Abstract
Objectives:
To estimate pain reporting among residents with cancer in relation to metropolitan area segregation and NH racial/ethnic composition.
Design:
Cross-sectional study.
Setting and Participants:
383,757 newly admitted Black (B), Hispanic (H), or White (W) residents with cancer in 12,096 US NHs (2011–2013)
Methods:
Using the Minimum Data Set 3.0, pain in past 5 days was determined by self-report or use of pain management. Theil’s entropy index, a measure of metropolitan area segregation, was categorized [high (up to 0.20), very high (0.20–0.30), or extreme (0.30–0.53)].
Results:
Pain prevalence decreased across segregation level [Black: high: 77%, very high: 75%, extreme: 72%; Hispanic: high: 79%, very high: 77%, extreme: 70%; White: high: 80%, very high: 77%, extreme: 74%)]. In extremely segregated areas, all residents were less likely to have recorded pain (adjusted prevalence ratios: B: 4.6% less likely, 95% Confidence interval (CI): 3.1%−6.1%; H: 6.9% less likely, 95% CI: 4.2%−9.6%; W: 7.4% less likely, 95% CI: 6.5%−8.2%) than in the least segregated areas. At all segregation levels, pain was recorded more frequently for residents (B or W) in predominantly White (>80%) NHs than in mostly Black (>50%) NHs or residents (H or W) in predominantly White NHs than mostly Hispanic (>50%) NHs.
Conclusions and Implications:
We observed decreased pain recording in metropolitan areas with greater racial/ethnic segregation. This may occur through the inequitable distribution of resources between NHs, resident-provider empathy, provider implicit bias, resident trust, and other factors.
Keywords: cancer, nursing homes, racial/ethnic segregation, pain
Brief Summary:
This study finds that Black, Hispanic, and White nursing home residents with cancer report pain less frequently in more segregated metropolitan areas, potentially reflecting unequal resource distribution.
Introduction
Racial/ethnic neighborhood segregation, measured at the metropolitan area level, reflects the degree to which racial/ethnic hierarchy has informed residential living patterns.1–3 These patterns tend to be maintained into the present,4,5 with ramifications for investments in medical care,6–8 schooling,9,10 policing,11 and environmental amenities.12
Resource allocation across nursing homes is likely more unequal in settings with greater social separation between racial/ethnic groups.13 Nursing home racial/ethnic composition may affect care quality, with nursing homes mostly occupied by Black and/or Hispanic residents under-resourced relative to homes with predominantly White residents.14 We expect residential segregation to be reiterated within nursing homes and that resource allocation is influenced by structural racial biases.
Nursing homes remain an important healthcare setting for older adults with cancer; approximately ten percent of residents have cancer.15 Pain is commonly experienced among residents with cancer.15–19 Twenty years have passed since the first major report on racial/ethnic disparities in cancer pain prevalence and management in US nursing homes.20 Recent studies document that racial/ethnic disparities persist.21 Nursing homes are highly racially segregated,13 and adverse impacts of racial segregation on Black and Hispanic nursing home residents have been reported.9,22
Racial/ethnic disparities in pain among nursing home residents with cancer may be affected by resident, provider, and resident-provider interaction factors.23 Black and Hispanic residents with cancer acclimated to a racially hostile social environment may downplay pain to avoid “standing out”.24 Black and Hispanic residents responding to a lifetime of discrimination in and outside healthcare settings may self-censor to avoid stereotype threat, or may not feel entitled to demand adequate treatment for pain.25 Providers, informed by implicit biases, may evoke fewer experiences of pain from Black and Hispanic residents,26,27 perhaps from social distance fostered in a segregated social milieu. Racial/ethnic “match” between residents and care staff may not overcome implicit biases.28 Providers may even have biases about racially differential “pain thresholds”, and in a more segregated setting have less incentive to question this dangerous myth.29,30 Trust between residents and staff may be influenced by racial/ethnic relations in and outside the nursing home setting; lack of trust may influence how Black and Hispanic residents express pain to providers.31
The study sought to examine the relationship between racial/ethnic segregation and nursing home racial/ethnic composition to pain reporting in residents with cancer and to provide exploratory analyses on potential mediating pathways. We hypothesize 1) Black and Hispanic residents are less likely to have pain documented in more segregated metropolitan areas; and 2) pain documentation among Black, Hispanic, and White residents with cancer will be lowest in mostly Black or mostly Hispanic nursing homes, and highest in predominantly White nursing homes.
Methods
The University of Massachusetts Medical School Institutional Review Board approved the study.
Data Sources
The Minimum Data Set 3.0 (MDS) is a comprehensive resident-level assessment conducted in US nursing homes.32 We identified newly admitted residents with cancer from 1/1/2011 to 12/31/2013 with assessed pain. Covariates (detailed below) included demographics and potentially painful conditions. We matched these data to neighborhood racial/ethnic segregation within metropolitan areas from Census data, and to nursing home-level measures from the Nursing Home Compare and Provider of Services files from the Centers for Medicare and Medicaid Services.
Study Population
The study population consisted of 384,048 newly admitted adult residents of 12,096 nursing homes in the United States and Puerto Rico who: 1) had an active cancer diagnosis, 2) had pain assessed, 3) were Hispanic of any race, non-Hispanic White, or non-Hispanic Black, 4) lived in a nursing home with at least 10 residents of known race/ethnicity, 5) in a metropolitan area,33 6) were able to make themselves understood (MDS item B0700 “Ability to express ideas and wants, consider both verbal and non-verbal expression” coded as “Understood”), and 7) had complete information on resident- and nursing home-level covariates. We restricted the analysis to Hispanic, non-Hispanic Black, and non-Hispanic White residents because these groups were widespread across metropolitan areas. We excluded nursing homes with populations who were mostly Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or multiracial. A flow chart describing population sizes at each of these stages is shown in Figure 1.
Figure 1.
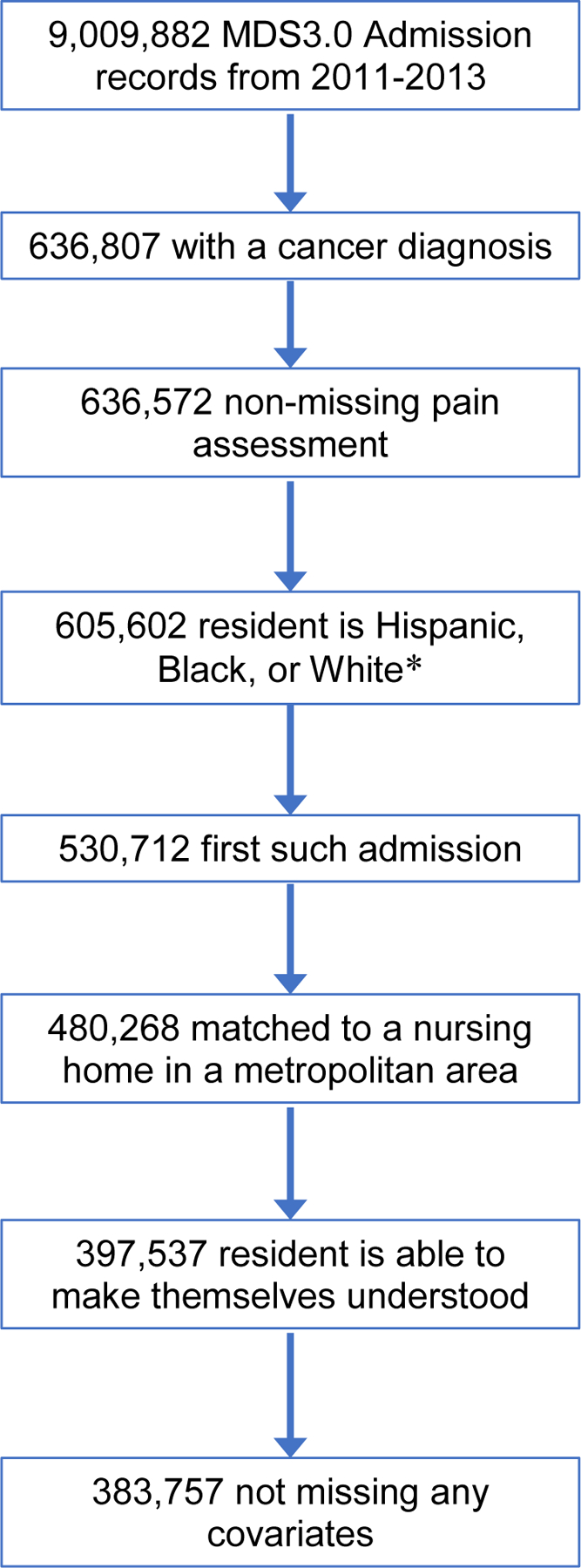
Flowchart depicting sample construction. *Hispanic residents of any race(s) were coded as “Hispanic, of any race(s)”; non-Hispanic residents who were black or white and no other race(s) were coded as “black only, not Hispanic” or “white only, not Hispanic,” respectively. All other residents, including non-Hispanic multiracial residents were excluded from further consideration.
Pain Prevalence
We used MDS item J0300 and items J0100 (a, b, and c) to create a binary variable indicating reported pain. Inter-rater agreement between staff nurses and gold-standard research nurses indicates excellent reliability (κ > 0.92).34 For residents able to self-report (≥90%)35, staff asked “Have you had pain or hurting at any time in the last 5 days?”. Affirmative answers indicated reported pain. Residents receiving non-medication intervention for pain (J0100c), scheduled pain medication (J0100a), or as needed pain medication (J0100b) in the 5 days before assessment (completed by nurses based on medical record review) were considered to have reported pain. Residents with non-missing information for all items were included.
Race/ethnicity
Race/ethnicity is collected on MDS item A1000 (a through f). The person administering the assessment is instructed to ask the resident or their proxy which racial/ethnic identifier “most closely corresponds to his or her race/ethnicity” and to check all that apply.32
Metropolitan Area Segregation
We used Theil’s entropy index to measure metropolitan area-level segregation across census tracts from the 2010 Census. Theil’s index measures unevenness, summarizing the degree to which racial/ethnic distributions within neighborhoods departs from the racial/ethnic distribution of a metropolitan area.36
Theil’s Entropy Index (H) is calculated by summing across 7 racial/ethnic groups (m) across census tracts (j) in a metropolitan area, where tj is the number of residents in tract j, T the number of residents in the metropolitan area, πjm the proportion of residents of race/ethnicity m in tract j, and Πm the proportion of residents in the metropolitan area of race/ethnicity m.
The lowest entropy index is 0 (all tracts in a metropolitan area have the same racial/ethnic distribution); the highest is 1, (each tract is occupied by members of one group). We arbitrarily classified metropolitan areas as highly segregated (entropy index <0.20), very highly segregated (0.20 to 0.30), or extremely segregated (0.30 to 0.45), using round number cutpoints near tertiles of the Black and Hispanic population.
On-line AppendixTable 1 shows select demographics of metropolitan areas in each segregation level. Larger metropolitan areas tend to be more segregated, as do those with larger Black populations, and those in the North central and Northeast regions.
Nursing Home Racial/Ethnic Composition
We characterized the racial/ethnic composition of nursing homes in each quarter by collapsing all assessments (Prospective Payment System, admission, quarterly, annual, and significant change in status assessments), randomly selecting one assessment (regardless of eligibility for this cancer pain analysis). We classified nursing homes into four mutually exclusive categories: predominantly White (>80% White residents), mostly Black (>50% Black residents), mostly Hispanic (>50% Hispanic residents), or integrated (<80% White, and <50% of any other racial/ethnic group). These categories represent arbitrary cutpoints intended to be readily interpretable.
Resident-level Covariates
We considered the following covariates: resident sex, age (19–64, 65–74, 75–84, 85 and older), activities of daily living,32 Cognitive Function Score (intact, mildly, moderately, or severely impaired),37 and life expectancy under 6 months. Potentially painful non-cancer conditions included: heart failure, respiratory failure, ulcerative colitis, wound infection, arthritis, osteoporosis, hip fracture, other fracture, mouth pain, high grade pressure ulcer, foot problems, surgical wounds, other open lesions, and burns.
Analytic Approach
Our theoretical framework rests on the supposition that racial/ethnic disparities in pain prevalence are largely due to reporting of pain, rather than the experience of pain. We estimated the prevalence of documented pain among Blacks, Hispanics, and Whites across metropolitan area segregation; and within levels of segregation across nursing home racial/ethnic composition. We adjusted for predictors of pain we judged unlikely to be the result of metropolitan area segregation or nursing home racial/ethnic composition. We used a Poisson model to estimate prevalence ratios directly,38 with a generalized estimating equation specification (exchangeable correlation matrix), to adjust for within-home correlation between residents. Using a nested model, we included race/ethnicity as a main effect and an interaction between race/ethnicity and the categorical segregation variable, with highly segregated as the reference group. We then nested nursing home racial/ethnic composition (mostly Black, mostly Hispanic, or integrated, relative to predominantly White) within metropolitan area segregation.
Sensitivity Analysis
We conducted two sensitivity analyses. Metropolitan areas with smaller populations tend to be predominantly White, and less highly segregated. We performed a subset analysis restricted to metropolitan areas with at least 250,000 population. Second, Puerto Rico’s history is quite different from that of the continental United States, we performed a subset analysis excluding Puerto Rico.
Results
Overall, 77% of adult nursing home residents with cancer had documented pain at admission (Table 1). Seventy-five percent of Blacks, 75% of Hispanics, and 78% of Whites reported pain. Pain was reported for 81% of women, 73% of men, 87% of residents aged <65 years, and 71% of those aged ≥85 years. In the least segregated metropolitan areas, 80% of residents with cancer reported pain, whereas in the most segregated metropolitan areas 73% of residents did. Seventy-nine percent of residents living in nursing homes with a predominantly White population reported pain, as did 75% of residents living in integrated nursing homes, 72% in mostly Black nursing homes, and 67% in mostly Hispanic nursing homes. While 80% of residents with intact cognitive function reported pain, 75% of residents with mild, 66% of residents with moderate, and 72% of residents with severe cognitive function reported pain. Eighty-seven percent of residents with a limited life expectancy reported pain. Reported pain was more common than the overall average of 77% in many of the potentially painful non-cancer conditions we considered (bowel disease, wound infection, arthritis, osteoporosis, fractures, mouth pain, high grade pressure ulcer, foot problems, open lesions, surgical wounds, and burns), but not all (heart failure, respiratory failure; Table 2).
Table 1.
Prevalence of Documented Pain among Residents with Cancer, by Resident Race/Ethnicity, Metropolitan Area Segregation, Nursing Home Racial/Ethnic Composition, and Demographic Characteristics
| Nursing Homes | Residents | Documented Pain | |
|---|---|---|---|
| Characteristic | N* | N | Percent |
| Total | 12,096 | 383,757 | 77.4 |
| Resident Race/Ethnicity | |||
| Black only, not Hispanic | 42,317 | 74.6 | |
| Hispanic, of any race(s) | 12,290 | 75.4 | |
| White only, not Hispanic | 329,150 | 77.8 | |
| Metropolitan area segregation† | |||
| Highly segregated (0.004–0.199) | 6,648 | 178,262 | 79.9 |
| Very highly segregated (0.200–0.299) | 3,481 | 108,719 | 76.8 |
| Extremely segregated (0.300–0.451) | 1,967 | 96,776 | 73.3 |
| Nursing home racial/ethnic composition‡ | |||
| Mostly Black | 781 | 18,974 | 72.3 |
| Mostly Hispanic | 182 | 3,295 | 66.9 |
| Integrated | 3,196 | 82,994 | 75.1 |
| Predominantly White | 8,649 | 278,494 | 78.5 |
| Resident sex | |||
| Male | 177,347 | 73.0 | |
| Female | 206,410 | 81.1 | |
| Resident age | |||
| 18 to 64 | 54,686 | 87.3 | |
| 65 to 74 | 87,547 | 81.5 | |
| 75 to 84 | 134,493 | 75.9 | |
| 85 and older | 107,031 | 70.8 | |
| Cognitive Function Score | |||
| Intact | 259,053 | 80.0 | |
| Mildly impaired | 86,837 | 74.5 | |
| Moderately impaired | 35,628 | 65.6 | |
| Severely impaired | 2,239 | 72.0 | |
| Activities of Daily Living (RUG-IV scale) | |||
| Independent (0–5) | 110,873 | 74.2 | |
| Moderately dependent (6–10) | 160,750 | 76.9 | |
| Dependent (11–16) | 112,134 | 81.2 | |
| Life expectancy less than 6 months | |||
| Yes | 20,089 | 87.2 | |
| No | 363,668 | 76.8 |
The number of nursing homes generally does not sum to the total, because any given nursing home may switch categories from one year to another.
Metropolitan area segregation measured with Theil’s Entropy Index across census tracts using the total 2010 Census population.
Mostly Black: ≥50% Black residents in a given quarter; Mostly Hispanic: ≥50% Hispanic residents in a given quarter; Predominantly White: ≥80% White residents in a given quarter; Integrated: <80% White residents, and <50% every other racial/ethnic group in a given quarter.
Table 2.
Prevalence of Documented Pain in Potentially Painful Conditions among Residents with Cancer
| Residents | Documented Pain | |
|---|---|---|
| Potentially Painful Condition | N | Percent |
| Total | 383,757 | 77.4 |
| Heart failure | 64,650 | 73.6 |
| Respiratory failure | 10,282 | 74.3 |
| Ulcerative colitis, Crohn’s disease, or inflammatory bowel disease | 5,286 | 80.2 |
| Wound infection | 8,960 | 86.2 |
| Arthritis | 91,996 | 83.8 |
| Osteoporosis | 37,356 | 82.2 |
| Hip fracture | 20,881 | 94.0 |
| Other fracture | 31,236 | 92.2 |
| Mouth pain | 7,417 | 86.6 |
| Grade 2–4 pressure ulcer(s) | 50,087 | 81.0 |
| Foot problems | 6,619 | 81.4 |
| Open lesions other than ulcers, rashes, or cuts | 13,542 | 80.1 |
| Surgical wound | 115,810 | 86.3 |
| Burns | 1,015 | 87.0 |
In unadjusted analyses (Table 3), pain reporting decreased as metropolitan area segregation increased, for Blacks (high: 77%; very high: 75%; extreme: 72%), Hispanics (high: 79%; very high: 77%; extreme: 70%) and Whites (high: 80%; very high: 77%; extreme: 74%). Within each category of metropolitan area segregation, Blacks were least likely to report pain in mostly Black nursing homes (extreme segregation: 69%; high segregation: 76%), somewhat more likely in integrated nursing homes (extreme: 74%; high: 76%), and most likely to have documented pain in predominantly White nursing homes (extreme: 77%; very high: 79%). Similarly, Hispanic residents were least likely to report pain in mostly Hispanic nursing homes (extreme: 57%; very high: 71%), somewhat more likely in integrated nursing homes (extreme: 72%; high: 79%), and most likely in predominantly White nursing homes (extreme: 78%; high: 83%). Among White residents, reported pain was also highest in predominantly White nursing homes (extreme: 75%; high: 81%), and lowest in mostly Hispanic nursing homes (very high: 66%; high: 73%).
Table 3.
Prevalence of Documented Pain among Residents with Cancer, by Race/Ethnicity, Metropolitan Area Segregation, and Nursing Home Racial/Ethnic Composition
| Black only residents, not Hispanic | Hispanic residents of any race(s) | White only residents, not Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metropolitan area segregation | Nursing homes | Residents | Documented pain | Residents | Documented pain | Residents | Documented pain | |||
| Nursing home composition | N | N | Percent | N | Percent | N | Percent | |||
| High segregation (0.004–0.199) | 6,648 | 11,464 | 76.8 | 5,351 | 78.5 | 161,447 | 80.2 | |||
| Mostly Black nursing home | 141 | 1,385 | 75.5 | * | 944 | 78.7 | ||||
| Mostly Hispanic nursing home | 121 | * | 1,012 | 70.8 | 533 | 73.2 | ||||
| Integrated nursing home | 1,486 | 5,839 | 76.1 | 2,712 | 78.7 | 23,745 | 78.1 | |||
| Predominantly White nursing home | 5,241 | 4,189 | 78.4 | 1,604 | 82.9 | 136,225 | 80.6 | |||
| Very high segregation (0.200–0.299) | 3,481 | 14,754 | 75.3 | 2,779 | 77.3 | 91,186 | 77.1 | |||
| Mostly Black nursing home | 351 | 5,109 | 72.7 | * | 2,083 | 47.7 | ||||
| Mostly Hispanic nursing home | 24 | * | 217 | 71.0 | 118 | 66.1 | ||||
| Integrated nursing home | 1,110 | 6,220 | 75.6 | 1,755 | 76.3 | 17,742 | 75.6 | |||
| Predominantly White nursing home | 2,248 | 3,394 | 78.8 | 686 | 81.2 | 71,243 | 77.5 | |||
| Extreme segregation (0.300–0.451) | 1,967 | 16,099 | 72.4 | 4,160 | 70.1 | 76,517 | 73.7 | |||
| Mostly Black nursing home | 289 | 6,915 | 69.4 | * | 2,047 | 71.6 | ||||
| Mostly Hispanic nursing home | 37 | * | 979 | 57.4 | 236 | 71.2 | ||||
| Integrated nursing home | 600 | 6,527 | 73.9 | 2,227 | 72.4 | 16,227 | 69.9 | |||
| Predominantly White nursing home | 1,160 | 2,539 | 76.8 | 607 | 78.1 | 58,007 | 74.8 | |||
Results for Black residents of mostly Hispanic nursing homes and Hispanic residents of mostly Black nursing homes are not reported due to unstable estimates.
After adjustment for resident sex, age, cognitive function, ADL, life expectancy under 6 months, and potentially painful conditions, these patterns remain (Table 4). In extremely segregated metropolitan areas, Black residents were 0.954 times as likely (4.6% less likely) to report pain (95% CI: 3.1% to 6.1% less likely), Hispanic residents 0.931 times as likely (6.9% less likely; 95% CI: 4.2% to 9.6% less likely), and White residents 0.926 times as likely (7.4% less likely; 95% CI: 6.5% to 8.2% less likely) than in the least segregated metropolitan areas. Within similarly segregated metropolitan areas, Black residents of mostly Black nursing homes reported pain less often than Black residents of predominantly White nursing homes (2.3% to 7.8% less likely). Hispanic residents of mostly Hispanic nursing homes were also less likely to report pain (8.0% to 21.7% less likely) than Hispanic residents of predominantly White nursing homes. Whites in mostly Black nursing homes also had a lower likelihood of reporting pain (6.0% to 2.1% less likely) than Whites in predominantly White nursing homes, as did Whites in mostly Hispanic nursing homes (7.1% to 11.1% less likely), and Whites in integrated nursing homes (2.9% to 6.1% less likely).
Table 4.
Adjusted* Prevalence Ratios of Documented Pain among Residents with Cancer, by Race/Ethnicity, Metropolitan Area Segregation, and Nursing Home Racial/Ethnic Composition
| Black only residents, not Hispanic | Hispanic residents of any race(s) | White only residents, not Hispanic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence ratio | 95% Confidence interval | Prevalence ratio | 95% Confidence interval | Prevalence ratio | 95% Confidence interval | ||||
| Metropolitan area segregation, not adjusted for nursing home racial/ethnic composition | |||||||||
| High segregation (0.004–0.199) | (ref) | (ref) | (ref) | ||||||
| Very high segregation (0.200–0.299) | 0.977 | (0.963–0.991) | 0.986 | (0.962–1.011) | 0.966 | (0.959–0.972) | |||
| Extreme segregation (0.300–0.451) | 0.954 | (0.939–0.969) | 0.931 | (0.904–0.958) | 0.926 | (0.918–0.935) | |||
| Nursing home racial/ethnic composition, within strata of metropolitan area segregation | |||||||||
| High segregation (0.004–0.199) | |||||||||
| Mostly Black nursing home | 0.977 | (0.943–1.013) | † | 0.979 | (0.944–1.015) | ||||
| Mostly Hispanic nursing home | † | 0.854 | (0.814–0.897) | 0.929 | (0.882–0.978) | ||||
| Integrated nursing home | 0.976 | (0.955–0.997) | 0.956 | (0.928–0.985) | 0.971 | (0.962–0.980) | |||
| Predominantly White nursing home | (ref) | (ref) | (ref) | ||||||
| Very high segregation (0.200–0.299) | |||||||||
| Mostly Black nursing home | 0.944 | (0.918–0.970) | † | 0.959 | (0.932–0.986) | ||||
| Mostly Hispanic nursing home | † | 0.920 | (0.839–1.009) | 0.889 | (0.763–1.036) | ||||
| Integrated nursing home | 0.968 | (0.946–0.991) | 0.939 | (0.898–0.982) | 0.970 | (0.959–0.982) | |||
| Predominantly White nursing home | (ref) | (ref) | (ref) | ||||||
| Extreme segregation (0.300–0.451) | |||||||||
| Mostly Black nursing home | 0.922 | (0.893–0.952) | † | 0.940 | (0.907–0.975) | ||||
| Mostly Hispanic nursing home | † | 0.783 | (0.698–0.878) | 0.924 | (0.852–1.001) | ||||
| Integrated nursing home | 0.959 | (0.933–0.985) | 0.938 | (0.893–0.985) | 0.939 | (0.922–0.956) | |||
| Predominantly White nursing home | (ref) | (ref) | (ref) | ||||||
Poisson models of prevalence ratios, adjusted for resident sex, age, cognitive function, dependence in activities of daily living, life expectancy under 6 months, potentially painful conditions, county urbanicity, and calendar year; a generalized estimating equation specification was used (exchangeable correlation matrix) to adjust for within-nursing home correlation.
Results for Black residents of mostly Hispanic nursing homes and Hispanic residents of mostly Black nursing homes are not reported due to unstable estimates.
Several additional factors not considered may lie on causal paths of interest; namely resident characteristics that may alter the pain experience (On-line Appendix Table 2), indicators of contact between staff and residents (On-line Appenidx Table 3), and nursing home characteristics that predict care quality or resources (On-line Appendix Table 4). Inclusion of these factors in a model tended to attenuate prevalence ratio estimates (On-line Appendix Table 5). However, because formal mediation analysis extends beyond the scope of this analysis, we did not assess which, if any, of these potential downstream mechanisms are dominant.
Neither restricting the analysis to larger metropolitan areas (n=310,584) (see On-line Appendix Table 6), nor exclusion of Puerto Rico (n=383,583) (see On-line Appendix Table 7) had an appreciable impact on prevalence ratio estimates.
Discussion
In a context where every metropolitan area in the United States is segregated, we found higher reporting of pain among nursing home residents with cancer in the least highly segregated metropolitan areas for Black, Hispanic, and White residents. Within comparably segregated contexts, pain among residents with cancer was most frequently documented for residents of predominantly White nursing homes, and least frequently documented for residents of mostly Hispanic nursing homes. In very highly and extremely segregated contexts, pain was reported less often in mostly Black nursing homes as well. Adjustment for age, sex, cognitive function, functional status, limited life expectancy, a wide range of potentially painful conditions, county urbanicity and calendar year did not materially alter these findings.
Previously, we conducted a systematic review to understand the role structural racism plays in explaining racial/ethnic disparities in quality outcomes in nursing homes.22 Of eight studies identified, most documented an adverse impact of racial segregation on racial/ethnic minority residents and quality outcomes,22 reinforcing the notion that segregation is a primary driver of care quality in nursing homes.6 Nursing home racial/ethnic composition is strongly associated with nursing home care quality.13 The separate experiences of White, Black, and Hispanic residents across nursing homes in more segregated metropolitan areas is likely accompanied by inequities in care for these residents.13
It may be tempting to conclude that racial/ethnic integration itself would reduce racial/ethnic disparities in the documentation of pain in nursing home residents with cancer. However, we caution that racial/ethnic segregation itself reflects structural racial biases.3,6,25
We found that reported pain among Whites was lower in more segregated metropolitan areas, to a similar degree as for Blacks and Hispanics. Adverse impacts of residential segregation among Whites have been documented for some health-related outcomes,12,39–42 but not all.43,44
The finding that pain reporting was lower in mostly Black and mostly Hispanic nursing homes, and lower for all three racial/ethnic groups studied, suggests that greater familiarity and affiliation with racial/ethnic minority residents may not suffice to prevent lower pain reporting for racial/ethnic minority groups.
Strengths and Limitations
The analysis was conducted in a national dataset of all eligible nursing home residents. It is the first to consider both metropolitan area segregation and nursing home racial/ethnic composition in a measure of nursing home care quality.To reduce measurement error due to reduced documentation of pain among residents with impaired communication, we restricted the analysis to residents able to make themselves understood. To the degree that resident-staff communication about pain is hampered, especially in ways related to resident race/ethnicity, it is possible that our findings do not fully reflect under-reported pain. Although racial/ethnic disparities in pain among residents with cancer persist,21 pain ascertainment in this setting is likely more complete than among residents without cancer.
We based our analysis on admission records, care should be exercised when extrapolating these findings to long-stay residents. Given the unique history of racial/ethnic biases in the United States and the structure of healthcare reimbursement in this country, extrapolation to other national settings should be avoided. We conjectured several potential mechanisms by which segregation may cause separation in nursing home composition (unequal distribution of resources, fraught resident-staff interactions, provider implicit bias and social distancing, resident self-censorship and lack of trust). Formal mediation analysis of these potential mechanisms was beyond the scope of this analysis because of the complexity of interpreting mediation analysis in the context of multi-level models. Mechanisms we did not discuss likely play a substantive role as well. Our measure of segregation was based on the most recent Census enumeration in 2010. Residential patterns have been resistant to change over recent decades.45
Policy Implications
Our findings provide evidence of unequal care in the presence of racial/ethnic segregation in the United States. Opportunities to improve access and quality include: (1) enforcing Title VI of the 1964 US Civil Rights Act, (2) Medicaid reimbursement, (3) value-based purchasing programs, and (4) quality improvement initiatives. Title IV bans racial discrimination in institutions receiving federal funding, but enforcement actions in nursing homes have been limited.6 Many nursing homes charge private payers more than Medicaid.46 Given that higher Medicaid payments are positively associated with nursing home quality,47 and Medicaid coverage increases with the proportion of racial/ethnic minority residents in nursing homes,48 increasing Medicaid payments could reduce resource gaps. Evidence of biased admission practices based on payer type exists.49 In Minnesota, legislation requires nursing homes to charge private payers the same as Medicaid to counter biased admission practices. A Federal equalization bill could help equalize access. Value-based purchasing programs provide incentives to improve quality across and within facilities, but may leave majority Black and Hispanic nursing homes at a structural disadvantage.50 These programs could be coupled with quality initiatives that target and support nursing homes disproportionately serving racial/ethnic minorities.51 The fact that we saw lower documentation of pain across the three largest racial/ethnic groups, and systematically across nursing home compositions and metropolitan areas, suggests a large burden of under-ascertained pain in this population, despite what may appear to be modest effect estimates.
Conclusions and Implications
This analysis supports the idea that cancer pain remains under-reported among Blacks, Hispanics, and Whites. This under-recognition of pain may be driven, in part, by racial/ethnic segregation resulting in more separate and less equal experiences for Black, Hispanic, and White residents. We were limited in our ability to directly assess pathways by which these effects may occur, such as resident-provider interactions, resident trust in medical care, or provider implicit bias.
Supplementary Material
Acknowledgements:
This work was funded by a grant to Dr. Lapane from the National Cancer Institute (1R21CA198172). Deborah Mack was funding as a pre-doctoral trainee (TR0041454). Sarah Forrester was funded as a post-doctoral trainee (HL120823). The funders had no role in the design, methods, subject recruitment, data collection, analysis or preparation of this paper.
Conflicts of Interest
The authors have no conflicts of interest with respect to the contents of this paper. Funding for this work was provided through an administrative supplement to a grant from the National Cancer Institute: R21 CA198172-02 Opioids and Adjuvants for Pain in Nursing Home Residents with Cancer.
References
- 1.Massey DS, Denton NA. Hypersegretation in U.S. metropolitan areas: black and Hispanic segregation along five dimensions. Demography 1989;26:373–391. [PubMed] [Google Scholar]
- 2.Reardon SF. A conceptual framework for measuring segregation and its association with population outcomes. In: Oakes JM & Kaufman JS, Eds. Methods in Social Epidemiology, Second Edition. San Francisco: Jossey-Bass, 2017:132–157. [Google Scholar]
- 3.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep 2001;116:404–416. doi: 10.1093/phr/116.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn RA, Truman BI, Williams DR. Civil rights as determinants of public health and racial and ethnic health equity: Health care, education, employment, and housing in the United States. SSM Popul Health 2018;4:17–24. doi: 10.1016/j.ssmph.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reardon SF, Farrell CR, Matthews SA, et al. Race and space in the 1990s: Changes in the geographic scale of racial residential segregation, 1990–2000. Soc Sci Res 2009;38:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams DR. Miles to go before we sleep: Racial inequalities in health. J Health Soc Behav 2012;53:279–295. doi: 10.1177/0022146512455804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol 2016;35:407–411. doi: 10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King CJ, Redwood Y. The health care institution, population health, and Black lives. J Nat Med Assoc 2016;108:131–136. doi: 10.1016/j.jnma.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 9.Reardon SF, Owens A. 60 years after Brown: Trends and consequences of school segregation. Ann Rev Sociol 2014;40:199–218. doi: 10.1146/annurev-soc-071913-043152 [DOI] [Google Scholar]
- 10.Davis T, Bhatt R, Schwarz K. School segregation in the era of accountability. Soc Currents 2015;2:239–259. doi: 10.1177/2329496515589852 [DOI] [Google Scholar]
- 11.Logan JR, Oakley D. Black lives and policing: The larger context of ghettoization. J Urb Affairs 2017;39:1031–1046. doi: 10.1080/07352166.2017.1328977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jesdale BM, Morello-Frosch R, Cushing L. The racial/ethnic distribution of heat risk-related land cover in relation to residential segregation. Environ Health Perspect 2013;121:811–817. doi: 10.1289/ehp.1205919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DB, Feng Z, Fennell ML, et al. Separate and unequal: Racial segregation and disparities in quality across U.S. nursing homes. Health Aff 2007;26:1448–1458. doi: 10.1377/hlthaff.26.5.1448 [DOI] [PubMed] [Google Scholar]
- 14.Mor V, Zinn J, Angelelli J, et al. Driven to tiers: socioeconomic and racial disparities in the quality of nursing home care. Milbank Q, 2004;82:227–56. doi: 10.1111/j.0887-378X.2004.00309.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pimentel C, Briesacher B, Gurwitz J, et al. Pain management among nursing home residents with cancer. J Am Geriatr Soc 2015;63:633–641. doi: 10.1111/jgs.13345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drageset J, Corbett A, Selbaek G, et al. Cancer-related pain and symptoms among nursing home residents: A systematic review. J Pain Symptom Manage. 2014;48:699–710.e1. doi: 10.1016/j.jpainsymman.2013.12.238 [DOI] [PubMed] [Google Scholar]
- 17.Buchanan RJ, Barkley J, Wang S, et al. Analyses of nursing home residents with cancer at admission. Cancer Nurs. 2005;28:406–414. [DOI] [PubMed] [Google Scholar]
- 18.Duncan JG, Bott MJ, Thompson SA, et al. Symptom occurrence and associated clinical factors in nursing home residents with cancer. Res Nurs Heal 2009;32:453–464. doi: 10.1002/nur.20331 [DOI] [PubMed] [Google Scholar]
- 19.Johnson VMP, Teno JM, Bourbonniere M, et al. Palliative care needs of cancer patients in U.S. nursing homes. J Palliat Med 2005;8:273–279. doi: 10.1089/jpm.2005.8.273 [DOI] [PubMed] [Google Scholar]
- 20.Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. J Am Med Assoc 1998;279:1877–1882. [DOI] [PubMed] [Google Scholar]
- 21.Mack DS, Hunnicutt JN, Jesdale BM, et al. Non-Hispanic Black-White disparities in pain and pain management among newly admitted nursing home residents with cancer. J Pain Res 2018;11:753–761. doi: 10.2147/JPR.S158128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack DS, Jesdale BM, Ulbricht CM, et al. Racial segregation measurement in nursing home facilities: A systematic review. Gerontologist 2019; [Epub ahead of print] doi: 10.1093/geront/gnz056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tait RC, Chibnall JT. Racial/ethnic disparities in the assessment and treatment of pain: Psychosocial perspectives. Am Psychol 2014;69:131–141. doi: 10.1037/a0035204 [DOI] [PubMed] [Google Scholar]
- 24.Merluzzi TV, Philip EJ, Zhang Z, et al. Perceived discrimination, coping, and quality of life for African-American and Caucasian persons with cancer. Cultur Divers Ethnic Minor Psychol 2015;21:337–344. doi: 10.1037/a0037543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams DR, Mohammed SA. Racism and health I: Pathways and scientific evidence. Am Behav Sci 2013;57(8). doi: 10.1177/0002764213487340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colón-Emeric CS, Corazzini K, McConnell E, et al. Study of individualization and bias in nursing home fall prevention practices. J Am Geriatr Soc 2017;65:815–821. doi: 10.1111/jgs.14675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haider AH, Schneider EB, Sriram N, et al. Unconscious race and class biases among registered nurses: vignette-based study using implicit association testing. J Am Coll Surg 2015;220:1077–1086. doi: 10.1016/j.jamcollsurg.2015.01.065 [DOI] [PubMed] [Google Scholar]
- 28.Tajeu GS, Halanych J, Juarez L, et al. Exploring the association of healthcare worker race and occupation with implicit and explicit racial bias. J Natl Med Assoc 2017;110:464–472. doi: 10.1016/j.jnma.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Trawalter S, Hoffman KM, Waytz A. Racial bias in perceptions of others’ pain. PLoS One 2012;7:e48546. doi: 10.1371/journal.pone.0048546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman KM, Trawalter S, Axt JR, et al. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between Blacks and Whites. Proc Natl Acad Sci U S A 2016;113:4296–4301. doi: 10.1073/pnas.1516047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson-Lane SG, Vallerand AH. Pain treatment practices of community-dwelling Black older adults. Pain Manag Nurs 2018;19:46–53. doi: 10.1016/j.pmn.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Centers for Medicare and Medicaid Services. Long-Term Care Facility Resident Assessment Instrument User’s Manual, Version 3.0. Washington: Department of Health and Human Services, 2011. [Google Scholar]
- 33.Ingram DD, Franco SJ. 2013 NCHS urban-rural classification scheme for counties. National Center for Health Statistics. Vital Health Stat 2014;2(166). [PubMed] [Google Scholar]
- 34.Saliba D, Buchanan J. Development & Validation of a Revised Nursing Home Assessment Tool: MDS 3.0. Los Angeles: RAND Health Corporation, 2008. [Google Scholar]
- 35.Thomas KS, Wysocki A, Intrator O, et al. Finding Gertrude: The resident’s voice in MDS 3.0. J Am Med Dir Assoc 2014;15:802–806. doi: 10.1016/j.jamda.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theil H Statistical Decomposition Analysis. Amsterdam: North-Holland Publishing Company, 1972. [Google Scholar]
- 37.Thomas KS, Dosa D, Wysocki A, et al. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care 2017;55:e68–e72. doi: 10.1097/MLR.0000000000000334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162:199–200. doi: 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 39.Collins CA. Racism and health: Segregation and causes of death amenable to medical intervention in major U.S. cities. Ann N Y Acad Sci 1999;896:396–398. [DOI] [PubMed] [Google Scholar]
- 40.Cooper RS, Kennelly JF, Durazo-Arvizu R, et al. Relationship between premature mortality and socioeconomic factors in Black and White populations of US metropolitan areas. Pub Health Rep 2001;116:464–473. doi: 10.1093/phr/116.5.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morello-Frosch R, Jesdale BM. Separate and unequal: Residential segregation and estimated cancer risks associated with ambient air toxics in U.S. metropolitan areas. Environ Health Perspect 2006;114:386–393. doi: 10.1289/ehp.8500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang VW. Racial residential segregation and weight status among US adults. Soc Sci Med 2006;63:1289–1303. doi: 10.1016/j.socscimed.2006.03.049 [DOI] [PubMed] [Google Scholar]
- 43.Hart KD, Kunitz SJ, Sell RR, et al. Metropolitan governance, residential segregation, and mortality among African Americans. Am J Public Health 1998;88:434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gould EI. Is segregation bad for your health? The case of low birth weight. In Brookings-Wharton Papers on Urban Affairs 2000. Washington: Brookings Institution Press; 2000. [Google Scholar]
- 45.Iceland J, Weinberg DH, Steinmetz E. Racial and Ethnic Residential Segregation in the United States: 1980–2000. U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; 2002. [Google Scholar]
- 46.Cohen R Nursing Home Rates—Private Pay versus Medicaid. Hartford: Connecticut Office of Legislative Research; 2000. [Google Scholar]
- 47.Grabowski DC, Angelelli JJ, Mor V. Medicaid payment and risk-adjusted nursing home quality measures. Health Aff 2004;23:243–252. doi: 10.1377/hlthaff.23.5.243 [DOI] [PubMed] [Google Scholar]
- 48.Gruneir A, Miller SC, Feng Z, et al. Relationship between state Medicaid policies, nursing home racial composition, and the risk of hospitalization for Black and White residents. Health Serv Res 2008;43:869–881. doi: 10.1111/j.1475-6773.2007.00806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer MH. Medicaid reimbursement rates and access to nursing homes: Implications for gender, race, and marital status. Res Aging 2001;23:532–551. doi: 10.1177/0164027501235002 [DOI] [Google Scholar]
- 50.Hefele JG, Wang X, Lim E. Fewer bonuses, more penalties at skilled nursing facilities serving vulnerable populations. Health Aff 2019;38:1127–1131. doi: 10.1377/hlthaff.2018.05393 [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Yin J, Cai X, et al. Association of race and sites of care with pressure ulcers in high-risk nursing home residents. JAMA 2011;306:179–186. doi: 10.1001/jama.2011.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


