Abstract
Objective
Epidural injection of local anaesthetics and intravenous opioid injection are two common analgesic strategies following major abdominal oncosurgery. However, epidural local anaesthetics may cause haemodynamic instability while opioid injection is associated with sedation and postoperative ileus. Intravenous lignocaine is also used for postoperative analgesia, and combined use of opioids plus lignocaine can reduce the doses and adverse effects of the individual drugs. This study therefore compared the analgesic efficacy of intravenous lignocaine–fentanyl (IV) to epidural ropivacaine–fentanyl (EPI) after major abdominal oncosurgery.
Methods
Sixty patients were randomised to IV and EPI groups. Patients in the IV group received preoperative intravenous bolus injections of lignocaine 1.5 mg kg−1 and fentanyl 0.5 μg kg−1, intraoperative infusions of lignocaine 1 mg kg−1 h−1 and fentanyl 0.5 μg kg−1 h−1, and postoperative infusions of lignocaine 0.5 mg kg−1 h−1 and fentanyl 0.25 μg kg−1 h−1. In the EPI group, patients received a 6-ml epidural bolus injection of ropivacaine 0.2% plus fentanyl 2 μg mL−1, intraoperative infusion of 5 mL·h−1 fentanyl and postoperative ropivacaine 0.1% plus fentanyl 1 μg mL−1 infusion at 5 mL h−1. All patients also received postoperative patient-controlled IV fentanyl as rescue analgesia. Patient-controlled fentanyl consumption was documented as the primary outcome for postoperative analgesic efficacy. Results were compared by Mann–Whitney U-test and Student’s t-test using Statistical Package for Social Science (SPSS) software.
Results
Median (min–max) rescue fentanyl requirement in the first 24 h postsurgery was comparable between IV and EPI groups [780 (340–2520) μg vs. 820 (140–2260) μg; p=0.6], as was postoperative pain score (p>0.05). The incidence of intraoperative hypotension requiring bolus mephenteramine injection was significantly higher in the EPI group than the IV group (36% vs. 17%; p<0.001).
Conclusion
Intravenous lignocaine–fentanyl and epidural ropivacaine–fentanyl have comparable postoperative analgesic efficacies after major open abdominal oncosurgery.
Keywords: Analgesic technique, epidural local anaesthetic, fentanyl, intravenous lignocaine, postoperative analgesia
Introduction
Effective pain management remains challenging after major abdominal oncosurgery. Despite the development of several multimodal analgesia regimens, about 41% of patients in the immediate postoperative period and 30% of patients on the first postoperative day experience moderate to severe pain (1). Numerous postoperative analgesic strategies are used for major abdominal surgeries, including epidural local anaesthetics (LAs) and intravenous opioids. However, both modalities have substantial limitations. Epidural LAs have been reported to provide better postoperative analgesia than intravenous opioids, but are associated with increased incidence of hypotension and bradycardia (2, 3). Therefore, epidural LAs are not well tolerated by patients undergoing surgical procedures with substantial blood loss or fluid shifts (3). Intravenous opioids also provide adequate postoperative analgesia but are frequently associated with itching, sedation, ileus, postoperative nausea and vomiting (PONV) and respiratory depression. Opioid use has also been associated with cancer recurrence (4).
Intravenous lignocaine infusion is an alternative analgesia technique with documented efficacy and opioid-sparing effects (5). In addition, lignocaine suppresses inflammation, postoperative thromboembolic episodes and postoperative ileus, resulting in shorter hospital stay and lower cost compared to epidural LA (6). Therefore, a combination of lignocaine and fentanyl infusion could be an effective strategy for postoperative analgesia. Further, patients with difficult catheter placement, who otherwise refuse epidural catheter placement, or have contraindications for epidural analgesia may benefit from this alternative strategy. However, it is not clear whether intravenous lignocaine infusion is as effective as epidural LAs for analgesia after major abdominal surgery (7).
In this study, we compared the analgesic efficacy of intravenous (IV) lignocaine–fentanyl infusion to epidural infusion of ropivacaine–fentanyl (EPI) after major open abdominal oncosurgery. The primary objective was to compare requirements for postoperative rescue analgesia by fentanyl. We hypothesised that the analgesic efficacy of intravenous lignocaine–fentanyl infusion would be comparable to epidural ropivacaine–fentanyl infusion.
Methods
Patient enrolment and study design
This study was approved by the institutional ethics committee of All India Institute of Medical Sciences, New Delhi, India and written informed consent was obtained from all subjects after full explanations of study protocols and aims. The trial was registered at ctri.nic.in (CTRI/2007/17/008987), and research conduct and reporting adhered to all CONSORT guidelines. From March 2017 to February 2018, patients scheduled for major open abdominal oncosurgery with American Society of Anesthesiologists physical status I, II or III and from 18 to 70 years of age were considered for enrolment. Patients with mental illnesses, uncontrolled heart disease, renal or hepatic disorders, contraindications for epidural catheter placement, or current opioid intake were excluded. All patients received instructions on use of the patient-controlled analgesia (PCA) device (CADD-Legacy® PCA, USA) and pain rating by a numeric rating scale (NRS).
Patients were monitored by plethysmography, non-invasive blood pressure (NIBP) measurement and five-lead ECG while in the operating room. Following assessment of baseline vital signs, patients were randomised to the IV or EPI group using computer-generated random numbers delivered in opaque, sequentially numbered, sealed envelopes. Patients and physicians were not blinded to randomisation as sham epidural catheters were not placed in patients of the IV group. However, as the primary outcome variable (requirement of postoperative rescue analgesic) was fully controlled by patients through the PCA device and the physician had no role in rescue analgesic administration, the result remained unbiased and objective.
Analgesic regimens
IV Group
An intravenous (i.v.) bolus of lignocaine 1.5 mg kg−1 was administered at least 10 min before induction of general anaesthesia (GA). Following GA (described below), patients received intraoperative lignocaine infusion at 1 mg kg−1 h−1. Patients also received a bolus dose of 0.5 μg kg−1 fentanyl before surgical incision, followed by 0.5 μg kg−1 h−1 intraoperative infusion. In the postoperative period, patients received continuous infusions of lignocaine 0.5 mg kg−1 h−1 and fentanyl 0.25 μg kg−1 h−1 in addition to patient-controlled fentanyl.
EPI Group
An epidural catheter was placed before GA induction. The catheter insertion site was planned according to the sensory dermatomes covered by the surgical incision. After successful placement of the epidural catheter, a 3-ml test dose of 2% lignocaine was administered with adrenaline (1:200000). Epidural catheter placement was deemed successful if an appropriate sensory band developed after epidural activation. Epidural analgesia was activated by 6 ml of ropivacaine 0.2% and fentanyl 2 μg mL−1, followed by intraoperative infusion of 5 mL h−1 fentanyl. GA was induced 10 min after epidural activation. During the postoperative period, patients received continuous epidural infusion of ropivacaine 0.1% and fentanyl 1 μg mL−1 at 5 mL h−1 in addition to patient-controlled fentanyl.
Surgery and patient care
In both groups, GA was induced with propofol 2–2.5 mg kg−1 (titrated until loss of verbal response) and fentanyl 2 μg kg−1. Endotracheal intubation was facilitated by rocuronium 0.6 mg kg−1. GA was maintained with desflurane in a mixture of 40% air and 60% oxygen. The end-tidal concentration of desflurane was adjusted to maintain a minimum alveolar concentration (MAC) of 0.6–1 MAC. Respiratory frequency and tidal volume were adjusted to maintain end-tidal carbon dioxide at 35–45 mmHg. During surgery, all patients received intravenous infusion of balanced salt solution at 6 mL kg−1 h−1. Intermittent bolus rocuronium injections were administered to ensure adequate neuromuscular blockade. All patients received intravenous paracetamol 15 mg kg−1 just before skin closure. At the end of surgery, residual neuromuscular block was reversed using neostigmine 0.06 mg kg−1 and glycopyrolate 0.01 mg kg−1. The endotracheal tube was removed when patients met institutional extubation criteria (Fully awake, follows verbal command, adequate oxygenation (SpO2 >92%), adequate ventilation) generating tidal volume of 5–6 mL kg−1, sustained 5 seconds head lift or hand grip, hemodynamically stable etc.).
During surgery, a 25-μg intravenous fentanyl bolus was administered if the patient’s heart rate (HR) increased above 20% or the surgical pleth index (SPI) (GE healthcare, Finland) rose above 60. Mean arterial pressure (MAP) was maintained within 20% of baseline and hypotension (MAP<65 mmHg) was treated by bolus injection of 200 mL balanced salt solution. If hypotension did not respond to fluid bolus injection, 6 mg bolus mephenteramine was injected. In case of persistent hypotension, noradrenaline infusion was started. The attending anaesthesiologist was also allowed to reduce analgesic infusion by up to 30% in case of persistent hypotension according to the individual clinical scenario. Bradycardia (HR<50 beats min−1) was treated using a 0.6 mg intravenous bolus of atropine.
During the intraoperative period, HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), MAC and SPI were recorded every 10 min. All intraoperative readings were averaged to obtain a mean value for each patient. The MAC hr was calculated for each patient by multiplying the average MAC value by the duration of anaesthesia. Haemodynamic responses to surgical incision (differences in HR, SBP and DBP between the pre-incision value and 1 min post-incision value), extubation time (time between skin closure and extubation), total rocuronium dose, fluid intake, urine output and blood loss were also documented and compared between groups.
In the postoperative period, all patients received the indicated analgesic infusion according to group allocation. Rescue analgesia was provided using fentanyl-based PCA (bolus dose 20 μg, lock out time 10 min, maximum dose of 120 μg h−1). Patients in both groups also received 6 hourly intravenous injections of paracetamol 10 mg kg−1.
Rescue analgesic (fentanyl) consumption via PCA during the first 24 h postsurgery was recorded as the primary outcome. Total rescue fentanyl was calculated as the number of delivered doses multiplied by the dose size (bolus dose of 20 mcg). Basal infusions of fentanyl (0.25 mcg kg−1 h−1 intravenously in the IV group and 5 mcg h−1 epidurally in the EPI group) were not included in the calculation of rescue fentanyl consumption as these were components of the fixed-dose analgesic regimens. Pain on coughing was self-evaluated using the NRS score, where 0 indicated the absence of pain and 10 the most severe pain. HR, MAP, respiratory rate (RR) and PONV were assessed just after transferring the patient to the post-anaesthesia care unit and 1, 2, 4, 8, 12, 18 and 24 h after completion of surgery. Total number of PCA activations, patient satisfaction score (using a 7-point Likert scale where ‘1’ is extremely dissatisfied and ‘7’ is extremely satisfied), postoperative 24 h fluid intake and urine output were also recorded. Patients were monitored for symptoms of lignocaine toxicity and opioid overdose, and sedation level was documented using the Ramsay sedation scale (RSS).
Statistical analysis
Sample size could not be calculated due to the lack of previous data comparing IV lignocaine–fentanyl to epidural ropivacaine–fentanyl for postoperative analgesic efficacy, so this is considered a pilot study. Continuous data with normal distributions are expressed as mean±SD and continuous data with non-normal distributions as median (min–max) or (interquartile range, IQR). Qualitative data are expressed as number of events (%). Ordinal qualitative variables and continuous variables with non-normal distributions were compared using the Mann–Whitney U-test. Continuous quantitative variables with normal distributions were compared using Student’s t-test. Nominal variables were compared between groups using the chi-square test. A p<0.05 (two-tailed) was considered statistically significant for all tests. All statistical calculations were conducted using Statistical Package for Social Science (SPSS Inc.; Chicago, IL, USA), release 16.0.
Results
Eighty-four patients were screened for eligibility, of which 24 were excluded based on the indicated inclusion and exclusion criteria. Of the 60 patients initially enrolled, 29 in the IV group and 28 in the EPI group completed the study protocol and were included in the data analysis (Figure 1). Patient demographic variables and distribution of surgery types were comparable between groups (Table 1).
Figure 1.
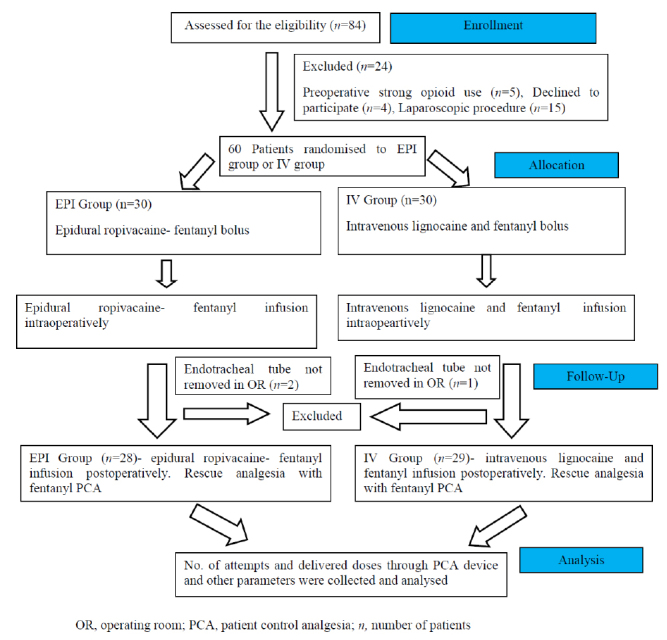
Flow diagram of the study showing participants flow
Table 1.
Comparison of patient demographics and surgery between intravenous lignocaine–fentanyl (IV) and epidural ropivacaine–fentanyl (EPI) analgesia groups
| Parameter | IV Group (n=29) | EPI Group (n=28) | p |
|---|---|---|---|
|
| |||
| Sex (n) (M/F) | 16/13 | 14/14 | 0.7 |
| Age (years) | 47±15 | 51±13 | 0.2 |
| BMI (kg m−2) | 22.77±3.97 | 22.65±4.04 | 0.9 |
| Duration of the procedure (min) | 246±95 | 215±120 | 0.3 |
| Type of surgical intervention | |||
| Radical hysterectomy (n) | 2 | 1 | |
| Radical cholecystectomy (n) | 5 | 6 | |
| Staging laparotomy (n) | 5 | 6 | |
| Colectomy (n) | 2 | 3 | |
| Gastrectomy (n) | 5 | 3 | |
| LAR/APR (n) | 4 | 5 | |
| Cytoreductive surgery+HIPEC (n) | 3 | 1 | |
| Retroperitoneal mass excision (n) | 1 | 0 | |
| Nephrectomy (n) | 2 | 1 | |
| Whipple’s procedure (n) | 0 | 2 | |
Data expressed as mean±SD or number of patients (n). BMI: body mass index; LAR: low anterior resection; APR: abdominoperineal resection; HIPEC: hyperthermic intraperitoneal chemotherapy
Postoperative analgesic and clinical parameters
The median number of rescue analgesia attempts (fentanyl bolus injections) using the PCA device was comparable between the IV and EPI groups during the first 24 h postsurgery [88 (19–1080) vs. 107 (7–772); p=0.9]. The median number of doses delivered was also comparable between IV and EPI groups [39 (17–126) vs. 41(7–113); p=0.6], and there was no significant difference in median total fentanyl consumption [780 (340–2520) μg vs. 820 (140–2260) μg; p=0.6] (Table 2). Note that the number of PCA attempts, number of delivered doses and fentanyl consumption were not normally distributed but are expressed as both median (min–max) and as mean±SD in Table 2 to facilitate sample size calculation in future studies.
Table 2.
Comparison of patient-controlled rescue analgesia requirements and clinical parameters during the first 24 h postsurgery
| Postoperative parameter | IV group (n=29) | EPI group (n=28) | p |
|---|---|---|---|
|
| |||
| No. of attempts in PCA device | 198±259; 88 (19–1080) | 154±162; 107 (7–772) | 0.9 |
| No. of delivered dose through PCA device | 52±38; 39 (17–126) | 44±30; 41 (7–113) | 0.6 |
| Rescue analgesic (fentanyl) consumption (μg) | 1040±760; 780 (340–2520) | 880±600; 820 (140–2260) | 0.6 |
| Fluid intake (mL) | 2258±368 | 2292±511 | 0.8 |
| Urine output (mL) | 1069±355 | 1084±376 | 0.9 |
| Satisfaction score | 6 (1–7) | 6 (3–7) | 0.9 |
| PONV (n) | 3 (10%) | 4 (14%) | 0.7 |
Continuous data expressed as mean±SD, median (min–max), or both. Qualitative events as number of patients [n (%)]. PCA: patient-controlled analgesia; PONV: postoperative nausea vomiting
Median pain scores on coughing at various times postsurgery did not differ between groups (p>0.1) (Figure 2). Although median pain score was higher in the EPI group than the IV group during the immediate postoperative period [5 (0–10) vs. 3 (0–9)], the difference did not reach significance (p>0.05). There was no significant difference in median patient satisfaction score between IV and EPI groups [6 (1–7) vs. 6 (3–7); p=0.9)] (Table 2). Similarly, there were also no significant group differences in HR, MAP and RR during the postoperative period (Figure 3).
Figure 2.
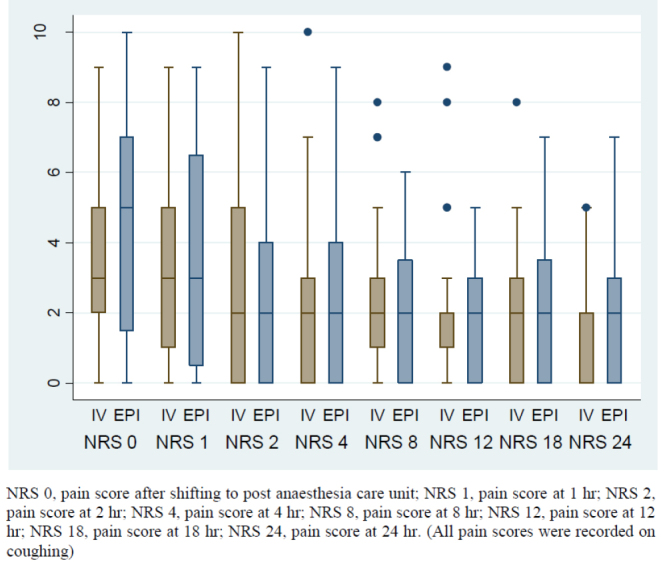
Box plot diagram showing median, IQR and range of pain scores (NRS) in the postoperative period in two groups
Figure 3.
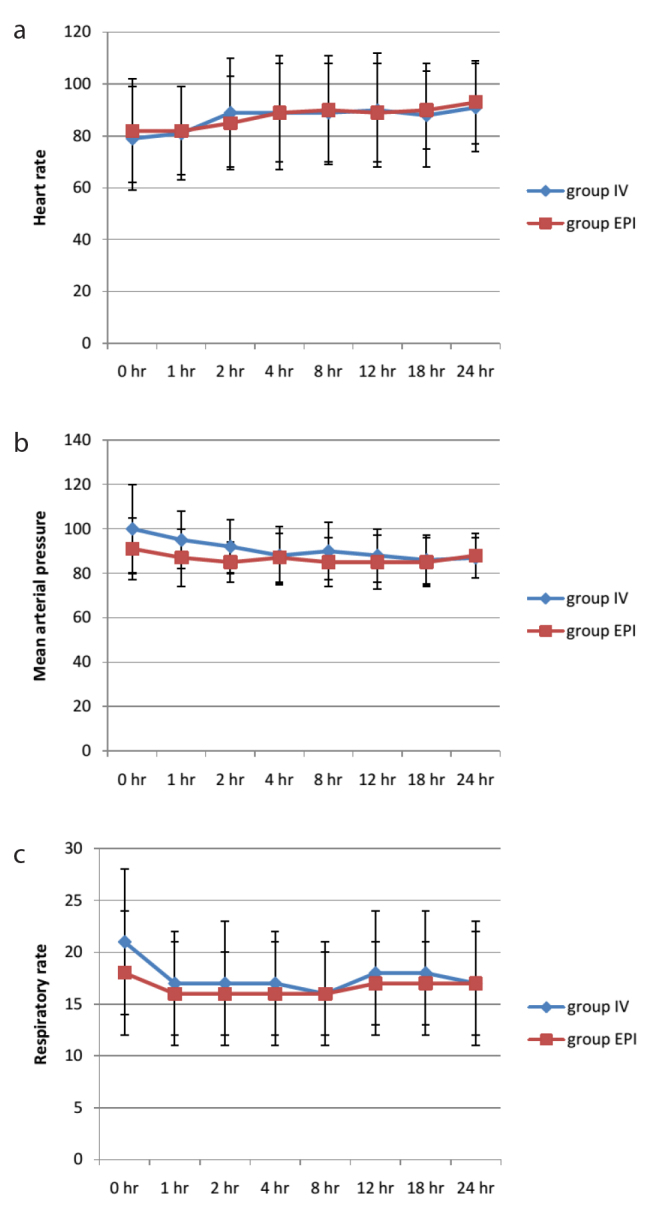
a–c. Mean heart rate, mean arterial pressure and respiratory rate in different postoperative time points in two groups
Intraoperative analgesic and clinical parameters
The median number of fentanyl rescue doses did not differ between IV and EPI groups [20 (0–100) mcg vs. 20 (0–125) mcg; p=0.4]. Similarly, mean HR, fluid intake, blood loss, hemodynamic response to surgical incision, extubation time and rocuronium use the during intraoperative period did not differ between groups (Table 3). Compared to the IV group, the EPI group demonstrated lower mean SBP (115±12 mmHg vs. 122±11 mmHg; p=0.028) and mean DBP (69±6 mmHg vs. 75±8 mmHg; p=0.004), while mean MAC hr value was significantly higher in the IV group than the EPI group (3.05±1.09 vs. 2.27±1.29; p=0.039). Intraoperative urine output was significantly lower in the EPI group than the IV group (254±212 mL vs. 359±191 mL; p=0.053) (Table 3), but output rate normalised to body weight did not markedly differ (1.47 mL kg−1 h−1 in IV group and 1.2 mL kg−1 h−1). Mean SPI level was significantly lower in the EPI group, but SPI was maintained between 40 and 60 in both groups, so the difference was not clinically significant (Table 3).
Table 2.
Comparison of intraoperative variables between analgesia groups
| Intraoperative parameter | IV group (n=29) | EPI group (n=28) | p |
|---|---|---|---|
|
| |||
| Mean HR (beats min−1) | 77±14 | 82±14 | 0.2 |
| Mean SBP (mmHg) | 122±11 | 115±12 | 0.028 |
| Mean DBP (mmHg) | 75±8 | 69±6 | 0.004 |
| Fluid Intake (mL) | 2245±847 | 2018±1037 | 0.4 |
| Urine output (mL) | 359±191 | 254±212 | 0.053 |
| Blood loss (mL) | 279±228 | 234±294 | 0.5 |
| Rescue fentanyl dose (mcg) | 20 (0–100) | 20 (0–125) | 0.4 |
| Mean MAC hour | 3.05±1.09 | 2.27±1.29 | 0.039 |
| HR changes on incision (beats min−1) | 3 (−14 to 22) | 2 (−14 to 35) | 0.7 |
| SBP changes on incision (mmHg) | 7 (−20 to 104) | 7.5 (−12 to 55) | 0.7 |
| DBP changes on incision (mmHg) | 8 (−10 to 62) | 6 (−4 to 45) | 0.3 |
| SPI | 55±7 | 50±8 | 0.021 |
| Extubation time (min) | 11±5 | 10±4 | 0.5 |
| Rocuronium use (mg h−1) | 23±6 | 26±8 | 0.1 |
| Intraoperative complications | |||
| No complication (n) | 16 (55) | 15 (54) | <0.001 |
| Bradycardia with hypotension (n) | 5 (17) | 2 (7) | |
| Hypertension requiring labetalol boluses (n) | 2 (7) | 0 | |
| Hypotension managed with mephenteramine boluses (n) | 5 (17) | 10 (36) | |
| Hypotension managed with noradrenaline infusion (n) | 1 (3) | 1 (4) | |
Data expressed as mean±SD, median (min–max), or number of patients [n (%)]. HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; SPI: surgical pleth index; MAC: minimum alveolar concentration
Adverse events and complications
One patient in the IV group developed hiccups and another developed sedation (RSS=4) following surgery. In the EPI group, one patient developed dizziness and one patient developed sedation (RSS=4). Three patients in the IV group and 4 in the EPI group developed PONV during the postoperative period (p=0.7) (Table 2).
During the intraoperative period, 5 IV group patients and 2 EPI group patients developed bradycardia and hypotension. Ten patients in the EPI group (36%) and 5 in the IV group (17%) developed hypotension requiring bolus mephenteramine (Table 3). Intraoperative hypertension requiring bolus labetalol was found in two IV group patients, whereas no patient in the EPI group exhibited intraoperative hypertension. The overall distribution of intraoperative complications differed significantly between groups (p<0.001). However, about half of the patients in both groups (16 in the IV group and 15 in the EPI group) exhibited no complications, including signs and symptoms of lignocaine toxicity in the IV group.
Discussion
This prospective randomised pilot study demonstrates that intravenous lignocaine–fentanyl infusion is equally effective for postoperative pain management during the first 24 h after major abdominal oncosurgery compared to traditional epidural ropivacaine–fentanyl infusion. Indeed, subjective pain scores and postoperative fentanyl requirements for rescue analgesia did not differ between analgesia groups. Moreover, overall adverse events frequency was similar, suggesting that intravenous lignocaine–fentanyl infusion is a safe and effective alternative to epidural ropivacaine–fentanyl infusion.
While this is the first study comparing intravenous lignocaine–fentanyl to epidural ropivacaine–fentanyl infusion for postoperative pain management, several previous reports have compared epidural LAs to intravenous lignocaine. Swenson et al reported equivalent rescue analgesic requirements and postoperative pain scores using epidural bupivacaine 0.125% and hydromorphone 6 μg mL−1 infused at 5 mL h−1 compared to intravenous lignocaine infusion at 1–2 mg min−1 (8). Alternatively, epidural LA has generally demonstrated superior analgesic efficacy compared to intravenous opioids (9–12). Epidural analgesia is also a safer technique for postoperative pain relief compared to intravenous opioids given the milder sedative and respiratory depressant effects (12). In our study, however, fentanyl was used at a reduced dose compared to previous trials (13, 14) as concomitant use of intravenous lignocaine is reported to reduce postoperative opioid requirements by approximately 50% (15). As a result, sedation was found in only one IV group patient and that instance was due to rescue fentanyl rather than basal lignocaine–fentanyl infusion. Therefore, intravenous lignocaine–fentanyl infusion appears no more likely to induce sedation than epidural ropivacaine–fentanyl infusion.
We also used lower doses of lignocaine. When used as the sole analgesic, intravenous lignocaine is administered as a 1.5–3 mg kg−1 bolus followed by 2–3 mg kg−1 h−1 infusion (8, 16, 17). In our study, an intravenous bolus of 1.5 mg kg−1 followed by 1 mg kg−1 h−1 infusion was administered intraoperatively until the completion of surgery, while only 0.5 mg kg−1 h−1 was infused during the postoperative period. Again, these lower lignocaine doses are possible due to the concomitant use of fentanyl.
During the intraoperative period, NIBP was significantly lower and bolus mephenteramine requirement to treat hypotension significantly higher in the EPI group (Table 3). However, the mean MAC hr of desflurane in the EPI group was significantly lower than in the IV group (Table 3). This discrepancy was probably due to a higher hypotension incidence in the EPI group, compelling the attending anaesthesiologists to maintain lower MAC. Moreover, other causes of hypotension like blood loss and compensatory fluid administration did not differ between groups (Table 3), so the higher incidence of hypotension in the EPI group was probably due to epidural local anaesthetic infusion. It was reported that haemodynamic instability associated with thoracic epidural analgesia can compromise enteric anastomosis and gastrointestinal recovery, suggesting that thoracic epidural analgesia may not be appropriate for pancreatoduodenectomy (18). In our study, urine output was significantly lower in the EPI group than the IV group, probably due to reduced perfusion pressure as there was no intergroup difference in intraoperative fluid administration or blood loss (Table 3). Despite these minor differences in physiological responses, intraoperative rescue fentanyl consumption was similar between the two groups, indicating equianalgesic potential of the two regimens during the intraoperative period.
Intravenous lignocaine–fentanyl infusion has several advantages over epidural analgesia while treating postoperative pain. For instance, routine use of anticoagulation for deep venous thrombosis (DVT) prophylaxis may reduce the safety of epidural catheter placement and removal. In addition, epidural placement carries serious risks of epidural hematoma formation, nerve injury and spinal cord injury. However, most of the ‘secondary effects’ of epidural LA (reduction of DVT, postoperative ileus, pulmonary embolism, myocardial infarction and stress response) have also been observed following systemic lignocaine administration (6, 19–23). Further, high-dose lignocaine can cause central nervous system and cardiac toxicity. Therefore, it is still critical to reduce lignocaine dose as in this study.
This study has several limitations. A minimum required sample size could not calculated due to the lack of similar comparative data. However, this new regimen (i.v. lignocaine–fentanyl) demonstrated analgesic efficacy comparable to the traditional epidural technique, suggesting sufficient efficacy and safety for clinical practice. Nonetheless, an adequately powered study is needed to assess differences in various clinical parameters. Concomitant use of fentanyl may mitigate some of the secondary benefits of intravenous lignocaine, so further research comparing these two analgesic techniques on primary outcomes like return of bowel function, length of hospital stay and incidence of DVT is required. Additional studies using different analgesic drug concentrations and enrolling other surgical populations are also required given that rescue fentanyl consumption was high in both groups. It is possible that patient heterogeneity influenced our results as we included all types of abdominal oncosurgery, although the distribution was comparable. The groups also differed in fentanyl infusion (5 mcg h−1 through the epidural route versus 0.25 mcg kg−1 h−1 through intravenous route), but this was to more accurately replicate the regimens used in routine clinical practice. We also used ropivacaine instead of lignocaine through the epidural route because ropivacaine is a commonly used local anaesthetic for epidural analgesia.
Conclusion
Intravenous lignocaine–fentanyl infusion appears to be an effective alternative to conventional epidural ropivacaine–fentanyl infusion for analgesia following major open abdominal oncosurgery. Further studies with adequate statistical power are needed to compare additional efficacy and safety outcomes.
Main Points.
Intravenous lignocaine-fentanyl has equivalent analgesic efficacy compared to gold standard epidural local anaesthetic after major abdominal oncosurgery.
Incidence of intraoperative hypotension is more when epidural local anaesthetic is used as analgesia.
Intravenous lignocaine can be administered safely in perioperative period specially when epidural catheter placement is difficult or contraindicated.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of All India Institute of Medical Sciences.
Informed Consent: Written informed consent was obtained from all subjects who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – R.N., S.M., R.G.; Design – R.N., S.M., R.G., V.K., N.G., S.J.B., S.B.; Supervision – R.N., S.M., R.G., V.K., S.J.B., N.G., S.B.; Resources – R.N., S.M., R.G.; Materials – R.N., S.M., R.G.; Data Collection and/or Processing – R.N., S.M.; Analysis and/or Interpretation – R.N., S.M., R.G., V.K., N.G., S.J.B., S.B.; Literature Search – R.N., S.M., R.G.; Writing Manuscript – R.N., S.M., R.G.; Critical Review – R.N., S.M., R.G., V.K., N.G., S.J.B., S.B.; Other – R.N., S.M., R.G., V.K., N.G., S.J.B., S.B.
Conflict of interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Sommer M, de Rijke JM, van Kleef M, Kessels AGH, Peters ML, Geurts JWJM, et al. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur J Anaesthesiol. 2008;25:267–74. doi: 10.1017/S0265021507003031. [DOI] [PubMed] [Google Scholar]
- 2.Moraca RJ, Sheldon DG, Thirlby RC. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg. 2003;238:663–73. doi: 10.1097/01.sla.0000094300.36689.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fotiadis RJ, Badvie S, Weston MD, Allen-Mersh TG. Epidural analgesia in gastrointestinal surgery. Br J Surg. 2004;91:828–41. doi: 10.1002/bjs.4607. [DOI] [PubMed] [Google Scholar]
- 4.Du KN, Feng L, Newhouse A, Mehta J, Lasala J, Mena GE, et al. Effects of Intraoperative Opioid Use on Recurrence-Free and Overall Survival in Patients With Esophageal Adenocarcinoma and Squamous Cell Carcinoma. Anesth Analg. 2018;127:210–6. doi: 10.1213/ANE.0000000000003428. [DOI] [PubMed] [Google Scholar]
- 5.Kranke P, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst Rev. 2015;7:CD009642. doi: 10.1002/14651858.CD009642.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Hollmann MW, Strümper D, Durieux ME. The poor man’s epidural: systemic local anesthetics for improving postoperative outcomes. Med Hypotheses. 2004;63:386–9. doi: 10.1016/j.mehy.2004.02.057. [DOI] [PubMed] [Google Scholar]
- 7.Dale GJ, Phillips S, Falk GL. The analgesic efficacy of intravenous lidocaine infusion after laparoscopic fundoplication: a prospective, randomized, double-blind, placebo-controlled trial. Local Reg Anesth. 2016;9:87–93. doi: 10.2147/LRA.S119483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swenson BR, Gottschalk A, Wells LT, Rowlingson JC, Thompson PW, Barclay M, et al. Intravenous lidocaine is as effective as epidural bupivacaine in reducing ileus duration, hospital stay, and pain after open colon resection: a randomized clinical trial. Reg Anesth Pain Med. 2010;35:370–6. doi: 10.1097/AAP.0b013e3181e8d5da. [DOI] [PubMed] [Google Scholar]
- 9.Wu CL, Cohen SR, Richman JM, Rowlingson AJ, Courpas GE, Cheung K, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103:1079–88. doi: 10.1097/00000542-200511000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA, Wu CL. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;290:2455–63. doi: 10.1001/jama.290.18.2455. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Wang C, Xu C, Cai Q. Influence of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia on postoperative pain control and recovery after gastrectomy for gastric cancer: a prospective randomized trial. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2013;16:193–200. doi: 10.1007/s10120-012-0168-z. [DOI] [PubMed] [Google Scholar]
- 12.Moslemi F, Rasooli S, Baybordi A, Golzari SEJ. A Comparison of Patient Controlled Epidural Analgesia With Intravenous Patient Controlled Analgesia for Postoperative Pain Management After Major Gynecologic Oncologic Surgeries: A Randomized Controlled Clinical Trial. Anesthesiol Pain Med. 2015;5:e29540. doi: 10.5812/aapm.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo A, Grieco DL, Bevilacqua F, Anzellotti GM, Scarano A, Scambia G, et al. Continuous intravenous analgesia with fentanyl or morphine after gynecological surgery: a cohort study. J Anesth. 2017;31:51–7. doi: 10.1007/s00540-016-2268-0. [DOI] [PubMed] [Google Scholar]
- 14.Hiro K, Sugiyama T, Kurata M, Oi Y, Okuda M. Postoperative Analgesia for Video-assisted Thoracoscopic Surgery--Continuous Intravenous Infusion of Fentanyl Combined with Intercostal Nerve Block v.s. Continuous Epidural Analgesia. Masui. 2016;65:114–8. [PubMed] [Google Scholar]
- 15.Kaba A, Laurent SR, Detroz BJ, Sessler DI, Durieux ME, Lamy ML, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106:11–8. doi: 10.1097/00000542-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kuo CP, Jao SW, Chen KM, Wong CS, Yeh CC, Sheen MJ, et al. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth. 2006;97:640–6. doi: 10.1093/bja/ael217. [DOI] [PubMed] [Google Scholar]
- 17.Staikou C, Avramidou A, Ayiomamitis GD, Vrakas S, Argyra E. Effects of intravenous versus epidural lidocaine infusion on pain intensity and bowel function after major large bowel surgery: a double-blind randomized controlled trial. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2014;18:2155–62. doi: 10.1007/s11605-014-2659-1. [DOI] [PubMed] [Google Scholar]
- 18.Pratt WB, Steinbrook RA, Maithel SK, Vanounou T, Callery MP, Vollmer CM. Epidural analgesia for pancreatoduodenectomy: a critical appraisal. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2008;12:1207–20. doi: 10.1007/s11605-008-0467-1. [DOI] [PubMed] [Google Scholar]
- 19.Groudine SB, Fisher HA, Kaufman RP, Patel MK, Wilkins LJ, Mehta SA, et al. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg. 1998;86:235–9. doi: 10.1097/00000539-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Tikuišis R, Miliauskas P, Samalavičius NE, Žurauskas A, Samalavičius R, Zabulis V. Intravenous lidocaine for post-operative pain relief after hand-assisted laparoscopic colon surgery: a randomized, placebo-controlled clinical trial. Tech Coloproctology. 2014;18:373–80. doi: 10.1007/s10151-013-1065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herroeder S, Pecher S, Schönherr ME, Kaulitz G, Hahnenkamp K, Friess H, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007;246:192–200. doi: 10.1097/SLA.0b013e31805dac11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park WY, Thompson JS, Lee KK. Effect of epidural anesthesia and analgesia on perioperative outcome: a randomized, controlled Veterans Affairs cooperative study. Ann surg. 2001;234:560–9. doi: 10.1097/00000658-200110000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holte K, Kehlet H. Epidural analgesia and risk of anastomotic leakage. Reg Anesth Pain Med. 2001;26:111–7. doi: 10.1053/rapm.2001.21241s. [DOI] [PubMed] [Google Scholar]


