Abstract
Background:
Antiepileptic drugs (AEDs) are commonly used by nursing home residents, both on and off label. The landscape of AED use has changed over the past two decades. Despite this, contemporaneous research on AED use in US nursing home residents is scant.
Objective:
To estimate the prevalence, describe prescribing patterns, identify factors associated with AED use, and assess whether these factors differ among AEDs with expanded indications in older adults (i.e., gabapentin, pregabalin, topiramate, and lamotrigine).
Methods:
We conducted a cross- sectional study among 549,240 long stay older residents who enrolled in fee- for- service Medicare and lived in 15,111 US nursing homes on September 1, 2016. Demographics and conditions associated with AED indications, epilepsy comorbidities, and safety came from the Minimum Data (MDS 3.0). Part D claims identified AED use. Robust Poisson models and multinomial logistic models for clustered data estimated adjusted prevalence ratios (aPR), adjusted odds ratios (aOR) and 95% confidence intervals (CI).
Results:
Overall, 24.0% used AEDs [gabapentin (13.3%), levetiracetam (4.7%) phenytoin (1.9%), pregabalin (1.8%), and lamotrigine (1.2%)]. AED use was associated with epilepsy (aPR = 3.73; 95% CI = 3.69–3.77), bipolar disorder (aPR = 1.20; 95% CI = 1.18–1.22), pain (aPRmoderate/ severe vs. no pain =1.42; 95% CI = 1.40–1.44), diabetes (aPR = 1.27; 95% CI = 1.26– 1.28), anxiety (aPR = 1.12; 95% CI = 1.11– 1.13), depression (aPR = 1.17; 95% CI = 1.15– 1.18), or stroke (aPR = 1.08; 95% CI = 1.06– 1.09). Residents with advancing age (aPR85+ vs. 65–74 years = 0.73; 95% CI= 0.73– 0.74), Alzheimer’s disease/ dementia (aPR = 0.87; 95% CI = 0.86– 0.88), or cognitive impairment (aPRsevere vs. no impairment = 0.62; 95% CI = 0.61– 0.63) had decreased AED use. Gabapentinoid use was highly associated with pain (aORmoderate/severe vs. no pain = 2.07; 95% CI = 2.01– 2.12) and diabetes (aOR = 1.79; 95% CI = 1.76– 1.82), but not with an epilepsy indication.
Conclusions:
AED use was common in nursing homes, with gabapentin most commonly used (presumably for pain). That multiple comorbidities were associated with AED use underscores the need for future studies to investigate the safety and effectiveness of AED use in nursing homes residents.
1. INTRODUCTION
From 1993 to 2016, the US Food and Drug Administration (FDA) approved 18 antiepileptic drugs (AEDs) [1–2] and expanded labeled indications for some AEDs (e.g., gabapentin and pregabalin for postherpetic neuralgia, topiramate for migraines, and lamotrigine for bipolar disorder) [3–4]. In nursing homes, AED use has increased and prescribing patterns have likely changed. In the 1990s, the prevalence of AEDs was approximately 10% in US nursing homes [5–7], with phenytoin, a first- generation AED, most commonly prescribed [5]. By 2013, the prevalence of AEDs in nursing homes in two states increased to 14.3% with gabapentin most commonly prescribed [8]. In an Italian nursing home, 32% used AEDs [48].Recent nationwide data regarding AED use in nursing home residents are lacking.
Historic data showed that factors associated with increased AED use in older nursing home residents include younger age, epilepsy, bipolar disorder, peripheral vascular disease and geographic area with greater use noted in the Northeast [5,9]. Given the newer AEDs available in the US, the expansion of indications, and lack of clinical guidelines navigating AED therapy in the nursing home setting, these findings are unlikely relevant to contemporary clinical practice. Because AED use may carry risks such as falls and fractures, adverse cognitive effects, and drug- drug interactions [10–17], a contemporary understanding of how AEDs are being used in nursing homes is warranted.
Using a contemporary national database of all US long stay older nursing home residents enrolled in fee- for- service Medicare, this study sought to estimate the prevalence, describe prescribing patterns, identify factors associated with AED use, and assess whether these factors differ among AEDs with expanded indications in older adults (i.e., gabapentin, pregabalin, topiramate, and lamotrigine). To our knowledge, this is the first nationwide study to assess the pattern of AED in the US nursing home settings since 1999.
2. METHODS
2.1. Data Sources
We linked three data sources including Minimum Data Set 3.0 (MDS 3.0, 2016), Master Beneficiary Summary File, and Medicare Part D pharmacy claims by residents’ unique beneficiary IDs. The MDS 3.0 is a standardized clinical assessment tool used on all residents of US Medicare and/ or Medicaid- certified nursing homes (over 96% of all US nursing homes). Comprehensive assessments are performed within 7 days of nursing home admission, every year, and whenever there are significant changes in residents’ health conditions [18]. An abbreviated assessment is administered quarterly [18]. During the assessment process, registered nurses conduct interviews with residents to collect information on residents’ demography and health conditions including comorbidities, functional status, and mood [19–20]. The MDS 3.0 items are valid and reliable capturing information on residents’ demographics and health conditions [19].
2.2. Study Design
We conducted a cross- sectional study among older adults who resided in US nursing homes on September 1, 2016 (the index date). Given the lack of seasonality in AED use [8], the point prevalence estimated on this date is intended to be representative of 2016 AED usage and patterns.
2.3. Study Sample
The inclusion criteria were: 1) residing in US nursing homes on the index date, i.e., September 1, 2016; 2) having an admission, annual, or quarterly MDS assessment between June 1, 2016 and September 1, 2016; 3) age ≥ 65 years. The exclusion criteria were: 1) in comatose state; 2) with skilled nursing facility stay; and 3) not continuously enrolled in Medicare Part D in June, July, August, and September, 2016. We excluded residents in a comatose state because the prescribing patterns for this population should be different from general nursing home residents. Medications are bundled into the per diem payment for skilled nursing facility stays. We excluded these residents because medication claims were not billed to Medicare Part D claims. If multiple assessments were identified within the 90- day time window, we used information in the assessment closest to the index date.
2.4. Measurements of Antiepileptic Drug Use
There were 27 AEDs available in the US market in September 2016, including 9 first- generation, 9 second- generation, and 9 third- generation AEDs (Supplemental Table 1 in Online Resource 1). Gabapentin, pregabalin, lamotrigine, and topiramate have expanded indications in older adults (Supplemental Table 1 in Online Resource 1). This classification of AEDs was based on guidelines developed by the American Academy of Neurology (AAN) [1–2], prior research [21], and year of initial US approval. AED use was determined based on Medicare Part D claims. Specifically, if residents had an AED claim on the index date, or if the prescription fill date plus day supply covered the index date, the resident was classified as an AED user. We created 27 binary variables to capture use of each AED on the index date. Next, four binary variables were created to indicate the use of first- generation, second-generation, third- generation, or any AEDs, respectively. A five- level categorical outcome variable was created to indicate the use of AEDs with expanded indications in older adults (gabapentinoids (i.e. gabapentin and pregabalin), topiramate, lamotrigine, other AEDs, or no AED use). The primary outcome variable was any AED use on the index date and the secondary one was use of AEDs with expanded indications.
2.5. Measurements of Covariates
Based on literature [5,9], we developed a list of covariates: demographic variables, conditions associated with AED indications, conditions associated with epilepsy comorbidities, and conditions associated with AED safety. The details of covariate assessments in MDS 3.0 were included in Supplemental Methods in Online Resource 1.
2.5.1. Demographic variables
Age was categorized into three groups, i.e., 65– 74, 75– 84, and ≥85 years. Gender was based on self- report and if needed, registered nurses assisted in correctly identifying gender of residents as men or women [18]. We collapsed some categories of race/ ethnicity in the MDS 3.0 assessments and created a four- category variable of race/ ethnicity, i.e., non- Hispanic white, non- Hispanic black, Hispanic, and others due to small number of respondents in some individual categories. Geographic area was determined based on locations of nursing home facilities, and categorized into West, Midwest, Northeast, and South according to US Census Divisions and Regions [22].
2.5.2. Conditions associated with AED indications/ contradictions
An active clinical diagnosis of epilepsy and bipolar disorder was defined based on Section I of the MDS 3.0 [3–4]. Pain was considered as a potentially appropriate use for antiepileptic drugs. Pain was assessed by the Pain Assessment Interview in the MDS 3.0, with both self- assessed and staff- assessed pain [18], from which we derived a three- level pain variable (no pain, mild/ infrequent pain, and moderate/ severe pain) [23–26]. We did not include other expanded indications of AEDs (Supplemental Table 1 in Online Resource 1) because these variables were not included as part of the MDS 3.0.
Hepatic or renal insufficiency/ failure can be potential contraindications for AED use in older nursing home residents. Hepatic insufficiency/failure was not assessed in MDS 3.0. Renal insufficiency/ failure was assessed but had a large proportion of missing responses. As such, we did not include hepatic or renal insufficiency/ failure as potential contraindications for AED use.
2.5.3. Conditions associated with epilepsy comorbidities
The following conditions often co-occur with epilepsy [5,27]: diabetes, anxiety, depression, stroke, Alzheimer’s disease/ dementia, peripheral vascular disease (PVD) and arthritis. All variables were binary, indicating the presence of the condition. Cognitive impairment was also included as a validated four- level categorical variable (i.e., no impairment/ intact, mild impairment, moderate impairment, and severe impairment) based on items in Brief Interview for Mental Status (BIMS) and Cognitive Performance Scale (CPS) [28].
2.5.4. Conditions associated with AED safety
The following conditions have been associated with AED safety [10–16]. Fracture was coded as a binary variable indicating the presence of any fractures. Physical functioning was determined from the validated MDS Activities of Daily Living (ADL) Self- Performance Hierarchy Scale, and categorized to no/ mild physical functioning impairment, moderate impairment, and severe impairment [29].
2.6. Statistical Analysis
We estimated the prevalence of AED use, overall, by generation and by individual agent. Then, we described the study sample by calculating proportions for categorical variables stratified by AED use. To examine AED prescribing patterns, we reported the five most commonly prescribed AEDs. Additional analyses were conducted to provide more detail on AED use including AEDs with expanded indications in older adults (i.e., gabapentin, pregabalin, topiramate, and lamotrigine), and proportions of residents with concurrent epilepsy who were treated with AEDs. To explore the factors associated with AED use, we first selected covariates whose prevalence differed among AED users and non- users by 5% or more (i.e., age, epilepsy, pain, diabetes, anxiety, depression, Alzheimer’s disease/ dementia, and cognitive impairment). Next, we included all demographic variables (age, gender and race), FDA- approved indications for some AEDs (epilepsy and bipolar disorder), and stroke. Stroke was included because it most frequently causes new- onset seizure in older adults [30]. Before entering covariates in the models, we calculated Cramer’s V coefficients and variance inflation factor (VIF) to assess pairwise correlation and overall multicollinearity of covariates, respectively. Cramer’s V was chosen over χ2 statistics in that we had a very large sample size, and χ2 test is likely to return all statistically significant results considering its test sensitivity. Covariates were considered highly correlated if their Cramer’s V coefficient equaled 0.5 or larger [31], but none met this threshold. VIFs for all covariates were less than 1.5, indicating multicollinearity was unlikely present. Lastly, we conducted two multivariable regression models including all covariates selected. To examine factors associated with any AED use, we conducted extensions of robust Poisson regression which estimated prevalence ratios (aPR) and 95% confidence intervals (CI) [32–33]. To identify factors associated with the use of AEDs with expanded indications in older adults, we performed multinomial logistic regression for clustered data which estimated odds ratios (aOR) and 95% confidence intervals (CI) [34]. The odds ratios (ORs) should approximate prevalence ratios (PRs) due to rare outcomes (Supplemental Table 2 in Online Resource 1). Both models accounted for the clustering of residents within nursing home facilities by using a generalized estimating equation (GEE) method and applied a sandwich variance estimator with an independent correlation structure. All statistical analyses were conducted in SAS, version 9.4 (SAS Institute, Inc., Cary, NC).
3. RESULTS
3.1. Prevalence and Prescribing Patterns of Antiepileptic Drugs
Our final study sample consisted of 549, 240 long stay residents who lived in 15,111 nursing homes (Figure 1). Overall, the prevalence of AED use was 24.0%, of which 81.6% were second- generation AED agents and 21.8% were first- generation AEDs. Gabapentin (13.3%), levetiracetam (4.7%), phenytoin (1.9%), pregabalin (1.8%), and lamotrigine (1.2%) were most commonly prescribed (Supplemental Table 2 in Online Resource 1). AEDs with expanded indications in older adults were used by 68.2% AED users. For gabapentin or pregabalin users, approximately half were in pain and one in ten had a diagnosis of epilepsy. About 38.3% of topiramate users had documented pain and 36.7% had epilepsy. Approximately 29.8% of lamotrigine users had bipolar disorder and 41.1% had a diagnosis of epilepsy. About one- third of residents with epilepsy were not treated with any AEDs, and this finding was consistent across age groups.
Figure 1.
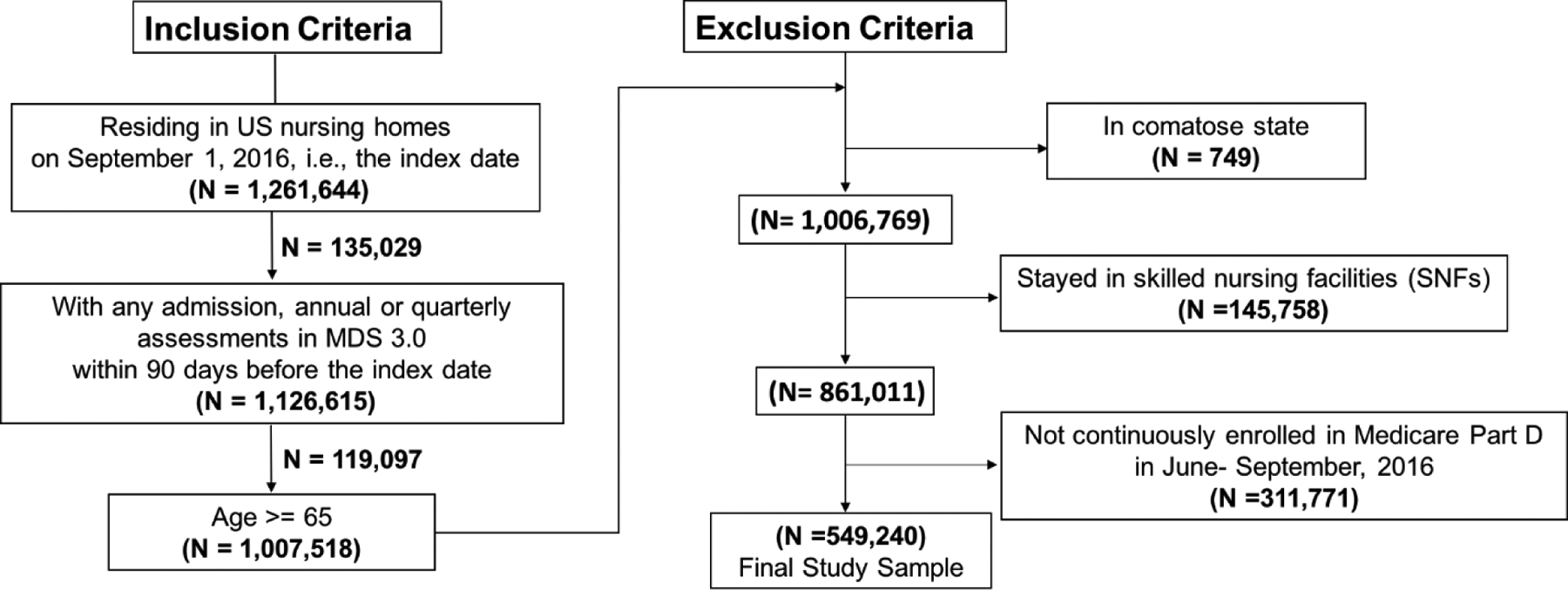
Study Sample Selection
3.2. Characteristics of the Sample by AED Use
The majority of residents were 75 years and older, women, and non- Hispanic white (Table 1). Overall, 9.6% of residents had diagnoses of epilepsy, 4.7% of residents had bipolar disorders, and 24.3% reported pain. Among AED users, 29.9% had epilepsy compared to 3.4% of non- users (Table 1). About 11.2% residents had stroke. Approximately 72.7% residents had cognitive impairment, and similar proportion of residents comorbid with Alzheimer’s disease or dementia. Compared to 65.8% non- users with Alzheimer’s disease or dementia, 51.7% AED users had the disease. Mood disorders were also prevalent, with 32.2% of residents having a diagnosis of anxiety, and 53.0% comorbid with depression Diabetes was prevalent among 41.7% AED users and 30.2% non- users (Table 1).
Table 1.
Characteristics of Residents in US Nursing Homes on September 1, 2016, Stratified by Antiepileptic Drug Use (n = 549,240)
| Characteristics | Antiepileptic Drug User (n =128,228) |
Non-User (n =421,012) |
|---|---|---|
| Age in years | ||
| 65– 74 | 31.5 | 16.6 |
| 75–84 | 34.2 | 29.8 |
| ≥ 85 | 34.3 | 53.6 |
| Gender | ||
| Women | 69.9 | 72.4 |
| Race/ ethnicitya | ||
| Non- Hispanic white | 80.8 | 82.8 |
| Non- Hispanic black | 14.5 | 12.1 |
| Hispanic | 1.9 | 2.0 |
| Others | 2.6 | 3.0 |
| Geographic area | ||
| West | 10.8 | 11.1 |
| Midwest | 26.6 | 28.1 |
| Northeast | 20.5 | 23.4 |
| South | 37.4 | 42.2 |
| Epilepsya | 29.9 | 3.4 |
| Bipolar disordera | 7.1 | 4.0 |
| Paina | ||
| No pain | 63.9 | 78.4 |
| Mild/ infrequent pain | 22.8 | 13.5 |
| Moderate/ severe pain | 12.3 | 7.2 |
| Diabetesa | 41.7 | 30.2 |
| Anxietya | 36.7 | 30.9 |
| Depressiona | 59.9 | 50.9 |
| Strokea | 14.6 | 10.2 |
| Alzheimer’s disease/ dementiaa | 51.7 | 65.8 |
| Peripheral vascular disease | 14.3 | 11.0 |
| Arthritis | 32.4 | 31.1 |
| Cognitive impairmenta | ||
| No impairment | 39.7 | 24.5 |
| Mild impairment | 23.9 | 21.3 |
| Moderate impairment | 27.0 | 39.3 |
| Severe impairment | 8.5 | 13.8 |
| Fracturesa | 3.5 | 3.7 |
| Physical functioninga | ||
| No/ mild impairment | 22.3 | 20.4 |
| Moderate impairment | 55.0 | 56.0 |
| Severe impairment | 22.6 | 23.6 |
Data are expressed as % unless otherwise specified.
Missing data on race/ ethnicity (nAED users = 399, nnon- users = 1,032), epilepsy (nAED users = 17, nnon- users = 58), bipolar disorder (nAED users = 15, nnon- users = 61), pain (nAED users = 1,254, nnon- users = 3,765), diabetes (nAED users = 20, nnon- users = 80), anxiety (nAED users = 37, nnon- users = 106), depression (nAED users = 28, nnon- users = 111), stroke (nAED users = 26, nnon- users = 98), Alzheimer’s disease / dementia (nAED users = 8, nnon- users = 39), cognitive impairment (nAED users = 1,302, nnon- users = 4,522), fractures (nAED users = 24, nnon- users = 99), and physical functioning (nAED users = 95, nnon- users = 250).
3.3. Factors Associated with Antiepileptic Drug Use
Table 2 and Table 3 shows the factors associated with the use of any AEDs and AEDs with expanded indications, respectively. The prevalence of AED use was less in those with advanced age. Compared to the prevalence of AED use in those aged 65– 74 years, the prevalence was 0.93 fold lower (95% CI = 0.92– 0.94) in residents aged 75– 84 years and 0.73 fold lower in those aged 85 years and older (95% CI= 0.73– 0.74).
Table 2.
Factors Associated with Any Antiepileptic Drug Usea
| Prevalence Ratios | ||
|---|---|---|
| Factor | Crude (95% Confidence Interval) |
Adjustedb (95% Confidence Interval) |
| Age group | ||
| ≥ 85 years | 0.59 (0.58– 0.60) | 0.73 (0.73– 0.74) |
| 75–84 years | 1.15 (1.14– 1.17) | 0.93 (0.92– 0.94) |
| 65 – 74 years | 1.00 (ref) | 1.00 (ref) |
| Gender | ||
| Women | 1.04 (1.03– 1.06) | 1.05 (1.04– 1.06) |
| Race/ ethnicity | ||
| Non- Hispanic black | 1.17 (1.16– 1.19) | 1.04 (1.03– 1.06) |
| Hispanic | 0.97 (0.93– 1.00) | 1.06 (1.03– 1.10) |
| Others | 0.89 (0.86– 0.93) | 0.98 (0.95– 1.01) |
| Non- Hispanic white | 1.00 (ref) | 1.00 (ref) |
| Epilepsy | 4.00 (3.97– 4.04) | 3.73 (3.69– 3.77) |
| Bipolar disorder | 1.61 (1.59– 1.64) | 1.20 (1.18– 1.22) |
| Pain | ||
| Moderate/ severe pain | 1.50 (1.48– 1.52) | 1.42 (1.40– 1.44) |
| Mild/ infrequent | 1.56 (1.54– 1.58) | 1.42 (1.41– 1.44) |
| No pain | 1.00 (ref) | 1.00 (ref) |
| Diabetes | 1.45 (1.43– 1.46) | 1.27 (1.26– 1.28) |
| Anxiety | 1.22 (1.21– 1.24) | 1.12 (1.11– 1.13) |
| Depression | 1.31 (1.29– 1.32) | 1.17 (1.15– 1.18) |
| Stroke | 1.35 (1.33– 1.36) | 1.08 (1.06– 1.09) |
| Alzheimer’s disease/ dementia | 0.66 (0.65– 0.67) | 0.87 (0.86– 0.88) |
| Cognitive impairment | ||
| Severe | 0.69 (0.68– 0.70) | 0.62 (0.61– 0.63) |
| Moderate | 0.66 (0.65– 0.66) | 0.69 (0.68– 0.70) |
| Mild | 1.10 (1.09– 1.11) | 0.86 (0.85– 0.87) |
| No impairment | 1.00 (ref) | 1.00 (ref) |
Complete case analysis was performed (N = 537,149).
The adjusted prevalence ratios were estimated from the extensions of robust Poisson regression model with all covariates selected, i.e., age, gender, race/ ethnicity, epilepsy, bipolar disorder, pain, diabetes, anxiety, depression, stroke, Alzheimer’s disease/ dementia, and cognitive impairment.
Table 3.
Factors Associated with the Use of Antiepileptic Drugs with Expanded Indications in Older Adultsa
| Adjustedb Odds Ratios (95% Confidence Interval) | |||
|---|---|---|---|
| Factor | Gabapentinoidsc | Topiramate | Lamotrigine |
| Age group | |||
| ≥ 85 years | 0.75 (0.73– 0.77) | 0.24 (0.20– 0.27) | 0.38 (0.34– 0.42) |
| 75–84 years | 1.08 (1.06– 1.11) | 1.85 (1.65– 2.07) | 1.38 (1.27– 1.49) |
| 65 – 74 years | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Gender | |||
| Women | 1.18 (1.15– 1.20) | 1.52 (1.35– 1.70) | 1.15 (1.06– 1.24) |
| Race/ ethnicity | |||
| Non- Hispanic black | 1.06 (1.03– 1.10) | 0.64 (0.54– 0.76) | 0.58 (0.51– 0.65) |
| Hispanic | 1.16 (1.08– 1.24) | 0.92 (0.62– 1.39) | 0.65 (0.48– 0.87) |
| Others | 1.03 (0.97– 1.09) | 0.92 (0.67– 1.26) | 0.59 (0.46– 0.77) |
| Non- Hispanic white | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Epilepsy | 1.09 (1.04– 1.14) | 7.35 (6.50– 8.32) | 11.13 (10.21– 12.13) |
| Bipolar disorder | 1.01 (0.97– 1.06) | 2.90 (2.53– 3.34) | 7.70 (7.07– 8.38) |
| Pain | |||
| Moderate/ severe pain | 2.07 (2.01– 2.12) | 1.45 (1.28– 1.64) | 1.08 (0.99– 1.19) |
| Mild/ infrequent | 2.07 (2.02– 2.11) | 1.43 (1.22– 1.67) | 0.99 (0.87– 1.12) |
| No pain | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Diabetes | 1.79 (1.76– 1.82) | 1.14 (1.02– 1.26) | 1.01 (0.94– 1.09) |
| Anxiety | 1.16 (1.14– 1.18) | 1.33 (1.20– 1.48) | 1.43 (1.33– 1.54) |
| Depression | 1.39 (1.37– 1.42) | 1.47 (1.32– 1.64) | 1.28 (1.20– 1.38) |
| Stroke | 1.14 (1.11– 1.17) | 0.85 (0.73– 1.00) | 0.87 (0.77– 0.97) |
| Alzheimer’s disease/ dementia | 0.81 (0.79– 0.83) | 0.88 (0.78– 0.98) | 1.18 (1.08– 1.28) |
| Cognitive impairment | |||
| Severe | 0.27 (0.26– 0.28) | 0.52 (0.43– 0.64) | 0.66 (0.58– 0.75) |
| Moderate | 0.45 (0.44– 0.47) | 0.76 (0.66– 0.87) | 0.73 (0.67– 0.80) |
| Mild | 0.72 (0.71– 0.74) | 1.02 (0.90– 1.16) | 0.90 (0.82– 0.98) |
| No impairment | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
Complete case (CC) analysis was performed (N = 517,981) among residents who only received one type of AED (N = 529,564).
The adjusted odds ratios were estimated from the multinomial regression model with clustered data including all covariates selected, i.e., age, gender, race/ ethnicity, epilepsy, bipolar disorder, pain, diabetes, anxiety, depression, stroke, Alzheimer’s disease/ dementia, and cognitive impairment. The reference group is ‘no AED use’.
Includes: gabapentin and pregabalin.
For AED indications, residents who had a diagnosis of epilepsy had 3.73- fold prevalence of AED use compared to those without the disorder (95% CI = 3.69– 3.77), though in gabapentinoid users, the prevalence of AEDs did not differ much by epilepsy indications. Residents with comorbid bipolar disorders were associated with 0.2- fold increased likelihood of AED use (95% CI = 1.18– 1.22). Those who in pain had higher prevalence of AED use compared to those without pain (aPRmild/ infrequent vs. no pain = 1.42; 95% CI = 1.41– 1.44; aPRmoderate/ severe vs. no pain = 1.42; 95% CI = 1.40– 1.44). Notably, pain was strongly associated with the use of gabapentinoids (aORmild/ infrequent vs. no pain = 2.07; 95% CI = 2.02– 2.11; aORmoderate/ severe vs. no pain = 2.07; 95% CI = 2.01– 2.12) and topiramate (aORmild/ infrequent vs. no pain = 1.43; 95% CI= 1.22– 1.67; aORmoderate/ severe vs. no pain = 1.45; 95% CI= 1.28– 1.64).
For epilepsy comorbidities, the prevalence of AED use was higher among residents with diabetes, anxiety, depression or stroke. Use of gabapentinoids was highly associated with diabetes (aOR = 1.79; 95% CI = 1.76– 1.82). Having Alzheimer’s disease/ dementia or cognitive impairment was associated with decreased use of any AEDs.
4. DISCUSSION
We found that one in four long stay older nursing home residents enrolled in fee- for- service Medicare received AEDs. Second- generation AED agents were the most commonly used. Factors associated with increased AED use included having a diagnosis of epilepsy, bipolar disorder, pain, diabetes, anxiety, depression, or stroke. Residents with advancing age, Alzheimer’s disease, dementia, or cognitive impairment were associated with decreased use of AEDs. Use of gabapentinoids was strongly associated with having pain but not with epilepsy indication.
The prevalence of AED use has increased from approximately 10% in all US nursing homes in the 1990s to 24.0% in 2016 [5–7]. The reasons for this increase are likely multifactorial. One explanation is the rise in epilepsy prevalence, i.e., from 4.7%- 6.3% in 1990s [35] to 9.1% in the current study. Another explanation is the growing use of newer- generation AEDs, with increased tolerability and reduced side effects including less drug interactions relative to first- generation AEDs [36]. The most likely cause is their growing use beyond the treatment of epilepsy. Gabapentin was the most commonly used AED in our study, and its use has increased for multiple types of pain [37]. Nevertheless, whether or not the increased use of AEDs in nursing home residents is cause for alarm is unclear. Longitudinal studies of AED use and adverse health outcomes in nursing home residents are warranted.
We found that 15.1% nursing home residents used gabapentinoids (13.3% of gabapentin and 1.8% of pregabalin). Our analyses suggest that the use of gabapentinoids was highly associated with pain and diabetes, but not with an epilepsy indication. Though gabapentinoids have expanded indications for neuropathic pain, and are often used in patients with diabetes, the observed strong association between gabapentinoid use and pain in this study was unlikely solely due to on- label prescribing of gabapentinoids for neuropathic pain. Rather, we suspect that gabapentinoids might also be prescribed off- label for other types of pain. Prior research has reported up to 95% of gabapentin being extensively prescribed off- label in clinical practices [38–39]. Furthermore, gabapentin and pregabalin has been prescribed for almost any type of pain [37]. The increasing use of gabapentinoids for pain may reflect clinicians’ desire to seek an alternative to opioids for pain management, partly in response to the opioid epidemic [40]. Nonetheless, the evidence regarding safety and effectiveness of off- label gabapentinoids prescribing is scant [37]. Gabapentinoids may cause sedation, dizziness, cognitive impairment when taken alone, and respiratory depression when taken with other central- nervous system depressants [37,41]. Research to understand the risks of gabapentin and pregabalin use in nursing homes settings is a pressing need.
Consistent with prior studies [5,7,9], decreased AED use was associated with advancing age, although the reason for this trend remains unclear. Greater AED use might be expected in older age groups in that the incidence of epilepsy increases with aging in community dwelling adults [42–43], i.e. from 4.7 per 1000 person- years among those aged 65– 69 years to 8.5 per 1000 person- years in those aged 85 and older [42]. Antiepileptic drug use was also found to decrease with conditions associated with decreased cognitive function. One hypothesis might be that physicians are less inclined to prescribe AEDs for those who already have impaired cognitive performance considering the adverse cognitive effects associated with AEDs [44]. Previous research has mixed findings on AED use and cognitive performance [5,45], perhaps due to differences in how interactions between cognitive function and other covariates were handled. Further research is required to investigate the extent to which reduced AED use with advancing age and cognitive decline in nursing home population represents cautious prescribing, or undertreatment of conditions for which AEDs may provide benefit.
As expected, the presence of epilepsy and bipolar disorder were strongly associated with greater AED use, in alignment with previous studies [5,9]. Having anxiety, depression or diabetes was associated with greater use, suggesting that they might be significant comorbidities among AED users. In support of this, a previous study found that nursing home residents with AED use had higher prevalence of depression compared to overall nursing home population [8]. Therefore, co- medications of antidepressants [43], antipsychotic medications [43], and antihyperglycemic drugs can be common among AED users. Considering that AEDs is susceptible to adverse drug- drug interactions in older adults [10], and that older nursing home residents with polypharmacy regimens have increased susceptibility to adverse drug events including fall -related injuries [46– 47], future work is needed to evaluate the safety of co- medications among AED users in nursing home residents.
This study has several strengths. First, using a comprehensive data source, it is the first national study on AED use in all Medicare/ Medicaid- certified US nursing homes since 1999, providing current data on cross- sectional AED use in this setting. Second, we focused on AEDs, a class of medications with safety concerns in older adults yet understudied in the vulnerable nursing home population. The study also has limitations inherent to administrative data sources. In community dwelling settings, concerns may be raised that drug records in administrative claims data do not always equate to drugs taken by patients because of issues with adherence. In the nursing home setting, this concern is unlikely because daily medication administration is conducted under supervision of nursing home staff. The prevalence of AEDs may be underestimated because we did not account for time spent in hospitals. We assumed no seasonal variations of AED use existed [8] among nursing home residents, therefore the point prevalence a single day in September, 2016 was assumed to be representative of the point prevalence throughout 2016. Information on drug indications were lacking. As such, we were not able to describe AED on/ off label use or assess the appropriateness of use.
5. CONCLUSIONS
One in four long stay older nursing home residents enrolled in fee- for- service Medicare used AEDs. Gabapentin was the most commonly prescribed agent, presumably for pain. Multiple comorbidities were associated with AED use. Nonetheless, little evidence exists to inform risk- benefit balance of AED regimens in this vulnerable population. Further study is required to investigate the safety and effectiveness of AED use in nursing homes.
Supplementary Material
Key Points:
This cross- sectional study of virtually all US long stay older nursing home residents enrolled in fee- for- service Medicare found that one in four residents used antiepileptic drugs (AED) in 2016.
Gabapentin was the most commonly prescribed agent, presumably for pain. Multiple comorbidities were associated with AED use.
Little evidence exists to inform risk- benefit balance of AED regimens in the nursing home population. Further study is required to investigate the safety and effectiveness of AED use in nursing homes.
Funding Sources:
This study was funded by a grant from the National Institute for Nursing Research (5R01NR016977, PI: Kate L. Lapane).
Footnotes
Ethical Approval: This study used routinely- collected administrative and claims dataset and was approved by the University of Massachusetts Medical School Institutional Review Board (protocal number H00011964).
Conflict of Interest: Danni Zhao, Divya Shridharmurthy, Matthew J. Alcusky, Yiyang Yuan, Anthony P. Nunes, Anne L. Hume, Jonggyu Baek, and Kate L. Lapane declare that they have no conflict of interest.
REFERENCES
- 1.Kanner AM, Ashman E, Gloss D, et al. Practice guideline update summary: Efficacy and tolerability of the new antiepileptic drugs I: Treatment of new-onset epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91(2):74–81. doi: 10.1212/WNL.0000000000005755 [DOI] [PubMed] [Google Scholar]
- 2.Kanner AM, Ashman E, Gloss D, et al. Practice guideline update summary: Efficacy and tolerability of the new antiepileptic drugs II: Treatment-resistant epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91(2):82–90. doi: 10.1212/WNL.0000000000005756 [DOI] [PubMed] [Google Scholar]
- 3.Patel H, Toe DC, Burke S, Rasu RS. Anticonvulsant use after formulary status change for brand-name second-generation anticonvulsants. Am J Manag Care. 2010;16(8):e197–204. [PubMed] [Google Scholar]
- 4.Drug Label: Pregabalin. US Food and Drug Administration; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021446s035,022488s013lbl.pdf. Accessed November 17, 2019.
- 5.Garrard J, Harms S, Hardie N, et al. Antiepileptic drug use in nursing home admissions. Annals of Neurology. 2003;54(1):75–85. doi: 10.1002/ana.10593 [DOI] [PubMed] [Google Scholar]
- 6.Cloyd JC, Lackner TE, Leppik IE. Antiepileptics in the elderly. Pharmacoepidemiology and pharmacokinetics. Arch Fam Med. 1994;3(7):589–598. [DOI] [PubMed] [Google Scholar]
- 7.Lackner TE, Cloyd JC, Thomas LW, Leppik IE. Antiepileptic drug use in nursing home residents: effect of age, gender, and comedication on patterns of use. Epilepsia. 1998;39(10):1083–1087. [DOI] [PubMed] [Google Scholar]
- 8.Bathena SPR, Leppik IE, Kanner AM, Birnbaum AK. Antiseizure, Antidepressant, and Antipsychotic Medication Prescribing in Elderly Nursing Home Residents. Epilepsy Behav. 2017;69:116–120. doi: 10.1016/j.yebeh.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrard J, Cloyd J, Gross C, et al. Factors Associated With Antiepileptic Drug Use Among Elderly Nursing Home Residents. J Gerontol A Biol Sci Med Sci. 2000;55(7):M384–M392. doi: 10.1093/gerona/55.7.M384 [DOI] [PubMed] [Google Scholar]
- 10.Johnell K, Fastbom J. Antiepileptic drug use in community-dwelling and institutionalized elderly: a nationwide study of over 1 300 000 older people. Eur J Clin Pharmacol. 2011;67(10):1069–1075. doi: 10.1007/s00228-011-1051-2 [DOI] [PubMed] [Google Scholar]
- 11.Halvorsen KH, Johannessen Landmark C, Granas AG. Prevalence of Different Combinations of Antiepileptic Drugs and CNS Drugs in Elderly Home Care Service and Nursing Home Patients in Norway. Epilepsy Res Treat. 2016;2016. doi: 10.1155/2016/5153093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perucca E, Berlowitz D, Birnbaum A, et al. Pharmacological and clinical aspects of antiepileptic drug use in the elderly. Epilepsy Research. 2006;68:49–63. doi: 10.1016/j.eplepsyres.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 13.Martin RC, Griffith HR, Faught E, Gilliam F, Mackey M, Vogtle L. Cognitive Functioning in Community Dwelling Older Adults with Chronic Partial Epilepsy. Epilepsia. 2005;46(2):298–303. doi: 10.1111/j.0013-9580.2005.02104.x [DOI] [PubMed] [Google Scholar]
- 14.Maximos M, Chang F, Patel T. Risk of falls associated with antiepileptic drug use in ambulatory elderly populations. Can Pharm J (Ott). 2017;150(2):101–111. doi: 10.1177/1715163517690744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergey GK. Initial treatment of epilepsy: special issues in treating the elderly. Neurology. 2004;63(10 Suppl 4):S40–48. doi: 10.1212/wnl.63.10_suppl_4.s40 [DOI] [PubMed] [Google Scholar]
- 16.Leppik IE, Birnbaum AK. Epilepsy in the elderly. Annals of the New York Academy of Sciences. 2010;1184(1):208–224. doi: 10.1111/j.1749-6632.2009.05113.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. 2011;4(6):385–407. doi: 10.1177/1756285611417920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long-Term Care Facility Resident Assessment Instrument 3.0 User’s Manual: Version 1.16. Centers for Medicare & Medicaid Services; 2018. https://downloads.cms.gov/files/1-MDS-30-RAI-Manual-v1-16-October-1-2018.pdf. Accessed November 17, 2019.
- 19.Saliba D, Buchanan J. Making the Investment Count: Revision of the Minimum Data Set for Nursing Homes, MDS 3.0. Journal of the American Medical Directors Association. 2012;13(7):602–610. doi: 10.1016/j.jamda.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 20.Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of Significant Changes in the Minimum Data Set for Nursing Homes Version 3.0. Journal of the American Medical Directors Association. 2012;13(7):595–601. doi: 10.1016/j.jamda.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 21.Marvanova M Pharmacokinetic characteristics of antiepileptic drugs (AEDs). Mental Health Clinician. 2016;6(1):8–20. doi: 10.9740/mhc.2015.01.00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Statistical Abstract of the United States 1995. Bureau of the Census; 1995. https://www.census.gov/prod/1/gen/95statab/preface.pdf. Accessed November 17, 2019.
- 23.Correspondence of Verbal Descriptor and Numeric Rating Scales for Pain Intensity: An Item Response Theory Calibration | The Journals of Gerontology: Series A | Oxford Academic. https://academic.oup.com/biomedgerontology/article/65A/7/778/558819. Accessed May 27, 2019. [DOI] [PubMed]
- 24.Nursing Home Data Compendium 2015. 2015:251.
- 25.Hunnicutt JN, Tjia J, Lapane KL. Hospice Use and Pain Management in Elderly Nursing Home Residents With Cancer. Journal of Pain and Symptom Management. 2017;53(3):561–570. doi: 10.1016/j.jpainsymman.2016.10.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S-H, Hunnicutt JN, Ulbricht CM, Dubé CE, Hume AL, Lapane KL. Adjuvant Use and the Intensification of Pharmacologic Management for Pain in Nursing Home Residents with Cancer: Data from a US National Database. Drugs Aging. 2019;36(6):549–557. doi: 10.1007/s40266-019-00650-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidenberg M, Pulsipher DT, Hermann B. Association of epilepsy and comorbid conditions. Future Neurol. 2009;4(5):663–668. doi: 10.2217/fnl.09.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubé CE, Mack DS, Hunnicutt JN, Lapane KL. Cognitive Impairment and Pain Among Nursing Home Residents With Cancer. Journal of Pain and Symptom Management. 2018;55(6):1509–1518. doi: 10.1016/j.jpainsymman.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wysocki A, Thomas KS, Mor V. Functional Improvement Among Short-Stay Nursing Home Residents in the MDS 3.0. J Am Med Dir Assoc. 2015;16(6):470–474. doi: 10.1016/j.jamda.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowan AJ. Common morbidities influence development, treatment strategies, and expected outcomes. Geriatrics 2005;60:30–34. [PubMed] [Google Scholar]
- 31.Jones CP, Papadopoulos C, Randhawa G. Who’s opting-in? A demographic analysis of the U.K. NHS Organ Donor Register. PLoS One. 2019;14(1). doi: 10.1371/journal.pone.0209161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou G A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 33.Zou G, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661–670. doi: 10.1177/0962280211427759 [DOI] [PubMed] [Google Scholar]
- 34.Hines RJO. Analysis of Clustered Polytomous Data Using Generalized Estimating Equations and Working Covariance Structures. Biometrics. 1997;53(4):1552–1556. doi: 10.2307/2533523 [DOI] [Google Scholar]
- 35.Moore T and White A Drug utilization and MDS-based outcomes for elderly nursing home residents — briefing paper. Abt Associates, Cambridge, MA; 1998 [Google Scholar]
- 36.Anderson GD, Hakimian S. Pharmacokinetic Factors to Consider in the Selection of Antiseizure Drugs for Older Patients with Epilepsy. Drugs Aging. 2018;35(8):687–698. doi: 10.1007/s40266-018-0562-2 [DOI] [PubMed] [Google Scholar]
- 37.Goodman CW, Brett AS. Gabapentin and Pregabalin for Pain — Is Increased Prescribing a Cause for Concern? New England Journal of Medicine. 2017;377(5):411–414. doi: 10.1056/NEJMp1704633 [DOI] [PubMed] [Google Scholar]
- 38.Hamer AM, Haxby DG, McFarland BH, Ketchum K. Gabapentin Use in a Managed Medicaid Population. JMCP. 2002;8(4):266–271. doi: 10.18553/jmcp.2002.8.4.266 [DOI] [PubMed] [Google Scholar]
- 39.Radley DC, Finkelstein SN, Stafford RS. Off-label Prescribing Among Office-Based Physicians. Arch Intern Med. 2006;166(9):1021–1026. doi: 10.1001/archinte.166.9.1021 [DOI] [PubMed] [Google Scholar]
- 40.Gostin LO, Hodge JG, Noe SA. Reframing the Opioid Epidemic as a National Emergency. JAMA. 2017;318(16):1539–1540. doi: 10.1001/jama.2017.13358 [DOI] [PubMed] [Google Scholar]
- 41.Peckham AM, Fairman KA, Sclar DA. All-Cause and Drug-Related Medical Events Associated with Overuse of Gabapentin and/or Opioid Medications: A Retrospective Cohort Analysis of a Commercially Insured US Population. Drug Safety. 2018;41(2):213–228. doi: 10.1007/s40264-017-0595-1 [DOI] [PubMed] [Google Scholar]
- 42.Ip Q, Malone DC, Chong J, Harris RB, Labiner DM. An update on the prevalence and incidence of epilepsy among older adults. Epilepsy Research. 2018;139:107–112. doi: 10.1016/j.eplepsyres.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 43.Leppik IE, Birnbaum AK. Epilepsy in the elderly. Annals of the New York Academy of Sciences. 2010;1184(1):208–224. doi: 10.1111/j.1749-6632.2009.05113.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. 2011;4(6):385–407. doi: 10.1177/1756285611417920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galimberti CA, Tartara E, Dispenza S, Marchese D, Bonizzoni E, Perucca E. Antiepileptic drug use and epileptic seizures in nursing home residents in the Province of Pavia, Italy: A reappraisal 12 years after a first survey. Epilepsy Research. 2016;119:41–48. doi: 10.1016/j.eplepsyres.2015.11.00946. [DOI] [PubMed] [Google Scholar]
- 46.Baranzini F, Poloni N, Diurni M, et al. Polypharmacy and psychotropic drugs as risk factors for falls in long-term care setting for elderly patients in Lombardy. Recenti Prog Med. 2009;100(1):9–16. [PubMed] [Google Scholar]
- 47.Baranzini F, Diurni M, Ceccon F, et al. Fall-related injuries in a nursing home setting: is polypharmacy a risk factor? BMC Health Services Research. 2009;9(1):228. doi: 10.1186/1472-6963-9-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Callegari C, Ielmini M, Bianchi L, Lucano M, Bertù L, Vender S. Antiepileptic drug use in a nursing home setting: a retrospective study in older adults. Funct Neurol. 2016;31(2):87–93. doi: 10.11138/FNeur/2016.31.2.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


