Abstract
Keywords: COVID-19; coronavirus; myocarditis; cardiac MRI; T1 mapping; T2 mapping
Keywords: COVID-19, coronavirus, myocarditis, cardiac MRI, T1 mapping, T2 mapping
Summary
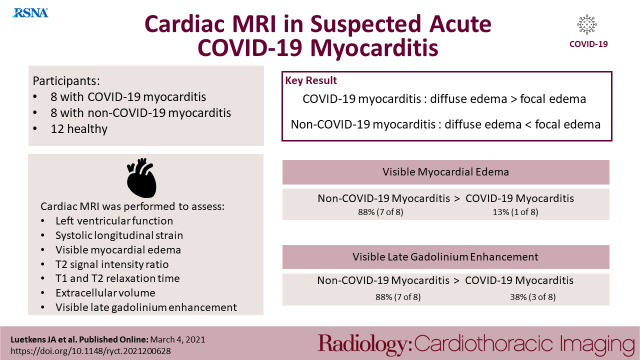
Participants with active and symptomatic coronavirus disease 2019 infection and suspected acute myocarditis had distinct pathologies on cardiac MRI with diffuse myocardial edema, which could be best depicted using myocardial mapping techniques.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and the resulting coronavirus disease 2019 (COVID-19) has become a worldwide pandemic. Although primarily affecting the respiratory system, COVID-19 associated myocardial injury is common and can occur directly due to myocardial viral infection or indirectly due to systemic inflammation, endothelial activation, and/or microvascular thrombosis (1, 2). Beside myocardial infarction, myocardial injury can also be a result of myocardial inflammation (2). However, current information about associated myocardial inflammation is mainly limited to case reports or series (3). As cardiac MRI is important for the diagnostic work up of patients with myocarditis, more data about MRI characteristics of COVID-19 associated acute inflammatory injury is needed. The aim of this study was to describe cardiac MRI findings in participants with active COVID-19 infection and suspected acute myocarditis.
Material and Methods
The institutional ethics commission approved this prospective study. All participants gave written informed consent. Participants with COVID-19 without structural heart disease and mechanical ventilatory support were included consecutively during the recruitment period from April 2020 to December 2020. Participants had a clinical suspicion for COVID-19 associated myocarditis with signs of acute myocardial injury (elevated troponin levels with or without electrocardiographic changes). Acute coronary syndromes were excluded by cardiac catheterization. The control groups consisted of healthy volunteers and participants with suspected acute non-COVID-19 myocarditis (4).
Cardiac MRI was performed at 1.5 Tesla in all participants using previously described acquisition parameters (4). Left ventricular function, average systolic longitudinal strain, T2 signal intensity ratio, T1 relaxation times, T2 relaxation times, extracellular volume, and quantitative late gadolinium enhancement (LGE) were determined. Focal myocardial edema and LGE were visually assessed. Measurements of the blinded readers (J.A.L. and A.I., with 8 and 4 years of experience, respectively, in cardiac MRI) were conducted as described previously (5).
Continuous variables between two groups were compared with Student’s t test. χ2 test was used to compare dichotomous variables. One-way analysis of variance followed by Tukey multiple comparison tests was performed to compare variables in the three participant groups. Statistical significance level was set to P < .05.
Results
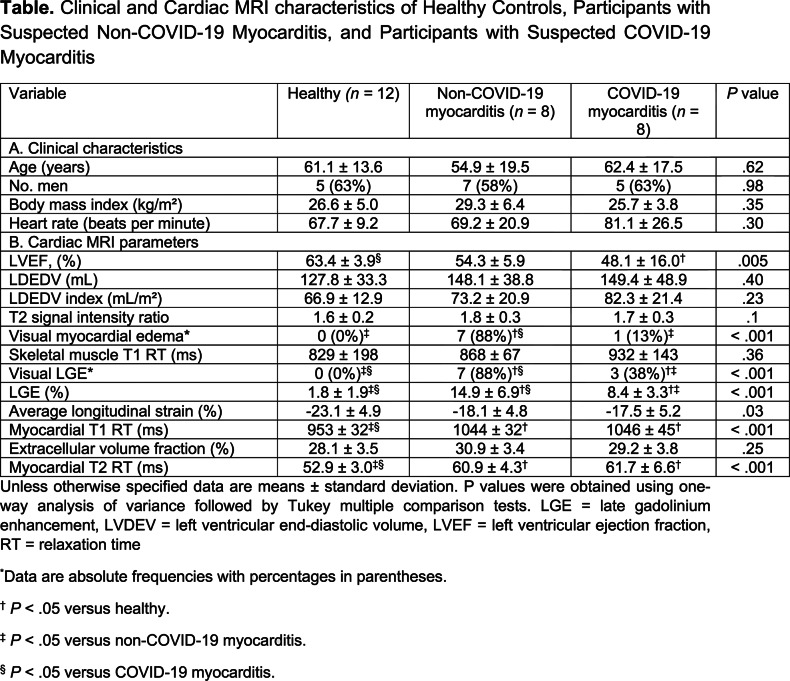
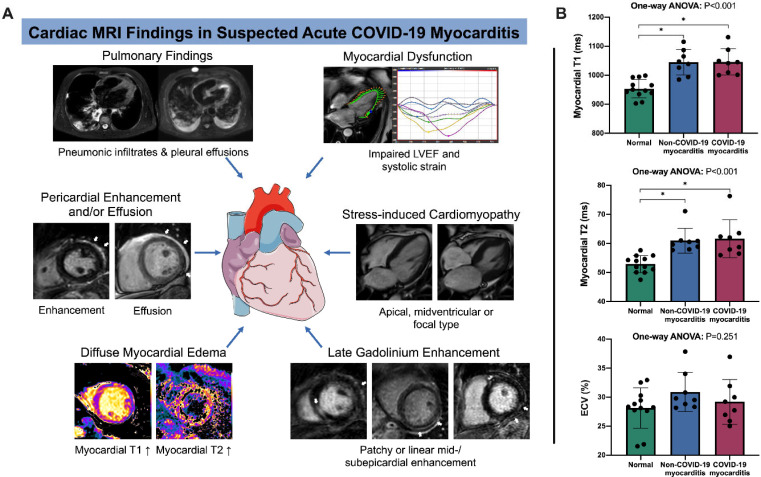
Participants with COVID-19 had dyspnea, fever and positive pneumonic infiltrates on chest CT scans or radiographs. Cardiac MRI was performed 7.6 ± 4.6 days after positive reverse transcriptase polymerase chain reaction. C-reactive protein (44.6 ± 37.1 mg/l) and troponin T (114 ± 249 ng/ml) levels were elevated in participants with COVID-19. Participants with COVID-19 had diffuse global higher T1 and T2 relaxation times compared to healthy participants (T1, 1046 ± 45 ms vs 953 ± 32 ms [P < .001]; T2, 61.7 ± 6.6 ms vs 52.9 ± 3.0 ms [P < .001]). Myocardial T1 and T2 were also prolonged in non-COVID-19 myocarditis, but these participants had a more focal disease with more visible myocardial edema (88% [7 of 8] vs. 13% [1 of 8]; P = .003) and LGE lesions (88% [7 of 8] vs. 38% [3 of 8]; P = .04). The T2 ratio was not significantly elevated in participants with COVID-19 myocarditis, however this was likely secondary to skeletal muscle edema as skeletal muscle T1 was also elevated in participants with COVID myocarditis (Table). A total of 38% (3 of 8) participants with COVID-19 had severe wall-motion abnormalities consistent with patterns of stressinduced cardiomyopathy (Figure). Additionally, 38% (3 of 8) of participants with COVID-19 had small pericardial effusions and/or pericardial enhancement.
Table:
Clinical and Cardiac MRI characteristics of Healthy Controls, Participants with Suspected Non-COVID-19 Myocarditis, and Participants with Suspected COVID-19 Myocarditis
Figure:
Summary of study results. (a) Composition of imaging findings found in our study cohort. Besides signs of active pulmonary coronavirus disease 2019 (COVID- 19) infection with pneumonic infiltrates and pleural effusions, participants with suspected acute COVID-19 associated myocarditis had an impaired left ventricular function, also with patterns of stress-induced cardiomyopathy in single cases (a midventricular type of stress-induced cardiomyopathy with corresponding diastolic and systolic images is shown). As a key finding a distinct diffuse myocardial edema (detected with myocardial T1 and T2 mapping) was present in most participants. Late gadolinium enhancement lesions (white arrows) were less pronounced, especially when compared to participants with non-COVID-19 myocarditis. Late gadolinium enhancement lesions were present in the subepicardium of the lateral wall or in the basal septal midmyocardium. Some participants displayed pericardial enhancement or small pericardial effusions (see white arrows on corresponding images). All image examples are from the described study cohort of participants. (b) Column graphs with individual plotted values show distribution of quantitative myocardial MRI parameters in healthy participants and in participants with suspected acute non-COVID-19 and COVID-19 myocarditis. Distributions are given for native myocardial T1 relaxation time, myocardial T2 relaxation time, and extracellular volume fraction. Data are presented as mean with standard deviation error bars. The figure contains a free medical image from Servier (https://smart.servier.com/). * indicates significant pairwise comparison (P < .05). LVEF = left ventricular ejection fraction; ANOVA = analysis of variance; ECV = extracellular volume fraction.
Discussion
We found a pattern of diffuse myocardial edema in participants with symptomatic COVID-19 infection and suspected myocarditis. Myocardial edema affects myocardial function and might be an expression of diffuse inflammation due to a systemic immune response, a direct myocardial damage of SARS-CoV-2, or a vascular leakage due to endothelial damage (6). Interestingly, the amount of LGE lesions, as a sign of myocyte necrosis was lower in participants with COVID-19 myocarditis when compared to participants with acute non-COVID-19 myocarditis, which is primarily induced by cardiotropic viruses (4). This result indicates that the pathomechanism of myocardial inflammation injury might be different in SARS-CoV-2 and is possibly based on an interplay of different broadly affections of the cardiovascular system (2). Also, we observed patterns of stress-induced cardiomyopathy as another potential mechanism of myocardial injury in participants with COVID-19. As the occurrence of myocardial injury and features of stress-induced cardiomyopathy are associated with fatal outcome of COVID-19 infection (2), the observed pronounced diffuse cardiac alterations are of particular interest.
These authors contributed equally to this work.
Funding: Supported by the German Heart Foundation/German Foundation of Heart Research (F/28/20)
Abbreviations:
- COVID-19
- coronavirus disease 2019
- LGE
- late gadolinium enhancement
- SARSCoV-2
- severe acute respiratory syndrome virus 2
References
- 1.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giustino G, Pinney SP, Lala A, Reddy VY, Johnston-Cox HA, Mechanick JI, Halperin JL, et al. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia: JACC Focus Seminar. JAm Coll Cardiol 2020;76:2011-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito A, Palmisano A, Natale L, Ligabue G, Peretto G, Lovato L, Vignale D, et al. Cardiac Magnetic Resonance Characterization of Myocarditis-Like Acute Cardiac Syndrome in COVID-19. JACC Cardiovasc Imaging 2020;13:2462-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, Schmeel FC, et al. Comparison of Original and 2018 Lake Louise Criteria for Diagnosis of Acute Myocarditis: Results of a Validation Cohort. Radiology: Cardiothoracic Imaging 2019;1:e190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isaak A, Praktiknjo M, Jansen C, Faron A, Sprinkart AM, Pieper CC, Chang J, et al. Myocardial Fibrosis and Inflammation in Liver Cirrhosis: MRI Study of the Liver-Heart Axis. Radiology 2020;297:51-61. [DOI] [PubMed] [Google Scholar]
- 6.Rafiee MJ, Babaki Fard F, Friedrich MG. COVID-19, myocardial edema and dexamethasone. Med Hypotheses 2020;145:110307. [DOI] [PMC free article] [PubMed] [Google Scholar]