Abstract
As efficacy and safety data emerge, differences between JAK inhibitor subclasses are appearing. JAK1 selective drugs, upadacitinib and filgotinib, have broadly come with the same overarching safety recommendations as other immunosuppressive drugs for RA: caution is needed regarding infection risk; monitoring for laboratory abnormalities, including lipids and muscle enzymes, is indicated. A distinguishing feature of JAK inhibitors is a risk for zoster reactivation. Numerically, overall rates of serious infection are similar among JAK inhibitor classes. There are currently no signals for diverticular perforation. VTE incidence rates were similar across comparator groups for the JAK1 selective agents. These observations are not yet conclusive evidence for different safety profiles between JAK1 selective agents and other JAK inhibitors. Differences in study population, design, and concomitant steroid use are examples of potential confounders. It is too early to draw conclusions on long-term outcomes such as malignancy and cardiovascular risk. Post-marketing pharmacovigilance studies will be essential.
Keywords: rheumatoid arthritis, JAK inhibitor, safety, zoster, trials, pharmacovigilance
Rheumatology key messages
JAK1 selective agents confer a small increase in risk of infection.
Herpes zoster reactivation can occur.
No signal seen for VTE or diverticular perforation in clinical trials.
Introduction
The 21st century has seen an astonishing evolution in the therapeutics available to treat immune mediate inflammatory diseases (IMIDs). Patients and clinicians now have a wide choice of medications available to help reduce inflammation and improve quality of life. The challenge is how to choose the right medicine for the right patient. The efficacy data for the therapeutic options spans anti-cytokine monoclonals, monoclonals against cellular targets and small molecule inhibitors of the Janus kinase (JAK) pathway. While some differences are starting to emerge, head-to-head comparisons are scarce and efficacy is impressive across the board. However, safety profiles are notably different across classes of treatment, and often safety takes priority over efficacy when selecting options for individual patients.
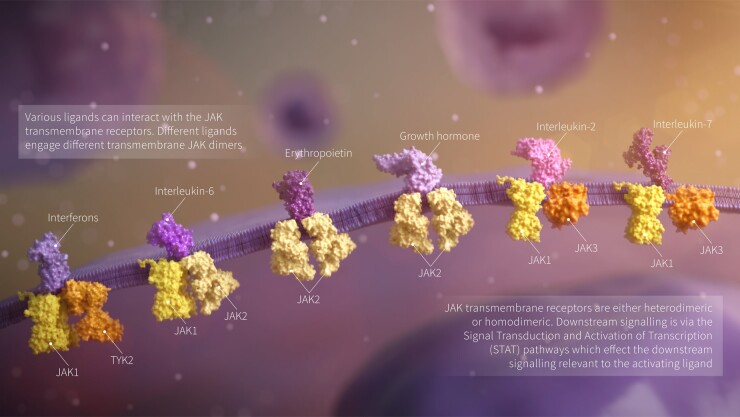
The JAK pathway is recognized as a key player in the immune dysregulation in many IMIDs. Four JAKs exist in humans: three are receptor bound protein kinases: JAK1, JAK2, JAK3, while the fourth member of the family, TYK2, is not receptor bound. The function of the JAK family is to send signals from extracellular cytokines and activate downstream cascades via the JAK-STAT pathway (Fig. 1). The JAK inhibitor tofacitinib was licensed in 2012, followed by baricitinib in 2017, upadacitinib in 2019, and filgotinib by the European Medical Agency (EMA) in 2020. The licensing of tofacitinib, baricitinib and filgotinib has been staggered between Europe and the US due to differences in opinions from the respective regulators about safety.
Fig. 1.
JAK transmembrane receptor signalling pathways
Upadacitinib and filgotinib distinguish themselves from baricitinib and tofacitinib as having greater selectivity for JAK-1. This review will consider the safety of selectively inhibiting the JAK-1 pathway.
Biological mechanisms of JAK inhibitors
The JAK family are responsible for the phosphorylation and activation of proteins involved in signal transduction from cell surface to the nucleus, thereby switching on transcription. Many pathways signal via JAK, including haematopoietic colony stimulating factors, hormones such as prolactin, and many cytokines. Therapeutic treatment of IMIDs benefits from the cytokine blockade effected by JAK inhibition. The JAK1 pathway is important for cytokines sharing the common gamma chain for type 1 cytokine receptors (e.g. IL-2, IL-6). JAK1 is also part of the canonical signalling pathway for type 1 and type 2 interferons. The implications of blocking JAK1 requires appreciation of two attributes of the available drugs: (i) the efficacy and safety of small molecular compounds that competitively inhibit JAK1 will be dose-dependent; and (ii) in vitro selectivity for JAK1 may not translate to in vivo selectivity in patients in the real world.
Safety of JAK inhibition
As a class, long-term JAK pharmacovigilance studies are very much in their preliminary stages. Information on the safety comes from the randomized controlled trials, with no post-marketing pharmacovigilance study data available yet. For upadacitinib, there is a large phase three trial programme to inform understanding, with the five SELECT (MONOTHERAPY, EARLY, COMPARE, NEXT and BEYOND) trials published [1–5]. Filgotinib has two large phase 2 trials (DARWIN 1 and 2) and three phase 3 trials (FINCH 1–3) of which only FINCH 2 is a full published trial to draw upon at the time of writing [6–8]. It will be several years before post-marketing surveillance data from the registries become available.
When considering the safety of a new therapy, it is imperative to consider clinical trial data in the right context. In general, absolute adverse event rates are lower in clinical trials than real-world cohorts. Trials select for patients with fewer comorbidities who are at inherently lower risk of infection. Trial populations vary, with some focussing on early disease participants who are treatment naïve, while other trials recruit patients who have failed csDMARDs and/or bDMARDs. Background concomitant DMARD and steroid use varies across and within individual trials. Finally, trial recruitment is global and overall event rates can mask significant country-to-country variation. All these factors need to be borne in mind when considering safety reporting.
It is reasonable to say that, in absolute terms, risks of adverse events are broadly similar for JAK inhibitors as for other bDMARD classes. However, concealed within a similar overall risk, there are important differences between JAK inhibitors and bDMARDs. Tofacitinib and baricitinib have adverse event profile characteristics that separate them out from the bDMARD classes, with signals including an increased risk of herpes zoster reactivation, elevations in lipids, decreases in haemoglobin and lymphocytes (including natural killer cells). Concerns have also emerged around a possible increased risk for venous thromboembolism (VTE) and arterial thromboembolism (ATE) [9–11]. The data for upadacitinib and filgotinib contain far fewer patient years of exposure compared with tofacitinib and bariticinib, and it is too early to make comparisons within the JAK class.
JAK1 and infection
The risk of serious infection, such as those resulting in hospitalization, is often viewed as the cardinal safety outcome for trials of immune modulators. The absolute event rates for serious infections were low (two to four events per 100 person years of follow-up) across the JAK1 trials [1–8]. Numerically higher event rates were seen for upadacitinib and filgotinib compared with placebo in a dose-dependent manner. Comparing event rates to those seen in the published trials of non-selective JAK inhibitors and bDMARDs, the serious infection rates for patients in the JAK1 trials appear similar and reassuring. Due to differences in patient populations, it is hard to compare between JAK classes. A systematic review and meta-analysis from 2019 acknowledged these challenges, highlighting that placebo infection incidence rates fluctuated between drugs (tofacitinib placebo arms 1.2, baricitinib placebo arms 4.1 and upadacitinib placebo arms 1.8 per 100 patient years) [12]. In meta-analysis, there was no significant difference in the infection rates between the three agents (filgotinib data were not available at the time of the analysis). Based upon the published filgotinib trial data now available, the absolute serious infections appear numerically lower than for upadacitinib. Given the limited data available for filgotinib and acknowledging differences in trial designs and populations, it is premature to speculate on differences within the JAK1 class.
While overall infection rates are broadly similar between the JAK inhibitors, there may be differences in patterns of infection, especially when considering non-serious events. Upper respiratory tract infections (including nasopharyngitis) are one of the most common non-serious infectious adverse events in the JAK1 trials. Upper respiratory tract infections are universally listed in Summary of Product Characteristics as ‘very common’ (occurring in ≥1/10) for all targeted therapies for RA (including TNF inhibitors, rituximab, abatacept and anti-IL6). Whether these non-serious events are attributable to a drug effect is hard to unpick. The clinical trial data infrequently perform objective statistical comparisons for non-serious events, and few of the larger registers routinely capture these outcomes.
Herpes zoster
The reactivation of Varicella zoster virus is the most recognized infectious complication with JAK inhibitors, with JAK-dependent functions implicated in various steps in the virus’s life cycle. Trial data have shown that herpes zoster infections are more frequent with upadacitinib compared with placebo, csDMARDs and biologics [1–5]. In the pooled population from the upadacitinib SELECT programme, herpes zoster was reported in 16 patients receiving the 15 mg dose vs seven patients in the placebo group. The higher 30 mg dose was associated with a greater number of infections, both serious and multidermatomal in nature. A systematic review and meta-analysis from 2019 reported an incidence of 2.41 per 100 patient-years with upadacitinib 15 mg, which is higher than seen with anti-TNF-therapy (incidence 1.6 per 100 patient-years) [12]. This study may have underestimated the incidence as it did not include three unpublished trials from the SELECT programme. In the phase II Darwin 1 and 2 studies, herpes zoster was reported in five patients receiving filgotinib vs only one patient in the placebo group [7, 8]. There were four cases reported across all filgotinib doses in the FINCH 2 trial with none reported in patients receiving placebo [6]. All zoster cases were uncomplicated and occurred in patients older than 55 years. With both drugs, there is no evidence on the incidence of post-herpetic neuralgia.
Long-term extension studies will be helpful in characterizing these events further. Real-world longitudinal data from analyses of tofacinib and baricitinib have reported higher incidence rates (4.4 and 3.2 per 100 patient-years, respectively) with substantially greater risk in older patients with co-prescription of glucocorticoids or methotrexate [13, 14]. The incidence is influenced by geography, with higher rates reported in Asian studies. Pooled data may suggest that selectivity for JAK1 to be associated with a lower risk of zoster, although data are still evolving.
Preventing zoster is a complex issue. For younger adults (under 50), it is reasonable to check varicella immunity prior to starting therapy. Anyone not already immune should be offered the chickenpox vaccine. For people over 50, a shingles vaccine should be considered. There is a live shingles vaccine widely available. This vaccine may reduce the incidence and severity of zoster, although there is a concern about the use of live vaccines in people already on (or about to begin) immune modulatory therapy [15]. The risks of a live vaccine are theoretically greater in a person who is naïve to the virus, so paradoxically it would be sensible to avoid this vaccine in someone without prior Varicella zoster exposure. A non-live subunit vaccine is available in some countries with superior immunogenicity. It is likely to become a standard of care for people beginning JAK inhibitor therapy once availability is widespread [16, 17].
Herpes simplex
The mechanisms behind reactivation of Herpes simplex and Varicella zoster virus are similar, and JAK inhibition associates with an increased susceptibility to both primary and reactivated HSV infections. Herpes simplex events are reported less often than zoster in trial data and overall has received less attention. These infections are an important consideration as they may be recurrent events and sometimes misdiagnosed as shingles.
Oral candidiasis
Oral candidiasis occurs in immunocompromised individuals due to the commensal microorganism Candida albicans. It is biologically plausible that JAK1 inhibition could reduce anti-fungal immunity. Neutrophil and macrophage anti-candida activity is driven in part by interferon-gamma, a pro-inflammatory cytokine that exerts its effect via the JAK-STAT pathway. In the upadacitinib trials there were 11 reported events of oral candidiasis in treatment arms, with one in the placebo arm [1–5]. It appears filgotinib may have a lower risk of oral candidiasis, with no events reported in the three trials [6–8]. A warning in comparing candidiasis infection between upadacitinib and filgotinib is that there may be differences in reporting thresholds. Most cases of candidiasis are non-serious and it is possible that there is a reporting bias accounting for variation.
Opportunistic infections
The risk of reactivation of latent tuberculosis (TB) with targeted therapy is well established. As a result, all JAK1 clinical trials have undertaken careful screening for TB prior to enrolment. There have been very few reported cases of active TB in the JAK1 trials. Screening in real-world clinical practice is likely to be less stringent than in the trial setting, so long-term extension studies and registries will be important. Mechanistically, and taking inference from the other JAK inhibitors, it is reasonable to assume that a risk of TB reactivation is present.
Pneumocystis jirovecii pneumonia was not reported in the JAK1 clinical trial programme. However, pneumonic illness with cryptococcus was reported in one patient in the SELECT-EARLY upadacitinib trial (in a patient on 15 mg daily) [5]. Cryptococcal lung infection is unusual and has also been described in patients on tofacitinib and baricitinib. Although a single event in the upadacitinib trials, the unusual nature of this organism suggests that there may be a mechanistic link. Fungal infections are a recognized risk with other classes of tyrosine kinase inhibitor and post-marketing pharmacovigilance will be important in clarifying this signal [18].
JAK1 and blood disorders
Several activating mutations in JAK proteins have been described as the cause of disorders of haematopoiesis. As a result, drugs blocking the JAK pathway have been developed for the treatment of myeloproliferative diseases. Ruxolitinib, a JAK2 selective inhibitor, is used to treat patients with myelofibrosis and polycythaemia rubra vera. Changes in haematological indices are therefore plausible with JAK inhibitors.
The upadacitinib trial programme saw no clinically important impact on haemoglobin levels at the licensed 15 mg daily dose [1, 2, 4]. Patients on a higher dose (30 mg daily) were more likely to develop anaemia (4.1% vs 2.8%) [5]. In contrast, a rise in haemoglobin was observed in the filgotinib trials [6–8]. Filgotinib’s effects also appear to be dose dependent. The rationale for the difference could be hypothesized by filgotinib having a minimal blockade of JAK2; in contrast, upadacitinib has a weak blockade effect.
Although there were no notable difference on lymphocyte count (including NK cells), one upadacitinib RCT reported three of seven patients with a change of grade 3–4 lymphocyte reduction developing viral infection (SELECT-NEXT) [1]. Dose-dependent neutropenia was reported, but is uncorrelated with lymphopaenia, and did not result in clinically significant events. The absence of an effect on NK cells is relevant, as this contrasts with changes seen with the other JAK inhibitors.
JAK1 and lipids
Active and untreated inflammatory arthritis is associated with raised non-traditional lipid profiles, namely high-density lipoprotein (HDL) and associated HDL proteins. The STAT1 pathway is crucial for cholesterol synthesis and it would be biologically expected to see changes in lipid levels with drugs that modulate the JAK pathways. Prior trials of tofacitinib have demonstrated dyslipidaemia, with rises in both HDL and low-density lipoprotein (LDL) levels [19].
The upadacitinib trials reported elevations of HDL and LDL. The changes appeared to not affect the HDL/LDL ratio at the end of the study period. To quantify changes, SELECT-MONO reported rises of 0.4 mmol/l for LDL and 0.3 mmol/l for HDL; these changes compared with <0.1 mmol/l changes for both HDL and LDL with methotrexate [4]. For RCTs involving filgotinib, there was a rise noted within both HDL and LDL levels within the first 4 weeks, which then led to a reduced LDL/HDL ratio over 24 weeks [6–8]. This change implied a preferential rise specific to the HDL component. The changes in lipid profile were dose dependent for both upadacitinib and filgotinib.
A crucial question about lipid changes is whether there is any subsequent change in cardiovascular risk. The experience from the IL-6 inhibitor class has been that the lipid changes do not translate into longer term cardiovascular risk (assuming the lipids are monitored and managed accordingly) [20]. However, there is a potential for substantial lag between lipid profile changes and cardiovascular disease. In humans, changes in lipids take many years to translate into clinical outcomes; this is demonstrated well from our understanding of the statin trials, albeit observing lipid changes in a reverse direction [21]. For the JAK1 inhibitors, long-term and registry data will be essential to characterizing any impact on cardiovascular risk.
Gastrointestinal safety
Gastrointestinal toxicity is familiar to rheumatologists in the form of peptic and duodenal ulceration attributable to corticosteroids or NSAIDs. Concerns of lower gastrointestinal toxicity have subsequently emerged with IL-6 inhibition, which has been associated with an increased risk of diverticular perforation [22]. In August 2020, regulators issued a warning around diverticular disease and perforation risk for patients on the JAK2 selective drug, baricitinib [23]. The observation was not entirely surprising as the cytokine IL-6 is a ligand for JAK2, and so a plausible mechanistic association exists. However, to date, the association between JAK inhibition and diverticular perforation has only been highlighted for baricitinib (with a regulatory update released in August 2020).
Only one trial from the upadacitinib or filgotinib programme reported any gastrointestinal perforations, and none of these were diverticular in origin. In the upadacitinib SELECT-COMPARE trial, three gastrointestinal perforations were reported in the upadacitinib arms, but none were spontaneous and were in the setting of a fallopian tube abscess, anal abscess and anal fistula [2]. In summary, the JAK1 class do not appear to share a risk of diverticular perforation.
Hepatotoxicity
In the upadacitinib and filgotinib trials, transient increases in AST and ALT levels were reported, mostly grade 1 and 2. Grade 3 and 4 elevations in AST and ALT were infrequent. No Hy’s law cases were reported suggesting that no drug-induced hepatocellular injury occurred. The incidence of grade 3 or 4 AST/ALT elevations was lower with JAK1 inhibitors than for methotrexate.
JAK1 and muscle enzymes
Non-selective JAK inhibitors have reported rises in CPK levels in rheumatoid cohorts. Graded as mild, none have previously been associated with rhabdomyolysis or renal injury. In vitro, JAK1 activation phosphorylates STAT3, which plays a role in skeletal muscle activation. Muscle-related symptoms are common reported side effects and are no different among the JAK1 trials compared with the other JAK inhibitors. The effects are dose dependent; for example, with higher levels of CPK recorded on higher doses of upadacitinib; 30 mg (11.1% vs 15 mg 2.8%) [5].
JAK1 and malignancy risk
Autoimmune diseases are believed to have a greater risk of malignancies including lymphoma [24, 25]. The clinical trials on JAK1 inhibitors are short-term studies in the context of considering malignancy, where long latency periods may exist. The double-blinded trials on upadacitinib were for 48 weeks or fewer, filgotinib trials were for 24 weeks. Long-term safety assessments studies and registry data will be essential to delineate any overall cancer risk related to JAK1 inhibitors.
Two trials on upadacitinib reported two patients who developed lymphoma and one patient with non-melanoma skin cancer [1, 4]. In SELECT-MONO, one patient developed non-Hodgkin’s lymphoma [4]. Two malignancies were reported in SELECT-NEXT, one basal cell carcinoma (in a patient with a previous history of skin cancer) and another who developed B-cell small lymphocytic lymphoma/chronic lymphocytic leukaemia [1]. The manufacturer recommends a periodic skin examination in patients with an increased risk of skin cancer. No malignancies were reported in filgotinib trials, although it is not appropriate to draw comparisons as the trial populations were much smaller for the filgotinib programme [6–8].
JAK1 and VTE risk
Concerns have been raised about venous thromboembolism (VTE) risk with JAK inhibitor therapy, with product warnings recommending caution in patients at increased risk of VTE. The JAK2 receptor role in myelopoiesis and platelet production is cited as a biological basis for the link between JAK inhibitor and VTE [26]. However, the connection is not straightforward. Direct inhibition of JAK2 with ruloxitinib in patients with myeloproliferative disorders is associated with fewer VTE events [27]. In addition, a meta-analysis of JAK inhibition across immune-mediated inflammatory diseases did not confirm any increase in VTE risk for the class of drug as a whole [28].
Across the four published upadacitinib RCTs with placebo arms, six VTE events were reported over 461 treatment-arm patient years, compared with one event in 366 placebo-arm patient years. An additional RCT with no placebo arm reported one VTE event in 116 patient years [4]. The three published filgotinib trials have reported one VTE event in 491 treatment-arm patient years compared with no events in 117 placebo-arm patient years, again showing no statistical imbalance [6–8]. Further VTE events may become known when the three filgotinib trials have publish their full findings on clinicaltrials.gov. Extension studies and registry data are required to further characterize VTE risk with JAK1 inhibition. If a risk of VTE does exist with JAK1 therapy, the effect size is likely to be small. No conclusions can be drawn to imply difference in rates within JAK inhibitor class.
Strengths and limitations of the data
The reader must bear in mind that the entirety of the information presented in this review is derived from the RCT programme for upadacitinib and filgotinib. Trials are designed to test efficacy over safety. As most safety outcomes are uncommon, it is important that the rheumatology community remain vigilant as new options for treating RA come to the market. Post-marketing surveillance is an essential responsibility of the clinical community and continued contribution to the global platform of pharmacovigilance registers is paramount.
Conclusions
The JAK1 inhibitor class has arrived with a favourable safety profile from the clinical trial programmes. There are suggestions that the adverse event profiles may differ between the JAK inhibitors subclasses. JAK1 inihibition appears to associate with fewer haematological effects compared with the other JAK inihibitors. Herpes zoster may be less frequent with filgotinib, although more data are needed before robust conclusions can be drawn. Different laboratory profiles of the drugs suggest that there may even be within-class differences between filgotinib and upadacitinib, although the clinical significance of these differences is uncertain.
Disclosure statement: The authors have declared no conflicts of interest.
Funding: J.G. has received speaker fees/honoraria from AbbVie, BMS, Celgene, Chugai, Gilead, Janssen, Lilly, Novartis, Pfizer, Sanofi and UCB. This paper was published as part of a supplement supported by an educational grant from Gilead.
InSight: The related InSight paper for this supplement can be accessed at https://academic.oup.com/rheumatology/article-lookup/doi/10.1093/rheumatology/keab341.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
References
- 1. Burmester GR, Kremer JM, Van den Bosch F. et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018;391:2503–12. [DOI] [PubMed] [Google Scholar]
- 2. Fleischmann RM, Genovese MC, Enejosa JV. et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis 2019;78:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Genovese MC, Fleischmann R, Combe B. et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 2018;391:2513–24. [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Pangan AL, Emery P. et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. The Lancet 2019;393:2303–11. [DOI] [PubMed] [Google Scholar]
- 5. Vollenhoven R, Takeuchi T, Pangan AL. et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately to severely active rheumatoid arthritis (SELECT-EARLY): a randomized, double-blind, active-comparator, multi-center, multi-country trial. Arthritis Rheumatol 2020;72:1607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Genovese MC, Kalunian K, Gottenberg JE. et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA 2019;322:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kavanaugh A, Kremer J, Ponce L. et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis 2017;76:1009–19. [DOI] [PubMed] [Google Scholar]
- 8. Westhovens R, Taylor PC, Alten R. et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis 2017;76:998–1008. [DOI] [PubMed] [Google Scholar]
- 9. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 2017;13:234–43. [DOI] [PubMed] [Google Scholar]
- 10. Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology 2019;58:ii34–i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mease P, Charles-Schoeman C, Cohen S. et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 2020;79:1400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bechman K, Subesinghe S, Norton S. et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology 2019;58:1755–66. [DOI] [PubMed] [Google Scholar]
- 13. Smolen JS, Genovese MC, Takeuchi T. et al. Safety profile of Baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019;46:7–18. [DOI] [PubMed] [Google Scholar]
- 14. Cohen SB, Tanaka Y, Mariette X. et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Annals of the Rheumatic Diseases 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cates M, Donati M, Gillet S, Ustianowski A, Galloway J.. Managing varicella zoster virus contact and infection in patients on anti-rheumatic therapy. Rheumatology 2018;57:596–605. [DOI] [PubMed] [Google Scholar]
- 16. Cunningham AL, Heineman TC, Lal H. et al. Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis 2018;217:1750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cunningham AL, Lal H, Kovac M. et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016;375:1019–32. [DOI] [PubMed] [Google Scholar]
- 18. Bechman K, Galloway JB, Winthrop KL.. Small-molecule protein kinases inhibitors and the risk of fungal infections. Curr Fungal Infect Rep 2019;13:229–43. [Google Scholar]
- 19. Wollenhaupt J, Silverfield J, Lee EB. et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 20. Castagné B, Viprey M, Martin J. et al. Cardiovascular safety of tocilizumab: A systematic review and network meta-analysis. PLOS ONE 2019;14:e0220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scandinavian Simvastatin Survival Study. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- 22. Strangfeld A, Richter A, Siegmund B. et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis 2017;76:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agency MaHpR. Baricitinib (olumiant▼): increased risk of diverticulitis, particularly in patients with risk factors. GOV.UK: Drug Safety Update, 2020. [Google Scholar]
- 24. Morton LM, Slager SL, Cerhan JR. et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014:130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y.. Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis 2001;60:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyakawa Y, Oda A, Druker BJ. et al. Thrombopoietin induces tyrosine phosphorylation of Stat3 and Stat5 in human blood platelets. Blood 1996;87:439–46. [PubMed] [Google Scholar]
- 27. Samuelson BT, Vesely SK, Chai-Adisaksopha C. et al. The impact of ruxolitinib on thrombosis in patients with polycythemia vera and myelofibrosis: a meta-analysis. Blood Coagul Fibrinolysis 2016;27:648–52. [DOI] [PubMed] [Google Scholar]
- 28. Yates M, Mootoo A, Adas M. et al. Venous thromboembolism risk with JAK inhibitors: A Meta-analysis. Arthritis Rheumatol 2020;doi:10.1002/art.41580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.