Abstract
Background/aims
SARS-CoV-2 is highly contagious. More evidence concerning extrapulmonary transmission routes such as the eyes is urgently needed. Although the humoral immune response is important in the viral containment, the local response in tears has not yet been studied. The aim of our study was twofold: to assess the prevalence of both SARS-CoV-2 RNA and antibodies in tear fluid.
Methods
In a first series, nasopharyngeal sampling and tear sampling by Schirmer test strips were performed in 26 acutely ill patients with COVID-19 to assess the presence of SARS-CoV-2 RNA by reverse transcription PCR. In a second series, IgG and IgA responses to SARS-CoV-2 spike protein in serum and tear fluid of convalescent individuals (n=22) were compared with control individuals (n=15) by ELISA.
Results
SARS-CoV-2 RNA was detected in tears of 7/26 (26.9%) patients with COVID-19. None of them had ocular symptoms. Convalescent individuals displayed a significant higher ratio of IgG (p<0.0001) and IgA (p=0.0068) in tears compared with control individuals. A sensitivity of 77.3% and specificity of 93.3% was observed for IgG, and 59.1% and 100% for IgA.
Conclusions
Our results demonstrate the presence of SARS-CoV-2 RNA and a local IgG and IgA immune response in tear fluid. These data confirm the possibility of SARS-CoV-2 transmission through tear fluid and the importance of the eye as a first defence against SARS-CoV-2, indicating the potential of tears as a non-invasive surrogate for serum in monitoring the host immune response.
Keywords: COVID-19, tears, immunology, diagnostic tests/investigation, microbiology
Key messages.
What is already known about this subject?
It is hypothesised that SARS-CoV-2 can be transmitted from the upper respiratory tract to the eyes. IgA antibodies have a crucial role in the immune defence of mucosal surfaces as the first point of entry of SARS-CoV-2.
What are the new findings?
The presence of SARS-CoV-2 RNA in tears of infected patients was demonstrated, confirming the possibility of transmission through tear fluid. Furthermore, convalescent individuals displayed a significant higher ratio of IgG and IgA in tears compared with control individuals, demonstrating the importance of the eye as a first defence against SARS-CoV-2.
How might these results change the focus of research or clinical practice?
These data are indicating the need for adjusted personal protective equipment, including goggles, when taking care of patients with COVID-19. Furthermore, tears have potential as a non-invasive surrogate for serum in monitoring the host immune response.
Introduction
SARS-CoV-2, a novel coronavirus discovered in Wuhan (China) mid-December 2019, has spread rapidly across the globe. This highly contagious virus mainly affects the lower respiratory tract, causing COVID-19. The pathogenesis of SARS-CoV-2 is complex, with multiple factors leading to injury of the lungs and dissemination to other organs. Although it is known that transmission occurs predominantly via respiratory droplets, extrapulmonary routes of transmission are not yet fully clarified. It is hypothesised that the virus can be transmitted from the upper respiratory tract through the nasolacrimal system to the eyes. ACE-2 receptors and the transmembrane protease serine type 2 are highly expressed on the ocular surface epithelium.1 2 As these are the receptor for SARS-CoV-2 and a cell surface-associated protease that facilitates viral entry following binding of the viral spike protein to ACE-2, it is suggested that the eye can be a portal of entry of the virus and that ocular fluid is a possible source of SARS-CoV-2.3 4 A limited number of studies have been published reporting conflicting data on ocular fluids as a potential source of spread.3 5–10 Therefore, in a first part of our study, we wanted to assess the presence of SARS-CoV-2 RNA in tear fluid of patients with COVID-19 to determine the need for adjusted personal protective equipment.3 11 12
During the SARS 2002–2003 epidemic, it was demonstrated that serological assays were a useful diagnostic tool in non-acute infections.13 Although more research is needed to assess the risk of reinfection in convalescing patients, recent data suggest that SARS-CoV-2 antibodies could protect at least for some time from subsequent viral exposure.14 Chao et al15 reported on the crucial role of IgA in the immune defence of mucosal surfaces as the first point of entry of SARS-CoV-2. IgA may serve as an indicator of host immune response and can be directly measured in saliva and tears. However, there is a lack of systematic studies on IgA production in patients with COVID-19 in general, and no in-depth investigation of the presence of SARS-CoV-2 antibodies in tears has been performed yet.15–18 Therefore, in a second part of our study, we investigated the presence of IgG and IgA antibodies against SARS-CoV-2 in tear fluid.
Thus, the objective of our study was twofold, namely to assess the prevalence of both SARS-CoV-2 RNA and IgG and IgA antibodies in tear fluid. By doing so, we aimed to investigate ocular involvement in the spread of the virus and the role of tears as a non-invasive (immunological) biomarker.
Materials and methods
Study population
The study consisted of different subgroups. We defined a study population to assess the prevalence of SARS-CoV-2 RNA in tear fluid on the one hand, and a study population to assess the prevalence of SARS-CoV-2 antibodies in tear fluid on the other hand. A written informed consent was obtained from the participants.
RNA
Between 10 August and 3 November 2020, adult patients aged 18 years or older with laboratory-confirmed COVID-19 infection (SARS-CoV-2 detected by reverse transcription PCR (RT-PCR) on a nasopharyngeal swab (UTM, Copan, Brescia, Italy)) that were admitted at University Hospital Brussels because of COVID-19 related symptoms, were recruited for tear fluid sampling.
Antibodies
Between 27 August and 13 October 2020, employees of University Hospital Brussels participating in a simultaneous study assessing the SARS-CoV-2 seroprevalence and seroconversion in employees (COVEMUZ study) were recruited for tear fluid sampling. For the COVEMUZ study, anti-SARS-CoV-2 IgG antibodies were assessed in each participant at three time points (May–June, July–August and October 2020). Controls were defined as employees without anti-SARS-CoV-2 IgG antibodies in serum at the three time points of the COVEMUZ study and without anti-SARS-CoV-2 IgG and IgA antibodies in serum at the moment of tear sampling. Two cohorts of convalescent employees were defined to study the presence of antibodies in tear fluid: a cohort with anti-SARS-CoV-2 IgG and a cohort with anti-SARS-CoV-2 IgA antibodies in serum at the moment of tear sampling.
Patient and public involvement
Patients were asked to assess the burden of the intervention. Patients or the public were not further involved in design, or conduct, or reporting, or dissemination plans of our research.
Sample collection
Collection of tear fluid was performed by application of a paper strip, called Schirmer Tear Test (HS Clement Clarke Ophthalmic, Harlow, UK) in both eyes. The bent end of a Schirmer test strip was placed in the lower eyelid of each eye for 5 min without anaesthesia. Insertion and removal of the paper strips was performed with gloves. Sterile gloves were used in case of RNA testing, besides personal protective equipment as it concerned acutely ill patients with COVID-19. The wetted strip was placed in an Eppendorf tube and analysed in the laboratory within 24 hours.
Tear fluid was collected within 48 hours of nasopharyngeal sampling or blood collection for the study of respectively RNA or antibodies. Blood samples were collected by venepuncture and centrifuged (3000 RPM, 10 min) to obtain serum.
Laboratory analysis
RNA
Nasopharyngeal swabs were analysed either by RealStar SARS-CoV-2 RT-PCR Kit 1.0 (Altona Diagnostics GmbH, Hamburg, Germany) targeting E-gene and S-gene or by Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, California, USA) targeting E-gene and N2-gene. All tear fluids were analysed by RealStar. The result was considered positive if both target genes were detected. RealStar results were expressed as crossing point (Cp) values and Xpert results as cycle threshold (Ct) values. RNA extraction was performed by easyMAG/eMAG (bioMérieux, Marcy l’Etoile, France) and eluted in 50 µL prior to RT-PCR by RealStar. The Xpert test was performed according to the manufacturer’s instructions.
Antibodies
Antibodies were analysed by anti-SARS-CoV-2 ELISA (IgG and IgA) (EUROIMMUN, Luebeck, Germany), an ELISA specific for the detection of antibodies against the S1 domain of the spike protein. Serum samples were analysed and interpreted according to the manufacturer’s instructions (negative: ratio <0.8; borderline: ratio ≥0.8–<1.1; positive: ratio ≥1.1). Borderline results were considered positive for analysis. The kit was used off-label for analysis of tear fluid samples as well. The antibodies were eluted by placing the Schirmer strips in 500 µL sample buffer, vortexing (1 min), centrifugation (1000 RPM, 5 min) and finally transferring 250 µL of the fluid to an Eppendorf tube that was loaded on the ANALYZER I-2P (EUROIMMUN) for fully automated processing.
Statistics
Demographic data are described by median (Mdn) and IQR. For continuous data, comparisons between groups were performed using the Mann-Whitney U test. Receiver operating characteristics (ROC) analyses were used to seek cut-offs for anti-SARS-CoV-2 IgG and IgA in tears and to report test accuracy, that is, area under the curve (AUC), sensitivity and specificity. The optimal cut-offs were determined by the Youden index. All statistical tests were performed two sided at a significance level of 0.05 using exact p values. Analyses were performed using GraphPad Prism V.8.4.3 (GraphPad Software, San Diego, California, USA).
Results
RNA
Twenty-six acutely ill patients with COVID-19 were included of which 20 were men (age Mdn 61 years, IQR 48–68) and six were women (age Mdn 53 years, IQR 47–74). SARS-CoV-2 was detected in tear fluid by RT-PCR in seven patients (26.9%). According to WHO categorisation, the included patients suffered from mild (n=3), moderate (n=21) or severe disease (n=2) (table 1).19 The median Cp value of the E-gene in positive tear fluids was 31.4 (IQR 28.7–32.0) and S-gene 30.8 (IQR 27.9–31.3). None of the patients had symptoms of ocular infection. No significant difference (p=0.3881) was observed in the E-gene value in nasopharyngeal swabs of patients with a positive result in tears (Mdn 18.7, IQR 17.3–25.8) compared with those without detection of SARS-CoV-2 in tears (Mdn 24.7, IQR 19.4–28.6). No significant difference (p=0.3068) was observed in the duration between onset of symptoms and tear sampling of patients with a positive result in tears (days Mdn 4, IQR 2.5–7) compared with those without detection of SARS-CoV-2 in tears (days Mdn 6, IQR 3.5–12).
Table 1.
Crossing point (Cp), respectively cycle threshold (Ct) values by RealStar (targeting E-gene and S-gene) and Xpert (targeting E-gene and N2-gene) analysis for SARS-CoV-2 in nasopharyngeal swabs and tear fluids. COVID-19 disease severity according to WHO categorisation and timing of tear sample collection in the patient's disease course.
| Nasopharyngeal swab | Tear fluid | COVID-19 disease severity | Timing of tear sampling in disease course (days after onset of symptoms*) |
||||
| E-gene | S-gene | N2-gene | E-gene | S-gene | |||
| Patient 1 | 20.55 | 20.09 | NA | – | – | Moderate | 8 |
| Patient 2 | 23.30 | 22.89 | NA | – | – | Moderate | 3 |
| Patient 3 | 16.98 | 16.59 | NA | – | – | Moderate | 14 |
| Patient 4 | 15.53 | 14.82 | NA | 34.57 | 33.92 | Moderate | 3 |
| Patient 5 | 28.50 | NA | 31.40 | – | – | Moderate | 20 |
| Patient 6 | 32.21 | 31.60 | NA | – | – | Moderate | 14 |
| Patient 7 | 28.79 | 28.46 | NA | – | – | Moderate | 2 |
| Patient 8 | 31.09 | 30.45 | NA | – | – | Moderate | 14 |
| Patient 9 | 28.02 | 27.58 | NA | – | – | Severe | 5 |
| Patient 10 | 21.95 | 21.49 | NA | – | – | Moderate | 2 |
| Patient 11 | 32.46 | 31.57 | NA | – | – | Severe | 6 |
| Patient 12 | 24.70 | 24.16 | NA | – | – | Moderate | 3 |
| Patient 13 | 29.20 | NA | 32.00 | 31.36 | 30.86 | Moderate | 14 |
| Patient 14 | 17.70 | NA | 20.20 | – | – | Moderate | 4 |
| Patient 15 | 18.50 | NA | 20.90 | – | – | Moderate | 10 |
| Patient 16 | 18.27 | 17.61 | NA | – | – | Moderate | 6 |
| Patient 17 | 20.30 | NA | 22.50 | – | – | Moderate | 7 |
| Patient 18 | 26.20 | NA | 29.10 | – | – | Moderate | 4 |
| Patient 19 | 13.40 | NA | 15.50 | – | – | Mild | 3 |
| Patient 20 | 18.70 | 18.12 | NA | 17.82 | 17.99 | Moderate | 6 |
| Patient 21 | 22.36 | 22.02 | NA | 31.73 | 30.76 | Moderate | 8 |
| Patient 22 | 32.98 | 32.27 | NA | 28.44 | 27.26 | Mild | 1 |
| Patient 23 | 17.68 | 16.98 | NA | 28.90 | 28.53 | Mild | 2 |
| Patient 24 | 26.20 | NA | 28.80 | – | – | Moderate | 6 |
| Patient 25 | 16.90 | 16.29 | NA | 32.19 | 31.64 | Moderate | 4 |
| Patient 26 | 32.72 | 31.57 | NA | – | – | Moderate | 14 |
*COVID-19 associated symptoms as defined by the WHO (eg, fever, cough, fatigue, anorexia, shortness of breath, myalgia, anosmia and ageusia).
NA, not available.
Antibodies
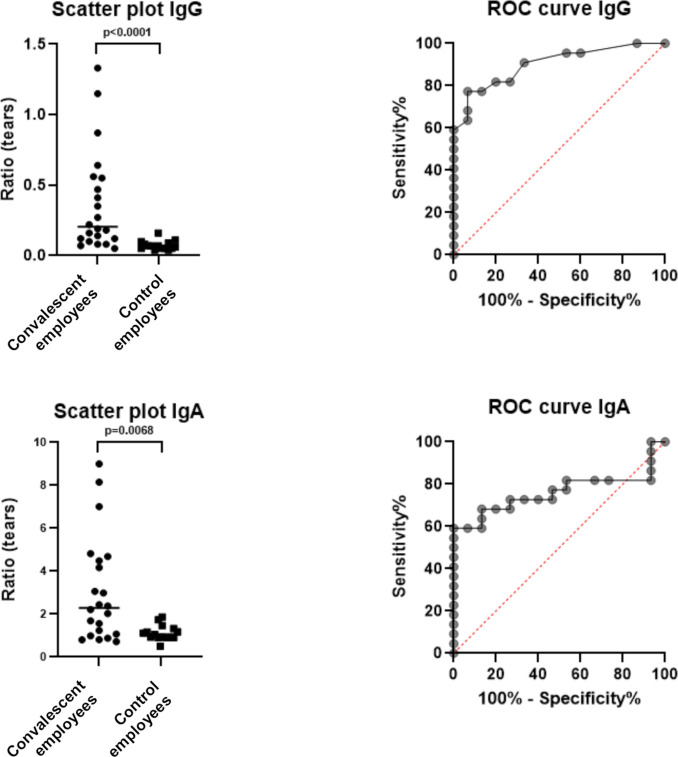
The control cohort consisted of 15 employees of our hospital of which two were men (age 31 and 39 years) and 13 women (age Mdn 35 years, IQR 31–59). The IgG positive cohort consisted of 22 convalescent employees of which 10 were men (age Mdn 52, IQR 30–60) and 12 women (age Mdn 37, IQR 27–52). The IgA positive cohort predominantly corresponded to the IgG cohort, consisting of 22 convalescent employees as well of which 10 were men (age Mdn 52, IQR 37–60) and 12 women (age Mdn 37, IQR 27–52). Compared with controls, convalescent employees displayed a significantly higher ratio of anti-SARS-CoV-2 IgG (p<0.0001) and IgA (p=0.0068) in tear fluid (figure 1; online supplemental tables 1–3). The diagnostic accuracy for IgG in tears was high (AUC=0.902) and for IgA moderate (AUC=0.761), with a sensitivity of 77.3% and specificity of 93.3% for IgG and a sensitivity of 59.1% and specificity of 100% for IgA, yielding the optimal cut-offs for detecting positive IgG and IgA cases above 0.115 and 1.95, respectively (figure 1).
Figure 1.
Left: scatter plots of anti-SARS-CoV-2 IgG and IgA ratios in tear fluid, indicating the median by a horizontal line and p value between convalescent (n=22) and control employees (n=15). Right: ROC curves of anti-SARS-CoV-2 IgG and IgA ratios in tear fluid of convalescent and control employees. ROC, receiver operating characteristic.
bmjophth-2021-000733supp001.pdf (94.8KB, pdf)
bmjophth-2021-000733supp002.pdf (94.8KB, pdf)
bmjophth-2021-000733supp003.pdf (94.7KB, pdf)
Four employees without IgG nor IgA antibodies in serum at the moment of tear sampling, but with known IgG antibodies in serum 2–3 months earlier were excluded. Interestingly, two of these tear fluid samples showed a positive result for IgA based on the above cut-off; IgG was negative in all four tear fluids.
Discussion
We investigated the prevalence of both SARS-CoV-2 RNA and antibodies in tear fluid.
Viral RNA was detected in tears of 26.9% (7/26) enrolled patients with COVID-19. Previous studies have shown varying results, detecting viral RNA mostly in less than 10% of patients, except for one study by Arora et al10 showing a positivity rate of 24%.3 5–10 In two large case series including 64 ocular samples from 17 patients (taken at different time points in the disease course), respectively 114 ocular samples (from as many patients), no SARS-CoV-2 could be detected.3 8 In three studies performed at Chinese hospitals, positivity rates of 3.3% (1/30), 5.3% (2/38) and 8.6% (3/35) were observed for tears in patients with COVID-19.5 6 9 Several factors can be responsible for the observed differences. We opted for Schirmer test strips instead of glass capillaries or conjunctival swabs as most convenient clinician and patient friendly tear sampling tool.20 The advantage of glass capillary tubes is that it does not induce local irritation and minimises transudation; however, it can be used only by trained personnel in cooperative patients.21 Schirmer strips are a non-invasive body fluid collection method commonly used in ophthalmological practice for testing the severity of dry eyes. The sampling of tears by Schirmer strips is highly accepted by patients in the primary healthcare setting as well.22 Twenty to twenty-five microlitres of tear fluid can be easily collected by these strips; however, the exact volume cannot be determined which impairs standardisation.21 Yan et al5 compared sampling by conjunctival swabbing and Schirmer strips, suggesting the positivity rate may be higher by using the latter; however, the difference was not statistically significant. The authors assume that a larger volume can be collected by Schirmer strips, resulting in a higher sensitivity of RNA detection. In the study of Arora et al,10 on the other hand, conjunctival swabbing appeared to be the best sampling method in comparison with Schirmer strips, although not significantly either. Thus, the sampling method probably cannot explain the observed differences in positivity rates; however, our study confirms the feasibility of sampling by Schirmer strips. Another inconsistency between published studies is the sampling of one or both eyes. In the study of Seah et al,3 samples from both eyes were analysed separately, and no SARS-CoV-2 RNA was detected in any tear sample. In studies with relatively high positivity rates, bilateral tear collection was performed.5 10 Our results confirm the importance of bilateral sampling to increase the sample volume and thus the diagnostic sensitivity. Furthermore, by adding the Schirmer strips directly from a dry tube to the lysis buffer used for extraction instead of collecting them in viral transport medium, additional dilution is avoided with possibly a beneficial effect on sensitivity. Finally, it is suggested that the time of sampling in the disease course may affect the results, but this has not been studied thoroughly yet.3 7 10 In our study, no significant difference in duration of COVID-19 symptoms was observed in patients with detection of SARS-CoV-2 in tears compared with those without.
There are few reports on the association of SARS-CoV-2 infection with ocular abnormalities.3 6 9 In a case series of 38 patients with COVID-19 studied by Wu et al,9 12 patients (31.6%) had ocular manifestations consistent with conjunctivitis. Viral RNA was detected in conjunctival swabs of two patients, both having ocular abnormalities.9 The presence of SARS-CoV-2 RNA on the ocular surface and ocular manifestations was studied in 29 hospitalised patients with COVID-19 by Meduri et al.23 RNA was not detected in any of the tear samples by RT-qPCR on Schirmer strips, in spite of a high prevalence of mild ocular symptoms (eye burning: n=4; foreign body sensation: n=3; tearing: n=3). Furthermore, mild conjunctival hyperaemia and/or chemosis (n=7) and blepharitis signs (n=11) were often observed. However, the presence of these ocular signs and symptoms being common in the general population was not checked prior to the onset of COVID-19 impeding the assessment of the correlation with COVID-19.23 Among three patients with a positive tear sample result studied by Karimi et al,7 only one patient had symptoms of conjunctivitis. No ocular abnormalities were observed in any patient with COVID-19 included in other studies concerning the ocular involvement, as was the case in our study.5 8 10 This implies that viral shedding in tears and thus infectivity is not related to ocular manifestation of infection.
As SARS-CoV-2 is a highly contagious virus, extrapulmonary routes of transmission and infection need to be investigated to mitigate the pandemic spread.24 SARS-CoV-2 gains entry to host cells via the ACE-2 receptor, and this receptor has been identified in the eye, explaining the ocular tropism of the virus.8 11 25–27 The eye and its adnexae represent a relatively large surface directly exposed to airborne viral particles and contaminated hands, which may thus serve as a portal of entry.11 24 26 27 The nasolacrimal system provides an anatomical bridge between the ocular and respiratory systems facilitating viral movement from tear fluid via the inferior meatus of the nose to the respiratory tract.11 Furthermore, the involvement of the eye besides symptoms such as dysgeusia, dysosmia, xerostomia and auditory discomfort in patients with COVID-19 may be a sign of the neurotropism of SARS-CoV-2 as is the case for other coronaviruses.28–30
Our results showing the presence of viral RNA in tears are confirming that the eye constitutes a site of virus replication and a possible reservoir for person-to-person transmission.4 11 24 25 These findings provide important insights in understanding infection and transmission. The importance of personal protective gear including good eye protection with goggles in addition to masks, gowns and gloves is highlighted.4 6 8 10 11 24 25 27 31
Besides mechanical and anatomical barriers against infection, the eye also possesses immunological defence mechanisms.8 25 The local mucosal response with antibody production is an important immune defence, and IgA is believed to be the major immunoglobulin in mucosal secretions.17 25 32 33 It is stated by Chao et al15 that secretory IgA can neutralise SARS-CoV-2 before it reaches and binds the epithelial cells. The immunoglobulin concentrations in tears from patients with different ocular diseases was determined by Sen and Sarin,34 showing that IgA was the predominant immunoglobulin, IgG could be detected in the majority of samples, IgM in a fewer. Viral antibodies in tears against cytomegalovirus, Epstein-Barr virus, herpes simplex virus (HSV) type I, varicella zoster virus, mumps and rubella were shown in previous studies, being mainly IgA class, but IgG antibodies were also detected.17 35 36 In a study by Hu et al,16 antibodies (predominantly IgA and IgG classes) against papillomavirus were demonstrated in tears, being 10-fold lower than in the corresponding serum samples but with a lower background noise. Given the presence of SARS-CoV-2 RNA in tears, it is reasonable to hypothesise that, like other viruses, the ocular surface serves as an initiation site of immune response with production of antibodies.37 However, no in-depth investigation has yet been performed determining the presence of SARS-CoV-2 antibodies in tear fluid. Our data are promising, showing the significant presence of anti-SARS-CoV-2 IgA as well as IgG antibodies in tear fluid of convalescent individuals compared with control individuals. This is a proof of the role of the eye as a first defence against SARS-CoV-2. Moreover, tears may serve as a surrogate for serum in monitoring the host immune response in epidemiological studies as well as in clinical practice. We have found only one study from the University Hospital of Zurich determining anti-SARS-CoV-2 IgA and IgG in tear fluid; however, no cut-off was established to determine the exact prevalence of both antibodies.18 Interestingly, we observed IgA antibodies in tears from two employees with a previous COVID-19, although at the time of tear sampling, no IgG nor IgA antibodies were detected in serum. In the study by Cervia et al,18 this phenomenon was observed as well: some of the SARS-CoV-2 exposed healthcare workers with negative SARS-CoV-2 specific IgA and IgG serum titres had detectable IgA in tears. In addition, in the above-mentioned study by Coyle and Sibony,17 15% of tears had antibodies to HSV-1 without detectable serum antibodies. In this study, tear viral antibodies were most often reflected in serum, although the immunoglobulin class differed sometimes. Performing a follow-up study determining ocular anti-SARS-CoV-2 IgA secretion in individuals with transient serum titres would be of great interest. Another interesting path to investigate is the association between total IgA, specific secretory IgA and viral RNA in tear fluid. Since IgA production is important as local immune defence, detecting IgA deficiency may probably play a role in identifying super spreaders.
Further research is necessary considering important parameters of antibody analysis in tears, such as the collection method and immunoassay used.16 21 We opted for Schirmer strips in accordance to the study of viral RNA. Importantly, we eluted the test strips immediately in the sample buffer used for the immunoassay minimising dilution. To the best of our knowledge, no commercial immunoassay has been approved for SARS-CoV-2 antibody testing in tear fluid yet. We opted for off-label use of the anti-SARS-CoV-2 ELISA from EUROIMMUN. However, further research including more tear fluid samples is needed to determine an optimal cut-off using this assay.
Our results demonstrating the presence of SARS-CoV-2 antibodies in tear fluid may be of great value in the diagnostic work-up of COVID-19. In contrast to the collection of blood samples, no trained personnel is required for collection of tears by Schirmer strips, and it causes no risk to patients making it an interesting tool in the development of a point-of-care test for monitoring the host immune response.16 22 Furthermore, these findings can be an aid in the search for a therapeutic strategy. Mucosal vaccination can induce local antibody secretion and thus local protective immunity within the mucosae, being the portal of entry.15
This study should be considered as a pilot study, and a follow-up study is needed to confirm the results and elaborate on the limitations. The main limitation of our study is the small sample size; our findings of SARS-CoV-2 RNA and antibodies in tear fluid should be validated in a larger study population including an evaluation of the correlation with COVID-19 disease severity and ocular symptoms. Moreover, only one tear fluid sample per patient was obtained in this study, but it would be interesting to collect multiple samples at different time points in the disease course to assess the kinetics of the viral load and antibodies in tears.
In conclusion, we observed SARS-CoV-2 RNA in tear fluid of 26.9% acutely ill patients with COVID-19 confirming the possibility of transmission through tear fluid, even in absence of ocular manifestations. These findings emphasise the need for protective eyewear by healthcare workers. However, because of the rather low detection rate, Schirmer test strips cannot be introduced as an alternative for nasopharyngeal swabbing in the diagnosis of COVID-19 so far.
The detection of SARS-CoV-2 IgG and IgA antibodies in tear fluid in our study is promising. It is a proof of the role of the eye as a first defence against SARS-CoV-2. Furthermore, tears may serve as a surrogate for serum in monitoring the host immune response in epidemiological studies as well as in clinical practice where the specimen can be taken by the patient himself.
Acknowledgments
The authors would like to thank the collaborators of the Department of Internal Medicine and Infectious Diseases for obtaining informed consents and sample collection. We would like to thank the lab technicians of the Department of Microbiology and Infection Control and the Laboratory of Neurochemistry for the specimen processing. This research has been conducted using the Central Biobank UZ Brussel.
Footnotes
Contributors: AM: design of the study, patient sampling, data analysis and interpretation, and writing of the manuscript. MB: data analysis and interpretation, and critical revision of the manuscript. TD and DDG: patient sampling and critical revision of the manuscript. IWy, IWe, RK and SDA: critical revision of the manuscript. OS: data analysis and critical revision of the manuscript. DP and PPMR: design of the study and critical revision of the manuscript.
Funding: Funding for this research was provided by the UZ Brussel Foundation.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical approval was obtained from the Medical Ethics Committee of UZ Brussel (B1432020000158 (RNA), B1432020000175 (antibodies)).
References
- 1.Zhou L, Xu Z, Castiglione GM, et al. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf 2020;18:537–44. 10.1016/j.jtos.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collin J, Queen R, Zerti D, et al. Co-Expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul Surf 2021;19:190–200. 10.1016/j.jtos.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology 2020;127:977–9. 10.1016/j.ophtha.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu C-W, Liu X-F, Jia Z-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet 2020;395:e39. 10.1016/S0140-6736(20)30313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Y, Zeng B, Zhang Z, et al. Detection of SARS-CoV-2 in simultaneously collected tear and throat swab samples from the patients with 2019- new SARS-CoV-2 infection disease: a single center cross-sectional study. Ophthalmic Epidemiol 2021:1–7. 10.1080/09286586.2021.1875011 [DOI] [PubMed] [Google Scholar]
- 6.Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol 2020;92:589–94. 10.1002/jmv.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimi S, Arabi A, Shahraki T, et al. Detection of severe acute respiratory syndrome Coronavirus-2 in the tears of patients with coronavirus disease 2019. Eye 2020;34:1220–3. 10.1038/s41433-020-0965-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng C, Yang Y, Chen H. Ocular detection of SARS-CoV-2 in 114 cases of COVID-19 pneumonia in Wuhan, China: an observational study. SSRNPublished Online First: 27 February 2020. [Google Scholar]
- 9.Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol 2020;138:575–8. 10.1001/jamaophthalmol.2020.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora R, Goel R, Kumar S, et al. Evaluation of SARS-CoV-2 in tears of patients with moderate to severe COVID-19. Ophthalmology 2021;128:494–503. 10.1016/j.ophtha.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Yu H, Mei T, et al. SARS-CoV-2 on the ocular surface: is it truly a novel transmission route? Br J Ophthalmol 2020:bjophthalmol-2020-316263. 10.1136/bjophthalmol-2020-316263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett BP, Wahlin K, Krawczyk M, et al. Potential of ocular transmission of SARS-CoV-2: a review. Vision 2020;4. 10.3390/vision4030040. [Epub ahead of print: 01 Sep 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Zhou B, Li M, et al. Serology of severe acute respiratory syndrome: implications for surveillance and outcome. J Infect Dis 2004;189:1158–63. 10.1086/380397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao L, Deng W, Gao H. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. bioRxiv 2020. [Google Scholar]
- 15.Chao YX, Rötzschke O, Tan E-K. The role of IgA in COVID-19. Brain Behav Immun 2020;87:182–3. 10.1016/j.bbi.2020.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Brendle S, Balogh K, et al. Antibody detection in tear samples as a surrogate to monitor host immunity against papillomavirus infections in vaccinated and naturally infected hosts. J Gen Virol 2014;95:2030–7. 10.1099/vir.0.064154-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyle PK, Sibony PA. Viral antibodies in normal tears. Invest Ophthalmol Vis Sci 1988;29:1552–8. [PubMed] [Google Scholar]
- 18.Cervia C, Nilsson J, Zurbuchen Y, et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol 2021;147:e9:545–57. 10.1016/j.jaci.2020.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . COVID-19 clinical management: living guidance, 2021. Available: https://apps.who.int/iris/handle/10665/338882 [Accessed 25 Jan 2021].
- 20.Raus P, Kumar BR, Pinkse M, et al. Bottom–up protein identifications from microliter quantities of individual human tear samples. important steps towards clinical relevance. EuPA Open Proteomics 2015;9:8–13. 10.1016/j.euprot.2015.06.005 [DOI] [Google Scholar]
- 21.Friedman MG. Antibodies in human tears during and after infection. Surv Ophthalmol 1990;35:151–7. 10.1016/0039-6257(90)90070-C [DOI] [PubMed] [Google Scholar]
- 22.Quah JHM, Tong L, Barbier S. Patient acceptability of tear collection in the primary healthcare setting. Optom Vis Sci 2014;91:452–8. 10.1097/OPX.0000000000000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meduri A, Oliverio GW, Mancuso G, et al. Ocular surface manifestation of COVID-19 and tear film analysis. Sci Rep 2020;10:20178. 10.1038/s41598-020-77194-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui KPY, Cheung M-C, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med 2020;8:687–95. 10.1016/S2213-2600(20)30193-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Güemes-Villahoz N, Burgos-Blasco B, Vidal-Villegas B, et al. Novel insights into the transmission of SARS-CoV-2 through the ocular surface and its detection in tears and conjunctival secretions: a review. Adv Ther 2020;37:4086–95. 10.1007/s12325-020-01442-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coroneo MT. The eye as the discrete but defensible portal of coronavirus infection. Ocul Surf 2021;19:176–82. 10.1016/j.jtos.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel KP, Vunnam SR, Patel PA, et al. Transmission of SARS-CoV-2: an update of current literature. Eur J Clin Microbiol Infect Dis 2020;39:2005–11. 10.1007/s10096-020-03961-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freni F, Meduri A, Gazia F, et al. Symptomatology in head and neck district in coronavirus disease (COVID-19): a possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol 2020;41:102612. 10.1016/j.amjoto.2020.102612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohmwald K, Gálvez NMS, Ríos M, et al. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci 2018;12:386. 10.3389/fncel.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2019;12. 10.3390/v12010014. [Epub ahead of print: 20 12 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J-PO, Lam DSC, Chen Y, et al. Novel coronavirus disease 2019 (COVID-19): the importance of recognising possible early ocular manifestation and using protective eyewear. Br J Ophthalmol 2020;104:297–8. 10.1136/bjophthalmol-2020-315994 [DOI] [PubMed] [Google Scholar]
- 32.Burns CA, Ebersole JL, Allansmith MR. Immunoglobulin A antibody levels in human tears, saliva, and serum. Infect Immun 1982;36:1019–22. 10.1128/IAI.36.3.1019-1022.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.TOMASI TB, Zigelbaum S. The selective occurence of GAMMA-1A globulins in certain body fluids. J Clin Invest 1963;42:1552–60. 10.1172/JCI104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen DK, Sarin GS. Immunoglobulin concentrations in human tears in ocular diseases. Br J Ophthalmol 1979;63:297–300. 10.1136/bjo.63.5.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox PD, Khaw PT, McBride BW, et al. Tear and serum antibody levels in ocular herpetic infection: diagnostic precision of secretory IgA. Br J Ophthalmol 1986;70:584–8. 10.1136/bjo.70.8.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozanova EB, Teplinskaia LE, Kaliberdina AF, et al. Cytomegalovirus antibodies in tear fluid of patients with retinitis. Arch Virol 2006;151:2407–17. 10.1007/s00705-006-0813-0 [DOI] [PubMed] [Google Scholar]
- 37.Isho B, Abe KT, Zuo M, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol 2020;5. 10.1126/sciimmunol.abe5511. [Epub ahead of print: 08 10 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2021-000733supp001.pdf (94.8KB, pdf)
bmjophth-2021-000733supp002.pdf (94.8KB, pdf)
bmjophth-2021-000733supp003.pdf (94.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.