Abstract
Oral delivery of protein drugs (PDs) made in plant cells could revolutionize current approaches of their production and delivery. Expression of PDs reduces their production cost by elimination of prohibitively expensive fermentation, purification, cold transportation/storage, and sterile injections and increases their shelf life for several years. Ability of plant cell wall to protect PDs from digestive acids/enzymes, commensal bacteria to release PDs in gut lumen after lysis of plant cell wall and role of GALT in inducing tolerance facilitate prevention or treatment allergic, autoimmune diseases or anti-drug antibody responses. Delivery of functional proteins facilitate treatment of inherited or metabolic disorders. Recent advances in making PDs free of antibiotic resistance genes in edible plant cells, long-term storage at ambient temperature maintaining their efficacy, production in cGMP facilities, IND enabling studies for clinical advancement and FDA approval of orally delivered PDs augur well for advancing this novel drug delivery platform technology.
Keywords: Molecular farming, chloroplast, protein drugs, oral tolerance, transplastomic plants, autoimmune diseases, metabolic disorders
Introduction
Molecular farming field refers to production of therapeutic proteins using plant cells as bioreactors with significant potential to improve the access of modern medicines worldwide, particularly in the developing world where affordability and accessibility are greater concerns. Plant cell wall polymers contain β 1,4 and β 1,6 linkages resistant to hydrolysis by human or animal digestive enzymes and therefore protect protein drugs from acids and enzymes in the stomach [1, 2]. When intact plant cells enter the intestine, commensal microbes present in the gut epithelium digest the plant cell wall and release the bioencapsulated protein. These two natural properties of plant cell wall and gut microbes offer unique opportunities for oral drug delivery, in addition to elimination of prohibitively expensive fermentation, purification, cold transportation/storage, short shelf life and sterile injections used in current protein therapeutics.
Glucocerebrosidase made in carrot cells was approved by FDA for treatment of Gaucher’s disease [3] but this protein drug (PD) is purified and injected. However, oral delivery of a protein drug made in plant cells was never utilized for FDA approval until now. Therefore, the first FDA approved orally delivered drug (Palforzia) made in plants is Ara h proteins expressed in peanut cells [4]**. Orally delivered plant vaccine antigens have been explored for decades to produce antibody against infectious diseases, including cholera Phase I clinical trials [5]. However, the gut immune system works towards immune suppression and inducing oral tolerance rather than immunity. Development of peanut allergy tolerance through the gut immune system is therefore a natural approach that suits this oral drug delivery approach. Failure of previous vaccine trials against infectious diseases could be due to a number of reasons including lack of oral adjuvants to prime the gut immune system [6]**. Therefore, plant-based vaccine is currently limited to booster vaccines [7], with injectable heterologous priming. Nevertheless, cold chain free booster vaccines have great value in the current epidemic because of need to vaccinate several billion people around the globe and sustain long-term immunity and protection.
Different routes for its delivery have been tested for protein drugs like insulin but each approach has certain advantages and limitations. For example, the use of absorption enhancers like Zinula Occludens toxin by modulation of intestinal tight junctions increased intestinal absorption by 72% but long term feasibility for repetitive drug delivery is questionable [8]. In addition, solid/oil/water (S/O/W) surfactant coated emulsion formulation of insulin enhanced intestinal absorption but efficiency was not quantified [9]. Also, pH-responsive insulin microsphere gels increased release and bioavailability but absorption efficiency was not quantified [10, 11]. In addition, polystyrene or bio-adhesive chitosan encapsulated nanoparticles resulted in 11.4% enhanced bioavailability of insulin after 6 hours [12]. However, despite several decades of research on oral protein drug delivery, injections remain the most common means for administering therapeutic proteins because of poor oral bioavailability.
In contrast to these chemical modification studies, fusion of protein drugs with transmucosal carriers are highly effective. For example, CTB fusion reduces dose of orally delivered insulin up to 500-fold, due to efficiency of transmucosal delivery across the gut [13–16].The fusion tags (receptor-binding proteins or cell-penetrating peptides) are therefore required for crossing of intestinal epithelium and efficient absorption. Fusion tags can also help to differentiate its delivery to the immune or circulatory system for more targeted drug delivery. When proteins are fused with the protein transduction domain (PTD) they are delivered to the circulatory system and not to the immune system; but when fused with Dendritic Cell Peptide (DC-Pep), they are delivered only to dendritic cells. However, when fused with CTB, they are efficiently delivered to the immune and circulatory system [1]. All the three tags deliver fused protein to the microfold cells (M cells). Although protein size could potentially influence efficiency of transmucosal delivery, both small and very large proteins have been delivered efficiently using fusion tags of proteins expressed in chloroplasts. For example, small proteins like insulin [17] or insulin like growth factor [18]** or very large proteins including blood clotting factors [19–21]** are delivered efficiently to facilitate functional evaluation studies.
This review primarily focuses on functional protein drugs required to treat inherited or metabolic disorders or induce tolerance to autoimmune disorders or food allergies that are currently advanced to the clinic or already approved by FDA [4]**. Therefore, in addition to basic concepts of transgene expression in plant cells and removal of antibiotic resistance genes, advances in production of plants under cGMP conditions, long-term storage free of cold chain and regulatory path for oral protein drug delivery are discussed.
Plant expression systems:
Direct and indirect methods of gene delivery including particle bombardment and Agrobacterium tumefaciens were established three decades ago for obtaining transgenic plants. Agrobacterium tumefaciens method is used for nuclear transformation of dicots but eventually advanced to a few monocots, including cereals [22]. However, particle bombardment is used to transform both the nuclear and chloroplast genomes. In addition, recombinant proteins are also targeted to various cellular organelles to minimize protein degradation and enhance yield. Different subcellular compartments including endoplasmic reticulum (ER), apoplast, or protein bodies have been explored in seed or leaf-based plant systems. Recombinant proteins that require glycosylation for its functionality, are targeted to ER in seeds and vegetative tissues via KDEL (Lysine-Aspartic acid-Glutamic Acid-Leucine) sequences. Besides plants as host, recombinant proteins are also expressed in plant cell cultures to facilitate regulatory approval [3], although anticipated cost reduction advantages were not accomplished and both plant cell culture and Chinese Hamster Ovary cells (CHO) produced protein drugs cost the same.
There are 10,000 copies of the chloroplast genomes in each plant cell. Therefore, foreign genes inserted into chloroplast genomes can potentially be expressed at very high levels up to 70% of the total leaf protein [23–26]**. The transgene is inserted into chloroplast genome through homologous recombination that eliminates the possibility of positional effects, thereby reducing the number of transplastomic lines for evaluation of expression level [25, 26]. There is no gene silencing reported so far after expression of numerous foreign genes. Multiple genes or proteins can be expressed in operons, facilitating metabolic engineering and production of small molecule drugs like artemisinin to treat malaria [27, 28]**. Several toxic proteins when present in the cytosol are expressed in chloroplasts to reduce their toxicity. Indeed, the first vaccine expressed in chloroplasts – Cholera toxin subunit B (CTB) was toxic to leaves when expressed via the plant nuclear genome.
Similar to mammalian cells, the chloroplast expression facilitates formation of disulfide bonds, proper folding and post-transcriptional modifications, and assembly to produce more complex protein drugs [23–30]. Production of recombinant proteins in chloroplast is among the strategies to avoid unwanted gene flow because expression in vegetative tissues eliminates reproductive structures and also due to maternal inheritance of chloroplast genomes [31, 32].
First established in tobacco, carrot and lettuce edible chloroplast systems have been developed subsequently [29, 33–35]. Thin lettuce leaves enable rapid removal of water through lyophilization and offer an ideal system for therapeutic protein expression and delivery. Therapeutic proteins ranging from very small regulatory proteins Ang1–7 [36, 37], exendin [38], antimicrobial peptides [39–41] insulin and growth hormones [42] to very large proteins [19, 20, 33, 43, 44]** have been successfully expressed in lettuce chloroplasts [6, 45, 46]**. Protein drugs expressed in lettuce chloroplasts are stable in freeze dried leaves for several years when stored at ambient temperature, maintaining folding, disulfide bonds and efficacy [33, 47]**. Several therapeutics proteins have been expressed to treat pulmonary hypertension[21, 36], hemophilia [20, 47, 48]**, retinopathy [49] and diabetes. More recently, this technology has been improved to express human genes through codon optimization using new algorithms [19, 50], eliminate the selectable marker antibiotic resistance gene [18, 21, 51, 52]** making it ideal for oral delivery of protein therapeutics in the clinic.
The presence of cellulose and lignin make plant cell wall undigestable for human/animal enzymes. The production of PD in the plant cell, its bioencapsulation, and on-site degradation for its release is the plant’s unique characteristic that is utilized for oral delivery of PD, which distinguishes it from other drug production/delivery systems. Plants as production host is now approved by FDA [4, 53]**. An enzyme i.e. Glucocerebrosidase produced in carrot cell suspension culture was the first example of FDA approved replacement therapy for treatment of Gaucher’s disease [54]. Several strategies are developed to stabilize PDs by targeting into different cellular organelles such as ER, apoplast, Golgi apparatus, or protein bodies [55]. The expression of PD in plant cells facilitates stability when stored at ambient temperature as freeze dried cells for many years [18, 21, 33, 47]**. Protein targeting has achieved certain objectives; however, increased protein yield remained a major challenge. In contrast, the PD expressed in plant chloroplast has unique advantages of higher yield, stability and offers the possibility of oral delivery. The PD when expressed in plants does not require further processing after lyophilization, and thereby eliminates the costly downstream processing, ultimately make PDs more affordable [23, 24, 50]. The freeze-drying process also minimizes bacterial contamination.
The plant-based system faced several hurdles until enzyme glucocerebrosidase for replacement therapy for Gaucher disease, known as Elelyso from Pfizer was commercialized [54]. In 2014, in response to the Ebola outbreak in Africa, another product ZMapp for post-infection therapy against the Ebola virus, a cocktail of three monoclonal antibodies was transiently expressed in Nicotiana benthamiana plant didn’t receive regulatory approval for human use [56]. Recently, the plant made oral immunotherapy drug to induce tolerance to peanut allergy has received FDA approval that paved a way for orally delivery of PDs to achieved tolerance to treat several incurable diseases [4]**. Several plant-made PD are in pre-clinical or clinical stages, awaiting FDA approval.
Mechanism of Oral Drug Delivery
The human intestine offers a larger mucosal area and therefore offers an ideal surface for oral delivery of plant-made PDs [24]. The gut-associated lymphoid tissue (GALT) is key source of regulatory T cell (Treg) that contains a large area of 300 m2 that accounts for the largest immune system tissue (>70%) in the human body [57]. The digestive enzymes present in humans or animals are unable to degrade the bioencapsulated protein drugs, and thus can pass through the digestive system. The intactness of plant-made protein and its release have been well documented in studies where green fluorescent protein (GFP) is found in the gut lumen within intact plant cells that withstands the enzymatic and acidic conditions (Figures 1, 2). The human enzymes present in the digestive system are unable to break the glycosidic bonds of carbohydrates of the plant cell wall. This unique characteristic facilitates protection of proteins bioencapsulated in plant cells. In contrast, the human gut bacteria are capable of digesting β1,4 and β1,6 linkages in the plant cell wall. Bacteriodetes and Firmicutes are the major microbes that facilitate the breakdown of the plant cell wall polymers [58, 59]**. The cellulosome, extracellular enzyme complex (present in anaerobic cellulolytic bacteria) contains the binding, structural and catalytic domains that help to make point of contact with the cell wall and break the glycosidic bonds. Furthermore, other bacteria present in the gut mucus layer also play a vital role in disrupting the cell wall. The mucous layer that also contains mucopolysaccharides acts as a substrate for gut bacteria. The penetration of the mucous layer is facilitated by Bacteroides fragilis that breakdown mucin glycoproteins [60]. This mechanism of cell wall degradation allows the oral delivery of encapsulated PD. The proof of concept is demonstrated by expression of fluorescent marker GFP among villi of the ileum (Figures 1, 2). This study provides direct evidence of PD protection from the digestive system and its absorbance by epithelial cells present in upper gut [1, 2].
Figure-1: Mechanism of tolerance induction by oral delivery of antigens bioencapsulated in plant cells.
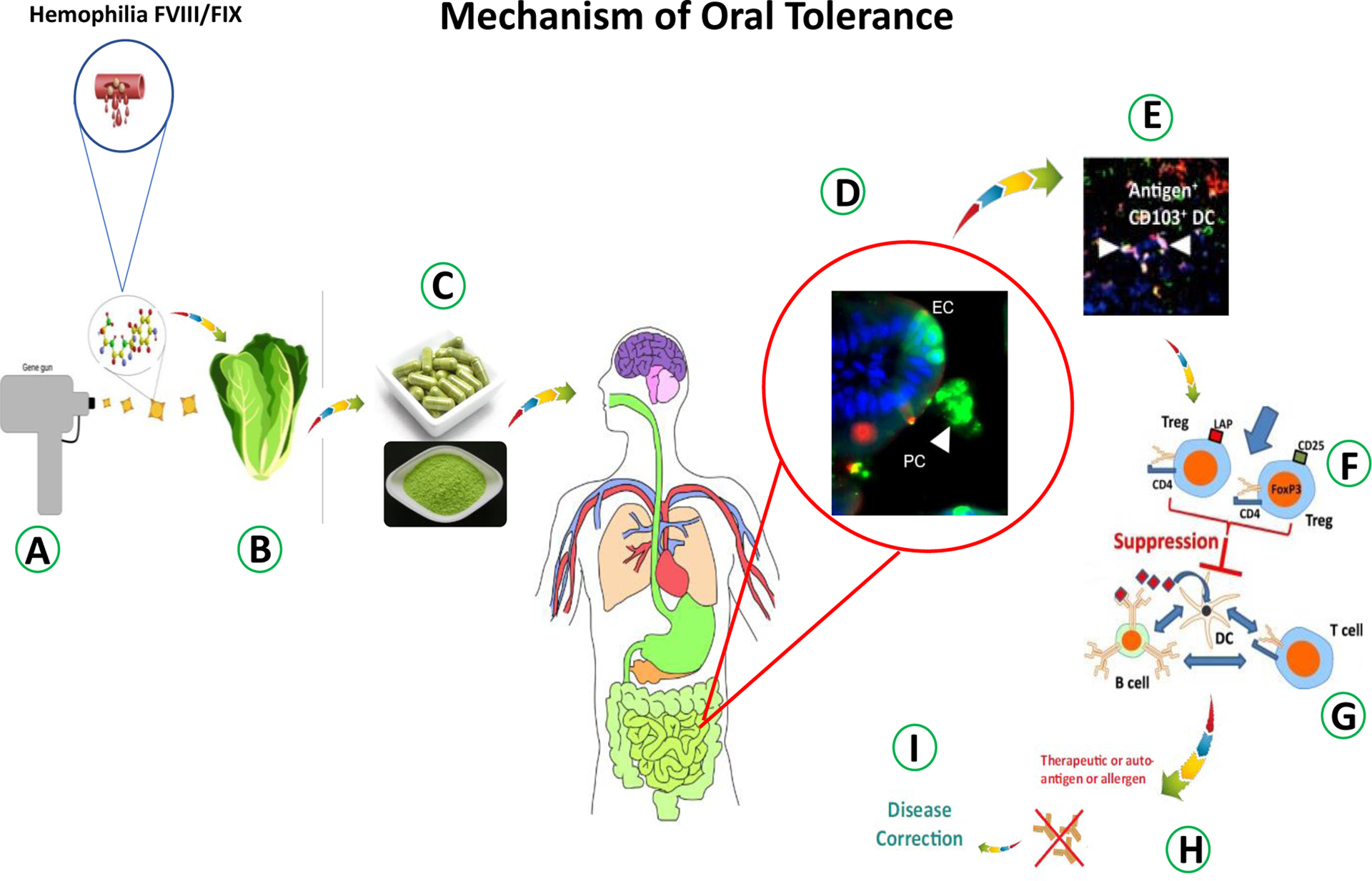
A. The gene gun used to shoot gold particles coated with genes into plant cells. B. Lettuce plant regenerated after selection and expressing antigens in chloroplasts. C. Lyophilized plant cells powder and capsules. D. Intact plant cells (PC) with GFP protected from digestion and GFP in the gut epithelial cell (EC) after lysis of plant cells and GFP uptake (Xiao et al., 2016). E. Antigen taken up by tolerogenic CD103+ DCs (white arrows). F. Induction of antigen specific regulatory T cells. G. Induced Tregs suppress B cell and T cell responses against the antigen. H, I. The disease prevention via oral tolerance of autoimmune diseases.
Figure-2: Oral delivery of functional protein drugs in lettuce lyophilized cells.
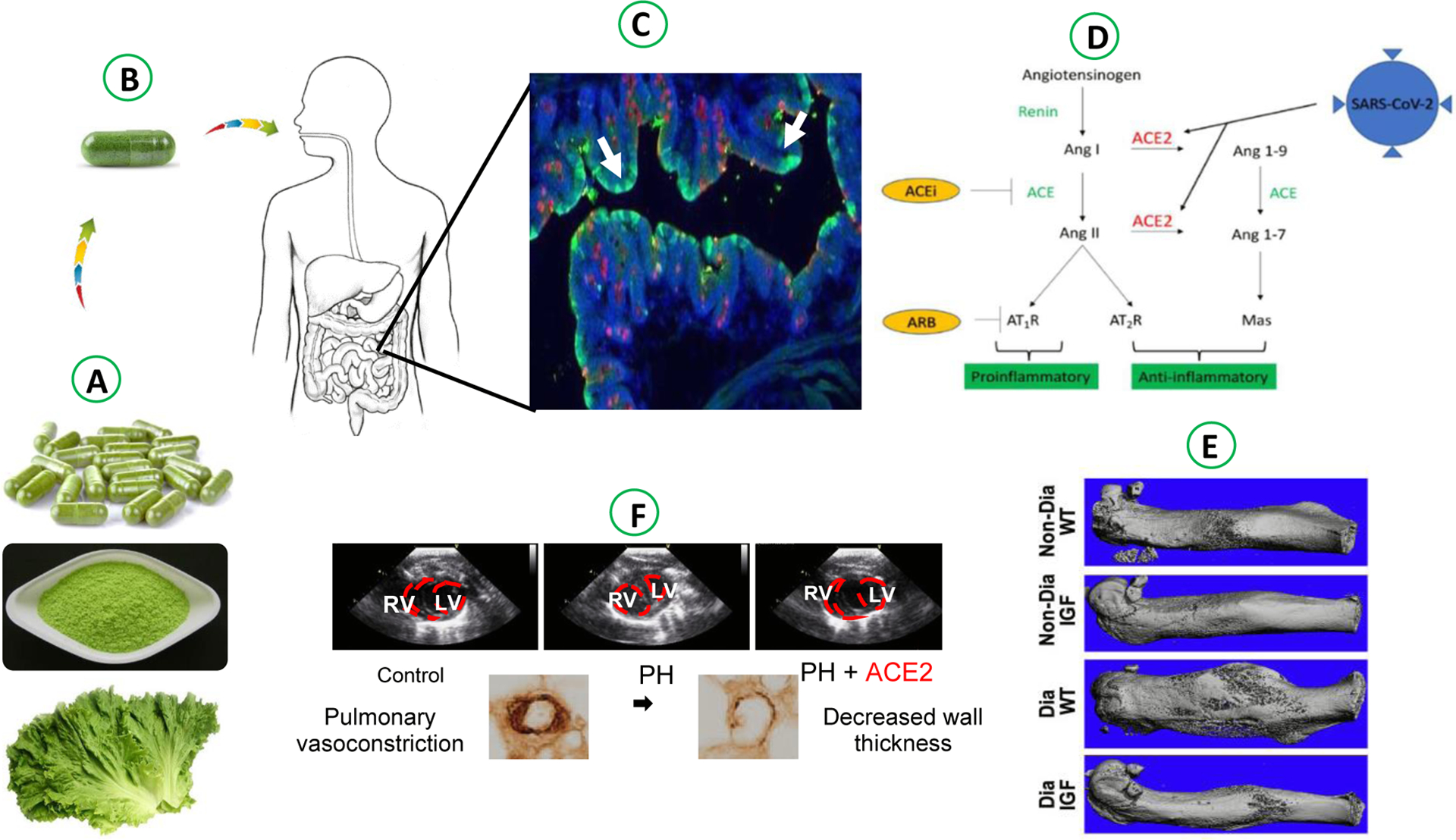
A. Lyophilization, grinding and capsule made from lettuce biomass containing protein drugs. B. Oral delivery of plant made PDs capsules . C. Intact plant cells (PC) with GFP avoided degradation and GFP in the gut epithelial cell (EC) after lysis of plant cells and GFP uptake (Xiao et al., 2016). D. Chloroplast bioencapsulated lettuce biomass expressing ACE2 (SARS-Cov-2 spike protein receptor) and Ang 1–7 in Renin-Angiotensin System (RAS) is potential therapeutic to treat SARS-Cov-2 infection (data not published) E. Bone regeneration in diabetic fracture healing in mice model via oral delivery of lettuce made Pro-IGF-1 (Park et al., 2019). F. Pulmonary vascular remodeling in mice model via oral delivery of ACE-2/Ang1–7 bioencaspsulated in lettuce cells (Daniell et al., 2020).
Upon delivery to gut mucosa and after its breakdown, PD must cross the intestinal epithelium and are transported to the circulatory or immune system. Such delivery is accomplished with tags (receptor-binding proteins) fused with PDs. The proof of concept has been documented in several studies, where PDs are efficiently delivered to the immune system. PD fused with CTB, protein transduction domain (PTD) and dendritic cell peptide (DCpep) are expressed and evaluated for their oral delivery efficacy [1]. While taking PDs to epithelial cells, fusion tags employ various endocytic sub-pathways. For instance, when CTB is tagged to PDs, it uses noncaveolar/nonclathrin, caveolar- or clathrin-mediated pathways, and then followed by retrograde transport [61]. The PTD does not use receptors but enter via direct translocation. The absorbance of PTD into epithelial cells involves electrostatic interactions with the plasma membrane, followed by endocytosis transport [62] . On the other hand, DCpep when fused to PDs facilitates the crossing of the epithelial barrier through M cells for entry, as well as they can directly enter by DCs in the intestinal lumen. When delivered orally, DCpep fused to GFP were observed in M cells [2]. After observing the DCpep-GFP mode of delivery, the evidence demonstrates that this fusion protein is delivered into circulation by transcytosis, involving M cells, along with the gut-liver axis [63]. DCpep tags also facilitate the delivery of fusion protein into the intestinal lumen by extended dendrites of DCs [64]. In addition to offering entry to the circulatory system, fusion tags also add to the stability of recombinant proteins. Tags provide N-terminal protection when fused to PDs, and therefore protect from degradation and increase their stability for several hours [36, 38]. The release of fusion protein is facilitated by cleaving tags by proteases like furin [65].
Protein drugs delivery to immune/nonimmune cells
GALT present in the human immune system accounts for 70% of the total immune system and also holds ~ 80% of immunoglobulin A-bearing cells [57]. In the human body, the exchange of gases and uptake of nutrients or waste excretion to the environment takes place by the mucosal system. Besides a gateway for exchange, it is also a point of connection for certain pathogens and toxins. Therefore, to combat the probable threat by pathogens, the mucosa-associated lymphoid tissues are provided with a large number of immune cells. To scrutinize the immune vs nonimmune cells, different strategies are adopted for the delivery of protein drugs to these cells. In order to treat metabolic disorders, delivery to immune cells is avoided, because the immune cells are heavily present throughout gastrointestinal tract. This is accomplished through PTD that can efficiently divert fusion PDs to the pancreas and kidney, and to avoid its delivery to immune cells [1]. In contrast, the PDs are delivered to immune cells to initiate specific immune responses. In the case of autoimmune diseases, a specific immune response is required to induce tolerance, and that is achieved by the delivery of therapeutic proteins to immune cells; DCs from Peyer’s patches induce tolerance to intestinal antigens. There are other factors to be considered to improve the oral vaccine efficacy such as M cells uptake of antigens and their transport to APCs. Therefore, immune and nonimmune cells play an important role in determining the efficacy of protein drugs.
There are several strategies to enhance the transmucosal delivery of antigen, and that can be achieved efficiently through the above-mentioned strategies. However, it is equally important that PDs are timely and on-site released and reach the gut epithelium. Different carriers such as CTB and PTD perform a vital role in determining the uptake of PDs across the gut epithelium. For this reason, functional CTB, a homopentamer, and relatively smaller size of 11.6 kDa polypeptide enter the cell by binding to the GM1 ganglioside receptor with a higher binding frequency of 15000 CTB molecules to each intestinal epithelial cell [66]. GM1 binding cells are found in intestinal epithelial cells, immune cells, neurons, etc. CTB along with fusion proteins forms pentamers within chloroplast irrespective of size of fused proteins [17, 18, 38, 43]** and transport the fusion protein into gut epithelial cells. The pentameric nature of CTB which is attributed to the presence of disulfide bonds, seven salt brides, and 30 hydrogen and other hydrophobic interactions among CTB monomers. The presence of such bonds, bridges, and interactions among CTB monomers causes its binding to GM1 receptors, and can carry any fused proteins as well to the circulatory system. The delivery of fusion protein is facilitated by cleavage of ubiquitous protease furin [50]. There are two known trafficking pathways that CTB follows for entry i.e. transcytosis and retrograde trafficking. In the latter case, the fusion proteins are trafficked to ER and Golgi network through recycling endosomes. Unfolding is followed by retro-translocation of fusion protein takes place once transported to ER before it is released to the cytosol [67]. An alternative pathway, CTB-PDs pass the epithelial cells through transcytosis. To cross through polarized epithelial cells by transcytosis, GM1 receptors with ceramid domain with short chains of fatty acid are required for CTB-PD-GM1 complexes.
The proof of concept for oral delivery of CTB tagged recombinant proteins was proposed by Limaye et al. (2006) [2]. In the study, the presence and assembly of pentameric CTB were confirmed in chloroplast with immunoblot and GM1 binding assay, respectively. The green fluorescent protein was tagged with CTB to track its assembly and delivery in mice fed with a CTB-GFP fusion protein [2]. The presence of CTB fusion protein in intestine was confirmed in epithelial cells and in M cells. Also, the fluorescence was also confirmed in other parts i.e. spleen cells, intestinal mucosa, hepatocytes demonstrating the delivery of fusion protein across the intestinal lumen. The retention of CTB epithelial cells until antigen is released is an important characteristic and that is performed by the inclusion of furin proteolytic cleavage sites. The delivery of CTB fusion protein was accomplished both in systemic circulation and the immune system. The concept of oral delivery of PDs has been demonstrated in numerous studies [6, 17–19, 21, 47, 50]** where PDs were detected in circulation in animal models as quickly as 30 minutes, and in some cases within 2–5 hours after oral delivery.
CTB fusion to PDs can facilitate the delivery of functional proteins to sera, or retinal barriers, and also can effectively induce oral tolerance [19, 24]**. CTB fusion protein forms aggregation because of pentamers formation. Quantitation of CTB fusion proteins remains a great challenge because of the complexity in the solubilization of CTB pentamers [50]. Such a pentamer exhibits strong resistance during protein extraction and its linearizing with denaturing agents such as sodium dodecyl sulfate and dithiothreitol. The CTB pentamers remain even after boiling to a certain degree and time where all other proteins solubilize. However, pentamers stability is important for oral delivery of CTB fused PDs. As an alternative, PTDs requires no specific receptors for oral delivery of PDs. PTDs are the macromolecules transporter of size 8–16 amino acids that can efficiently carry the PDs for delivery into living cells by fluid-phase micropinocytosis, a form of endocytosis [68]. In addition, DCpep that binds to DC-specific receptors, are ideal for transporting high weight molecules into DCs. PTDs can efficiently deliver PDs in mammalian cells and animals, both in vivo and in vitro [69]. The mode of action and its efficient binding properties can be explored for the delivery of PDs in plant cells [1].
The plant made PDs confer tolerance
Every day through diverse food intake, the human digestive system is exposed to numerous foreign antigens. Antigen-specific regulatory T cells (Tregs) are critical in eliminating unwanted immune response against microbiota. In addition, IL-10 production by Treg is important for the induction of tolerance. Tregs are critical to induce the suppression of the immune response to a specific antigen in the small intestine [70]. The mammalian intestine consists of lamina propria (LP) and an epithelium, where Peyer’s patches (PP) are present in the middle section of the small intestine called the ileum. The epithelial and M cells of PP are responsible for the transportation of antigen from the gut lumen to dendritic cells (DCs) and macrophages, collectively known as specialized antigen-presenting cells (APCs). Among conventional DC, CD103+ DCs present in the LP are critical for inducing oral tolerance. CD103+ plays an important role in delivering antigen to mesenteric lymph nodes (MLNs), where they are converted to FoxP3 expressing Treg by producing Vit-A and TGF- β. The gut homing reactor and IL-10 are also promoted by tans retinoic acid (RA) [71]. All LAP+CD4+, CD4+CD25+FoxP3+ work in Treg dependent manner. For mucosal immunity, the secretion of immunoglobin IgA into the intestinal lumen is critical, and herein the TGF-β has a critical role in switching in B cells into IgA. Treg, CD8+ T cells, and type-1 regulatory T cells are among the other subsets of Treg that can be induced in GALT. To prevent inflammation of mucosal interphases, IL-10 production is required by Treg.
There are several examples of the proteins expressed in plant cells to induce tolerance against allergy or drug antibody responses. Plants are grown in FDA approved cGMP facilities [33, 43, 53]. Several plant-based oral tolerance protocols have been developed, in clinical trials, or approved by FDA to treat autoimmune, allergic, or genetic diseases such as hemophilia peanut allergy, rheumatoid arthritis, and asthma and pollen allergies [19, 20, 29, 47, 48, 53]**. After successful clinical trials of peanut product AR101 [4, 53]**, the first FDA approved treatment to prevent peanut allergy has been reported with orally delivered peanut powder led the foundation for orally immunotherapy. In the twelve months long clinical trials the subject tolerated 443mg of peanut exposure with no more than mild symptoms at exit DBPCFC, in plants grown in cGMP facility. The plant made recombinant factor IX have already tested to prevent anti-drug antibodies production in hemophilia dogs [47]**. The hemophilia mice exhibit tolerance when gavaged with FVIII expressed in chloroplast and suppressed the inhibitor formation. The other FVIII domains i.e. heavy chain and light chain i.e. (HC) and (LC), were expressed in a higher amount after codon optimization. The HC, LC, and SC fused to CTB upon oral delivery of lyophilized cells were successful in reducing inhibitor titers in hemophilia A mice [19]**. The lyophilized CTB-FIX accumulated in a higher amount in plant cells. when orally gavaged to mice blocked the formation of inhibitors generated by FIX injections, decreased IgE, fatal anaphylaxis and enhanced life span of hemophilia B mice [48].
Other examples of functional protein drugs include rheumatoid arthritis (RA) which is an autoimmune disease that causes hyperplasia, synovial inflammation, and auto-antibody production. Such inflammation can further spread that can affect cardiovascular, psychological, and pulmonary functions. Extracellular protein i.e. Type-II collagen (CII) is normal practice for treatment to ensure smooth joints movement. However, some patients encounter a high level of autoimmune antibodies production against CII. Oral delivery of CII fused to glutelin expressed in rice seeds showed a reduction in IgG2a response against type-II collagen injections [72]. Also, rice seeds containing CII256–271 presented inhibition in the initial onset of arthritis in mice, apparently mediated by CD4+CD25− T cells [73]. Also, inflammatory bowel disease is another example of autoimmune disorder which is characterized by injury of color and small intestine and mucosal inflammation. The gut histology revealed that oral delivery of plant leaves material (tobacco) containing human interleukin-10 exhibited a reduction in the severity of colitis at the inflammatory site in IL-10 deficit mice [74]. The oral delivery was found equally effective in treating chronic inflammatory disease asthma characterized by bronchospasm, shortness of breath, etc. The life-threatening disease triggered by allergens affects over 200 million people worldwide. The disease prevention was achieved by oral tolerance induction by vaccinating mice with rice seed expressing mite allergen [75]. The allergen-immunized mice feed with rice seed expressing (p45–145) fragment of mite allergen (Der p1) encapsulated in ER showed reduction in serum levels of allergen-specific IgE and IgG. These examples of successful immunotherapy for allo, auto, and allergic disorders demonstrate the efficacy of oral delivery of therapeutics as a promising strategy for its treatment.
Case studies of plant made therapeutics
Hemophilia
Hemophilia A is an X-linked bleeding disorder is a challenging complication which occurs in approx. one in 5000 newborn boys worldwide due to absence of coagulation factor FVIII proteins [1]. FVIII is a glycoprotein that consists of several domains i.e. A1-A2-B-ap-A3-C1-C2 that circulates in a non-covalently bound complex with von Willebrand factor (VWF). In the circulations, the FVIII is protected by VWF from enzymatic degradation, and after activation (FVIIIa) through release from VWF react to FIX as cofactor after activation by releasing from VWF [76]. The blood clotting factor IX and VII (as cofactor) inactively circulate in the bloodstream. The blood clotting is dependent on the formation of an enzymatic complex that is an important component for the coagulation cascade. Both the FIX and FVIII are critical in case of any vascular injury repair and to stop any life-threatening bleeding. The deficiency is overcome by therapeutic FVIII infusions which result in neutralizing alloantibodies against factor VIII proteins, where no prophylactic tolerance protocols are available to-date. The formation of inhibitors is a challenging impediment for the hemophilia patient which results in drastic increases in morbidity besides the cost for its treatment. To treat or prevent the bleeding event, FVIII is injected intravenously into hemophilia A patients that result in FVIII inhibitor formation in the patients (30% to 5%) with severe and mild hemophilia. The inhibitor formation occurs in the beginning 50 days after the infusion of therapeutic FVIII protein, and the reversal of inhibitors formation is not only costly but also not equally effective in all patients. The immune tolerance induction (ITI) protocols can eliminate the inhibitors formation with moderate success rate of 60% [77], however, this daily dosage intravenous FVIII protocols are time-consuming and take years to complete that ultimately affects patient’s quality of life. In addition, the patient deal with high medical bills as treatment often cost up to $100,000.
Immune tolerance induction protocols against inhibitor formation are required to address such complications. Humans have evolved immune regulatory pathways in the small intestine that helps the human body to induce tolerance to daily food antigens. The oral route for antigen administration has prospect as an alternative feasible solution to avoid inhibitor formation [70, 78]. Taking advantage of the gut immune system that has naturally evolved to avoid pathogenic immune response that enters orally through food intake, Daniell and Herzog’s labs have introduced an innovative approach to induce FVIII, FIX specific immune tolerance in hemophilia A, B animal models (mice, dogs) by expressing FVIII, FIX antigens in plant chloroplasts [19, 20, 33, 47, 48, 76]**. The concept of immune suppression of bioencapsulated FVIII and FIX proteins has been applied successfully in the hemophilia B animal model [47]**. The oral route for delivery of FIX proteins encapsulated in plant cells has prevented inhibitor formation in hemophilia B mice during protein replacement therapy [48]. Also, elimination of inhibitor formation in mice and dog models has been achieved by chloroplast based oral delivery of FVIII and full-length FIX expressed in lyophilized plant material [20, 47, 48]**. In addition, the role of the gut microbiome is reported in inducing oral tolerance in the hemophilia animal model [58]**.
Pulmonary Hypertension
The elevated pulmonary artery pressure and vascular resistance result in pulmonary arterial hypertension (PAH) which is an incurable disease that causes human death. Besides its high treatment cost of up to $60–200k a year per patient, it also contains several side effects; and, with a survival rate of only 55% at three years [79]. The current medication available to-date requires more frequent intravenous delivery because the drugs for treatment are with a short half-life, cause continuous discomfort for patients. The Renin-angiotensin-aldosterone system (RAAS), which is up-regulated by PAH [80], is critical in the cardiovascular system by its roles in the regulation of pathogenesis pathways. The Angiotensin-Converting Enzyme (ACE) are critical in RAAS by converting Angiotensin-I (Ang-I) into Angiotensin-II (Ang-II) which has a further role in stimulating AT1 receptor (AT1R). The ACE homolog Angiotensin-Converting Enzyme-2 (ACE2) produces Angiotensin-(1–7) from Ang-II that further activates the Mas receptor. Together with G protein-coupled receptor Mas, ACE2/Ang-1(1–7) acts as a homeostatic regulator of vascular function to protect blood vessels, hearth, kidney as well as central nervous system; where such protection is attributed to neutralizing ACE/Ang-II/AT1R axis. The ACE2/Ang-1(1–7)/Mas axis also exerts protection in diabetes, hypertension, and cardiovascular disorders [81]. Therefore, to explore an alternative approach of cost-effective production system for delivery of drug against PH is pressing unmet medical need.
To address patient compliance and ease of administration, and to eliminating long term repetitive intravenous delivery of a drug, ACE-2 and its enzymatic product Angiotensin-(1–7) were expressed in lettuce chloroplast [21]** for its oral delivery. Combination therapy of orally delivered CTB-ACE2/ANG-(1–7) was detected in high quantities in lyophilized biomass with proven stability and functionality more than two years at room temperature are found effective in treating PH. New technology for removing the antibiotic resistance gene was found successful in yet another study by Daniell laboratory[18, 21]**. Also, the plants containing no antibiotic resistance gene for Ang1–7 were generated with high protein quantity that is also stable in subsequent generations for years at ambient temperature with stability and functionality including proper folding. When the monocrotaline-induced PAH rats were orally gavaged; dose-dependent delivery of both ACE-2 and Ang-(1–7) was recorded in plasma or tissues. More importantly, the oral delivery of both CTB-ACE2/ANG-(1–7) reduced the PAH development with a decrease up to 30–50% in right ventricular RV hypertrophy, total pulmonary resistance index (TPRI) and RV systolic pressure, and pulmonary artery remodeling in comparison to sham animals [21]**. Both the PDs also reduced cardiac index. In another study, human ACE2/Ang-(1–7) that contained the aadA gene was expressed in plant chloroplast [36] to attenuate PH. As expected, combination therapy with the oral delivery ACE2 and Ang-(1–7) was effective in the rat model to halt the development of monocrotaline-induced PH and also boosted associated cardiopulmonary pathophysiology, in addition to its advantageous impact against monocrotaline-induced lung injury. Considering the aforementioned promising efficacy in animal models, pre-clinical trials for the development of CTB-ACE2/Ang(1–7) based needle-less oral drug for the treatment of PH is planned in the near future.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects host respiratory cells via the angiotensin converting enzyme 2 (ACE2) receptor with high affinity and causes COVID-19 disease. Infection of alveolar cells, results in lung injury, and dramatically lowers ACE2 levels. ACE2 is expressed in type II alveolar epithelial cells in healthy individuals and produce surfactants to protect alveoli from collapsing. Most importantly, ACE2 produces anti-inflammatory, cytoprotective Ang (1–7) peptide via cleavage of the vasoconstrictor angiotensin II (AngII). As shown above in case of hypertension, ACE2 supplementation could be beneficial in COVID-19 patients to serve as a decoy to reduce SARS-CoV-2 entry into type II cells and to protect against lung injury via the anti-inflammatory actions of Ang(1–7). Preclinical studies with full-length ACE2 showed that it accumulated10-fold higher in the lungs than plasma. Therapeutic efficacy and safety of supplementing ACE2 and Ang(1–7) with this existing product in non-critically ill COVID-19 patients is currently pursued, awaiting FDA approval of IND.
Human Insulin-like growth factor-1
Human Insulin-like growth factor-1 (IGF-1) is an anabolic hormone essential for cell proliferation that is also effective in treating multiple muscle disorders. It also mediates the growth effects of growth hormones and performs a vital role in the regeneration and development of skeletal muscles and bones, however, requires more frequent intravenous administration for drug delivery, also requires surgical implantation. Improving IGF-1 levels in the serum helps in decreasing serum glucose, downgrade lipolysis, facilitate intracellular transport of glucose, decrease protein degradation and also help in enhancing protein synthesis and bone growth [82]. The production of IGF-1 in the liver is a deficit in quantifying enough to improve muscle hypertrophy. When administered IGF-1 in the sera, it translocate to muscle tissues, and therefore improving IGF-1 levels in the circulatory system is recommended. Testing the animal models with the oral delivery of IGF-1 without tag resulted in fewer IGF-1 detection in sera. Currently, the only means for its delivery to muscles is through injections in clinical studies.
Oral delivery of plant made IGF-1 is a promising alternative medication to replace injectable therapy by delivering PDs to the circulatory system intact in the plant cell wall, a natural bioencapsulation that helps in bypassing acid hydrolysis until it reaches the gut lumen. The comparison analysis of circulatory levels of rhIGF-1 and oral delivery of lyophilized biomass containing Pro-IGF-1 clearly demonstrates the efficacy. In a study conducted by Fouque and colleagues (2000) [83] observed a 100% increase of rhIGF-1 over 20 days of regular injections (12 hours) in malnourished CAPD patients. However, the orally delivered with IGF-1 achieved the same level of increase in a relatively shorter time of 2 hours after a single dose in the mice model [18]**. The codon-optimized IGF-1 and with e-peptide (Pro-IGF1) showed its efficacy by its delivery to muscle tissues. More importantly, plant cell wall encapsulated CTB-Pro-IGF1 fused to CTB or PTD fusion proteins has a significant impact on promoting bone volume, area, and density when femoral fractured diabetic mice were orally gavaged. The selectable marker-free PTD-Pro-IGF-1 showed equal expression and efficacy in subsequent generations. The oral delivery of rhIGF-1 showed biological activity to promote musculoskeletal cell proliferation, differentiation and fracture healing in diabetic mice.
Diabetes
Diabetes is distinguished by β-cell destruction and loss of function or ability of insulin-producing pancreatic β-cell, leading to an abnormal hike in blood glucose levels that accounts for the 7th foremost cause of death in the United States. The diabetic symptoms include frequent urination, weight loss, hike in hunger or thirst, and irritability. The rapid urbanization worldwide, and changing lifestyle in response to technological advancement that adds more comfort in daily life has reduced physical activities that resulted in increased obesity. Diabetes patients are on the rise worldwide, and it is estimated to reach more than half a billion by 2030 [84]. As of 2007, total expenses on diabetes care and indirect cost account for $ 174 billion in the US alone. According to the American Diabetes Association, 2008, approx. $1 out of every $10 in the US is attributed to diabetes medication, which can reach up to $396 billion by 2025 [85]. Recombinant insulin is predominantly produced in Saccharomyces cerevisiae expression systems containing engineered insulin sequences with native A and B chains that lack B30 threonine fused to synthetic C-peptide [86] Type-I diabetes patients rely on subcutaneous injections as standard management, approx. more than 60 thousand times throughout their lives, in addition to Type-2 patients who require insulin therapy. The patients, sometimes, also encounter on-site injuries due to continuous injections. Affordability and access to insulin is a major issue where only 23% of low-income countries have access to insulin [87]. The formulation of oral insulin is hampered because of low stomach pH causes its poor bio-stability. Also, the thick mucus layer of the intestine decreases its bioavailability causes greater challenges for oral delivery. In addition, several other formulations are under development or in clinical trials for its oral delivery such as hepatic-directed vesicle insulin, nano-encapsulated insulin, and enteric-coated insulin [88]. However, none of the delivery systems has achieved success to meet primary efficacy endpoints. Also, all oral formulations have failed to lower the pronounced metabolic effects, and its faster removal from circulation than subcutaneous injections are greater concerns. The insulin currently used in insulin therapy does not contain C-peptide that can result in highly biologically active, monomeric insulin [89]. Also, insulin is 51 amino acids short-lived polypeptide that also require a cold chain for its storage and transport that hamper its access to medication, especially in the development world. Therefore, as an alternative method for insulin administration, the edible plant made insulin contains potential benefit to eliminate the inconvenience of injections to improve patient compliance
In contrast to the bacterial system, a plant-based system can make up to 20 million insulin doses in one acre of tobacco growing space offer affordability and safety with enhanced patient compliance [17]. Several plants have been engineered in the nucleus and chloroplast for insulin production as potential innovative drugs for the treatment of diabetes to improve low-cost access. The early investigators expressed insulin in plant tissue was primarily on treating the autoimmune aspect of diabetics by oral tolerance induction. The earlier insulin expression in potato tubers and CTB fused to B-chain in tobacco via nuclear insertion showed a relatively lower amount of functional protein of up to 0.1% TSP [90]. Also, lower expression of pro-insulin (with or without C-peptide) was reported in Arabidopsis. Insulin analog SCI-57 was also transiently expressed in Nicotiana Benthamiana it’s for cost-effective production [87]. Taking advantage of large copies number of chloroplast per cell, transplastomic lettuce leaves accumulated a significant amount of CTB fused pro-insulin (A, B, C peptides) up to 53% TLP showed a reduction in blood glucose levels in mice model, obtained similar results to commercially available insulin [17]. The diabetic mice when fed with lyophilized lettuce biomass containing pro-insulin showed lower blood glucose levels within two hours after gavage [17]. Glucagon-like peptide (GLP-1) is helpful in insulin secretion, however rapid degradation reduces its half-life to only 2 min in circulation, in contrast to exenatide (Byetta) which has a relatively longer half-life of 4 min with insulinotropic effects. Oral delivery of Exenatide can be an ideal candidate because its insulinotropism is glucose-dependent and tends to eliminate the risk of hypoglycemia at higher doses. The CTB fused exendin-4 (EX4) was expressed (14.3% TLP) in the tobacco chloroplast to achieve the goal of its oral delivery. The lyophilized biomass containing EX4 was effective in insulin secretion similar fashion to commercial EX4 in mouse pancreatic cell line, beta-TC6. The bioencapsulated CTB-EX4 maintained its pentameric structure and functionality 15 months after storage at ambient temperature exhibiting its perceived advantages of eliminating painful injections and cold chain requirements, making it affordable and accessible [38].
Oral therapeutics for Peanut Allergy
Food allergy is a life-threatening medical disorder and increasingly common in developed countries that affect 5–10% population. Peanut allergy is a more prevalent food allergy affects 1 out of 5 children to total 1 million, and 1 in 160 adults in the United States [91]. It is difficult to identify individuals at higher risk; however, the food allergies begin in the early stage of life, with 80% chances of its persistence into adulthood [48]. Allergic reactions related to peanut can be triggered in unpredictable fashion by a minute amount of exposure (mg) and can be severe enough to cause fatal food-related anaphylaxis. Peanut is widely used in a variety of foods, and individuals with peanut allergy found exposed to accidental ingestions (50%) over the two years. Before writing this review, there was no specific treatment that is approved by the FDA or European Medicines Agency. Such a large allergic population worldwide must rely on the current standard treatment of strictly avoiding exposure to allergic food to prevent life-threatening reactions, however, besides avoiding allergic food, inadvertent exposure is probable to happen. Early efforts of subcutaneous immunotherapy as a treatment for peanut allergy resulted in severe adverse reactions attributed to the route of administration. Therefore, considering the oral route as a strategy for oral immunotherapy (OIT) might be a better option with broader safety as reported earlier in egg and milk allergy.
Oral route is preferred for PDs delivery because of its ease of delivery, enhanced patience compliance and safety profile [92]. There is a wealth of other data related to pre-clinical trials that demonstrate oral delivery of protein-induced immune tolerance [70, 78, 93, 94]. Also, success has been achieved in OIT for treating life-threatening food allergies in animal models, demonstrating desensitization or unresponsiveness [4]**. Recently, using pharmaceutical-grade peanut powder, AR101 demonstrated desensitization of the dosage exceeding the level that normally triggers a reaction with accidental ingestions. In the phase-2 clinical trials, the product showed tolerance to 443mg in all subjects, the amount equal to one and a half peanut. The 22 weeks clinical trial showed promising results where 78% of subjects tolerated 600mg single highest dose, with no mild symptoms. The phase 2 trials also included the placebo control group (19%) and entry and exit DBCFCs, which was missing in phase 1 clinical trial for AR101. During the AR101 clinical trials, the dosage was discontinued for those 21% of the subjects who showed no tolerance. The product AR101 was manufactured in cGMP conditions with no microbial contamination or other related allergens. AR101 is an effective OIT to provide meaningful means with safety for desensitization of peanut allergy to reduce risk of allergy reactions in children older than four years [53].
Recently, as a major breakthrough, product Palforzia received FDA approval for human use as the first oral biologic drug product for the treatment of peanut allergy. After successful tolerance induction in phase-2 clinical trials [53], and reviewing data of phase-3 clinical trials, the FDA granted approval considering data regarding the effectiveness and safety in randomized, double-blind, placebo-controlled food challenges (DBPCFC) clinical model study performed in the U.S., Canada and Europe [4]**. Palforzia is a pharmaceutical-grade peanut powder that is manufactured from peanuts plants grown in cGMP facility showed efficacy by suppressing SPT reactivity and enhancing IgG4 production to modify peanut-specific immune responses [4]**. In ARC003 phase-3 clinical trials that enrolled patients aged 4–17 years along with a smaller population aged 18–55 years assessed the efficacy and safety of AR101. The treatment was divided into three phases: Initial dose escalation given on a single day with 20–30 minutes intervals. Dosage of 0.5–12mg orally delivered peanut powder with placebo in initial dose escalation. Followed by up-dosing that consisted of 11 stages for ascending peanut powder dosages from 3mg to 300mg over 24 months with a two-week interval. All the dosages of peanut powder packed capsules under the supervision of professional healthcare in a health care facility during Initial and up-dosing. During treatment, professional healthcare is to monitor any potential mild to severe allergic reactions including anaphylaxis during all phases of oral dosage. The patients may continue oral delivery of the product provided in the sachet for maintenance treatment at home. However, the treatment may be discontinued if any allergic reactions appear during all three phases. The product Palforzia showed the effectiveness of tolerating 600mg dosage (twice daily maintenance dose) in 500 allergic subjects (67.2%) for six months, in comparison to 4.0% of placebo recipients. All subjects showed lower mild allergic symptoms. The DBPCFC study reported side effects of vomiting, cough, throat irritation, runny nose, and abdominal pain in approx. 700 subjects with peanut-allergy. The FDA also requires Risk Evaluation and Mitigation Strategy (REMS) for Palforzia treatment under the supervision of certified healthcare in a health care facility to mitigate the risk of associated anaphylaxis.
Conclusions
Aforementioned examples show that protein drugs made in plant cells could revolutionize current approaches of their production and delivery and make them affordable. This is based on the simple concept that plant cell wall could protect antigens or protein drugs from digestive acids/enzymes. Upon reaching the gut, commensal bacteria to release protein drugs by lysis of plant cell wall GALT plays a major role in inducing tolerance to treat treatment allergy or autoimmune diseases, genetic disorders, or anti-drug antibody responses that are faced by hemophilia or other patients. In addition, several examples are provided to deliver functional proteins to treat inherited or metabolic disorders. Expression of PDs reduces their production cost by elimination of prohibitively expensive fermentation, purification, cold transportation/ storage and sterile injections and increase their shelf life from days/weeks to several years. Recent advances in making PDs free of antibiotic resistance genes in edible plant cells, long term storage at ambient temperature maintaining their efficacy, production in cGMP facilities, IND enabling studies for clinical advancement and FDA approval of orally delivered PDs augur well for advancing this novel drug delivery platform technology.
Table-1:
Oral delivery of therapeutic proteins for induction of tolerance, enhance metabolic functions or immunity through booster vaccines.
| Disease | Plant | Antigen | Antibiotic | Expression system | Expression level | Animal model /human | Functional Evaluation | References |
|---|---|---|---|---|---|---|---|---|
| Peanut allergy | Peanut | AR003 | n/a | n/a | n/a | Human | Oral delivery of Arah proteins showed tolerating 600mg peanut flour dose in 500 allergic subjects (67.2%)- suppressed SPT reactivity and enhanced IgG4 production to modify peanut-specific immune responses | [4] |
| Pollen allergy | Rice | Destructed Cry j 1 and Cry j 2 | n/a | Seed | n/a | Mice | Oral delivery of rice seeds suppressed allergen-specific Cd4 (+) T-Cell proliferation in mice and reduced allergy symptoms including sneezing, eosinophils etc. | [95] |
| Pollen allergy | Rice | Cry j 1 and Cry j 2 | n/a | Seed | n/a | Mice | Oral immunotherapy decreased allergic conjunctivitis and IFN-γ production in mice splenocytes | [96] |
| Allergic asthma | Rice | Mite allergen (Der p 1) | n/a | Seed | 7.5% TSP | Mice | Oral gavage of rice mite allergen expressed in rice seed reduced serum level allergen Especific IgE and IgG without bystander suppression. | [75] |
| Rheumatoid arthritis | Rice | Type II collagen (CII) | mALS | Seed | Up to 24 mg/g | Mice | Oral delivery of APL7 transgenic rice suppressed the development of arthritis and delayed onset of disease in mice model | [73] |
| Hemophilia A | Lettuce Tobacco |
FVIII | Marker free | Chloroplast | 3622 μg/g DW | Mouse | Oral gavage of C2, LC and HC fused with CTB suppressed inhibitor formation in animal model | [19, 20] |
| Hemophilia B | Lettuce | FIX | Marker free | Chloroplast | 1000 μg/g DW | Mice/dog | Orally delivered FIX inhibited formation of anti-drug antibodies in hemophilia A mice | [33, 47] |
| Pompe disease | Tobacco | GAA | aadA | Chloroplast | 190 μg/g DW | Mice | Oral delivery of CTB-GAA suppressed significantly Immunoglobulin formation against GAA in Pompe mice | [43] |
| Diabetes fracture | Lettuce | Pro-IGF-1 | Marker free | Chloroplast | 370 μg/g DW | Mice | The oral delivery of IGF-1 promoted musculoskeletal cell proliferation, differentiation and fracture healing in diabetic mice. | [18] |
| Diabetes | Lettuce | Extendin-1 | aadA | Chloroplast | 14.3% TLP | mice | Oral delivery of extendin-4 lowered blood glucose levels and stimulated insulin secretion in mice | [38] |
| Diabetes | Lettuce Tobacco |
Pro-insulin | aadA | Chloroplast | Tobacco: ~16%TSP Lettuce: 72% TLP |
Mice | Oral delivery of pro-insulin resulted in lower blood or urine glucose levels, and preserved insulin-producing β-cells in mice | [29, 35] |
| Hypertension | Lettuce | CTB-ACE2 | aadA | Chloroplast | 8600 μg/g DW | Mice | Oral delivery of CTB-ACE2/ANG-(1–7) reduced development of pulmonary hypertension, decreased right ventricular RV hypertrophy, total pulmonary resistance index, RV systolic pressure and pulmonary artery remodeling | [21] |
| Hypertension | Lettuce | CTB-Ang1–7 | Marker free | Chloroplast | 1900 μg/g DW | Rats | ||
| Alzheimer’s disease | Tobacco | Myelin basic protein (MBP) | aadA | Chloroplast | 2% TSP | Mice | Oral delivery of CTB-MBP resulted in increased level of MBP levels in brain, and amyloid plaque intensity was reduced up to 70% in mice | [97] |
| Gaucher’s disease | Carrot cells | glucocerebrosidase | n/a | Plant cell | 3 mg kg−1 | Rat and pig | The orally delivered dose was inadequate due to low levels of expression. | [98] |
| Cancer | Tobacco | IFN-α2b | aadA | Chloroplast | 20% TSP | Mice | Increased expression of MHC-I on splenocytes, and improved number of natural killer (NK) cells. The IFN-α2b also protected mice from metastatic tumor line. | [99] |
| Polio | Tobacco | VP1 | aadA | Chloroplast | 5.5mg/g DW | Mice | VP1-specific IgG1, VP1-IgA titres and neutralization (80%−100% seropositivity of Sabin-1,2, 3). VP1-specific IgG1, VP1-IgA titres and neutralization (80%−100% seropositivity of Sabin-1,2, 3). VP1-specific IgG1, VP1-IgA titres and neutralization (80%−100% seropositivity of Sabin-1,2, 3). Oral boost in mice resulted in high levels of VP1 specific IgG1 and VP1-IgA titers and neutralization and seropostivity against all three polio virus serotypes. |
[6, 7] |
| Dengue | Lettuce | EDIII-1–4 | aadA | Chloroplast | 3.5% TSP | Rabbit | The injection of vaccine antigen EDIII-1–4 produced in lettuce elicited antibody production. | [100] |
| Plague | Lettuce | F1-V | aadA | Chloroplast | 14.1% TSP | Mice | Oral gavage of lettuce produced F1-V showed survival rate of 88% in aerosolized Y. pestis challenge. | [101] |
| Tuberculosis | Carrot | ESAT6 | nptII | Root | 0.056% TSP | Mice | Mice fed with ESAT6 induced cell-mediated and humoral immunities | [102] |
| Anthrax | Lettuce | pagA | aadA | Chloroplast | 14.2% | Mice | Subcutaneous immunization of lettuce produced pagA resulted in 100% survival of lethal dose of lethal toxin doses. | [103] |
| Hepatitis B | Lettuce | HBcAg | n/a | Nuclear | 3.5 μg/g DW | Mice | Oral delivery of lyophilized lettuce containing HBcAg elicited a specific and efficient response in mice model | [104] |
| Hepatitis C | Lettuce | E1E2 | nptII | Nuclear | n/a | Mice | Injections with oral boosts with the lettuce E1E2 developed systemic response. | [105] |
Acknowledgement
Most of the recent publications on chloroplast biotechnology in the Daniell laboratory reviewed here were supported by funding from NIH grant R01 HL 107904, R01 HL 109442 and R01 HL 133191.
Conflict of interest
Although there is no specific financial conflict of interest to report, the corresponding author discloses that he is inventor on several patents that describe expression of protein drugs in chloroplasts and their oral delivery. In addition, he was funded in the past by several pharmaceutical companies including Bayer, Novo Nordisk, Johnson & Johnson and is currently funded by Shire/Takeda.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Xiao YH, Kwon KC, Hoffman BE, Kamesh A, Jones NT, Herzog RW, et al. Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials. 2016;80:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Limaye A, Koya V, Samsam M, Daniell H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. Faseb Journal. 2006;20:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fox JL. First plant-made biologic approved. Nat. Biotechnol 2012;30:472-. [Google Scholar]
- [4]**.Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, Hourihane JO, et al. AR101 Oral Immunotherapy for Peanut Allergy. New Engl. J. Med 2018;379:1991–2001. [DOI] [PubMed] [Google Scholar]; This is the first example of FDA approved orally delivered protein drug expressed in plant cells. The phase-3 clinical trial reported here ia a landmark study for oral tolerance induction through orally delivered peanut powder that tested up to 600mg peanut flour in 500 allergic subjects in DBPCFC. This study opens the door to orally deliver therapeutic proteins without need for any purification or cold chain storage.
- [5].Tacket CO, Mason HS, Losonsky G, Clements JD, Levine MM, Arntzen CJ. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat. Med 1998;4:607–9. [DOI] [PubMed] [Google Scholar]
- [6]**.Daniell H, Rai V, Xiao Y. Cold chain and virus-free oral polio booster vaccine made in lettuce chloroplasts confers protection against all three poliovirus serotypes. Plant Biotechnol. J 2019;17:1357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]; This double blinded study evaluated by the Center for Disease Control, USA and immunization performed in the Daniell lab (University of Pennsylvania) supported by the Gates Foundation offers direct evidence for requirement of an injectable priming for vaccine antigens to develop neutralizing antibody against three poliovirus serotypes. However, lettuce booster vaccines after injectable priming conferred prolonged mucosal and systemic immnity and much higher levels of protection against all three poliovirus serotypes. Therefore, orally delivered plant cells expressing antigens are suitable for cold chain free booster vaccines but do not work primary vaccines.
- [7].Chan HT, Daniell H. Plant-made oral vaccines against human infectious diseases-Are we there yet? Plant Biotechnol. J 2015;13:1056–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fasano A, Uzzau S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J .Clin. Invest 1997;99:1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Toorisaka E, Ono H, Arimori K, Kamiya N, Goto M. Hypoglycemic effect of surfactant-coated insulin solubilized in a novel solid-in-oil-in-water (S/O/W) emulsion. Int. J. Pharmaceut 2003;252:271–4. [DOI] [PubMed] [Google Scholar]
- [10].Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J. Pharm. Sci 1999;88:933–7. [DOI] [PubMed] [Google Scholar]
- [11].Kyriakides TR, Cheung CY, Murthy N, Bornstein P, Stayton PS, Hoffman AS. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J. Cont. Rel 2002;78:295–303. [DOI] [PubMed] [Google Scholar]
- [12].Carino GP, Jacob JS, Mathiowitz E. Nanosphere based oral insulin delivery. J Control Release. 2000;65:261–9. [DOI] [PubMed] [Google Scholar]
- [13].Czerkinsky C, Sun JB, Lebens M, Li BL, Rask C, Lindblad M, et al. Cholera toxin B subunit as transmucosal carrier-delivery and immunomodulating system for induction of antiinfectious and antipathological immunity. Ann. Ny. Acad. Sci 1996;778:185–93. [DOI] [PubMed] [Google Scholar]
- [14].Bergerot I, Arreaza GA, Cameron MJ, Burdick MD, Strieter RM, Chensue SW, et al. Insulin B-chain reactive CD4(+) regulatory T-cells induced by oral insulin treatment protect from type 1 diabetes by blocking the cytokine secretion and pancreatic infiltration of diabetogenic effector T-cells. Diabetes. 1999;48:1720–9. [DOI] [PubMed] [Google Scholar]
- [15].Ploix C, Bergerot I, Durand A, Czerkinsky C, Holmgren J, Thivolet C. Oral administration of cholera toxin B-insulin conjugates protects NOD mice from autoimmune diabetes by inducing CD4(+) regulatory T-cells. Diabetes. 1999;48:2150–6. [DOI] [PubMed] [Google Scholar]
- [16].Petersen JS, Bregenholt S, Apostolopolous V, Homann D, Wolfe T, Hughes A, et al. Coupling of oral human or porcine insulin to the B subunit of cholera toxin (CTB) overcomes critical antigenic differences for prevention of type I diabetes. Clin. Exp. Immunol 2003;134:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boyhan D, Daniell H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol. J 2011;9:585–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]**.Park J, Yan G, Kwon KC, Liu M, Gonnella PA, Yang S, et al. Oral delivery of novel human IGF-1 bioencapsulated in lettuce cells promotes musculoskeletal cell proliferation, differentiation and diabetic fracture healing. Biomaterials. 2020;233. 119591. [DOI] [PMC free article] [PubMed] [Google Scholar]; his study evaluated different fusion tags to orally deliver human IGF-1 expressed in marker free edible plant cells. Oral delivery of IGF-1 promoted musculoskeletal cell proliferation, differentiation and fracture healing in diabetic mice. The lettuce chloroplast made pro-IGF showed longer shelve life up to 31 months after storage at ambient temperature.
- [19]**.Kwon KC, Sherman A, Chang WJ, Kamesh A, Biswas M, Herzog RW, et al. Expression and assembly of largest foreign protein in chloroplasts: oral delivery of human FVIII made in lettuce chloroplasts robustly suppresses inhibitor formation in haemophilia A mice. Plant Biotechnol. J 2018;16:1148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports the largest recombinant protein expressed in chloroplasts (FVIII-SC); proper pentamer assebly, disulfide bond formation and function, regardless of largest size is demonstrated. Different domains of FVIII (HC, C2, SC) when orally gavaged showed reduced inhibitor titres in hemophilia mice.
- [20].Sherman A, Su J, Lin S, Wang X, Herzog RW, Daniell H. Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood. 2014;124:1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]**.Daniell H, Mangu V, Yakubov B, Park J, Habibi P, Shi Y, et al. Investigational new drug enabling angiotensin oral-delivery studies to attenuate pulmonary hypertension. Biomaterials. 2020;233. 119750. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows the first Investigational New Drug enabling toxicology, pharmacokinetic studies and creation of antibiotic resistance gene free protein drug made in an edible plant system. Oral delivery of CTB-ACE2/ANG-(1–7) reduced development of pulmonary hypertension, decreased right ventricular RV hypertrophy, total pulmonary resistance index, RV systolic pressure and pulmonary artery remodeling. ACE2/ANG-(1–7) is unique for its binding affinity with SARS-CoV-2 spike protein and is potential therapeutic candidate for treatment of COVID-19.
- [22].Imani J, Kogel KH. Plant Transformation Techniques: Agrobacterium- and Microparticle-Mediated Gene Transfer in Cereal Plants. Methods Mol. Biol 2020;2124:281–94. [DOI] [PubMed] [Google Scholar]
- [23].Daniell H, Jin SX, Zhu XG, Gitzendanner MA, Soltis DE, Soltis PS. Green giant-a tiny chloroplast genome with mighty power to produce high-value proteins: history and phylogeny. Plant Biotechnol. J 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Daniell H, Kulis M, Herzog RW. Plant cell-made protein antigens for induction of Oral tolerance. Biotechnol. Adv 2019;37;107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Verma D, Daniell H. Chloroplast vector systems for biotechnology applications. Plant Physiol. 2007;145:1129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jin SX, Daniell H. The Engineered Chloroplast Genome Just Got Smarter. Trends Plant Sci. 2015;20:622–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].De Cosa B, Moar W, Lee SB, Miller M, Daniell H. Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat. Biotechnol 2001;19:71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]**.Malhotra K, Subramaniyan M, Rawat K, Kalamuddin M, Qureshi MI, Malhotra P, et al. Compartmentalized Metabolic Engineering for Artemisinin Biosynthesis and Effective Malaria Treatment by Oral Delivery of Plant Cells. Mol. Plant 2016;9:1464–77. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows highest expression of Artemisining outside Artemesia and demonstrated that orally delivered plant cells reduced parasitemia in challenged animals than clinically used purified drug.
- [29].Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts - oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol. J 2007;5:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Daniell H Transgene containment by maternal inheritance: Effective or elusive? Proc. Natl. Acad. Sci. USA 2007;104:6879–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Clark M, Maselko M. Transgene Biocontainment Strategies for Molecular Farming. Front. Plant. Sci 2020;11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Daniell H Transgene containment by maternal inheritance: effective or elusive? Proc. Natl. Acad. Sci. U S A 2007;104:6879–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Su J, Zhu L, Sherman A, Wang X, Lin S, Kamesh A, et al. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials. 2015;70:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kumar S, Dhingra A, Daniell H. Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol. 2004;136:2843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ruhlman T, Verma D, Samson N, Daniell H. The Role of Heterologous Chloroplast Sequence Elements in Transgene Integration and Expression. Plant Physiol. 2010;152:2088–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shenoy V, Kwon KC, Rathinasabapathy A, Lin S, Jin G, Song C, et al. Oral delivery of Angiotensin-converting enzyme 2 and Angiotensin-(1–7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension. 2014;64:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shil PK, Kwon KC, Zhu P, Verma A, Daniell H, Li Q. Oral delivery of ACE2/Ang-(1–7) bioencapsulated in plant cells protects against experimental uveitis and autoimmune uveoretinitis. Mol. Ther 2014;22:2069–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kwon KC, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol J. 2013;11:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].DeGray G, Rajasekaran K, Smith F, Sanford J, Daniell H. Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol. 2001;127:852–62. [PMC free article] [PubMed] [Google Scholar]
- [40].Gupta K, Kotian A, Subramanian H, Daniell H, Ali H. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget. 2015;6:28573–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lee SB, Li BC, Jin SX, Daniell H. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol. J 2011;9:100–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Daniell H, Ruiz G, Denes B, Sandberg L, Langridge W. Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol. 2009;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Su J, Sherman A, Doerfler PA, Byrne BJ, Herzog RW, Daniell H. Oral delivery of Acid Alpha Glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol. J 2015;13:1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang X, Su J, Sherman A, Rogers GL, Liao G, Hoffman BE, et al. Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood. 2015;125:2418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Daniell H, Chan H-T, Pasoreck EK. Vaccination via chloroplast genetics: affordable protein drugs for the prevention and treatment of inherited or infectious human diseases. Annu. Rev. Genet 2016;50:595–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Daniell H, Lin C-S, Yu M, Chang W-J. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]**.Herzog RW, Nichols TC, Su J, Zhang B, Sherman A, Merricks EP, et al. Oral Tolerance Induction in Hemophilia B Dogs Fed with Transplastomic Lettuce. Mol. Ther 2017;25:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first large animal study using edible plant cells expressing a human therapeutic protein. The oral delivery of CTB-FIX resulted in robust suppression of IgE and immunoglobulin G formation, and coagulation time was markedly shortened when hemophilia dogs were orally fed with lyophilized lettuce biomass containing FIX.
- [48].Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, et al. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc. Natl. Acad. Sci. U S A 2010;107:7101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shil PK, Kwon KC, Zhu P, Verma A, Daniell H, Li QH. Oral Delivery of ACE2/Ang-(1–7) Bioencapsulated in Plant Cells Protects against Experimental Uveitis and Autoimmune Uveoretinitis. Mol. Ther 2014;22:2069–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kwon KC, Daniell H. Oral Delivery of Protein Drugs Bioencapsulated in Plant Cells. Mole. Ther 2016;24:1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Daniell H, Ribeiro T, Lin S, Saha P, McMichael C, Chowdhary R, et al. Validation of leaf and microbial pectinases: commercial launching of a new platform technology. Plant Biotechnol. J 2019;17:1154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kumari U, Singh R, Ray T, Rana S, Saha P, Malhotra K, et al. Validation of leaf enzymes in the detergent and textile industries: launching of a new platform technology. Plant Biotechnol. J 2019;17:1167–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bird JA, Spergel JM, Jones SM, Rachid R, Assa’ad AH, Wang J, et al. Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J. Allergy. Clin. Immunol. Pract 2018;6:476–85 e3. [DOI] [PubMed] [Google Scholar]
- [54].Shaaltiel Y, Bartfeld D, Hashmueli S, Baum G, Brill-Almon E, Galili G, et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol. J 2007;5:579–90. [DOI] [PubMed] [Google Scholar]
- [55].Khan I, Twyman RM, Arcalis E, Stoger E. Using storage organelles for the accumulation and encapsulation of recombinant proteins. Biotechnol. Journal 2012;7. [DOI] [PubMed] [Google Scholar]
- [56].Gao J, Yin L. Drug development for controlling Ebola epidemic - a race against time. Drug Discov. Ther 2014;8:229–31. [DOI] [PubMed] [Google Scholar]
- [57].Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin. Exp. Immunol 2008;153:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]**.Kumar SRP, Wang XM, Avuthu N, Bertolini TB, Terhorst C, Guda C, et al. Role of Small Intestine and Gut Microbiome in Plant-Based Oral Tolerance for Hemophilia. Front. Immunol 2020;11. 00844. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first in depth investigation of gut microbiome in relation to digestion of plant cells and induction of oral tolerance. The study is of outstanding interest for reporting diverse bacterial species to produce equally diverse enzymes capable of digesting plant cell wall to facilitate oral drug delivery.
- [59].Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol 2012;10:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wernick NLB, Chinnapen DJF, Cho JA, Lencer WI. Cholera Toxin: An Intracellular Journey into the Cytosol by Way of the Endoplasmic Reticulum. Toxins. 2010;2:310–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Noguchi H, Matsushita M, Matsumoto S, Lu YF, Matsui H, Bonner-Weir S. Mechanism of PDX-1 protein transduction. Biochem. Bioph. Res. Co 2005;332:68–74. [DOI] [PubMed] [Google Scholar]
- [63].Ohno H Intestinal M cells. J. Biochem 2016;159:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang XM, Sherman A, Liao GX, Leong KW, Daniell H, Terhorst C, et al. Mechanism of oral tolerance induction to therapeutic proteins. Adv. Drug. Deliver. Rev 2013;65:759–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Thomas G Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Bio 2002;3:753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sanchez J, Holmgren J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell Mol. Life Sci 2008;65:1347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Saslowsky DE, te Welscher YM, Chinnapen DJF, Wagner JS, Wan J, Kern E, et al. Ganglioside GM1-mediated Transcytosis of Cholera Toxin Bypasses the Retrograde Pathway and Depends on the Structure of the Ceramide Domain. J. Biol. Chem 2013;288:25804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zahid M, Robbins PD. Protein Transduction Domains: Applications for Molecular Medicine. Curr. Gene Ther 2012;12:374–80. [DOI] [PubMed] [Google Scholar]
- [69].Khaja K, Robbins P. Comparison of Functional Protein Transduction Domains Using the NEMO Binding Domain Peptide. Pharmaceuticals (Basel). 2010;3:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rezende RM, Weiner HL. History and mechanisms of oral tolerance. Semin. Immunol 2017;30:3–11. [DOI] [PubMed] [Google Scholar]
- [71].Bakdash G, Vogelpoel LTC, van Capel TMM, Kapsenberg ML, de Jong EC. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Muco. Immunol 2015;8:265–78. [DOI] [PubMed] [Google Scholar]
- [72].Hashizume F, Hino S, Kakehashi M, Okajima T, Nadano D, Aoki N, et al. Development and evaluation of transgenic rice seeds accumulating a type II-collagen tolerogenic peptide. Trans. Res 2008;17:1117–29. [DOI] [PubMed] [Google Scholar]
- [73].Iizuka M, Wakasa Y, Tsuboi H, Asashima H, Hirota T, Kondo Y, et al. Suppression of collagen-induced arthritis by oral administration of transgenic rice seeds expressing altered peptide ligands of type II collagen. Plant Biotechnol. J 2014;12:1143–52. [DOI] [PubMed] [Google Scholar]
- [74].Menassa R, Du CG, Yin ZQ, Ma SW, Poussier P, Brandle J, et al. Therapeutic effectiveness of orally administered transgenic low-alkaloid tobacco expressing human interleukin-10 in a mouse model of colitis. Plant Biotechnol. J 2007;5:50–9. [DOI] [PubMed] [Google Scholar]
- [75].Suzuki K, Kaminuma O, Yang LJ, Takai T, Mori A, Umezu-Goto M, et al. Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnol. J 2011;9:982–90. [DOI] [PubMed] [Google Scholar]
- [76].Batsuli G, Meeks SL, Herzog RW, Lacroix-Desmazes S. Innovating immune tolerance induction for haemophilia. Haemophilia. 2016;22:31–5. [DOI] [PubMed] [Google Scholar]
- [77].Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124:3365–72. [DOI] [PubMed] [Google Scholar]
- [78].Kuhn C, Weiner HL. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy-Uk. 2016;8:889–906. [DOI] [PubMed] [Google Scholar]
- [79].Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Survival in Patients With Idiopathic, Familial, and Anorexigen-Associated Pulmonary Arterial Hypertension in the Modern Management Era. Circulation. 2010;122:156–63. [DOI] [PubMed] [Google Scholar]
- [80].Maron BA, Leopold JA. Emerging Concepts in the Molecular Basis of Pulmonary Arterial Hypertension: Part II: Neurohormonal Signaling Contributes to the Pulmonary Vascular and Right Ventricular Pathophenotype of Pulmonary Arterial Hypertension. Circulation. 2015;131:2079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bader M ACE2, angiotensin-(1–7), and Mas: the other side of the coin. Pflug. Arch. Eur. J. Phy 2013;465:79–85. [DOI] [PubMed] [Google Scholar]
- [82].Samokhin AO, Stephens T, Wertheim BM, Wang RS, Vargas SO, Yung LM, et al. NEDD9 targets COL3A1 to promote endothelial fibrosis and pulmonary arterial hypertension. Sci. Transl. Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fouque D, Peng SC, Shamir E, Kopple JD. Recombinant human insulin-like growth factor-1 induces an anabolic response in malnourished CAPD patients. Kidney Int. 2000;57:646–54. [DOI] [PubMed] [Google Scholar]
- [84].Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pr 2011;94:311–21. [DOI] [PubMed] [Google Scholar]
- [85].Giannini C, Mohn A, Chiarelli F. Technology and the issue of cost/benefit in diabetes. Diabetes Metab. Res. Rev 2009;25 Suppl 1:S34–44. [DOI] [PubMed] [Google Scholar]
- [86].Baeshen NA, Baeshen MN, Sheikh A, Bora RS, Ahmed MMM, Ramadan HAI, et al. Cell factories for insulin production. Microb. Cell Fact 2014;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Munoz-Talavera A, Gomez-Lim MA, Salazar-Olivo LA, Reinders J, Lim K, Escobedo-Moratilla A, et al. Expression of the Biologically Active Insulin Analog SCI-57 in Nicotiana Benthamiana. Front. in Pharm 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zijlstra E, Heinemann L, Plum-Morschel L. Oral insulin reloaded: a structured approach. J. Diabetes Sci. Technol 2014;8:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wahren J, Larsson C. C-peptide: New findings and therapeutic possibilities. Diabetes Res. Clin. Pr 2015;107:309–19. [DOI] [PubMed] [Google Scholar]
- [90].Li D, O’Leary J, Huang Y, Huner NP, Jevnikar AM, Ma S. Expression of cholera toxin B subunit and the B chain of human insulin as a fusion protein in transgenic tobacco plants. Plant Cell Rep. 2006;25:417–24. [DOI] [PubMed] [Google Scholar]
- [91].Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immun 2010;125:1322–6. [DOI] [PubMed] [Google Scholar]
- [92].Hu X, Yang G, Chen S, Luo S, Zhang J. Biomimetic and bioinspired strategies for oral drug delivery. Biomater. Sci 2020;8:1020–44. [DOI] [PubMed] [Google Scholar]
- [93].Fukuda K, Ishida W, Harada Y, Wakasa Y, Takagi H, Takaiwa F, et al. Efficacy of oral immunotherapy with a rice-based edible vaccine containing hypoallergenic Japanese cedar pollen allergens for treatment of established allergic conjunctivitis in mice. Allergol. Int 2018;67:119–23. [DOI] [PubMed] [Google Scholar]
- [94].Mowat AM. To respond or not to respond - a personal perspective of intestinal tolerance (vol 18, pg 405, 2018). Nat. Rev. Immunol 2018;18:535-. [DOI] [PubMed] [Google Scholar]
- [95].Wakasa Y, Takagi H, Hirose S, Yang LJ, Saeki M, Nishimura T, et al. Oral immunotherapy with transgenic rice seed containing destructed Japanese cedar pollen allergens, Cry j 1 and Cry j 2, against Japanese cedar pollinosis. Plant Biotechnol. J 2013;11:66–76. [DOI] [PubMed] [Google Scholar]
- [96].Fukuda K, Ishida W, Wakasa Y, Takagi H, Takaiwa F, Fukushima A. Oral Immunotherapy for Allergic Conjunctivitis Using Transgenic Rice Expressing Hypoallergenic Antigens. Cornea. 2018;37 Suppl 1:S67–S73. [DOI] [PubMed] [Google Scholar]
- [97].Kohli N, Westerveld DR, Ayache AC, Verma A, Shil P, Prasad T, et al. Oral delivery of bioencapsulated proteins across blood-brain and blood-retinal barriers. Mol. Ther 2014;22:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Shaaltiel Y, Gingis-Velitski S, Tzaban S, Fiks N, Tekoah Y, Aviezer D. Plant-based oral delivery of beta-glucocerebrosidase as an enzyme replacement therapy for Gaucher’s disease. Plant Biotechnol. J 2015;13:1033–40. [DOI] [PubMed] [Google Scholar]
- [99].Arlen PA, Falconer R, Cherukumilli S, Cole A, Cole AM, Oishi KK, et al. Field production and functional evaluation of chloroplast-derived interferon-alpha 2b. Plant Biotechnol. J 2007;5:511–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].van Eerde A, Gottschamel J, Bock R, Hansen KEA, Munang’andu HM, Daniell H, et al. Production of tetravalent dengue virus envelope protein domain III based antigens in lettuce chloroplasts and immunologic analysis for future oral vaccine development. Plant Biotechnol. J 2019;17:1408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect. Immun 2008;76:3640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Uvarova EA, Belavin PA, Permyakova NV, Zagorskaya AA, Nosareva OV, Kakimzhanova AA, et al. Oral Immunogenicity of plant-made Mycobacterium tuberculosis ESAT6 and CFP10. Biomed. Res. Int 2013;2013:316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Koya V, Moayeri M, Leppla SH, Daniell H. Plant-based vaccine: Mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge\. Infect. Immun 2005;73:8266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pyrski M, Mieloch AA, Plewinski A, Basinska-Barczak A, Gryciuk A, Bociag P, et al. Parenteral-Oral Immunization with Plant-Derived HBcAg as a Potential Therapeutic Vaccine against Chronic Hepatitis B. Vaccines-Basel. 2019;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Clarke JL, Paruch L, Dobrica MO, Caras I, Tucureanu C, Onu A, et al. Lettuce-produced hepatitis C virus E1E2 heterodimer triggers immune responses in mice and antibody production after oral vaccination. Plant Biotechnol. J 2017;15:1611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


