Abstract
Although US of the lungs is increasingly used clinically, diagnostic radiologists are not routinely trained in its use and interpretation. Lung US is a highly sensitive and specific modality that aids in the evaluation of the lungs for many different abnormalities, including pneumonia, pleural effusion, pulmonary edema, and pneumothorax. This review provides an overview of lung US to equip the diagnostic radiologist with knowledge needed to interpret this increasingly used modality.
Supplemental material is available for this article.
© RSNA, 2021
Summary
Compared with chest radiography, lung US may have higher sensitivity for detection of pleural effusion, pneumonia, pneumothorax, and pulmonary edema. It is increasingly used in the intensive care unit to detect these conditions.
Key Points
■ US of the lungs is increasingly being used clinically and is simple to learn, perform, and interpret.
■ Lung US is predominantly artifact-based as opposed to most US examinations, which allow direct visualization of the target region of interest. At lung US, the A-line artifact is seen in air-filled lung, the B-line artifact is seen in conditions such as pulmonary edema and/or fibrosis, and consolidation and effusion are directly visualized.
■ Meta-analyses suggest that lung US has higher sensitivity with similar specificity compared to chest radiography for some pulmonary conditions, including pneumonia, pleural effusion, and pulmonary edema.
Introduction
Lung US has dramatically increased in popularity over the last decade and is routinely performed at the patient’s bedside, especially in the emergency department and the intensive care unit (ICU) (1–3). Formal training in the performance and interpretation of lung US, however, is not a traditional component of radiology residency education in the United States. As this modality becomes an imaging staple, the diagnostic radiologist should be fluent in lung US performance and interpretation to maintain relevance and assist the ordering clinician. In this imaging essay, the essentials of lung US are described and radiographic correlation for lung US imaging findings on chest radiographs and chest CT images is provided. A discussion of the role of lung US in health care delivery and the future directions of lung US is also included.
Utility of Lung US
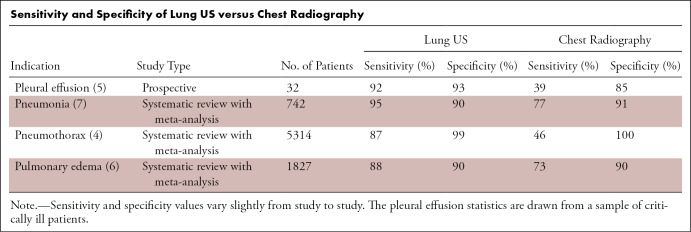
Lung US is radiation-free, low-cost, rapid, and portable, allowing real-time examination of pulmonary structures. Meta-analyses suggest that compared with chest radiography, lung US may have higher sensitivity and similar specificity for detection of pleural effusion, pneumonia, pneumothorax, and pulmonary edema (Table) (4–7). It is increasingly used in the ICU to detect these diseases. Critical care providers have adopted the bedside lung US in emergency (BLUE) protocol as a standardized approach to lung US in the ICU. This protocol can be performed in less than 3 minutes at the bedside and has a diagnostic accuracy greater than 90% for asthma and/or chronic obstructive pulmonary disease, pneumonia, pneumothorax, pulmonary edema, and pulmonary embolism (1,8). The accuracy for pulmonary embolism derives from the protocol directing the provider to examine the deep venous structures if there is no sign of pulmonary disease. Many practitioners have advocated for the regular use of lung US in the ICU to decrease the use of chest radiography, which is associated with increased cost and nontrivial cumulative radiation exposure (especially in pediatric patients) (9–17). Lung US also has a well-established role in guiding interventional procedures, including thoracentesis and biopsy, and has been shown to improve outcomes in these procedures by reducing complications (eg, pneumothorax) (18,19). Finally, lung US has a special role in pediatrics, including improved visualization of abnormalities in the thorax owing to the small thoracic diameters of children and the lack of ionizing radiation to produce diagnostic imaging results (11,20–31).
Sensitivity and Specificity of Lung US versus Chest Radiography
The limitations of lung US should also be noted when considering its general utility. Lung US is operator dependent, and its quality varies by practitioner. Advanced technical skill and clinical knowledge of the operator increase diagnostic yield; however, studies have shown that the rudimentary skills of the modality can be learned with relative ease (32–34). A limitation of lung US compared with chest radiography is the time needed to perform the examination. Depending on the thoroughness of the examination, complete lung US can take 20 minutes to perform, whereas chest radiography can be completed in a few minutes (9,28). Another limitation in lung US is that findings such as A-lines and B-lines can be seen in a variety of conditions (1,2,14,35). For example, in pediatric patients with bronchiolitis, studies have shown the lung US findings of atelectasis and pneumonia overlap (22,25,36). However, this is not a problem unique to US, as similar issues with specificity exist with both chest radiography and, to a lesser extent, with CT. Lung US is predominantly an artifact-based imaging modality, typically visualizing only abnormalities abutting the pleural line as discussed herein in the Physics of Lung US section (2,35,37). For a complete examination of the deeper thoracic structures, chest radiography and/or CT imaging are preferred. Similarly, in the ICU, lung US is more limited in evaluating lines and tubes compared with chest radiography. Thus, rather than being rival modalities, chest radiography and lung US function as complementary modalities that can be used in conjunction to benefit patient care.
Image Acquisition at Lung US
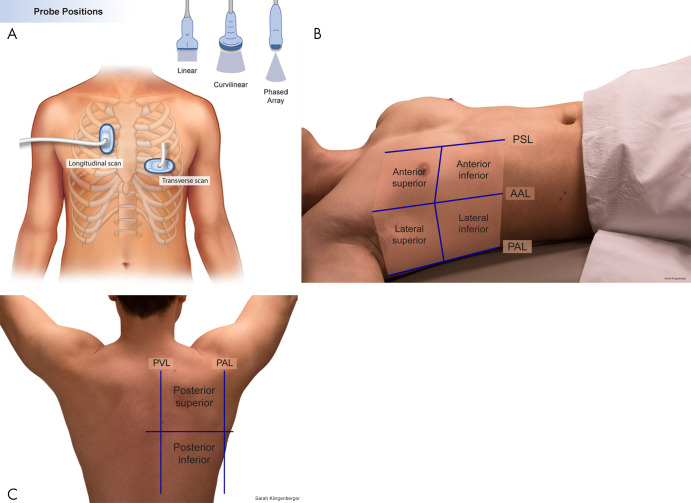
Complete lung US involves examining each hemithorax in the anterior, lateral, and posterior lung zones (Fig 1) (35,38). All lung fields should also be examined in transverse and longitudinal orientations, as failure to include both orientations may lead to missed abnormalities (39). The patient may be imaged in both the supine and upright position. As with all US examinations, proper technique is essential to ensure adequate imaging. Because much of lung US is artifact based, the probe must be held perpendicular to the skin to ensure perpendicular orientation to the pleural line to produce the requisite artifacts for interpretation. In addition, larger body habitus may limit evaluation of the pleural line as well as structures at depth. A variety of probes may be used in this application depending on the patient’s age and the indication for the examination (35). Curvilinear or phased-array 5–9-MHz probes are well equipped to examine the lung. Linear 7–12-MHz probes offer the best resolution of superficial structures and are especially useful in children (low thoracic diameter) and for assessing pneumothorax. In the point-of-care setting, lung US may be abbreviated and tailored to only transverse and sagittal views of the area of interest. Contrast-enhanced US of the lungs has been investigated and may provide additional benefit to assess peripheral pulmonary lesions and guide biopsies (40–44). Color Doppler US has an established role in the evaluation of abscess and empyema as well as a lesser known role in the evaluation of pneumothorax (37,45–49). Some evidence also suggests that Doppler US can be helpful in distinguishing benign and malignant peripheral lung lesions, but this is somewhat controversial (37,46,50–52).
Figure 1:
Process of performing lung US. A, Illustration demonstrates basic longitudinal and transverse probe orientations. B, C, A complete lung US examination includes transverse and longitudinal scans through the anterior, lateral, and posterior lungs. AAL = anterior axillary line, PAL = posterior axillary line, PSL = parasternal line, PVL = paravertebral line. (Reprinted, with permission, from University of Rochester, Rochester, New York © 2021; medical illustration by Gwen Mack and Nadezhda D. Kiriyak and photographs by Sarah Klingenberger).
Physics of Lung US
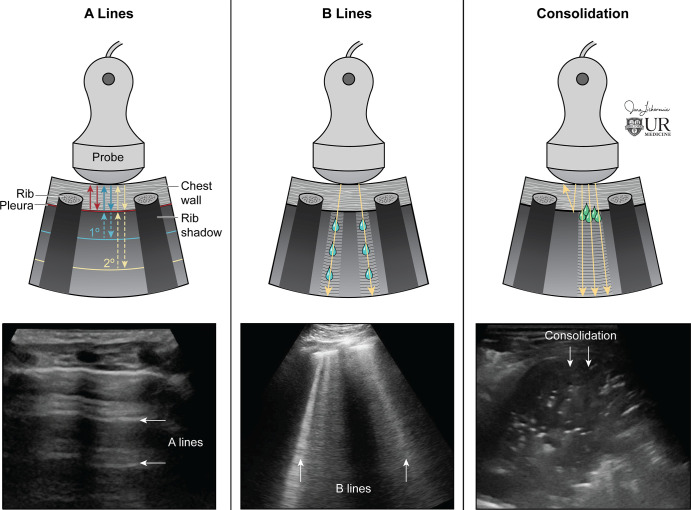
The physics of lung US is complicated and is briefly summarized here in simple terms. Lung US is unique among other US examinations, because it is predominantly artifact based, in contrast to other US examinations in which anatomy is directly visualized. Most ultrasound waves are reflected at the pleura in an air-filled lung owing to the acoustic impedance mismatch at the air and soft-tissue interface that results in a hyperechoic pleural line (53). Thus, the air-filled lung parenchyma cannot be directly visualized at US. Instead, A-line artifacts, which are horizontal reverberation artifacts of the hyperechoic pleural line, are reflected from the air-filled lung, allowing the observer to infer its existence (Fig 2). When the lung interstitium is thickened (ie, pulmonary edema, interstitial inflammation and/or infection, or pulmonary fibrosis), B-line artifacts (hyperechoic vertical lines traversing the imaging field below the pleural line) replace the normal A-lines (Fig 2). The physics of B-lines is not entirely understood (54–57). Lung collapse or consolidation removes the A-line artifact and allows for direct visualization of the parenchyma (Fig 2). Pleural effusions, both complex and simple, are also directly visualized. If a pulmonary abnormality does not touch the pleural line, it is not visualized owing to the acoustic impedance mismatch between the air and soft-tissue interface. The majority of clinically significant abnormalities, especially life-threatening abnormalities, typically abuts the pleural line, therefore permitting its detection at US (1).
Figure 2:
Physics of lung US. (Left) Ultrasound waves reflected at the pleural line creating A-line reverberation artifacts. (Center) As the interstitium thickens, the artifact pattern changes, with B-line artifacts obliterating A-lines. B-lines are hyperechoic vertical artifacts arising from the pleural line extending to the bottom of the field of view. (Right) Consolidation is directly penetrated by US, resulting in visualization without artifact if the consolidation is touching the pleural line. (Reprinted, with permission, from University of Rochester, Rochester, New York © 2021; medical illustration by Jane Lichorowic).
Radiographic Correlation of Lung US Findings
Air-Filled Lung
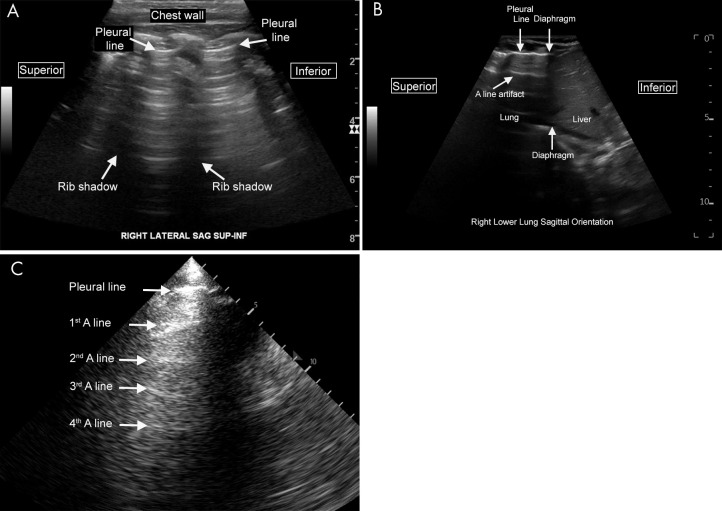
The A-line artifact predominates in normal air-filled lungs (Fig 3) (1,35). In addition, during respiration the sliding visceral and parietal pleura is visualized as shimmering motion of the pleural line, referred to as lung sliding (Movies 1 and 2). Pathologic conditions with air-filled lungs also have A-line artifacts; these conditions include asthma, chronic obstructive pulmonary disease, mild viral illness, and pulmonary embolism without focal infarct. In suspected pulmonary embolism, in which A-lines predominate, a US examination of the deep venous structures can be performed (1,35). In addition, if a pulmonary infarct is present, it will appear as a consolidation abutting the pleural surface (46).
Figure 3:
Normal lung US anatomy. A, Labeled US image of normal lung in a pediatric patient (scanning performed in the sagittal orientation with a curvilinear abdominal probe). The pleural line is the labeled hyperechoic line that represents the junction of the visceral and the parietal pleura. The A-line artifacts are clearly visualized as horizontal reverberation artifacts of the hyperechoic pleural line. The rib shadows separate the intercostal spaces. B, Labeled US image of normal lung in a neonate (scanning performed in the sagittal orientation at the lower lung). The interface between the liver and lung is clearly visualized. C, Labeled lung image from a lower-end ultrasound machine with a suboptimal acoustic window. Even on such limited examinations, normal A-line artifact can often still be appreciated on careful examination, as seen here. Cine clips show normal lung sliding (Movie 1) and absent lung sliding (Movie 2).
Movie 1:
Cine US clip showing normal lung sliding.
Movie 2:
Cine US clip showing absent lung sliding.
Interstitial Thickening: Edema and Fibrosis
When the pulmonary interstitium thickens (secondary to fibrosis or fluid), B-line artifacts replace the normal A-lines (1,35). B-line artifact consists of well-defined, laserlike, vertical, echogenic lines arising from the pleural line and extending to the bottom of the image. Scattered B-lines (fewer than two per intercostal space) can be present in normal lung (2,56). The number of B-lines directly correlates to disease severity. B-lines are diffusely present in the setting of pulmonary edema, pulmonary fibrosis, and pneumonitis (including vaping injury) (Figs 4 and 5) (Movies 3 and 4). The presence of B-lines on US is sensitive for pulmonary edema and may be more sensitive compared to chest radiography for its detection (6). Focal and/or unilateral B-lines suggest a localized process such as atypical pneumonia.
Figure 4:
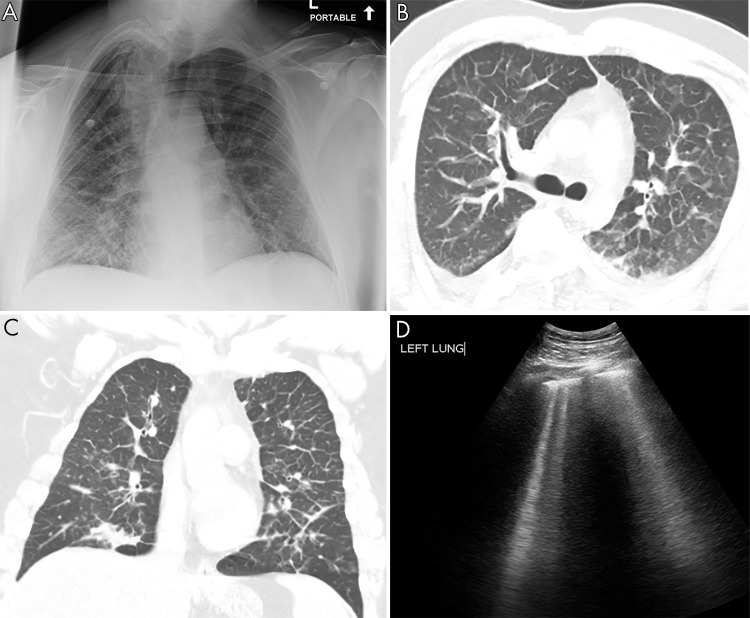
Pulmonary edema on lung US. A, Anteroposterior chest radiograph shows nonspecific prominent interstitial markings bilaterally in a 27-year-old man with pulmonary edema. B, Axial and C, coronal CT images show marked bilateral septal thickening, scattered consolidation, and ground-glass opacity. D, Lung US shows B-line artifacts arising from the pleural line and loss of A-lines. Movie 3 shows B-line artifacts in this same patient.
Figure 5:
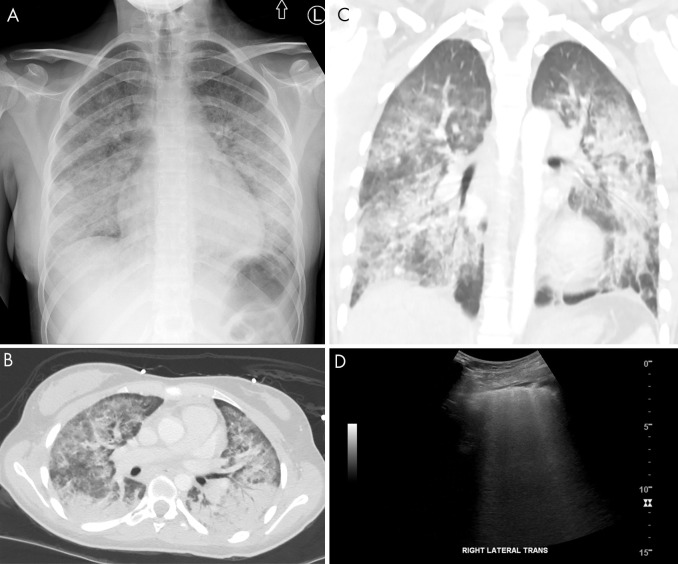
Vaping lung injury on lung US. A, Anteroposterior chest radiograph demonstrates nonspecific bilateral interstitial and consolidative pulmonary opacity in a 17-year-old adolescent girl with a vaping-induced lung injury. B, Axial and C, coronal CT images demonstrate diffuse bilateral consolidation, interstitial thickening, and ground-glass opacity. D, Lung US demonstrates loss of A-lines with confluent B-line artifacts better appreciated on the cine clip (Movie 4) Trans = transverse.
Movie 3:
Cine US clip showing B-lines from the same patient with pulmonary edema as in Figure 4.
Movie 4:
Cine US clip showing B-lines from the same patient with vaping lung injury as in Figure 5.
Pneumothorax
A pneumothorax separates the visceral and parietal pleura, eliminating normal lung sliding between these layers on lung US. The point of transition between the pneumothorax and normal lung is known as the lung point. Identification of a lung point on lung US yields 100% specificity for pneumothorax (58). Pneumothorax can also be identified at M-mode US, a technique that depicts motion. In a healthy lung, the tissue superficial to the pleural line remains stationary, with smooth horizontal lines at M-mode imaging. Deep to the pleura, the lung motion interrupts the lines, creating a finely interrupted granular or “sandy” pattern. This normal pattern is called the seashore sign as it depicts the boundary between the stationary chest wall (“ocean”) and moving lung (“sand”) (Fig 6). When a pneumothorax is examined at M-mode US, the smooth horizontal lines are uninterrupted, as the chest wall and air deep to the pleura are both stationary in pneumothorax (1). This appearance of pneumothorax at M-mode examination has been dubbed the bar code sign (Fig 7) (Movies 5 and 6). Lung US surpasses chest radiography in sensitivity for pneumothorax (Table) (4). The addition of color Doppler US can improve detection of pneumothorax, as color signal from the lung is absent owing to the air barrier of the pneumothorax (48).
Figure 6:

In healthy lung, M-mode US shows the seashore sign in which tissue superficial to the pleural line remains stationary creating smooth horizontal lines, and deep to the pleura, the lung motion interrupts the lines, creating a finely interrupted granular or “sandy” pattern. RT = right.
Figure 7:
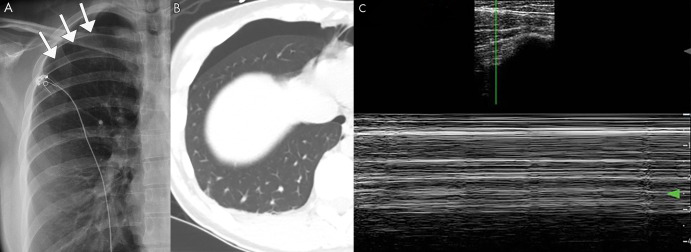
Pneumothorax on lung US. A, Posteroanterior chest radiograph and B, axial CT image of a spontaneous pneumothorax in a 26-year-old patient. C, M-mode US image shows the barcode sign, in which the smooth horizontal lines corresponding to the stationary chest wall are uninterrupted owing to lack of lung sliding, which is diagnostic for pneumothorax. Cine clips show absent lung sliding in the right lung (Movie 5) and the normal lung sliding in the left lung (Movie 6), which cannot be appreciated on still imaging. Arrows indicate location of the pleura in the setting of a pneumothorax.
Movie 5:
Cine US clip showing absent lung sliding in right lung from the same patient in Figure 7 with pneumothorax. The absent lung sliding corresponds to pneumothorax.
Movie 6:
Cine US clip provided for comparison showing lung sliding in the left lung without pneumothorax.
Pleural Effusion
US directly images pleural fluid (59). Simple effusions commonly present as anechoic fluid in the posterior dependent lung (1,5,35,49). Complex pleural fluid collections, including chronic effusions, malignant effusions, hemithorax, and empyema, are more heterogeneous in appearance on US depending on extent of debris, septations, and pleural thickening. Given the ability to visualize this level of detail, lung US is often better than conventional chest radiography for assessing complicated pleural effusions and assists in interventional management. Lung US can help to identify loculated areas for drainage or indicate the need for more aggressive therapy, including surgical washout or tissue plasminogen activator administration. A large effusion creates an acoustic window, allowing visualization of the vertebral bodies, also known as the spine sign (Fig 8).
Figure 8:
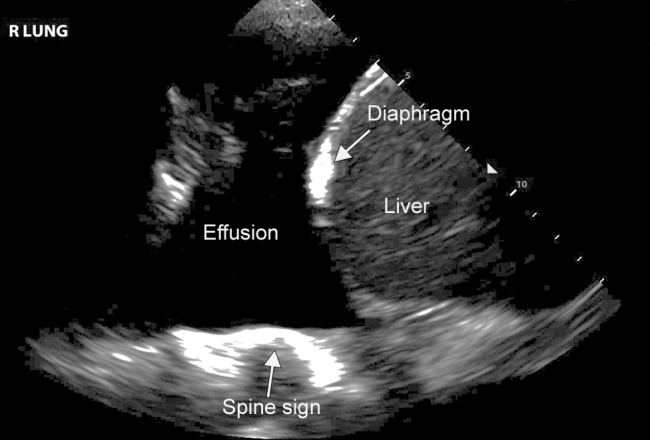
Pleural effusion on lung US. Lung US image of a patient with a moderate-sized pleural effusion. The acoustic window created by the effusion allows visualization of the spine (spine sign), as can be seen in this image. Normally, the vertebral bodies are not apparent on US.
Infection
Lung US is an excellent modality to evaluate and monitor known or suspected pulmonary infection (7,20,28,30,60–62). Pneumonia has several imaging appearances depending on the extent of consolidation or interstitial involvement (1,5,7,35). A completely consolidated lung mimics the solid appearance of the liver; this is known as hepatization (Fig 9) (Movies 7 and 8). In consolidation, fluid or cells fill the alveoli, and the normal A-lines or air-filled lung are lost. If air bronchograms are present, they are manifested as hyperechoic foci within the consolidated lung (Fig 9, B). The interface between consolidated abnormal lung and aerated normal lung is identified by an irregular hyperechoic line termed the shred sign (Fig 9, C). Smaller infections may manifest as a focal subpleural hypoechoic area. In early infection, focal B-lines may be present, indicating that the affected interstitium is becoming thickened and/or inflamed. Color Doppler imaging is useful for the evaluation of lung abscesses and empyema. A lung abscess demonstrates internal vascularity, because there are residual portions of the necrotic lung parenchyma in the lesion (Fig 10). In comparison, pleural-based empyema demonstrates no internal flow (Fig 11) (45). Foci of gas within a collection are hyperechoic and demonstrate twinkle artifact on color Doppler US. Complex conditions containing mixed areas of pneumonia, atelectasis, and pleural fluid often are better assessed at lung US than at chest radiography (Fig 12).
Figure 9:
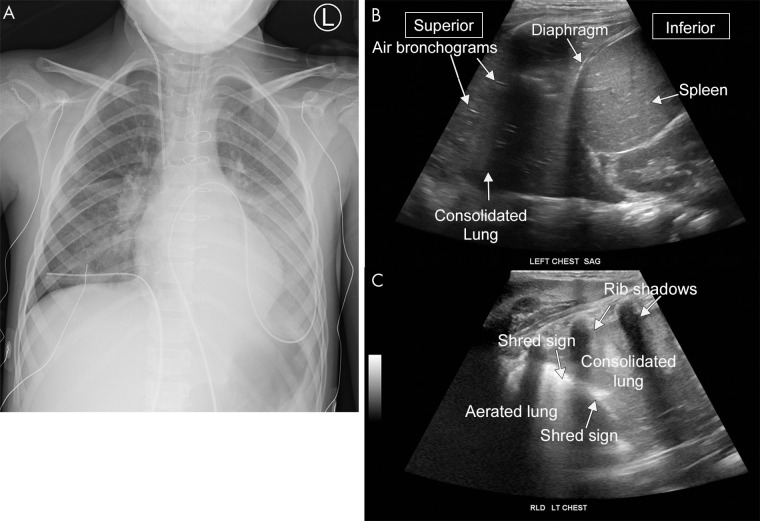
Pneumonia on lung US. A, Anteroposterior chest radiograph and B, C, US images from a 6-year-old patient with pneumonia. The lower lung on US appears similar in appearance to the liver, representing so-called hepatization of the pulmonary parenchyma consistent with consolidation. B, The hyperechoic foci within the consolidation are air bronchograms. C, The shred sign is the irregular hyperechoic line separating the consolidated and aerated lung. A sagittal cine clip (Movie 7) and a transverse cine clip (Movie 8) show more detail. LT = left, RLD = right lateral decubitus.
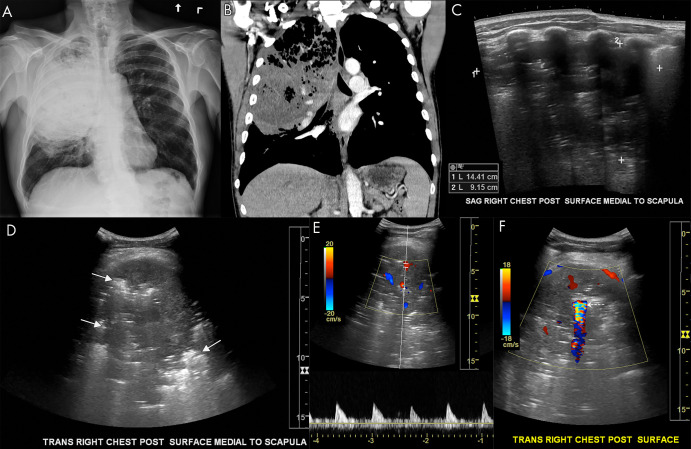
Figure 10:
Pulmonary abscess on lung US. A, Anteroposterior chest radiograph and B, coronal chest CT image show a large pulmonary abscess in the right upper lung containing foci of air in a 56-year-old smoker. C, Sagittal and D, transverse grayscale US images demonstrate a large 14-cm abscess. Hyperechoic foci are seen throughout the abscess corresponding to air (arrows, D). E, F, Associated color Doppler images show internal Doppler flow consistent with abscess. There is a large focus of twinkle artifact corresponding to air within the abscess (dashed arrow, F).
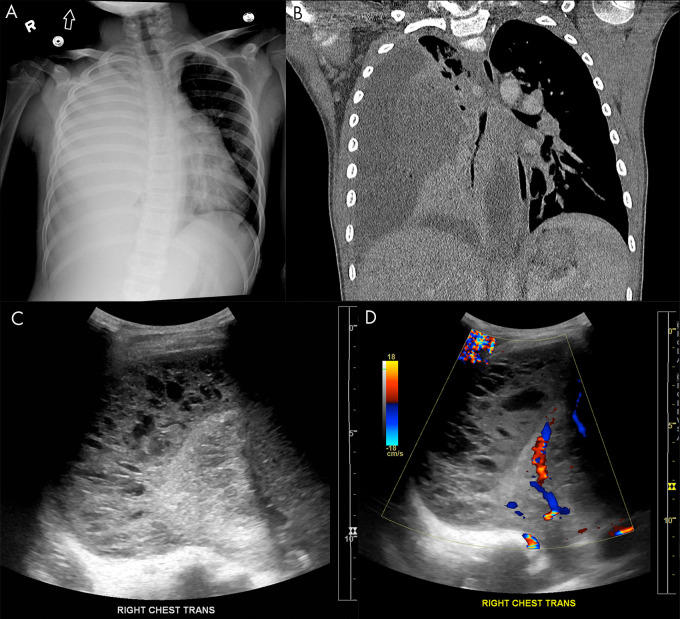
Figure 11:
Empyema on lung US. A, Anteroposterior chest radiograph and B, coronal chest CT image show a large, right-sided empyema occupying the pleural space with associated compression of the pulmonary parenchyma in a 9-year-old boy. Lung US C, grayscale and D, Doppler images demonstrate a multiloculated complex-appearing fluid collection in the pleural space with septations and internal debris without internal color flow.
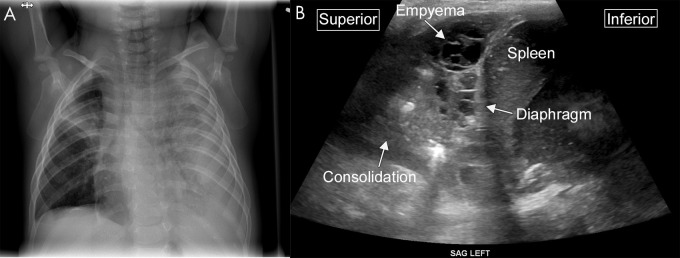
Figure 12:
Complex collection on lung US. A, Anteroposterior chest radiograph demonstrates near complete opacification of the left hemithorax, with loculated pleural fluid tracking along the left lateral chest wall in an 8-month-old male infant. In addition, a focal consolidation is present in the right upper lobe. B, Sagittal US image from the same patient shows consolidation within the lung and a multiseptated complex pleural fluid collection consistent with empyema.
Movie 7:
Sagittal cine US clip of the same patient in Figure 9 with lower lobe pneumonia.
Movie 8:
Transverse cine US clip of the same patient in Figure 9 with lower lobe pneumonia.
Viral infections, including COVID-19 and bronchiolitis, can also be assessed and monitored using lung US (54,63–66). There is subjective enthusiasm for the ability of lung US to diagnose, stratify risk, and monitor COVID-19 infection, although findings at lung US can lack specificity (63). B-line artifact of varying severity, consolidations, and pleural irregularities have all been visualized in COVID-19 infection (Fig 13) (Movies 9 and 10). In areas of focal ground-glass opacity, diffuse confluent B-lines are present with loss of A-lines. Pleural effusion is rare in these patients. During the recovery phase, the B-lines decrease and the A-lines typically return. It is important to note that lung US performed on patients with COVID-19 presents a risk to the operator; this risk can be minimized with the proper use of protective equipment (63). Outside of COVID-19, lung US is effective in evaluating viral infection in pediatric patients (22,25,28,36).
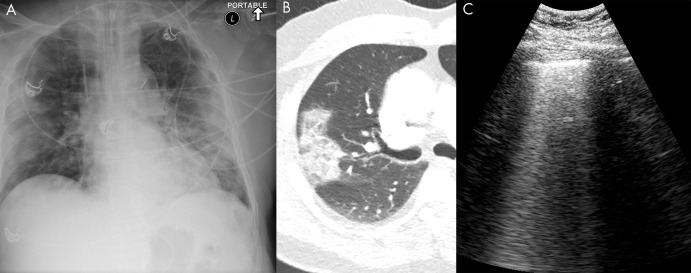
Figure 13:
COVID-19 infection on lung US. A, Anteroposterior chest radiograph, B, axial chest CT image, and C, lung US image from the same 74-year-old man, who tested positive for COVID-19 5 days prior to imaging. The chest radiograph shows bilateral peripheral opacity, which presents with a ground-glass appearance on the chest CT image. Lung US imaging in this patient demonstrated numerous B-lines throughout the parenchyma which were diffusely confluent in some sections. These are better seen in a cine clip (Movie 9). A small consolidation in a patient with COVID-19 is shown in Movie 10.
Movie 9:
Cine US clip of B-lines from the same patient in Figure 13 with COVID-19 infection. LT = left
Movie 10:
Cine US clip shows a small consolidation in a COVID-19 positive patient.
Future Directions and Medical-Legal Perspectives
As the use of lung US increases, diagnostic radiologists stand at a crossroads. Should we leave lung US to the clinicians? Or, perhaps, might we stake our own claim to the modality? We feel that there is a role for both clinicians and radiologists in performing and interpreting lung US. In general, lung US and chest radiography should be understood as complementary examinations, each providing unique clinical data with its own strengths and limitations. One could consider initially assessing patients with diagnostic lung US, because it is radiation-free, and then in cases of clinical uncertainty, follow up with radiography. However, the advantages of diagnostic US must be weighed against the increased time and personnel resources needed to perform and interpret lung US compared with those for routine chest radiography. Perhaps in children, for whom radiation is a greater concern, the benefits of lung US outweigh this drawback. Known infections, including pneumonias and viral illnesses, can be safely monitored with lung US, thus eliminating the need for repeat radiation exposure. As to who should perform and interpret lung US, there may be a role for both point-of-care examinations and more formal involvement by a radiologist. Given the time cost of a full lung US examination, routine use as a point-of-care screening tool in the emergency department may be best performed by clinicians. In more complicated cases, however, a radiologist may offer expertise that is best suited to a formal diagnostic examination.
As we embark into new territory with lung US, some concerns exist regarding the potential additional malpractice exposure to a clinician performing lung US. In general, point-of-care US is already in widespread use and is generally accepted (67). Review of the literature shows no known lawsuits related directly to point-of-care US; however, litigation has occurred secondary to the failure to perform US, which suggests that use of point-of-care lung US may reduce the risk of litigation (68,69). There are published professional standards for quality assurance, archiving, and documenting US procedures that should be followed (70,71).
With the recent improvements in point-of-care portable US, lung US has the potential to replace the stethoscope at the bedside (11,14,72). Auscultation often has limited sensitivity and specificity, and US allows for a focused examination of the area of interest (73). Another obvious application of lung US is in rural or economically impoverished areas, where access to medical imaging may be limited, and in low- and middle-income countries where the modality has already demonstrated efficacy (74). Similar to other uses for US, lung US is also well-suited to tele-US and remote reading (75–77). Because pneumonia is the leading killer of children from birth to 5 years of age worldwide, with nearly 1 million associated deaths per year and over 100 million hospitalizations per year, the use of lung US could be a powerful tool in promoting global health for aid organizations (74,78–83).
For years, chest radiography and chest CT have been the staples of regular thoracic diagnostic imaging. As a versatile and highly accurate imaging modality, lung US has the potential to substantially alter the thoracic diagnostic imaging milieu for the better. With its high sensitivity and specificity for a variety of pulmonary conditions, lung US appears to have a bright future, and its increasing use has the potential to positively affect health care worldwide.
Acknowledgments
Acknowledgments
We would like to thank Solomon Kim, Nadezhda Kiriyak, Sarah Klingenberger, Jane Lichorowic, and Gwen Mack for their assistance with our illustrations and figures. We would like to thank Nancy Carson, MBA, RDMS, RVT, for her manuscript edits.
Disclosures of Conflicts of Interest: T.J.M. disclosed no relevant relationships. D.J.R. disclosed no relevant relationships. Y.T.Z. disclosed no relevant relationships. J.W. disclosed no relevant relationships. T.P.O. disclosed no relevant relationships. W.H.N. disclosed no relevant relationships. K.A.K.J. disclosed no relevant relationships.
Abbreviations:
- BLUE
- bedside lung US in emergency
- ICU
- intensive care unit
References
- 1.Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care 2014;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtenstein DA. Current Misconceptions in Lung Ultrasound: A Short Guide for Experts. Chest 2019;156(1):21–25. [DOI] [PubMed] [Google Scholar]
- 3.Saraogi A. Lung ultrasound: Present and future. Lung India 2015;32(3):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebrahimi A, Yousefifard M, Mohammad Kazemi H, et al. Diagnostic Accuracy of Chest Ultrasonography versus Chest Radiography for Identification of Pneumothorax: A Systematic Review and Meta-Analysis. Tanaffos 2014;13(4):29–40. [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004;100(1):9–15. [DOI] [PubMed] [Google Scholar]
- 6.Maw AM, Hassanin A, Ho PM, et al. Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults With Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis. JAMA Netw Open 2019;2(3):e190703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye X, Xiao H, Chen B, Zhang S. Accuracy of Lung Ultrasonography versus Chest Radiography for the Diagnosis of Adult Community-Acquired Pneumonia: Review of the Literature and Meta-Analysis. PLoS One 2015;10(6):e0130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015;147(6):1659–1670. [DOI] [PubMed] [Google Scholar]
- 9.Bouhemad B, Zhang M, Lu Q, Rouby JJ. Clinical review: Bedside lung ultrasound in critical care practice. Crit Care 2007;11(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brogi E, Bignami E, Sidoti A, et al. Could the use of bedside lung ultrasound reduce the number of chest x-rays in the intensive care unit? Cardiovasc Ultrasound 2017;15(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantinotti M, Giordano R, Valverde I. Lung ultrasound: a new basic, easy, multifunction imaging diagnostic tool in children undergoing pediatric cardiac surgery. J Thorac Dis 2017;9(6):1396–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganapathy A, Adhikari NK, Spiegelman J, Scales DC. Routine chest x-rays in intensive care units: a systematic review and meta-analysis. Crit Care 2012;16(2):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gargani L, Picano E. The risk of cumulative radiation exposure in chest imaging and the advantage of bedside ultrasound. Crit Ultrasound J 2015;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung Ultrasound for Critically Ill Patients. Am J Respir Crit Care Med 2019;199(6):701–714 [Published corrections appear in Am J Respir Crit Care Med 2020;201(8):1015 and Am J Respir Crit Care Med 2020;201(11):1454.]. [DOI] [PubMed] [Google Scholar]
- 15.Tolsma M, Rijpstra TA, Rosseel PM, et al. Defining indications for selective chest radiography in the first 24 hours after cardiac surgery. J Thorac Cardiovasc Surg 2015;150(1):225–229. [DOI] [PubMed] [Google Scholar]
- 16.Touw HR, Parlevliet KL, Beerepoot M, et al. Lung ultrasound compared with chest X-ray in diagnosing postoperative pulmonary complications following cardiothoracic surgery: a prospective observational study. Anaesthesia 2018;73(8):946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trumbo SP, Iams WT, Limper HM, et al. Deimplementation of Routine Chest X-rays in Adult Intensive Care Units. J Hosp Med 2019;14(2):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez Lima DR, Yepes AF, Birchenall Jiménez CI, Mercado Díaz MA, Pinilla Rojas DI. Real-time ultrasound-guided thoracentesis in the intensive care unit: prevalence of mechanical complications. Ultrasound J 2020;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto N, Watanabe T, Yamada K, et al. Efficacy and safety of ultrasound (US) guided percutaneous needle biopsy for peripheral lung or pleural lesion: comparison with computed tomography (CT) guided needle biopsy. J Thorac Dis 2019;11(3):936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambroggio L, Sucharew H, Rattan MS, et al. Lung Ultrasonography: A Viable Alternative to Chest Radiography in Children with Suspected Pneumonia? J Pediatr 2016;176:93–98.e7. [DOI] [PubMed] [Google Scholar]
- 21.Berce V, Tomazin M, Gorenjak M, Berce T, Lovrenčič B. The Usefulness of Lung Ultrasound for the Aetiological Diagnosis of Community-Acquired Pneumonia in Children. Sci Rep 2019;9(1):17957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biagi C, Pierantoni L, Baldazzi M, et al. Lung ultrasound for the diagnosis of pneumonia in children with acute bronchiolitis. BMC Pulm Med 2018;18(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boursiani C, Tsolia M, Koumanidou C, et al. Lung Ultrasound as First-Line Examination for the Diagnosis of Community-Acquired Pneumonia in Children. Pediatr Emerg Care 2017;33(1):62–66. [DOI] [PubMed] [Google Scholar]
- 24.Buonsenso D, Brancato F, Valentini P, Curatola A, Supino M, Musolino AM. The Use of Lung Ultrasound to Monitor the Antibiotic Response of Community-Acquired Pneumonia in Children: A Preliminary Hypothesis. J Ultrasound Med 2020;39(4):817–826. [DOI] [PubMed] [Google Scholar]
- 25.Di Mauro A, Ammirabile A, Quercia M, et al. Acute Bronchiolitis: Is There a Role for Lung Ultrasound? Diagnostics (Basel) 2019;9(4):E172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendaus MA, Jomha FA, Alhammadi AH. Lung ultrasound for the diagnosis of childhood pneumonia: a safe and accurate imaging mode. Ther Clin Risk Manag 2015;11:1817–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iorio G, Capasso M, De Luca G, et al. Lung ultrasound in the diagnosis of pneumonia in children: proposal for a new diagnostic algorithm. PeerJ 2015;3:e1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovrenski J. Pediatric lung ultrasound - pros and potentials. Pediatr Radiol 2020;50(3):306–313. [DOI] [PubMed] [Google Scholar]
- 29.Omran A, Eesai S, Ibrahim M, El-Sharkawy S. Lung ultrasound in diagnosis and follow up of community acquired pneumonia in infants younger than 1-year old. Clin Respir J 2018;12(7):2204–2211. [DOI] [PubMed] [Google Scholar]
- 30.Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics 2015;135(4):714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbankowska E, Krenke K, Drobczyński Ł, et al. Lung ultrasound in the diagnosis and monitoring of community acquired pneumonia in children. Respir Med 2015;109(9):1207–1212. [DOI] [PubMed] [Google Scholar]
- 32.Lim JS, Lee S, Do HH, Oh KH. Can Limited Education of Lung Ultrasound Be Conducted to Medical Students Properly? A Pilot Study. BioMed Res Int 2017;2017:8147075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marini TJ, Castaneda B, Baran T, et al. Lung Ultrasound Volume Sweep Imaging for Pneumonia Detection in Rural Areas: Piloting Training in Rural Peru. J Clin Imaging Sci 2019;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.See KC, Ong V, Wong SH, et al. Lung ultrasound training: curriculum implementation and learning trajectory among respiratory therapists. Intensive Care Med 2016;42(1):63–71. [DOI] [PubMed] [Google Scholar]
- 35.Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound 2014;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buonsenso D, Musolino AM, Gatto A, Lazzareschi I, Curatola A, Valentini P. Lung ultrasound in infants with bronchiolitis. BMC Pulm Med 2019;19(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sartori S, Tombesi P. Emerging roles for transthoracic ultrasonography in pulmonary diseases. World J Radiol 2010;2(6):203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38(4):577–591. [DOI] [PubMed] [Google Scholar]
- 39.Milliner BHA, Tsung JW. Lung Consolidation Locations for Optimal Lung Ultrasound Scanning in Diagnosing Pediatric Pneumonia. J Ultrasound Med 2017;36(11):2325–2328. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen N, Pietersen PI, Nolsoe C, Konge L, Graumann O, Laursen CB. Clinical Applications of Contrast-Enhanced Thoracic Ultrasound (CETUS) Compared to Standard Reference Tests: A Systematic Review. Ultraschall Med 2020. 10.1055/a-1143-3141. Published online April 7, 2020. [Google Scholar]
- 41.Laursen CB, Graumann O, Møller TV, Davidsen JR. Contrast-enhanced Ultrasound-guided Transthoracic Lung Biopsy. Am J Respir Crit Care Med 2016;194(5):e5–e6. [DOI] [PubMed] [Google Scholar]
- 42.Petkov R, Yamakova Y, Petkova E, Petrov D, Yamkov G. Contrast-enhanced ultrasound examination of pulmonary lesions. Eur Respir J 2012;40(Suppl 56):264. https://erj.ersjournals.com/content/40/Suppl_56/P264.22753835 [Google Scholar]
- 43.Quarato CMI, Lacedonia D, Venuti M, Simeone A, Sperandeo M. Contrast-Enhanced Ultrasound in COVID-19 Pneumonia: The Pulmonary Circulation Is a Highly Specialized Vascular System. J Ultrasound Med 2020. 10.1002/jum.15452. Published online August 31, 2020. [DOI] [PubMed] [Google Scholar]
- 44.Sperandeo M, Rea G, Grimaldi MA, Trovato F, Dimitri LM, Carnevale V. Contrast-enhanced ultrasound does not discriminate between community acquired pneumonia and lung cancer. Thorax 2017;72(2):178–180. [DOI] [PubMed] [Google Scholar]
- 45.Chen HJ, Yu YH, Tu CY, et al. Ultrasound in peripheral pulmonary air-fluid lesions. Color Doppler imaging as an aid in differentiating empyema and abscess. Chest 2009;135(6):1426–1432. [DOI] [PubMed] [Google Scholar]
- 46.Koh DM, Burke S, Davies N, Padley SP. Transthoracic US of the chest: clinical uses and applications. RadioGraphics 2002;22(1):e1. [DOI] [PubMed] [Google Scholar]
- 47.Kurian J, Levin TL, Han BK, Taragin BH, Weinstein S. Comparison of ultrasound and CT in the evaluation of pneumonia complicated by parapneumonic effusion in children. AJR Am J Roentgenol 2009;193(6):1648–1654. [DOI] [PubMed] [Google Scholar]
- 48.Richards JR, Awrey JM, Medeiros SE, McGahan JP. Color and Power Doppler Sonography for Pneumothorax Detection. J Ultrasound Med 2017;36(10):2143–2147. [DOI] [PubMed] [Google Scholar]
- 49.Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med 2015;10(12):811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu WH, Ikezoe J, Chen CY, et al. Color Doppler ultrasound signals of thoracic lesions. Correlation with resected histologic specimens. Am J Respir Crit Care Med 1996;153(6 Pt 1):1938–1951. [DOI] [PubMed] [Google Scholar]
- 51.Sripathi S, Mahajan A. Comparative study evaluating the role of color Doppler sonography and computed tomography in predicting chest wall invasion by lung tumors. J Ultrasound Med 2013;32(9):1539–1546. [DOI] [PubMed] [Google Scholar]
- 52.Yuan A, Chang DB, Yu CJ, Kuo SH, Luh KT, Yang PC. Color Doppler sonography of benign and malignant pulmonary masses. AJR Am J Roentgenol 1994;163(3):545–549. [DOI] [PubMed] [Google Scholar]
- 53.Miller A. Practical approach to lung ultrasound. BJA Educ 2016;16(2):39–45. [Google Scholar]
- 54.World Health Organization . Millennium Development Goals (MDGs). https://www.who.int/topics/millennium_development_goals/en/. Published 2019. Accessed June 2020 [Google Scholar]
- 55.Doerschug KC, Schmidt GA. Intensive care ultrasound: III. Lung and pleural ultrasound for the intensivist. Ann Am Thorac Soc 2013;10(6):708–712. [DOI] [PubMed] [Google Scholar]
- 56.Neto FMJ, Rahal AJ, Vieira FA, da Silva PS, de Gusmão Funari MB. Advances in lung ultrasound. Einstein (Sao Paulo) 2016;14(3):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soldati G, Demi M, Inchingolo R, Smargiassi A, Demi L. On the Physical Basis of Pulmonary Sonographic Interstitial Syndrome. J Ultrasound Med 2016;35(10):2075–2086. [DOI] [PubMed] [Google Scholar]
- 58.Lichtenstein D, Mezière G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med 2000;26(10):1434–1440. [DOI] [PubMed] [Google Scholar]
- 59.Brogi E, Gargani L, Bignami E, et al. Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Crit Care 2017;21(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bourcier JE, Braga S, Garnier D. Lung Ultrasound Will Soon Replace Chest Radiography in the Diagnosis of Acute Community-Acquired Pneumonia. Curr Infect Dis Rep 2016;18(12):43. [DOI] [PubMed] [Google Scholar]
- 61.Xia Y, Ying Y, Wang S, Li W, Shen H. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis 2016;8(10):2822–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yadav KK, Awasthi S, Parihar A. Lung Ultrasound is Comparable with Chest Roentgenogram for Diagnosis of Community-Acquired Pneumonia in Hospitalised Children. Indian J Pediatr 2017;84(7):499–504. [DOI] [PubMed] [Google Scholar]
- 63.Gargani L, Soliman-Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M. Why, when, and how to use lung ultrasound during the COVID-19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging 2020;21(9):941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med 2020;46(5):849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sultan LR, Sehgal CM. A Review of Early Experience in Lung Ultrasound in the Diagnosis and Management of COVID-19. Ultrasound Med Biol 2020;46(9):2530–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volpicelli G, Lamorte A, Villén T. What’s new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med 2020;46(7):1445–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sorensen B, Hunskaar S. Point-of-care ultrasound in primary care: a systematic review of generalist performed point-of-care ultrasound in unselected populations. Ultrasound J 2019;11(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med 2012;30(2):338–341. [DOI] [PubMed] [Google Scholar]
- 69.Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med 2015;16(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.American College of Radiology . Practice Parameters for Documentation and Reporting. https://www.acr.org/Clinical-Resources/Practice-Parameters-and-Technical-Standards/Practice-Parameters-for-Documentation-and-Reporting. Published 2020. Accessed June 2020. [Google Scholar]
- 71.European Society of Radiology (ESR) . Good practice for radiological reporting. Guidelines from the European Society of Radiology (ESR). Insights Imaging 2011;2(2):93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lichtenstein D. Novel approaches to ultrasonography of the lung and pleural space: where are we now? Breathe (Sheff) 2017;13(2):100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wipf JE, Lipsky BA, Hirschmann JV, et al. Diagnosing pneumonia by physical examination: relevant or relic? Arch Intern Med 1999;159(10):1082–1087. [DOI] [PubMed] [Google Scholar]
- 74.Amatya Y, Rupp J, Russell FM, Saunders J, Bales B, House DR. Diagnostic use of lung ultrasound compared to chest radiograph for suspected pneumonia in a resource-limited setting. Int J Emerg Med 2018;11(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Britton N, Miller MA, Safadi S, Siegel A, Levine AR, McCurdy MT. Tele-Ultrasound in Resource-Limited Settings: A Systematic Review. Front Public Health 2019;7:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marini TJ, Oppenheimer DC, Baran TM, et al. New Ultrasound Telediagnostic System for Low-Resource Areas: Pilot Results From Peru. J Ultrasound 2021;40(3):583-595. [DOI] [PubMed] [Google Scholar]
- 77.Marini TJ, Castaneda B, Baran T, et al. Lung Ultrasound Volume Sweep Imaging for Pneumonia Detection in Rural Areas: Piloting Training in Rural Peru. J Clin Imaging Sci 2019;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health 2019;7(1):e47–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008;86(5):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudan I, O’Brien KL, Nair H, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health 2013;3(1):010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H; WHO Child Health Epidemiology Reference Group . Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ 2004;82(12):895–903. [PMC free article] [PubMed] [Google Scholar]
- 82.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381(9875):1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wardlaw T, Salama P, Johansson EW, Mason E. Pneumonia: the leading killer of children. Lancet 2006;368(9541):1048–1050. [DOI] [PubMed] [Google Scholar]