Abstract
Background.
Patients with pulpal and periapical conditions often seek treatment for pain, intraoral swelling, or both. Even when definitive, conservative dental treatment (DCDT) is an option, antibiotics are often prescribed. The purpose of this review was to summarize available evidence regarding the effect of antibiotics, either alone or as adjuncts to DCDT, to treat immunocompetent adults with pulpal and periapical conditions, as well as additional population-level harms associated with antibiotic use.
Type of Studies Reviewed.
The authors updated 2 preexisting systematic reviews to identify newly published randomized controlled trials. They also searched for systematic reviews to inform additional harm outcomes. They conducted searches in MEDLINE, Embase, the Cochrane Library, and the Cumulative Index to Nursing and Allied Health Literature. Pairs of reviewers independently conducted study selection, data extraction, and assessment of risk of bias and certainty in the evidence using the Grading of Recommendations Assessment, Development, and Evaluation approach.
Results.
The authors found no new trials via the update of the preexisting reviews. Ultimately, 3 trials and 8 additional reports proved eligible for this review. Trial estimates for all outcomes suggested both a benefit and harm over 7 days (very low to low certainty evidence). The magnitude of additional harms related to antibiotic use for any condition were potentially large (very low to moderate certainty evidence).
Conclusions and Practical Implications.
Evidence for antibiotics, either alone or as adjuncts to DCDT, showed both a benefit and a harm for outcomes of pain and intraoral swelling and a large potential magnitude of effect in regard to additional harm outcomes. The impact of dental antibiotic prescribing requires further research.
Keywords: Antibiotics, pulpitis, abscesses, American Dental Association, evidence-based dentistry
Orofacial pain and swelling, often derived from pulpal and periapical conditions, are common reasons for visiting a dentist.1 Although the national prevalence of pulpal and periapical orofacial pain and intraoral swelling in the dental setting is unknown, Horst and colleagues1 reported in 2015 that among a sample of 1,688 adult dental patients, 9% reported dentoalveolar pain during the past 12 months. Sometimes, patients cannot access a dentist when they experience symptoms and seek out emergency care in nondental settings. From 2011 through 2015, more than 400,000 patients treated in US hospital emergency departments (EDs) had diagnostic codes related to pulpal and periapical conditions, which accounted for 19% of all ED visits associated with a dental diagnosis.2 In 2015, diseases of the teeth and gingiva were among the top 20 reasons for any ED visit in patients aged 15 through 64 years.3
Dental pain associated with pulpal and periapical conditions usually results from caries. As caries progresses into the pulp, the patient can develop reversible pulpitis, in which the pulp becomes inflamed causing either stimulated (for example, response to cold) or unstimulated (for example, spontaneous) pain. If the pulp is incapable of healing and the patient experiences lingering or spontaneous pain with thermal changes, this is known as symptomatic irreversible pulpitis (SIP). Once the inflammation spreads beyond the canal system and into the periodontal ligament space around the root, the patient will experience pain with mastication, percussion, or palpation, with or without evidence of radiographic periapical pathosis, referred to as symptomatic apical periodontitis (SAP). If the pulp does not respond to pulp testing, this is usually a sign that pulp vitality is compromised irreversibly (pulp necrosis). If necrotic pulp is not treated endodontically, it may become infected, and the patient can develop a localized acute apical abscess (LAAA) with formation of purulent material and localized swelling.4,5 If the abscess is left untreated, the infection may spread into adjacent fascial space or local lymph nodes, and the patient may seek treatment for systemic involvement (for example, fever, chills, malaise, or cellulitis) (Table 1).5
Table 1.
Pulpal and periapical target conditions and their clinical signs and symptoms.
| TARGET CONDITION | CHARACTERISTICS OF CLINICAL SIGNS AND SYMPTOMS |
|---|---|
| Symptomatic Irreversible Pulpitis | Spontaneous pain that may linger with thermal changes owing to vital inflamed pulp that is incapable of healing |
| Symptomatic Apical Periodontitis | Pain with mastication, percussion, or palpation, with or without evidence of radiographic periapical pathosis, and without intraoral swelling |
| Pulp Necrosis and Symptomatic Apical Periodontitis | Nonvital pulp, with pain with mastication, percussion, or palpation, with or without evidence of radiographic periapical pathosis, and without intraoral swelling |
| Pulp Necrosis and Localized Acute Apical Abscess | Nonvital pulp, with spontaneous pain with or without mastication, percussion, or palpation; with formation of purulent material and localized swelling; and without evidence of fascial space or local lymph node involvement, fever, or malaise |
| Acute Apical Abscess with Systemic Involvement | Necrotic pulp with spontaneous pain, with or without mastication, percussion, or palpation, with formation of purulent material, swelling, evidence of fascial space or local lymph node involvement, fever, or malaise |
Source: American Association of Endodontists.5
Definitive, conservative dental treatment (DCDT), or tooth-preserving treatments, includes a range of effective strategies to manage the pulpal and periapical conditions described above. DCDT cannot always be provided immediately, and antibiotics are prescribed frequently as an attempt to temporarily manage distressing patient symptoms such as pain and intraoral swelling. From 2011 through 2015, antibiotics were prescribed in 85% of ED visits for pulpal and periapical conditions.2 Antibiotics may be necessary for some patients, and although there is published literature on appropriate versus inappropriate antibiotic types and regimen durations used in dentistry, to our knowledge, no comprehensive guidance exists for United States general dental practitioners on when it may be appropriate versus inappropriate to prescribe antibiotics for pulpal and periapical conditions.6-9 It is also important to note that although antibiotics can be life-saving drugs, their use, whether inappropriate or appropriate, can result in unintended consequences including antibiotic resistance and adverse patient outcomes.10,11
The purpose of this review is to present evidence on the effect of antibiotic therapy compared with no antibiotic therapy, used alone or as adjuncts to DCDT for the treatment of SIP with or without SAP, pulp necrosis and symptomatic apical periodontitis (PN-SAP), or pulp necrosis and localized acute apical abscess (PN-LAAA) in immunocompetent patients (that is, patients with the ability to mount a bacterial challenge). This review was developed by methodologists at the American Dental Association (ADA) Center for Evidence-Based Dentistry and a multidisciplinary group of subject matter experts convened by the ADA Council on Scientific Affairs. Its content informed the development of a clinical practice guideline on the appropriate use of antibiotics for the urgent management of pulpal- and periapical-related pain and intraoral swelling published in The Journal of the American Dental Association.12
METHODS
The Cochrane Collaboration published systematic reviews in 2014 and 2016 on the effects of systemic antibiotics for SAP and LAAA and for SIP in immunocompetent adults, respectively.13,14 We chose to update and integrate both Cochrane reviews as part of our review and followed guidance from the Preferred Reporting Items of Systematic Reviews and Meta-Analyses15 checklist to write this article.
Selection criteria
For the update of the Cochrane reviews,13,14 we adhered to the selection criteria described below.
Type of Studies
Randomized controlled trials (RCTs) with any follow-up time.
Participants
Immunocompetent adults 18 years of age or older, with SIP with or without SAP, PN-SAP, or PN-LAAA, with no other comorbidities. Immunocompromised patients were excluded.
Intervention and Comparison
Administration of any oral systemic antibiotic at any dosage compared with no antibiotic administration, with or without any analgesics at any dosage, with or without DCDT immediately available. DCDT refers to pulpectomy, pulpotomy, nonsurgical root canal treatment, or incision and drainage. Extractions are not considered conservative management (that is, the goal of treatment is to preserve the tooth) and hence were excluded from the scope of this review.
Outcomes
Pain, intraoral swelling, total number of analgesics used, progression of the disease to a more severe state, allergic reactions, and adverse events, including endodontic flare-up, diarrhea, Clostridioides difficile infection (CDI), and repeat procedure. A full listing of outcomes is in the appendix, available at the end of this article.
Additional selection criteria
Anticipating paucity of evidence from RCTs informing harm or undesirable outcomes, we defined additional criteria to expand our review and include observational data. We used the selection criteria described below.
Type of Studies
Systematic reviews of observational studies, defined as explicit reporting of a systematic search including at least 2 databases, published within the past 5 years. We also retrieved individual observational studies, with no date limit, from key health care and government agencies monitoring harms related to antibiotic use. We prioritized studies reporting U.S. national estimates over single-center studies.
Participants
Any person of any age seeking treatment in any dental setting in the United States. If data directly collected from dental settings were not available, we prioritized available data in the following order:
patients seeking treatment in any outpatient setting in the United States;
patients seeking treatment in any health care setting in the United States (for example, hospital or long-term care facility).
Exposures
Patients receiving any systemic antibiotic for the management of any health condition, including the conditions of interest. When the studies included populations of both patients exposed and not exposed to antibiotics, we prioritized the inclusion of those who received antibiotics. When unable to distinguish these 2 populations, we included the study and acknowledged this limitation.
Outcomes
Any harm or undesirable outcome, including but not limited to community-associated CDI, antibiotic-resistant infections, costs, hospitalizations, and anaphylaxis. A full listing of outcomes is in the Appendix, available at the end of this article.
Literature search
In conjunction with the expert panel and methodologists, an informationist (K.K.O.) developed an inclusive search strategy consisting of 3 components:
an update of the 2013 Cochrane review by Cope and colleagues13;
an update of the 2016 Cochrane review by Agnihotry and colleagues14;
a search for systematic reviews on outcomes of harm (undesireable effects) related to antibiotic use.
The published search strategy for the Cope and colleagues13 review was translated into and replicated in all databases being used for this search (search strategy 1 in the Appendix, available at the end of this article). The published search strategy for the Agnihotry and colleagues14 review was adapted for inclusivity by means of combining the antibiotics search string used in the Cope and colleagues review13 with a new, simple pulpectomy and dental pulp concept (search strategy 2 in the Appendix). Database-supplied publication date limits were used to limit from the date of last update onward for both systematic reviews. The informationist used the clinical queries filter to limit to systematic reviews in PubMed,22 and the SIGN filter23 was used to limit to systematic reviews in all other databases for the search for systematic reviews on outcomes of harms related to antibiotic use (search strategy 3 in the Appendix). To limit to adult humans, the informationist used filters based on the model outlined in the Cochrane Handbook for Systematic Reviews of Interventions, chapter 6.4.11.24 Database-supplied limits were applied to restrict to items published within the past 5 years.
We ran all 3 searches in 4 databases: MEDLINE via PubMed, Embase via embase.com, the Cochrane Library 2018, issue 6; and the Cumulative Index to Nursing and Allied Health Literature Complete via EBSCO. We also searched the gray literature (World Health Organization International Clinical Trials Registry Platform, ClinicalTrials.gov, and OpenGrey). We did not apply any restriction on language to any of the searches. In addition, we searched health care and government agencies Web sites and databases and contacted the panel representative, Michele Neuburger, from the Centers for Disease Control and Prevention for additional information on published resources. All searches were completed in late May and early June 2018. In September 2019, and before we submitted this manuscript for publication, we updated the search strategies for MEDLINE via PubMed.
Selection of primary studies and data extraction
The authors of this review independently and in duplicate conducted title and abstract screening of references retrieved from the 3 search strategies:
the update of the Cope and colleagues13 review (H.C., L.P.);
the update of the Agnihotry and colleagues14 review (L.P., O.U.);
the search for outcomes on harms (E.K., L.P., M.P.T., O.U.).
Pairs of reviewers (E.K., H.C., L.P., M.P.T., O.U.) screened the full-text articles of all potentially relevant studies independently and in duplicate. When disagreements occurred and consensus was not achieved, alternate reviewers (A.C.-L., M.P.T.) decided final eligibility (Table 2 shows the excluded studies).
Table 2.
Excluded studies.
| UPDATED SEARCH FOR AGNIHOTRY AND COLLEAGUES,14 2016 | |
|---|---|
| Citation | Reason for Exclusion |
| 1. Segura-Egea JJ, Martín-González J, Jiménez-Sánchez MC, et al. Worldwide pattern of antibiotic prescription in endodontic infections. Int Dent J. 2017;67(4):197-205. | Abstract |
| 2. Agnihotry A, Fedorowicz Z, van Zuuren EJ, Farman AG, Al-Langawi JH. Antibiotic use for irreversible pulpitis. Cochrane Database Syst Rev. 2016;2:CD004969. | Not an RCT* |
| 3. Beus H, Fowler S, Drum M, et al. What is the outcome of an incision and drainage procedure in endodontic patients? A prospective, randomized, single-blind study. J Endod. 2018;44(2):193-201. | Intervention not of interest |
| 4. Haritha N, Lavanya A. A study comparing the effectiveness of two agents with infection reducing properties. 2017. CTRI/2017/05/00847. Available at: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=16477. Accessed September 2, 2018. | Intervention not of interest; study in progress |
| 5. Priya S. Effect of pulpal medicine on periodontal healing. 2017. CTRI/2017/05/008660. Available at: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=16358. Accessed September 2, 2018. | Intervention not of interest; study in progress |
| 6. Del Fabbro M, Corbella S, Sequeira-Byron P, et al. Endodontic procedures for retreatment of periapical lesions. Cochrane Database Syst Rev. 2016;10:CD005511. | Not an RCT |
| 7. Gottlieb M, Khishfe B. Are antibiotics necessary for dental pain without overt infection? Ann Emerg Med. 2017;69(1):128-130. | Review article |
| 8. Noorollahian, N. Evaluation of clinical and radiographic success rate of lesion sterilization and tissue repair in non-vital primary molars. 2016. IRCT2013112615558N1. Available at: https://en.irct.ir/trial/14794. Accessed September 2, 2018. | Intervention not of interest; study in progress |
| 9. Karim K, Kumar K, Naz S, Kumar N. Clinical effect of augmentin as intracanal medicament compared with no any medication on endodontic flare-up in cases of symptomatic apical periodontitis: a pilot study. Med Forum. 2016;27(9):28-31. | Intervention not of interest |
| 10. Lee, MB. Antibiotic use [letter]. JADA. 2016;147(8):601-602. | Letter to the editor |
| 11. Miyashita H, Worthington HV, Qualtrough A, Plasschaert A. Pulp management for caries in adults: maintaining pulp vitality. Cochrane Database Syst Rev. 2016;11:CD004484. | Withdrawn article |
| 12. Miyashita H, Worthington HV, Qualtrough A, Plasschaert A. Pulp management for caries in adults: maintaining pulp vitality. Cochrane Database Syst Rev. 2016;11:CD004484. | Duplicate |
| 13. Huang X, Wu M. Effect of photodynamic therapy on deep caries in permanent tooth: a controlled clinical trial. 2016. NCT02929914. Available at: https://clinicaltrials.gov/ct2/show/nct02929914. Accessed September 2, 2018. | Intervention not of interest; study in progress |
| 14. Tolby N, Olkkola S, Chea I. The effects of dexamethasone on the time to pain resolution in dental periapical abscess. NCT03005522. Available at: https://clinicaltrials.gov/ct2/show/nct03005522. Accessed September 2, 2018. | Intervention not of interest; study in progress |
| 15. Iorio Lopes Pontes Póvoa, N. Antimicrobial photodynamic therapy associated with the conventional endodontic treatment: a clinical and microbiological study. 2017. NCT03212729. Available at: https://clinicaltrials.gov/ct2/show/nct03212729. Accessed September 2, 2018. | Intervention not of interest |
| 16. Oclay K. Postoperative pain in single-visit and multiple-visit retreatment cases. 2017. NCT03042377. Available at: https://clinicaltrials.gov/ct2/show/nct03042377. Accessed September 2, 2018. | Intervention not of interest |
| 17. Sevekar SA, Gowda SHN. Postoperative pain and flare-ups: comparison of incidence between single and multiple visit pulpectomy in primary molars. J Clin Diagn Res. 2017;11(3):ZC09-ZC12. | Intervention not of interest |
| 18. Singh RK, Shakya VK, Khanna R, et al. Interventions for managing immature permanent teeth with necrotic pulps. Cochrane Database Syst Rev. 2017;6:CD012709. | Study protocol |
| 19. Sheesh F. Effect of occlusal reduction on post-operative pain. 2017. NCT03189771. Available at: https://clinicaltrials.gov/ct2/show/NCT03189771. Accessed September 2, 2018. | Intervention not of interest; study in progress |
| 20. Jia Z, Yu DU, Yuan DU, Jiang C. Interleukin-17 in apical exudates of periapical periodontitis treated with minocycline controlled-release formulation. Chin J Tissue Eng Res. 2017:21(10):1508-1513. | Intervention not of interest |
| UPDATED SEARCH FOR COPE AND COLLEAGUES,13 2014 | |
| Citation | Reason for Exclusion |
| 21. Parfenov SA. Therapy of chronic apical periodontitis in the elderly age. Adv Gerontol. 2013;26(3):553-557. | Population not of interest |
| 22. Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. Pediatr Dent. 2016;38(6):402-411. | Review article |
| 23. Albandar JM. Aggressive and acute periodontal diseases. Periodontol. 2000. 2014;65(1):7-12. | Review article |
| 24. Asmar G, Cochelard D, Mokhbat J, Lemdani M, Haddadi A, Ayoubz F. Prophylactic and therapeutic antibiotic patterns of Lebanese dentists for the management of dentoalveolar abscesses. J Contemp Dent Pract. 2016;17(6):425-433. | Outcomes reported not of interest |
| 25. Cope A, Francis N, Wood F, Mann MK, Chestnutt IG. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Syst Rev. 2014;6:CD010136. | Not an RCT |
| 26. Deffez JP, Scheimberg A, Rezvani Y. Multicenter double-blind study of the efficacy and tolerance of roxithromycin versus erythromycin ethylsuccinate in acute orodental infection in adults. Diagn Microbiol Infect Dis. 1992;15(4 suppl):133S-137S. | Population not of interest |
| 27. Del Fabbro M, Corbella S, Sequeira-Byron, et al. Endodontic procedures for retreatment of periapical lesions. Cochrane Database Syst Rev. 2016;10:CD005511. | Not an RCT |
| 28. Enezei HH, Alam MK. Survival analysis for the use of two types of antibiotics in the remedy of mandibular third molar deep abscess. J Int Med. 2015;22(5):430-432. | Not an RCT |
| 29. Herrera D, Alonso B, de Arriba L, Santa Cruz I, Serrano C, Sanz M. Acute periodontal lesions. Periodontol 2000. 2014;65(1):149-177. | Review article |
| 30. Hodgdon A. Dental and related infections. Emerg Med Clin North Am. 2013;31(2):465-480. | Review article |
| 31. Holmes CJ, Pellecchia R. Antimicrobial therapy in management of odontogenic infections in general dentistry. Dent Clin North Am. 2016;60(2):497-507. | Review article |
| 32. Iheozor-Ejiofor Z, Middleton P, Esposito M, Glenny AM. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database Syst Rev. 2017;6:CD005297. | Not an RCT |
| 33. Keine KC, Kuga MC, Pereira KF, et al. Differential diagnosis and treatment proposal for acute endodontic infection. J Contemp Dent Pract. 2015;16(12):977-983. | Review article |
| 34. Li C, Lv Z, Shi Z, et al. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. 2014;11:CD009197. | Not an RCT |
| 35. Manfredi M, Figini L, Gagliani M, Lodi G. Single versus multiple visits for endodontic treatment of permanent teeth. Cochrane Database Syst Rev. 2016;12:CD005296. | Not an RCT |
| 36. Meschi N, Fieuws S, Vanhoenacke, A, et al. Root-end surgery with leucocyte-and platelet-rich fibrin and an occlusive membrane: a randomized controlled clinical trial on patients’ quality of life. Clin Oral Investig. 2018;(22):2401-2411. | Intervention not of interest |
| 37. Gartshore L, Youngston CC. Comparison of two dental techniques used to treat teeth which have become infected or painful following trauma. 2013. NCT01817413. Available at: https://clinicaltrials.gov/ct2/show/nct01817413. Accessed September 2, 2018. | Study in progress |
| 38. Gomaa A, Ezzat K, Amin SAW. Effect of amoxicillin/clavulanic acid combination on postoperative endodontic pain. 2017. NCT03007342. Available at: https://clinicaltrials.gov/ct2/show/nct03007342. Accessed September 2, 2018. | Study in progress |
| 39. Moushtaha NNT. Effect of preoperative amoxicillin/clavulanic acid combination on postoperative endodontic pain. 2017. NCT03033147. Available at: https://clinicaltrials.gov/ct2/show/nct03033147. Accessed September 2, 2018. | Study in progress |
| 40. El Sedawy NSA, Wanees SAW, Gawdat S. Effect of preoperative clindamycin on postoperative endodontic pain. 2017. NCT03033472. Available at: https://clinicaltrials.gov/ct2/show/nct03033472. Accessed September 2, 2018. | Study in progress |
| 41. Robertson DP, Keys W, Rautemaa-Richardson R, Burns R, Smith AJ. Management of severe acute dental infections. BMJ. 2015;350:h1300. | Review article |
| 42. Segura-Egea JJ, Martín-González J, Jiménez-Sánchez MDC, Crespo-Gallardo I, Saúco-Márquez JJ, Velasco-Ortega E. Worldwide pattern of antibiotic prescription in endodontic infections. Int Dent J. 2017;67(4):197-205. | Review article |
| 43. Simpson TC, Weldon JC, Worthington HV, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015;11:CD004714. | Not an RCT |
| 44. Singh RK, Shakya VK, Khanna R, at al. Interventions for managing immature permanent teeth with necrotic pulps. Cochrane Database Systemat Rev. 2017;6:CD12709. | Study protocol |
| 45. Tichter A, Perry K. Are antibiotics beneficial for the treatment of symptomatic dental infections? Ann Emerg Med. 2015;65(3):332-333. | Review article |
| 46. Veitz-Keenan A, De Bartolo AM. Insufficient evidence of the effect of systemic antibiotics on adults with symptomatic apical periodontitis or acute apical abscess. Evid Based Dent. 2014;15(4):104-105. | Review article |
| NON-COCHRANE SYSTEMATIC REVIEWS WITH OUTCOMES ON HARMS RELATED TO ANTIBIOTIC USE | |
| Citation | Reason for Exclusion |
| 47. Bassetti M, Poulakou G, Ruppe E, Bouza E, Van Hal SJ, Brink A. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. 2017;4310:1464-1475. | Outcomes reported not of interest |
| 48. Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014;14(1):13. | Outcomes reported not of interest |
| 49. Birgand G, Moore LS, Bourigault C, et al. Measures to eradicate multidrug-resistant organism outbreaks: how much do they cost? Clin Microbiol Infect. 2016;22(2):162.e1-162.e9. | Population included not of interest |
| 50. Drekonja DM, Filice GA, Greer N, et al. Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol. 2015;36(2):142-152. | Outcomes reported not of interest |
| 51. Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One. 2017;12(12):e0189621. | Population not of interest |
| 52. Lang PM, Jacinto RC, Dal Pizzol TS, Ferreira MBC, Montagner F. Resistance profiles to antimicrobial agents in bacteria isolated from acute endodontic infections: systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48(5):467-474. | Outcomes reported not of interest |
| 53. Löffler C, Böhmer F. The effect of interventions aiming to optimise the prescription of antibiotics in dental care: a systematic review. PLoS One. 2017;12(11):e0188061. | Outcomes reported not of interest |
| 54. McGowan K, McGowan T, Ivanovski S. Optimal dose and duration of amoxicillin-plus-metronidazole as an adjunct to non-surgical periodontal therapy: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Periodontol. 2018;45(1):56-67. | Population not of interest |
| 55. Moraes LC, Só MVR, Dal Pizzol TDS, Ferreira MB., Montagner F. Distribution of genes related to antimicrobial resistance in different oral environments: a systematic review. J Endod. 2015;41(4):434-441. | Outcomes reported not of interest |
RCT: Randomized controlled trial.
Pairs of reviewers (L.P., M.P.T., O.U.) independently extracted outcome data from the relevant studies using standardized forms. Abstracted study characteristics from reports included country, study design, patient characteristics, follow-up time, intervention characteristics, description of included study population, observation and data collection period, methods, conflicts of interest, and funding source. We contacted primary study authors when clarification was needed.
Outcome measures
We analyzed pain as continuous outcomes and dichotomized ordinal scales and analyzed intraoral swelling as dichotomized ordinal scales (Appendix, available at the end of this article).
We presented dichotomous outcomes using relative risks and continuous outcomes using mean differences, both accompanied by their 95% confidence intervals (Appendix). For beneficial outcomes, we calculated absolute measures for all relative measures using baseline risks (control group risk). For harm outcomes, we presented data using a common denominator of 10,000 or 100,000 for ease of comparison between outcomes, if possible.
Statistical analysis
We conducted meta-analysis using a random-effects model to obtain pooled estimates using Review Manager, Version 5.3 (Cochrane Collaboration). When meta-analysis was not possible (for example, owing to population differences between studies), we attempted to calculate and report relative risks and mean differences at an individual study level. When data directly informing the impact of antibiotic prescriptions in dentistry were not available, we calculated both the overall estimate for all prescriptions in the health care system and illustrated the potential impact of antibiotics prescribed by dentists via attributing 10% of the burden of harm outcomes to dental prescriptions. This was based on estimations that suggest that dentistry accounts for approximately one-tenth of total outpatient antibiotic prescriptions by all providers in the United States (third highest prescribers among all health care specialties).8,25,26 We also calculated the national CDI burden estimates to specify burden of CDIs and hospitalizations that are community associated and, if possible, community-associated CDIs attributable to antibiotic prescribing and consumption; we adjusted our analysis considering that 64% of community-associated CDIs are associated with antibiotic consumption and that 12% of community-associated CDIs are the primary reason for hospital admissions.27
Assessment of risk of bias and methodological quality
Two pairs of reviewers (L.P., M.P.T., O.U.) independently assessed the risk of bias of the included studies and the quality of any preexisting reviews, using the Cochrane Risk of Bias tool, Hoy and colleagues,19 and AMSTAR 2 appraisal tool.20 Any disagreements in judgments were resolved by a third reviewer (A.C.-L.) (Appendix, available at the end of this article).
Certainty in the evidence
We assessed the certainty in the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach across studies at an outcome level (Appendix, available at the end of this article).21
RESULTS
Characteristics of included studies
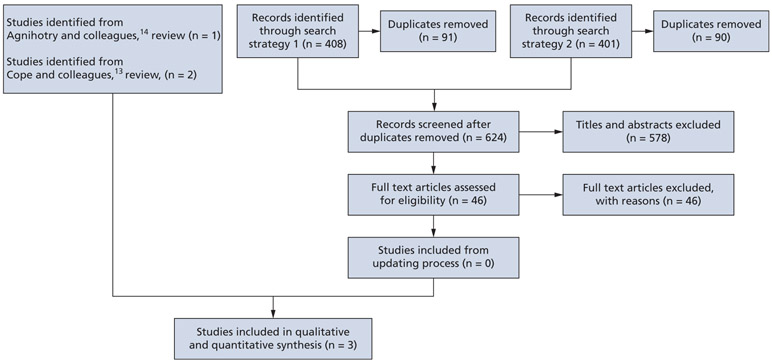
We used the reviews by Agnihotry and colleagues14 and Cope and colleagues13 containing 3 RCTs to inform benefits and harms of antibiotic use for the target conditions. In our search to update both reviews,13,14 we screened 628 titles and abstracts and 46 citations for full-text screening and found no studies meeting our selection criteria (Figure 1). These 3 RCTs were conducted in the United States and included adult patients seeking emergency treatment of the target conditions (number of patients who completed the trials, 111) (Table 3).16-18 Patients in the intervention groups received antibiotics with or without DCDT, whereas those in the control groups received either no antibiotics or placebo, with or without DCDT. Patients in both intervention and control groups received analgesics (ibuprofen) with or without rescue analgesics (acetaminophen plus codeine), as well as written and verbal instructions for the management of pain.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses15 flowchart of the screening and study-selection process for randomized controlled trials.
Table 3.
Characteristics of included randomized controlled trials.*
| STUDY, STUDY ARM | DESCRIPTION OF INCLUDED PATIENT POPULATION |
AGE, Y, MEAN (STANDARD DEVIATION) |
SEX, % FEMALE |
FOLLOW-UP TIMES |
DENTAL INTERVENTION PERFORMED |
FUNDING SOURCE |
NOTES |
|---|---|---|---|---|---|---|---|
| Fouad and Colleagues,16 1996 | |||||||
| Endodontic treatment (partial or total pulpectomy) and drainage (if necessary) plus 500 milligrams of penicillin (2 tablets at the end of the visit, followed by 1 tablet 4 times daily, for 7 d) plus 600 mg of ibuprofen (before endodontic treatment and 4 times daily for 24 h after treatment, then as needed) Endodontic treatment (partial or total pulpectomy) and drainage (if necessary) plus placebo tablets (2 tablets at the end of the visit, followed by 1 tablet 4 times daily, for 7 d) or no medicine plus 600 mg of ibuprofen (before endodontic treatment and 4 times daily for 24 h after treatment, then as needed)† |
Healthy adults seeking emergency treatment and diagnosed with acute apical abscess Patients had pulp necrosis with periapical pain, swelling |
34.92 (17.33) (1 age not recorded) 35.57 (9.43) (4 ages not recorded)† |
33.3% (1 sex not recorded) 50% (3 sex not recorded)† |
6 h, 12 h,1 d,‡ 2 d,‡ 3 d‡ | "All were then treated as follows: after local anesthesia, the offending tooth was accessed, the working length determined and cleaning and shaping of the canals was either partially or completely done (depending on the availability of time) with copious irrigation with 2.6% sodium hypochlorite. Canals were dried, medicated with calcium hydroxide paste, and then temporized with Cavit or IRM. When indicated, a localized intraoral swelling was incised for drainage with a drain inserted for 24 to 48 hours."16 | Not reported | Reporting in the study did not allow for ascertaining the timing of the initiation of antibiotic therapy in relation to definitive, conservative dental treatment. During the 3-day follow-up period, 1 participant in the placebo group reported diarrhea. One patient in the penicillin group experienced fatigue and reduced energy postoperatively. Two people in the placebo group experienced flare-ups, and 2 in the no placebo group experienced flare-ups. |
| Nagle and Colleagues,18 2000 | |||||||
| 500 mg capsule of penicillin (every 6 h for 7 d) plus 600 mg tablet of ibuprofen (1 tablet every 4-6 h, as needed) plus 300 mg acetaminophen with 30 mg of codeine (2 tablets, every 4-6 h, as needed if ibuprofen did not work) 500 mg capsule of placebo control with lactose (every 6 h for 7 d) plus 600 mg tablet of ibuprofen (1 tablet every 4-6 h, as needed) plus 300 mg acetaminophen with 30 mg of codeine (2 tablets, every 4-6 h, as needed if ibuprofen did not work) |
Healthy adult patients seeking emergency treatment with a clinical diagnosis of irreversible pulpitis Experienced spontaneous moderate to severe pain and percussion sensitivity associated with the tooth | 30 (9.8) 34 (11.6) |
42.5% | 1 d,‡ 2 d,‡ 3 d,‡ 4 d, 5 d, 6 d, 7 d‡ | None | Supported by research funding from the Endodontic Graduate Student Research Fund and the Steve Goldberg Memorial Fund, The Ohio State University | No assessment of adverse effects to either the antibiotics or analgesics were reported by the investigators. |
| Henry and Colleagues,17 2001 | |||||||
| Endodontic treatment (total pulpectomy) plus 500 mg of penicillin (28 capsules total, taken every 6 h for 7 d) plus 200 mg tablets of ibuprofen (2 tablets every 4-6 h as needed) plus 300 mg acetaminophen with 30 mg codeine (1 or 2 tablets every 4 h, as needed if ibuprofen did not work) Endodontic treatment (total pulpectomy) plus 500 mg of placebo (lactose) (28 capsules total, taken every 6 h for 7 d) 200 mg tablets of ibuprofen (2 tablets every 4-6 h as needed) plus 300 mg acetaminophen with 30 mg codeine (1 or 2 tablets every 4 h, as needed if ibuprofen did not work) |
Healthy adult patients seeking emergency treatment with clinical diagnosis of symptomatic necrotic teeth who actively had spontaneous pain | 37 (16.5) 38 (18.8) |
48.8% | 1 d,‡ 2 d,‡ 3 d,‡ 4 d, 5 d, 6 d, 7 d‡ | "The canals were prepared using a stepback preparation and K-type files (L.D. Caulk, Inc., Milford, DE). The canals were irrigated with 2.62% sodium hypochlorite initially and after every other file placed to working length. Complete biomechanical preparation of all canals was accomplished. The canals were dried and a sterile cotton pellet was placed over the canal orifices, and the access opening was sealed with Cavit. The occlusion was not adjusted."17 | Funding from the Graduate Endodontic Student Research Fund and the Goldberg Memorial Fund, Graduate Endodontics, College of Dentistry, The Ohio State University | Reporting in the study did not allow for ascertaining of the timing of the initiation of antibiotic therapy in relation to definitive, conservative dental treatment. No assessment of adverse effects to either the antibiotics or analgesics were reported by the investigators. |
There were no conflicts of interest reported by the authors in the 3 studies. All of the studies were conducted in the United States.
Due to a lack of clinical difference, any placebo or no medication arms described by study authors were considered as “no antibiotics” for data analysis.
Follow-up time analyzed.
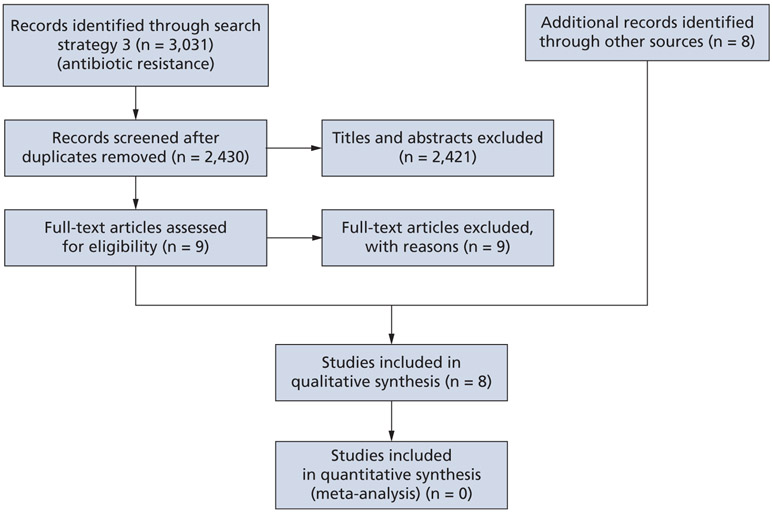
To collect additional harm outcome data not available through RCTs, we screened 2,430 titles and abstracts from search strategy 3 (Appendix, available at the end of this article) and selected 9 reports for full-text screening; ultimately, none were included. We found 8 individual reports through searching in health care and government agencies databases and resources (Figure 2).11,26-32 These studies, published between 2011 and 2019, were all conducted in the United States and used either a cross-sectional, active population and laboratory-based surveillance, or systematic review methodology to obtain their results (Table 4).11,26-32
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses15 flowchart of the screening and study-selection process for systematic reviews.
Table 4.
Characteristics of included observational studies.
| STUDY | STUDY DESIGN | DESCRIPTION OF INCLUDED PATIENT OR STUDY POPULATION |
AGE, Y | SEX, % FEMALE |
|---|---|---|---|---|
| Mainous and Colleagues,31 2011 | Active population- and laboratory-based surveillance | Patient hospitalization associated with antibiotic-resistant infections in the United States | Not reported | Not reported |
| Centers for Disease Control and Prevention,11 2013 | Not reported | Not reported | Not reported | Not reported |
| Chitnis and Colleagues,27 2013 | Active population- and laboratory-based surveillance | "Sequential sample of patients with putative community-associated CDI† was contacted by telephone for an interview in 8 of 10 US surveillance sites … patients not reporting an overnight stay were classified as confirmed patients with community-associated CDI and were asked additional questions …"27 | Median (range), 51 (1-97) | 66.6% |
| Hicks and Colleagues,26 2015 | Cross-sectional | Patients who were prescribed systemic oral antibiotics in the United States during 2011 | All age groups were included in the sample | 60% |
| OBSERVATION (DATA COLLECTION) PERIOD |
METHODS | PERTINENT OUTCOMES |
DESCRIPTION OF THE DATA | CONFLICTS OF INTEREST |
FUNDING SOURCE |
|---|---|---|---|---|---|
| January 1, 1997 through December 31, 2006 | Conducted an analysis of the NHDS* of 1997-2006 | Admission to hospital due to antibiotic-resistant infection | "Discharge survey data (NHDS) during 1997 to 2006. The NHDS covers approximately 270,000 patients per year in 500 short-stay hospitals by using a stratified, multistage survey to create a nationally representative annual sample of discharge records. Children’s and general hospitals are included; federal, military, Veterans Affairs, and institutional hospitals are not included. Each discharge record contains up to seven different International Classification of Diseases, Ninth Revision (ICD-9), Clinical Modification discharge diagnosis codes; is population-weighted on the basis of the probability of sample selection; and is adjusted for nonresponse. Nationally representative estimates of hospitalizations in the U.S. can be computed with the NHDS. We included all acute-care hospitalizations in the analysis."31 | Not reported | Supported in part by contract HHSA290 2007 10015 from the Agency for Healthcare Research and Quality |
| Not reported | Not reported | Antibiotic-resistant infections, mortality due to antibiotic-resistant infections, antibiotic-resistant infection related costs | Not reported | Not reported | Not reported |
| January 1, 2009, through May 31, 2011 | "Medical records were reviewed and interviews performed to assess outpatient, household, and food exposures among patients with community-associated CDI (i.e., toxin or molecular assay positive for C. difficile and no overnight stay in a health care facility within 12 weeks). Molecular characterization of C. difficile isolates was performed."27 | Hospitalizations in which CDI was the primary reason; antibiotic use within 12 wk before CDI | Clinical characteristics, outcomes, demographics, and exposures among patients with community-associated infections. | None reported | "This work was funded by the Emerging Infections Program Cooperative Agreement between study sites and the Centers for Disease Control and Prevention under the following grants: U50CK000201 (California), U50CK000194 (Colorado), U50CK000195 (Connecticut), U50CK000196 (Georgia), U50CK000203 (Maryland), U50CK000204 (Minnesota), U50CK000199 (New York), and U50CK000198 (Tennessee)."27 |
| January 1, 2011, through December 31, 2011 | "Systemic, oral antibiotic prescriptions dispensed by US county during 2011 were extracted from the IMS Health Xponent database. IMS Health captures >70% of all outpatient prescriptions in the United States, reconciles them to wholesale deliveries, and projects to 100% coverage of all prescription activity using a patented projection method based on a comprehensive sample of patient deidentified prescription transactions, collected from pharmacies that report their entire pharmacy business to IMS Health each week."26 | Antibiotic prescribing rate of general dentists | "These data represent all outpatient antibiotic prescriptions, across all payers, including community pharmacies and nongovernmental mail service pharmacies."26 | "R.J.H. is an employee of IMS Health. All other authors report no potential conflicts."26 | Not reported |
| STUDY | STUDY DESIGN | DESCRIPTION OF INCLUDED PATIENT OR STUDY POPULATION |
AGE, Y | SEX, % FEMALE |
|---|---|---|---|---|
| Lessa and Colleagues,30 2015 | Active population- and laboratory-based surveillance | Patients with CDI in 10 Centers for Disease Control EIP‡ sites, which spanned across 34 counties | ≥ 1 y | Not reported |
| Zhang and Colleagues,32 2016 | Systematic review and meta-analysis | "Most studies (n = 15) investigated economic outcomes in all age inpatients. Three studies reported cost data in children less than 20 years old. Other studies investigated complicated CDI in high-risk patient groups, such as those with major surgery (n = 16), inflammatory bowel diseases (n = 2), liver or renal disease (n = 4), elderly (n = 2) and ICU patients (n = 1). There was 1 study each in nonsurgical inpatients, sepsis inpatients, and patients with prolonged acute mechanical ventilation. There was 1 study focusing only on recurrent CDI in the general population."32 | "The mean/median age of the CDI patient groups ranged from 47.4 to 73.0 years."32 | Not reported |
| Dhopeshwarkar and Colleagues,28 2019 | Cross-sectional | Patients who visited Brigham and Women’s Hospital or Massachusetts General Hospital and who had allergies that were either observed by clinicians directly in the health care setting or reported by patients as having occurred previously. | Not reported | 57.92% |
| OBSERVATION (DATA COLLECTION) PERIOD |
METHODS | PERTINENT OUTCOMES | DESCRIPTION OF THE DATA |
CONFLICTS OF INTEREST |
FUNDING SOURCE |
|---|---|---|---|---|---|
| January 1, 2011, through December 31, 2011 | "Performed an initial medical-record review to collect data on demographic characteristics, the location of stool collections, and health care exposures on all cases of C. difficile infection in 8 of the 10 EIP sites … Classified cases as either ‘community-associated’ or ‘health-care’ associated … A convenience sample of clinical laboratories across the EIP sites (37 laboratories) submitted all C. difficile—positive stool specimens from cases with full medical-record review for culture … Between November 2011 and January 2012, all laboratories serving the surveillance population were surveyed to assess the type of C. difficile diagnostic tests that were used during 2011."30 | Community-associated CDI, mortality due to community-associated CDI, community-associated CDI related costs, admission to hospital due to community-associated CDI | “This surveillance was expanded to 10 sites in 2011 to provide better national estimates of disease burden, incidence, recurrence, and mortality by capturing data across the spectrum of health care delivery and community settings.”30 | "Disclosure forms provided by the authors are available with the full text of the article."30 | EIP Cooperative Agreement between 10 EIP sites and the Centers for Disease Control and Prevention |
| Search conducted July 2015 (studies were published from 1997-2012) | Conducted a systematic review and meta-analysis of available evidence regarding health care costs attributed to CDI | Community-associated CDI related costs, length of hospital stay due to community-associated CDI | "Most studies (n = 27) used national level databases, with 17 used National Independent Sample (NIS) database and the remaining 10 studies extracted data from various national databases. Fifteen studies were conducted at state level, of which 6 studies only collected data in single hospital. All studies reported cost in hospital level of care, no articles identified in LTCF and community. Nearly all identified references were retrospective hospital database studies (n = 40) and only 1 study was a prospective observational study and another study was a decision tree model."32 | “Three of the six study authors are employees of Sanofi Pasteur.”32 | Sanofi Pasteur |
| January 1, 1995, through December 31, 2013 | "Data were collected from Partners HealthCare System (PHS), an integrated healthcare delivery network in the Greater Boston area … . At PHS, patient allergy information captured by the EHR allergy module was integrated into the Partners’ Enterprise-wide Allergy Repository (PEAR), resulting in a longitudinal allergy record accessible across the healthcare network. Included patients had allergies that were either observed by clinicians directly in the healthcare setting or reported by patients as having occurred previously … . Patients were considered to have reported anaphylaxis if the reaction recorded in PEAR was either coded ‘anaphylaxis’ or a free-text entry that mapped to ‘anaphylaxis’ because of synonyms (e.g., anaphylactic reaction, anaphylactic) or a misspelling (e.g., anaphylactic, anaphylaxis)."28 | Anaphylaxis due to antibiotic drugs and drug classes | Prevalence and incidence rates of drug-induced anaphylaxis by drug class | “ND is a St. John’s University postdoctoral fellow with Daiichi Sankyo, Inc. RD is an MCPHS University postdoctoral fellow with Sanofi Genzyme. AS, MT, DWB, KGB, and LZ report no conflicts of interest.”28 | “Agency for Healthcare Research and Quality (AHRQ) R01HS022728, the National Institute of Allergy and Infectious Diseases (NIAID) K01AI125631, and the American Academy of Allergy, Asthma and Immunology (AAAAI) Foundation.”28 |
| STUDY | STUDY DESIGN | DESCRIPTION OF INCLUDED PATIENT OR STUDY POPULATION |
AGE, Y | SEX, % FEMALE |
|---|---|---|---|---|
| Johnston and Colleagues,29 2019 | Cross-sectional | “We identified patients with a discharge diagnosis of one or more of the bacterial infections… during their inpatient stay using ICD-9-CM codes. Similar approaches have been previously validated for identification of patients with bacterial infection during inpatient hospitalization.”29 | Range of means (standard deviation), 56.6 (21.9)-65.2 (19.3) | Range, 45.6-61.6 |
| OBSERVATION (DATA COLLECTION) PERIOD | METHODS | PERTINENT OUTCOMES |
DESCRIPTION OF THE DATA | CONFLICTS OF INTEREST |
FUNDING SOURCE |
|---|---|---|---|---|---|
| January 1, 2014, through December 31, 2014 | Conducted an analysis of the National Inpatient Sample for 2014 | Length of hospital stay due to antibiotic-resistant infections | "Clinical characteristics inpatient stays for patients with bacterial infection.”29 | “K.J.J. holds an academic appointment at SLUCOR. K.E.T. serves as Chairman of the Partnership to Fight Chronic Disease. D.J.M.”29 | “This work was supported by the Saint Louis University Center for Outcomes Research (SLUCOR) as well as Merck and Co. SLUCOR purchased and provided access to the data used in this study. Merck and Co. provided an unrestricted grant to the Partnership to Fight Chronic Disease to support the analysis.”29 |
NHDS: National Hospitalization Discharge Survey.
CDI: Clostridioides difficile infection.
EIP: Emerging Infections Program.
Risk of bias and methodological quality assessment
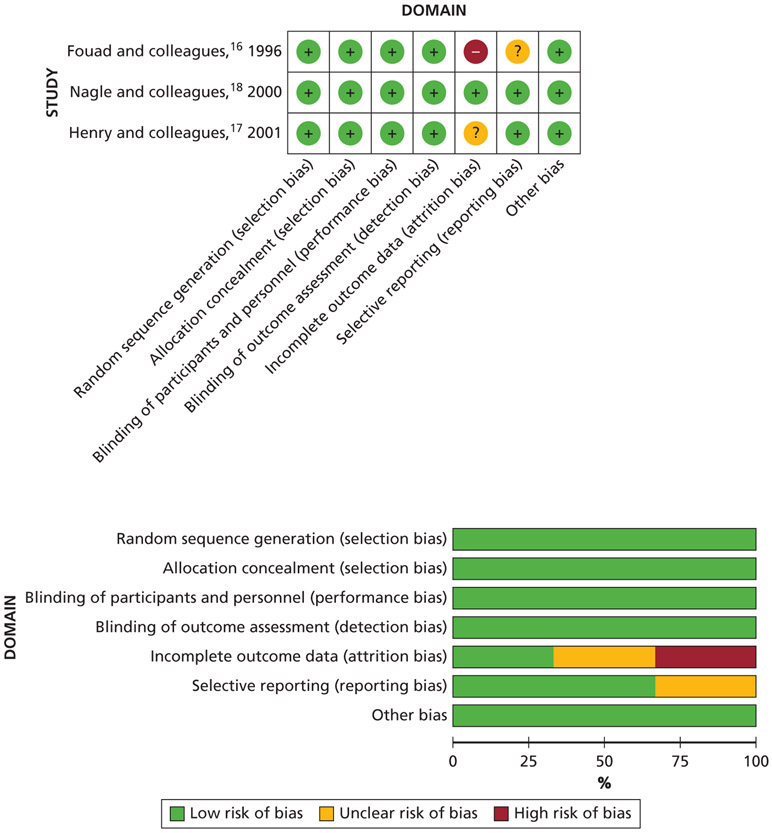
For the included RCTs, a full risk of bias assessment was not possible because reporting issues forced unclear judgments for selective reporting and incomplete outcome data. We determined that the domain of incomplete outcome data was the most serious methodological concern among the 3 studies (Figure 3).16-18
Figure 3.
Risk of bias analysis of included randomized controlled trials. (+): Low risk of bias. (−): High risk of bias. (?): Unclear risk of bias.
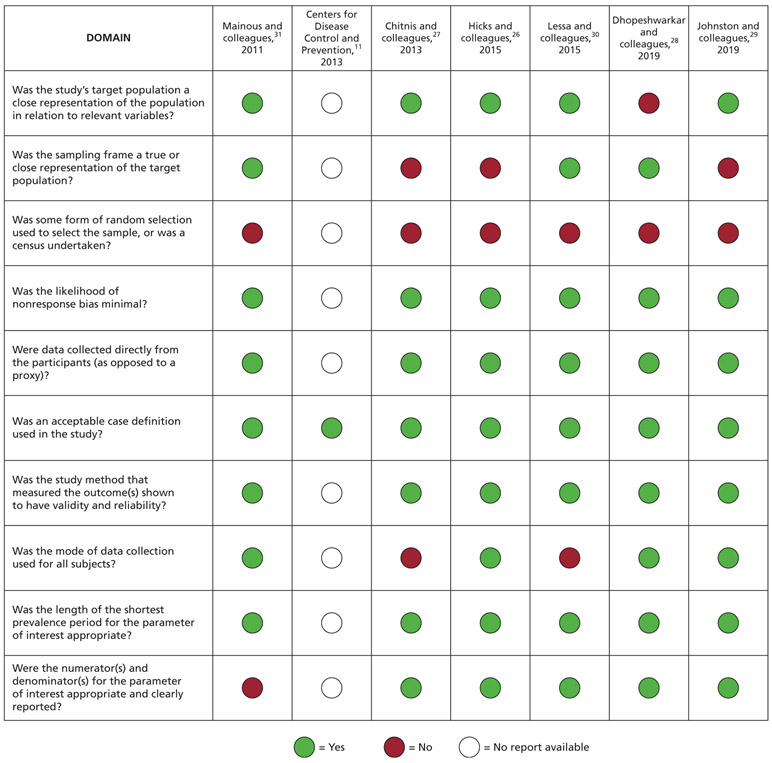
For the observational reports informing additional harm or undesirable outcomes, 1 systematic review32 was judged to be of poor methodological quality and 6 individual studies26-31 were judged as at low risk of bias. For the systematic review,32 the most serious methodological concerns were lack of a protocol, limited risk of bias assessment, and limited information on meta-analytical methods. For the 6 remaining reports,26-31 random sample selection did not occur among most of the included studies. A full risk of bias assessment was not possible for 1 study11 owing to poor reporting and, therefore, we were unable to assess most of the risk of bias domains (Figure 4).26-31
Figure 4.
Risk of bias of included observational studies.
Effects of interventions
No DCDT Available: Oral Systemic Antibiotics Compared With the Nonuse of Oral Systemic Antibiotics
SIP with or without SAP
One study (N = 40, 7-day follow-up) informed the effect of antibiotics for improving the following beneficial outcomes in immunocompetent adults with SIP with or without SAP.18 We located data for all outcomes except endodontic flare-up, diarrhea, CDI, allergic reaction, repeat procedure, and progression of disease to a more severe state such as malaise and trismus (Appendix, available at the end of this article) for this population. The study authors did report intraoral swelling, but owing to symptom inconsistencies with a clinical diagnosis of SIP with or without SAP, we disregarded these data (Table 1).18
Patient-reported pain intensity and experience
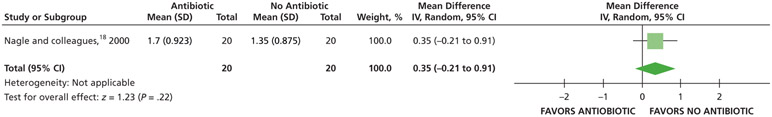
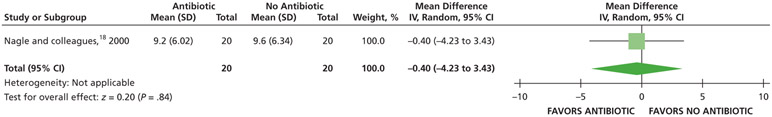
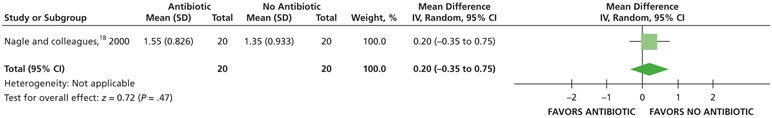
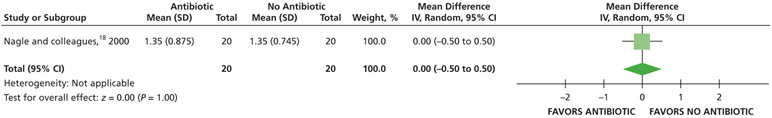
When receiving antibiotics, patients may experience differences of less than one-half a point on a visual analog scale (VAS) of pain, ranging from 0 through 3, compared with patients who did not receive antibiotics over 7 days (24, 48, and 72 hours and 7 days) (low certainty) (Table 5; Figures 5-8).18
Table 5.
Relative and absolute desirable and undesirable effects (95% confidence interval) from randomized controlled trials and certainty in the evidence for systemic antibiotics compared with no systemic antibiotics for symptomatic irreversible pulpitis with or without symptomatic apical periodontitis in immunocompetent adults when definitive, conservative dental treatment is not available.
| OUTCOMES* | PARTICIPANTS (STUDIES), NO. |
CERTAINTY OF THE EVIDENCE ACCORDING TO GRADE† |
RR‡ (95% CONFIDENCE INTERVAL) |
ANTICIPATED ABSOLUTE EFFECTS | |
|---|---|---|---|---|---|
| Risk With No Systemic Antibiotic§ (No. of People) |
Risk Difference With Systemic Antibiotics (Range) |
||||
| Pain Intensity at 24 H | 40 (1 RCT¶,#) | Low** | Not applicable | Mean pain intensity at 24 h, 1.35 | MD,†† 0.35 higher (0.21 lower - 0.91 higher) |
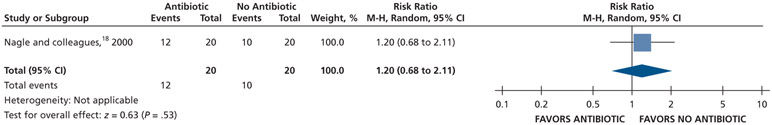
| Pain Experience at 24 H | 40 (1 RCT#) | Low‡‡ | RR, 1.20, (0.68 to 2.11)§§ |
500 per 1,000 | 100 more per 1,000 (160 fewer - 555 more)§§ |
| Pain Intensity at 48 H | 40 (1 RCT#) | Low** | Not applicable | Mean pain intensity at 48 h, 1.35 | MD, 0.2 higher (0.35 lower - 0.75 higher) |
| Pain Experience at 48 H | 40 (1 RCT#) | Low‡‡ | RR, 1.22 (0.65 to 2.29)§§ |
450 per 1,000 | 99 more per 1,000 (158 fewer - 581 more)§§ |
| Pain Intensity at 72 H | 40 (1 RCT#) | Low** | Not applicable | Mean pain intensity at 72 h, 1.35 | MD, 0 (0.5 lower - 0.5 higher) |
| Pain Experience at 72 H | 40 (1 RCT#) | Low‡‡ | RR, 1.00 (0.47 to 2.14)§§ |
400 per 1,000 | 0 fewer per 1,000 (212 fewer - 456 more)§§ |
| Pain Intensity at 7 D | 40 (1 RCT#) | Low** | Not applicable | Mean pain intensity at 7 d, 1.35 | MD, 0.15 lower (0.75 lower - 0.45 higher) |
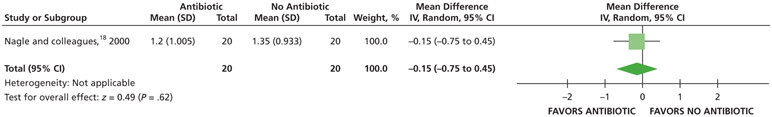
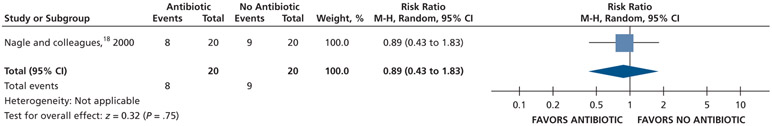
| Pain Experience at 7 D | 40 (1 RCT#) | Low‡‡ | RR, 0.89 (0.43 to 1.83)§§ |
450 per 1,000 | 49 fewer per 1,000 (257 fewer - 374 more)§§ |
| Total Number of Nonsteroidal Anti-inflammatory Drugs (Tablets) Used | 40 (1 RCT#) | Low** | Not applicable | Mean total number of nonsteroidal antiinflammatory drugs (tablets) used, 9.6 | MD, 0.4 lower (4.23 lower - 3.43 higher) |
| Total Number of Acetaminophen with Codeine (Tablets) Used | 40 (1 RCT#) | Low¶¶ | Not applicable | Mean total number of acetaminophen with codeine (tablets) used, 4.45 | MD 2.45 higher (1.23 lower - 6.13 higher) |
Selection criteria: patient or population: immunocompetent adults with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis; setting: dental settings in which definitive, conservative dental treatment is not immediately available; intervention: systemic antibiotics; comparison: no systemic antibiotic. No studies meeting the selection criteria reported data on malaise, trismus, fever, cellulitis, additional dental visit, additional medical visit, allergic reaction, endodontic flare-up, diarrhea, Clostridioides difficile infection, or repeat procedure for this population. Nagle and colleagues18 did report intraoral swelling, but owing to symptom inconsistencies with a clinical diagnosis of symptomatic irreversible pulpitis with or without symptomatic apical periodontitis, the guideline authors did not extract this data.
GRADE: Grading of Recommendations Assessment, Development and Evaluation. GRADE Working Group grades of evidence: high certainty: we are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect; very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
RR: Risk ratio.
For dichotomous outcomes, the guideline authors calculated absolute treatment effects via using the control group’s baseline risk as the assumed control intervention risk.
RCT: Randomized controlled trial.
Nagle and colleagues.18
Serious issues of imprecision due to small sample size.
MD: Mean difference.
There were serious issues of imprecision due to small sample size, and the confidence interval suggests a large benefit and a large harm.
For Nagle and colleagues,18 the data for the outcome of pain were dichotomized (visual analog scale from 0-3) as follows: “no pain” and “mild pain” were coded as “no pain,” and “moderate pain” and “severe pain” were coded as “pain.”
There were serious issues of imprecision due to small sample size, and the confidence interval suggests both a small benefit and a large harm.
Figure 5.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of pain intensity at 24 h. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Figure 8.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of pain intensity at 7 d. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
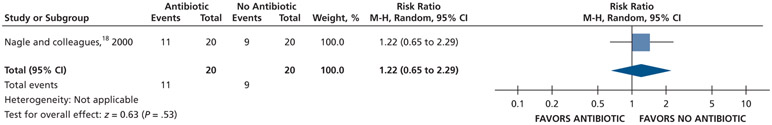
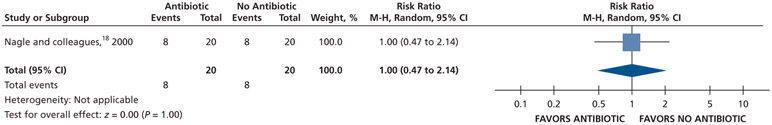
Patients who received antibiotics may experience an increased risk of experiencing pain at 24 hours (20% increase) and 48 hours (22% increase), whereas no difference and a reduction (11% reduction) in pain were observed at 72 hours and 7 days follow-up, respectively, compared with patients who did not receive antibiotics (low certainty) (Table 5; Figures 9-12).18
Figure 9.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of pain experience at 24 h. M-H: Mantel-Haenszel test. CI: Confidence interval.
Figure 12.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of pain experience at 7 d. M-H: Mantel-Haenszel test. CI: Confidence interval.
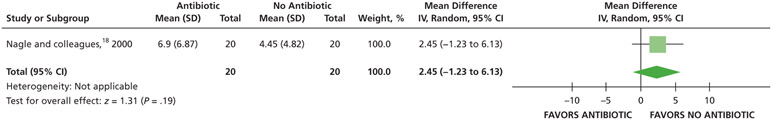
Analgesic use
Patients who received antibiotics may use, on average, one-half of a 600 milligram ibuprofen tablet less and 2 more 300 mg tablets of acetaminophen with 30 mg of codeine rescue analgesic tablets over 7 days compared with patients who did not receive antibiotics (low certainty) (Table 5; Figures 13-15).18
Figure 13.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of total number of ibuprofen tablets used. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Figure 15.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of pain intensity at 24 h. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
For all of these outcomes, the described differences were not statistically significant. For the outcomes of patient-reported pain intensity and total analgesics used, these differences were also not clinically significant.
Pulp necrosis and SAP or LAAA
No studies met our selection criteria.
DCDT Available: Oral Systemic Antibiotics Compared With the Nonuse of Oral Systemic Antibiotics as Adjuncts to DCDT
SIP with or without SAP
No studies met our selection criteria.
PN-SAP or PN-LAAA
Two studies informed the effectiveness of antibiotics as adjuncts to DCDT for the following beneficial outcomes in immunocompetent adults with PN-SAP (N = 41, 7-day follow-up)17 or PN-LAAA (N = 31, 3-day follow-up).16 We found data for all outcomes except trismus, fever, cellulitis, allergic reaction, CDI, repeat procedure, additional dental visit, or additional medical visit for this population.
Patient-reported pain intensity and experience
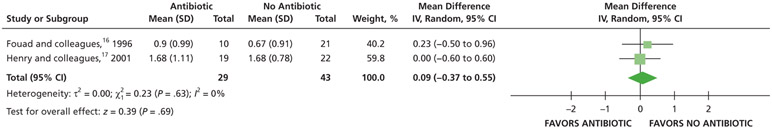
When given antibiotics as adjuncts to DCDT, patients may experience differences of less than one-half point on a pain VAS, ranging from 0 through 3, compared with patients who did not receive antibiotics as adjuncts to DCDT over 24, 48, and 72 hours (low certainty) (Table 6; Figures 15-17).16,17
Table 6.
Relative and absolute desirable and undesirable effects (95% confidence interval) from randomized controlled trials and certainty in the evidence for systemic antibiotics as adjuncts to definitive, conservative dental treatment compared with no systemic antibiotics as adjuncts to definitive, conservative dental treatment for pulp necrosis and symptomatic apical periodontitis and pulp necrosis and localized acute apical abscess in immunocompetent adults.
| OUTCOMES* | PARTICIPANTS (STUDIES), NO. |
CERTAINTY OF THE EVIDENCE ACCORDING TO GRADE† |
RR‡ (95% CONFIDENCE INTERVAL) |
ANTICIPATED ABSOLUTE EFFECTS | |
|---|---|---|---|---|---|
| Risk With No Systemic Antibiotic as Adjuncts to Definitive, Conservative Dental Treatment§ (No. of People) |
Risk Difference With Systemic Antibiotics as Adjuncts to Definitive, Conservative Dental Treatment (Range) |
||||
| Pain Intensity at 24 H | 72 (2 RCTs¶)#,** | Very low††,‡‡ | Not applicable | The mean pain intensity at 24 h ranged from 0.67-1.68 | MD,§§ 0.09 higher (0.37 lower to 0.55 higher) |
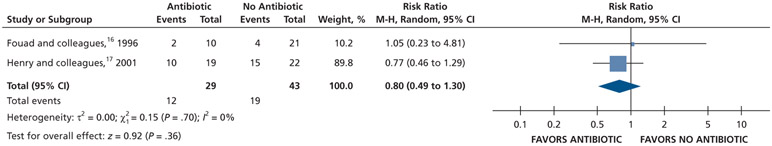
| Pain Experience at 24 H | 72 (2 RCTs)#,** | Very low††,¶¶ | RR, 0.80 (0.49 to 1.30)## | 442 per 1,000 | 88 fewer per 1,000 (225 fewer to 133 more) |
| Pain Intensity at 48 H | 72 (2 RCTs)#,** | Very low††,‡‡ | Not applicable | The mean pain intensity at 48 h ranged from 0.52-0.96 | MD, 0.39 higher (0.13 lower to 0.91 higher) |
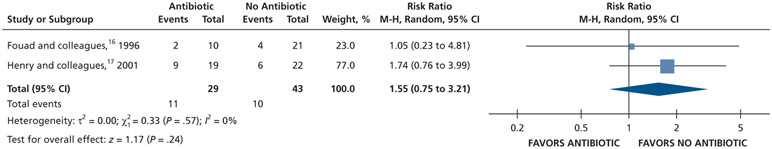
| Pain Experience at 48 H | 72 (2 RCTs)#,** | Very low††,¶¶ | RR, 1.55 (0.75 to 3.21)## | 233 per 1,000 | 128 more per 1,000 (58 fewer to 514 more) |
| Pain Intensity at 72 H | 72 (2 RCTs)#,** | Very low††,‡‡ | Not applicable | The mean pain intensity at 72 h ranged from 0.29-0.82 | MD, 0.12 higher (0.32 lower to 0.56 higher) |
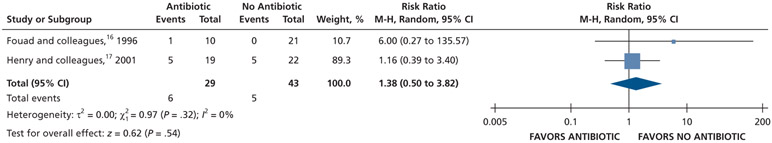
| Pain Experience at 72 H | 72 (2 RCTs)#,** | Very low††,¶¶ | RR, 1.38 (0.50 to 3.82)## | 116 per 1,000 | 44 more per 1,000 (58 fewer to 328 more) |
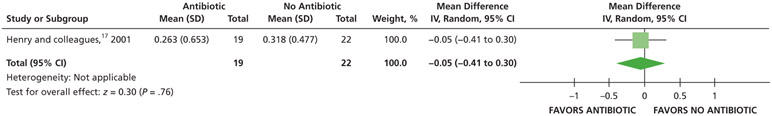
| Pain Intensity at 7 D | 41 (1 RCT)# | Low‡‡ | Not applicable | The mean pain intensity at 7 d was 0.32 | MD, 0.05 lower (0.41 lower to 0.3 higher) |
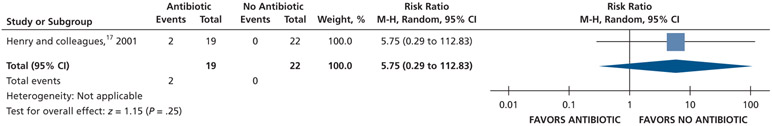
| Pain Experience at 7 D | 41 (1 RCT)# | Low¶¶ | RR, 5.75 (0.29 to 112.83)## | 23 per 1,000 | 108 fewer per 1,000 (16 fewer to 2,542 more) |
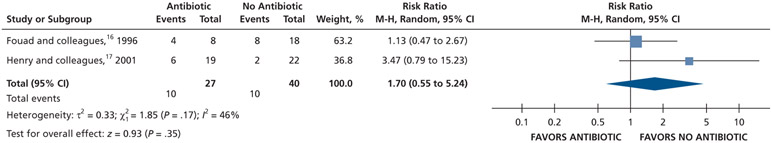
| Intraoral Swelling at 24 H | 67 (2 RCTs)#,**,*** | Very low††,¶¶ | RR, 1.70 (0.55 to 5.24)†††,‡‡‡ | 250 per 1,000 | 175 more per 1,000 (112 fewer to 1,060 more) |
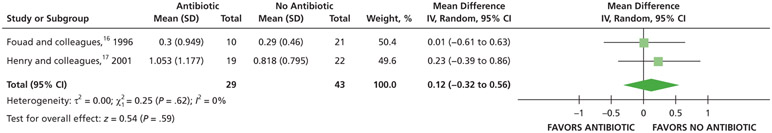
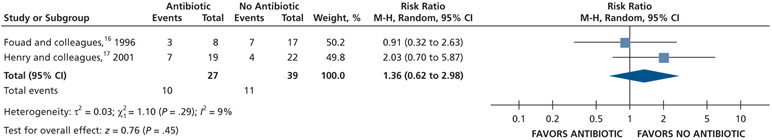
| Intraoral Swelling at 48 H | 66 (2 RCTs)#,**,§§§ | Very low††,¶¶ | RR, 1.36 (0.62 to 2.98)†††,‡‡‡ | 282 per 1,000 | 102 more per 1,000 (107 fewer to 558 more) |
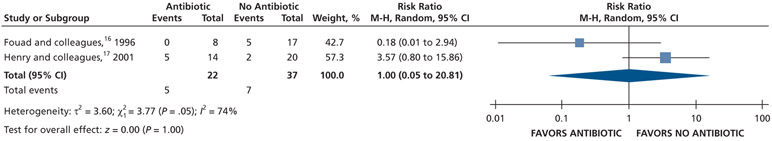
| Intraoral Swelling at 72 H | 59 (2 RCTs)#,**,§§§ | Very low††,### | RR, 1.00 (0.05 to 20.81)†††,‡‡‡ | 189 per 1,000 | 0 fewer per 1,000 (180 fewer to 3,748 more) |
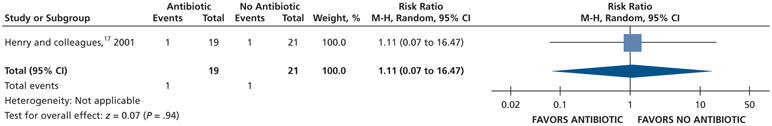
| Intraoral Swelling at 7 D | 40 (1 RCT)# | Low### | RR, 1.11 (0.07 to 16.47)‡‡‡ | 48 per 1,000 | 5 more per 1,000 (44 fewer to 737 more) |
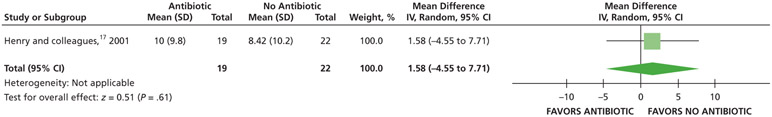
| Total Number of Nonsteroidal Anti-inflammatory Drugs (Tablets) Used | 41 (1 RCT)# | Low### | Not applicable | The mean total number of nonsteroidal antiinflammatory drugs (tablets) used was 8.42 | MD, 1.58 higher (4.55 lower to 7.71 higher) |
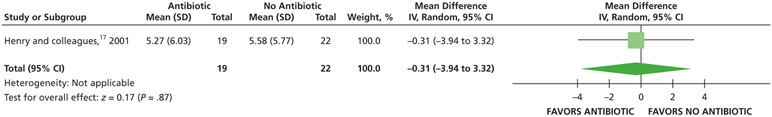
| Total Number of Acetaminophen with Codeine (Tablets) Used | 41 (1 RCT)# | Low### | Not applicable | The mean total number of acetaminophen with codeine (tablets) used was 5.58 | MD, 0.31 lower (3.94 lower to 3.32 higher) |
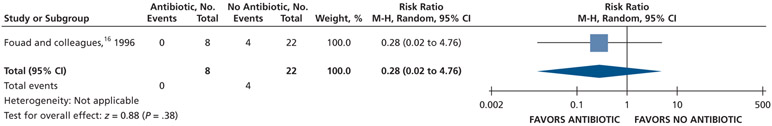
| Endodontic Flare-up | 30 (1 RCT)** | Very low††,¶¶ | RR, 0.28 (0.02 to 4.76) | 182 per 1,000 | 131 fewer per 1,000 (178 fewer to 684 more) |
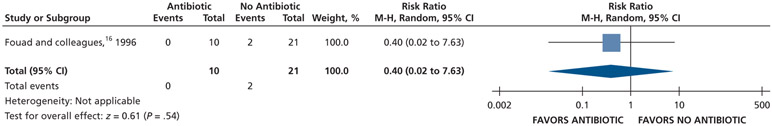
| Diarrhea | 31 (1 RCT)**,**** | Very low††,¶¶ | RR, 0.40 (0.02 to 7.63) | 95 per 1,000 | 57 fewer per 1,000 (93 fewer to 631 more) |
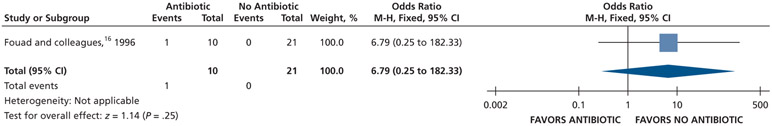
| Malaise | 32 (1 RCT)**,**** | Very low††,¶¶ | RR, 6.79 (0.25 to 182.33) | 24 per 1,000 | 138 fewer per 1,000 (18 fewer to 4,317 more) |
Selection criteria: patient or population: immunocompetent adults with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess; setting: dental setting in which definitive, conservative dental treatment is immediately available; intervention: systemic antibiotics as adjuncts to definitive, conservative dental treatment; comparison: no systemic antibiotic as adjunct to definitive, conservative dental treatment. No studies meeting the selection criteria reported data on trismus, fever, cellulitis, additional dental visit, additional medical visit, allergic reaction, Clostridioides difficile infection, or repeat procedure for this population.
GRADE: Grading of Recommendations Assessment, Development and Evaluation. GRADE Working Group grades of evidence: high certainty: we are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect; very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
RR: Risk ratio.
For dichotomous outcomes, the guideline authors calculated absolute treatment effects via using the control group’s baseline risk as the assumed control intervention risk.
RCT: Randomized controlled trial.
Henry and colleagues.17
Fouad and colleagues16
Serious issues of risk of bias (attrition bias and selective reporting).
Serious issues of imprecision due to small sample size.
MD: Mean difference.
Very serious issues of imprecision owing to small sample size and the confidence interval suggests a large benefit and a large harm.
For included studies, the data for the outcome of pain were dichotomized (visual analog scale from 0-3) as follows: “no pain” and “mild pain” were coded as “no pain” and “moderate pain” and “severe pain” were coded as “pain.”
In Fouad and colleagues,16 14 participants were excluded from the analysis because they either did not report their baseline swelling or they did not report swelling data at follow up.
In Fouad and colleauges,16 the data for the outcome of intraoral swelling were dichotomized (visual analog scale from 0-4) as follows: “no swelling,” “much less swelling,” and “slightly less swelling,” when compared with swelling at baseline, were coded as “no swelling.” The options of “same swelling” and “more swelling,” when compared with swelling at baseline, were coded as “swelling.”
In Henry and colleagues,17 the data for the outcome of intraoral swelling were dichotomized (visual analog scale from 0-3) as follows: “no swelling” and “mild swelling” were coded as “no swelling” and “moderate swelling” and “severe swelling” were coded as “swelling.”
In Fouad and colleagues,16 15 participants were excluded from the analysis because they either did not report their baseline swelling or they did not report swelling data at follow up.
Serious issue of imprecision owing to small sample size and the confidence interval suggests both a small benefit and a small harm.
Owing to the total number of participants in Fouad and colleagues16 informing this outcome, the total number of participants for the outcome of pain at 72 h was used.
Figure 17.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of pain intensity at 72 h. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Patients who received antibiotics as adjuncts to DCDT may experience a decreased risk of experiencing pain at 24 hours (20% decrease) and an increased risk of experiencing pain at 48 hours (55% increase) and 72 hours (38% increase) compared with patients who did not receive antibiotics as adjuncts to DCDT (low certainty) (Table 6; Figures 18-20).16,17 After 7 days, patients receiving antibiotics as adjuncts to DCDT may experience no difference in points on a VAS for pain compared with those not receiving antibiotics as adjuncts to DCDT (low certainty) (Table 6; Figure 21).16,17 In addition, after 7 days, patients receiving antibiotics as adjuncts to DCDT may be 6 times more likely to experience pain than those who did not receive antibiotics as adjuncts to DCDT (low certainty) (Table 6; Figure 22).17
Figure 18.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of pain experience at 24 h. M-H: Mantel-Haenszel test. CI: Confidence interval.
Figure 20.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of pain experience at 72 h. M-H: Mantel-Haenszel test. CI: Confidence interval.
Figure 21.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of pain intensity at 7 d. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Figure 22.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of pain experience at 7 d. M-H: Mantel-Haenszel test. CI: Confidence interval.
Patient-reported intraoral swelling
Patients receiving antibiotics as adjuncts to DCDT may have an increased risk of developing intraoral swelling at 24 hours (70% increase) and 48 hours (36% increase) compared with patients who did not receive antibiotics as adjuncts to DCDT. However, at 72 hours, there was no difference in intraoral swelling between the 2 groups (low to very low certainty) (Table 6; Figures 23-25).16,17
Figure 23.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of intraoral swelling at 24 h. M-H: Mantel-Haenszel test. CI: Confidence interval.
Figure 25.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of swelling at 72 h. M-H: Mantel-Haenszel test. CI: Confidence interval.
After 7 days, patients receiving antibiotics as adjuncts to DCDT may have an increased risk (11% increase) of intraoral swelling compared with patients who did not receive antibiotics as adjuncts to DCDT (low certainty) (Table 6; Figure 26).17
Figure 26.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of intraoral swelling at 7 d. M-H: Mantel-Haenszel test. CI: Confidence interval.
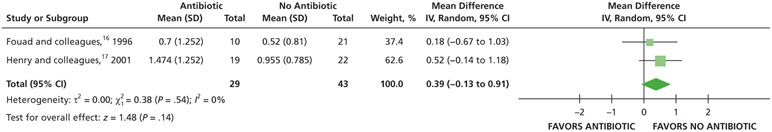
Analgesic use
When given antibiotics as adjuncts to DCDT, patients may use on average 2 more 200 mg ibuprofen tablets and one-half of a 300 mg of acetaminophen with 30 mg of codeine rescue analgesic less compared with patients not receiving antibiotics as adjuncts to DCDT after 7 days (low certainty) (Table 6; Figures 27-28).17
Figure 27.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of total number of ibuprofen tablets used. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Figure 28.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of total number of acetaminophen with codeine tablets used. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Harms related to the use of systemic antibiotics (endodontic flare-up, diarrhea, and malaise)
Patients receiving antibiotics as adjuncts to DCDT may have a decreased risk of experiencing an endodontic flare-up (72% decrease) and diarrhea (60% decrease) and an increased risk of experiencing malaise (679% increase) compared with patients not receiving antibiotics as adjuncts to DCDT over 3 days (very low certainty) (Table 6; Figures 29-31).16
Figure 29.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of endodontic flare-up. M-H: Mantel-Haenszel test. CI: Confidence interval.
Figure 31.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of malaise. M-H: Mantel-Haenszel test. CI: Confidence interval.
For all outcomes, the differences were not statistically significant. For the outcomes of patient-reported pain intensity and total analgesics used, the differences were also not clinically significant.
Additional Outcomes of Harm (Adverse Effects) Related to the Use of Systemic Antibiotics
For additional harm outcomes of interest not reported in the included RCTs, we extracted estimates that were as close as possible when no direct evidence was reported on a specific a priori defined outcome. We found data for all outcomes except for mortality due to community-associated CDIs related to a dental prescription for antibiotics; mortality due to antibiotic-resistant infections associated with a dental prescription for antibiotics; cost-effectiveness of antibiotics to treat SIP with or without SAP, PN-SAP or PN-LAA in any outpatient setting; admission to hospital due to community-associated CDIs related to a dental prescription for antibiotics; length of hospital stay due to community-associated CDI related to a dental prescription for antibiotics; length of hospital stay due to antibiotic-resistant infections associated with a dental prescription for antibiotics; allergic reaction and fatal anaphylaxis due to antibiotics; and allergic reaction and fatal anaphylaxis due to antibiotics associated with a dental prescription.
Community-associated C. difficile infections
Data suggest that approximately 6,400 cases of 10,000 total cases of community-associated CDI may be associated with an exposure to antibiotics (moderate certainty).27,30 From a dental perspective, this translates into an estimated 640 cases of community-associated CDIs of 10,000 total community-associated CDI cases that may be associated with patients consuming antibiotics received from a dentist (very low certainty).26,27,30 Furthermore, of 10,000 total cases of community-associated CDIs, approximately 80 people died after a possible exposure to antibiotics (moderate certainty) (Tables 7-8).27,30
Table 7.
Magnitude of undesirable effects related to use of any antibiotic by any patient in any setting from observational studies and certainty in the evidence.
| OUTCOME* | STUDIES, NO. | CERTAINTY OF THE EVIDENCE ACCORDING TO GRADE† |
IMPACT |
|---|---|---|---|
| Community-Associated Clostridioides difficile Infections | 2 observational studies‡,§ | Moderate¶ | Of 10,000 people with a community-associated C. difficile infection in 2011, approximately 6,400 probably were exposed to antibiotics.# |
| Community-Associated C. difficile Infection Related to a Dental Prescription for Antibiotics | 3 observational studies‡,§,** | Very low†† | Of 10,000 people with a community-associated C. difficile infection in 2011, approximately 640 may have been exposed to antibiotics received from a dentist.#,‡‡,§§ |
| Mortality Due to Community-Associated C. difficile Infections | 2 observational studies‡,§ | Moderate¶ | Of 10,000 people with a community-associated C. difficile infection in 2011, approximately 80 people probably died due to exposure to antibiotics.# |
| Antibiotic-Resistant Infections | 1 observational study¶¶ | Low | At least 2 million people may experience an antibiotic-resistant infection annually in the United States. |
| Mortality Due to Antibiotic-Resistant Infections | 1 observational study¶¶ | Low | Annually, there may have been approximately 23,000 deaths due to antibiotic-resistant infections. |
| Community-Associated C. difficile Infection Related Costs | 2 observational studies‡,## | Moderate¶ | In 2011, the mean community-associated C. difficile—attributable cost was likely $3 billion. |
| Community-Associated C. difficile Infection Costs Associated With a Dental Prescription for Antibiotics | 2 observational studies‡,** | Very low†† | The guideline authors approximated that in 2011 $300 million may have been related to community-associated C. difficile infections that were associated with a dental prescription for antibiotics.‡‡,§§,*** |
| Antibiotic-Resistant Infection Related Costs | 1 observational study¶¶ | Low | In 2008, antibiotic resistance may have caused $20 billion in direct costs with an additional $35 billion associated with productivity losses. |
| Antibiotic-Resistant Infection Related Costs Associated With a Dental Prescription for Antibiotics | 2 observational studies**,¶¶ | Very low†† | The guideline authors approximate that $2 billion in direct costs with an additional $3.5 billion associated with productivity losses may have been related to antibiotic resistance associated with a dental prescription for antibiotics.‡‡,§§,*** |
| Admission to Hospital Due to Community-Associated C. difficile Infection | 2 observational studies‡,§ | Moderate¶ | Of 10,000 people with a community-associated C. difficile infection, 1,270 patients probably listed community-associated C. difficile infection as the primary reason for admission to the hospital. |
| Admission to Hospital Due to Antibiotic-Resistant Infection | 1 observational study††† | Low | In 2006, infection-related hospitalizations associated with antibiotic-resistant infections may have accounted for 2.4% of all infection-related hospitalizations. |
| Admission to Hospital Due to Antibiotic-Resistant Infection Associated With a Dental Prescription for Antibiotics | 2 observational studies**,††† | Very low†† | The guideline authors approximated that in 2006, 0.24% of infection-related hospitalizations due to antibiotic-resistant infections may have been associated with a dental prescription for antibiotics.‡‡,§§,*** |
| Length of Hospital Stay Due to Community-Associated C. difficile Infection | 1 observational study## | Low | The average community-associated C. difficile—attributable length of stay due to community-associated C. difficile infection may be 5.7 d (range, 2.1-33.4). |
| Length of Hospital Stay Due to Antibiotic-Resistant Infections | 1 observational study‡‡‡ | Low | In 2014, the average (standard deviation) length of hospital stay due to bacterial infections and infections associated with multidrug-resistant organisms (that is, methicillin-resistant Staphylococcus aureus and other multidrug-resistant organisms) may have ranged from 9.45 (11.81) d to 9.47 (11.59) d. |
| Anaphylaxis Due to Antibiotics | 1 observational study§§§ | Low | Of 10,000 hospitalizations from 1995 through 2013, approximately 46 patients may have reported anaphylaxis due to a penicillin drug class; 2 patients may have reported anaphylaxis due to amoxicillin; 6 patients may have reported anaphylaxis due to a cephalosporin drug class#; and 1 patient may have reported anaphylaxis due to cephalexin.# |
| Anaphylaxis Due to Antibiotics Associated with a Dental Prescription | 2 observational studies**,§§§ | Very low†† | Of 100,000 hospitalizations from 1995 through 2013, approximately 46 patients may have reported anaphylaxis due to a penicillin drug class and received the antibiotic from a dentist; 2 patients may have reported anaphylaxis due to amoxicillin and received the antibiotic from a dentist; 6 patients may have reported anaphylaxis due to a cephalosporin drug class and received the antibiotic from a dentist; and 1 patient may have reported anaphylaxis due to cephalexin and received the antibiotic from a dentist.#,‡‡,§§ |
Selection criteria: patient or population: any person of any age seeking treatment in any dental setting in the United States; setting: any dental setting in the United States; exposure: any systemic antibiotics; nonexposure: no systemic antibiotic. No studies meeting the selection criteria reported data on mortality due to community-associated Clostridioides difficile infections related to a dental prescription for antibiotics; mortality due to antibiotic-resistant infections associated with a dental prescription for antibiotics; cost-effectiveness of antibiotics to treat symptomatic irreversible pulpitis with or without symptomatic apical periodontitis, pulp necrosis and symptomatic apical periodontitis, or pulp necrosis and localized acute apical abscess; admission to hospital due to community-associated C. difficile infections related to a dental prescription for antibiotics; length of hospital stay due to community-associated C. difficile infection related to a dental prescription for antibiotics; length of hospital stay due to antibiotic-resistant infections associated with a dental prescription for antibiotics; allergic reaction due to antibiotics; allergic reaction due to antibiotics associated with a dental prescription; fatal anaphylaxis due to antibiotics; or fatal anaphylaxis due to antibiotics associated with a dental prescriptions.
GRADE: Grading of Recommendations Assessment, Development and Evaluation. GRADE Working Group grades of evidence: high certainty: we are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect; very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Considerations for Lessa and colleagues30: the case definition of C. difficile infection relying only on positive test results for C. difficile toxin or molecular assay from unformed samples sent to laboratories may lead to an underestimation of the true burden (that is, partially formed samples being untested); there is the possibility for an underestimation of “both recurrence and mortality, given that [they] assessed only first recurrences and deaths that were documented in the medical record”; there is a potential overdiagnosis or an overestimation of the burden of C. difficile infection owing to diagnostic tests being highly sensitive (that is, a poor distinction between colonization and the disease); The authors estimated the recurrence of and mortality due to C. difficile infection via using a random sample of cases that may or may not be representative of the US rates.
Considerations for Chitnis and colleagues27: there are potential issues of generalizability to the US population given that patients included in the analysis with community-associated C. difficile infection were more likely to be white and female; only a convenience sample of stools were sent for definitive testing (40%); although antibiotic use within 12 weeks was adjudicated on the basis of a telephone interview (self-reported) and medical records, it is unclear as to how many cases were confirmed using both methods; hospitalization in which C. difficile infection was the primary reason for admission was ascertained through medical records.
Upgraded due to a large effect on the basis of observational studies without important risk of bias or other limitations.
This is likely an overestimation of the effect of dental prescriptions for antibiotics because the provided information and data did not differentiate between inpatient and outpatient antibiotic prescriptions. The guideline authors assume that prescribing for dental conditions rarely occurs in inpatient settings.
Considerations for Hicks and colleagues26: dentistry accounts for 10% of the total outpatient antibiotic prescriptions in the United States; the magnitude of antibiotic prescriptions may not necessarily represent the magnitude of antibiotic consumption by patients; there is possible underestimation owing to the total number of prescriptions from other nondental professionals (for example, emergency medicine services) for any dental condition not being included in the estimate; estimates related to antibiotic prescribing practices reported by Hicks and colleagues26 correspond to that of general dentists and dental specialties combined.
Downgraded owing to serious issues of indirectness related to estimates being extrapolated to illustrate the burden in a dental setting.
Data were adjusted considering that dentistry accounts for 10% of total outpatient antibiotic prescriptions in the United States.
The presented estimate assumes that dental prescriptions for any antibiotic has the same potential of inducing antibiotic resistance as nondental related prescriptions.
Considerations for Centers for Disease Control and Prevention3: no reports containing methods or results are linked to this report; estimates used from this report are likely an underestimation of the true burden of antibiotic resistance related outcomes; the magnitude of antibiotic resistance related outcomes may not necessarily represent the magnitude of antibiotics prescribed for and consumed by patients.
Considerations for Zhang and colleagues32: all included studies in the review reported direct medical costs from a hospital perspective; indirect costs to patients and society and costs of additional care after hospital discharge have not been captured (for example, productivity loss due to work day losses and costs in long-term care facilities). Approximately 9% of patients with C. difficile infections were discharged to a long-term care facility for an average of 24 d of after-care, which would result in an additional $141 million burden on the health care system and society due to long-term care facility transfers; primary C. difficile infections were not separated for the estimation of recurrent C. difficile infection costs; there was discrepancy in case definitions in cost studies versus surveillance and epidemiological studies (for example, community-versus health care—associated C. difficile infections); the total costs of C. difficile infection in the United States may be higher than the reported estimate.
This is likely an overestimation of the effect of dental prescriptions for antibiotics owing to the primary study not measuring or reporting antibiotic exposure.
Considerations for Mainous and colleagues31: the methods did not allow the guideline authors to determine whether the infection arose in the hospital or the patients were colonized or infected before admission; International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes were used instead of laboratory results on bacterial cultures; “Greater awareness of drug resistance among hospital coding departments may have prompted more attention to adding these codes to discharge records of patients who were relatively healthy and discharged without incident."
Considerations for Johnston and colleagues29: International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes were used instead of laboratory results on bacterial cultures; the authors were unable to distinguish between hospital-acquired and community-acquired infections; 10% of the eligible population was excluded due to missing data.
Considerations for Dhopeshwarkar and colleagues28: the estimates presented in this study only included penicillin and cephalosporin drug classes and amoxicillin and cephalexin drugs and did not include other individual drugs commonly prescribed by dentists such as clindamycin. Considerations for: Durkin and colleagues:6 there may be issues of generalizability as only patients from 2 Boston-area hospitals were included in this analysis, which may not be representative of inpatient populations admitted to other US hospitals; there was a potential overestimate of the occurrence of anaphylaxis owing to reported cases not being confirmed by tryptase tests; there was possible underestimation owing to exclusion of codes listed in electronic health records not directly linking to anaphylaxis; there was uncertainty surrounding whether the estimates of the reported or observed cases of anaphylaxis resulted in death.
Table 8.
Calculations of the magnitude of undesirable effects related to use of any antibiotic by any patient in any setting from observational studies.
| OUTCOME* | STUDIES, NO. | CERTAINTY OF THE EVIDENCE ACCORDING TO GRADE† |
CALCULATION OF IMPACT |
|---|---|---|---|
| Community-Associated Clostridioides difficile Infections | 2 observational studies‡,§ | Moderate¶ | Of the estimated cases of community-associated C. difficile infections, approximately 64% were exposed to antibiotics in 2011. This represents 102,409 cases of 159,700 total C. difficile infections (95% CI,# 85,056 to 119,040).** |
| Community-Associated C. difficile Infection Related to a Dental Prescription for Antibiotics | 3 observational studies‡,§,†† | Very low‡‡ | The guideline authors approximated that 6.4% of people with community-associated C. difficile infections who were exposed to antibiotics received the prescription from a dentist. This represents 10,221 cases of 159,700 total C. difficile infections in 2011 (95% CI, 8,506 to 11,904).**,§§,¶¶ |
| Mortality Due to Community-Associated C. difficile Infections | 2 observational studies‡,§ | Moderate¶ | In 2011, approximately 2,000 of 159,700 people infected with community-associated C. difficile infection died within 30 d of diagnosis (95% CI, 1,200 to 2,800). Of the estimated cases of community-associated C. difficile infection, approximately 64% were exposed to antibiotics, and 1,280 people died due to community-associated C. difficile infection related to exposure to antibiotics (95% CI, 768 to 1,792). This represents a 0.8% mortality rate due to community-associated C. difficile infection related to exposure to antibiotics.** |
| Antibiotic-Resistant Infections | 1 observational study## | Low | Estimate taken directly from report. |
| Mortality Due to Antibiotic-Resistant Infections | 1 observational study## | Low | Estimate taken directly from report. |
| Community-Associated C. difficile Infection Related Costs | 2 observational studies‡,*** | Moderate¶ | The estimated cost due to community-associated C. difficile infection in 2015, as reported by Zhang and colleagues,32 was $20,085. The estimated cases of community-associated C. difficile infection in 2011, as reported by Lessa and colleagues,30 was 159,700 cases. The US Department of Labor33 inflation calculator was used to convert the value of a 2015 US dollar to the value of a 2011 US dollar, which equates to $19,163.40. $19,163.40 x 159,700 cases of C. difficile infection in 2011 = $3,060,394,980. |
| Community-Associated C. difficile Infection Costs Associated with a Dental Prescription for Antibiotics | 2 observational studies‡,†† | Very low‡‡ | The total cost due to community-associated C. difficile infections was adjusted by 10%.§§,¶¶,††† |
| Antibiotic-Resistant infection Related Costs | 1 observational study## | Low | Estimate taken directly from report. |
| Antibiotic-Resistant Infection Related Costs Associated with a Dental Prescription for Antibiotics | 2 observational studies††,## | Very low‡‡ | The total cost related to antibiotic-resistance infections was adjusted by 10%.§§,¶¶,††† |
| Admission to Hospital Due to Community-Associated C. difficile Infection | 2 observational studies‡,§ | Moderate¶ | Of the estimated cases of community-associated C. difficile infections in 2011, approximately 12.7% of the patients were admitted to the hospital owing to community-associated C. difficile infections being the primary reason for admission. This represents 20,287 (95% CI, 16,878 to 23,622) of 159,700 total cases with community-associated C. difficile infections. |
| Admission to Hospital Due to Antibiotic-Resistant Infection | 1 observational study‡‡‡ | Low | Estimate taken directly from report. |
| Admission to Hospital Due to Antibiotic-Resistant Infection Associated with a Dental Prescription for Antibiotics | 1 observational study‡‡‡ | Very low‡‡ | Admissions to the hospital due to antibiotic-resistant infections was adjusted by 10%.§§,¶¶,††† |
| Length of Hospital Stay Due to Community-Associated C. difficile Infection | 1 observational study*** | Low | Estimate taken directly from report. |
| Length of Hospital Stay Due to Antibiotic-Resistant Infections | 1 observational study§§§ | Low | Estimate taken directly from report. |
| Anaphylaxis Due to Antibiotics | 1 observational study¶¶¶ | Low | Estimates taken directly from report.** |
| Anaphylaxis Due to Antibiotics Associated with a Dental Prescription | 2 observational studies††,¶¶¶ | Very low‡‡ | Reported anaphylaxis due to antibiotics occurrences was adjusted by 10%.**,§§,¶¶ |
Selection criteria: patient or population: any person of any age seeking treatment in any dental setting in the United States; setting: any dental setting in the United States; exposure: any systemic antibiotics; nonexposure: no systemic antibiotic. No studies meeting the selection criteria reported data on mortality due to community-associated Clostridioides difficile infections related to a dental prescription for antibiotics, length of hospital stay due to community-associated C. difficile infection related to a dental prescription for antibiotics, length of hospital stay due to antibiotic-resistant infections associated with a dental prescription for antibiotics, allergic reaction due to antibiotics, allergic reaction due to antibiotics associated with a dental prescription, fatal anaphylaxis due to antibiotics, or fatal anaphylaxis due to antibiotics associated with a dental prescriptions.
GRADE: Grading of Recommendations Assessment, Development and Evaluation. GRADE Working Group grades of evidence: high certainty: we are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect; very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Considerations for Lessa and colleagues30: the case definition of C. difficile infection relying only on positive test results for C. difficile toxin or molecular assay from unformed samples sent to laboratories may lead to an underestimation of the true burden (that is, partially formed samples being untested); there is the possibility for an underestimation of “both recurrence and mortality, given that [they] assessed only first recurrences and deaths that were documented in the medical record”; there is a potential over-diagnosis or an overestimation of the burden of C. difficile infection owing to diagnostic tests being highly sensitive (that is, a poor distinction between colonization and the disease); the authors estimated the recurrence of and mortality due to C. difficile infection via using a random sample of cases that may or may not be representative of the US rates.
Considerations for Chitnis and colleagues27: there are potential issues of generalizability to the US population given that patients included in the analysis with community-associated C. difficile infection were more likely to be white and female; only a convenience sample of stools were sent for definitive testing (40%); although antibiotic use within 12 weeks was adjudicated on the basis of a telephone interview (self-reported) and medical records, it is unclear as to how many cases were confirmed using both methods; hospitalization in which C. difficile infection was the primary reason for admission was ascertained through medical records.
Upgraded due to a large effect based on observational studies without important risk of bias or other limitations.
CI: Confidence interval.
This is likely an overestimation of the effect of dental prescriptions for antibiotics because the provided information and data did not differentiate between inpatient and outpatient antibiotic prescriptions. The guideline authors assume that prescribing for dental conditions rarely occurs in inpatient settings.
Considerations for Hicks and colleagues26: dentistry accounts for 10% of the total outpatient antibiotic prescriptions in the United States; the magnitude of antibiotic prescriptions may not necessarily represent the magnitude of antibiotic consumption by patients; there is possible underestimation owing to the total number of prescriptions from other nondental professionals (for example, emergency medicine services) for any dental condition not being included in the estimate; estimates related to antibiotic prescribing practices reported by Hicks and colleagues26 correspond to that of general dentists and not all dental specialties combined.
Downgraded owing to serious issues of indirectness related to estimates being extrapolated to illustrate the burden in a dental setting.
Data were adjusted considering that dentistry accounts for 10% of total outpatient antibiotic prescriptions in the United States.
The presented estimate assumes that dental prescriptions for any antibiotic has the same potential of inducing antibiotic resistance as nondental related prescriptions.
Considerations for Centers for Disease Control and Prevention3: no reports containing methods or results is linked to this report; estimates used from this report are likely an underestimation of the true burden of antibiotic resistance related outcomes; the magnitude of antibiotic resistance related outcomes may not necessarily represent the magnitude of antibiotics prescribed for and consumed by patients.
Considerations for Zhang and colleagues32: all included studies in the Zhang and colleagues review reported direct medical costs from a hospital perspective; indirect costs to patients and society and costs of additional care after hospital discharge were not captured (for example, productivity loss due to work day losses and costs in long-term care facilities). Approximately 9% of patients with C. difficile infections were discharged to a long-term care facility for an average of 24 d of after-care, which would result in an additional $141 million burden on the health care system and society due to long-term care facility transfers; primary C. difficile infections were not separated for the estimation of recurrent C. difficile infection costs; there was discrepancy in case definitions in cost studies versus surveillance and epidemiologic studies (for example, community-versus health care—associated C. difficile infections); the total costs of C. difficile infection in the United States may be higher than the reported estimate.
This is likely an overestimation of the effect of dental prescriptions for antibiotics owing to the primary study not measuring or reporting antibiotic exposure.
Considerations for Mainous and colleagues31: the methods did not allow the guideline authors to determine whether the infection arose in the hospital or if patients were colonized or infected prior to admission, International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes were used instead of laboratory results on bacterial cultures; “Greater awareness of drug resistance among hospital coding departments may have prompted more attention to adding these codes to discharge records of patients who were relatively healthy and discharged without incident.”
Considerations for Johnston and colleagues: International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes were used instead of laboratory results on bacterial cultures; the authors were unable to distinguish between hospital-acquired and community-acquired infections; 10% of the eligible population was excluded owing to missing data.
Considerations for Dhopeshwarkar and colleagues28: the estimates presented in this study only included penicillin and cephalosporin drug classes and amoxicillin and cephalexin drugs and did not include other individual drugs commonly prescribed by dentists such as clindamycin. Source: Durkin and colleagues;6 there may be issues of generalizability as only patients from 2 Boston-area hospitals were included in this analysis, which may not be representative of inpatient populations admitted to other US hospitals; there was a potential overestimate of the occurrence of anaphylaxis owing to reported cases not being confirmed by tryptase tests; there was possible underestimation owing to exclusion of codes listed in electronic health records not directly linking to anaphylaxis; there was uncertainty surrounding whether the estimates of the reported or observed cases of anaphylaxis resulted in death.
Antibiotic-resistant infections
Annually, 2 million people may be affected by antibiotic-resistant infections in the United States, and there are approximately 23,000 deaths due to these infections (low certainty) (Tables 7-8).11
Costs
In 2008, $20 billion in direct costs may have been attributable to antibiotic-resistant infections and an additional $35 billion in associated productivity losses (low certainty).11 This translates into an estimated $2 billion in direct costs and $3.5 billion in productivity loss associated with dental prescriptions for antibiotics (very low certainty).11,26 In 2015, community-associated CDIs were associated with approximately $3 billion in costs (moderate certainty),30,32,33 which may translate into approximately $300 million in costs being associated with a dental prescription for antibiotics (very low certainty) (Tables 7-8).26,30,32
Hospitalizations
Of 10,000 people with community-associated CDIs, 1,270 may have been admitted to a hospital with community-associated CDI as the primary reason for admission (moderate certainty).27,30 In 2006, 2.4% of all infection-related hospitalizations could be attributed to antibiotic-resistant infections (low certainty).31 This translates into approximately 0.24% of infection-related hospitalizations due to antibiotic resistance being associated with dental prescriptions for antibiotics (very low certainty).26,31 In addition, evidence suggests patients were hospitalized on average for 5.7 days owing to community-associated CDIs32 and approximately 9 days for bacterial infections associated with multidrug-resistant microorganisms (low certainty) (Tables 7-8).29
Anaphylaxis
Evidence suggests that from 1995 through 2013, for every 10,000 hospitalizations, about 46 were attributed to anaphylaxis associated with the use of a penicillin drug class and another 6 anaphylaxis-related hospitalizations were associated with a cephalosporin drug class (low certainty).34 From a dental perspective, this is approximately 46 and 6 of 100,000 hospitalizations due to a penicillin or cephalosporin drug class prescribed from a dentist, respectively (very low certainty) (Tables 7-8).26,28
DISCUSSION
Summary of the main results
Evidence on the effect of antibiotics versus no antibiotics, with or without DCDT, for outcomes of pain and intraoral swelling showed both a small to large benefit and a small to large harm. Data on outcomes of endodontic flare-up, diarrhea, and malaise suggest that there may be a reduced risk of experiencing an endodontic flare-up and diarrhea and an increased risk of experiencing malaise associated with the use of antibiotics as adjuncts to DCDT.16-18
Evidence suggests a large magnitude of effect for additional harm outcomes such as CDI, mortality, and hospitalization associated with the use of antibiotics for any condition, medical or dental.11,26-32
Certainty in the evidence
The certainty in the evidence ranged from very low to low across all outcomes informed by RCT data and from very low to moderate for all harm outcomes informed by observational data. We downgraded the certainty for RCT data owing to issues of risk of bias (attrition bias and selective reporting), imprecision (confidence intervals showing both a large benefit and a large harm), and failure to meet the optimal information size. We upgraded additional data collected from observational reports on harm outcomes owing to a potentially large magnitude of effect.
Comparison with other reviews
Although our review is partially an update of 2 preexisting Cochrane reviews,13,14 a 2016 review assessed the effects of antibiotics to treat endodontic infections and pain.35 Unlike the Cochrane reviews13,14 and our updated review, in the 2016 review the study authors included patients with pulp necrosis and asymptomatic apical periodontitis along with symptomatic patients. Two 2003 systematic reviews assessed the effects of antibiotics for the management of PN-SAP and PN-LAAA in adult patients.36,37 Unlike the Cochrane reviews13,14 and our updated review, these reviews included trials that provided head-to-head comparisons of antibiotics with other antibiotics and other management options, included extractions as a dental treatment of interest, and did not use GRADE to assess certainty in the evidence. Similar to our review, these 4 previously published reviews evaluated local and systemic symptom relief in patients with pulpal and periapical conditions, and their estimates also suggest that antibiotics are associated with both benefits and harms.13,14,36,37 Unlike our review, none of these reviews included additional harm outcomes informed by observational data.13,14,36,37 In addition, the 2014 review by Cope and colleagues13 and the 2016 review by Agnihotry and colleagues14 were updated and published concurrently by the Cochrane Collaboration in 201838 and 2019,39 respectively, during our update process, and the authors did not find any new eligible studies to be included in their reviews. Other reviews have summarized harms associated with antibiotic use, but their patient populations were too narrow (for example, urinary tract infection patients) for us to use these reviews to inform our outcomes.40
Strengths and limitations of this review
The strengths of our review include that we used methodology in line with recommendations from the Cochrane Handbook for Systematic Reviews of Interventions24 and that we screened and performed data abstraction independently and in duplicate, contacted authors for data clarification, and assessed the certainty in the evidence using GRADE. A major limitation of this review is the lack of accurate estimates quantifying the direct impact of dental antibiotic prescribing on health outcomes. Although we attempted to provide an estimation of this impact via attributing 10% of the magnitude of harm outcomes to dental prescriptions (on the basis of reports approximating that 10% of all antibiotic prescriptions are made by dentists),8,41 this exercise has a number of limitations. It assumes that for all antibiotic prescriptions made by medical and oral health care professionals, all antibiotic types may contribute equally to outcomes (such as antibiotic resistance), disregards the duration of antibiotic regimens, and does not account for antibiotic prescribing versus consumption (that is, even if an antibiotic is prescribed, the amount consumed by a patient is not measured in relation to the measured outcome). These adjusted estimates may reflect the impact of antibiotics used for any dental indication and are not limited to the conditions of interest in this review. Additional limitations of our review include a paucity of large, robustly designed trials in this subject area, inclusion of studies with poorly defined conditions of interest, and inconsistencies between target conditions and patient signs and symptoms in the primary trials.
CONCLUSIONS
Evidence on the effects of antibiotics, either alone or as adjuncts to DCDT, suggests both a benefit and a harm for the outcomes of pain and intraoral swelling. Evidence also suggests large potential harms associated with antibiotic use for any condition for the outcomes of community-associated CDI, mortality due to community-associated CDI, antibiotic-resistant infections, and mortality due to antibiotic-resistant infections. Clinical decision making should include this summary of benefits and harms along with other pertinent considerations, including the patient’s values and preferences, acceptability, and feasibility. We conducted this review in collaboration with an expert panel during the development of an associated clinical practice guideline.12
Figure 6.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of pain intensity at 48 h. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Figure 7.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of pain intensity at 72 h. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Figure 10.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of pain experience at 48 h. M-H: Mantel-Haenszel test. CI: Confidence interval.
Figure 11.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of pain experience at 72 h. M-H: Mantel-Haenszel test. CI: Confidence interval.
Figure 14.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics in adult patients with symptomatic irreversible pulpitis with or without symptomatic apical periodontitis for the outcome of total number of acetaminophen with codeine tablets used. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Figure 16.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of pain intensity at 48 h. SD: Standard deviation. IV: Inverse variance. CI: Confidence interval.
Figure 19.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of pain experience at 48 h. M-H: Mantel-Haenszel test. CI: Confidence interval.
Figure 24.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of intraoral swelling at 48 h. M-H: Mantel-Haenszel test. CI: Confidence interval.
Figure 30.
Forest plot of comparison of oral systemic antibiotics versus nonuse of oral systemic antibiotics as adjuncts to definitive, conservative dental treatment in adult patients with pulp necrosis and symptomatic apical periodontitis or pulp necrosis and localized acute apical abscess for the outcome of diarrhea. M-H: Mantel-Haenszel test. CI: Confidence interval.
Acknowledgments
Disclosures. Dr. Durkin receives salary support from the Centers for Disease Control and Prevention, the National Institutes for Health, and the Missouri Department of Health and Senior Services. Dr. Fouad receives funding from the Foundation for Endodontics. Dr. Lockhart receives funding from the National Institutes of Health. Dr. Patton receives grant funding from the National Institute of Dental and Craniofacial Research; she is the President of the American Academy of Oral Medicine, a member of the ADA Council on Scientific Affairs, the co-editor of The ADA Practical Guide to Patients with Medical Conditions, and the oral medicine section editor of the journal Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. Dr. Suda receives funding from the Agency for Healthcare Research and Quality, the Centers for Disease Control and Prevention, the National Institutes of Health, the Veteran’s Affairs Health Services Research and Division, the Veterans Affairs Rehabilitation Research and Development, and the Veteran Affairs Quality Enhancement Research Initiative. None of the other authors reported any disclosures.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Methodologists from the ADA Center for Evidence-Based Dentistry led the development and authorship of the systematic review and clinical practice guideline in collaboration with the expert panel. The ADA Council on Scientific Affairs commissioned this work. This study was funded by the American Dental Association.
ABBREVIATION KEY
- ADA
American Dental Association
- CDI
Clostridioides difficile infection
- DCDT
Definitive, conservative dental treatment
- ED
Emergency department
- EIP
Emerging Infections Program
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- LAAA
Localized acute apical abscess
- NHDS
National Hospitalization Discharge Survey
- PN-LAAA
Pulp necrosis and localized acute apical abscess
- PN-SAP
Pulp necrosis and symptomatic apical periodontitis
- RCT
Randomized controlled trial
- SAP
Symptomatic apical periodontitis
- SIP
Symptomatic irreversible pulpitis
- VAS
Visual analog scale
APPENDIX
METHODS
Selection criteria
A complete list of outcomes for total analgesics used includes the total number of nonsteroidal antiinflammatory drugs used and the total number of rescue analgesics used. A complete list of outcomes for progression of disease to a more severe state includes malaise, trismus, fever, cellulitis, additional dental visit, and additional medical visit.
A complete list of outcomes for community-associated Clostridioides difficile infection (CDI) includes community-associated CDI, community-associated CDI related to a dental prescription for antibiotics, and mortality due to community-associated CDI.
A complete list of outcomes for antibiotic-resistant infections includes antibiotic-resistant infections and mortality due to antibiotic-resistant infections.
A complete list of outcomes for costs includes community-associated CDI related costs; community-associated CDI related costs associated with a dental prescription for antibiotics; antibiotic-resistant infections related costs; antibiotic-resistant infections related costs associated with a dental prescriptions for antibiotics; and cost-effectiveness of antibiotics to treat symptomatic irreversible pulpitis with or without symptomatic apical periodontitis, pulp necrosis and symptomatic apical periodontitis, or pulp necrosis and localized acute apical.
A complete list of outcomes of hospitalizations includes admission to hospital due to community associated CDI, admission to hospital due to community-associated CDI related to a dental prescription for antibiotics, admission to hospital due to antibiotic-resistant infection, admission to hospital due to antibiotic-resistant infection associated with dental prescriptions for antibiotics, length of hospital stay due to community-associated CDI, length of hospital stay due to community-associated CDI related to a dental prescription for antibiotics, length of hospital stay due to antibiotic-resistant infection, and length of hospital stay due to antibiotic-resistant infections associated with a dental prescription for antibiotics.
A complete list of outcomes of anaphylaxis includes allergic reaction to antibiotics, allergic reaction to antibiotics associated with a dental prescription, anaphylaxis due to antibiotics, anaphylaxis due to antibiotics associated with a dental prescription, fatal anaphylaxis due to antibiotics, and fatal anaphylaxis due to antibiotics associated with a dental prescription.
Literature Search
Search Strategy #1 (Update of Cope 2014). Search conducted in this database on June 5, 2018.
Embase. Database: Embase via embase.com
#1 ‘antiinfective agent’/exp
#2 ‘penicillin derivative’/exp
#3 antibiotic* OR ‘anti-biotic*’ OR ‘anti biotic*’
#4 antibacterial* OR ‘anti-bacterial*’ OR ‘anti bacterial*’
#5 antiinfect* OR ‘anti-infect*’ OR ‘anti infect*’
#6 antimicrobial* OR ‘anti-microbial*’ OR ‘anti microbial*’
#7 penicillin* OR amox?cillin OR ampicillin OR erythromycin OR clindamycin* OR doxycycline* OR metronidazole OR azithromycin OR ‘co amoxiclav’ OR oxytetracycline OR cefalexin OR cephalexin OR cefradine OR cephradine OR clarithromycin OR tetracycline
#8 actimoxi OR amoxicilline OR amoxil OR ‘brl 2333’ OR clamoxyl OR hydroxyampicillin OR penamox OR polymox OR trimox OR wymox OR ‘amoxi-clav’ OR ‘amoxi-clavulanate’ OR augmentin OR ‘brl 25000’ OR clavulanate OR clavulin OR coamoxiclav OR spektramox OR synulox
#9 phenoxymethylpenicillin OR apocillin OR beromycin OR berromycin OR betapen OR fenoxymethylpenicillin OR ‘pen vk’ OR ‘v-cillin k’ OR vegacillin
#10 clont OR danizol OR trichazol* OR trichapol OR trivazol OR satric OR metrogyl OR flagyl OR gineflavir OR metrodzhil OR nidagyl
#11 chlolincocin OR chlorlincocin OR cleocin OR ‘dalacin c’
#12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11
#13 ‘tooth periapical disease’/exp
#14 dental* NEXT/5 absces*
#15 (tooth OR teeth) NEXT/5 absces*
#16 (periapical NEXT/5 absces*) OR (‘peri-apical’ NEXT/5 absces*) OR (apical NEXT/5 absces*)
#17 (periapical NEXT/5 periodont*) OR (‘peri-apical’ NEXT/5 periodont*) OR (apical NEXT/5 periodont*)
#18 (periapical NEXT/5 inflam*) OR (‘peri-apical’ NEXT/5 inflam*) OR (apical NEXT/5 inflam*)
#19 (periapical NEXT/5 infect*) OR (‘peri-apical’ NEXT/5 infect*) OR (apical NEXT/5 infect*)
#20 (dentoalveol* NEXT/5 absces*) OR (‘dento-alveol*’ NEXT/5 absces*) OR (alveol* NEXT/5 absces*)
#21 (periradicular NEXT/5 absces*) OR (‘peri-radicular’ NEXT/5 absces*) OR (radicular NEXT/5 absces*)
#22 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
#23 #12 AND #22
#24 random*
#25 factorial*
#26 (crossover* OR cross) AND over* OR ‘cross over*’
#27 placebo
#28 doubl* NEXT/1 blind*
#29 singl* NEXT/1 blind*
#30 assign*
#31 allocat*
#32 volunteer*
#33 ‘crossover procedure’/exp
#34 ‘double blind procedure’/exp
#35 ‘randomized controlled trial’/exp
#36 ‘single blind procedure’/exp
#37 #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR
#35 OR #36
#38 (‘animal’/exp OR ‘nonhuman’/exp) NOT (‘human’/exp OR ‘human cell’/exp OR ‘human’:ti OR ‘humans’:ti)
#39 #23 AND #37
#40 #39 NOT #38
#41 #40 AND (2013:py OR 2014:py OR 2015:py OR 2016:py OR 2017:py OR 2018:py)
Search Strategy #2 (Adapted update of Agnihotry 2016). Search conducted in this database on June 5, 2018.
Embase. Database: Embase via embase.com
#1 ‘antiinfective agent’/exp
#2 ‘penicillin derivative’/exp
#3 antibiotic* OR ‘anti-biotic*’ OR ‘anti biotic*’
#4 antibacterial* OR ‘anti-bacterial*’ OR ‘anti bacterial*’
#5 antiinfect* OR ‘anti-infect*’ OR ‘anti infect*’
#6 antimicrobial* OR ‘anti-microbial*’ OR ‘anti microbial*’
#7 penicillin* OR amox?cillin OR ampicillin OR erythromycin OR clindamycin* OR doxycycline* OR metronidazole OR azithromycin OR ‘co amoxiclav’ OR oxytetracycline OR cefalexin OR cephalexin OR cefradine OR cephradine OR clarithromycin OR tetracycline
#8 actimoxi OR amoxicilline OR amoxil OR ‘brl 2333’ OR clamoxyl OR hydroxyampicillin OR penamox OR polymox OR trimox OR wymox OR ‘amoxi-clav’ OR ‘amoxi-clavulanate’ OR augmentin OR ‘brl 25000’ OR clavulanate OR clavulin OR coamoxiclav OR spektramox OR synulox
#9 phenoxymethylpenicillin OR apocillin OR beromycin OR berromycin OR betapen OR fenoxymethylpenicillin OR ‘pen vk’ OR ‘v-cillin k’ OR vegacillin
#10 clont OR danizol OR trichazol* OR trichapol OR trivazol OR satric OR metrogyl OR flagyl
OR gineflavir OR metrodzhil OR nidagyl
#11 chlolincocin OR chlorlincocin OR cleocin OR ‘dalacin c’
#12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11
#13 ‘pulpectomy’/exp
#14 ‘pulpitis’/exp
#15 pulp*
#16 #13 OR #14 OR #15
#17 #12 AND #16
#18 random*
#19 factorial*
#20 (crossover* OR cross) AND over* OR ‘cross over*’
#21 placebo
#22 doubl* NEXT/1 blind*
#23 singl* NEXT/1 blind*
#24 assign*
#25 allocat*
#26 volunteer*
#27 ‘crossover procedure’/exp
#28 ‘double blind procedure’/exp
#29 ‘randomized controlled trial’/exp
#30 ‘single blind procedure’/exp
#31 #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30
#32 #17 AND #31
#33 ‘animal’/exp
#34 ‘nonhuman’/exp
#35 ‘human’/exp
#36 ‘human cell’/exp
#37 ‘human’:ti OR ‘humans’:ti
#38 #33 OR #34
#39 #35 OR #36 OR #37
#40 #38 NOT #39
#41 #32 NOT #40
#42 #41 AND (2016:py OR 2017:py OR 2018:py)
Search Strategy #3 (Systematic review on harms related to antibiotic use). Search conducted in this database on June 5, 2018.
Embase. Database: Embase via embase.com
#1 ‘antibiotic resistance’/exp
#2 ‘antibiotic resistance’:ti,ab OR ‘antibiotic resistant’:ti,ab OR ‘antibiotic resistances’:ti,ab OR ‘antibiotics resistance’:ti,ab OR ‘antibiotics resistances’:ti,ab OR ‘antibiotics resistant’:ti,ab OR ‘antimicrobial resistant’:ti,ab OR ‘antimicrobial resistance’:ti,ab OR ‘antimicrobial resistances’:ti,ab OR ‘antimicrobials resistant’:ti,ab OR ‘antimicrobials resistance’:ti,ab OR ‘antimicrobials resistances’:ti,ab OR ‘bacterial resistant’:ti,ab OR ‘bacterial resistance’:ti,ab OR ‘bacterial resistances’:ti,ab OR ‘bacterials resistant’:ti,ab OR ‘bacterials resistance’:ti,ab OR ‘bacterials resistances’:ti,ab OR ‘antibacterial resistant’:ti,ab OR ‘antibacterial resistance’:ti,ab OR ‘antibacterial resistances’:ti,ab OR ‘antibacterials resistant’:ti,ab OR ‘antibacterials resistance’:ti,ab OR ‘antibacterials resistances’:ti,ab OR ‘microbial drug resistant’:ti,ab OR ‘microbial drug resistance’:ti,ab OR ‘microbial drug resistances’:ti,ab OR ‘microbial drugs resistant’:ti,ab OR ‘microbial drugs resistance’:ti,ab OR ‘microbial drugs resistances’:ti,ab OR ‘antibiotic stewardship’:ti,ab OR ‘antibiotics stewardship’:ti,ab OR ‘antibiotic surveillance’:ti,ab OR ‘antibiotics surveillance’:ti,ab OR ‘antimicrobial stewardship’:ti,ab OR ‘antimicrobials stewardship’:ti,ab OR ‘antimicrobial surveillance’:ti,ab OR ‘antimicrobials surveillance’:ti,ab OR ‘resistance to antibiotics’:ti,ab OR ‘resistant to antibiotics’:ti,ab OR ‘resistance to microbial drugs’:ti,ab OR ‘resistant to microbial drugs’:ti,ab OR ‘antibacterial drug resistance’:ti,ab
#3 #1 OR #2
#4 ‘meta analysis’/exp
#5 (meta NEXT/1 analy*) OR metaanalys*
#6 systematic NEXT/1 (review*1 OR overview*1)
#7 #4 OR #5 OR #6
#8 cancerlit:ab
#9 cochrane:ab
#10 embase:ab
#11 psychlit:ab OR psyclit:ab
#12 psychinfo:ab OR psycinfo:ab
#13 cinahl:ab OR cinhal:ab
#14 ‘science citation index’:ab
#15 bids:ab
#16 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
#17 ‘reference lists’:ab
#18 bibliograph*:ab
#19 ‘hand-search*’:ab
#20 ‘manual search*’:ab
#21 ‘relevant journals’:ab
#22 #17 OR #18 OR #19 OR #20 OR #21
#23 ‘data extraction’:ab
#24 ‘selection criteria’:ab
#25 #23 OR #24
#26 ‘review’:it
#27 #25 AND #26
#28 letter:it
#29 editorial:it
#30 ‘animal’/exp
#31 ‘human’/exp
#32 #30 NOT (#30 AND #31)
#33 #28 OR #29 OR #32
#34 #7 OR #16 OR #22 OR #27
#35 #34 NOT #33
#36 #3 AND #35
#37 ‘child’/exp
#38 ‘adult’/exp
#39 #36 NOT (#37 NOT #38)
#40 #39 AND (2013:py OR 2014:py OR 2015:py OR 2016:py OR 2017:py OR 2018:py)
Selection of primary studies and data extraction
We conducted a sensitivity analysis for 1 3-arm study16 (arm 1: antibiotics, arm 2: placebo, arm 3: no medication) to determine if it was appropriate to combine arms 2 and 3 owing to similarities in their clinical mechanism. In comparing the treatment effect of arm 1 versus arm 2 and arm 2 versus arm 3, the confidence intervals overlapped substantially. Therefore, we deemed it acceptable to combine the placebo and no medication arms into a single “no antibiotics” arm. Combining these arms allowed us to increase the power and precision in our pooled estimates (Table 9).16,17
Table 9.
Sensitivity analysis for the outcomes of pain and intraoral swelling.
| OUTCOME, FOLLOW-UP TIME, COMPARISON | RISK RATIO | 95% CONFIDENCE INTERVAL |
|---|---|---|
| Pain* | ||
| 24 h | ||
| Antibiotics versus placebo | 0.76 | 0.47 to 1.24 |
| Antibiotics versus no medicine | 0.81 | 0.49 to 1.34 |
| 48 h | ||
| Antibiotics versus placebo | 1.63 | 0.77 to 3.45 |
| Antibiotics versus no medicine | 1.84 | 0.84 to 4.00 |
| 72 h | ||
| Antibiotics versus placebo | 1.34 | 0.51 to 3.53 |
| Antibiotics versus no medicine | 1.66 | 0.40 to 6.83 |
| Intraoral Swelling* | ||
| 24 h | ||
| Option 1† | 1.70 | 0.55 to 5.24 |
| Option 2‡ | 1.74 | 0.46 to 6.59 |
| 48 h | ||
| Option 1† | 1.36 | 0.62 to 2.98 |
| Option 2‡ | 0.96 | 0.11 to 8.24 |
| 72 h | ||
| Option 1† | 1.00 | 0.05 to 20.81 |
| Option 2‡ | 1.35 | 0.11 to 15.95 |
The estimates were calculated with the data from Fouad and colleagues16 and Henry and colleagues.17
In dichotomizing the outcome of intraoral swelling, option 1 categorized “no swelling” and “mild swelling” used in Henry and colleagues17 and “no swelling,” “much less swelling,” and “slightly less swelling” used in Fouad and colleagues16 as “no swelling.” “Moderate swelling” and “severe swelling” used in Henry and colleagues17 and “same swelling” and “more swelling” used in Fouad and colleagues16 were categorized as “swelling.”
In dichotomizing the outcome of intraoral swelling, option 2 categorized “no swelling” and “mild swelling” used in Henry and colleagues17 and “no swelling” and “much less swelling” used in Fouad and colleagues16 as “no swelling.” “Moderate swelling” and “severe swelling” used in Henry and colleagues17 and “slightly less swelling,” “same swelling,” and “more swelling” used in Fouad and colleagues16 were categorized as “swelling.”
Outcome measures
Included studies informing pain outcomes used the same 0 to 3 visual analog scale (VAS), in which 0 is “no pain,” 1 is “mild pain,” 2 is “moderate pain,” and 3 is “severe pain.”16-18 Dichotomous results for pain experience were categorized as follows: 0 and 1 are “no pain,” and 2 and 3 are “pain.”
Included studies informing the outcome of intraoral swelling used 2 different VASs. One study used a VAS ranging from 0 to 3, in which 0 is no swelling, 1 is mild swelling, 2 is moderate swelling, and 3 is severe swelling.17 Another study used a VAS ranging from 0 to 4 that asked patients to compare their current swelling with preoperative swelling, in which 0 is no swelling, 1 is much less swelling, 2 is slightly less swelling, 3 is same swelling, and 4 is more swelling.16
We conducted a sensitivity analysis to determine the threshold to dichotomize this outcome. In dichotomizing the outcome of intraoral swelling for Henry and colleagues,17 0 and 1 were categorized as “no swelling,” and 2 and 3 were categorized as “swelling.” Because the 0 to 4 VAS used in Fouad and colleagues16 contained 5 possible choices, there were 2 options to dichotomize this scale. Option 1 categorized 0, 1, and 2 as “no swelling” and 3 and 4 as “swelling.” Option 2 categorized 0 and 1 as “no swelling” and 2, 3, and 4 as “swelling.” When the treatment effect of these 2 options, along with the dichotomized data from Henry and colleagues,17 were compared, the confidence intervals overlapped substantially (Table 9), indicating that the results would be similar irrespective of the threshold chosen. In presenting these choices to the expert panel, methodologists communicated that option 1 indicated that even a small reduction in swelling would be relevant to the patient and that option 2 meant that although this might represent a small change in swelling, it is likely not important to patient. By majority vote, the decision was made to use option 1 for data analysis for the outcome of intraoral swelling.
For dichotomous outcomes (for example, pain experience and intraoral swelling), we interpreted a relative risk above 1 as having not favored antibiotics, whereas we interpreted a relative risk below 1 as favoring antibiotics. For continuous outcomes (for example, pain intensity), we interpreted a positive mean difference as the average increase in an outcome.
Assessment of risk of bias and methodological quality
Two reviewers (M.P.T., O.U.) independently assessed the risk of bias of the included studies informing beneficial outcomes using the Cochrane Risk of Bias tool (Review Manager, Version 5.3, Cochrane Collaboration) for the domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Judgments that were assigned to each study were either low, high, or unclear risk of bias. Two reviewers (L.P., M.P.T.) independently assessed the risk of bias of included studies informing harm outcomes using a tool developed by Hoy and colleagues.19 This 10-item tool assessed the internal and external validity of prevalence studies. Responses to each question can be yes, no, or not reported. Reviewers also independently assessed the quality of systematic reviews informing harm outcomes using the AMSTAR 2 critical appraisal tool.20 Any disagreements in judgments were resolved by a third reviewer (A.C.-L.).
Certainty in the evidence
We assessed the certainty in the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach across studies at an outcome level.21 The certainty in the evidence can either be high, moderate, low, or very low. In GRADE, RCTs start as high certainty in the evidence, whereas observational studies start as low certainty in the evidence. Certainty can be reduced when serious or very serious issues of risk of bias, inconsistency, indirectness, imprecision, and publication bias are identified. We assessed inconsistency using the χ2 test and I2 statistic and via visual assessment of forest plots. We assessed indirectness via considering to what extent each included study’s population, interventions, comparators, and outcomes differed from our clinical questions. We assessed imprecision via
evaluating the width of confidence intervals (appreciable benefit or harm) and using the optimal information size for both dichotomous and continuous outcomes;
for dichotomous outcomes, considering 10% reduction or increase in pain experience or intraoral swelling as clinically significant;
for continuous outcomes, considering a 1 point change in pain intensity and a 6 pill change in total number of analgesics as clinically significant.
We planned to evaluate publication bias by means of using a funnel plot when 10 or more studies were available. Certainty in the evidence can be upgraded when a large magnitude of effect, opposing plausible residual bias or confounding that reduces a treatment effect, or dose-response gradient is observed.
Contributor Information
Malavika P. Tampi, Center for Evidence-Based Dentistry, Science Institute, American Dental Association, Chicago, IL..
Lauren Pilcher, Center for Evidence-Based Dentistry, Science Institute, American Dental Association, Chicago, IL..
Olivia Urquhart, Evidence Synthesis and Translation Research, Science Institute, American Dental Association, Chicago, IL..
Erinne Kennedy, Alliance Dental Center, Quincy, MA..
Kelly K. O’Brien, ADA Library & Archives, American Dental Association, Chicago, IL..
Peter B. Lockhart, Department of Oral Medicine, Carolinas Medical Center-Atrium Health, Charlotte, NC..
Elliot Abt, Department of Oral Medicine, College of Dentistry, University of Illinois at Chicago, Chicago, IL..
Anita Aminoshariae, School of Dental Medicine, Case Western Reserve University, Cleveland, OH..
Michael J. Durkin, Infectious Diseases Division, Department of Internal Medicine, School of Medicine, Washington University, St. Louis, MO..
Ashraf F. Fouad, Division of Comprehensive Oral Health, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC..
Prerna Gopal, Science Institute, American Dental Association, Chicago, IL..
Benjamin W. Hatten, Department of Emergency Medicine, School of Medicine, University of Colorado, Aurora, CO..
Melanie S. Lang, Department of Oral and Maxillofacial Surgery, University of Washington, Seattle, WA..
Lauren L. Patton, Department of Dental Ecology, and program director of the General Practice Residency program, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC..
Thomas Paumier, Mercy Medical Center, Canton, OH.
Katie J. Suda, Center of Innovation for Complex Chronic Healthcare, Edward Hines, Jr. Hopsital; College of Pharmacy, University of Illinois at Chicago, Chicago, IL..
Hannah Cho, College of Dental Medicine-Illinois, Midwestern University, Downers Grove, IL..
Alonso Carrasco-Labra, Evidence Synthesis and Translation Research, Science Institute, American Dental Association, Chicago, IL; Department of Oral and Craniofacial Health Sciences, School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC..
References
- 1.Horst OV, Cunha-Cruz J, Zhou L, Manning W, Mancl L, DeRouen TA. Prevalence of pain in the orofacial regions in patients visiting general dentists in the Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry research network. JADA. 2015; 146(10):721–728 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts RM, Hersh AL, Shapiro DJ, Fleming-Dutra KE, Hicks LA. Antibiotic prescriptions associated with dental-related emergency department visits. Ann Emerg Med. 2018;74(1):45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey: 2015 Emergency Department Summary Tables—Table 14. Available at: https://www.cdc.gov/nchs/data/nhamcs/web_tables/2015_ed_web_tables.pdf. Accessed January 28, 2019.
- 4.Zero DT, Zandona AF, Vail MM, Spolnik KJ. Dental caries and pulpal disease. Dent Clin North Am. 2011;55(1): 29–46. [DOI] [PubMed] [Google Scholar]
- 5.American Association of Endodontists. Glossary of endodontic terms. Available at: http://www.nxtbook.com/nxtbooks/aae/endodonticglossary2016/index.php. Accessed December 10, 2018.
- 6.Durkin MJ, Feng Q, Warren K, et al. ; Centers for Disease Control and Prevention Epicenters. Assessment of inappropriate antibiotic prescribing among a large cohort of general dentists in the United States. JADA. 2018; 149(5):372–381 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockhart PB, Hanson NB, Ristic H, Menezes AR, Baddour L. Acceptance among and impact on dental practitioners and patients of American Heart Association recommendations for antibiotic prophylaxis. JADA. 2013; 144(9):1030–1035. [DOI] [PubMed] [Google Scholar]
- 8.Roberts RM, Bartoces M, Thompson SE, Hicks LA. Antibiotic prescribing by general dentists in the United States, 2013. JADA. 2017;148(3):172–178 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suda KJ, Calip GS, Zhou J, et al. Assessment of the appropriateness of antibiotic prescriptions for infection prophylaxis before dental procedures, 2011 to 2015. JAMA Netw Open. 2019;2(5):e193909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornhill MH, Dayer MJ, Prendergast B, Baddour LM, Jones S, Lockhart PB. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother. 2015;70(8):2382–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Anti-btiotic resistance threats in the United States, 2013. Available at: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed November 22, 2018.
- 12.Lockhart P, Tampi M, Abt E, et al. Evidence-based clinical practice guideline on antibtioc use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: a report from the American Dental Association. JADA. 2019;150(11):906–921.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cope A, Francis N, Wood F, Mann MK, Chestnutt IG. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Syst Rev. 2014;6:CD010136. [DOI] [PubMed] [Google Scholar]
- 14.Agnihotry A, Fedorowicz Z, van Zuuren EJ, Farman AG, Al-Langawi JH. Antibiotic use for irreversible pulpitis. Cochrane Database Syst Rev. 2016;2:CD004969. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 16.Fouad AF, Rivera EM, Walton RE. Penicillin as a supplement in resolving the localized acute apical abscess. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996; 81(5):590–595. [DOI] [PubMed] [Google Scholar]
- 17.Henry M, Reader A, Beck M. Effect of penicillin on postoperative endodontic pain and swelling in symptomatic necrotic teeth. J Endod. 2001;27(2):117–123. [DOI] [PubMed] [Google Scholar]
- 18.Nagle D, Reader A, Beck M, Weaver J. Effect of systemic penicillin on pain in untreated irreversible pulpitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(5):636–640. [DOI] [PubMed] [Google Scholar]
- 19.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. [DOI] [PubMed] [Google Scholar]
- 20.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. National Library of Medicine. Search strategy used to create the PubMed systematic reviews filter. Available at: https://www.nlm.nih.gov/bsd/pubmed_subsets/sysreviews_strategy.html. Accessed August 1, 2018. [Google Scholar]
- 23.Healthcare Improvement Scotland SIGN. Search filters. Available at: https://www.sign.ac.uk/search-filters.html. Accessed August 1, 2018.
- 24.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Available at: http://handbook-5-1.cochrane.org. Accessed October 31, 2019. [Google Scholar]
- 25.Durkin MJ, Hsueh K, Sallah YH, et al. ; Centers for Disease Control and Prevention Epicenters. An evaluation of dental antibiotic prescribing practices in the United States. JADA. 2017;148(12):878–886 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. [DOI] [PubMed] [Google Scholar]
- 27.Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173(14):1359–1367. [DOI] [PubMed] [Google Scholar]
- 28.Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston KJ, Thorpe KE, Jacob JT, Murphy DJ. The incremental cost of infections associated with multidrug-resistant organisms in the inpatient hospital setting: a national estimate. Health Serv Res. 2019;54(4):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lessa FC, Winston LG, McDonald LC. Emerging infections program CdST. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015; 372(24):2369–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mainous AG 3rd, Diaz VA, Matheson EM, Gregorie SH, Hueston WJ. Trends in hospitalizations with antibiotic-resistant infections: U.S., 1997–2006. Public Health Rep. 2011;126(3):354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1): 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Department of Labor Bureau of Labor Statistics. CPI inflation calculator. Available at: https://www.bls.gov/data/inflation_calculator.htm. Accessed May 20, 2019.
- 34.Higgins JP, Altman DG, Gotzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aminoshariae A, Kulild JC. Evidence-based recommendations for antibiotic usage to treat endodontic infections and pain: a systematic review of randomized controlled trials. JADA. 2016;147(3):186–191. [DOI] [PubMed] [Google Scholar]
- 36.Matthews DC, Sutherland S, Basrani B. Emergency management of acute apical abscesses in the permanent dentition: a systematic review of the literature. J Can Dent Assoc. 2003;69(10):660. [PubMed] [Google Scholar]
- 37.Sutherland S, Matthews DC. Emergency management of acute apical periodontitis in the permanent dentition: a systematic review of the literature. J Can Dent Assoc. 2003;69(3):160. [PubMed] [Google Scholar]
- 38.Cope AL, Francis N, Wood F, Chestnutt IG. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Syst Rev. 2018;9:CD010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agnihotry A, Thompson W, Fedorowicz Z, van Zuuren EJ, Sprakel J. Antibiotic use for irreversible pulpitis. Cochrane Database Syst Rev. 2019;5:CD004969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions: United States, 2016. Available at: https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-Report-2016-H.pdf. Accessed November 15, 2018.