Abstract
Background:
Ischemic optic neuropathy (ION) is a rare complication of anesthesia and surgery that causes vision loss in spine fusion. We sought to develop a predictive model based upon known pre-operative risk factors for perioperative ION to guide patient and physician pre-operative decision-making.
Methods:
In the National Inpatient Sample (NIS) for 1998–2012, discharges for posterior thoracic, lumbar, and sacral spine fusion were identified and classified by ION status. Variables were selected without weighting via variable clustering using Principal Component Analysis of Mixed Data (PCA-MIX). Hierarchical clustering with four clusters was performed, and the variable with largest squared loading in each cluster was chosen. By splitting our sample into a training and testing data set, we developed and internally validated a predictive model. The final model using variables known pre-operatively was constructed to allow determination of relative and absolute risk of developing perioperative ION, and was tested for calibration and discrimination.
Results:
The final predictive model based upon hierarchical clustering contained three pre-operative factors, age, male or female sex, and the presence of obstructive sleep apnea (OSA). The predictive model based upon these factors had an area under the receiver operating characteristic curve of 0.65 and good calibration. A score cutoff of > 1 had 100% sensitivity, while score of 3 had 96.5% specificity. The highest estimated absolute risk (844.5/million) and relative risk of ION (46.40) was for a male, age 40–64, with OSA.
Conclusions:
The predictive model could enable screening for patients at higher risk of ION in order to provide more accurate risk assessment and surgical and anesthetic planning for perioperative ION in spine fusion.
Introduction
Perioperative Vision Loss (POVL) associated with non-ophthalmic surgery remains a poorly understood and rare, though severely disabling injury, most commonly caused by ischemic optic neuropathy (ION), or retinal artery occlusion (RAO).1 Patients undergoing spine and cardiac surgery are at greatest risk, and ION, which can result in bilateral blindness, remains the most frequent and serious visual complication in spine fusion.2 The incidence of ION after spine fusion surgery has been decreasing, possibly secondary to greater use of minimally invasive surgery and staging, and attention to practice advisories. This notwithstanding, the United States continues to have a large and increasing utilization of spine fusion surgery.2 Therefore it remains of continuing importance to understand the disease burden, identify risk factors, and develop risk stratification strategies to prevent this devastating complication. However, there is currently no predictive model to assist physicians and patents in estimating the risk of ION based upon pre-operative characteristics.
Prior studies have identified possible pre-operative risk factors for ION in spine surgery including increasing age, obesity, and male sex. Intra-operative risk factors include Wilson frame use, anesthesia duration, estimated blood loss, and colloid as percent of non-blood replacement.2,3
A predictive model for ION in spine fusion patients is the logical extension of previous studies of clinical associations, because such a model would consider the impact of multiple risk factors and could guide physicians in pre-operative risk stratification. Similar models have stratified patients for more common complications after surgery such as post-operative nausea and vomiting, showing the feasibility of these tools to assess risk and intervene to decrease incidence.4 Patients are also significant stakeholders in risk stratification. In a survey, 80% wished to be informed of the risk of blindness in spine fusion.5 However, in contrast to prediction modeling for more common, serious perioperative complications,6–8 development of a risk prediction model for perioperative ION based upon known pre-operative characteristics has been hindered by its rare occurrence.
Building upon previous studies, our primary objective was to develop and validate a predictive model for perioperative ION using hospital discharge data in the National Inpatient Sample (NIS). This study concentrates on predicting ION from known pre-operative risk factors, as these factors should generally be readily available and verifiable. As we and others previously described,9–11 the NIS, because of its size, random sampling, diagnosis coverage, and wide coverage of US hospitals,12 appears ideally and uniquely suited for studying rare disorders among hospital discharges, including serious events associated with inpatient hospitalization, e.g., specific perioperative conditions with significant morbidity of interest to anesthesia providers. 13
Methods
Data Set
The requirement for written informed consent was waived by The University of Illinois at Chicago Institutional Review Board, with the study deemed “exempt” since the datasets contain no specific patient identifiers.
Discharge data from the NIS, of the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality (AHRQ), were analyzed.2,11 The NIS randomly samples a large range of hospitals (20% of all non-federal hospitals in the US) and includes a large set of diagnosis and procedure fields. These factors render the NIS highly suitable for testing hypotheses concerning the role of common pre-existing diseases in the development of rare conditions such as perioperative ION. NIS contains demographics, discharge status, outcomes, diagnoses (up to 30) and procedures (up to 15) coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Quality control and reliability of the NIS have been verified. We used the same data set (1998 to 2012, Table 1) as in our previous analysis.2
Table 1:
Characteristics of Spine Fusion Patients with and without Ischemic Optic Neuropathy in the Nationwide Inpatient Sample (NIS) 1998 to 2012, Based upon Population Estimates
| ION | Unaffected | ICD9CM Code | ||
|---|---|---|---|---|
| Number of Discharges | 257 | 2,510,816 | ||
| Mean Age in years (95% CI) | 60.0 (56.8–63.1) | 56.1 (55.8–56.3) | ||
| Sex | Male (%) | 179 (70) | 1,122,459 (45) | |
| Female (%) | 78 (30) | 1,387,191 (55) | ||
| Unknown (%) | 0 (0) | 1,116 (0) | ||
| Race | White (%) | 181 (71) | 1,675,794 (67) | |
| Non-white (%) | 9 (4) | 319,095 (13) | ||
| Unknown (%) | 66 (26) | 515,297 (21) | ||
| Hospital size based on Number of Beds | Small (%) | 33 (13) | 317,452 (13) | |
| Medium (%) | 39 (15) | 558,084 (22) | ||
| Large (%) | 185 (72) | 1,622,759 (65) | ||
| Unknown (%) | 0 (0) | 12,522 (1) | ||
| Teaching Status of Hospital | Teaching (%) | 169 (66) | 1,205,418 (48) | |
| Non-Teaching (%) | 78 (30) | 1,057,526 (42) | ||
| Unknown (%) | 10 (4) | 247,872 (10) | ||
| Hospital Region | Northeast (%) | 38 (15) | 373,055 (15) | |
| Midwest (%) | 95 (37) | 599,514 (24) | ||
| South (%) | 91 (35) | 1,044,241 (42) | ||
| West (%) | 33 (13) | 494,006 (20) | ||
| Number of Vertebral Levels Operated Upon | 2–3 (%) | 77 (30) | 1,473,999 (59) | 81.62 |
| 4–8 (%) | 65 (25) | 295,335 (12) | 81.63 | |
| 9+ (%) | 15 (6) | 32,942 (1) | 81.64 | |
| Unknown (%) | 100 (39) | 708,539 (28) | ||
| Anemia (%) | 107 (42) | 567,229 (23) | 280.x, 285.x | |
| Atherosclerosis (%) | 0 (0) | 7,023 (0) | 440.x | |
| Coronary Artery Disease (%) | 34 (13) | 218,537 (9) | 414.x, 411.8x | |
| Carotid Artery Stenosis (%) | 0 (0) | 5,996 (0) | 433.1x | |
| Chronic Kidney Disease (%) | 10 (4) | 38,732 (2) | 585.x | |
| Congestive Heart Failure (%) | 18 (7) | 46,568 (2) | 428.x | |
| Diabetes with Neurological Complications (%) | 10 (4) | 23,764 (1) | 250.6x, 249.6x | |
| Diabetes Mellitus, no complications listed (%) | 43 (11) | 331,624 (13) | 250.x | |
| Hypercoagulability (%) | 0 (0) | 3,556 (0) | 289.81, 289.82 | |
| Hyperlipidemia (%) | 84 (33) | 523,578 (21) | 272.x | |
| Hypertension (%) | 125 (49) | 1,083,801 (43) | 401.x, 402.x, 405.x, 997.91, 404.x | |
| Obesity (%) | 44 (17) | 247,176 (10) | 278.x | |
| Obstructive Sleep Apnea (%) | 20 (8) | 78,081 (3) | 327.23 | |
| Peripheral Vascular Disease (%) | 37,653 (2) | 443.x | ||
| Previous Cerebrovascular Disease (%) | 4,062 (0) | 997.02, 434.x | ||
| Smoking (%) | 34 (13) | 377,368 (15) | V15.82, 305.1, 989.84 | |
| Thrombocytopenia (%) | 23 (9) | 36,590 (2) | 287.3x, 287.4, 287.49, 287.5 | |
| Bleeding complicating a procedure (%) | 10 (4) | 19,927 (1) | 998.1, 998.11, E870 | |
| Transfusion (%) | 65 (25) | 269,397 (11) | 99.00, 99.04 | |
Variables in bold were not analyzed in Rubin et al, 2016. Imputation resulted in some slight differences in numbers and percentages for some parameters from Rubin et al 2016.
Legend:
95% CI: 95% Confidence Interval
ION: Ischemic Optic Neuropathy
The AHRQ provided updated discharge weights to ensure accurate weighting and enable analysis across multiple years.14 We used the split-sample method to temporally validate our prediction model.15,16 The first two-thirds of the data meeting inclusion criteria were temporally assigned to the training data set (1998–2008) in order to generate the predictive model, and the remaining one-third (“the test set,” 2009–2012) was used for testing the predictive model.
Data Classification in NIS
We used similar methods to those we previously applied to NIS analysis except as detailed below.2,11 Adult (>18 y) discharges with an ICD-9-CM procedure code for posterior thoracic, lumbar, or sacral spine fusion (81.05, 81.07, 81.08, 81.35, 81.37, 81.38) from 1998–2012 in NIS were included.2 ION rarely occurs in children, hence those < 18 y of age were excluded. Cervical spinal fusion, rarely associated with ION, was also excluded.17 A discharge with a principal or secondary diagnostic ICD-9 code for ION (377.41) was considered to have developed ION during the hospitalization. In our previous studies, we also established the basis for considering diagnoses of ION to have developed during the hospitalization for the surgical procedure.2,11 Except as described below, derivation of the ICD-9-CM diagnosis codes was as detailed in our previous study.2
Patient and Surgical Characteristics in NIS
Potential risk factors were identified prior to analysis based upon previous case series, large database reviews, and case reports as recommended by STROBE, and as explained below. Guidelines were followed to maintain scientific rigor in studies using NIS in particular.18 Demographics included age in years, sex, and race. Based upon our previous studies of ION in spine fusion and ION in cardiac surgery,2,11 medical diagnoses that were potential risk factors were defined by ICD-9 diagnosis codes. (Table 1)
Changes from previous studies resulting from related research findings since our previous publication2 included studying factors found to increase risk of perioperative ION in our study of cardiac surgery,11or suggested as risk factors in spontaneous, not operatively-associated ION.19 Additional factors that were not considered in the previous study2 are shown in bold in Table 1, and include congestive heart failure, hypercoagulability, hyperlipidemia, thrombocytopenia, and diabetes with associated complications, which more accurately classifies the disorder according to severity.20 Although we also collected data for the ICD-9-CM codes for peri-operative factors including bleeding complicating a procedure, and transfusion, to realistically simulate the information that would ordinarily be available to the clinician pre-operatively, we only considered pre-operative factors in the risk model. Vertebral levels operated on were considered as part of the expected pre-operative surgical plan. To examine for uneven distribution of ION, we studied hospital size, region, and teaching status.
Statistical Analysis
Data were analyzed using STATA v14.0-MP and R (v3.4.1; R Core Team, Vienna, Austria). Trend weights were used to derive population-level estimates and population-level regressions using the STATA Survey function.21 Multiple imputation was conducted by using fully conditional specification to minimize bias,22 and imputed data was used for cluster analysis and multivariable logistic regression. Characteristics were tabulated over the entire data set for all spinal fusion discharges with or without ION for 1998–2012 using the weighted national estimates. Our previous reports provided the basis for NIS having appropriate sample size to test our hypotheses.2,11
Parameters in the training and test data sets were compared by chi-square or weighted 2-sample t-test. Variables were pre-selected without weighting via variable clustering by using the imputed data and Principal Component Analysis of Mixed Data (PCA-MIX) algorithm, which allows for both numerical and categorical variables for selection at the same time.23 In the selection process, hierarchical clustering with 4 clusters was chosen due to its stability and the variable with largest squared loading in each cluster was chosen. Thus, the variable which had the strongest association with the cluster center was selected as the representative of that cluster and was added to the multivariable model. As this approach identifies variables with most independent information, it does not ignore important predictors which may be shadowed by other related variables. Thus, it is possible that some of the selected variables show no statistical significance in the multivariable model. Moreover, since this selection process does not consider the association between predictors and the outcome of interest, it may be more beneficial to use the entire data set for the selection and validate the association in the model with testing data later.
Multivariable logistic regression created a predictive model from the training data set using selected variables from the PCA-MIX output. We then developed a scoring scheme using the point estimates of the β-coefficients (or log of odds ratios) as previously described.16 For the predictive model, the β-coefficients were rounded to the nearest integer. The sum of this predictive score was then evaluated in the test data set for sensitivity, specificity, and area under the receiver operator characteristic curve (AUC).24
From the final model, predicted values were used to construct a table of relative and absolute risks of each combination of the selected variables. To confirm the calibration of the model, we used Spiegelhalter’s Z-statistic from the Brier test.25
Results
Table 1 demonstrates the characteristics of discharges with ION and those without. There were no differences between affected and un-affected discharges in hospital size or region of the country, but the risk of developing ION was significantly higher in teaching hospitals. There were significant differences in demographic characteristics between the training data set (2/3s of the data, 1998–2008), and the remaining one-third (“the test set,” 2009–2012), e.g., in the latter, older age, fewer cases of ION, and greater percentage having lesser vertebral levels operated upon, as shown in Supplemental Table 1.
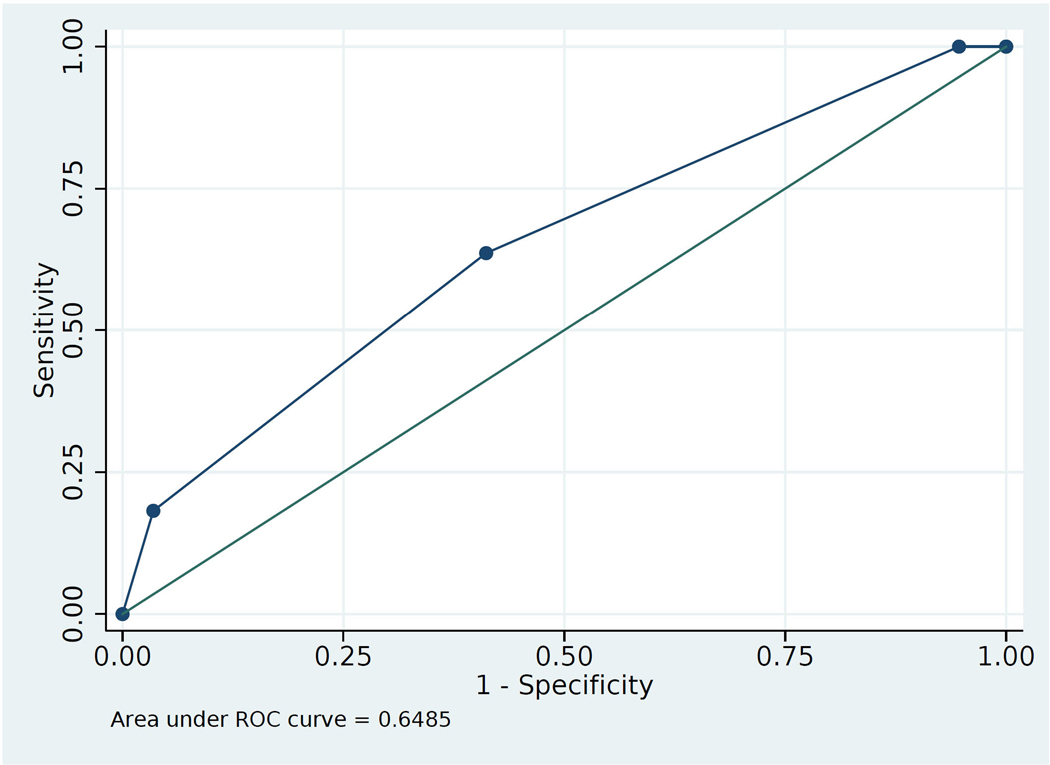
From the cluster analysis, the resulting model contained known preoperative factors of sex, obstructive sleep apnea, age, and chronic kidney disease (CKD, Table 2). β-coefficients and odds ratios for the variables in this model (F-statistic 4.29, P=0.0018) from multivariable analysis are shown in Table 3. CKD was omitted from analysis at this point due to insufficient number of subjects with CKD and ION in the training data. The β-coefficients were rounded to the nearest integer, resulting in the Variable Score. This model had Spiegelhalter’s Z statistic of −2.46, and p=0.99, indicating good calibration.25 If a discharge contained any one of the variables then the respective Variables Scores were added together for an overall Predictive Score, ranging from 0 to 6 (age range scores were not cumulative). As demonstrated in Table 4, a score ≥ 1 had 100% sensitivity, while score = 3 had 96.7% specificity. The Receiver Operator Characteristic Curve based on these values (Figure 1), had AUC of 0.65 for discrimination.
Table 2:
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | ||||
|---|---|---|---|---|---|---|---|
| Variable | Squared loading | Variable | Squared loading | Variable | Squared loading | Variable | Squared loading |
| Age | 0.49 | Male | 0.53 | Chronic Kidney Disease | 0.42 | Obstructive Sleep Apnea | 0.48 |
| Hypertension | 0.49 | Smoke | 0.35 | Congestive Heart Failure | 0.34 | Obesity | 0.46 |
| Hyperlipidemia | 0.40 | Race | 0.18 | Diabetes with Neurological Complications | 0.25 | Number of Vertebral Levels Operated Upon | 0.30 |
| Coronary Artery Disease | 0.29 | Peripheral Vascular Disease | 0.17 | ||||
| Diabetes, uncomplicated | 0.25 | ||||||
The squared loadings on first principal component of PCAMIX is a measure of the association between each variable and the cluster center.
When more than one variable shares the same highest squared loading in one cluster, one variable is randomly selected.
Table 3:
Predictive Model for Ischemic Optic Neuropathy in all Spinal Fusion Patients in the Nationwide Inpatient Sample, 1998–2012 using the Population Weighted Sample. Selected variables chosen based on cluster analysis from list of all variables that would be known pre-operatively
| Variable | β-coefficient (SE) | Odds Ratio (95% CI) | P value | Variable Score | |
|---|---|---|---|---|---|
| Male | 1.16 (0.36) | 3.18 (1.58–6.41) | 0.001 | 1 | |
| Obstructive Sleep Apnea | 1.26 (0.72) | 3.53 (0.86–14.55) | 0.080 | 1 | |
| Age | 18–39 | Reference | |||
| 40–64 | 1.45 (0.75) | 4.28 (0.99–18.59) | 0.052 | 1 | |
| 65+ | 1.35 (0.78) | 3.86 (0.84–17.76) | 0.082 | 1 | |
Legend:
SE: Standard Error
95% CI: 95% Confidence Interval
Table 4:
Diagnostic Characteristics of the Predictive Score based on all preoperative variables in the Test Dataset
| Score Cutoff | Sensitivity (%) | Specificity (%) | Positive Likelihood Ratio | Negative Likelihood ratio |
|---|---|---|---|---|
| ≥1 | 100.00 | 5.33 | 1.0563 | 0.0000 |
| ≥2 | 63.64 | 58.85 | 1.5465 | 0.6179 |
| =3 | 18.18 | 96.52 | 5.2197 | 0.8477 |
The scores are derived from the b-coefficients in Table 3. Note that age scores are not cumulative, that is, age > 65 y does not receive 2 + 2 = 4 points.
Figure 1:
Area under the Receiver Operator Characteristic Curve for the predictive model as tested on the testing data set.
Absolute and relative risk for each possible combination of the three variables in the pre-operative risk model are shown in Table 5. The lowest absolute (18.2/million procedures) and relative risk (1.00) was for female, age 18–39, and no OSA, while the highest absolute risk (844.5/million) and relative risk (46.40) was for male, age 40–64, and with OSA. The reference category is female patients aged 18–39 without OSA.
Table 5:
Risk Prediction for ION after Spinal Fusion Surgery: Effect of changes in variables known pre-operatively on ION risk
| Age | Sex | OSA | Absolute Risk of ION per 1 million procedures | Relative Risk |
|---|---|---|---|---|
| 18–39 | F | N | 18.2 | 1.00 |
| 18–39 | M | N | 57.6 | 3.16 |
| 18–39 | F | Y | 62.5 | 3.43 |
| 65+ | F | N | 69.0 | 3.79 |
| 40–64 | F | N | 77.5 | 4.26 |
| 18–39 | M | Y | 198.1 | 10.88 |
| 65+ | M | N | 218.8 | 12.02 |
| 65+ | F | Y | 237.1 | 13.03 |
| 40–64 | M | N | 245.8 | 13.51 |
| 40–64 | F | Y | 266.4 | 14.64 |
| 65+ | M | Y | 751.7 | 41.30 |
| 40–64 | M | Y | 844.5 | 46.40 |
The absolute risk was calculated using the incidence values from 1998–2012 in Rubin et al, Anesthesiology, 2016. OSA = obstructive sleep apnea
Discussion
In this study we developed a predictive model for ION associated with spine fusion based on NIS data, using pre-existing co-morbidities expected to be detected on pre-operative evaluation, with internal validation that yields promise for reasonable risk stratification. The capacity to predict risk of rare and serious perioperative complications has been limited, due to factors including lack of large datasets necessary for diseases with rare outcomes, and inadequate prediction methodology. In the predictive model, which was based upon pre-operative factors, and generated and internally validated with a split-sample approach, each variable, age, sex, and history of OSA, were assigned an integer point value. These point values can be added together easily by the clinician to generate a predictive score. Moreover, we provide a simple table which enables relative and absolute risk to be estimated based upon these parameters. This allows a clinician to rapidly identify a higher-risk patient and offer counseling about the increased risk of developing ION based on parameters that would be known from the pre-operative history. Identification of a higher risk patient would also be expected to prompt discussion amongst the anesthesiologist and surgeon with respect to perioperative management to mitigate risks, e.g., potentially performing a less complex procedure.
A novel contribution of this study was constructing a predictive model using the NIS data and internally validating it. PCA-MIX is a machine learning technique which is increasingly accepted for accuracy in prediction and forecasting from big data. For big data sets, it reduces the dimensionality, enhances interpretability and minimizes information loss.26 In our previous study,3 a model was constructed from mainly intra-operative variables from an anonymous non-randomly collected patient registry and controls derived exclusively from academic medical institutions. Data on many pre-existent diseases were not solicited or not recorded. Our present study uses a nationally representative data collection with a vastly greater number of subjects and provides a simple risk calculation for the clinician based upon data expected to be readily available prior to surgery. It is interesting in this regard that the risk of ION was higher in discharges from teaching hospitals, suggesting caution in the interpretation of results heavily weighted with those institutions, which is not the case for the NIS. Our earlier study included intra-operative components not available in NIS including hours of anesthesia, blood loss, and amounts of blood and fluid given,3 none of which can be exactly predicted pre-operatively. Hence, we suggest a rapid pre-operative assessment of risk can be obtained with our present model, and the clinician, if aware of a high likelihood of blood loss and lengthy surgery, can use the earlier prediction for further fine-tuning of the risk calculation and/or discussion with the patient.
Our analysis was performed with the same data set as used in previous studies on ION.2 Slight differences in some of the percentages of discharges with certain pre-operative factors were caused by imputation to account for missing values in the dataset. We also used updated rationale 27,28 to study additional possible risk factors in ION, such as risk factors associated with non-operative ION. For the split sample method, it would be reasonable to either split the entire dataset randomly, or use a temporal sequence, the latter meaning that the testing data set will be taken from discharges in later years in the database. The advantage of a temporally split-sample is that the test set is then expected to more closely mirror current clinical practices and patient populations. An interesting outcome of this splitting of the data was that the test set (2009–2012) in comparison to the training set (1998–2008) had a higher percentage with 2–3 vertebral levels operated upon (78 vs 46%), and more obesity and OSA. These likely reflect the trends toward increasing use of minimally invasive spine surgery as well as increasing obesity and OSA in the US population.29,30 In this regard, somewhat surprisingly, the number of vertebral levels operated upon was eliminated as a predictor in the cluster analysis.
Our study is not without limitations. Its major strengths are the use of a large database reflecting a population sample of the United States with the application of novel prediction strategy to a rare complication.2 With respect to studying risk factors for rare disorders such as ION, NIS has significant advantages over all other presently existing large data collections (reviewed elsewhere)31 such as NSQIP (National Surgical Quality Improvement Program), where the patient population is much smaller, ION diagnosis has not been collected, and the patient population is mostly derived from large academic centers and therefore may not be representative of trends across the population of the United States.32
With regard to accuracy of diagnosis data in NIS, and as an example, a diagnosis of obesity, large studies of different and common disease entities in NIS have reported relatively lower incidence of obesity compared to other databases including NSQIP, and in our study, the incidence was lower than in our previous report on spine fusion and ION using data from a registry.2 The reason for these discrepancies are not clear. Since NIS uses diagnostic codes and other databases such as NSQIP use patient data to calculate BMI, they may be more likely to report a subject as obese.33–35 Also, in Table 1, no discharges were reported with atherosclerosis in the ION group, while 13% had coronary artery disease. This is not necessarily a contradiction, as the NIS does not collect every diagnosis on every discharge. The incidence of coronary artery disease in the ION and unaffected discharges is nonetheless consistent with reports from large epidemiological studies.36 Similarly, anemia was common in discharges in this study, 23% in unaffected, and 42% in affected. It is possible that the anemia diagnosis was acquired post-operatively, but this would not affect our results.
Newer, large patient databases including Marketscan (Truven Health Analytics), and Optum,37 are derived from large commercial health insurers and enable cross-sectional follow-up studies, but these have fewer patients than NIS, and may suffer from bias in that they include only commercially insured patients mostly from large employers.37 NACOR (National Anesthesia Clinical Outcomes Registry) may be nationally representative, but has insufficient cases of ION for analysis, hence it could not be applied for our study question.38 Medicare data contains a large population, but is generally available only as a small 5–10% sample, significantly limiting research on rare disorders, and also is unsuited for our study, because spine surgery contains a large proportion of younger patients that would not be represented; thus determination of the role of age in the disorder would be effectively non-feasible.39 Additionally, there is a lack of intraoperative data in NIS and most of these datasets, however, this limitation is mitigated as our approach is centered upon identification of pre-operative risk. As in any data collection, there are limitations of predictive modeling for rare disorders, which we mitigated by using the novel combination of PCA-MIX and logistic regression.40
In conclusion, our study demonstrated preoperative variables including age, sex and the presence of OSA to generate a risk prediction model with good calibration, acceptable AUC and adequate sensitivity to predict perioperative ION in patients undergoing posterior spinal fusion surgery. For the clinician and patient, knowledge of these factors that confer higher risk, together with the predictive score, should assist to identify and help counsel patients at high risk to develop ION. Further research is needed to determine if the prediction model would help to facilitate surgical planning, e.g., whether high risk patients should undergo staged procedures. In addition, our novel prediction modeling may be a useful paradigm to stratify risks for other rare complications in the perioperative period.
Supplementary Material
Supplemental Table 1: Testing set and training set comparison
Key Points Summary:
Question: Can Ischemic Optic Neuropathy after spinal fusion surgery be predicted?
Findings: Certain known factors, including male or female sex, patient age, and the presence of obstructive sleep apnea can be used to pre-operatively identify patients at higher risk of this adverse event.
Meaning: We provide a risk score for perioperative ION to stratify patients before elective spinal fusion surgery.
Acknowledgments
Financial Disclosures: This research was supported by National Institutes of Health (Bethesda, MD) grants R21 EY027447 (Dr. Roth), R21 EY027447-01S1 (Dr. Roth), K23 EY 024345 (Dr. Moss), P30 EY 026877 to the Department of Ophthalmology at Stanford University, P30 EY001792 to the Department of Ophthalmology at the University of Illinois at Chicago, unrestricted grants from Research to Prevent Blindness, Inc. (New York, NY) to the Stanford University Department of Ophthalmology and to the University of Illinois at Chicago Department of Ophthalmology and Visual Sciences. The project was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grants UL1 TR002003 to the Center for Clinical and Translational Sciences at the University of Illinois at Chicago and UL1 TR002389 to the University of Chicago Institute for Translational Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or of the United States Department of the Army.
Glossary of Abbreviations and Terms:
- AHRQ
Agency for Healthcare Research and Quality
- AUC
Area under the receiver operating characteristic curve
- CKD
Chronic kidney disease
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- ION
Ischemic optic neuropathy
- NACOR
National Anesthesia Clinical Outcomes Registry
- NIS
National Inpatient Sample
- NSQIP
National Surgical Quality Improvement Program
- OSA
Obstructive sleep apnea
- POVL
Perioperative vision loss
- PCA-MIX
Principal component analysis of mixed data
- RAO
Retinal artery occlusion
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
Footnotes
Trial Registrations: Not applicable
Shikhar H. Shah: This author helped with conception and design, data analysis, drafting the article, and final approval of manuscript.
Yi-Fan Chen: This author helped with conception and design, data analysis, drafting the article, and final approval of manuscript.
Heather E. Moss: This author helped with conception and design, drafting the article, and final approval of manuscript.
Daniel S. Rubin: This author helped with conception and design, data analysis, drafting the article, and final approval of manuscript.
Charlotte E. Joslin: This author helped with conception and design, drafting the article, and final approval of manuscript.
Steven Roth: This author helped with conception and design, data analysis, drafting the article, and final approval of manuscript
Conflicts of Interest: Dr. Roth has received compensation for expert witness evaluation and testimony in cases of perioperative visual loss on behalf of patients, hospitals, and health care providers.
Contributor Information
Shikhar H. Shah, Department of Anesthesiology, Walter Reed National Military Medical Center.
Yi-Fan Chen, The Center for Clinical & Translational Sciences, University of Illinois at Chicago.
Heather E. Moss, Departments of Ophthalmology, and Neurology & Neurological Sciences, Stanford University.
Daniel S. Rubin, Department of Anesthesia and Critical Care, University of Chicago.
Charlotte E. Joslin, Department of Ophthalmology and Visual Sciences, College of Medicine; Department of Epidemiology and Biostatistics, College of Public Health, University of Illinois at Chicago.
Steven Roth, Departments of Anesthesiology, and Ophthalmology and Visual Sciences, College of Medicine, University of Illinois at Chicago.
References
- 1.Roth S, Thisted RA, Erickson JP, Black S, Schreider BD. Eye injuries after non ocular surgery: A study of 60, 965 anesthetics from 1988 to 1992. Anesthesiology. 1996;85:1020–1027. [DOI] [PubMed] [Google Scholar]
- 2.Rubin DS, Parakati I, Lee LA, Moss HE, Joslin CE, Roth S. Perioperative Visual Loss in Spine Fusion Surgery: Ischemic Optic Neuropathy in the United States from 1998 to 2012 in the Nationwide Inpatient Sample. Anesthesiology. 2016;125(3):457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee LA, Roth S, Todd MM, et al. The Postoperative Visual Loss Study Group. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology. 2012;116(1):15–24. [DOI] [PubMed] [Google Scholar]
- 4.Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700. [DOI] [PubMed] [Google Scholar]
- 5.Corda DM, Dexter F, Pasternak JJ, Trentman TL, Nottmeier EW, Brull SJ. Patients’ perspective on full disclosure and informed consent regarding postoperative visual loss associated with spinal surgery in the prone position. Mayo Clin Proc. 2011;86(9):865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glance LG, Faden E, Dutton RP, et al. Impact of the Choice of Risk Model for Identifying Low-risk Patients Using the 2014 American College of Cardiology/American Heart Association Perioperative Guidelines. Anesthesiology. 2018;129(5):889–900. [DOI] [PubMed] [Google Scholar]
- 7.Lee CK, Hofer I, Gabel E, Baldi P, Cannesson M. Development and Validation of a Deep Neural Network Model for Prediction of Postoperative In-hospital Mortality. Anesthesiology. 2018;129(4):649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russotto V, Sabate S, Canet J. Development of a prediction model for postoperative pneumonia: A multicentre prospective observational study. Eur J Anaesthesiol. 2018. [DOI] [PubMed] [Google Scholar]
- 9.Cauley CE, Anderson G, Haynes AB, Menendez M, Bateman BT, Ladha K. Predictors of In-hospital Postoperative Opioid Overdose After Major Elective Operations: A Nationally Representative Cohort Study. Ann Surg. 2017;265(4):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulkerson Schaeffer S, Gimovsky AC, Aly H, Mohamed MA. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32(5):798–803. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DS, Matsumoto MM, Moss HE, Joslin CE, Tung A, Roth S. Ischemic Optic Neuropathy in Cardiac Surgery: Incidence and Risk Factors in the United States from the National Inpatient Sample 1998 to 2013. Anesthesiology. 2017;126(5):810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dexter F, Epstein RH. Reductions in Average Lengths of Stays for Surgical Procedures Between the 2008 and 2014 United States National Inpatient Samples Were Not Associated With Greater Incidences of Use of Postacute Care Facilities. Anesth Analg. 2018;126(3):983–987. [DOI] [PubMed] [Google Scholar]
- 13.Burton BN, Lin TC, Said ET, Gabriel RA. National Trends and Factors Associated With Inpatient Mortality in Adult Patients With Opioid Overdose. Anesth Analg. 2019;128(1):152–160. [DOI] [PubMed] [Google Scholar]
- 14.HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 1996–2005. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed 06/2008.
- 15.Mazumdar M, Smith A, Bacik J. Methods for categorizing a prognostic variable in a multivariable setting. Stat Med. 2003;22(4):559–571. [DOI] [PubMed] [Google Scholar]
- 16.Bang H, Vupputuri S, Shoham DA, et al. SCreening for Occult REnal Disease (SCORED): a simple prediction model for chronic kidney disease. Arch Intern Med. 2007;167(4):374–381. [DOI] [PubMed] [Google Scholar]
- 17.Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652–659. [DOI] [PubMed] [Google Scholar]
- 18.Khera R, Angraal S, Couch T, et al. Adherence to Methodological Standards in Research Using the National Inpatient Sample. Jama. 2017;318(20):2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cestari DM, Gaier ED, Bouzika P, et al. Demographic, Systemic, and Ocular Factors Associated with Nonarteritic Anterior Ischemic Optic Neuropathy. Ophthalmology. 2016. [DOI] [PubMed] [Google Scholar]
- 20.Pearce I, Simo R, Lovestam-Adrian M, Wong DT, Evans M. Association between diabetic eye disease and other complications of diabetes: Implications for care. A systematic review. Diabetes Obes Metab. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houchens R, Ross D, Elixhauser A, Jiang J. Nationwide Inpatient Sample Redesign Final Report.. HCUP NIS Related Reports ONLINE. 2014. http://www.hcupus.ahrq.gov/db/nation/nis/nisrelatedreports.jsp. Published April 4 2014. Accessed 26December18.
- 22.Sullivan TR, Lee KJ, Ryan P, Salter AB. Multiple imputation for handling missing outcome data when estimating the relative risk. BMC medical research methodology. 2017;17(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chavent M, Kuentz-Simonet V, Saracco J. Orthogonal rotation in PCAMIX. Advances in Data Analysis and Classification. 2012;6(2):131–146. [Google Scholar]
- 24.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. [DOI] [PubMed] [Google Scholar]
- 25.Rufibach K. Use of Brier score to assess binary predictions. Journal of Clinical Epidemiology. 2010;63(8):938–939. [DOI] [PubMed] [Google Scholar]
- 26.Jolliffe Ian T, Cadima J. Principal component analysis: a review and recent developments. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2016;374(2065):20150202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calway T, Rubin DS, Moss HE, Joslin CE, Mehta AI, Roth S. Perioperative Retinal Artery Occlusion: Incidence and Risk Factors in Spinal Fusion Surgery From the US National Inpatient Sample 1998–2013. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2018;38:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calway T, Rubin DS, Moss HE, Joslin CE, Beckmann K, Roth S. Perioperative Retinal Artery Occlusion: Risk Factors in Cardiac Surgery from the United States National Inpatient Sample 1998–2013. Ophthalmology. 2017;124(2):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae J, Lee SH. Minimally Invasive Spinal Surgery for Adult Spinal Deformity. Neurospine. 2018;15(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundeen EA, Park S, Pan L, O’Toole T, Matthews K, Blanck HM. Obesity Prevalence Among Adults Living in Metropolitan and Nonmetropolitan Counties - United States, 2016. MMWR Morbidity and mortality weekly report. 2018;67(23):653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss HE, Joslin CE, Rubin DS, Roth S. Big Data Research in Neuro-Ophthalmology: Promises and Pitfalls. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eamer G, Al-Amoodi MJH, Holroyd-Leduc J, Rolfson DB, Warkentin LM, Khadaroo RG. Review of risk assessment tools to predict morbidity and mortality in elderly surgical patients. Am J Surg. 2018;216(3):585–594. [DOI] [PubMed] [Google Scholar]
- 33.Abdullah A, Eigbire G, Salama A, Wahab A, Nadkarni N, Alweis R. Relation of Obstructive Sleep Apnea to Risk of Hospitalization in Patients With Heart Failure and Preserved Ejection Fraction from the National Inpatient Sample. The American journal of cardiology. 2018;122(4):612–615. [DOI] [PubMed] [Google Scholar]
- 34.Schlussel AT, Delaney CP, Maykel JA, Lustik MB, Nishtala M, Steele SR. A National Database Analysis Comparing the Nationwide Inpatient Sample and American College of Surgeons National Surgical Quality Improvement Program in Laparoscopic vs Open Colectomies: Inherent Variance May Impact Outcomes. Diseases of the colon and rectum. 2016;59(9):843–854. [DOI] [PubMed] [Google Scholar]
- 35.Pratt TS, Hudson CO, Northington GM, Greene KA. Obesity and Perioperative Complications in Pelvic Reconstructive Surgery in 2013: Analysis of the National Inpatient Sample. Female pelvic medicine & reconstructive surgery. 2018;24(1):51–55. [DOI] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics−−2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. [DOI] [PubMed] [Google Scholar]
- 37.Walsh JA, Adejoro O, Chastek B, Palmer JB, Hur P. Treatment Patterns Among Patients with Psoriatic Arthritis Treated with a Biologic in the United States: Descriptive Analyses from an Administrative Claims Database. J Manag Care Spec Pharm. 2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glance LG, Dutton RP, Feng C, Li Y, Lustik SJ, Dick AW. Variability in Case Durations for Common Surgical Procedures. Anesth Analg. 2018;126(6):2017–2024. [DOI] [PubMed] [Google Scholar]
- 39.Mues KE, Liede A, Liu J, et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clinical epidemiology. 2017;9:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grauer JN, Leopold SS. Editorial: large database studies--what they can do, what they cannot do, and which ones we will publish. Clin Orthop Relat Res. 2015;473(5):1537–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Testing set and training set comparison