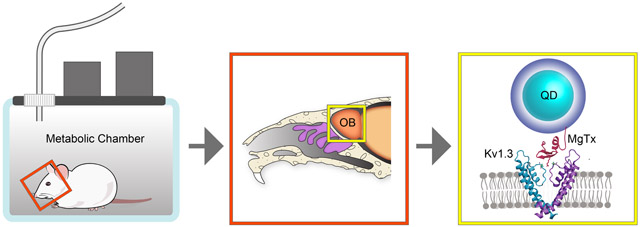
Abstract
The olfactory system is a driver of feeding behavior, whereby olfactory acuity is modulated by the metabolic state of the individual. The excitability of the major output neurons of the olfactory bulb (OB) can be modulated through targeting a voltage-dependent potassium channel, Kv1.3, which responds to changes in metabolic factors such as insulin, glucose, and glucagon-like peptide-1. Because gene-targeted deletion or inhibition of Kv1.3 in the periphery has been found to increase energy metabolism and decrease body weight, we hypothesized that inhibition of Kv1.3 selectively in the OB could enhance excitability of the output neurons to evoke changes in energy homeostasis. We thereby employed metal-histidine coordination to self-assemble the Kv1.3 inhibitor margatoxin (MgTx) to fluorescent quantum dots (QDMgTx) as a means to label cells in vivo and test changes in neuronal excitability and metabolism when delivered to the OB. Using patch-clamp electrophysiology to measure Kv1.3 properties in heterologously-expressed cells and native mitral cells in OB slices, we found that QDMgTx had a fast rate of inhibition, but with a reduced IC50, and increased action potential firing frequency. QDMgTx was capable of labeling cloned Kv1.3 channels but was not visible when delivered to native Kv1.3 in the OB. Diet-induced obese mice were observed to reduce body weight and clear glucose more quickly following osmotic mini-pump delivery of QDMgTx/MgTx to the OB, and following MgTx delivery, they increased the use of fats as fuels (reduced respiratory exchange ratio). These results suggest that enhanced excitability of bulbar output neurons can drive metabolic responses.
Keywords: potassium channel, margatoxin, RER, metabolism, obesity, olfaction
Graphical Abstract
The excitability of the major output neurons of the olfactory bulb (OB) can be modulated through targeting a voltage-dependent potassium channel, Kv1.3, to elicit changes in metabolism. We bound the Kv1.3 inhibitor margatoxin (MgTx) to fluorescent quantum dots (QDMgTx) as a means to label cells in vivo while enhancing neuronal excitability when delivered to the OB. Diet-induced obese mice had an improved metabolic health following osmotic mini-pump delivery of QDMgTx/MgTx to the OB, supporting that enhanced excitability of bulbar output neurons can act as a driver of fuel utilization, glucose homeostasis, and body weight set point.
1. INTRODUCTION
The olfactory bulb (OB) processes olfactory information relayed peripherally from the main olfactory epithelium to communicate to the brain concerning external chemical environment cued by odorant molecules (Kleene 2008; Antunes et al. 2014). The integrity of that information can be altered depending upon the nutritional state of the animal or individual, whereby fasting or excess nutrition can modify electrical signaling or the olfactory sensory structures in the OB (Palouzier-Paulignan et al. 2012; Aime et al. 2014; Thiebaud et al. 2014;Julliard et al. 2017; Fadool and Kolling 2020). One of the drivers of excitability in the major projection neurons of the OB, the mitral cells, is a voltage-gated potassium channel, Kv1.3. Action potential firing frequency, shape, and spike train periodicity is known to be driven by metabolic hormones and important energy factors that decrease Kv1.3 current amplitude, suggesting that the ion channel can differentially regulate excitability based upon nutritional status and energy homeostasis (Fadool et al. 2000; Marks and Fadool 2007; Marks et al. 2009; Tucker et al. 2010a; Tucker et al. 2010b; Tucker et al. 2013; Kovach et al. 2016; Thiebaud et al. 2016; Fadool et al. 2011; Palouzier-Paulignan et al. 2012; Julliard et al. 2017; Thiebaud et al. 2019; Fadool and Kolling 2020). Global gene-targeted deletion of Kv1.3 channel or pharmacological block of the channel peripherally in mice decreases body weight and reduces diet-induced obesity (DIO) (Xu et al. 2003; Fadool et al. 2004; Tucker et al. 2008; Tucker et al. 2012a; Tucker et al. 2012b; Upadhyay et al. 2013). Herein, our goal was to design a chemical probe to block Kv1.3 specifically in the OB, to determine if mice made obese through diet could lose body weight by changing excitability centrally.
We selected the pore-blocker margatoxin (MgTx) to inhibit Kv1.3 current flow and label cells expressing the channel because of its reported selectivity (Garcia et al. 1997; Garcia-Calvo et al. 1993; Knaus et al. 1995) and applications in the OB (Fadool and Levitan 1998; Colley et al. 2004; Mast and Fadool 2012; Schwartz et al. 2017). Luminescent quantum dots (QDs) were utilized due to their intense photo properties and ability to tune their interactions with biomolecules via modification of the QDs polymer ligand (Sundberg et al. 1974; Wang et al. 2016). QDs have unique optical and spectroscopic properties (Medintz et al., 2005; Mattoussi et al., 2012) that make them advantageous drug vectors for bioimaging. MgTx can be covalently conjugated to polyethyleneglycol (PEG)-modified Zn2+ quantum dots (QDs) and still retain ability to inhibit Kv1.3 (Schwartz et al. 2017), so that the conjugated molecule can be visually tracked in vivo. However, controlling the valence and orientation of MgTx on the conjugates is difficult via this strategy (Mout et al. 2012), and significant loss of material during the conjugation limits in vivo applications (Schwartz et al. 2017). We have now, alternatively, produced recombinant MgTx with an N-terminal polyhistidine tag that allows self-assembly of a controlled number of peptides per QDs. This approach has previously been reported to allow conjugation of a variety of biomolecules to both QDs and gold nanoparticles and can be attributed to the strong affinity of histidine for metal rich surfaces (Sundberg and Martin 1974; Goldman et al. 2005a; Goldman et al. 2005b; Anikeeva et al. 2006; Medintz et al. 2006a; Medintz et al. 2006b; Sapsford et al. 2007; Aldeek et al. 2013a; Aldeek et al. 2013b; Wang et al. 2015; Wang et al. 2016b). Compared to covalent conjugation, self-assembly driven by metal-His coordination is highly efficient and prevents significant loss of material while providing control over the number and orientation of biomolecules bound to the QD surface (Medintz et al. 2003; Medintz et al. 2004; Medintz et al. 2006a; Medintz et al. 2006b; Goldman et al. 2005a; Goldman et al. 2005b; Anikeeva et al. 2006; Wang et al. 2007; Wang et al. 2016a).
Following production of MgTx with an N-terminal polyhistidine tag (HisMgTx) and conjugation of this peptide to QDs using polyhistidine self-assembly (QDMgTx), we electrophysiologically measured the bioconjugate’s ability to inhibit Kv1.3 in a heterologous expression system. We then tested its ability to increase excitability by blocking the native channel in mitral cell neurons of the OB. Due to our ability to synthesize the bioconjugate in sufficient quantity for in vivo applications, we delivered HisMgTx and QDMgTx to DIO mice using osmotic mini-pumps cannulated into to the OB such that we could measure the ability of the inhibitor to modulate whole-body metabolism and confirm targeted inhibition of Kv1.3 in the OB. Block of Kv1.3 in the OB in the obese mice caused a reduction in body weight, modified fuel utilization, and altered ingestive behaviors. These results provide evidence that modulating OB physiology, centrally, through targeting Kv1.3 in the brain can change energy utilization and eating behaviors.
2. MATERIALS AND METHODS
Our study design was not pre-registered as a clinical trial.
2.1. Ethical approval
All animal experiments were approved by the Florida State University (FSU) Institutional Animal Care and Use Committee (IACUC) under protocol #1427 and were conducted in accordance with the American Veterinary Medicine Association (AVMA) and the National Institutes of Health (NIH). For preparation of OB slices, mice were anaesthetized with isoflurane (Aerrane, Baxter, Deerfield, IL) using the IACUC-approved drop method and were then sacrificed by decapitation (AVMA Guidelines on Euthanasia, June 2007).
2.2. Animal care
All mice (C57BL/6J background strain, RRID:IMSR_JAX:000644, The Jackson Laboratory, Bar Harbor, ME) were housed individually in conventional style open cages at the Florida State University vivarium on a standard 12 h/12 h light/dark cycle and were allowed ad libitum access to 5001 Purina Chow (Purina, Richmond, VA) or a moderately-high fat diet (MHF, Cat. #D12266B, Research Diets, 32% fat, 17% protein, 51% carbohydrate) and water. Due to need for metabolic assessment and surgical recovery, all mice were individually housed and provided with two sources of enrichment – a house and a nestlet. Postnatal day (P) 15-30 mice of both sexes were used for electrophysiology. Mice that were used for metabolic assessment were maintained on MHF-diet upon weaning (P24) for an average of 7.5 to 8 months that was not significantly different between treatment groups (Control = 240 ± 20 days, MgTx = 232.5 ± 19 days, and QDMgTx = 232.2 ± 21 days; one-way ANOVA, F(2,19) = 0.01547, p = 0.9847). Assignment of animals to a treatment group was arbitrary and the mean body weight for a treatment group following induction of obesity and prior to surgery was not significantly different (Control = 37 ± 1.6 g, MgTx = 35.1 ± 1.5 g, and QDMgTx = 39.2 ± 1.7 g; one-way ANOVA, F(2,19) = 1.411, p = 0.2682). Because experiments were designed to test the metabolic effect of QD conjugates following diet-induced obesity, only male mice were used in these studies given our previous report that female mice do not gain adiposity or body weight (Tortoriello et al. 2004; Tortoriello et al. 2007). A total of 55 mice were used in our study. Our experiments were exploratory and the study design did not contain pre-determined exclusion criteria. Four mice were excluded in the study’s results (one was excluded as a statistical outlier (2-sided Grubbs’ test), two developed dermatitis due to oily fat diet, and one did not survive surgery) and an additional five mice were used to optimize placement of the osmotic mini-pump using stereotactically-guided surgery. The number of subjects per experiments are specified in the figures and figure legends. Animal subjects were not randomized, rather assignment of animals to a treatment group was arbitrary – the surgeon blindly selected which pump to implant while a separate investigator loaded and retained the knowledge of pump contents. Each of the pumps (PBS control, HisMgTx, and QDMgTx) had an equal chance of being selected. Animals were metabolically screened in cohort sizes of four because this number of surgeries was achievable in a day’s duration. Each day contained mouse surgery using all treatment groups so that data collection was not biased.
2.3. Solutions and reagents
All salts and sugars were purchased from Sigma-Aldrich (St. Louis, MO) or ThermoFisher Scientific (Waltham, MA). Solutions used for expression and purification of the His-conjugated margatoxin peptide (HisMgTx) included Luria broth (LB, Cat. #244620, BD Biosciences, Sparks, MD) supplemented with 1% glucose, 0.5 M isopropyl β-D-1-thiogalactopyranoside (IPTG, #5800, EM Science, Gibbstown, NJ), equilibration buffer (25 mM Tris, 300 mM NaCl), 50 mM EDTA, 100 mM NiSO4, and 300 mM imidazole. Phosphate-buffered saline (PBS) was used for conjugation (pH 8.0), prepared as previously described (Biju et al. 2008). For whole-cell electrophysiology experiments in HEK293 cells, the bath solution consisted of (in mM): 150 NaCl, 5 KCl, 2.6 CaCl2, 2 MgCl2, and 10 Hepes, pH 7.4; 305 mOsm. The intracellular pipette solution consisted of (in mM): 145 KCl, 10 HEPES, 10 EGTA, 2 MgCl2, 3 NaATP, 0.4 GTP, pH7.4; 295 mOsm. The artificial cerebrospinal fluid (ACSF), sucrose-modified ACSF, and intracellular pipette solution for slice electrophysiology experiments were carefully monitored for osmolarity and prepared as previously reported (Fadool et al. 2011; Tucker et al. 2013; Thiebaud et al. 2016).
2.4. cDNA constructs
All Shaker channel coding regions were downstream from a cytomegalovirus promoter. Rat Kv1.3 (rKv1.3) was subcloned into the multiple cloning region of pcDNA3 (now available only as 3.1; RRID:Addgene_23252; originally 3.0 Invitrogen, Carlsbad, CA) at the unique HindIII restriction site as previously described (Holmes et al. 1996). DNA encoding human CD8 was amplified from pCDM8 (RRID:Addgene_11588) and subcloned into the pcDNA3 vector between the BamH1 and EcoR1 restriction sites (Mast et al. 2010). DNA encoding MgTx was subcloned into a PET-28b vector (Cat. # 69865-3, EMD Biosciences, San Diego, CA) containing a His6 tag on the N-terminus.
2.5. Experiment 1. Quantum dot (QD) synthesis and conjugation to margatoxin (MgTx)
2.5.1. QD growth and phase transfer
The luminescent CdSe-ZnS core-shell quantum dots (QDs) used in this study had an approximate diameter of ~6 nm and emitted in the green region of the visible spectrum (peak at 533 nm) (Yu and Peng 2002; Clapp et al. 2006). These nanocrystals were grown stepwise, starting with the core, followed by ZnS-overcoating, using high temperature reduction of cadmium, selenium, zinc, and sulfur precursors in coordinating solvent mixtures made of alkylphosphines, alkyphosphine-carboxyl, and alkylamines, following previous protocols (Clapp et al. 2006). The native capping ligands, made of a mixture that included trioctylphosphine/triocylphosphineoxide (TOP/TOPO) and alkylphosphonic acid, were exchanged with poly(isobutylene-alt-maleic anhydride) (PIMA) based polymeric ligand presenting several imidazole and zwitterionic moieties (His-PIMA-ZW) to promote dispersion of the QDs in buffer media and allow conjugation to biomolecules. Additional details on the phase transfer, conjugation steps, and purification of the conjugate can be found in previous reports (Wang et al. 2015; Wang et al. 2016a). The final concentration of the QD dispersion was determined from the absorbance at 350 nm, using an extinction coefficient of 2.467 × 10−6 M−1cm−1 for the QDs (Leatherdale et al. 2002).
2.5.2. Recombinant expression of HisMgTx
His-conjugated margatoxin (HisMgTx) was sublconed into the multiple cloning region of PET-28b+ vector (EMD Biosciences, Cat. #69865-3, San Diego, CA) using NdeI and XhoI restriction sites (Addgene Vector Database). The construct was transformed into BL21 SHuffle T7 Express Escherichia coli (New England BioLabs, Cat.#C3029J, Ipswitch, MA). The PET-28b vector included an N-terminal His6 tag, which allowed for affinity purification and conjugation to QDs and a thrombin cleavage site thrombin cleavage site (underlined) between the His6 tag and the MgTx coding sequence (MGSSHHHHHH-SSGLVPRGS-HMTIINVKCTSPKQCLPPCKAQ FGQSAGAKCMNGKCKCYPH). BL21 SHuffle T7 Express E. coli contain an oxidizing cytoplasm that promotes proper disulfide bond formation in HisMgTx. Transformed E. coli were grown in LB with 1% glucose at 37°C to OD600 = 0.8 while shaking at 250 RPM, then expression was induced with 0.5 mM IPTG at 16°C while shaking at 250 RPM (Figure 1a). Following overnight expression, bacterial cells were lysed using a microfluidizer (110L, Microfluidics, Newton, MA), then HisMgTx was purified from the clarified lysate using nickel affinity chromatography (Gold Biotechnology, St. Louis, MO, #H-320-50) followed by reversed-phase high performance liquid chromatography (RP-HPLC), as shown in Figure 1b. RP-HPLC (Shimadzu Prominence HPLC, Kyoto, Japan) was performed with a C18 analytical column (Cat. #651286-8, Phenomenex, Torrence, CA) using a flow rate of 1 ml/min and a gradient of 15-30% solvent B (0.075% trifluoroacetic acid (TFA) in 90% acetonitrile) in solvent A (0.1% TFA in water) over 50 min. HisMgTx was detected from peptide absorbance at 220 nm. The monoisotopic mass of the purified recombinant HisMgTx determined using mass spectrometry was 6475.9 kDa (Figure 1c). The purified HisMgTx was quantified using a Qubit Fluorimeter (Model #Q32857, ThermoFisher Scientific) then lyophilized (Model #MODULYOD-115, ThermoFisher Scientific) and stored at −20°C. Prior to use, the peptide was reconstituted in PBS with 0.05% bovine serum albumin (BSA).
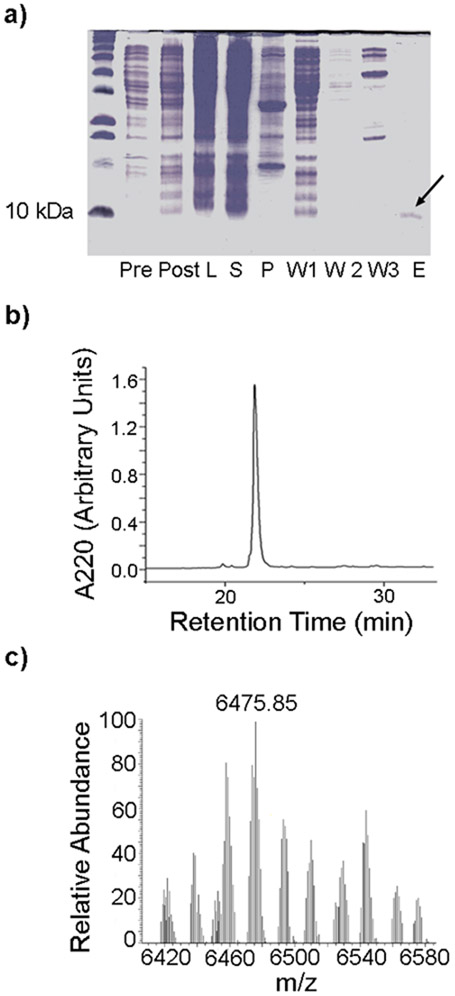
FIGURE 1.
Purification of HisMgTx using T7 Shuffle Express E. coli. a) 20% acrylamide gel illustrating various stages in the expression and purification of recombinant HisMgTx. Pre = pre-induction sample, Post = post induction sample, L = cell lysate, S = supernatant, P = pellet, W1 = wash 1 from affinity column, W2 = wash 2 from affinity column, E = elution with 50 mM imiadzole. Arrow = eluted protein product. b) RP-HPLC chromatogram with single peak at ~23 min retention time corresponding to recombinant HisMgTx. c) LC-MS/MS spectrum of purified HisMgTx, showing a monoisotopic mass of 6475.85 Da.
2.5.3. Preparation of QDMgTx
HisMgTx was conjugated to QDs (referred to as QDMgTx) by mixing the peptide with His-PIMA-ZW capped QDs in PBS followed by incubation at 4° C for 1 h. Addition of the His6 tag to the N-terminus of MgTx allowed facile binding to Zn2+ due to high affinity of histidine for Zn2+. Altered electrophoretic mobility of QDMgTx in comparison to non-conjugated QDs on a 0.8% agarose gel confirmed successful interaction of HisMgTx with the QDs (Figure 2). For the agarose gel experiments, several QDMgTx dispersions were prepared where the molar ratio of QD:HisMgTx varied from 1:10 to 1:60. QD concentration was maintained at 100 nM. For all cell labeling, electrophysiology, and animal studies, the QDMgTx was prepared at molar ratio of 1:15 and concentrations described for QDMgTx are with respect to the QD.
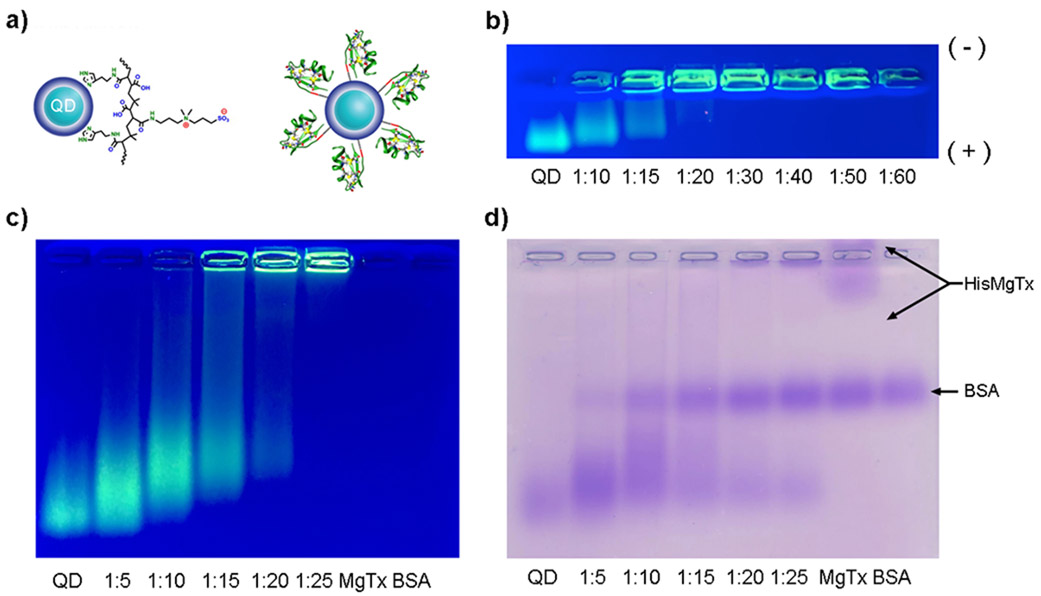
FIGURE 2.
Conjugation of HisMgTx to green CdSe-ZnS core-shell QDs. These QDs were modified with a polyimidazole-based zwitterionic ligand containing the short chain poly(isobutylene-alt-maleic anhydride) (His-PIMA-ZW). a) Schematic representation of a QD modified with His-PIMA-ZW (Wang et al., 2016b) and of the same QD with HisMgTx bound to its surface. b) 0.8% agarose gel confirming HisMgTx binding to QDs (20 min run time). As a larger ratio of HisMgTx is bound to the surface, the constructs’ mass increases. [QD] = 100 nM. (+) and (−) indicate positive and negative poles of gel. c) Same as b, however the sample was electrophoresed for longer (70 min) and includes HisMgTx (MgTx = HisMgTx) and BSA (carrier protein for HisMgTx). d) Coomassie stain of c, to probe the presence of peptide not bound to QDs following conjugation (MgTx = HisMgTx).
2.6. Experiment 2. Using heterologously-expressed Kv1.3 channels to test QD targeting and function
2.6.1. Maintenance and transfection of HEK293 cells
We used Human Embryonic Kidney Cells (HEK293; RRID:CVCL_0045) that are not listed as a commonly misidentified cell line at the International Cell Line Authentication Committee Registry (ICLACR). Our HEK293 cells were contributed by Dr. Robert Margolski (Monell Chemical Senses Center, Philadelphia, PA) and have been authenticated for cross-contamination and misidentification by profiling short tandem repeats. Moreover, cell culture practices at Florida State University have followed “Guidance on Good Cell Culture Practices (GCCP)”(Coecke et al. 2005). Our last cryopreserved batch of HEK293 cells was prepared in February of 2016 and each vial is experimentally used between passages 4 and 25. HEK293 cells were grown in Modified Eagle’s Medium (MEM, Cat. #12360-038, ThermoFisher Scientific/Gibco) supplemented with 2% penicillin/streptomycin (Cat. #P0781, Sigma) and 10% fetal bovine serum (FBS, Cat. #16000-044, ThermoFisher Scientific/Gibco). Plating to low density and transfection procedures were as previously described (Cook and Fadool 2002). Briefly, when cells reached 70–80% confluency they were transiently co-transfected with 0.3 μg Kv1.3 and 0.2 μg pCDM8 cDNAs for 4 h using lipofectamine (Cat. #18324–012, ThermoFisher Scientific/Invitrogen) in serum-reduced media (OptiMEM, Cat. #31985-070, ThermoFisher Scientific/Gibco). Including cDNA encoding the human CD8 transmembrane protein permitted panning for transfected cells by labeling cells with a red polypropylene-antibody-linked bead prior to patch-clamping (Mast and Fadool 2012). The investigator was not blind to which cDNA was transfected into the HEK 293 cells.
2.6.2. Electrophysiology of HEK 293 cells
Thirty-six hours post transfection, cells were incubated with Dynabeads® CD8 microparticles (Cat. #11147D, Thermo Fisher Scientific /Gibco) for 2 min to visualize channel-transfected cells (Mast and Fadool 2012). Hoffman modulation contrast optics was used to visualize cells at 40× magnification (Axiovert 135, Carl Zeiss, Thornwood, NY). Patch electrodes were fabricated from Sutter glass (BF150-86-15, Sutter Instrument Co., Novato CA) using a vertical puller (Model PP-830, Narishige, Tokyo, Japan) to achieve pipette resistances of 7-10 MΩ. Outward currents were recorded at room temperature (rt; 20–22°C) as acquired from the whole-cell configuration using an Axopatch 200B patch-clamp amplifier (Molecular Devices/Axon Instruments, Sunnyvale, CA). All voltage signals were generated and data acquired with the use of an Axon Digidata 1200 A/D converter in conjunction with pClamp v10.3 software (Molecular Devices/Axon Instruments, Silicon Valley, CA). Recordings were filtered at 2 kHz and digitized at 2–5 kHz. Typically, cells were held (Vh) at −90 mV and stepped to a depolarizing potential of +40 mV (Vc) using a pulse duration (Pd) of 250 ms. Cells were stimulated with an interpulse interval (IPI) of 45 s to prevent cumulative inactivation, a property of Kv1.3 channels (Marom and Levitan, 1993; Kupper et al., 1995; Cook and Fadool, 2002). HisMgTx, QDs, or QDMgTx were applied directly to the bath to measure change in Kv1.3 current properties in response to peptide inhibitor, nanoparticle, or conjugate.
2.6.3. Nano-labeling of Kv1.3 channel in HEK293 cells
HEK293 cells were grown on 12 mm glass coverslips (Cat. #12-545-90, Thermo-Fisher Scientific) and were transfected with Kv1.3 or pcDNA3 cDNA at 70–80% confluency. At 48 h post transfection, cells were washed twice with HEK293 bath solution to ensure that channels were in an open state so that QDMgTx could bind the open pore of the channel. Cells were then incubated with the QDMgTx conjugate in PBS (1:15; 50 nM QD, 750 nm HisMgTx) for 30 min, permeabilized with 0.2% Triton X-100 in PBS (Cat. #9002-93-1, Thermo Fisher Scientific) for 10 min, fixed with freshly prepared 4% paraformaldehyde in PBS (Thermo-Fisher Scientific, Cat. #BP531-25) for 10 min, and then nuclear stained with 4′,6-diamidino-2-phenylindole (DAPI, Cat. #D1306, Thermo-Fisher Scientific). All incubations were performed at rt. Cells were rinsed with PBS twice between each step. Coverslips were mounted to glass slides for imaging using Fluoromount-G mounting media (Cat. #0100-01, Southern Biotech, Birmingham, AL). Slides were viewed by light and fluorescent microscopy (Axioert S100, Zeiss, Oberkochen, Germany) and signals were detected from the emissions of DAPI (340–380 nm excitation /435–485 nm emission) and QDs (532–587 nm excitation /608–683 nm emission). Individual digital images were acquired using a Zeiss Axiocam digital camera and AxioVision software, then were overlaid using Adobe Photoshop (Adobe Systems, San Jose, CA). Fluorescence intensity was determined using ImageJ (NIH, Bethesda, MD) using a protocol described by Luke Hammond at The University of Queensland (https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html), whereby the mean gray value (fluorescent intensity) was measured across a uniform region of interest (ROI) and the corrected total cell fluorescence (CTCF) was determined as CTCF = integrated density – (ROI area x mean fluorescence of background readings). Data were averaged over a sample of five sets of transfected HEK293 cells.
2.7. Experiment 3. QD functional effect on olfactory bulb using ex vivo slice electrophysiology
P21–P35 C57Bl/6J mice were anesthetized by inhalation of isoflurane (see Ethics section) and quickly decapitated, then the OBs were exposed by removing the dorsal and lateral portions of the skull between the lambda suture and cribriform plate. The OBs were harvested and 300-μm coronal sections prepared using ice-cold oxygenated sucrose-modified ACSF solutions during vibratome sectioning (Vibratome/Leica Model 1000, Wetzlar, Germany), as previously described (Fadool et al. 2011; Thiebaud et al. 2016). Then slices were allowed to recover in an interface chamber containing oxygenated ACSF ((Krimer and Goldman-Rakic 1997) for 20–30 min at 33°C and then were maintained at room temperature for 60 min before recording. Recordings from OB slices were made at rt using a continuously-perfused (Ismatec; 1 ml/min, Wertheim, Germany), submerged-slice recording chamber (RC-26, Warner Instruments, Hamden, CT) with ACSF. Slices were visualized at 10× and 40× using an Axioskop 2FS Plus microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY) equipped with infrared detection capabilities (Dage MT1, CCD100, Michigan City, IN). Electrodes were fabricated from borosilicate glass (Hilgenberg Cat. #1405002, Mansfield, Germany) to a pipette resistance of 4–7 MΩ. Following determination of spike threshold, cells were stimulated with a long, perithreshold current step (typically ranging from 5 to 50 pA) for 5000 ms every 10 s to acquire spike frequency data under ASCF conditions (Control). After recording for a minimum of 10 min, the bath was switched to QDs in ASCF (QD) and spike firing frequency was measured using the same current injection for 10 min. Finally, the bath was switched a third time to apply QDMgTx to the slice and the spike firing frequency was again measured for a minimum of 10 min. Final working concentration of QDMgTx added to the bath for these slice experiments was 10 nM.
2.8. Experiment 4. QDMgTx/HisMgTx effect on metabolism determined using Comprehensive Laboratory Animal Metabolic System (CLAMS)
2.8.1. Osmotic mini-pump surgery
Mice were weaned at P24 to MHF diet for a mean of 7.5-8 months prior to surgical placement of an osmotic mini-pump to deliver QDMgTx/HisMgTx or control treatments. We assembled cannulated Alzet micro-osmotic mini-pumps (Cat. #1002, Durect Corporation, Cupertino, CA) to allow for continuous delivery at a rate of 0.25 μl per hour over a 14-day period. The mini-pumps were filled with control PBS (2% BSA), 375 nM HisMgTx, 25 nM QD, or 25 nM QDMgTx 1:15 diluted PBS (2% BSA), and were prepared per manufacturer’s protocols the evening prior to surgeries. Animals were anesthetized with a continuous flow of isofluorane and were given 0.1 mg/kg buprenorphine HCl (Cat. #NDC42023-179-01, PAR Pharmaceuticals, Woodcliff Lake, NJ) for pain management. Mice were restrained in a Stoelting/Kopf Instruments stereotaxic apparatus (Chicago, IL) and two holes were drilled (Stoelting, micromotor high-speed drill model 51449) into the skull of the animal above the OB at the mitral cell layer coordinates (AP – 0.25 mm from bregma, M/L +/− 0.075 and D/V − 2.25 mm from dura). Cannula (Cat. #3280PD-1.5-SPC OP CONN28 gauge DBL, Plastics One, Roanoke, VA) were bilaterally inserted (inter-cannula distance = 1.5 mm; cannula depth = 2.25 mm) into the holes and then a fitted Y connector (21Y connector Cat. # 37200 81003720001F, Plastics One) delivered the pump contents to the cannula. The mini-pump itself was inserted in a pocket created under the skin on the back of the animal. The cannula were adhered to the skull using cement and the skin was closed on the back and neck using surgical staples (Clay Adams wound clips Cat. # 427631, 9 mm, Becton Dickinson, Sparks MD). Mice were closely monitored on a heating pad in their home cage while recovering from initial anesthesia and were administered a second dose of buprenorphine HCl under anesthesia during the first 12 h of recovery. Mice were allowed to fully recover for 24 h in the Comprehensive Laboratory Metabolic System (CLAMS; Columbus Instruments, Columbus, OH; RRID:SCR_016718) home cages, where they were provided moistened food out of the hopper on the cage floor.
Following CLAMS assessment, mice were histologically prepared to confirm cannula placement. Briefly, mice were anesthesized with a ketamine/xylazine mix (80 mg/kg and 10 mg/kg) to reach anesthetic plane 2, and then sacrificed via intracardial perfusion with PBS and 4% paraformaldehyde (PFA, Cat. #411678, ThermoFisher Scientific). The skulls were postfixed in 4% PFA/PBS overnight and decalcified for 6–9 days in 0.3 M EDTA (Cat. #5311, ThermoFisher Scientific). Extracted brains were cryoprotected in 10% then 30% sucrose/PBS at 4°C before being frozen in OCT compound-embedding medium (Cat. #4585, Fisher Healthcare). Frozen tissue was stored at −80°C until day of cryosectioning. Cryosectioning was performed on a LEICA model CM1850 cryostat (Leica Microsystems, Wetzlar, Germany) to yield OB coronal sections at 16-μm thickness that were transferred to 1% gelatin-coated Superfrost slides (Cat. #48311, VWR International, Radnor, PA) and stored at −20°C. OB sections were stained with cresyl violet as previously (Fadool et al. 2000), then viewed by light microscopy (Zeiss Axiovert, Model #S100) to confirm cannula precision.
2.8.1. Glucose tolerance, indirect calorimetry and metabolic assessment
The experimental workflow of the glucose tolerance testing, CLAMS metabolic assessment, and surgical implantation of osmotic mini-pumps is diagramed in Figure 6a. The strategy was to perform an intraperitoneal glucose tolerance test (IPGTT) prior and subsequent to drug delivery and allow mice to acclimate to the CLAMS chambers prior to surgery to achieve a baseline, thereby collecting pre and post metabolic parameters in response to the QD drug delivery within subjects. For the IPGTT, mice were fasted during the dark phase for 12 h and then tail blood samples were acquired to monitor clearance of serum glucose over time in response to 2.5 μg/ml glucose per kg body weight (University of Virginia Vivarium Protocols, Susanna R. Keller). Glucose levels were measured using an Ascensia Contour Blood Glucose Monitoring System (Ascensia Diabetes Care US, Inc., Parsippany, NJ).
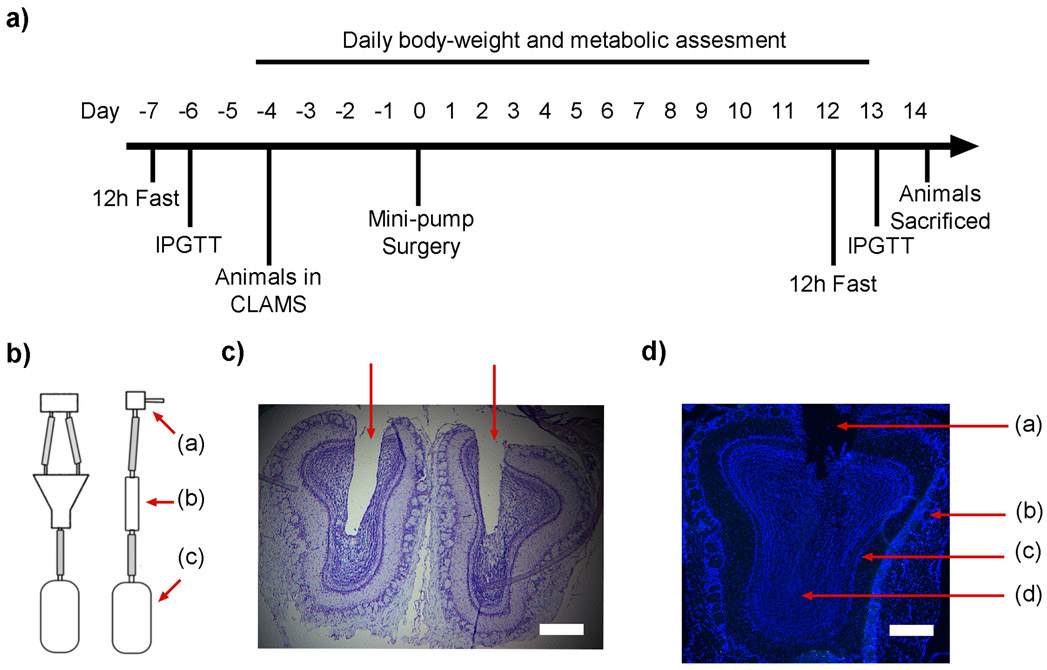
FIGURE 6.
Cannula-guided osmotic mini-pump delivery of QDMgTx to the OB. a) Schematic of experimental timeline. CLAMS = comprehensive laboratory animal metabolic system, IPGTT = intraperitoneal glucose tolerance test. b) An Alzet osmotic mini-pump (c) was connected to bifurcated (b) cannula (a) and implanted into the OB of C57J/B6 obese mice using stereotactic guidance. c) Photomicrograph of a pair of OBs confirming placement of the bifurcated cannula; bright field, cresyl violet stain. d) Photomicrograph of an OB following incubation with 25 nM QDMgTx; DAPI stained, (a) = cannula placement, (b) = glomerular cell layer, (c) = mitral cell layer, (d) = granule cell layer; c) and d) scale bar = 5 μm.
Metabolic parameters, oxygen consumption (VO2; ml/kg/min), carbon dioxide production (VCO2; ml/kg/min), respiratory exchange ratio (RER), energy expenditure (EE; kcal/h), locomotor activity, and caloric and water intake were measured using the CLAMS system (Bell and Fadool 2017; Fadool and Kolling, 2020). Mice had ad libitum access to food and water in overhead feeders attached to electronic balances that could detect both disturbance (meal duration) and decrease in food mass (meal size). The design of the feeder (Cat. # CLAMS – HC, Homecage) allows placement of the food in a familiar location, and in pellet form, so that there is a catch tray that accounts for weight of crumbs with minor spillage. VO2 and VCO2 were normalized with respect to body weight in kilograms. RER was calculated as VCO2/VO2. EE was calculated according to the Lusk Equation (3.815 + 1.232 x RER) x VO2 (Lusk 1924). Further information concerning the calculation of these metabolic variables by the CLAMS can be found online as a helpful resource (https://www.slideshare.net/InsideScientific/measuring-energy-balance-in-mice-from-vo2vco2-food-intake-and-activity-data). Locomotor activity was continuously recorded using optical beams along the x-axis of the cage so that consecutive photo beam breaks could be scored as ambulatory movement. All data were recorded in intervals using Oxymax software (CLAX; Columbus Instruments) and each interval measurement represented the average value during a 30 s sampling period per cage. In total, mice were housed in the CLAMS for 19 days (−4 to +14; see Figure 6a). No data were acquired in the CLAMS during the first two days in the chambers (−4 to −2) to permit acclimation to the environment or during the 24 h recovery from surgery (0 to 1). The 48 h after acclimation were used for baseline determination (−2 to 0) and this average was used to normalize data acquired for individual mice for the 11 days subsequent to drug delivery (1 to 11). Data are graphed in a normalized fashion in order to visually compare the response of drug treatment across mouse subjects for a particular metabolic variable, and non-normalized data were used in a paired-subject analysis (repeated measure) as described in the statistical analyses below.
2.9. Data and statistical analyses
Statistical analyses were performed using Prism 8 software (Graph Pad, La Jolla, CA). This software tests for normality using the D'Agostino-Pearson normality test (omnibus K2). Significantly-different means were calculated at the 95% confidence level or α ≤ 0.05, unless otherwise specified. Sample sizes of our studies were determined by A Priori Type Power Analysis applied for ANOVA and t-test, and tested for a range of estimated means and SD for biophysical and metabolic parameters that were adequate for a power of 0.80, α ≤ 0.05, G*Power Cohen ranges f ≤ 0.68 - 0.81.
2.9.1. Experiment 2a.
In order to quantify the fluorescent intensity of QDMgTx binding to Kv1.3-transfected HEK293 cells, images at 40X magnification were analyzed and averaged across a total of five different transfection rounds. A F-max test for homogeneity of variance was performed and then a Student’s t-test was applied to determine if there were significantly-different means in the fluorescent intensity of mock transfected cells (pcDNA3 alone) versus that of Kv1.3 transfected cells.
2.9.2. Experiment 2b.
Electrophysiological records were analyzed using Clampex v10.6 software (Molecular Devices/Axon Instruments), Origin v8.0 (Microcal, Borland International, Scotts Valley, CA), and Excel v2013 (Microsoft Office, Redmond, WA). HEK 293 cells were allowed to stabilize for 3–5 sweeps after achieving the whole-cell configuration. Following stabilization (taken as Time 0), peak current amplitude was measured (Ii, initial current), and then normalized as In/Ii (In, current at time n) in order to compare change in peak current amplitude prior and subsequent to peptide inhibitor or QD conjugate channel interaction. For generation of the concentration-response curves, the I5/Ii was used to measure the drop in current amplitude for concentrations from 101 to 104 pM from a mean of 4–6 recordings at each tested concentration. In order to observe change in peak current amplitude and time course of inhibition, Kv1.3 peak current amplitude was measured prior to and following application of HisMgTx, QD, or QDMgTx. Changes in peak current amplitude (I5/Ii) in the presence of the peptide inhibitor, nanoparticle, or conjugate at the fifth time point (T5), 225s, compared with that at the start of the recording (T0) were statistically analyzed using non-normalized data in a two-way mixed-design analysis of variance (ANOVA) using time and treatment as factors, with a Bonferroni’s post-hoc test. The sample size (N) noted in the figure legend represents number of cells recorded that were sampled across a minimum of three transfection batches. In mitral cells, changes in action potential firing frequency were tested for statistical significance using a repeated measure, one-way ANOVA and Bonferroni’s post-hoc test. The sample size (N) noted in the figure legend represents the number of cells recorded that were recorded across three mice. Changes in metabolic variables (VO2, RER, IPGTT, bodyweight) were compared using non-normalized data in a two-way, mixed design, repeated-measures ANOVA using time and treatment as factors, followed by a Dunnett’s post-hoc test. Differences in iAUC were statistically analyzed using an ordinary one-way ANOVA with a Dunnett’s post-hoc test. All reported values are mean ± standard error of the mean (SEM) with indicated sample size (N) being the number of mice.
3. RESULTS
3.1. QD synthesis and conjugation to MgTx
3.1.1. Production of recombinant HisMgTx
MgTx was previously produced via recombinant expression in the bacterium E. coli and the yeast Pichia pastoris (Garcia-Calvo et al. 1993;Anangi et al. 2012). We explored production via P. pastoris and chemical synthesis, and constructs prepared using these methods were used for preliminary experiments. However, recombinant expression in E. coli proved to be the most efficient method for our purposes. Here, recombinant HisMgTx was produced using a PET-28b vector transformed into BL21 SHuffle T7 Express E. coli. The PET-28b vector enabled expression of MgTx with an N-terminal His6 tag, which was used for affinity purification and QD binding. An acrylamide gel summarizing the expression and affinity purification is shown in Figure 1a, and the affinity-purified product was purified to homogeneity using C18 RP-HPLC (Figure 1b). The mass of HisMgTx estimated from the gel is higher than expected (~9 kDa), likely due its high isoelectric point (pI 9.46) reducing its electrophoretic mobility. The mass of the peptide determined using LC-MS/MS (Figure 1c) was found to be equivalent to the predicted mass (experimental = 6475.85 Da, predicted = 6473.53 Da).
3.1.2. Metal-histidine-mediated self-assembly of QDMgTx
Metal-histidine-mediated self-assembly of QDMgTx allowed control over the orientation as well as the valence of peptide per QD. The addition of a terminal His6 tag is commonly used during protein expression in bacteria or during protein synthesis, thus can be easily applied to conjugate these biomolecules to QDs via polyimadazole-to metal coordination (Dennis et al. 2010;Zhan et al. 2013). Here, we used QDs capped with a His-PIMA-ZW ligand, a compact capping ligand that allowed HisMgTx to directly access the QD surface (Figure 2a) as previously noted (Wang et al. 2015). The conjugation was characterized using gel electrophoresis in which greater amounts of QD bound to HisMgTx were identified as increased mass (Figure 2b). A decrease in mobility of QDs was observed with an increase in the amount of HisMgTx conjugated per QD. Negligible migration of conjugates was observed as the molar ratio of QD:HisMgTx was increased to and above 1:30, suggesting that the QD surface was saturated with peptide. In Figure 2c-d, the gel was stained with Coomassie blue to identify any unbound peptide following the conjugation. A lane containing HisMgTx alone or BSA alone (carrier protein for HisMgTx) served as controls. No HisMgTx bands were observed in the 1:5, 1:10, or 1:15 conjugates, indicating that all peptide used for the conjugation was bound to the QDs. HisMgTx bands were observed in the 1:20 and 1:25 conjugations, indicating that following conjugation in these conditions, there was excess peptide not bound to the surface of the QDs. This is in agreement with previous studies in which metal-histidine-mediated self-assembly was similarly used (Medintz et al 2004). Because the 1:15 QDMgTx conjugate had the largest amount of bound HisMgTx with no observable unbound peptide, our electrophysiological and metabolic analyses used this version of the conjugate.
3.2. Testing QD targeting and function using heterologously-expressed Kv1.3 channels
3.2.1. QDMgTx can label HEK293 cells expressing Kv1.3
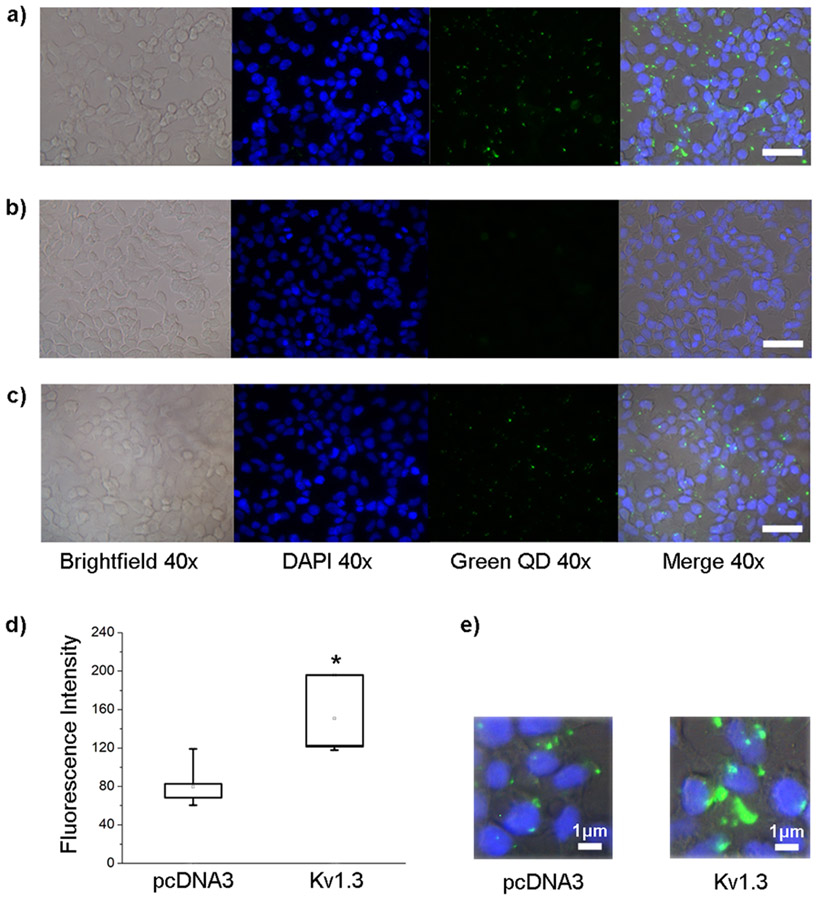
Kv1.3 or pcDNA3 transfected HEK293 cells were incubated with bath-applied QDMgTx to determine labeling of the conjugate in Kv1.3-transfected cells. QDMgTx labeling was strongly observed in Kv1.3-transfected cells (Figure 3a) and less so under pcDNA3 transfection conditions (Figure 3c). The conjugate appeared to non-specifically bind to pcDNA3-transfected cells with a significantly lower fluorescence intensity than that of Kv1.3-transfected cells (Figure 3d; 79.7 ± 10.4 for pcDNA3 vs. 129.0 ± 18.0 for Kv1.3, n=5, Student’s t-test, * p = 0.0227). When Kv1.3-transfected cells were incubated with QDs alone (Figure 3b), no measurable labeling was observed.
FIGURE 3.
QDMgTx can label HEK293 cells expressing Kv1.3. Photomicrographs of HEK293 cells transfected with Kv1.3 and incubated with a) 50 nM QDMgTx 1:15 or b) 50 nM QDs. c) Photomicrographs of HEK293 cells transfected with pcDNA3 and incubated with 50 nM QDMgTx, scale bar = 10 μm. d) Box plot and e) enlarged photomicrographs to quantify/visualize fluorescence intensity of the labeling found for experiments in a) and c), respectively. Box determined by the 25th and 75th percentiles and whiskers indicate 5th and 95th percentiles; n = 5 transfection batches for each condition. Student’s t-test, * significantly different, p = 0.0227.
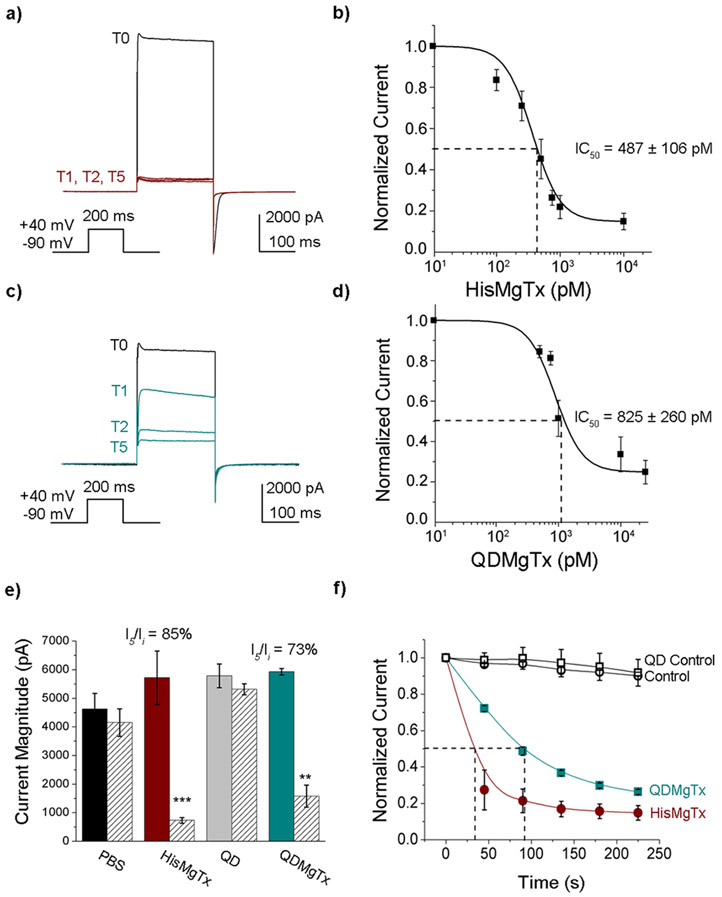
3.2.2. Inhibition of Kv1.3 by HisMgTx and QDMgTx
MgTx is reported to block Kv1.3 with an IC50 of 11.7–119 pM (Garcia-Calvo et al. 1993; Knaus et al. 1995), and therefore we measured the ability of HisMgTx and QDMgTx to block Kv1.3 in HEK293 cells (Figure 4). Addition of the epitope tag lowered the affinity of the peptide inhibitor, but HisMgTx still blocked Kv1.3 with picomolar affinity (IC50 = 487 ± 106 pM, Hill coefficient = 1). Compared to HisMgTx, the 1:15 QDMgTx blocked Kv1.3 at a lower, but still picomolar affinity (IC50 = 825 ± 260 pM, Hill coefficient = 1; Figure 4b, d). When applied at equal concentrations (10 nM), HisMgTx and QDMgTx each significantly decreased Kv1.3 peak current amplitude (Figure 4e; two-way mixed-design analysis of variance (ANOVA) using time as a factor, F(1,50) = 20.61, p < 0.0001, and using treatment as a factor, F(3,50) = 3.26, p = 0.0290). Application of QD alone or control bath solution did not elicit a decrease in voltage-activated current over time (I5/Ii; Figure 4e). The mean percent inhibition (at I5/Ii) by HisMgTx vs QDMgTx was not significantly different (85 ± 3.9% for HisMgTx, n = 5 vs. 73 ± 7.4% for QDMgTx, n = 4, ArcSin Transformation Student’s t-test, p > 0.05; Figure 4e). We measured the time required to achieve 50% inhibition of voltage-activated currents in the whole-cell patch configuration to be less than 2 min for 10 nM HisMgTx or QDHisMgTx, and the times were not significantly different (Figure 4f, 32.7 ±10.9 s for HisMgTx, n = 5 vs. 73.4 ± 17.7 s QDMgTx, n = 4, Student’s t-test, p > 0.05).
FIGURE 4.
Inhibition of Kv1.3 currents by HisMgTx and its QD conjugate. Representative whole-cell patch recording of a HEK293 cell transfected with Kv1.3 (Time 0; T0) and monitored for block of voltage-activated currents following bath application of a) HisMgTx or c) QDMgTx. Inhibitors were bath applied using a 10 nM concentration. Vh = −90 mV, Vc = + 40 mV, IPI = 45 s. b, d) Concentration-response curves for inhibition of Kv1.3 under conditions a and c, respectively; HisMgTx = IC50 = 487 ± 106 pM. n = 5 for each concentration tested. QDMgTx = IC50 = 825 ± 260 pM, n = 4–6 for all concentrations tested. e) Peak current amplitude at time 0 (T0) vs time 5 (T5) for a population of recordings, where HisMgTx, unconjugated QDs, or QDMgTx was applied to the bath solution. Values represent mean ± SEM, n = 16 for PBS control, n = 4–5 for all other conditions. Two-way mixed-design analysis of variance (ANOVA) using time as a factor; Bonferroni’s post hoc test. *** p < 0.001; ** p < 0.01. f) Normalized peak current amplitude (Ii = current initial; In = current at n time point) versus time for the population of recordings represented in panel e. Open circle (black) = control, closed circle (red) = HisMgTx, open square (black) = QD Control, closed square (green) = QDHisMgTx. Dotted line = time to half-maximum inhibition. Sample size (n) = number of cell recordings.
3. QD functional effect on OB using ex vivo slice electrophysiology
3.3.1. QDMgTx enhances mitral cell action potential (AP) firing in olfactory bulb slices
The major output neuron of the OB is the mitral cell, with Kv1.3 making up 60–80% of outward currents in these projection cells (Fadool and Levitan 1998). When applied via bath application, QDMgTx significantly increased the AP firing frequency over that of control ACSF or QDs (Figure 5; one-way repeated measure (RM) ANOVA, F(2,15) = 8.88, **p = 0.0029). Spike analysis demonstrated that QDMgTx and QDs decreased the pause duration between AP trains (Control, 2511.0 ± 899.5 ms, n = 46; QD, 1270.0 ± 477.9 ms, n = 97; QDMgTx; 1069.0 ± 93.7 ms, n = 43; one-way RM ANOVA, F(2,183) = 6.304, p = 0.0167), while QDMgTx but not QD increased the interspike interval (ISI) within the AP train (Control, 57.4 ± 4.7 ms, n = 73; QD, 59.6.0 ± 8.1 ms, n = 116; QDMgTx; 68.0 ± 12.4 ms, n = 61; one-way RM ANOVA, F(2,247) =6.304, p = <0.0001). Spike analysis was acquired over a subset of 2–4 bursting cells (Control, n = 4; QD, n = 3; QDMgTx, n = 2). Previous reports show that MgTx (Mast and Fadool 2012; Thiebaud et al. 2016) and other neuromodulators (Fadool et al. 2011) that act to decrease Kv1.3 activity, increase the spike firing frequency and reduce the pause duration between AP trains, but have less effect on ISI.
FIGURE 5.
Mitral cell AP firing frequency is enhanced following application of QDMgTx. a) Representative APs recorded in a cell sequentially introduced to bath application of ACSF, QD, and then QDMgTx. b) Box plot of the normalized firing frequency for a population of MCs. One-way repeated measures ANOVA, ** p < 0.05; Bonferonni’s post hoc test, Control vs. QD - p > 0.05; QD vs. QDMgTx * p < 0.05; Control vs. QDMgTx * p < 0.05); Box plot and whiskers notation as in Figure 3. Sample size (n) = number of cell recordings = 5.
3.3.2. QDMgTx/HisMgTx delivery to the OB in obese mice via cannula-guided osmotic mini-pumps
Due to our ability to inhibit Kv1.3 currents using QDMgTx in a heterologous system or in native neurons in ex-vivo OB slices, we next wanted to test in-vivo delivery of the inhibitor in mice by targeting native Kv1.3 channels in OB mitral cells. We initially attempted delivery of the QDMgTx/HisMgTx using an intranasal delivery (IND) technique (Thorne et al. 2004; Hanson and Frey 2008) that we had previously used to deliver insulin to the OB in awake mice (Marks et al. 2009). Because we could not observe fluorescent labeling in the OB following IND, we were not able to determine if the lack of label was due to failed delivery across the cribiform plate or failed detection of signal beyond the resolution limit (data not shown). Therefore, we alternatively examined whether we could localize iron-oxide magnetic nanoparticles (IOMNP) that would allow us to track delivery by ex vivo magnetic resonance imaging (MRI). A concentration of 5 mg/ml of IOMNP alone was prepared in PBS and 3 x 10 μl naris deliveries were made three times with a 2 min periodicity for a total volume of 90 μl per mouse (Marks et al. 2009). Images, as shown in Supplemental Figure 1, were acquired using a widebore 11.75-T equipped with a Bruker Avance console and Micro2.5 imaging gradients (Bruker Biospin, Billerica, MA) at the FAMU-FSU College of Engineering (Fu et al. 2005; Rosenberg et al. 2013) using a 3D gradient-recalled echo sequence at an isotropic resolution of 50 μm. Both coronal and sagittal views of the combined OB and epithelium revealed that the IOMNP were not able to penetrate the axonal spaces in the cribiform plate to gain access to the OB neurolamina. We tried altering the time of animal sacrifice following IND, varying the concentration of IOMNP, and including known permeabilizing agents (Triton X100, hyalronidase, and chitosan), but even these variations failed to provide visualization of the magnetic nanoparticles beyond the nasal cavity, epithelium, or periphery of the brain (Supplemental Figure 2).
Instead of using the IND technique, we used stereotaxically-guided placement of osmotic mini-pumps to assure successful and targeted delivery of QDMgTx/HisMgTx and controls to the OB. The experimental work for the glucose tolerance testing, CLAMS metabolic assessment, and surgical implantation of osmotic mini-pumps is diagramed in Figure 6a as previously described in the methods. Each obese mouse was studied for 22 days (−7 to +14) with surgical placement of the pump after 7 days (0) (Figure 6b). Histological examination of the brains following metabolic testing was performed (Figure 6c) to confirm proper placement of the cannula using cresyl violet staining. One mouse was excluded from the data set due to a mistargeted cannula. In a separate cohort of non-obese mice (n = 3) for which cannula were clearly perforating the OB, no fluorescent QD labeling could be observed in the OB or anywhere in the brain, despite the empty osmotic capsule (Figure 6d). This result suggested that the drug treatments were osmotically delivered, but were beyond the resolution of detection of the fluorescent label.
3.4. Assessment of QDMgTx/HisMgTx effect on metabolism using CLAMS
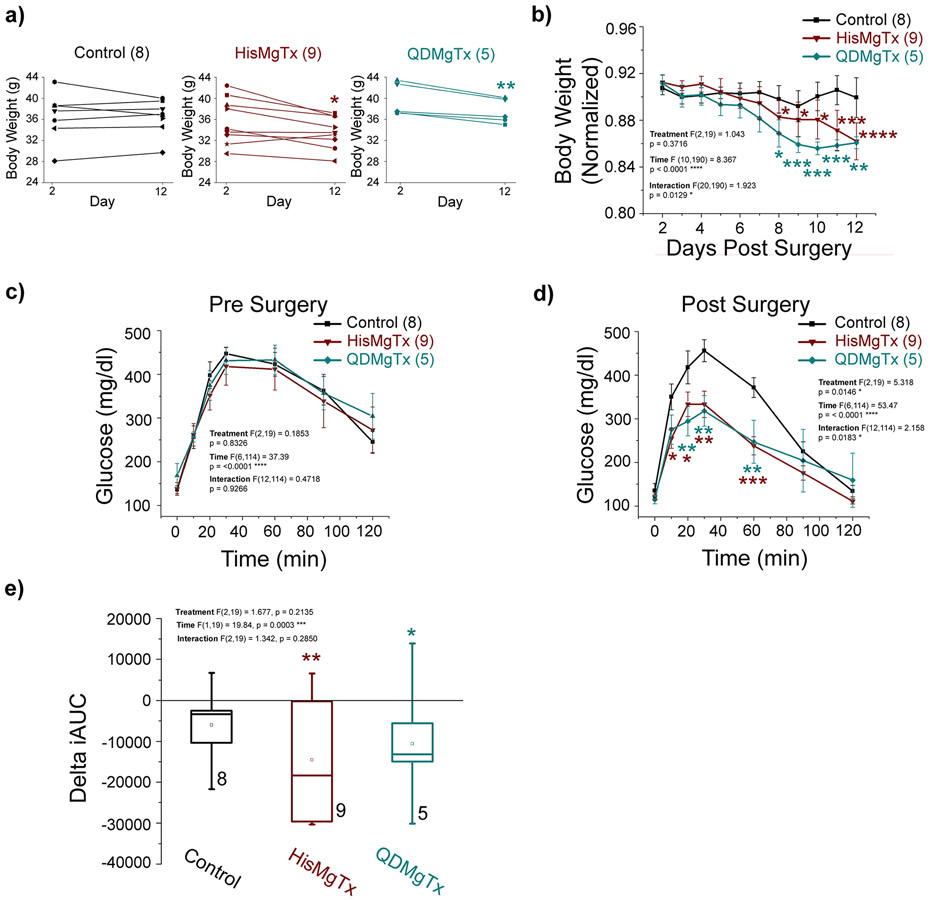
3.4.1. Delivery of a Kv1.3 inhibitor to the OB of obese mice leads to a reduction in body weight and enhanced glucose clearance
Body weight and glucose clearance were monitored in obese mice receiving PBS (control), HisMgTx, or QDMgTx via cannulated delivery to the OB. HisMgTx- and QDMgTx-treated mice had a significantly-reduced body weight compared to PBS control mice (Figure 7a; paired t-test, p < 0.05). We compared the normalized change in body weight over time (+2 to +12) following implantation of the cannulated osmotic mini-pump (at 0; Figure 7b; 2-way RM ANOVA, using time as the factor: F (10,190) = 8.367, **** p < 0.0001). There was a significant interaction of time x treatment: F (20, 190) = 1.923, * p < 0.05. An IPGTT was performed twice on each mouse (−6, +13) to monitor their fasted ability to clear glucose prior to (Figure 7c) and after drug delivery (Figure 7d). Prior to drug treatment (−6) all mice cleared glucose similarly (2-way RM ANOVA, p > 0.05). Following drug treatment (+13), mice receiving HisMgTx or QDMgTx cleared glucose significantly faster than control animals (Figure 7d; 2-way RM ANOVA using treatment: F(2, 19) = 5.318, * p = 0.0146 and time: F (6,114) = 53.47, ***p < 0.0001 as factors). The integrated area under the curve (iAUC) is an additional measure of an animals’ ability to clear glucose, and therefore we also examined how iAUC changed following drug delivery. Comparison of the iAUC before (−6) and following (+13) drug delivery is shown in Figure 7e as a normalized change (IPGTT AUC delta). Mice receiving HisMgTx or QDMgTx had a significant change in iAUC (Figure 7e; 2-way RM ANOVA using time as the factor: F (1,19) = 19.84, *** p = 0.0003), whereas control animals had no change in iAUC p > 0.05).
FIGURE 7.
Obese mice receiving a Kv1.3 inhibitor delivered to the OB had reduced body weight and cleared glucose more quickly. a) Body weight on day 2 vs. day 12 for individual mice. b) Normalized body weight versus time. Glucose clearance on c) Pre (−6) and d) Post Surgery (13). e) Box plot of the change in iAUC for data in (c-d). Data represent mean ± SEM. PBS: n = 8; HisMgTxFSU: n =9; QDMgTx: n = 5. a) paired t-test within treatment, * p < 0.05. b-e) Two-way RM ANOVA with factors of treatment, time, and interaction as noted. b) Bonferroni’s post hoc test * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 significantly-different from day 2. c-d) Bonferoni’s post-hoc test * p < 0.05, ** p < 0.01, *** p < 0.001 significantly-different from PBS Control. e) one-way ANOVA, Bonferroni’s post-hoc test significantly-different than Day −6, * p < 0.05, ** p < 0.01. Sample size (n) = number of mice.
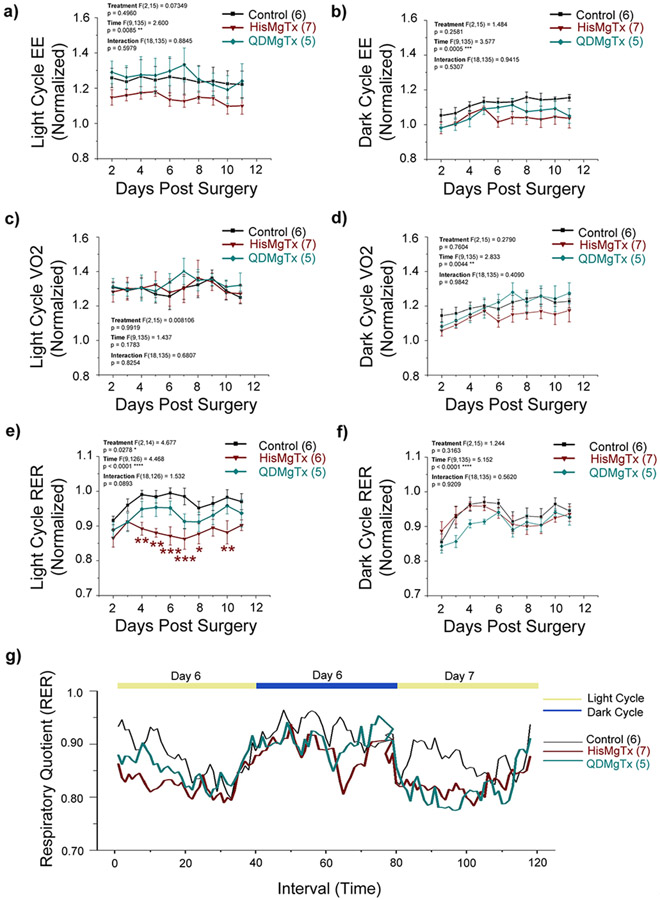
3.4.2. Inhibition of Kv1.3 in the OB of obese mice does not alter the EE but reduces RER
Energy expenditure (EE), or the amount of calories burned per hour, is a compilation of resting metabolic rate, the thermic effect of eating, and energy expended during voluntary and involuntary physical movement. Following delivery of HisMgTx or QDMgTx (+2 to +12), there was no change in EE in either the light or dark cycle (Figure 8a, b; 2-way RM ANOVA using treatment as the factor: light cycle: F(2, 15) = 0.7349, p = 0.496; dark cycle, F(2, 15) =1.484, p = 0.2581). It is well known that EE can be transiently increased due to the trauma or inflammation resulting from surgery (Clark 1973) and we observed this increase in the light cycle following surgery on Day 0 (not shown; 0.20 – 0.28 fold increase) and a subsequent lowering of EE over time (F (9, 135) = 2.60, * p = 0.0085) for all treatments without a significance in the Bonferroni’s post-hoc test (p > 0.05). VO2 is also a primary measure of metabolism. We observed no difference in VO2 with respect to delivery of HisMgTx or QDMgTx (+2 to +12), in either the light or dark cycle (Figure 8c, d; 2-way RM ANOVA using treatment as the factor: light cycle: F(2, 15) = 0.0081, p = 0.9919; dark cycle, F(2, 15) =0.2790, p = 0.7604). We did observe an elevation of VO2 over time that in the dark cycle that was independent of treatment (2-way RM ANOVA using time as the factor: F (9, 135) = 2.833, ** p = 0.0044). Likewise, there was no difference in carbon dioxide production (VCO2) with respect to any treatment (Supplementary Figure 3; 2-way RM ANOVA using treatment as the factor: light cycle: F(2, 15) = 0.4245, p = 0.6617 dark cycle, F(2, 15) = 1.035, p = 0.3793). Interestingly, we observed a treatment effect with a measured reduction in the respiratory exchange ratio (RER) that was restricted to the light cycle (Figure 8e-f). RER is the ratio of the volume of CO2 produced to the volume of O2 consumed (VCO2/VO2) and is as indicator of which nutrients are being metabolized for energy. RER is 0.7 when lipids are being metabolized, 0.8 for proteins and 1.0 for carbohydrates (Ramos-Jimenez et al. 2008). When comparing changes in RER during the light cycle (+2 to +12) there was a significant reduction in RER for animals receiving HisMgTx compared to PBS (Figure 8e, 2-way RM ANOVA using with treatment as the factor: F(2,14) = 4.677, * p = 0.0278. There was not a significant effect for QDMgTx nor a time x treatment interaction, p = 0.0893. Figure 8g illustrates the mean RER for all mice (+6 to +7) to incorporate a visualization of the specific RER effect associated with the light cycle.
FIGURE 8.
Inhibition of Kv1.3 in the OB of obese mice reduces RER without changing EE and VO2. Normalized EE in the a) light and b) dark cycle, VO2 in the c) light and d) dark cycle, and RER in the e) light and f) dark cycle. Data represent mean ± SEM with number of animals per treatment group as noted. Notations and statistical analyses as in Figure 7b. g) Mean RER of all mice over time across the light and dark cycle of day 6 and 7.
3.4.3. Inhibition of Kv1.3 in the OB of obese mice may change ingestive behavior without differentially affecting locomotor activity
To complement measurements of EE, VO2 and RER we measured changes in total calories consumed by mice or changes in their locomotor activity by comparing baseline values prior to and after drug delivery (−1 vs. +11, Table 1). The total caloric intake and water consumption were not significantly different for mice in any drug treatment group (paired t-test, p > 0.05). Despite eating the same amount of calories, mice that received either HisMgTx or QDMgTx reduced their number of feeding bouts while either increasing bout duration or increasing amount of food/bout (paired t-test, p < 0.05), neither of which were observed in the PBS control mice. HisMgTx-treated mice decreased their number of feed bouts roughly 50% while increasing the feeding duration approximately 66%. QDMgTx-treated mice also decreased their number of feed bouts roughly 50%, but had no change in feeding duration while increasing food/bout by about 30%. All mice exhibited greater ambulatory locomotor activity in the dark cycle over that of the light cycle, as anticipated for a nocturnal animal. Although individual mice varied in their baseline ambulatory activity in the dark cycle (−1), all mice exhibited significantly reduced activity regardless of drug treatment, inferring less overall locomotor activity attributed to surgery or acclimation to the CLAMS extended environment (−1 vs. 11; Table 1, paired t-test, * p < 0.05, ** p < 0.01).
Table 1.
Locomotor and Feeding behavior of obese mice treated with PBS, HisMgTx or QDMgTx via cannulated osmotic-minipump. All values ± standard error of mean (SEM). Paired t-test, Day −1 vs. Day 11
| PBS | HisMgTx | QDMgTx | ||||
|---|---|---|---|---|---|---|
| Day −1 | Day 11 | Day −1 | Day 11 | Day −1 | Day 11 | |
| X-Axis Beam Breaks | ||||||
| 12h Light (Cumulative) | 4656 ± 3037 (6) | 1552 ± 342 | 6783 ± 2403 (7) | 2667 ± 1296 | 4590 ± 2205 (5) | 1216 ± 551 |
| 12h Dark (Cumulative) | 12495 ± 2056 (6) | 6892 ± 1627 * | 25376 ± 4464 (7) | 8649 ± 3073** | 14935 ± 1993 (5) | 5376 ± 466 * |
| Water Intake (g) | 2.6 ± 0.3 (5) | 3.1 ± 0.3 | 3.3 ± 0.3 (7) | 3.8 ± 0.5 | 3.6 ± 0.5 (4) | 3.2 ± 0.2 |
| Caloric Intake (kcal) | 13.9 ± 1.2 (6) | 14.6 ± 0.3 | 14.7 ± 1.0 (7) | 12.6 ± 1.5 | 16.0 ± 1.2 (5) | 15.1 ± 0.9 |
| Feeding Bouts | 21.3 ± 1.9 (6) | 18.5 ± 2.0 | 37.3 ± 4.1 (7) | 20.0 ± 2.3 * | 44.6 ± 7.0 (5) | 18.8 ± 3.2 * |
| Grams/Feeding Bout | 0.2 ± 0.01 (6) | 0.2 ± 0.01 | 0.15 ± 0.01(7) | 0.17 ± 0.02 | 0.17 ± 0.02 (5) | 0.25 ± 0.01* |
| Feeding Duration (s) | 177.4 ± 65.4 (6) | 256.8 ± 60.4 | 133.6 ± 24.1 (7) | 222.5 ± 42.9* | 242.9 ± 60.6 (5) | 320.3 ± 39.8 |
p = 0.05
p < 0.01
4. DISCUSSION
Herein, we produced a polyhistidine-modified peptide that inhibits Kv1.3 and can be self-assembled to fluorescent QDs through a one-step metal-histidine coordination. We demonstrated that this coupling strategy did not significantly alter the inhibitor interaction with the channel as determined by electrophysiological analyses, and it allowed sufficient conjugation to permit in vivo investigation of targeted delivery to a brain area that highly expresses Kv1.3. Most significantly, we discovered that osmotic pump administered HisMgTx and QDMgTx to the OB caused a loss of body weight in DIO mice and increased their ability to clear a glucose challenge. The loss in body weight was not attributed to a change in oxygen consumption or total energy expenditure. Treatment with the peptide inhibitor alone (HisMgTx) caused a reduction in RER in the light cycle, suggesting a shift in fuel utilization to fats over that of carbohydrates by blocking Kv1.3 conductance selectively in the OB. Interestingly, this did not cause a change in total caloric consumption, rather a change in ingestive behavior whereby mice significantly decreased feeding bouts while eating longer or larger meals.
Recombinant expression of the Kv1.3 inhibitor (HisMgTx) permitted conjugation to the Zn2+ surface of QDs using polyhistidine-mediated self-assembly, while retaining a majority of predicted biophysical properties of the channel in a blocked state. Addition of a polyhistidine tag only slightly decreased the inhibitory potency of the peptide (IC50 of 487 pM compared to reported IC50 values of 112–119 pM for unconjugated peptide (Garcia-Calvo et al. 1993; Knaus et al. 1995)). Despite this small reduction in potency, block of the channel occurred quickly. MgTx is amenable as a peptide blocker due to the fact that it has an extremely slow koff of 2 h, and yet its kon is also rather slow for a vestibule blocker (Knaus et al. 1995; Fadool and Levitan 1998). Conjugation of the inhibitor to the QD (QDMgTx) reduced the potency of channel inhibition (IC50 = 894 pM), however, the binding kinetics were equally as strong as that of HisMgTx, taking an average of 32 and 73 s to reach half block, respectively. Regardless of the measured inhibitory potency of either form of the inhibitor, both had rapid association with the channel and were equally effective blockers, causing a loss of 73-85% of the outward current in Kv1.3-expressing HEK293 cells. When tested on ex vivo slices of the OB, QDMgTx, but not QDs alone, increased AP firing frequency by decreasing the pause duration between spike trains. Although we observed that QDMgTx binds nonspecifically to mock-transfected HEK293 cells, functionally, we found that QDs did not enhance excitability of mitral cells, which is consistent with their inability to block Kv1.3 voltage-activated conductance in HEK293 cells. These results suggest that the conjugated HisMgTx, not the QD, is acting to increase mitral cell excitability.
It is not clear why QDMgTx was effective in labeling Kv1.3-expressing HEK293 cells but could not be visualized in mitral cells given the slow koff for the MgTx interaction with the channel. Given the confirmation of the accuracy of the cannula placement and the calculated volume of the OB with determined osmotic delivery rates, it is unlikely that the inhibitors traveled beyond the OB region. Moreover, dye tracking and viral infection at similar flow rates and injection volumes to the OB in our hands did not reveal labeling outside the OB. Certainly, catabolism or clearance of the QD is feasible given the duration of delivery, and would be a factor to reduce sustained visibility of the label in vivo. Previous studies have shown that QDs are cleared within hours or days, depending on route of delivery (Fischer et al. 2006; Medintz et al. 2008). Likewise, a greater concentration of the conjugate may also be required for in vivo delivery to visualize QDs with fluorescence microscopy, while transmission electron or confocal microscopy, as others have used (Dante et al. 2017; Brunetti et al. 2018) may be a more appropriate technique for visualizing signal at low or therapeutic concentrations.
Immobilization of polyhistidine-containing biomolecules on the Zn2+ surface of QDs has been widely applied and is an attractive route of conjugation (Clapp et al. 2004; Dif et al. 2009; Wang et al. 2016b). We previously covalently conjugated MgTx to QDs by activating carboxyl groups on the designed QDs such that they would form an amide bond with basic residues found on the surface of MgTx (Schwartz et al. 2017). Despite this method was not capable of producing sufficient yields to perform in-vivo studies, it produced a conjugate that inhibited voltage-activated Kv1.3 currents (66% inhibition) in HEK293 cells and was selective to Kv1.2 and 1.3 Shaker family members when screened for selectivity (Kv1.1 to 1.7). Although we did not retest for selectivity with our newly synthesized HisMgTx and QDMgTx conjugate, herein, we did not expect the selectivity to be different than native MgTx peptide because primary surface interaction of the peptide has been identified via NMR and mutational and molecular dynamic studies to be on the peptides 3 β-sheets (Johnson et al., 1994; Yu et al., 2004; Khabiri et al., 2011; Nikouee et al., 2012) that we did not modify.
Having now produced therapeutic quantities of HisMgTx and QDMgTx, we were interested if pharmacological inhibition of Kv1.3 in the OB could alter metabolic function in DIO mice. Previous work has shown that pharmacological inhibition of Kv1.3 or whole-body deletion of the channel can significantly alter metabolism in mice. In the periphery, Kv1.3 is expressed in T-lymphocytes, brown fat, and white adipose tissue, whereas in the CNS, Kv1.3 is found in the dentate gyrus of the hippocampus, mitral cells in the OB, and in pyramidal cells of the pyriform cortex (Kues and Wunder 1992; Fadool and Levitan 1998; Marks and Fadool 2007; Colley et al. 2009; Upadhyay et al. 2013; Al Koborssy et al. 2018). Whether there are differential mechanisms to reduce body weight by inhibiting Kv1.3 peripherally versus centrally is not known. When the Kv1.3 inhibitor ShK-186 was delivered to the periphery via intraperitoneal injection every other day over a 10-week period, DIO mice had improved glucose clearance and a reduction in body weight that was attributed to an activation of brown fat that increased β-oxidation of fatty acids. Mice had enhanced VO2 and EE without a change in total caloric consumption or locomotor activity (Upadhyay et al. 2013). Upadhyay et al. (2013) reported that a peripherally administered Kv1.3 inhibitor increased RER, selectively in the light cycle, which in turn increased brown adipose tissue metabolism that may or may not have an effect on thermogenesis in humans (Carpentier et al. 2018). Although it is not clear if peripherally administered Kv1.3 blockers cross the blood-brain barrier, especially at the level of the olfactory system that is known to be leaky (Palouzier-Paulignan et al. 2012), our data demonstrate that direct application of Kv1.3 inhibitors to the OB reduces body weight in DIO mice by oppositely changing fuel metabolism (decreasing RER), improving glucose clearance and altering ingestive behavior, rather than modifying VO2 or EE. Our measured decrease in RER indicates an increased lipid oxidation over that of carbohydrates, but the mechanism of how this might be activated by the OB is unresolved.
In addition to increased metabolism, Kv1.3−/− mice also have elevated olfactory ability, in terms of both odor discrimination and odor threshold (referred to as super-smeller mice) (Xu et al. 2003; Fadool et al. 2004; Tucker et al. 2008; Tucker et al. 2012a; Tucker et al. 2012b; Thiebaud et al. 2014). Kv1.3−/− mice have smaller, supernumerary glomeruli, increased density of odorant receptors, and can discriminate odor molecules that differ by only one carbon atom (Fadool et al. 2004; Biju et al. 2008). While super-smeller mice do not have changed caloric intake, they have irregular ingestive behaviors (increased meal frequency and decreased water bolus) and elevated locomotor activity (Fadool et al. 2004). Interestingly, genetic ablation of olfactory sensory neurons, resulting in a loss of olfactory ability, was shown to selectively reduce body weight in DIO but not control fed mice (Riera et al. 2017). The reduction in body weight occurred without a change in caloric intake but rather a significant increase in VO2 and EE in both the light and dark cycle. The reduction in body weight was thought to be caused by an activation of white and brown fat to increase lipolysis. A recent study (Mutlu et al. 2020) identified select olfactory neurons in C. elegans that could regulate fat metabolism without altering ingestive behavior. Inhibition of neuropeptide olfactory circuits in the worm revealed a molecular mechanism using glucocorticoid-inducible kinase to regulate fat metabolism in an odor-specific fashion. This further suggests that olfactory physiology can influence metabolic function.
Nutritional status is well understood to influence olfactory physiology. In the OB, Kv1.3 is expressed in mitral cells, the primary projection neurons of the OB that project to higher regions of the brain via the lateral olfactory tract. Mitral cell activity is primarily stimulated by olfactory sensory input, however feeding state and nutritional status play a role as well (Aime et al. 2014; Thiebaud et al. 2014). GLP-1, insulin, and glucose all increase the excitability of mitral cells by reducing Kv1.3 activity (Fadool and Levitan 1998; Fadool et al. 2000; Tucker et al. 2010a; Tucker et al. 2013; Thiebaud et al. 2016). In obesity, GLP-1 secretions are known to be reduced from sources outside of the OB (Ranganath et al. 1996; Faerch et al. 2015) and the ability to clear glucose is limited (Thiebaud et al. 2014). Likewise, a concurrent reduction in olfactory ability is observed (Thiebaud et al. 2014). At the level of the OB, the ability of insulin to increase mitral cell activity is significantly reduced (Fadool et al. 2011) following diet-induced obesity. An altered action of GLP-1, insulin, and glucose on Kv1.3 in obese mice, brought on by altered levels or signaling, could lead to changed excitability of the output neurons to higher olfactory cortical and hypothalamic regions to change metabolic set-point.
Herein, we delivered HisMgTx/QDMgTx to the OB in obese mice, essentially jumpstarting a pathway dampened by nutritional state, and observed a reduction in body weight and improved glucose clearance, and with HisMgTx, a significant reduction in RER. RER is an indicator of which nutrients are being metabolized for energy and a reduction suggests a shift in fuel utilization to fats rather than carbohydrates. This identifies for the first time a unique role of the OB in energy regulation. Interestingly, central infusions of GLP-1 have similar impacts on energy regulation. GLP-1R activation in the CNS has been shown to reduce RER (Hwa et al. 1998; Kooijman et al. 2015), improve glucose clearance (Sandoval et al. 2008a; Sandoval et al. 2008b; Kooijman et al. 2015), and reduce caloric intake (Tang-Christensen et al. 1996; Dossat et al. 2011). Here, we observed an effect on ingestive behavior due to HisMgTx and QDMgTx delivery to the OB, however caloric intake was difficult to monitor due to the cannulated surgery having a significant influence on this variable. Likewise, we previously identified the unique presence of GLP-1 secreting cells known as preproglucagon neurons in the OB, however it is unclear if these cells act as a driver of energy regulation (Thiebaud et al. 2016; Thiebaud et al. 2019). The exact mechanism by which neuromodulation of Kv1.3 influences metabolism and fuel utilization is still under exploration, but it is clear that direct inhibition of Kv1.3 in the OB has a significant effect on metabolic function.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Ms. Abigail Thomas and Carley Huffstetler for exceptional technical assistance, mouse husbandry, and assistance with our citations. We are gracious for the epitope tagging and subcloning performed by Dr. Brian Washburn in the FSU Molecular Cloning Facility and HPLC support by Margarete Seavy in the FSU Analytical Facility. A portion of this work was performed at the National High Magnetic Field Laboratory, which is supported by the National Science Foundation (NSF) grant DMR-1644779 and state of Florida. This work was supported by research and training awards administered by the National Institute on Deafness and other Communication Disorders (NIDCD) at the National Institutes of Health (NIH): R01DC013080, T32DC000044, and F31DC014638 and National Science Foundation (NSF-CHE, Grant #1508501. We are appreciative of the award of an East Asia and Pacific Summer Institutes (EAPSI) Australian Fellowship from the NSF (Award #1613988). This work was also supported by a FSU Legacy Fellowship, a Robinson Endowment Award from Tallahassee Memorial Hospital, and by the Stanley and Shirley Marshall Endowment Fund. All constructs produced in this work (HisMgTx, QDs, QDMgTx) can be shared upon request.
Abbreviations Used:
- ACSF
artificial cerebral spinal fluid
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- CLAMS
comprehensive laboratory animal metabolic system
- cDNA
copy DNA
- DAPI
4′,6-diamidino-2-phenylindole
- Da
2alton
- DIO
diet-induced obesity
- EE
energy expenditure
- FBS
fetal bovine serum
- GLP-1
glucagon-like peptide 1
- h
hour
- HEK293 cells
human embryonic kidney 293 cells
- HisMgTx
histidine epitope-tagged margatoxin
- HPLC
high performance liquid chromatography
- iAUC
integrated area under the curve
- Ii
initial current
- In
current at the nth pulse
- IND
intranasal delivery
- IPGTT
intraperitoneal glucose tolerance test
- IPI
interpulse interval
- kHz
kilohertz
- Kv1.3
voltage-dependent potassium channel 1.3
- Kv1.3−/−
global knockout of Kv1.3
- MgTx
margatoxin
- min
minutes
- ml
milliliter
- mOsm
milliosmole
- mM
millimole
- mV
millivolt
- MRI
magnetic resonance imaging
- N-terminal
amino terminus
- OB
olfactory bulb
- OSNs
olfactory sensory neurons
- P
postnatal day
- PBS
phosphate-buffered saline
- Pd
pulse duration
- PEG
polyethylene glycol
- PFA
paraformaldehyde
- pI
isoelectric point
- RER
respiratory exchange ratio
- RRID
Research Resource Identifier
- rt
room temperature
- QD
quantum dot
- QDMgTx
quantum dot margatoxin conjugate
- RM
repeated measure
- SEM
standard error of the mean
- T
time
- Vc
command voltage
- VCO2
volume of carbon dioxide produced through metabolism
- VO2
volume of oxygen consumed
Footnotes
CONFLICT OF INTEREST
The authors have no interests to declare, scientific or financial.
REFERENCES
- Aime P, Palouzier-Paulignan B, Salem R, Al KD, Garcia S, Duchamp C, Romestaing C and Julliard AK (2014) Modulation of olfactory sensitivity and glucose-sensing by the feeding state in obese Zucker rats. Front Behav. Neurosci 8, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Koborssy D, Palouzier-Paulignan B, Canova V, Thevenet M, Fadool DA and Julliard AK (2018) Modulation of olfactory-driven behavior by metabolic signals: role of the piriform cortex. Brain Struct Funct. 224(1), 315–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldeek F, Muhammed MA, Palui G, Zhan N and Mattoussi H (2013a) Growth of highly fluorescent polyethylene glycol- and zwitterion-functionalized gold nanoclusters. ACS Nano. 7, 2509–2521. [DOI] [PubMed] [Google Scholar]
- Aldeek F, Safi M, Zhan N, Palui G and Mattoussi H (2013b) Understanding the self-assembly of proteins onto gold nanoparticles and quantum dots driven by metal-histidine coordination. ACS Nano. 7, 10197–10210. [DOI] [PubMed] [Google Scholar]
- Anangi R, Koshy S, Huq R, Beeton C, Chuang WJ and King GF (2012) Recombinant expression of margatoxin and agitoxin-2 in Pichia pastoris: an efficient method for production of Kv1.3 channel blockers. PLoS One 7, e52965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anikeeva N, Lebedeva T, Clapp AR, Goldman ER, Dustin ML, Mattoussi H and Sykulev Y (2006) Quantum dot/peptide-MHC biosensors reveal strong CD8-dependent cooperation between self and viral antigens that augment the T cell response. Proc. Natl. Acad. Sci. U. S. A 103, 16846–16851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes G, Sebastião AM and Simoes de Souza FM (2014) Mechanisms of regulation of olfactory transduction and adaptation in the olfactory cilium. PLoS One. 9(8), e105531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GA and Fadool DA (2017) Awake, long-term intranasal insulin treatment does not affect object memory, odor discrimination, or reversal learning in mice. Physiol Behav. 174, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biju KC, Marks DR, Mast TG and Fadool DA (2008) Deletion of voltage-gated channel affects glomerular refinement and odorant receptor expression in the mouse olfactory system. J. Comp Neurol 506, 161–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti J, Riolo G, Gentile M, Bernini A, Paccagnini E, Falciani C, Lozzi L, Scali S, Depau L, Pini A, Lupetti P and Bracci L (2018) Near-infrared quantum dots labelled with a tumor selective tetrabranched peptide for in vivo imaging. J. Nanobiotechnology 16, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F and Turcotte EE (2018) Brown Adipose Tissue Energy Metabolism in Humans. Front Endocrinol. (Lausanne) 9, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp AR, Goldman ER and Mattoussi H (2006) Capping of CdSe-ZnS quantum dots with DHLA and subsequent conjugation with proteins. Nat. Protoc 1, 1258–1266. [DOI] [PubMed] [Google Scholar]
- Clapp AR, Medintz IL, Mauro JM, Fisher BR, Bawendi MG and Mattoussi H (2004) Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J. Am. Chem. Soc 126, 301–310. [DOI] [PubMed] [Google Scholar]
- Clark RG (1973) Metabolic states in relation to surgery and anesthesia., pp. 765–767. [Google Scholar]
- Coecke S, Balls M, Bowe G, Davis J, Gstraunthaler G, Hartung T, Hay R, Merten OW, Price A, Schechtman L, Stacey G and Stokes W (2005) Guidance on good cell culture practice. a report of the second ECVAM task force on good cell culture practice. Altern. Lab Anim 33, 261–287. [DOI] [PubMed] [Google Scholar]
- Colley B, Tucker K and Fadool DA (2004) Comparison of modulation of Kv1.3 channel by two receptor tyrosine kinases in olfactory bulb neurons of rodents. Receptors Channels 10, 25–36. [PMC free article] [PubMed] [Google Scholar]
- Colley BS, Cavallin MA, Biju K, Marks DR and Fadool DA (2009) Brain-derived neurotrophic factor modulation of Kv1.3 channel is disregulated by adaptor proteins Grb10 and nShc. BMC Neurosci 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook KK and Fadool DA (2002) Two adaptor proteins differentially modulate the phosphorylation and biophysics of Kv1.3 ion channel by SRC kinase. J. Biol. Chem 277, 13268–13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dante S, Petrelli A, Petrini EM, Marotta R, Maccione A, Alabastri A, Quarta A, De DF, Ravasenga T, Sathya A, Cingolani R, Proietti ZR, Berdondini L, Barberis A and Pellegrino T (2017) Selective targeting of neurons with inorganic nanoparticles: Revealing the crucial role of nanoparticle surface charge. ACS Nano. 11, 6630–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis AM, Sotto DC, Mei BC, Medintz IL, Mattoussi H and Bao G (2010) Surface ligand effects on metal-affinity coordination to quantum dots: implications for nanoprobe self-assembly. Bioconjug. Chem 21, 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dif A, Boulmedais F, Pinot M, Roullier V, Baudy-Floc'h M, Coquelle FM, Clarke S, Neveu P, Vignaux F, Le BR, Dahan M, Gueroui Z and Marchi-Artzner V (2009) Small and stable peptidic PEGylated quantum dots to target polyhistidine-tagged proteins with controlled stoichiometry. J. Am. Chem. Soc 131, 14738–14746. [DOI] [PubMed] [Google Scholar]
- Dossat AM, Lilly N, Kay K and Williams DL (2011) Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 31, 14453–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA and Kolling LJ (2020) Role of Olfaction for Eating Behavior, in The Senses: A Comprehensive Reference, (Fritzsch B and Meyerhoff W, eds), pp. 43 pages. Elsevier, Netherlands. [Google Scholar]
- Fadool DA and Levitan IB (1998) Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. J Neurosci 18, 6126–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K and Pedarzani P (2011) Mitral cells of the olfactory bulb perform metabolic sensing and are disrupted by obesity at the level of the Kv1.3 ion channel. PLoS One 6, e24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA and Kaczmarek LK (2004) Kv1.3 channel gene-targeted deletion produces "Super-Smeller Mice" with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron 41, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Phillips JJ and Simmen JA (2000) Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1.3. J Neurophysiol 83, 2332–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faerch K, Torekov SS, Vistisen D, Johansen NB, Witte DR, Jonsson A, Pedersen O, Hansen T, Lauritzen T, Sandbaek A, Holst JJ and Jorgensen ME (2015) GLP-1 Response to Oral Glucose Is Reduced in Prediabetes, Screen-Detected Type 2 Diabetes, and Obesity and Influenced by Sex: The ADDITION-PRO Study. Diabetes 64, 2513–2525. [DOI] [PubMed] [Google Scholar]
- Fischer HC, Liu L, Pang KS and Chan WCW (2006) Pharmacokinetics of nanoscale quantum dots: In vivo distribution, sequestration, and clearance in the rat. Adv. Funct. Materials 16(10), 1299–1305. [Google Scholar]
- Fu R, Brey WW, Shetty K, Gor'kov P, Saha S, Long JR, Grant SC, Chekmenev EY, Hu J, Gan Z, Sharma M, Zhang F, Logan TM, Bruschweller R, Edison A, Blue A, Dixon IR, Markiewicz WD and Cross TA (2005) Ultra-wide bore 900 MHz high-resolution NMR at the National High Magnetic Field Laboratory. J. Magn Reson 177, 1–8. [DOI] [PubMed] [Google Scholar]
- Garcia ML, Hanner M, Knaus HG, Koch R, Schmalhofer W, Slaughter RS and Kaczorowski GJ (1997) Pharmacology of potassium channels. Adv. Pharmacol 39, 425–471. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer W, Kaczorowski GJ and Garcia ML (1993) Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J. Biol. Chem 268, 18866–18874. [PubMed] [Google Scholar]
- Goldman ER, Mattoussi H, Anderson GP, Medintz IL and Mauro JM (2005a) Fluoroimmunoassays using antibody-conjugated quantum dots. Methods Mol. Biol 303, 19–34. [DOI] [PubMed] [Google Scholar]
- Goldman ER, Medintz IL, Whitley JL, Hayhurst A, Clapp AR, Uyeda HT, Deschamps JR, Lassman ME and Mattoussi H (2005b) A hybrid quantum dot-antibody fragment fluorescence resonance energy transfer-based TNT sensor. J. Am. Chem. Soc 127, 6744–6751. [DOI] [PubMed] [Google Scholar]
- Hanson LR and Frey WH (2008) Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC. Neurosci 9 Suppl 3, S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TC, Fadool DA and Levitan IB (1996) Tyrosine phosphorylation of the Kv1.3 potassium channel. J Neurosci 16, 1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Williams P, Witten MB, Tedesco R and Strader CD (1998) Differential effects of intracerebroventricular glucagon-like peptide-1 on feeding and energy expenditure regulation. Peptides 19, 869–875. [DOI] [PubMed] [Google Scholar]
- Johnson BS, Stevens SP and Williamson JM (1994) Determination of the three-dimensional structure of margatoxin by 1H, 13C, 15N triple-resonance nuclear magnetic resonance spectroscopy. Biochemistry 33, 15061–15070. [DOI] [PubMed] [Google Scholar]
- Julliard AK, Al Koborssy D, Fadool DA and Palouzier-Paulignan B (2017) Nutrient sensing: another chemosensitivity of the olfactory system. Front Physiol 8, 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabiri M, Nikouee A, Cwiklik L, Grissmer S and Ettrich R (2011). Charybdotoxin unbinding from the mKv1.3 potassium channel: a combined computational and experimental study. J Phys Chem B 115, 11490–11500. [DOI] [PubMed] [Google Scholar]
- Kleene SJ (2008) The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses. 33(9), 839–859. [DOI] [PubMed] [Google Scholar]
- Knaus HG, Koch RO, Eberhart A, Kaczorowski GJ, Garcia ML and Slaughter RS (1995) [125I]margatoxin, an extraordinarily high affinity ligand for voltage-gated potassium channels in mammalian brain. Biochemistry. 34(41), 13627–13634. [DOI] [PubMed] [Google Scholar]
- Kooijman S, Wang Y, Parlevliet ET, Boon MR, Edelschaap D, Snaterse G, Pijl H, Romijn JA and Rensen PC (2015) Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 58, 2637–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CP, Al KD, Huang Z, Chelette BM, Fadool JM and Fadool DA (2016) Mitochondrial ultrastructure and glucose signaling pathways attributed to the Kv1.3 ion channel. Front Physiol 7, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS and Goldman-Rakic PS (1997) An interface holding chamber for anatomical and physiological studies of living brain slices. J Neurosci Methods 75, 55–58. [DOI] [PubMed] [Google Scholar]
- Kues WA and Wunder F (1992) Heterogeneous expression patterns of mammalian potassium channel genes in developing and adult rat brain. Eur. J. Neurosci 4, 1296–1308. [DOI] [PubMed] [Google Scholar]
- Kupper J, Bowlby MR, Marom S and Levitan IB (1995). Intracellular and extracellular amino acids that influence C-type inactivation and its modulation in a voltage-dependent potassium channel. Pflugers Arch. 430(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Leatherdale CA, Woo W-K, Mikulec FV and Bawendi MG (2002) On the absorption cross section of CdSe nanocrystal quantum dots. J Phys. Chem B 106, 7619–7622. [Google Scholar]
- Lusk G (1924) Analysis of the oxidation of mixtures of carbohydrates and fat., pp. 41–42. [Google Scholar]
- Marks DR and Fadool DA (2007) Post-synaptic density 95 (PSD-95) affects insulin-induced Kv1.3 channel modulation of the olfactory bulb. J Neurochem 103, 1608–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DR, Tucker K, Cavallin MA, Mast TG and Fadool DA (2009) Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci 29, 6734–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marom S and Levitan IB (1994) State-dependent inactivation of the Kv3 potassium channel. Biophys J. 67(2), 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast TG, Brann JH and Fadool DA (2010) The TRPC2 channel forms protein-protein interactions with Homer and RTP in the rat vomeronasal organ. BMC Neurosci 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast TG and Fadool DA (2012) Mature and precursor brain-derived neurotrophic factor have individual roles in the mouse olfactory bulb. PLoS One 7, e31978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoussi H, Palui G, and Na HB (2012) Luminescent quantum dots as platforms for probing in vitro and in vivo biological processes. Adv Drug Deliv Rev 64(2), 138–166. [DOI] [PubMed] [Google Scholar]
- Medintz IL, Clapp AR, Brunel FM, Tiefenbrunn T, Uyeda HT, Chang EL, Deschamps JR, Dawson PE and Mattoussi H (2006a) Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat. Mater 5, 581–589. [DOI] [PubMed] [Google Scholar]
- Medintz IL, Clapp AR, Mattoussi H, Goldman ER, Fisher B and Mauro JM (2003) Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat. Mater 2, 630–638. [DOI] [PubMed] [Google Scholar]
- Medintz IL, Konnert JH, Clapp AR, Stanish I, Twigg ME, Mattoussi H, Mauro JM, and Deschamps JR (2004) A fluorescence resonance energy transfer-derived structure of a quantum dot-protein bioconjugate nanoassembly. Proc Nat. Acad. Sci.USA 101(26), 9612–9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medintz IL, Mattoussi H and Clapp AR (2008) Potential clinical applications of quantum dots. Int J Nanomed. 3(2), 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medintz IL, Sapsford KE, Clapp AR, Pons T, Higashiya S, Welch JT and Mattoussi H (2006b) Designer variable repeat length polypeptides as scaffolds for surface immobilization of quantum dots. J. Phys. Chem. B 110, 10683–10690. [DOI] [PubMed] [Google Scholar]
- Medintz IL, Uyeda HT, Goldman ER, and Mattoussi H (2005) Quantum dot bioconjugates for imaging, labeling and sensing. Nat Mater 4(6), 435–446. [DOI] [PubMed] [Google Scholar]
- Mout R, Moyano DF, Rana S and Rotello VM (2012) Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev 41, 2539–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu AS, Gao SM, Zhang H and Wang MC (2020) Olfactory specificity regulates lipid metabolism through neuroendocrine signaling in Caenorhabditis elegans. Nat. Commun. 11, 1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikouee A, Khabiri M Grissmer S and Ettrich R (2012). Charybdotoxin and margatoxin acting on the human voltage-gated potassium channel hKv1.3 and its H399N mutant: an experimental and computational comparison. J Phys Chem B 116, 5132–5240. [DOI] [PubMed] [Google Scholar]
- Palouzier-Paulignan B, Lacroix MC, Aime P, Baly C, Caillol M, Congar P, Julliard AK, Tucker K and Fadool DA (2012) Olfaction under metabolic influences. Chem Senses 37, 769–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Jimenez A, Hernandez-Torres RP, Torres-Duran PV, Romero-Gonzalez J, Mascher D, Posadas-Romero C and Juarez-Oropeza MA (2008) The respiratory exchange ratio is associated with fitness indicators both in trained and untrainedmMen: A possible application for people with reduced exercise tolerance. Clin Med Circ. Respirat. Pulm. Med 2, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R and Marks V (1996) Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 38, 916–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera CE, Tsaousidou E, Halloran J, Follett P, Hahn O, Pereira MMA, Ruud LE, Alber J, Tharp K, Anderson CM, Bronneke H, Hampel B, Filho CDM, Stahl A, Bruning JC and Dillin A (2017) The sense of smell impacts metabolic health and obesity. Cell Metab 26, 198–211. [DOI] [PubMed] [Google Scholar]
- Rosenberg JT, Sellgren KL, Sachi-Kocher A, Calixto BF, Baird MA, Davidson MW, Ma T and Grant SC (2013) Magnetic resonance contrast and biological effects of intracellular superparamagnetic iron oxides on human mesenchymal stem cells with long-term culture and hypoxic exposure. Cytotherapy. 15, 307–322. [DOI] [PubMed] [Google Scholar]
- Sandoval D, Cota D and Seeley RJ (2008a) The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu. Rev. Physiol 70, 513–535. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Bagnol D, Woods SC, D'Alessio DA and Seeley RJ (2008b) Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57, 2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapsford KE, Pons T, Medintz IL, Higashiya S, Brunel FM, Dawson PE and Mattoussi H (2007) Kinetics of metal-affinity driven self-assembly between proteins or peptides and CdSe-ZnS quantum dots. J Phys Chem C 111, 11528–11538. [Google Scholar]
- Schwartz AB, Kapur A, Wang W, Huang Z, Fardone E, Palui G, Mattoussi H and Fadool DA (2017) Margatoxin-bound quantum dots as a novel inhibitor of the voltage-gated ion channel Kv1.3. J Neurochem. 140, 404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg RJ and Martin RB (1974) Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chemical Reviews. 74(4), 471–517. [Google Scholar]
- Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M and Sheikh SP (1996) Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am. J Physiol 271, R848–R856. [DOI] [PubMed] [Google Scholar]
- Thiebaud N, Gribble F, Reimann F, Trapp S and Fadool DA (2019) A unique olfactory bulb microcircuit driven by neurons expressing the precursor to glucagon-like peptide 1. Sci Rep. 9(1), 15542–51880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud N, Johnson MC, Butler JL, Bell GA, Ferguson KL, Fadool AR, Fadool JC, Gale AM, Gale DS and Fadool DA (2014) Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J Neurosci 34, 6970–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud N, Llewellyn-Smith I, Gribble F, Reimann F, Trapp S and Fadool DA (2016) The incretin hormone glucagon-like peptide 1 increases mitral cell excitability by decreasing conductance of a voltage-dependent potassium channel. J Physiol 594, 2607–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V and Frey WH (2004) Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neurosci 127, 481–496. [DOI] [PubMed] [Google Scholar]
- Tortoriello DV, McMinn J and Chua SC (2004) Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology 145, 1238–1247. [DOI] [PubMed] [Google Scholar]
- Tortoriello DV, McMinn JE and Chua SC (2007) Increased expression of hypothalamic leptin receptor and adiponectin accompany resistance to dietary-induced obesity and infertility in female C57BL/6J mice. Int J Obes. (Lond) 31, 395–402. [DOI] [PubMed] [Google Scholar]
- Tucker K, Cavallin MA, Jean-Baptiste P, Biju KC, Overton JM, Pedarzani P and Fadool DA (2010a) The olfactory bulb: A metabolic sensor of brain insulin and glucose concentrations via a voltage-gated potassium channel. Results Probl Cell Differ 52, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K, Cavallin MA, Overton JM and Fadool DA (2010b) Ion channel in the olfactory bulb serves as a metabolic sensor, Chemical Senses Poster 217, 99. [Google Scholar]
- Tucker K, Cho S, Thiebaud N, Henderson MX and Fadool DA (2013) Glucose sensitivity of mouse olfactory bulb neurons is conveyed by a voltage-gated potassium channel 26. J. Physiol 591, 2541–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K, Overton JM and Fadool DA (2008) Kv1.3 gene-targeted deletion alters longevity and reduces adiposity by increasing locomotion and metabolism in melanocortin-4 receptor-null mice. Int. J. Obes. (Lond) 32, 1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K, Overton JM and Fadool DA (2012a) Diet-induced obesity resistance of Kv1.3−/− mice is olfactory bulb dependent. J. Neuroendocrinol 24, 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KR, Godbey SJ, Thiebaud N and Fadool DA (2012b) Olfactory ability and object memory in three mouse models of varying body weight, metabolic hormones, and adiposity. Physiol Behav. 107, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay SK, Eckel-Mahan KL, Mirbolooki MR, Tjong I, Griffey SM, Schmunk G, Koehne A, Halbout B, Iadonato S, Pedersen B, Borrelli E, Wang PH, Mukherjee J, Sassone-Corsi P and Chandy KG (2013) Selective Kv1.3 channel blocker as therapeutic for obesity and insulin resistance. Proc Natl Acad Sci U S A 110, E2239–E2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Ji X, Burns H and Mattoussi H (2016a) A multi-coordinating polymer ligand optimized for the functionalization of metallic nanocrystals and nanorods. Faraday Discuss. 191, 481–494. [DOI] [PubMed] [Google Scholar]
- Wang W, Ji X, Du L and Mattoussi H (2007) Enhanced colloidal stability of various gold nanostructures wsing a multicoordinating polymer coating. J. Phys Chem. C 121(41), 22901–22913. [Google Scholar]
- Wang W, Ji X, Kapur A, Zhang C and Mattoussi H (2015) A multifunctional polymer combining the imidazole and zwitterion motifs as a biocompatible compact coating for quantum dots. J Am Chem Soc. 137(44), 14158–14172. [DOI] [PubMed] [Google Scholar]
- Wang W, Kapur A, Ji X, Zeng B, Mishra D and Mattoussi H (2016b) Multifunctional and high affinity polymer ligand that provides bio-orthogonal coating of quantum dots. Bioconjug. Chem 27(9), 2024–2036. [DOI] [PubMed] [Google Scholar]
- Xu J, Koni PA, Wang P, Li G, Kaczmarek LK, Wu Y, Li Y, Flavell RA and Desir GV (2003) The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight Hum Mol Genet 12, 551–559. [DOI] [PubMed] [Google Scholar]
- Yu K, Fu W, Liu H, Luo X Chen KX, Ding J, Shen J and Jiang H (2004) Computational simulations of interactions of scorpion toxins with the voltage-gated potassium ion channel. Biophys J 86, 3542–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WW and Peng X (2002) Formation of high-quality CdS and other II-VI semiconductor nanocrystals in noncoordinating solvents: tunable reactivity of monomers. Angew. Chem. Int. Ed Engl 41, 2368–2371. [DOI] [PubMed] [Google Scholar]
- Zhan N, Palui G, Safi M, Ji X and Mattoussi H (2013) Multidentate zwitterionic ligands provide compact and highly biocompatible quantum dots. J. Am. Chem. Soc 135, 13786–13795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.