Abstract
The uptake of Jak inhibitors in the RA space has been among the most rapid in rheumatology, based on the results of comprehensive clinical trial programmes of five agents. Newer generations of Jak inhibitors, like upadacitinib and filgotinib, target Jak 1 selectively with the aim of maximizing efficacy and to improve safety. This article will review the clinical significance of evidence on: (i) Jak 1 selectivity; (ii) efficacy from the SELECT and FINCH clinical trial programmes including patient intolerant or inadequately responding to MTX (MTX-IR) and other csDMARDs patients who are bDMARD-IR) and those using monotherapy when MTX is not tolerated or contraindicated and those treated when methotrexate naive; and (iii) safety from the clinical trial programmes of these two agents will be discussed.
Keywords: Jak 1 selectivity, upadacitinib, filgotinib, efficacy, safety
Rheumatology key messages
Jak 1 selective agents, upadacitinib and filgotinib, show strong efficacy across RA groups.
Reduced venous thromboembolism (VTE) risk and a lesser zoster signal (filgotinib) needs confirming outside of clinical trials.
Introduction
Despite more effective use of conventional synthetic DMARDs (csDMARDs) and a proliferation of approved biologic DMARDs (bDMARDs) for the management of RA, including five TNF inhibitors, two IL-6 inhibitors, an IL-1 inhibitor, two anti-CD20 mAbs and a CTLA4 inhibitor, a clear unmet need remains in the management of RA patients as remission is achieved in only 40–50% of patients, less in patients with long-standing disease and time spent in remission is rarely prolonged [1]. Primary non-response and secondary loss of response over time or discontinuation due to adverse effects is commonplace. Agents with a novel mechanism of action continue to be required. The cloning of Janus kinases (JAKs) by 1991, the function of JAK identified and the elucidation of JAK-signal transducers and activators of transcription (STAT) biology by 1995 led to the successful development of JAK inhibitors with increasing selectivity of kinase inhibition ranging initially from pan-JAK (e.g. tofacitinib) to predominantly Jak 1 selectivity (e.g. upadacitinib and filgotinib), JAK 3 selectivity (e.g. decernotinib), Tyk2 selectivity (e.g. BMS-986165), combined Jak1/Tyk2 selective inhibition (e.g. bepocitinib) and JAK3/TEC kinase inhibitor (e.g. ritlecitinib) [2].
Upadacitinib was first studied in humans in 2012, and by 2013, and using a compound screening kinase-focused library to a triazolopyridine-based series of Jak1 inhibitors, filgotinib with a 30-fold selectivity for Jak 1 was developed [3, 4]. Focusing on these Jak 1 selective inhibitors, other articles in this supplement will detail the science of Jak1 inhibition, efficacy from the phase 3 clinical trial programme and appropriate safety considerations in detail but what is the practicing clinician to make of this evidence? This article will discuss the clinical utility of Jak 1 inhibitors for RA patients.
Supplementary Table S1, available at Rheumatology online, details the distinguishing characteristics of Jak inhibitors when compared with TNF inhibitors—the ‘gold standard’ for many clinicians for many years. In particular, Jak inhibitors provide an oral rather than parenteral option, with short half-life in case of adverse effects like infection, need for elective surgery or planning for pregnancy. These are agents that have no immunogenicity, are conveniently taken once or twice a day, able to inhibit a number of cytokines of pathogenic importance in rheumatic diseases including IL2, IL6, IL12 and IL23 among others such as GMCSF, erythropoietin and IFN but not TNF, IL1, IL17 and TGF-β. These therapies do not have TNF inhibitor associated issues such as aggravation of congestive cardiac failure, psoriasis induction, induction of SLE-type syndromes or demyelination. Jak inhibitors have been shown to be efficacious as monotherapy (previously the domain of the IL6 inhibitors such as tocilizumab and sarilumab) or in combination with methotrexate, and are either renally excreted or undergo hepatic metabolism (e.g. CyP3A4 pathways, rather than the reticulo-endothelial degradation of monoclonal antibodies). In contrast to bDMARDs, metabolites can have activity, e.g. carboxylesterases metabolise filgotinib to a major JAK1 selective metabolite (GS-829845) which has one-tenth the potency of the parent compound. [4]. Further, Jak inhibitors have potential for the development of generics when patents expire, which may lead to significantly cheaper options. Indeed, if Jak inhibitors are low-cost therapies, with or without methotrexate and prednisone (already low-cost medications) then the rheumatic poor would have access to effective therapy currently unaffordable for much of the world’s population. Indeed, in some Asian countries Jak inhibitor mimics already exist at the cost of cents per tablet.
Market share
The uptake of JAK inhibitors in the RA space has been among the most rapid in rheumatology. Fig. 1 demonstrates the rapid uptake of Jakinibs in the Australian market using Pharmaceutical Benefits Scheme data [5]. Supplementary Table S2, available at Rheumatology online lists current indication for upadacitinib and filgotinib. While comprehensive data is difficult to ascertain, in the USA for new commencements and the switch RA market, the Jakinibs have in the order of 29% market share while the TNF class have about 40%, and other mechanisms of action roughly 10%. Jakinib usage is predominantly as monotherapy in two of three patients.
Fig. 1.
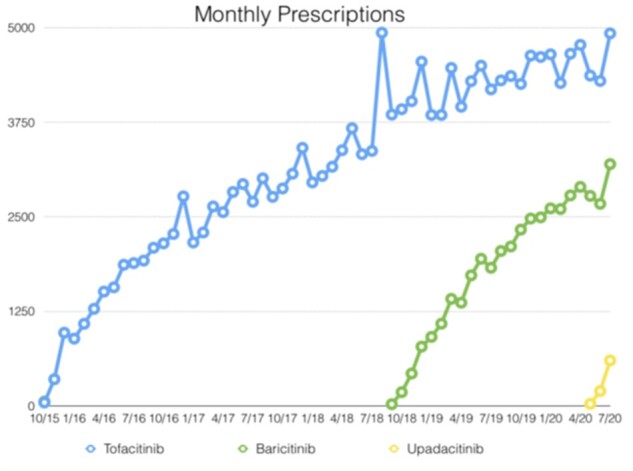
Jakinibs Australian Pharmaceutical Benefits Scheme
Selectivity
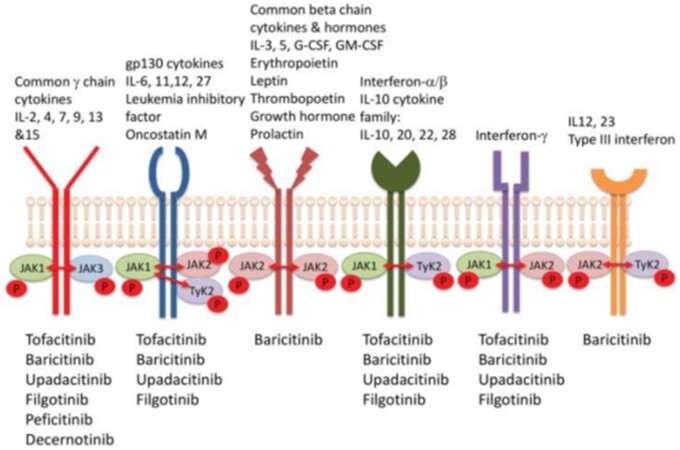
Fig. 2 highlights the homo- and hetero-dimeric nature of the JAK pathway and the overlap in cytokine signalling [6]. Further, the seven STATs have functional overlap so that each member of the STAT family can be activated by multiple JAKs so that a single STAT can transmit signals normally transduced by an alternate STAT [7]. Other clinically important variables include duration of the dosing period reversibly inhibited by the JAK inhibitor in addition to variables in hepatic metabolism (with accompanying drug interactions as detailed elsewhere) or renal excretion.
Fig. 2.
Jak selectivity
Refer to numerous examples in other chapters of this supplement that show a jak selectivity figure.
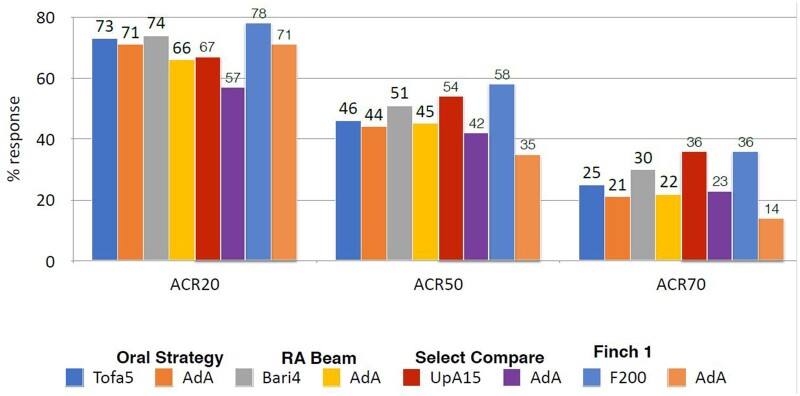
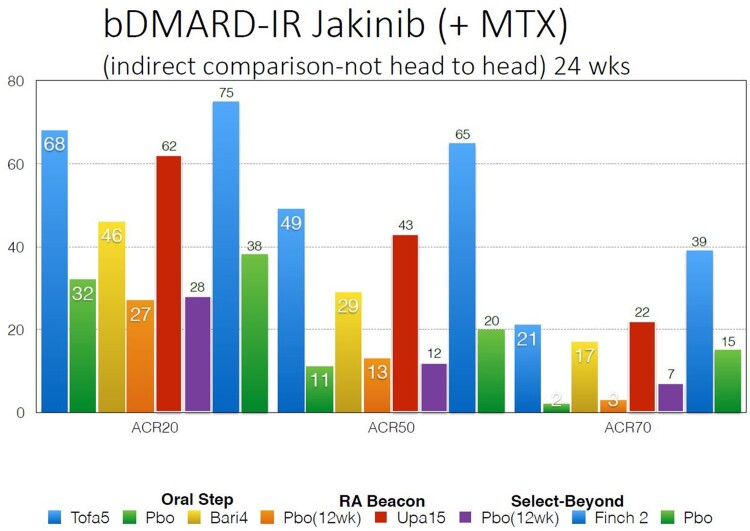
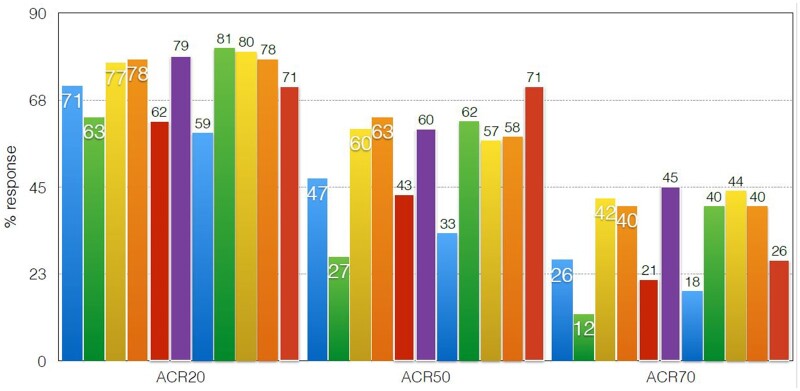
Although it was predicted that Jak 1 selectivity would enable inhibition of IL6 and gamma cytokine-mediated signalling while avoiding Jak 2 inhibition, thus reducing EPO (anaemia) GM-CSF (neutropaenia) inhibition, selectivity is dose- and tissue-dependent. Indeed, it has become apparent that it is difficult to separate the Jak inhibitors, either pan-Jak or selective Jaks, on the basis of efficacy [8, 9]. Indirect comparisons, which are not from head-to-head trials, demonstrate in Fig. 3 comparable ACR responses in the MTX-IR population, in Fig. 4 comparable responses in the bDMARD-IR population and in Fig. 5 in the monotherapy population.
Fig. 3.
MTX-IR Jakinib vs adalimumab (indirect comparison not head-to-head) 24 weeks
Fig. 4.
bDMARD-IR Jakinib (+ MTX) (indirect comparison not head-to-head) 24 weeks
Fig. 5.
Jakinib monotherapy (indirect comparison) corrected for MTX 24 weeks
Efficacy of Jak I selective inhibitors
The methodology and results of the SELECT (Upadacitinib) and FINCH (Filgotinib) clinical trial programmes have been detailed in other papers in this supplement, but what is the clinician to make of these trials?
MTX-IRs
In this space and rare for RA studies, active comparator and studies powered for superiority/non-inferiority have been performed.
Baricitinib with background MTX (RA-Beam trial) for the first time showed superiority to the then ‘gold standard’ therapy in many countries of adalimumab and MTX, a result that has led to rapid uptake of this drug across European countries in particular [10].
Tofacitinib has been studied with an active comparator arm in the ORAL Standard study as well as a powered non-inferiority study ORAL Strategy, which showed tofacitinib with MTX was non-inferior to adalimumab and MTX and monotherapy was quite effective (with ACR 20, 50 and 70 responses of 65, 38, 18%—that expected for all bDMARDs plus MTX). These studies laid the benchmark for all subsequent Jak inhibitors to equal [11, 12].
RA patients in the SELECT Compare study, on a background of MTX, were randomized to placebo or 15 mg upadacitinib or adalimumab 40 mg fortnightly and the study was powered to test superiority for selected measures. All ranked primary and secondary endpoints were met but, in particular, upadacitinib 15 mg with MTX was superior to adalimumab and MTX at ACR50, and superior for change from baseline in HAQ as well as change in pain from baseline to week 12 with differences maintained through 26 weeks. Non-inferiority was also demonstrated for DAS28-CRP low disease activity (LDA) vs adalimumab on background MTX. Patient reported outcomes like quality of life, pain and fatigue were all significant in favour of upadacitinib 15 mg vs placebo and retardation of radiological progression while an insensitive measure of damage compared with, e.g. MRI was significant when compared with placebo and no different to adalimumab confirming Jak inhibitors are not expensive NSAIDs. Onset of efficacy was rapid.
Significance for remission was seen for all measures from DAS-28 through SDAI, the high bar of Boolean remission as well as for Clinical Disease Activity Index (CDAI) (which does not include CRP or ESR to refute those who suggest the DAS28-CRP superiority results from IL6 inhibition and its effect on CRP and ESR). Further, the SELECT-Compare trial included a switch study for non-responders (which was not powered for superiority) from upadacitinib non-responders to adalimumab and also from adalimumab non-responders to upadacitinib with immediate switch (without washout). Efficacy was achieved with either switch, numerically higher switching to upadacitinib and there was no safety penalty.
The expected safety signals for upadacitinib were seen including herpes zoster, Creatine Phosphokinase (CPK) elevation, lymphopaenia and hepatic transaminitis through to 72 weeks. Importantly, serious infection, major adverse cardiac events(MACE), VTE and malignancy were balanced across the groups [13, 14]. The SELECT NEXT trial confirmed efficacy and safety for upadacitinib in combination with other csDMARDs such as SSZ and LEF as alternatives to combination with MTX [15].
In the FINCH 1 trial, on a background of MTX, filgotinib 100 mg, or 200 mg daily, adalimumab 40 mg fortnightly or placebo was studied for 52 weeks. For filgotinib 200 mg with MTX, DAS28CRP LDA showed non-inferiority to adalimumab with MTX at 200 mg but not at the 100 mg filgotinib dose with MTX so that subsequent analyses in the ranked hierarchy were nominal only.
Efficacy was confirmed vs placebo for ACR 20 at week 12, DAS28-CRP LDA, HAQ-D and other patient reported outcomes as well as modified Total Sharp Score for radiological outcomes for filgotinib 200 and 100 mg [16].
A serious issue for this study was the very high placebo responses seen in patients recruited from eastern Europe and Central and South America—instead of the expected 30–35% placebo response rates, in these groups rates were 56–59% making superiority a difficult bar to achieve. A number of factors played a role, such as non-responders were not switched to one of the two doses of filgotinib as in most trials but withdrawn to ‘standard of care’—a strong incentive for patients including patients in the placebo arm to stay in the trial. More importantly, all patients including the placebo arm were given (and supervised for compliance) MTX therapy in patients who were supposedly MTX-IR. Their HAQ response of twice the minimal clinically significant difference confirmed these patients were not truly MTX-IR, perhaps receiving regular MTX for the first time magnifying the placebo response.
Most importantly, both filgotinib doses showed, for this patient group including Asians, lower than expected rates of herpes zoster and no signal for VTE, gastric perforation, malignancy or MACE events but a small increase in high-grade lymphopaenia and neutropenia.
When the ACR components are individually examined, the superiority for Jak inhibitors including baricitinib and filgotinib is driven not by swollen joint count changes but by pain scores and patient and physician global scores. Given Jak inhibitors are thought not to cross the blood barrier, this opens the path for studies both oral and topical to be tested in a number of chronic pain conditions, for example diabetic peripheral neuropathy and complex regional pain syndromes.
bDMARD-IR studies
Efficacy and safety post-bDMARD is an important consideration for any therapy with a novel mechanism of action. Tofacitinib in the Oral Step study and baricitinib in the RA Beacon study have laid the platform for efficacy predominantly post-TNF [17, 18].
In the SELECT-BEYOND study, patients were randomized to upadacitinib 15 mg, 30 mg or placebo with 28% having failed two bDMARDs and in 25%, three or more. An important issue to address was to show efficacy for a Jak inhibitor post-parenteral IL-6 inhibition, predominantly tocilizumab as seen in 18% of entrants. Efficacy was demonstrated against placebo for ACR 20, 50 and DAS28CRP LDA but the response rates for ACR 20, 50 and 70 were not the 50/30/15% levels seen with most drugs with other mechanism of actions in TNF-IR trials (except for IL6 inhibitors which show this level of response) but in a higher 65/34/12% range at the 12-week primary end point for upadacitinib 15 mg daily. Efficacy was shown regardless of number of failed bDMARDs and efficacy was demonstrated post parenteral-IL-6 inhibition. Zoster rates were upadacitinib dose dependent and four adjudicated VTE were seen in the upadacitinib and switch placebo to upadacitinib arms vs 0 in the placebo arm to 12–24 weeks [19].
In the FINCH 2 bDMARD-IR study, 200 mg, 100 mg filgotinib and placebo were studied. ACR 20/50/70 response rates at week 12 for filgotinib 200 mg were 66/34/12%, again a response numerically higher than expected post-bDMARD compared with drugs with other mechanisms of action. DAS28-CRP Remission (REM) dose response was 31% for filgotinib 200 mg at week 24. All secondary endpoints met significance compared with placebo. Improvement was evident by 2 weeks. ACR20 responses were independent of the number of prior bDMARDs. Low zoster rates were seen in 1.3–1.4% range to 24 weeks [20].
MTX monotherapy
Up to 30% of RA patients are MTX intolerant or in whom MTX is contra-indicated, so efficacy as monotherapy is a common and important advantage as TNF inhibitors do not have in general monotherapy superiority when compared with MTX alone [21]. SELECT monotherapy and SELECT Early (see below) examined monotherapy. In SELECT monotherapy, 15 and 30 mg upadacitinib were compared with continuation of MTX at the prior stable dose—an unusual trial design. Significance vs stable MTX for primary and secondary endpoints including DAS28-CRP LDA, CDAI <10, ACR 20/50/70 and were all met. Interestingly, 41% of patients continuing the same therapy unchanged and entering a clinical trial met ACR 20 criteria and 19% achieved DAS28-CRP LDA [22].
MTX naïve
Although rarely an option for most clinicians as regulators and reimbursors and recommendations such as EULAR 2019 update all calls for MTX as initial therapy for RA patients, with the future possibility of cheaper generics there may well come a time when cost is not an issue and head-to-head studies initiating either a Jak inhibitor or MTX in early MTX-naive RA patients is an important study comparing efficacy safety and tolerability. The tofacitinib ORAL Start study and baricitinib RA Begin study have demonstrated that Jak inhibitor monotherapy resulted in clinically and statistically significant reductions in signs and symptoms of RA, improvements in patient reported outcomes (PROs) and statistically significant inhibition of progression of structural damage compared with MTX [23, 24].
In the SELECT Early study, 15 and 30 mg upadacitinib were compared with MTX. Both doses of upadacitinib were superior to methotrexate in all efficacy outcomes, including multiple definitions of clinical remission and PROs. The clinical remission response rates were similar between the 15 and 30 mg doses (∼30% for CDAI and SDAI at week 24; ≥24% for Boolean at week 24). Responses favouring upadacitinib were rapid (as early as the first post-baseline visit: week 2, at which methotrexate had not been fully titrated), and persisted through 24 weeks across both patient- and physician-reported measures. Additionally, both upadacitinib doses prevented progression of structural damage over 24 weeks in ∼90% of patients.
VTE rates were balanced across groups, there was a dose response for CPK elevation and serious infection. Grades 3 and 4 anaemia and Grades 3 and 4 neutropenia increased at higher doses suggesting loss of Jak1 selectivity with the higher dose [25].
The Finch 3 trial, examined over 24 weeks, filgotinib 100 and 200 mg in combination with MTX, and MTX monotherapy and filgotinib 200 mg monotherapy in early RA MTX naïve patients. Statistically significantly, more patients achieved ACR 50/70/DAS28 LDA and REM for filgotinib 200 mg monotherapy vs MTX. Clinical efficacy for filgotinib was seen by 2 weeks. Imaging superiority was seen for filgotinib 200 mg vs MTX. There were no new safety signals and again, no signal for VTE, MACE, malignancy, balanced serious infections and low herpes zoster rates (0.5%) [26].
Safety
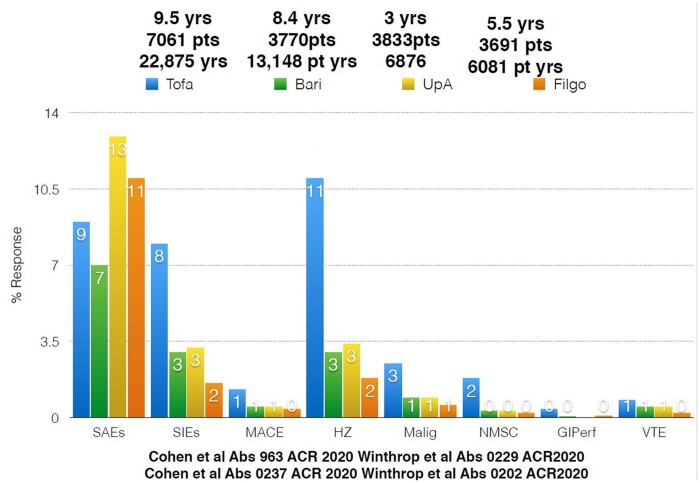
Clinicians request long-term follow-up of large numbers of patients with co-morbidities that exclude them from clinical trials for reassurance on rarer safety issues, but having appropriate controls is vital. Yet in clinical trials the placebo arms and comparator arms such as MTX and Adalimumab are relatively small in patient numbers and patient years of follow-up. To date, 9.5 years of tofacitinib clinical trial safety has been presented and 5.5 years of baricitinib trial safety [27, 28] (see Fig. 6 for an indirect and not head-to-head comparison of major adverse effects from the safety of long-term databases of the four major Jakinibs).
Fig. 6.
Safety profile clinical trial exposure
Upadacitinib safety data has been presented to 3 years of follow-up including 3833 patients with 6878 patient years of follow-up compared with 456 patient years for MTX and 769 patient years of adalimumab follow-up.
A dose response was seen for 15–30 mg upadacitinib for serious infections, CPK elevations and herpes zoster [the majority non-serious (96%) and involved a single dermatome (74%)]. VTE rates (0.3–0.5/100 patient years), MACE events and malignancies excluding non-melanoma skin cancer (numerically higher at upadacitinib 30 mg) were comparable across groups with previously demonstrated numerical increases for zoster, hepatic transaminitis and CPK elevation [29].
The filgotinib Finch 4 long-term extension trial for patients enrolled in filgotinib clinical trials is actively underway. Presented at the recent EULAR 2020, safety analysis of seven randomized clinical trials including Finch 1–4 and Darwin 1–3 studies included 4057 RA patients with 5493 patient years of follow-up. There was little in the way of dose response 100–200 mg, serious infection rates balanced across groups, lower than expected zoster rates and signal for VTE, MACE and malignancy including non-melanoma skin cancer [30]. An important issue that has affected Food and Drug Administration approval for filgotinib remains the animal studies (rats and dogs) showing decreased fertility, impaired spermatogenesis and histopathological effects on male reproductive organs with the histological effects being dose dependent. The potential effects on human patients are unknown and the results of studies such as MANTA-RAy to answer the question of human effects and reversibility are awaited. The main active metabolite (GS-829845) has no similar effects. The high intra-individual variability in sperm counts in normal males let alone chronically ill males may make interpretations of the results difficult [31].
Conclusion
Five Jak inhibitors have been avidly taken up by rheumatologists in many countries, having shown efficacy in a number of immune-mediated inflammatory diseases. Indeed, only TNF-inhibitors have a comparable spread of potential indications. In RA, Jak 1 selective inhibitors, upadacitinib and filgotinib have been comprehensively studied across the clinical spectrum from MTX-IR, bDMARD-IR, monotherapy to MTX-naive settings. Superiority to ‘gold standard’ therapy (for many TNF inhibitors combined with MTX), has been demonstrated in certain instances as described above as well as elevated remission rates, numerically higher responses post-bDMARD (including three or more failed bDMARDs) and ‘may’ have safer profiles as far as VTE and zoster (filgotinib) is concerned. Safety follow-up in long-term trial extension in clinical trials ranges from 3 years to a decade, but it will only be when large numbers of patients are included with all the usual co-morbidities that exclude patients from clinical trials (and with multiple co-medications followed for prolonged periods of time) before safety can be confirmed and touted. It is already apparent that a clear need exists for a JAK-IR trial to give guidance in those difficult patients who are Jak inhibitor non-responders.
Jak inhibitors are here to stay and clinicians need to carefully consider the evidence as presented from a very comprehensive clinical trial programme, weigh up risks and benefits and utilise this class of therapies for the benefit of their patients as the field searches for a ‘cure’.
Funding: This paper was published as part of a supplement supported by an educational grant from Gilead.
Disclosure statement: P.N. received research grant funding and honoraria for clinical trials and lectures on behalf of and advice to Abbvie and Gilead/Galapagos.
InSight: The related InSight paper for this supplement can be accessed at https://academic.oup.com/rheumatology/article-lookup/doi/10.1093/rheumatology/keab341.
Data availability statement
No data available.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Yu C, Jin S, Wang Y. et al. Remission rate and predictors of remission in patients with rheumatoid arthritis under treat-totarget strategy in real-world studies: a systematic review and meta-analysis. Clin Rheumatol 2019;38:727–38. [DOI] [PubMed] [Google Scholar]
- 2. Stark GR, Darnell JE Jr.. The JAK-STAT pathway at twenty. Immunity 2012;36:503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parmentier JM, Voss J, Graff C. et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol 2018;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor PC, Azeez MA, Kiriakidis S.. Filgotinib for the treatment of rheumatoid arthritis. Exp Opin Invest Drugs 2017;26:1181–7. [DOI] [PubMed] [Google Scholar]
- 5. www.medicareaustralia.com.au.
- 6. Choy EH. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology 2019;58:953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartze DM.. JAK–STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 2017;77:521–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biggioggero M, Becciolini A, Crotti C, Agape E, Favalli EG.. Upadacitinib and filgotinib: the role of JAK1 selective inhibition in the treatment of rheumatoid arthritis. Drugs Context 2019;8:212595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bechman K, Yates M, Galloway JB.. The new entries in the therapeutic armamentarium: the small molecule JAK inhibitors. Pharmacol Res 2019;147:104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor PC, Keystone EC, van der Heijde D. et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med 2017;376:652–62. [DOI] [PubMed] [Google Scholar]
- 11. van Vollenhoven RF, Fleischmann R, Cohen S. et al. ; ORAL Standard Investigators. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 12. Fleischmann R, Mysler E, Hall S. et al. ORAL Strategy investigators. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390:457–68. [DOI] [PubMed] [Google Scholar]
- 13. Fleischmann R, Pangan AL, Song IH. et al. Genovese MC upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase iii, double-blind, randomized controlled trial. Arthritis Rheumatol 2019;71:1788–800. [DOI] [PubMed] [Google Scholar]
- 14. Fleischmann RM, Genovese MC, Enejosa JV. et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis 2019;78:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burmester GR, Kremer JM, Van den Bosch F. et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018;391:2503–12. [DOI] [PubMed] [Google Scholar]
- 16. Combe B, Kivitz A, Tanaka Y. et al. Efficacy and safety of Filgotinib for patients with rheumatoid arthritis with inadequate response to methotrexate: FINCH 1 52 week results. 2020.
- 17. Burmester GR, Blanco R, Charles-Schoeman C. et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 18. Genovese MC, Kremer JM, Kartman CE. et al. Response to baricitinib based on prior biologic use in patients with refractory rheumatoid arthritis. Rheumatology 2018;57:900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Genovese MC, Fleischmann R, Combe B. et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 2018;391:2513–24. [DOI] [PubMed] [Google Scholar]
- 20. Genovese MC, Kalunian K, Gottenberg JE. et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 Randomized Clinical Trial. JAMA 2019;322:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nash P, Nicholls D.. Perceptions of methotrexate use in rheumatoid arthritis by rheumatologists and their patients: an Australian survey study. Int J Rheum Dis 2013;16:652–61. [DOI] [PubMed] [Google Scholar]
- 22. Smolen JS, Pangan AL, Emery P. et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019;393:2303–11. [DOI] [PubMed] [Google Scholar]
- 23. Lee EB, Fleischmann R, Hall S. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 24. Fleischmann R, Schiff M, van der Heijde D. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol 2017;69:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Vollenhoven R, Takeuchi T, Pangan AL. et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naïve patients with moderately to severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol 2020;72:1607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Westhovens R, Rigby W, van der Heijde D. et al. Efficacy and safety of filgotinib for patients with rheumatoid arthritis naïve to methotrexate therapy: FINCH 3 Primary outcome results. Ann Rheum Dis 2021;annrheumdis-2020-219213.
- 27. Wollenhaupt J, Lee EB, Curtis JR. et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther 2019;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winthrop KL, Harigai M, Genovese MC, Lindsey S. et al. Infections in baricitinib clinical trials for patients with active rheumatoid arthritis. Ann Rheum Dis 2020;79:1290–7. [DOI] [PubMed] [Google Scholar]
- 29. Cohen S, Van Vollenhoven R, Curtis J. et al. Safety profile of Uoadacitinib up to 3 years of exposure with RA. Ann Rheum Dis 2020;80:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Genovese MC, Winthrop K, Tanaka Y. et al. Integrated safety analysis of Filgotinib treatment for rheumatoid arthritis from 7 clinical trials [abstract]. EULAR 2020. [Google Scholar]
- 31.US National Institutes of Health. MANTA: https://www.clinicaltrials.gov/ct2/show/NCT03201445. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data available.