Abstract
During the COVID-19 pandemic, donor hematopoietic stem cell grafts are frequently cryopreserved to ensure the availability of graft before starting a conditioning regimen. However, the safety of cryopreservation has been controversial in unrelated hematopoietic stem cell transplantation (HSCT), especially for bone marrow (BM) grafts. In addition, in unrelated HSCT, the effect of the time from harvest to cryopreservation of donor grafts required for the transportation of donor graft has not been fully clarified. In this study, we retrospectively analyzed the first 112 patients with available data who underwent cryopreserved unrelated blood and marrow transplantation through the Japan Marrow Donor Program during the COVID-19 pandemic. There were 112 patients, including 83 who received BM grafts and 29 who received peripheral blood stem cell (PBSC) grafts. The median time from stem cell harvest to cryopreservation was 9.9 hours (range, 2.6 to 44.0 hours), and the median time from cryopreservation to infusion was 231.2 hours. The incidence of neutrophil engraftment at day 28 after HSCT was 91.1%, and among 109 patients (excluding 3 patients with early death), all but 1 patient achieved neutrophil engraftment within 60 days after HSCT. The time to neutrophil engraftment and time to platelet engraftment were shorter in PBSC transplantation compared with BM transplantation (BMT), but the differences were not statistically significant (P = .064 and .18). Multivariate analysis among BM recipients revealed that a higher number of frozen nucleated cells and the absence of HLA mismatch were associated with faster neutrophil engraftment. The time to neutrophil engraftment after unrelated cryopreserved BMT was not different from that after unrelated BMT without cryopreservation. Our findings suggest that unrelated donor BM and PBSC grafts can be safely cryopreserved even after transit from the harvest center to the transplantation center. In the current COVID-19 pandemic, cryopreservation can be considered as an option while balancing the risks and benefits of the procedure.
Key Words: Cryopreservation, COVID-19, Bone marrow transplantation, Peripheral blood stem cell transplantation, Japan Marrow Donor Program
INTRODUCTION
Hematopoietic stem cell transplantation (HCT) has been established as a curative treatment strategy for a variety of hematologic disorders. In autologous HCT, hematopoietic stem cell grafts are obtained from the patients themselves, whereas in allogeneic HCT. they are obtained from healthy donors. In autologous HCT, cryopreservation techniques are required, as patients should receive conditioning regimens between the stem cell harvest and stem cell infusion. Dimethyl sulfoxide (DMSO) has been successfully used as a cryoprotectant in the cryopreservation of hematopoietic stem cells [1]. The combination of hydroxyethyl starch and a lower concentration of DMSO was also investigated for the cryopreservation of hematopoietic stem cells without rate-controlled freezing [2,3]. In Japan, CP-1 (Kyokutoseiyaku, Tokyo, Japan), a freezing medium that enables cryopreservation with a final concentration of hydroxyethyl starch at 6% and DMSO at 5%, is commercially available and widely used in HCT [4,5]. In contrast to autologous HCT, cryopreservation is not necessary in allogeneic HCT; however, in allogeneic peripheral blood stem cell (PBSC) transplantation (PBSCT), donor grafts are often cryopreserved to start a conditioning regimen after confirming a sufficient collection of stem cells [6]. On the other hand, donor grafts are rarely cryopreserved in allogeneic bone marrow (BM) transplantation (BMT) to avoid unnecessary mononuclear cell separation and cryopreservation [6]. In addition, cryopreservation is generally not permitted in unrelated BMT through the Japan Marrow Donor Program (JMDP), because the cryopreservation of donor grafts may increase the number of unused grafts.
In 2020, however, during the COVID-19 pandemic, the JMDP introduced the cryopreservation of donor grafts as an exception, because donor cell collection may be practically impossible during this period. Although the cryopreservation of allogeneic donor grafts has been shown to be safe and effective in small case series, sufficient information is still not available, especially for the preservation of donor BM grafts [7,8]. In addition, in unrelated HCT, the effect of the time from harvest to cryopreservation of donor grafts required for the transportation of donor graft has not been fully clarified. Therefore, in this study, we retrospectively analyzed the first 112 patients with available data who underwent cryopreserved unrelated HCT through the JMDP during the COVID-19 pandemic.
METHODS
Patients
All the requests for the cryopreservation of stem cells were reviewed by the JMDP Central Office. After approval, stem cells were collected at harvest centers and then shipped to transplantation centers under room temperature. All the stem cells were cryopreserved at the transplantation centers on receipt.
Questionnaires were sent to transplantation centers to retrospectively collect data on unrelated cryopreserved HCT performed through the JMDP between April and November 2020, and all but 1 patient without response to the questionnaire were included in this study. This study was approved by the Ethics Committee of the JMDP.
Statistical Analysis
The study's primary endpoint was neutrophil engraftment, defined as the first of the 3 consecutive days with an absolute neutrophil count of at least 0.5 × 103/μL, and platelet engraftment, defined as the first day with a platelet count exceeding 20 × 103/μL without platelet transfusion for at least 7 days. Fisher's exact test was used to compare categorical variables, and the Mann–Whitney U test was used to compare continuous variables. Time to engraftment data were analyzed while treating death without engraftment as a competing risk and compared between groups with Gray's test. Multivariate analysis was performed using Fine–Gray proportional hazards modeling based on an available case analysis for missing data, including variables with P < .15 on univariate analysis as independent variables. Information about the use of granulocyte colony-stimulating factor (G-CSF) was not obtained, and thus background diseases were grouped into myeloid malignancies and others. This was also included as an independent variable as a substitute for the use of G-CSF, because the use of G-CSF was avoided for myeloid malignancies in some centers.
In addition, the patients who received cryopreserved BM grafts were compared with those who received BM grafts without cryopreservation between January 2016 and December 2018 with and without matching for recipient age, donor age, background disease, background disease status, and HLA mismatch. Categorical variables were strictly matched, whereas continuous variables were matched using caliper widths equal to 0.2 SD.
All P values were 2-sided, and P<.05 was considered to indicate statistical significance. All statistical analyses were performed with EZR version 1.54 (Saitama Medical Center, Jichi Medical University, Shimotsuke, Japan) [9].
RESULTS
Patients and Stem Cell Grafts
The study cohort comprised 112 patients (64 males and 48 females) with a median age of 54 years (range, 12-69 years). Eighty-three patients underwent BMT and 29 underwent PBSCT. In this period, 456 BM grafts and 134 PBSC grafts were transplanted through the JMDP, and thus 18.2% of the BM grafts and 21.6% of the PBSC grafts had been cryopreserved. The median age of the donors was 41.5 years (range, 20-54 years). The background disease was acute myelogenous leukemia in 32 patients, acute lymphoblastic leukemia in 18, chronic myelogenous leukemia in 4, myelodysplastic syndrome in 30, myeloproliferative neoplasms in 7, adult T cell leukemia/lymphoma in 7, lymphoma in 7, plasma cell neoplasms in 2, and nonmalignant diseases in 5. There was an HLA mismatch between the donor and the recipient in 43 HCTs (38.3%). CP-1 was used for cryopreservation in 106 patients, and DMSO was used in 6 patients. Frozen grafts were stored in a deep freezer in 77 patients and in liquid nitrogen in 35 patients. The median time from stem cell harvest to cryopreservation was 9.9 hours (range, 2.6-44.0 hours), and the median time from cryopreservation to infusion was 231.2 hours (range, 36.0-664.7 hours). The patient characteristics grouped according to stem cell source are shown in Table 1 .
Table 1.
Patient characteristics according to type of stem cell graft
 |
Neutrophil and Platelet Engraftment
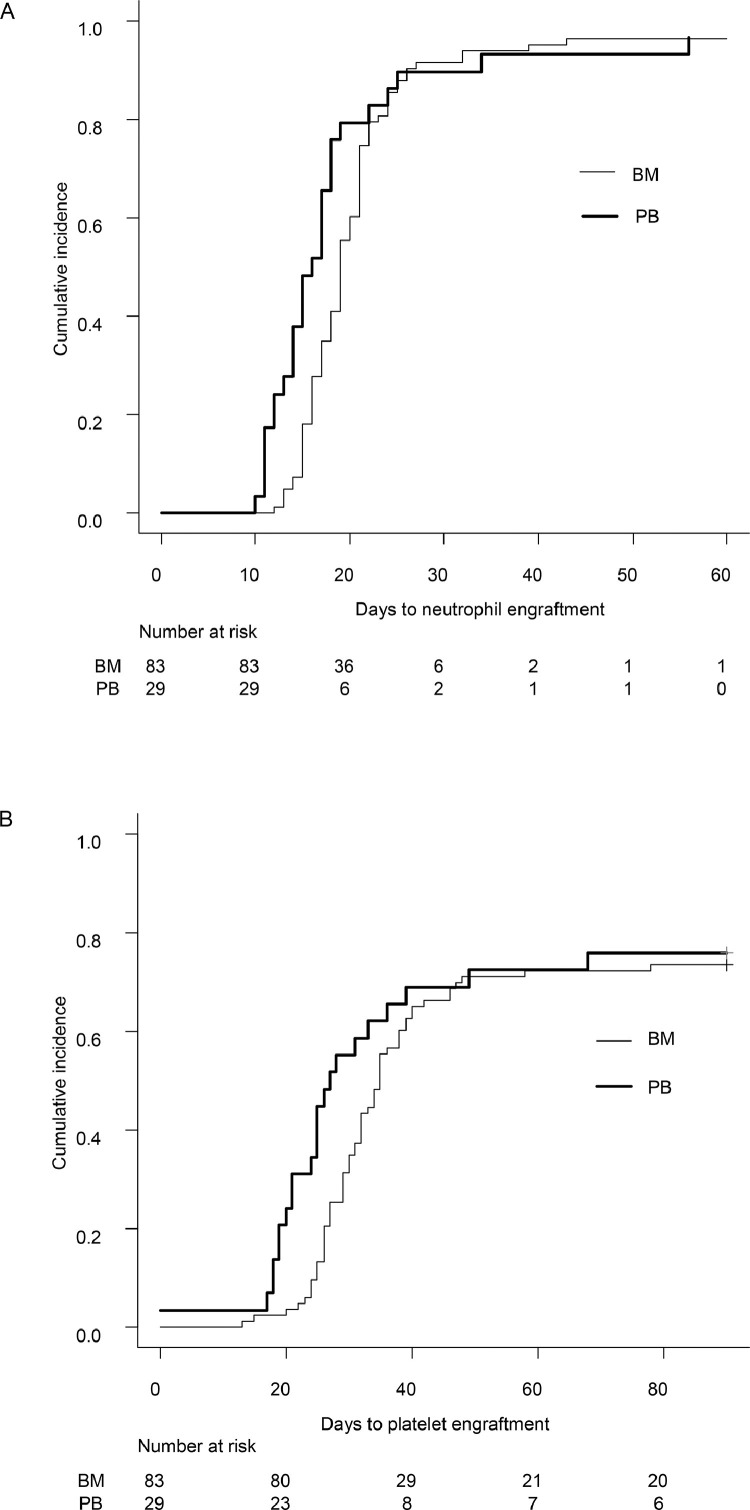
The incidence of neutrophil engraftment at day 28 after HCT was 91.1% (95% confidence interval [CI], 83.8%-95.2%), treating death without engraftment as a competing risk, and among 109 patients (excluding 3 patients who died early, at 16, 23, and 35 days after HCT), all but 1 patient achieved neutrophil engraftment within 60 days after HCT. The incidence of platelet engraftment was 33.0% (95% CI, 24.5%-41.8%) at day 28 post-HCT and 72.3% (95% CI, 62.9%-79.7%) at day 60 post-HCT. The time to neutrophil engraftment and time to platelet engraftment were shorter in PBSCT than in BMT (median, 16 days [range, 14-18 days] versus 19 days [range, 18-21 days] for neutrophil engraftment and 27 days [range, 24-49 days] versus 35 days [range, 32-39 days] for platelet engraftment), but the differences were not statistically significant (P = .064 and .18, respectively) (Figure 1 ).
Figure 1.
Time to neutrophil (A) and platelet (B) engraftment according to the stem cell source.
Predictive Factors of Neutrophil Engraftment in BMT
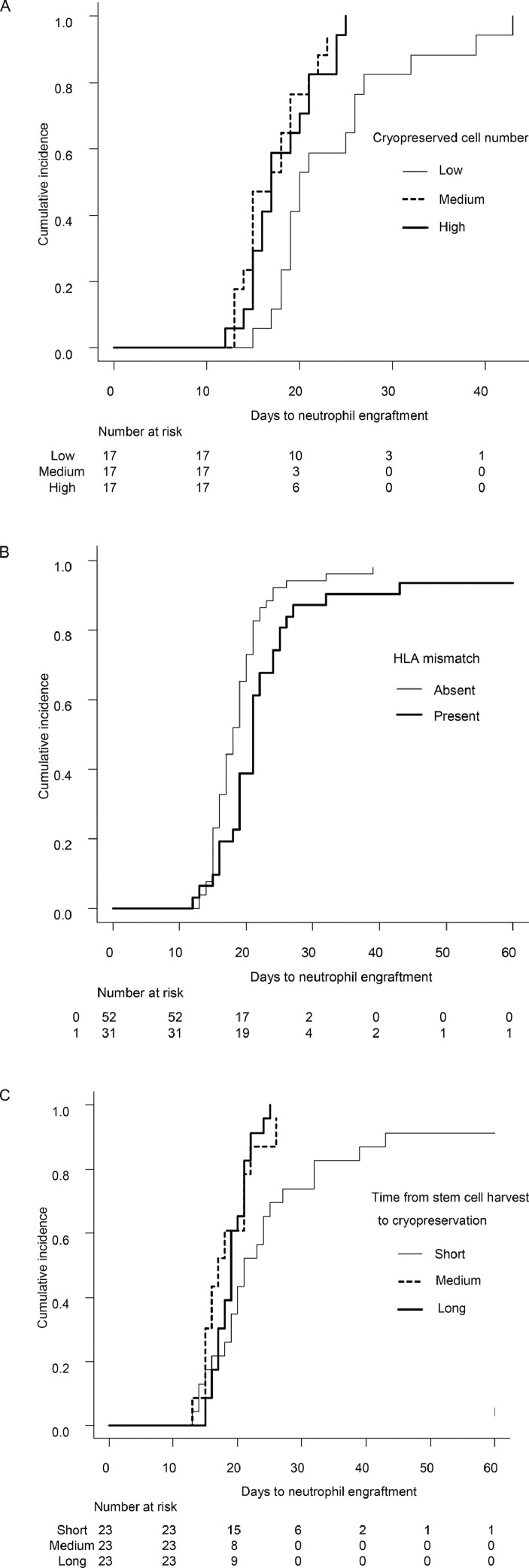
Factors that predicted neutrophil engraftment after HCT were analyzed only in BMT, because the number of PBSCT recipients was limited. Continuous variables were divided equally into 3 groups and treated as categorical variables. Univariate analysis revealed that the number of frozen nucleated cells, not harvested cells, and the presence of HLA mismatch were significantly associated with neutrophil engraftment (Table 2 , Figure 2 A, B). The time from stem cell harvest to cryopreservation was also significant, but, contrary to our expectations, the shortest duration was associated with delayed neutrophil engraftment (Figure 2C).
Table 2.
Univariate and multivariate analyses for neutrophil engraftment after unrelated BMT
 |
Figure 2.
Time to neutrophil engraftment after unrelated BMT, according to the number of cryopreserved nucleated cells (A), the presence of HLA mismatch (B), and the time from stem cell harvest to cryopreservation (C).
Multivariate analysis revealed that a higher number of frozen nucleated cells and the presence of HLA mismatch were independently associated with neutrophil engraftment. The time from stem cell harvest to cryopreservation was not significant in the multivariate analysis.
Comparison of BMT with Cryopreservation and without Cryopreservation
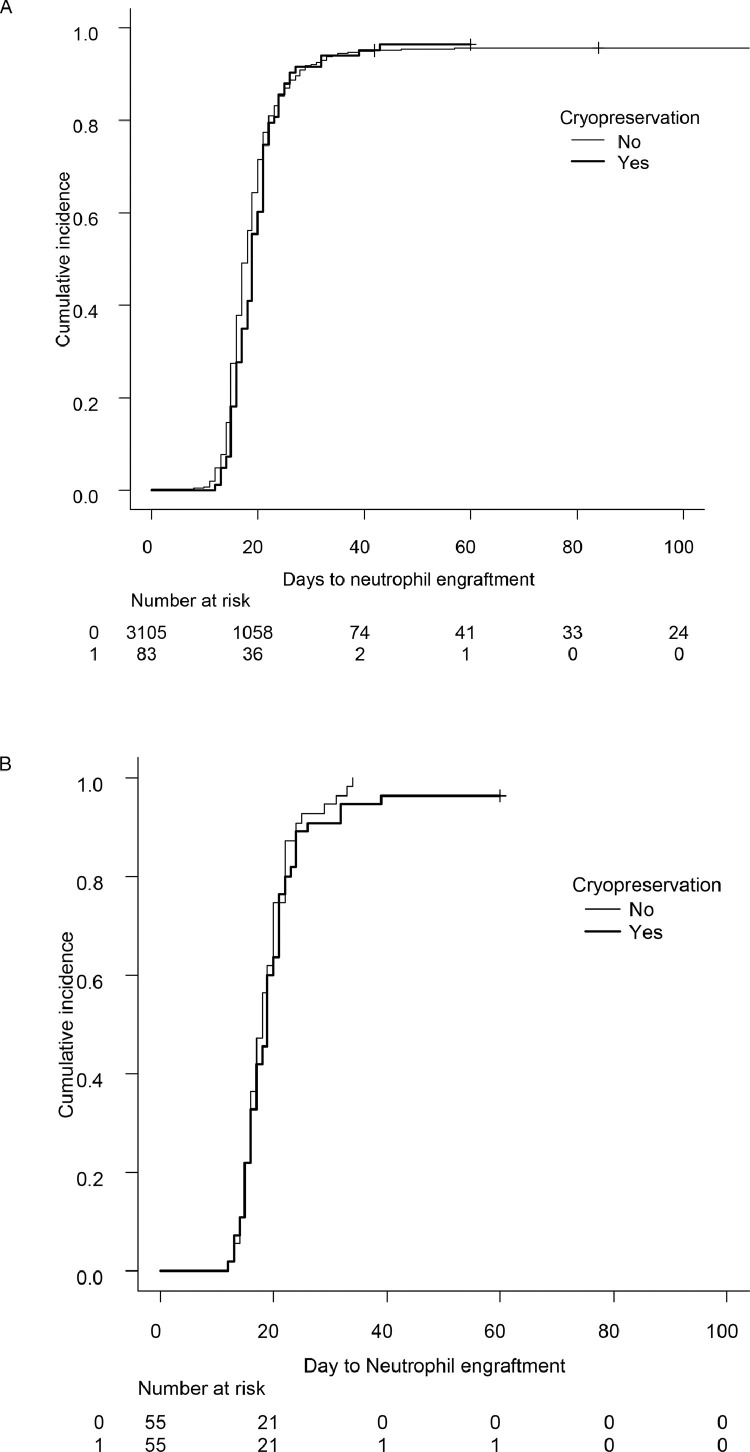
We compared neutrophil engraftment between the 83 patients who received cryopreserved BM grafts and 3105 patients who received BM grafts without cryopreservation. The median day to engraftment was 18 in the cryopreserved BM group and 19 days in the noncryopreserved BM group (P = .32), and the engraftment curves were superimposed (Figure 3 A). After matching for recipient age, donor age, background disease, background disease status, and HLA mismatch, 55 pairs were matched, and no significant difference in neutrophil engraftment was observed (median 18 and 19 days, respectively; P = .32) (Figure 3B).
Figure 3.
Time to neutrophil engraftment after unrelated BMT with or without cryopreservation, before (A) and after (B) matching for recipient age, donor age, background disease, background disease status, and HLA mismatch.
DISCUSSION
During the COVID-19 pandemic, donor grafts are more frequently cryopreserved to ensure graft availability before starting a conditioning regimen [10]. The safety of cryopreservation has been established in allogeneic PBSCT, and most allogeneic PBSCTs are safely performed using cryopreserved grafts [6]. In addition, a recent large retrospective study from Princess Margaret Cancer Centre showed no difference in engraftment or survival between cryopreserved and fresh PBSC grafts [11]. On the other hand, there has been less experience with allogeneic BMT using cryopreserved grafts. Hamadani et al. [12] recently reported that graft cryopreservation did not affect the outcomes of HCT using post-transplantation cyclophosphamide, but most recipients received PBSC grafts. In allogeneic HCT for severe aplastic anemia, the use of cryopreserved graft was associated with increased graft failure and inferior overall survival [13]. In this study, two-thirds of the patients received BM grafts. Therefore, the safety of cryopreservation of allogeneic BM grafts has not been established. In addition, most previous large studies analyzed HCT from related donors, and thus in unrelated HCT, in which the time from stem cell harvest to cryopreservation is longer than in related HCT, even the safety of the cryopreservation of PBSC grafts is unclear. Lioznov et al. [14] suggested that PBSC grafts are more sensitive to cryopreservation after transport and storage. A recent analysis from Australia showed that a longer transit time before cryopreservation was associated with inferior post-thaw viability of CD34+ cells [15].
In this study, however, when we excluded 3 patients with early death, all but 1 patient achieved neutrophil engraftment. Therefore, both unrelated BM and PBSC grafts appeared to be safely cryopreserved. The transit time before cryopreservation in the longest group was between 11.0 and 32.1 hours in the BMT recipients and between 26.6 and 44.0 hours in the PBSCT recipients, suggesting that a transit time of up to 24 hours before cryopreservation may be acceptable. An unexpected finding in this study was the relationship between the shorter time from stem cell harvest to cryopreservation and delayed engraftment in the univariate analysis. However, this relationship was not confirmed in the multivariate analysis. We considered that the coincidentally higher proportion of patients with nonremission diseases and the lower number of cryopreserved nucleated cells in the shortest transit time group, although not statistically significant, resulted in the significantly delayed engraftment in the univariate analysis, but not in the multivariate analysis adjusted for these factors.
The cryopreserved nucleated cell number, rather than the harvested nucleated cell number, was associated with neutrophil engraftment after unrelated cryopreserved BMT. This may reflect that the number of cryopreserved cells is more closely related to the number of hematopoietic precursor cells, by condensing mononuclear cells before cryopreservation. The number of cryopreserved mononuclear cells in the lowest group ranged between 0.31 and 0.54 × 1010. Although we did not have information on patient body weight, if we assume an average body weight of 60 kg and consider that the median recovery rate of mononuclear cells before cryopreservation was 0.50 in this study, the number of cryopreserved mononuclear cells in the lowest group corresponds to a range of 1.0 to 1.8 × 108/kg harvested nucleated cells. A limitation of this study is the lack of data on the viability of graft cells before and after cryopreservation, but the fact that the number of mononuclear cells before cryopreservation was significantly associated with neutrophil engraftment suggests that the cryopreservation procedure might not have strongly affected graft quality.
In conclusion, unrelated donor BM and PBSC grafts can be safely cryopreserved even after the transit time from the harvest center to the transplantation center. In the current COVID-19 pandemic, cryopreservation can be considered as an option while balancing the risks and benefits of the procedure.
ACKNOWLEDGMENTS
Financial disclosure: Nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
Footnotes
Members of the Medical Committee of the Japan Marrow Donor Program: Yoshinobu Kanda, Masami Inoue, Naoyuki Uchida, Yasushi Onishi, Reiko Kamata, Mika Kotaki, Ryoji Kobayashi, Junji Tanaka, Takahiro Fukuda, Nobuharu Fujii, Koichi Miyamura, Shin-Ichiro Mori, Yasuo Mori, Yasuo Morishima, and Hiromasa Yabe.
Financial disclosure: See Acknowledgments on page 664.e6.
REFERENCES
- 1.Appelbaum FR, Herzig GP, Ziegler JL, Graw RG, Levine AS, Deisseroth AB. Successful engraftment of cryopreserved autologous bone marrow in patients with malignant lymphoma. Blood. 1978;52:85–95. [PubMed] [Google Scholar]
- 2.Katayama Y, Yano T, Bessho A, et al. The effects of a simplified method for cryopreservation and thawing procedures on peripheral blood stem cells. Bone Marrow Transplant. 1997;19:283–287. doi: 10.1038/sj.bmt.1700644. [DOI] [PubMed] [Google Scholar]
- 3.Takaue Y, Abe T, Kawano Y, et al. Comparative analysis of engraftment after cryopreservation of peripheral blood stem cell autografts by controlled- versus uncontrolled-rate methods. Bone Marrow Transplant. 1994;13:801–804. [PubMed] [Google Scholar]
- 4.Stiff PJ, Murgo AJ, Zaroulis CG, DeRisi MF, Clarkson BD. Unfractionated human marrow cell cryopreservation using dimethylsulfoxide and hydroxyethyl starch. Cryobiology. 1983;20:17–24. doi: 10.1016/0011-2240(83)90054-8. [DOI] [PubMed] [Google Scholar]
- 5.Makino S, Harada M, Akashi K, et al. A simplified method for cryopreservation of peripheral blood stem cells at -80 degrees C without rate-controlled freezing. Bone Marrow Transplant. 1991;8:239–244. [PubMed] [Google Scholar]
- 6.Ikeda K, Ohto H, Okuyama Y, et al. Adverse events associated with infusion of hematopoietic stem cell products: a prospective and multicenter surveillance study. Transfus Med Rev. 2018;32:186–194. doi: 10.1016/j.tmrv.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Stockschläder M, Krüger W, tom Dieck A, et al. Use of cryopreserved bone marrow in unrelated allogeneic transplantation. Bone Marrow Transplant. 1996;17:197–199. [PubMed] [Google Scholar]
- 8.Stockschläder M, Krüger W, Kroschke G, et al. Use of cryopreserved bone marrow in allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995;15:569–572. [PubMed] [Google Scholar]
- 9.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devine SM. Transplantation of allogeneic cryopreserved hematopoietic cell grafts during the COVID-19 pandemic: a National Marrow Donor Program perspective. Am J Hematol. 2021;96:169–171. doi: 10.1002/ajh.26052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alotaibi AS, Prem S, Chen S, et al. Fresh vs. frozen allogeneic peripheral blood stem cell grafts: a successful timely option. Am J Hematol. 2021;96:179–187. doi: 10.1002/ajh.26033. [DOI] [PubMed] [Google Scholar]
- 12.Hamadani M, Zhang MJ, Tang XY, et al. Graft cryopreservation does not impact overall survival after allogeneic hematopoietic cell transplantation using post-transplantation cyclophosphamide for graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2020;26:1312–1317. doi: 10.1016/j.bbmt.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eapen M, Zhang MJ, Tang XY, et al. Hematopoietic cell transplantation with cryopreserved grafts for severe aplastic anemia. Biol Blood Marrow Transplant. 2020;26:e161–e166. doi: 10.1016/j.bbmt.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lioznov M, Dellbrügger C, Sputtek A, Fehse B, Kröger N, Zander AR. Transportation and cryopreservation may impair haematopoietic stem cell function and engraftment of allogeneic PBSCs, but not BM. Bone Marrow Transplant. 2008;42:121–128. doi: 10.1038/bmt.2008.93. [DOI] [PubMed] [Google Scholar]
- 15.Purtill D, Antonenas V, Chiappini P, et al. Variable CD34+ recovery of cryopreserved allogeneic HPC products: transplant implications during the COVID-19 pandemic. Blood Adv. 2020;4:4147–4150. doi: 10.1182/bloodadvances.2020002431. [DOI] [PMC free article] [PubMed] [Google Scholar]