Abstract
Aim
To estimate the mortality rate, the rate of return of spontaneous circulation (ROSC) and survival with favorable neurological outcome in patients with COVID-19 after in-hospital cardiac arrest (IHCA) and attempted cardiopulmonary resuscitation (CPR).
Methods
PubMed, EMBASE, Web of Science, bioRxiv and medRxiv were surveyed up to 8th February 2021 for studies reporting data on mortality of patients with COVID-19 after IHCA. The primary outcome sought was mortality (in-hospital or at 30 days) after IHCA with attempted CPR. Additional outcomes were the overall rate of IHCA, the rate of non-shockable presenting rhythms, the rate of ROSC and the rate of survival with favorable neurological status.
Results
Ten articles were included in the systematic review and meta-analysis, for a total of 1179 COVID-19 patients after IHCA with attempted CPR. The estimated overall mortality rate (in-hospital or at 30 days) was 89.9% (95% Predicted Interval [P.I.] 83.1%–94.2%; 1060/1179 patients; I2 = 82%). The estimated rate of non-shockable presenting rhythms was 89% (95% P.I. 82.8%–93.1%; 1022/1205 patients; I2 = 85%), and the estimated rate of ROSC was 32.9% (95% P.I. 26%–40.6%; 365/1205 patients; I2 = 82%). The estimated overall rate of survival with favorable neurological status at 30 days was 6.3% (95% P.I. 4%–9.7%; 50/851 patients; I2 = 48%). Sensitivity analysis showed that COVID-19 patients had higher risk of death after IHCA than non COVID-19 patients (OR 2.34; 95% C.I. 1.37–3.99; number of studies = 3; 1215 patients).
Conclusions
Although one of three COVID-19 patients undergoing IHCA may achieve ROSC, almost 90% may not survive at 30 days or to hospital discharge.
Keywords: COVID-19, Cardiac arrest, cpr, Mortality
Introduction
Patients with coronavirus disease 2019 (COVID-19) can develop severe acute respiratory failure and hypoxemia.1 Indeed, cardiorespiratory comorbidities have been identified as risk factors for the development of the most severe forms of the disease.1 Cardiac arrest may thus complicate the in-hospital clinical course of these patients, as a consequence of various triggers, both pneumological (e.g. severe hypoxemia, pneumothorax) and cardiological ones (e.g pulmonary embolism, QT interval prolongation).2, 3 Moreover, COVID-19 pandemic has determined the hospitalization of an unprecedented number of patients which has been associated with a high number of in-hospital cardiac arrests (IHCA).4
Poor outcomes have been described in patients with COVID-19 undergoing IHCA, despite cardiopulmonary resuscitation (CPR) attempts.5 At the same time, cardiopulmonary resuscitation of a patient with COVID-19 carries inherent risks for healthcare workers as resuscitation procedures may disperse aerosol.6, 7 Much public and scientific debate has therefore surrounded the ethics of CPR and do-not-attempt resuscitation (DNAR) orders in patients with COVID-19.8 At this time, the rates of mortality and other relevant clinical outcomes in patients with COVID-19 after IHCA remain unclear and the data surrounding this topic is fragmented.9
The aim of this systematic review and meta-analysis was to estimate the mortality rate in patients with COVID-19 after IHCA and attempted CPR from the available literature. We also sought to estimate the rate of return of spontaneous circulation (ROSC) and survival with favorable neurological outcome in the same population of patients.
Methods
The protocol of this systematic review and meta-analysis was prospectively registered in Open Science Framework (osf.io/45n2c). A comprehensive search of PubMed, EMBASE and Web of Science publication databases was conducted on 8th February 2021 without limiting the results by date filters. BioRxiv and medRxiv pre-print servers were also searched using ‘cardiac arrest’, ‘cpr’ and ‘COVID-19’ as search terms and keywords. No language restriction was applied. The retrieved records were considered relevant if they provided data on mortality after in-hospital cardiac arrest occurring in adult patients with COVID-19. Nonrandomized studies, both prospective and retrospective, and case series were included. Abstracts and case reports were excluded. The full search strategy is provided as Supplementary material 1. The retrieved records were independently screened using the titles and abstracts by two authors (MI, CM). The articles selected during this screening were then downloaded in full-text and independently reviewed by the same two authors. Studies were included if the two screening authors agreed regarding eligibility and, in case of disagreement, a third author adjudicated (AC). The reference lists of relevant articles were also searched for additional potentially relevant papers (i.e. snowballing method). The data were extracted in duplicate by two authors (MI, GC) using a standard data extraction form. Discrepancies in the extracted data were also adjudicated by a third author (AC). To reduce heterogeneity from studies that also provided data on patients developing IHCA who had not received CPR, we extracted only the data on those patients who received CPR. The final version of the database was validated by all the authors involved in data collection (MI, GC, CM, AC). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and the checklist for meta-analysis of observational studies (MOOSE) are provided in Tables S1 and S2, Supplementary material 2.
Outcomes
The primary outcome sought was mortality after IHCA with attempted CPR (in-hospital or at 30 days if the former was not reported). Additional outcomes were the overall rate of IHCA, the rate of non-shockable presenting rhythms, the rate of return of spontaneous circulation (ROSC) and the rate of survival with favorable neurological functional status, defined as a Cerebral Performance Category (CPC) score of 1 or 2 (CPC 1 – Good cerebral performance: conscious, alert, able to work, might have mild neurologic or psychologic deficit; CPC 2 – Moderate cerebral disability: conscious, sufficient cerebral function for independent activities of daily life. Able to work in sheltered environment; CPC 3 – Severe cerebral disability: conscious, dependent on others for daily support because of impaired brain function. Ranges from ambulatory state to severe dementia or paralysis; CPC 4 – Coma or vegetative state: any degree of coma without the presence of all brain death criteria. Unawareness, even if appears awake (vegetative state) without interaction with environment; may have spontaneous eye opening and sleep/awake cycles. Cerebral unresponsiveness; CPC 5 – Brain death: apnea, areflexia, EEG silence, etc.).10
Qualitative analysis
Two investigators (AC, MI) performed a qualitative analysis of the included studies independently and in duplicate. Disagreements over assessments were resolved by consensus or, if necessary, adjudicated by a third author (SE). As our primary outcome was an incidence, the Methodological Index for Non Randomized studies (MINORS)11 was used for the qualitative assessment, due to its ability to evaluate the methodological quality of single arm studies. The eight items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). The global ideal score is 16 for non-comparative studies.11
Quantitative analysis
Meta-analysis was performed if two or more of the included studies reported data on the outcomes of interest. To maintain symmetry, the summary estimates were calculated using logit transformation of individual study proportions and presented along with the corresponding 95% prediction interval (P.I.). Random effect models (DerSimonian-Laird) were used for all the analyses. For the outcome of mortality, we performed sub-group analyses based on the level of care (i.e. ICU and non-ICU) and on the number of centres per study (i.e. multicentre, single centre). We also performed a sensitivity analysis on papers comparing the mortality rates of patients with COVID-19 to those of non COVID-19 after IHCA, providing odds ratio as a measure of risk.
The I-squared (I2) statistical model was used to describe the percentage of variation across the included studies due to heterogeneity. All the analyses were performed by AC and MI, with input from SE, using OpenMeta-Analyst.12
Results
Characteristics of included studies and patients
The search identified a total of 2088 records. After the exclusion of duplicates and not relevant records, ten articles were included, evaluating a total of 1179 COVID-19 patients after IHCA and attempted CPR.5, 13, 14, 15, 16, 17, 18, 19, 20, 21 The process of inclusion and exclusion, detailed in a the PRISMA flow diagram, is presented as Fig. S1 in Supplementary material 2. None of the included articles was published in pre-print servers.
All the included articles reported retrospective studies. Three were multicentre and seven were single centre studies. Nine studies reported data on a mixed cohort of patients in both the intensive care unit (ICU) and other wards. One study included only ICU patients. Eight studies were conducted in the U.S., one was conducted in China and one in Sweden. All the included studies specifically included patients with IHCA and CPR, except one that also provided data on patients with IHCA that did not undergo CPR.13 All the studies specifically included patients with COVID-19, but three studies also reported data on historical cohorts of non COVID-19 patients.15, 19, 21
The mean age of the patients ranged from 61 to 69 years. Overall, 64.9% (782/1205) of the patients developed cardiac arrest in ICUs, 35.1% (423/1205) in non-ICU settings. The characteristics of the included studies, and the overall qualitative assessment (MINORS score) are provided as Table 1 .
Table 1.
Main characteristics of the included studies.
| Authors (year) [ref.] | Design (country) | Setting | Populationa | Presenting rhythm | Outcomes | Qualitative assessmentb |
|---|---|---|---|---|---|---|
| Bhatla et al. (2020)18 | Single centre retrospective study (US) | Both ICU and non-ICU | 9 ICU patients with COVID-19 (PCR testing) who developed IHCA and received CPR ICU: 100% (9/9) |
Non-shockable: 89% (8/9) Shockable: 11% (1/9) |
In-hospital mortality: 44% (4/9) ROSC: 66% (6/9) Discharged alive: 22% (2/9) Still hospitalized: 33% (3/9) |
12/16 |
| Hayek et al. (2020)13 | Multicentre retrospective study (US) | 68 ICUs | 400 ICU patients with COVID-19 (laboratory confirmed) who developed IHCA and received CPR ICU: 100% (400/400) Age: 61 ± 14 y.o. Male: 66.5% (266/400) SOFA: 5.9 ± 3.3 On vasopressors: 56.5% (226/400) On IMV: 64% (257/400) |
Non-shockable: 73% (294/400) Shockable: 12% (48/400) |
In-hospital mortality: 88% (352/400) ROSC: 34% (135/400) Favorablecneurological status: 7% (28/400) |
14/16 |
| Miles et al. (2020)21d | Single centre retrospective study (US) | Both ICU and non-ICU | 125 patients who developed IHCA and received CPR during pandemic (99 COVID-19 positive at PCR testing, 12 negative, 14 indeterminate) ICU: 33% (41/125) Age: 67 (IQR 57–76) Male: 66% (82/125) |
Non-shockable: 90% (113/125) Shockable: 3% (4/125) Unknown: 6% (8/125) |
In-hospital mortality: 98% (97/99) ROSC: 3.6% (45/125) |
13/16 |
| Mitchell et al. (2020)16 | Multicentre retrospective study (US) | Both ICU and non-ICU (11 hospitals) | 260 hospitalized patients with COVID-19 (at PCR testing) who developed IHCA and received CPR ICU: 64% (166/260) Age: 69 y.o. (IQR 60−77) Male: 71.5% (186/260) |
Non-shockable: 90% (233/260) Shockable: 8% (22/260) Unknown: 2% (5/260) |
In-hospital mortality: 88% (229/260) 30-day mortality: 88% (228/260) ROSC: 22% (58/260) Favorablecneurological status at 30 day: 6% (16/260) |
13/16 |
| Shah et al. (2021)5 | Single centre retrospective study (US) | Both ICU and non-ICU | 63 hospitalized patients with COVID-19 (PCR testing) who developed IHCA and received CPR ICU: 84% (53/63) Age: 66 y.o. (IQR 59–74) Male: 49.2% (31/63) CCI ≥ 5: 40% (29/63) On vasopressors: 60% (38/63) |
Non-shockable: 92% (58/63) Shockable: 8% (5/63) |
In-hospital mortality: 100% (63/63) ROSC: 29% (18/63) |
13/16 |
| Shao et al. (2020)14 | Single centre retrospective study (China) | Both ICU and non-ICU | 136 hospitalized patients with severe COVID-19 (WHO definitions) who developed IHCA and received CPR ICU: 17% (23/136) Age 69 y.o. (IQR 61−77) Male: 66% (90/136) |
Non-shockable: 94% (128/136) Shockable: 6% (8/136) |
30-day mortality: 97% (132/136) ROSC: 13% (18/136) Favorablecneurological status at 30day: 0.7% (1/136) |
13/16 |
| Sheth et al. (2020)17 | Single centre retrospective case series (US) | Both ICU and non-ICU | 31 hospitalized patients with COVID-19 (PCR testing) who developed IHCA and received CPR ICU: 77% (24/31) Age: 69 y.o. (IQR 57−76) Male: 71% (22/31) SOFA: 9 (IQR 4−13) On IMV: 58% (18/31) |
Non-shockable: 87% (27/31) Shockable: 13% (4/31) |
In-hospital mortality: 100% (31/31) ROSC: 42% (13/31) |
12/16 |
| Sultanian et al. (2021)19 | Multicentre retrospective registry-based study (Sweden) | 72 emergency wards connected to the Swedish National registry | 72 hospitalized patients with COVID-19 (confirmed, suspected or recent) who developed IHCA and received CPR ICU: 14% (10/72) Age: 67.8 ± 13.0 y.o. Male: 68% (49/72) |
Non-shockable: 83% (60/72) Shockable: 18% (12/72) |
30-day mortality: 75% (54/72) ROSC: 31% (22/72) |
13/16 |
| Thapa et al. (2021)20 | Single centre retrospective study (US) | Both ICU and non-ICU | 54 hospitalized patients with COVID-19 who developed IHCA and received CPR ICU: 18.5% (10/54) Age: 61 y.o. (IQR 50−68) Male: 61% (33/54) On vasopressors: 46% (25/54) On MV: 79% (43/54) |
Non-shockable: 96% (52/54) Shockable: 4% (2/54) |
In-hospital mortality: 100% (54/54) ROSC: 54% (29/54) |
12/16 |
| Yuriditsky et al. (2020)15 | Single centre retrospective observational study (US) | Both ICU and non-ICU | 55 hospitalized patients with COVID-19 (PCR testing) who developed IHCA and received CPR ICU: 83.6% (46/55) Age: 69 y.o. (IQR 64−77) Male: 87% (48/55) On vasopressors/inotropes: 67% (37/55) On IMV: 76% (42/55) |
Non-shockable: 89% (49/55) Shockable: 11% (6/55) |
30-day mortality: 80% (44/55) ROSC: 38% (21/55) Favorablecneurological status at 30 day: 9% (5/55) |
13/16 |
The table shows the main characteristics of the included studies, as reported by the authors, and the qualitative assessment performed using the MINORS tool.
COVID-19, coronavirus disease 2019; CPC, Cerebral Performance Category; CPR, cardiopulmonary resuscitation; ICU, intensive care unit; IHCA, in-hospital cardiac arrest; IMV, invasive mechanical ventilation; IQR, interquartile range; MINORS, Methodological Index for Non-Randomized studies; MV, mechanical ventilation; PCR, polymerase chain reaction; ROSC, return of spontaneous circulation; US, United States.
The column shows the characteristics of the cohort of COVID-19 patients who developed IHCA and received attempts of CPR.
Qualitative assessment was performed using the MINORS score. The eight items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). The global ideal score being 16 for non-comparative studies.
Defined according to the Cerebral Performance Category (CPC) scoring system, as a CPC score of 1 or 2.
For this study, when disaggregated data were not available to describe the cohort of COVID-19 patients developing IHCA and receiving CPR, data were reported from the cohort of hospitalized patients during the pandemic, including 99 patients tested positive for COVID-19, but also 14 patients undergone to IHCA before the arrival of test result for COVID-19 (indeterminate), and 12 patients tested negative for COVID-19.
Six studies had 13/16 as overall qualitative assessment score,5, 14, 15, 16, 19, 21 three studies had 12/1617, 18, 20 and one had 14/16.13 The detailed qualitative assessment with individual domain score per study is provided as Table S3, Supplementary material 2.
Outcomes
Seven studies provided data on the rate of IHCA with attempted CPR in cohorts of hospitalized patients with COVID-19,5, 13, 14, 15, 18, 20 with an estimated overall rate of IHCA of 4.1% (95% P.I. 2.1%–7.8%; 816/17,028 patients; I2 = 99%; Fig. S2, Supplementary material 2).
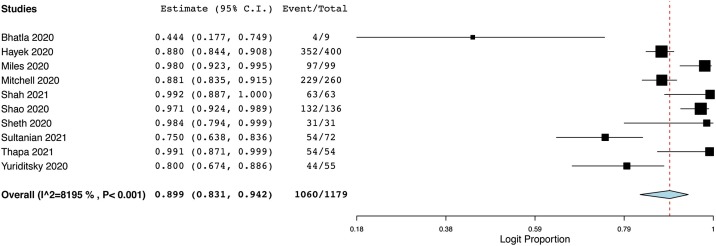
Among COVID-19 patients with IHCA and CPR the estimated overall mortality rate (in-hospital or at 30 days) was 89.9% (95% P.I. 83.1%–94.2%; 1060/1179 patients; I2 = 82%). Fig. 1 presents the forest plot depicting the results of the meta-analysis for mortality.
Fig. 1.
Forest plot reporting the results of the meta-analysis for mortality of COVID-19 patients undergoing in-hospital cardiac arrest with cardiopulmonary resuscitation.
The figure shows the forest plots reporting the results of meta-analysis for mortality (in-hospital or at 30-day when the former was not reported) of COVID-19 patients undergoing in-hospital cardiac arrest with cardiopulmonary resuscitation.
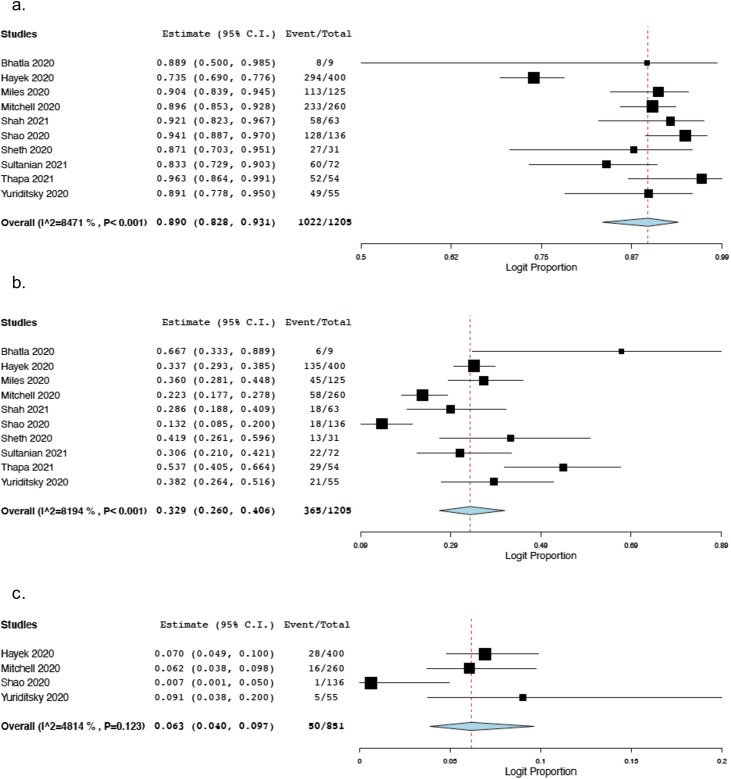
All the included studies provided information on the presenting rhythms. The estimated overall rate of non-shockable presenting rhythms was 89% (95% P.I. 82.8%–93.1%; 1022/1205 patients; I2 = 85%; Fig. 2 , panel a).
Fig. 2.
Forest plots reporting the results of the meta-analysis for additional outcomes of COVID-19 patients undergoing in-hospital cardiac arrest with cardiopulmonary resuscitation.
The figure shows the forest plots reporting the results of meta-analysis for non-shockable presentation rhythm (panel a), ROSC (panel b) and 30-day favorable neurological status (defined as a CPC score of 1 or 2) (panel c) of COVID-19 patients undergoing in-hospital cardiac arrest with cardiopulmonary resuscitation. In this analysis, the total number of patients in the study by Miles et al. included 12 patients tested negative for COVID-19 and 14 patients with indeterminate result at COVID-19 testing.
All the included studies provided information on ROSC. The estimated overall ROSC rate was 32.9% (95% P.I. 26%–40.6%; 365/1205 patients; I2 = 82%; Fig. 2, panel b).
Four studies provided data on the neurological status of survivors at 30 days.13, 14, 15, 16 The estimated overall rate of a favorable neurological status (defined as a CPC score of 1 or 2) at 30 days was 6.3% (95% P.I. 4%–9.7%; 50/851 patients; I2 = 48%; Fig. 2, panel c).
Subgroup analyses
When the arrest occurred in an ICU, the estimated mortality rate was 85.8% (95% P.I. 76%–92%; number of studies = 6; 594/675 patients; I2 = 70%; Fig. S3, panel a, Supplementary material 2).5, 13, 14, 16, 17, 18 When the arrest occurred in non ICU settings, the estimated mortality rate was 95.5% (95% P.I. 88.2–98.4%; number of studies: 4; 216/224 patients; I2 = 28%; Fig. S3, panel b, Supplementary material 2)5, 14, 16 ,17 in non-ICU settings.
In multicentre studies the estimated mortality rate was 85.1% (95% P.I. 77.8%–90.2%; number of studies = 3; 635/732 patients; I2 = 78%; Fig. S4, panel a, Supplementary material 2).13, 16, 19
In single centre studies the estimate mortality rate was 94.8% (95% P.I. 82.2%–98.7%; number of studies = 7; 425/447 patients; I2 = 85%; Fig. S4, panel b, Supplementary material 2).5, 14, 15, 17 , 18, 20, 21
Sensitivity analysis: COVID-19 vs. non COVID-19
Three studies reported the data on mortality of COVID-19 versus non-COVID-19 patients who developed IHCA.15, 19, 21 Patients with COVID-19 had a significantly higher risk of death after IHCA with CPR than non-COVID-19 (OR 2.34; 95% C.I. 1.37–3.99; 1215 patients; Fig. S5, Supplementary material 2).
Discussion
To the best of our knowledge, this is the most updated systematic review and meta-analysis on mortality rate after IHCA with attempted CPR in patients with COVID-19. The main finding of our study was that although one in three patients with COVID-19 may achieve ROSC after IHCA with attempted CPR, only one in ten may survive 30 days or to hospital discharge and 6% may survive with favorable neurological status (defined as CPC score of 1 or 2). The mortality rates seem to be similarly high, regardless of whether the arrest occurred in the ICU or in a ward, although the latter finding is highly heterogeneous.
Recently, an intense debate regarding the ethics of universal do-not-resuscitate orders for patients with COVID-19 arose.22 Pre pandemic data showed the rate of ROSC after IHCA in adult patients has increased from 50% to 60–70% dependent on age group, while the rate of survival to hospital discharge has increased from 15 to 25% over the last decade in all age groups.23 Less than 30% were discharged from hospital with more than moderate neurological dysfunction, only one in three of these was severely neurologically impaired.24 These data are very different from the estimated rates found in our analysis; poorer outcomes seem much more likely in COVID-19 patients who undergo IHCA, despite CPR. This finding is corroborated by our sensitivity analysis, that showed that patients with COVID-19 may have a significantly higher risk of death than non COVID-19 patients after IHCA with CPR.
COVID-19 patients that undergo cardiac arrest are probably critically ill. It is therefore only fair to compare the outcomes of these patients to other critically ill patients. Using data from the get with the guidelines database, Girotra et al. studied a cohort of patients with pneumonia or sepsis, admitted to an ICU and receiving mechanical ventilation at the time of IHCA. These patients did not have COVID-19 but the authors specifically selected this cohort to simulate the clinical characteristics of critically ill patients with COVID-19. Only 12.5% of these critically ill non-COVID patients survived to hospital discharge, the rate of survival with a CPC of 1 or 2 was 9.2% and the rate of survival with CPC of 1 was 6.2%.25 Our analysis provides even stronger grounds for survival expectations, showing that the outcomes of COVID-19 patients that undergo IHCA and CPR are indeed similar to the simulation cohort selected. COVID-19 may be complicated by pulmonary embolism,2 myocarditis,26 myocardial injury27 and myocardial infarction due to the pro-thrombotic state accompanying the disease.28 All of these are potential underlying causes of IHCA and each may influence the chance of survival of patients with COVID-19. When discussing possible patients’ outcomes, the emphasis should therefore not be on the fact that the patient has COVID-19, but rather on the overall severity of their clinical condition.
Another factor contributing to the debate on universal do-not-resuscitate orders for patients with COVID-19 was the high risk of exposure to infections among the healthcare workers when performing CPR.22, 29, 30 The World Health Organization lists CPR as an aerosol generating procedure.6 The American Heart Association guidelines on advanced cardiovascular life support provided a modified algorithm for suspected or confirmed COVID-19 patients with cardiac arrest.31 The algorithm suggests that personal protective equipment (PPE) should be donned before initiation of CPR, and the risk of aerosolization should be minimized by avoidance of closed-circuit disconnection, performance of intubation by an experienced provider and using video laryngoscopy if such is available.31, 32 The overwhelming number of patients contemporarily hospitalized during COVID-19 outbreaks, plus the need to wear PPE may have also influenced the CPR performance and may have also increased the time from cardiac arrest to the start of CPR, as also demonstrated in patients with cardiac arrest out of the hospital, and these aspects could have ultimately affected the chance of survival.33, 34
While avoiding CPR in all patients with COVID-19 is not supported by the available evidence, futility can still be argued on the basis of ongoing clinical deterioration despite provision of intensive care. CPR does not reverse the cause of cardiac arrest in most critically ill patients, therefore the benefit from CPR may be low.35, 36 Conversely, when IHCA occurs in suboptimal conditions in the wards, the early disadvantages accompanying this situation (e.g. unwitnessed arrest) may be balanced by a greater likelihood of reversal of acute disease components (e.g. hypoxemia). This assumption was reflected by our subgroup analysis on ICU and non-ICU settings, which showed nearly similar outcomes regardless of location. The fact that the overall rate of mortality in non-ICU settings was as high as 95.5% may reflect crisis allocation of resources (e.g. not all the most severe patients could be admitted to an ICU).
Our analysis has several limitations. Any analysis is only as good as the studies included in it. We chose to study incidence without a comparator because early review of the literature suggested that most of the studies we would identify would have no comparator. We also identified only ten studies (1179 patients). These numbers are probably too low to provide a definitive estimation of the rate of mortality and other outcomes. Most of the data came from the U.S. We found no data from European countries other than Sweden (one study). Only one study provided data from China during the first COVID-19 outbreak in Wuhan, including a relatively small sample size (n = 136 patients). Our data has large statistical heterogeneity which probably stems from differences in the characteristics of the study cohorts (e.g. severity or setting) and the timing of the studies during the course of the pandemic (e.g. early pandemic, late pandemic). Lastly, all the included studies refer to the first pandemic peak. It cannot be excluded that the outcome of IHCA in COVID-19 patients could be different during the second pandemic peak, considering a different organization of the hospitals, increased availability of ICU beds and increased preparedness of physicians to treat COVID-related complications that can lead to cardiac arrest.
Conclusions
Data from the available evidence suggests an estimated mortality rate of nearly 90% among patients with COVID-19 after IHCA with CPR, regardless of arrest location, even though one patient out of three may achieve ROSC. COVID-19 patients seem to have a significantly higher risk of death after IHCA with CPR than non-COVID-19 patients. The decision to provide CPR, even in critically ill patients, requires a patient centered approach that acknowledges advance directives along with input from healthcare proxies. Certainly, when CPR is deemed futile or when the risk to those engaged in resuscitation exceed the potential benefit, the decision to proceed with CPR becomes more complicated. More data are needed to clarify for which COVID-19 patients, the benefit derived from CPR may not justify the risk incurred by this procedure to the treating healthcare staff. There is an urgent need for data regarding the outcomes of COVID-19 patients outside the U.S. Future studies should also compare the outcomes of patients with versus without COVID-19 after IHCA.
CRediT authorship contribution statement
All the authors have made substantial contributions to the conception and design of the study, acquisition or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content. All the authors approved the final version of the manuscript to be submitted.
Mariachiara Ippolito: Conceptualization, Methodology, Formal analysis, Validation, Data curation, Writing - original draft. Giulia Catalisano: Validation, Data curation, Writing - review & editing. Claudia Marino: Validation, Data curation, Writing - review & editing. Rosa Fucà: Validation, Data curation, Writing - review & editing. Antonino Giarratano: Supervision, Validation, Writing - review & editing. Enrico Baldi: Supervision, Validation, Writing - review & editing. Sharon Einav: Supervision, Validation, Formal analysis, Data curation, Writing - original draft. Andrea Cortegiani: Conceptualization, Methodology, Formal analysis, Validation, Data curation, Writing - original draft, Project administration.
Funding
This research did not receive any funds from agencies or sponsors in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Acknowledgements
None.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resuscitation.2021.04.025.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 3.Vicentini A., Masiello L., D’Amore S., et al. QTc interval and mortality in a population of SARS-2-CoV infected patients. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008890. [DOI] [PubMed] [Google Scholar]
- 4.University of Oxford. Coronavirus (COVID-19) Hospitalizations – Statistics and Research – Our World in Data. (Accessed 20 February 2021, at https://ourworldindata.org/covid-hospitalizations).
- 5.Shah P., Smith H., Olarewaju A., et al. Is cardiopulmonary resuscitation futile in coronavirus disease 2019 patients experiencing in-hospital cardiac arrest? Crit Care Med. 2021;49:201–208. doi: 10.1097/CCM.0000000000004736. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Epidemic-prone and pandemic-prone acute respiratory diseases Infection Control Strategies for Specifi c Procedures in Health-Care Facilities A Quick Reference Guide. (Accessed 20 February 2021, at http://www.who.int/csr/resources/publications/WHO_CD_EPR_2007_6/en/index.html).
- 7.Moscarelli A., Iozzo P., Ippolito M., et al. Cardiopulmonary resuscitation in prone position: a scoping review. Am J Emerg Med. 2020;38:2416–2424. doi: 10.1016/j.ajem.2020.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hospitals consider universal do-not-resuscitate orders for coronavirus patients. Washington Post. (Accessed 20 February 2021, at https://www.washingtonpost.com/gdpr-consent/?next_url=https%3A%2F%2Fwww.washingtonpost.com%2Fhealth%2F2020%2F03%2F25%2Fcoronavirus-patients-do-not-resucitate%2F).
- 9.Mir T., Sattar Y., Ahmad J., et al. Outcomes of in-hospital cardiac arrest in COVID-19 patients: a proportional prevalence meta-analysis. Catheter Cardiovasc Interv. 2021 doi: 10.1002/ccd.29525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grenvik A., Safar P. Churchill; New York: 1981. Brain failure and resuscitation. [Google Scholar]
- 11.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 12.Wallace, Byron C, Dahabreh IJ, et al. Open Meta Analyst; Closing the gap between methodologists and end-users: R as a computational back-end.
- 13.Hayek S.S., Brenner S.K., Azam T.U., et al. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. BMJ. 2020;371:m3513. doi: 10.1136/bmj.m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao F., Xu S., Ma X., et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuriditsky E., Mitchell O.J.L., Brosnahan S.B., et al. Clinical characteristics and outcomes of in-hospital cardiac arrest among patients with and without COVID-19. Resusc Plus Elsevier B.V. 2020;4 doi: 10.1016/j.resplu.2020.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell O.J.L., Yuriditsky E., Johnson N.J., et al. In-hospital cardiac arrest in patients with coronavirus 2019. Resuscitation. 2021;160:72–78. doi: 10.1016/j.resuscitation.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheth V., Chishti I., Rothman A., et al. Outcomes of in-hospital cardiac arrest in patients with COVID-19 in New York City. Resuscitation. 2020;155:3–5. doi: 10.1016/j.resuscitation.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Hear Rhythm Elsevier Inc. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sultanian P., Lundgren P., Stro A., et al. Cardiac arrest in COVID-19: characteristics and outcomes of in- and out-of-hospital cardiac arrest. A report from the Swedish Registry for Cardiopulmonary Resuscitation. Eur Heart J. 2021;42:1094–1106. doi: 10.1093/eurheartj/ehaa1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thapa S.B., Kakar T.S., Mayer C., Khanal D. Clinical outcomes of in-hospital cardiac arrest in COVID-19. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miles J.A., Mejia M., Rios S., et al. Characteristics and outcomes of in-hospital cardiac arrest events during the COVID-19 pandemic: a single-center experience from a New York City Public Hospital. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.007303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz Z., Perkins G.D. Cardiopulmonary resuscitation after hospital admission with covid-19. BMJ. 2020;369:m1387. doi: 10.1136/bmj.m1387. [DOI] [PubMed] [Google Scholar]
- 23.Wiberg S., Holmberg M.J., Donnino M.W., et al. Age-dependent trends in survival after adult in-hospital cardiac arrest. Resuscitation. 2020;151:189–196. doi: 10.1016/j.resuscitation.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girotra S., Nallamothu B.K., Spertus J.A., Li Y., Krumholz H.M., Chan P.S. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girotra S., Tang Y., Chan P.S., Nallamothu B.K. Survival after in-hospital cardiac arrest in critically ill patients: implications for COVID-19 outbreak? Circ Cardiovasc Qual Outcomes. 2020;13:446–449. doi: 10.1161/CIRCOUTCOMES.120.006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Hear Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19 — a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cappuccio F.P. Confusion over CPR in patients with covid-19. BMJ. 2020;369:m1805. doi: 10.1136/bmj.m1805. [DOI] [PubMed] [Google Scholar]
- 30.Mahase E., Kmietowicz Z. Covid-19: doctors are told not to perform CPR on patients in cardiac arrest. BMJ. 2020;368:m1282. doi: 10.1136/bmj.m1282. [DOI] [PubMed] [Google Scholar]
- 31.AHA. ACLS Cardiac Arrest Algorithm for Suspected or Confirmed COVID-19 Patients. (Accessed 20 February 2021, at https://cpr.heart.org/-/media/cpr-files/resources/covid-19-resources-for-cpr-training/english/algorithmacls_cacovid_200406.pdf?la=en).
- 32.Ippolito M., Vitale F., Accurso G., et al. Medical masks and respirators for the protection of healthcare workers from SARS-CoV-2 and other viruses. Pulmonology. 2020;26:204–212. doi: 10.1016/j.pulmoe.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim T.H., Kim C.H., Do Shin S., Haam S. Influence of personal protective equipment on the performance of life-saving interventions by emergency medical service personnel. Simulation. 2016;92:893–898. [Google Scholar]
- 34.Baldi E., Sechi G.M., Mare C., et al. COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020;41:3045–3054. doi: 10.1093/eurheartj/ehaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer D.B., Lo B., Dickert N.W. CPR in the Covid-19 era — an ethical framework. N Engl J Med. 2020;383:e6. doi: 10.1056/NEJMp2010758. [DOI] [PubMed] [Google Scholar]
- 36.Einav S., Cortegiani A., Marcus E.L. Cardiac arrest in older adult patients. Curr Opin Anaesthesiol. 2021;34:40–47. doi: 10.1097/ACO.0000000000000942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.