Enoyl-CoA hydratase (ECH) catalyzes the second step of the β-oxidation pathway. Here, the crystal structure of ECH from T. thermophilus HB8 is reported at 2.85 Å resolution.
Keywords: β-oxidation pathway, crotonases, enoyl-CoA hydratase, crystal structure, fatty-acid metabolism, Thermus thermophilus HB8
Abstract
Fatty-acid degradation is an oxidative process that involves four enzymatic steps and is referred to as the β-oxidation pathway. During this process, long-chain acyl-CoAs are broken down into acetyl-CoA, which enters the mitochondrial tricarboxylic acid (TCA) cycle, resulting in the production of energy in the form of ATP. Enoyl-CoA hydratase (ECH) catalyzes the second step of the β-oxidation pathway by the syn addition of water to the double bond between C2 and C3 of a 2-trans-enoyl-CoA, resulting in the formation of a 3-hydroxyacyl CoA. Here, the crystal structure of ECH from Thermus thermophilus HB8 (TtECH) is reported at 2.85 Å resolution. TtECH forms a hexamer as a dimer of trimers, and wide clefts are uniquely formed between the two trimers. Although the overall structure of TtECH is similar to that of a hexameric ECH from Rattus norvegicus (RnECH), there is a significant shift in the positions of the helices and loops around the active-site region, which includes the replacement of a longer α3 helix with a shorter α-helix and 310-helix in RnECH. Additionally, one of the catalytic residues of RnECH, Glu144 (numbering based on the RnECH enzyme), is replaced by a glycine in TtECH, while the other catalytic residue Glu164, as well as Ala98 and Gly141 that stabilize the enolate intermediate, is conserved. Their putative ligand-binding sites and active-site residue compositions are dissimilar.
1. Introduction
Acetyl-coenzyme A (acetyl-CoA) is a central metabolic intermediate from archaebacteria to mammals (Nitschke & Russell, 2013 ▸). It is the molecule through which glycolytic pyruvate enters the tricarboxylic acid (TCA) cycle. Acetyl-CoA occupies a critical position in multiple cellular processes as a metabolic intermediate. It is a key precursor in both anabolic and catabolic reactions and is an allosteric regulator of enzymatic activities (Pietrocola et al., 2015 ▸). In most mammalian cells, acetyl-CoA is predominantly generated in the mitochondrial matrix by various metabolic circuitries, namely glycolysis, β-oxidation and the catabolism of branched amino acids.
Fatty acids are a major source of metabolic energy in living organisms. Their degradation is an oxidative process that involves four enzymatic steps and is usually referred to as the β-oxidation pathway (Schulz, 1991 ▸). During this process, the bond between C2 and C3 of a long-chain acyl-CoA is broken during each cycle, resulting in the formation of acetyl-CoA as a byproduct. This acetyl-CoA then enters the mitochondrial tricarboxylic acid (TCA) cycle, resulting in the production of NADH, which in turn is fed into the electron-transport chain to produce energy in the form of ATP (Hamed et al., 2008 ▸).
The crotonases are a superfamily of metabolic enzymes that are highly conserved from prokaryotes to eukaryotes. Members of this family include enoyl-CoA hydratase (ECH) and enoyl-CoA isomerase (ECI) and they share similar catalytic reactions with regard to substrate molecules and enzyme mechanisms (Müller-Newen et al., 1995 ▸; Lohans et al., 2017 ▸). ECH catalyzes the second step of the physiologically important β-oxidation pathway, with nearly diffusion-controlled reaction rates for the best substrates (Agnihotri & Liu, 2003 ▸). This enzyme facilitates the syn addition of water molecules across the double bond of a trans-2-enoyl-CoA thioester to produce an S-3-hydroxyacyl-CoA. These typical syn-specific ECHs (S-ECHs) are also known as ECH-1s. ECHs that specifically generate anti hydration to produce R-3-hydroxyacyl-CoAs are known as ECH-2s (or R-ECHs). ECH-2 plays an important role in the metabolism of lipid intermediates in eukaryotes and in the biosynthesis of polyhydroxyalkanoates in bacteria (Liu et al., 2016 ▸; Abdel-Mawgoud et al., 2013 ▸). In humans, ECH has been reported to be associated with the progression, metastasis and drug resistance of cancers (Zhang et al., 2015 ▸). It might function as a tumour promoter or as a tumour suppressor for certain cancers depending on the particular type or stage of tumour cells/tissues. Decreased activity of mitochondrial β-oxidation of fatty acids decreases the formation of the energetically important acetyl-CoA substrate, thereby increasing the susceptibility to energy deficiency and the dysfunction of organs that depend on fatty-acid ketone bodies as their energy source. Moreover, it affects the post-translational acetylation of mitochondrial proteins, a mechanism that is emerging as a critical regulator of mitochondrial function (Shi & Tu, 2015 ▸).
Detailed structural and biochemical studies of ECH have been performed on the enzyme from rat (Engel et al., 1996 ▸). The first crystal structure to be reported was that of Rattus norvegicus ECH (RnECH) co-crystallized with the inhibitor octanoyl-CoA and in complex with the substrate DAC-CoA (Engel et al., 1998 ▸). The structure revealed that the ECH monomers form a tightly associated trimeric structure and two such trimers then form the hexameric complex. The hexamer contains six distinct active sites that are fully contained within the individual monomeric units, except for contact of residues from three helices at the C-terminal end with the neighbouring subunit. Structural and mutagenetic studies of RnECH revealed that Ala98, Gly141, Glu144 and Glu164 are vital residues that are important for catalysis (Feng et al., 2002 ▸).
In this study, we report the crystal structure of ECH from Thermus thermophilus HB8 (TtECH) at 2.85 Å resolution determined by molecular replacement using RnECH (PDB entry 2dub; Engel et al., 1998 ▸) as a search model. Overall, the TtECH monomer assembles into a hexamer similar to that of RnECH with significant structural changes around the active-site region.
2. Materials and methods
2.1. Protein expression and purification
The TtECH gene was cloned into pET-11a (Novagen) at the NdeI and BamHI sites. The recombinant plasmid was transformed into Escherichia coli BL21 (DE3) cells, and after induction with isopropyl β-d-1-thiogalactopyranoside, the transformant was grown at 37°C for 20 h in LB medium containing ampicillin. The cells were collected, suspended in 20 mM Tris–HCl pH 8.0 containing 0.5 M NaCl and 5 mM 2-mercaptoethanol and disrupted by sonication. The cell lysate was heat-treated at 70°C for 13 min, and cell debris and denatured protein were removed by centrifugation for 30 min at 4°C and 14 000 rev min−1. The resultant supernatant solution was further purified in four steps as follows: (i) Super Q TOYOPEARL column chromatography (0–0.3 M NaCl gradient), (ii) RESOURCE S (Amersham Biosciences) column chromatography (0–0.3 M NaCl gradient), (iii) Bio-Scale CHT-20-I (Bio-Rad) column chromatography (10–150 mM sodium phosphate gradient) and (iv) HiLoad 16/60 Superdex 75 pg column chromatography (Amersham Biosciences). The protein was visualized on SDS–PAGE using Quick-CBB. Protein concentrations were measured according to the Bradford method using bovine serum albumin as a standard (Bradford, 1976 ▸).
2.2. Protein crystallization
We tried to crystallize native and selenomethionine (SeMet)-derivatized TtECH protein and only obtained crystals of SeMet-derivatized protein. The native protein failed to crystallize. The recombinant system produced close to >90% selenium-substituted protein without any structural changes. Crystallization was performed by the microbatch method (Chayen et al., 1990 ▸) using a TERA crystallization robot with a proprietary crystallization screen reagent set (Sugahara et al., 2008 ▸). 0.5 µl screen solution was mixed with 0.5 µl protein solution (protein concentration of 18 mg ml −1 in 20 mM Tris–HCl pH 8.0, 50 mM NaCl) and covered with 15 µl of a mixture of silicone and paraffin oils. Thin plate-shaped crystals of approximate dimensions 0.25 × 0.14 × 0.04 mm were obtained in a month at 22°C. The crystals were soaked in a storage solution consisting of 0.1 M acetate and 0.72 M sodium citrate pH 5.9. Before data collection, the crystal was transferred to a cryoprotectant [20%(v/v) glycerol was added to the storage buffer].
2.3. Data collection and processing
X-ray data for the SeMet-derivatized form of TtECH were collected at a wavelength of 0.98 Å (the peak in the fluorescence spectrum for selenium) on beamline BL44B2 at SPring-8, Japan using a crystal cooled to 100 K. A thin crystal was mounted in a nylon-fibre loop and flash-cooled in a nitrogen-gas stream. Data were collected to 2.85 Å resolution (180 images of 1° oscillation) and were processed using HKL-2000 (Otwinowski & Minor, 1997 ▸).
2.4. Structure determination and refinement
The TtECH structure was solved by molecular replacement with CNS (Brünger et al., 1998 ▸) using the structure of RnECH (PDB entry 2dub) as the search model (32% sequence identity; Engel et al., 1998 ▸). Structure factors in the 15.0–4.0 Å resolution range were used for both the cross-rotation and translation searches. The data set gave a straightforward solution for the monomeric search model. The structure was then refined and water molecules were added using CNS (Brünger et al., 1998 ▸). Rigid-body refinement was used to improve the initial phases derived from the monomer model. Model building was carried out using QUANTA (Accelrys, San Diego, California, USA) and showed clear density for the monomer. The final model was produced after several rounds of model building and energy minimization followed by individual B-factor refinement. Water molecules were added to the model and inspected manually during refinement. The progress of refinement was monitored using the cross-validation indicator R free calculated using 5% of the reflections as a test set (Brünger, 1992 ▸). PROCHECK was used to inspect and correct the model within a refinement cycle (Laskowski et al., 1993 ▸). PyMOL was used to visualize and generate figures (DeLano, 2002 ▸).
3. Results and discussion
Here, we present crystals of ECH from T. thermophilus HB8 with the symmetry of the rhombohedral space group R32 that diffracted to 2.85 Å resolution. The unit-cell dimensions of the TtECH crystals are a = b = 131.23, c = 110.61 Å and they contain one molecule per asymmetric unit. Analysis of the Matthews coefficient indicates the presence of one molecule in the asymmetric unit (V M = 3.28 Å3 Da−1; 62.2% solvent content; Matthews, 1968 ▸). Statistics of crystal structure solution and refinement are summarized in Table 1 ▸. The final model of TtECH has a working R factor of 20.3% and an R free of 26.7%. The Ramachandran plot for TtECH shows 87.1% of the residues in the most favoured regions, with 12.4% in additionally allowed regions, 0.5% in generously allowed regions and no outlier residues, indicating that this structure represents a good-quality model. The solved monomer model structure contains 253 amino acids, two glycerol molecules, one 1,4-diethylene dioxide molecule and 72 water molecules.
Table 1. Data-collection and refinement statistics for TtECH (PDB entry 1uiy).
Values in parentheses are for the outer shell.
| Data collection and processing | |
| Diffraction source | BL44B2, SPring-8 |
| Detector | CCD, MAR Research |
| Wavelength (Å) | 0.980 [12651 eV] |
| Temperature (K) | 100 |
| Space group | R32 |
| a, b, c (Å) | 131.23, 131.23, 110.61 |
| α, β, γ (°) | 90, 90, 120 |
| R merge (%) | 6.8 (45.2) |
| Resolution range (Å) | 40–2.85 (2.98–2.85) |
| No. of unique reflections | 14983 (1684) |
| Completeness (%) | 95.0 (86.2) |
| Multiplicity | 7.69 (5.00) |
| 〈I/σ(I)〉 | 13.2 (5.7) |
| Mosaicity (°) | 0.7 |
| Structure refinement | |
| Refinement program | CNS 1.1 |
| Resolution range (Å) | 40–2.85 |
| R/R free (%) | 20.3/26.7 |
| No. of non-H atoms | |
| Protein | 1907 |
| Ligand | 18 |
| Water | 72 |
| Mean B factor (Å2) | |
| Overall | 58.1 |
| Main chain | 57.2 |
| Side chains | 57.2 |
| R.m.s.d., bond lengths (Å) | 0.005 |
| R.m.s.d., angles (°) | 1.2 |
| Ramachandran plot | |
| Most favoured (%) | 87.1 |
| Additionally allowed (%) | 12.4 |
| Generously allowed (%) | 0.5 |
3.1. The overall structure of T. thermophilus enoyl-CoA hydratase
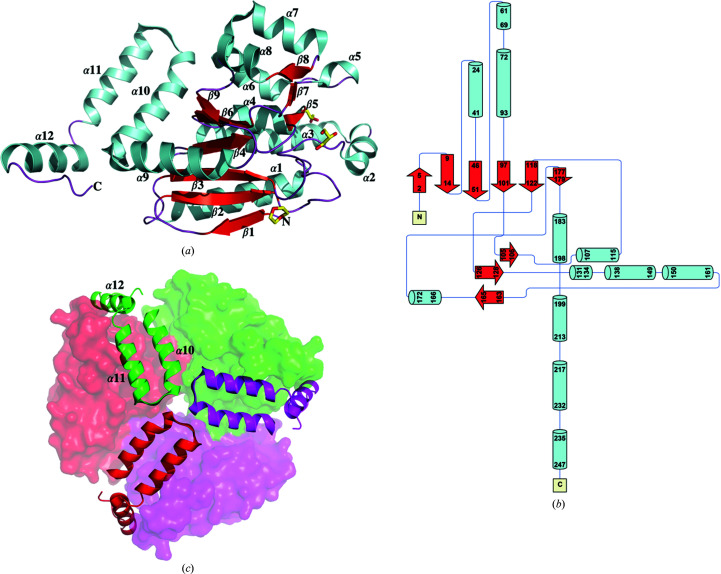
TtECH is an α/β protein with 12 α-helices and nine β-strands. Monomeric TtECH is shown in a ribbon representation and as a schematic diagram in Figs. 1 ▸(a) and 1 ▸(b), respectively. The structure of TtECH is similar to those of crotonase family members, consisting of a globular domain and a trimerization domain at the N- and C-terminus, respectively. The globular domain contains nine parallel β-strands in the centre surrounded by nine α-helices. The trimerization domain at the C-terminal end of TtECH includes three α-helices (α10–α12). It mediates tight association specifically with α2, α3, α5, α6 and α7 in the globular domain of the adjacent subunit, thereby forming a trimer with threefold symmetry (Fig. 1 ▸ c). Within the trimerization domain, α12 also acts as a lid to cover the active site of the neighbouring subunit.
Figure 1.
Cartoon (a) and topology (b) representation of ECH from T. thermophilus HB8. (c) Top view. The threefold axis is perpendicular to the paper. The N-terminal globular domain is shown in surface representation and the trimerization domain is shown in ribbon representation, with helices α10–α12 labelled. (a) and (c) were made using PyMOL (DeLano, 2002 ▸). The topology diagram of the TtECH structure was made using Pro-origami (Stivala et al., 2011 ▸).
3.2. Enoyl-CoA hydratase assembly
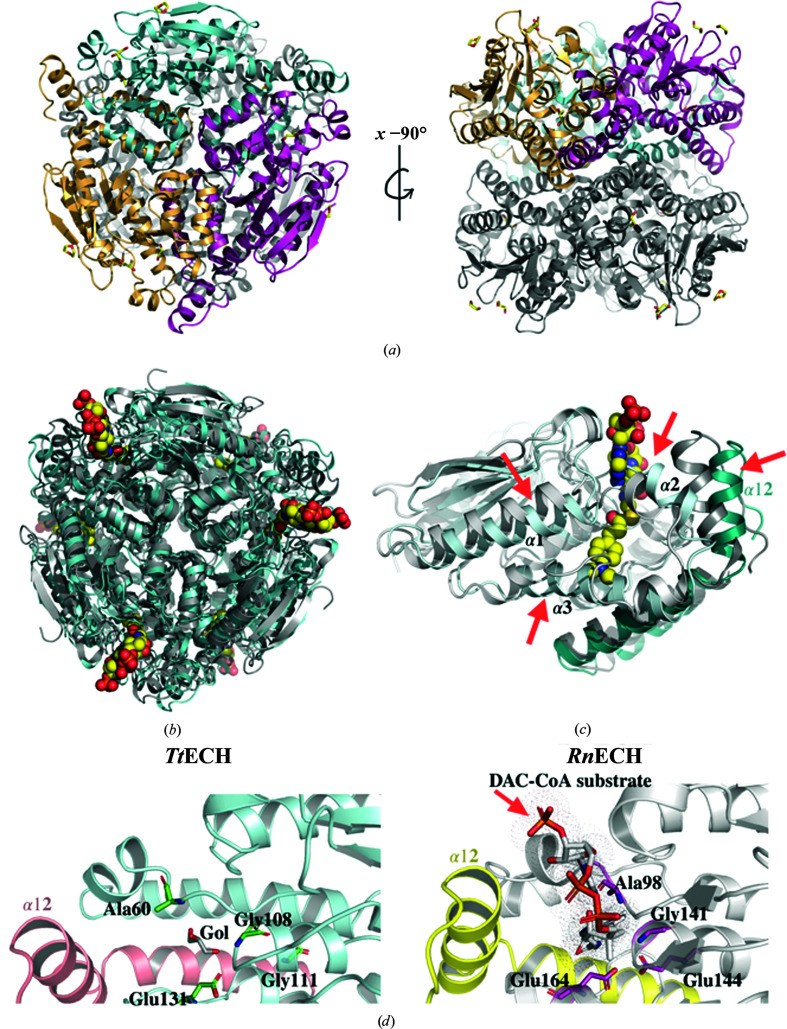
In its native state, ECH is a homohexamer with 32 symmetry consisting of two stacked trimers (Engel et al., 1996 ▸). Unlike the well characterized RnECH, the TtECH protein crystallized with one monomer per asymmetric unit, and its functional hexamer was generated by applying twofold rotation perpendicular to the threefold axis of the trimer (Fig. 2 ▸ a). The N-terminal globular domain of TtECH (residues 1–198) without the C-terminal trimerization domain superimposed with the corresponding domain of RnECH (PDB entry 2dub; residues 30–232) with a root-mean-square deviation (r.m.s.d.) of 1.21 Å. In contrast, full-length TtECH (residues 1–253) superimposed with RnECH (residues 30–292) with an r.m.s.d. of 1.64 Å, suggesting a major shift in the position of the trimerization domain. Likewise, the TtECH trimer and hexamer superimposed with the trimer and hexamer of RnECH with r.m.s.d.s of 1.74 and 2.52 Å, respectively. PISA analysis suggests that TtECH assembles into a stable hexamer similar to that of RnECH, with a total molecular mass of 162.5 kDa and with 36 390 Å2 buried surface area (Krissinel & Henrick, 2007 ▸). The interaction between the two subunits to form the trimer involves 12 hydrogen bonds, six salt bridges and several hydrophobic interactions involving many residues. PISA analysis (Krissinel & Henrick, 2007 ▸) indicates that the buried surface area between two subunits during trimer formation is 2051.9 Å2, in which most of the interaction is mediated through the trimerization domain of one subunit and the globular domain of the adjacent subunit. In the case of hexamer formation, the region between residues 205 and 234 (α10 and α11) is mainly involved in the stacking between two trimer subunits. Helices α10 and α11 face the partner trimers and are arranged symmetrically around the threefold axis, making head-to-head stacking with the subunit in the adjacent stacked trimer, and thereby bringing two trimers together to form a hexamer. PISA analysis indicated that a surface area of about 652.1 Å2 was buried between two adjacent subunits during hexamer formation.
Figure 2.
(a) Cartoon representation of the TtECH hexamer with each subunit in one stacked trimer shown in a different colour (left) and a 90° rotation around the x axis (right). (b) Superimposition of the TtECH hexamer (cyan) with the RnECH hexamer containing the substrate 4-dimethylaminocinnamoyl-CoA (DAC-CoA) (grey; PDB entry 1ey3). The bound substrate is shown as a space-filling model. (c) Superimposition of the TtECH monomer (cyan) with the RnECH monomer containing DAC-CoA substrate (grey; PDB entry 1ey3). The red arrow indicates a region with significant changes between the two structures. The trimerization domain (α10–α12) shown in dark cyan is from the neighbouring subunit. (d) Left: TtECH catalytic site residues (Ala60, Gly108, Gly111 and Glu131) are shown in green with bound glycerol (Gol). Right: RnECH catalytic site residues (Ala98, Gly141, Glu144 and Glu164) are shown in magenta with bound substrate.
3.3. Enoyl-CoA hydratase active site
The hexamer contains six distinct active sites, which are fully contained within each individual monomeric subunit, except for contact of the α11 and α12 helices from the neighbouring subunit (Fig. 2 ▸ b). The overall structure of TtECH is well structured compared with RnECH, and their superposition showed a notable shift in the positions of three helices and the loops surrounding the active-site region (Fig. 2 ▸ c). More specifically, the short α-helix and 310-helix in RnECH are replaced by a well ordered single long helix α3 (with B factors in the range 36–61 Å2 for Cα atoms) in TtECH. The above changes make the active-site pocket less flexible in TtECH than that in RnECH, and may sterically hinder the binding of long-chain fatty acids. Another major shift was seen for the α11 and α12 helices, which cover the active site of an adjacent subunit, when compared with RnECH (Fig. 2 ▸ c).
In the case of RnECH, two catalytic glutamate residues, Glu144 and Glu164, are proposed to act as the catalytic acid and base in concert to facilitate the hydration reaction and are conserved in most species. Previous studies have shown that the mutation of Glu164 in RnECH to Gln164 resulted in a large decrease in the enzyme activity (Feng et al., 2002 ▸). Furthermore, the importance of Glu164 was further substantiated by the three-dimensional structure of RnECH, which showed that this residue was placed in a position to catalyze the protonation/deprotonation of the α-carbon of the substrate (Engel et al., 1998 ▸). The crystal structure of RnECH with octanoyl-CoA revealed that Glu164 protonates the C2 atom of the fatty acid and Glu144 activates a water molecule for attack on the C3 atom of the enoyl-CoA substrate (Engel et al., 1998 ▸; Fig. 2 ▸ d). In TtECH, the glycerol used as a cryoprotectant is seen bound in the active site very close to the conserved Glu131 residue (at a position equivalent to Glu164 in RnECH; Fig. 2 ▸ d). Overall, the CoA moieties of the substrate are allocated a binding pocket formed from two adjacent monomers, while the active-site catalytic residues are provided by a single monomer.
3.4. Enoyl-CoA hydratase sequence alignment
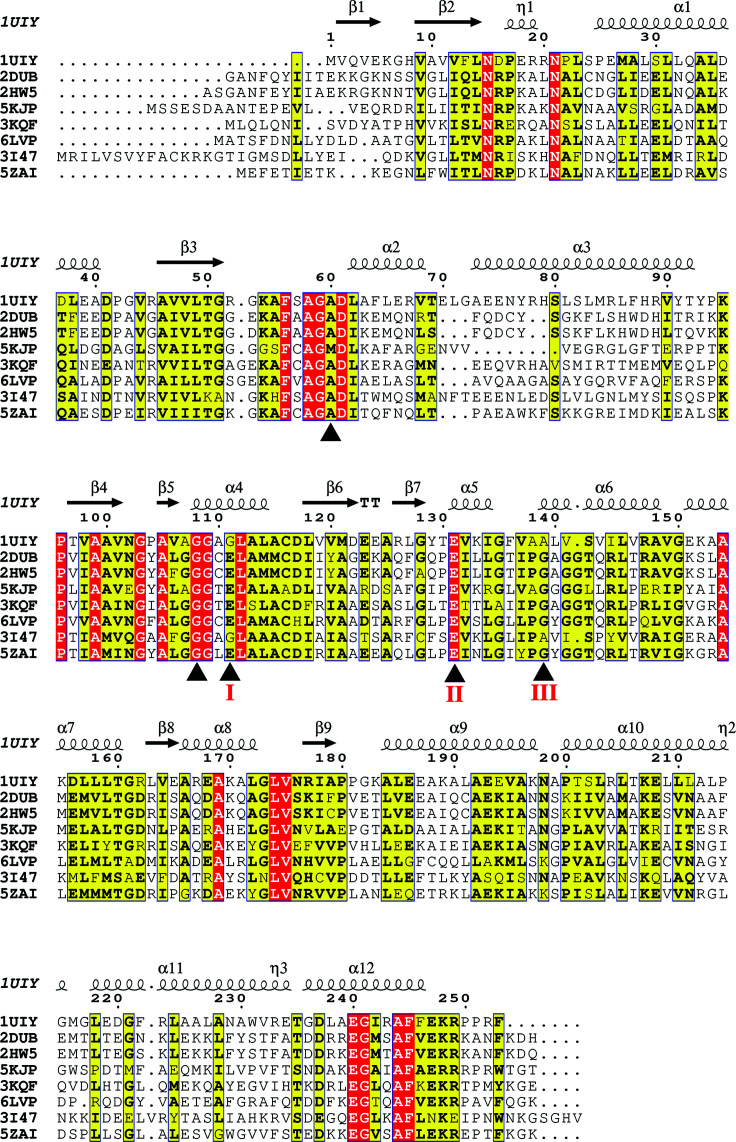
Multiple sequence alignment using Clustal Omega (Sievers et al., 2011 ▸) and ESPript (http://espript.ibcp.fr/ESPript/ESPript/) was carried out between the TtECH sequence and those of other known ECH structures. Here, we have carried our sequence analysis using sequences from diverse organisms ranging from bacteria to humans. The sequence of TtECH (PDB entry 1uiy) was aligned with those of ECHs from Rattis norvegicus (RnECH; PDB entry 2dub; 32.8% sequence identity; Engel et al., 1998 ▸), human (PDB entry 2hw5; 32.4% identity; Structural Genomics Consortium, unpublished work), Mycobacterium tuberculosis H37Rv (PDB entry 5kjp; 30.8% identity; Center for Structural Genomics of Infectious Diseases, unpublished work), Bacillus anthracis (PDB entry 3kqf; 32.4% identity; Center for Structural Genomics of Infectious Diseases, unpublished work), Hymenobacter sp. PAMC 26628 (PDB entry 6lvp; 31.6% identity; Hwang et al., 2020 ▸) and Legionella pneumophila (PDB entry 3i47; 32.4% identity; New York SGX Research Center for Structural Genomics, unpublished work), and that of 3-hydroxypropionyl-CoA dehydratase (3HPCD) from Metallosphaera sedula (PDB entry 5zai; 32.0% identity; Lee & Kim, 2018 ▸).
Based on the sequence- and structure-based alignment of ECH superfamily members, the key three positions delineating the ligand-binding site within the active site, which determine the ECH/ECI function, are indicated (labelled I–III in Fig. 3 ▸; Srivastava et al., 2015 ▸). In the vicinity of the catalytic site, the conserved Ala60 and Gly111 residues (TtECH residue numbering) that are positioned to form an oxyanion hole during enzyme catalysis are also indicated (Fig. 3 ▸). The structural and mutagenic studies revealed that the hydration and isomerization functions of ECH can be attributed to the presence of glutamate residues in the active site (Müller-Newen et al., 1995 ▸; Srivastava et al., 2015 ▸). Bifunctional ECH/ECI enzymes contain two glutamate residues in the same active site, whereas monofunctional ECIs contain only one glutamate residue. RnECH has Glu144 and Glu164 residues at positions I and II, respectively, whereas TtECH has one conserved glutamate residue at position II and this is the only possible catalytic residue involved in substrate activation. The sequence alignment shows that the three key TtECH residues are Gly111, Glu13 and Ala139, which match Mycobacterium tuberculosis ECHA7, which is predicted to function as an ECI (Srivastava et al., 2015 ▸). Therefore, we suspect that TtECH catalyzes the enzyme reaction in a mode similar to that of other ECI enzymes.
Figure 3.
Multiple sequence alignment of TtECH with its homologs performed using Clustal Omega (Sievers et al., 2011 ▸) and ESPript. TtECH (PDB entry 1uiy) is alignmed with RnECH (PDB entry 2dub), human ECH (PDB entry 2hw5), Mycobacterium tuberculosis H37Rv ECH (PDB entry 5kjp), Bacillus anthracis ECH (PDB entry 3kqf), Hymenobacter sp. PAMC 26628 ECH (PDB entry 6lvp), Legionella pneumophila ECH (PDB entry 3i47) and 3-hydroxypropionyl-CoA dehydratase (3HPCD) from Metallosphaera sedula (PDB entry 5zai). The catalytic residues are indicated by black triangles below the sequence. The residues that determine ECH/ECI activity are marked I–III below the sequence.
3.5. Enoyl-CoA hydratase substrate recognition
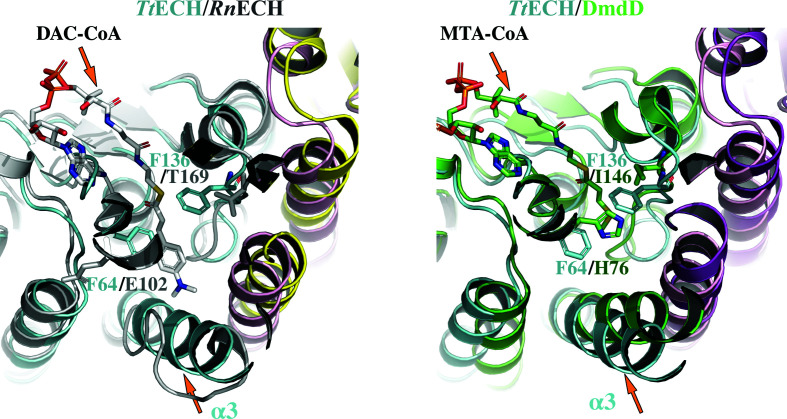
It is known that the ECH family enzymes present similar overall structures but utilize various types of enoyl-CoAs, with carbon chain lengths of four to 16, as substrates (Waterson & Hill, 1972 ▸). Structural analysis revealed that the conformation of the α3 helix and the residues constituting the enoyl-binding pocket are the key elements that determine the substrate specificity of the ECH family enzymes (Lee & Kim, 2018 ▸). The enzyme accommodates long-chain enoyl-CoAs as substrates with a highly flexible helix–loop–helix conformation at the corresponding region of the α3 helix. In contrast, an ECH enzyme with a tightly formed α3 helix would accommodate short-chain enoyl-CoAs as substrates. When we compared TtECH with RnECH, the former has a tightly formed rigid α3 helix with three aromatic residues, Phe64, Phe136 and Phe245, in the enoyl-group binding site. Among the three aromatic residues, Phe64 and Phe136 are located in the substrate-binding tunnel. RnECH exhibits a highly flexible short helix–loop–helix conformation in the corresponding region to the TtECH α3 helix and is known to accommodate long-chain enoyl-CoAs as substrates (Fig. 4 ▸ a). We then compared the structure of DmdD–MTA-CoA (PDB entry 5zai; Tan et al., 2013 ▸), which recognizes a shorter enoyl-CoA as a substrate, with that of TtECH. The TtECH structure is analogous to that of DmdD, with a rigid α3 helix. The substrate-binding pocket of DmdD is relatively smaller in size and it was classified as a bacterial short-chain ECH (Fig. 4 ▸ b). Based on the above analysis, we speculate that TtECH recognize a shorter substrate.
Figure 4.
Comparison of the active sites in chain A of TtECH with the RnECH–DAC-CoA and DmdD–MTA-CoA structures. The structures are overlaid with TtECH in cyan (chain A) and magenta (chain B), RnECH in dark white (chain A) and yellow (chain B) and DmdD in green (chain A) and purple (chain B). The residues Phe64, Phe136 and Phe245 of TtECH point towards the active site and reduce the size of the active-site pocket. For structural clarity, only Phe64 and Phe136 of TtECH and the corresponding residues in RnECH and DmdD are shown (stick representation). The α3 helix in TtECH and DmdD is tightly formed, while in RnECH it transforms to a helix–loop–helix.
4. Conclusion
We have crystallized and solved the X-ray structure of ECH from T. thermophilus HB8. ECH is a metabolically important enzyme that is conserved from bacteria to mammals. The structure of TtECH is similar to those of other ECH/ECI members of the crotonase family. TtECH has only one conserved glutamate residue (position II) in the catalytic site, mimicking a monofunctional enzyme with ECI activity. The tightly formed α3 helix and the bulky aromatic amino acids constituting the enoyl-binding pocket in the TtECH enzyme distinguish it as a member of the bacterial short-chain ECH subgroup.
Supplementary Material
PDB reference: enoyl-CoA hydratase from T. thermophilus HB8, 1uiy
Acknowledgments
We thank the staff of beamline BL44B2 at SPring-8 for assistance during the X-ray experiment. Author contributions were as follows: BB solved the crystal structure, SJ and HG helped with figure preparation, and SP and BB wrote the manuscript. The authors declare no conflicts of interest with regard to this manuscript.
Funding Statement
This work was funded by Ministry of Education, Culture, Sports, Science and Technology grant . Department of Biotechnology, Ministry of Science and Technology, India grant .
References
- Abdel-Mawgoud, A. M., Lépine, F. & Déziel, E. (2013). J. Chromatogr. A, 1306, 37–43. [DOI] [PubMed]
- Agnihotri, G. & Liu, H. (2003). Bioorg. Med. Chem. 11, 9–20. [DOI] [PubMed]
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. [DOI] [PubMed]
- Brünger, A. T. (1992). Nature, 355, 472–475. [DOI] [PubMed]
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. [DOI] [PubMed]
- Chayen, N. E., Shaw Stewart, P. D., Maeder, D. L. & Blow, D. M. (1990). J. Appl. Cryst. 23, 297–302.
- DeLano, W. L. (2002). PyMOL. http://www.pymol.org.
- Engel, C. K., Kiema, T. R., Hiltunen, J. K. & Wierenga, R. K. (1998). J. Mol. Biol. 275, 847–859. [DOI] [PubMed]
- Engel, C. K., Mathieu, M., Zeelen, J. P., Hiltunen, J. K. & Wierenga, R. K. (1996). EMBO J. 15, 5135–5145. [PMC free article] [PubMed]
- Feng, Y., Hofstein, H. A., Zwahlen, J. & Tonge, P. J. (2002). Biochemistry, 41, 12883–12890. [DOI] [PubMed]
- Hamed, R. B., Batchelar, E. T., Clifton, I. J. & Schofield, C. J. (2008). Cell. Mol. Life Sci. 65, 2507–2527. [DOI] [PMC free article] [PubMed]
- Hwang, J., Jeong, C.-S., Lee, C. W., Shin, S. C., Kim, H.-W., Lee, S. G., Youn, U. J., Lee, C. S., Oh, T.-J., Kim, H. J., Park, H., Park, H. H. & Lee, J. H. (2020). J. Microbiol. 58, 606–613. [DOI] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. (1993). J. Appl. Cryst. 26, 283–291.
- Lee, D. & Kim, K.-J. (2018). Sci. Rep. 8, 10692. [DOI] [PMC free article] [PubMed]
- Liu, G., Cai, S., Hou, J., Zhao, D., Han, J., Zhou, J. & Xiang, H. (2016). Sci. Rep. 6, 24015. [DOI] [PMC free article] [PubMed]
- Lohans, C. T., Wang, D. Y., Wang, J., Hamed, R. B. & Schofield, C. J. (2017). ACS Catal. 7, 6587–6599.
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Müller-Newen, G., Janssen, U. & Stoffel, W. (1995). Eur. J. Biochem. 228, 68–73. [DOI] [PubMed]
- Nitschke, W. & Russell, M. J. (2013). Philos. Trans. R. Soc. B, 368, 20120258. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J. M., Madeo, F. & Kroemer, G. (2015). Cell Metab. 21, 805–821. [DOI] [PubMed]
- Schulz, H. (1991). Biochim. Biophys. Acta, 1081, 109–120. [DOI] [PubMed]
- Shi, L. & Tu, B. P. (2015). Curr. Opin. Cell Biol. 33, 125–131. [DOI] [PMC free article] [PubMed]
- Sievers, F., Wilm, A., Dineen, D., Gibson, T. J., Karplus, K., Li, W., Lopez, R., McWilliam, H., Remmert, M., Söding, J., Thompson, J. D. & Higgins, D. G. (2011). Mol. Syst. Biol. 7, 539. [DOI] [PMC free article] [PubMed]
- Srivastava, S., Chaudhary, S., Thukral, L., Shi, C., Gupta, R. D., Gupta, R., Priyadarshan, K., Vats, A., Haque, A. S., Sankaranarayanan, R., Natarajan, V. T., Sharma, R., Aldrich, C. C. & Gokhale, R. S. (2015). Chem. Biol. 22, 1577–1587. [DOI] [PubMed]
- Stivala, A., Wybrow, M., Wirth, A., Whisstock, J. C. & Stuckey, P. J. (2011). Bioinformatics, 27, 3315–3316. [DOI] [PubMed]
- Sugahara, M., Asada, Y., Shimizu, K., Yamamoto, H., Lokanath, N. K., Mizutani, H., Bagautdinov, B., Matsuura, Y., Taketa, M., Kageyama, Y., Ono, N., Morikawa, Y., Tanaka, Y., Shimada, H., Nakamoto, T., Sugahara, M., Yamamoto, M. & Kunishima, N. (2008). J. Struct. Funct. Genomics, 9, 21–28. [DOI] [PubMed]
- Tan, D., Crabb, W. M., Whitman, W. B. & Tong, L. (2013). PLoS One, 8, e63870. [DOI] [PMC free article] [PubMed]
- Waterson, R. M. & Hill, R. L. (1972). J. Biol. Chem. 247, 5258–5265. [PubMed]
- Zhang, J., Ibrahim, M. M., Sun, M. & Tang, J. (2015). Clin. Chim. Acta, 448, 13–17. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: enoyl-CoA hydratase from T. thermophilus HB8, 1uiy