Abstract
Objectives:
Frailty affects an estimated 15% of community dwelling older adults. Few studies look at psychosocial variables like self-efficacy (confidence to perform well at a particular task or life domain) in relation to frailty. The purpose of this study was to evaluate associations between pre-frailty/frailty and self-efficacy.
Methods:
This cross-sectional study enrolled community dwelling older adults 65 and older (N = 146) with at least one chronic condition. Scales included: 5-item FRAIL scale (including measures of Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight); coping self-efficacy used to measure confidence in one’s ability to problem solve, emotionally regulate and ask for support when problems in life occur; illness intrusiveness; patient health questionnaire to assess depressive symptoms; financial strain; life events count; social support; heart rate; tobacco use and body mass index. Logistic regression was used for model development.
Results:
Roughly half (49.3%) of the participants were frail/pre-frail. High coping self-efficacy was associated with a 92% decreased odds of pre-frailty/frailty after adjustment for age, sex, race, co-morbidities, heart rate, a life events count, and body mass index. This relationship remained significant when illness intrusiveness and depression scores were added to the model (OR: 0.10; p-value = 0.014). Increases in age, co-morbidities, heart rate and body mass index were also significantly associated with higher adjusted odds of pre-frailty/frailty.
Conclusions:
High coping self-efficacy was associated with greater odds of a robust state. Further consideration should be given to coping self-efficacy in frailty research and intervention development.
Keywords: Frailty, coping self-efficacy, chronic disease, healthy aging, older adults
Background and significance
Frailty is characterized as a state of decreased physiologic reserve and vulnerability to negative health outcomes, especially in the event of a stressor (Walston et al., 2006). Frailty involves multiple inter-related homeostatic mechanisms, including the immune and autonomic nervous systems. It is associated with decreased function, increased disability and increasing co-morbid conditions (Fried et al., 2009; Gill et al., 2002; Varadhan et al., 2014). This results in frail persons requiring more frequent hospitalizations and residence in nursing homes (Morley et al., 2013). It is estimated that 15% of older community dwelling adults in the US are frail with increased prevalence in older ages, multi-morbidity, tobacco use, women, racial and ethnic minority communities and those with lower incomes (Bandeen-Roche et al., 2015; Kojima, Iliffe, & Walters, 2015). Frailty is also influenced by other social determinants of health, including traumatic life events (such as the death of a spouse), living arrangements, and perceived stress (Desrichard, Vallet, Agrigoroaei, Fagot, & Spini, 2018; Gobbens, van Assen, Luijkx, Wijnen-Sponselee, & Schols, 2010). As the US population ages, it is crucial to identify specific risk factors for frailty to provide appropriate care and develop optimal interventions.
Not all individuals experience the same degree or progression of frailty. It is thought that in addition to physical factors, environmental and psychosocial factors play a role in frailty development (Kirkwood, 2005). One such factor is self-efficacy, which is defined as confidence to perform a specific behavior or succeed in a specific life domain. Self-efficacy is attained through four distinct mechanisms: personal mastery, physiologic feedback, verbal persuasion and vicarious experience (Bandura, 1997). The latter two mechanisms rely heavily on one’s social ties, making social support another key concept to consider in self-efficacy research. Of particular interest for this study, coping self-efficacy is a domain-specific construct of confidence to cope effectively with difficulties in the areas of problem solving, emotional regulation and obtaining support from one’s social network (Chesney, Neilands, Chambers, Taylor, & Folkman, 2006).
Self-efficacy is malleable and was successfully targeted in health-related interventions, making it a potentially useful intervention goal for the treatment of frailty. Increases in self-efficacy are linked to better health behavior (such as exercise and diet) and decreases in the progression of multiple disease processes including diabetes and cardiovascular disease (Alharbi et al., 2017; Schüz, Wurm, Warner, & Ziegelmann, 2012; Scult et al., 2015). In the 2017 American Heart Association’s Heart Disease and Stroke update, increasing self-efficacy was identified as 1 of 8 evidence-based individual approaches for improving health behaviors and health factors in the clinic setting (Benjamin et al., 2017).
Some associations between frailty and self-efficacy are previously established. Doba et al.’s cross sectional results suggested that higher frailty was associated with significantly lower self-efficacy (Doba et al., 2016). This study used a general self-efficacy instrument specifically designed for their study and not previously validated. They used the 7-point clinical frailty scale which is based on clinician judgement to rate each participant’s frailty status. Stretton et al. found that falls self-efficacy was the single best predictor of physical functioning in frail older adults (Stretton, Latham, Carter, Lee, & Anderson, 2006). Similarly, frailty was assessed using a clinician-based rapid clinical screening. In one 12-month randomized control pilot with 117 participants, a self-efficacy based problem solving therapy resulted in a short term 44% improvement in frailty status, but the improvement was not sustained over the course of the 12 month study (Chan et al., 2012). None of these studies included US older adult populations.
Although researchers have reported an association between frailty and self-efficacy, this association has not been evaluated with a US older adult population with validated self-efficacy instruments and participant-based assessments of frailty. Therefore, the purpose of this study was to evaluate the association between pre-frailty/frailty and self-efficacy in a sample of US older adults using the FRAIL scale (Morley, Malmstrom, & Miller, 2012) and the self-efficacy of chronic disease management (Ritter, Lorig, Ritter, & Lorig, 2014) and coping self-efficacy (Chesney et al., 2006) scales.
We also hypothesized that self-efficacy is mediated by depressive symptoms and illness intrusiveness on frailty. Illness intrusiveness is the degree to which chronic disabling and life threatening medical conditions and their treatments affect perceived quality of life (DeCoster, Killian, & Roessler, 2013). In Devin’s conceptual framework, illness intrusiveness is conceptualized as a response stressor driven by one’s chronic disease and treatment. Devin notes the importance of the appraisal process affecting perceived illness intrusiveness and, we hypothesize that self-efficacy is a part of that appraisal process (Devins, 2010). Illness-caused changes to activity, function and lifestyle can lead to emotional distress and depressive symptoms (Devins, 1994). The self-efficacy theory of depression asserts that low self-efficacy beliefs lead to apathy, feelings of worthlessness and inadequacy and, decrease rates of initiation of positive behaviors- all of which contribute to a depressed mood state (Maddux, 1995; Schwarzer, 1992). In this way, self-efficacy beliefs are an integral part of the appraisal processes for both perceived depressive symptoms and illness intrusiveness. We chose to look at both of these constructs in order to examine a more global emotional distress state and a more chronic illness specific perceived state of distress.
Methods
Study design
This was a cross sectional study using convenience sampling. The study was approved by the Johns Hopkins institutional review board (IRB00123553).
Participants
Data collection took place at retirement communities for older adults in Silver Spring, Maryland (n = 134), Laurel, Maryland (n = 10) and Baltimore, Maryland (n = 2) from May 2017 through November 2017. Recruitment involved flyer placement and informational sessions where potential participants could learn more about the study. A mutually agreeable time was found to meet with each participant, obtain consent, take a heart rate measurement, obtain co-morbidity data and review the survey. Each participant filled out the survey at their leisure over a 3-day period, then returned it to a research team member. Inclusion criteria required participants to be 65 years old or older, have at least one chronic condition, and English proficiency. Exclusion criteria included diagnosis of a progressive neurological condition, terminal illness diagnosis, or active cancer treatment.
Measures
Frailty was measured by self-report using the 5-item FRAIL scale, which consists of 5 domains including fatigue, resistance, ambulation, illnesses and loss of weight and has been associated longitudinally with morbidity and mortality in specific populations (Malmstrom, Miller, & Morley, 2014; Morley et al., 2012; Susanto, Hubbard, & Gardiner, 2018). Participants were asked: (1) about their level of fatigue over the past 4 weeks; (2) any difficulty walking up 10 steps without rest (e.g. resistance); (3) any difficulty walking several hundred yards (e.g. ambulation), both without the use of aids; (4) loss of weight over the past year and (5) out of 11 common chronic illnesses, the total number for which each participant had been diagnosed. If the participant had 5 or more of those chronic illnesses, they were given one point for that criteria. Total scores ranged from 0 to 5 with one point for each positive response to the above items. Frailty was represented with a score of 3–5, pre-frailty with a score of 1–2 and robust with a score of 0.
Self-efficacy was measured using two different instruments both with Likert type scales from 1 to 10 with 1 being no confidence at all, 5 being moderately confident and 10 being totally confident. The coping self-efficacy scale, a 13-item measure specifically evaluating one’s ability to cope in the domains of problem-solving, emotional regulation and social support with Cronbach’s internal consistency coefficient alpha of 0.95 for this sample (Chesney et al., 2006). An example question is: ‘When things aren’t going well for you, or when you’re having problems, how confident or certain are you that you can think about one part of the problem at a time?’ The self-efficacy of chronic disease scale uses 6-items to assess one’s ability to manage chronic conditions (Ritter et al., 2014). Internal consistency for this sample was 0.94. Both coping self-efficacy and self-efficacy of chronic disease management were dichotomized at a mean score of 5, which indicated ‘moderate confidence’ to create low self-efficacy and high self-efficacy groups.
Other psychosocial variables were also measured. Literature review revealed that the below variables were important to evaluate in research involving self-efficacy and frailty in older adults. Depressive symptoms were measured using the 8-item patient health questionnaire (PHQ8) which is clinically valid and a potential mediator of self-efficacy on frailty (Maddux, 1995). It asks questions like: ‘Over the past 2 weeks, how often have you been bothered by feeling down, depressed or hopeless?’(Kroenke et al., 2009) Internal consistency for this sample was 0.79. The illness intrusiveness scale was used to measure the degree to which a person’s current medical condition(s) influences their ability to function in 3 domains (relationships and personal development, intimacy and instrumental) (Devins, 2010). Internal consistency for this sample was 0.87. On a scale of 1 to 7 (not very much to very much), it asks questions like: ‘How much does your illness(es) and/or its treatment interfere with your feeling of being healthy?’ Like depressive symptoms, it was hypothesized that illness intrusiveness may mediate the association of self-efficacy on frailty (DeCoster et al., 2013; Devins, 2010). The ENRICHD 7-item social support instrument was used to assess 4 domains of social support including: emotional, instrumental, informational and appraisal (Vaglio et al., 2004) with internal consistency of 0.86 for this sample. The Holmes Rahe Social Readjustment Scale (Life Events) measured recently occurring life events in a weighted manner where the higher the measure, the greater likelihood of illness in the near future, and used successfully in older adult populations. Coefficient of correlation between discrete groups within the validating sample were ≥ 0.82 with the majority of coefficients ≥ 0.90 (Holmes & Rahe, 1967; Holt et al., 2012). The perceived stress scale was used to measure the perceptions of stress in life over the past month and has been used successfully in older adults (Ezzati et al., 2014) with internal consistency of 0.89 for this sample. The financial strain instrument was used to measure difficulty paying bills, buying food or affording medical care (Cornoni-Huntley et al., 1993). Financial strain was dichotomized into no financial strain or some financial strain. Internal consistency for this sample was 0.70.
Socio-demographic variables included age, sex, race and years of education. Co-morbidities were evaluated using an adaptation of the Self-Administered Co-Morbidity Questionnaire which asked ‘yes/no’ style questions about a variety of disease processes as well as ‘yes/no’ questions about treatments and functional limitations for each disease process (Katz, Chang, Sangha, Fossel, & Bates, 1996). Diseases were added to the questionnaire to more extensively ask about cardiovascular disease and risk factors. A composite score was calculated based on yes answers. Tobacco use, height and weight were self-reported. Tobacco use was dichotomized into current/past use and never used. Heart rate was also measured as a marker of medical control of common conditions in older adults.
Statistical analyses
The main outcome measure of frailty was dichotomized into two categories: ‘Robust’ and ‘Pre-Frail/Frail’ to perform logistic regressions. Combining the frail and pre-frail groups was done because there were few participants identified as frail (n = 8). In order to keep participants with frailty in the study for analysis, a combined category of pre-frail/frail was developed. We hypothesize that the category represents an emerging frailty (Ahmed, Mandel, & Fain, 2007). Fifteen candidate covariates were considered for the analysis including: age, sex, race, education, co-morbidities, tobacco use, heart rate, body mass index, depression, coping self-efficacy or self-efficacy of chronic disease management, perceived stress, illness intrusiveness, social support, life events and financial strain.
Differences in the characteristics between robust and pre-frailty/frailty groups were analyzed using T-tests and Chi-square tests (Table 1). Results with 2-tailed p < 0.05 were considered significant. Simple logistic regression was also performed between each candidate variable and pre-frailty/frailty to obtain odds ratios (Table 2). Collinearity was assessed by calculating variance inflation factors, which were all below 2.0. Logit-linearity was checked between the binomial outcome variable, pre-frailty/frailty, and continuous candidate variables resulting in the addition of a linear spline term for life events at a score of 20. All variables were also checked for correlation with the primary predictor, self-efficacy. Coping self-efficacy and self-efficacy of chronic disease management were evaluated in separate models.
Table 1.
Totals and comparison of robust vs. pre-frail/frail participants.
| Variables | Total | Robust | Pre-frail/frail |
|---|---|---|---|
| N | 146 | 74 | 72 |
| Socio-demographics | |||
| Age** | 81.62 (6.14) | 79.18(5.40) | 84.13 (5.86) |
| Caucasian | 131 (92.9) | 65 (92.9) | 66 (92.96) |
| Female | 110 (75.3) | 57 (77.0) | 53 (73.6) |
| Education | 17.05 (3.12) | 17.30 (3.07) | 16.80 (3.17) |
| Medical history | |||
| Co-morbidities compositea** | 10.68 (5.06) | 8.53 (3.80) | 12.90 (5.25) |
| Tobacco use | 65 (46.4) | 27 (38.0) | 38 (55.1) |
| Body mass index | 27.16 (5.38) | 26.62 (4.48) | 27.72 (6.16) |
| Heart rate* | 68.04 (7.88) | 66.63 (7.33) | 69.34 (8.19) |
| Psychosocial factors | |||
| Depression Score* | 3.78 (3.51) | 2.78 (2.53) | 4.81 (4.06) |
| Coping self-efficacy, N high SE* | 129 (88.4) | 70 (94.6) | 59 (81.9) |
| Self-efficacy of chronic disease management, N high SE* | 130 (89.0) | 70 (94.6) | 60 (83.3) |
| Social support | 24.25 (4.68) | 24.23 (4.65) | 24.26 (4.75) |
| Perceived stress | 2.36 (0.28) | 2.36 (0.27) | 2.36 (0.29) |
| Life events* | 85.02 (68.31) | 72.26 (64.98) | 98.14 (69.60) |
| Illness intrusiveness** | 21.83 (13.01) | 17.41 (10.31) | 26.32 (13.96) |
| Financial strain, N w/strain | 16 (11.03) | 8 (10.81) | 8 (11.27) |
Note: Values displayed as means (SD) for continuous variables and total numbers (percent of total) for categorical variables. SD, standard deviation; SE, self-efficacy.
Composite score of disease count, treatment count and functional limitations.
p < 0.05 for significant difference between the robust and prefrail/frail groups.
p < 0.0001 for significant difference between the robust and prefrail/frail groups.
Table 2.
Odds ratios of pre-frailty/frailty in unadjusted and two adjusted models.
| Unadjusteda | Model 1b | Model 2b,c | |
|---|---|---|---|
| Socio-demographics | |||
| Age | 1.17 (1.09–1.25)** | 1.22 (1.11–1.34)** | 1.23 (1.12–1.36)** |
| Race | 0.98 (0.27–3.56) | 1.73 (0.19–15.81) | 1.75 (0.18–16.79) |
| Sex | 0.83 (0.39–1.77) | 0.51 (0.16–1.58) | 0.63 (0.20–2.04) |
| Education | 0.95 (0.85–1.05) | ||
| Medical history | |||
| Co-morbidities composite | 1.23 (1.13–1.34)** | 1.21 (1.09–1.36)* | 1.18 (1.05–1.33)* |
| Tobacco use | 2.00 (1.02–3.92)* | ||
| Body mass index | 1.04 (0.98–1.11) | 1.12 (1.01–1.24)* | 1.12 (1.01–1.25)* |
| Heart rated | 1.58 (1.00–2.48)* | 2.03 (1.08–3.80)* | 2.32 (1.18–4.56)* |
| Psychosocial factors | |||
| Depression | 1.21 (1.08–1.36)* | 0.98 (0.83–1.16) | |
| Coping self-efficacy | 0.26 (0.08–0.84)* | 0.08 (0.02–0.44)* | 0.10 (0.02–0.63)* |
| Social support | 1.00 (0.93–1.07) | ||
| Perceived stress | 0.91 (0.28–2.91) | ||
| Life events | 1.01 (1.00–1.01)* | 1.00 (0.996–1.01) | 1.00 (0.99–1.00) |
| Life events 0–20e | 0.97 (0.90–1.05) | 0.96 (0.89–1.06) | |
| Life events 21 + | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | |
| Illness intrusiveness | 1.06 (1.03–1.10)** | 1.05 (0.99–1.10) | |
| Financial strain | 1.05 (0.37–2.96) | ||
Note: Values reported as odds ratios (95% confidence intervals).
Simple linear regressions.
Multiple logistic regressions.
Model 2 includes illness intrusiveness and depression.
Odds of frailty/pre-frailty per 10 unit increase in heart rate.
Life events variable splined at 20 to fit the data more accurately.
p < 0.05.
p < 0.0001.
Model building was primarily guided by literature review. Variables of age, race, sex, BMI, heart rate, life events and co-morbidities were included because of their clear associations with frailty in the literature (Dent, Kowal, & Hoogendijk, 2016; Leng, Chen, & Mao, 2014). The financial strain and education variables were excluded due to the lack of variation in those variables. Social support and perceived stress variables were excluded because they were not bivariately associated with frailty/pre-frailty. Tobacco use was excluded despite being significantly associated with pre-frailty/frailty because of the redundancy of the tobacco variable when combined with the co-morbidities variable. Therefore, to aid in model parsimony, tobacco use was excluded. Consequently, the first model included age, sex, race, BMI, co-morbidities, heart rate, coping self-efficacy, and life events. The second model included all aforementioned variables as well as depressive symptoms and illness intrusiveness. These two adjusted models were developed to evaluate associations with and without depressive symptoms and illness intrusiveness, which could both be considered mediators between self-efficacy and frailty (Devins, 2010; Maddux, 1995). To evaluate both the direct and indirect associations of self-efficacy with frailty, Model 1, without depressive symptoms and illness intrusiveness, is considered the primary outcome model for this study. Given that these constructs can also be considered bi-directional, Model 2 is also listed, which includes depressive symptoms and illness intrusiveness.
Multiple logistic regression was used to determine odds ratios for pre-frailty/frailty adjusted for the final model variables (Table 2). Hosmer-Lemeshow goodness-of-fit testing was performed to determine adequate model fit. Data was analyzed using STATA version 14 (STATA Corp, College Station, TX).
Results
Of the 146 participants, 74 (50.7%) were robust and 72 (49.3%) were frail/pre-frail. Mean age was 81.62 ± 6.14 with 92.9% of the sample identifying as white and 75.3% female. Mean education was just over one year of graduate school. Table 1 lists baseline characteristics of the total sample and comparisons between robust and pre-frail/frail participants.
The robust and pre-frail/frail groups differed by many characteristics including age, co-morbidities, tobacco use, heart rate, depressive symptoms, coping self-efficacy, self-efficacy of chronic disease management, life events, and illness intrusiveness (Table 1).
Table 2 lists results from both analyses as well as simple logistic regressions for each of the candidate variables and the main outcome variable of pre-frailty/frailty.
High coping self-efficacy was inversely associated with pre-frailty/frailty. There was a 91% reduction (p = 0.003; CI 0.02–0.44) in the adjusted odds of pre-frailty/frailty in the presence of high coping self-efficacy. See Figure 1 for forest plot of Model 1 co-variates. This relationship remained significant when illness intrusiveness and depression scores were added in Model 2. Also expected, for each additional composite co-morbidity, there was a 21% increase in the adjusted odds of pre-frailty/frailty (p = 0.001; 95% CI 1.09–1.36). For each one unit increase in BMI, the adjusted odds of pre-frailty/frailty increased by 12% (p = 0.037; CI 1.01–1.24) and remained significant in Model 2. Additionally, with each 10 unit increase in heart rate, the unadjusted odds of having pre-frailty/frailty increased by 58% (p = 0.048; 95% CI 1.00–2.48). In Models 1 and 2, for every 10 unit increase in heart rate, there was a significant 2 and 2.3 fold increase in the adjusted odds of pre- frailty/frailty (Model 1: p = 0.027; CI 1.08–3.80; Model 2: p = 0.015; CI 1.18–4.56). Neither Illness intrusiveness or depressive symptoms were significantly associated with pre-frailty/frailty in Model 2. Self-efficacy of Chronic Disease Management was not significant once adjusted and, therefore, values reported here concentrate on coping self-efficacy findings.
Figure 1.
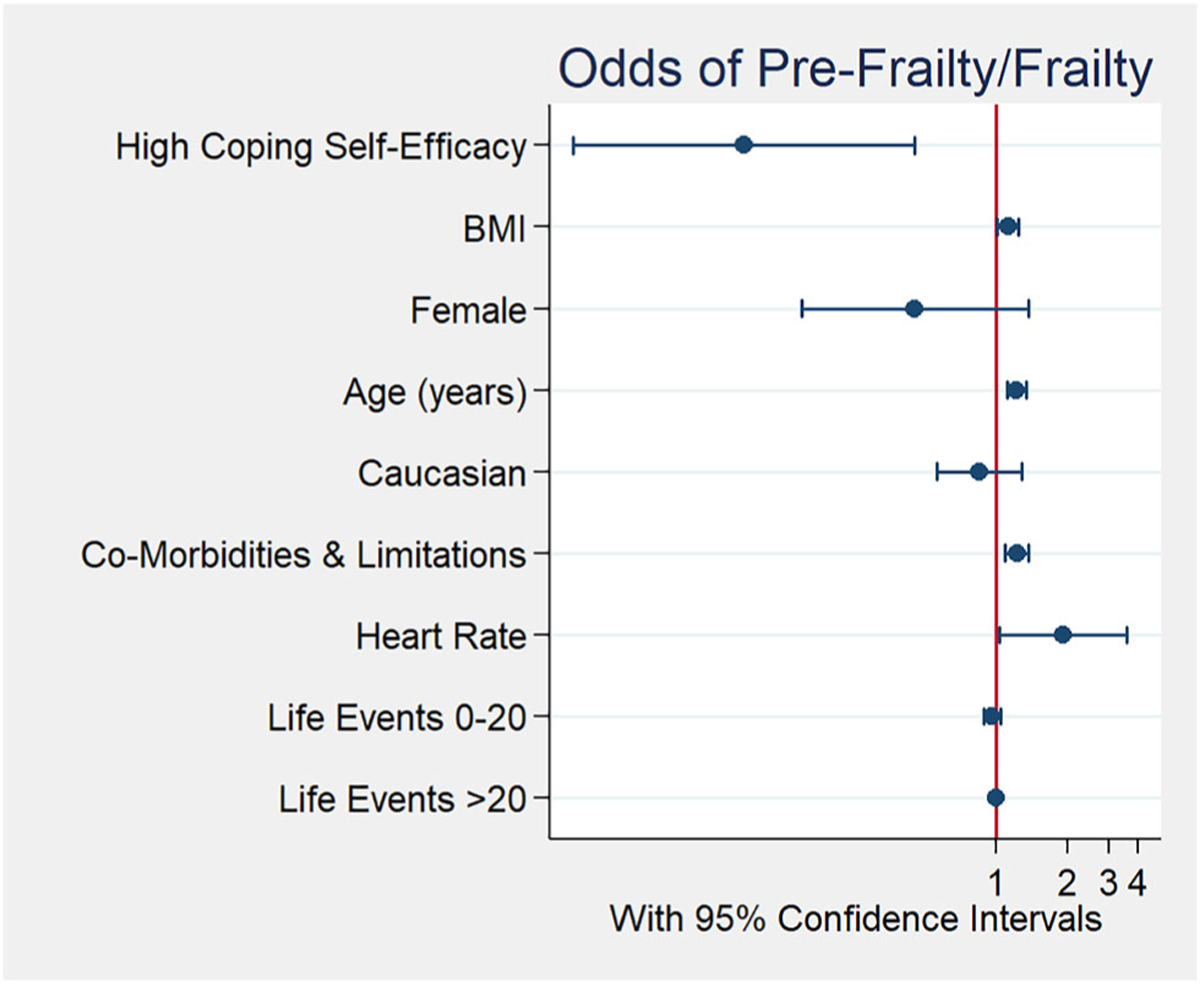
Forest plot of odds for pre-frailty/frailty by co-variates for Model 1.
Hosmer-Lemeshow goodness-of-fit testing was performed. This indicated adequate model fit for both Model 1 and Model 2 with p-values of 0.34 and 0.22, respectively. Cronbach’s alpha for the coping self-efficacy scale was 0.952.
Sensitivity analyses
Extensive sensitivity analyses were also conducted. To address possible endogeneity, the frailty variable was evaluated without the inclusion of fatigue, which can be indicative of more depressive symptomatology (Hawker et al., 2011). Coping self-efficacy remained significant showing a 90% and 89% reduction in the adjusted odds of frailty for Model 1 and Model 2, respectively. Depressive symptoms and illness intrusiveness were not significantly associated with pre-frailty/frailty in Model 2 without fatigue as well. Analysis of the model while excluding frail participants (n = 8) was conducted and, coping self-efficacy remained significantly associated with pre-frailty in both Models 1 and 2, respectively (β = 0.009, p = 0.004; β = 0.009, p = 0.013).
Given the potential overlap of the co-morbidities variable (which somewhat accounts for function) and frailty (which also accounts for function), analyses were also performed using a simple disease count and no chronic disease variable. No changes in associations between any of the model variables were found with the exception of illness intrusiveness, which became significant in both models when a simple disease count or no chronic disease variable was in the model, indicating a potential interaction between illness intrusiveness and functional status. Coping self-efficacy remained significantly associated with pre-frailty/frailty throughout this analysis.
We also evaluated the model with each mediator, depressive symptoms and illness intrusiveness, separately, and found no significant changes in findings. To further evaluate the potential confounding of depressive symptoms and self-efficacy, the main analysis was performed with participants with no significant depressive symptoms (PHQ8 < 10) and, coping self-efficacy remained significantly associated with pre-frailty/frailty (83% reduction in adjusted odds of frailty, p = 0.049) in Model 1. Interestingly, the inclusion of illness intrusiveness in this model resulted in both coping self-efficacy and illness intrusiveness being no longer significantly associated with pre-frailty/frailty.
Discussion
In this sample of community dwelling older adults living with chronic conditions, we found that the odds of pre-frailty/frailty were decreased by 91% in the presence of high coping self-efficacy. The majority of the frailty literature focuses on physiologic associations. Although psychosocial factors have broadly been acknowledged in frailty models, this study identifies one particular and significant factor, coping self-efficacy. Overall, these findings suggest that coping self-efficacy may be an important psychosocial factor to account for in frailty research.
Coping self-efficacy is a global domain that evaluates confidence in problem-solving, emotional regulation, and social coping abilities. Each of these perceived skills are directly applicable to how someone adapts and adjusts to change, in this case the change in physical capabilities and characteristics consistent with the frailty phenotype (Maddux, 1995). These coping perceptions also influence health behaviors and habits- both effecting frailty status and possibly progression (Brinkman et al., 2018). As such, our findings are consistent with other self-efficacy research in older adults with chronic disease (Gobeil-Lavoie, Chouinard, Danish, & Hudon, 2019; Lorig et al., 2001; Marks, Allegrante, & Lorig, 2005).
It is hypothesized that frailty can be slowed or reversed. A recent systematic review identified intervention studies from 1990 to 2016 that have slowed or reversed pre-frailty in a community-dwelling sample of older adults (Frost et al., 2017). The studies mainly focused on exercise and nutrition-based interventions and had mixed outcomes on overall function. Another systematic review of 22 home-based behavior change interventions for frailty or pre-frailty showed generally positive effects on physical function and behavior (Gardner et al., 2017). The greatest effect included interventions with both physical function components and behavioral education. Self-efficacy was not specifically tested in these studies but was shown to influence both behavior and physical function in other disease specific contexts (DePew, Karpman, Novotny, & Benzo, 2013). More research is needed to determine if increases in self-efficacy can also positively influence, buffer or reverse frailty status. Neither of our hypothesized mediators, depressive symptoms nor illness intrusiveness, showed significance in our final models. The mean depression score for this sample was 3.78 (robust participants’ mean of 2.78, pre-frail/frail mean of 4.81). These numbers are well below the cut off for clinically significant depressive symptoms (PHQ8 of 10 or higher). Therefore, this sample may not have enough variability to properly evaluate depressive symptomatology as a mediator. That said, when those participants with clinically significant depressive symptoms were removed from the analysis, coping self-efficacy was still significantly (p = 0.049) associated with pre-frailty/frailty. The relationship between depression, coping self-efficacy and frailty should be further explored in other participant samples.
With the addition of our sensitivity analyses, a greater understanding of illness intrusiveness with coping self-efficacy and pre-frailty/frailty was obtained. We hypothesize that because the co-morbidities measure included questions about function, illness intrusiveness was not significantly associated with pre-frailty/frailty. When function was not accounted for in co-morbidities (using a simple disease count), illness intrusiveness was significantly related to pre-frailty/frailty except when fatigue was removed from the frailty scale scoring. This leads us to conclude that illness intrusiveness could be conceptualized as both a cognitive and functional measure. Importantly, throughout the sensitivity analyses, coping self-efficacy remained significantly associated with pre-frailty/frailty except for the analysis of participants with no depressive symptoms and the inclusion of the illness intrusiveness variable. This represents the mediation of illness intrusiveness on coping self-efficacy and pre-frailty/frailty. We hypothesize that this finding may represent an issue of statistical power due to sample size decreases with the exclusion of participants with significant depressive symptoms. These relationships should also be explored in other samples with more variation in functional status. These sensitivity analyses are broadly consistent with the original analyses.
This study has a number of limitations that should be considered. First, as a cross-sectional study, the directionality of the relationship between frailty and coping self-efficacy cannot be assessed. Second, while frailty is a widely accepted construct, the measurement is still debated. In this study, we used the FRAIL scale which accounts for the 5 domains thought to be important in a frailty phenotype assessment (Bandeen-Roche et al., 2015; Fried et al., 2001). However, this scale needs further validation in an older adult population because it has only been validated in middle-aged communities (Morley et al., 2012; Susanto et al., 2018) and favors disability and disease domains in assessing frailty. Third, this population was less racially diverse, more robust, highly educated and with fewer average numbers of chronic conditions compared to the general older adult population making generalizability to the larger US older population more challenging.
Despite these limitations, the relationship between coping self-efficacy and frailty remained significant after adjustment and throughout our sensitivity analyses, which is an important finding. It suggests that self-efficacy, particularly coping self-efficacy become another pathway to explore in frailty research.
Acknowledgement
The authors would like to thank Dr. Gayle Page, Svetlana Bautista and Christine Leyden for their contributions.
Funding
This work was supported by: (1) National Institute of Health Pre-doctoral Fellowship, Clinical and Translational Science Award (4TL1TR001078–04), (2) Dr. Scholl Foundation Fellowship, (3) National Institute of Health, National Institute of Nursing Research Pre-doctoral Fellowship, Interdisciplinary Cardiovascular Health Research (T32NR012704), (4) National Institute of Health, National Institute of Nursing Research Intramural Research Program and (5) Johns Hopkins University Provost’s Post-Doctoral Fellowship.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ahmed N, Mandel R, & Fain MJ (2007). Frailty: An Emerging Geriatric Syndrome. The American Journal of Medicine, 120(9), 748–753. [DOI] [PubMed] [Google Scholar]
- Alharbi M, Gallagher R, Neubeck L, Bauman A, Prebill G, Kirkness A, & Randall S (2017). Exercise barriers and the relationship to self-efficacy for exercise over 12 months of a lifestyle-change program for people with heart disease and/or diabetes. European Journal of Cardiovascular Nursing, 16(4), 309–317. [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, … Kasper JD (2015). Frailty in older adults: A nationally representative profile in the United States. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 70(11), 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A (1997). Self-efficacy: The exercise of control. New York, NY: W.H. Freeman and Company. [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, … Muntner P (2017). Heart Disease and Stroke Statistics—2017 update: A report from the American Heart Association. Circulation, 135(10), e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman S, Voortman T, Kiefte-de Jong JC, van Rooij FJA, Ikram MA, Rivadeneira F, … Schoufour JD (2018). The association between lifestyle and overall health, using the frailty index. Archives of Gerontology and Geriatrics, 76, 85–91. [DOI] [PubMed] [Google Scholar]
- Chan D-CD, Tsou H-H, Yang R-S, Tsauo J-Y, Chen C-Y, Hsiung CA, & Kuo KN (2012). A pilot randomized controlled trial to improve geriatric frailty. BMC Geriatrics, 12(1), 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney MA, Neilands TB, Chambers DB, Taylor JM, & Folkman S (2006). A validity and reliability study of the coping self-efficacy scale. British Journal of Health Psychology, 11(3), 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornoni-Huntley J, Ostfeld AM, Taylor JO, Wallace RB, Blazer D, Berkman LF, … Scherr PA (1993). Established populations for epidemiologic studies of the elderly: Study design and methodology. Aging Clinical and Experimental Research, 5(1), 27–37. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8481423 [DOI] [PubMed] [Google Scholar]
- DeCoster VA, Killian T, & Roessler RT (2013). Diabetes intrusiveness and wellness among elders: A test of the illness intrusiveness model. Educational Gerontology, 39(6), 371–385. [Google Scholar]
- Dent E, Kowal P, & Hoogendijk EO (2016). Frailty measurement in research and clinical practice: A review. European Journal of Internal Medicine, 31, 3–10. [DOI] [PubMed] [Google Scholar]
- DePew ZS, Karpman C, Novotny PJ, & Benzo RP (2013). Correlations between gait speed, 6-minute walk distance, physical activity, and self-efficacy in patients with severe chronic lung disease. Respiratory Care, 58(12), 2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrichard O, Vallet F, Agrigoroaei S, Fagot D, & Spini D (2018). Frailty in aging and its influence on perceived stress exposure and stress-related symptoms: Evidence from the Swiss Vivre/Leben/Vivere study. European Journal of Ageing, 15(4), 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devins GM (1994). Illness intrusiveness and the psychosocial impact of lifestyle disruptions in chronic life-threatening disease. Advances in Renal Replacement Therapy, 1(3), 251–263. [DOI] [PubMed] [Google Scholar]
- Devins GM (2010). Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. Journal of Psychosomatic Research, 68(6), 591–602. [DOI] [PubMed] [Google Scholar]
- Doba N, Tokuda Y, Saiki K, Kushiro T, Hirano M, Matsubara Y, & Hinohara S (2016). Assessment of self-efficacy and its relationship with frailty in the elderly. Internal Medicine, 55(19), 2785–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A, Jiang J, Katz JM, Sliwinski JM, Zimmerman EM, & Lipton BR (2014). Validation of the Perceived Stress Scale in a community sample of older adults. International Journal of Geriatric Psychiatry, 29(6), 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, … McBurnie MA (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 56(3), M146–56. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11253156 [DOI] [PubMed] [Google Scholar]
- Fried LP, Xue Q-L, Cappola AR, Ferrucci L, Chaves P, Varadhan R, … Bandeen-Roche K (2009). Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 64(10), 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R, Belk C, Jovicic A, Ricciardi F, Kharicha K, Gardner B, … Walters K (2017). Health promotion interventions for community-dwelling older people with mild or pre-frailty: A systematic review and meta-analysis. BMC Geriatrics, 17(1), 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner B, Jovicic A, Belk C, Kharicha K, Iliffe S, Manthorpe J, … Walters K (2017). Specifying the content of home-based health behaviour change interventions for older people with frailty or at risk of frailty: An exploratory systematic review. BMJ Open, 7(2), e014127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, & Byers A (2002). A program to prevent functional decline in physically frail, elderly persons who live at home. New England Journal of Medicine, 347(14), 1068–1074. [DOI] [PubMed] [Google Scholar]
- Gobbens RJJ, van Assen MALM, Luijkx KG, Wijnen-Sponselee MT, & Schols JMGA (2010). Determinants of frailty. Journal of the American Medical Directors Association, 11(5), 356–364. [DOI] [PubMed] [Google Scholar]
- Gobeil-Lavoie A-P, Chouinard M-C, Danish A, & Hudon C (2019). Characteristics of self-management among patients with complex health needs: A thematic analysis review. BMJ Open, 9(5), e028344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker GA, Gignac MAM, Badley E, Davis AM, French MR, Li Y, … Lou W (2011). A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care & Research, 63(10), 1382–1390. [DOI] [PubMed] [Google Scholar]
- Holmes TH, & Rahe RH (1967). The Social Readjustment Rating Scale. Journal of Psychosomatic Research, 11(2), 213–218. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6059863 [DOI] [PubMed] [Google Scholar]
- Holt EW, Muntner P, Joyce C, Morisky DE, Webber LS, & Krousel-Wood M (2012). Life events, coping, and antihypertensive medication adherence among older adults: The cohort study of medication adherence among older adults. American Journal of Epidemiology, 176 Suppl, S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, & Bates DW (1996). Can comorbidity be measured by questionnaire rather than medical record review? Medical Care, 34(1), 73–84. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8551813 [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL (2005). Understanding the odd science of aging. Cell, 120(4), 437–447. [DOI] [PubMed] [Google Scholar]
- Kojima G, Iliffe S, & Walters K (2015). Smoking as a predictor of frailty: A systematic review. BMC Geriatrics, 15(1), 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, & Mokdad AH (2009). The PHQ-8 as a measure of current depression in the general population. Journal of Affective Disorders, 114(1–3), 163–173. [DOI] [PubMed] [Google Scholar]
- Leng S, Chen X, & Mao G (2014). Frailty syndrome: An overview. Clinical Interventions in Aging, 9, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Bandura A, … Holman HR (2001). Chronic disease self-management program: 2-year health status and health care utilization outcomes. Medical Care, 39(11), 1217–1223. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11606875 [DOI] [PubMed] [Google Scholar]
- Maddux JE (1995). Self-efficacy, adaptation, and adjustment : Theory, research, and application. New York, NY: Plenum Press. Retrieved from https://catalyst.library.jhu.edu/catalog/bib_1891576 [Google Scholar]
- Malmstrom TK, Miller DK, & Morley JE (2014). A comparison of four frailty models. Journal of the American Geriatrics Society, 62(4), 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks R, Allegrante JP, & Lorig K (2005). A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: Implications for health education practice (part I). Health Promotion Practice, 6(1), 37–43. [DOI] [PubMed] [Google Scholar]
- Morley JE, Malmstrom TK, & Miller DK (2012). A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. The Journal of Nutrition, Health & Aging, 16(7), 601–608. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22836700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, … Walston J (2013). Frailty consensus: A call to action. Journal of the American Medical Directors Association, 14(6), 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter LP, Lorig K, Ritter PL, & Lorig K (2014). The English and Spanish Self-Efficacy to Manage Chronic Disease Scale measures were validated using multiple studies. Journal of Clinical Epidemiology, 67(11), 1265–1273. [DOI] [PubMed] [Google Scholar]
- Schüz B, Wurm S, Warner LM, & Ziegelmann JP (2012). Self-efficacy and multiple illness representations in older adults: A multilevel approach. Psychology & Health, 27(1), 13–29. [DOI] [PubMed] [Google Scholar]
- Schwarzer R (1992). Self-efficacy : Thought control of action. Washington, DC: Hemisphere Pub. Corp. Retrieved from https://catalyst.library.jhu.edu/catalog/bib_897056 [Google Scholar]
- Scult M, Haime V, Jacquart J, Takahashi J, Moscowitz B, Webster A, … Mehta DH (2015). A healthy aging program for older adults: Effects on self-efficacy and morale. Advances in Mind-Body Medicine, 29(1), 26–33. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25607120 [PMC free article] [PubMed] [Google Scholar]
- Stretton CM, Latham NK, Carter KN, Lee AC, & Anderson CS (2006). Determinants of physical health in frail older people: The importance of self-efficacy. Clinical Rehabilitation, 20(4), 357–366. [DOI] [PubMed] [Google Scholar]
- Susanto M, Hubbard RE, & Gardiner PA (2018). Validity and responsiveness of the FRAIL scale in middle-aged women. Journal of the American Medical Directors Association, 19(1), 65–69. [DOI] [PubMed] [Google Scholar]
- Vaglio J, Conard M, Poston WS, O’Keefe J, Haddock CK, House J, & Spertus JA (2004). Testing the performance of the ENRICHD Social Support Instrument in cardiac patients. Health and Quality of Life Outcomes, 2(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadhan R, Yao W, Matteini A, Beamer BA, Xue Q-L, Yang H, … Walston J (2014). Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(2), 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, … Fried LP (2006). Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. Journal of the American Geriatrics Society, 54(6), 991–1001. [DOI] [PubMed] [Google Scholar]


