Abstract
Objective
Given that the coronavirus disease 2019 (COVID-19) mainly spreads through the respiratory system and is associated with severe pulmonary complications, lung cancer patients may have worse outcomes than those with other tumors. There is no confirmed evidence about the mortality comparison between COVID-19 patients with lung cancer and other tumors. We performed a systematic review and pooled analysis to provide precise estimates of the mortality rate of COVID-19 patients with lung cancer and other tumors.
Materials and Methods
Our study systemically included and reviewed 13 studies on the characteristics of COVID-19 patients with lung cancer published up to November 1, 2020. The primary endpoint was all-cause mortality. We also compared the all-cause mortality rates in China and other regions as a secondary endpoint. The mortality rate was assessed with a fixed-effects model, which was used to derive the pooled mortality and 95 % confidence interval (CI).
Results
Thirteen studies from different countries, involving 1,229 patients with both COVID-19 and cancer, were selected for the pooled analysis. A total of 343 deaths were recorded in this population: 86 for lung cancers and 257 for other tumors. The mortality rate varies from 18 % to 60 % for patients with lung cancer and COVID-19 and 10%–41% for other tumor patients with COVID-19. The overall meta-analysis did not show a significant mortality difference for the lung cancer and other tumor subgroups (OR = 1.47, 95 %CI = 0.98–2.20, p = 0.06, I2 = 23 %). Nevertheless, in regions other than China, the pooled mortality of lung cancer patients with COVID-19 was 42 %, which was significantly higher than that of other tumors (24 %) (OR = 2.73, 95 % CI = 1.54–4.86, p = 0.0006, I2 = 16 %).
Conclusion
Appropriate and aggressive preventive measures should be implemented to reduce the risk of COVID-19 in patients with cancer and optimally manage those who contract the infection.
Keywords: Lung cancer, COVID-19, Mortality, SARS-CoV-2, Pandemic
1. Introduction
The COVID-19 pandemic has had a significant impact on patients with cancer. Patients with cancer are susceptible to COVID-19 because immunosuppressive treatments can weaken the immune system, and COVID-19 complications, such as acute respiratory distress syndrome and mechanical ventilation, may lead to worse survival. Second, a sharp reduction in cancer screening and the postponement of ongoing or planned therapy during the pandemic's initial months may have also resulted in excess deaths from cancer. Additionally, cancers occur often in the older adult population, and cancer patients commonly have other comorbidities, which may increase the risk of poor outcomes. Therefore, it has been presumed that cancer is a risk factor for severe COVID-19. Among the types of cancer, lung cancer has been the most studied. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) contaminates the respiratory system and causes respiratory complications of the lungs, which worsens the prognosis of lung cancer patients. Ziegle et al. demonstrated that angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) on the alveolar epithelial cells are the receptors for SARS-CoV-2 to invade the human body, and the abnormal respiratory epithelium is likely to be more prone to rapid virus entry into the lungs [1]. The most controversial point was determining whether lung cancer patients have a higher risk of contagiousness or lung cancer was more severe than other cancer types when complicated by COVID-19.
In an observational study conducted in China, most COVID-19 cases had lung cancer (19.6 %) compared with gastrointestinal (18.7 %) and genitourinary (18.7 %) cancers [2]. Other results from a multi-center study in the early days of the coronavirus outbreak indicated that patients with lung cancer or lung metastasis showed a considerably higher risk of death (OR, 2.34; 95 % confidence interval [CI], 1.15–4.77); P = 0.03), higher ICU admission rates (OR, 2.84; 95 % CI [1.59–5.08]; P < 0.01), and higher risks of other critical events than patients with other cancers without lung metastasis [3]. Another systemic review reported that patients with hematological malignancies (OR, 2.39; 95 % CI, 1.17–4.87); P = 0.02) and lung cancer (OR, 1.83; 95 % CI, (1.00–3.37); P = 0.05] who were infected by COVID-19 had the highest mortality [4]; in contrast, another study by Liang reported that lung patients with lung cancer did not have a higher probability of severe events [5].
In the above studies, the sample sizes varied; the samples included 7–102 patients with lung cancer. The enrollment time spanned various stages of the epidemic, which resulted in heterogeneous and inconsistent opinions. Therefore, we conducted this systematic review to assess the mortality of lung cancer patients infected by COVID-19.
2. Materials and methods
All systematic review and meta-analysis procedures were conducted according to the Systematic Reviews and Meta-Analyses (PRISMA) Statement relevant to healthcare [6]. We followed an established protocol registered in the International Prospective Register of Systematic Reviews (PROSPERO), and the record is available at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020222523(7).
2.1. Literature search
A systematic literature search and review of studies published in PubMed, Google Scholar, Web of Science, and MedRxiv between February 1 and October 31, 2020, were performed by two investigators (Yue Y. and Haike L.). To amply search the literature, we conducted the literature searches using a combination of medical subject headings (MeSH) and free terms, such as “COVID-19″ and “lung cancer.” For COVID-19, the search items were as follows: “novel coronavirus” OR “SARS-CoV-2″ OR “COVID-19.″ For lung cancer, the search term was “cancer” OR “carcinoma” OR “tumor” OR “NSCLC” OR “lung cancer.” We used “AND” to combine the items of the two keywords.
2.2. Study selection
The selected data were screened independently by two investigators (Yue Y. and Haike L.). During the preliminary screening, studies that were irrelevant to the study, based on their titles and abstracts, were excluded. The full text was required for further screening. All included studies need to be checked for duplicate medical records from the same or overlapping cohort of patients. Subsequently, all procedures were cross-checked by the two investigators.
The inclusion criteria were as follows: (i) studies enrolling patients with COVID-19 and lung cancer, and COVID-19 should have been diagnosed clinically or based on laboratory tests; the diagnosis of lung cancer or other tumors should have had no restriction of pathological type and stage, primary or secondary; (ii) studies reporting mortality rate in patients with lung cancer and COVID-19; (iii) any kind of study (including retrospective studies, randomized controlled trials, prospective cohort studies, and case series); (iv) studies published in the English language.
The exclusion criteria were as follows: (i) publication types including conference abstracts, case reports, letters, comments, non-human studies, and studies with incomplete data; (ii) studies published in languages other than those mentioned above.
2.3. Data extraction and quality assessment
Two investigators independently extracted the data. The extracted data consisted of the first author’s name, type of publication (i.e., peer-reviewed, preprint, or conference proceedings), population country, single-center or multi-center study type (retrospective or prospective, RCT, case series, cohort control), diagnostic methods of COVID-19, reported number of patients with lung cancer and COVID-19, the number of deaths among the study population, and other characteristics of patients.
Two reviewers (Yang S. and Mengyang Z.) independently assessed the quality of the included studies. The Newcastle–Ottawa Scale (NOS) and NICE guideline (NG45) were used to determine the quality of case-control studies and case series, respectively.
2.4. Statistical analysis
The primary outcome was all-cause mortality in patients with lung cancer and COVID-19 infection, and the secondary endpoint was all-cause mortality in China and other regions. Odds ratios (ORs) were calculated. A 95 % confidence interval (CI) was reported for the outcomes. The value of I2 and the result of the chi-squared test were used to assess statistical heterogeneity. High heterogeneity was considered when I2> 50 %. A fixed-effects model was used, and p < 0.05 denoted statistical significance. The meta-analysis was performed using RevMan 5.3 (The Cochrane Collaboration, London, United Kingdom).
3. Results
A flowchart of the literature search is presented in Fig. 1 . The initial implementation of the search strategy yielded 15,632 potentially relevant citations, of which 15,429 were excluded because of duplicate exclusion and irrelevant titles or abstracts. The remaining 190 studies were excluded for various reasons after the full-text screening. According to the predetermined criteria, 13 retrospective studies, including 1,229 patients, were included in the meta-analysis.
Fig. 1.
PRISMA flow diagram for the selection of studies to be included in the systematic review and meta-analysis.
The overall descriptions of the 13 studies are summarized in Table 1 . Of the 13 retrospective studies, four were from China, and nine were from other regions (USA, Spain, Brazil, France, and Italy). Eligible RCT studies were not included, and case series were the most common, accounting for 7. According to the scales mentioned above, all included studies were of high quality, with no less than five points. For the above 13 studies, 10 studies [[1], [2], [3],[7], [8], [9], [10], [11], [12], [13]] reported the number of deaths in patients with lung cancer and other tumors, whereas the other 3(4–6) studies only reported on lung cancer populations and could not provide OR. Therefore, we included the first 10 studies in the meta-analysis and discussed all 13 studies in the systematic review for more information on the outcomes.
Table 1.
Characteristics of the included studies.
| No. | Study publication year | country | Single/multi-center | Study design | Study type | Period | Diagnosis method for covid-19 | Lung cancer |
Other tumors |
Quality Score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Deaths | Total | Deaths | |||||||||
| 1 | Zhang, 2020 [2] | China | Multi-center | Retrospective | Case-series study | 2020.1.5−3.18 | RT-PCR | 21 | 5 | 86 | 18 | NICE 7 |
| 2 | Yarza, 2020 [8] | Spain | Single | Retrospective | Case-series study | 2020.3.9−4.19 | RT-PCR/clinical | 15 | 6 | 48 | 10 | NICE 6 |
| 3 | Yang, 2020 [9] | China | Multi-center | Retrospective | Case-control study | 2020.1.13−3.18 | RT-PCR | 24 | 6 | 181 | 74 | NICE 7 |
| 4 | Luo, 2020 [10] | USA | Single | Retrospective | Case-control study | 2020.3.12−5.6 | RT-PCR | 102 | 25 | NR | NR | NICE 6 |
| 5 | Rogado, 2020 [11] | Spain | Single | Retrospective | Case-series study | 2020.3.5−4.7 | RT-PCR | 17 | 9 | NR | NR | NOS 7 |
| 6 | Calles, 2020 [12] | Spain | Single | Retrospective | Case-series study | 2020.2.24−5.12 | RT-PCR | 23 | 8 | NR | NR | NICE 6 |
| 7 | de Melo, 2020 [13] | Brazil | Single | Retrospective | Case-series study | 2020.4−30-5.26 | RT-PCR | 7 | 4 | 174 | 56 | NICE 6 |
| 8 | Yu, 2020 [14] | China | Single | Retrospective | Case-series study | 2019.12.30−2.17 | RT-PCR/clinical | 7 | 2 | 5 | 1 | NICE 6 |
| 9 | Mehta, 2020 [15] | USA | Single | Retrospective | Cohort study | 2020.3.18−4.8 | NR | 11 | 6 | 207 | 55 | NOS 5 |
| 10 | Basse, 2020 [16] | France | Single | Retrospective | Cohort study | 2020.3.13−4.25 | RT-PCR/CT | 18 | 6 | 123 | 20 | NOS 5 |
| 11 | Dai, 2020 [3] | China | Multi-center | Retrospective | Case-control study | 2020.1.1−2.24 | RT-PCR | 22 | 4 | 83 | 8 | NOS 7 |
| 12 | Stroppa, 2020 [17] | Italy | Single | Retrospective | Case-series study | 2020.2.21−3.18 | RT-PCR | 8 | 2 | 17 | 7 | NOS 6 |
| 13 | Hogan, 2020 [18] | UK | Multi-center | Retrospective | Cohort study | 2020.3.1−4.30 | RT-PCR | 5 | 3 | 25 | 8 | NOS 7 |
Abbreviations: RT-PCR: Reverse transcription-polymerase chain reaction. CT: Computed tomography. NOS: Newcastle–Ottawa Scale (NOS). NICE: National Institute for Health and Care Excellence.
3.1. A meta-analysis of patients with lung cancer and other tumors with COVID-19
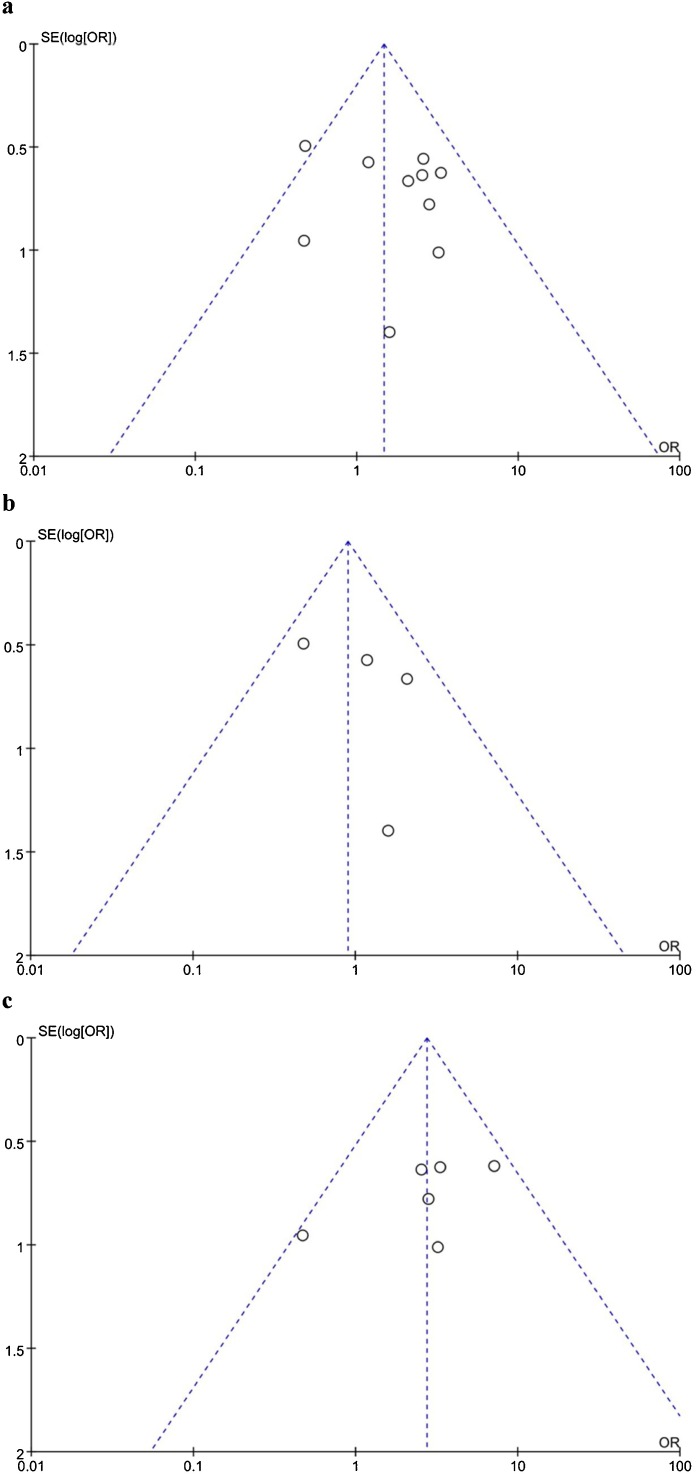
Ten studies involving 1,087 patients (138, lung cancer group; 949, other tumor group) have reported mortality comparisons (Fig. 2 ). There were 44 deaths and 257 deaths in the lung cancer and other tumor groups, respectively. The mortality rate in the lung cancer group was 32 %, which was higher than the 27 % for the other tumors group (OR = 1.47, 95 %CI = 0.98–2.20). Heterogeneity testing revealed minimal heterogeneity, with I2 = 23 % (p = 0.24). Therefore, we used the fixed-effects model. The overall mortality was not significantly different (p = 0.06). Publication bias was evaluated using a funnel plot (Fig. 5a), which showed no substantial evidence of asymmetry.
Fig. 2.
Forest plot of pooled all-cause mortality rates of lung cancer patients and other tumors patients with COVID-19.
Fig. 5.
Funnel plot for the results from (a) all studies and those from (b) China and (c) other regions.
3.2. Subgroup analysis
Four Chinese studies (1.3.8.11) included 74 lung cancer patients and 355 other tumor patients, of which 17 and 101 died, respectively (OR = 0.9, 95 %CI = 0.49–1.65). Six studies from developed countries (USA, France, Spain, Brazil, Italy) reported 27 deaths in 64 lung cancer patients and 144 deaths in 594 patients with other tumors (OR = 2.73, 95 %CI = 1.54–4.86). Heterogeneity testing in these two subgroups revealed minimal heterogeneity, with I2 = 16 % (Fig. 3 ) and I2 = 16 % (Fig. 4 ). We used a fixed-effects model. Mortality in the Chinese subgroup showed no significant differences (p = 0.72), significant differences were observed in the other regions (p = 0.0006); the mortality of the lung cancer group was 42 %, which was higher than that for other tumors (24 %). Publication bias was evaluated using a funnel plot (Fig. 5b and c), and it showed no significant evidence of asymmetry.
Fig. 3.
Forest plot of pooled all-cause mortality rates of lung cancer patients and other tumors patients with COVID-19 in China.
Fig. 4.
Forest plot of pooled all-cause mortality rates of lung cancer patients and other tumors patients with COVID-19 in other regions.
4. Discussion
In this study, we performed a systematic review and pooled analysis to estimate the mortality rates of COVID-19 patients with lung cancer and other tumors. This systematic review and meta-analysis of 1,229 COVID-19 admitted patients from 13 studies, including 280 lung cancer patients and 949 other tumor patients, is the first, to the best of our knowledge, to comprehensively compare the mortality rates of the two groups of patients.
Considering the characteristics of lung cancer patients, their greater predisposition to respiratory infections, and the previous diagnosis of metastatic disease in most of them, we expected to observe more significant mortality. However, our study shows that there is no significant difference between the mortality rates of lung cancer patients with COVID-19 and patients with other tumors who have COVID-19. Luo compared the following cohorts to explore the contribution of coronavirus to severe outcomes: COVID-19 infection plus lung cancer, lung cancer without COVID-19. This study concluded that patient-specific features, rather than cancer-specific features or treatments, are the most significant determinants of severity. Furthermore, COVID-19 only accounted for a minority of the overall lung cancer deaths during the pandemic (11 % overall) [10]. Since different regions have different primary care settings, lung cancer incidence and mortality, and treatments for COVID-19, we divided China (developing countries) and other areas into two subgroups for comparison. Generally, after allowing for differences in population size and age structures, developing countries were more likely to have higher lung cancer incidence and fewer medical resources than developed countries. In the developed countries, the mortality rate for lung cancer with COVID-19 was significantly higher than that for other tumors. However, in China, the mortality rate was still not significant. One interpretation is that primary care and treatment for cancer contribute the most to the mortality of patients with cancer and COVID-19. The low primary care settings for cancer in China lead to more tumor-related mortality. In contrast, the mortality difference in developed countries may be due to the complications of COVID-19.
There are some limitations to our study. Since most of the studies included in our analysis reported the all-cause mortality rate other than the case fatality rate, the evidence for the cause of death, including COVID-19, cancer, or other comorbidities, was insufficient. More prospective and well-organized studies should be conducted to investigate the main cause of death in these patients and evaluate the impact of different etiologies and clinical factors on prognosis, and more balanced strategies may be adopted. Moreover, although we planned to take age, sex, cancer stage, and comorbidities into consideration, only a few included studies provided these detailed data for a pooled analysis.
As is customary with the included studies, our findings are relatively intuitive; nonetheless, having objective data to confirm our suspicions about the mortality of lung cancer patients with COVID-19 should help in the development of evidence-based recommendations for the deployment of limited resources within healthcare environments.
5. Conclusion
There was no significant difference between the lung cancer and other tumor subgroups. Appropriate and aggressive preventive measures should be implemented to reduce the risk of COVID-19 in patients with cancer and optimally manage those who contract the infection. More data are needed to clarify some of these associations.
Funding statement
This work was supported by the Chongqing Performance Incentive and Guidance Project for Scientific Research Institutions (cstc2020jxjl130016), Chongqing Key Disease Prevention and Control Technology Project (2019ZX002), and the Chongqing Science and Technology Innovation Guidance Project Led by Academician (2018).
CRediT authorship contribution statement
Haike Lei: Literature search,Study selection. Yue Yang: Literature search,Study selection. Wei Zhou: Writing - Original Draft. Mengyang Zhang: Data extraction and quality assessment, Results. Yang Shen: Data extraction and quality assessment. Dan Tao: Writing - Review & Editing. Lulu Wang: Writing - Original Draft. Qianqian Lei: Writing - Review & Editing. Ying Wang: Ideas; formulation or evolution of overarching research aims. Yongzhong Wu: Supervision and Project administration.
Transparency document
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Cai C., Ahmed O.A., Shen H., et al. Which cancer type has the highest risk of COVID-19 infection? J. Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang H., Wang L., Chen Y., et al. Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan, China. Cancer. 2020;(126):4023–4031. doi: 10.1002/cncr.33042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai M., Liu D., Liu M., et al. Patients with Cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatesulu B.P., Chandrasekar V.T., Girdhar P., et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. medRxiv. 2020 doi: 10.1093/jncics/pkaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knobloch K., Yoon U., Vogt P.M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J. Craniomaxillofac. Surg. 2011;39:91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Booth A., Clarke M., Dooley G., et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst. Rev. 2012;1:2. doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarza R., Bover M., Paredes D., et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur. J. Cancer. 2020;135:242–250. doi: 10.1016/j.ejca.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang K., Sheng Y., Huang C., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo J., Rizvi H., Preeshagul I.R., et al. COVID-19 in patients with lung cancer. Ann. Oncol. 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogado J., Pangua C., Serrano-Montero G., et al. Covid-19 and lung cancer: A greater fatality rate? Lung Cancer. 2020;146:19–22. doi: 10.1016/j.lungcan.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calles A., Aparicio M.I., Alva M., et al. Outcomes of COVID-19 in patients with lung Cancer Treated in a tertiary hospital in Madrid. Front. Oncol. 2020;10:1777. doi: 10.3389/fonc.2020.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Melo A.C., Thuler L.C.S., Da Silva J.L., et al. Cancer inpatients with COVID-19: a report from the Brazilian National Cancer institute. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241261. e0241261-e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J., Ouyang W., Chua M.L., et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta V., Goel S., Kabarriti R., et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basse C., Diakite S., Servois V., et al. Characteristics and outcome of SARS-CoV-2 infection in cancer patients. Jnci Cancer Spectr. 2020 doi: 10.1093/jncics/pkaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroppa E.M., Toscani I., Citterio C., et al. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy) Future Oncol. 2020;16:1425–1432. doi: 10.2217/fon-2020-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joharatnam-Hogan N., Hochhauser D., Shiu K.K., et al. Outcomes of the 2019 novel coronavirus in patients with or without a history of cancer: a multi-centre North London experience. Ther. Adv. Med. Oncol. 2020;(12) doi: 10.1177/1758835920956803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.