Abstract
Significance: Alzheimer's disease (AD) is the most common cause of dementia in the elderly. AD is currently ranked as the sixth leading cause of death, but some sources put it as third, after heart disease and cancer. Currently, there are no effective therapeutic approaches to treat or slow the progression of chronic neurodegeneration. In addition to the accumulation of amyloid-β (Aβ) and tau, AD patients show progressive neuronal loss and neuronal death, also high oxidative stress that correlates with abnormal levels or overload of brain metals.
Recent Advances: Several promising compounds targeting oxidative stress, redox metals, and neuronal death are under preclinical or clinical evaluation as an alternative or complementary therapeutic strategy in mild cognitive impairment and AD. Here, we present a general analysis and overview, discuss limitations, and suggest potential directions for these treatments for AD and related dementia.
Critical Issues: Most of the disease-modifying therapeutic strategies for AD under evaluation in clinical trials have focused on components of the amyloid cascade, including antibodies to reduce levels of Aβ and tau, as well as inhibitors of secretases. Unfortunately, several of the amyloid-focused therapeutics have failed the clinical outcomes or presented side effects, and numerous clinical trials of compounds have been halted, reducing realistic options for the development of effective AD treatments.
Future Directions: The focus of research on AD and related dementias is shifting to alternative or innovative areas, such as ApoE, lipids, synapses, oxidative stress, cell death mechanisms, neuroimmunology, and neuroinflammation, as well as brain metabolism and bioenergetics.
Keywords: Alzheimer's, neurodegeneration, oxidative stress, ferroptosis, redox, metals
Introduction
Alzheimer's disease (AD) is a chronic degenerative brain disease and the most common cause of dementia in the elderly (sporadic late-onset AD). AD stands as the sixth leading cause of death in the United States and is currently incurable with no effective therapeutic approaches to treat, reverse, or slow down its progression (9, 102). The real cause of AD is still a controversy, but old age is the most significant risk factor. Etiology of AD is a complex combination of behavioral, genetic, and environmental risk factors that derive in pathogenic mechanisms, most notably the abnormal accumulation of amyloid-β (Aβ), hyperphosphorylated tau, neuroinflammation, progressive synaptic loss, and neuronal death. Consequently, therapeutic strategies have focused on the key components of the amyloid cascade: Aβ and tau. Small drugs and immunotherapies have been designed to inhibit Aβ production or aggregation, clearance or neutralization of neurotoxic Aβ oligomers/plaques, blocking extracellular spread and toxicity of tau (67).
In almost three decades of research, only five prescription drugs have been approved by the Food and Drug Administration (FDA) for AD, and none are based on amyloid or tau (Table 1) (2a). Although none of these medications cures or stops the disease, they are prescribed to alleviate dementia symptomatology, including memory loss and confusion. Patients diagnosed with mild to moderate AD receive galantamine, rivastigmine, and donepezil, whereas memantine and memantine/donepezil are prescribed to treat moderate to severe AD.
Table 1.
Food and Drug Administration Approved Medications for Alzheimer's Diseasea
| Drug | Mechanism of action | Dosage | Side effects |
|---|---|---|---|
| Donepezil | Cholinesterase inhibitor | Oral 5–10 mg/day, maximum 23 mg/day | Nausea, vomiting, diarrhea, muscle cramps, fatigue, weight loss. |
| Rivastigmine | Cholinesterase inhibitor | Oral 3–12 mg/day | Nausea, vomiting, diarrhea, weight loss, indigestion, muscle weakness. |
| Patch 4.6–13.3 mg/day | |||
| Galantamine | Cholinesterase inhibitor | Oral 8–24 mg/day | Nausea, vomiting, diarrhea, loss of appetite, dizziness, headache. |
| Memantine | NMDA antagonist | Oral 5–28 mg/day | Dizziness, headache, diarrhea, constipation, confusion. |
| Memantine+Donepezil | NMDA antagonist and cholinesterase inhibitor | Variable (7/10, 14/10, 21/10 or 28/10 mg) depending on whether patients are stabilized on memantine and/or donepezil. | Nausea, vomiting, diarrhea, headache, dizziness, loss of appetite. |
Ref. (2a).
NMDA, N-methyl-d-aspartate.
Unfortunately, more than 200 phase II/III clinical trials assessing more than 100 different drugs have dramatically failed, with the cost of several billion dollars and decades of research (67, 115). Some methodological factors in the design of AD clinical trials have been correlated with the unsuccessful outcomes rather than the effectiveness of the drug itself, including inadequate dosing, recruitment/selection of patients, inconsistency in cognitive scoring procedures, inappropriate time of intervention, and assessment of target engagement, among others (67, 115). This raises the question as to whether current therapies are targeting the wrong pathological components or whether a multitarget approach is needed, for effective disease modification rather than symptom remission. Herein, we review therapeutic approaches for AD and related dementia that identify alternative targets and brain mechanisms beyond the centralized Aβ and tau strategies. Some of them are in early or conceptual stages, tested on in vitro or animal models, and others are already under scrutiny in clinical trials.
Alterations of Redox Homeostasis
The course of AD is accompanied by a progressive alteration or detriment of brain metabolism. Patients with AD present alterations of the neuroendocrine system, glucose metabolism, antioxidants, neuroinflammation, and reactive oxygen species (ROS), affecting cognitive functions, behavior, and functionality of the brain. Overall, this state is commonly known as oxidative stress, characterized by an impaired response to oxidant or electrophile stress (Fig. 1). Oxidative stress affects many metabolic pathways in the brain; the interventions that counteract oxidative damage include antioxidants, polyphenols, glucose metabolism, diet, and lifestyle (18).
FIG. 1.
Imaging of ROS in neurons. Live cell imaging of N27 rat dopaminergic neurons treated with 250 μM of ammonium iron (III) citrate for 3 h. (A) Bright-field imaging. (B) DCFDA fluorescence as an indicator of ROS in cells. DCFDA, 2′,7′-dichlorofluorescin diacetate; ROS, reactive oxygen species. Color images are available online.
Antioxidants
Vitamin E (α-tocopherol) has been proposed as a neuroprotective agent and an antioxidant. Supplementation of vitamin E in AD models reduced deficits of learning and memory, attenuation of oxidative stress, decreased Aβ deposits, and improved cognition (75). Trials with AD patients receiving doses of 800, 1000, or 2000 IU/day of vitamin E for 6–48 months showed a slow functional decline in comparison with placebo, lower oxidative stress but no effects over the progression from mild cognitive impairment (MCI) to AD (51, 75). This antioxidant is also supplemented in combination with selenium or memantine, but with no significant changes in dementia progression, suggesting that vitamin E is beneficial only in mild-to-moderate AD to reduce functional decline (47, 97).
The most extensively consumed antioxidant is vitamin C (ascorbic acid). Ascorbic acid supplementation showed neuroprotective properties, reducing free radicals and neuroinflammation, also iron chelation and Aβ reduction (118). Ascorbate participates in neuronal maturation and differentiation, myelin formation, modulation of neurotransmission, and redox homeostasis (95). APP/PSEN1 mice with vitamin C deficiency showed increased levels of oxidative stress (malondialdehyde, protein carbonyls, F2-isoprostanes) in the brain cortex and decreased glutathione (GSH) compared with wild-type controls and also increased levels of Aβ (38). In a randomized phase I clinical trial with 78 subjects (NCT00117403), supplements were administered for 16 weeks, 800 IU/day of vitamin E + 500 mg/day of vitamin C + 900 mg/day of α-lipoic acid or 400 mg/day CoQ; the compounds did not affect cerebrospinal fluid (CSF) AD biomarkers (Aβ or tau) but caused reduction of oxidative stress. A longitudinal study of 600 elderly patients reported that around 8% of the subjects used vitamins C and/or E (normal dose or high dose), but the vitamins showed no correlation to reduce the time to develop dementia (52).
Vitamin B supplementation in elderly patients may reduce cognitive decline, by lowering levels of serum homocysteine, which has been linked as a potential risk factor for cognitive impairment. Clinical trials in phase III (NCT00056225) evaluating the efficacy of high-dose vitamin B12 (1 mg/day), B6 (25 mg/day), and B9 (folic acid, 5 mg/day) with AD patients reported reduction in homocysteine levels, but no significant improvement in cognition (6, 188). Some adverse effects of depression were observed in patients taking vitamin B. Vitamin supplementation can be included in nutritional formulations enriched in folate, α-tocopherol, B12, S-adenosyl methionine, N-acetyl cysteine, and acetyl-l-carnitine; in a phase II trial, the formulation was administered for 3–6 months to 106 individuals with AD (NCT01320527). The evaluation indicated that the formulation helped to maintain or improve cognitive performance and mood behavior (142). Vitamins B1/B6/B9/B12 modify brain metabolism, oxidative stress, inflammation, and cognition in AD. A 3-month pilot study of vitamin supplementation reduced levels of oxidized proteins (carbonyl groups) (145), whereas folic acid (1.25 mg/day for 6 months) reduced Aβ and inflammation biomarkers (TNFα, IL6) (23). Remarkably, a high B-vitamin dose for 2 years (folic acid 0.8 mg, vitamin B6 20 mg, vitamin B12 0.5 mg) slowed brain shrinkage and atrophy (45).
Serum levels of carotenoids in AD patients indicated that β-carotene was significantly lower in the demented group, whereas α-carotene was similar in comparison with controls (88). This deficiency could be related to a dietary deficiency, since AD patients usually have a weight and body mass index lower than controls. Low levels of β-carotene correlate with higher levels of CSF Aβ and tau and increased oxidative stress (165). The oxidative damage is related to low levels of blood antioxidants (uric acid, vitamins, and carotenes), since none of the major antioxidant enzymes is significantly decreased in AD (150). Remarkably, institutionalized AD patients receiving nutritional supplements showed reduced morbidity and mortality (65).
Caffeine has antioxidant properties, and its consumption reduced Aβ in an AD mouse model. The administration of 0.5 and 30 mg/day of caffeine was shown to have important effects in the sporadic AD-like rabbit model with a cholesterol-enriched diet, including reduction of cholesterol-induced Aβ levels and p-tau, control oxidative stress, and normalize levels of adenosine A(1) receptors (136). Lifelong consumption of caffeinated drinks (coffee and tea) has been linked with the prevention of cognitive decline and reducing the risk of neurodegeneration, but the recommended intake should be around 200–400 mg (2.5–5 cups of coffee) daily (122). Other benefits of caffeine could be linked to increasing alertness, concentration, improved mood, reduced depression, and even potentiation of the effect of other regular drugs such as analgesics. Caffeine trials have determined a lack of association with the prevention of AD and related dementia, but others showed the potential benefits of coffee, tea, and caffeine consumption (131). New studies testing the potential uses of caffeinated products for symptomatology control or prevention of AD will continue over the next few years.
Production of melatonin (N-acetyl-5-metoxytryptamine) is reduced with aging, suggesting that this decline is related to AD progression. Its mechanism of action is by stimulation of nonamyloidogenic processing of AβPP, activation of α-secretase, and downregulation of β- and γ-secretases (157). The neuroprotective effect of melatonin is an antioxidant, free radical scavenger, and mitochondrial protectant, stimulating the synthesis of antioxidant enzymes (SOD, GPx, and glutathione reductase) and production of glutathione, modulating aggregation of Aβ and tau, and regulating tau phosphorylation (10, 130). APP/PS1 mice supplemented with melatonin for 12 months showed a significant reduction of plasma levels and Aβ deposits (126). Clinical trials using melatonin (50–100 mg/day) for 10 days to 24 weeks showed safety, but no improvement of cognitive abilities in AD patients only improved sleep quality (178).
Among the herbal extract used for cognitive disorders and AD, Ginkgo biloba is one of the most popular. Standardized G. biloba extract is a supplement with antioxidant properties, improving memory and cognitive functions (161). In cultured neurons, Neuro2A overexpressing AβPP G. biloba extract was added at 100 μg/mL for up to 10 h, observing a reduction of ROS and malondialdehyde, enhancement antioxidant enzymes, and reduction of Aβ (24). The standardized extract of G. biloba was administered to rats, observing an increase in catalase and superoxide dismutase (SOD) activities in the hippocampus, striatum, and substantia nigra, and reduced lipid peroxidation, reducing overall oxidative damage (16). At least 21 clinical trials have been performed for G. biloba, indicating potential benefits in cognitive functions but with inconsistencies among the trials and with mild adverse events (186). A most recent trial with standardized G. biloba extract (EGb 761) tested its effectiveness for treating behavioral and psychological symptoms of dementia; after 22–24 weeks with a daily dose of 240 mg, the patients showed better results in symptom scores except for delusions, hallucinations, and elation/euphoria (148). The efficacy of G. biloba in AD remains controversial but it could be beneficial to control symptoms.
Polyphenols
Epigallocatechin-3-gallate (EGCG or Sunphenon EGCg) is the key bioactive polyphenolic flavonoid from green tea, with antioxidant, anti-inflammatory, and neuroprotective effects. The APP/PS1 mice treated with EGCG and/or ferulic acid (30 mg/kg) showed reversed cognitive impairment and reduced Aβ, promoting nonamyloidogenic AβPP processing, reducing neuroinflammation and oxidative stress (119). EGCG treatment decreased p-tau in rat primary cortical neurons, this independent of Nrf2 activation but with enhanced autophagy (26). Preclinical and translational studies of EGCG have shown anti-inflammatory and neuroprotective effects against neuronal damage and brain edema, but there are reports of a negative association between green tea and the prevalence of MCI (20). The benefits of EGCG seem to be related to improving mood and work performance, modulation of cerebral blood flow, and reduced stress. A phase III trial is testing the safety and tolerability of Sunphenon EGCg (high-purity EGCg, NCT00951834) with a dosage of 200–800 mg, but no results are published.
Resveratrol (trans-3,4′,5-trihydroxystilbene) is a polyphenol with antioxidant properties found in red grapes (wine), blueberries, peanuts, soybeans, pomegranates, and dark chocolate. The mechanisms attributed to resveratrol are extensive, from cell signaling in cytokines, caspases, metalloproteins, inflammation, and glucose metabolism, among others. The utility of resveratrol for treating oxidative stress in neurodegeneration was tested in mice that were fed with 300 mg/kg resveratrol for 45 days, observing reduced Aβ plaque formation (48% reduction in the cortex, 89% reduction in the striatum, and 90% reduction in the hypothalamus), but glutathione declined by 21% and cysteine increased by 54% (90). A phase II trial with 119 participants analyzed a 500 mg/day of resveratrol; the supplement was safe and tolerated (with minor side effects); and after 1 year, the CSF Aβ and plasma Aβ showed a decline. Interestingly, the group that received resveratrol showed increased brain volume loss (169). Resveratrol has been extensively tested with 244 trials; in 2019, there were 27 ongoing clinical trials (phase III for AD and phase IV for MCI) (160).
Curcumin is a polyphenol from turmeric herb. Its properties include inhibition of Aβ aggregation, reduction of p-tau, copper-binding, reduction of cholesterol, microglia modulation, and antioxidant and anti-inflammatory effects (141, 167). In vitro and animal studies with curcumin determined its utility as an antioxidant and anti-inflammatory agent, but clinical trials showed limited effects due to its low solubility and bioavailability (69). The elderly receiving 1500 mg/day of curcumin for 12 months showed no significant differences between treated and placebo, as measured by cognitive tests (139). At least six clinical trials have used curcumin (alone or combined), reaching phase II.
Glucose metabolism
The brain requires a high amount of energy in the form of ATP, but in MCI and AD, glucose metabolism is significantly impaired contributing to oxidative damage (18). The therapeutic strategy has been to repurpose antidiabetics such as intranasal insulin, pioglitazone, rosiglitazone, metformin, sitagliptin, and liraglutide.
Intranasal insulin (commercial names: Detemir, Levemir, Humulin, Novolin) is currently in phase II/III for AD and phase II for MCI. A pilot study monitored the effects of 8 weeks of intranasal insulin (4 × 40 IU/day), indicating enhanced mood, improving memory, and the absence of systemic side effects (12). A phase II trial (NCT00438568) with 104 participants tested 20–40 IU of insulin for 4 months, indicating improved memory or preserved general cognition; CSF biomarkers did not change, but in an exploratory analysis, the ratio of tau/Aβ changed in insulin-treated patients (31). The response to insulin seems to differ by gender and ApoE genotype; both men and women showed cognitive improvement with low-dose insulin, but only men responded positively to high-dose insulin. Remarkably, men with ApoE4− improved but women with ApoE4− did not, whereas all ApoE4+ remained cognitively stable (28). Pioglitazone (commercial names: AD4833, Actos, Glustin, Piozone) is an insulin sensitizer or PPARγ agonist. Phase II trial NCT00982202 with 29 nondiabetic AD subjects was shown to be safe and well tolerated with a dose of 45 mg/day (58, 64). A phase III trial is ongoing to confirm observations of the potential use of pioglitazone for MCI and AD. Rosiglitazone (commercial name: Avandia) is another antidiabetic compound or PPARγ agonist that reached phase III until its discontinuation by GlaxoSmithKline due to no significant improvement and side effects. Two phase III studies evaluated its efficacy and safety (2–8 mg), indicating no statistical differences in cognition or global function between groups, with adverse events of edema in 14%–19% of patients (NCT00428090 and NCT00550420) (66, 79). Metformin is a common drug prescribed for type 2 diabetes. The antioxidant properties of the drug were probed in neuronal PC12 cells and primary hippocampal neurons dosed with H2O2, observing a reduction of cell death, ROS, and mitochondrial damage (192). A pilot study with 80 patients with MCI tested 1000 mg metformin twice per day for 12 months, showing differences in ADAS-Cog but no significant differences in memory, glucose uptake PET, or plasma Aβ (106). A crossover study with 20 nondiabetic subjects not only explored an 8-week dosage of metformin, showing safety and tolerability, but also improved executive functioning, learning/memory, and attention (96). Sitagliptin is a dipeptidyl peptidase (DPP4) inhibitor, with antioxidant, anti-inflammatory, and antiapoptotic properties (182). The APP/PS1 mice treated with 20 mg/kg of sitagliptin for 8 weeks showed reduced Aβ deposition and protected cognitive function (43). A pilot study with 205 elderly diabetic patients with or without cognitive impairment showed improvement of cognitive function after a 6-month treatment with sitagliptin, comparable to metformin treatment (83). The glucagon-like peptide receptor stimulating drug Liraglutide (commercial names: Victoza) works by modulating endoplasmic reticulum stress response and stimulation of autophagy (129). An intervention study with AD patients treated for 6 months with liraglutide showed no effects on Aβ deposition (48). In another trial, 38 AD patients received a 26-week dose of liraglutide. The treatment prevented cognitive impairment but with no effects on Aβ deposition or cognition (63). There is an active phase II trial of liraglutide (NCT01843075) with 204 patients that is monitoring cerebral glucose metabolic rate and Aβ and tau by MRI.
Lifestyle interventions in AD
Nonpharmacological therapies or lifestyle interventions aim at preventing or slowing AD progression and reducing symptomatology, proposed as alternatives to pharmacological therapies (Fig. 2).
FIG. 2.
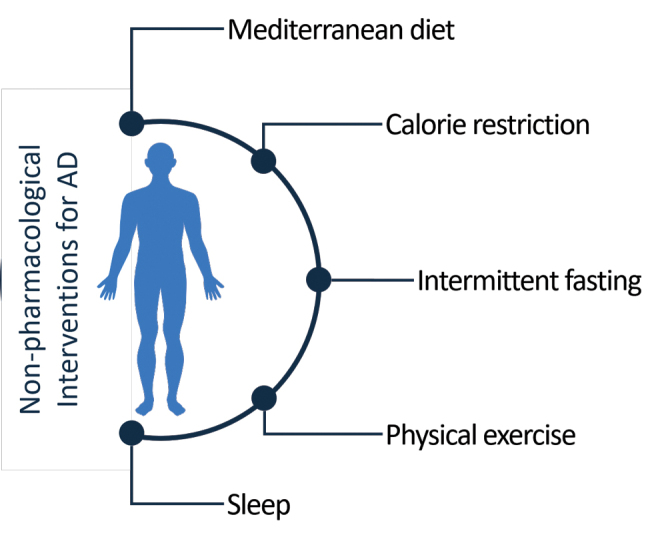
Lifestyle nonpharmacological interventions for AD. AD, Alzheimer's disease. Color images are available online.
The Mediterranean diet has been correlated with a lower risk of chronic diseases, cardiovascular disease, cancer, and lower mortality. A community-based study monitored 2258 individuals for 4 years, and the subjects following a Mediterranean diet showed a lower risk for AD (149). The Mediterranean diet includes a variety of fresh fruits and vegetables, olive oil, fish, and moderate wine intake that contribute essential vitamins, minerals, polyphenols, and unsaturated fatty acids that may reduce oxidative stress and inflammation (116). A cohort of 82 elderly patients was examined: The group following the Mediterranean diet showed better learning and memory performance and even neuroimaging indicated that dentate gyri were larger in comparison with the control group (89). Interestingly, a change from a traditional Japanese diet to a Western diet increased the prevalence of AD, increasing rates from 1% in 1985 to 7% in 2008 (40) and reaching 11.3% in 2012 (128). The Western diet has been linked to a higher risk of cognitive decline and dementia: This diet is high in red meat, processed meat, processed foods, saturated fat, and fried foods. This dietary pattern showed a high correlation with neurodegeneration, including elevated amyloid levels, neuritic plaques, and small vessel disease (5, 49). Caloric restriction is defined as a dietary regime with a strong limitation on calorie intake without facing a lack of nutrients or malnutrition. A reduction in calories is linked with an extended lifespan, slowdown of aging, improved memory, and reduction of AD progression (170). Intermittent fasting is an eating pattern in which individuals go over extended periods (usually 16–48 h) with little or no food intake followed by a period of normal food intake (113). Animal models under intermittent fasting demonstrated benefits in age-related chronic diseases, such as diabetes, cardiovascular, cancer, and neurological disorders including AD. This, by activation of adaptive cellular stress signaling pathways, enhances mitochondrial metabolism, promoting DNA repair and autophagy (113). An AD rat model under intermittent fasting (3 h per day of food consumption) showed elevated fat oxidation as an energy source, decreased serum glucose levels, lowered cortisol levels, and overall preserved spatial memory function compared with normal diet (156). Physical exercise has many health benefits and may reduce the risk of developing chronic diseases, but there is no conclusive evidence to confirm that physical exercise prevents MCI or AD. Nevertheless, cumulative evidence from observational studies suggests the benefits of exercise for the brain, including slowdown of cognitive decline and fewer Aβ plaque and tau tangles (121a). Trials with MCI or AD patients demonstrated that physical activity may have a positive impact on the development of the disease, but until now there is inconclusive evidence that exercise improves cognition in AD (19). Sleep changes correlate with AD pathology (64a). Alterations in quality sleep have an inverse relationship with cognitive impairment, and it may be an early indicator of AD and Aβ/tau deposition (105). In particular, chronic sleep deprivation increases Aβ plaques and may promote tau accumulation (80).
Biometals in the AD Brain
Iron and copper are essential metals required for normal brain function: They serve mainly as co-factors in the synthesis of neurotransmitters, oxygen transportation, synapses, and ATP production. The course of AD seems to be correlated with a marked dyshomeostasis or overload of metals, causing oxidative stress (Figs. 3 and 4). Amyloid plaques can deposit or accumulate Fe and Cu, and this phenomenon is more evident when compared with healthy age-matched individuals (Fig. 4) (162). Therapeutic strategies to deal with abnormalities of metals in the brain have been proposed and tested in AD models, at both preclinical and clinical levels. These are described as metal-protein-attenuating compounds or as metal chelators.
FIG. 3.
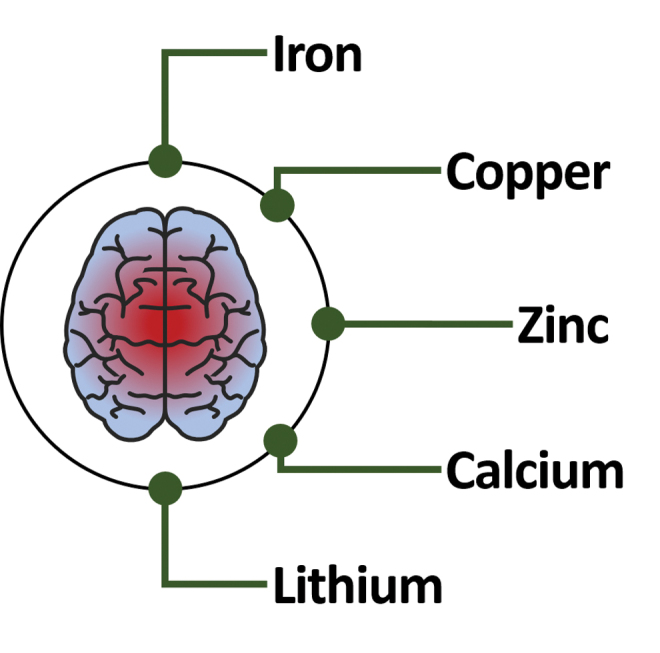
Biometals in the brain. Human brain affected by AD suffers from altered homeostasis of essential biometals such as iron, copper, zinc, and calcium, affecting the overall redox state of the neurons. Color images are available online.
FIG. 4.
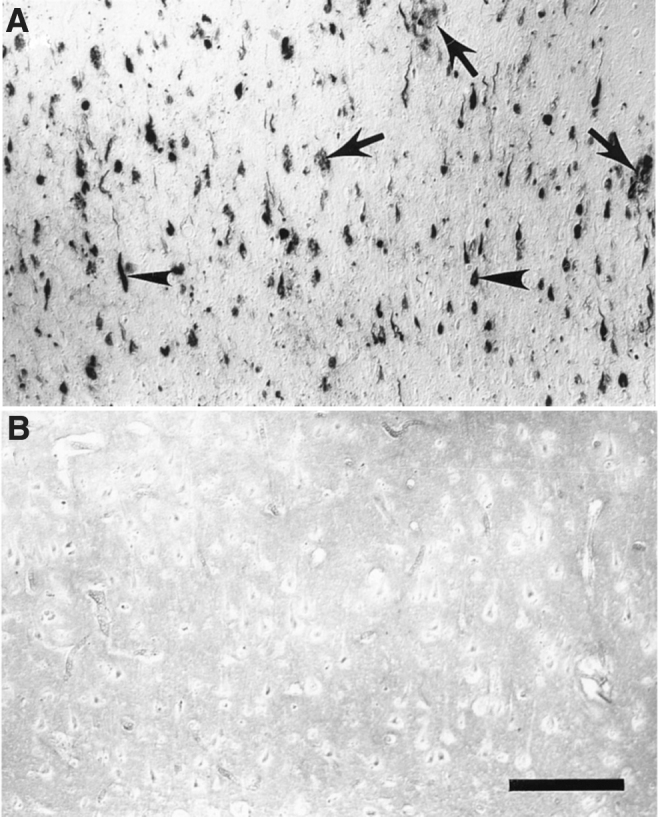
Histochemical location of redox-active iron in AD tissue sections. (A) Tissue section from AD brain. (B) Control. Arrowheads indicate neurofibrillary tangle and arrows indicate amyloid senile plaques. Scale bar = 200 μm. From Smith et al. (162).
Iron dyshomeostasis
The iron chelator deferoxamine or desferrioxamine was tested in AD patients (125 mg twice daily for up to 24 months), showing a significant reduction in progression, but no improvement in cognition or memory and presenting side effects such as appetite and weight loss (32). Intranasal deferoxamine in mice and rat models showed inhibition of Aβ aggregation and decreased memory loss (54, 76). Deferoxamine decreases protein oxidation and promotes the expression of the insulin receptor in the brain (53). The APP/PS1 mice treated with deferoxamine showed a reduction of Aβ and neuronal death accompanied by microglial activation (191).
Deferasirox and deferiprone have been used for iron chelation therapy in neurodegeneration. Deferasirox administered for 4 months to aged rats caused a reduction in iron accumulation and expression of TfR1 and ferritin, also reducing Aβ, oxidative stress, and inflammation biomarkers (11). Treatment with 10–50 mg/kg/day of deferiprone in rabbits caused a reduction in Aβ, BACE1, and p-tau, but no reduction in ROS (137). Deferiprone is currently under clinical trial (phase II, NCT03234686) with 171 subjects with prodromal AD or mild AD receiving 15 mg/kg to test the effect in cognitive decline. Similarly, deferiprone followed a phase II trial (10–15 mg/kg, for 6 months), showing to be well tolerated and reducing iron accumulation, but with no significance in the improvement of motor scores or cognitive function (110).
Clioquinol (iodochlorhydroxyquin or PBT1) has been proposed as an AD treatment. Its activity is through disruption of the interactions between Aβ and metal ions (copper and zinc) and, consequently, reducing Aβ plaques and oxidative stress. Preclinical studies with transgenic AD mice showed a 49% reduction in Aβ deposition after a 9-week course of clioquinol treatment and benefits in memory (25, 73). A phase II trial with 36 subjects (125–375 mg twice daily, 36 weeks) showed tolerability, reduction in Aβ, no effect in cognition, and no effect in plasma copper levels (143). Since clioquinol showed no significant effect in cognition, the phase III trial was terminated due to the presence of toxic contaminants and side effects (147).
PBT2 is an 8-hydroxyquinoline analog with activity as a translocator of copper and zinc and reducing Aβ. The preclinical analysis of PBT2 (30 mg/kg/day) restored dendritic spine density and reduced 13% Aβ (3, 35). A phase II trial with 78 patients (50–250 mg/day) revealed safety, reduction of Aβ, and improvement in cognition (98). A follow-up phase II trial with 40 subjects over 12-month treatment (250 mg/day) confirmed safety and tolerability, but with no significant difference in Aβ (174).
Copper dislocation
Lipophilic copper-containing complexes have been tested for antibacterial, antimycotic, or anticancer properties, but copper bis(thiosemicarbazonate) complex (Cu-GTSM) was explored as a neuroprotective agent. In vitro analysis revealed that Cu-GTSM promoted neurogenesis and neurite elongation (14), and a dose-dependent reduction of Aβ and p-tau, with an increase of intracellular copper (34, 44). A similar compound Cu-ATSM [diacetylbis(N(4)-methylthiosemicarbazonato) copper(II)] showed anti-inflammatory effects on microglia and astrocytes (27), but with limited effectiveness in AD mice (2). Cu(II)-orotate-dihydrate was tested in mild AD patients during a phase II trial. After 12-month administration of 8 mg, this compound reduced Aβ by 30%, but without effect on tau or cognition (92, 93).
Aggregation of Aβ is influenced by Fe and Cu. The metal-chelating tripeptide GHK (glycyl-l-histidyl-l-lysine) is a copper-binding ligand with antiaging properties (134). GHK interacts with Aβ and sequesters Cu2+ to prevent the formation of toxic aggregates and ROS (140). In fact, the Aβ aggregates contain copper and these are destabilized with chelators. We tested bathocuproinedisulfonic acid (BCDS) and bicinchoninic acid (BCA) chelators with amyloid plaque cores. The presence of both Cu chelators caused disassembly of high-order amyloid structures, obtaining mainly small and fibrillar aggregates (Fig. 5). BCDS binds Cu+, whereas BCA interacts with Cu2+; the fact that both compounds showed activity indicates that copper is present in both oxidation states within amyloid plaques. Interruption of Aβ–Cu interactions is an alternative approach to avoid or reduce the formation of neurotoxic protein aggregates. For example, APP/PS1 mice treated with BCDS showed nonamyloidogenic processing of AβPP and reduced Aβ deposition (179). A pilot study with 34 AD subjects testing the copper-chelating agent d-penicillamine showed that a 6-month treatment reduced oxidative stress but with no differences in cognitive decline (164). Triethylenetetramine (TETA or trientine) was approved for the treatment of Wilson's disease and diabetes: This compound is a Cu(II) chelator with potential therapeutic uses in AD. In APP/PS1 mice, a 3-month treatment of TETA reduced Aβ deposition and synapse loss (29, 175). In the same direction, tetrathiomolybdate [(NH4)2MoS4] is a copper-chelating agent approved for Wilson's disease. This compound reduced inflammation biomarkers and increased SOD activity (146). In Tg2576 mice, the treatment with tetrathiomolybdate reduced copper in the brain and reduced Aβ deposition; here, the compound was proposed as prevention and not a treatment for AD (138). Similarly, APP/PS1 mice receiving tetrathiomolybdate showed a lower formation of Aβ plaques (179).
FIG. 5.
Amyloid plaque cores treated with Cu chelators. Electron microscopy imaging of (A) amyloid-β plaque without Cu chelators, (B) amyloid-β plaques with bathocuproine disulfonic acid, (C) amyloid-β plaques with bicinchoninic acid.
Zinc deficiency, calcium, and lithium
A marked zinc deficiency is linked with neurodegeneration, presumably as a dietary deficiency. The imbalance of zinc in the brain could be related to the interaction of Zn2+ with Aβ aggregates (17), but contradictory results indicate that zinc modulates the nucleation of Aβ retarding its aggregation (1). Zinc supplementation was tested in AD subjects with contradictory results. Compounds such as ZnS04, zinc bis-dl-hydrogen aspartate, zinc methionine, reaZin (zinthionein), zinc oxide, and zinc gluconate showed some signs of improvement in memory and cognitive outcomes, but in other cases they lacked significant benefits [reviewed in Adlard and Bush (4)].
Calcium suffers age-related dysregulation, playing a role in AD progression; it does this by causing synaptic deficits, and aggregation of Aβ and tau (168). Several compounds targeting calcium channels or proteins related to calcium dysregulation are tested as a therapeutic approach for AD (168). Memantine is an antagonist of glutamatergic NMDA receptors inhibiting Ca2+ influx, and it is one of the few FDA approved drugs for AD (Table 1). Another target for calcium dysregulation is the ionotropic transmembrane receptor AMPA. The antagonists of the AMPA receptor include LY451395, LY450108, and S18986. A clinical trial of LY450108 and LY451395 showed that single and multiple doses (1 and 5 mg) were safe and well tolerated (86). A clinical trial with LY451395 (0.2 mg for 28 days, 1.0 mg thereafter for 8-weeks) showed no significant changes in cognition, and the majority of patients showed mild adverse effects (22). Aged mice dosed with S18986 (0.03 and 0.1 mg/kg) showed a restoration of memory impairment. The utility of S18986 was compared with memantine to treat age-related cognitive dysfunction in mild AD (171). Nimodipine (or Nimotop) is a blocker of L-type VGCC calcium channels, targeting calcium dyschondrosteosis in dementia. The usefulness of nimodipine is not clear but sometimes it is prescribed for cognitive impairment and dementia (103). Clinical trials of nimodipine showed benefit when administered 90 mg/day (12 weeks) by improving cognitive function. A phase I trial showed no benefits or effects on secondary outcomes (CSF Aβ, tau, and p-tau) after an 8-week treatment of nimodipine (153). Nilvadipine (or Nilvad, Nivadil, ARC029) is a blocker of L-type VGCC calcium channels, initially prescribed for hypertension. Aged hTau mice treated with nilvadipine presented reduced inflammatory responses and significantly improved spatial memory (120). An initial clinical trial showed that nilvadipine reduced Aβ, reduced inflammation, and improved cerebral blood flow. A phase III clinical trial tested the effectiveness of nilvadipine in slowing cognitive decline (NCT02017340). Here, 511 subjects received 8 mg/day for 78 weeks, indicating that the compound was safe and well tolerated, but unfortunately without benefits in cognitive decline (99). ST101 (or ZSET1446) modulates T-type voltage-gated calcium channels. This compound induces AβPP processing in the nonamyloidogenic pathway, improves memory, and reduces Aβ in transgenic mice and nonhuman primates (71). ST101 was tested in a phase II trial with 210 AD patients dosed with 10, 60, or 120 mg for 12 weeks; the results indicated a dose-response in cognitive measures, especially in patients treated with donepezil (62). Hyperforin is an acylphloroglucinol isolated from Hypericum perforatum (St John's Wort), being an agonist of TRPC6 (transient receptor potential cation channel subfamily C member 6) with antioxidant and anti-inflammatory properties. This compound decreased Aβ in the hippocampus of a rat AD model, preventing neurotoxicity and reducing ROS (37). Hyperforin also showed dose-dependent neuroprotective effects against Aβ and H2O2 (87). Another TRPC6 activator is NSN21778, which works as a positive modulator of the neuronal-store-operated Ca2+ influx. This pathway is compromised in AD, and treatment with NSN21778 was able to rescue hippocampal long-term potentiation impairment in an AD mouse model (189).
Cytosolic calcium is regulated by pumping Ca2+ into the endoplasmic reticulum by sarco ER Ca2+-ATPase (SERCA), which also interacts with presenilins altering Aβ production (70). Thapsigargin is an SERCA inhibitor that causes downregulation of γ-secretase presenilin-2 but not presenilin-1, but thapsigargin also increases oxidative stress and induces apoptosis in vitro (33, 144). Dantrolene is an antagonist of ryanodine receptors that release Ca2+ from the sarco/endoplasmic reticulum. This compound showed neuroprotective properties in AD animal models (101). Long-term treatment of dantrolene on 3 × Tg-AD mice (5 mg/kg for 6 months) decreased the accumulation of Aβ and p-tau in the hippocampus, but with no changes in motor functions or cognition (183). Carvedilol (or Coreg, Artist, Aucardic, Dilatrend, Kredex) is a nonselective α/β-adrenergic receptor antagonist and α-adrenergic receptor blocker, currently prescribed for high blood pressure and cardiovascular problems. Treatment with carvedilol increased basal synaptic transmission in the TgCRND8 mice model, also enhancing neuronal plasticity and suppression of neuronal hyperexcitability (8). A phase IV trial with 29 participants is testing the effect of 25 mg/day dose of carvedilol on AD patients (NCT01354444). The trial was completed in January 2017, but no reports have been published. Xestospongin C is as an antagonist of the calcium channel activated by inositol triphosphate. When tested in APP/PS1 mice, intracerebroventricular injection of 5 μmol modulated intracellular Ca2+, improved cognition, reduced Aβ plaques, and reduced neuronal apoptosis (180).
Lithium is recognized as a neuroprotective and neurotrophic compound and is mainly prescribed to treat bipolar disorder. A study of the Danish population reported a nonlinear association of lithium consumption in drinking water with the diagnosis of dementia. Here, the authors found that elderly individuals (median age 80.3 years) with a higher low-term lithium exposure had a lower incidence of dementia (91). These conclusions are controversial, since other factors such as age, lifestyle, and health care were not associated. Another population study reported an inverse relationship between lithium in drinking water and AD (50). A most recent study did not find any significant benefit of groundwater lithium exposure in mental illnesses (132). This can be explained, because doses of therapeutic lithium are orders of magnitude higher than its concentration in drinking water. Lithium formulations (lithium carbonate, lithium salicylate, lithium salicylate-proline) have shown efficacy in reducing AD. In APP/PS1 mice, these compounds served as prophylactic to prevent cognitive decline, memory decline, and irritability when administered for 4 months (77). SK-N-SH neurons were treated with LiCl; the transcriptomic analysis revealed that the core AD pathways did not suffer significant changes, but they affected noncoding nucleolar RNA and microRNAs connected to the AβPP pathway (107). However, mixed results of therapeutic lithium, micro-dose, and low-dose lithium treatments (300–600 mg) seem to improve symptoms in AD subjects, in particular, by stabilizing cognitive impairment and reducing agitation/aggression.
Neuronal Death Through Ferroptosis
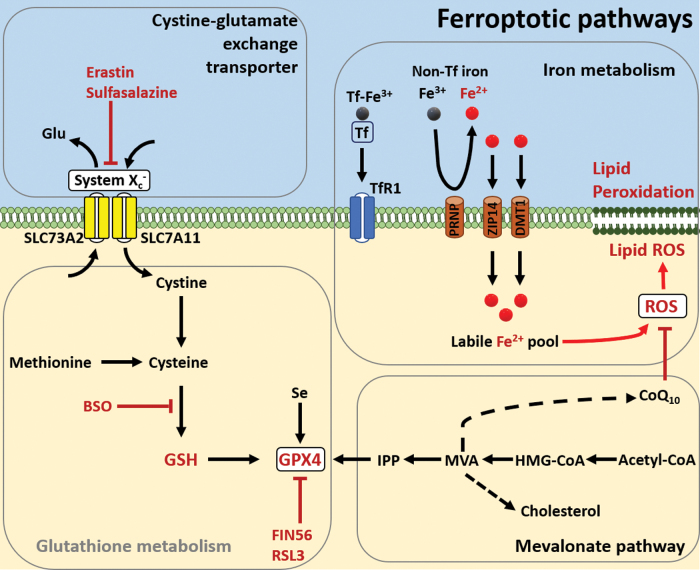
Oxidative stress and altered metals have been implicated in activating cell death. Examination of AD brains has revealed a notable reduction in the brain volume and overall cell number in comparison with healthy individuals. Nevertheless, the specific mechanisms that trigger neuronal death in AD still remain undefined. Recently, a novel form of regulated cell death mediated by iron was described and termed ferroptosis, which is morphologically, biochemically, and genetically distinctive from apoptosis and necrosis (59, 79a, 184). Ferroptosis has similar characteristics as oxytosis, a non-excitotoxic pathway for glutamate-induced cell death previously described (146a, 150a, 166a). Oxytosis is characterized by glutathione depletion, lipoxygenase activation, ROS accumulation, and calcium influx. Ferroptosis results from dysfunction of redox homeostasis, ROS overproduction, and lipid peroxidation. Neurodegeneration is closely correlated with high oxidative stress and abnormal levels or overload of redox-active and nonredox metals, consequently promoting Aβ/tau aggregation, mitochondrial dysfunction, inflammation responses, and synaptic failure. Misregulated ferroptosis pathways have been speculated to trigger neurodegeneration, but the cellular mechanisms are not completely understood. Different small drugs have been designed as ferroptosis inductors or ferroptosis inhibitors. These compounds target four main pathways: system xc−, glutathione metabolism, lipid peroxidation, and iron metabolism (Fig. 6). Erastin, erastin2, IKE, sorafenib, sulfasalazine, and glutamate serve as inhibitors of system xc− (SLC73A2/SLC7A11), which operates as glutamate/cystine transport and regulation of intracellular glutathione precursors. The compound l-buthionine-(S,R)-sulfoximine (BSO) inhibits the enzyme γ-glutamylcysteine synthetase, which catalyzes an ATP-dependent condensation of cysteine and glutamate, leading to glutathione depletion. The selenoprotein glutathione peroxidase 4 (GPX4) has been identified as a critical component of ferroptosis, because GPX4 functions as phospholipid hydroperoxidase reducing lipid peroxidation. The compounds (1S,3R)-RSL3, ML-162, and ML-210 bind to GPX4, serving as potent inhibitors; whereas FIN56 reduces GPX4 expression and also interferes with the mevalonate pathway (cholesterol synthesis). An excessive amount of free iron (ferric Fe3+/ferrous Fe2+) may trigger ferroptosis. The labile pool of Fe is redox active and generates an excessive amount of hydroxyl radicals that cause lipid peroxidation. Ferroptosis is a topic that is actively evolving, and novel ferroptosis inductors have been designed or discovered with potential therapeutic uses.
FIG. 6.
Overview of ferroptotic pathways. Diagram indicating the main pathways in ferroptosis, cystine-glutamate exchange transporter, glutathione metabolism, iron metabolism, and mevalonate. Ferroptosis induces erastin; sulfasalazine inhibits system Xc−; BSO inhibits production of GSH; Fin56 and RSL3 inhibit GPX4; and Fe2+ promotes overproduction of ROS and lipid ROS. BSO, l-buthionine-(S,R)-sulfoximine; GPX4, glutathione peroxidase 4; GSH, glutathione. Color images are available online.
Ferroptosis inhibitors may prevent cell death by restoring cellular functions, being ROS scavengers or metal chelators. For example, liproxstatin-1, ferrostatin-1, and its analogs interfere with peroxyl radicals, and the activity of GPX4 is potentiated by compounds SRS16-86 and SRS11-92. The redox-active Fe can form complexes with deferasirox, deferiprone, deferoxamine mesylate, or ciclopirox to reduce oxidative stress that is mediated by ROS. The first recognized antiferroptotic gene is the flavoprotein apoptosis-inducing factor mitochondria-associated 2 (AIFM2), now known as ferroptosis suppressor protein 1 (FSP1) (41). This protein confers protection against ferroptosis mediated by GPX4 deletion, and it achieves this by catalyzing regeneration of CoQ10 using NAD(PH)H and, consequently, trapping lipid peroxyl radicals.
Some ferroptosis-like biochemical and morphological features have been observed in AD in clinical, in vivo and in vitro studies, including GSH depletion (108), marked lipid peroxidation (15, 61, 127, 166), mitochondrial dysfunction (56, 60, 173, 177), GPX4 downregulation (78, 187), and overall altered redox homeostasis. Ferroptosis presents biochemical and morphological features that are different from apoptosis and necrosis (Table 2 and Fig. 7), but since ferroptosis is a newly described and accepted mechanism of programmed cell death, new molecular pathways, proteins, and genes are continuously described. The activation of ferroptosis has been described as ferrosenescence and correlated to iron dyshomeostasis observed in aging and neurodegenerative disorders (152). Ferroptosis can be prevented through selenium donors, lipoxygenase inhibitors, iron chelators, and ferroptosis inhibitors. The use of ferroptosis inhibitors is an alternative to prevent or treat iron-dependent disorders in neurodegenerative diseases, delay or prevent neuronal failure, block lipid peroxidation, reduce oxidative stress, and reduce cell death. The ferroptosis inhibitors function by targeting key molecules in the main ferroptotic pathways (Fig. 6).
Table 2.
Ferroptosis in Neurodegeneration
| Morphology changes | Biochemical changes | Inductors | Inhibitors |
|---|---|---|---|
| Rounding up and detachment (cultured cells) Lack of membrane rupture and no blebbing Small mitochondria and changes in its morphology Normal size of nucleus and no chromatin condensation |
Iron overload Overproduction of ROS Inhibition system Xc− Glutathione depletion Lipid peroxidation Inflammatory markers |
Erastin Erastin2 Sorafebib Sulfasalazine Glutamate l-Buthionine-sulfoximine 1S,3R-RSL3 ML-162, ML-210 FIN56 Free iron (III)/iron (II) Artemisin, Artesunate |
Liproxsatatin-1 Ferrostatin-1 UAMC-3203 SRS16, SRS11-92 Deferasinox Deferiprone Deferoxamine Ciclopirox CoQ10 Selenium Glutathione |
FIG. 7.
Ferroptotic neurons. Live cell imaging of SH5Y5Y neuronal cells treated with 100 mM of ferroptosis inductors for 24 h. (A) Control. (B) Buthionine sulfoximine. (C) Sulfasalazine.
Redox-active iron
Iron chelators such as deferasirox, deferiprone, and deferoxamine have shown potential to inhibit ferroptosis (39, 76). The relationship of these compounds with AD is discussed in the Alterations of Redox Homeostasis section. Ciclopirox possesses iron-binding properties. This compound showed neuroprotective properties and significantly reduced the age-dependent acceleration of mortality rate in Caenorhabditis elegans (104). The mechanism of ciclopirox and deferoxamine is a G1/S cell cycle blocker. Low-affinity iron chelators are proposed as an alternative to reduce the disruption of iron metabolism in AD. Examples of these natural iron chelators are curcumin, kolaviron, procyanidins, baicalein, tetramethylpyrazine, ferulic acid, polyphenols, catechins, epigallocatechin-3-gallate, phytate, epimedium, astragalus, kudzu root (Radix puerariae), and neem tree (Azadirachta indica) (152). The copper chelator Cu(II)-ATSM inhibits ferroptosis, by delaying the progression of degeneration caused by erastin and RSL3. The efficacy of Cu(II)-ATSM was similar to ferroptosis inhibitor liproxstatin-1 (163). Similar to iron chelators, copper-binding agents have potential as ferroptosis inhibitors in neurodegeneration.
Glutathione peroxidase
Ferrostatin-1 is a potent ferroptosis inhibitor: It is a radical scavenger by blocking erastin-induced ROS production and lipid peroxidation (39). This compound is one of the first ferroptosis inhibitors identified: Its activity was confirmed in cancer models and also by inhibition of glutamate-induced cell death in organotypic rat brain slices (39). Analogs of ferrostatin-1, such as ferrostatin-1 dyine and UAMC-3203, are new therapeutic compounds with the potential for enhanced stability and solubility. The use of ferrostatin and liproxstatin-1 helped to prevent mitochondrial dysfunction and neuronal death in mouse hippocampal neurons dosed with erastin (123).
Glutathione depletion in ferroptosis is caused by the disruption of GPX4. Consequently, the redox system fails, and ROS accumulates causing protein and lipid oxidation. The compound RSL3 inhibits GPX4 when RSL3 is added to neuronal HT22 cells and mouse fibroblasts; it causes dose-dependent mitochondrial damage and lipid peroxidation. Ferroptotic response in HT22 neurons was reversed with the use of deferoxamine, ferrostatin-1 and liproxstatin-1, and mitochondrial ROS scavenger MitoQ or by genetic modification of BID receptor (85). GPX4 depletion in neurons caused neurodegeneration in mice. The inactivation of this enzyme could be prevented with α-tocopherol, lipoxygenase inhibitors or through siRNA-mediated silencing of AIF (151). Another important characteristic of GPX4 is that it is a selenoprotein. Site-directed mutation in GPX4 of the selenocysteine residue activated ferroptosis (82). Selenium is essential for a healthy brain: It is inversely associated with DNA hypomethylation, protects against DNA damage, and is used by neuronal proteins such as GPX4 (152). Elevated blood levels of iron and copper correlate with a selenium decline and cognitive impairment in AD (172). Selenium-based supplements are alternative interventions for ferroptosis and neurodegeneration (152). Ebselen is a lipid-soluble antioxidant with GPX-like activity. In AD models, Ebselen showed inhibition of oxidative stress, reducing Aβ and BACE; it also reduced p-tau and improved postsynaptic density. Further, the spatial and memory test of 3 × Tg mice treated with Ebselen showed significant improvement (185). In a mice model of sporadic AD, Ebselen treatment (1–10 mg/kg) helped to reverse memory impairment, and it reduced hippocampal oxidative stress and apoptosis (111). Se compounds have been proposed as supplements for neurodegeneration, maintaining functional levels of selenoproteins in the brain. Additional compounds with potential therapeutic applications are selenoneine, organo-Se-compounds- (PhSe)2, sodium selenite, sodium selenate, selenomethionine, Selol, and selenoglutathione (42).
Lipid peroxidation in ferroptosis
The lipid content in the human brain is extremely high, being 36%–40% in gray matter, 49%–66% in white matter, and 78%–81% in myelin (125). Under oxidative stress conditions, the brain lipids may suffer a series of complex modifications that will have an impact on normal neuronal and brain functions (Fig. 8). Abnormally elevated levels of lipid peroxidation have been observed in the brain and body fluids of AD patients (15, 133). The formation of oxidized lipids is mediated by acyl-CoA synthetase long-chain family member 4 (ACSL4), lysophosphatidylcholine acyltransferase 3 (LPCAT3), and lipoxygenases. Thiazolidinediones are inhibitors of ACSL4. Currently, there are three compounds of this family approved for treatment in humans (pioglitazone, rosiglitazone, and lobeglitazone); at this time, none of these treatments focuses on neurodegenerative diseases. Interestingly, the ApoE gene polymorphism influences the efficacy of thiazolidinediones. In a clinical trial with 2381 subjects, rosiglitazone significantly decreased cognitive scores in ApoE4− patients, but it increased in ApoE+ carriers (81).
FIG. 8.
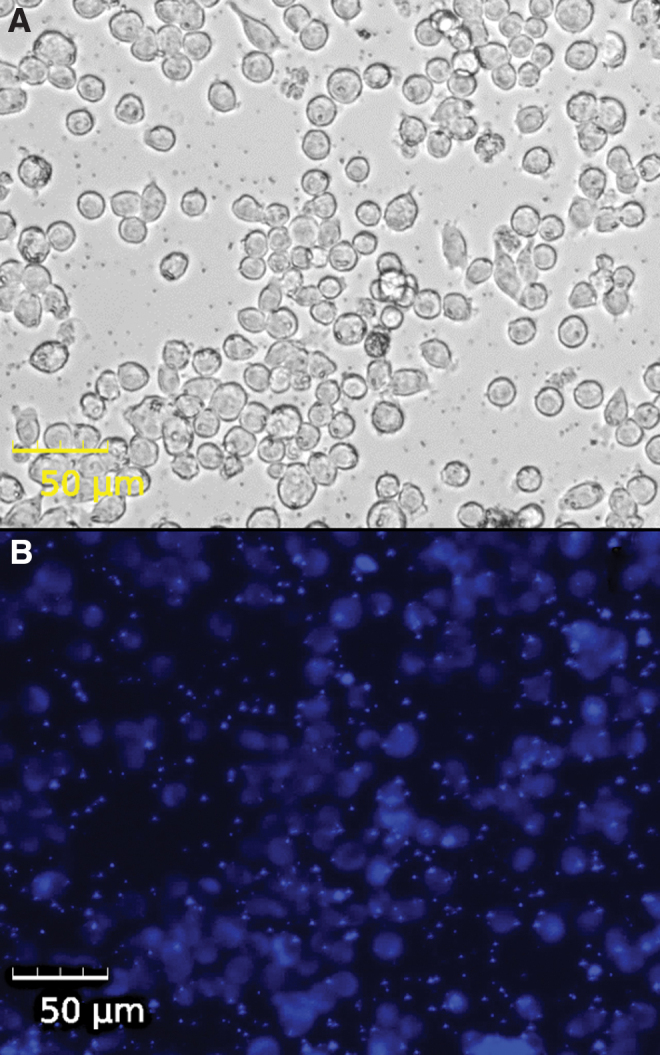
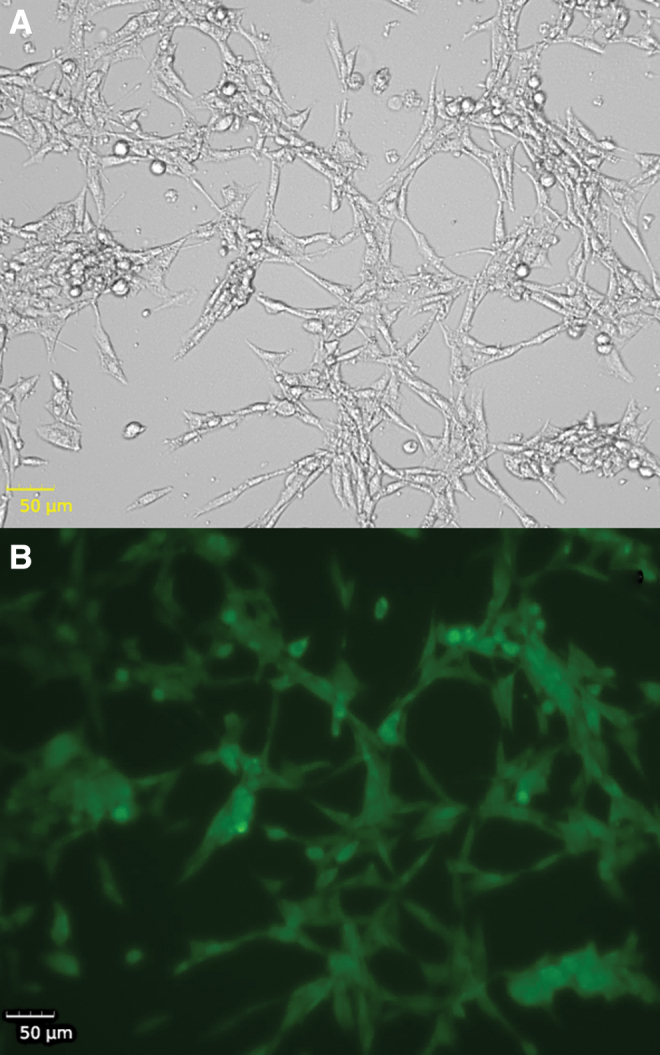
Lipid peroxidation in microglia. Live cell imaging of BV2 mouse microglia treated with 250 μM of ammonium iron (III) citrate for 3 h. (A) Bright-field imaging. (B) DPPP fluorescence as an indicator of lipid peroxidation in cells. Color images are available online.
Lipoxygenases (LOX) catalyzes the deoxygenation of polyunsaturated fatty acids (PUFA) into lipids. In particular, 12/15-lipoxygenase is upregulated in the AD brain and linked to the oxidation of phospholipids. Since PUFA are prone to oxidative damage through both enzymatic and nonenzymatic processes, overproduction of lipid peroxides can be ameliorated through inhibition of free-radicals ROS or inhibition of lipoxygenases. Small molecules such as the flavonoid baicalein can inhibit LOX with therapeutic potential by reducing oxidative stress, as an anti-inflammatory and neuroprotectant (100). The APP/PS1 mice fed with baicalein for 2 months showed inhibition of 12/15-LOX, reduced BACE1 activity, and decreased Aβ and p-tau (74). There were similar observations in an AD rat model, where baicalein improved the behavioral test and promoted overexpression of antioxidant proteins, stress response, phospholipid metabolism, and antiapoptosis in the hippocampus (181). Nordihydroguaiaretic acid (NGDA) is another LOX inhibitor; this phenolic compound is obtained from the leaves of the evergreen desert shrub Larrea tridentata (Creosote bush). When NGDA was administered to a transgenic Drosophila model of AD for 30 days at 20–80 μM, it promoted reduced oxidative stress, prevented memory loss, and increased the life span of flies, but without effect on Aβ (158). The NGDA showed neuroprotective properties in rat hippocampal neurons dosed with Aβ. Moreover, the treatment suppressed the accumulation of ROS induced by iron (68). Zileuton inhibits LOX5; in the 3 × Tg mice model of AD, a 3-month treatment of zileuton reduced γ-secretase, Aβ, and tau (36). In addition, there was a decrease in brain damage and a reduction of inflammatory cytokine expression (TNFα, IFNγ, IL1β, IL6, CXCL1, CCL3, and CCL5) (159).
The substrates of LOX are PUFA, such as omega-3 and omega-6. Another therapeutic strategy consists of dietary supplements to replace those that have suffered peroxidation. Long-chain PUFA from omega-3, -6, or -9 series are administered as supplements. The beneficial effects of omega-3 acids of fish oil, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) in AD stand as antioxidants, anti-inflammatory, antiapoptotic, and neurotrophic, also improving cognitive function (7). In vitro and in vivo neuronal models revealed a reduced production of lipid peroxides and ROS, also a reduced number of apoptotic cells after omega-3 supplementation (176). In vitro neurons showed neuroprotective effects of DHA, because oxidized lipids may cause a shift from nonamyloidogenic to amyloidogenic AβPP processing (72). In a clinical trial with 40 AD subjects, a daily dose of 1.7 g DHA and 0.6 g EPA for 6 months showed no significant effects in oxidative stress and inflammation biomarkers in comparison with controls (55). Additional trials focusing on supplements and interventions of the Mediterranean diet are running; the results will provide new information on the therapeutic use of omega acids to manage AD. However, the epidemiological and nutritional studies of DHA/EPA in AD are still controversial and with no reliable conclusions.
CoQ10 plays a critical role in the ferroptosis pathway as a potent antioxidant ROS scavenger preventing or reducing lipid peroxidation (Fig. 6). CoQ10 supplementation was tested in old mice, using a dose of 0.72–2.81 mg/g for 15 weeks. CoQ10 treatment improved spatial learning and decreased oxidative damage, even delaying senescence (154). CoQ10 alone (123 mg/kg/day) or in combination with α-tocopherol (vitamin E, 200 mg/kg/day) improved brain function in old mice, suggesting a synergistic effect of these compounds in brain metabolism (114). A combination of CoQ10+α-tocopherol ameliorated age-related impairment and overall protein oxidation; these two supplements showed better performance when administered together (155). Tg19959 mice treated with CoQ10 showed decreased levels of Aβ in the hippocampus and cortex, also a reduction in oxidative stress biomarkers (46). A phase I clinical trial with 75 subjects (60–85 years old) evaluated vitamin E (800 IU), vitamin C (200 mg), alpha-lipoic acid (200 mg), and CoQ10 (400 mg) administered three times per day for 4 months (NCT00117403). The results indicated no changes in AD biomarkers (Aβ, tau, and p-tau), but a 19% reduction of oxidative stress (biomarker F2-isoprostane) (57). Mechanistically, the FSP1 function as an oxidoreductase that uses CoQ10 as a lipophilic radical-trapping antioxidant that stops the lipid peroxidation reactions (13). In conjunction, FSP1+CoQ10 and glutathione+GPX4 serve as a ferroptosis-resistance antioxidant mechanism in the cells.
GSH is a major antioxidant that counteracts oxidative stress. The levels of GSH suffer a significant reduction in the hippocampus and cortex in MCI and AD (109). Glutathione supplementation has been proposed as a therapeutic strategy for MCI and AD. Elevation of GSH is possible through supplementation with the precursors N-acetyl-cysteine and γ-glutamylcysteine ethyl ester, which are the substrates for GSH synthesis (135). Intranasal administration of GSH (100–200 mg, 3 times per day for 3 months) followed a phase IId clinical trial with 45 patients with Parkinson's, indicating improvements in symptomatology but no significance in outcome measures (117). The use of GSH supplementation in clinical trials with MCI or AD patients has not been evaluated yet (109).
Ferroptosis and senescence in AD
The mechanisms, cellular pathways, and biomolecules involved in ferroptosis are still partially described, but there is an overlap with other mechanisms of cell arrest and programmed death. Cellular senescence is a potential contributor to the accumulation of iron in AD and related dementia; astrocytes, microglia, and even neurons display senescent features (112). The iron content is increased during senescence, and this phenomenon can be induced by a low dose of H2O2, causing increased oxidative stress and cellular dysfunction (94). Nonferritin-bound iron showed a marked dysregulation, cytosolic accumulation, and induced both a senescent phenotype and ferroptosis in induced pluripotent human fibroblast and differentiated neurons (30). Transcriptomics of AD transgenic mouse revealed an expression pattern consistent with senescence, including stress response, aberrant cell cycle, and β-galactosidase activity; similar observations were confirmed in postmortem human AD brain (121). Analysis of the brain of AD patients and AD mice exhibited senescence-like phenotype in oligodendrocyte progenitor cells, with overexpression of p21/CDKN1A, p16/INK4/CDKN2A, and detection of senescence-associated β-galactosidase (190). A potential treatment for senescence is the FDA-approved senolytic cocktail of dasatinib-quercetin, which reduces inflammation and ameliorates cognitive deficits. Overall, senolytics promote clearance of senescent cells: This therapy may help to remove cells with altered or irreversible cell cycle arrest that once accumulated in the tissues could trigger inflammation and oxidative stress. Currently, a phase II clinical trial is testing senolytic therapy for modulating the progression of AD (NCT04063124). Around 40 patients >65 years old are receiving dasatinib (D) and quercetin (Q) (D + Q). These compounds are administered intermittently for 2 days on/14 days off for 12 weeks (6 cycles), and the dosage of D + Q was not disclosed. The trials will be completed at the end of 2020, with assessments of CSF biomarkers (Aβ, tau, IL6, p16) and cognitive tests.
Conclusions
Due to recent failures of some amyloid-focused therapeutic compounds evaluated in several clinical trials, researchers, public health/funding agencies, and pharmaceutical companies are seeking AD drugs targeting other mechanisms than Aβ, tau, and secretases. AD is a complex and mostly multifactorial chronic condition; it is extremely complicated that a single drug or intervention can avoid, reduce, or reverse the disease. Here, we presented and discussed potential therapeutic compounds and interventions that focused on re-establishing redox homeostasis, biometal alterations, and neuronal failure in Alzheimer's. Some of these strategies may be beneficial in maintaining the mental function, management of symptoms, or slowdown the progression of neurodegeneration.
Abbreviations Used
- Aβ
amyloid-β
- AβPP
amyloid-β precursor protein
- ACSL4
acyl-CoA synthetase long-chain family member 4
- AD
Alzheimer's disease
- AIF
apoptosis-inducing factor
- AIFM2
apoptosis-inducing factor mitochondria-associated 2
- BACE
β-secretase
- BCA
bicinchoninic acid
- BCDS
bathocuproinedisulfonic acid
- BSO
l-buthionine-(S,R)-sulfoximine
- CSF
cerebrospinal fluid
- Cu-GTSM
copper bis(thiosemicarbazonate) complex
- DCFDA
2′,7′-dichlorofluorescin diacetate
- DHA
docosahexaenoic acid
- EGCG
epigallocatechin-3-gallate
- EPA
eicosapentaenoic acid
- FDA
Food and Drug Administration
- FSP1
ferroptosis suppressor protein 1
- GHK
glycyl-l-histidyl-l-lysine
- GPX4
glutathione peroxidase 4
- GSH
glutathione
- IKE
imidazole ketone erastin
- LOX
lipoxygenases
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- NGDA
nordihydroguaiaretic acid
- NMDA
N-methyl-d-aspartate
- MCI
mild cognitive impairment
- PUFA
polyunsaturated fatty acids
- p-tau
hyperphosphorylated tau
- ROS
reactive oxygen Species
- SERCA
sarco ER Ca2+-ATPase
- SOD
superoxide dismutase
- TETA
triethylenetetramine
- TRPC6
transient receptor potential cation channel subfamily C member 6
Funding Information
This research project was possible by the generous support from Alzheimer's Association (AARFD-17-529742), The Lowe Foundation, Semmes Foundation, and NIH R01AG066749.
References
- 1. Abelein A, Graslund A, and Danielsson J. Zinc as chaperone-mimicking agent for retardation of amyloid beta peptide fibril formation. Proc Natl Acad Sci U S A 112: 5407–5412, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Acevedo K, Masaldan S, Opazo CM, and Bush AI. Redox active metals in neurodegenerative diseases. J Biol Inorg Chem 24: 1141–1157, 2019 [DOI] [PubMed] [Google Scholar]
- 2a. ADEAR Alzheimer's & Related Dementias Education & Referral Center. Treatment of Alzheimer's disease. How Is Alzheimer's disease treated? https://www.nia.nih.gov/health/how-alzheimers-disease-treated (accessed January15, 2020)
- 3. Adlard PA, Bica L, White AR, Nurjono M, Filiz G, Crouch PJ, Donnelly PS, Cappai R, Finkelstein DI, and Bush AI. Metal ionophore treatment restores dendritic spine density and synaptic protein levels in a mouse model of Alzheimer's disease. PLoS One 6: e17669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adlard PA and Bush AI. Metals and Alzheimer's disease: how far have we come in the clinic? J Alzheimers Dis 62: 1369–1379, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aggarwal NT, Schneider JA, Holland T, Wang Y, Cherian LJ, and Morris MC. P1–P199: Western diet is related to ad and vascular brain neuropathologies in older adults. Alzheimer's & Dementia 14: P355–P356, 2018 [Google Scholar]
- 6. Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, Bottiglieri T, Jin S, Stokes KT, Thomas RG, and Thal LJ. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 300: 1774–1783, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ajith TA. A recent update on the effects of omega-3 fatty acids in Alzheimer's disease. Curr Clin Pharmacol 13: 252–260, 2018 [DOI] [PubMed] [Google Scholar]
- 8. Arrieta-Cruz I, Wang J, Pavlides C, and Pasinetti GM. Carvedilol reestablishes long-term potentiation in a mouse model of Alzheimer's disease. J Alzheimers Dis 21: 649–654, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Association A. 2018 Alzheimer's disease facts and figures. Alzheimers Dement 14: 367–429, 2018 [Google Scholar]
- 10. Balmik AA and Chinnathambi S. Multi-faceted role of melatonin in neuroprotection and amelioration of tau aggregates in Alzheimer's disease. J Alzheimers Dis 62: 1481–1493, 2018 [DOI] [PubMed] [Google Scholar]
- 11. Banerjee P, Sahoo A, Anand S, Bir A, and Chakrabarti S. The oral iron chelator, deferasirox, reverses the age-dependent alterations in iron and amyloid-beta homeostasis in rat brain: implications in the therapy of Alzheimer's disease. J Alzheimers Dis 49: 681–693, 2016 [DOI] [PubMed] [Google Scholar]
- 12. Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, and Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29: 1326–1334, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, and Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575: 688–692, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bica L, Liddell JR, Donnelly PS, Duncan C, Caragounis A, Volitakis I, Paterson BM, Cappai R, Grubman A, Camakaris J, Crouch PJ, and White AR. Neuroprotective copper bis(thiosemicarbazonato) complexes promote neurite elongation. PLoS One 9: e90070, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bradley-Whitman MA and Lovell MA. Biomarkers of lipid peroxidation in Alzheimer disease (AD): an update. Arch Toxicol 89: 1035–1044, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bridi R, Crossetti FP, Steffen VM, and Henriques AT. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytother Res 15: 449–451, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, and Tanzi RE. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265: 1464–1467, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Butterfield DA and Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20: 148–160, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cammisuli DM, Innocenti A, Fusi J, Franzoni F, and Pruneti C. Aerobic exercise effects upon cognition in Alzheimer's Disease: a systematic review of randomized controlled trials. Arch Ital Biol 156: 54–63, 2018 [DOI] [PubMed] [Google Scholar]
- 20. Cascella M, Bimonte S, Muzio MR, Schiavone V, and Cuomo A. The efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer's disease: an overview of pre-clinical studies and translational perspectives in clinical practice. Infect Agent Cancer 12: 36, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. This reference has been deleted
- 22. Chappell AS, Gonzales C, Williams J, Witte MM, Mohs RC, and Sperling R. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology 68: 1008–1012, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Chen H, Liu S, Ji L, Wu T, Ji Y, Zhou Y, Zheng M, Zhang M, Xu W, and Huang G. Folic acid supplementation mitigates Alzheimer's disease by reducing inflammation: a randomized controlled trial. Mediators Inflamm 2016: 5912146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen L, Zhang C, Han Y, Meng X, Zhang Y, Chu H, and Ma H. Gingko biloba extract (EGb) inhibits oxidative stress in neuro 2A cells overexpressing APPsw. J Biomed Res Int 2019: 9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, and Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30: 665–676, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Chesser AS, Ganeshan V, Yang J, and Johnson GV. Epigallocatechin-3-gallate enhances clearance of phosphorylated tau in primary neurons. Nutr Neurosci 19: 21–31, 2016 [DOI] [PubMed] [Google Scholar]
- 27. Choo XY, Liddell JR, Huuskonen MT, Grubman A, Moujalled D, Roberts J, Kysenius K, Patten L, Quek H, Oikari LE, Duncan C, James SA, McInnes LE, Hayne DJ, Donnelly PS, Pollari E, Vähätalo S, Lejavová K, Kettunen MI, Malm T, Koistinaho J, White AR, and Kanninen KM. CuII(atsm) attenuates neuroinflammation. Front Neurosci 12: 668, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, Cholerton B, Plymate SR, Arbuckle M, and Craft S. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer's disease. J Alzheimers Dis 35: 789–797, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooper GJ. Therapeutic potential of copper chelation with triethylenetetramine in managing diabetes mellitus and Alzheimer's disease. Drugs 71: 1281–1320, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Cozzi A, Orellana DI, Santambrogio P, Rubio A, Cancellieri C, Giannelli S, Ripamonti M, Taverna S, Di Lullo G, Rovida E, Ferrari M, Forni GL, Fiorillo C, Broccoli V, and Levi S. Stem cell modeling of neuroferritinopathy reveals iron as a determinant of senescence and ferroptosis during neuronal aging. Stem Cell Rep 13: 832–846, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, and Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69: 29–38, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, and Andrews DF. Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet 337: 1304–1308, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Crestini A, Piscopo P, Iazeolla M, Albani D, Rivabene R, Forloni G, and Confaloni A. Rosuvastatin and thapsigargin modulate gamma-secretase gene expression and APP processing in a human neuroglioma model. J Mol Neurosci 43: 461–469, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Crouch PJ, Hung LW, Adlard PA, Cortes M, Lal V, Filiz G, Perez KA, Nurjono M, Caragounis A, Du T, Laughton K, Volitakis I, Bush AI, Li QX, Masters CL, Cappai R, Cherny RA, Donnelly PS, White AR, and Barnham KJ. Increasing Cu bioavailability inhibits Abeta oligomers and tau phosphorylation. Proc Natl Acad Sci U S A 106: 381–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crouch PJ, Savva MS, Hung LW, Donnelly PS, Mot AI, Parker SJ, Greenough MA, Volitakis I, Adlard PA, Cherny RA, Masters CL, Bush AI, Barnham KJ, and White AR. The Alzheimer's therapeutic PBT2 promotes amyloid-beta degradation and GSK3 phosphorylation via a metal chaperone activity. J Neurochem 119: 220–230, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Di Meco A, Lauretti E, Vagnozzi AN, and Pratico D. Zileuton restores memory impairments and reverses amyloid and tau pathology in aged Alzheimer's disease mice. Neurobiol Aging 35: 2458–2464, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dinamarca MC, Cerpa W, Garrido J, Hancke JL, and Inestrosa NC. Hyperforin prevents beta-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer's amyloid-beta-deposits. Mol Psychiatry 11: 1032–1048, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Dixit S, Bernardo A, Walker JM, Kennard JA, Kim GY, Kessler ES, and Harrison FE. Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice. ACS Chem Neurosci 6: 570–581, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, and Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dodge HH, Buracchio TJ, Fisher GG, Kiyohara Y, Meguro K, Tanizaki Y, and Kaye JA. Trends in the prevalence of dementia in Japan. Int J Alzheimers Dis 2012: 956354, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Grocin AG, Xavier da Silva TN, Panzilius E, Scheel CH, Mourao A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O'Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, and Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575: 693–698, 2019 [DOI] [PubMed] [Google Scholar]
- 42. Dominiak A, Wilkaniec A, Wroczyński P, and Adamczyk A. Selenium in the therapy of neurological diseases. Where is it going? Curr Neuropharmacol 14: 282–299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dong Q, Teng SW, Wang Y, Qin F, Li Y, Ai LL, and Yu H. Sitagliptin protects the cognition function of the Alzheimer's disease mice through activating glucagon-like peptide-1 and BDNF-TrkB signalings. Neurosci Lett 696: 184–190, 2019 [DOI] [PubMed] [Google Scholar]
- 44. Donnelly PS, Caragounis A, Du T, Laughton KM, Volitakis I, Cherny RA, Sharples RA, Hill AF, Li QX, Masters CL, Barnham KJ, and White AR. Selective intracellular release of copper and zinc ions from bis(thiosemicarbazonato) complexes reduces levels of Alzheimer disease amyloid-beta peptide. J Biol Chem 283: 4568–4577, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, and Smith AD. Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A 110: 9523–9528, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dumont M, Kipiani K, Yu F, Wille E, Katz M, Calingasan NY, Gouras GK, Lin MT, and Beal MF. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis 27: 211–223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, Love S, Schellenberg GD, McCarten JR, Malphurs J, Prieto S, Chen P, Loreck DJ, Trapp G, Bakshi RS, Mintzer JE, Heidebrink JL, Vidal-Cardona A, Arroyo LM, Cruz AR, Zachariah S, Kowall NW, Chopra MP, Craft S, Thielke S, Turvey CL, Woodman C, Monnell KA, Gordon K, Tomaska J, Segal Y, Peduzzi PN, and Guarino PD. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 311: 33–44, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Egefjord L, Gejl M, Moller A, Braendgaard H, Gottrup H, Antropova O, Moller N, Poulsen HE, Gjedde A, Brock B, and Rungby J. Effects of liraglutide on neurodegeneration, blood flow and cognition in Alzheimer s disease—protocol for a controlled, randomized double-blinded trial. Dan Med J 59: A4519, 2012 [PubMed] [Google Scholar]
- 49. Elizabeth de Sousa Rodrigues M, Houser MC, Walker DI, Jones D, Chang J, Barnum C, and Tansey MG. P4–P480: Western diet promotes central insulin impairment and the dysregulation of metabolites associated with Alzheimer's disease: the role of soluble TNF. Alzheimer's & Dementia 15: P1496–P1497, 2019 [Google Scholar]
- 50. Fajardo VA, Fajardo VA, LeBlanc PJ, and MacPherson REK. Examining the relationship between trace lithium in drinking water and the rising rates of age-adjusted Alzheimer's disease mortality in Texas. J Alzheimers Dis 61: 425–434, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Farina N, Llewellyn D, Isaac M, and Tabet N. Vitamin E for Alzheimer's dementia and mild cognitive impairment. Cochrane Database Syst Rev 4: CD002854, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fillenbaum GG, Kuchibhatla MN, Hanlon JT, Artz MB, Pieper CF, Schmader KE, Dysken MW, and Gray SL. Dementia and Alzheimer's disease in community-dwelling elders taking vitamin C and/or vitamin E. Ann Pharmacother 39: 2009–2014, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Fine JM, Forsberg AC, Stroebel BM, Faltesek KA, Verden DR, Hamel KA, Raney EB, Crow JM, Haase LR, Knutzen KE, Kaczmarczek KD, Frey WH, and Hanson LR. Intranasal deferoxamine affects memory loss, oxidation, and the insulin pathway in the streptozotocin rat model of Alzheimer's disease. J Neurol Sci 380: 164–171, 2017 [DOI] [PubMed] [Google Scholar]
- 54. Fine JM, Forsberg AC, Stroebel BM, Verden DR, Hamel KA, Raney EB, Frey WH, II, and Hanson LR. Intranasal deferoxamine prevents memory loss in the intracerebroventricular streptozotocin rat model of Alzheimer's disease. Alzheimers Dement (N Y) 11: P613–P614, 2015 [DOI] [PubMed] [Google Scholar]
- 55. Freund-Levi Y, Vedin I, Hjorth E, Basun H, Faxen Irving G, Schultzberg M, Eriksdotter M, Palmblad J, Vessby B, Wahlund LO, Cederholm T, and Basu S. Effects of supplementation with omega-3 fatty acids on oxidative stress and inflammation in patients with Alzheimer's disease: the OmegAD study. J Alzheimers Dis 42: 823–831, 2014 [DOI] [PubMed] [Google Scholar]
- 56. Friedland-Leuner K, Stockburger C, Denzer I, Eckert GP, and Muller WE. Mitochondrial dysfunction: cause and consequence of Alzheimer's disease. Prog Mol Biol Transl Sci 127: 183–210, 2014 [DOI] [PubMed] [Google Scholar]
- 57. Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B, Montine TJ, Thomas RG, and Aisen P. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol 69: 836–841, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Galimberti D and Scarpini E. Pioglitazone for the treatment of Alzheimer's disease. Expert Opin Investig Drugs 26: 97–101, 2017 [DOI] [PubMed] [Google Scholar]
- 59. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jaattela M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Munoz-Pinedo C, Nagata S, Nunez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, and Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25: 486–541, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gan X, Huang S, Wu L, Wang Y, Hu G, Li G, Zhang H, Yu H, Swerdlow RH, Chen JX, and Yan SS. Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer's disease cybrid cell. Biochim Biophys Acta 1842: 220–231, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gaschler MM and Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun 482: 419–425, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gauthier S, Rountree S, Finn B, LaPlante B, Weber E, and Oltersdorf T. Effects of the acetylcholine release agent ST101 with donepezil in Alzheimer's disease: a randomized phase 2 study. J Alzheimers Dis 48: 473–481, 2015 [DOI] [PubMed] [Google Scholar]
- 63. Gejl M, Gjedde A, Egefjord L, Moller A, Hansen SB, Vang K, Rodell A, Braendgaard H, Gottrup H, Schacht A, Moller N, Brock B, and Rungby J. In Alzheimer's disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci 8: 108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Geldmacher DS, Fritsch T, McClendon MJ, and Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol 68: 45–50, 2011 [DOI] [PubMed] [Google Scholar]
- 64a. George J. Untangling the Alzheimer's-sleep connection. Med Page Today. https://www.medpagetoday.com/neurology/alzheimersdisease/84140 (accessed January15, 2020)
- 65. Gil Gregorio P, Ramirez Diaz SP, and Ribera Casado JM. Dementia and Nutrition. Intervention study in institutionalized patients with Alzheimer disease. J Nutr Health Aging 7: 304–308, 2003 [PubMed] [Google Scholar]
- 66. Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, Craft S, Landreth G, Linnamagi U, and Sawchak S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer's disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord 30: 131–146, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Golde TE, DeKosky ST, and Galasko D. Alzheimer's disease: the right drug, the right time. Science 362: 1250–1251, 2018 [DOI] [PubMed] [Google Scholar]
- 68. Goodman Y, Steiner MR, Steiner SM, and Mattson MP. Nordihydroguaiaretic acid protects hippocampal neurons against amyloid beta-peptide toxicity, and attenuates free radical and calcium accumulation. Brain Res 654: 171–176, 1994 [DOI] [PubMed] [Google Scholar]
- 69. Goozee KG, Shah TM, Sohrabi HR, Rainey-Smith SR, Brown B, Verdile G, and Martins RN. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer's disease. Br J Nutr 115: 449–465, 2016 [DOI] [PubMed] [Google Scholar]
- 70. Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, and LaFerla FM. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J Cell Biol 181: 1107–1116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Green KN, Khashwji H, Estrada T, and Laferla FM. ST101 induces a novel 17 kDa APP cleavage that precludes Abeta generation in vivo. Ann Neurol 69: 831–844, 2011 [DOI] [PubMed] [Google Scholar]
- 72. Grimm MO, Haupenthal VJ, Mett J, Stahlmann CP, Blumel T, Mylonas NT, Endres K, Grimm HS, and Hartmann T. Oxidized docosahexaenoic acid species and lipid peroxidation products increase amyloidogenic amyloid precursor protein processing. Neurodegener Dis 16: 44–54, 2016 [DOI] [PubMed] [Google Scholar]
- 73. Grossi C, Francese S, Casini A, Rosi MC, Luccarini I, Fiorentini A, Gabbiani C, Messori L, Moneti G, and Casamenti F. Clioquinol decreases amyloid-beta burden and reduces working memory impairment in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis 17: 423–440, 2009 [DOI] [PubMed] [Google Scholar]
- 74. Gu XH, Xu LJ, Liu ZQ, Wei B, Yang YJ, Xu GG, Yin XP, and Wang W. The flavonoid baicalein rescues synaptic plasticity and memory deficits in a mouse model of Alzheimer's disease. Behav Brain Res 311: 309–321, 2016 [DOI] [PubMed] [Google Scholar]
- 75. Gugliandolo A, Bramanti P, and Mazzon E. Role of vitamin E in the treatment of Alzheimer's disease: evidence from animal models. Int J Mol Sci 18: 2504, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guo C, Wang T, Zheng W, Shan ZY, Teng WP, and Wang ZY. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer's disease. Neurobiol Aging 34: 562–575, 2013 [DOI] [PubMed] [Google Scholar]
- 77. Habib A, Shytle RD, Sawmiller D, Koilraj S, Munna SA, Rongo D, Hou H, Borlongan CV, Currier G, and Tan J. Comparing the effect of the novel ionic cocrystal of lithium salicylate proline (LISPRO) with lithium carbonate and lithium salicylate on memory and behavior in female APPswe/PS1dE9 Alzheimer's mice. J Neurosci Res 97: 1066–1080, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hambright WS, Fonseca RS, Chen L, Na R, and Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12: 8–17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Harrington C, Sawchak S, Chiang C, Davies J, Donovan C, Saunders AM, Irizarry M, Jeter B, Zvartau-Hind M, van Dyck CH, and Gold M. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer's disease: two phase 3 studies. Curr Alzheimer Res 8: 592–606, 2011 [DOI] [PubMed] [Google Scholar]
- 79a. Hirschhorn T and Stockwell BR.. The development of the concept of ferroptosis. Free Radic Biol Med 133: 130–143, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, and Holtzman DM. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363: 880–884, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Iketani R, Ohno K, Kawasaki Y, Matsumoto K, Yamada H, and Kishino S. Apolipoprotein E gene polymorphisms affect the efficacy of thiazolidinediones for Alzheimer's disease: a systematic review and meta-analysis. Biol Pharm Bull 41: 1017–1023, 2018 [DOI] [PubMed] [Google Scholar]
- 82. Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, Mehr L, Aichler M, Walch A, Lamp D, Jastroch M, Miyamoto S, Wurst W, Ursini F, Arner ESJ, Fradejas-Villar N, Schweizer U, Zischka H, Friedmann Angeli JP, and Conrad M. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172: 409–422.e21, 2018 [DOI] [PubMed] [Google Scholar]
- 83. Isik AT, Soysal P, Yay A, and Usarel C. The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer's disease. Diabetes Res Clin Pract 123: 192–198, 2017 [DOI] [PubMed] [Google Scholar]
- 84. This reference has been deleted
- 85. Jelinek A, Heyder L, Daude M, Plessner M, Krippner S, Grosse R, Diederich WE, and Culmsee C. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic Biol Med 117: 45–57, 2018 [DOI] [PubMed] [Google Scholar]
- 86. Jhee SS, Chappell AS, Zarotsky V, Moran SV, Rosenthal M, Kim E, Chalon S, Toublanc N, Brandt J, Coutant DE, and Ereshefsky L. Multiple-dose plasma pharmacokinetic and safety study of LY450108 and LY451395 (AMPA receptor potentiators) and their concentration in cerebrospinal fluid in healthy human subjects. J Clin Pharmacol 46: 424–432, 2006 [DOI] [PubMed] [Google Scholar]
- 87. Jiang X, Kumar M, and Zhu Y. Protective effect of hyperforin on beta amyloid protein induced apoptosis in PC12 cells and colchicine induced Alzheimer's disease: an anti-oxidant and anti-inflammatory therapy. J Oleo Sci 67: 1443–1453, 2018 [DOI] [PubMed] [Google Scholar]
- 88. Jimenez-Jimenez FJ, Molina JA, de Bustos F, Orti-Pareja M, Benito-Leon J, Tallon-Barranco A, Gasalla T, Porta J, and Arenas J. Serum levels of beta-carotene, alpha-carotene and vitamin A in patients with Alzheimer's disease. Eur J Neurol 6: 495–497, 1999 [DOI] [PubMed] [Google Scholar]
- 89. Karstens AJ, Tussing-Humphreys L, Zhan L, Rajendran N, Cohen J, Dion C, Zhou XJ, and Lamar M. Associations of the Mediterranean diet with cognitive and neuroimaging phenotypes of dementia in healthy older adults. Am J Clin Nutr 109: 361–368, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, and Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int 54: 111–118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kessing LV, Gerds TA, Knudsen NN, Jorgensen LF, Kristiansen SM, Voutchkova D, Ernstsen V, Schullehner J, Hansen B, Andersen PK, and Ersboll AK. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry 74: 1005–1010, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kessler H, Bayer TA, Bach D, Schneider-Axmann T, Supprian T, Herrmann W, Haber M, Multhaup G, Falkai P, and Pajonk FG. Intake of copper has no effect on cognition in patients with mild Alzheimer's disease: a pilot phase 2 clinical trial. J Neural Transm (Vienna) 115: 1181–1187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kessler H, Pajonk FG, Bach D, Schneider-Axmann T, Falkai P, Herrmann W, Multhaup G, Wiltfang J, Schafer S, Wirths O, and Bayer TA. Effect of copper intake on CSF parameters in patients with mild Alzheimer's disease: a pilot phase 2 clinical trial. J Neural Transm (Vienna) 115: 1651–1659, 2008 [DOI] [PubMed] [Google Scholar]
- 94. Killilea DW, Wong SL, Cahaya HS, Atamna H, and Ames BN. Iron accumulation during cellular senescence. Ann N Y Acad Sci 1019: 365–367, 2004 [DOI] [PubMed] [Google Scholar]
- 95. Kocot J, Luchowska-Kocot D, Kielczykowska M, Musik I, and Kurzepa J. Does vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients 9: 659, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Koenig AM, Mechanic-Hamilton D, Xie SX, Combs MF, Cappola AR, Xie L, Detre JA, Wolk DA, and Arnold SE. Effects of the insulin sensitizer metformin in Alzheimer disease: pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis Assoc Disord 31: 107–113, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kryscio RJ, Abner EL, Caban-Holt A, Lovell M, Goodman P, Darke AK, Yee M, Crowley J, and Schmitt FA. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer's Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 74: 567–573, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, and Ritchie CW. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol 7: 779–786, 2008 [DOI] [PubMed] [Google Scholar]
- 99. Lawlor B, Segurado R, Kennelly S, Olde Rikkert MGM, Howard R, Pasquier F, Borjesson-Hanson A, Tsolaki M, Lucca U, Molloy DW, Coen R, Riepe MW, Kalman J, Kenny RA, Cregg F, O'Dwyer S, Walsh C, Adams J, Banzi R, Breuilh L, Daly L, Hendrix S, Aisen P, Gaynor S, Sheikhi A, Taekema DG, Verhey FR, Nemni R, Nobili F, Franceschi M, Frisoni G, Zanetti O, Konsta A, Anastasios O, Nenopoulou S, Tsolaki-Tagaraki F, Pakaski M, Dereeper O, de la Sayette V, Senechal O, Lavenu I, Devendeville A, Calais G, Crawford F, and Mullan M. Nilvadipine in mild to moderate Alzheimer disease: a randomised controlled trial. PLoS Med 15: e1002660, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li Y, Zhao J, and Holscher C. Therapeutic potential of baicalein in Alzheimer's disease and Parkinson's disease. CNS Drugs 31: 639–652, 2017 [DOI] [PubMed] [Google Scholar]
- 101. Liang L and Wei H. Dantrolene, a treatment for Alzheimer disease? Alzheimer Dis Assoc Disord 29: 1–5, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Long JM and Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179: 312–339, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lopez-Arrieta JM and Birks J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst Rev 2002: CD000147, 2002 [DOI] [PubMed] [Google Scholar]
- 104. Lublin A, Isoda F, Patel H, Yen K, Nguyen L, Hajje D, Schwartz M, and Mobbs C. FDA-approved drugs that protect mammalian neurons from glucose toxicity slow aging dependent on cbp and protect against proteotoxicity. PLoS One 6: e27762, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, Fagan AM, McCue L, Xiong C, Morris JC, Benzinger TLS, and Holtzman DM. Reduced non–rapid eye movement sleep is associated with tau pathology in early Alzheimer's disease. Sci Transl Med 11: eaau6550, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Luchsinger JA, Perez T, Chang H, Mehta P, Steffener J, Pradabhan G, Ichise M, Manly J, Devanand DP, and Bagiella E. Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis 51: 501–514, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Maloney B, Balaraman Y, Liu Y, Chopra N, Edenberg HJ, Kelsoe J, Nurnberger JI, and Lahiri DK. Lithium alters expression of RNAs in a type-specific manner in differentiated human neuroblastoma neuronal cultures, including specific genes involved in Alzheimer's disease. Sci Rep 9: 18261, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mandal PK, Saharan S, Tripathi M, and Murari G. Brain glutathione levels—a novel biomarker for mild cognitive impairment and Alzheimer's disease. Biol Psychiatry 78: 702–710, 2015 [DOI] [PubMed] [Google Scholar]
- 109. Mandal PK, Shukla D, Tripathi M, and Ersland L. Cognitive improvement with glutathione supplement in Alzheimer's disease: a way forward. J Alzheimers Dis 68: 531–535, 2019 [DOI] [PubMed] [Google Scholar]
- 110. Martin-Bastida A, Ward RJ, Newbould R, Piccini P, Sharp D, Kabba C, Patel MC, Spino M, Connelly J, Tricta F, Crichton RR, and Dexter DT. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson's disease. Sci Rep 7: 1398, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Martini F, Rosa SG, Klann IP, Fulco BCW, Carvalho FB, Rahmeier FL, Fernandes MC, and Nogueira CW. A multifunctional compound ebselen reverses memory impairment, apoptosis and oxidative stress in a mouse model of sporadic Alzheimer's disease. J Psychiatr Res 109: 107–117, 2019 [DOI] [PubMed] [Google Scholar]
- 112. Masaldan S, Belaidi AA, Ayton S, and Bush AI. Cellular Senescence and Iron Dyshomeostasis in Alzheimer's Disease. Pharmaceuticals (Basel) 12: 93, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mattson MP, Longo VD, and Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev 39: 46–58, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. McDonald SR, Sohal RS, and Forster MJ. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic Biol Med 38: 729–736, 2005 [DOI] [PubMed] [Google Scholar]
- 115. Mehta D, Jackson R, Paul G, Shi J, and Sabbagh M. Why do trials for Alzheimer's disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin Investig Drugs 26: 735–739, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Miranda A, Gomez-Gaete C, and Mennickent S. Role of Mediterranean diet on the prevention of Alzheimer disease [in Spanish]. Rev Med Chil 145: 501–507, 2017 [DOI] [PubMed] [Google Scholar]
- 117. Mischley LK, Lau RC, Shankland EG, Wilbur TK, and Padowski JM. Phase IIb study of intranasal glutathione in Parkinson's disease. J Parkinsons Dis 7: 289–299, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Monacelli F, Acquarone E, Giannotti C, Borghi R, and Nencioni A. Vitamin C, aging and Alzheimer's disease. Nutrients 9: 670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mori T, Koyama N, Tan J, Segawa T, Maeda M, and Town T. Combined treatment with the phenolics (-)-epigallocatechin-3-gallate and ferulic acid improves cognition and reduces Alzheimer-like pathology in mice. J Biol Chem 294: 2714–2731, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Morin A, Mouzon B, Ferguson S, Paris D, Saltiel N, Lungmus C, Mullan M, and Crawford F. Treatment with nilvadipine mitigates inflammatory pathology and improves spatial memory in aged hTau mice after repetitive mild TBI. Front Aging Neurosci 10: 292, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, and Orr ME. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17: e12840, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121a. National Institute on Aging. Basics of Alzheimer's disease and dementia. Preventing Alzheimer's disease: what do we know?. https://www.nia.nih.gov/health/preventing-alzheimers-disease-what-do-we-know (accessed January15, 2020)
- 122. Nehlig A. Effects of coffee/caffeine on brain health and disease: what should I tell my patients? Pract Neurol 16: 89–95, 2016 [DOI] [PubMed] [Google Scholar]
- 123. Neitemeier S, Jelinek A, Laino V, Hoffmann L, Eisenbach I, Eying R, Ganjam GK, Dolga AM, Oppermann S, and Culmsee C. BID links ferroptosis to mitochondrial cell death pathways. Redox biology 12: 558–570, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. This reference has been deleted
- 125. O'Brien JS and Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res 6: 537–544, 1965 [PubMed] [Google Scholar]
- 126. O'Neal-Moffitt G, Delic V, Bradshaw PC, and Olcese J. Prophylactic melatonin significantly reduces Alzheimer's neuropathology and associated cognitive deficits independent of antioxidant pathways in AβPPswe/PS1 mice. Mol Neurodegener 10: 27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Obulesu M, Venu R, and Somashekhar R. Lipid peroxidation in Alzheimer's disease: emphasis on metal-mediated neurotoxicity. Acta Neurol Scand 124: 295–301, 2011 [DOI] [PubMed] [Google Scholar]
- 128. Ohara T, Hata J, Yoshida D, Mukai N, Nagata M, Iwaki T, Kitazono T, Kanba S, Kiyohara Y, and Ninomiya T. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology 88: 1925–1932, 2017 [DOI] [PubMed] [Google Scholar]
- 129. Panagaki T, Michael M, and Holscher C. Liraglutide restores chronic ER stress, autophagy impairments and apoptotic signalling in SH-SY5Y cells. Sci Rep 7: 16158, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, Kaur C, Hardeland R, and Cardinali DP. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res 23: 267–300, 2013 [DOI] [PubMed] [Google Scholar]
- 131. Panza F, Solfrizzi V, Barulli MR, Bonfiglio C, Guerra V, Osella A, Seripa D, Sabba C, Pilotto A, and Logroscino G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging 19: 313–328, 2015 [DOI] [PubMed] [Google Scholar]
- 132. Parker WF, Gorges RJ, Gao YN, Zhang Y, Hur K, and Gibbons RD. Association between groundwater lithium and the diagnosis of bipolar disorder and dementia in the United States. JAMA Psychiatry 75: 751–754, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pena-Bautista C, Vigor C, Galano JM, Oger C, Durand T, Ferrer I, Cuevas A, Lopez-Cuevas R, Baquero M, Lopez-Nogueroles M, Vento M, Hervas D, Garcia-Blanco A, and Chafer-Pericas C. Plasma lipid peroxidation biomarkers for early and non-invasive Alzheimer Disease detection. Free Radic Biol Med 124: 388–394, 2018 [DOI] [PubMed] [Google Scholar]
- 134. Pickart L, Vasquez-Soltero JM, and Margolina A. The effect of the human peptide GHK on gene expression relevant to nervous system function and cognitive decline. Brain Sci 7: 20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pocernich CB, and Butterfield DA. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim Biophys Acta 1822: 625–630, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Prasanthi JR, Dasari B, Marwarha G, Larson T, Chen X, Geiger JD, and Ghribi O. Caffeine protects against oxidative stress and Alzheimer's disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic Biol Med 49: 1212–1220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Prasanthi JR, Schrag M, Dasari B, Marwarha G, Dickson A, Kirsch WM, and Ghribi O. Deferiprone reduces amyloid-beta and tau phosphorylation levels but not reactive oxygen species generation in hippocampus of rabbits fed a cholesterol-enriched diet. J Alzheimers Dis 30: 167–182, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Quinn JF, Harris CJ, Cobb KE, Domes C, Ralle M, Brewer G, and Wadsworth TL. A copper-lowering strategy attenuates amyloid pathology in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis 21: 903–914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rainey-Smith SR, Brown BM, Sohrabi HR, Shah T, Goozee KG, Gupta VB, and Martins RN. Curcumin and cognition: a randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br J Nutr 115: 2106–2113, 2016 [DOI] [PubMed] [Google Scholar]
- 140. Rajasekhar K, Madhu C, and Govindaraju T. Natural tripeptide-based inhibitor of multifaceted amyloid β toxicity. ACS Chem Neurosci 7: 1300–1310, 2016 [DOI] [PubMed] [Google Scholar]
- 141. Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Tonk S, Kuruva CS, Bhatti JS, Kandimalla R, Vijayan M, Kumar S, Wang R, Pradeepkiran JA, Ogunmokun G, Thamarai K, Quesada K, Boles A, and Reddy AP. Protective effects of Indian spice Curcumin against amyloid-beta in Alzheimer's disease. J Alzheimers Dis 61: 843–866, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Remington R, Bechtel C, Larsen D, Samar A, Doshanjh L, Fishman P, Luo Y, Smyers K, Page R, Morrell C, and Shea TB. A phase II randomized clinical trial of a nutritional formulation for cognition and mood in Alzheimer's disease. J Alzheimers Dis 45: 395–405, 2015 [DOI] [PubMed] [Google Scholar]
- 143. Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, and Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 60: 1685–1691, 2003 [DOI] [PubMed] [Google Scholar]
- 144. Rivabene R, Visentin S, Piscopo P, De Nuccio C, Crestini A, Svetoni F, Rosa P, and Confaloni A. Thapsigargin affects presenilin-2 but not presenilin-1 regulation in SK-N-BE cells. Exp Biol Med (Maywood) 239: 213–224, 2014 [DOI] [PubMed] [Google Scholar]
- 145. Rommer PS, Fuchs D, Leblhuber F, Schroth R, Greilberger M, Tafeit E, and Greilberger J. Lowered levels of carbonyl proteins after vitamin B supplementation in patients with mild cognitive impairment and Alzheimer's disease. Neurodegener Dis 16: 284–289, 2016 [DOI] [PubMed] [Google Scholar]
- 146. Sabayan B, Farshchi S, Zamiri N, and Sabayan B. Can tetrathiomolybdate be a potential agent against Alzheimer disease? A hypothesis based on abnormal copper homeostasis in brain. Alzheimer Dis Assoc Disord 24: 309–310, 2010 [DOI] [PubMed] [Google Scholar]
- 146a. Sagara Y, Dargusch R, Chambers D, Davis J, Schubert D, and Maher P. Cellular mechanisms of resistance to chronic oxidative stress. Free Radic Biol Med 24: 1375–1389, 1998 [DOI] [PubMed] [Google Scholar]
- 147. Sampson EL, Jenagaratnam L, and McShane R. Metal protein attenuating compounds for the treatment of Alzheimer's dementia. Cochrane Database Syst Rev 2014: CD005380, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Savaskan E, Mueller H, Hoerr R, von Gunten A, and Gauthier S. Treatment effects of Ginkgo biloba extract EGb 761(R) on the spectrum of behavioral and psychological symptoms of dementia: meta-analysis of randomized controlled trials. Int Psychogeriatr 30: 285–293, 2018 [DOI] [PubMed] [Google Scholar]
- 149. Scarmeas N, Stern Y, Tang MX, Mayeux R, and Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol 59: 912–921, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Schrag M, Mueller C, Zabel M, Crofton A, Kirsch WM, Ghribi O, Squitti R, and Perry G. Oxidative stress in blood in Alzheimer's disease and mild cognitive impairment: a meta-analysis. Neurobiol Dis 59: 100–110, 2013 [DOI] [PubMed] [Google Scholar]
- 150a. Schubert D, Kimura H, and Maher P. Growth factors and vitamin E modify neuronal glutamate toxicity. Proc Natl Acad Sci USA 89: 8264–8267, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Bornkamm GW, Schweizer U, and Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab 8: 237–248, 2008 [DOI] [PubMed] [Google Scholar]
- 152. Sfera A, Bullock K, Price A, Inderias L, and Osorio C. Ferrosenescence: the iron age of neurodegeneration? Mech Ageing Dev 174: 63–75, 2018 [DOI] [PubMed] [Google Scholar]
- 153. Sha SJ, Miller ZA, Min S-w, Zhou Y, Brown J, Mitic LL, Karydas A, Koestler M, Tsai R, Corbetta-Rastelli C, Lin S, Hare E, Fields S, Fleischmann KE, Powers R, Fitch R, Martens LH, Shamloo M, Fagan AM, Farese RV Jr., Pearlman R, Seeley W, Miller BL, Gan L, and Boxer AL.. An 8-week, open-label, dose-finding study of nimodipine for the treatment of progranulin insufficiency from GRN gene mutations. Alzheimers Dement (N Y) 3: 507–512, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Shetty RA, Forster MJ, and Sumien N. Coenzyme Q(10) supplementation reverses age-related impairments in spatial learning and lowers protein oxidation. Age (Dordr) 35: 1821–1834, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Shetty RA, Ikonne US, Forster MJ, and Sumien N. Coenzyme Q10 and alpha-tocopherol reversed age-associated functional impairments in mice. Exp Gerontol 58: 208–218, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Shin BK, Kang S, Kim DS, and Park S. Intermittent fasting protects against the deterioration of cognitive function, energy metabolism and dyslipidemia in Alzheimer's disease-induced estrogen deficient rats. Exp Biol Med (Maywood) 243: 334–343, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Shukla M, Govitrapong P, Boontem P, Reiter RJ, and Satayavivad J. Mechanisms of melatonin in alleviating Alzheimer's disease. Curr Neuropharmacol 15: 1010–1031, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Siddique YH and Ali F. Protective effect of nordihydroguaiaretic acid (NDGA) on the transgenic Drosophila model of Alzheimer's disease. Chem Biol Interact 269: 59–66, 2017 [DOI] [PubMed] [Google Scholar]
- 159. Silva BC, de Miranda AS, Rodrigues FG, Silveira AL, Resende GH, Moraes MF, de Oliveira AC, Parreiras PM, Barcelos Lda S, Teixeira MM, Machado FS, Teixeira AL, and Rachid MA. The 5-lipoxygenase (5-LOX) inhibitor zileuton reduces inflammation and infarct size with improvement in neurological outcome following cerebral ischemia. Curr Neurovasc Res 12: 398–403, 2015 [DOI] [PubMed] [Google Scholar]
- 160. Singh AP, Singh R, Verma SS, Rai V, Kaschula CH, Maiti P, and Gupta SC. Health benefits of resveratrol: evidence from clinical studies. Med Res Rev 39: 1851–1891, 2019 [DOI] [PubMed] [Google Scholar]
- 161. Singh SK, Srivastav S, Castellani RJ, Plascencia-Villa G, and Perry G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 16: 666–674, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Smith MA, Harris PL, Sayre LM, and Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A 94: 9866–9868, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Southon A, Szostak K, Acevedo KM, Dent KA, Volitakis I, Belaidi AA, Barnham KJ, Crouch PJ, Ayton S, Donnelly PS, and Bush AI. CuII (atsm) inhibits ferroptosis: implications for treatment of neurodegenerative disease. Br J Pharmacol 177: 656–667, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Squitti R, Rossini PM, Cassetta E, Moffa F, Pasqualetti P, Cortesi M, Colloca A, Rossi L, and Finazzi-Agro A. d-Penicillamine reduces serum oxidative stress in Alzheimer's disease patients. Eur J Clin Invest 32: 51–59, 2002 [DOI] [PubMed] [Google Scholar]
- 165. Stuerenburg HJ, Ganzer S, and Muller-Thomsen T. Plasma beta carotene in Alzheimer's disease. Association with cerebrospinal fluid beta-amyloid 1–40, (Abeta40), beta-amyloid 1–42 (Abeta42) and total Tau. Neuro Endocrinol Lett 26: 696–698, 2005 [PubMed] [Google Scholar]
- 166. Sultana R, Perluigi M, and Allan Butterfield D. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med 62: 157–169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166a. Tan S, Schubert D, and Maher P. Oxytosis: A novel form of programmed cell death. Curr Top Med Chem 1: 497–506, 2001 [DOI] [PubMed] [Google Scholar]
- 167. Tang M and Taghibiglou C. The mechanisms of action of Curcumin in Alzheimer's disease. J Alzheimers Dis 58: 1003–1016, 2017 [DOI] [PubMed] [Google Scholar]
- 168. Tong BC, Wu AJ, Li M, and Cheung KH. Calcium signaling in Alzheimer's disease & therapies. Biochim Biophys Acta Mol Cell Res 1865: 1745–1760, 2018 [DOI] [PubMed] [Google Scholar]
- 169. Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R, and Aisen PS. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 85: 1383–1391, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Van Cauwenberghe C, Vandendriessche C, Libert C, and Vandenbroucke RE. Caloric restriction: beneficial effects on brain aging and Alzheimer's disease. Mamm Genome 27: 300–319, 2016 [DOI] [PubMed] [Google Scholar]
- 171. Vandesquille M, Krazem A, Louis C, Lestage P, and Beracochea D. S 18986 reverses spatial working memory impairments in aged mice: comparison with memantine. Psychopharmacology (Berl) 215: 709–720, 2011 [DOI] [PubMed] [Google Scholar]
- 172. Vaz FNC, Fermino BL, Haskel MVL, Wouk J, de Freitas GBL, Fabbri R, Montagna E, Rocha JBT, and Bonini JS. The relationship between copper, iron, and selenium levels and Alzheimer disease. Biol Trace Elem Res 181: 185–191, 2018 [DOI] [PubMed] [Google Scholar]
- 173. Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, Cuzzoni G, Venturini L, Ricevuti G, and Bongiorno AI. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer's disease. Int J Immunopathol Pharmacol 25: 345–353, 2012 [DOI] [PubMed] [Google Scholar]
- 174. Villemagne VL, Rowe CC, Barnham KJ, Cherny R, Woodward M, Bozinosvski S, Salvado O, Bourgeat P, Perez K, Fowler C, Rembach A, Maruff P, Ritchie C, Tanzi R, and Masters CL. A randomized, exploratory molecular imaging study targeting amyloid β with a novel 8-OH quinoline in Alzheimer's disease: the PBT2–204 IMAGINE study. Alzheimers Dement (N Y) 3: 622–635, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wang CY, Xie JW, Xu Y, Wang T, Cai JH, Wang X, Zhao BL, An L, and Wang ZY. Trientine reduces BACE1 activity and mitigates amyloidosis via the AGE/RAGE/NF-kappaB pathway in a transgenic mouse model of Alzheimer's disease. Antioxid Redox Signal 19: 2024–2039, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wang L, Fan H, He J, Wang L, Tian Z, and Wang C. Protective effects of omega-3 fatty acids against Alzheimer's disease in rat brain endothelial cells. Brain Behav 8: e01037, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wang X, Wang W, Li L, Perry G, Lee HG, and Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim Biophys Acta 1842: 1240–1247, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wang YY, Zheng W, Ng CH, Ungvari GS, Wei W, and Xiang YT. Meta-analysis of randomized, double-blind, placebo-controlled trials of melatonin in Alzheimer's disease. Int J Geriatr Psychiatry 32: 50–57, 2017 [DOI] [PubMed] [Google Scholar]
- 179. Wang Z, Zhang YH, Zhang W, Gao HL, Zhong ML, Huang TT, Guo RF, Liu NN, Li DD, Li Y, Wang ZY, and Zhao P. Copper chelators promote nonamyloidogenic processing of AbetaPP via MT1/2/CREB-dependent signaling pathways in AbetaPP/PS1 transgenic mice. J Pineal Res 65: e12502, 2018 [DOI] [PubMed] [Google Scholar]
- 180. Wang ZJ, Zhao F, Wang CF, Zhang XM, Xiao Y, Zhou F, Wu MN, Zhang J, Qi JS, and Yang W. Xestospongin C, a reversible IP3 receptor antagonist, alleviates the cognitive and pathological impairments in APP/PS1 mice of Alzheimer's disease. J Alzheimers Dis 72: 1217–1231, 2019 [DOI] [PubMed] [Google Scholar]
- 181. Wei D, Tang J, Bai W, Wang Y, and Zhang Z. Ameliorative effects of baicalein on an amyloid-beta induced Alzheimer's disease rat model: a proteomics study. Curr Alzheimer Res 11: 869–881, 2014 [PubMed] [Google Scholar]
- 182. Wicinski M, Wodkiewicz E, Slupski M, Walczak M, Socha M, Malinowski B, and Pawlak-Osinska K. Neuroprotective activity of sitagliptin via reduction of neuroinflammation beyond the incretin effect: focus on Alzheimer's disease. Biomed Res Int 2018: 6091014, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wu Z, Yang B, Liu C, Liang G, Eckenhoff MF, Liu W, Pickup S, Meng Q, Tian Y, Li S, and Wei H. Long-term dantrolene treatment reduced intraneuronal amyloid in aged Alzheimer triple transgenic mice. Alzheimer Dis Assoc Disord 29: 184–191, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, and Tang D. Ferroptosis: process and function. Cell Death Differ 23: 369–379, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Xie Y, Tan Y, Zheng Y, Du X, and Liu Q. Ebselen ameliorates beta-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer's disease mice. J Biol Inorg Chem 22: 851–865, 2017 [DOI] [PubMed] [Google Scholar]
- 186. Yang G, Wang Y, Sun J, Zhang K, and Liu J. Ginkgo biloba for mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Curr Top Med Chem 16: 520–528, 2016 [DOI] [PubMed] [Google Scholar]
- 187. Yoo MH, Gu X, Xu XM, Kim JY, Carlson BA, Patterson AD, Cai H, Gladyshev VN, and Hatfield DL. Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer's disease. Antioxid Redox Signal 12: 819–827, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zhang DM, Ye JX, Mu JS, and Cui XP. Efficacy of vitamin B supplementation on cognition in elderly patients with cognitive-related diseases. J Geriatr Psychiatry Neurol 30: 50–59, 2017 [DOI] [PubMed] [Google Scholar]
- 189. Zhang H, Sun S, Wu L, Pchitskaya E, Zakharova O, Fon Tacer K, and Bezprozvanny I. Store-operated calcium channel complex in postsynaptic spines: a new therapeutic target for Alzheimer's disease treatment. J Neurosci 36: 11837–11850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, and Mattson MP. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci 22: 719–728, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zhang Y and He ML. Deferoxamine enhances alternative activation of microglia and inhibits amyloid beta deposits in APP/PS1 mice. Brain Res 1677: 86–92, 2017 [DOI] [PubMed] [Google Scholar]
- 192. Zhao X, Zeng Z, Gaur U, Fang J, Peng T, Li S, and Zheng W.. Metformin protects PC12 cells and hippocampal neurons from H2O2-induced oxidative damage through activation of AMPK pathway. J Cell Physiol 2019. [Epub ahead of print]; DOI: 10.1002/jcp.28337 [DOI] [PubMed] [Google Scholar]