Abstract
Objective
To perform a follow-up study of the quality of life in patients with epilepsy in the era of the COVID-19 crisis.
Methods
Two months before the first case of the COVID-19 in Serbia, we obtained the Serbian Version of Quality of Life Inventory for Epilepsy 31 (SVQOLIE-31) and Neurological Disorders Depression Inventory for Epilepsy scores (SVNDDI-E) for another study. We retested the same patients one year after in COVID-19 pandemic. In addition to SVQOLIE-31, and SVNDDI-E we used a generic questionnaire compiled from items related to the COVID-19.
Results
We retested 97 out of 118 patients (82.2%) for the follow-up analysis. The average age was 36.1 ± 12.2 (range: 18–69), and 49 were women (50.5%). The median duration of epilepsy was 13 years (range: 1.5–48). The structural etiology of epilepsy was noted in 41 (42.3%), unknown etiology in 41 (42.3%), and genetic etiology in 15 (15.4%) patients. Fewer patients (27.8%) experienced at least one seizure three months before follow-up testing when compared to patients who experienced at least one seizure three months in initial testing (36.0%) (p = 0.15). All patients reported full compliance with anti-seizure medication in the follow-up. The SVQOLIE-31 score during the COVID-19 pandemic visit (64.5 ± 14.6) was significantly lower than the SVQOLIE-31 score before the pandemic (p < 0.001). The SVNDDI-E score during the COVID-19 pandemic (10.5 ± 3.5) was significantly higher than the SVNDDI-E score before it (p < 0.001). Multiple linear regression analyses revealed fear of seizures, and fear of a reduction in household income, significantly associated with SVQOLIE-31 and SVNDDI-E overall score. These variables accounted for 66% and 27% of the variance of SVQOLIE-31 and SVNDDI-E overall score.
Significance
Lower quality of life, higher prevalence of depression, healthcare availability issues, and perceived fears during pandemic all suggest COVID-19 has negatively impacted lives of patients with epilepsy.
Keywords: Epilepsy, COVID-19; Quality of life; QOLIE-31; NDDI-E; Depression
1. Introduction
Newly identified coronavirus first reported in Wuhan, China, in December 2019, causing acute respiratory distress syndrome [1] spread worldwide, and caused a pandemic unprecedented in the modern world. While the focus for health authorities is resolutely on the physical consequence of the virus, for many, the current outbreak of coronavirus has produced with it strong feelings of anxiety, panic, and isolation. There are several possible reasons for those mentioned above. First, the lack of efficient treatment and knowledge gaps in virus origin, virulence, and case fatality rates generated draconian governmental responses such as social distancing and lockdowns previously unexplored in many present-day societies. Further, uncertainties linked to pandemic created lively debate among prominent scientists, and some criticized drastic epidemiological countermeasures adopted in many countries as a “once-in-a-century evidence fiasco” [2]. Simultaneously, foremost economic strategists publicly questioned the value of human life relative to a unit of economic activity [3]. Finally, mass-media has an imperative role in today’s world and significantly shaped the COVID-19 pandemic's public understanding as a significant global threat by disease tracking and updates through live updates dashboard. Nevertheless, media coverage of coronavirus news during lockdowns, quarantines, and financial and social hardships induced fear and caused psychological stress [4].
Data about the impact of the COVID-19 pandemic on patients with epilepsy are scarce. Prevalent belief convinces that patients with epilepsy are neither more likely to be infected by the coronavirus nor are they more likely to have severe COVID-19 manifestations because they have epilepsy [5], [6]. However, according to a Spanish study, patients with tumor-related, drug-resistant epilepsy, insomnia, and economic difficulties are at a higher risk of increased seizure frequency during the COVID-19 pandemic [7]. In a Chinese study that compared the severity of psychological distress between patients with epilepsy and healthy controls during the COVID-19 outbreaks, patients with epilepsy demonstrated higher scores on the psychological distress scale. Additionally, they spent more time paying attention to media coverage of the COVID-19 outbreak. As in the Spanish study, drug-resistant patients with epilepsy were the most vulnerable group having a significant risk for severe psychological distress [8].
In the realm of epilepsy, there is growing acceptance that traditional patterns, such as seizure count measures, do not circumscribe a full variety of outcomes that may be relevant in evaluating the clinical impact of different factors in patients with epilepsy. Health-related quality-of-life trials assess patients' perception of their disease's impact on their social, mental, and physical health. Furthermore, epilepsy-targeted health-related quality-of-life measures may be preferable to generic ones in longitudinal studies [9].
From its beginning, the COVID-19 epidemic in Serbia showed a similar pattern as in the vast majority of the European countries, with almost half a million confirmed cases and 4200 deaths among 3 million tested up to the middle of February 2021[10]. Meanwhile, the country went through different epidemiological countermeasures such as total lockdown followed by partial openings and the current decreasing tendency of COVID-19 incidence rate in all parts of the country amid intense vaccination (Serbia has one of the highest rates of vaccination in the world) [11]. Almost two months prior to the first case of the COVID-19 in Serbia, reported by health authorities on March 6th, 2020, we obtained Quality of Life Inventory for Epilepsy 31 (QOLIE-31) [12] and Neurological Disorders Depression Inventory for Epilepsy scores (NDDI-E) [13] in the group of 118 patients with epilepsy for the purpose of another study [14]. We retested patients after one year and consequently performed a follow-up study of the quality of life in patients with epilepsy obtaining data in the era of the COVID-19 crisis.
2. Material and methods
2.1. Patient population
Our initial study [14] included consecutive Serbian patients with epilepsy attending the outpatient clinic at the Clinic for Neurology University Clinical Center of Serbia between 15.12.2019 and 15.01.2020. In total 118 patients with the following inclusion criteria participated in the study: (1) confirmed diagnosis of epilepsy according to International League Against Epilepsy (ILAE) criteria [15], (2) over 17 years of age, (3) have been receiving stable doses of antiepileptic medication for three months before study enrollment, and (4) have formal written informed consent for participation in the study. We excluded 48 patients when the following criteria were met: (1) progressive or untreated, neurological, psychiatric, or other comorbidities (diabetes, chronic obstructive pulmonary disease, active psychiatric or malignant disease, etc.); (2) patients who did not cooperate; (3) cognitive or other neurological disorders (dementia, hemiplegia, or aphasia) which prevented participants from completing the questionnaire on their own despite detailed explanation; and (4) psychiatry comorbidities that required the use of other psychoactive medication (depression, anxiety, insomnia, psychosis, bipolar disorder, suicidality, chronic alcoholism), without overt clinical signs of psychopathological or behavioral dysfunction.
We retested the same patients attending the outpatient clinic at the clinic for Neurology university clinical Center of Serbia between 14.12.2020 and 15.01.2021.
2.2. Instruments
For determining the quality of life, the Serbian version of the QOLIE-31 (SVQOLIE-31 scale) was used [16]. The SVQOLIE-31 questionnaire consists of 31 questions divided into the following seven groups: Seizure Worry, Overall Quality of Life, Emotional Well-Being, Energy/Fatigue, Cognitive Function, Medication Effect, and Social Function. The total score summarizes the average values of scores of the subscales and then linearly transformed into a range from 0 to 100. We defined clinically significant change of SVQOLIE-31 as a difference of more than 10 points between test and retest on final scores. The prevalence of depression symptoms was self-assessed with the Serbian version of the neurological disorders depression inventory for epilepsy – NDDI-E (SVNDDI-E) [17]. The SVNDDI-E scale has six questions, of which every question has four offered answers on the Likert scale. The final score of the SVNDDI-E questionnaire is the summation of points from the answers, and it varies from a minimum of 6 points (lowest frequency of depressive mood) up to a maximum of 24 points (highest frequency of depressive mood). We defined clinically significant change of SVQOLIE-31 as a difference of more than 2 points between test and retest on final scores. For the collection of primary clinical data, we used a generic questionnaire. The patients gave full written consent for their clinical data to be used in this study. The local ethics committee of the Clinical Center of Serbia approved this study.
The researcher filled out the generic questionnaire consisting of questions regarding COVID-19 compiled from two previous studies [18], [19] and two questions from the initial study (occurrence and number of seizures in last three months). Afterward, the patient filled the SVQOLIE-31 and the SVNDDI-E scales. The entire procedure for completing the generic questionnaire takes up to 10 min.
2.3. Statistical analysis
Statistical analysis was conducted using International Business Machines Corporation (IBM) Statistical Package for the Social Sciences (SPSS) version 22. The statistical finding was significant for value p < 0.05 (2-tailed). All demographic data were analyzed descriptively, with nominal data presented as frequencies and percentages and continuous data presented as means and standard variations. For continuous data, paired t-tests were used for group comparison. The following tests were run: paired sample t-test, and McNemar test. We performed univariate linear regression analysis to identify variables that were associated with QOLIE-31 and SVNDDI-E overall score. Variables found to have significant correlation with QOLIE-31 and SVNDDI-E overall score were then included in a multiple linear regression model.
3. Results
3.1. Demographics
In total, we retested 97 out of 118 patients (82.2%) for the longitudinal analysis of the quality of life and depression screening in the era of COVID-19. Only retested patients entered further analysis. The average years of age was 36.1 ± 12.2 (range: 18–69), and 49 were women (50.5%). Their educational level was as follows: 3 patients had no education or finished only primary school (3.1%); 56 patients finished high school (57.7%); and 37 patients graduated from college or university (38.2%). The median duration of epilepsy was 13 years (range: 1.5–48). The structural etiology of epilepsy was noted in 41 (42.3%), unknown etiology in 41 (42.3%), and genetic etiology in 15 (15.4%) patients. Focal seizures were noted in 75 (77.3%), generalized in 20 (20.6%) and unclassified in 2 (2.1%) patients.
3.2. Impact of confinement measures and exposure to COVID-19
All patients reported full compliance with anti-seizure medication in the year of follow-up. In the follow-up year, only 8 (8.2%) patients reported at least one regular outpatient visit to their neurologist. Of those who did not make regular outpatient consultation, 70 (72.3%) patients reported they could not reach their neurologist due to health systems restriction during the COVID-19 epidemic, 16 (16.5%) patients reported other reasons, and 3 (3.1%) patients reported fear of viral exposure as the main reason.
At the follow-up visit 22 patients were employed (22.7%). Temporary suspension or permanent termination of employment was reported by 13 (13.4%), and significant reduction in household income was reported by 36 (37.1%) patients in the year of follow-up.
Exposure to a person with COVID-19 lasted longer than 10 minutes was reported in 34 patients (35.1%). Sixteen patients (16.5%) tested PCR positive for SARS-COV-2 virus on a nasopharyngeal swab, and 14/16 (87.5%) experienced symptoms related to COVID-19. Neither of the tested patients exhibited COVID-19 sequelae. The prevalence of perceived fears among patients with epilepsy is presented in Table 1 .
Table 1.
Perceived fears among patients with epilepsy.
| No | Mild to moderate | High | Extremely High | Total | |
|---|---|---|---|---|---|
| Fear of seizures | 55 (56.7%) | 25 (25.8%) | 12 (12.4%) | 5 (5.1%) | 97 (100%) |
| Fear of drug supply disruption | 64 (66.0%) | 16 (16.5%) | 12 (12.4%) | 5 (5.1%) | |
| Fear of inability to reach neurologist | 48 (49.5%) | 14 (14.4%) | 19 (19.6%) | 16 (16.5%) | |
| Fear of reduction in household income | 53 (54.6%) | 24 (24.7%) | 14 (14.4%) | 6 (6.3%) | |
| Fear of Covid-19 | 43 (44.3%) | 24 (24.7%) | 20 (20.6%) | 10 (10.3%) |
3.3. SVQOLIE31 and SVNDDI-E during COVID-19 pandemic
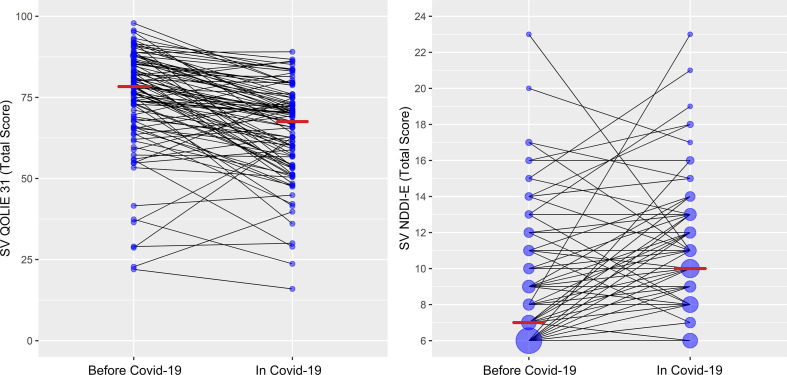
The SVQOLIE-31 score during COVID-19 pandemic visit (64.4 ± 14.6) was significantly lower than SVQOLIE-31 score before the pandemic (74.2 ± 16.0) (t(96) = 7.276, p < 0.001) (Fig. 1 ). Significant decrease in the SVQOLIE-31 score on follow-up test compared to initial SVQOLIE-31 (less than 10 points) was noted in 47 (48.5%) patients. The SVNDDI-E score during COVID-19 pandemic visit (10.5 ± 3.5) was significantly higher than SVNDDI-E score before the pandemic (8.8 ± 3.5) (t(96) = -4.777, p < 0.001) (Fig. 1). Significant increase in the SVNDDI-E score on follow-up test when compared to initial SVNDDI-E score (more than 2 points) was noted in 36 (37.1%) patients.
Fig. 1.
Median of the Serbian Version of Quality of Life in Epilepsy – 31 and Serbian Version of Neurological Disorders Depression Inventory in Epilepsy before COVID-19 and in COVID-19 pandemic.
Fewer patients (27.8%) experienced at least one seizure 3 months prior to follow-up testing in COVID-19 era when compared to patients who experienced at least one seizure 3 months prior to COVID-19 pandemic (36.0%)but this was not significantly different (McNemar, p = 0.15). Average number of seizures in both groups did not significantly differ (t(96) = −0.156, p = 0.875).
After multiple linear regression analyses only, fear of seizures and fear of reduction in household income remained significantly associated with SVQOLIE-31 overall score. These variables accounted for 66% of the variance of SVQOLIE-31 overall score (Table 2 ).
Table 2.
Variable associated with SVQOLIE-31 overall score in linear regression.
| Factors | Univariate linear regression |
Multivariate linear regression |
||
|---|---|---|---|---|
| B (95% CI) | p-value | B (95% CI) | p-value | |
| Age | −0.041 (−0.283, 0.201) | 0.739 | ||
| Gender | −2.143 (−8.045, 3.758) | 0.473 | ||
| Educational Level | 6.357 (1.013, 11.701) | 0.020 | 3.038 (−1.127, 7.203) | 0.151 |
| Epilepsy Duration | −0.02 (−0.278, 0.238) | 0.880 | ||
| Achieved regular outpatient consultation | 5.315 (−5.386, 16.015) | 0.327 | ||
| Treatment change in the last year | −10,105 (−16,863, −3,346) | 0.004 | −2.494 (−7.923, 2.935) | 0.364 |
| Patient is currently employed | −0.467 (−6.391, 5.458) | 0.876 | ||
| Temporary suspension or permanent termination of employment | −5.140 (−13.761, 3.482) | 0.240 | ||
| Significant reduction in household income | −9.293 (−15.117, −3.469) | 0.002 | 2.426 (−2.910, 7.762) | 0.369 |
| Fear of seizures | −10.271 (−12.885, −7.658) | <0.001 | −8.562 (−11.379, −5.745) | <0.001 |
| Fear of drug supply disruption | −3.377 (−6.608, −0.146) | 0.041 | −0.633 (−3.544, 2.279) | 0.667 |
| Fear of inability to reach neurologist | −4.699 (−7.059, −2.339) | <0.001 | −2.085 (−4.474, 0.303) | 0.086 |
| Fear of reduction in household income | −5.946 (−8.896, −2.996) | <0.001 | −4.376 (−7.030, −1.723) | 0.001 |
| Fear of COVID-19 | −2.014 (−4.857, 0.829) | 0.163 | ||
| Contact with COVID-19 positive person | 0.180 (−6.020, 6.381) | 0.954 | ||
| History of PCR confirmed COVID-19 | 2.371 (−5.586, 10.329) | 0.555 | ||
| History of recognizable symptoms of COVID-19 | 0.186 (−8.233, 8.605) | 0.965 | ||
SVQOLIE-31 - Serbian version of the Quality of Life in Epilepsy-31.
After multiple linear regression analyses only fear of seizures and fear of reduction in household income remained significantly associated with SVNDDI-E overall score. These variables accounted for 27.1% of the variance of SVNDDI-E overall score (Table 3 ).
Table 3.
Variable associated with SVNDDI-E overall score in linear regression.
| Factors | Univariate linear regression |
Multivariate linear regression |
||
|---|---|---|---|---|
| B (95% CI) | p-value | B (95% CI) | p-value | |
| Age | −0,041 (−0,099, 0,016) | 0,159 | ||
| Gender | 0,918 (−0,49, 2,325) | 0,199 | ||
| Educational Level | −0,825 (−2,134, 0,483) | 0,214 | ||
| Epilepsy Duration | −0,017 (−0,079, 0,045) | 0,594 | ||
| Achieved regular outpatient consultation | −1,504 (−4,067, 1,058) | 0,247 | ||
| Treatment change in the last year | 1,228 (−0,449, 2,905) | 0,149 | ||
| Patient is currently employed | −0,238 (−1,659, 1,183) | 0,740 | ||
| Temporary suspension or permanent termination of employment | 0,660 (−1,419, 2,74) | 0,530 | ||
| Significant reduction in household income | 1,405 (−0,036, 2,847) | 0,056 | ||
| Fear of seizures | 1,724 (1,002, 2,447) | <0,001 | 1.392 (0.654, 2.130) | <0.001 |
| Fear of drug supply disruption | 0,607 (−0,176, 1,39) | 0,127 | ||
| Fear of inability to reach neurologist | 0,806 (0,217, 1,394) | 0,008 | 0.427 (−0.128, 0.983) | 0.130 |
| Fear of reduction in household income | 1,182 (0,455, 1,908) | 0,002 | 0.933 (0.263, 1.602) | 0.007 |
| Fear of COVID-19 | 0,297 (−0,39, 0,983) | 0,393 | ||
| Contact with COVID-19 positive person | 0,173 (−1,314, 1,661) | 0,818 | ||
| History of PCR confirmed COVID-19 | −0,53 (−2,44, 1,38) | 0,583 | ||
| History of recognizable symptoms of COVID-19 | −0,173 (−2,193, 1,847) | 0,865 | ||
SVNDDI-E – Serbian version of the Neurological Disorders Depression Inventory for Epilepsy.
4. Discussion
The entire world is dealing with detrimental consequences of COVID-19, including economic difficulties, negative impact of confinement measures, and challenges that the global healthcare system is facing. Since epilepsy is a chronic condition that requires regular outpatient visits, drug-supplementation, and psychological support from doctors, family, and friends, changes caused by COVID-19 could potentially negatively influence the needs of patients with epilepsy. Our follow-up study directly evaluates the quality of life in the era of COVID-19 in the same group of patients previously evaluated at baseline in a period before the COVID-19 pandemic. Since the seizure occurrence as a most significant predictor of quality of life and mood disorders in patients with epilepsy did not differ in our cohort, while testing before and in the COVID-19 time, our results might prove the direct causal effect of COVID-19 and quality of life decrement.
4.1. Quality of life and mental health during pandemic
Deterioration of mental health and quality of life is in the COVID-19 pandemic is already well documented in different neurological conditions such as Parkinson's disease [20] and other non-neurological chronic conditions [21]. Compared to a recently published study focusing on COVID-19 impact on the patients with epilepsy quality of life [22], our data show consistency. Previous studies explored the influence of COVID-19 on psychosocial aspects via web-based platforms [22], [23]. However, those studies failed to investigate the causal relationship due to the absence of surveying the pandemic because the pandemic had been unpredictable. In other words, the strength of our study is performing the survey before the pandemic. Additionally, our study's design enables us to reveal that public emergency brought by COVID-19 produces a severe decline in quality of life score (>10 points) in almost half of the patients.
Previously published studies already showed the expected negative impact of the COVID-19 on anxiety and depression [22], [23], i.e., the ongoing COVID-19 pandemic puts the population with epilepsy under critical psychosocial distress. Our study might confirm that COVID-19 increases the rate of depression. However, there is a limitation to this kind of conclusion. Even though an NDDI-E score of more than 15 had a specificity of 90%, a sensitivity of 81%, and a positive predictive value of 0.62 for a diagnosis of major depression [13], it is still a screening instrument not intended to replace the clinical judgment which we did not systematically perform in our sample. Furthermore, an increment in the NDDI-E score may show a trend, and we do not know whether this is reversible. Therefore, further work is inevitable to reveal direct causal relation between Covid-19 and depression in patients with epilepsy.
Apart from depression, fear of seizures, fear of inability to reach consultant neurologist, and fear of reducing household income during the COVID-19 period significantly affect quality of life in our multiple logistic regression. Our results are strongly correlated with recently published data from Croatia [24]. Due to the pandemic, negative impacts on patients with epilepsy mental health could be anticipated. Nevertheless, perceived fears are most probable. Interestingly, as in the previously mentioned study, we found that fear of inability to reach a consultant neurologist might be a crucial risk factor because it appears to be notably adjusted.
4.2. COVID-19 in patients with epilepsy
The number of COVID-19 positive patients with epilepsy (16.5%) in our study is slightly lower than the proportion of cumulative confirmed cases per the total number tested in Serbia (18%) at the time of the study performance [10]. None of our 14 patients who experienced COVID-19 symptoms reported a severe clinical presentation, which correlates to the statement that patients with epilepsy are not a higher risk of contracting coronavirus and neither are they prone to develop serious clinical manifestations [5], [6]. More than a half of our patients (55.7%) declared having fear of COVID-19 infection and 24.7% of them considered it to be mild to moderate. An intriguing finding of our study is that two patients, who have been seizure-free for 1 and 5 years respectively, both suffered one seizure after COVID-19 infection. However, there is no proof that seizing was triggered by the virus.
5. Conclusion
This study highlights the significant consequence of the COVID-19 on patients with epilepsy. It also emphasizes the importance of understanding the psychological insufficiencies detected in patients with epilepsy brought by the actual pandemic. Lower quality of life, higher prevalence of depression, healthcare availability issues, and perceived fears during pandemic all suggest COVID-19 has negatively impacted lives of patients with epilepsy. The current findings should be evaluated in the circumstances of possible liability to vary in pandemic severity, period and size of lockdown, patients' judgment, and cultural traits. Therefore, further work is necessary to settle the relationship between COVID-19 and quality of life in patients with epilepsy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by project No 175087 Ministry of Education, Science and Technological Development of the Republic of Serbia.
References:
- 1.WHO-China Joint Mission. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). https://reliefweb.int/sites/reliefweb.int/files/resources/who-china-joint-mission-on- covid-19-final-report.pdf. Accessed February 11, 2021
- 2.Ioannidis J. A fiasco in the making? As the coronavirus pandemic takes hold, we are making decisions without reliable data. https://www.statnews.com/2020/03/17/a-fiasco-in-the-making-as-the-coronavirus-pandemic-takes-hold-we-are-making-decisions-without-reliable-data/. Accessed April 1, 2020
- 3.Dalio R. The three biggest questions we need to answer now. https://www.linkedin.com/pulse/three-biggest-questions-we-need-answer-now-ray-dalio/?. Accessed March 29, 2020.
- 4.Anwar A, Malik M, Raees V, Anwar A. Role of mass media and public health communications in the COVID-19 pandemic. Cureus 12(9): 2020; e10453. [DOI] [PMC free article] [PubMed]
- 5.Asadi-Pooya A.A., Attar A., Moghadami M., Karimzadeh I. Management of COVID-19 in people with epilepsy: drug considerations. Neurol Sci. 2020;41(8):2005–2011. doi: 10.1007/s10072-020-04549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuroda N. Epilepsy and COVID-19: Updated evidence and narrative review. Epilepsy Behav. 2021;13(116) doi: 10.1016/j.yebeh.2021.107785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonseca E., Quintana M., Lallana S., Luis Restrepo J., Abraira L., Santamarina E., et al. Epilepsy in time of COVID-19: A survey-based study. Acta Neurol Scand. 2020;142(6):545–554. doi: 10.1111/ane.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao X., Zhou D., Li Z., Zeng G., Hao N., Li E., et al. Severe psychological distress among patients with epilepsy during the COVID-19 outbreak in southwest China. Epilepsia. 2020;61(6):1166–1173. doi: 10.1111/epi.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birbeck G.L., Kim S., Hays R.D., Vickrey B.G. Quality of life measures in epilepsy: how well can they detect change over time? Neurology. 2000;54(9):1822–1827. doi: 10.1212/wnl.54.9.1822. [DOI] [PubMed] [Google Scholar]
- 10.Homepage COVID-19 in the Republic of Serbia. https://covid19.rs/homepage-english/. Accessed February 15, 2020.
- 11.Delauney Guy. Covid: How Serbia soared ahead in vaccination campaign. https://www.bbc.com/news/world-europe-55931864. Accessed February 13, 2021
- 12.Cramer J.A., Perrine K., Devinsky O., Bryant-Comstock L., Meador K., Hermann B. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia. 1998;39(1):81–88. doi: 10.1111/j.1528-1157.1998.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilliam F.G., Barry J.J., Hermann B.P., Meador K.J., Vahle V., Kanner A.M. Rapid detection of major depression in epilepsy: a multicentre study. Lancet Neurol. 2006;5(5):399–405. doi: 10.1016/S1474-4422(06)70415-X. [DOI] [PubMed] [Google Scholar]
- 14.Sokić N., Ristić A.J., Bukumirić Z., Vojvodić N., Kovačević M., et al. Validation of the Serbian version of the Liverpool Adverse Events Profile of antiseizure therapy in patients with epilepsy. Epilepsy Behav. 2020;111 doi: 10.1016/j.yebeh.2020.107309. [DOI] [PubMed] [Google Scholar]
- 15.Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 16.Martinović Z., Milovanović M., Tosković O., Jovanović M., Buder N., Simonović P., et al. Psychometric evaluation of the Serbian version of the Quality of Life in Epilepsy Inventory-31 (QOLIE-31) Seizure. 2010;19(8):517–524. doi: 10.1016/j.seizure.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Ristić A.J., Pjevalica J., Trajković G., Parojčić A., Mihajlović A., Vojvodić N., et al. Validation of the Neurological Disorders Depression Inventory for Epilepsy (NDDI-E) Serbian version. Epilepsy Behav. 2016;57(Pt A):1–4. doi: 10.1016/j.yebeh.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Huang S., Wu C., Jia Y., Li G., Zhu Z., Lu K., et al. COVID-19 outbreak: The impact of stress on seizures in patients with epilepsy. Epilepsia. 2020;61(9):1884–1893. doi: 10.1111/epi.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamoto M., Carrazana E., Viereck J., Liow K. Epilepsy in the time of COVID-19. Acta Neurol Scand. 2021;143(3):333–335. doi: 10.1111/ane.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shalash A., Roushdy T., Essam M., Fathy M., Dawood N.L., Abushady E.M., et al. Mental health, physical activity, and quality of life in Parkinson's disease during COVID-19 PANDEMIC. Mov Disord. 2020;35(7):1097–1099. doi: 10.1002/mds.28134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciążyńska M., Pabianek M., Szczepaniak K., Ułańska M., Skibińska M., Owczarek W., et al. Quality of life of cancer patients during coronavirus disease (COVID-19) pandemic. Psychooncology. 2020;29(9):1377–1379. doi: 10.1002/pon.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh M.Y., Lim K.S., Fong S.L., Khor S.B., Tan C.T. Impact of COVID-19 on quality of life in people with epilepsy, and a multinational comparison of clinical and psychological impacts. Epilepsy Behav. 2021;10(117) doi: 10.1016/j.yebeh.2021.107849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Hees S, Siewe Fodjo JN, Wijtvliet V, Van den Bergh R, Faria de Moura Villela E, da Silva CF, et al. Access to healthcare and prevalence of anxiety and depression in persons with epilepsy during the COVID-19 pandemic: a multicountry online survey. Epilepsy Behav 2020;112:107350. [DOI] [PMC free article] [PubMed]
- 24.Friedrich L., Sruk A., Bielen I. Responses of people with epilepsy to the COVID-19 pandemic in the time of national lockdown. Epilepsy Behav. 2021;116 doi: 10.1016/j.yebeh.2021.107790. [DOI] [PMC free article] [PubMed] [Google Scholar]