoma patients treated with ASCT (497 conditioned with BEAM) in a population-based single-center study.
Methods
All lymphoma patients who underwent ASCT at the Hematology Unit, Karolinska University Hospital, from 1 January 1994 until 31 October 2019 were included in this retrospective single-center observational study (excluding patients with primary CNS lymphoma). The material is population-based, since the Hematology Unit is the only site conducting this procedure in the Stockholm-Gotland Healthcare Region (population, 2.4 million). Patient data were extracted from electronic medical files. BEAM was dosed day − 7: BCNU 300 mg/m2 4-h infusion, intrathecal methotrexate 12 mg; days − 6, − 5, − 4, − 3: etoposide 200 mg/m2 2-h infusion, cytarabine 400 mg/m2 12-h infusion (day − 3 also intrathecal methotrexate 12 mg); day − 2: melphalan 140 mg/m2 (intrathecal methotrexate was omitted in patients with indolent, T-cell and Hodgkin lymphoma). BEAC was dosed day − 6: BCNU 300 mg/m2 4-h infusion; days − 5, − 4, − 3, − 2: etoposide 100 mg/m2 twice daily, cytarabine 100 mg/m2 0.5-h infusion twice daily, cyclophosphamide 35 mg/kg once and mesna 14 mg/kg four times daily. BCNU-thiotepa was dosed BCNU 400 mg/m2 1-h infusion day − 6; thiotepa 5 mg/kg twice daily days − 5 and − 4. Investigations of stem-cell harvests (median, 6 million CD34+ cells per kg), NRM, and procedure-related toxicities have been published before [2]. Refractory disease was defined as a previous failure of chemotherapy or relapse within 3 months of treatment.
CT and PET/CT
Complete and partial response (CR; PR) were defined as responses with at least 50% reduction of lymphoma size and separated by the absence or presence of a lymph node or focal lesion in any organ ≥1 cm, or as specified by the reviewing radiologist. PET/CT responses were defined according to the Lugano classification (PET/CT-CR was Deauville score ≤ 3) [3]. PET/CT-PR denotes a shrinking of lymphoma mass but still metabolically active disease.
Statistical analysis
Patients were followed from ASCT until death or last follow-up (November 2019). Depending on the nature of the independent variables, relationships between them were investigated using the χ2, Wilcoxon or Spearman test. Overall, lymphoma-specific, and progression-free survivals (OS; LSS; PFS) were calculated from the day of ASCT until the day of death (OS), death from lymphoma (LSS), death or progression of disease (PFS). Univariate and multivariate analyses were conducted using Kaplan-Meier curves and Cox regression; the proportional hazards assumption was checked with graphs based on Schoenfeld residuals. All P values are two-tailed and calculated using Stata 14.2 (StataCorp, College Station, TX, USA). P < 0.05 was considered significant.
This study was approved by the Ethics Committee, Stockholm (Ref no. 2012/783–31/3 with Amendments 2015/327–32 and 2016/2379–32).
Results
Five hundred twenty-one lymphoma patients underwent ASCT between 1994 and 2019 (Table 1). Most patients were male (63%) and the median age was 57 years (range, 18–72). At last follow-up (November 2019), the median follow-up time in survivors was 5.3 years (range, 0.1–24.3). The median OS, LSS, and PFS were 13.2, not reached, and 5.7 years (Fig. 1a). OS and PFS were 74 and 66% at 2 years and 64 and 52% at 5 years (Table 2). The conditioning regimen was BEAM in 497 patients. Because of occasional melphalan shortages from 2015 onwards, BEAC was used in 20 patients. Three patients with CNS relapse of systemic lymphoma were conditioned with BCNU-thiotepa and the first patient in 1994 received cyclophosphamide-total body irradiation. There was improved outcome over the three calendar periods 1994–2004, 2005–2014, 2015–2019 (2-year OS: 66, 73, 83%; P = 0.018; Fig. 1b), partly explained by decreasing NRM (at 100 days, 9.8, 3.9, 2.9%, respectively; previously thoroughly described [2]). The OS improvement between 1994 and 2004 and 2005–2014 was due to lower NRM (P = 0.027), but the 10-percentage point OS advance between 2005 and 2014 and 2015–2019 (P = 0.029) was not accompanied by a significant reduction in NRM (P = 0.6). Rituximab use increased in B-cell disease (30, 96, 100% over the three calendar periods; P < 0.00005) but could not explain the better outcome (Table 1). Neither was it caused by changes in conditioning because the BEAM regimen never changed, and, in the last calendar period, there was a clear tendency for inferior outcome in patients treated with BEAC compared with BEAM (Fig. 1c), with P = 0.059 for OS, P = 0.034 for LSS, and P = 0.062 for PFS. When BEAC patients were excluded, not only OS and NRM, but also LSS was significantly better in the last calendar period (P = 0.040), suggesting the emergence of a new lymphoma-specific survival factor.
Table 1.
Clinical characteristics
| N | per cent | OS | LSS | PFS | |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Sex | |||||
| Female | 193 | 37% | 1 | 1 | 1 |
| Male | 328 | 63% | 1.12 (0.83–1.50) | 1.06 (0.72–1.54) | 1.01 (0.79–1.30) |
| Age, years | |||||
| Median (range) 57 (18–72) | |||||
| 18–45 | 126 | 24% | 1 | 1 | 1 |
| 46–55 | 114 | 22% | 2.04 (1.26–3.31) | 2.08 (1.09–3.94) | 1.70 (1.15–2.51) |
| 56–65 | 189 | 36% | 2.99 (1.94–4.61) | 2.87 (1.62–5.08) | 2.09 (1.47–2.93) |
| 66–72 | 92 | 18% | 3.26 (1.97–5.40) | 3.43 (1.81–6.52) | 2.32 (1.54–3.50) |
| Lymphoma entity | |||||
| Aggressive B cell | 121 | 23% | 1 | 1 | 1 |
| Transformed B cell | 107 | 21% | 0.80 (0.53–1.20) | 0.66 (0.40–1.09) | 1.02 (0.72–1.46) |
| Indolent B cell | 49 | 9% | 0.88 (0.56–1.40) | 0.59 (0.32–1.10) | 1.15 (0.76–1.74) |
| Mantle cell | 99 | 19% | 0.50 (0.32–0.78) | 0.38 (0.21–0.66) | 0.65 (0.45–0.95) |
| T cell | 85 | 16% | 0.71 (0.47–1.10) | 0.51 (0.29–0.90) | 0.80 (0.55–1.18) |
| Classical Hodgkin | 60 | 12% | 0.37 (0.21–0.66) | 0.19 (0.08–0.49) | 0.54 (0.33–0.87) |
| Indication for ASCT | |||||
| Upfront | 183 | 35% | 1 | 1 | 1 |
| Relapsed disease | 249 | 48% | 1.85 (1.31–2.62) | 1.82 (1.17–2.84) | 1.50 (1.13–2.00) |
| Refractory disease | 88 | 17% | 2.48 (1.64–3.73) | 2.58 (1.53–4.36) | 1.95 (1.37–2.77) |
| Calendar period | |||||
| 1994–2004 | 112 | 21% | 1 | 1 | 1 |
| 2005–2014 | 239 | 46% | 0.93 (0.67–1.29) | 1.15 (0.74–1.77) | 0.92 (0.58–1.23) |
| 2015–2019 | 170 | 33% | 0.54 (0.34–0.87) | 0.74 (0.41–1.33) | 0.82 (0.57–1.19) |
| Number of prior lines of chemotherapy | |||||
| 1 | 201 | 41% | 1 | 1 | 1 |
| 2 | 256 | 52% | 1.65 (1.19–2.29) | 1.80 (1.18–2.76) | 1.44 (1.09–1.89) |
| 3 or more | 37 | 7% | 2.32 (1.33–4.07) | 2.70 (1.36–5.39) | 1.81 (1.11–2.96) |
| Conditioning regimen | |||||
| BEAM | 497 | 95% | 1 | 1 | 1 |
| BEAC | 20 | 4% | 1.22 (0.57–2.61) | 1.57 (0.69–3.59) | 1.59 (0.88–2.84) |
| BCNU-Thiotepa | 3 | 1% | NA | NA | NA |
| Cy-TBI | 1 | 0% | NA | NA | NA |
| Rituximab prior to ASCT in B-cell disease | |||||
| No | 63 | 17% | 1 | 1 | 1 |
| Yes | 313 | 83% | 0.61 (0.42–0.87) | 0.76 (0.47–1.22) | 0.73 (0.53–1.02) |
Abbreviations: OS overall survival, LSS lymphoma-specific survival, PFS progression-free survival, ASCT autologous stem-cell transplantation, NA not analyzable
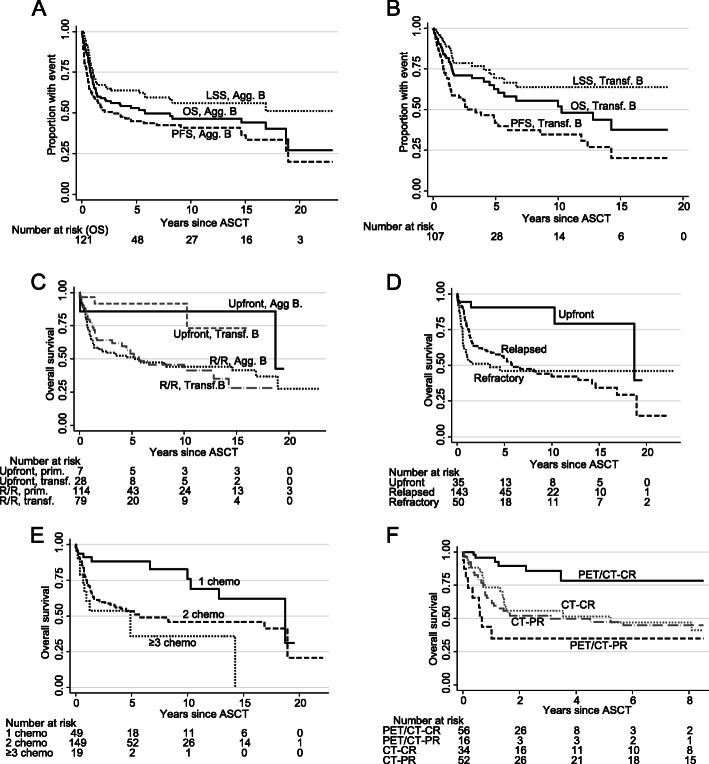
Fig. 1.
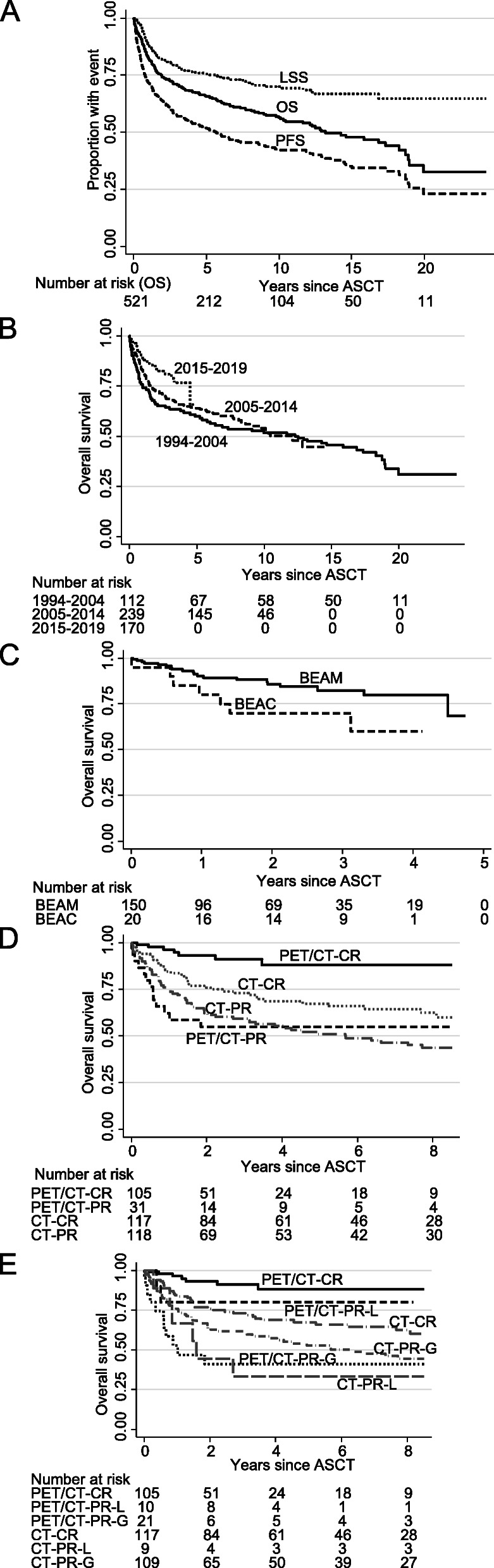
Kaplan-Meier graphs of (a) overall, lymphoma-specific, and progression-free survival in all patients (OS; LSS; PFS), b OS by calendar period, c BEAM or BEAC conditioning in the last calendar period, d partial or complete remission (PR; CR) status according to computerized tomography (CT) or 18-flourodeoxyglucose positron emission tomography (PET)/CT, e PR or CR status according to CT and PET/CT with PR patients locally treated after ASCT (PR-L) as a separate group, compared with generalized PR (PR-G); the last two graphs are cut at 8 years due to the different observation times between CT and PET/CT patients. Abbreviation: ASCT, autologous stem-cell transplantation
Table 2.
Lymphoma entities
| N | OS | LSS | PFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 y | 5y | 10y | P | 2y | 5y | 10y | P | 2y | 5y | 10y | P | ||
| All lymphomas | 521 | 74% | 66% | 56% | 82% | 76% | 70% | 64% | 52% | 42% | |||
| Primary aggressive B-cell lymphomas | 121 | 59% | 53% | 47% | 67% | 64% | 56% | 52% | 45% | 41% | |||
| DLBCL or high-grade B-cell lymphoma | 105 | 59% | 52% | 44% | 68% | 64% | 55% | 50% | 42% | 38% | |||
| Not-DLBCL or high-grade B-cell lymphoma | 16 | 60% | 60% | 60% | 0.28 | 64% | 64% | 64% | 0.55 | 61% | 61% | 61% | 0.14 |
| FL grade 3B | 2 | ||||||||||||
| Burkitt lymphoma | 3 | ||||||||||||
| PTLD | 2 | ||||||||||||
| PMBCL or greyzone lymphoma | 3 | ||||||||||||
| Plasmablastic lymphoma | 5 | ||||||||||||
| Lymphomatoid granulomatosis grade III | 1 | ||||||||||||
| Upfront patients | 7 | 86% | 86% | 86% | 100% | 100% | 100% | 86% | 86% | 86% | |||
| Relapsed/refractory patients | 114 | 58% | 51% | 44% | 0.19 | 65% | 62% | 53% | 0.070 | 50% | 43% | 38% | 0.09 |
| Transformed B-cell lymphomas | 107 | 71% | 63% | 52% | 78% | 69% | 64% | 59% | 42% | 35% | |||
| Transformed FL | 72 | 76% | 71% | 56% | 81% | 75% | 68% | 65% | 48% | 38% | |||
| Transformed not-FL | 35 | 61% | 41% | 41% | 0.22 | 73% | 49% | 49% | 0.35 | 48% | 30% | 30% | 0.28 |
| Transformed MZL | 18 | ||||||||||||
| Transformed CLL/SLL (Richter) | 5 | ||||||||||||
| Transformed WM | 3 | ||||||||||||
| Transformed NLPHL | 2 | ||||||||||||
| Transformed indolent B-cell lymphoma | 7 | ||||||||||||
| Upfront patients | 28 | 92% | 92% | 92% | 95% | 95% | 95 | 68% | 55% | 41% | |||
| Relapsed/refractory patients | 79 | 64% | 54% | 41% | 0.006 | 73% | 61% | 55% | 0.009 | 55% | 38% | 33% | 0.13 |
| Primary and transformed aggressive B-cell lymphomas | 228 | 64% | 58% | 50% | 72% | 67% | 60% | 55% | 44% | 39% | |||
| Upfront patients | 35 | 90% | 90% | 90% | 96% | 96% | 96% | 72% | 63% | 54% | |||
| Relapsed/refractory patients | 193 | 60% | 53% | 44% | 0.002 | 68% | 63% | 54% | 0.001 | 52% | 42% | 37% | 0.028 |
| Indolent B-cell lymphomas | 49 | 80% | 59% | 44% | 90% | 75% | 65% | 60% | 45% | 26% | |||
| FL grade 1-3A | 39 | 78% | 57% | 43% | 88% | 76% | 69% | 60% | 44% | 31% | |||
| Not-FL grade 1-3A | 10 | 89% | 63% | 51% | 0.55 | 100% | 71% | 57% | 0.24 | 57% | 46% | 11% | 0.48 |
| MZL | 1 | ||||||||||||
| CLL/SLL | 6 | ||||||||||||
| WM | 1 | ||||||||||||
| NLPHL | 2 | ||||||||||||
| All indolent lymphomas | 156 | 74% | 61% | 48% | 82% | 71% | 64% | 59% | 43% | 31% | |||
| Not transformed | 49 | 80% | 59% | 44% | 90% | 75% | 65% | 60% | 45% | 26% | |||
| Transformed | 107 | 71% | 63% | 52% | 0.77 | 78% | 69% | 64% | 0.65 | 59% | 42% | 35% | 0.77 |
| All follicular lymphomas | 111 | 77% | 65% | 50% | 83% | 75% | 68% | 63% | 47% | 35% | |||
| Not transformed | 39 | 78% | 57% | 43% | 88% | 76% | 69% | 60% | 44% | 31% | |||
| Transformed | 72 | 76% | 71% | 56% | 0.58 | 81% | 75% | 68% | 0.63 | 65% | 48% | 38% | 0.69 |
| Mantle cell lymphoma | 99 | 86% | 75% | 73% | 91% | 82% | 82% | 80% | 62% | 43% | |||
| Classical | 83 | 90% | 78% | 78% | 95% | 86% | 86% | 84% | 67% | 47% | |||
| Non-classical | 16 | 66% | 58% | 43% | 0.021 | 70% | 62% | 62% | 0.025 | 61% | 37% | 18% | 0.005 |
| Blastic | 9 | ||||||||||||
| Pleomorphic or P53+ or Ki67 > 60% | 7 | ||||||||||||
| Upfront patients | 87 | 90% | 78% | 78% | 93% | 84% | 84% | 83% | 66% | 45% | |||
| Relapsed/refractory patients | 12 | 58% | 49% | 32% | 0.006 | 81% | 67% | 67% | 0.30 | 58% | 40% | 40% | 0.054 |
| T-cell lymphomas | 85 | 74% | 69% | 53% | 83% | 79% | 71% | 65% | 54% | 45% | |||
| PTCL | 78 | 79% | 73% | 56% | 87% | 83% | 74% | 70% | 58% | 48% | |||
| Non-PTCL | 7 | 18% | 18% | ND | 0.004 | 25% | 25% | ND | 0.013 | 0% | 0% | 0% | 0.002 |
| Sézary/ATLL | 3 | ||||||||||||
| ENKTCL | 4 | ||||||||||||
| PTCL, ALCL | 25 | 79% | 79% | 74% | 91% | 91% | 91% | 80% | 75% | 70% | |||
| PTCL, non-ALCL | 53 | 78% | 69% | 41% | 0.08 | 85% | 78% | 63% | 0.09 | 65% | 47% | 33% | 0.023 |
| AITL/FHTCL | 29 | ||||||||||||
| PTCL-NOS | 17 | ||||||||||||
| EATCL/HSTCL/SPTCL/Lennert | 7 | ||||||||||||
| Upfront PTCL patients | 56 | 81% | 75% | 67% | 86% | 80% | 71% | 70% | 60% | 55% | |||
| Relapsed/refractory PTCL patients | 22 | 73% | 68% | 39% | 0.07 | 89% | 89% | 80% | 0.44 | 68% | 54% | 38% | 0.21 |
| Classical Hodgkin lymphoma | 60 | 85% | 83% | 74% | 92% | 90% | 90% | 71% | 66% | 60% | |||
Abbreviations: OS overall survival, LSS lymphoma-specific survival, PFS progression-free survival, DLBCL diffuse large B-cell lymphoma, FL follicular lymphoma, PTLD post-transplantation lymphoproliferative disorder, PMBCL primary mediastinal B-cell lymphoma, MZL marginal zone lymphoma, CLL chronic lymphocytic leukemia, SLL small lymphocytic lymphoma, WM Waldenström macroglobulinemia, NLPHL nodular lymphocyte-predominant Hodgkin lymphoma, PTCL peripheral T-cell lymphoma, ALCL anaplastic large cell lymphoma, AITL angioimmunoblastic T-cell lymphoma, FHTCL follicular helper T-cell lymphoma, EATCL enteropathy-associated T-cell lymphoma, HSTCL hepatosplenic T-cell lymphoma, SPTCL subcutaneous panniculitis-like T-cell lymphoma, ATLL adult T-cell leukemia/lymphoma, ENKTCL extranodal NK/T-cell lymphoma, nasal type
The emergence of PET/CT
We compared the prognostic value of pre-transplant CT and PET/CT. Cases were excluded if the last radiology evaluation prior to ASCT was PR but conducted interim (before the last course of chemotherapy prior to conditioning). All assessments showing CR in the last evaluation were included, either when conducted interim or directly before the ASCT. Thus, the last evaluation of response prior to ASCT was CT in 235 patients (62%) and PET/CT in 136 (36%), the remaining 8 (2%) were assessments using MRI, bone marrow biopsy, or palpation. Over time, PET/CT became increasingly common: PET/CT constituted 2% and CT 98% of the final evaluations 1994–2004, the corresponding numbers were 24 and 75% 2005–2014, and 60 and 37% 2015–2019 (P < 0.0005). Of patients evaluated with PET/CT prior to ASCT, 77% were in CR and 23% in PR (Table 3).
Table 3.
Remission status by PET/CT and CT
| N | percent | OS | LSS | PFS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2y | 5y | P | 2y | 5y | P | 2y | 5y | P | |||
| PET/CT | 136 | 100% | |||||||||
| PET/CT-CR | 105 | 77% | 93% | 88% | 94% | 92% | 81% | 67% | |||
| PET/CT-PR | 31 | 23% | 55% | 55% | 0.0003 | 68% | 68% | 0.0009 | 45% | 41% | 0.004 |
| PET/CT-PR-local | 10 | 7% | 80% | 80% | 90% | 90% | 60% | 50% | |||
| PET/CT-PR-general | 21 | 15% | 41% | 41% | < 0.00005 | 56% | 56% | 0.0002 | 36% | 36% | 0.003 |
| Aggressive B-cell lymphoma | 72 | ||||||||||
| PET/CT-CR | 50 | 69% | 90% | 76% | 90% | 85% | 73% | 55% | |||
| PET/CT-PR | 22 | 31% | 50% | 50% | 0.004 | 59% | 59% | 0.006 | 45% | 39% | 0.032 |
| Relapsed/refractory | 54 | ||||||||||
| PET/CT-CR | 36 | 67% | 87% | 64% | 87% | 80% | 75% | 48% | |||
| PET/CT-PR | 18 | 33% | 43% | 43% | 0.013 | 50% | 50% | 0.008 | 44% | 44% | 0.15 |
| Peripheral T-cell lymphoma | 18 | ||||||||||
| PET/CT-CR | 15 | 83% | 91% | 91% | 91% | 91% | 78% | 62% | |||
| PET/CT-PR | 3 | 17% | 33% | NA | 0.024 | 50% | NA | 0.19 | 33% | NA | 0.27 |
| Classical Hodgkin lymphoma | 25 | ||||||||||
| PET/CT-CR | 21 | 84% | 100% | 100% | 100% | 100% | 89% | 67% | |||
| PET/CT-PR | 4 | 16% | 75% | 75% | 0.083 | 100% | 100% | 1 | 50% | 50% | 0.37 |
| CT | 235 | ||||||||||
| CT-CR | 117 | 50% | 76% | 67% | 83% | 78% | 66% | 55% | |||
| CT-PR | 118 | 50% | 62% | 51% | 0.035 | 70% | 62% | 0.022 | 51% | 36% | 0.004 |
| Aggressive B-cell lymphoma | 86 | ||||||||||
| CT-CR | 34 | 40% | 59% | 52% | 69% | 69% | 50% | 35% | |||
| CT-PR | 52 | 60% | 52% | 47% | 0.81 | 57% | 52% | 0.25 | 46% | 37% | 0.85 |
| Relapsed/refractory | 77 | ||||||||||
| CT-CR | 30 | 39% | 50% | 45% | 64% | 64% | 46% | 30% | |||
| CT-PR | 47 | 61% | 49% | 44% | 0.99 | 54% | 49% | 0.38 | 43% | 35% | 0.99 |
| Peripheral T-cell lymphoma | 44 | ||||||||||
| CT-CR | 27 | 61% | 74% | 63% | 77% | 71% | 67% | 56% | |||
| CT-PR | 17 | 39% | 76% | 76% | 0.93 | 92% | 92% | 0.22 | 65% | 44% | 0.25 |
| Classical Hodgkin lymphoma | 13 | ||||||||||
| CT-CR | 3 | 23% | 100% | 100% | 100% | 100% | 67% | 67% | |||
| CT-PR | 10 | 77% | 70% | 70% | 0.21 | 70% | 70% | 0.32 | 50% | 50% | 0.34 |
Abbreviations: PET/CT positron emission tomography/computerized tomography, CT computerized tomography, CR complete remission, PR partial remission, NA not analyzable
Both with CT and PET/CT, PR compared with CR was a poor prognostic marker (OS, P = 0.035, P = 0.0003, respectively). However, PET/CT was markedly more powerful than CT (Table 3; Fig. 1d). For example, at 2 years, the OS rates were in CT-CR 76% and CT-PR 62%, and in PET/CT-CR 93% and PET/CT-PR 55% (Table 3). Because CT had been conducted more in the earlier times with higher NRM, the analysis was repeated with restriction to the last calendar period (2015–2019) with similar results (2-year OS: CT-CR 81%, CT-PR 70%, PET/CT-CR 96%, PET/CT-PR 63%). At the start of conditioning, in patients with PET/CT-CR, 68% had normal C-reactive protein (< 3 mg/L), 25% 3–10 mg/L, and 7% ≥10 mg/L, whereas in PET/CT-PR, the corresponding numbers were 41, 38, and 21% (P = 0.005). There were no C-reactive protein differences between CT-CR and CT-PR (P = 0.65).
We further investigated the patients transplanted in PR: 19 were given a planned local therapy after ASCT (18, irradiation; 1, splenectomy). That approach appeared useful in the 10 patients where the local therapy was PET-guided (2-year OS/PFS 80%/60%) but not in those 9 where CT defined PR (2-year OS/PFS 44%/44%; Fig. 1e). The PET/CT-PR patients given local therapy (PET/CT-PR-local) was an intermediate prognostic group between PET/CT-CR and PET/CT-PR-general (several FDG-avid sites; Table 3). With respect to OS and PFS, PET/CT-PR compared with PET/CT-CR showed inferior outcome with hazard ratios (HR) 4.0 (95% confidence interval [CI], 1.8–9.1) and 2.4 (95% CI, 1.3–4.6). For comparison, CT-PR compared with CT-CR had HR 1.5 (95% CI, 1.0–2.2) for OS and HR 1.6 (95% CI, 1.2–2.3) for PFS.
Primary and transformed aggressive B-cell lymphomas
In total, 228 (121 primary and 107 transformed) patients were transplanted for aggressive B-cell lymphoma. Outcome did not differ between diffuse large B-cell or other types of primary aggressive lymphoma; likewise, there were no differences between transformed follicular or other transformed indolent lymphomas, nor, overall, between primary or transformed aggressive lymphomas (Table 2), and the curves were roughly similar (Fig. 2a, b). There were no outcome differences between primary or transformed aggressive lymphoma in the upfront or relapsed/refractory setting (Fig. 2c), why the 228 aggressive B-cell patients were subsequently grouped for statistical power. Patients transplanted upfront in first remission had better outcome than those who had had relapsed or refractory disease (Table 2; Fig. 2d), similarly, the number of prior lines of chemotherapy was highly predictive for outcome (OS, P = 0.001; LSS, P = 0.0002; PFS, P = 0.0002; Fig. 2e). PET/CT was a valuable predictive tool for OS (P = 0.004), LSS (P = 0.006), and PFS (P = 0.032), while remission status by CT had no value (Fig. 2f; Table 3).
Fig. 2.
Kaplan-Meier graphs of overall, lymphoma-specific, and progression-free survival (OS; LSS; PFS) in (a) primary aggressive (Agg. B) and (b) transformed indolent (Transf. B) B-cell lymphoma, c OS by upfront and relapsed/refractory status in primary aggressive and transformed indolent B-cell lymphoma, d OS in primary aggressive or transformed indolent B-cell lymphoma patients by upfront, relapsed, or refractory status, e OS by number of preceding lines of chemotherapy, f OS by PR or CR status according to CT and PET/CT (with PET/CT-PR patients locally treated after ASCT grouped with PET/CT-CR); the last graph is cut at 8 years due to the different observation times between CT and PET/CT patients. Abbreviation: ASCT, autologous stem-cell transplantation
Upfront transplanted primary or transformed aggressive B-cell lymphoma
As shown in Table 2, 35 patients were transplanted upfront, with good OS and PFS (at 2 years 90 and 72%). The small number of events made analysis of the predictive value of PET/CT difficult, but there was a trend for inferior outcome (OS, P = 0.061; PFS, P = 0.078) in patients with PET/CT-PR. CR or PR according to CT had no prognostic value (P ≥ 0.4).
Relapsed/refractory transplanted primary or transformed aggressive B-cell lymphoma
There were 193 patients transplanted with relapsed or refractory disease: OS and PFS at 2 years were 60 and 52% (Table 2). The 2-year OS and PFS in patients in PET/CT-CR prior to ASCT were 87 and 75%, but in patients with PET/CT-PR 43 and 44% (P = 0.013 and P = 0.15); with CT-CR, the 2-year OS and PFS were 50 and 46% and with CT-PR, 49 and 43% (P = 0.99 and P = 0.99; Table 3). Thus, PET/CT exhibited much stronger predictive value than CT.
Indolent B-cell lymphomas
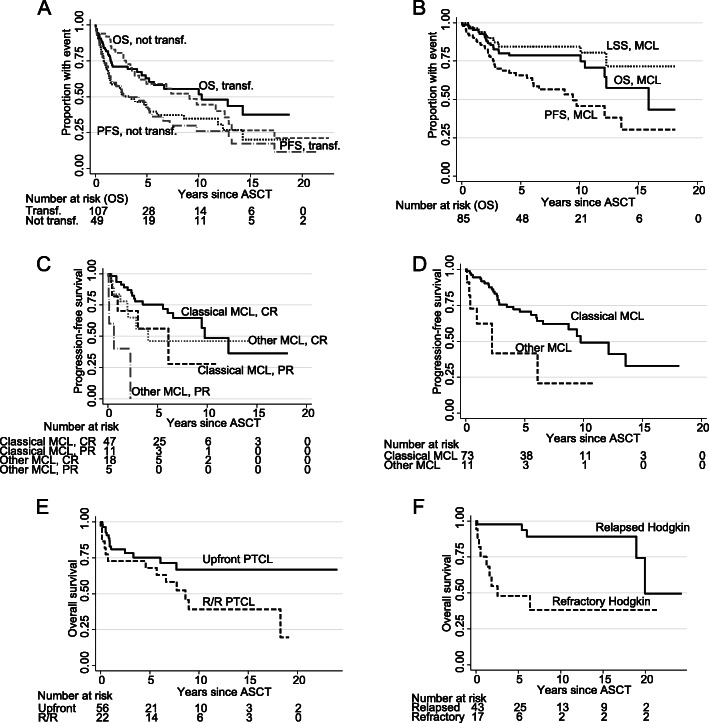
Short- and long-term outcome of the 49 patients with indolent lymphoma are shown in Table 2; late relapses were seen. In follicular lymphoma, 31% were alive and in remission after 10 years. Outcome was almost identical (Fig. 3a; Table 2) in transformed and not-transformed indolent lymphoma, apart from an expected initial higher mortality in transformed disease. There were too few events for investigating the PET/CT method in not-transformed indolent lymphoma. When combining transformed and not-transformed disease, PET/CT was highly predictive for OS (P = 0.0003) and PFS (P = 0.013), while CT was not predictive (P > 0.5 for both comparisons).
Fig. 3.
Kaplan-Meier graphs of (a) overall and progression-free survival (OS; PFS) in not-transformed (not transf.) and transformed (transf.) indolent B-cell lymphoma, b OS, lymphoma-specific survival (LSS), and PFS in mantle cell lymphoma (MCL), c PFS by remission status in classical and other types of MCL, d PFS in classical and other types of MCL transplanted upfront, e OS in peripheral T-cell lymphoma (PTCL) by upfront or relapsed/refractory (R/R) status, f OS in Hodgkin lymphoma by relapsed or refractory status. Abbreviation: ASCT, autologous stem-cell transplantation
Mantle cell lymphoma
Eighty-five out of 99 mantle cell lymphoma patients were transplanted upfront in or according to the Nordic MCL2 trial [19]. These patients showed excellent outcome with median PFS 9.4 years (Fig. 3b). Patients with more aggressive variants of MCL (blastic, pleomorphic, TP53+, or Ki67 > 60%) showed poor outcome (Table 2), also after upfront ASCT according to the MCL2 protocol (Fig. 3c). CR compared with PR, regardless whether informed from PET/CT or CT, was highly predictive of outcome (OS, P = 0.039; LSS, P = 0.044; PFS = 0.025; Fig. 3d). Also in the subset of upfront transplanted patients, CR according to PET/CT or CT predicted outcome (OS, P = 0.042; LSS, P = 0.049; PFS, P = 0.030). Late relapses were seen.
T-cell lymphoma
Seventy eight out of 85 patients had peripheral T-cell lymphoma, and 56/78 were transplanted upfront in or according to the Nordic NLG-T-01 trial [20]; these patients showed good outcome (Fig. 3e) with 5-year OS at 75% (Table 2). The two-year OS/PFS in patients with peripheral T-cell lymphoma were 91%/78% if PET/CT-CR, 33%/33% if PET/CT-PR (Table 3). In contrast, CT could not predict outcome. Also in upfront transplanted peripheral T-cell lymphoma patients, PET/CT predicted OS (P = 0.005) and PFS (P = 0.045) but CT did not (P ≥ 0.5 for both comparisons).
Classical Hodgkin lymphoma
These 60 patients, all relapsed/refractory, showed excellent outcome (OS at 5 years, 83%; Table 2). However, refractory disease portended poorer survival than relapsed disease (Fig. 3f; OS, P = 0.002; LSS, P = 0.022; PFS, P = 0.021). In PET/CT-CR, 2-year OS/PFS were 100%/89% and in PET/CT-PR 75%/50%, but there were too few events to achieve statistical significance (Table 3).
Relapse after ASCT
The estimated fraction of relapses at 2 years were with PET/CT-CR 18%, PET/CT-PR 41%, CT-CR 29%, CT-PR 43%. Two hundred five out of 521 patients relapsed after ASCT, at a median time of 308 days. Of these 205 patients 118 died from lymphoma, 3 from treatment toxicity, 18 from other causes, while 66 stayed alive. Relapse was most common in indolent (53%), transformed (42%), and primary aggressive (44%) B-cell lymphoma. It was seen in 36, 35, and 25% of mantle cell, T-cell, and Hodgkin lymphoma. Five-year OS after relapse was 30%, and particularly poor in aggressive B-cell lymphoma (16%), but 51% in Hodgkin lymphoma. The 44 patients who underwent allogeneic transplantation for relapsed disease showed better survival than those who did not (5-year OS 63% vs 21%; P < 0.00005), particularly in aggressive or transformed B-cell lymphoma (5-year OS 70% vs 11%; P < 0.00005). No patient had undergone CAR-T cell therapy at last follow-up. Long-term adverse events in our patients, including myeloid disease, have been described previously [2].
Discussion
This population-based single-center study shows that outcome after ASCT has improved with increasing PET/CT use. PET/CT may verify metabolic remission, identifying patients with truly chemosensitive disease, who have excellent outcome with ASCT. Hence, BEAM followed by ASCT remains an effective treatment option in lymphoma.
Two-year OS and PFS in relapsed/refractory primary or transformed aggressive B-cell lymphoma were 60 and 52%. These figures are not inferior to those reported from the large CAR-T cell trials. In the axicabtagene ciloleucel trial, PFS was 42% at a median follow-up of 15.4 months [21]. In the tisagenlecleucel trial, the intention-to-treat analysis showed OS to be 40% at 1 year, and, in the patients who did proceed to receive CAR-T cell therapy, PFS was about 35% at 18 months [22]. Lastly, the lisocabtagene maraleucel trial showed a 1-year PFS of 44% [23]. Granted, these two modalities are not directly comparable, because only responders to chemotherapy proceed to ASCT and the follow-up times differ; on the other hand, many patients were excluded from therapy in the CAR-T cell trials because of disease progression between leukapheresis and CAR-T cell infusion, and the CAR-T numbers come from clinical trials, not real-world practice, although emerging real-world CAR-T data appear to be equally good [24]. Furthermore, CAR-T cells are vastly more useful than ASCT for patients with stable disease after salvage induction therapy, and, of course, for those who relapse after ASCT. However, with PET/CT, one may identify those patients with aggressive B-cell lymphomas who will benefit from ASCT consolidation: in relapsed/refractory patients with PET/CT-CR, 2-year OS/PFS was 87%/75%. This excellent outcome for PET-negative relapsed/refractory patients is not inferior to the outcome of patients who attained complete remission in the CAR-T cell trials (PFS between 65 and 85% at 1 year) [21–23]—again the comparison limps somewhat because of differences in remission status and patient selection (particularly chemosensitivity) prior to the respective procedure. In our material, it also appears that the small proportion of patients who only have one site of PET positivity might be cured by with ASCT followed by local irradiation or surgery conducted after the bone-marrow regeneration. Involved-field radiotherapy to PET-positive lesions prior to ASCT has been shown to be a successful approach [25]. In PET/CT-CR patients with peripheral T-cell lymphoma, 2-year OS/PFS after ASCT was 91%/78%. These figures compare favourably with other series, including one in which some of our patients participated [20, 26, 27]. Outcome in PET/CT-CR patients with Hodgkin lymphoma was excellent: 2-year OS/PFS 100%/89%.
In mantle cell lymphoma and indolent lymphoma, OS and PFS after ASCT were similar to previous reports, and ASCT remains part of standard therapy [19, 28]. However, late relapses do occur after ASCT; the 10-year PFS was in follicular lymphoma 31% and in mantle cell lymphoma 43%. We believe that CAR-T cells [29, 30] and bispecific antibodies [31, 32] might improve long-term outcome for these slow-growing entities.
In our patients, survival after ASCT improved from 2015, without any improvement of NRM, thus this is probably due to a better identification of patients who should and should not proceed to ASCT. Karolinska’s standard today is upfront ASCT in first remission for patients with transformed indolent B-cell, mantle cell, and peripheral T-cell lymphoma (except allogeneic SCT for hepatosplenic lymphoma), and ASCT in remitting relapsed/refractory aggressive B-cell lymphoma and (if first-line immunochemotherapy was < 2 years ago) for relapsed/refractory follicular and nodal marginal zone lymphoma. A PET/CT is done before every ASCT. Patients who have attained PET/CT-CR proceed to ASCT, having a good chance for cure in aggressive lymphoma (2-year PFS: relapsed/refractory aggressive B-cell lymphoma 75%, relapsed/refractory classical Hodgkin lymphoma 89%, peripheral T-cell lymphoma 78%). In the small number of patients with PET/CT-PR-local (a solitary active tumour), patients are given local irradiation after ASCT, with an acceptable cure rate (2-year PFS, 60%). Patients who do not attain PET/CT-CR or PET/CT-PR-local are considered for treatment with CAR-T cells or trials with bispecific antibodies, or, if not available, experimental salvage regimens to induce better remission status and then ASCT.
With ASCT, the real treatment for lymphoma is the preceding conditioning regimen. Several conditioning regimens exist. Between 2015 and 2019, we used BEAC as an alternative to BEAM because of melphalan shortages. The patients treated with BEAC showed inferior LSS, compared with those who received BEAM. It should be noted that Karolinska’s BEAM regimen, used throughout this 25-year period, has its own cytarabine schedule, with daily 400 mg/m2 12-h infusions, instead of the more common [33] two daily 200 mg/m2 1-h infusions. Infusion times correlate with cytarabine efficacy and toxicity, since the exposure to ara-C triphosphate (the active metabolite) is increased with longer infusions (cytarabine disappears from the plasma with a half-life of 7–13 min) [34, 35].
Conclusion
We conclude that an increasingly PET/CT-based selection of patients prior to ASCT has improved outcome after ASCT for lymphoma. For patients with PET/CT-CR, conditioning with BEAM followed by ASCT remains a strong standard treatment for several lymphoma entities. Further research is needed to identify the place of ASCT among other emerging salvage techniques.
Acknowledgments
The authors thank all patients, colleagues, and coworkers at the Hematology Unit, Karolinska.
Abbreviations
- ASCT
Autologous stem-cell transplantation
- CAR
Chimeric antigen receptor
- CR
Complete response
- CT
Computerized tomography
- LSS
Lymphoma-specific survival
- NRM
Non-relapse mortality
- OS
Overall survival
- PET/CT
Positron emission tomography/computerized tomography
- PFS
Progression-free survival
- PR
Partial response
- TRM
Treatment-related mortality
Authors’ contributions
BEW planned the study. KN, MC and BEW collected data. BEW analyzed data. KN, MC, KS, and BEW interpreted data. KN, MC, KS, and BEW (i.e., all authors) wrote and approved the manuscript.
Funding
This work was supported by Stockholm County Council (clinical research appointment), Cancerfonden, and Svenska Sällskapet för Medicinsk Forskning (SSMF). The funders had no role in the research or manuscript writing. Open Access funding provided by Karolinska Institute.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee, Stockholm (Ref no. 2012/783–31/3 with Amendments 2015/327–32 and 2016/2379–32). Consent was waived. No administrative permissions were required to access the raw data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuruvilla J. The role of autologous and allogeneic stem cell transplantation in the management of indolent B-cell lymphoma. Blood. 2016;127(17):2093–2100. doi: 10.1182/blood-2015-11-624320. [DOI] [PubMed] [Google Scholar]
- 2.Carlsten M, Jadersten M, Hellstrom A, et al. The Karolinska experience of autologous stem-cell transplantation for lymphoma: a population-based study of all 433 patients 1994-2016. Exp Hematol Oncol. 2019;8(1):7. doi: 10.1186/s40164-019-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance, Australasian Leukaemia and Lymphoma Group. Eastern Cooperative Oncology Group. European Mantle Cell Lymphoma Consortium. Italian Lymphoma Foundation. European Organisation for Research. Treatment of Cancer/Dutch Hemato-Oncology Group. Grupo Español de Médula Ósea. German High-Grade Lymphoma Study Group. German Hodgkin’s Study Group. Japanese Lymphorra Study Group. Lymphoma Study Association. NCIC Clinical Trials Group. Nordic Lymphoma Study Group. Southwest Oncology Group. United Kingdom National Cancer Research Institute Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Maertens J, Bormans G, Thomas J́, Balzarini J, de Wolf-Peeters C, Mortelmans L, Verhoef G. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102(1):53–59. doi: 10.1182/blood-2002-12-3842. [DOI] [PubMed] [Google Scholar]
- 5.Schot B, van Imhoff G, Pruim J, Sluiter W, Vaalburg W, Vellenga E. Predictive value of early 18F-fluoro-deoxyglucose positron emission tomography in chemosensitive relapsed lymphoma. Br J Haematol. 2003;123(2):282–287. doi: 10.1046/j.1365-2141.2003.04593.x. [DOI] [PubMed] [Google Scholar]
- 6.Alousi AM, Saliba RM, Okoroji GJ, Macapinlac HA, Hosing C, Korbling M, Samuels BI, Popat U, Kebriaei P, Anderlini P, Qazilbash MH, de Lima M, Giralt SA, Champlin RE, Khouri IF. Disease staging with positron emission tomography or gallium scanning and use of rituximab predict outcome for patients with diffuse large B-cell lymphoma treated with autologous stem cell transplantation. Br J Haematol. 2008;142(5):786–792. doi: 10.1111/j.1365-2141.2008.07277.x. [DOI] [PubMed] [Google Scholar]
- 7.Schot BW, Zijlstra JM, Sluiter WJ, et al. Early FDG-PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood. 2007;109(2):486–491. doi: 10.1182/blood-2005-11-006957. [DOI] [PubMed] [Google Scholar]
- 8.Derenzini E, Musuraca G, Fanti S, Stefoni V, Tani M, Alinari L, Venturini F, Gandolfi L, Baccarani M, Zinzani PL. Pretransplantation positron emission tomography scan is the main predictor of autologous stem cell transplantation outcome in aggressive B-cell non-Hodgkin lymphoma. Cancer. 2008;113(9):2496–2503. doi: 10.1002/cncr.23861. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson M, Hoyt R, Roberts AW, Grigg A, Seymour JF, Prince HM, Szer J, Ritchie D. Improved survival for relapsed diffuse large B cell lymphoma is predicted by a negative pre-transplant FDG-PET scan following salvage chemotherapy. Br J Haematol. 2010;150(1):39–45. doi: 10.1111/j.1365-2141.2010.08162.x. [DOI] [PubMed] [Google Scholar]
- 10.Devillier R, Coso D, Castagna L, Brenot Rossi I, Anastasia A, Chiti A, Ivanov V, Schiano JM, Santoro A, Chabannon C, Balzarotti M, Blaise D, Bouabdallah R. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin's lymphoma responding to prior salvage therapy. Haematologica. 2012;97(7):1073–1079. doi: 10.3324/haematol.2011.056051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roland V, Bodet-Milin C, Moreau A, Gastinne T, Mahé B, Dubruille V, Maisonneuve H, Juge-Morineau N, Moreau P, Jardel H, Planche L, Mohty M, Moreau P, Harousseau JL, Kraeber-Bodéré F, le Gouill S. Impact of high-dose chemotherapy followed by auto-SCT for positive interim [18F] FDG-PET diffuse large B-cell lymphoma patients. Bone Marrow Transplant. 2011;46(3):393–399. doi: 10.1038/bmt.2010.130. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz CH, Matasar MJ, Zelenetz AD, Nimer SD, Gerecitano J, Hamlin P, Horwitz S, Moskowitz AJ, Noy A, Palomba L, Perales MA, Portlock C, Straus D, Maragulia JC, Schoder H, Yahalom J. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665–1670. doi: 10.1182/blood-2011-10-388058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armand P, Welch S, Kim HT, LaCasce AS, Jacobsen ED, Davids MS, Jacobson C, Fisher DC, Brown JR, Coughlin E, Freedman AS, Chen YB. Prognostic factors for patients with diffuse large B cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation in the positron emission tomography era. Br J Haematol. 2013;160(5):608–617. doi: 10.1111/bjh.12176. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JB, Hall NC, Ruppert AS, Jones JA, Porcu P, Baiocchi R, Christian BA, Penza S, Benson DM, Jr, Flynn J, Andritsos LA, Devine SM, Blum KA. Association of pre-transplantation positron emission tomography/computed tomography and outcome in mantle cell lymphoma. Bone Marrow Transplant. 2013;48(9):1212–1217. doi: 10.1038/bmt.2013.46. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson E, Cao Q, Linden MA, Frolich J, Anand V, Burns LJ, Bachanova V. Hematopoietic cell transplantation for mantle cell lymphoma: predictive value of pretransplant positron emission tomography/computed tomography and bone marrow evaluations for outcomes. Clin Lymphoma Myeloma Leuk. 2014;14(2):114–121. doi: 10.1016/j.clml.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailly C, Carlier T, Berriolo-Riedinger A, Casasnovas O, Gyan E, Meignan M, Moreau A, Burroni B, Djaileb L, Gressin R, Devillers A, Lamy T, Thieblemont C, Hermine O, Kraeber-Bodéré F, le Gouill S, Bodet-Milin C. Prognostic value of FDG-PET in patients with mantle cell lymphoma: results from the LyMa-PET project. Haematologica. 2020;105(1):e33–e36. doi: 10.3324/haematol.2019.223016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn SY, Jung SY, Jung SH, Ahn JS, Lee JJ, Kim HJ, Kang SR, Han YH, Kwak JY, Yhim HY, Yang DH. Prognostic significance of FDG-PET/CT in determining upfront autologous stem cell transplantation for the treatment of peripheral T cell lymphomas. Ann Hematol. 2020;99(1):83–91. doi: 10.1007/s00277-019-03867-9. [DOI] [PubMed] [Google Scholar]
- 18.Kedmi M, Avivi I, Ribakovsky E, Benyamini N, Davidson T, Goshen E, Tadmor T, Nagler A, Avigdor A. Is there a role for therapy response assessment with 2-[fluorine-18] fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in mantle cell lymphoma? Leuk Lymphoma. 2014;55(11):2484–2489. doi: 10.3109/10428194.2014.882506. [DOI] [PubMed] [Google Scholar]
- 19.Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, Eriksson M, Nordström M, Kimby E, Boesen AM, Kuittinen O, Lauritzsen GF, Nilsson-Ehle H, Ralfkiær E, Åkerman M, Ehinger M, Sundström C, Langholm R, Delabie J, Karjalainen-Lindsberg ML, Brown P, Elonen E, for the Nordic Lymphoma Group Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic lymphoma group. Blood. 2008;112(7):2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, Holte H, Österborg A, Merup M, Brown P, Kuittinen O, Erlanson M, Østenstad B, Fagerli UM, Gadeberg OV, Sundström C, Delabie J, Ralfkiaer E, Vornanen M, Toldbod HE. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 21.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak Ö, Salles G, Maziarz RT. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 23.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, Sehgal A, Solomon SR, Ghosh N, Albertson TM, Garcia J, Kostic A, Mallaney M, Ogasawara K, Newhall K, Kim Y, Li D, Siddiqi T. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 24.Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607–5615. doi: 10.1182/bloodadvances.2020003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoppe BS, Moskowitz CH, Zhang Z, et al. The role of FDG-PET imaging and involved field radiotherapy in relapsed or refractory diffuse large B-cell lymphoma. Bone Marrow Transplant. 2009;43(12):941–948. doi: 10.1038/bmt.2008.408. [DOI] [PubMed] [Google Scholar]
- 26.Reimer P, Rüdiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, Engert A, Einsele H, Müller-Hermelink HK, Wilhelm M. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27(1):106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 27.Corradini P, Vitolo U, Rambaldi A, et al. Intensified chemo-immunotherapy with or without stem cell transplantation in newly diagnosed patients with peripheral T-cell lymphoma. Leukemia. 2014;28(9):1885–1891. doi: 10.1038/leu.2014.79. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Ubieto A, Grande C, Caballero D, et al. Autologous stem cell transplantation for follicular lymphoma: favorable long-term survival irrespective of Pretransplantation rituximab exposure. Biol Blood Marrow Transplant. 2017;23(10):1631–1640. doi: 10.1016/j.bbmt.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, McSweeney PA, Miklos DB, Pagel JM, Kersten MJ, Milpied N, Fung H, Topp MS, Houot R, Beitinjaneh A, Peng W, Zheng L, Rossi JM, Jain RK, Rao AV, Reagan PM. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Pender BS, Hawkins RM, Vakil A, Steinmetz RN, Riddell SR, Maloney DG, Turtle CJ. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood. 2019;134(7):636–640. doi: 10.1182/blood.2019000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster SJ, Bartlett NL, Assouline S, Yoon SS, Bosch F, Sehn LH, Cheah CY, Shadman M, Gregory GP, Ku M, Wei MC, Yin S, Kwan A, Yousefi K, Hernandez G, Li CC, O'Hear C, Budde LE. Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and is active in treatment through multiple lines. Blood. 2019;134(Supplement_1):6–6. doi: 10.1182/blood-2019-123742. [DOI] [Google Scholar]
- 32.Topp MS, Arnason J, Advani R, Brown JR, Allan J, Ansell S, O'Brien S, Chavez J, Duell J, Rosenwald A, Charnas R, Ambati SR, Adriaens L, Ufkin M, Zhu M, Li J, Gasparini P, Jankovic V, Fiaschi N, Zhang W, Hamon S, Thurston G, Murphy AJ, Yancopoulos GD, Lowy I, Sternberg D, Bannerji R. Clinical activity of REGN1979, an anti-CD20 x anti-CD3 BISPECIFIC antibody (AB) in patients (PTS) with (w/) relapsed/refractory (r/r) b-cell non-HODGKIN lymphoma (b-NHL) Hematol Oncol. 2019;37:90–92. doi: 10.1002/hon.58_2629. [DOI] [Google Scholar]
- 33.Ferme C, Mounier N, Divine M, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin's disease in relapse or failure after initial chemotherapy: results of the Groupe d’Etudes des Lymphomes de l’Adulte H89 trial. J Clin Oncol. 2002;20(2):467–475. doi: 10.1200/JCO.2002.20.2.467. [DOI] [PubMed] [Google Scholar]
- 34.Breithaupt H, Pralle H, Eckhardt T, von Hattingberg M, Schick J, Löffler H. Clinical results and pharmacokinetics of high-dose cytosine arabinoside (HD ARA-C) Cancer. 1982;50(7):1248–1257. doi: 10.1002/1097-0142(19821001)50:7<1248::AID-CNCR2820500705>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Stentoft J. The toxicity of cytarabine. Drug Saf. 1990;5(1):7–27. doi: 10.2165/00002018-199005010-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.