To the editor:
Although the pathogenesis of minimal change disease (MCD) has not yet been clearly elucidated, immune dysregulation is probable,1 and the risk of relapse after the administration of various vaccines (especially meningococcal C conjugate vaccines) has been suggested.2 , 3 Here, we report a case of MCD relapse following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination.
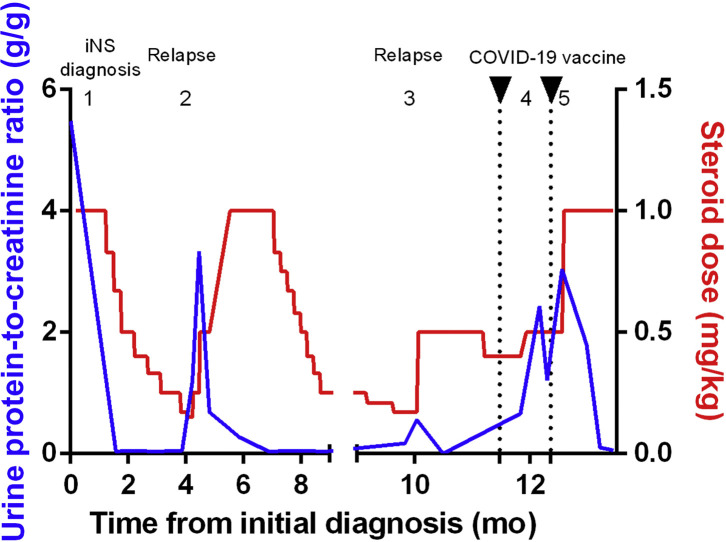
The patient was a 34-year-old woman with a recent history of nephrotic syndrome diagnosed as MCD. Steroids allowed complete remission, but she was cortico-dependent, as 2 relapses occurred during steroid tapering (Figure 1 ). A second-line treatment (rituximab) was thus planned, after vaccination against SARS-CoV-2 with the BNT162b2 vaccine (Pfizer-BioNTech). Six weeks before the first injection, steroids had been increased (0.5 mg/kg) due to the onset of low-grade proteinuria, leading to remission. Ten days after the first vaccine injection (ongoing steroid dose 0.4 mg/kg), she presented with full-blown relapse (edema; protein-to-creatinine ratio [PCR] of 2.4 g/g), leading to an increase of steroid dose to 0.5 mg/kg, achieving a decrease in proteinuria (PCR 1.2 g/g). She received the second injection (27 days after the first one), and a few days later, proteinuria increased again to a nephrotic range (PCR 3 g/g), leading to a new increase of steroid dose to 1 mg/kg that finally allowed complete remission. Response to the vaccine was satisfying, with a positive SARS-CoV-2 serology (anti-S protein antibodies assessed by electrochemiluminescence immunoassay [Roche Elecsys] before second injection at 55.2 U/ml), and no other immediate or delayed adverse event occurred following vaccination. This observation suggests that the BNT162b2 vaccine could favor MCD relapse, potentially through the stimulation of the immune response and the cytokine release it induces. Steroids seem efficient in achieving prompt remission.
Editor’s Note.
The RNA-platform COVID-19 vaccines strongly stimulate the immune system. Many of us who care for GN patients were concerned that these vaccines could cause disease flares in our patients. While this has not yet been studied systematically, it appears that most patients with GN who have been vaccinated have not had kidney disease exacerbations. Nonetheless, starting with one letter to the Journal describing gross hematuria in 2 patients with known IgA nephropathy after the second dose of a COVID-19 vaccine, Kidney International has received many similar reports. While IgA nephropathy seems to be the most frequently exacerbated GN, the following letters offer the experiences of our readers who have observed other types of glomerular disease worsened or uncovered after vaccination. Undoubtedly, these patients are trying to tell us something about the immunopathogenesis of their glomerular diseases.
Figure 1.
Urine protein-to-creatinine ratio and steroid dose during follow-up. (1) Diagnosis of idiopathic nephrotic syndrome (iNS; minimal change disease diagnosed on kidney biopsy). (2) First relapse when tapering steroids from 10 mg to 9 mg; increase of steroid dose to 1 mg/kg (60 mg), leading to remission of NS. (3) Second relapse when tapering steroids from 12.5 mg to 10 mg; increase of steroid dose to 0.5 mg/kg, leading to remission. Coronavirus disease 2019 (COVID-19) mRNA vaccine was injected while the patient was treated with 0.4 mg/kg steroids (25 mg; ongoing tapering from last relapse occurring 6 weeks before first vaccine injection; no proteinuria on urine dipstick test at the time of vaccine injection). (4) Proteinuria after first mRNA COVID-19 vaccine injection; increase of steroid dose from 25 mg to 30 mg (0.5 mg/kg). (5) Proteinuria increase after second mRNA COVID-19 vaccine injection; increase of steroids to 60 mg (1 mg/kg), leading to remission.
References
- 1.Vivarelli M., Massella L., Ruggiero B., Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12:332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel C., Shah H.H. Vaccine-associated kidney diseases: a narrative review of the literature. Saudi J Kidney Dis Transpl. 2019;30:1002. doi: 10.4103/1319-2442.270254. [DOI] [PubMed] [Google Scholar]
- 3.Abeyagunawardena A., Goldblatt D., Andrews N., Trompeter R. Risk of relapse after meningococcal C conjugate vaccine in nephrotic syndrome. Lancet. 2003;362:449–450. doi: 10.1016/s0140-6736(03)14072-x. [DOI] [PubMed] [Google Scholar]