Abstract
The insulin and insulin-like growth factor (IGF) family of proteins are part of a complex network that regulates cell proliferation and survival. While this system is undoubtedly important in prenatal development and postnatal cell growth, members of this family have been implicated in several different cancer types. Increased circulating insulin and IGF ligands have been linked to increased risk of cancer incidence. This observation has led to targeting the IGF system as a therapeutic strategy in a number of cancers. This chapter aims to describe the well-characterized biology of the IGF1R system, outline the rationale for targeting this system in cancer, summarize the clinical data as it stands, and discuss where we can go from here.
1. Introduction
The insulin and insulin-like growth factor (IGF) family of proteins was first identified in the middle of the 20th century. Over the following decades, it was discovered how critical the IGF family is for normal development, cell metabolism, and cell survival. As our understanding of the expansive network of proteins and processes involved in and controlled by the IGF family expanded, so too did our understanding of how the downstream pathways could contribute to development and survival of cancer.
Epidemiological and promising preclinical data led to much enthusiasm for the targeting of IGF family members in several cancer types in the 1990s and early 2000s. Early phase clinical trials showed promise as well, however, larger phase III clinical trials failed to show clinical benefit and most therapies directed against the type I IGF receptor (IGF1R) were discontinued. Since then there has been much debate as to whether these trials were abandoned too early as data collected from these same trials has given us better insight into IGF1R biology.
This chapter will review normal IGF family protein biology and signaling, as well as examine the rationale for developing therapeutics against this system in cancer treatment, how they have been targeted in the clinic, and discuss where we go from here.
2. The insulin-like growth factor (IGF) family
2.1. Insulin and IGF ligands
The IGF family of proteins includes three ligands: IGF1, IGF2, and insulin. All three ligands are derived from unique genes on chromosomes 12 (IGF1) and 11 (IGF2 and INS). Both IGF1 and IGF2 are primarily synthesized in the liver, however, they are also produced by several other tissues and can act in an endocrine, autocrine, or paracrine manner (Livingstone & Borai, 2014; Yakar et al., 1999). While hepatic IGF1 is produced in response to pituitary-derived growth hormone (GH) and changes with age, circulating levels of both IGF1 and IGF2 can be affected equally by hormones, genetic factors, and lifestyle factors (Frystyk, Skjaerbaek, Dinesen, & Orskov, 1994; Harrela et al., 1996; Miller, Schalch, & Draznin, 1981; Schwander, Hauri, Zapf, & Froesch, 1983). Insulin expression is regulated by many factors, however, glucose is the most important as it has been shown to play a role in insulin transcription, translation, and secretion from pancreatic β-cells to act in an endocrine fashion (Giddings, Chirgwin, & Permutt, 1982; Kulkarni et al., 2011; Layden, Durai, & Lowe, 2010; Poitout et al., 2006).
IGF1 and IGF2 are single chain polypeptides. After synthesis, they are processed in the endoplasmic reticulum where the E-peptide is cleaved, leaving the mature form containing A and B domains. Insulin is first synthesized as a single polypeptide (preproinsulin) in β-cells in the pancreas and is processed into proinsulin in the endoplasmic reticulum. However, unlike the IGF ligands, insulin is then processed into three peptides—the A and B chain peptides, which are covalently linked via two disulfide bonds and form the mature form of insulin, and the C-peptide. As indicated by their names, insulin and IGFs have substantial homology. After processing, IGF1 and IGF2 share about 70% homology in their amino acid sequence and have about 50% homology with the A and B chains in mature insulin (Bach et al., 1993). Further, the 3D structures of both IGF1 and IGF2 closely resemble that of proinsulin (Blundell, Bedarkar, Rinderknecht, & Humbel, 1978). Despite this close homology, the ligands have substantially different affinities for each of the receptors in the IGF family (discussed below).
2.2. IGF binding proteins
Though insulin is found only in a “free” state in circulation, only about 0.4% of IGF1 and 0.2% of IGF2 exists in an unbound state (Frystyk et al., 1994). Instead, IGFs in extracellular fluids are primarily found bound to six structurally related binding proteins, IGFBP-1 through IGFBP-6, which bind IGFs with equal or higher affinity to the IGF receptors, indicating that this binding is an important regulator of IGF signaling (Sitar, Popowicz, Siwanowicz, Huber, & Holak, 2006). In a free state, both insulin and IGFs have a half-life of about 10min; however, when IGFs are bound to IGFBPs this time is increased to between 25min and 15h, depending on the IGFBP complex (Guler, Zapf, Schmid, & Froesch, 1989). IGFBPs share about 50% of their amino acid sequence and contain three distinct domains: an N-terminal domain, a cysteine-rich C-terminal domain, and a central linker domain. The N- and C-terminal domains are highly conserved among family members and facilitate IGF binding. The central linker domain is less conserved and is the site of the majority of the post-translational modifications found on IGFBPs (Baxter, 2000). All six members of the family have high affinity for IGF1 and IGF2, with most Kd values in the 10−10 to 10−11 M range (Baxter, 2000). IGFBPs are synthesized in a number of different cell types and their genes, which are spread across four chromosomes, are regulated independently.
The gene encoding IGFBP-1 is found on chromosome 7 and its expression is rapidly inhibited by insulin via suppression of transcription (Chahal et al., 2008). IGFBP-1 only binds a small fraction of circulating IGF1, however, this binding reduces bioavailability of IGF1 thereby inhibiting its activity (Brismar, Fernqvist-Forbes, Wahren, & Hall, 1994). Additionally, IGFBP-1 contains an arginine-glycine-aspartate (RGD) domain, which was found to interact with integrins, a family of cell adhesion receptors involved in cell-cell and cell-extracellular matrix interactions (Jones, Gockerman, Busby, Wright, & Clemmons, 1993). The IGFBP2 gene is located on chromosome 2 and, similar to IGFBP-1, it contains an RGD motif (Jones, Doerr, & Clemmons, 1995). IGFBP-2 protein expression in human plasma inversely correlates with fasting insulin levels and body mass index (BMI), indicating it could be used as an early marker of insulin resistance (Blum et al., 1993; Ko et al., 2012). IGFBP-3, whose gene is also found on chromosome 2, is the most abundant family member. IGFBP-3 does not contain an RGD motif, however, it does contain a basic heparin-binding motif in its C-terminus that also allows interaction with the extracellular matrix (ECM) (Fowlkes, Thrailkill, George-Nascimento, Rosenberg, & Serra, 1997). While all IGFBPs can form binary complexes with IGFs, the majority of circulating IGFs (approximately 75%–80%) form a ternary complex with IGFBP-3 and acid-labile subunit (ALS) (Leong, Baxter, Camerato, Dai, & Wood, 1992). These 150-kDa complexes are highly stable with a half-life of 12–15h, however, they are unable to cross capillary endothelium (Guler et al., 1989; Lewitt, Saunders, Phuyal, & Baxter, 1994). Similar to IGF1, IGFBP-3 is also produced in the liver in response to GH, likely to ensure there are sufficient levels to bind and stabilize hepatically-derived IGF1 (Blum et al., 1993). IGFBP4 is found on chromosome 17, though not much is known about its regulation. Its protein product binds to IGF1 and IGF2 with similar affinities, however, unlike other IGFBPs, there is no evidence that IGFBP-4 is able to tether to cell surfaces as it lacks both RGD and heparin-binding motifs (Zhou, Diehl, Hoeflich, Lahm, & Wolf, 2003). IGFBP5 is found on chromosome 2, and its expression is regulated by glucocorticoids. IGFBP-5 is the most evolutionarily conserved binding protein and, similar to IGFBP-3, it has a heparin-binding motif and can form ternary complexes with IGFs and ALS though at much lower frequency (Andress, 1995; Twigg & Baxter, 1998). IGFBP-6 has the highest affinity for IGF2 of all the IGFBPs and also contains a heparin-binding motif (Bach et al., 1993; Fowlkes et al., 1997). Its gene resides on chromosome 12 and not much is known about the regulation of its expression.
IGFs are released from IGFBPs through methods that reduce their mutual affinity such as by binding of IGFBPS to molecules in the ECM, phosphorylation of IGFBPs, or following proteolytic cleavage of the IGFBP central linker domain by one of several proteases including pregnancy-associated plasma protein A (PAPP-A) and prostate specific antigen (PSA) (reviewed in Bunn & Fowlkes, 2003). The resulting C-terminal and N-terminal domains have substantially lower affinity for IGFs compared to the IGF receptors, but these peptides are still able to partially inhibit IGF activity (Payet, Wang, Baxter, & Firth, 2003). IGFBPs are protected from proteolytic cleavage via post-translational modifications in their linker domain (Coverley, Martin, & Baxter, 2000; Gibson, Aplin, White, & Westwood, 2001; Neumann, Marinaro, & Bach, 1998). Though IGFBPs are typically thought of as inhibitory of IGF actions, their ability to form complexes with IGFs and accumulate in the extracellular space via interactions between the heparin binding or RGD domains and the ECM allows them to act as a pericellular reservoir of IGFs, which can be released via activation of IGFBP-targeting proteases. In this way, IGFBPs can, in fact, promote IGF action by stabilizing the ligands and allowing them to be released in close proximity to their receptors, through which they exert their cellular effects.
2.3. The IGF family of receptors
IGF1, IGF2, and insulin bind to and elicit their effects through the cell surface transmembrane receptors, IGF1R, IGF2R, and insulin receptor (IR) (Fig. 1). IGF1R is found on chromosome 15 and its expression is regulated by several growth factors including GH, thyroxine, platelet-derived growth factor, and fibroblast growth factor. The mature receptor is comprised of two dimerized hemi-receptors, each made of an extracellular α-subunit and a membrane spanning β-subunit (De Meyts et al., 1994). Each α- and β-subunit are encoded by a single gene and are synthesized as a single polypeptide, known as a preproreceptor, which is processed into a proreceptor in the endoplasmic reticulum. Additional processing includes addition of a disulfide bond between the α- and β-subunits, dimerization of two proreceptors via disulfide bonds in their α-subunits, and proteolytic cleavage in order to create a mature α2β2 tetrameric receptor. IGF1R has two splice isoforms differing by three nucleotides that ultimately code for either an Arg or Thr-Gly in the extracellular portion of the β-subunit of the receptor (Condorelli, Bueno, & Smith, 1994; Tobin, Yee, Brunner, & Rotwein, 1990).
Fig. 1.
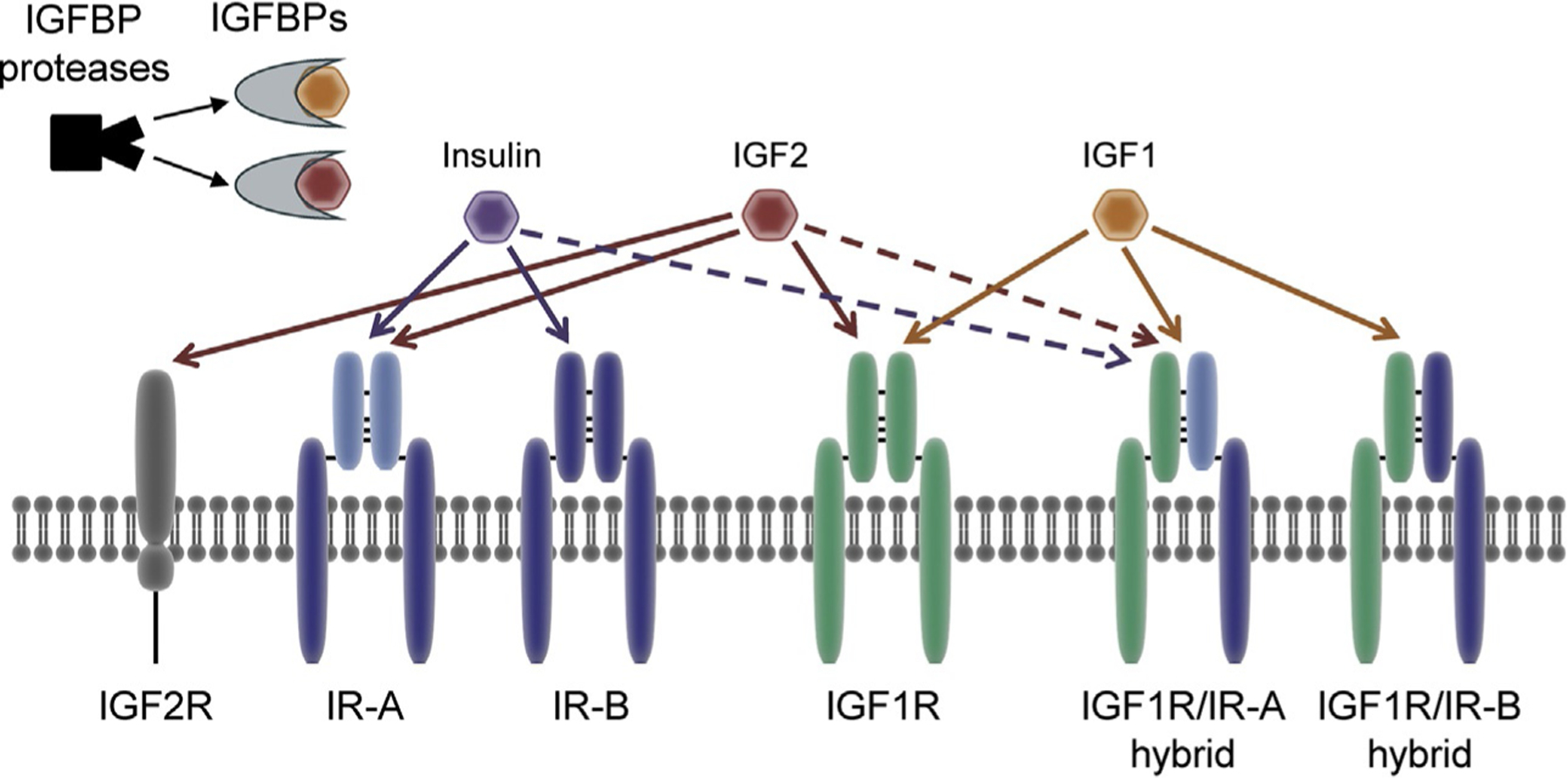
Insulin and IGF system components. Components of the IGF system are discussed extensively in the text. Briefly, the IGF system is comprised of IR and IGF1R homo- and hetero-dimers which are stimulated via binding of their ligands, insulin, IGF1, and IGF2. Each ligand has differing affinities for each of the receptors—solid lines denote high affinity interactions while dashed lines denote low affinity interactions. IGF1 and IGF2 are both primarily found in circulation bound to IGF binding proteins (IGFBP). IGFs are released from IGFBPs upon proteolytic cleavage by an IGFBP protease. Adapted from Sachdev, D. & Yee, D. (2007). Disrupting insulin-like growth factor signaling as a potential cancer therapy. Molecular Cancer Therapeutics, 6(1), 1–12.
IGF2R, also called the mannose 6-phosphate receptor, is found on chromosome 6 in humans and is structurally and functionally unrelated to IGF1R and IR. Comprised of an N-terminal extracellular domain, a single membrane-spanning region, and a small cytoplasmic tail, IGF2R lacks the intracellular tyrosine kinase activity of the other two receptors in this family. It does, however, play a role in many IGF-independent biological processes including trafficking of lysosomal enzymes (Ghosh, Dahms, & Kornfeld, 2003). IGF2R expression is ubiquitous across human tissues and the receptor is also found in a soluble form in circulation (Lobel, Dahms, & Kornfeld, 1988; Oshima, Nolan, Kyle, Grubb, & Sly, 1988). In regard to IGF signaling, IGF2R predominantly acts as a decoy receptor for IGF2, as binding of IGF2 to IGF2R leads to its endocytosis and lysosomal degradation without the activation of signaling cascades associated with IGF1R and IR activation (Kornfeld, 1992).
InsR, the gene encoding IR, is located on chromosome 19. Similar to IGF1R, a mature IR is composed of two α-subunits and β-subunits, which are processed and dimerized in the endoplasmic reticulum (Knutson, 1991). Two splice variants of IR exist—full length IR-B and the short form IR-A, which lacks the 36 base pair exon 11 and is primarily expressed in fetal tissues (Frasca et al., 1999). The presence of the 12 amino acid domain encoded by exon 11 in the C-terminus of the α-subunit decreases insulin binding affinity by two to threefold, and reduces receptor internalization and downregulation (Yamaguchi et al., 1991).
The existence of these three unique receptors for IGF1, IGF2, and insulin was originally suggested in 1982, however, the relationship among these receptors has since been found to be rather complex (Sara et al., 1982). IGF1R and IR share a high degree of homology with 84% overlapping amino acid sequence in the tyrosine kinase domain, 45%–65% in the ligand-binding domain, 100% in the ATP binding domain, and over 50% in overall sequence. IGF1R and IR also share structural similarities. Both receptors have six domains in their extracellular regions—L1, CR, L2, Fn1, Fn2, and Fn3 domains—followed by a transmembrane region, an intracellular juxtamembrane region, a tyrosine kinase domain, and a C-terminal tail (De Meyts & Whittaker, 2002; Ullrich et al., 1985). Further, IGF1R and both IR variants can form functional hybrid receptors in all tissues in which the receptors are expressed (Bailyes et al., 1997; Entingh-Pearsall & Kahn, 2004; Moxham, Duronio, & Jacobs, 1989; Valensise et al., 1996). IGF2R, however, is not able to participate in these heterodimers.
Each of the insulin and IGF ligands has vastly different binding affinities for IGF1R, IR, and hybrid receptors (Fig. 1). IGF1 has greater affinity for IGF1R compared to the hybrid receptor, and almost no affinity for IR (Slaaby et al., 2006). Similarly, insulin binds to IR with far higher affinity than the hybrid receptor and has very low affinity for IGF1R (Soos, Field, & Siddle, 1993). IGF2 has equal affinity for IGF1R and IGF2R, and can also bind the IR-A/IGF1R hybrid receptor and IR-A, however, it has lower affinity for IR-B and IR-B/IGF1R hybrid receptors.
In the absence of ligand, IGF1R, IR, and hybrid receptors on the cell surface are held in an auto-inhibitory inverted “v” conformation via reciprocal interaction between the L2-Fn1 domain pairs from opposing α-subunits (Xu et al., 2018). In this state the intracellular components of the β-subunits are spatially separated preventing autophosphorylation. Binding of one molecule of ligand induces an asymmetrical conformational change that both reduces affinity for ligand in the second ligand binding pocket and induces a shift in the intracellular domain such that the kinase domains in the β-subunits come in close enough proximity to trans-phosphorylate tyrosine residues in the kinase domains, leading to intracellular signaling (Kavran et al., 2014; Pang & Shafer, 1984; Ward, Menting, & Lawrence, 2013).
2.4. Signaling
Binding of ligand to IGF1R and IR leads to autophosphorylation on tyrosine residues Y1131, Y1135, and Y1136 on IGF1R or Y1158, Y1162, and Y1163 on IR in their respective kinase domains, followed by phosphorylation of tyrosine residues in the juxtamembrane region (Fig. 2). These residues allow subsequent docking and phosphorylation of adaptor molecules such as insulin receptor substrate (IRS)-1, IRS-2, and SHC (Wei, Hubbard, Hendrickson, & Ellis, 1995). Additional residues allow for alternate adaptor interactions. The downstream consequences of activation of these adaptors have been well characterized. In normal tissues, IRS-1 and IRS-2 are ubiquitously expressed and are critical mediators of insulin and IGF-dependent mitogenesis and metabolic regulation (Bruning, Winnay, Cheatham, & Kahn, 1997; White, 2002; Yamauchi et al., 1996). It is important to note that different normal tissues use these signals in different ways. In the liver, activation of this pathway leads to activation of glycogen storage (Clemmons, 2004). In epithelial cells, however, phosphorylation of IRS-1 leads to activation of the PI3K/Akt/mTOR signaling cascade, ultimately promoting proliferation and pro-survival pathways (Jackson, White, & Yee, 1998). IRS-2 also leads to signaling through PI3K, however this signaling leads to alterations in cell adhesion and motility (Jackson, Zhang, Yoneda, & Yee, 2001). Activation of SHC promotes activation of proliferation via the Ras/Raf/MAPK signaling cascade. While these pathways downstream of IGF and insulin receptors are essential during both prenatal and postnatal growth, aberrant activation of these pathways has also been strongly associated with cancer development and prognosis (Pollak, 2012).
Fig. 2.
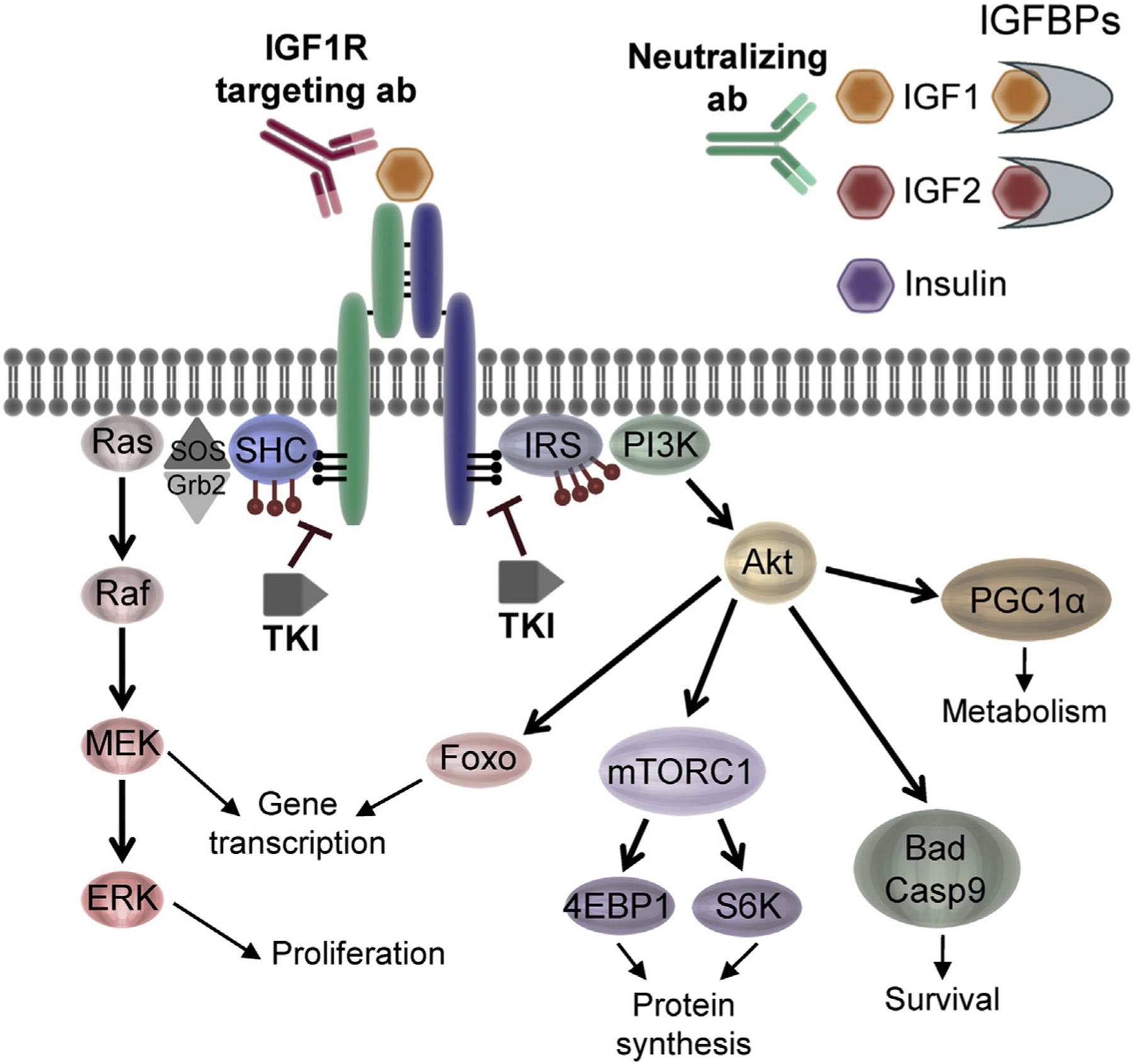
Downstream pathways activated by insulin and IGF receptors. Receptor activation by insulin and IGF ligands primarily activates two major pathways—Akt and Ras/ERK. Actions of these downstream mediators induce numerous responses important in normal tissue and cancer including proliferation, survival, metabolic changes, protein synthesis, and gene transcription. IGF system-targeting methods include ligand-neutralizing antibodies, IGF1R targeting antibodies, and tyrosine kinase inhibitors (TKIs) which all target important nodes to disrupt the system. Adapted from Sachdev, D. & Yee, D. (2007). Disrupting insulin-like growth factor signaling as a potential cancer therapy. Molecular Cancer Therapeutics, 6(1), 1–12.
3. The IGF family in cancer
3.1. Epidemiological evidence
Members of the IGF family of proteins are widely expressed in normal and cancerous tissues (Badzio et al., 2010; Cox et al., 2009; Kim et al., 2012; Law et al., 2008). Several epidemiological studies have established a link between the IGF family of proteins and an increased risk of cancer incidence or worse prognosis. In normal development, IGF1 produced in response to GH released during puberty is responsible for skeletal development and height. Analysis of data from the Million Women Study found that increased height was significantly associated with an increased risk of cancer incidence in 10 of the 17 cancers assessed (Green et al., 2011). Conversely, patients that have IGF1 deficiency have a significantly lower incidence of cancer (Guevara-Aguirre et al., 2011; Shevah & Laron, 2007). More directly, data from the Nurses’ Health Study cohort showed that, in premenopausal women, there was a very strong positive correlation between circulating IGF1 levels and breast cancer risk (Hankinson et al., 1998). This finding has been further confirmed in breast cancer, as well as extended to other cancer types (Shi, Yu, McLarty, & Glass, 2004). High plasma levels of IGF1 were also associated with an increased risk of lung cancer (OR = 2.06, 95% confidence interval = 1.19–3.56), and methylation of the IGFBP-3 promoter was associated with decreased survival (Chang et al., 2002; Yu et al., 1999). Even more strikingly, the Physicians’ Health Study found that men in the highest quartile of plasma IGF1 levels had a risk of developing prostate cancer that was 4.3 times higher than men in the lowest quartile, independent of PSA levels (Chan et al., 1998). Subsequent studies failed to replicate these results, as did studies looking at the correlation between IGFBP-3 levels and prostate cancer development (Cutting et al., 1999; Koistinen et al., 2002). In colorectal cancer, men and women with the highest levels of circulating IGF1 had a significantly greater risk of cancer development compared to the lowest levels and, similar to lung cancer, higher expression of IGFBP-3 was associated with a reduced relative risk (Giovannucci et al., 2000; Ma et al., 1999).
It is important to note that other studies have provided conflicting results to this relationship between IGFBP-3 and lung cancer risk, likely due to the complex relationship between IGFBPs and receptor activation (Hankinson et al., 1998; Harman et al., 2000; Shi et al., 2004). Another consideration when evaluating IGFBPs is the availability of the proteases that allow release of the IGF ligands from their binding proteins. Circulating PAPP-A levels, for example, are significantly higher in lung cancer patients compared to healthy subjects (Bulut et al., 2009). Further, another study showed that, in advanced lung cancer, pleural fluid had a 47-fold increased in PAPP-A compared to serum levels (Espelund et al., 2017). PAPP-A was also shown to be overexpressed in ovarian cancer, Ewing sarcoma, and several other cancers (Alexiadis, Mamers, Chu, & Fuller, 2006; Guo, Bao, Guo, & Yang, 2018; Kalli et al., 2004; Kirschner et al., 2017). In breast cancer, not only is PAPP-A overexpressed, particularly in the luminal B subtype, but is also an independent predictor of early recurrence for patients with stage 1 and 2 disease (Kuhajda, Abeloff, & Eggleston, 1985; Kuhajda & Eggleston, 1985; Mansfield et al., 2014). Similarly, elevated PSA levels strongly correlate with prostate cancer development, despite the lack of consistent association between prostate cancer and IGF1 or IGFBP-3 levels (Lojanapiwat, Anutrakulchai, Chongruksut, & Udomphot, 2014). Together these data indicate that, in some instances, circulating levels of IGFs or their binding proteins may be less important than the ability to release ligand in proximity to the tumor.
Additional studies looking at the effects of high serum insulin levels have uncovered similar results to elevated IGF1. A meta-analysis of several population studies that found that hyperinsulinemia and high C-peptide levels are correlated with increased risk of breast, colorectal, and pancreatic cancers (Giovannucci, 2001; Pollak, 2012). Hyperinsulinemia often occurs in the context of diabetes, obesity, and other metabolic syndrome, and these conditions all promote several additional factors that have been shown to support tumor growth including hyperglycemia, hyperlipidemia, chronic inflammation, and expression of several growth factors and cytokines (Belardi, Gallagher, Novosyadlyy, & LeRoith, 2013). While it is difficult to tease apart the contributions of each of these factors, evidence suggests that the IGF system independently supports cancer initiation and development (reviewed in Stone, McPherson, & Gail Darlington, 2018).
In addition to circulating ligands and binding proteins, increased receptor expression has also been found to correlate with prognosis. While the prognostic value of IGF1R in lung cancer has been controversial, a recent meta-analysis of 22 studies found that high expression of IGF1R in fact does predict shorter disease-free survival in non-small cell lung cancer (NSCLC) patients, however, is does not have predictive value for overall survival in NSCLC, or disease free survival or overall survival in small cell lung cancer (SCLC) (Xu et al., 2019). In prostate cancer samples, high staining for IGF1R, but not IR, was associated with increased proliferation, decreased apoptosis, and an increased risk of lethal disease (Ahearn et al., 2018; Zu et al., 2013). Higher levels of tumor IGF1R expression have also been positively correlated with worse disease outcome in gastric cancer and renal cell carcinoma (Matsubara et al., 2008; Sichani et al., 2010). In breast cancer, reports on IGF1R as a prognostic factor have been mixed. Multiple studies found that IGF1R expression correlates with good prognostic markers in patients with early breast cancer and with the luminal B subtype of breast cancer, however, its expression was associated with worse outcome in patients with the HER2-enriched subtype and shorter disease free survival in patients with triple negative disease (Fu et al., 2011; Hartog et al., 2011; Yerushalmi et al., 2012).
Based on the cumulative weight of these epidemiological studies, several methods for targeting the IGF system have been developed for use in preclinical and clinical settings. These include antibodies to neutralize IGF1 and IGF2, antibodies targeting IGF1R, and tyrosine kinase inhibitors (TKI) to prevent activation of downstream targets.
3.2. Targeting the IGF family
3.2.1. Ligand neutralization
Unlike insulin, many cell types express IGF ligands and, in fact, autocrine production by transformed cells is common. The pro-proliferative properties of IGF1 and IGF2 in mammalian epithelial cells were first identified in 1982, and not long after their effects in driving proliferation in other tissue and cancers types followed (Reddan & Dziedzic, 1982). Elevated IGF2 levels were shown to increase the number of colorectal lesions in ApcMin mice and nearly 70% of transgenic mice overexpressing IGF2 developed lung adenocarcinomas by 18 months of age (Hassan & Howell, 2000; Moorehead, Sanchez, Baldwin, & Khokha, 2003). Further, the structure of IGF1R makes it difficult to activate kinase signaling pathways without ligand binding to the receptor (Kaleko, Rutter, & Miller, 1990).
These and other preclinical observations led to the development of several antibodies to neutralize IGF ligands (Dransfield et al., 2010; Feng et al., 2006; Gao et al., 2011; Zhao, Feng, Zhu, & Dimitrov, 2011). One such antibody, MEDI-573 (dusigitumab), has higher affinity for IGF2 over IGF1, but has no affinity for insulin, and was shown to suppress growth of mouse embryonic fibroblast cell line xenografts engineered to overexpress either human IGF2 and IGF1R, or human IGF1 and IGF1R (Gao et al., 2011). Further, MEDI-573 was shown to inhibit sarcoma cell line growth in vitro and in xenograft models (Zhong et al., 2014). With these promising data, MEDI-573 was taken into clinical trials for breast cancer and hepatocellular carcinoma (HCC). The breast cancer study looked at the efficacy of MEDI-573 plus aromatase inhibitor (AI) versus AI alone in women with metastatic hormone-sensitive breast cancer. Unfortunately no clinical benefit was seen in this patient population (NCT01446159). Phase II of the HCC study was not launched at the discretion of the sponsor (NCT01498952). A second IGF neutralizing antibody, xentuzumab (BI 836845), has higher affinity for IGF1 over IGF2 and also showed great promise in preclinical models (Friedbichler et al., 2014). Xentuzumab is also being tested in a phase I study in patients with NSCLC in combination with the EGFR inhibitor afatinib (completed not published, NCT02191891) and in a phase Ib/II study in patients with metastatic or locally advanced ER+ breast cancer in combination with a abemaciclib, a CDK4/6 inhibitor, and endocrine therapy (NCT03099174). An additional study combining xentuzumab with an AI (exemestane) and the mTOR inhibitor everolimus in metastatic breast cancer to bone is ongoing (NCT03659136).
3.2.2. IGF1R targeted monoclonal antibodies
Much preclinical data has supported the targeting of IGF1R. One striking example comes from a study where mice engineered to express constitutively active IGF1R developed salivary and mammary adenocarcinomas as early as 8 weeks of age (Carboni et al., 2005). The success of monoclonal antibodies targeting other receptors, such as trastuzumab for HER2 expressing cancers, led to their use becoming one of the dominant strategies for targeting the IGF system in clinical trials. These IGF1R-directed monoclonal antibodies most often elicit their effects by binding the extracellular region of the receptor and causing its internalization and degradation (Cohen et al., 2005; Sachdev, Singh, Fujita-Yamaguchi, & Yee, 2006). While these antibodies do not cross-react with IR, they can bind and inhibit the activity of hybrid receptors (Pandini et al., 2007).
As early as 1989, antibodies targeting IGF1R in breast cancer showed promise in preclinical studies (Arteaga et al., 1989). The monoclonal antibody AVE1642 inhibited breast cancer xenograft growth in preclinical studies, and even exhibited anti-tumor activity in solid tumors in phase I trials (Sachdev, Zhang, Matise, Gaillard-Kelly, & Yee, 2010; Zeng, Sachdev, Zhang, Gaillard-Kelly, & Yee, 2009). AVE1642 treatment led to partial response or stable disease in 25 of 57 patients in one study and 11 of 27 patients in another (Macaulay et al., 2013; Soria et al., 2013). Unfortunately AVE1642 and additional IGF1R antibodies teprotumumab (R1507) and robatumumab all failed in phase II trials in solids tumors (Lin et al., 2014; Pappo et al., 2014; Ramalingam et al., 2011). Phase I clinical trial testing of dalotuzumab as a single agent in solid tumors showed that some patients saw clinical benefit, while in a separate trial in breast cancer, the combination of dalotuzumab plus the mTOR inhibitor ridaforolimus showed partial response or stable disease in 6 of 11 patients with ER+ highly proliferative disease (Atzori et al., 2011; Di Cosimo et al., 2015). Similar to other phase II trials with this type of agent, however, a phase II trial testing blockade of the same pathway components using cixutumumab and the mTOR inhibitor temsirolimus found no efficacy with this combination in patients who had received multiple prior lines of chemotherapy (Ma et al., 2013).
Use of monoclonal antibodies in NSCLC have resulted in equally disappointing results. One phase II study using cisplatin and pemetrexed with or without cixutumumab as first-line therapy in stage IV nonsquamous NSCLC found that patients derived no clinical benefit from addition of cixutumumab but did experience increased incidence of hyperglycemia (Novello et al., 2017). Further, despite promising phase II trial data, a phase III trial testing the IGF1R-directed monoclonal antibody figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone was closed early due to lack of clinical benefit and increased rates hyperglycemia and serious adverse events (Goto et al., 2012; Langer et al., 2014). It is important to note, however, that a small subset of patients did respond to treatment and response was associated with higher baseline levels of circulating IGF1 (Goto et al., 2012).
Clinical trials targeting IGF1R in other cancers have shown some promise. In metastatic pancreatic cancer patients, use of the IGF1R-directed antibody ganitumab plus gemcitabine showed no effect on overall survival, however, data from this trial suggest that a subset of patients may derive benefit; further a later trial showed that there may in fact be improved overall survival with this treatment combination (Fuchs et al., 2015; Kindler et al., 2012). In a phase I study in patients with sarcoma or Ewing’s sarcoma, almost half of patients had a reduction in tumor volume over baseline, with one patient showing a complete response, one showing a partial response, and eight patients having prolonged stable disease (Olmos et al., 2010). In a separate phase I study in Ewing’s sarcoma, cixutumumab combined with an mTOR inhibitor led to tumor regression in 29% (5/17) patients (Naing et al., 2012). Further, a phase II study in refractory Ewing sarcoma showed 10% of patients had a complete or partial response to another IGF1R targeting antibody, teprotumumab (Pappo et al., 2011). A phase III trial combining ganitumab with chemotherapy in metastatic Ewing sarcoma has completed enrollment (NCT02306161).
3.2.3. Tyrosine kinase inhibitors
In contrast to the IGF1R-directed antibodies, the small molecule tyrosine kinase inhibitors (TKI) block downstream IGF1R signaling by competing for the ATP-binding site in the catalytic domain of the receptor, and most often elicit their effect without causing receptor internalization (Ji et al., 2007). Due to the close homology between IGF1R and IR, currently available TKIs not only bind and inhibit IGF1R, but IR as well. Unfortunately, because of this cross-reactivity, some in vivo studies have shown this method of targeting the IGF system leads to insulin resistance and hyperglycemia (King, Aleksic, Haluska, & Macaulay, 2014; Pollak, 2008). Additionally, off target effects on other kinases have been seen with use of some of these TKIs, which may influence their therapeutic potential (Carboni et al., 2009).
Despite this, the efficacy of TKIs has been shown in preclinical studies in multiple cancer types. In breast, endometrial, and neuroblastoma, NVP-AEW541 showed antiproliferative effects in vitro and in vivo (Attias-Geva, Bentov, Fishman, Werner, & Bruchim, 2011; Fagan, Uselman, Sachdev, & Yee, 2012; Garcia-Echeverria et al., 2004; Tanno et al., 2006). Studies in pancreatic cancer cell lines showed that BMS-754807 enhanced gemcitabine activity and reduced xenograft tumor growth in a dose dependent manner by reducing activation of IGF1R and downstream mediators, and inducing apoptosis (Awasthi, Zhang, Ruan, Schwarz, & Schwarz, 2012). Similar effects on signaling and cell proliferation in vitro, plus efficacy as a single agent and in combination with other treatments on xenograft growth were found in several cancer types (Carboni et al., 2009; Franks, Jones, Briah, Murray, & Moorehead, 2016).
Clinical trials using these agents, however, have had less successful results. A study looking at the efficacy of letrozole in combination with BMS-754807 was discontinued early by the sponsor without reaching the primary endpoint of progression free survival at 24 weeks (NCT01225172). In a phase I dose escalation study using linsitinib (OSI-906) in combination with EGFR inhibitor erlotinib in patients with advanced solid tumors, disease control occurred in 51% (38/75) patients (Macaulay et al., 2016). Unfortunately in a phase III trial in patients with adrenocortical carcinoma, linsitinib showed no benefit over placebo (Fassnacht et al., 2015). It is important to note, however, that 3% of patients had a partial response indicating that there may be a subpopulation of patients that may derive benefit. Unfortunately in advanced NSCLC, patients treated with AXL1717 showed no difference in progression free survival compared to the docetaxel treated group in a phase II trial (Bergqvist et al., 2017).
4. Lessons learned and where we go from here
Epidemiological and preclinical data provide a strong rationale for targeting the IGF system as it has cumulatively shown that every aspect of the IGF system can be altered in cancer. Despite this potential, clinical trial data has been less compelling. Negative phase II and phase III trial data have led some to believe that there is sufficient evidence to halt the investigation of targeting the IGF system in most cancers. However, several studies did show that certain populations of patients derived clinical benefit from treatment, and IGF system-targeting therapies were well tolerated. Before completely discounting the targeting of this system, it may be worth asking ourselves who benefitted, who did not, and why.
4.1. Biomarkers
Due to the desire for rapid clinical development of new therapeutic strategies, most of the discussed clinical trials were initiated in unselected patient populations as no predictive biomarkers had been identified. In the most simplistic approach, one might hypothesize that IGF1R expression may be sufficient to predict response to these therapies. Unfortunately, while low levels of IGF1R were shown in preclinical studies to be a strong negative predictor of response, IGF1R expression itself is necessary but not sufficient to accurately predict sensitivity (Lee & Yee, 2011; Zha et al., 2009). In some trials, patients were given IGF1R-targeting treatment regardless of the levels of receptor expression in their tumors, indicating that some of the negative results could be due to a lack of target expression.
Data from initial negative trials have been used to identify candidate predictive biomarkers. For example, circulating ligand levels may prove to be useful in stratifying patients who may benefit. In patients with metastatic pancreatic cancer, high baseline levels of IGF1 or IGF2 identified patients who were likely respond to IGF1R targeting therapies, as were low levels of IGFBP-2 or high IGFBP-3 (McCaffery et al., 2013). Pretreatment circulating levels of IGF1 have also shown some promise as a potential marker in NSCLC, however, some of these studies have been retracted due to a lack of reproducibility (Goto et al., 2012; Gualberto et al., 2011). In vitro, Huang et al. examined gene signatures in sarcoma and neuroblastoma cell lines sensitive and resistant to the TKI BMS-536924 (Huang et al., 2009). Interestingly, high IGF1 and IGF2 expression levels were associated with sensitivity, as was IGF1R expression, and IGFBP-3 and IGFBP-6 were both highly expressed in the resistant lines. Similar results were found in colorectal cancer explants, whereby increased levels of IGF2 mRNA associated with better response to the IGF1R-targeting antibody h10H5 (Zha et al., 2009). It has been proposed that high levels of circulating ligands might have predictive value, as tumors that arise in these patients may be pushed to become dependent on these signals and therefore may be more likely to respond to their blockade. Measuring expression profiles to identify IGF driven breast cancers has also been shown in preclinical models (Litzenburger et al., 2011).
Other unexpected candidate predictive biomarkers under investigation are transforming fusion proteins. Preclinical studies in breast cancer show that activation of the IGF system is a requirement for transformation in cells harboring an ETV6-NTRK3 fusion protein, a translocation that is found in virtually all secretory breast cancers (Tognon et al., 2011). Further, in Ewing’s sarcoma a common translocation, EWS-FLI1, leads to increased IGF1 expression and requires expression of IGF1R in order to be transforming (Toretsky, Kalebic, Blakesley, LeRoith, & Helman, 1997).
Additional preclinical studies using patient-derived xenografts looking at gene expression and mutational profiles found that KRAS mutational status may also be predictive of response, and highlighted the concept that rational combination therapies could be another useful approach to increase the efficacy of IGF system targeting agents (Pitts et al., 2010).
4.2. Combination therapies
Results from clinical trials suggest that targeting the IGF system alone is likely not sufficient for sustained tumor inhibition. Several targeted therapies have been used successfully in combination with other treatments, and in fact dual inhibition of mTOR and IGF1R in Ewing’s sarcoma patients has shown great promise (Naing et al., 2012). There have been several other studies using IGF family inhibitors in combination with other agents, however, many of these combinations have not been selected by examining preclinical evidence. However, preclinical data exists that may help in identifying clinically useful combinations.
In vitro studies have pointed to overexpression of EGFR and its ligands as a possible mechanism of resistance to IGF family-targeted therapies, indicating that, for certain cancers, dual targeting may be beneficial (Huang et al., 2009). Additional data suggest that, in melanoma, addition of BRAF inhibitors may work cooperatively with IGF1R inhibition to induce apoptosis (Villanueva et al., 2010; Yeh, Bohula, & Macaulay, 2006). Beyond Ewing sarcoma, other cancers may also benefit from dual inhibition of mTOR and the IGF system (Friedbichler et al., 2014).
Another important factor to consider when discussing combination therapies is the timing and order of treatment administration. In breast cancer trials, for example, almost all women had previously been treated with endocrine therapy before receiving IGF1R-targeting antibodies. Preclinical data has shown that endocrine therapy resistance is associated with loss of IGF1R expression; this trend was also seen in patients, indicating that from the start these trials were set to fail (Drury et al., 2011; Fagan et al., 2012).
4.3. IR compensation
Some evidence suggests that the failure of IGF1R targeting therapies has been, at least in part, due to continued activation of downstream pathways through IR. Preclinical studies have shown that targeting of IGF1R can in fact lead to an increase in IR expression and may confer inherent resistance to IGF1R-targeting therapies (Ulanet, Ludwig, Kahn, & Hanahan, 2010). Early trials using the IGF1R targeting antibody figitumumab showed that patients taking the drug had increased circulating insulin, indicating this may be a potential mechanism for resistance (Haluska et al., 2007). This observation also led to the concern that tumors in patients with hyperinsulinemia may have an intrinsic resistance to IGF1R targeted therapies due to activation of IR. Similar trends have been seen in vitro and in vivo, as hyperinsulinemic mice have increased xenograft growth compared to their normal counterparts; however blocking this increased insulin signaling using BMS-536924 reduced tumor burden (Novosyadlyy et al., 2010).
While targeting of IR was previously avoided due to the potential for detrimental metabolic side effects, there has recently been renewed interest. Optimism has been driven by data showing that small molecule inhibitors of IGF1R/IR were tolerated far better than expected, possibly due to their differential distribution to insulin target tissues (Dool et al., 2011; Fassnacht et al., 2015). This suggests that targeting IR may be possible without inducing severe hypoglycemia. Further, upregulation of the IR-A isoform in several cancer types may provide a better, more specific cancer target. This method is also appealing as IGF2 has equal affinity for IR-A as insulin. However, isoform specific modulators have yet to be developed.
5. Conclusions
It is clear that single agent targeting of the IGF system does not work. This does not rule out the possible use of these agents for all indications. Thoughtful investigation into combination therapies and biomarker identification are needed in order to illuminate where these therapies will be useful in the clinic.
Acknowledgment
This work was supported by grants from the NIH T32 CA009138 (L.M.F.), 5P30 CA077598 (D.Y.) and P50 CA116201 (D.Y.).
References
- Ahearn TU, Peisch S, Pettersson A, Ebot EM, Zhou CK, Graff RE, et al. (2018). Expression of IGF/insulin receptor in prostate cancer tissue and progression to lethal disease. Carcinogenesis, 39(12), 1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexiadis M, Mamers P, Chu S, & Fuller PJ (2006). Insulin-like growth factor, insulin-like growth factor-binding protein-4, and pregnancy-associated plasma protein-A gene expression in human granulosa cell tumors. International Journal of Gynecological Cancer, 16(6), 1973–1979. [DOI] [PubMed] [Google Scholar]
- Andress DL (1995). Heparin modulates the binding of insulin-like growth factor (IGF) binding protein-5 to a membrane protein in osteoblastic cells. The Journal of Biological Chemistry, 270(47), 28289–28296. [PubMed] [Google Scholar]
- Arteaga CL, Kitten LJ, Coronado EB, Jacobs S, Kull FC Jr., Allred DC, et al. (1989). Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. The Journal of Clinical Investigation, 84(5), 1418–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attias-Geva Z, Bentov I, Fishman A, Werner H, & Bruchim I (2011). Insulin-like growth factor-I receptor inhibition by specific tyrosine kinase inhibitor NVP-AEW541 in endometrioid and serous papillary endometrial cancer cell lines. Gynecologic Oncology, 121(2), 383–389. [DOI] [PubMed] [Google Scholar]
- Atzori F, Tabernero J, Cervantes A, Prudkin L, Andreu J, Rodriguez-Braun E, et al. (2011). A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clinical Cancer Research, 17(19), 6304–6312. [DOI] [PubMed] [Google Scholar]
- Awasthi N, Zhang C, Ruan W, Schwarz MA, & Schwarz RE (2012). BMS-754807, a small-molecule inhibitor of insulin-like growth factor-1 receptor/insulin receptor, enhances gemcitabine response in pancreatic cancer. Molecular Cancer Therapeutics, 11(12), 2644–2653. [DOI] [PubMed] [Google Scholar]
- Bach LA, Hsieh S, Sakano K, Fujiwara H, Perdue JF, & Rechler MM (1993). Binding of mutants of human insulin-like growth factor II to insulin-like growth factor binding proteins 1–6. The Journal of Biological Chemistry, 268(13), 9246–9254. [PubMed] [Google Scholar]
- Badzio A, Wynes MW, Dziadziuszko R, Merrick DT, Pardo M, Rzyman W, et al. (2010). Increased insulin-like growth factor 1 receptor protein expression and gene copy number in small cell lung cancer. Journal of Thoracic Oncology, 5(12), 1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailyes EM, Nave BT, Soos MA, Orr SR, Hayward AC, & Siddle K (1997). Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: Quantification of individual receptor species by selective immunoprecipitation and immunoblotting. The Biochemical Journal, 327(Pt. 1), 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC (2000). Insulin-like growth factor (IGF)-binding proteins: Interactions with IGFs and intrinsic bioactivities. American Journal of Physiology Endocrinology and Metabolism, 278(6), E967–E976. [DOI] [PubMed] [Google Scholar]
- Belardi V, Gallagher EJ, Novosyadlyy R, & LeRoith D (2013). Insulin and IGFs in obesity-related breast cancer. Journal of Mammary Gland Biology and Neoplasia, 18(3–4), 277–289. [DOI] [PubMed] [Google Scholar]
- Bergqvist M, Holgersson G, Bondarenko I, Grechanaya E, Maximovich A, Andor G, et al. (2017). Phase II randomized study of the IGF-1R pathway modulator AXL1717 compared to docetaxel in patients with previously treated, locally advanced or metastatic non-small cell lung cancer. Acta Oncologica, 56(3), 441–447. [DOI] [PubMed] [Google Scholar]
- Blum WF, Horn N, Kratzsch J, Jorgensen JO, Juul A, Teale D, et al. (1993). Clinical studies of IGFBP-2 by radioimmunoassay. Growth Regulation, 3(1), 100–104. [PubMed] [Google Scholar]
- Blundell TL, Bedarkar S, Rinderknecht E, & Humbel RE (1978). Insulin-like growth factor: A model for tertiary structure accounting for immunoreactivity and receptor binding. Proceedings of the National Academy of Sciences of the United States of America, 75(1), 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar K, Fernqvist-Forbes E, Wahren J, & Hall K (1994). Effect of insulin on the hepatic production of insulin-like growth factor-binding protein-1 (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes. The Journal of Clinical Endocrinology and Metabolism, 79(3), 872–878. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Winnay J, Cheatham B, & Kahn CR (1997). Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Molecular and Cellular Biology, 17(3), 1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut I, Coskun A, Ciftci A, Cetinkaya E, Altiay G, Caglar T, et al. (2009). Relationship between pregnancy-associated plasma protein-A and lung cancer. The American Journal of the Medical Sciences, 337(4), 241–244. [DOI] [PubMed] [Google Scholar]
- Bunn RC, & Fowlkes JL (2003). Insulin-like growth factor binding protein proteolysis. Trends in Endocrinology and Metabolism, 14(4), 176–181. [DOI] [PubMed] [Google Scholar]
- Carboni JM, Lee AV, Hadsell DL, Rowley BR, Lee FY, Bol DK, et al. (2005). Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Research, 65(9), 3781–3787. [DOI] [PubMed] [Google Scholar]
- Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, et al. (2009). BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Molecular Cancer Therapeutics, 8(12), 3341–3349. [DOI] [PubMed] [Google Scholar]
- Chahal J, Chen CC, Rane MJ, Moore JP, Barati MT, Song Y, et al. (2008). Regulation of insulin-response element binding protein-1 in obesity and diabetes: Potential role in impaired insulin-induced gene transcription. Endocrinology, 149(10), 4829–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. (1998). Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science, 279(5350), 563–566. [DOI] [PubMed] [Google Scholar]
- Chang YS, Wang L, Liu D, Mao L, Hong WK, Khuri FR, et al. (2002). Correlation between insulin-like growth factor-binding protein-3 promoter methylation and prognosis of patients with stage I non-small cell lung cancer. Clinical Cancer Research, 8(12), 3669–3675. [PubMed] [Google Scholar]
- Clemmons DR (2004). Role of insulin-like growth factor in maintaining normal glucose homeostasis. Hormone Research, 62(Suppl. 1), 77–82. [DOI] [PubMed] [Google Scholar]
- Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, et al. (2005). Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clinical Cancer Research, 11(5), 2063–2073. [DOI] [PubMed] [Google Scholar]
- Condorelli G, Bueno R, & Smith RJ (1994). Two alternatively spliced forms of the human insulin-like growth factor I receptor have distinct biological activities and internalization kinetics. The Journal of Biological Chemistry, 269(11), 8510–8516. [PubMed] [Google Scholar]
- Coverley JA, Martin JL, & Baxter RC (2000). The effect of phosphorylation by casein kinase 2 on the activity of insulin-like growth factor-binding protein-3. Endocrinology, 141(2), 564–570. [DOI] [PubMed] [Google Scholar]
- Cox ME, Gleave ME, Zakikhani M, Bell RH, Piura E, Vickers E, et al. (2009). Insulin receptor expression by human prostate cancers. Prostate, 69(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Cutting CW, Hunt C, Nisbet JA, Bland JM, Dalgleish AG, & Kirby RS (1999). Serum insulin-like growth factor-1 is not a useful marker of prostate cancer. BJU International, 83(9), 996–999. [DOI] [PubMed] [Google Scholar]
- De Meyts P, Wallach B, Christoffersen CT, Urso B, Gronskov K, Latus LJ, et al. (1994). The insulin-like growth factor-I receptor. Structure, ligand-binding mechanism and signal transduction. Hormone Research, 42(4–5), 152–169. [DOI] [PubMed] [Google Scholar]
- De Meyts P, & Whittaker J (2002). Structural biology of insulin and IGF1 receptors: Implications for drug design. Nature Reviews Drug Discovery, 1(10), 769–783. [DOI] [PubMed] [Google Scholar]
- Di Cosimo S, Sathyanarayanan S, Bendell JC, Cervantes A, Stein MN, Brana I, et al. (2015). Combination of the mTOR inhibitor ridaforolimus and the anti-IGF1R monoclonal antibody dalotuzumab: Preclinical characterization and phase I clinical trial. Clinical Cancer Research, 21(1), 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dool CJ, Mashhedi H, Zakikhani M, David S, Zhao Y, Birman E, et al. (2011). IGF1/insulin receptor kinase inhibition by BMS-536924 is better tolerated than alloxan-induced hypoinsulinemia and more effective than metformin in the treatment of experimental insulin-responsive breast cancer. Endocrine-Related Cancer, 18(6), 699–709. [DOI] [PubMed] [Google Scholar]
- Dransfield DT, Cohen EH, Chang Q, Sparrow LG, Bentley JD, Dolezal O, et al. (2010). A human monoclonal antibody against insulin-like growth factor-II blocks the growth of human hepatocellular carcinoma cell lines in vitro and in vivo. Molecular Cancer Therapeutics, 9(6), 1809–1819. [DOI] [PubMed] [Google Scholar]
- Drury SC, Detre S, Leary A, Salter J, Reis-Filho J, Barbashina V, et al. (2011). Changes in breast cancer biomarkers in the IGF1R/PI3K pathway in recurrent breast cancer after tamoxifen treatment. Endocrine-Related Cancer, 18(5), 565–577. [DOI] [PubMed] [Google Scholar]
- Entingh-Pearsall A, & Kahn CR (2004). Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I. The Journal of Biological Chemistry, 279(36), 38016–38024. [DOI] [PubMed] [Google Scholar]
- Espelund US, Bjerre M, Hjortebjerg R, Rasmussen TR, Lundby A, Hoeflich A, et al. (2017). Insulin-like growth factor bioactivity, stanniocalcin-2, pregnancy-associated plasma protein-A, and IGF-binding protein-4 in pleural fluid and serum from patients with pulmonary disease. The Journal of Clinical Endocrinology and Metabolism, 102(9), 3526–3534. [DOI] [PubMed] [Google Scholar]
- Fagan DH, Uselman RR, Sachdev D, & Yee D (2012). Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: Implications for breast cancer treatment. Cancer Research, 72(13), 3372–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, et al. (2015). Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. The Lancet Oncology, 16(4), 426–435. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhu Z, Xiao X, Choudhry V, Barrett JC, & Dimitrov DS (2006). Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Molecular Cancer Therapeutics, 5(1), 114–120. [DOI] [PubMed] [Google Scholar]
- Fowlkes JL, Thrailkill KM, George-Nascimento C, Rosenberg CK, & Serra DM (1997). Heparin-binding, highly basic regions within the thyroglobulin type-1 repeat of insulin-like growth factor (IGF)-binding proteins (IGFBPs) −3, −5, and −6 inhibit IGFBP-4 degradation. Endocrinology, 138(6), 2280–2285. [DOI] [PubMed] [Google Scholar]
- Franks SE, Jones RA, Briah R, Murray P, & Moorehead RA (2016). BMS-754807 is cytotoxic to non-small cell lung cancer cells and enhances the effects of platinum chemotherapeutics in the human lung cancer cell line A549. BMC Research Notes, 9, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, et al. (1999). Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Molecular and Cellular Biology, 19(5), 3278–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedbichler K, Hofmann MH, Kroez M, Ostermann E, Lamche HR, Koessl C, et al. (2014). Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Molecular Cancer Therapeutics, 13(2), 399–409. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Skjaerbaek C, Dinesen B, & Orskov H (1994). Free insulin-like growth factors (IGF-I and IGF-II) in human serum. FEBS Letters, 348(2), 185–191. [DOI] [PubMed] [Google Scholar]
- Fu P, Ibusuki M, Yamamoto Y, Hayashi M, Murakami K, Zheng S, et al. (2011). Insulin-like growth factor-1 receptor gene expression is associated with survival in breast cancer: A comprehensive analysis of gene copy number, mRNA and protein expression. Breast Cancer Research and Treatment, 130(1), 307–317. [DOI] [PubMed] [Google Scholar]
- Fuchs CS, Azevedo S, Okusaka T, Van Laethem JL, Lipton LR, Riess H, et al. (2015). A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: The GAMMA trial. Annals of Oncology, 26(5), 921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Chesebrough JW, Cartlidge SA, Ricketts SA, Incognito L, Veldman-Jones M, et al. (2011). Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Research, 71(3), 1029–1040. [DOI] [PubMed] [Google Scholar]
- Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, et al. (2004). In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell, 5(3), 231–239. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Dahms NM, & Kornfeld S (2003). Mannose 6-phosphate receptors: New twists in the tale. Nature Reviews Molecular Cell Biology, 4(3), 202–212. [DOI] [PubMed] [Google Scholar]
- Gibson JM, Aplin JD, White A, & Westwood M (2001). Regulation of IGF bioavailability in pregnancy. Molecular Human Reproduction, 7(1), 79–87. [DOI] [PubMed] [Google Scholar]
- Giddings SJ, Chirgwin J, & Permutt MA (1982). Effects of glucose on proinsulin messenger RNA in rats in vivo. Diabetes, 31(7), 624–629. [DOI] [PubMed] [Google Scholar]
- Giovannucci E (2001). Insulin, insulin-like growth factors and colon cancer: A review of the evidence. The Journal of Nutrition, 131(11 Suppl), 3109S–3120S. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Pollak MN, Platz EA, Willett WC, Stampfer MJ, Majeed N, et al. (2000). A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiology, Biomarkers & Prevention, 9(4), 345–349. [PubMed] [Google Scholar]
- Goto Y, Sekine I, Tanioka M, Shibata T, Tanai C, Asahina H, et al. (2012). Figitumumab combined with carboplatin and paclitaxel in treatment-naive Japanese patients with advanced non-small cell lung cancer. Investigational New Drugs, 30(4), 1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral V, et al. (2011). Height and cancer incidence in the Million Women Study: Prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. The Lancet Oncology, 12(8), 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto A, Hixon ML, Karp DD, Li D, Green S, Dolled-Filhart M, et al. (2011). Pre-treatment levels of circulating free IGF-1 identify NSCLC patients who derive clinical benefit from figitumumab. British Journal of Cancer, 104(1), 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, et al. (2011). Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Science Translational Medicine, 3(70), 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler HP, Zapf J, Schmid C, & Froesch ER (1989). Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinologica, 121(6), 753–758. [DOI] [PubMed] [Google Scholar]
- Guo Y, Bao Y, Guo D, & Yang W (2018). Pregnancy-associated plasma protein a in cancer: Expression, oncogenic functions and regulation. American Journal of Cancer Research, 8(6), 955–963. [PMC free article] [PubMed] [Google Scholar]
- Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, et al. (2007). Phase I dose escalation study8 of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clinical Cancer Research, 13(19), 5834–5840. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. (1998). Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet, 351(9113), 1393–1396. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB, & Baltimore Longitudinal Study on Aging (2000). Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. The Journal of Clinical Endocrinology and Metabolism, 85(11), 4258–4265. [DOI] [PubMed] [Google Scholar]
- Harrela M, Koistinen H, Kaprio J, Lehtovirta M, Tuomilehto J, Eriksson J, et al. (1996). Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. The Journal of Clinical Investigation, 98(11), 2612–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartog H, Horlings HM, van der Vegt B, Kreike B, Ajouaou A, van de Vijver MJ, et al. (2011). Divergent effects of insulin-like growth factor-1 receptor expression on prognosis of estrogen receptor positive versus triple negative invasive ductal breast carcinoma. Breast Cancer Research and Treatment, 129(3), 725–736. [DOI] [PubMed] [Google Scholar]
- Hassan AB, & Howell JA (2000). Insulin-like growth factor II supply modifies growth of intestinal adenoma in Apc(Min/+) mice. Cancer Research, 60(4), 1070–1076. [PubMed] [Google Scholar]
- Huang F, Greer A, Hurlburt W, Han X, Hafezi R, Wittenberg GM, et al. (2009). The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Research, 69(1), 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JG, White MF, & Yee D (1998). Insulin receptor substrate-1 is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. The Journal of Biological Chemistry, 273(16), 9994–10003. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Zhang X, Yoneda T, & Yee D (2001). Regulation of breast cancer cell motility by insulin receptor substrate-2 (IRS-2) in metastatic variants of human breast cancer cell lines. Oncogene, 20(50), 7318–7325. [DOI] [PubMed] [Google Scholar]
- Ji QS, Mulvihill MJ, Rosenfeld-Franklin M, Cooke A, Feng L, Mak G, et al. (2007). A novel, potent, and selective insulin-like growth factor-I receptor kinase inhibitor blocks insulin-like growth factor-I receptor signaling in vitro and inhibits insulin-like growth factor-I receptor dependent tumor growth in vivo. Molecular Cancer Therapeutics, 6(8), 2158–2167. [DOI] [PubMed] [Google Scholar]
- Jones JI, Doerr ME, & Clemmons DR (1995). Cell migration: Interactions among integrins, IGFs and IGFBPs. Progress in Growth Factor Research, 6(2–4), 319–327. [DOI] [PubMed] [Google Scholar]
- Jones JI, Gockerman A, Busby WH Jr., Wright G, & Clemmons DR (1993). Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proceedings of the National Academy of Sciences of the United States of America, 90(22), 10553–10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleko M, Rutter WJ, & Miller AD (1990). Overexpression of the human insulin like growth factor I receptor promotes ligand-dependent neoplastic transformation. Molecular and Cellular Biology, 10(2), 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli KR, Chen BK, Bale LK, Gernand E, Overgaard MT, Oxvig C, et al. (2004). Pregnancy-associated plasma protein-A (PAPP-A) expression and insulin-like growth factor binding protein-4 protease activity in normal and malignant ovarian surface epithelial cells. International Journal of Cancer, 110(5), 633–640. [DOI] [PubMed] [Google Scholar]
- Kavran JM, McCabe JM, Byrne PO, Connacher MK, Wang Z, Ramek A, et al. (2014). How IGF-1 activates its receptor. eLife, 3, e03772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Kim ES, Liu D, Lee JJ, Solis L, Behrens C, et al. (2012). Prognostic impact of insulin receptor expression on survival of patients with nonsmall cell lung cancer. Cancer, 118(9), 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ Jr., Rocha-Lima CM, et al. (2012). A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Annals of Oncology, 23(11), 2834–2842. [DOI] [PubMed] [Google Scholar]
- King H, Aleksic T, Haluska P, & Macaulay VM (2014). Can we unlock the potential of IGF-1R inhibition in cancer therapy? Cancer Treatment Reviews, 40(9), 1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner A, Thiede M, Grunewald TG, Alba Rubio R, Richter GH, Kirchner T, et al. (2017). Pappalysin-1 T cell receptor transgenic allo-restricted T cells kill Ewing sarcoma in vitro and in vivo. Oncoimmunology, 6(2), e1273301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson VP (1991). Cellular trafficking and processing of the insulin receptor. The FASEB Journal, 5(8), 2130–2138. [DOI] [PubMed] [Google Scholar]
- Ko JM, Park HK, Yang S, Kim EY, Chung SC, & Hwang IT (2012). Association between insulin-like growth factor binding protein-2 levels and cardiovascular risk factors in Korean children. Endocrine Journal, 59(4), 335–343. [DOI] [PubMed] [Google Scholar]
- Koistinen H, Paju A, Koistinen R, Finne P, Lovgren J, Wu P, et al. (2002). Prostate-specific antigen and other prostate-derived proteases cleave IGFBP-3, but prostate cancer is not associated with proteolytically cleaved circulating IGFBP-3. Prostate, 50(2), 112–118. [DOI] [PubMed] [Google Scholar]
- Kornfeld S (1992). Structure and function of the mannose 6-phosphate/insulin like growth factor II receptors. Annual Review of Biochemistry, 61, 307–330. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP, Abeloff MD, & Eggleston JC (1985). Pregnancy-associated plasma protein A: A clinically significant predictor of early recurrence in stage II breast carcinoma. Human Pathology, 16(3), 228–235. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP, & Eggleston JC (1985). Pregnancy-associated plasma protein A and extensive necrosis. Clinically significant predictors of early recurrence in stage I estrogen receptor-negative breast carcinoma. Laboratory Investigation, 53(1), 101–107. [PubMed] [Google Scholar]
- Kulkarni SD, Muralidharan B, Panda AC, Bakthavachalu B, Vindu A, & Seshadri V (2011). Glucose-stimulated translation regulation of insulin by the 5’ UTR-binding proteins. The Journal of Biological Chemistry, 286(16), 14146–14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer CJ, Novello S, Park K, Krzakowski M, Karp DD, Mok T, et al. (2014). Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. Journal of Clinical Oncology, 32(19), 2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, et al. (2008). Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Research, 68(24), 10238–10246. [DOI] [PubMed] [Google Scholar]
- Layden BT, Durai V, & Lowe WL Jr. (2010). G-protein-coupled receptors, pancreatic islets, and diabetes. Nature Education, 3(9), 13. [Google Scholar]
- Lee AV, & Yee D (2011). Targeting IGF-1R: At a crossroad. Oncology (Williston Park), 25(6), 535–536, Discussion 551. [PMC free article] [PubMed] [Google Scholar]
- Leong SR, Baxter RC, Camerato T, Dai J, & Wood WI (1992). Structure and functional expression of the acid-labile subunit of the insulin-like growth factor-binding protein complex. Molecular Endocrinology, 6(6), 870–876. [DOI] [PubMed] [Google Scholar]
- Lewitt MS, Saunders H, Phuyal JL, & Baxter RC (1994). Complex formation by human insulin-like growth factor-binding protein-3 and human acid-labile subunit in growth hormone-deficient rats. Endocrinology, 134(6), 2404–2409. [DOI] [PubMed] [Google Scholar]
- Lin EH, Lenz HJ, Saleh MN, Mackenzie MJ, Knost JA, Pathiraja K, et al. (2014). A randomized, phase II study of the anti-insulin-like growth factor receptor type 1 (IGF-1R) monoclonal antibody robatumumab (SCH 717454) in patients with advanced colorectal cancer. Cancer Medicine, 3(4), 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzenburger BC, Creighton CJ, Tsimelzon A, Chan BT, Hilsenbeck SG, Wang T, et al. (2011). High IGF-IR activity in triple-negative breast cancer cell lines and tumor grafts correlates with sensitivity to anti-IGF-IR therapy. Clinical Cancer Research, 17(8), 2314–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone C, & Borai A (2014). Insulin-like growth factor-II: Its role in metabolic and endocrine disease. Clinical Endocrinology, 80(6), 773–781. [DOI] [PubMed] [Google Scholar]
- Lobel P, Dahms NM, & Kornfeld S (1988). Cloning and sequence analysis of the cation-independent mannose 6-phosphate receptor. The Journal of Biological Chemistry, 263(5), 2563–2570. [PubMed] [Google Scholar]
- Lojanapiwat B, Anutrakulchai W, Chongruksut W, & Udomphot C (2014). Correlation and diagnostic performance of the prostate-specific antigen level with the diagnosis, aggressiveness, and bone metastasis of prostate cancer in clinical practice. Prostate International, 2(3), 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. (1999). Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. Journal of the National Cancer Institute, 91(7), 620–625. [DOI] [PubMed] [Google Scholar]
- Ma CX, Suman VJ, Goetz M, Haluska P, Moynihan T, Nanda R, et al. (2013). A phase I trial of the IGF-1R antibody Cixutumumab in combination with temsirolimus in patients with metastatic breast cancer. Breast Cancer Research and Treatment, 139(1), 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay VM, Middleton MR, Eckhardt SG, Rudin CM, Juergens RA, Gedrich R, et al. (2016). Phase I dose-escalation study of linsitinib (OSI-906) and erlotinib in patients with advanced solid tumors. Clinical Cancer Research, 22(12), 2897–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay VM, Middleton MR, Protheroe AS, Tolcher A, Dieras V, Sessa C, et al. (2013). Phase I study of humanized monoclonal antibody AVE1642 directed against the type 1 insulin-like growth factor receptor (IGF-1R), administered in combination with anticancer therapies to patients with advanced solid tumors. Annals of Oncology, 24(3), 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield AS, Visscher DW, Hart SN, Wang C, Goetz MP, Oxvig C, et al. (2014). Pregnancy-associated plasma protein-A expression in human breast cancer. Growth Hormone & IGF Research, 24(6), 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara J, Yamada Y, Hirashima Y, Takahari D, Okita NT, Kato K, et al. (2008). Impact of insulin-like growth factor type 1 receptor, epidermal growth factor receptor, and HER2 expressions on outcomes of patients with gastric cancer. Clinical Cancer Research, 14(10), 3022–3029. [DOI] [PubMed] [Google Scholar]
- McCaffery I, Tudor Y, Deng H, Tang R, Suzuki S, Badola S, et al. (2013). Putative predictive biomarkers of survival in patients with metastatic pancreatic adenocarcinoma treated with gemcitabine and ganitumab, an IGF1R inhibitor. Clinical Cancer Research, 19(15), 4282–4289. [DOI] [PubMed] [Google Scholar]
- Miller LL, Schalch DS, & Draznin B (1981). Role of the liver in regulating somatomedin activity: Effects of streptozotocin diabetes and starvation on the synthesis and release of insulin-like growth factor and its carrier protein by the isolated perfused rat liver. Endocrinology, 108(4), 1265–1271. [DOI] [PubMed] [Google Scholar]
- Moorehead RA, Sanchez OH, Baldwin RM, & Khokha R (2003). Transgenic overexpression of IGF-II induces spontaneous lung tumors: A model for human lung adenocarcinoma. Oncogene, 22(6), 853–857. [DOI] [PubMed] [Google Scholar]
- Moxham CP, Duronio V, & Jacobs S (1989). Insulin-like growth factor I receptor beta-subunit heterogeneity. Evidence for hybrid tetramers composed of insulin-like growth factor I and insulin receptor heterodimers. The Journal of Biological Chemistry, 264(22), 13238–13244. [PubMed] [Google Scholar]
- Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, et al. (2012). Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clinical Cancer Research, 18(9), 2625–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann GM, Marinaro JA, & Bach LA (1998). Identification of O-glycosylation sites and partial characterization of carbohydrate structure and disulfide linkages of human insulin-like growth factor binding protein 6. Biochemistry, 37(18), 6572–6585. [DOI] [PubMed] [Google Scholar]
- Novello S, Scagliotti G, de Castro G Jr., Kiyik M, Kowalyszyn R, Deppermann KM, et al. (2017). An open-label, multicenter, randomized, phase II study of cisplatin and pemetrexed with or without cixutumumab (IMC-A12) as a first-line therapy in patients with advanced nonsquamous non-small cell lung cancer. Journal of Thoracic Oncology, 12(2), 383–389. [DOI] [PubMed] [Google Scholar]
- Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, et al. (2010). Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Research, 70(2), 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, et al. (2010). Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: A phase 1 expansion cohort study. The Lancet Oncology, 11(2), 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A, Nolan CM, Kyle JW, Grubb JH, & Sly WS (1988). The human cation-independent mannose 6-phosphate receptor. Cloning and sequence of the full-length cDNA and expression of functional receptor in COS cells. The Journal of Biological Chemistry, 263(5), 2553–2562. [PubMed] [Google Scholar]
- Pandini G, Wurch T, Akla B, Corvaia N, Belfiore A, & Goetsch L (2007). Functional responses and in vivo anti-tumour activity of h7C10: A humanised monoclonal antibody with neutralising activity against the insulin-like growth factor-1 (IGF-1) receptor and insulin/IGF-1 hybrid receptors. European Journal of Cancer, 43(8), 1318–1327. [DOI] [PubMed] [Google Scholar]
- Pang DT, & Shafer JA (1984). Evidence that insulin receptor from human placenta has a high affinity for only one molecule of insulin. The Journal of Biological Chemistry, 259(13), 8589–8596. [PubMed] [Google Scholar]
- Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, et al. (2011). R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: Results of a phase II Sarcoma Alliance for Research Through Collaboration Study. Journal of Clinical Oncology, 29(34), 4541–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappo AS, Vassal G, Crowley JJ, Bolejack V, Hogendoorn PC, Chugh R, et al. (2014). A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: Results of a Sarcoma Alliance for Research Through Collaboration Study. Cancer, 120(16), 2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payet LD, Wang XH, Baxter RC, & Firth SM (2003). Amino- and carboxyl-terminal fragments of insulin-like growth factor (IGF) binding protein-3 cooperate to bind IGFs with high affinity and inhibit IGF receptor interactions. Endocrinology, 144(7), 2797–2806. [DOI] [PubMed] [Google Scholar]
- Pitts TM, Tan AC, Kulikowski GN, Tentler JJ, Brown AM, Flanigan SA, et al. (2010). Development of an integrated genomic classifier for a novel agent in colorectal cancer: Approach to individualized therapy in early development. Clinical Cancer Research, 16(12), 3193–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitout V, Hagman D, Stein R, Artner I, Robertson RP, & Harmon JS (2006). Regulation of the insulin gene by glucose and fatty acids. The Journal of Nutrition, 136(4), 873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M (2008). Insulin and insulin-like growth factor signalling in neoplasia. Nature Reviews Cancer, 8(12), 915–928. [DOI] [PubMed] [Google Scholar]
- Pollak M (2012). The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nature Reviews Cancer, 12(3), 159–169. [DOI] [PubMed] [Google Scholar]
- Ramalingam SS, Spigel DR, Chen D, Steins MB, Engelman JA, Schneider CP, et al. (2011). Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. Journal of Clinical Oncology, 29(34), 4574–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddan JR, & Dziedzic DC (1982). Insulin-like growth factors, IGF-1, IGF-2 and somatomedin C trigger cell proliferation in mammalian epithelial cells cultured in a serum-free medium. Experimental Cell Research, 142(2), 293–300. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Singh R, Fujita-Yamaguchi Y, & Yee D (2006). Down-regulation of insulin receptor by antibodies against the type I insulin-like growth factor receptor: Implications for anti-insulin-like growth factor therapy in breast cancer. Cancer Research, 66(4), 2391–2402. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Zhang X, Matise I, Gaillard-Kelly M, & Yee D (2010). The type I insulin-like growth factor receptor regulates cancer metastasis independently of primary tumor growth by promoting invasion and survival. Oncogene, 29(2), 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara VR, Hall K, Von Holtz H, Humbel R, Sjogren B, & Wetterberg L (1982). Evidence for the presence of specific receptors for insulin-like growth factors 1 (IGE-1) and 2 (IGF-2) and insulin throughout the adult human brain. Neuroscience Letters, 34(1), 39–44. [DOI] [PubMed] [Google Scholar]
- Schwander JC, Hauri C, Zapf J, & Froesch ER (1983). Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: Dependence on growth hormone status. Endocrinology, 113(1), 297–305. [DOI] [PubMed] [Google Scholar]
- Shevah O, & Laron Z (2007). Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: A preliminary report. Growth Hormone & IGF Research, 17(1), 54–57. [DOI] [PubMed] [Google Scholar]
- Shi R, Yu H, McLarty J, & Glass J (2004). IGF-I and breast cancer: A meta-analysis. International Journal of Cancer, 111(3), 418–423. [DOI] [PubMed] [Google Scholar]
- Sichani MM, Yazdi FS, Moghaddam NA, Chehrei A, Kabiri M, Naeimi A, et al. (2010). Prognostic value of insulin-like growth factor-I receptor expression in renal cell carcinoma. Saudi Journal of Kidney Diseases and Transplantation, 21(1), 69–74. [PubMed] [Google Scholar]
- Sitar T, Popowicz GM, Siwanowicz I, Huber R, & Holak TA (2006). Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proceedings of the National Academy of Sciences of the United States of America, 103(35), 13028–13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaaby R, Schaffer L, Lautrup-Larsen I, Andersen AS, Shaw AC, Mathiasen IS, et al. (2006). Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. The Journal of Biological Chemistry, 281(36), 25869–25874. [DOI] [PubMed] [Google Scholar]
- Soos MA, Field CE, & Siddle K (1993). Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. The Biochemical Journal, 290(Pt. 2), 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria JC, Massard C, Lazar V, Ozoux ML, Mery-Mignard D, Deslandes A, et al. (2013). A dose finding, safety and pharmacokinetic study of AVE1642, an anti-insulin-like growth factor-1 receptor (IGF-1R/CD221) monoclonal antibody, administered as a single agent and in combination with docetaxel in patients with advanced solid tumours. European Journal of Cancer, 49(8), 1799–1807. [DOI] [PubMed] [Google Scholar]
- Stone TW, McPherson M, & Gail Darlington L (2018). Obesity and cancer: Existing and new hypotheses for a causal connection. eBioMedicine, 30, 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno B, Mancini C, Vitali R, Mancuso M, McDowell HP, Dominici C, et al. (2006). Down-regulation of insulin-like growth factor I receptor activity by NVP-AEW541 has an antitumor effect on neuroblastoma cells in vitro and in vivo. Clinical Cancer Research, 12(22), 6772–6780. [DOI] [PubMed] [Google Scholar]
- Tobin G, Yee D, Brunner N, & Rotwein P (1990). A novel human insulin-like growth factor I messenger RNA is expressed in normal and tumor cells. Molecular Endocrinology, 4(12), 1914–1920. [DOI] [PubMed] [Google Scholar]
- Tognon CE, Somasiri AM, Evdokimova VE, Trigo G, Uy EE, Melnyk N, et al. (2011). ETV6-NTRK3-mediated breast epithelial cell transformation is blocked by targeting the IGF1R signaling pathway. Cancer Research, 71(3), 1060–1070. [DOI] [PubMed] [Google Scholar]
- Toretsky JA, Kalebic T, Blakesley V, LeRoith D, & Helman LJ (1997). The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. The Journal of Biological Chemistry, 272(49), 30822–30827. [DOI] [PubMed] [Google Scholar]
- Twigg SM, & Baxter RC (1998). Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. The Journal of Biological Chemistry, 273(11), 6074–6079. [DOI] [PubMed] [Google Scholar]
- Ulanet DB, Ludwig DL, Kahn CR, & Hanahan D (2010). Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proceedings of the National Academy of Sciences of the United States of America, 107(24), 10791–10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A, Bell JR, Chen EY, Herrera R, Petruzzelli LM, Dull TJ, et al. (1985). Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature, 313(6005), 756–761. [DOI] [PubMed] [Google Scholar]
- Valensise H, Liu YY, Federici M, Lauro D, Dell’anna D, Romanini C, et al. (1996). Increased expression of low-affinity insulin receptor isoform and insulin/insulin-like growth factor-I hybrid receptors in term placenta from insulin-resistant women with gestational hypertension. Diabetologia, 39(8), 952–960. [DOI] [PubMed] [Google Scholar]
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. (2010). Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell, 18(6), 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CW, Menting JG, & Lawrence MC (2013). The insulin receptor changes conformation in unforeseen ways on ligand binding: Sharpening the picture of insulin receptor activation. Bioessays, 35(11), 945–954. 10.1002/bies.201370111. [DOI] [PubMed] [Google Scholar]
- Wei L, Hubbard SR, Hendrickson WA, & Ellis L (1995). Expression, characterization, and crystallization of the catalytic core of the human insulin receptor proteintyrosine kinase domain. The Journal of Biological Chemistry, 270(14), 8122–8130. [DOI] [PubMed] [Google Scholar]
- White MF (2002). IRS proteins and the common path to diabetes. American Journal of Physiology Endocrinology and Metabolism, 283(3), E413–E422. [DOI] [PubMed] [Google Scholar]
- Xu J, Bie F, Wang Y, Chen X, Yan T, & Du J (2019). Prognostic value of IGF-1R in lung cancer: A PRISMA-compliant meta-analysis. Medicine (Baltimore), 98(19), e15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Kong GK, Menting JG, Margetts MB, Delaine CA, Jenkin LM, et al. (2018). How ligand binds to the type 1 insulin-like growth factor receptor. Nature Communications, 9(1), 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. (1999). Normal growth and development in the absence of hepatic insulin-like growth factor I. Proceedings of the National Academy of Sciences of the United States of America, 96(13), 7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Flier JS, Yokota A, Benecke H, Backer JM, & Moller DE (1991). Functional properties of two naturally occurring isoforms of the human insulin receptor in Chinese hamster ovary cells. Endocrinology, 129(4), 2058–2066. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Tobe K, Tamemoto H, Ueki K, Kaburagi Y, Yamamoto-Honda R, et al. (1996). Insulin signalling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Molecular and Cellular Biology, 16(6), 3074–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh AH, Bohula EA, & Macaulay VM (2006). Human melanoma cells expressing V600E B-RAF are susceptible to IGF1R targeting by small interfering RNAs. Oncogene, 25(50), 6574–6581. [DOI] [PubMed] [Google Scholar]
- Yerushalmi R, Gelmon KA, Leung S, Gao D, Cheang M, Pollak M, et al. (2012). Insulin-like growth factor receptor (IGF-1R) in breast cancer subtypes. Breast Cancer Research and Treatment, 132(1), 131–142. [DOI] [PubMed] [Google Scholar]
- Yu H, Spitz MR, Mistry J, Gu J, Hong WK, & Wu X (1999). Plasma levels of insulin-like growth factor-I and lung cancer risk: A case-control analysis. Journal of the National Cancer Institute, 91(2), 151–156. [DOI] [PubMed] [Google Scholar]
- Zeng X, Sachdev D, Zhang H, Gaillard-Kelly M, & Yee D (2009). Sequencing of type I insulin-like growth factor receptor inhibition affects chemotherapy response in vitro and in vivo. Clinical Cancer Research, 15(8), 2840–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha J, O’Brien C, Savage H, Huw LY, Zhong F, Berry L, et al. (2009). Molecular predictors of response to a humanized anti-insulin-like growth factor-I receptor monoclonal antibody in breast and colorectal cancer. Molecular Cancer Therapeutics, 8(8), 2110–2121. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Feng Y, Zhu Z, & Dimitrov DS (2011). Human monoclonal antibody fragments binding to insulin-like growth factors I and II with picomolar affinity. Molecular Cancer Therapeutics, 10(9), 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Fazenbaker C, Breen S, Chen C, Huang J, Morehouse C, et al. (2014). MEDI-573, alone or in combination with mammalian target of rapamycin inhibitors, targets the insulin-like growth factor pathway in sarcomas. Molecular Cancer Therapeutics, 13(11), 2662–2673. [DOI] [PubMed] [Google Scholar]
- Zhou R, Diehl D, Hoeflich A, Lahm H, & Wolf E (2003). IGF-binding protein-4: Biochemical characteristics and functional consequences. The Journal of Endocrinology, 178(2), 177–193. [DOI] [PubMed] [Google Scholar]
- Zu K, Martin NE, Fiorentino M, Flavin R, Lis RT, Sinnott JA, et al. (2013). Protein expression of PTEN, insulin-like growth factor I receptor (IGF-IR), and lethal prostate cancer: A prospective study. Cancer Epidemiology, Biomarkers & Prevention, 22(11), 1984–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


